Abstract
Objective
To provide a comprehensive quantitative review of biological, environmental, and behavioral correlates across domains of cognitive function in sickle cell disease (SCD).
Methods
Forty-seven studies were identified in PubMed, MedLine, and PsycINFO involving 2573 participants with SCD.
Results
Meta-analytic findings across all identified samples indicate that hemoglobin and hematocrit were positively correlated with Full Scale IQ [FSIQ; r = .15, 95% confidence interval (CI) = .10 to .21], language and verbal reasoning (r = .18, 95% CI = .11 to .24), and executive function (r = .10, 95% CI = .01 to .19) with small effects and significant heterogeneity. Transcranial Doppler velocity was negatively associated with visual spatial and perceptual reasoning (r = −.18, 95% CI = −.31 to −.05). Socioeconomic status was positively associated with FSIQ (r = .23, 95% CI = .17 to .28), language and verbal reasoning (r = .28, 95% CI = .09 to .45), visual spatial and perceptual reasoning (r = .26, 95% CI = .09 to .41), and executive function (r = .18, 95% CI = .07 to .28) with small to medium effects. Finally, total behavioral problems were negatively associated with FSIQ (r = −.12, 95% CI = −.21 to −.02) such that participants with lower FSIQ exhibited greater behavioral and emotional problems.
Conclusions
Findings provide evidence for biological, environmental, and psychosocial corelates across multiple domains of cognitive function in SCD. More research on more specific cognitive domains and psychosocial correlates is needed in addition to assessments of interactional models among risk factors.
Keywords: behavioral problems, cognitive function, emotional problems, hematocrit, hemoglobin, sickle cell disease, socioeconomic status, TCD velocity
Introduction
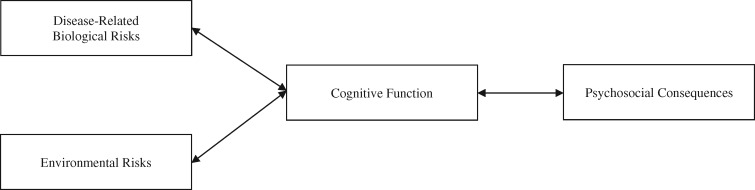
Deficits in cognitive function are clinically significant sequalae of sickle cell disease (SCD; DeBaun, 2014). Although there have been several narrative and quantitative reviews of cognitive function in this population (e.g., Berkelhammer et al., 2007; Prussien, Jordan, DeBaun, & Compas, 2019; Schatz, Finke, Kellett, & Kramer, 2002), there are no systematic reviews of correlates of cognitive deficits in SCD. The heuristic model that guides the current review poses that correlates of cognitive function can be conceptualized as risks or consequences (see Figure 1). Potential risks to cognitive function include biological and environmental characteristics, and psychosocial consequences of cognitive deficits include behavioral and emotional functioning.
Figure 1.
Heuristic model of biological, demographic, and environmental risks of cognitive function and psychosocial consequences in SCD. Note. SCD = sickle cell disease.
In a recent comprehensive meta-analysis of cognitive function in SCD, Prussien et al. (2019) found significant deficits in several domains of cognitive function across the lifespan. This quantitative review also showed that individuals with the most severe phenotypes of the disease [i.e., sickle cell anemia (SCA)] that have a history of silent cerebral infarcts (SCIs) showed large deficits across each domain of cognitive function (Hedges’ g = −.63 to −1.03), and those with a history of overt stroke had significantly larger deficits than the sample with a history of SCI (g = −1.28 to −1.82). Although the degree of cognitive deficits in samples without a history of infarcts were lower than those with SCI or stroke, samples without a history of infarcts showed medium to large deficits relative to the normative mean (g = −.55 to −.74) and relative to healthy or sibling controls (g = −.48 to −.76), suggesting that chronic disease-related biological characteristics may also contribute to cognitive impairment in patients without an acute injury to the brain. For example, chronic anemia is significantly associated with lower Verbal IQ, Performance IQ, and Full Scale IQ (FSIQ; Bernaudin, Verlhac, & Freard, 2000; Hijmans et al., 2011; Steen et al., 2003). Steen et al. (2003) found that the degree of chronic anemia explained 23% of the variance in predicting FSIQ in children with SCD without a history of stroke, and there is no data to suggest that anemia does not continue to affect patients with a history of infarct or stoke. Researchers have hypothesized that the pathway through which chronic anemia affects cognitive functioning may be through prolonged reduced oxygenation of the brain (Steen et al., 2003). Hemoglobin, hematocrit, and cerebral blood flow (CBF) velocity, typically measured using transcranial Doppler ultrasound (TCD), are all indices of oxygenation in the blood and brain. One narrative review has examined the association between TCD velocity and cognition in SCD, and it showed mixed findings (Bakker et al., 2014). However, no meta-analysis has quantitatively assessed the association between TCD velocity and hematological characteristics with cognitive function in this population.
In addition to biological risks, environmental factors are also significantly associated with IQ and executive function in SCD. Children with SCD often grow up in low socioeconomic status (SES) homes, and factors such as parent education, household income, and home environment are related to cognitive function in this population (King et al., 2014; Tarazi, Grant, Ely, & Barakat, 2007). Specifically, King et al. (2014) found that children living with a parent who had some college education scored 6.2 IQ points higher than children living with a parent who had no college education. This finding remained significant even when controlling for history of SCI. Results for household income showed that each additional $1,000 per capita income was associated with a 0.33-point increase in children’s FSIQ (King et al., 2014). Yarboi et al. (2017) found that increased maternal financial stress was the strongest predictor of deficits across all domains of cognitive functioning and academic achievement (i.e., Verbal IQ, Performance IQ, FSIQ, academic achievement, and measures of executive function). Further, Bills, Schatz, Hardy, and Reinman (2020) found that parent and family functioning predicted language scores in children with SCD even after controlling for SES. Based on the evidence for the association of SES with cognitive function in this population, understanding the influence of environmental factors on multiple domains of cognitive function in SCD is essential; yet, no meta-analysis to date has quantitatively assessed the association between these environmental risks and cognition in this population.
