ABSTRACT
Introduction
Upon injury, skeletal muscle undergoes a multiphase process beginning with degeneration of the damaged tissue, which is accompanied by inflammation and finally regeneration. One consequence of an injured microenvironment is excessive production of reactive oxygen species, which results in attenuated regeneration and recovery of function ultimately leading to fibrosis and disability. The objective of this research was to test the potential of the antioxidant, N-Acetyl-L-Cysteine (NAC), as a mediator of reactive oxygen species damage that results from traumatic muscle injury in order to support repair and regeneration of wounded muscle tissue and improve function recovery.
Materials and Methods
Adult female Lewis rats were subjected to compartment syndrome injury as previously published by our group. Rats received intramuscular injections of NAC or vehicle at 24, 48, and 72 hours postinjury. Muscle function, tissue fibrosis, and the expression of myogenic and angiogenic markers were measured.
Results
Muscle function was significantly improved, and tissue fibrosis was significantly decreased in NAC-treated muscles.
Conclusions
These results suggest that NAC treatment of skeletal muscle after injury may be a viable option for the prevention of long-term fibrosis and scar formation, facilitating recovery of muscle function.
1. INTRODUCTION
Musculoskeletal disorders are a primary cause of disability in the military and civilian populations.1 Extremity trauma resulting from high-energy explosives is a devastating consequence of combat injury. Studies showed that 54% of evacuated wounded service members have extremity injuries. More than one-quarter of all extremity war injuries involve fractures; 82% of these are open.1,2 The Military Extremity Trauma Amputation/Limb Salvage Study showed that major lower-limb trauma sustained in the military results in significant disability.3 Acute compartment syndrome (ACS) is a condition in which increased pressure within the fascicles of the skeletal muscle compromises the circulation and function of the tissues therein, resulting in tissue ischemia and necrosis. ACS is a major contributor to long-term morbidity and disability as a result of combat-related injuries to the extremities. Sequelae of an increase in intracompartmental pressure include ischemia, necrosis, and nerve damage. If untreated, ACS may lead to limb amputation, multiple organ failure, and death.
Skeletal muscle has an innate ability to heal after injury; however, this regenerative capacity is compromised in cases of severe injury, disease, or age. Despite the remarkable regenerative capacity of skeletal muscle, the healing process is often slow resulting in incomplete functional recovery and nonresolved tissue fibrosis.4 Despite the prevalence of musculoskeletal injuries and decades of medical research, our understanding of the pathophysiology of musculoskeletal injuries is limited, and as such has hindered the development of new therapeutics.
The formation of dense scar tissue after injury is a major impairment to the recovery of muscle function after injury and can lead to muscle contracture and chronic pain, resulting in decreased mobility and quality of life. Irrespective of the primary cause of injury (contusion, strain, or laceration), the basic biological processes occurring in scar formation are identical.4 Fibrotic lesions are the result of abnormal healing and are dependent on a cascade of multiple processes induced by injury such as poor perfusion and the persistent activation of inflammatory cells and cytokines.5,6 Transforming growth factor β (TGFβ) is a major mediator of the fibrotic response after injury.7,8 The use of antifibrotic agents, such as Losartan, an angiotensin II receptor blocker, and TGFβ inhibitor, have been purported to reduce fibrosis and improve both muscle regeneration and function after minor injuries such as contusion and lacerations.9,10 However, there are conflicting data on the long-term beneficial effects of Losartan and its ability to improve muscle function after severe injury or disease.11,12 Moreover, Losartan may have off target effects on nonskeletal muscle tissues because of its ability to modulate the renin-angiotensin system.13
N-Acetyl-L-Cysteine (NAC) is a cysteine precursor of glutathione synthesis and a potent antioxidant, which has been US Food and Drug Administration approved for over 40 years to treat acetaminophen toxicity, acute liver failure, and diseases characterized by low glutathione levels.14,15 The microenvironment of injured tissue is characterized by an excessive presence of reactive oxygen species (ROS), which play a crucial role in the pathophysiology of scar formation.16–19 There is overwhelming in vitro and in vivo evidence for the use of NAC as a protective agent against pathologic tissue fibrosis and cell death caused by oxidative stress in injured tissue.20–23 In this study, we examined the effect of NAC treatment on the recovery of skeletal muscle function using a rat model ACS.24 We found that NAC treatment resulted in a significant decrease in ROS production, decreased fibrotic deposition, and a corresponding significant increase in the restoration of muscle function. These results suggest that early treatment with NAC after an acute injury may benefit long-term muscle function and improve patient mobility and quality of life.
2. MATERIALS AND METHODS
2.1. Injury Model
All animal studies were approved by the Wake Forest University Institutional Animal Care and Use Committee and were conducted in accordance with National Institute of Health (NIH) guidelines. Adult female Lewis rats (11–12 months of age; Harlan Laboratories, Indianapolis, Indiana) were anesthetized with isoflurane before injury. ACS muscle injury was performed as previously described.24 Briefly, neonatal blood pressure cuffs (Tempa-Kuff, size #2; Trimline Medical Products, Branchburg, New Jersey) were tightened around the left hind limb, proximal to the tibialis anterior (TA) muscle, and held at a pressure of 120 to 140 mmHg for 3 hours. Rats were euthanized at 4, 7, 14, and 28 days after injury.
