Abstract
Prions are molecular pathogens, able to convert a normal cellular prion protein (PrPC) into a prion (PrPSc). The information necessary for this conversion is contained in the conformation of PrPSc. Mass spectrometry (MS) and small-molecule covalent reactions have been used to study prions. Mass spectrometry has been used to detect and quantitate prions in the attomole range (10−18 mole). MS-based analysis showed that both possess identical amino acid sequences, one disulfide bond, a GPI anchor, asparagine-linked sugar antennae, and unoxidized methionines. Mass spectrometry has been used to define elements of the secondary and tertiary structure of wild-type PrPSc and GPI-anchorless PrPSc. It has also been used to study the quaternary structure of the PrPSc multimer. Small molecule reagents react differently with the same lysine in the PrPC conformation than in the PrPSc conformation. Such differences can be detected by Western blot using mAbs with lysine-containing epitopes, such as 3F4 and 6D11. This permits the detection of PrPSc without the need for proteinase K pretreatment and can be used to distinguish among prion strains. These results illustrate how two important chemical tools, mass spectrometry and covalent modification by small molecules, are being applied to the detection and structural study of prions. Furthermore these tools are or can be applied to the study of the other protein misfolding diseases such as Alzheimer Disease, Parkinson Disease, or ALS.
Keywords: Western blot, covalent modification, lysine, mass spectrometry, monoclonal antibody, prion
Mass Spectrometry-Based Study of the Structure of PrPSc
Mass spectrometry has played a significant role in determining the primary covalent structures of PrPC and PrPSc. A combination of genetic analysis,1 amino acid sequencing,2-5 chemical,6 and MS analysis3,7-9 was used to show that PrPC and PrPSc possess identical amino acid sequences, a single disulfide bond, two sites for the attachment of sugar antennae, and a covalently bound glycosylphosphatidylinisotol (GPI) anchor (Fig. 1). A detailed mass spectrometry-based comparison of the sugar antennae that were present in purified PrPC and PrPSc showed that the antennae of both isoforms were highly varied (>30 different forms) and that they varied similarly in both PrPC and PrPSc.10–12 Mass spectrometric analysis of the GPI anchor showed that several variants were found in similar proportions in both PrPC and PrPSc. 13, 14 These results indicated that any post-translational differences between PrPC and PrPSc were not in the primary structure and were not covalent.
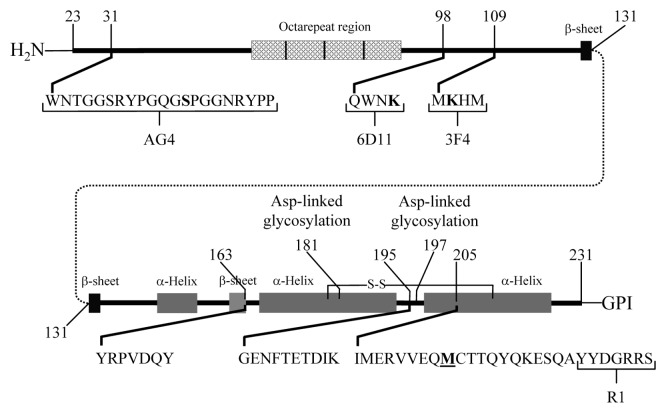
Figure 1. Cartoon of the hamster PrPC. The location of the epitopes of the AG4, 3F4, R1, and 6D11 monoclonal antibodies and tryptic peptides GENFTETDIK, VVEQMCTTQYQK, ESQAYYDGR, and YPGQGSPGGNR and the chymotryptic peptide YRPVDQY are indicated. The position of Met 213 is indicated M.
A variety of mass spectrometry-based approaches have been used to study the chemistry and structure of PrP. The location of methionines oxidized by hydrogen peroxide in rPrP was determined by mass spectrometry.15 More recently, mass spectrometry was used to study the structure of rPrP and rPrPβ by measuring the difference in the hydrogen peroxide reactivity of methionines present in the two isoforms.16 Copper-mediated oxidation of the histidine residues in PrP was studied by mass spectrometry-based analysis of rPrP.17 The reaction products of lysines and reactive oxygen species in hamster PrPSc were quantitated by mass spectrometry.18 The differences in secondary structure of the product from the seeded polymerization of rPrP starting with PrPSc or rPrP were examined using hydrogen/deuterium (H/D) exchange and MS analysis.19 Mass spectrometry-based analysis of the reaction of cross-linking reagents and PrPSc has been used to study the tertiary structure of hamster PrPSc by measuring the spatial proximity of terminal glycines in hamster PrP 27–30.20 Other researchers used different cross-linking reagents and PK digestion to study the structural differences between rPrP and rPrPβ.21 These approaches used recombinant PrP or regions of PrPSc that were not glycosylated.
Mass spectrometry-based study of PrPSc structure has mainly focused on the non-glycosylated N-terminal region of PrPSc. Mass spectrometry has been used to study copper-mediated oxidative cleavage of PrPC.22 MS was used to identify N-terminal ragged ends and prion strain-dependent variations in those N-terminal ragged ends.23-26 The PK cleavage sites between cysteine 179 and the N-terminus of the Dy and 263K strains of PrPSc were identified by a MS-based analysis.27 This analysis showed that the PK sensitive and PK resistant forms of PrPSc were infectious and shared a common structure.28,29 Due to the glycoform variability, these approaches have been limited to the N-terminal region of the protein.
The PrP produced by transgenic GPI-anchorless (GPI-) mice was shown to have no GPI anchor and only a limited amount of glycosylation.30,31 Such limited glycosylation permitted a more complete MS analysis of H/D exchange that occurred in this region. It showed only a limited amount of exchange, which suggested that GPI- PrPSc was composed mostly of β-sheet secondary structure.32 Other researchers used mass spectrometry to identify the products from PK digested GPI- PrPSc.33,34 This analysis led the researchers to conclude that PrPSc was composed of largely PK resistant β-sheet strands that were linked by identifiable short, flexible, and PK sensitive loops. Mass spectrometry was used to detect variations in the extent of H/D exchange as rPrP was converted into infectious rPrPSc.35 Recent MS-related work has led to the reassessment of the relative proportion of α-helix and β-sheet in PrPSc samples, whose purity was assessed by mass spectrometry.36 These studies concluded that the secondary structure of PrPSc was composed almost entirely of β-sheet.
Mass Spectrometry-Based Analysis of Oxidized PrPSc
During the MS analysis of the primary structure of hamster PrPSc, one of the peptides (IMERVVEQMCTTQYQK; Hamster PrP 205–220) was observed to contain oxidized methionine. The origin of this modification was unclear, since it could have been the consequence of a biological process or an artifact.3 When methionines in human rPrP were systematically replaced by a more polar analog, methoxinine, the methoxinine containing rPrP was found to be more susceptible to fibrilization than the native human rPrP.37 Using different model systems, other researchers obtained results which suggested that the oxidation of methionines in this region of rPrP would make it more susceptible to fibrilization.38,39 Western blot-based analysis of PrPSc showed that methionines from this region were oxidized to a significant extent.40-42 Oxidized methionine was proposed to be a covalent signature of PrPSc, which would be the first demonstrated covalent difference between PrPC and PrPSc.40
A large body of work suggested that oxidized methionines were unlikely to have a role in prion propagation. Mammals were demonstrated to express methionine sulfoxide reductases (Msr) as a housekeeping protein whose purpose was to reduce any oxidized methionines.43-46 The incubation period for prion infected Msr-ablated mice was shown to be the same as that of the corresponding wild type mice, which suggested that oxidized methionines do not have a role in prion propagation.47 Peptides spanning this region of PrP were shown to fibrilize without the presence of oxidized methionine.3,48 Other studies showed that the presence of oxidized methionines actually inhibited PrP fibrilization.49-51 When rPrP (29-231) was oxidized by hydrogen peroxide, enzymatically cleaved, and then analyzed by MS, the methionines present in the peptide IMERVVEQMCTTQYQK were shown to be remarkably resistant to hydrogen peroxide-mediated oxidation,15 which was consistent with predictions from a more general study of hydrogen peroxide-mediated oxidation of methionines.52 These results suggested that significant biological oxidation of this region of PrPSc was unlikely to occur.
Since a methionine could be oxidized by a biological process or by artifact, it was important to have a method that minimized artifactual oxidation when quantitating methionine oxidation. SDS-PAGE separation was essential for a Western-blot analysis of PrP, but it was also shown to be an inherently oxidative process.53,54 Although methionine oxidation from SDS-PAGE could be minimized by rigorous use of chemical reductants,53 this approach was not used in the analysis of PrP by Western blot analysis. Methionines were shown to be oxidized by exposure to air during general handling55-57 and even, to a limited extent, by the ionization necessary for MS analysis.58,59 These reports indicated that artifactual oxidation of methionine caused by common laboratory manipulation could be significant.