Deficits in cognitive function have far reaching effects, including impairment in emotional and behavioral functioning. Studies within clinical child psychology have shown that deficits in cognitive function are related to internalizing and externalizing symptoms (Moran, 2016; Schoemaker, Mulder, Dekovic, & Matthys, 2013; Snyder, 2013). Children and adolescents with SCD may be at risk for increased internalizing symptoms relative to healthy peers (Benton, Boyd, Ifeagwu, Feldtmose, & Smith-Whitley, 2011; Jonassaint, Jones, Leong, & Frierson, 2016; Trzepacz, Vannatta, Gerhardt, Ramey, & Noll, 2004), and deficits in cognitive function may contribute to increased symptoms. Several studies have focused on the relation between executive function and emotional problems (e.g., Allen, Anderson, Rothman, & Bonner, 2017; Hardy, Bills, Wise, & Hardy, 2018). While causal links and directionality have yet to be determined in longitudinal studies, cross-sectional analyses in pediatric populations show that the association between cognition and emotional functioning may be through the use of fewer adaptive coping mechanisms (e.g., Campbell et al., 2008; Hocking et al., 2011). Prussien et al. (2018) found that in addition to a direct association between working memory deficits and depressive symptoms, deficits in verbal reasoning had an indirect effect on depression through the use of secondary control coping strategies (i.e., acceptance, cognitive reappraisal, positive thinking, and distraction) to adapt to stress. However, there has not yet been a quantitative review of the relation between cognitive function and emotional, behavioral, and emotional problems in SCD.
The current study is the first quantitative meta-analysis to examine correlates of cognitive function in individuals with SCD, and it expands upon findings of cognitive function and SCD across the lifespan (e.g., Prussien et al., 2019) by analyzing correlates, including potential risks for and consequences of cognitive deficits in this population. A comprehensive meta-analysis can help to provide a broad quantitative summary of the significance, magnitude, and direction of cognitive correlates reported in the literature in SCD, and findings can be used to inform future research and clinical intervention. This meta-analysis focuses on biological risks related to hematological (i.e., hemoglobin and hematocrit) and cerebral hemodynamic (i.e., TCD velocity) characteristics in order to examine the hypothesis that chronic deoxygenation of the brain is a significant pathway through which SCD affects cognitive function in this population. Infarct status and age were primary correlates investigated in the Prussien et al. (2019); therefore, they will not be assessed in the current review. Further, potential environmental risks are also assessed in order to determine additional influences on cognitive function in individuals with SCD. Finally, in order to determine potential psychosocial consequences of cognitive deficits, behavioral and emotional correlates are assessed. Thus, the primary aims of this review are to (a) assess biological, environmental, and psychosocial correlates of cognitive function in SCD; (b) examine correlates across multiple domains of cognitive function; and (c) identify gaps in the literature on risks and consequences of cognitive deficits in SCD.
Methods
Literature Search
PubMed, PsycINFO, and MedLine were searched for empirical studies reported prior to January 2019 to identify articles that examined cognitive function in children, adolescents, and adults with SCD using standardized assessments. Identical search terms and strategy used in Prussien et al. (2019) were utilized (see prior meta-analysis for more detail).
Inclusion Criteria for Meta-Analysis
Studies were included if they (a) assessed children, adolescents, or adults of all ages diagnosed with any SCD phenotype, (b) used a valid and reliable performance-based assessment of cognitive function, and (c) assessed and reported biological, demographic, environmental, or psychosocial correlates of cognitive function.
Exclusion Criteria for Meta-Analysis
Studies were excluded if they (a) only reported questionnaire-based assessments of cognitive function, (b) reported statistics that are incompatible with the meta-analytic aims, or (c) involved experimental manipulation of cognitive function (e.g., intervention studies). However, if pre-intervention correlates of the cognitive function were reported, these were included in the meta-analysis.
Data Coding Procedure
The following information was extracted from each study where available: (a) sample demographic variables (i.e., mean age, percentage of sample that were female, mean parent education, mean parent/household income); (b) sample disease characteristics (i.e., percentage of sample with SCA, percentage of sample with history of SCI, and percentage of sample with a history of overt stroke); (c) domains and measures of cognitive function; (d) correlates and measures used; (e) sample size; and (f) summary statistics for the calculation of effect sizes. Cognitive function scores were categorized into one of five domains: (a) FSIQ, defined as “the aggregate or global capacity of the individual to act purposefully, to think rationally, and to deal effectively with his or her environment” (Wechsler, 1939); (b) language and verbal reasoning, defined as “the ability to access and apply acquired word knowledge. The application of this knowledge involves verbal concept formation, reasoning, and expression” (Wechsler, 2014); (c) visual spatial and perceptual reasoning, defined as “the ability to evaluate visual details and to understand visual spatial relationships to construct geometric designs from a model … and to detect the underlying conceptual relationship among visual objects and to use reasoning to identify and apply rules” (Wechsler, 2014); (d) executive function defined as “inhibiting dominant responses, updating working memory representations, and shifting between task sets” (Friedman et al., 2008); and (e) Processing speed, defined as “the speed and accuracy of visual identification, decision making, and decision implementation” (Wechsler, 2014).
Cognitive tasks were coded into each domain based on how they aligned with the definition. Although more specific domains of cognition are often examined by neuropsychologists, the current review aimed to assess a consistent set of domains of cognition across correlates in order to maintain structure and quantitatively compare effect sizes. Due to the limited number of studies in this population, and the format of the reported data in the included studies, which predominately used Wechsler scales (75.5%), these broader domains of analyses were chosen (see Supplementary Table 2 for complete list of measures of cognitive function within categories).