2.2. Intramuscular NAC Administration
NAC (80 mg/kg/day) (Sigma Aldrich, St. Louis, Missouri) was dissolved in phosphate buffered saline (PBS) and adjusted to physiological pH (7.35–7.45) with 10 M sodium hydroxide.25 Rats were randomized to receive either intramuscular injection of NAC or an equivalent volume of vehicle (PBS) into the TA muscle.26 A total of three doses were given 24, 48, and 72 hours after injury.27
2.3. In Vivo Muscle Function Test
The contractile function (ie, torque-frequency relationship) of the left anterior crural muscles was measured in vivo before and 7, 14, and 28 days after injury via stimulation of the peroneal nerve, as previously described.24 Anterior crural muscle function was assessed by measuring maximal isometric torque as a function of stimulation frequency (1–200 Hz). Data were analyzed using a custom-made Labview-based program (provided by the US Army Institute of Surgical Research, Houston, Texas).
2.4. Tissue Analysis
Muscle tissues were harvested, weighed, and processed for histology at the indicated time points. Fibrosis was quantified from Masson’s trichrome stained sections using the Threshold Color plugin for ImageJ software version 1.44 (NIH, Bethesda, Maryland). The same set threshold was used for all samples analyzed. The data were calculated in pixels as percentage fibrotic regions/total tissue area. At least six different animals were used per group, and 10 to 12 muscle sections were analyzed per animal.
Nitro-tyrosine staining was used for an indirect measurement of the presence of ROS28 and quantified using ImageJ software. At least three different animals were used per group, and 10 to 12 muscle sections were analyzed per animal. CD146 staining was used to assess the total number of blood vessels and the diameter of blood vessels per high powered field at 7 days postinjury. At least three different animals were used per group, and six muscle sections were analyzed per animal.
2.5. Analysis of Myofiber Cross-Sectional Area
To assess muscle fiber cross-sectional area, transverse muscle sections (8 μM) were stained with hematoxylin and eosin (H&E). Stained sections were visualized, and pictures were captured using an Olympus DP80 microscope. Fiber cross-sectional area was measured using ImageJ software. Three different animals were used per group, and 6 muscle sections were analyzed per animal.
2.6. Real-Time Quantitative Polymerase Chain Reaction
RT-QPCR was performed on muscles isolated at 4 and 14 days after injury. Day 28 was not analyzed because of the finding of no functional differences between the NAC- and PBS-treated groups. Total ribonucleic acid (RNA) was isolated from the harvested TA muscles using the PerfectPure Fibrosis Tissue Kit (5 PRIME, Gaithersburg, Maryland) according to the manufacture’s protocol. Quantitative polymerase chain reaction (qPCR) was performed in 20-mL reactions in 96-well plates using cDNA samples generated from 12.5 ng of total RNA. The TaqMan probes (Applied Biosystems, Foster City, California) specific for rat genes are as follows: Transforming Growth Factor β1 (TGFβ1) (Rn01475963_m1), Myostatin (MSTN) (Rn00569683_m1), CD31 (Rn01467262_m1), Vascular Endothelial Growth Factor (VEGF) (Rn01511601_m1), Paired box (Pax)7 (Rn01518732_m1), myoblast determination protein 1(MyoD) (Rn00598571_m1), Myogenin (MyoG) (Rn00567418_m1), and Glyceraldehyde-2-Phosphate Dehydrogenase (GAPDH) (Rn01775763_g1) genes (Applied Biosystems/Life Technologies, Carlsbad, California). Superoxide dismutase (Sod1) primers (catalog# RQP049577) were obtained from GeneCopoeia (Rockville, Maryland). A qPCR for using rat HIF1 primers (F: gtcaccacaggacagtacagga; R: gaagggagaaaatcaagtcgtg) was performed using SYBR Green PCR kit (Qiagen, Carlsbad, California). A qPCR was performed using the Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems/Life Technologies, Carlsbad, California). Relative quantification of PCR products were based upon the value difference between the target gene and GAPDH.
2.7. Statistical Analyses
All tissue analyses were performed by one individual blinded to the experimental groups. Data were analyzed using Prism Software (GraphPad Software, San Diego, California) or Microsoft Excel (Redmon, Washington). Each functional and morphological measures (dependent variables) were compared among groups in response to changes in treatment and time (independent variables). Since these outcome measures could be expressed on a continuous scale, we examined all outcomes of interest using a 2-way analysis of variance (ANOVA) model with these two factors (treatment and time) included. In the event of a significant ANOVA, post-hoc means comparison testing was performed with Fisher’s least significant difference correction or with Tukey’s honestly significance difference tests. Data are presented as mean ± standard error of mean. A p-value of < 0.05 was considered to be statistically significant.
3. RESULTS
3.1. Effect of NAC on Reactive Oxygen Species in Injured Muscle
Prior studies have shown that the lipid peroxidation product nitro-tyrosine can be used as an indirect marker of ROS levels in injured tissue.28 Therefore, nitro-tyrosine was used as an indicator of the activity of NAC on the ROS levels in the compartment syndrome injured muscles. Immunohistochemical analysis of TA muscles stained with anti–nitro-tyrosine antibodies (red arrows) showed that treatment with NAC significantly reduced the amount of ROS in the injured tissue compared to PBS-treated injured muscles at 4 days postinjury (Fig. 1).
Figure 1.