MS-based analysis was determined to be well suited for the detection and quantitation of the amounts of oxidized methionine present in PrP.60,61 Solution-based digestion was used instead of a SDS-PAGE-based digestion to eliminate that source of artifactual oxidation.62,63 The well established multiple reaction monitoring (MRM) and solution-based digestion methods were used to determine the ratio of the oxidized and unoxidized forms of the tryptic peptide VVEQMCTTQYQK (Hamster PrP 209–220).62-66 The levels of artifactual oxidation as a result of in-solution digestion and MS analysis was determined to be detectable, but negligible.60,61 This work indicated that a MRM-based analysis introduced a minimal amount of artifactual methionine oxidation.
A MRM-based approach was used to analyze three strains of hamster-adapted scrapie, ten field cases of sheep scrapie and ten cases of elk CWD.60,61 The prions were purified according to the method of Bolton et al.,67 which isolated both the PK sensitive and resistant fractions of PrPSc in high yield.68 The results from a 10-week time course experiment showed that the proportion of oxidized methionine in the analyte peptide actually decreased from the earliest time point (3 wk; ~13%) to the final time point (10 wk, ~8%) (Fig. 2).61 Analysis of brains from hamsters infected with the 263K, 139H or drowsy (Dy) prion strain showed that the proportion of oxidized methionine present in PrPC (uninfected hamsters) was similar (~2–10%) to that found in PrPSc derived from hamsters infected with those strains of hamster-adapted scrapie.61 Samples from ten field cases of scrapie-infected sheep and ten field cases of CWD-infected elk showed a similarly low proportion of methionine oxidation in the homologous peptide (VVEQMCITQYQR; Sheep PrP 212–223) in both the sheep and elk PrPSc.60 Even though the sheep and elk were much older than the hamsters and presumably contained PrPSc that had more time to be exposed to biological oxidation, the proportion of oxidized methionine was similar in sheep, elk, and hamsters.60,61 These data indicated that the observed amounts of oxidation present in these methionines was a consequence of handling proteins in the presence of air and not evidence of a PrPSc specific covalent signature.60,61
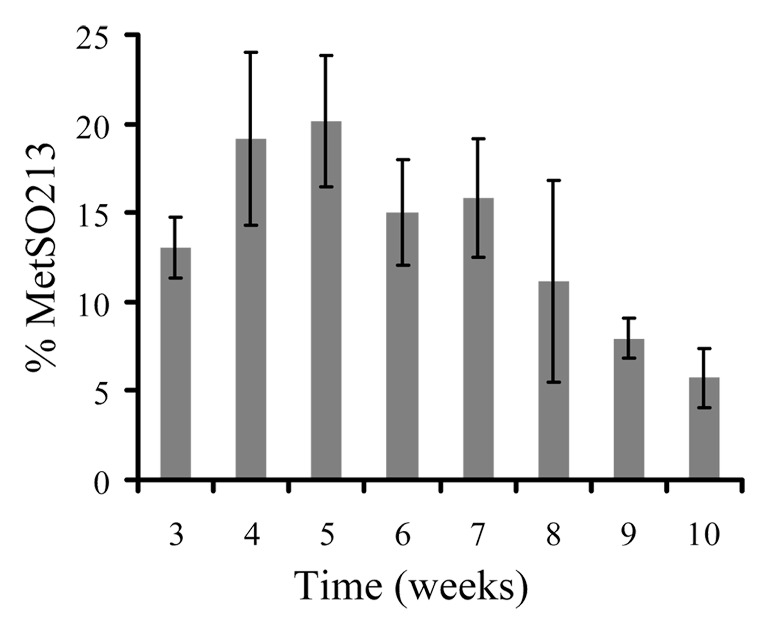
Figure 2. Percentage of oxidized Met213 present in PrP27–30 isolated from the brains of hamsters during the course of an ic challenge (263K), as measure by mass spectrometry. Results are reported as means (± SD) for each time point (n = 4).
The relationship between polymorphisms in the VVEQMCITQYQR peptide and the extent of methionine oxidation was determined using a model system.60,69 Unnatural sheep rPrP polymorphisms (I replaced by T [hamster analog] or V [mouse analog]) were isolated, digested, mixed and subjected to air oxidation.60,69 MRM analysis of the peptide mixtures showed that peptides containing isoleucine were oxidized in a higher proportion than in analogs containing valine or threonine (I > V > T), even though all three were exposed to air oxidation under the same conditions. These results showed that the chemistry of air oxidation was different from that of peroxide mediated oxidation. Furthermore, it indicated that sheep and elk PrPC were more susceptible to oxidation than was hamster PrPC. Even though sheep and elk PrP were intrinsically more susceptible to oxidation, sheep, elk and hamster PrPSc all had similarly low levels of oxidized methionine. This body of work provided further evidence that post-translational differences between PrPC and PrPSc were purely conformational and not covalent.
Mass Spectrometry-Based Detection of PrPSc
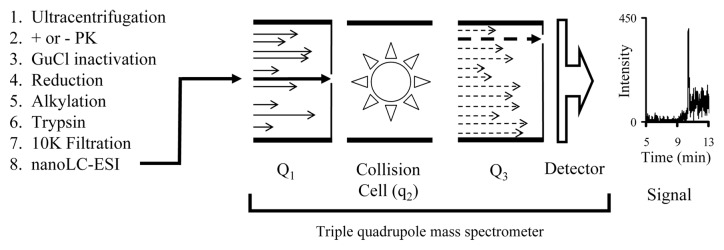
The MRM approach was used to detect and quantitate the PrPSc present in sheep, elk, deer, mouse and hamsters.60,62,63,70,71 The set of tryptic peptides from the digestion of rPrP was analyzed and the peptides VVEQMCTTQYQK (Hamster PrP 209–220), VVEQMCITQYQR (Sheep PrP 212–223), and VVEQMCVTQYQK (Mouse PrP 208–219) were empirically determined to be suitable for a MRM-based analysis.62,71 These analyte peptides were detectable in the attomole (10−18 mole) range and present in both PrPSc and PrP 27–30. MS analysis of tissue from uninfected controls showed that they contained no peptides that would interfere with the MRM analysis. Stable isotope-labeled (15N and 13C) analogs of these analyte peptides were chemically synthesized and used as internal standards.60-63,71 Adding a known amount of an internal standard to the tryptic digest of a sample permitted the quantitation of the analyte peptide relative to the known amount of the added internal standard. In hamsters, this approach was used to detect PrPSc one day post ic inoculation (Fig. 3).63 In addition PrPSc was easily detectable in non-obex brain tissue from field cases of sheep scrapie and elk CWD.60,71 Furthermore this approach was used to detect and quantitate PrPSc in non-brain tissue (spleen, tonsil and RAMALT).
Figure 3. Scheme showing the process of analyzing a prion sample by a MS-based MRM method. An aliquot (~1/5) of a hamster brain (1 d post ic inoculation) was processed for mass spectrometry (Steps 1–7; +PK). The sample was chromatographed using a nano-LC system and then continuously sprayed by electrospray ionization (ESI) (Step 8) into the mass spectrometer. The first quadrupole (Q1) was set to permit only the ions with a mass/charge ratio (m/z; z = 2) of the analyte peptide (VVEQMCTTQYQK) to enter the collision cell (q2). In the collision cell the filtered ions were fragmented. These fragments entered the third quadrupole which was set to permit ions with an m/z corresponding to an optimized fragment of the analyze peptide (b2 ion [VV]) to enter the detector. The resulting signal from the detector was recorded.
The tryptic peptides GENFTETDIK (Hamster PrP 195–204) and ESQAYYDGR (Hamster PrP 221–229) were used to confirm the diagnosis of prion diseases.62,70,71 They were not suitable for use as analyte peptides, since the asparagine in the GENFTETDIK peptide was only present in those PrP molecules that were not glycosylated (N-197) and ESQAYYDGR was not present in all forms of PrPSc.72 These two peptides were shown to have a greater MRM signal intensity than the analyte peptide, so they could be used to confirm the presence of PrPSc without a loss of sensitivity.62,71 This approach was used to detect prions in five strains of hamster-adapted scrapie and four strains of murine-adapted scrapie in asymptomatic preclinical animals.70,71 Furthermore, it was used to confirm the presence of prions in field cases of sheep infected with scrapie and elk infected with CWD.60,71 These results demonstrate the utility of mass spectrometry to diagnose prion diseases.70,71
MRM-based methods of detecting PrP were not limited to three tryptic peptides. The peptides YPGQGSPGGNR (Hamster PrP 38–48) and YRPVDQY (Hamster PrP 163–169) were found to be common to virtually all known mammalian PrP proteins and could be used to detect PrP, using rPrP as a model.73,74 Both of these peptides were detected in digested PrPSc from the brains of prion-infected hamsters (Silva CJ, unpublished results). Mass spectrometry-based analysis of N-terminal cleavage sites (ragged ends) was used to distinguish between different types of human prion diseases.23 The MS-based analysis of the ragged ends from sheep PrPSc showed utility in distinguishing among strains of sheep scrapie.24-26 The variety of MS-based prion detection methods was illustrated by these reports.
