Abstract
BACKGROUND:
Comprehensive longitudinal studies are important for understanding the complex risk factors, pathways, exposures and interactions that lead to the development and persistence of asthma. We aimed to examine associations between use of household cleaning products in early life and childhood respiratory and allergic disease using data from the Canadian Healthy Infant Longitudinal Development (CHILD) Cohort Study.
METHODS:
We summed responses from parental questionnaires that indicated the frequency of use of 26 household cleaning products in the homes of 2022 children from this birth cohort when they were 3–4 months of age to create a cumulative Frequency of Use Score (FUS). We used multivariable logistic regression models to assess whether frequent compared with less frequent use was associated with recurrent wheeze, atopy or asthma diagnosis, as defined by the questionnaire and clinical assessments at age 3 years. Data were collected between 2008 and 2015.
RESULTS:
Children in homes with a higher frequency of use of cleaning products in infancy, as determined by an interquartile range increase, had higher odds of recurrent wheeze (adjusted odds ratio [OR] 1.35, 95% confidence interval [CI] 1.11–1.64), recurrent wheeze with atopy (adjusted OR 1.49, 95% CI 1.02–2.16) and asthma diagnosis (adjusted OR 1.37, 95% CI 1.09–1.70), but no increase in the odds of atopy at age 3 years (adjusted OR 1.14, 95% CI 0.96–1.35). Compared with the lowest tertile of FUS exposure, infants in the highest tertile had higher odds of acquiring asthma. Stratification of the results showed that females had higher ORs than males for all outcomes, although the p values for this sex difference did not reach statistical significance.
INTERPRETATION:
Frequent use of household cleaning products in early life was associated with an increased risk for childhood wheeze and asthma but not atopy at age 3 years. Our findings add to the understanding of how early life exposures to cleaning products may be associated with the development of allergic airway disease and help to identify household behaviours as a potential area for intervention.
The prevalence of childhood asthma has steadily increased over the past several decades and is now a leading cause of childhood chronic disease and admissions to hospital in developed countries, making it a priority for clinicians, researchers and the public.1 The first months of life are critical for the development of the immune and respiratory systems. 2 By identifying hazardous exposures and behaviours during infancy, preventive measures could be implemented to potentially reduce childhood asthma and allergy risk.
Most of the evidence that chronic low-level exposure to the irritants in cleaning products causes chronic inflammation, triggers asthma symptoms and worsens asthma control3 comes from domestic and occupational studies in adult populations.4–7 Young children, who spend 80%–90% of their time indoors in early life,8,9 are especially vulnerable because of their increased respiration rate and proximity to the ground, which increases gaseous and dermal exposures. Analyzing the effects of exposures to cleaning products is complex and challenging. Many approaches for the estimation of indoor pollution can be found in the existing literature.10 These include odour recognition, physical presence, questionnaires and composite scoring,6,11–14 ambient air measures of volatile organic compounds8,11,15 or a combination of assessments.16
We aimed to examine the association between the frequency of use of cleaning products in early infancy (3–4 months of age) and childhood asthma or its precursors at 3 years of age, using data from the Canadian Healthy Infant Longitudinal Development (CHILD) Cohort Study.17
Methods
Study design and population
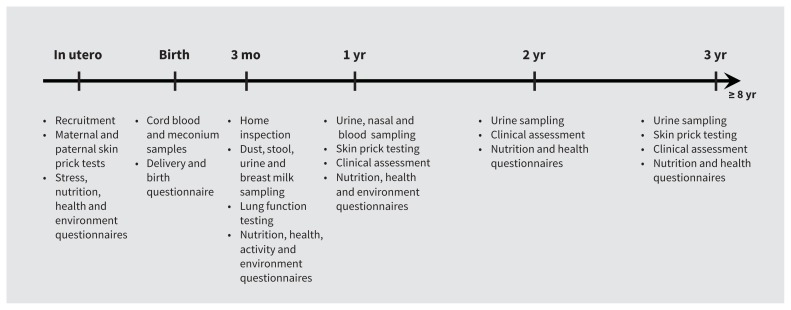
The CHILD Study is an ongoing longitudinal prospective evaluation of a birth cohort following infants from pregnancy through childhood that involves 3455 children recruited from largely urban centres in 4 Canadian provinces (Vancouver; Edmonton; Winnipeg, Morden and Winkler, Manitoba; and Toronto) to reflect the Canadian, largely urban, population.17 Questionnaires relating to environmental exposures, psychosocial stresses, nutrition and general health were completed by parents at recruitment in the second or third trimesters of pregnancy and when their children are 3, 6, 12, 18, 24, 30 and 36 months of age.17 Periodic clinical assessments, skin prick tests and biological sampling of children are also conducted (Figure 1). For our evaluation, we used data that were collected between 2008 and 2015.
Figure 1:
Data collection in the first 3 years of the Canadian Healthy Infant Longitudinal Development (CHILD) Cohort Study.
