Abstract
Dietary vitamin A is metabolized into bioactive retinoic acid (RA) in vivo and regulates the development of many embryonic tissues. RA signaling is active in the oral ectoderm-derived tissues of the neuroendocrine system, but its role there has not yet been fully explored. We show here that RA signaling is active during pituitary organogenesis and dependent on the pituitary transcription factor Prop1. Prop1-mutant mice show reduced expression of the aldehyde dehydrogenase gene Aldh1a2, which metabolizes the vitamin A–intermediate retinaldehyde into RA. To elucidate the specific function of RA signaling during neuroendocrine development, we studied a conditional deletion of Aldh1a2 and a dominant-negative mouse model of inhibited RA signaling during pituitary organogenesis. These models partially phenocopy Prop1-mutant mice by exhibiting embryonic pituitary dysmorphology and reduced hormone expression, especially thyrotropin. These findings establish the role of RA in embryonic pituitary stem cell progression to differentiated hormone cells and raise the question of gene-by-environment interactions as contributors to pituitary development and disease.
Keywords: pituitary, development, retinoic acid, PROP1, ALDH1A2
Retinoic acid (RA) is a vitamin A–derived molecule that plays an important role during the development of many organs, including the eye, heart, and limb, by binding the retinoic acid receptors (RAR) and initiating gene transcription (1). Fate-mapping studies showed that all Rathke’s pouch–derived cells are exposed to RA signaling between e14.5 and e17.5 in mice (2), but little is known about the specific role of RA signaling in neuroendocrine organogenesis.
The pituitary gland is a neuroendocrine gland that releases hormones into the bloodstream to regulate growth, metabolism, stress response, reproduction, and pregnancy and lactation, and its development is tightly regulated by signaling pathways (3). Aldehyde dehydrogenase enzymes (ALDH1A1, ALDH1A2, ALDH1A3) that synthesize biologically active RA from retinaldehyde are expressed in the mouse and rat pituitary gland (4-7). Aldh1a2 mutant mice are embryonic lethal at e10.5 and have Rathke’s pouch aplasia and gross craniofacial defects (8), features that are also observed in quails born from vitamin A–deficient hens (9). Dietary vitamin A deficiency in humans primarily affects the eye and can impair growth (10), although there have not been focused studies on pituitary function in vitamin A–deficiency patients.
There are multiple in vitro observations that suggest that RA acts on pituitary cells to increase hormone production: There are RAR binding sites upstream of the Pou1f1, Gh, and Tshb genes (11-13); RA stimulates Pou1f1 and Gh expression in pituitary endocrine cell lines (14-16); RA induces differentiation of Pou1f1-only committed nonhormonal cells into Gh-expressing endocrine cells (17); RA activates expression of the pituitary-specific transcription factor Prop1 in vitro and ex vivo (18); and a K216E mutation in POU1F1 found in a patient with combined pituitary hormone deficiency (CPHD) abrogates Pou1f1 interaction with RARs, which appears to prevent RA-dependent autoregulation of Pou1f1 (19). There have been some contradictory observations, however. For example, RA inhibits pro-opiomelanocortin (POMC) and adrenocorticotropin (ACTH) expression in AtT20 mouse ACTH tumor cells and human primary ACTH tumor cells but not normal rat pituitary cells (20); and an RARα agonist increases Pomc expression in AtT20 cells (21). Additionally, vitamin A–deficiency in rats causes an elevation in thyrotropin β-subunit (Tshb) messenger RNA (mRNA) but does not affect growth hormone (Gh) expression (22). With these various effects on distinct endocrine cell types in primary and transformed cells, an in vivo study is needed to determine whether RA performs these roles simultaneously during pituitary development.
In this study, we identify Aldh1a2 as a novel downstream target of Prop1 in vivo and show that Prop1 is required for Aldh1a2 expression and activation of RA signaling during pituitary organogenesis. We reduce RA signaling during pituitary development using both a conditional deletion of Aldh1a2 and a conditional dominant-negative RA knockdown model, and show that RA is important for proper pituitary morphogenesis and hormone expression. Together, our work demonstrates that Prop1 activates pituitary RA signaling to regulate fundamental aspects of pituitary development. Because PROP1 is the most commonly mutated gene that causes CPHD in humans (23), our findings raise the question of whether modulation of the RA pathway or some dietary factors can interact with genetic risk factors to influence the severity of pituitary developmental disorders.
Materials and Methods
Mice
Mice were housed under 12-hour light and 12-hour dark cycles and food and water provided ad libitum as approved by the University of Michigan’s Institutional Animal Care and Use Committee. All mice used have been previously described. Mice carrying the Prop1df allele were originally obtained from Dr Andrzej Bartke (Southern Illinois University) (24) and maintained on a mixed background. RARE-LacZ mice on an outbred ICR background (JAX stock #008477) (25) were obtained from the Jackson Laboratory and crossed with the Prop1df strain. Hesx1Cre knock-in mice on a C57BL/6 background (26) were obtained from Dr Juan-Pedro Martinez-Barbera (Institute of Child Health). Mice bearing loxP sites flanking exon 4 of Aldh1a2 (Aldh1a2F) mice were previously generated by Dr Joseph Napoli (University of California, Berkeley) at University of California, Davis, and acquired from the Mutant Mouse Resource and Research Center at University of California, Davis, on a C57BL/6J background (MMRRC_041423-UCD) and crossed with Hesx1Cre/+ mice. Excision of exon 4 creates a frameshift and premature stop codon (ALDH1A2 p.T121Mfs*26) leading to nonsense-mediated decay. A schematic of the genetic construct used to generate these mice is available at http://hdl.handle.net/2027.42/151759. R26dnRAR mice (27) on a mixed/ C57BL/6 backcross were obtained from the Jackson Laboratory (JAX stock #029812) and crossed with Hesx1Cre/+ mice. Genotyping protocols are available in the referenced articles or on the repository facility websites.
Quantitative polymerase chain reaction
mRNA was extracted using the Qiagen RNeasy kit (Qiagen) and complementary DNA (cDNA) made using the Superscript II Reverse Transcriptase kit (ThermoFisher) according to manufacturer instructions. Quantitative polymerase chain reaction (qPCR) using Taqman probes (Thermo Fisher) and the ΔΔCT method was performed as previously described (28) with Gapdh (TaqMan Rodent Gapdh Control Reagents [VIC Probe], Applied Biosystems 4 308 313) or Hprt (see as follows) as housekeeping controls. The Taqman probes used were: Aes mm00507851_g1; Aldh1a1 mm_00657317_m1; Aldh1a2 mm00501306_m1; Aldh1a3 mm_00474049_m1; Cga mm00438189_m1; Gata2 mm00492301_m1; Gh mm00433590_g1; Hprt mm03024075_m1; Pomc mm00435874_m1; Pou1f1 mm00476852_m1; Prop1 mm00839471_m1; Sox2 mm03053810_s1; Tshb mm00437190_m1; and Zeb2 mm00497193_m1.
RNA in situ hybridization
The Aldh1a2 in situ probe containing the full-length mouse Aldh1a2 sequence (~2.3 kb) cloned into the pBluescript KS vector was obtained from Dr Donna Martin (University of Michigan). We performed nonradioactive in situ hybridization as previously described (28) on frozen sections and counterstained with HP yellow (Anatech Ltd) following the Allen Brain Atlas protocol.
LacZ/X-Gal staining
Staining for β-galactosidase activity in RARE-LacZ frozen sections was performed as previously described (29) and counterstained with HP yellow.
Transcript detection of LacZ
Primers used to detect mRNA/cDNA transcript expression were LacZ forward: CGTGGCCTGATTCATTCC, LacZ reverse: ATCCTCTGCATGGTCAGG, Hprt forward: GTCGGTGAAAAGGACCTCT, Hprt reverse: CACAGGA CTAGAACACCTGC. PCR was performed with an initial 2-minute hold at 94°C, then 30 cycles of 30 seconds at 94°C, 30 seconds at 55°C, and 30 seconds at 72°C, and a final 10-minute extension at 72°C.