Mass spectrometry has been employed to analyze complex intact protein, proteomic, and peptidomic mixtures and to detect differences between prion-infected animals and healthy controls. The urine of BSE-infected cattle was analyzed by this method and shown to be subtly different from uninfected control animals.75-77 A proteomic analysis of glycosylated serum proteins from prion infected mice showed differences in the relative amounts of these proteins.78 Other researchers used a whole protein approach to show that there were differences in the protein expression profiles present in the brains of prion infected mice and uninfected controls.79 A more focused approach used MS to examine differences in the hippocampal proteome in control mice and those in the late stage of an infection (Me7 prion strain).80 An analogous whole protein comparison of serum from scrapie-infected sheep showed differential protein expression profiles in infected animals examined at early and later stages of the disease.81 A peptidomics-based approach has been used to distinguish between prion infected and healthy animals by examining the peptidome of hamsters infected with prions.82 A MS-based peptidomic analysis was able to distinguish differences in the relative amounts of tryptic peptides derived from the small proteins (<10 kDa) present in the CSF fluid from CJD and non-CJD patients.83 A proteomics-based approach revealed that urine-derived fertility products were contaminated with PrP.84,85 Proteomic approaches have been used to identify proteins that co-purify with PrPSc as part of a search for PrPSc binding partners.86-88 Mass spectrometry has been used to determine the binding partners of GPI- PrP.89 Other researchers used mass spectrometry to identify peptides from bovine brain tissue that accelerate conformational changes in rPrP.90 These approaches reveal the myriad ways in which mass spectrometry has been used to detect prions directly or to infer their presence indirectly.
Small Molecule Based Study of PrPSc
Mass spectrometry-based and other forms of analysis have clearly demonstrated that the post-translational differences between PrPC and PrPSc are not covalent and must be conformational (non-covalent).9 These results meant that the same amino acid could react differently with the same reagent, depending on whether it was in the PrPC or PrPSc conformation. A variety of reagents have been used to covalently modify PrP, but not to distinguish between PrPC and PrPSc.20,21,91–95 These results indicated that there are amino acid residues on PrPSc that could be covalently modified by a variety of reagents and these covalent bonds would remain intact after PrPSc was denatured. Thus, some information about the PrPSc conformation would be retained after the reacted PrPSc was denatured.
Purified PrPSc has been reacted with commercially available reagents to obtain structural information about its C-terminal region.96 Hamster PrPSc was reacted with tetranitromethane (TNM) or acetic anhydride (Ac2O), which nitrated the tyrosine or acetylated the ϵ-amino groups of lysine, respectively. After reaction, the samples were analyzed by mass spectrometry to determine which tyrosine or lysine was covalently modified. The extent of tyrosine nitration was also monitored by Western blot, using the antibody R1 (Hamster PrP 225–231 epitope).97 Covalent modification of the tyrosine interfered with the binding of the antibody, resulting in a reduction of signal relative to the unmodified tyrosine. In this way antibodies were used to detect covalent modifications of PrPSc.96
The lysines present in the PrPC conformer reacted completely with synthetic reagents. The extent of this reaction was monitored by Western blot using antibodies that did and did not have lysine-containing epitopes.98 The NHS esters of acetic acid (Ac-NHS) and 4-trimethylamoniumbutyric acid ([3-carboxypropyl]trimethylammonium chloride) (tMAB-NHS) were used and covalently converted the ϵ-amino group of an accessible lysine into the corresponding amide. These reagents were reacted with detergent solubilized hamster brain homogenates (DSHBH) from uninfected animals and analyzed by Western blot using the antibodies 3F4 (Hamster PrP 109–112 epitope) and AG4 (Hamster PrP 31–51 epitope) (Fig. 4). The epitope of the 3F4 antibody was shown to contain a lysine, while the epitope of the AG4 antibody was shown not to (Fig. 1). Blots probed with the 3F4 antibody showed no signals for PrPC after reaction with either of these reagents. In contrast blots probed with AG4 showed signals from PrPC after reaction with both of the reagents. These results indicated that covalent modification of a lysine that was part of an antibody’s epitope (3F4) would prevent the binding of that antibody. They also indicated that non lysine-containing epitopes (AG4) were not affected by the reagents.
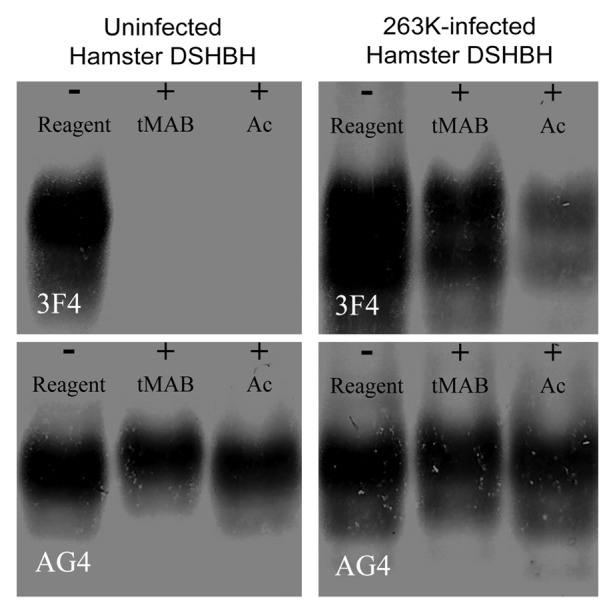
Figure 4. Western blots of uninfected or 263K-infected hamster DSHBH. Samples consist of uninfected or 263K-infected DSHBH reacted with nothing (- reagent), 20 mM tMAB-NHS (+tMAB), or 20 mM Ac-NHS (+Ac). Identical amounts of the same reaction mixtures were probed with either the 3F4 or AG4 monoclonal antibody.
These reagents were used to distinguish between the PrPC and PrPSc present in prion infected DSHBH.98 The Ac-NHS and tMAB-NHS reagents were reacted with DSHBH from animals infected with the 263K strain of hamster-adapted scrapie. The reaction mixtures were analyzed by Western blot and probed with either AG4 or 3F4. The blot probed with AG4 was qualitatively similar to the blot of the uninfected DSHBH (Fig. 4B). When the same blot was probed with 3F4 signals were apparent in both the unreacted and reacted DSHBH from infected animals, but not from uninfected animals. Since the 3F4 epitope was hidden in the PrPSc conformation, it was less able to react with the reagents.99 When the reacted PrPSc was denatured for Western blot analysis, the now exposed and unreacted epitope could bind to the 3F4 antibody and would generate the observed signal. Since the 3F4 epitope was exposed in the PrPC conformation, this approach could be used to detect PrPSc in the presence of PrPC without the need to remove the PrPC first.
The Ac-NHS and the tMAB-NHS reagents both reacted with lysines, but to a different extent that was influenced by the non-NHS portions of their chemical structures. These differences in chemical structure meant that they interacted differently with the chemical environment of the lysine prior to reacting with it. Such reagent-dependent differences in reactivity could be seen by Western blot analysis (Fig. 4A; lanes 4–6), where the signal for the Ac-NHS reaction was noticeably less intense than that of the tMAB-NHS reagent. The tMAB-NHS reagent was positively charged and the Ac-NHS reagent was not, so such differences were not surprising. Differences in the extent of reactivity were also observed when the non-NHS portion of the reagents used were bulkier, but uncharged.100 This indicated that the extent of the reaction was dependent upon the choice of reagents.
Since these reagents could be used to distinguish between the PrPC and PrPSc conformations, they might be useful in distinguishing among PrPSc conformers or strains.98 The drowsy (Dy) and 263K strains were reacted with the Ac-NHS, tMAB-NHS, and other related reagents and analyzed by Western blot. The blots were probed with 3F4 or 6D11 (Hamster PrP 98–101 epitope), another antibody shown to have a lysine-containing epitope. The blots showed that the reactivity of the Dy conformation was different from that of the 263K conformation.98,100 This showed that these two lysines (3F4 or 6D11 epitope) are in different chemical environments depending upon their PrPSc conformation (Dy or 263K).
Only a limited number of PrP lysines were demonstrated to be a component of an available antibody’s epitope. Covalent modification of lysine would also prevent its cleavage by trypsin, so a measurement of the absence of tryptic peptides was determined to be a means to detect covalently modified lysines. The absence of tryptic peptides was measured by MS using stable isotope-labeled internal standards. This approach has been used to detect covalently modified lysines in recombinant hamster PrP.100,101 Such a MS–based approach could be used to analyze PrPSc lysines until appropriate antibodies become available.
Mass Spectrometry and Small Molecule-Based Study of Other Protein Misfolding Diseases
Other, non-TSE, neurodegenerative diseases have been associated with pathological protein misfolding and discussed in recent reviews.102,103 These include the amyloid-β deposits associated with Alzheimer, the accumulation of tau associated with Alzheimer disease or other tauopathies, α-synuclein associated with Parkinson disease, and the SOD1 associated with amyotrophic lateral sclerosis (ALS). The proteins associated with these diseases show protein misfolding and progressive pathological aggregation that are analogous to those seen in prion diseases. As with prions, researchers have used small molecule modification and mass spectrometry to study these proteinopathies.