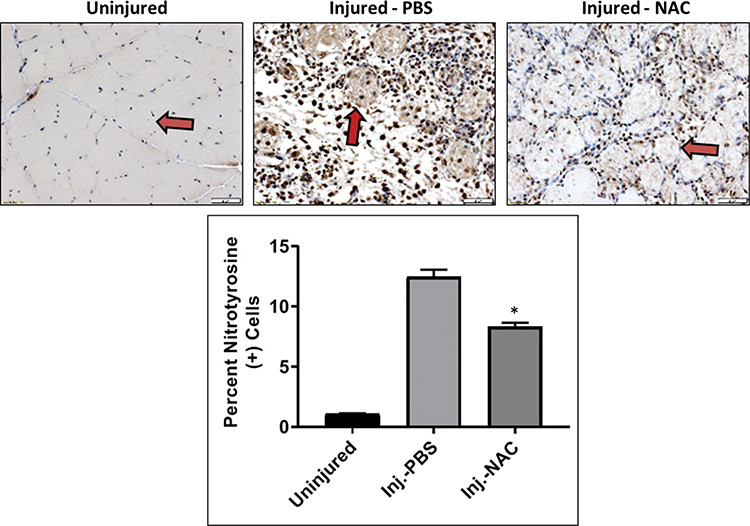
Effect of NAC on reactive oxygen species in injured muscle. Nitro-tyrosine staining of TA muscles harvested 4 days after injury. Red arrows indicate individual myofibers in order to highlight staining. The number of nitro-tyrosine (+) cells was quantified using ImageJ software. All data are expressed as mean ± SEM. Asterisk indicated significant difference (p < 0.001) between phosphate buffered saline (PBS) and NAC-treated injured muscles.
3.2. Effect of NAC on Skeletal Muscle Function after Injury
Isometric torque was examined as a measure of muscle function before and on days 7, 14, and 28 postinjury. As expected, injured muscles showed a significant decrease in force generation compared to contralateral uninjured muscles regardless of treatment at days 7 and 14 and 28 days after injury (Fig. 2A). However, NAC treatment resulted in significantly higher maximum force production 7 and 14 days after injury, as compared to PBS-treated muscles (Fig. 2B). By 28 days after injury, approximately 75% of muscle function was restored regardless of treatment with no difference in function between NAC- and PBS-treated groups (Fig. 2A and B).
Figure 2.
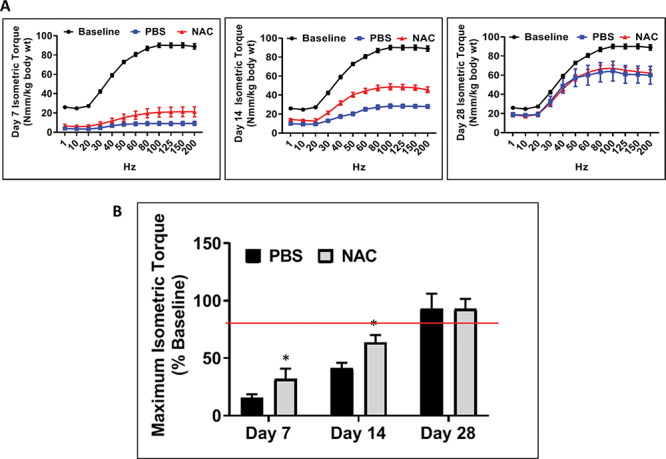
Skeletal muscle function after injury. Isometric torque curves (A) per Nmm/kg/body weight 7, 14, and 28 days after injury. (B) Maximum isometric torque measured 7, 14, and 28 days postinjury. All data are expressed as mean ± SEM. Asterisks indicate significance between PBS- and NAC-treated muscles. Single asterisks refer to significant differences between PBS- and NAC-treated injured muscles. Day 7: *p < 0.0295, day 14: *p < 0.048. All data are expressed as mean ± SEM.
3.3. Effect of NAC on Muscle Fibrosis Following Injury
Masson’s trichrome stained muscle tissue sections were used to quantify tissue fibrosis in uninjured, NAC-treated and PBS-treated TA muscles 14 and 28 days postinjury. Treatment with NAC resulted in a significant reduction in tissue fibrosis compared to PBS-treated injured muscles (Fig. 3A). There was no significant difference between NAC-treated injured muscles and uninjured muscles. TGFβ, MSTN, and SOD1 are genes known to play an important role in tissue fibrosis and scar formation.29,30 Quantitative reverse-transcription polymerase chain reaction was used to examine the expression of these genes 4 and 14 days after injury. At 4 days postinjury, treatment with NAC resulted in an increase in expression of TGFβ approaching significance (p < 0.051) and a significant increase in SOD1 expression (p < 0.017). No change in MSTN expression in comparison to PBS-treated injured muscles was detected (Fig. 3B–D, left column). In contrast, 14 days after injury, TGFβ expression was decreased in NAC-treated muscles as compared to PBS-treated muscles (p < 0.09). The expression of MSTN and SOD1 was significantly decreased (p < 0.039 and p < 0.022 respectively) in the NAC-treated injured muscles as compared to the PBS-treated injured muscles (Fig. 3B–D, right column).
Figure 3.