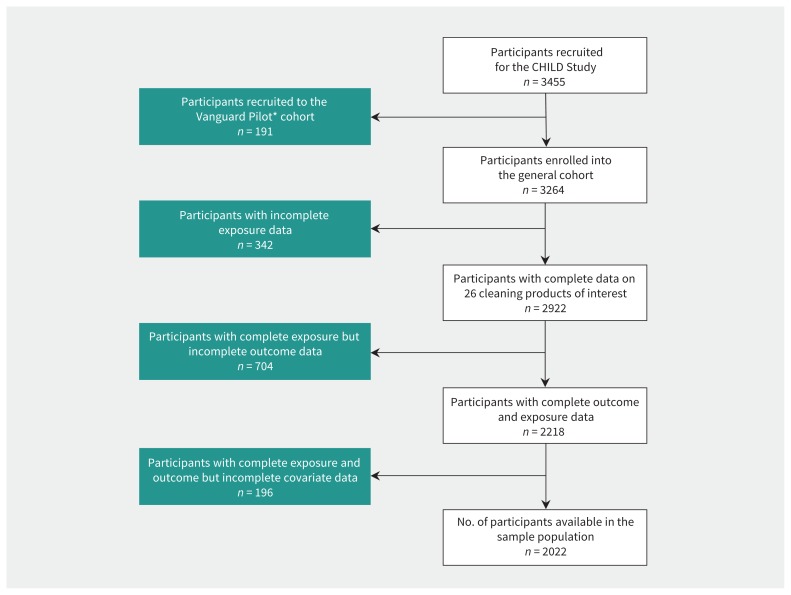
We excluded responses for children in the Vanguard cohort because the questionnaires were not identical to those used for the General cohort, and we also excluded responses for children with incomplete data for exposure, outcomes or covariates ( Figure 2). In total, 1242 participants were excluded from our available General cohort of 3264 because of missing data ( Appendix 1, Supplement Tables S2a and S2b, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190819/-/DC1). We included 2022 participants in our analysis.
Figure 2:
Participant selection for the study. CONSORT diagram illustrating sequential exclusion of children with incomplete data for exposures, outcomes and covariates. Note: CHILD = Canadian Healthy Infant Longitudinal Development Cohort. *Participants in the Vanguard Pilot study were a subset of the CHILD cohort study used to test the exposure assessment approaches. Questionnaires used in the Vanguard Pilot group differed slightly from the questionnaires used for the rest of the CHILD Cohort Study.
Exposure assessment
When infants were 3–4 months of age, parents completed a questionnaire on household exposures to cleaning products and indicated how often, on average, specified categories of products were used within the home. Scores were assigned for each product to reflect whether they used the product: never (score = 0), less than monthly (score = 1), monthly (score = 2), weekly (score = 3), or daily (score = 4). We derived a Frequency of Use Score (FUS) for each participant by summing the scores assigned to their questionnaire responses for the 26 categories of products. Each of the 26 products had the same values assigned. Therefore, different participants can have the same FUS score with a different mix of products. For example, someone who uses only 4 products but uses them all daily will have the same FUS as someone who uses 8 products on a monthly basis. The purpose of the FUS is to assign each participant a score that acts as a proxy to reflect the cumulative frequency of cleaning products used in the home.
Outcomes
We assessed asthma diagnosis, atopy, recurrent wheeze and a combination of recurrent wheeze and atopy at 3 years of age. We considered a child as having asthma if their parent responded “Yes” to “Was your child diagnosed with asthma?” in the questionnaire or if asthma was diagnosed by a CHILD clinician at the 3-year face-to-face clinical assessment.
Because asthma diagnosis at age 3 years is often difficult or unreliable, we considered additional outcome measures. We determined atopic status by skin tests, as previously detailed,18 for 13 inhalant and 4 food allergens. We considered a child to have recurrent wheeze if they had 2 or more episodes of wheeze in the previous 12 months, as determined by parent-reported health questionnaire or by presentation in a clinical or hospital setting. We defined an episode of wheeze as “a whistling sound coming from his/her chest with or without a cold for at least 15 minutes at a time, with episodes separated from each other by at least 7 days.” We characterized those patients who had both atopy and recurrent wheeze as having “recurrent wheeze with atopy.” Wheezing in early childhood, particularly if recurrent and accompanied by atopy, is an important predictor of future risk for childhood asthma, with abnormal airway function and a higher responsiveness to environmental exposures throughout life.19–23
Covariates
We selected covariates for inclusion based on a priori assessment of the literature for confounding,12,20,21,24,25 statistically significant bivariate analysis for association with the exposure variable and at least 1 of our health outcomes, and overall model fit. Covariates used included parental history of asthma, child sex and ethnicity, household income, city/location, prenatal or early life smoking exposure, pet ownership and visible mould in the home. Appendix 1 details other covariates that we considered but did not include in the final model.
We considered a patient to have a parental history of asthma if at least 1 parent responded when asked “Have you ever had asthma?”. Household income was reported using 5 levels: 1 = $0–$49 999, 2 = $50 000–99 999, 3 = $100 000–149 999, 4 = $150 000 or more and 5 = prefer not to say. If both parents reported that they were white in the questionnaire, we classified their child as white; for any other combination, child ethnicity was categorized as other. If responses for children up to 3 months of age indicated that the child had been exposed to tobacco smoke in or around the home, or an odour of tobacco smoke was detected during the home assessment at 3–4 months of age by the CHILD research assistant, we classified the child as exposed to tobacco smoke. Visible mould in the home was noted during the home assessment by the research assistant when the child was 3–4 months of age. We determined pet ownership based on parental response to “Have you had any furry pets living in your home since you completed this questionnaire during your pregnancy?”.
Statistical analysis
We used multivariable logistic regression models adjusted for all covariates to examine the relation between the FUS and health outcomes of interest among participants at 3 years of age. This analysis was repeated after stratification by sex.