Correlates of cognitive function were categorized as (a) biological, (b) demographic, (c) environmental, or (d) psychosocial. Biological correlates included hemoglobin and hematocrit, fetal hemoglobin, oxygen saturation, and TCD velocity measured at or near the time of the cognitive assessment. Correlations of hemoglobin level and hematocrit percentage with domains of cognitive function were coded into the same category, as laboratory complete blood cell count measures of hematocrit is indirect and approximated using the direct measurement of hemoglobin. Demographic correlates included participant gender. Participant age was not assessed in this review, as it was a primary correlate investigated in the prior meta-analysis on cognitive function in SCD across the lifespan (Prussien et al., 2019). Environmental correlates included SES composite (i.e., Hollingshead index, or a composite of household income and parent education); household income; parent education; home environment, measured using the Home Observation for Measurement of the Environment (Caldwell & Bradley, 2003); and parent stress, measured using the Pediatric Inventory for Parents (Streisand, Braniecki, Tercyak, & Kazak, 2001) or the Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983).
Finally, psychosocial correlates were extracted within two domains: (a) emotional and behavioral problems and (b) academic achievement. Emotional and behavioral problems included total problems, internalizing problems, and externalizing problems. Total problems included both the Child Behavior Checklist or Youth Self Report (Achenbach and Rescorla, 2001) Total Problems scale and a reversed effect direction of the PedsQL (Bastiaansen, Koot, Bongers, Varni, & Verhulst, 2004). Academic achievement included total academic skills, reading, writing/spelling, and math. With regard to tests on Woodcock–Johnson, only those on the Tests of Achievement were coded into academic achievement domains. Assessments on the Woodcock–Johnson Tests of Cognition were coded into domains of cognitive function (described above).
All data were double-extracted by two of the study authors. Rater agreement across the first 15 studies was 94%, and any disagreements on the remaining studies were discussed and corrected during weekly consensus meetings. Analyses were conducted using random effects models, as the studies varied in methodology and design, using study as the unit of analysis and the mean of the selected outcomes. When studies selectively reported only significant associations (e.g., “All other correlations were nonsignificant”), the corresponding authors were contacted for information, and missing effect sizes were conservatively coded as r = .00. Although this conservative approach may bias findings in the nonsignificant direction, excluding these nonsignificant effect sizes would also generate biased the meta-analytic findings, potentially overestimating the mean effect size for the association between cognitive function and demographic, biological, and environmental factors. We utilized Cohen’s (1988) guidelines to interpret the magnitude of the effect size for significant correlations (i.e., r = .10 as small, .30 as medium, and above .50 as large).
Computation of Effect Sizes
In analyses, we used correlations or mean differences, where reported. In line with other recent meta-analyses (e.g., Compas et al., 2017; Robles, Slatcher, Trombello, & McGinn, 2014), if the only statistic reported was from a multivariate analysis (standardized β), we converted the β to r using the formula (r = β + .05λ), where λ equals 0 when the β is negative and λ equals 1 when the β is nonnegative as recommended by Peterson and Brown (2005).
All analyses were conducted with the Comprehensive Meta-Analysis Program version 3 (Borenstein, Hedges, Higgins, & Rothstein, 2014), using random effects models. The mean effect size for each study (r) was used as the level of analysis; therefore, if a single study reported correlations with the same correlate across different measures of a domain of cognitive function, the within-study mean effect size was calculated. When authors published different studies using the same sample or a smaller subset of the same sample, a mean effect size was used in analysis. For example, mean effect sizes within each domain of cognitive function were used for all studies reporting effects from the large multisite Cooperative Study of Sickle Cell Disease (k = 5, k’ = 1). Effect sizes in meta-analyses based on very small number of studies are subject to problems in data synthesis (Davey, Turner, Clarke, & Higgins, 2011); therefore, a minimum of five studies (k’ = 5) was set to estimate effect sizes. Due to the large impact of overt stroke on cognition, separate effect sizes were computed across studies that explicitly excluded patients with overt stroke when the minimum number of studies was met.
Weighted mean effect sizes (r), 95% confidence intervals (CIs), and estimated heterogeneity statistics (Q) were calculated for each cognitive function domain using the procedures outlined by Hedges and Olkin (1985). The 95% CIs on the effect sizes represent the range in which the mean effect size falls in 95% of cases; calculation for CIs (lower and upper limit) is as follows:
where M* is the mean effect size in the sample, Z is the critical z-value representing the confidence level, and VM* is the variance of M*. A mean effect is considered significant when the CI does not include zero. The number of studies (k), total sample size (N), mean effect size (r) and significance value of the effect, 95% CI for the mean effect size, and Q statistic and its significance value are reported for analyses. The Q statistic is the most widely used estimate of heterogeneity, and it provides information on the presence or absence of heterogeneity. Using data computed in Comprehensive Meta-Analysis Program, forest plots for each effect size were generated in R studio using the “rmeta” and “forestplot” packages.
Post hoc exploratory categorical moderation analyses were conducted to determine the effect of SCD phenotype on the mean effect size for the association between hemoglobin and hematocrit with domains of cognitive function across all samples. Moderation effects for SCD versus SCA group status were analyzed in mixed effects models when enough data was available. A significant mixed effects total between groups factor (Qb) indicates that effect size of a particular domain differs significantly between groups.
Study Quality Assessment
Criteria from the National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (National Heart, Lung, and Blood Institute, 2014) were adapted for the current review, excluding items that were irrelevant to or inconsistent with the aims and inclusion/exclusion criteria. Studies were assigned one point per criterion met, which were summed for a total quality score of 0 to 7 (0 indicating lowest quality and 7 highest quality).
