Abstract
Chronic wasting disease (CWD) is a horizontally transmissible prion disease of free ranging deer, elk and moose. Recent experimental transmission studies indicate caribou are also susceptible to the disease. CWD is present in southeast Alberta and southern Saskatchewan. This CWD-endemic region is expanding, threatening Manitoba and areas of northern Alberta and Saskatchewan, home to caribou. Soil can serve as a stable reservoir for infectious prion proteins; prions bound to soil particles remain infectious in the soils for many years. Soils of western Canada are very diverse and the ability of CWD prions to bind different soils and the impact of this interaction on infectivity is not known. In general, clay-rich soils may bind prions avidly and enhance their infectivity comparable to pure clay mineral montmorillonite. Organic components of soils are also diverse and not well characterized, yet can impact prion-soil interaction. Other important contributing factors include soil pH, composition of soil solution and amount of metals (metal oxides). In this review, properties of soils of the CWD-endemic region in western Canada with its surrounding terrestrial environment are described and used to predict bioavailability and, thus, potential spread of CWD. The major soils in the CWD-endemic region of Alberta and Saskatchewan are Chernozems, present in 60% of the total area; they are generally similar in texture, clay mineralogy and soil organic matter content, and can be characterized as clay loamy, montmorillonite (smectite) soils with 6–10% organic carbon. The greatest risk of CWD spread in western Canada relates to clay loamy, montmorillonite soils with humus horizon. Such soils are predominant in the southern region of Alberta, Saskatchewan and Manitoba, but are less common in northern regions of the provinces where quartz-illite sandy soils with low amount of humus prevail.
Keywords: CWD expanding, prion, soil texture, soil organic matter, soil pH
Introduction
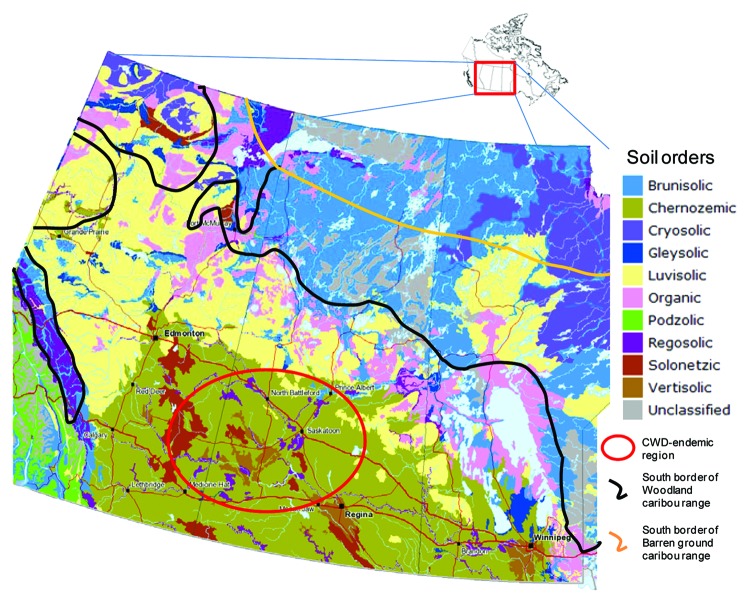
Chronic wasting disease (CWD) is a fatal prion disease affecting free range white-tailed deer, mule deer, elk and moose as well as farmed cervids. It first appeared in North America in the western USA in the 1960s. Over the past decades, the geographic range has expanded. In Canada CWD was initially identified in a captive elk and, subsequently, in free-ranging mule deer in Saskatchewan in 2000. The first free-ranging case of CWD in Alberta was identified in mule deer in 2005, in white-tailed deer in 2007; a CWD-infected moose was identified in Alberta in 2012 (http://esrd.alberta.ca/fish-wildlife/wildlife-diseases/chronic-wasting-disease/). CWD has not been identified in Manitoba; however, southwestern regions of that province are clearly at risk. Spread of CWD further north jeopardizes caribou herds and may trigger a new wave of prion disease among this cervid species. Prion protein (PrP) of Caribou (Rangifer tarandus spp.) has an identical amino acid sequence as the common allele of mule deer and white-tailed deer.1 Recent transmission studies have demonstrated the ability of CWD to transmit to reindeer by the oral route.2 The Woodland (R.t. caribou) and Barren ground (R.t. groenlandicus) caribou range extends south to central parts of Alberta, Saskatchewan and Manitoba3 (Fig. 1). The physical distance between caribou and the CWD-endemic region appears to be the sole factor currently limiting the exposure and transmission of CWD to caribou herds.
Figure 1. Soil orders map of Western Canada (Alberta, Saskatchewan and Manitoba) with range of caribou (Rangifer tarandus spp.) and CWD-endemic region. (Map source: Agriculture and Agri-Food Canada, 2010, v.3.1).
The routes of CWD transmission remain unclear. CWD is a contagious prion disease; the infectious agent is released in various body fluids including saliva, feces, blood and urine.4 Although the majority of studies suggest an oral route of exposure to be responsible for environmental transmission,5 there is also evidence for intranasal and aerosol transmission6,7 as contributing factors. In all transmission routes, soils can serve as a stable reservoir of prion diseases (transmissible spongiform encephalopathies, TSEs). Prions bound to soil particles can remain infectious in the soils for many years.8,9 Therefore, soil properties are an important factor for PrPTSE preservation and transmission in the environment.10-13 Analysis of soil-prion interactions and the impact on infectivity is a complicated task because soils are multicomponent systems consisting of mineral particles (clay, silt, sand); soil organic matter (humic, fulvic acids and humin); humus or/and Fe-Mn films and cutans interacting with mineral particles. The enormous complexity of soils indicates a need to examine a variety of soils and their separated compounds (mineral and organic) to identify the ability of prions to bind the soil, what the effect of binding is on infectivity and what components of soil bind prions.