Histology and immunofluorescence
Hematoxylin and eosin staining and immunofluorescence of paraffin sections were performed as previously described (28). Antibodies used were guinea pig anti-TSHB (National Hormone and Peptide Program [NHPP]) at 1:100 (30); rabbit anti-chorionic gonadotropin alpha (CGA) (NHPP) at 1:100 (31); rabbit anti-POU1F1 (kindly provided by Dr Simon Rhodes, Indiana University–Purdue University Indianapolis) at 1:100 (32); rabbit anti-POMC/ACTH (NHPP) at 1:100 (33), monkey anti-GH (NHPP) at 1:100 (34), rabbit antiprolactin (NHPP) at 1:100 (35), and guinea pig anti-LHB (NHPP) at 1:100 (36).
Imaging and statistical analyses
Microscopy images were captured using a Leica Leitz DMB microscope and Leica DFC310 FX camera. Images were analyzed and compiled in Adobe Photoshop CS6. Quantitative data show individual values, mean, and standard error of the mean, and were analyzed by Student’s unpaired t test. Conventional P value annotations were used: *P less than .05; **P less than .01; ***P less than .001.
Single-cell RNA sequencing
Pituitary glands were dissected from 1 P4 Hesx1Cre/+ and 1 P4 female Hesx1Cre/+; R26dnRAR/dnRAR mouse and dispersed by enzymatic mix for 4 hours, and single-cell 3’ RNA libraries were generated at the University of Michigan Advanced Genomics Core using the 10x Genomics Chromium controller following manufacturers’ instructions for v2 reagents and sequenced on a HiSeq4000 paired-end kit as previously described (37). Samples were demultiplexed and barcode processed using Cell Ranger Single Cell Software Suite 3.0.2. Demultiplexed fastq files were aligned to the University of California Santa Cruz mouse reference genome mm10 (GRCm38) amended for the extended 3’ untranslated region of Prop1 as previously described (37). Cell filtering, sample integration, uniform manifold approximation and projection (UMAP), and gene expression visualization were performed using the Seurat (v3.0.2) package in R (38) and figures compiled in Abode Photoshop CS6. Single-cell RNA sequencing (ScRNAseq) data described here are available on the National Center for Biotechnology Information Gene Expression Omnibus (39) using accession number GSE142074.
Results
Prop1 is required for retinoic acid signaling during pituitary development
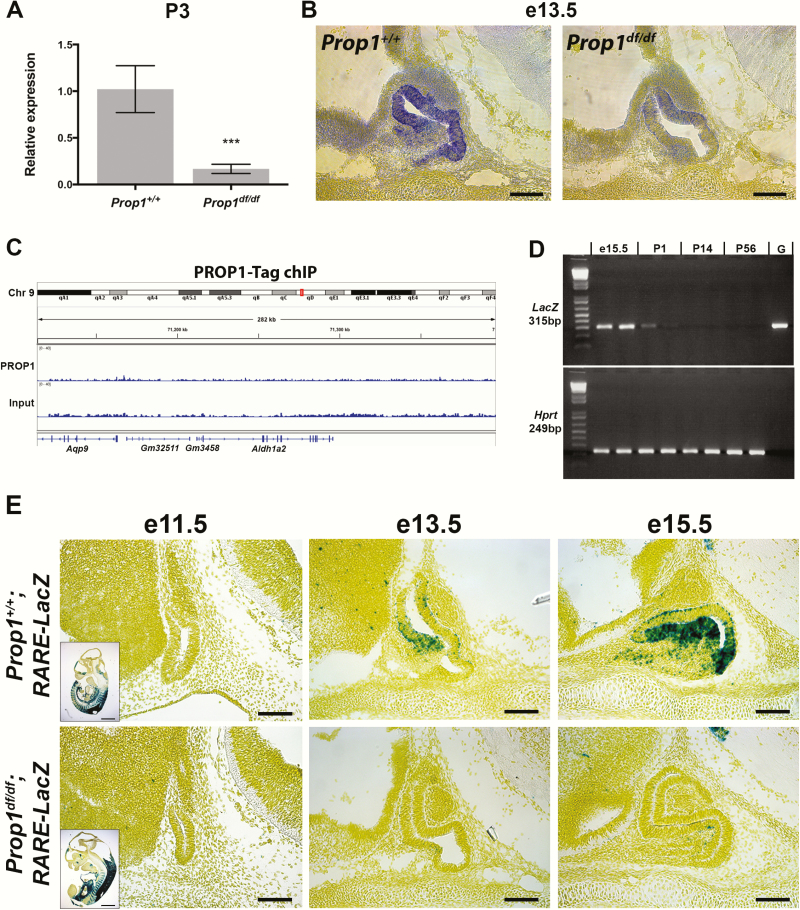
The mouse Prop1df allele is a spontaneous S83P missense mutation that inactivates the DNA binding domain, and homozygous mice are functionally equivalent to null mutants (40, 41). Downregulation of Aldh1a2 in newborn Prop1df/df mice was previously indicated from a cDNA microarray study (42) and was confirmed by qPCR at P3 (Fig. 1A). Expression of Aldh1a2 in the developing mouse pituitary was previously reported at e18.5, but a detailed analysis of pituitary expression was not conducted (5). We performed mRNA in situ hybridization and demonstrate that Aldh1a2 expression is detectable at e13.5 in the stem cell niche of normal pituitaries and strongly reduced in Prop1-mutant pituitaries (Fig. 1B). In wild-type e13.5 pituitaries, the reporter expression is highest both in the dorsal and ventral sides of the niche, with little expression detected in the transitional zone of the ventral side, where progenitors delaminate and migrate to form the anterior lobe. Previously published chromatin immunoprecipitation (ChIP) data from pituitary GHFT1 cells stably transfected with tagged Prop1 (43) do not show direct Prop1 binding within 100 kb in either direction of the Aldh1a2 genomic locus (Fig. 1C).
Figure 1.
Prop1 regulates pituitary Aldh1a2 expression and is required for proper retinoic acid signaling during pituitary development. A, Aldh1a2 expression is reduced in Prop1df/df mice as detected by quantitative polymerase chain reaction at P3 (n = 4 and 4). B, Aldh1a2 expression as detected by in situ hybridization is reduced at e13.5 in the developing pituitary of Prop1-mutants (n = 3 and 3). C, Chromatin immunoprecipitation using a tagged PROP1 in GHFT1 cells (data set previously published by Pérez Millán et al [43]) did not find direct PROP1 binding with 100 kb in either direction of the mouse Aldh1a2 locus. D, Reverse-transcriptase PCR for LacZ transcript in pituitary glands from RARE-LacZ reporter mice from different ages showed strongest LacZ expression (ie, RA signaling activity) during pituitary development. P14 and P56 samples are from male mice. Hprt transcript shown as positive control. Lane G, genomic DNA control. E, RA signaling, as reported by transgenic RARE-LacZ activity, is active between e13.5 and e15.5 in the normal pituitary. RA signaling activity is lost in Prop1-mutant pituitary glands at e13.5 and e15.5 (n = 3-7). Scale bars = 100 μm.
