Abstract
Aims
We aimed to evaluate the associations of body fat distribution with cardiovascular function and geometry in the middle-aged general population.
Methods and results
Four thousand five hundred and ninety participants of the UK Biobank (54% female, mean age 61.1 ± 7.2 years) underwent cardiac magnetic resonance for assessment of left ventricular (LV) parameters [end-diastolic volume (EDV), ejection fraction (EF), cardiac output (CO), and index (CI)] and magnetic resonance imaging for body composition analysis [subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT)]. Body fat percentage (BF%) was assessed by bioelectrical impedance. Linear regressions were performed to assess the impact of visceral (VAT) and general (SAT and BF%) obesity on cardiac function and geometry. Visceral obesity was associated with a smaller EDV [VAT: β −1.74 (−1.15 to −2.33)], lower EF [VAT: β −0.24 (−0.12 to −0.35), SAT: β 0.02 (−0.04 to 0.08), and BF%: β 0.02 (−0.02 to 0.06)] and the strongest negative association with CI [VAT: β −0.05 (−0.06 to −0.04), SAT: β −0.02 (−0.03 to −0.01), and BF% β −0.01 (−0.013 to −0.007)]. In contrast, general obesity was associated with a larger EDV [SAT: β 1.01 (0.72–1.30), BF%: β 0.37 (0.23–0.51)] and a higher CO [SAT: β 0.06 (0.05–0.07), BF%: β 0.02 (0.01–0.03)]. In the gender-specific analysis, only men had a significant association between VAT and EF [β −0.35 (−0.19 to −0.51)].
Conclusion
Visceral obesity was associated with a smaller LV EDV and subclinical lower LV systolic function in men, suggesting that visceral obesity might play a more important role compared to general obesity in LV remodelling.
Keywords: Obesity, Body fat distribution, Visceral adipose tissue, Left ventricular function, Left ventricular geometry, Magnetic resonance imaging
Introduction
The disease burden related to obesity increased significantly over the last decades, making excess body weight one of the most challenging public health problems.1 Most previous studies investigating the relation between obesity and cardiovascular function used indirect anthropomorphic obesity measures such as body mass index (BMI), rather than measures of body fat distribution such as subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT).2 VAT is located around the abdominal organs, and metabolically distinct from SAT as VAT produces more pro-inflammatory adipokines and is related to insulin resistance.3 In addition, visceral obesity has been linked to concentric remodelling, whereas SAT and other general obesity measures have been associated with eccentric remodelling, suggesting a different effect of visceral obesity on left ventricular (LV) structure.4 Previous studies have suggested that obesity in heart failure is more likely related to the negative impact of obesity on diastolic dysfunction rather than systolic function.5 However, so far only few large scale studies have investigated the association between visceral obesity and systolic function, with different parameters of systolic function and in vastly different populations than the UK Biobank population.4,6 In addition, the impact of body fat distribution on vascular function assessed by the augmentation index (AIx) remains to be elucidated as there is limited evidence on the potential impact of visceral obesity on AIx.7
The purpose of this study was to evaluate the associations of body fat distribution [VAT, SAT, and body fat percentage (BF%)] with LV systolic function, geometry, and arterial stiffness in the middle-aged general population. We hypothesize that the negative associations of obesity with cardiovascular function and structure are more pronounced for visceral fat (VAT) than for general obesity measures (SAT and BF%).
Methods
UK Biobank
The UK Biobank study (see www.ukbiobank.ac.uk for more information) is a large population-based cohort that includes 503 325 individuals aged 45–69 years old.8 Participants were recruited across the United Kingdom for participation in the UK Biobank over 5 years period beginning in 2006. The study protocol was approved by the National Research Ethics Service Committee North West–Haydock (reference 11/NW/0382). Informed consent was obtained from all participants, and all procedures were performed in accordance with the ethical principles for medical research declared in the World Medical Association Declaration of Helsinki. Questionnaire-based data were obtained on ethnicity, socioeconomic status assessed by the Townsend Deprivation Index, smoking (never, former, and current), alcohol consumption (never, special occasions, 1–3x/month, 1–2x/week, 3–4x/week, and daily), and self-reported history of cardiovascular disease and diabetes. For the current study, only data of individuals who underwent cardiac and abdominal magnetic resonance imaging (MRI) at the release date of 30 January 2018 was included.