Western Canada soils vary widely from Chernozems in the south to Luvisols and Brunisols in the north (Fig. 1; Table 1). These diverse soils are characterized by differing texture, mineralogical composition, pH, and soil organic matter amount and composition (Table 1). Thus, western Canadian soils could influence the bioavailability, persistence and transmissibility of infectious prions in the environment. A recent study in southern Saskatchewan has suggested the importance of landscape position for CWD transmission: croplands have a higher risk compared with coulees and creek valleys.13 This correlation may be related to soil properties because soil texture, organic matter content, pH etc. usually vary with topography. To date, only a few soil characteristics have been investigated with respect to their influence on infectious and uninfected agents: some common soil minerals (montomorillonite, kaolinite and quartz),14-16 organic polyanions and humic acids (as soil organic matter compounds),17-20 and some metals and their oxides (Al2O3, SiO2, MnO).21-25 The main focus of this review is the characterization of soil properties in the CWD-endemic region and its surrounding areas from Alberta to Manitoba to estimate their potential for binding prions and maintaining bioavailability.
Table 1. Characteristics of soils in prairie provinces.
| Regions of prairie provinces | Great Groups | pH | SOM content in surface horizon | Texture | Diagnostic Horizon | Comments |
|---|---|---|---|---|---|---|
| Southern Alberta, Saskatchewan and Manitoba | Black Chernozems | 6.5–7.5 | 5–9.5% | Sandy loam to clay loam | Ah, Ap, Ahe | A grassland soil whose diagnostic horizon is formed by high levels of organic matter additions from the roots of grasses. |
| Brown Chernozems | 6.5–7.5 | 2.5–3.4% | Loam, clay loam | |||
| Vertisolic soils | 7.2–8.5 | 1–6% | Clay loam, clay | Bss | Associated with high clay glacio-lacustrine landscapes; characterized by shrinking and swelling of clays. | |
| Solonetzic soils | 8–9 | 1–2% | Clay | Bn or Bnt | A grassland soil with high sodium levels in the B horizon; usually associated with a clay-rich B horizon and high Na content. | |
| Western and Northern Alberta and Manitoba | Regosolic soils | 4–8 | < 1% | Sandy, loamy sand | No B hor. | Weakly developed soils found throughout prairie provinces wherever pedogenic conditions prevent the formation of B horizons. |
| Central and Northern Alberta | Gray Luvisols | 4.5–5.5 | 1–2% in mineral horizon | Sandy loam, loam | Bt | A forest soils where dominant process is eluviation of clay (and sometimes organic substances) from the Ae horizon and its deposition in the Bt horizon. Above-ground SOM input: LFH horizon. |
| Western Alberta, Northern Saskatchewan and Manitoba | Brunisols | 5–6.5 | ~2% | Sandy loam, loam | Bm | A forest soil whose properties are not strongly enough developed to meet the criteria for the Luvisolic or Podzolic Orders. |
| Northern-east Manitoba | Cryosols | 4–7 | < 1% in min. hor. or 10–30% in org. hor. | Mostly fine-textured from sandy to silt loam | Bcr | Common in the subarctic forest area in Cryosolic soils are formed in either mineral or organic materials that have permafrost. |
| Central Manitoba | Gleysols | < 5 | < 1% in min. hor. or 10–30% in org. hor. | Sandy-loam | G | Diagnostic bluish-gray color reflects the occurrence of a reducing (anoxic) environment |
| Northern Alberta and Central Saskatchewan and Manitoba | Organic soils | 4.5–6.5 | > 30% | NA | O | Soils of the Organic order are composed largely of organic materials. They include most of the soils commonly known as peat, muck, or bog and fen. |
Prion Interactions with Different Soil Compounds
Soil minerals and texture
Soil texture and mineralogical composition are the most well-studied soil properties in regard to prion binding capacity.14-16,19,26-28 Prions have a strong affinity for clay minerals commonly found in soil; PrPSc binds clay minerals (montmorillonite, Mte) avidly in comparison with quartz sand, an interaction that considerably enhances prion infectivity.14,15 Similar results were described using as prion source the hamster-adapted scrapie strain hyper (PrPHY); silty clay loam soil bound prions more avidly than the sandy loam soil.16 Our data also confirmed that soils with clay texture bind PrPHY more avidly than the soils with lighter texture (Fig. 2). Clay texture soil (soil S1) binds twice as much PrPHY as soil matrix with a sandy loam texture (soil S2). Soil S1 has higher binding capacity compared with soil S2 because of its heavier texture and also the presence of Mte: bound PrPHY signal is stronger in S1 soil. Most of the PrP was, however, unbound likely due to presence of other minerals which have a lower binding capacity compared with pure mineral Mte. The clay content of Canadian soils can play an integral role in the spread of CWD in the environment similar to that of western USA. It is estimated in northern Colorado that the risks of CWD in free-ranging deer increased by 8.9% for every 1% increase in surface soil clay content.11 Therefore, clay content as well as soil texture may control prion bioavailability and the subsequent extension of the disease range.
Figure 2. Hamster scrapie prions (PrPHY) bind to mineral fractions of soils S1 and S2. Identical amounts of 10% brain homogenate (BHHY) were incubated with soils overnight at room temperature. Samples were fractionated through a sucrose cushion to separate bound PrPHY from unbound; both pellet (bound PrPHY) and supernatant (unbound PrPHY) were analyzed for presence of PK-resistant PrPHY by western blot with 3F4 antibody. Soil S1 with clay texture and montmorillonite-kaolinite mineralogical composition binds more PrPHY compared with soil S2 with sandy-loam texture and Illite-kaolinite composition.