Publication Bias
Several steps were taken to investigate possible publication bias. In addition to including unpublished dissertations, we examined funnel plots for each effect and calculated Egger’s tests, which is a statistical test used to detect funnel plot asymmetry (Egger, Smith, Schneider, & Minder, 1997); however, it is important to note that the Egger’s test may have lower power when used for effects with fewer than 10 studies (Higgins & Green, 2011). Second, we conducted trim and fill analyses (Duval & Tweedie, 2000) to determine how many studies would need to be included above or below the meta-analytic mean to make the funnel plot symmetrical. A higher number of studies denotes greater publication bias. Trim and fill analyses also impute the missing studies and calculate adjusted meta-analytic effect sizes that account for publication bias.
Results
Description of Included Studies
Based on the inclusion criteria, a total of 62 studies were deemed eligible for inclusion; however, 15 studies were further excluded from quantitative analysis. Although these studies met the inclusion criteria and assessed either biological, environmental, or psychosocial correlates of cognitive function, we were unable to conduct a meta-analysis on the primary correlate(s) reported within these studies due to k’ < 5 for correlate(s) (e.g., fetal hemoglobin, oxygen saturation, gender, home environment, parent stress, total academic skills, reading, spelling, math, externalizing problems, and internalizing problems) or the cognitive domain across correlates (i.e., processing speed; see Supplementary Figure 1 for number of studies assessing each correlate across cognitive domains). Forty-seven studies (37 independent samples) with 2,573 participants with SCD were included (see Supplementary File for PRISMA diagram).
Studies included in the quantitative analyses had a mean sample age of 11.1 years. Five samples assessed preschool-aged children, 28 assessed school-aged children and adolescents, and four assessed adults. Mean sample age within studies ranged from 8.8 months to 36.5 years, and the mean proportion of female participants across samples was 52.14%. Seventeen of the 37 independent samples included 100% SCA (HbSS and HbSβ0 Thalassemia), and all other samples included a variety of SCD phenotypes including, but not limited to, SCA. Of samples that reported these disease-related characteristics, 15.2% of participants (286 of 1,759 total participants) had a history of a SCI, and 5.6% of participants (111 of 1,981 total participants) had a history of an overt stroke. See Supplementary Table 1 for complete study descriptions and sample characteristics.
Biological Risks for Cognitive Deficits
Twenty-seven samples (35 studies) assessed cognitive correlates of hematological characteristic in SCD. Meta-analytic findings showed that hemoglobin and hematocrit were significantly correlated with FSIQ (r = .15, 95% CI = .10 to .21), language and verbal reasoning (r = .18, 95% CI = .11 to .24), and executive function (r = .10, 95% CI = .01 to .19), with small to medium effects and significant heterogeneity, as shown by the significant Qw statistic; however, hemoglobin and hematocrit were not related to visual spatial and perceptual reasoning (r = .06, 95% CI = −.01 to .12; see Table I). A similar pattern of significant correlations were found across samples that excluded patients with overt stroke, also shown in Table I. Exploratory categorical moderation analyses across all samples showed that hemoglobin/hematocrit were only significantly correlated with FSIQ and executive function in heterogeneous samples of SCD phenotypes, and they were not related to these cognitive domains in homogenous SCA samples. This difference in correlations across SCD samples was only significant for FSIQ (see Table II).
Table I.
Cross-Sectional Effect Sizes for Correlates of Cognitive Function in SCD
| FSIQ |
Language and Verbal Reasoning |
Visual Spatial and Perceptual Reasoning |
Executive Function |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlates | k' | n | r [95% CI] | Q w | k' | n | r [95% CI] | Q w | k' | n | r [95% CI] | Q w | k' | n | r [95% CI] | Q w | |
| All samples | |||||||||||||||||
| Biological | |||||||||||||||||
| Hgb/Hct | 17 | 1181 | .15 [.10, .21] | 47.24 | 12 | 787 | .18 [.11, .24] | 30.51 | 11 | 715 | .06 [−.01, .12] | 16.48 | 19 | 1147 | .10 [.01, .19] | 37.44 | |
| TCD velocity | 7 | 589 | .04 [−.12, .04] | 8.10 | 8 | 470 | −.14 [−.30, .03] | 26.92 | 6 | 419 | −.18 [−.31, −.05] | 10.33 | 7 | 486 | −.02 [−.09, .05] | 4.66 | |
| Environmental | |||||||||||||||||
| SES | 13 | 737 | .23 [.17, .28] | 14.84 | 7 | 359 | .28 [.09, .45] | 29.22 | 5 | 292 | .26 [.09, .41] | 11.25 | 5 | 300 | .18 [.07, .28] | 5.48 | |
| Psychosocial | |||||||||||||||||
| Total problems | 6 | 563 | −.12 [−.21, −.02] | 8.35 | |||||||||||||
| Samples excluding patients with overt stroke | |||||||||||||||||
| Biological | |||||||||||||||||
| Hgb/Hct | 12 | 722 | .23 [.10, .35] | 34.35 | 10 | 570 | .19 [.04, .33] | 28.85 | 10 | 582 | .06 [−.04, .16] | 16.03 | 14 | 786 | .14 [.03, .25] | 28.25 | |
| TCD velocity | 5 | 240 | .09 [−.07, .24] | 7.23 | 7 | 314 | −.14 [−.35, .09] | 23.43 | 5 | 263 | −.20 [−.37, −.03] | 9.34 | |||||
| Environmental | |||||||||||||||||
| SES | 10 | 462 | .28 [.21, .35] | 7.89 | 5 | 268 | .28 [.06, .47] | 17.53 | 5 | 300 | .18 [.07, .28] | 5.48 | |||||
| Psychosocial | |||||||||||||||||
| Total Problems | |||||||||||||||||
Note. FSIQ = full scale IQ; Hgb/Hct = hemoglobin/hematocrit; TCD = transcranial Doppler ultrasound; SES = socioeconomic status; k’ = number of independent samples; n = number of children, adolescents, or adults; r = aggregated correlation coefficient; CI = confidence interval of correlation coefficient; Qw = Q statistic for total within heterogeneity.
Bolded effect sizes represent significant effects.
Table II.