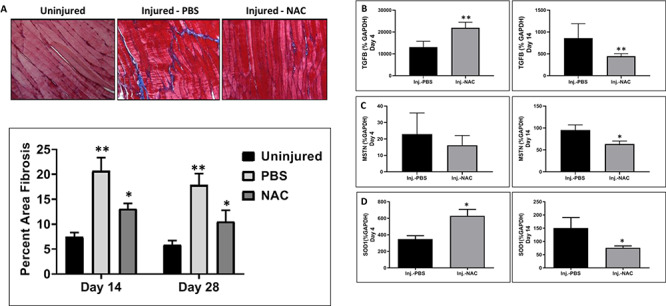
Effect of NAC on muscle fibrosis following injury. (A) Masson’s trichrome stain was used to visualize collagen deposition in tissue 14 and 28 days after injury. The amount of collagen staining per tissue was determined using ImageJ software as described in methods. The double asterisk refers to significance between PBS-treated injured muscles and uninjured muscles (**p < 0.002), and the single asterisk refers to significance between PBS- and NAC-treated injured muscles (*p < 0.012). (B) The expression of genes involved in fibrosis (TGF(B), MSTN(C), and SOD1(D)) was assessed by qPCR at 4 (left column) and 14 (right column) days after injury. The double asterisks refer to data approaching significance (Day 4: TGFβ**p < 0.051; day 14: TGFβ**p < 0.09), and single asterisks refer to significant data (Day 4: SOD1*p < 0.017; day 14: MSTN*p < 0.039; SOD1*p < 0.022). All data are expressed as mean ± SEM.
3.4. Effect of NAC on Vascularization of Injured Tissue
Early neovascularization is necessary for tissue regeneration and functional recovery after injury. CD146 staining was used to quantify the number and diameter of blood vessels per high powered field in PBS- and NAC-treated injured muscles 7 days postinjury (Fig. 4A–C). NAC treatment resulted in increased numbers of blood vessels compared to PBS-treated animals as well as increased blood vessel diameter between NAC- and PBS-treated animals (Fig. 4B and C). Expression of the angiogenic markers HIF1, CD31, and VEGF was determined by qPCR in PBS- and NAC-treated muscles 4 and 14 days post injury. Treatment with NAC resulted in a significant increase in these angiogenic markers compared to PBS-treated muscles 4 days postinjury (Fig. 4D–F, left column). At 14 days postinjury, HIF1 and CD31 were significantly decreased, or approaching significance, in the NAC-treated muscles as compared to the PBS-treated muscles (Fig. 4D–F, right column).
Figure 4.
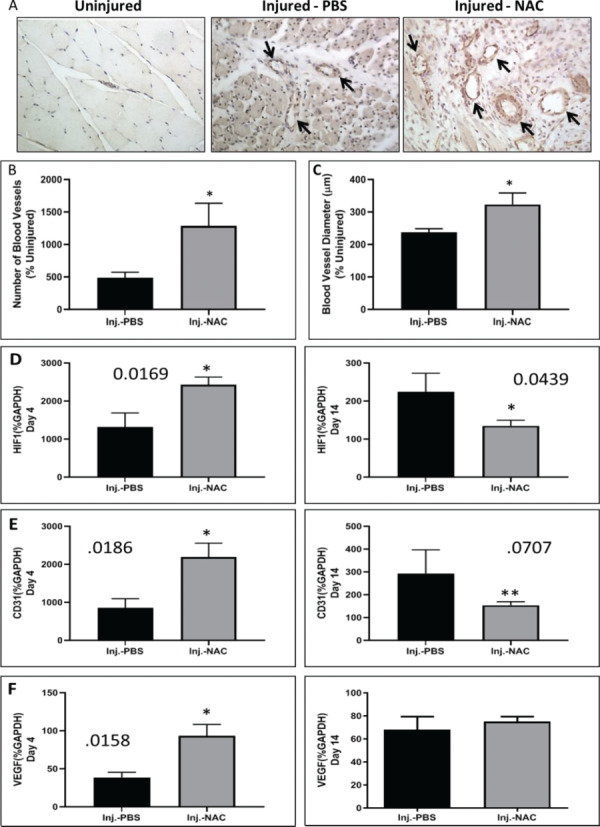
Effect of NAC on the vasculature of injured tissue. (A) CD146 stain of representative sections of muscle from uninjured, PBS-, and NAC-treated injured muscles 7 days postinjury. CD146 stained sections were used to quantitate (B) the number of blood vessels per high powered field (percent uninjured) and (C) the diameter of blood vessels (percent uninjured). Asterisks refer to significant changes between NAC- and phosphate buffered saline (PBS)-treated injured muscles (B—*p < 0.050; C—*p < 0.043). Expression of genes involved in angiogenesis (D) HIF1, (E) CD31, and (F) VEGF were measured using qPCR 4 and 14 days postinjury. Single asterisks refer to significant differences between PBS- and NAC-treated injured muscles (Day 4: HIF1*p < 0.017; CD31*p < 0.019; VEGF*p < 0.012; day 14: HIF1*p < 0.043). The double asterisk refers to data approaching significance (Day 14: CD31**p < 0.071). All data are expressed as mean ± SEM.
3.5. Effect of NAC on Muscle Regeneration
Immature and regenerating myofibers have a smaller cross-sectional area (CSA) than mature myofibers. To further analyze the state of muscle tissue regeneration, myofiber CSA was determined from H&E stained muscle tissue samples 14 days postinjury (Fig. 5A and B). The NAC-treated muscles contained larger myofibers, which are characteristic of more mature skeletal muscle fibers as compared to the PBS-treated muscles.
Figure 5.