Participants were ranked into categories of exposure risk based on tertiles of FUS distribution, which yielded 3 groups of roughly equal size. We calculated odds ratios (ORs), 95% confidence intervals (CIs) and p values comparing participants with moderate and high frequencies of exposure to those with a low frequency of exposure using multivariable logistic regression models. Additionally, we analyzed FUS as a continuous predictor to compare participants at the 75th percentile to those at the 25th percentile of the FUS distribution, also known as an interquartile range increase (IQRI). We conducted a 1-sided Cochran–Armitage test for trend in continuous and categorical models. We also used multivariable logistic regression models to compare different frequencies of use for each product with health outcomes, comparing participants with frequent exposure (daily or weekly) with those with infrequent exposure (monthly, less than monthly or never).
We adjusted all models for prenatal or early life exposure to tobacco smoke, sex, child’s ethnicity, parental asthma history, household income, city/location, visible mould in the home and pet ownership. We used R version 3.3.1 for all statistical analyses.
Ethics approval
This study was approved by research ethics boards at each recruitment site and through the Hamilton Integrated Research Ethics Board (certificate No. 07–2929).
Results
Most of the 2022 included participants were white (n = 1313, 64.9%), had no tobacco smoke exposure up to 3–4 months of age (n = 1545, 76.4%) and did not have a parental history of asthma (n = 1308, 64.7%). Pets were present in 57% (n = 862) of homes, visible mould was seen in 41.9% (n = 847) of homes, and 51.8% (n = 1047) of families had an annual income over $100 000 (Appendix 1). The most frequently used products were hand dishwashing detergent, dishwasher detergent, multisurface cleaners, glass cleaners and laundry detergents (Table 1). Among our sample population, 13.8% (n = 280) were atopic, 8.6% (n = 174) had recurrent wheeze, 6.4% (n = 129) had received an asthma diagnosis and 2.1% (n = 42) had recurrent wheeze with atopy at 3 years of age, with a higher proportion of all outcomes except atopy present at a higher exposure level (Table 2). The FUS ranged from 5 to 76 units, with a median score of 31 (IQR 24–37) units. The FUS had a 25th percentile of 24 units and a 75th percentile of 37 units (Appendix 1). An IQRI in FUS (13 units) was associated with significantly higher odds of recurrent wheeze (adjusted OR 1.35, 95% CI 1.11–1.64), recurrent wheeze with atopy (adjusted OR 1.49, 95% CI 1.02–2.16), and asthma diagnosis (adjusted OR 1.37, 95% CI 1.09–1.70), but not the odds of atopy (adjusted OR 1.14, 95% CI 0.96–1.35) (Table 3). The association between the IQRI and outcomes was stronger for girls than boys for all outcomes, but the p values for interaction were not statistically significant (Table 3). Analysis by tertiles of frequency of exposure showed nonsignificant trends toward associations with recurrent wheeze (adjusted OR 1.26, 95% CI 0.85–1.88), recurrent wheeze with atopy (adjusted OR 1.76, 95% CI 0.81–4.06) and asthma (adjusted OR 1.57, 95% CI 0.98–2.53), but not with atopy alone (adjusted OR 1.17, 95% CI 0.85–1.63) when compared with participants with a low frequency of exposure (Table 4). Again, effect estimates were larger in girls than in boys. The risks of these outcomes were higher for those in households that were frequent users of liquid or solid air fresheners, spray air fresheners, plug-in deodorizers, dusting sprays, antimicrobial hand sanitizers and oven cleaners compared with infrequent users (Appendix 1).
Table 1:
Use of cleaning products as reported by parents for children at 3–4 months of age
| Cleaning product | No. (%) of responses reporting use of cleaning products n = 2022 |
Average use score* | ||||
|---|---|---|---|---|---|---|
| Never | < Monthly | Monthly | Weekly | Daily | ||
| Hand dishwashing detergent | 44 (2.2) | 9 (0.4) | 17 (0.8) | 124 (6.1) | 1828 (90.4) | 3.82 |
| Dishwasher detergent | 343 (17.0) | 23 (1.1) | 39 (1.9) | 506 (26.0) | 1111 (54.9) | 3.00 |
| Multisurface cleaner | 404 (20.0) | 139 (6.9) | 329 (16.3) | 891 (44.1) | 259 (12.8) | 2.23 |