To elucidate the time points at which RA signaling is active in the pituitary gland, we used a transgenic reporter strain for RA signaling, which carries 3 RAR elements that bind RARs upstream of a minimal promoter and β-galactosidase expression cassette (RARE-LacZ) (25). Reverse-transcriptase PCR showed LacZ expression at e15.5 in the embryonic pituitary that rapidly decreases by postnatal day 1 (Fig. 1D). We could not detect the LacZ transcript in 2- and 8-week-old male RARE-LacZ pituitaries, suggesting that RA signaling is an important component of pituitary organogenesis (Fig. 1D). To assess whether the downregulation in Aldh1a2 in Prop1df/df embryos expression leads to a functional reduction in RA synthesis and signaling, we crossed the RARE-LacZ reporter mice into the Prop1 mutant strain. In reporter control mice, RA signaling in Rathke’s pouch is not detected at e11.5, but it begins at e13.5 in the transitional zone of Rathke’s pouch and is high at e15.5 in the same area (Fig. 1E, top row). Prop1df/df; RARE-LacZ embryos showed a near-total loss of RA signaling activity at e13.5 and e15.5 (Fig. 1E, bottom row). This demonstrates that Prop1 induction of Aldh1a2 expression during pituitary development correlates with activation of RA signaling in pituitary progenitor cells. Neither the RARE-LacZ mice in this study nor lineage-tracing in the RARE-Cre mice (2) showed detectable RA signaling activity in the posterior lobe or ventral diencephalon, indicating that RA generated by Aldh1a2-expressing cells in the oral ectoderm-derived Rathke’s pouch acts locally on cells fated to become hormone-producing cells of the anterior and intermediate lobes.
Conditional deletion of Aldh1a2 during pituitary development causes dysmorphology and reduction in thyrotropin
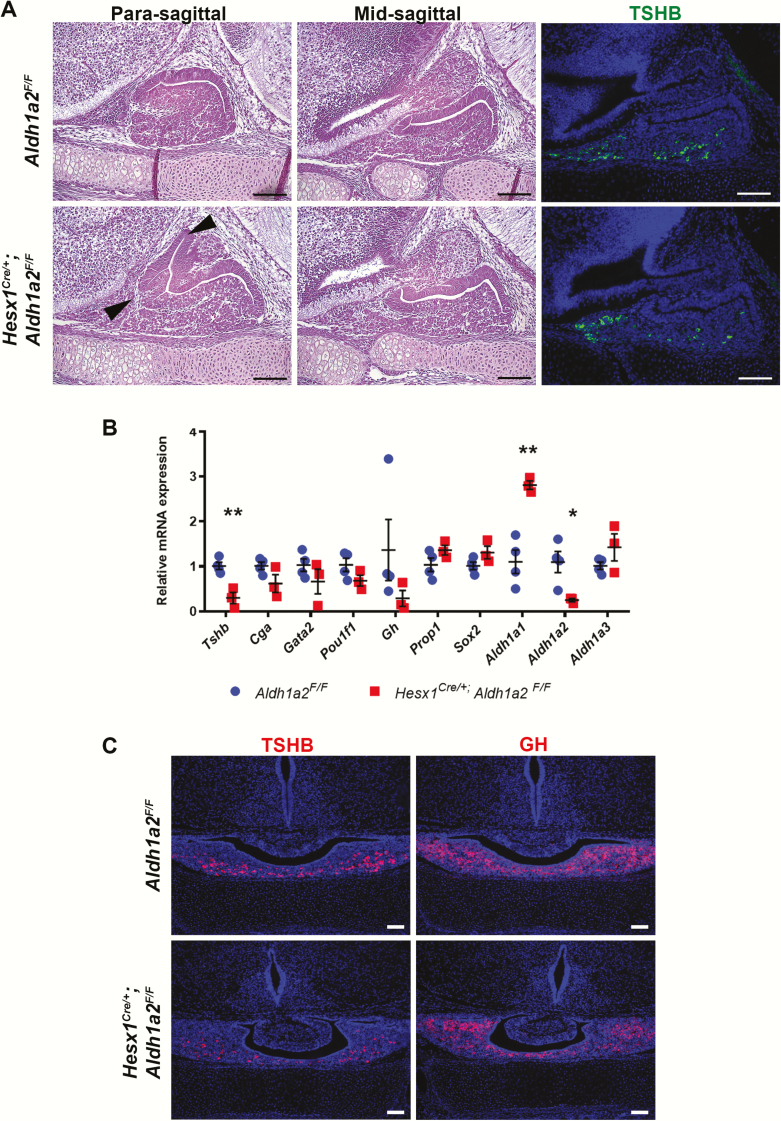
PROP1 is a transcription factor that is necessary for establishing the stem cell pool and driving pituitary stem cells to undergo and epithelial to mesenchymal-like transition process, and it directly regulates endocrine cell specification by activating Pou1f1 expression, which is essential for 3 hormone-producing cell types that produce growth hormone (GH), thyrotropin (TSH), and prolactin (40, 43). The spatial and temporal expression pattern of RA signaling suggested it could have a role in these Prop1-mediated processes. We investigated the role of RA signaling during pituitary development by using the Hesx1Cre knock-in strain (26) to conditionally delete Aldh1a2 during pituitary development. Aldh1a2–/– mice are embryonic-lethal at e10.5 (44) and show Rathke’s pouch formation defects (8). Hesx1Cre/+; Aldh1a2F/F embryos at e15.5 show intermediate lobe and marginal zone dysmorphology (Fig. 2A). Immunofluorescence for TSHB showed the presence of thyrotropes (Fig. 2A), whereas qPCR showed reduced Aldh1a2 expression as expected and a significant reduction in Tshb mRNA (Fig. 2B). No significant alterations in expression of other characteristic thyrotrope genes, such as Cga, Gata2, or Pou1f1, were observed. Aldh1a1 is upregulated, possibly to compensate for loss of Aldh1a2 expression (Fig. 2B). Immunofluorescence in P0 Hesx1Cre/+; Aldh1a2F/F mice show dysmorphology of the intermediate lobe, similar GH, and reduced TSHB (Fig. 2C).
Figure 2.
Conditional deletion of Aldh1a2 in the pituitary causes embryonic dysmorphology and reduced Tshb expression. A, E15.5 Hesx1Cre/+; Aldh1a2F/F embryos showed dysmorphology of the intermediate lobe and marginal zone (arrowheads), whereas the anterior lobe parenchyma appears normal. Thyrotropin β-subunit (TSHB) hormone protein is detected in the rostral tip and parenchyma of Hesx1Cre/+; Aldh1a2F/F embryos (n = 3 and 3). B, Quantitative polymerase chain reaction (qPCR) of e15.5 Hesx1Cre/+; Aldh1a2F/F pituitaries confirmed robust reduction of Aldh1a2. Tshb transcripts are reduced, whereas other thyrotrope markers such as Cga, Gata2, and Pou1f1 are not significantly altered (n = 3 and 4). C, Hesx1Cre/+; Aldh1a2F/F mice display mild dysmorphology of the intermediate lobe, comparable growth hormone (GH), and reduced TSHB at P0 (n = 3 and 3). Scale bars = 100 μm.
Inhibition of pituitary retinoic acid signaling by conditional overexpression of a dominant-negative retinoic acid receptor causes dysmorphology and reduction in thyrotropin
To overcome possible compensatory mechanisms in the conditional Aldh1a2 deletion model, such as increases in other RA-synthesizing enzymes or non-pituitary sources of RA, we used Hesx1Cre mice (26) to conditionally overexpress a dominant-negative truncated human RARα from the Rosa26 locus (27) during embryonic pituitary organogenesis. This truncated RAR acts as a dominant-negative on all 3 mouse RARs, thereby inhibiting RA signaling in pituitary cells from any source (45). We refer to this allele as R26dnRAR; it is also called RAR403 or RAR* in previous literature. This allele has previously been used effectively to inhibit RA signaling in vivo (27, 46-50).