Some recent reports showed that mass spectrometry could be used to study the structure of these misfolded proteins and to detect them in tissues. The structure of α-synuclein oligomers and a pathogenic variant of SOD1 have been studied using mass spectrometry to monitor differences in hydrogen/deuterium exchange.104,105 MS has been used to study the aggregation and post-translational modifications of SOD1.106 It was used to identify novel α-synuclein isoforms in brain tissue and amyloid-β in cerebral spinal fluid.107-109 These results demonstrated the utility of MS for structural studies.
These proteins have been shown to have varied post-translational modifications, which have been detected and characterized by mass spectrometry. The increase in nitration of tyrosine-39 of α-synuclein was monitored by mass spectrometry.110 Phosphorylation sites of tau and other proteins were identified in the brains of Alzheimer disease patients using a novel mass spectrometry-based approach.111 Components of the Lewy body proteome were determined using mass spectrometry.112 A mass spectrometry-based analysis has been used to study the structure α-synuclein by analyzing the reaction products of small molecule crosslinkers with α-synuclein.113 Antibodies have been used to detect covalent modification of these proteins. Nitrated forms of α-synuclein associated with Parkinson disease have been identified using antibodies specific for the nitrated form of the protein.114,115 These results indicate that MS or antibodies can be used to detect the specific post-translational modifications associated with these proteinopathies.
In principle the covalent modification by small molecules approach used to study prions (vide supra) could be applied to the study of amyloid-β, tau, α-synuclein, or SOD1. The reagents have been described and a number of monoclonal antibodies (mAbs) have been produced. Anti-amyloid-β (1–40) monoclonal antibodies were shown to bind to a lysine-containing region of the protein.116 Several anti-tau mAbs, with lysine-containing eptiopes, have been produced.117,118 The anti-α-synuclein mAb Syn-17 was demonstrated to have a lysine-containing epitope.119 Some of the lysine residues of SOD1 were shown to be components of the epitopes of anti-SOD1 monoclonal antibodies.120 As has been shown with prions, these or other antibodies (with lysine-containing epitopes) may be useful in developing a Western blot-based detection of conformation dependent differences in small molecule reactivity between the normal and pathogenic forms of amyloid-β, tau, α-synuclein, or SOD1.
Summary
Mass spectrometry has played a crucial role in the study of prions and other protein misfolding diseases and will continue to do so. Mass spectrometry-based analysis provides the strongest evidence for conformation being the sole difference between PrPSc and PrPC. More recently mass spectrometry has been used to study the structure of PrPSc, by analyzing the peptide mixtures resulting from proteolytic digestion. The widespread availability of GPI- PrPSc and rPrPSc means a ready supply of non-glycosylated PrP protein that is suitable for MS analysis.
Using antibodies to detect small molecule modifications of PrPSc is another means of studying PrPSc. This approach does not require a mass spectrometer, the reagents are reasonably easy to prepare, and it uses equipment that is available in any molecular biology laboratory. It relies on the well established technique of Western blotting. This approach can be used to distinguish between PrPC, PrPSc, and different strains of PrPSc. Furthermore it can be used to study other protein misfolding diseases (Alzheimer’s disease, Parkinson’s disease, etc.). In principle it can be used instead of MS analysis when the appropriate antibodies are available.
Two important chemical tools, mass spectrometry and covalent modification by small molecules, are being successfully applied to the detection and structural study of these molecular pathogens.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Basler K, Oesch B, Scott M, Westaway D, Wälchli M, Groth DF, McKinley MP, Prusiner SB, Weissmann C. . Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell 1986; 46:417 - 28; http://dx.doi.org/ 10.1016/0092-8674(86)90662-8; PMID: 2873895 [DOI] [PubMed] [Google Scholar]
- 2.Hope J, Morton LJ, Farquhar CF, Multhaup G, Beyreuther K, Kimberlin RH. . The major polypeptide of scrapie-associated fibrils (SAF) has the same size, charge distribution and N-terminal protein sequence as predicted for the normal brain protein (PrP). EMBO J 1986; 5:2591 - 7; PMID: 3096712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stahl N, Baldwin MA, Teplow DB, Hood L, Gibson BW, Burlingame AL, Prusiner SB. . Structural studies of the scrapie prion protein using mass spectrometry and amino acid sequencing. Biochemistry 1993; 32:1991 - 2002; http://dx.doi.org/ 10.1021/bi00059a016; PMID: 8448158 [DOI] [PubMed] [Google Scholar]
- 4.Prusiner SB, Groth DF, Bolton DC, Kent SB, Hood LE. . Purification and structural studies of a major scrapie prion protein. Cell 1984; 38:127 - 34; http://dx.doi.org/ 10.1016/0092-8674(84)90533-6; PMID: 6432339 [DOI] [PubMed] [Google Scholar]
- 5.Turk E, Teplow DB, Hood LE, Prusiner SB. . Purification and properties of the cellular and scrapie hamster prion proteins. Eur J Biochem 1988; 176:21 - 30; http://dx.doi.org/ 10.1111/j.1432-1033.1988.tb14246.x; PMID: 3138115 [DOI] [PubMed] [Google Scholar]
- 6.Bolton DC, Meyer RK, Prusiner SB. . Scrapie PrP 27-30 is a sialoglycoprotein. J Virol 1985; 53:596 - 606; PMID: 3918176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin MA. . Mass spectrometric analysis of prion proteins. Adv Protein Chem 2001; 57:29 - 54; http://dx.doi.org/ 10.1016/S0065-3233(01)57017-5; PMID: 11447694 [DOI] [PubMed] [Google Scholar]
- 8.Stahl N, Borchelt DR, Hsiao K, Prusiner SB. . Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell 1987; 51:229 - 40; http://dx.doi.org/ 10.1016/0092-8674(87)90150-4; PMID: 2444340 [DOI] [PubMed] [Google Scholar]
- 9.Stahl N, Baldwin M, Teplow DB, Hood LE, Beavis R, Chait B, Gibson BW, Burlingame AL, Prusiner SB. Cataloging post-translational modifications of the scrapie prion protein by mass spectrometry. In: Prusiner SB, Collinge J, Powell J, Anderton B, eds. Prion diseases of humans and animals. New York: Ellis Horwood, 1992:361-79. [Google Scholar]
- 10.Endo T, Groth D, Prusiner SB, Kobata A. . Diversity of oligosaccharide structures linked to asparagines of the scrapie prion protein. Biochemistry 1989; 28:8380 - 8; http://dx.doi.org/ 10.1021/bi00447a017; PMID: 2574992 [DOI] [PubMed] [Google Scholar]
- 11.Rudd PM, Endo T, Colominas C, Groth D, Wheeler SF, Harvey DJ, Wormald MR, Serban H, Prusiner SB, Kobata A, et al. . Glycosylation differences between the normal and pathogenic prion protein isoforms. Proc Natl Acad Sci U S A 1999; 96:13044 - 9; http://dx.doi.org/ 10.1073/pnas.96.23.13044; PMID: 10557270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stimson E, Hope J, Chong A, Burlingame AL. . Site-specific characterization of the N-linked glycans of murine prion protein by high-performance liquid chromatography/electrospray mass spectrometry and exoglycosidase digestions. Biochemistry 1999; 38:4885 - 95; http://dx.doi.org/ 10.1021/bi982330q; PMID: 10200178 [DOI] [PubMed] [Google Scholar]
- 13.Stahl N, Baldwin MA, Prusiner SB. . Electrospray mass spectrometry of the glycosylinositol phospholipid of the scrapie prion protein. Cell Biol Int Rep 1991; 15:853 - 62; http://dx.doi.org/ 10.1016/0309-1651(91)90037-J; PMID: 1686992 [DOI] [PubMed] [Google Scholar]
- 14.Stahl N, Baldwin MA, Hecker R, Pan KM, Burlingame AL, Prusiner SB. . . Glycosylinositol phospholipid anchors of the scrapie and cellular prion proteins contain sialic acid. Biochemistry 1992; 31:5043 - 53; http://dx.doi.org/ 10.1021/bi00136a600; PMID: 1350920 [DOI] [PubMed] [Google Scholar]