Categorical Moderator Analyses of Cross-Sectional Effect Sizes for the Association of Hemoglobin & Hematocrit with Cognitive Function in SCD in All Samples
| FSIQ |
Language and Verbal Reasoning |
Executive Function |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Correlates | Qb | k' | r [95% CI] | Qb | k' | r [95% CI] | Qb | k' | r [95% CI] |
| Hgb/Hct | 19.92 | 1.91 | 5.64 | ||||||
| SCA | 6 | .04 [−.11, .20] | 3 | .11 [−.23, .43] | 8 | −.00 [−.09, .09] | |||
| SCD | 10 | .19 [.12, .25] | 7 | .13 [−.01, .27] | 9 | .15 [.05, .25] | |||
Note. FSIQ = full scale IQ; Qb = Q statistic for total between heterogeneity; k’ = number of independent samples; r = aggregated correlation coefficient; CI = confidence interval of correlation coefficient.
Bolded effect sizes represent significant effects.
Thirteen samples (17 studies) assessed cognitive correlates of TCD velocity. TCD velocity had a significant negative association with visual spatial and perceptual reasoning (r = −.18, 95% CI = −.31 to −.05), such that higher velocity was related to lower scores. TCD velocity, however, was not significantly related to any other domain. A visual assessment of the forest plot for the association between TCD velocity and cognitive function (see Supplementary Figure 8) showed that although each study showed a negative effect size, only one of the six effects included were statistically significant. Therefore, this mean effect may have been strongly driven by one study (Winrow, 2000) with a sample of 10 participants. A similar pattern of significant correlations was also found across samples that excluded patients with overt stroke.
Environmental Risks for Cognitive Deficits
Sixteen samples (17 studies) assessed SES in relation to domains of cognitive function. All measures of SES (i.e., SES composites, parent education, and household income) were collapsed into one category in order to have a sufficient number of samples within each analysis. Meta-analytic findings showed that indicators of SES were significantly and positively associated with FSIQ (r = .23, 95% CI = .17 to .28), language and verbal reasoning (r = .28, 95% CI = .09 to .45), visual spatial and perceptual reasoning (r = .26, 95% CI = .09 to .41), and executive function (r = .18, 95% CI = .07 to .28), with small to medium effects. Similar results were also found across samples that excluded patients with overt stroke.
Psychosocial Consequences of Cognitive Deficits
Six samples and studies assessed total problems in relation to FSIQ. Total problems had a significant negative association with FSIQ (r = −.12, 95% CI = −.21 to −.02), such that participants with lower scores exhibited greater problems. There was an insufficient number of studies to assess this finding across samples that excluded patients with overt stroke
Forest plots for each effect are shown in Supplementary Figures 2–14.
Study Quality
Quality ratings are depicted in Supplementary Table 1 and Figure 15. Seven criteria were used, and study quality ranged from 4 to 7 (M = 5, SD = 1). Overall, studies infrequently included sample size justification/power analyses and rarely reported participation rate or had a participation rate greater than 50%.
Publication Bias
Of the nine significant effect sizes, no effect sizes produced significant Egger’s tests using two-tailed criterion at p < .05 (Egger et al., 1997). Trim and fill analyses were also conducted for all significant effects. Adjusted effect sizes for five effects (i.e., hematocrit with executive function, TCD with visual spatial and perceptual reasoning, SES with FSIQ, SES with visual spatial and perceptual reasoning, and total problems with FSIQ) indicated from trim and fill analyses are presented in Supplementary Table 3. Each of these effects remained statistically significant and of similar magnitude.
Discussion
The current review is the first quantitative meta-analysis to assess correlates of cognitive function in SCD, and it builds on findings from a recent meta-analysis of cognitive function in this population (Prussien et al., 2019). While the previous quantitative review assessed group differences of cognitive function across age and infarct status, findings presented here provide evidence that both biological and environmental characteristics are associated with cognitive function in this population. Hemoglobin level and hematocrit percentage were the primary biological characteristics that were associated with cognitive function across domains, such that higher hemoglobin or hematocrit was related to higher scores. Further, findings suggest that measures of anemia are primarily associated with FSIQ and executive function in samples of SCD rather than SCA. TCD velocity was only related to visual spatial and perceptual reasoning. Finally, findings showed that indicators of SES were positively related to each cognitive domain, and higher FSIQ was related to lower total behavioral and emotional problems.
Findings provide evidence for biological predictors of cognitive function. First, hemoglobin and hematocrit were positively related to FSIQ, language and verbal reasoning, and executive function, particularly in phenotypically heterogenous samples of SCD. Higher TCD velocity, one compensatory mechanism that occurs in the brain to accommodate for chronic anemia, was negatively related to visual spatial and perceptual reasoning. This association, however, was likely highly influenced by results of one study with a small sample size; and all other domains showed mixed findings with nonsignificant aggregated effects. TCD velocity is a proxy for oxygen delivery to the brain, whereas CBF (ml blood/100 g tissue/min) assesses the actual rate of blood that is delivered to brain tissue. Nevertheless, few studies have assessed this more precise measure in SCD samples. In one study to date, Strouse et al. (2006) found that left, right, anterior, and global CBF was significantly related to Performance IQ in a small sample of children with SCA. Although both elevated TCD velocity and CBF are adaptive compensatory mechanisms in the brain to accommodate for hypoxic and anemic conditions, they are strong indicators for risk of stroke. Increased baseline TCD velocity and CBF suggest that the brain is exerting significant effort to maintain minimum oxygen consumption, and this might make it difficult to meet additional demands during cognitive tasks. A unique relation between TCD velocity and visual spatial and perceptual reasoning was not hypothesized, and therefore, more research is needed to replicate and extend findings from Strouse et al. (2006) using larger samples, as CBF is a stronger measure of oxygen delivery to the brain compared to TCD.