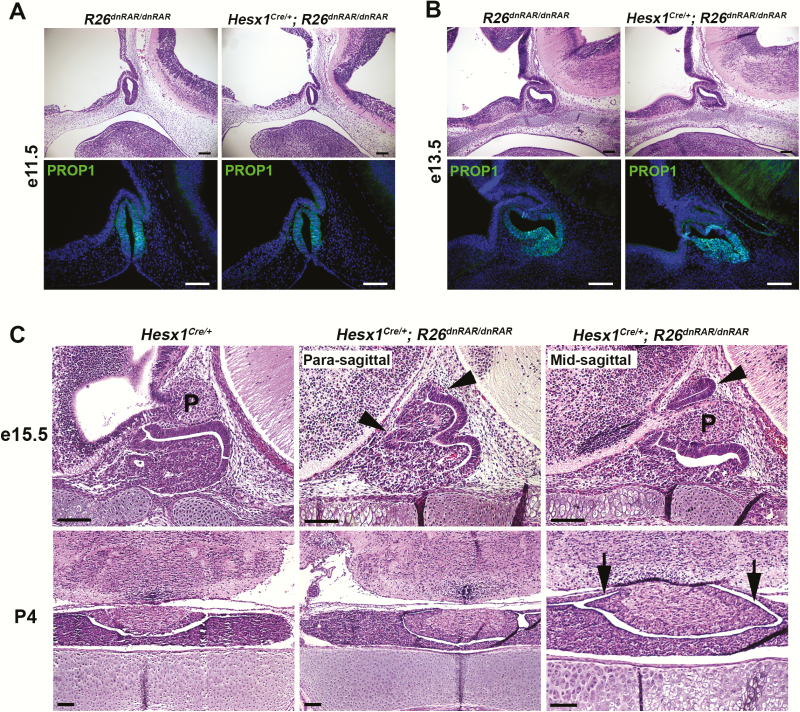
We examined Hesx1Cre/+; R26dnRAR/dnRAR embryos at e15.5 and found pituitary dysmorphology, as we did previously in Hesx1Cre/+; Aldh1a2F/F embryos, with the stem cell niche and prospective intermediate lobe extending dorsally around the posterior lobe (Fig. 3A). This intermediate lobe dysmorphology is more severe than in Hesx1Cre/+; Aldh1a2F/F embryos, and it is similar to Prop1df/df pituitary dysmorphology. However, neither Hesx1Cre/+; R26dnRAR/dnRAR nor Hesx1Cre/+; Aldh1a2F/F embryos showed obvious anterior lobe hypoplasia (Figs. 2A and 3). Intermediate lobe dysmorphology around the posterior lobe in Hesx1Cre/+; R26dnRAR/dnRAR mice persists at P4. Hesx1Cre/+; R26dnRAR/dnRAR mice are viable and do not show growth insufficiency, infertility, or other apparent signs of pituitary dysfunction.
Figure 3.
Conditional inhibition of pituitary retinoic acid (RA) signaling causes pituitary dysmorphology after e13.5. A and B, Hesx1Cre/+; R26dnRAR/dnRAR embryos at e11.5 and e13.5 show normal histology and PROP1 expression (e11.5: n = 3 and 3. e13.5: n = 2 and 2). C, Hesx1Cre/+; R26dnRAR/dnRAR embryos exhibit pituitary dysmorphology of the anterior and intermediate lobes at e15.5 (arrowheads), extending ventrally around the infundibulum/posterior lobe (P) (n = 3 and 3). Pituitary dysmorphology persists at P4, showing intermediate lobe dysmorphology around the posterior lobe (arrows) but normal anterior pituitary parenchyma (n = 2 and 3). Scale bars = 100 μm.
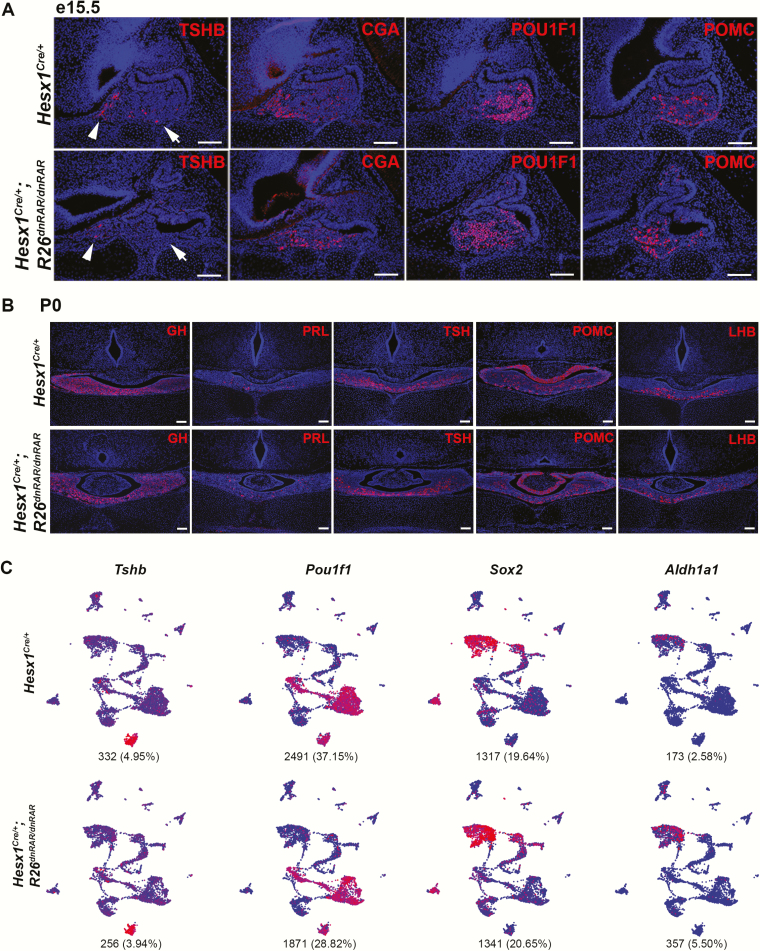
Immunofluorescence for lineage specification and hormone markers in this RA-inhibition model revealed a transient suppression of POU1F1-lineage cells. TSHB subunit protein is greatly diminished at e15.5 in Hesx1Cre/+; R26dnRAR/dnRAR embryonic pituitaries (Fig. 4A). Although low TSHB staining is detectable in the POU1F1-independent rostral tip region in conditional mutants, little or no staining is detected in the caudomedial region of the anterior lobe parenchyma where POU1F1-dependent thyrotropes reside. The glycoprotein α subunit CGA, expressed in (pre)-thyrotropes and (pre)-gonadotropes, was detected in the rostral tip region and caudomedial areas. The endocrine markers POU1F1 and POMC are also detected, indicating that the inhibition of RA signaling was not sufficient to prevent specification of those lineages. However, TSHB and all other anterior pituitary hormones are detectable at comparable expression to control animals at P0 (Fig. 4B).
Figure 4.
Inhibition of retinoic acid (RA) signaling during pituitary development delays pituitary hormone expression. A, Hesx1Cre/+; R26dnRAR/dnRAR embryos at e15.5 exhibit significant reduction in thyrotropin β-subunit (TSHB) expression both in the rostral tip (arrowheads) and anterior lobe (arrows). CGA and POU1F1 expression are detectable by immunofluorescence, indicating that thyrotrope specification has occurred (n = 3 and 3). B, Newborn Hesx1Cre/+; R26dnRAR/dnRAR mice display normal expression of anterior pituitary hormones, including TSHB, indicating a recovery of expression between e15.5 and P0 (n = 3 and 3). Scale bars = 100 μm. C, Uniform manifold approximation and projection (UMAP) of single-cell RNA sequencing of pituitary cells from 1 P4 Hesx1Cre/+; R26dnRAR/dnRAR mouse (6705 cells) and 1 female Hesx1Cre/+ mouse (6492 cells). Blue = low expression, red = high expression. Number and percentage of cells per genotype expressing each gene are under each plot. The conditional mutant possesses all cell populations present in the Cre-control animal. Cells expressing Tshb and Pou1f1, 2 genes significantly affected at e15.5 in the conditional mutant, are comparable in number between the P4 Hesx1Cre/+; R26dnRAR/dnRAR and control animal. The proportion of Sox2-expressing stem cells remains the same in the conditional mutant, although more cells express Aldh1a1.
scRNAseq was performed on pituitary cells from a P4 female Hesx1Cre/+ and a P4 female Hesx1Cre/+; R26dnRAR/dnRAR mouse and analyzed and visualized by UMAP in Seurat. We recovered 13 197 total cells, consisting of 6705 Hesx1Cre/+ cells and 6492 Hesx1Cre/+; R26dnRAR/dnRAR cells (Fig. 4C). The UMAP split by genotype shows that all cell populations detected in the Cre control are also detected in the conditional mutant. Cells expressing Tshb and Pou1f1, which were significantly reduced in Hesx1Cre/+; R26dnRAR/dnRAR embryos at e15.5, are comparable in number between the conditional mutant and control at P4. The number of cells expressing Aldh1a1 is increased, whereas the number of Sox2-expressing stem cells remains unchanged.