| Scented laundry detergent | 611 (30.2) | 40 (2.0) | 66 (3.3) | 975 (48.2) | 330 (16.3) | 2.18 |
| Toilet bowl cleaner | 377 (18.6) | 83 (4.1) | 440 (21.8) | 1084 (53.6) | 38 (1.9) | 2.16 |
| Unscented laundry detergent | 696 (34.4) | 14 (0.7) | 26 (1.3) | 856 (42.3) | 430 (21.3) | 2.15 |
| Glass cleaner | 549 (27.2) | 178 (8.8) | 479 (23.7) | 751 (37.1) | 65 (3.2) | 1.80 |
| Eco and organic cleaners | 800 (39.6) | 76 (3.8) | 222 (11.0) | 647 (32.0) | 277 (13.7) | 1.77 |
| Alcohol-type hand sanitizer | 928 (45.9) | 218 (10.8) | 181 (9.0) | 356 (17.6) | 339 (16.8) | 1.49 |
| Floor cleaner | 778 (38.5) | 228 (11.3) | 459 (22.7) | 530 (26.2) | 27 (1.3) | 1.41 |
| Fabric softener | 1080 (53.4) | 48 (2.4) | 77 (3.8) | 631 (31.2) | 186 (9.2) | 1.40 |
| Disinfectant in home | 1059 (52.4) | 161 (8.0) | 302 (14.9) | 443 (21.9) | 57 (2.8) | 1.15 |
| Bleach | 944 (46.7) | 426 (21.1) | 355 (17.6) | 272 (13.4) | 25 (1.2) | 1.01 |
| Bathroom tile cleaner | 1153 (57.0) | 153 (7.6) | 361 (17.9) | 344 (17.0) | 11 (0.5) | 0.96 |
| Spray air freshener | 1280 (63.3) | 256 (12.7) | 113 (5.6) | 253 (12.5) | 120 (5.9) | 0.85 |
| Solvent (paint or polish remover) | 1074 (53.1) | 572 (28.3) | 294 (14.5) | 82 (4.1) | – | 0.70 |
| Liquid or solid air freshener | 1550 (76.7) | 140 (6.9) | 83 (4.1) | 106 (5.2) | 143 (7.1) | 0.59 |
| Disinfectant in bedrooms | 1538 (76.1) | 94 (4.7) | 154 (7.6) | 218 (10.8) | 18 (0.9) | 0.56 |
| Dusting polish or spray | 1593 (78.8) | 122 (6.0) | 145 (7.2) | 156 (7.7) | 6 (0.3) | 0.45 |
| Plug-in air freshener | 1697 (83.9) | 92 (4.5) | 46 (2.3) | 37 (1.8) | 150 (7.4) | 0.44 |
| Furniture polish | 1631 (80.7) | 140 (6.9) | 144 (7.1) | 106 (5.2) | 1 (< 0.1) | 0.37 |
| Oven cleaner | 1554 (76.9) | 344 (17.0) | 98 (4.8) | 25 (1.2) | 1 (< 0.1) | 0.31 |
| Drain cleaner | 1526 (75.5) | 399 (19.7) | 67 (3.3) | 21 (1.0) | 9 (0.4) | 0.31 |
| Chemical hand cleaner | 1840 (91.0) | 93 (4.6) | 42 (2.1) | 31 (1.5) | 16 (0.8) | 0.17 |
| Floor polish | 1875 (92.7) | 63 (3.1) | 51 (2.5) | 33 (1.6) | – | 0.13 |
| Silver or brass polish | 1927 (95.3) | 74 (3.7) | 16 (0.8) | 4 (0.2) | 1 (< 0.1) | 0.06 |
Note: FUS = Frequecy of Use Score, – = no response for use of a particular product on a daily basis. The distribution of responses for self-reported average frequency of use for each cleaning product that we considered in creation of the FUS is shown.
We calculated an average use score for each product by summing the total number of frequency of use points (never = 0, < monthly = 1, monthly = 2, weekly = 3 and daily = 4) that were generated by the product, divided by the number of participants in our sample (n = 2022), then rounded to 2 decimal places. Smaller values indicate a lower overall frequency of use for that product.
Table 2:
Distribution of health outcomes in participants at 3 years of age, by frequency of use of cleaning products
| Health outcome | Total no. (%) of participants n = 2022 |
No. (%) of participants | ||
|---|---|---|---|---|
| Households with low use of cleaning products (FUS < 27) n = 666 |
Households with moderate use of cleaning products (FUS ≥ 27 and ≤ 35) n = 700 |
Households with high use of cleaning products (FUS > 35) n = 656 |
||
| Recurrent wheeze | 174 (8.6) | 51 (7.7) | 52 (7.4) | 71 (10.8) |
| Recurrent wheeze and atopy* | 42 (2.1) | 10 (1.5) | 12 (1.7) | 20 (3.0) |
| Asthma diagnosis | 129 (6.4) | 32 (4.8) | 45 (6.4) | 52 (7.9) |
| Atopy* | 280 (13.8) | 90 (13.5) | 93 (13.3) | 97 (14.8) |
Note: FUS = Frequency of Use Score. The Distribution of health outcomes among FUS catagories is shown with crude values and percentages for each tertile of the exposure group. The distributions of covariates by tertiles are shown in Appendix 1, Table S1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190819/-/DC1.
We determined atopic status as a positive response to at least 1 of 13 inhalant allergens: Alternaria alternata, cat hair, dog epithelium, house dust mites (Dermatophagoides pteronyssinus and Dermatophagoides farina), grass, Midwest trees, ragweed, weeds, cladosporium, penicillium, aspergillus and German cockroach, and 4 food allergens (whole cow’s milk, egg white, soybean and peanuts).