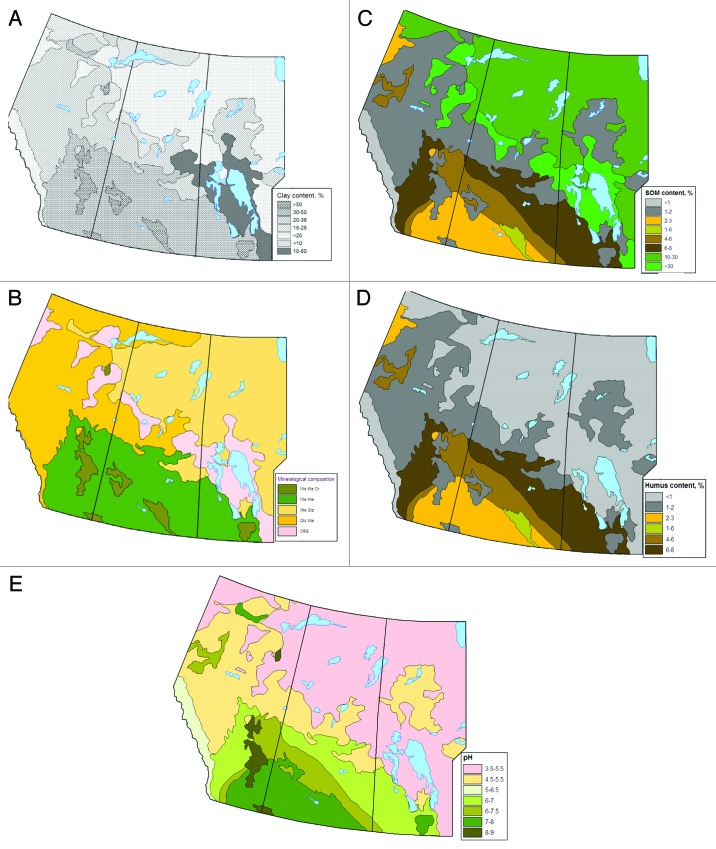
The texture of the prairie provinces’ soils varies in full spectra from sand-dominant (light texture) to clay-rich (heavy texture). The majority of soils (up to 90%) in the CWD-endemic region are loam and increasingly finer.29 The uppermost mineral layer (soil horizon) of the CWD-endemic region and surrounding area soils are primarily fine-textured silts and clay loams but in the northern regions of the prairie provinces, the surface horizons change to loamy or sandy loamy (Fig. 3A). The texture of the surface soil horizons is lighter in the northern soils and mountain soils suggesting a reduced prion binding capacity of these soils compared with soils of the CWD-endemic regions. The most common soils of northern parts of prairie provinces are Luvisols. The diagnostic feature of Luvisolic soils is eluviations-illuviation processes where soil material (e.g., clay, humus, iron, or aluminum) transfers out from eluvial horizon Ae and deposits into illuvial horizons Bt. Luvisols have eluvial horizons (Ae) from which clay has been leached after snowmelt or heavy rains; this process creates a textural contrast between the Ae and the illuvial Bt horizon. Lessivage (or clay translocation), the major soil-forming process responsible for the formation of Luvisols,30 also can promote transport of clay-bound prions into the lower soil horizons. Considering the soil’s texture and clay translocation with possible transportation of binding prions from surface horizons, the maintenance of CWD prion bioavailability through soils is not as favorable in the caribou regions of western Canada. The northern and mountain soil textures differ from soils in the CWD-endemic region and PrPCWD might not bind or persist on the surface to the same extent as on clay loamy Chernozems, which prevail in the southern parts of the prairie provinces.
Figure 3. Distribution of some soil characteristics in the soil surface horizons across western Canada: (A) clay content in the surface soil horizons, % of texture; (B) mineralogical composition of surface soil horizons: Mte, montmorillonite; Kte, kaolinite; Qtz, quartz; Ch, chlorite; ORG, organic horizon without mineral fraction. First is dominant mineral followed by remaining occurred minerals; (C) soil organic matter content in the surface soil horizons, %; (D) humus content in the surface soil horizons, %; (E) soil pH.
Mineralogical composition of surface horizons is also important for estimation of whole soil prion binding capacity. Different minerals bind prions with different binding capacity, quartz < kaolinite < Mte-Ca < Mte-Na,31 and mineral particle size specific surface area also change binding capacity. Cooke et al.27 have shown that soils with similar textures can exhibit remarkably different protein recoveries, and explained this discrepancy by a difference in mineralogy and/or soil pH. Our studies with infectious prions have shown that soils with different mineralogical composition can vary in prion binding capacities (Fig. 2). For this binding experiment, only the mineral fractions were used (soil organic matter was removed with 30% hydrogen peroxide treatment). The soils with Mte in the mineral fraction bind prions better than soils where kaolinite is the dominant mineral.
The clay mineralogy of soils across the CWD-endemic region of the prairie provinces is dominated by smectites (mostly Mte), with smaller amounts of mica, kaolinite and chlorite29,32-36 (Fig. 3B). The fine clay fractions (< 2 µm) are composed almost entirely of Mte and illite (mica) which occur in the ratio of approximately two to one.37 In the coarse clay fractions (2–5 µm), Mte and illite occur in approximately equal amounts with kaolinite; chlorite and primary minerals making up the remainder of the fractions.30,38 Quartz is ubiquitous in coarse fractions (mostly sand; > 0.05 mm), with chlorite, Mte and illite present in small amounts.39 The main clay mineral of the major soils of the CWD-endemic region is, therefore, Mte; the soils differ by illite, mica, kaolinite, and chlorite contents. The major soils in southern parts of the prairie provinces have similar mineralogical composition of soil clay fractions as in the CWD-endemic region. Further north, the mineralogical composition of soils changes: the level of smectite in the clay fraction is decreased while the quantity of illite and mica is increased (Fig. 3B). Thus mineralogical composition as well as soil texture of northern soils (Luvisols and Brunisols) differ from southern soils which may affect binding to prions as well as their ability to preserve and transport prions.