- 15.Requena JR, Dimitrova MN, Legname G, Teijeira S, Prusiner SB, Levine RL. . Oxidation of methionine residues in the prion protein by hydrogen peroxide. Arch Biochem Biophys 2004; 432:188 - 95; http://dx.doi.org/ 10.1016/j.abb.2004.09.012; PMID: 15542057 [DOI] [PubMed] [Google Scholar]
- 16.Serpa JJ, Makepeace KA, Borchers TH, Wishart DS, Petrotchenko EV, Borchers CH. . Using isotopically-coded hydrogen peroxide as a surface modification reagent for the structural characterization of prion protein aggregates. J Proteomics 2013; Forthcoming http://dx.doi.org/ 10.1016/j.jprot.2013.11.020; PMID: 24316355 [DOI] [PubMed] [Google Scholar]
- 17.Requena JR, Groth D, Legname G, Stadtman ER, Prusiner SB, Levine RL. . Copper-catalyzed oxidation of the recombinant SHa(29-231) prion protein. Proc Natl Acad Sci U S A 2001; 98:7170 - 5; http://dx.doi.org/ 10.1073/pnas.121190898; PMID: 11404462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pamplona R, Naudí A, Gavín R, Pastrana MA, Sajnani G, Ilieva EV, Del Río JA, Portero-Otín M, Ferrer I, Requena JR. . Increased oxidation, glycoxidation, and lipoxidation of brain proteins in prion disease. Free Radic Biol Med 2008; 45:1159 - 66; http://dx.doi.org/ 10.1016/j.freeradbiomed.2008.07.009; PMID: 18703134 [DOI] [PubMed] [Google Scholar]
- 19.Smirnovas V, Kim JI, Lu X, Atarashi R, Caughey B, Surewicz WK. . Distinct structures of scrapie prion protein (PrPSc)-seeded versus spontaneous recombinant prion protein fibrils revealed by hydrogen/deuterium exchange. J Biol Chem 2009; 284:24233 - 41; http://dx.doi.org/ 10.1074/jbc.M109.036558; PMID: 19596861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onisko B, Fernández EG, Freire ML, Schwarz A, Baier M, Camiña F, García JR, Rodríguez-Segade Villamarín S, Requena JR. . Probing PrPSc structure using chemical cross-linking and mass spectrometry: evidence of the proximity of Gly90 amino termini in the PrP 27-30 aggregate. Biochemistry 2005; 44:10100 - 9; http://dx.doi.org/ 10.1021/bi0501582; PMID: 16042387 [DOI] [PubMed] [Google Scholar]
- 21.Petrotchenko EV, Serpa JJ, Hardie DB, Berjanskii M, Suriyamongkol BP, Wishart DS, Borchers CH. . Use of proteinase K nonspecific digestion for selective and comprehensive identification of interpeptide cross-links: application to prion proteins. Mol Cell Proteomics 2012; 11:013524; http://dx.doi.org/ 10.1074/mcp.M111.013524; PMID: 22438564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald AJ, Dibble JP, Evans EG, Millhauser GL. . A New Paradigm for Enzymatic Control of alpha-Cleavage and beta-Cleavage of the Prion Protein. J Biol Chem 2014; Forthcoming http://dx.doi.org/ 10.1074/jbc.M113.502351; PMID: 24247244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen SG, Zou W, Parchi P, Gambetti P. . PrP(Sc) typing by N-terminal sequencing and mass spectrometry. Arch Virol Suppl 2000; 209 - 16; PMID: 11214924 [PubMed] [Google Scholar]
- 24.Gielbert A, Davis LA, Sayers AR, Hope J, Gill AC, Sauer MJ. . High-resolution differentiation of transmissible spongiform encephalopathy strains by quantitative N-terminal amino acid profiling (N-TAAP) of PK-digested abnormal prion protein. J Mass Spectrom 2009; 44:384 - 96; http://dx.doi.org/ 10.1002/jms.1516; PMID: 19053160 [DOI] [PubMed] [Google Scholar]
- 25.Gielbert A, Davis LA, Sayers AR, Tang Y, Hope J, Sauer MJ. . Quantitative profiling of PrP(Sc) peptides by high-performance liquid chromatography mass spectrometry to investigate the diversity of prions. Anal Biochem 2013; 436:36 - 44; http://dx.doi.org/ 10.1016/j.ab.2013.01.015; PMID: 23357236 [DOI] [PubMed] [Google Scholar]
- 26.Howells LC, Anderson S, Coldham NG, Sauer MJ. . Transmissible spongiform encephalopathy strain-associated diversity of N-terminal proteinase K cleavage sites of PrP(Sc) from scrapie-infected and bovine spongiform encephalopathy-infected mice. Biomarkers 2008; 13:393 - 412; http://dx.doi.org/ 10.1080/13547500801903719; PMID: 18484354 [DOI] [PubMed] [Google Scholar]
- 27.Sajnani G, Pastrana MA, Dynin I, Onisko B, Requena JR. . Scrapie prion protein structural constraints obtained by limited proteolysis and mass spectrometry. J Mol Biol 2008; 382:88 - 98; http://dx.doi.org/ 10.1016/j.jmb.2008.06.070; PMID: 18621059 [DOI] [PubMed] [Google Scholar]
- 28.Sajnani G, Silva CJ, Ramos A, Pastrana MA, Onisko BC, Erickson ML, Antaki EM, Dynin I, Vázquez-Fernández E, Sigurdson CJ, et al. . PK-sensitive PrP is infectious and shares basic structural features with PK-resistant PrP. PLoS Pathog 2012; 8:e1002547; http://dx.doi.org/ 10.1371/journal.ppat.1002547; PMID: 22396643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva CJ, Sajnani G, Ramos A, Pastrana MA, Onisko BC, Erickson ML, Antaki EM, Dynin I, Vazquez-Fernandez E, Sigurdson CJ, et al. . A comparison of the structure of the PK-sensitive and PK-resistant forms of PrPSc. Prion 2013; 7:Supplement 90 - 1 [Google Scholar]
- 30.Chesebro B, Trifilo M, Race R, Meade-White K, Teng C, LaCasse R, Raymond L, Favara C, Baron G, Priola S, et al. . Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 2005; 308:1435 - 9; http://dx.doi.org/ 10.1126/science.1110837; PMID: 15933194 [DOI] [PubMed] [Google Scholar]
- 31.Chesebro B, Race B, Meade-White K, Lacasse R, Race R, Klingeborn M, Striebel J, Dorward D, McGovern G, Jeffrey M. . Fatal transmissible amyloid encephalopathy: a new type of prion disease associated with lack of prion protein membrane anchoring. PLoS Pathog 2010; 6:e1000800; http://dx.doi.org/ 10.1371/journal.ppat.1000800; PMID: 20221436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smirnovas V, Baron GS, Offerdahl DK, Raymond GJ, Caughey B, Surewicz WK. . Structural organization of brain-derived mammalian prions examined by hydrogen-deuterium exchange. Nat Struct Mol Biol 2011; 18:504 - 6; http://dx.doi.org/ 10.1038/nsmb.2035; PMID: 21441913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vázquez-Fernández E, Alonso J, Pastrana MA, Ramos A, Stitz L, Vidal E, Dynin I, Petsch B, Silva CJ, Requena JR. . Structural organization of mammalian prions as probed by limited proteolysis. PLoS One 2012; 7:e50111; http://dx.doi.org/ 10.1371/journal.pone.0050111; PMID: 23185550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vazquez-Fernandez E, Pastrana M, Ramos A, Alonso J, Stitz L, Vidal E, Dynin I, Silva C. . Probing the structure of GPI-less PrPSc by limited proteolysis. Prion 2012; 6:supplement 26 - 7; PMID: 22453174 22453174 [Google Scholar]
- 35.Miller MB, Wang DW, Wang F, Noble GP, Ma J, Woods VL Jr., Li S, Supattapone S. . Cofactor molecules induce structural transformation during infectious prion formation. Structure 2013; 21:2061 - 8; http://dx.doi.org/ 10.1016/j.str.2013.08.025; PMID: 24120764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore RA, Timmes AG, Wilmarth PA, Safronetz D, Priola SA. . Identification and removal of proteins that co-purify with infectious prion protein improves the analysis of its secondary structure. Proteomics 2011; 11:3853 - 65; http://dx.doi.org/ 10.1002/pmic.201100253; PMID: 21805638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolschner C, Giese A, Kretzschmar HA, Huber R, Moroder L, Budisa N. . Design of anti- and pro-aggregation variants to assess the effects of methionine oxidation in human prion protein. Proc Natl Acad Sci U S A 2009; 106:7756 - 61; http://dx.doi.org/ 10.1073/pnas.0902688106; PMID: 19416900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colombo G, Meli M, Morra G, Gabizon R, Gasset M. . Methionine sulfoxides on prion protein Helix-3 switch on the alpha-fold destabilization required for conversion. PLoS One 2009; 4:e4296; http://dx.doi.org/ 10.1371/journal.pone.0004296; PMID: 19172188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Younan ND, Nadal RC, Davies P, Brown DR, Viles JH. . Methionine oxidation perturbs the structural core of the prion protein and suggests a generic misfolding pathway. J Biol Chem 2012; 287:28263 - 75; http://dx.doi.org/ 10.1074/jbc.M112.354779; PMID: 22654104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canello T, Engelstein R, Moshel O, Xanthopoulos K, Juanes ME, Langeveld J, Sklaviadis T, Gasset M, Gabizon R. . Methionine sulfoxides on PrPSc: a prion-specific covalent signature. Biochemistry 2008; 47:8866 - 73; http://dx.doi.org/ 10.1021/bi800801f; PMID: 18680312 [DOI] [PubMed] [Google Scholar]