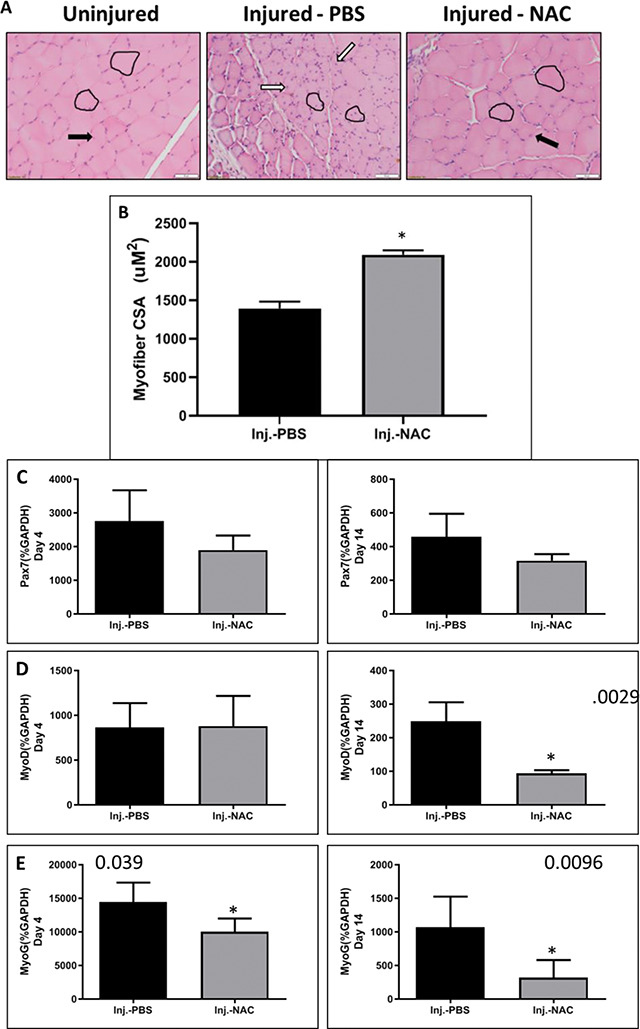
Effect of NAC on muscle regeneration. (A) H&E staining was used to observe the morphology of uninjured, phosphate buffered (PBS)-, and NAC-treated injured muscles at 14 days postinjury. Single myofibers are outlined in black. Black arrows indicate mature myofibers, and white arrows indicate immature regenerating myofibers. (B) Myofiber CSA was determined from H&E stained sections. The single asterisk refers to significant differences in myofiber size between PBS- and NAC-treated injured muscle (*p < 0.003). Expression of myogenic markers (C) Pax7, (D) MyoD, and (E) MyoG were measured using qPCR at 4 and 14 days postinjury. Single asterisks refer to significant differences in expression between PBS- and NAC-treated injured muscles (Day 4: MyoG*p < 0.039; day 14: MyoD*p < 0.003; MyoG*p < 0.001). All data are expressed as mean ± SEM.
Skeletal muscle regeneration depends on activation of satellite cells and their potential to differentiate and mature into new fibers.24,31 The myogenic transcription factors Pax7, MyoD, and MyoG were analyzed via qPCR in PBS- and NAC-treated muscles 4 and 14 days postinjury. Treatment with NAC resulted in a significant decrease in MyoG gene expression compared to PBS-treated muscles at 4 days after injury (Fig. 5C–E, left column). At 14 days postinjury, the expression of both MyoD and MyoG was reduced in NAC-treated muscles as compared to PBS-treated muscles (Fig. 5C–E, right column). No significant difference in Pax7 expression was detected at either time point.
4. DISCUSSION
Skeletal muscle has a tremendous innate regenerative capacity; however, after a severe injury or an injury to a frail or ill patient, the regenerative capacity is attenuated resulting in a slower healing process, which often culminates in incomplete functional recovery and favors the formation of dense scar tissue. Despite recent advances in medicine and our growing understanding of the pathophysiology of muscle injury, there has been little progress in the development of new therapeutic approaches to enhance regeneration and recovery of function. The presence of excessive unresolved fibrotic scar tissue is a major hindrance to the recovery of full muscle function. Prior data examining the efficacy of antifibrotic agents to treat skeletal muscle fibrosis is controversial. Antifibrotic agents that have shown encouraging results in preclinical studies, but early clinical studies have limited success in improving muscle function.7,12 For example, Losartan has been shown to reduce fibrosis in skeletal muscle but was shown to lack efficacy in the fibrotic model of volumetric muscle loss.12 Limitations in the efficacy of current treatments for skeletal muscle injuries might be due to the hostile microenvironment caused by injury or diseases, which often lead to inflammatory infiltrates promoting ROS production and further tissue damage. Therefore, antifibrotic treatments that include antioxidants may prove to have greater efficacy than an antifibrotic alone.