Soil organic matter
As with the mineral compounds of soil, soil organic matter (SOM) plays a significant role in affecting soil properties such as soil structure and its stability, cation-exchange and water-holding capacity, specific surface area, aeration, and aggregation. SOM amounts range from 0.5 to 9% in the surface mineral horizon to 100% in the organic soils. In general, the SOM is a complicated mixture of humic (humic acids (HA), fulvic acids (FA) and humin) and non-humic substances (plant debris, lignins, proteins, polysaccharides) and its composition varies from soil to soil. Therefore, total amount of SOM is not an adequate indicator of soil properties as the composition of SOM and the ratio of its different compounds can affect prion binding. Soils of parkland and grassland zones (Chernozems) accumulate organic matter primarily underground (in the root zone) and form the Ah horizon, which accumulates a high amount of organic matter as a humus (mixture of FA, HA and humin) and is usually expressed morphologically by a darkening of the surface soil and a well-developed structure. Accumulation of organic matter in soils of boreal forests (Luvisols and Brunisols) occurs on the surface and organic litter (LHF horizon) is formed such that the SOM is represented mostly by plant residues with varying degrees of decomposition. Prion-SOM interactions are not well characterized.40 In studies where the ability of SOM compounds to bind uninfectious recombinantly generated PrP was investigated, the affinity of SOM for recPrP was almost equivalent to that offered by the clay mineral surfaces.17-20 In addition, pure HA not only exhibited the strongest affinity for recPrP but also increased (about 10 times) the sorption capacity of kaolinite and Mte clays.19 However, differences in the structure of infectious and non-infectious prion proteins require more judicious analyses of these results to estimate SOM-infectious prion interactions.40 The binding mechanism of SOM and its influence on prion infectivity is still poorly understood. Some results suggest that dissolved organic matter influences inactivation of infectious prions (PrPSc) in water;41 and that soil humic acids inhibit the transformation of PrPc into PrPSc-like structure.42 At the same time, total organic carbon in topsoils of the British Isles has been correlated with incidence rates of scrapie.43 Our data also show a change in PrPCWD recovery and molecular weight during incubation with HA (Fig. 4A). Higher concentrations of HA alter PrPCWD causing a reduction in molecular weight as well as reduction in signal. This loss of signal could be attributed to encapsulation of PrPCWD and subsequent difficulties of migration of prions in PAGE; or, alternatively, partial degradation of PrPCWD or changing its structure so only a portion of the PrPCWD may be detectable. HA may also alter the binding capacity of Mte for infectious prion proteins (Fig. 4B). Low HA concentration (1 g L−1) increases binding capacity of Mte: less unbound PrPHY remains in the supernatant after centrifugation through a sucrose cushion. A high concentration of HA (25 g L−1) also increases binding capacity of Mte but recovery of PrPHY is dramatically reduced. Not only bound (pellet, p) and unbound (supernatant, s) PrP are detected but also PrP adsorbed to the tube (residual, r). We suggest that recovery of PrPHy is poor due to its interactions with HA. The total signal (r+p+s) of PrPHy with Mte (without HA) is equal to the signal of BH-PrPPK+ but, in the presence of HA, the additive signal (r+p+s) declines (Fig. 4B). PrPCWD has changed by binding with HA but the exact mechanism of this process is still unclear.40 In other studies,44 it was suggested that negatively-charged HA could encapsulate positively-charged proteins and preserve their activity. A more recent study20 has shown HA-like substances copolymerize with recPrP and irreversibly involve the PrP in their structure creating complexes which decrease recPrP recovery.
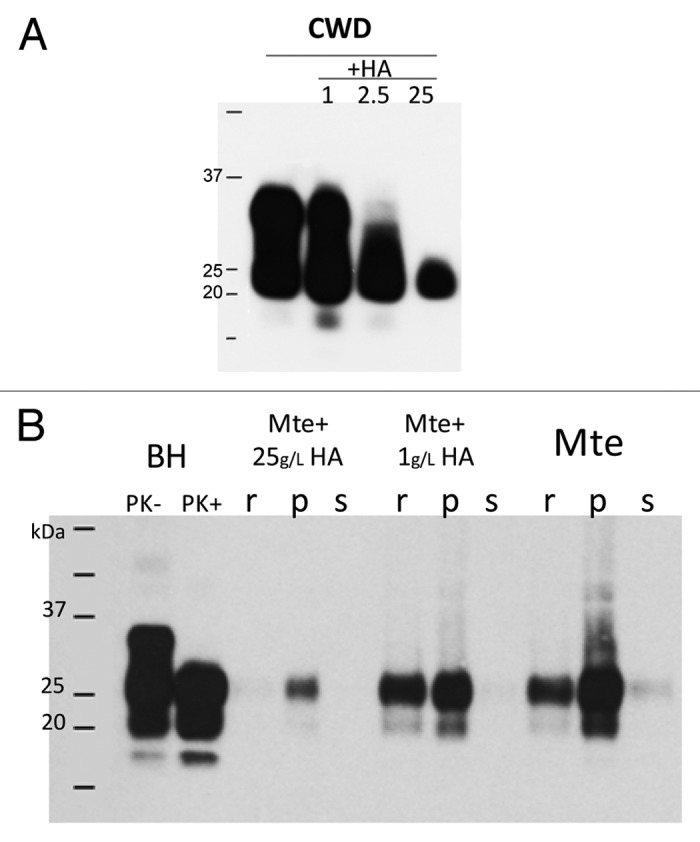
Figure 4. Influence of HA on PrPSc: (A) interactions between HA and PrPCWD affect PrPCWD recovery and molecular weight. Identical amounts of 10% brain homogenate (BHCWD) were incubated with water (control) and HA (1g/L, 2.5 g/L and 25 g/L) overnight at room temperature. Samples were analyzed for presence of PK-resistant PrPCWD by western blot with Bar224 antibody. (B) HA affect hamster scrapie prion (PrPHY) binding to Mte. 10% brain homogenate (BHHY) was incubated with Mte ± HA (1 g/L and 25 g/L) overnight at room temperature. Samples were fractionated through a sucrose cushion to separate bound PrPHY from unbound; residual (r; adsorbed on the Eppendorf tube PrPHY), pellet (p; bound PrPHY) and supernatant (s; unbound PrPHY) were analyzed for presence of PK-resistant PrPHY by western blot with 3F4 antibody.