Inhibition of RA signaling alters embryonic pituitary stem cell marker expression but not stem cell clonogenic capacity
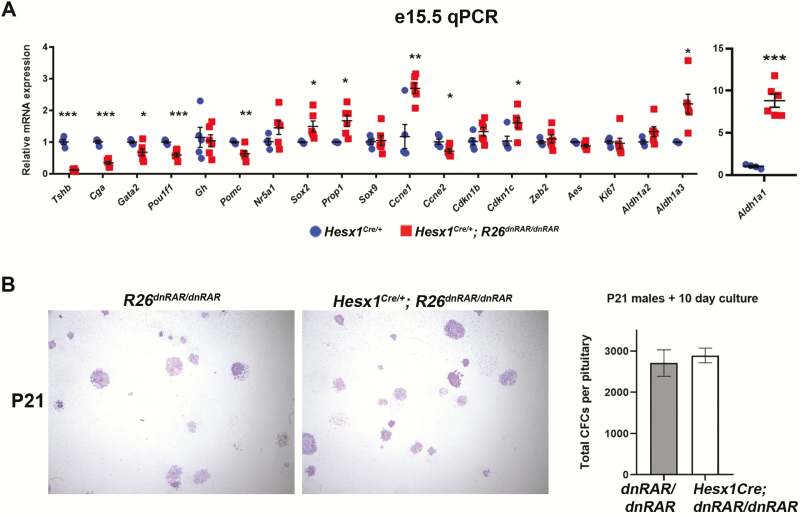
qPCR of e15.5 Hesx1Cre/+; R26dnRAR/dnRAR pituitary glands and littermate controls confirmed that inhibition of RA signaling severely reduces Tshb expression (Fig. 5A), and further showed quantitative reductions in the other thyrotrope genes Cga, Pou1f1, and Gata2 (Fig. 4A). Pomc expression is reduced by approximately 50%, whereas Gh expression is unchanged, but mRNA levels of the (pre)-gonadotrope marker Nr5a1 (formerly Sf1) are not statistically different.
Figure 5.
Inhibition of pituitary retinoic acid (RA) signaling upregulates expression of stem cell and transitional markers but does not increase clonogenic potential. A, Quantitative polymerase chain reaction (qPCH) in e15.5 Hesx1Cre/+; R26dnRAR/dnRAR embryos confirms severe downregulation of Tshb seen by immunofluorescence. Other thyrotrope markers such as Cga, Gata2, and Pou1f1 are reduced but to a lesser degree than Tshb. Expression of the melanotrope/corticotrope marker Pomc is also reduced. Expression of the stem cell markers Sox2 and Prop1, as well as cell cycle progression markers Ccne1 and Cdkn1c, are elevated at e15.5 by qPCR, whereas EMT/migration genes Zeb2 and Aes are not. Aldh1a1 and Aldh1a3 are upregulated in the conditional mutant pituitaries. (n = 4 and 6). B, Three-week-old R26dnRAR/dnRAR and Hesx1Cre/+; R26dnRAR/dnRAR male pituitary glands were dispersed and assessed for colony-forming potential after 10 days in culture. No significant differences were noted between genotypes. Conditional mutant stem cells form both densely packed and sparse-flat types of colonies and comparable numbers of colonies per pituitary gland (n = 3 and 3).
We found transcriptional changes in a number of genes associated with pituitary stem cells and the transition to differentiation (Fig. 5A). Sox2 and Prop1 are elevated in the absence of RA signaling, as is the transition marker Cdkn1c (ie, p57/Kip2). Cyclin E regulates cell cycle progression into S phase: Confusingly, Ccne1 is increased, whereas Ccne2 is reduced in Hesx1Cre/+; R26dnRAR/dnRAR pituitaries. Expression of the transition and migration regulators Zeb2 and Aes are not changed. Expression both of Aldh1a1 and Aldh1a3 are upregulated in Hesx1Cre/+; R26dnRAR/dnRAR embryos.
To determine the effect of these stem cell gene expression changes, we performed pituitary stem cell colony–forming assays to assess their clonogenic potential in vitro (43, 51). Three-week-old male pituitary glands from Hesx1Cre/+; R26dnRAR/dnRAR mice and control littermates were dispersed and cultured for 10 days, and then stained and counted. Conditional mutant pituitary stem cells formed colonies normal in number and histology comparable to controls (Fig. 5B).
Discussion
In this study, we demonstrated that PROP1 is required for robust embryonic pituitary RA signaling. A recent in vitro study showed that RAR agonists can increase Prop1 expression in rat pituitary cell lines (18). Using the RARE-LacZ reporter strain, we first detected evidence of pituitary RA signaling at e13.5, several days after Prop1 expression is initiated. Expression of Aldh1a2, the major RA synthetic enzyme gene expressed in the developing pituitary gland, was not detected at e11.5, before Prop1 expression, and Aldh1a2 expression was reduced in Prop1df/df mutants based on in situ hybridization during gestation and by microarray analysis (42) and qPCR of gene expression in newborns. Finally, the RARE-LacZ reporter revealed no evidence of RA signaling during pituitary development in Prop1df/df mutants. This places Prop1 gene expression upstream of RA signaling genetically. It is possible that RA could feedback and upregulate Prop1 gene expression.
PROP1 may regulate Aldh1a2 transcription indirectly. We do not detect direct binding of PROP1 without 200 kb of the genomic locus surrounding Aldh1a2 on mouse chromosome 9 in ChIP data from a GHFT1 cell line stably transfected to express a tagged PROP1 (43), but these immortalized cells have many differences relative to normal pituitary cells in vivo. The limited amount of embryonic pituitary tissue makes ChIP difficult and there are no ChIP-grade PROP1 antibodies currently available.
We first used Hesx1Cre/+; Aldh1a2F/F mice to study the role of pituitary RA signaling as a direct test of the reduced Aldh1a2 expression and subsequent RA signaling in Prop1-mutant mice. Hesx1Cre/+; Aldh1a2F/F embryos partially phenocopy Prop1-mutant mice with dysmorphology of the stem cell niche and reduced TSH. The related Aldh1a1 gene was upregulated, potentially providing compensation for Aldh1a2 deficiency. We continued our studies using a dominant-negative model that should ablate RAR activation regardless of the source of RA (27, 46-50). Hesx1Cre/+; R26dnRAR/dnRAR embryos display more severe features of phenotypes seen in Hesx1Cre/+; Aldh1a2F/F embryos, indicating that it is likely more effectively reducing RA signaling during pituitary development. The Hesx1Cre/+; R26dnRAR/dnRAR animals demonstrate that RA signaling is required for maximal embryonic expression of Pou1f1, Tshb, and Gh. The observed reductions in Pou1f1 and Gh are in line with predictions from previous in vitro data that demonstrated RA is required for RAR/POU1F1 interaction and binding to the distal Pou1f1 enhancer that maintains Pou1f1 expression after Prop1-mediated activation (13, 19).