Table 3:
Health outcomes in children at 3 years of age per interquartile range increase in the Frequency of Use Score for household use of cleaning products*†
| Outcome phenotype | OR (95% CI) | Sex‡ FUS p value |
Trend¶ p value |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Overall sample population (IQRI = 13 points) n = 2022 |
Boys (IQRI = 14 points) n = 1047 |
Girls (IQRI = 13 points) n = 975 |
|||||||
|
|
|
|
|
||||||
| Unadj. | Adj. | Unadj. | Adj. | Unadj. | Adj. | Unadj. | Adj. | ||
| Recurrent wheeze | 1.45 (1.20–1.75) | 1.35 (1.11–1.64) | 1.27 (0.97–1.66) | 1.17 (0.89–1.55) | 1.78 (1.33–2.38) | 1.66 (1.22–2.25) | 0.07 | 0.09 | 0.0001 |
|
| |||||||||
| Recurrent wheeze and atopy | 1.65 (1.15–2.35) | 1.49 (1.02–2.16) | 1.37 (0.81–2.29) | 1.19 (0.98–1.05) | 2.18 (1.26–3.66) | 2.14 (1.17–3.86) | 0.18 | 0.18 | 0.003 |
|
| |||||||||
| Asthma diagnosis | 1.44 (1.16–1.78) | 1.37 (1.09–1.70) | 1.36 (1.00–1.85) | 1.32 (0.96–1.81) | 1.60 (1.14–2.23) | 1.48 (1.03–2.09) | 0.40 | 0.52 | 0.0004 |
|
| |||||||||
| Atopy | 1.10 (0.94–1.29) | 1.14 (0.96–1.35) | 1.03 (0.81–1.29) | 1.11 (0.87–1.41) | 1.20 (0.94–1.51) | 1.17 (0.90–1.50) | 0.34 | 0.29 | 0.12 |
Note: Adj. = adjusted, CI = confidence interval, FUS = Frequecy of Use Score, IQRI = interquartile range increase, OR = odds ratio, Unadj. = unadjusted.
We derived all values using multivariable logistic regression models.
Models were adjusted for prenatal or early-life exposure to tobacco smoke, sex, child’s ethnicity, parental asthma history, household income, city/location, visible mould in the home and pet ownership.
For the sex-stratified analysis, we adjusted for all covariates except sex. The p value of the interaction term when included in the regression model for the overall sample is reported.
We used a 1-sided Cochran–Armitage test (unadjusted) to evaluate increasing trend, by continuous FUS score for each health outcome.
Table 4:
Results for health outcomes at 3 years, by tertiles of Frequency of Use Score for cleaning products*†
| Outcome phenotype | OR (95% CI) | Sex‡ FUS p value |
Trend¶ p value |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Overall sample population n = 2022 |
Boys n = 1047 |
Girls n = 975 |
|||||||
|
|
|
|
|
||||||
| Unadj. | Adj. | Unadj. | Adj. | Unadj. | Adj. | Unadj. | Adj. | ||
| Recurrent wheeze | 0.02 | ||||||||
|
| |||||||||
| Moderate | 0.97 (0.65–1.45) | 0.93 (0.61–1.40) | 1.05 (0.64–1.71) | 0.96 (0.57–1.60) | 0.89 (0.43–1.89) | 0.91 (0.43–1.95) | 0.47 | 0.62 | |
|
| |||||||||
| High | 1.46 (1.01–2.14) | 1.26 (0.85–1.88) | 1.03 (0.63–1.69) | 0.88 (0.52–1.49) | 2.52 (1.35–4.94) | 2.33 (1.20–4.75) | 0.04 | 0.04 | |
|
| |||||||||
| Recurrent wheeze and atopy | 0.02 | ||||||||
|
| |||||||||
| Moderate | 1.14 (0.49–2.73) | 1.13 (0.47–2.74) | 1.53 (0.56–4.53) | 1.43 (0.51–4.36) | 1.58 (0.31–11.45) | 1.83 (0.34–13.49) | 0.27 | 0.32 | |
|
| |||||||||
| High | 2.06 (0.98–4.63) | 1.76 (0.81–4.06) | 1.75 (0.66–5.14) | 1.46 (0.53–4.41) | 4.45 (1.14–29.38) | 4.15 (0.97–28.63) | 0.65 | 0.73 | |
|
| |||||||||
| Asthma diagnosis | 0.01 | ||||||||
|
| |||||||||
| Moderate | 1.36 (0.86–2.19) | 1.34 (0.83–2.17) | 1.63 (0.92–2.98) | 1.60 (0.88–2.97) | 1.00 (0.45–2.29) | 0.98 (0.43–2.28) | 0.26 | 0.33 | |
|
| |||||||||
| High | 1.71 (1.09–2.71) | 1.57 (0.98–2.53) | 1.53 (0.85–2.83) | 1.47 (0.80–2.76) | 2.01 (0.98–4.37) | 1.79 (0.83–4.07) | 0.63 | 0.67 | |
|
| |||||||||
| Atopy | 0.25 | ||||||||
|
| |||||||||
| Moderate | 0.98 (0.71–1.34) | 1.05 (0.76–1.45) | 1.09 (0.72–1.65) | 1.20 (0.78–1.86) | 0.76 (0.47–1.23) | 0.82 (0.50–1.34) | 0.42 | 0.63 | |
|
| |||||||||
| High | 1.11 (0.81–1.51) | 1.17 (0.85–1.63) | 1.12 (0.74–1.69) | 1.34 (0.86–2.09) | 1.02 (0.63–1.65) | 0.94 (0.57–1.57) | 0.92 | 0.88 | |
Note: Adj. = adjusted, CI = confidence interval, FUS = Frequecy of Use Score, OR = odds ratio, Unadj. = unadjusted.
We derived all values using multivariable logistic regression models. These regression models used those participants with homes in the low group of FUS as the reference group to which participants from moderate and high households were compared.
Models were adjusted for prenatal or early life exposure to tobacco smoke, sex, child’s ethnicity, parental asthma history, household income, city/location, visible mould in the home and pet ownership.
For the sex-stratified analysis, we adjusted for all covariates except sex. The p value of the interaction term when included in the regression model for the overall sample is reported.