Findings for hemoglobin level and hematocrit percentage and TCD velocity suggest that changes in modifiable biological targets could lead to improvements in cognitive function. For example, the primary medical interventions to reduce symptoms of SCD (i.e., hydroxyurea, chronic transfusions, and apheresis) have been shown to increase hematocrit percentage, lower TCD velocity, and reduce risk for stroke (DeBaun et al., 2014; Ghafuri et al., 2017). Nevertheless, few studies have assessed cognition before and after the implementation of these interventions. Current medical guidelines in the United States recommend that children with SCA begin hydroxyurea at age 2 years, making it challenging or unrealistic to construct large-scale randomized control trials to test the effect of the drug on cognition in the United States. Nevertheless, adherence is a major concern in this population (Walsh et al., 2014), which provides an opportunity for cooperative investigations on increasing adherence and determining if improved cognition follows. Further, intervention studies could be both feasible and necessary in more developing nations where access to hydroxyurea and transfusions are limited, and the prevalence rate of SCA is large, as is the case in sub-Saharan Africa. Although access to advanced imaging techniques are limited, studies have begun to use less-expensive techniques such as TCD ultrasound to assess CBF velocity and risks for neurological impairment while assessing the implementation of hydroxyurea in Nigeria (Galadanci et al., 2015). Finally, pre-post assessments during infancy and preschool years are both feasible and important, yet only one small study to date has assessed this with nine preschool children with SCA in the United States (Thornburg et al., 2009). Findings from this study suggested nonsignificant effects for changes in neurocognitive function after 2 years on hydroxyurea.
Chronic transfusion therapy is another biological intervention that may have downstream effects on cognitive function through these variables. In a recent study on the effect of transfusion on cognitive function in school-aged children with SCA, Hood et al. (2019) used the NIH Toolbox (Weintraub et al., 2013) to measure cognitive function close to and far from the time of transfusions. Findings showed that children performed better on executive function tasks near the time of transfusion compared to far from transfusion, and, despite having more severe disease characteristics, children receiving transfusion also had higher scores near transfusion compared to participants receiving hydroxyurea. Replication of these findings and assessing effects across multiple domains of cognitive function is needed to further understand biological interventions for cognitive remediation in this population.
This study is the first quantitative review to assess the association of SES with cognitive function in SCD. In a meta-analysis of cognitive function in SCD across the lifespan, Prussien et al. (2019) found that there were significant cognitive deficits relative to both the normative mean and to sibling or healthy controls, and deficits were much greater relative to the normative mean. Although the deficit relative to healthy controls corroborate the important findings for biological risks, both samples of SCD and healthy matched controls performed significantly lower than the normative mean across studies (Schatz et al., 2002). Shared environmental and socioeconomic disadvantage among SCD and healthy matched samples may account for the additional deficit in functioning. Indicators and measures of SES showed significant effects across domains of cognitive function; however, there was an insufficient number of studies to assess income and parent education separately. Further, these indicators of SES may be proxies for more complex socioenvironmental influences. Although studies are beginning to assess the influence of parenting on neurocognitive function in SCD (e.g., Fields et al., 2016; Yarboi et al., 2019), future research should continue to build upon the limited research on the potential mediating role of home environment, parent stress and parenting in the association between SES and cognitive function, and environmental interventions aimed to improve cognitive function in SCD should be designed and tested.
A final aim of the current review was to assess potential psychosocial correlates and consequences of deficits in cognitive function in SCD. Findings showed that lower FSIQ was significantly related to greater behavioral and emotional problems. Although we hypothesize that these psychosocial problems are consequences of cognitive deficits, as shown in the heuristic model (Figure 1), this significant association could be reciprocal. Findings of the systematic review showed that few studies assess theses correlates, and this area of research is arguably weak and requires more attention. Few studies have directly assessed the association between cognitive function and internalizing problems, and only one study has assessed externalizing problems as a correlate of cognitive function in this population (Allen et al., 2017; Prussien et al., 2018). Further, no quantitative analysis was done on academic achievement due to an insufficient number of studies. More studies used measures of achievement alongside cognitive function but did not show how they were related. Although there are portions of some achievement assessments that mirror tasks on assessments of cognitive function, intelligence and achievement are separate constructs. Finally, only one study assessed the association between cognitive function and employment status in adults with SCD (Sanger et al., 2016) . More studies are needed to understand the downstream social and financial consequences of cognitive deficits in adolescence and adulthood.
The current meta-analysis had several strengths and limitations. This was the first quantitative review to assess correlates of multiple domains of cognitive function in SCD, and it assessed biological, environmental, and psychosocial correlates. Nevertheless, one primary limitation of the current review is that broad domains of cognitive function were assessed, comprised of several different tests and tasks, potentially hiding specific correlates of more narrow domains of cognition. Future research should continue to assess more specific domains of cognitive and executive function in order to get a more comprehensive understanding of specific cognitive correlates. Further, all age groups were included across effect sizes. Although the magnitude of correlations with domains of cognitive function may differ with age, there was an insufficient number of studies to assess age as a categorical moderator of the correlates assessed, as the vast majority of studies investigated cognitive correlates in school-aged samples. In addition to this, we were unable to assess several other moderators of effects due to the limited number of studies across each potential moderator, and understanding environmental and psychosocial moderators of the association of SCD with cognitive function is essential. Future research should assess interactional models across biological and environmental correlates of cognitive function, assess specific clinical significance of correlations, and test biological and environmental intervention designs for cognitive remediation.
Funding
Preparation of this manuscript was supported in part by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (F31 HD095592) and a training grant from the National Institute of Mental Health (T32 MH18921).
Conflicts of interest: None declared.