Hesx1 Cre/+; R26dnRAR/dnRAR mice overcome the defect in Pou1f1-lineage activation and do not display physiological symptoms of CPHD, in contrast to the reported K216E mutation in POU1F1 that affected RA signaling and was found in a human case of CPHD (19). There are multiple plausible explanations for the lack of persistent hypopituitarism in Hesx1Cre/+; R26dnRAR/dnRAR mice: GH deficiency in mice must be severe to cause growth insufficiency and dwarfism (52), and growth in humans may be more sensitive to milder reduction in GH. The lack of postnatal CPHD phenotypes in Hesx1Cre/+; R26dnRAR/dnRAR mice could result from compensatory gene expression changes, incomplete blockade of RA signaling, and/or feedback regulation from target organs such as the thyroid gland. The truncated dnRAR heterodimerizes with endogenous RARs and retinoid X receptors (RXRs) to interfere with their ability respond to RA (45), and alteration of RA signaling in the developing inner ear feeds back to alter expression of some RAR and RXR proteins (46). Aldh1a1 and Aldh1a3 expression is upregulated in the developing pituitary gland of conditional mutants, potentially increasing RA biosynthesis. Additionally, PROP1 directly activates Pou1f1 (40), which then autoregulates its own expression (13), so it is likely that the initial reduction in Pou1f1, Tshb, and other hormone markers observed in Hesx1Cre/+; R26dnRAR/dnRAR embryos is gradually overcome with accumulating POU1F1 protein. Although RA inhibition in these 2 models does not appear to lead to permanent, physiologically significant pituitary dysfunction in mice, they clearly reveal a functional role for RA signaling in pituitary progenitor cells, for maintenance of proper developmental morphology, and for timely activation of the Pou1f1-lineage. Aldh1a1/2 expression in the postnatal rodent pituitary is concentrated in stem cells (6, 37), suggesting RA signaling may play a role in those cells under certain circumstances
Multiple genes are required for normal behavior of pituitary stem cells as they transition to differentiation. The zinc-finger homeodomain transcription factor ZEB2 is a downstream target of Prop1 that regulates Gli2 and Cdh1 expression in pituitary stem cells (43). It is not known whether defects in Zeb2 alter RA signaling or cause dysmorphology because Zeb2-null mice are embryonic lethal (53) and pituitary-conditional Zeb2 knockout mice have not yet been described. Mutant mice lacking the Groucho/TLE transcriptional co-activator/co-repressor Aes display pituitary dysmorphology and reduced GH immunostaining (54). The molecular mechanisms causing pituitary dysmorphology in Aes–/– mutants are not known. Because RA inhibition does not alter Aes or Zeb2 expression, these 2 genes do not appear to account for pituitary dysmophology in the RA inhibition model. Ultimately, there are likely to be converging mechanisms that result in proper pituitary morphogenesis, including RA signaling.
The elevation of Prop1 expression in Hesx1Cre/+; R26dnRAR/dnRAR mice may result from blocked differentiation of Prop1- and Sox2-expressing progenitors. The spatiotemporal expression pattern of RA signaling in normal mice, together with the dysmorphology and gene expression changes in Hesx1Cre/+; R26dnRAR/dnRAR mice, is consistent with RA signaling stimulating embryonic pituitary progenitor migration and the transition to differentiation.
We observed a reduction in Pomc expression at e15.5, indicating that RA positively regulates embryonic Pomc expression, similar to in vitro data showing RARα agonists increase Pomc expression in AtT20 cells (21). Treatment with RA in the same AtT20 cell line conversely decreases Pomc expression (20), suggesting RA may be reducing Pomc/ACTH expression in these ACTH-tumor cells through additional mechanisms than the direct binding of RA to its receptors and RAR-mediated transcriptional activity.
Defects in genes that cause CPHD can manifest with severe developmental abnormalities affecting craniofacial development, sensory perception, and intellectual development, and there are no obvious genotype-phenotype correlations (23). For example, patients with loss-of-function mutations in HESX1 and GLI2 can present with septo-optic dysplasia, holoprosencephaly, or nonsyndromic CPHD. This variability is likely attributable to variation in other genes that affect pituitary development (ie, oligogenic contribution), environmental factors, and/or epigenetic events. Our demonstration that RA plays a role in the developing pituitary leads us to consider the role of exogenous and environmental factors on pituitary development. Whereas many signaling ligands (eg, Notch, Wnt) are encoded by endogenous genes, bioactive RA is wholly derived from dietary vitamin A. Vitamin A deficiency is especially common in developing regions, and 1 in 6 pregnant women globally are vitamin A deficient (World Health Organization Global Database on Vitamin A Deficiency, 2009). Vitamin A deficiency remains a prevalent health burden in sub-Saharan Africa, south Asia, and rural China (55, 56), and maternal vitamin A deficiency increases the risk for visual impairments and facial clefting in children (57, 58). Although pituitary gland dysfunction was not specifically assessed in these epidemiology studies, it often presents together with visual impairment and midline defects such as clefting (59). Studies in birds and mammalian model organisms have demonstrated that maternal vitamin A deficiency causes developmental defects in the progeny, including craniofacial, vision, and pituitary gland abnormalities (9, 60-63). Our data in genetically engineered mice show a direct effect of diminished RA signaling on pituitary development, which is more tractable than nutritional studies. There is intrinsic merit in establishing the basic molecular mechanism whereby RA regulates pituitary development. This study sets the stage for future preclinical studies that assess whether subclinical vitamin A deficiency increases the penetrance or severity of hypopituitarism in genetically susceptible mice.
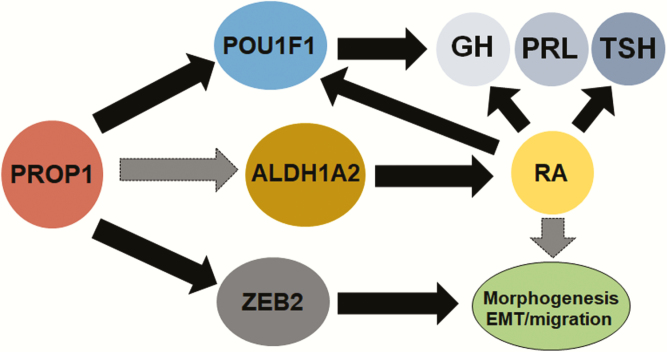
In conclusion, we show that a novel function of Prop1 is to activate the RA signaling pathway through the induction of the RA-synthesizing enzyme ALDH1A2 (Fig. 6). Using a conditional Aldh1a2 knockout and a dominant-negative model, we demonstrate that RA signaling contributes to proper pituitary progenitor migration, organ morphogenesis, and expression of Pou1f1 and hormone genes. Our study unveils a novel aspect of pituitary development and leads us to consider the influence of exogenous and environmental factors on neuroendocrine development and whether they may account for any unexplained cases of congenital pituitary hormone deficiency in humans.
Figure 6.
Model schematic integrating retinoic acid (RA) signaling with other pathways dependent on PROP1. In this study, we show that PROP1 is required for proper Aldh1a2 expression and RA signaling during embryonic pituitary development, although we do not yet have evidence of whether PROP1 direct or indirectly regulates Aldh1a2 gene expression. RA signaling is required for timely expression of lineage markers and pituitary hormones, especially for thyrotropin (TSH), in conjunction with other known mechanisms such as Pou1f1-regulated hormone expression. RA signaling also regulates pituitary morphogenesis, likely together with other converging pathways regulating proper pituitary morphogenesis.
Acknowledgments
We thank our collaborators for generously providing the materials as indicated. We thank Judy Opp, Bob Lyons, and other members of the University of Michigan Advanced Genomics Core for help with the single-cell RNA sequencing.