In the present study, we investigated the effect of the antioxidant NAC on skeletal muscle regeneration after injury using a rat compartment syndrome injury model. The biological action of NAC is mediated via glutathione, which is a free radical scavenger leading to reduction of O2− and H2O2. The NAC has been shown to reduce oxidative stress and inflammation in different tissues and disease states, such as ischemia, leading to better outcomes.32–35 For example, NAC use during liver inflammation due to injury has been associated with a reduction in liver damage.33 Additionally, NAC has shown clinical efficacy for the treatment of idiopathic pulmonary fibrosis.22 Our data showed that administration of NAC was associated with decreased ROS production (Fig. 1), decreased fibrosis (Fig. 3), and increased recovery of muscle function (Fig. 2) in a rat model of experimental stroke (30-minute MCA occlusion and 24 hour of reperfusion) when administered at the time of reperfusion (0 hour) and then 6 hour later. The rationale of using NAC at these times was based on previously published data showing beneficial effects when used before ischemia.35 The focus of this research is post-insult (ischemia) NAC treatment, as that would be more relevant in the clinical scenario. NAC reduced infarction very significantly in all six consecutive coronal sections measured as infarct area (Fig. 1A). Furthermore, treatment of NAC decreased infarct volume in both cortex and striatum, although more significantly in cortex (Fig. 1B). NAC has been used in various studies as an antioxidant because NAC is a precursor of glutathione.36 Glutathione is well known to protect brain from ischemic injury37. Treatment with NAC resulted in a significant increase in glutathione content in both cortex and striatum regions. The significance of glutathione enrichment has been investigated in the focal cerebral ischemia model38, and therapeutic use of NAC in other disease states has been reviewed.39 The NAC seems to have some benefits for certain cancers but may also have side effects, such as causing oxidative damage to cellular and isolated DNA.40 In our model, NAC did not protect at very low (50 mg/kg) or high (500 mg/kg) doses administered at 0 hour and repeated at 6 hour after reperfusion. Highly protective doses were 150 and 250 mg/kg. Because NAC has a short half-life and crosses the blood-brain barrier41, we chose to use repeated, lower doses effective in a rat model.
The body’s response to injury is to limit the extent of tissue damage due to the toxic microenvironment (immune infiltrates and necrotic tissue) by walling off the damaged areas with collagen, resulting in fibrotic depositions that limit regeneration of healthy tissue.34 Under normal conditions, the profibrotic pathways are terminated after the initial damage is controlled.42,43 However, in abnormal regeneration, the profibrotic signaling pathways persist and uncontrolled synthesis of collagen results in prolonged fibrotic scar formation and functional impairment.34,44
Transforming growth factor β is a potent mediator of the fibrotic response in various tissues.45 Myostatin, a member of the TGFβ superfamily, is known to induce fibrotic pathways in skeletal muscle while inhibiting skeletal muscle maturation.29 In contrast, SOD1 has been shown to be induced in postischemic tissues and is involved in regulating anti-inflammatory pathways as indicated by the SOD1−/− knock out mice that show an accelerated aging phenotype.46,47 In the present study, TGF-β and SOD1 levels were found to be significantly increased in the NAC-treated muscles at 4 days after injury (Fig. 3B–D, left panels). Transforming growth factor β, MTSN and SOD1, expression was suppressed in the NAC-treated muscles at 14 days after injury as compared to PBS-treated muscles (Fig. 3B–D, right panels). These data suggest that NAC treatment after injury accelerates the resolution of the inflammatory phase after injury leading to reduced tissue fibrosis (Fig. 3) and increased functional recovery (Fig. 2).
Although administration of NAC was associated with an indirect measure of decreased ROS production, decreased profibrotic gene expression, and decreased fibrotic tissue, it is unclear if the reduction in ROS due to NAC treatment directly resulted in these changes. We cannot exclude the possibility that NAC might indirectly act to enhance muscle regeneration. Further investigation is required to determine the mechanism by which NAC affects these changes.
Vascular injuries lead to cessation of blood flow resulting in ischemia, hypoxia, and tissue necrosis. Rapid revascularization and tissue perfusion is essential to maintain tissue integrity.48 One potential mechanism with which NAC could improve tissue regeneration is through the enhancement of neovascularization. Studies have demonstrated that NAC can promote angiogenesis and clearance of ROS during wound healing.49 In this study, we showed that NAC treatment significantly increased the numbers and diameter of blood vessels as well as the expression of vascular markers (HIF1, CD31, VEGF) after injury as compared to PBS-treated muscles. These data suggest that process of neovascularization was accelerated by NAC treatment (Fig. 5). Further study is required to determine the mechanism by which NAC increased angiogenesis.
Although this study provides important mechanistic insights into the protective role of NAC on muscle injury, several questions still remain. First, this study was limited to adult female rats and did not examine the effect of NAC on their adult male counterparts. Older rats were used because of their prolonged regenerative capacity as compared to young rats as previously demonstrated.50 Female rats were chosen because of the demonstration of increased tissue fibrosis after compartment syndrome injury as compared to age-matched male rats (data not shown). Further experiments will involve repeating the NAC treatment in adult male rats. Second, despite the fact that NAC-treated groups had improved function at day 14 postinjury compared to PBS, we were unable to detect any differences in function at day 28 postinjury between PBS- and NAC-treated rats, respectively. The tremendous regeneration capacity of rats results in resolution of injury within weeks, resulting in a small window for therapeutic improvement.24,50 However, treatment with NAC resulted in decreased MyoD and MyoG expression and larger muscle fiber cross section, suggesting that NAC-treated muscle is at a more progressive stage of regeneration.
In conclusion, our data revealed that administration of NAC after injury was associated with lower levels of ROS and tissue fibrosis, along with an increase in myofibers size (maturity) and vascularization. Enhanced tissue regeneration was associated with improved skeletal muscle function. NAC may be a viable option as a target for future therapeutic intervention in skeletal muscle injury. Further work needs to be done to determine the molecular mechanisms underlying these alterations in regeneration.
Presented as a poster at the 2018 Military Health System Research Symposium, August 2018, Kissimmee, FL (Abstract # MHSRS-18-0155).
The views expressed are solely those of the authors and do not reflect the official policy or position of the U.S. Army, U.S. Navy, U.S. Air Force, the Department of Defense, or the U.S. Government
FUNDING
NIH T32 Training Grant (5T32GM099606-02) and the Department of Defense (USAMRAA AFIRM W81XWH-08-2-0032).