We used a 1-sided Cochran–Armitage test (unadjusted) to evaluate increasing trends by FUS level for each health outcome. For boys (n = 1047), the FUS was less than 27 for the low-use group (n = 331), between 27 and 35 for the moderate-use group (n = 365) and greater than 35 for the high-use group (n = 351). For girls (n = 975), the FUS was less than 26 for the low-use group (n = 295), between 26 and 35 for the moderate-use group (n = 375) and greater than 35 for the high-use group (n = 305).
Interpretation
Children living in a home with a higher frequency of use of any cleaning product during infancy had higher odds of recurrent wheeze, recurrent wheeze with atopy and asthma diagnosis at age 3 years; however, we found little difference in atopy when we compared these children with children living in homes with a lower frequency of product use. These findings were consistent when treating exposure as either a categorical or continuous variable. Many spray cleaning products that were scented showed strong associations with these health outcomes and may be important drivers of this risk.
The absence of an association between cleaning products and atopy is consistent with previous findings.12,26 A 2015 longitudinal study found that early life indoor exposure to volatile organic compounds increased the risk of atopic dermatitis in children at 3 years of age,27 which may facilitate subsequent sensitization to common allergens. However, allergic sensitivities are still developing and evolving in childhood,18 and frequent use of cleaning products may hinder the growth of and contact with indoor microbes that are needed to prime the immune system and protect against allergic disease.28
A proposed mechanism for our findings is that chemicals in cleaning products damage the respiratory epithelium by affecting inflammatory pathways of the innate immune system rather than allergic pathways. Studies involving adults have shown that household cleaning products may target the respiratory epithelium, causing bronchial hyperresponsiveness and wheeze ( irritant-induced asthma) as a result of chronic exposure.6 This damage worsens with long-term exposure, causing remodelling, reduced lung function, and increased airway reactivity and sensitivity to future exposures, including allergens.12
Although we corroborated that boys have more childhood allergy and asthma than girls,29 in this study we found a trend to a stronger association between use of cleaning products and outcomes in girls than boys, although this sex difference was not statistically significant. A study involving adults and children with asthma found that males had higher eosinophil and immunoglobulin E levels than females,30 raising interest in sex-dependent biological mechanisms for asthma development. Some researchers have postulated the role of hormones as a biological explanation for these differences,29,31 but understanding nonhormonal biological differences can inform interventions. A 2005 study involving school children in Saskatchewan found the relation between home mould exposure and asthma to be more prominent in girls than in boys.32 Some studies have reported that women are more susceptible to the effects of cigarette smoke than men,33,34 consistent with a more heightened innate immune response than in men. In addition, there are sex differences in microbiome diversity,35 with women showing greater diversity.36,37 Microbiome diversity has been linked to disinfectant use.38 When we included a sex–FUS interaction term in the models, there was borderline significance for recurrent wheeze but not for the other outcomes, which suggests caution be used when interpreting these sex differences (Tables 3 and 4). These results are hypothesis generating, and more investigation of male versus female biological response to inflammatory exposures in early life is needed.
Limitations
The FUS is an index of frequent use of cleaning products. Our FUS weights all cleaning products equally in the scoring, which allows for objectivity in the interpretation of the results but does not account for the differing potency of ingredients found in different products that may have more potent oxidizing properties. 15,26 We elected to keep our analysis strategy consistent with that of the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort12 (Appendix 1). The relation between FUS and outcomes was also determined through an alternative scoring method in a sensitivity analysis, which found that this altered scoring approach only slightly attenuated overall results.
Mixing cleaning products is a common cause of inhalation accidents that increases the risk of asthma and reactive airway dysfunction syndrome.15,26 With more than 100 different volatile organic compounds identified in a study of cleaning products,39 the extent of secondary exposures created when common cleaning products are mixed40 is not fully understood and remains a challenge for researchers to measure accurately.
The use of data from the questionnaires has the potential for recall bias as well as social desirability bias. Owing to the limited number of participants in each nonwhite ethnicity group, the child’s ethnicity was classified as a binary variable (white v. other ethnicities) to allow for analysis.
We assumed that each child was exposed to the products being used for cleaning, although we do not have data on the child’s location during the cleaning, whether the cleaned areas were rinsed or ventilated afterward, and other factors that would influence persistent exposure. Although the questionnaire did not specify who was using the cleaning product, cleaners persist in the air causing exposure both to direct users and those who are secondarily exposed over time. For example, a single use of a kitchen degreaser can affect indoor aerosolized concentrations of irritant chemicals for several days.40 Data for use of cleaning products from maternal prenatal questionnaires correlated well with the more robust measures from early childhood used in our study, suggesting that these measures are reflective of prenatal exposure. This is consistent with results from the ALSPAC cohort.12
Finally, the CHILD cohort has a higher average household income, parental educational level, and proportion of parental asthma and allergy than the general Canadian population, potentially limiting the generalizability of our results.
Conclusion
Our report involving a national Canadian birth cohort showed that frequent use of household cleaning products in early infancy is associated with an increased risk for childhood wheeze and asthma but not atopy at 3 years and that there may be sex differences in this association. Our results do not prove causation, but it is possible that initial effects on the airway in early life from frequent use of cleaning products may be due to an inflammatory rather than an acquired allergic response. The potential for these common household products to prime the airway for future allergic response is an area of future investigation for researchers.