The SOM content of the major soils in the CWD endemic regions of the prairie provinces is high because Chernozems are the dominant soils. Uppermost Chernozemic humic horizons have more than 6–8% of organic carbon and this is mostly humus (Fig. 3C). The humic horizons of both Solonetzic soils and Vertisols are also enriched with organic matter (to 5%). Only light textured Regosols on sandy deposits in the CWD-endemic regions (Fig. 1) have low amounts of SOM (1–3%). Most high organic carbon content correlates with clay-clay loam texture (40 - 50% of clay) of the soils as well as amount of smectite clay minerals (smectite > 50%). All soils in the southern parts (not only CWD-endemic region) of the prairie provinces have high humus content. The humus content decreases north and west of the CWD-endemic region but total amount of SOM may increase due to differences in its accumulation. Soils in regions with caribou populations accumulate SOM mostly as organic LHF horizon where initial plant residues are weakly degraded and have not yet transformed into humic substances (Fig. 3C and 3D). More prion-binding experiments with SOM compounds (humic and fulvic acids, humin, plant litter) and their detailed analyses are necessary to understand the fate of prions in different soil types. Binding mechanisms will depend not only on the amount and composition of SOM but also on the ratio between different compounds. SOM, as well as inorganic compounds (e.g., Mte), may play a relevant role in prion persistence and infectivity in soil environment.
pH and solution conditions
Soil pH determines general soil properties and can change binding capacity of minerals and SOM, and the “bioavailability” and adsorption of metals. pH conditions can directly influence prion structure and properties45 as well as altering the binding capacities of mineral and organic compounds. For example, kaolinite and humus have mostly negatively charged, pH-dependent surfaces which may significantly change binding capacity of whole soil as pH decreases or increases. Prion binding can be directly affected by soil pH: PrPSc adsorption to quartz sand was maximal around the isoelectric point at pH ∼4 for prions, corresponding to maximal PrPSc aggregation.28 Soil pH of surface horizons in the Canadian prairie provinces has a strong south-north trend for acidification: pH drops from 8.5–9 in Solontzetic soils to 3.5–4.5 in Luvisolic soils (Fig. 3E). Thus, a difference in prion-binding capacity between southern and northern soils, as a result of acidification, is anticipated.
The soil pH is closely related to the concentration and composition of soil ions. Saunders et al.46 reported that the solution ionic strength and composition did not significantly affect PrPSc adsorption but they did note binding differences between phosphate buffer solution (PBS), CaCl2 solution and water. Differrent ionic conditions could alter properties of adsorbing surfaces and their binding capacity; phosphate ions from PBS absorb irreversibly on clay particles,47 and can affect with protein sorption.48 The sorption capacities for organic matter and clay minerals significantly depend on the ionic strength of the solution (as well as the pH) and the homoionic salt species (e.g., Na+, Ca2+). Different cations affect height of the interlayer space and proteins associated with the interlayer areas of expandable clays (e.g., smectites) potentially decreasing their bioavailability.49 Cooke et al.27 found lower recovery of recPrP in the presence of soil cations, a finding related to the strong interactions between clay particles and exchangeable cations. Soil solution composition may, in addition to pH, change the binding capacity of prions to whole soils. Additional studies are needed to examine influence of ions in soil solution on prion binding and their impact on infectivity. In soils with high pH, the solution ionic strength is higher with Ca2+, Na+, Mg2+ as dominant ions. Alkaline (Solontzetic, Vertisolic and some Brown Chernozemic subgroups) soils are commonly associated with areas of active saline ground water discharge. In more acidic soils (Luvisols and organic soils in the north of provinces), the ionic strength decreases and ion composition also changes with H+ becoming the dominant ion.
Metals in soil
Soil metals are present as ions in the soil solution, as part of minerals (oxides or salts) or as exchangeable cations adsorbed on clay and organic particles. Metal mobility and availability depend on many soil properties including pH, humus content, and mineralogical composition. Ca and Mg are predominant exchangeable cations in the southern soils of the prairie provinces (Chernozems) with high pH; in the saline soils (Solontzetic soils), Na is the prevalent ion. In the northern soils (Luvisols, Brunisols and Organic soils) with acidic pH, the most abundant metal ions are Al, Fe and sometimes Mn.
Soil metals can influence PrPSc stabilization on soil particle surfaces and may create an additional risk factor for prion infectivity persistence in soil. There is contradictory data about role of Mn in prion fate; some results support a role for Mn in the formation and stabilization of PrPSc while other data suggests that Mn (particularly Mn oxides) can degrade prions. The association of PrPSc with manganese potentially makes prions more infectious as cell culture studies showed increased susceptibility to infection in the presence of elevated manganese.50 A dramatic increase in PrPSc survival (10 times) over a two year period was observed in the presence of manganese-ions in a soil solution.22 Mn-associated prions are 100 times more likely to cause infection than a “metal-free” prion.21 We have recently shown that low levels of copper and high level of manganese also were associated with abnormal prion protein in the brains of infected animals.25 The normal isoform (non-infectious) of the prion protein binds copper; the protein conversion to the infectious isoform depends on the presence of manganese.23 These results suggest that manganese is a risk factor for both the survival of the infectious agent in soil and its transmissibility.