- 41.Canello T, Frid K, Gabizon R, Lisa S, Friedler A, Moskovitz J, Gasset M, Gabizon R. . Oxidation of Helix-3 methionines precedes the formation of PK resistant PrP. PLoS Pathog 2010; 6:e1000977; http://dx.doi.org/ 10.1371/journal.ppat.1000977; PMID: 20625387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oien DB, Canello T, Gabizon R, Gasset M, Lundquist BL, Burns JM, Moskovitz J. . Detection of oxidized methionine in selected proteins, cellular extracts and blood serums by novel anti-methionine sulfoxide antibodies. Arch Biochem Biophys 2009; 485:35 - 40; http://dx.doi.org/ 10.1016/j.abb.2009.01.020; PMID: 19388147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moskovitz J. . Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim Biophys Acta 2005; 1703:213 - 9; http://dx.doi.org/ 10.1016/j.bbapap.2004.09.003; PMID: 15680229 [DOI] [PubMed] [Google Scholar]
- 44.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. . Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A 2001; 98:12920 - 5; http://dx.doi.org/ 10.1073/pnas.231472998; PMID: 11606777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boschi-Muller S, Gand A, Branlant G. . The methionine sulfoxide reductases: Catalysis and substrate specificities. Arch Biochem Biophys 2008; 474:266 - 73; http://dx.doi.org/ 10.1016/j.abb.2008.02.007; PMID: 18302927 [DOI] [PubMed] [Google Scholar]
- 46.Weissbach H, Resnick L, Brot N.. . Methionine sulfoxide reductases: history and cellular role in protecting against oxidative damage. Biochim Biophys Acta 2005; 1703:203 - 12 [DOI] [PubMed] [Google Scholar]
- 47.Tamgüney G, Giles K, Glidden DV, Lessard P, Wille H, Tremblay P, Groth DF, Yehiely F, Korth C, Moore RC, et al. . Genes contributing to prion pathogenesis. J Gen Virol 2008; 89:1777 - 88; http://dx.doi.org/ 10.1099/vir.0.2008/001255-0; PMID: 18559949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gasset M, Baldwin MA, Lloyd DH, Gabriel JM, Holtzman DM, Cohen F, Fletterick R, Prusiner SB. . Predicted α-helical regions of the prion protein when synthesized as peptides form amyloid. Proc Natl Acad Sci U S A 1992; 89:10940 - 4; http://dx.doi.org/ 10.1073/pnas.89.22.10940; PMID: 1438300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergstrom AL, Chabry J, Bastholm L, Heegaard PM. . Oxidation reduces the fibrillation but not the neurotoxicity of the prion peptide PrP106-126. Biochim Biophys Acta 2007; 1774:1118 - 27 [DOI] [PubMed] [Google Scholar]
- 50.Breydo L, Bocharova OV, Makarava N, Salnikov VV, Anderson M, Baskakov IV. . Methionine oxidation interferes with conversion of the prion protein into the fibrillar proteinase K-resistant conformation. Biochemistry 2005; 44:15534 - 43; http://dx.doi.org/ 10.1021/bi051369+; PMID: 16300402 [DOI] [PubMed] [Google Scholar]
- 51.Grabenauer M, Wu C, Soto P, Shea JE, Bowers MT. . Oligomers of the prion protein fragment 106-126 are likely assembled from beta-hairpins in solution, and methionine oxidation inhibits assembly without altering the peptide’s monomeric conformation. J Am Chem Soc 2010; 132:532 - 9; http://dx.doi.org/ 10.1021/ja905595k; PMID: 20020713 [DOI] [PubMed] [Google Scholar]
- 52.Ghesquière B, Jonckheere V, Colaert N, Van Durme J, Timmerman E, Goethals M, Schymkowitz J, Rousseau F, Vandekerckhove J, Gevaert K. . Redox proteomics of protein-bound methionine oxidation. Mol Cell Proteomics 2011; 10:006866; http://dx.doi.org/ 10.1074/mcp.M110.006866; PMID: 21406390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun G, Anderson VE. . Prevention of artifactual protein oxidation generated during sodium dodecyl sulfate-gel electrophoresis. Electrophoresis 2004; 25:959 - 65; http://dx.doi.org/ 10.1002/elps.200305800; PMID: 15095433 [DOI] [PubMed] [Google Scholar]
- 54.Swiderek KM, Davis MT, Lee TD. . The identification of peptide modifications derived from gel-separated proteins using electrospray triple quadrupole and ion trap analyses. Electrophoresis 1998; 19:989 - 97; http://dx.doi.org/ 10.1002/elps.1150190614; PMID: 9638945 [DOI] [PubMed] [Google Scholar]
- 55.Kotiaho T, Eberlin MN, Vainiotalo P, Kostiainen R. . Electrospray mass and tandem mass spectrometry identification of ozone oxidation products of amino acids and small peptides. J Am Soc Mass Spectrom 2000; 11:526 - 35; http://dx.doi.org/ 10.1016/S1044-0305(00)00116-1; PMID: 10833026 [DOI] [PubMed] [Google Scholar]
- 56.Chowdhury SK, Eshraghi J, Wolfe H, Forde D, Hlavac AG, Johnston D. . Mass spectrometric identification of amino acid transformations during oxidation of peptides and proteins: modifications of methionine and tyrosine. Anal Chem 1995; 67:390 - 8; http://dx.doi.org/ 10.1021/ac00098a026; PMID: 7856883 [DOI] [PubMed] [Google Scholar]
- 57.Guan Z, Yates NA, Bakhtiar R. . Detection and characterization of methionine oxidation in peptides by collision-induced dissociation and electron capture dissociation. J Am Soc Mass Spectrom 2003; 14:605 - 13; http://dx.doi.org/ 10.1016/S1044-0305(03)00201-0; PMID: 12781462 [DOI] [PubMed] [Google Scholar]
- 58.Chen M, Cook KD. . Oxidation artifacts in the electrospray mass spectrometry of Abeta Peptide. Anal Chem 2007; 79:2031 - 6; http://dx.doi.org/ 10.1021/ac061743r; PMID: 17249640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morand K, Talbo G, Mann M. . Oxidation of peptides during electrospray ionization. Rapid Commun Mass Spectrom 1993; 7:738 - 43; http://dx.doi.org/ 10.1002/rcm.1290070811; PMID: 8374164 [DOI] [PubMed] [Google Scholar]
- 60.Silva CJ, Dynin I, Erickson ML, Requena JR, Balachandran A, Hui C, Onisko BC, Carter JM. . Oxidation of methionine 216 in sheep and elk prion protein is highly dependent upon the amino acid at position 218 but is not important for prion propagation. Biochemistry 2013; 52:2139 - 47; http://dx.doi.org/ 10.1021/bi3016795; PMID: 23458153 [DOI] [PubMed] [Google Scholar]
- 61.Silva CJ, Onisko BC, Dynin I, Erickson ML, Vensel WH, Requena JR, Antaki EM, Carter JM. . Assessing the role of oxidized methionine at position 213 in the formation of prions in hamsters. Biochemistry 2010; 49:1854 - 61; http://dx.doi.org/ 10.1021/bi901850n; PMID: 20121218 [DOI] [PubMed] [Google Scholar]
- 62.Onisko B, Dynin I, Requena JR, Silva CJ, Erickson M, Carter JM. . Mass spectrometric detection of attomole amounts of the prion protein by nanoLC/MS/MS. J Am Soc Mass Spectrom 2007; 18:1070 - 9; http://dx.doi.org/ 10.1016/j.jasms.2007.03.009; PMID: 17446085 [DOI] [PubMed] [Google Scholar]
- 63.Onisko BC, Silva CJ, Dynin I, Erickson M, Vensel WH, Hnasko R, Requena JR, Carter JM. . Sensitive, preclinical detection of prions in brain by nanospray liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2007; 21:4023 - 6; http://dx.doi.org/ 10.1002/rcm.3310; PMID: 18000838 [DOI] [PubMed] [Google Scholar]
- 64.Domon B, Aebersold R. . Mass spectrometry and protein analysis. Science 2006; 312:212 - 7; http://dx.doi.org/ 10.1126/science.1124619; PMID: 16614208 [DOI] [PubMed] [Google Scholar]
- 65.Pan S, Aebersold R, Chen R, Rush J, Goodlett DR, McIntosh MW, Zhang J, Brentnall TA. . Mass spectrometry based targeted protein quantification: methods and applications. J Proteome Res 2009; 8:787 - 97; http://dx.doi.org/ 10.1021/pr800538n; PMID: 19105742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Picotti P, Aebersold R. . Selected reaction monitoring-based proteomics: workflows, potential, pitfalls and future directions. Nat Methods 2012; 9:555 - 66; http://dx.doi.org/ 10.1038/nmeth.2015; PMID: 22669653 [DOI] [PubMed] [Google Scholar]