A precautionary approach to using cleaning products and targeted hygiene is reasonable for housekeeping where young children are present. In the pursuit of obtaining a clean and healthy home (i.e., low allergen, free of mould and with good air quality), it is important that parents read labels and be informed about the risks associated with the use of cleaning products. The benefits of alternatives to spray and aerosol cleaners, the effect of cleaning products on the microbiome38 and identification of improved exposure measurement methods warrant further research.
Acknowledgements
The authors are grateful to all the families who took part in this study, and the entire Canadian Healthy Infant Longitudinal Development (CHILD) Cohort Study team. Additional support was provided by Health Canada, Environment Canada, Canada Mortgage and Housing Corporation, the Sick Children’s Hospital Foundation, Don and Debbie Morrison, the Silver Thread Foundation and the Childhood Asthma Foundation. The authors acknowledge the generosity of ALK Abello, Mississauga, Ont., for supplying all allergens for the study, and Lincoln Diagnostics Inc., Decatur, Ill., for supplying the Duotip-Test II devices and skin testing kits. Malcolm Sears holds the AstraZeneca endowed Chair in Respiratory Epidemiology. The authors also thank the CHILD study team for access to their data sets, and their assistance and analytical advice.
See related article at www.cmaj.ca/lookup/doi/10.1503/cmaj.200025
Footnotes
CMAJ Podcasts: author interview at https://soundcloud.com/cmajpodcasts/190819-res
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Jaclyn Parks, Lawrence McCandless and Tim Takaro contributed to the original conception and design of this work. Jeffrey Brook, Stuart Turvey, Piush Mandhane, Allan Becker, Anita Kozyrskyj, Meghan Azad, Diana Lefebvre, Malcolm Sears, Padmaja Subbarao, James Scott, Tim Takaro, Theo Moraes and Christoffer Dharma were responsible for collecting or making the data available for analysis. Jaclyn Parks and Christoffer Dharma analyzed the data. Jaclyn Parks and Tim Takaro drafted the manuscript. All of the authors contributed to interpretation of the data, reviewed and revised the manuscript for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: Canadian Institutes of Health Research and Allergy, Genes and Environment Network of Centers of Excellence.
Data sharing: Researchers interested in collaborating on a project and accessing Canadian Healthy Infant Longitudinal Development (CHILD) Cohort Study data should contact the study's National Coordinating Centre (NCC) to discuss their needs before initiating a formal request. To contact the NCC, please email child@mcmaster.ca. More information about data access for the CHILD Cohort Study can be found at www.childstudy.ca/ForScientists/DataAccess.
References
- 1.Sears MR. Trends in the prevalence of asthma. Chest 2014;145:219–25. [DOI] [PubMed] [Google Scholar]
- 2.Menzies-Gow A, McBrien CN, Baker JR, et al. Update in asthma and airway inflammation 2018. Am J Respir Crit Care Med 2019;200:14–19. [DOI] [PubMed] [Google Scholar]
- 3.Quirce S, Barranco P. Cleaning agents and asthma. J Investig Allergol Clin Immunol 2010;20:542–50. [PubMed] [Google Scholar]
- 4.Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis 2009;61:337–41. [DOI] [PubMed] [Google Scholar]
- 5.Weinmann T, Gerlich J, Heinrich S, et al. Association of household cleaning agents and disinfectants with asthma in young German adults. Occup Environ Med 2017;74:684–90. [DOI] [PubMed] [Google Scholar]
- 6.Zock J-P, Plana E, Jarvis D, et al. The use of household cleaning sprays and adult asthma: an international longitudinal study. Am J Respir Crit Care Med 2007;176:735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svanes Ø, Bertelsen RJ, Lygre SHL, et al. Cleaning at home and at work in relation to lung function decline and airway obstruction. Am J Respir Crit Care Med 2018;197:1157–63. [DOI] [PubMed] [Google Scholar]
- 8.Fedoruk MJ, Bronstein R, Kerger BD. Ammonia exposure and hazard assessment for selected household cleaning product uses. J Expo Anal Sci Environ Epidemiol 2005;15:534–44. [DOI] [PubMed] [Google Scholar]
- 9.Al horr Y, Arif M, Katafygiotou M, et al. Impact of indoor environmental quality on occupant well-being and comfort: a review of the literature. Int J Sustain Built Environ 2016;5:1–11. [Google Scholar]
- 10.Billionnet C, Sherrill D, Annesi-Maesano IGERIE study. Estimating the health effects of exposure to multi-pollutant mixture. Ann Epidemiol 2012;22:126–41. [DOI] [PubMed] [Google Scholar]
- 11.Choi H, Schmidbauer N, Sundell J, et al. Common household chemicals and the allergy risks in pre-school age children. PLoS One 2010;5:e13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson J, Sherriff A, Farrow A, et al. Household chemicals, persistent wheezing and lung function: Effect modification by atopy? Eur Respir J 2008;31:547–54. [DOI] [PubMed] [Google Scholar]
- 13.Nickmilder M, Carbonnelle S, Bernard A. House cleaning with chlorine bleach and the risks of allergic and respiratory diseases in children. Pediatr Allergy Immunol 2007;18:27–35. [DOI] [PubMed] [Google Scholar]
- 14.Bably M, Arif AA, Post A. Prenatal use of cleaning and scented products and its association with childhood asthma, asthma symptoms, and mental health and developmental comorbidities. J Asthma 2019;August 26:1–6. [DOI] [PubMed] [Google Scholar]