Conversely, the soil mineral compound, manganese oxide, may serve as a reactive burial material for the disposal of prions because it is the strongest natural oxidant in soil.22 Russo et al. have shown the pathogenic prion protein was degraded by a synthetic manganese oxide mineral after several hours incubation at room temperature. In the environment, a link between prion disease and these metals in soils has been suggested: significantly higher concentrations of manganese in forage on scrapie-free farms were found, compared with scrapie-affected farms.51,52 The presence of high concentrations of manganese in the soil may affect the fate of infectious prions (probably oxidation and degradation) in the environment. Iron or aluminum oxides may also influence binding and stabilization of prions in soils53 but data related to interaction of iron oxides with prion proteins in soil environment are not available. Iron oxides may affect PrP fate in soil by altering their adsorption surfaces as well as increasing the sorption capacity of silicate clays through surface coatings.54 Distribution of Fe and Mn oxides in western Canadian soils has a south-north trend: lower concentrations in Chernozems in southern part and more in northern Luvisols and Cryosols. In northern soils, these metals become more mobile and create complexes with SOM compounds because of the low pH. A more detailed study of iron, along with manganese, in soils of CWD-endemic regions might be the key to understanding prion protein and infectivity persistence in the natural environment.
Conclusions
Soils can serve as an environmental reservoir for infectious prions and contribute to CWD expansion. Soil compounds (organic and inorganic) can bind and intercalate with infectious prions; many of these interactions affect maintenance of prion bioavailability and infectivity. The diversity of soil types in western Canada with their extreme variability in both organic and inorganic composition would suggest distinct outcomes of interactions of soil with PrPCWD. The potential contribution of northern soils (Luvisols and Brunisols) in the maintenance and bioavailability of CWD is unknown. Vertical transport of bound prions with clay particles in Luvisols into underlying horizons could limit transmission of prions through soil consumption. Differences in mineralogical composition, clay content, pH, amount and composition of SOM and other soil properties indicate a large number of variables in soil/prion interaction, a complexity that can impact prion persistence and infectivity levels in the environment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by Alberta Innovates BioSolutions through the Alberta Prion Research Institute grants 200900188, 201300025, and PAA13007.
References
- 1.Robinson SJ, Samuel MD, O’Rourke KI, Johnson CJ. . The role of genetics in chronic wasting disease of North American cervids. Prion 2012; 6:153 - 62; http://dx.doi.org/ 10.4161/pri.19640; PMID: 22460693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell GB, Sigurdson CJ, O’Rourke KI, Algire J, Harrington NP, Walther I, Spraker TR, Balachandran A. . Experimental oral transmission of chronic wasting disease to reindeer (Rangifer tarandus tarandus). PLoS One 2012; 7:e39055; http://dx.doi.org/ 10.1371/journal.pone.0039055; PMID: 22723928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COSEWIC Designatable Units for Caribou (Rangifer tarandus) in Canada. Committe on the Status of Endangered Wildlife in Canada 2011; 1 - 88 [Google Scholar]
- 4.Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes-Klug J, Seelig DM, et al. . Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 2006; 314:133 - 6; http://dx.doi.org/ 10.1126/science.1132661; PMID: 17023660 [DOI] [PubMed] [Google Scholar]
- 5.Miller MW, Williams ES. . Chronic wasting disease of cervids. Curr Top Microbiol Immunol 2004; 284:193 - 214; http://dx.doi.org/ 10.1007/978-3-662-08441-0_8; PMID: 15148993 [DOI] [PubMed] [Google Scholar]
- 6.Nichols TA, Spraker TR, Rigg TD, Meyerett-Reid C, Hoover C, Michel B, Bian J, Hoover E, Gidlewski T, Balachandran A, et al. . Intranasal inoculation of white-tailed deer (Odocoileus virginianus) with lyophilized chronic wasting disease prion particulate complexed to montmorillonite clay. PLoS One 2013; 8:e62455; http://dx.doi.org/ 10.1371/journal.pone.0062455; PMID: 23671598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denkers ND, Hayes-Klug J, Anderson KR, Seelig DM, Haley NJ, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mathiason CK, et al. . Aerosol transmission of chronic wasting disease in white-tailed deer. J Virol 2013; 87:1890 - 2; http://dx.doi.org/ 10.1128/JVI.02852-12; PMID: 23175370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown P, Gajdusek DC. . Survival of scrapie virus after 3 years’ interment. Lancet 1991; 337:269 - 70; http://dx.doi.org/ 10.1016/0140-6736(91)90873-N; PMID: 1671114 [DOI] [PubMed] [Google Scholar]
- 9.Georgsson G, Sigurdarson S, Brown P. . Infectious agent of sheep scrapie may persist in the environment for at least 16 years. J Gen Virol 2006; 87:3737 - 40; http://dx.doi.org/ 10.1099/vir.0.82011-0; PMID: 17098992 [DOI] [PubMed] [Google Scholar]
- 10.Miller MW, Williams ES. . Prion disease: horizontal prion transmission in mule deer. Nature 2003; 425:35 - 6; http://dx.doi.org/ 10.1038/425035a; PMID: 12955129 [DOI] [PubMed] [Google Scholar]