- 67.Bolton DC, Rudelli RD, Currie JR, Bendheim PE. . Copurification of Sp33-37 and scrapie agent from hamster brain prior to detectable histopathology and clinical disease. J Gen Virol 1991; 72:2905 - 13; http://dx.doi.org/ 10.1099/0022-1317-72-12-2905; PMID: 1684986 [DOI] [PubMed] [Google Scholar]
- 68.Pastrana MA, Sajnani G, Onisko B, Castilla J, Morales R, Soto C, Requena JR. . Isolation and characterization of a proteinase K-sensitive PrPSc fraction. Biochemistry 2006; 45:15710 - 7; http://dx.doi.org/ 10.1021/bi0615442; PMID: 17176093 [DOI] [PubMed] [Google Scholar]
- 69.Silva CJ, Dynin I, Erickson ML, Hui C, Carter JM. . Oxidation of methionine in PrP is dependent upon the oxidant and the amino acid two positions removed. Prion 2013; 7:Supplement 81 29095079 [Google Scholar]
- 70.Silva CJ, Erickson ML, Dynin IA, Onisko BC, Carter JM. . Diagnosing Prion Diseases. Mass Spectrometry-Based Approaches Prion 2011; 5:Supplement 85 [Google Scholar]
- 71.Silva CJ, Onisko BC, Dynin I, Erickson ML, Requena JR, Carter JM. . Utility of mass spectrometry in the diagnosis of prion diseases. Anal Chem 2011; 83:1609 - 15; http://dx.doi.org/ 10.1021/ac102527w; PMID: 21288014 [DOI] [PubMed] [Google Scholar]
- 72.Stahl N, Baldwin MA, Burlingame AL, Prusiner SB. . Identification of glycoinositol phospholipid linked and truncated forms of the scrapie prion protein. Biochemistry 1990; 29:8879 - 84; http://dx.doi.org/ 10.1021/bi00490a001; PMID: 1980209 [DOI] [PubMed] [Google Scholar]
- 73.Douma MD, Kerr GM, Brown RS, Keller BO, Oleschuk RD. . Mass spectrometric detection of proteins in non-aqueous media - The case of prion proteins in biodiesel. Can J Chem 2008; 86:774 - 81; http://dx.doi.org/ 10.1139/v08-083 [DOI] [Google Scholar]
- 74.Sturm R, Sheynkman G, Booth C, Smith LM, Pedersen JA, Li L. . Absolute quantification of prion protein (90-231) using stable isotope-labeled chymotryptic peptide standards in a LC-MRM AQUA workflow. J Am Soc Mass Spectrom 2012; 23:1522 - 33; http://dx.doi.org/ 10.1007/s13361-012-0411-1; PMID: 22714949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lamoureux L, Simon SL, Plews M, Ruddat V, Brunet S, Graham C, Czub S, Knox JD. . Urine proteins identified by two-dimensional differential gel electrophoresis facilitate the differential diagnoses of scrapie. PLoS One 2013; 8:e64044; http://dx.doi.org/ 10.1371/journal.pone.0064044; PMID: 23704971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lamoureux L, Simon SL, Plews M, Stobart M, Groschup M, Czub S, Graham C, Knox JD. . Analysis of clusterin glycoforms in the urine of BSE-infected Fleckvieh-Simmental cows. J Toxicol Environ Health A 2011; 74:138 - 45; http://dx.doi.org/ 10.1080/15287394.2011.529063; PMID: 21218342 [DOI] [PubMed] [Google Scholar]
- 77.Simon SL, Lamoureux L, Plews M, Stobart M, LeMaistre J, Ziegler U, Graham C, Czub S, Groschup M, Knox JD. . The identification of disease-induced biomarkers in the urine of BSE infected cattle. Proteome Sci 2008; 6:23; http://dx.doi.org/ 10.1186/1477-5956-6-23; PMID: 18775071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei X, Herbst A, Ma D, Aiken J, Li L. . A quantitative proteomic approach to prion disease biomarker research: delving into the glycoproteome. J Proteome Res 2011; 10:2687 - 702; http://dx.doi.org/ 10.1021/pr2000495; PMID: 21469646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barr JB, Watson M, Head MW, Ironside JW, Harris N, Hogarth C, Fraser JR, Barron R. . Differential protein profiling as a potential multi-marker approach for TSE diagnosis. BMC Infect Dis 2009; 9:188; http://dx.doi.org/ 10.1186/1471-2334-9-188; PMID: 19943924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Asuni AA, Gray B, Bailey J, Skipp P, Perry VH, O’Connor V. . Analysis of the hippocampal proteome in ME7 prion disease reveals a predominant astrocytic signature and highlights the brain-restricted production of clusterin in chronic neurodegeneration. J Biol Chem 2013; Forthcoming http://dx.doi.org/ 10.1074/jbc.M113.502690; PMID: 24366862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Batxelli-Molina I, Salvetat N, Andréoletti O, Guerrier L, Vicat G, Molina F, Mourton-Gilles C. . Ovine serum biomarkers of early and late phase scrapie. BMC Vet Res 2010; 6:49; http://dx.doi.org/ 10.1186/1746-6148-6-49; PMID: 21044301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herbst A, McIlwain S, Schmidt JJ, Aiken JM, Page CD, Li L. . Prion disease diagnosis by proteomic profiling. J Proteome Res 2009; 8:1030 - 6; http://dx.doi.org/ 10.1021/pr800832s; PMID: 19133784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen C, Xiao D, Zhou W, Zhang YC, Shi Q, Tian C, Zhang J, Zhou CX, Zhang JZ, Dong XP. . Comparative peptidome analyses of the profiles of the peptides ranging from 1-10 KD in CSF samples pooled from probable sporadic CJD and non-CJD patients. Prion 2012; 6:46 - 51; http://dx.doi.org/ 10.4161/pri.6.1.18082; PMID: 22453178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van Dorsselaer A, Carapito C, Delalande F, Schaeffer-Reiss C, Thierse D, Diemer H, McNair DS, Krewski D, Cashman NR. . Detection of prion protein in urine-derived injectable fertility products by a targeted proteomic approach. PLoS One 2011; 6:e17815; http://dx.doi.org/ 10.1371/journal.pone.0017815; PMID: 21448279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuwabara Y, Mine K, Katayama A, Inagawa T, Akira S, Takeshita T. . Proteomic analyses of recombinant human follicle-stimulating hormone and urinary-derived gonadotropin preparations. J Reprod Med 2009; 54:459 - 66; PMID: 19769189 [PubMed] [Google Scholar]
- 86.Giorgi A, Di Francesco L, Principe S, Mignogna G, Sennels L, Mancone C, Alonzi T, Sbriccoli M, De Pascalis A, Rappsilber J, et al. . Proteomic profiling of PrP27-30-enriched preparations extracted from the brain of hamsters with experimental scrapie. Proteomics 2009; 9:3802 - 14; http://dx.doi.org/ 10.1002/pmic.200900085; PMID: 19637240 [DOI] [PubMed] [Google Scholar]
- 87.Petrakis S, Malinowska A, Dadlez M, Sklaviadis T. . Identification of proteins co-purifying with scrapie infectivity. J Proteomics 2009; 72:690 - 4; http://dx.doi.org/ 10.1016/j.jprot.2009.01.025; PMID: 19367687 [DOI] [PubMed] [Google Scholar]
- 88.Schmitt-Ulms G, Hansen K, Liu J, Cowdrey C, Yang J, DeArmond SJ, Cohen FE, Prusiner SB, Baldwin MA. . Time-controlled transcardiac perfusion cross-linking for the study of protein interactions in complex tissues. Nat Biotechnol 2004; 22:724 - 31; http://dx.doi.org/ 10.1038/nbt969; PMID: 15146195 [DOI] [PubMed] [Google Scholar]
- 89.Zafar S, Asif AR, Ramljak S, Tahir W, Schmitz M, Zerr I. . Anchorless 23-230 PrP(C) Interactomics for Elucidation of PrP(C) Protective Role. Mol Neurobiol 2014; Forthcoming http://dx.doi.org/ 10.1007/s12035-013-8616-2; PMID: 24390569 [DOI] [PubMed] [Google Scholar]
- 90.Nokihara K, Yajima S, Hitara A, Sogon T, Yasuhara T. . Characterization of peptides obtained from digests of bovine brain which accelerate structural conversions of the recombinant bovine prion protein. FEBS Lett 2013; 587:673 - 6; http://dx.doi.org/ 10.1016/j.febslet.2013.01.033; PMID: 23376025 [DOI] [PubMed] [Google Scholar]
- 91.Kouassi GK, Irudayaraj J. . A nanoparticle-based immobilization assay for prion-kinetics study. J Nanobiotechnology 2006; 4:8; http://dx.doi.org/ 10.1186/1477-3155-4-8; PMID: 16916458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McKinley MP, Bolton DC, Prusiner SB. . A protease-resistant protein is a structural component of the scrapie prion. Cell 1983; 35:57 - 62; http://dx.doi.org/ 10.1016/0092-8674(83)90207-6; PMID: 6414721 [DOI] [PubMed] [Google Scholar]
- 93.Pimenova T, Meier L, Roschitzki B, Paraschiv G, Przybylski M, Zenobi R. . Polystyrene beads as an alternative support material for epitope identification of a prion-antibody interaction using proteolytic excision-mass spectrometry. Anal Bioanal Chem 2009; 395:1395 - 401; http://dx.doi.org/ 10.1007/s00216-009-3119-8; PMID: 19787344 [DOI] [PubMed] [Google Scholar]