- 15.Singer BC, Coleman BK, Destaillats H, et al. Indoor secondary pollutants from cleaning product and air freshener use in the presence of ozone. Atmos Environ 2006;40:6696–710. [DOI] [PubMed] [Google Scholar]
- 16.Golding J, Pembrey M, Jones RALSPAC Study Team. ALSPAC-The Avon Longitudinal Study of Parents and Children. I. study methodology. Paediatr Perinat Epidemiol 2001;15:74–87. [DOI] [PubMed] [Google Scholar]
- 17.Takaro TK, Scott JA, Allen RW, et al. The Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort study: assessment of environmental exposures. J Expo Sci Environ Epidemiol 2015;25:580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran MM, Lefebvre DL, Dharma C, et al. Predicting the atopic march: results from the Canadian Healthy Infant Longitudinal Development Study. J Allergy Clin Immunol 2018;141:601–607.e8. [DOI] [PubMed] [Google Scholar]
- 19.Jedrychowski W, Perera FP, Maugeri U, et al. Early wheezing phenotypes and severity of respiratory illness in very early childhood: study on intrauterine exposure to fine particle matter. Environ Int 2009;35:877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klinnert MD, Nelson HS, Price MR, et al. Onset and persistence of childhood asthma: predictors from infancy. Pediatrics 2001;108:E69. [DOI] [PubMed] [Google Scholar]
- 21.Sherriff A, Peters TJ, Henderson J, et al. Risk factor associations with wheezing patterns in children followed longitudinally from birth to 3 1/2 years. Int J Epidemiol 2001;30:1473–84. [DOI] [PubMed] [Google Scholar]
- 22.Sherriff A, Farrow A, Golding J, et al. Frequent use of chemical household products is associated with persistent wheezing in pre-school age children. Thorax 2005;60:45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jartti T, Kuusipalo H, Vuorinen T, et al. Allergic sensitization is associated with rhinovirus-, but not other virus-induced wheezing in children. Pediatr Allergy Immunol 2010;21:1008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bobolea I, Arismendi E, Valero A, et al. Early life origins of asthma: a review of potential effectors. J Investig Allergol Clin Immunol 2019;29:168–79. [DOI] [PubMed] [Google Scholar]
- 25.Henderson AJ, Sherriff A, Northstone K, et al. Pre- and postnatal parental smoking and wheeze in infancy: cross cultural differences. Avon Study of Parents and Children (ALSPAC) Study Team, European Longitudinal Study of Pregnancy and Childhood (ELSPAC) Co-ordinating Centre. Eur Respir J 2001;18:323–9. [DOI] [PubMed] [Google Scholar]
- 26.Siracusa A, De Blay F, Folletti I, et al. Asthma and exposure to cleaning products — a European Academy of Allergy and Clinical Immunology task force consensus statement. Allergy 2013;68:1532–45. [DOI] [PubMed] [Google Scholar]
- 27.Kwon JH, Kim E, Chang M-H, et al. Indoor total volatile organic compounds exposure at 6 months followed by atopic dermatitis at 3 years in children. Pediatr Allergy Immunol 2015;26:352–8. [DOI] [PubMed] [Google Scholar]
- 28.Hesselmar B, Hicke-Roberts A, Wennergren G. Allergy in children in hand versus machine dishwashing. Pediatrics 2015;135:e590–7. [DOI] [PubMed] [Google Scholar]
- 29.Koper I, Hufnagl K, Ehmann R. Gender aspects and influence of hormones on bronchial asthma — secondary publication and update. World Allergy Organ J 2017;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oryszczyn M-P, Bouzigon E, Maccario J, et al. Interrelationships of quantitative asthma-related phenotypes in the epidemiological study on the genetics and environment of asthma, bronchial hyperresponsiveness, and atopy. J Allergy Clin Immunol 2007;119:57–63. [DOI] [PubMed] [Google Scholar]
- 31.Zein JG, Erzurum SC. Asthma is different in women. Curr Allergy Asthma Rep 2015;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawson JA, Rennie DC, Senthilselvan Phd A, et al. Regional variations in risk factors for asthma in school children. Can Respir J 2005;12:321–6. [DOI] [PubMed] [Google Scholar]
- 33.Kamil F, Pinzon I, Foreman MG. Sex and race factors in early-onset COPD. Curr Opin Pulm Med 2013;19:140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sørheim I-C, Johannessen A, Gulsvik A, et al. Gender differences in COPD: Are women more susceptible to smoking effects than men? Thorax 2010;65:480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YS, Unno T, Kim B-Y, et al. Sex differences in gut microbiota. World J Mens Health 2019. March 25 10.5534/wjmh.190009 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de la Cuesta-Zuluaga J, Kelley ST, Chen Y, et al. Age- and sex-dependent patterns of gut microbial diversity in human adults. mSystems 2019;4:e00261–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jašarević E, Morrison KE, Bale TL. Sex differences in the gut microbiome–brain axis across the lifespan. Philos Trans R Soc Lond B Biol Sci 2016;371: 20150122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tun MH, Tun HM, Mahoney JJ, et al. Postnatal exposure to household disinfectants, infant gut microbiota and subsequent risk of overweight in children. CMAJ 2018;190:E1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolkoff P, Schneider T, Kildesø J, et al. Risk in cleaning: chemical and physical exposure. Sci Total Environ 1998;215:135–56. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz J, Makeš O, Ondráček J, et al. Single usage of a kitchen degreaser can alter indoor aerosol composition for days. Environ Sci Technol 2017;51: 5907–12. [DOI] [PubMed] [Google Scholar]