- 11.David Walter W, Walsh DP, Farnsworth ML, Winkelman DL, Miller MW. . Soil clay content underlies prion infection odds. Nat Commun 2011; 2:200; http://dx.doi.org/ 10.1038/ncomms1203; PMID: 21326232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders SE, Bartz JC, Bartelt-Hunt SL. . Soil-mediated prion transmission: is local soil-type a key determinant of prion disease incidence?. Chemosphere 2012; 87:661 - 7; http://dx.doi.org/ 10.1016/j.chemosphere.2011.12.076; PMID: 22265680 [DOI] [PubMed] [Google Scholar]
- 13.Silbernagel ER, Skelton NK, Waldner CL, Bollinger TK. . Interaction among deer in a chronic wasting disease endemic zone. J Wildl Manage 2011; 75:1453 - 61; http://dx.doi.org/ 10.1002/jwmg.172 [DOI] [Google Scholar]
- 14.Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM. . Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathog 2007; 3:e93; http://dx.doi.org/ 10.1371/journal.ppat.0030093; PMID: 17616973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. . Prions adhere to soil minerals and remain infectious. PLoS Pathog 2006; 2:e32; http://dx.doi.org/ 10.1371/journal.ppat.0020032; PMID: 16617377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saunders SE, Bartz JC, Bartelt-Hunt SL. . Influence of prion strain on prion protein adsorption to soil in a competitive matrix. Environ Sci Technol 2009; 43:5242 - 8; http://dx.doi.org/ 10.1021/es900502f; PMID: 19708348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao MA, Russo F, Granata V, Berisio R, Zagari A, Gianfreda L. . Fate of prions in soil: Interaction of a recombinant ovine prion protein with synthetic humic-like mineral complexes. Soil Biol Biochem 2007; 39:493 - 504; http://dx.doi.org/ 10.1016/j.soilbio.2006.08.020 [DOI] [Google Scholar]
- 18.Pucci A, D’Acqui LP, Calamai L. . Fate of prions in soil: interactions of RecPrP with organic matter of soil aggregates as revealed by LTA-PAS. Environ Sci Technol 2008; 42:728 - 33; http://dx.doi.org/ 10.1021/es071314q; PMID: 18323094 [DOI] [PubMed] [Google Scholar]
- 19.Polano M, Anselmi C, Leita L, Negro A, De Nobili M. . Organic polyanions act as complexants of prion protein in soil. Biochem Biophys Res Commun 2008; 367:323 - 9; http://dx.doi.org/ 10.1016/j.bbrc.2007.12.143; PMID: 18174023 [DOI] [PubMed] [Google Scholar]
- 20.Pucci A, Russo F, Rao MA, Gianfreda L, Calamai L, D'Acqui LP. . Location and stability of a recombinant ovine prion protein in synthetic humic-like mineral complexes. Biol Fertil Soils 2012; 48:443 - 51; http://dx.doi.org/ 10.1007/s00374-011-0639-0 [DOI] [Google Scholar]
- 21.Davies P, Marken F, Salter S, Brown DR. . Thermodynamic and voltammetric characterization of the metal binding to the prion protein: insights into pH dependence and redox chemistry. Biochemistry 2009; 48:2610 - 9; http://dx.doi.org/ 10.1021/bi900170n; PMID: 19196019 [DOI] [PubMed] [Google Scholar]
- 22.Russo F, Johnson CJ, Johnson CJ, McKenzie D, Aiken JM, Pedersen JA. . Pathogenic prion protein is degraded by a manganese oxide mineral found in soils. J Gen Virol 2009; 90:275 - 80; http://dx.doi.org/ 10.1099/vir.0.003251-0; PMID: 19088299 [DOI] [PubMed] [Google Scholar]
- 23.Davies P, Brown DR. Prion diseases, metals and antioxidants. Brain Dis. and Metalloproteins 2012, 249-293. [Google Scholar]
- 24.Jacobson KH, Kuech TR, Pedersen JA. . Attachment of pathogenic prion protein to model oxide surfaces. Environ Sci Technol 2013; 47:6925 - 34; PMID: 23611152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson CJ, Gilbert PUPA, Abrecht M, Baldwin KL, Russell RE, Pedersen JA, Aiken JM, McKenzie D. . Low copper and high manganese levels in prion protein plaques. Viruses 2013; 5:654 - 62; http://dx.doi.org/ 10.3390/v5020654; PMID: 23435237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rigou P, Rezaei H, Grosclaude J, Staunton S, Quiquampoix H. . Fate of prions in soil: adsorption and extraction by electroelution of recombinant ovine prion protein from montmorillonite and natural soils. Environ Sci Technol 2006; 40:1497 - 503; http://dx.doi.org/ 10.1021/es0516965; PMID: 16568762 [DOI] [PubMed] [Google Scholar]
- 27.Cooke CM, Rodger J, Smith A, Fernie K, Shaw G, Somerville RA. . Fate of prions in soil: detergent extraction of PrP from soils. Environ Sci Technol 2007; 41:811 - 7; http://dx.doi.org/ 10.1021/es0618189; PMID: 17328187 [DOI] [PubMed] [Google Scholar]
- 28.Ma X, Benson CH, McKenzie D, Aiken JM, Pedersen JA. . Adsorption of pathogenic prion protein to quartz sand. Environ Sci Technol 2007; 41:2324 - 30; http://dx.doi.org/ 10.1021/es062122i; PMID: 17438782 [DOI] [PubMed] [Google Scholar]
- 29.Pennock D, Bedard-Haughn A, Viaud V. . Chernozemic soils of Canada: Genesis, distribution, and classification. Can J Soil Sci 2011; 91:719 - 47; http://dx.doi.org/ 10.4141/cjss10022 [DOI] [Google Scholar]
- 30.Lavkulich LM, Arocena JM. . Luvisolic soils of Canada: Genesis, distribution, and classification. Can J Soil Sci 2011; 91:781 - 806; http://dx.doi.org/ 10.4141/cjss2011-014 [DOI] [Google Scholar]
- 31.Saunders SE, Bartelt-Hunt SL, Bartz JC. . Prions in the environment: occurrence, fate and mitigation. Prion 2008; 2:162 - 9; http://dx.doi.org/ 10.4161/pri.2.4.7951; PMID: 19242120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kodama H. . Clay minerals in Canadian soils: Their origin, distribution and alteration. Can J Soil Sci 1979; 59:37 - 85; http://dx.doi.org/ 10.4141/cjss79-005 [DOI] [Google Scholar]