- 94.Qin K, Yang Y, Mastrangelo P, Westaway D. . Mapping Cu(II) binding sites in prion proteins by diethyl pyrocarbonate modification and matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometric footprinting. J Biol Chem 2002; 277:1981 - 90; http://dx.doi.org/ 10.1074/jbc.M108744200; PMID: 11698407 [DOI] [PubMed] [Google Scholar]
- 95.Pimenova T, Nazabal A, Roschitzki B, Seebacher J, Rinner O, Zenobi R. . Epitope mapping on bovine prion protein using chemical cross-linking and mass spectrometry. J Mass Spectrom 2008; 43:185 - 95; http://dx.doi.org/ 10.1002/jms.1280; PMID: 17924399 [DOI] [PubMed] [Google Scholar]
- 96.Gong B, Ramos A, Vázquez-Fernández E, Silva CJ, Alonso J, Liu Z, Requena JR. . Probing structural differences between PrP(C) and PrP(Sc) by surface nitration and acetylation: evidence of conformational change in the C-terminus. Biochemistry 2011; 50:4963 - 72; http://dx.doi.org/ 10.1021/bi102073j; PMID: 21526750 [DOI] [PubMed] [Google Scholar]
- 97.Peretz D, Williamson RA, Matsunaga Y, Serban H, Pinilla C, Bastidas RB, Rozenshteyn R, James TL, Houghten RA, Cohen FE, et al. . A conformational transition at the N terminus of the prion protein features in formation of the scrapie isoform. J Mol Biol 1997; 273:614 - 22; http://dx.doi.org/ 10.1006/jmbi.1997.1328; PMID: 9356250 [DOI] [PubMed] [Google Scholar]
- 98.Silva CJ. . Using small molecule reagents to selectively modify epitopes based on their conformation. Prion 2012; 6:163 - 73; http://dx.doi.org/ 10.4161/pri.18795; PMID: 22436143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB. . Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 1998; 4:1157 - 65; http://dx.doi.org/ 10.1038/2654; PMID: 9771749 [DOI] [PubMed] [Google Scholar]
- 100.Silva C, Erickson M, Dynin I, Carter M. . Using synthetic small molecule reagents and antibodies to distiguish among PrP conformers–New uses for old antibodies. Prion 2012; 6:Supplement 90 [Google Scholar]
- 101.Silva CJ, Erickson ML, Dynin I, Carter JM. . Distinguishing between PrPC and PrPSc using small molecule reagents. Prion 2013; 7:Supplement 90 [Google Scholar]
- 102.Jucker M, Walker LC. . Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 2013; 501:45 - 51; http://dx.doi.org/ 10.1038/nature12481; PMID: 24005412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grad L, Cashman NR. . Prion-like activity of Cu/Zn superoxide dismutase: Implications for amyotrophic lateral sclerosis. Prion 2014; Forthcoming PMID: 24394345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mysling S, Betzer C, Jensen PH, Jorgensen TJ. . Characterizing the Dynamics of α-Synuclein Oligomers Using Hydrogen/Deuterium Exchange Monitored by Mass Spectrometry. Biochemistry 2013; 52:9097 - 103; http://dx.doi.org/ 10.1021/bi4009193; PMID: 24191706 [DOI] [PubMed] [Google Scholar]
- 105.Shaw BF, Durazo A, Nersissian AM, Whitelegge JP, Faull KF, Valentine JS. . Local unfolding in a destabilized, pathogenic variant of superoxide dismutase 1 observed with H/D exchange and mass spectrometry. J Biol Chem 2006; 281:18167 - 76; http://dx.doi.org/ 10.1074/jbc.M600623200; PMID: 16644738 [DOI] [PubMed] [Google Scholar]
- 106.Borges-Alvarez M, Benavente F, Vilaseca M, Barbosa J, Sanz-Nebot V. . Characterization of superoxide dismutase 1 (SOD-1) by electrospray ionization-ion mobility mass spectrometry. J Mass Spectrom 2013; 48:60 - 7; http://dx.doi.org/ 10.1002/jms.3128; PMID: 23303748 [DOI] [PubMed] [Google Scholar]
- 107.Brinkmalm G, Portelius E, Öhrfelt A, Mattsson N, Persson R, Gustavsson MK, Vite CH, Gobom J, Månsson JE, Nilsson J, et al. . An online nano-LC-ESI-FTICR-MS method for comprehensive characterization of endogenous fragments from amyloid β and amyloid precursor protein in human and cat cerebrospinal fluid. J Mass Spectrom 2012; 47:591 - 603; http://dx.doi.org/ 10.1002/jms.2987; PMID: 22576872 [DOI] [PubMed] [Google Scholar]
- 108.Brinkmalm G, Brinkmalm A, Bourgeois P, Persson R, Hansson O, Portelius E, Mercken M, Andreasson U, Parent S, Lipari F, et al. . Soluble amyloid precursor protein α and β in CSF in Alzheimer’s disease. Brain Res 2013; 1513:117 - 26; http://dx.doi.org/ 10.1016/j.brainres.2013.03.019; PMID: 23541617 [DOI] [PubMed] [Google Scholar]
- 109.Ohrfelt A, Zetterberg H, Andersson K, Persson R, Secic D, Brinkmalm G, Wallin A, Mulugeta E, Francis PT, Vanmechelen E, et al. . Identification of novel α-synuclein isoforms in human brain tissue by using an online nanoLC-ESI-FTICR-MS method. Neurochem Res 2011; 36:2029 - 42; http://dx.doi.org/ 10.1007/s11064-011-0527-x; PMID: 21674238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Danielson SR, Held JM, Schilling B, Oo M, Gibson BW, Andersen JK. . Preferentially increased nitration of alpha-synuclein at tyrosine-39 in a cellular oxidative model of Parkinson’s disease. Anal Chem 2009; 81:7823 - 8; http://dx.doi.org/ 10.1021/ac901176t; PMID: 19697948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xia Q, Cheng D, Duong DM, Gearing M, Lah JJ, Levey AI, Peng J. . Phosphoproteomic analysis of human brain by calcium phosphate precipitation and mass spectrometry. J Proteome Res 2008; 7:2845 - 51; http://dx.doi.org/ 10.1021/pr8000496; PMID: 18510355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xia Q, Liao L, Cheng D, Duong DM, Gearing M, Lah JJ, Levey AI, Peng J. . Proteomic identification of novel proteins associated with Lewy bodies. Front Biosci 2008; 13:3850 - 6; http://dx.doi.org/ 10.2741/2973; PMID: 18508479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Borsarelli CD, Falomir-Lockhart LJ, Ostatná V, Fauerbach JA, Hsiao HH, Urlaub H, Paleček E, Jares-Erijman EA, Jovin TM. . Biophysical properties and cellular toxicity of covalent crosslinked oligomers of α-synuclein formed by photoinduced side-chain tyrosyl radicals. Free Radic Biol Med 2012; 53:1004 - 15; http://dx.doi.org/ 10.1016/j.freeradbiomed.2012.06.035; PMID: 22771470 [DOI] [PubMed] [Google Scholar]
- 114.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. . Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 2000; 290:985 - 9; http://dx.doi.org/ 10.1126/science.290.5493.985; PMID: 11062131 [DOI] [PubMed] [Google Scholar]
- 115.Good PF, Hsu A, Werner P, Perl DP, Olanow CW. . Protein nitration in Parkinson’s disease. J Neuropathol Exp Neurol 1998; 57:338 - 42; http://dx.doi.org/ 10.1097/00005072-199804000-00006; PMID: 9600227 [DOI] [PubMed] [Google Scholar]
- 116.Ida N, Hartmann T, Pantel J, Schröder J, Zerfass R, Förstl H, Sandbrink R, Masters CL, Beyreuther K. . Analysis of heterogeneous A4 peptides in human cerebrospinal fluid and blood by a newly developed sensitive Western blot assay. J Biol Chem 1996; 271:22908 - 14; http://dx.doi.org/ 10.1074/jbc.271.37.22908; PMID: 8798471 [DOI] [PubMed] [Google Scholar]
- 117.Kosik KS, Orecchio LD, Binder L, Trojanowski JQ, Lee VM, Lee G. . Epitopes that span the tau molecule are shared with paired helical filaments. Neuron 1988; 1:817 - 25; http://dx.doi.org/ 10.1016/0896-6273(88)90129-8; PMID: 2483104 [DOI] [PubMed] [Google Scholar]
- 118.Carmel G, Mager EM, Binder LI, Kuret J. . The structural basis of monoclonal antibody Alz50’s selectivity for Alzheimer’s disease pathology. J Biol Chem 1996; 271:32789 - 95; http://dx.doi.org/ 10.1074/jbc.271.51.32789; PMID: 8955115 [DOI] [PubMed] [Google Scholar]
- 119.Choi JY, Park HJ, Seong YM, Choi EY, Min BR, Rhim H. . Fine epitope mapping of monoclonal antibodies specific to human alpha-synuclein. Neurosci Lett 2006; 397:53 - 8; http://dx.doi.org/ 10.1016/j.neulet.2005.11.058; PMID: 16380207 [DOI] [PubMed] [Google Scholar]
- 120.Broering TJ, Wang H, Boatright NK, Wang Y, Baptista K, Shayan G, Garrity KA, Kayatekin C, Bosco DA, Matthews CR, et al. . Identification of human monoclonal antibodies specific for human SOD1 recognizing distinct epitopes and forms of SOD1. PLoS One 2013; 8:e61210; http://dx.doi.org/ 10.1371/journal.pone.0061210; PMID: 23613814 [DOI] [PMC free article] [PubMed] [Google Scholar]