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- CGA
chorionic gonadotropin alpha
- ChIP
chromatin immunoprecipitation
- CPHD
combined pituitary hormone deficiency
- dnRAR
dominant-negative retinoic acid receptor
- GH
growth hormone
- POMC
pro-opiomelanocortin
- R26
Rosa26 genomic locus
- RA
retinoic acid
- RAR
retinoic acid receptors
- scRNAseq
single-cell RNA sequencing
- TSH
thyrotropin
- UMAP
uniform manifold approximation and projection
Financial Support: This work was supported by the National Institutes of Health (Grants R01HD34283 and R01HD30428 to S.A.C.). Aldh1a2F mice were generated by Dr Joseph Napoli at University of California, Davis (National Institutes of Health Grant DK112754).
Author Contributions: L.Y.M.C. and S.A.C. designed the experiments. L.Y.M.C. performed the experiments and analyzed the data. L.Y.M.C. and S.A.C. interpreted the data. L.Y.M.C. and S.A.C. wrote the manuscript.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
References
- 1. Cunningham TJ, Duester G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat Rev Mol Cell Biol. 2015;16(2):110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dollé P, Fraulob V, Gallego-Llamas J, Vermot J, Niederreither K. Fate of retinoic acid-activated embryonic cell lineages. Dev Dyn. 2010;239(12):3260–3274. [DOI] [PubMed] [Google Scholar]
- 3. Cheung LY, Davis SW, Brinkmeier ML, Camper SA, Pérez-Millán MI. Regulation of pituitary stem cells by epithelial to mesenchymal transition events and signaling pathways. Mol Cell Endocrinol. 2017;445:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Niederreither K, Fraulob V, Garnier JM, Chambon P, Dollé P. Differential expression of retinoic acid-synthesizing (RALDH) enzymes during fetal development and organ differentiation in the mouse. Mech Dev. 2002;110(1-2):165–171. [DOI] [PubMed] [Google Scholar]
- 5. Niederreither K, McCaffery P, Dräger UC, Chambon P, Dollé P. Restricted expression and retinoic acid-induced downregulation of the retinaldehyde dehydrogenase type 2 (RALDH-2) gene during mouse development. Mech Dev. 1997;62(1):67–78. [DOI] [PubMed] [Google Scholar]
- 6. Fujiwara K, Kikuchi M, Takigami S, Kouki T, Yashiro T. Expression of retinaldehyde dehydrogenase 1 in the anterior pituitary glands of adult rats. Cell Tissue Res. 2007;329(2):321–327. [DOI] [PubMed] [Google Scholar]
- 7. Fujiwara K, Maekawa F, Kikuchi M, Takigami S, Yada T, Yashiro T. Expression of retinaldehyde dehydrogenase (RALDH)2 and RALDH3 but not RALDH1 in the developing anterior pituitary glands of rats. Cell Tissue Res. 2007;328(1):129–135. [DOI] [PubMed] [Google Scholar]
- 8. Ribes V, Wang Z, Dollé P, Niederreither K. Retinaldehyde dehydrogenase 2 (RALDH2)-mediated retinoic acid synthesis regulates early mouse embryonic forebrain development by controlling FGF and sonic hedgehog signaling. Development. 2006;133(2):351–361. [DOI] [PubMed] [Google Scholar]
- 9. Maden M, Blentic A, Reijntjes S, Seguin S, Gale E, Graham A. Retinoic acid is required for specification of the ventral eye field and for Rathke’s pouch in the avian embryo. Int J Dev Biol. 2007;51(3):191–200. [DOI] [PubMed] [Google Scholar]
- 10. Sommer A. Vitamin A deficiency and clinical disease: an historical overview. J Nutr. 2008;138(10):1835–1839. [DOI] [PubMed] [Google Scholar]
- 11. Breen JJ, Hickok NJ, Gurr JA. The rat TSHbeta gene contains distinct response elements for regulation by retinoids and thyroid hormone. Mol Cell Endocrinol. 1997;131(2):137–146. [DOI] [PubMed] [Google Scholar]
- 12. García-Villalba P, Au-Fliegner M, Samuels HH, Aranda A. Interaction of thyroid hormone and retinoic acid receptors on the regulation of the rat growth hormone gene promoter. Biochem Biophys Res Commun. 1993;191(2):580–586. [DOI] [PubMed] [Google Scholar]
- 13. Rhodes SJ, Chen R, DiMattia GE, et al. A tissue-specific enhancer confers Pit-1-dependent morphogen inducibility and autoregulation on the pit-1 gene. Genes Dev. 1993;7(6):913–932. [DOI] [PubMed] [Google Scholar]
- 14. Bedo G, Santisteban P, Aranda A. Retinoic acid regulates growth hormone gene expression. Nature. 1989;339(6221):231–234. [DOI] [PubMed] [Google Scholar]
- 15. Morita S, Fernandez-Mejia C, Melmed S. Retinoic acid selectively stimulates growth hormone secretion and messenger ribonucleic acid levels in rat pituitary cells. Endocrinology. 1989;124(5):2052–2056. [DOI] [PubMed] [Google Scholar]
- 16. Sánchez-Pacheco A, Palomino T, Aranda A. Retinoic acid induces expression of the transcription factor GHF-1/Pit-1 in pituitary prolactin- and growth hormone-producing cell lines. Endocrinology. 1995;136(12):5391–5398. [DOI] [PubMed] [Google Scholar]
- 17. Mogi C, Goda H, Mogi K, et al. Multistep differentiation of GH-producing cells from their immature cells. J Endocrinol. 2005;184(1):41–50. [DOI] [PubMed] [Google Scholar]
- 18. Yoshida S, Fujiwara K, Nishihara H, Kato T, Yashiro T, Kato Y. Retinoic acid signalling is a candidate regulator of the expression of pituitary-specific transcription factor Prop1 in the developing rodent pituitary. J Neuroendocrinol. 2018;30(3):e12570. [DOI] [PubMed] [Google Scholar]
- 19. Cohen LE, Zanger K, Brue T, Wondisford FE, Radovick S. Defective retinoic acid regulation of the Pit-1 gene enhancer: a novel mechanism of combined pituitary hormone deficiency. Mol Endocrinol. 1999;13(3):476–484. [DOI] [PubMed] [Google Scholar]
- 20. Páez-Pereda M, Kovalovsky D, Hopfner U, et al. Retinoic acid prevents experimental Cushing syndrome. J Clin Invest. 2001;108(8):1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uruno A, Saito-Hakoda A, Yokoyama A, et al. Retinoic acid receptor-α up-regulates proopiomelanocortin gene expression in AtT20 corticotroph cells. Endocr J. 2014;61(11):1105–1114. [DOI] [PubMed] [Google Scholar]
- 22. Breen JJ, Matsuura T, Ross AC, Gurr JA. Regulation of thyroid-stimulating hormone beta-subunit and growth hormone messenger ribonucleic acid levels in the rat: effect of vitamin A status. Endocrinology. 1995;136(2):543–549. [DOI] [PubMed] [Google Scholar]
- 23. Fang Q, George AS, Brinkmeier ML, et al. Genetics of combined pituitary hormone deficiency: roadmap into the genome Era. Endocr Rev. 2016;37(6):636–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bartke A. The response of two types of dwarf mice to growth hormone, thyrotropin, and thyroxine. Gen Comp Endocrinol. 1965;5(4):418–426. [DOI] [PubMed] [Google Scholar]
- 25. Rossant J, Zirngibl R, Cado D, Shago M, Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5(8):1333–1344. [DOI] [PubMed] [Google Scholar]
- 26. Andoniadou CL, Signore M, Sajedi E, et al. Lack of the murine homeobox gene Hesx1 leads to a posterior transformation of the anterior forebrain. Development. 2007;134(8):1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rajaii F, Bitzer ZT, Xu Q, Sockanathan S. Expression of the dominant negative retinoid receptor, RAR403, alters telencephalic progenitor proliferation, survival, and cell fate specification. Dev Biol. 2008;316(2):371–382. [DOI] [PubMed] [Google Scholar]
- 28. Cheung LYM, Okano H, Camper SA. Sox21 deletion in mice causes postnatal growth deficiency without physiological disruption of hypothalamic-pituitary endocrine axes. Mol Cell Endocrinol. 2017;439:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davis SW, Keisler JL, Pérez-Millán MI, Schade V, Camper SA. All hormone-producing cell types of the pituitary intermediate and anterior lobes derive from Prop1-expressing progenitors. Endocrinology. 2016;157(4):1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SciCrunch. Guinea Pig Anti-Mouse Thyroid-stimulating hormone antibody. RRID: AB_2756856. https://scicrunch.org/resolver/AB_2756856. SciCrunch website. Accessed January 22, 2020.