Supplementary Material
References
- Achenbach T. M.& Rescorla L. A. (2001). Manual forthe ASEBA school-age forms and profiles. Burlington, VT: Research Center forChildren, Youth, and Families, University of Vermont. [Google Scholar]
- Allen T. M., Anderson L. M., Rothman J. A., Bonner M. J. (2017). Executive functioning and health-related quality of life in pediatric sickle cell disease. Child Neuropsychology, 23, 889–906. [DOI] [PubMed] [Google Scholar]
- Bakker M., Hofmann J., Churches O. F., Badcock N. A., Kohler M., Keage H. A. D. (2014). Cerebrovascular function and cognition in childhood: A systematic review of transcranial Doppler studies. BMC Neurology, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen D., Koot H. M., Bongers I. L., Varni J. W., Verhulst F. C. (2004). Measuring quality of life in children referred for psychiatric problems: Psychometric properties of the PedsQL 4.0 generic core scales. Quality of Life Research, 13, 489–495. [DOI] [PubMed] [Google Scholar]
- Benton T., Boyd R., Ifeagwu J., Feldtmose E., Smith-Whitley K. (2011). Psychiatric diagnosis in adolescents with sickle cell disease: A preliminary report. Current Psychiatry Reports, 13, 111–115. [DOI] [PubMed] [Google Scholar]
- Berkelhammer L. D., Williamson A. L., Sanford S. D., Dirksen C. L., Sharp W. G., Margulies A. S., Prengler R. A. (2007). Neurocognitive sequelae of pediatric sickle cell disease: A review of the literature. Child Neuropsychology, 13, 120–131. [DOI] [PubMed] [Google Scholar]
- Bernaudin F., Verlhac S., Fréard F., Roudot-Thoraval F., Benkerrou M., Thuret I., Brugières P. (2000). Multicenter prospective study of children with sickle cell disease: Radiographic and psychometric correlation. Journal of Child Neurology, 15, 333–343. [DOI] [PubMed] [Google Scholar]
- Bills S. E., Schatz J., Hardy S. J., Reinman L. (2020). Social-environmental factors and cognitive and behavioral functioning in pediatric sickle cell disease. Child Neuropsychology, 26, 83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M., Hedges L., Higgins J., Rothstein H. (2014). Comprehensive meta-analysis version 3. Englewood, NJ: Biostat. [Google Scholar]
- Caldwell B., Bradley R. (2003). HOME inventory early adolescent version. Little Rock, AR: University of Arkansas for Medical Sciences. [Google Scholar]
- Campbell L. K., Scaduto M., Van Slyke D., Niarhos F., Whitlock J. A., Compas B. E. (2008). Executive function, coping, and behavior in survivors of childhood acute lymphocytic leukemia. Journal of Pediatric Psychology, 34, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd edn). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. [PubMed] [Google Scholar]
- Compas B. E., Jaser S. S., Bettis A. H., Watson K. H., Gruhn M., Dunbar J. P., Thigpen J. C. (2017). Coping, emotion regulation and psychopathology in childhood and adolescence: A meta-analytic and narrative review. Psychological Bulletin, 143, 939–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J., Turner R. M., Clarke M. J., Higgins J. P. (2011). Characteristics of meta-analyses and their component studies in the Cochrane Database of Systematic Reviews: A cross-sectional, descriptive analysis. BMC Medical Research Methodology, 11, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun M. R. (2014). Perspective: Thinking beyond survival. Nature, 545, S16. [DOI] [PubMed] [Google Scholar]
- DeBaun M. R., Gordon M., McKinstry R. C., Noetzel M. J., White D. A., Sarnaik S. A., Casella J. F. (2014). Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. New England Journal of Medicine, 371, 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S., Tweedie R. (2000). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56, 455–463. [DOI] [PubMed] [Google Scholar]
- Egger M., Smith G. D., Schneider M., Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. British Medical Journal, 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields M. E., Hoyt-Drazen C., Abel R., Rodegheir M. J., Yarboi J. M., Compas B. E., King A. A. (2016). A pilot study of parent education intervention improces early childhood development among toddlers with sickle cell disease. Pediatric Blood & Cancer, 63, 2131–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman N. P., Miyake A., Young S. E., Defries J. C., Corley R. P.& Hewitt,J K. (2008). Individual differences in executive functions are almost entirelygenetic in origin. Journal of Experimental Psychology, 137, 201–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galadanci N. A., Abdullahi S. U., Tabari M. A., Abubakar S., Belonwu R., Salihu A., DeBaun M. R. (2015). Primary stroke prevention in Nigerian children with sickle cell disease (SPIN): Challenges of conducting a feasibility trial. Pediatric Blood and Cancer, 62, 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafuri D. L., Chaturvedi S., Rodeghier M., Stimpson S. J., McClain B., Byrd J., DeBaun M. R. (2017). Secondary benefit of maintaining normal transcranial Doppler velocities when using hydroxyurea for prevention of severe sickle cell anemia. Pediatric Blood and Cancer, 64, e26401–e26405. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Bills S. E., Wise S. M., Hardy K. K. (2018). Cognitive abilities moderate the effect of disease severity on health-related quality of life in pediatric sickle cell disease. Journal of Pediatric Psychology, 43, 882–894. [DOI] [PubMed] [Google Scholar]
- Hedges L., Olkin I. (1985). Statistical methods for meta-analysis. New York, NY: Academic Press. [Google Scholar]
- Higgins J .P. T., Green S. (2011). Cochrane handbook for systematic reviews of interventions Retrieved from www.cochrane-handbook.org
- Hijmans C. T., Grootenhuis M. A., Oosterlaan J., Heijboer H., Peters Marjolein P., Fijnvandraat K. (2011). Neurocognitive deficits in children with sickle cell disease are associated with the severity of anemia. Pediatric Blood and Cancer, 57, 297–302. [DOI] [PubMed] [Google Scholar]
- Hocking M. C., Barnes M., Shaw C., Lochman J. E., Madan-Swain A., Saeed S. (2011). Executive function and attention regulation as predictors of coping success in youth with functional abdominal pain. Journal of Pediatric Psychology, 36, 64–73. [DOI] [PubMed] [Google Scholar]