References
- 1. Belmont PJ, Schoenfeld AJ, Goodman G: Epidemiology of combat wounds in operation Iraqi freedom and operation enduring freedom: orthopaedic burden of disease. J Surg Orthop Adv; 2010; 19: 2–7. [PubMed] [Google Scholar]
- 2. Owens BD, Kragh JF, Wenke JC, et al. : Combat wounds in operation Iraqi freedom and operation enduring freedom. J Trauma; 2008; 64: 295–9. [DOI] [PubMed] [Google Scholar]
- 3. Doukas WC, Hayda RA, Frisch HM, et al. : The military extremity trauma amputation/limb salvage (METALS) study: outcomes of amputation versus limb salvage following major lower-extremity trauma. J Bone Joint Surg Am; 2013; 95: 138–45. [DOI] [PubMed] [Google Scholar]
- 4. Jarvinen TA, Jarvinen M, Kalimo H: Regeneration of injured skeletal muscle after the injury. Muscles Ligaments Tendons J; 2013; 3: 337–45. [PMC free article] [PubMed] [Google Scholar]
- 5. Ghaly A, Marsh DR: Ischaemia-reperfusion modulates inflammation and fibrosis of skeletal muscle after contusion injury. Int J Exp Pathol; 2010; 91: 244–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mendias CL, Gumucio JP, Davis ME, et al. : Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve; 2012; 45: 55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garg K, Ward CL, Hurtgen BJ, et al. : Volumetric muscle loss: persistent functional deficits beyond frank loss of tissue. J Orthop Res; 2015; 33: 40–6. [DOI] [PubMed] [Google Scholar]
- 8. Kim J, Lee J: Role of transforming growth factor-beta in muscle damage and regeneration: focused on eccentric muscle contraction. J Exerc Rehabil; 2017; 13: 621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huard J, Bolia I, Briggs K, et al. : Potential usefulness of losartan as an antifibrotic agent and adjunct to platelet-rich plasma therapy to improve muscle healing and cartilage repair and prevent adhesion formation. Orthopedics; 2018; 41: e591–e7. [DOI] [PubMed] [Google Scholar]
- 10. Kobayashi M, Ota S, Terada S, et al. : The combined use of losartan and muscle-derived stem cells significantly improves the functional recovery of muscle in a young mouse model of contusion injuries. Am J Sports Med; 2016; 44: 3252–61. [DOI] [PubMed] [Google Scholar]
- 11. Bish LT, Yarchoan M, Sleeper MM, et al. : Chronic losartan administration reduces mortality and preserves cardiac but not skeletal muscle function in dystrophic mice. PLoS One; 2011; 6: e20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garg K, Corona BT, Walters TJ: Losartan administration reduces fibrosis but hinders functional recovery after volumetric muscle loss injury. J Appl Physiol; 2014; 117: 1120–31. [DOI] [PubMed] [Google Scholar]
- 13. Walton SL, Mazzuca MQ, Tare M, et al. : Angiotensin receptor blockade in juvenile male rat offspring: implications for long-term cardio-renal health. Pharmacol Res; 2018; 134: 320–31. [DOI] [PubMed] [Google Scholar]
- 14. Abe M, Takiguchi Y, Ichimaru S, Tsuchiya K, Wada K: Comparison of the protective effect of N-acetylcysteine by different treatments on rat myocardial ischemia-reperfusion injury. J Pharmacol Sci; 2008; 106: 571–7. [DOI] [PubMed] [Google Scholar]
- 15. Khanna G, Diwan V, Singh M, Singh N, Jaggi AS: Reduction of ischemic, pharmacological and remote preconditioning effects by an antioxidant N-acetyl cysteine pretreatment in isolated rat heart. Yakugaku Zasshi; 2008; 128: 469–77. [DOI] [PubMed] [Google Scholar]
- 16. Staiculescu MC, Foote C, Meininger GA, Martinez-Lemus LA: The role of reactive oxygen species in microvascular remodeling. Int J Mol Sci; 2014; 15: 23792–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andre-Levigne D, Modarressi A, Pepper MS, Pittet-Cuenod B: Reactive oxygen species and NOX enzymes are emerging as key players in cutaneous wound repair. Int J Mol Sci; 2017; 18: 2149–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pirotte N, Stevens AS, Fraguas S, et al. : Reactive oxygen species in planarian regeneration: an upstream necessity for correct patterning and brain formation. Oxidative Med Cell Longev; 2015; 2015: 392476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luo L, Sun Z, Zhang L, et al. : Effects of low-level laser therapy on ROS homeostasis and expression of IGF-1 and TGF-beta1 in skeletal muscle during the repair process. Lasers Med Sci; 2013; 28: 725–34. [DOI] [PubMed] [Google Scholar]
- 20. Aslan GI, Otgun I, Acer T, Tepeoglu M, Hicsonmez A: The effect of intraperitoneal n-acetylcysteine on postoperative adhesions in rat models. Ann Ital Chir; 2017; 88: 258–62. [PubMed] [Google Scholar]
- 21. Nogueira GB, Punaro GR, Oliveira CS, et al. : N-acetylcysteine protects against diabetic nephropathy through control of oxidative and nitrosative stress by recovery of nitric oxide in rats. Nitric Oxide; 2018; 78: 22–31. [DOI] [PubMed] [Google Scholar]
- 22. Rogliani P, Calzetta L, Cavalli F, Matera MG, Cazzola M: Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Pulm Pharmacol Ther; 2016; 40: 95–103. [DOI] [PubMed] [Google Scholar]
- 23. Shen Y, Miao NJ, Xu JL, et al. : N-acetylcysteine alleviates angiotensin II-mediated renal fibrosis in mouse obstructed kidneys. Acta Pharmacol Sin; 2016; 37: 637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Criswell TL, Corona BT, Ward CL, et al. : Compression-induced muscle injury in rats that mimics compartment syndrome in humans. Am J Pathol; 2012; 180: 787–97. [DOI] [PubMed] [Google Scholar]