Anthropomorphic obesity measurements
Anthropometric measurements were obtained by trained research clinic staff. Weight was measured using the Tanita BC418 body composition analyser and height was measured using the wall-mounted SECA 240 height measure.8 Body surface area was calculated using Du Bois formula.9 BMI was calculated as weight/height2 (kg/m2). BF% was measured using the Tanita BC418MA body composition analyser using electrical impedance. Healthy weight was defined as BMI 18.0–24.9 kg/m2, overweight as BMI 25.0–29.9 kg/m2, and obese as BMI ≥ 30.0 kg/m2.
Magnetic resonance imaging
During the imaging visit participants underwent a combined protocol of cardiac magnetic resonance (CMR) and abdominal MRI on a clinical wide bore 1.5-T scanner (MAGNETOM Aera, Syngo Platform VD13A, Siemens Healthcare, Erlangen, Germany).10,11 Specific scan parameters are presented in the Supplementary data.
Body composition by MRI
A dual-echo Dixon Vibe protocol was used to obtain body composition data, a 6-min protocol that covered neck to knees and was divided over six 3D spoiled gradient-echo axial slabs.10 Using the integrated scanner software, fusion of the axial slabs provided a dataset of isolated water and fat volumes. VAT and SAT were calculated by automatic segmentation using AMRA Profiler (AMRA Medical AB, Linköping, Sweden).
Cardiac magnetic resonance
A 20-min CMR protocol was used to obtain four long axis as well as a stack of short-axis cines based on balanced steady-state free precession, to assess LV cardiac function [ejection fraction (EF), cardiac output (CO), and index (CI)] and geometry [end-diastolic volume (EDV) and end-systolic volume (ESV)].11 In this protocol, four long-axis cines were obtained (horizontal long axis, vertical long axis, and LV outflow tract cines both sagittal and coronal) and a short-axis stack of balanced steady-state free precession cines (flip angle 80°, repetition and echo time of 2.7 ms, and 1.16 ms for the long axis and 2.6 ms and 1.10 ms for the short axis, respectively, slice thickness 6 mm for the long axis and 8 mm for the short axis with a 2 mm gap, matrix 208 × 187 and a typical field of view of 380 × 274 for the long axis and 380 × 252 for the short axis).
Augmentation index by pulse wave analysis
Brachial blood pressure readings were obtained using a manual sphygmomanometer for calibrating peripheral waveforms. The Vicorder (Skidmore Medical, Bristol, UK) digitally computes a brachial pressure wave trace with the cuff inflated to a static 70 mmHg using a volume displacement technique. A brachial-to-aortic transfer function was used to calculate central blood pressure using Vicorder software.11 The AIx is derived from the central pressure waveform, which consists of two peaks. The first peak arises from the output of the left ventricle and the second originates from the reflection of the pulse wave of the peripheral arteries, the AIx is defined as the percentage of increase in pulse pressure from the reflected waveform.12 Negative values of AIx were excluded from the analysis because these do not represent true negative values, which could falsely distort the analysis.13
Statistical analysis
All variables were checked for normal distribution and potential outliers were excluded (more than four times the standard deviation of the mean). Linear regression models were constructed to calculate regression coefficients (β) with corresponding 95% confidence interval for the association between obesity measures (VAT, SAT, and BF%) as determinants and LV parameters (EDV, ESV, EF, CO, and CI) and arterial stiffness as outcome variables. Adjustment was done as following; the basic model (Model 1) consisted of age and sex, in Model 2 ethnicity, socioeconomic status, alcohol consumption, smoking, hypertension, and diabetes were added. Because visceral obesity is highly correlated with total body fat, to study the specific contribution of VAT it is important to adjust for overall obesity. Therefore, VAT was additionally adjusted for BF% in Model 3.14 AIx is known to be influenced by height, heart rate, and mean arterial pressure, therefore, AIx was additionally adjusted for these variables in Models 2 and 3.15 To test whether obesity measures were more strongly associated with cardiovascular function in males or females, interaction term sex was added to the regression models. To assess whether associations between obesity measures and LV geometry were influenced by body size, regressions were also performed for EDV and ESV indexed to height2.7. Sensitivity analysis was performed by excluding diabetics and patients with cardiovascular disease. Analyses were performed using R (version 3.5.0), P-values of < 0.05 were considered statistically significant.
Results
Out of a total of 502 617 participants of the UK Biobank, 5995 participants had available CMR data of whom 4590 participants also had abdominal MRI and BF% measurement data (flowchart shown in Figure 1). Baseline characteristics, stratified according to gender, are presented in Table 1. Included participants had a mean age of 61.1 ± 7.2 years, 54% was female, 52% of the women, and 68% of the men were overweight or obese.