- Hood A. M., King A. A., Fields M. E., Ford A. L., Guilliams K. P., Hulbert M. L., White D. E. (2019). Higher executive abilities following a blood transfusion in children and young adults with sickle cell disease. Pediatric Blood & Cancer, 66, e27899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassaint C. R., Jones V. L., Leong S., Frierson G. M. (2016). A systematic review of the association between depression and healthcare utilization in children and adults with sickle cell disease. British Journal of Haematology, 174, 136–147. [DOI] [PubMed] [Google Scholar]
- King A. A., Strouse J. J., Rodeghier M. J., Compas B. E., Casella J. F., McKinstry R. C., DeBaun M. R. (2014). Parent education and biologic factors influence on cognition in sickle cell anemia. American Journal of Hematology, 89, 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran T. P. (2016). Anxiety and working memory capacity: A meta-analysis and narrative review. Psychological Bulletin, 142, 831–864. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute. (2014). Study quality assessment tools: quality assessment tool for case series studies. Washington, DC: NHLBI. [Google Scholar]
- Peterson R. A., Brown S. P. (2005). On the use of beta coefficients in meta-analysis. Journal of Applied Psychology, 90, 175–181. [DOI] [PubMed] [Google Scholar]
- Prussien K. V., DeBaun M. R., Yarboi J., Bemis H., McNally C., Williams E., Compas B. E. (2018). Cognitive function, coping, and depressive symptoms in children with sickle cell disease. Journal of Pediatric Psychology, 43, 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussien K. V., Jordan L. C., DeBaun M. R., Compas B. E. (2019). Cognitive function in Sickle Cell Disease across domains, cerebral infarct status, and the lifespan: A meta-analysis. Journal of Pediatric Psychology, 44, 948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles T. F., Slatcher R. B., Trombello J. M., McGinn M. M. (2014). Marital quality and health: A meta-analytic review. Psychological Bulletin, 140, 140–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger M., Jordan L., Pruthi S., Day M., Covert B., Merriweather B., Kassim A. (2016). Cognitivedeficits are associated with unemployment in adults with sickle cell anemia. Journal of Clinical and Experimental Neuropsychology, 38, 661–671. [DOI] [PubMed] [Google Scholar]
- Schatz J., Finke R. L., Kellett J. M., Kramer J. H. (2002). Cognitive functioning in children with sickle cell disease: A meta-analysis. Journal of Pediatric Psychology, 27, 739–748. [DOI] [PubMed] [Google Scholar]
- Schoemaker K., Mulder H., Dekovic M., Matthys W. (2013). Executive functions in preschool children with externalizing behaviors: A meta-analysis. Journal of Abnormal Child Psychology, 41, 457–471. [DOI] [PubMed] [Google Scholar]
- Snyder H. R. (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139, 81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen R. G., Miles M. A., Helton K. J., Strawn S., Wang W., Xiong X., Mulhern R. K. (2003). Cognitive impairment in children with hemoglobin SS sickle cell disease: Relationship to MR imaging findings and hematocrit. AJNR American Journal of Neuroradiology, 24, 382–389. [PMC free article] [PubMed] [Google Scholar]
- Streisand R., Braniecki S., Tercyak K. P., Kazak A. E. (2001). Childhood illness-related parenting stress: The pediatric inventory for parents. Journal of Pediatric Psychology, 26, 155–162. [DOI] [PubMed] [Google Scholar]
- Strouse J. J., Cox C. S., Melhem E. R., Lu H., Kraut M. A., Razumovsky A., Casella J. F. (2006). Inverse correlation between cerebral blood flow measured by continuous arterial spin-labeling (CASL) MRI and neurocognitive function in children with sickle cell anemia (SCA). Blood, 108, 379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi R. A., Grant M. L., Ely E., Barakat L. P. (2007). Neuropsychological functioning in preschool-age children with sickle cell disease: The role of illness-related and psychosocial factors. Child Neuropsychology, 13, 155–172. [DOI] [PubMed] [Google Scholar]
- Thornburg C. D., Dixon N., Burgett S., Mortier N. A., Schultz W. H., Zimmerman S. A., Ware R. E. (2009). A pilot study of hydroxyurea to prevent chronic organ domage in young children with sickle cell anemia. Pediatric Blood & Cancer, 52, 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzepacz A. M., Vannatta K., Gerhardt C. A., Ramey C., Noll R. B. (2004). Emotional, social, and behavioral functioning of children with sickle cell disease and comparison peers. Journal of Pediatric Hematology/Oncology, 26, 642–648. [DOI] [PubMed] [Google Scholar]
- Walsh K. E., Cutrona S. L., Kavanagh P. L., Crosby L. E., Malone C., Lobner K., Bundy D. G. (2014). Medication adherence among pediatric patients with sickle cell disease: A systematic review. Pediatrics, 134, 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (1939). The measurement of adult intelligence. Baltimore: Williams & Wilkins. [Google Scholar]
- Wechsler D. (2014). Wechsler intelligence scale for children (5th edn WISC-V). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weintraub, S., Dikmen, S. S., Heaton, R. K., Tulsky, D. S., Zelazo, P. D., Bauer, P. J., … Gershon, R. C. (2013). Cognition assessment using the NIH Toolbox. Neurology, 80, S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winrow, N. (2000). Neuropsychological and pathophysiological correlates of sickle cell disease in a pediatric population (Doctoral dissertation). Retrieved from PsycINFO Database. (Accession No. 2000-95018-239). [Google Scholar]
- Yarboi J., Compas B. E., Brody G. H., White D., Patterson J. R., Ziara K., King A. (2017). Association of social-environmental factors with cognitive function in children with sickle cell disease. Child Neuropsychology, 23, 343–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarboi J., Prussien K. V., Bemis H., Williams E., Watson K. H., McNally C., Compas B. E. (2019). Responsive parenting behaviors and cognitive function in children with sickle cell disease. Journal of Pediatric Psychology, 44, 1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.