- 33.Abder-Ruhman M. Mineralogical characteristics of soils from east central Alberta. M.Sc. thesis. 1980, 1-120. [Google Scholar]
- 34.Dudas MJ, Pawluk S. . Reevaluation of the occurrence of interstratified clays and other phyllosilicates in southern Alberta soils. Can J Soil Sci 1982; 62:61 - 9; http://dx.doi.org/ 10.4141/cjss82-007 [DOI] [Google Scholar]
- 35.Miller JJ, Brierley JA. . Solonetzic soils of Canada: Genesis, distribution, and classification. Can J Soil Sci 2011; 91:889 - 902; http://dx.doi.org/ 10.4141/cjss10040 [DOI] [Google Scholar]
- 36.Brierley JA, Stonehouse HB, Mermut AR. . Vertisolic soils of Canada: Genesis, distribution, and classification. Can J Soil Sci 2011; 91:903 - 16; http://dx.doi.org/ 10.4141/cjss10060 [DOI] [Google Scholar]
- 37.Kohut CK, Dudas MJ. . Layer charge characteristics of smectites in salt-affected soils in Alberta, Canada. Clays Clay Miner 1995; 43:78 - 84; http://dx.doi.org/ 10.1346/CCMN.1995.0430109 [DOI] [Google Scholar]
- 38.Smith CAS, Webb KT, Kenney E, Anderson A, Kroetsch D. . Brunisolic soils of Canada: Genesis, distribution, and classification. Can J Soil Sci 2011; 91:695 - 717; http://dx.doi.org/ 10.4141/cjss10058 [DOI] [Google Scholar]
- 39.Kohut CK, Dudas MJ. . Characteristics of clay minerals in saline alkaline soils in Alberta, Canada. Soil Sci Soc Am J 1994; 58:1260 - 9; http://dx.doi.org/ 10.2136/sssaj1994.03615995005800040038x [DOI] [Google Scholar]
- 40.Smith CB, Booth CJ, Pedersen JA. . Fate of prions in soil: a review. J Environ Qual 2011; 40:449 - 61; http://dx.doi.org/ 10.2134/jeq2010.0412; PMID: 21520752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miles SL, Takizawa K, Gerba CP, Pepper IL. Survival of infectious prions in water. Water Distrib. Syst. Anal. - Proc. Int. Conf., WDSA 2012, 454-458.
- 42.Corsaro A, Anselmi C, Polano M, Aceto A, Florio T, De Nobili M. . The interaction of humic substances with the human prion protein fragment 90-231 affects its protease K resistance and cell internalization. J Biol Regul Homeost Agents 2010; 24:27 - 39; PMID: 20385069 [PubMed] [Google Scholar]
- 43.Imrie CE, Korre A, Munoz-Melendez G. . Spatial correlation between the prevalence of transmissible spongiform diseases and British soil geochemistry. Environ Geochem Health 2009; 31:133 - 45; http://dx.doi.org/ 10.1007/s10653-008-9172-y; PMID: 18427934 [DOI] [PubMed] [Google Scholar]
- 44.Tomaszewski JE, Schwarzenbach RP, Sander M. . Protein encapsulation by humic substances. Environ Sci Technol 2011; 45:6003 - 10; http://dx.doi.org/ 10.1021/es200663h; PMID: 21678916 [DOI] [PubMed] [Google Scholar]
- 45.van der Kamp MW, Daggett V. . Influence of pH on the human prion protein: insights into the early steps of misfolding. Biophys J 2010; 99:2289 - 98; http://dx.doi.org/ 10.1016/j.bpj.2010.07.063; PMID: 20923664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saunders SE, Yuan Q, Bartz JC, Bartelt-Hunt S. . Effects of solution chemistry and aging time on prion protein adsorption and replication of soil-bound prions. PLoS One 2011; 6:e18752; http://dx.doi.org/ 10.1371/journal.pone.0018752; PMID: 21526178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sparks DL. Environmental soil chemistry. Academic press Elsevier Science: CA, USA, 2003; pp. 352. [Google Scholar]
- 48.Docoslis A, Rusinski LA, Giese RF, Van Oss CJ. . Kinetics and interaction constants of protein adsorption onto mineral microparticles - Measurement of the constants at the onset of hysteresis. Colloids Surf B Biointerfaces 2001; 22:267 - 83; http://dx.doi.org/ 10.1016/S0927-7765(01)00150-3 [DOI] [Google Scholar]
- 49.Yu WH, Li N, Tong DS, Zhou CH, Lin CX, Xu CY. . Adsorption of proteins and nucleic acids on clay minerals and their interactions: A review. Appl Clay Sci 2013; 80-81:443 - 52; http://dx.doi.org/ 10.1016/j.clay.2013.06.003 [DOI] [Google Scholar]
- 50.Davies P, Brown DR. . Manganese enhances prion protein survival in model soils and increases prion infectivity to cells. PLoS One 2009; 4:e7518; http://dx.doi.org/ 10.1371/journal.pone.0007518; PMID: 19844576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gudmundsdóttir KB, Sigurdarson S, Kristinsson J, Eiríksson T, Jóhannesson T. . Iron and iron/manganese ratio in forage from Icelandic sheep farms: relation to scrapie. Acta Vet Scand 2006; 48:16; http://dx.doi.org/ 10.1186/1751-0147-48-16; PMID: 16987395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eiríksson T, Björnsson H, Gudmundsdóttir KB, Kristinsson J, Jóhannesson T. . The distribution of four trace elements (Fe, Mn, Cu, Zn) in forage and the relation to scrapie in Iceland. Acta Vet Scand 2010; 52:34; http://dx.doi.org/ 10.1186/1751-0147-52-34; PMID: 20492671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fusi P, Ristori GG, Calamai L, Stotzky G. . Adsorption and binding of protein on “clean” (homoionic) and “dirty” (coated with Fe oxyhydroxides) montmorillonite, illite and kaolinite. Soil Biol Biochem 1989; 21:911 - 20; http://dx.doi.org/ 10.1016/0038-0717(89)90080-1 [DOI] [Google Scholar]
- 54.Saidy AR, Smernik RJ, Baldock JA, Kaiser K, Sanderman J. . The sorption of organic carbon onto differing clay minerals in the presence and absence of hydrous iron oxide. Geoderma 2013; 209-210:15 - 21; http://dx.doi.org/ 10.1016/j.geoderma.2013.05.026 [DOI] [Google Scholar]