- 31.SciCrunch. RRID: AB_2814823. Rabbit anti-rat glycoprotein hormones, alpha (CGA/aGSU) antibody. https://scicrunch.org/resolver/AB_2814823. SciCrunch website. Accessed January 22, 2020.
- 32.SciCrunch. RRID: AB_2722652. Anti Pit-1 antibody. https://scicrunch.org/resolver/AB_2722652. SciCrunch website. Accessed January 22, 2020.
- 33.SciCrunch. RRID: AB_2665562. Rabbit anti-Human ACTH antibody. https://scicrunch.org/resolver/AB_2665562. SciCrunch website. Accessed January 22, 2020.
- 34.SciCrunch. RRID: AB_2665564. Monkey anti-Rat GH antibody. https://scicrunch.org/resolver/AB_2665564. SciCrunch website. Accessed January 22, 2020.
- 35.SciCrunch. RRID: AB_2629220. Rabbit anti-Rat Prolactin Antibody. https://scicrunch.org/resolver/AB_2629220. SciCrunch website. Accessed January 22, 2020.
- 36.SciCrunch. RRID: AB_2665565. Guinea Pig anti-Rat LHβ antibody. https://scicrunch.org/resolver/AB_2665565. SciCrunch website. Accessed January 22, 2020.
- 37. Cheung LYM, George AS, McGee SR, et al. Single-Cell RNA sequencing reveals novel markers of male pituitary stem cells and hormone-producing cell types. Endocrinology. 2018;159(12):3910–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stuart T, Butler A, Hoffman P, et al. Comprehensive integration of single-cell data. Cell. 2019;177(7):1888–1902.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sornson MW, Wu W, Dasen JS, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384(6607):327–333. [DOI] [PubMed] [Google Scholar]
- 41. Nasonkin IO, Ward RD, Raetzman LT, et al. Pituitary hypoplasia and respiratory distress syndrome in Prop1 knockout mice. Hum Mol Genet. 2004;13(22):2727–2735. [DOI] [PubMed] [Google Scholar]
- 42. Brinkmeier ML, Davis SW, Carninci P, et al. Discovery of transcriptional regulators and signaling pathways in the developing pituitary gland by bioinformatic and genomic approaches. Genomics. 2009;93(5):449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pérez Millán MI, Brinkmeier ML, Mortensen AH, Camper SA. PROP1 triggers epithelial-mesenchymal transition-like process in pituitary stem cells. Elife. 2016;5:e14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21(4):444–448. [DOI] [PubMed] [Google Scholar]
- 45. Damm K, Heyman RA, Umesono K, Evans RM. Functional inhibition of retinoic acid response by dominant negative retinoic acid receptor mutants. Proc Natl Acad Sci U S A. 1993;90(7):2989–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brown CC, Esterhazy D, Sarde A, et al. Retinoic acid is essential for Th1 cell lineage stability and prevents transition to a Th17 cell program. Immunity. 2015;42(3):499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li P, Pashmforoush M, Sucov HM. Mesodermal retinoic acid signaling regulates endothelial cell coalescence in caudal pharyngeal arch artery vasculogenesis. Dev Biol. 2012;361(1):116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pino-Lagos K, Guo Y, Brown C, et al. A retinoic acid-dependent checkpoint in the development of CD4+ T cell-mediated immunity. J Exp Med. 2011;208(9):1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ruane D, Brane L, Reis BS, et al. Lung dendritic cells induce migration of protective T cells to the gastrointestinal tract. J Exp Med. 2013;210(9):1871–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shen H, Cavallero S, Estrada KD, et al. Extracardiac control of embryonic cardiomyocyte proliferation and ventricular wall expansion. Cardiovasc Res. 2015;105(3):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gaston-Massuet C, Andoniadou CL, Signore M, et al. Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc Natl Acad Sci U S A. 2011;108(28):11482–11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Waite E, Lafont C, Carmignac D, et al. Different degrees of somatotroph ablation compromise pituitary growth hormone cell network structure and other pituitary endocrine cell types. Endocrinology. 2010;151(1):234–243. [DOI] [PubMed] [Google Scholar]
- 53. Higashi Y, Maruhashi M, Nelles L, et al. Generation of the floxed allele of the SIP1 (Smad-interacting protein 1) gene for Cre-mediated conditional knockout in the mouse. Genesis. 2002;32(2):82–84. [DOI] [PubMed] [Google Scholar]
- 54. Brinkmeier ML, Potok MA, Cha KB, et al. TCF and Groucho-related genes influence pituitary growth and development. Mol Endocrinol. 2003;17(11):2152–2161. [DOI] [PubMed] [Google Scholar]
- 55. Abolurin OO, Adegbola AJ, Oyelami OA, Adegoke SA, Bolaji OO. Prevalence of vitamin A deficiency among under-five children in South-Western Nigeria. Niger Postgrad Med J. 2018;25(1):13–16. [DOI] [PubMed] [Google Scholar]
- 56. Hamer DH, Keusch GT. Vitamin A deficiency: slow progress towards elimination. Lancet Glob Health. 2015;3(9):e502–e503. [DOI] [PubMed] [Google Scholar]
- 57. Mitchell LE, Murray JC, O’Brien S, Christensen K. Retinoic acid receptor alpha gene variants, multivitamin use, and liver intake as risk factors for oral clefts: a population-based case-control study in Denmark, 1991-1994. Am J Epidemiol. 2003;158(1):69–76. [DOI] [PubMed] [Google Scholar]
- 58. Johansen AM, Lie RT, Wilcox AJ, Andersen LF, Drevon CA. Maternal dietary intake of vitamin A and risk of orofacial clefts: a population-based case-control study in Norway. Am J Epidemiol. 2008;167(10):1164–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McCabe MJ, Dattani MT. Genetic aspects of hypothalamic and pituitary gland development. Handb Clin Neurol. 2014;124:3–15. [DOI] [PubMed] [Google Scholar]
- 60. Jackson B, Kinsey VE. The relation between maternal vitamin-A intake, blood level, and ocular abnormalities in the offspring of the rat. Am J Ophthalmol. 1946;29:1234–1242. [DOI] [PubMed] [Google Scholar]
- 61. Warkany J, Roth CB, Wilson JG. Multiple congenital malformations; a consideration of etiologic factors. Pediatrics. 1948;1(4):462–471. [PubMed] [Google Scholar]
- 62. Warkany J, Schraffenberger E. Congenital malformations induced in rats by maternal vitamin A deficiency; defects of the eye. Arch Ophthal. 1946;35:150–169. [DOI] [PubMed] [Google Scholar]
- 63. Wilson JG, Barch S. Fetal death and maldevelopment resulting from maternal vitamin A deficiency in the rat. Proc Soc Exp Biol Med. 1949;72(3):687–693. [DOI] [PubMed] [Google Scholar]