Figure 1.
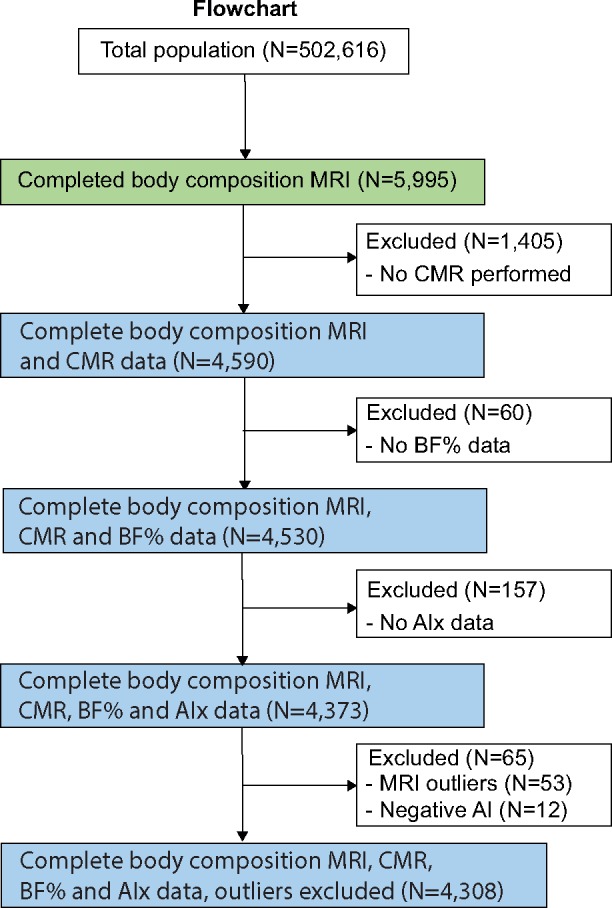
A flowchart describing sample selection. AIx, augmentation index; BF%, body fat percentage; CMR, cardiac magnetic resonance; MRI, magnetic resonance imaging.
Table 1.
Baseline characteristics stratified by gender
| Gender |
Total population (n = 4590) | ||
|---|---|---|---|
| Men (n = 2126) | Women (n = 2464) | ||
| Age (years) | 61.7 ± 7.1 | 60.6 ± 7.2 | 61.1 ± 7.2 |
| Ethnicity (% Whites) | 2050 (97.1) | 2397 (98.0) | 4447 (97.5) |
| Townsend deprivation index | −1.9 (2.8) | −1.9 (2.7) | −1.9 ± 2.7 |
| Smoking (%) | |||
| Never | 1201 (57.0) | 1576 (64.8) | 2777 (61.1) |
| Former | 794 (37.7) | 767 (31.5) | 1561 (34.4) |
| Current | 112 (5.3) | 90 (3.7) | 202 (4.4) |
| Alcohol | |||
| Never | 121 (5.7) | 170 (7.0) | 291 (6.4) |
| Special occasions | 148 (7.0) | 360 (14.8) | 508 (11.2) |
| 1–3x/month | 181 (8.6) | 341 (14.0) | 522 (11.4) |
| 1–2x/week | 539 (25.5) | 653 (26.8) | 1192 (26.2) |
| 3–4x/week | 635 (30.1) | 565 (23.2) | 1200 (26.4) |
| Daily | 489 (23.1) | 349 (14.3) | 838 (18.4) |
| Systolic blood pressure (mmHg) | 135.8 ± 16.9 | 136.2 ± 21.5 | 136.0 ± 19.5 |
| Diastolic blood pressure (mmHg) | 71.4 ± 10.0 | 66.9 ± 11.2 | 69.0 ± 10.9 |
| History of | |||
| Hypertension (%) | 581 (27.5) | 492 (20.2) | 1073 (23.6) |
| Diabetes (%) | 117 (5.5) | 78 (3.2) | 195 (4.3) |
| Angina (%) | 47 (2.2) | 32 (1.3) | 79 (1.7) |
| Myocardial infarction (%) | 65 (3.1) | 16 (0.7) | 81 (1.8) |