- 25. Eakin K, Baratz-Goldstein R, Pick CG, et al. : Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One; 2014; 9: e90617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Topcu-Tarladacalisir Y, Tarladacalisir T, Sapmaz-Metin M, et al. : N-acetylcysteine counteracts oxidative stress and protects alveolar epithelial cells from lung contusion-induced apoptosis in rats with blunt chest trauma. J Mol Histol; 2014; 45: 463–71. [DOI] [PubMed] [Google Scholar]
- 27. Prabhu A, Sujatha DI, Kanagarajan N, Vijayalakshmi MA, Ninan B: Effect of N-acetylcysteine in attenuating ischemic reperfusion injury in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Ann Vasc Surg; 2009; 23: 645–51. [DOI] [PubMed] [Google Scholar]
- 28. Liou GY, Storz P: Detecting reactive oxygen species by immunohistochemistry. Methods Mol Biol; 2015; 1292: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li ZB, Kollias HD, Wagner KR: Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem; 2008; 283: 19371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lieber RL, Ward SR: Cellular mechanisms of tissue fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am J Physiol Cell Physiol; 2013; 305: C241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bentzinger CF, Wang YX, Rudnicki MA: Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol; 2012; 4: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Drowley L, Okada M, Beckman S, et al. : Cellular antioxidant levels influence muscle stem cell therapy. Mol Ther; 2010; 18: 1865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baumgardner JN, Shankar K, Hennings L, et al. : N-acetylcysteine attenuates progression of liver pathology in a rat model of nonalcoholic steatohepatitis. J Nutr; 2008; 138: 1872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duffield JS, Lupher M, Thannickal VJ, Wynn TA: Host responses in tissue repair and fibrosis. Annu Rev Pathol; 2013; 8: 241–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sekhon B, et al. : N-Acetyl cysteine protects against injury in a rat model of focal cerebral ischemia. Brain Res; 2003; 971: 1–8. [DOI] [PubMed] [Google Scholar]
- 36. Santangelo L, et al. : Evaluation of the antioxidant response in the plasma of healthy or hypertensive subjects after short-term exercise. J Hum Hypertens; 2003; 17: 791–798. [DOI] [PubMed] [Google Scholar]
- 37. Tyurin V, et al. : Oxidative stress following traumatic brain injury in rats: quantitation of biomarkers and detection of free radical intermediates. J Neurochem; 2000; 75: 2178–2189. [DOI] [PubMed] [Google Scholar]
- 38. Anderson M, et al. : Glutathione monoethylester prevents mitochondrial glutathione depletion during focal cerebral ischemia. Neurochem Int; 2004; 44: 153–159. [DOI] [PubMed] [Google Scholar]
- 39. Sochman J, et al. : N-acetylcysteine in acute cardiology: 10 years later: what do we know and what would we like to know? J Am Coll Cardiol; 2002; 39: 1422–1428. [DOI] [PubMed] [Google Scholar]
- 40. Oikawa S, et al. : N-acetylcysteine, a cancer chemopreventive agent, causes oxidative damage to cellular and isolated DNA. Carcinogenesis; 1999; 20: 1485–1490. [DOI] [PubMed] [Google Scholar]
- 41. Farr S, et al. : The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem; 2003; 84, 1173–1183. [DOI] [PubMed] [Google Scholar]
- 42. Eming SA, Martin P, Tomic-Canic M: Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med; 2014; 6: 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mauck RL, Burdick JA: From repair to regeneration: biomaterials to reprogram the meniscus wound microenvironment. Ann Biomed Eng; 2015; 43: 529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wynn TA: Cellular and molecular mechanisms of fibrosis. J Pathol; 2008; 214: 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pohlers D, Brenmoehl J, Löffler I, et al. : TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta; 2009; 1792: 746–56. [DOI] [PubMed] [Google Scholar]
- 46. Tsang CK, Chen M, Cheng X, et al. : SOD1 phosphorylation by mTORC1 couples nutrient sensing and redox regulation. Mol Cell; 2018; 70: 502–15 e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Y, Unnikrishnan A, Deepa SS, et al. : A new role for oxidative stress in aging: the accelerated aging phenotype in Sod1(−/)(−) mice is correlated to increased cellular senescence. Redox Biol; 2017; 11: 30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nauta TD, van Hinsbergh VW, Koolwijk P: Hypoxic signaling during tissue repair and regenerative medicine. Int J Mol Sci; 2014; 15: 19791–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aktunc E, Ozacmak VS, Ozacmak HS, et al. : N-acetyl cysteine promotes angiogenesis and clearance of free oxygen radicals, thus improving wound healing in an alloxan-induced diabetic mouse model of incisional wound. Clin Exp Dermatol; 2010; 35: 902–9. [DOI] [PubMed] [Google Scholar]
- 50. Zhou Y, Lovell D, Bethea M, et al. : Age-dependent changes cooperatively impact skeletal muscle regeneration after compartment syndrome injury. Am J Pathol; 2014; 184: 2225–36. [DOI] [PubMed] [Google Scholar]