| Stroke (%) | 37 (1.8) | 18 (0.7) | 55 (1.2) |
| Body surface area (m²) | 2.0 ± 0.2 | 1.7 ± 0.2 | 1.9 ± 0.2 |
| Body mass index (kg/m2) | 26.8 ± 3.5 | 26.0 ± 4.4 | 26.4 ± 4.0 |
| Overweight (%) | 1092 (51.4) | 864 (35.1) | 1956 (42.7) |
| Obese (%) | 358 (16.8) | 401 (16.3) | 759 (16.6) |
| Body fat percentage (%) | 24.1 ± 5.2 | 35.4 ± 6.5 | 30.2 ± 8.2 |
| Visceral adipose tissue (L) | 4.7 ± 2.2 | 2.5 ± 1.4 | 3.5 ± 2.1 |
| Subcutaneous adipose tissue (L) | 5.7 ± 2.3 | 7.8 ± 3.3 | 6.9 ± 3.1 |
| LV ejection fraction (%) | 54.6 ± 6.2 | 57.6 ± 5.4 | 56.2 ± 6.0 |
| LV end-diastolic volume (mL) | 157.7 ± 33.6 | 123.0 ± 24.8 | 139.1 ± 34.0 |
| LV end-systolic volume (mL) | 72.2 ± 21.2 | 52.4 ± 13.7 | 61.6 ± 20.1 |
| LV stroke volume (mL) | 85.4 ± 18.9 | 70.5 ± 14.8 | 77.4 ± 18.4 |
| LV cardiac output (L/min) | 5.1 ± 1.1 | 4.4 ± 1.0 | 4.7 ± 1.1 |
| LV cardiac index (L/min/m²) | 2.6 ± 0.5 | 2.5 ± 0.5 | 2.5 ± 0.5 |
| Augmentation index (%) | 19.3 ± 8.0 | 22.8 ± 8.8 | 21.2 ± 8.6 |
Data are shown as n (%) or mean ± SD. Overweight = 25–29.9 kg/m2, Obese >30 kg/m2.
LV, left ventricular.
Associations between obesity measures and LV geometry
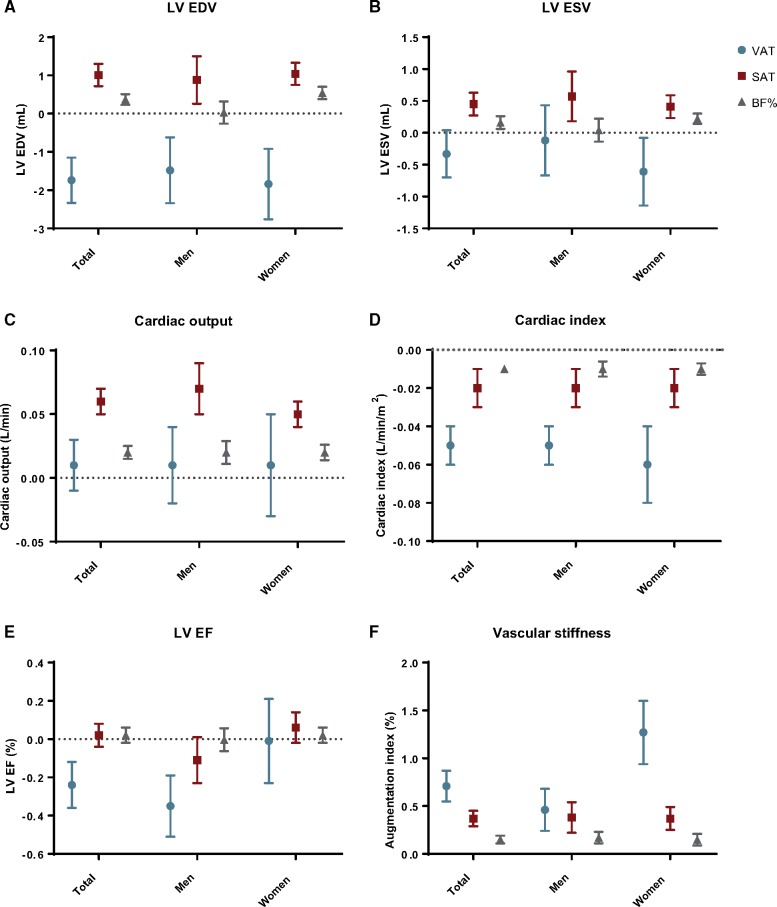
In the crude association, visceral obesity (VAT) was positively associated with EDV and general obesity measures (SAT and BF%) were negatively associated with EDV. After correction for age and gender (Model 1) this association reversed, showing a negative association of visceral obesity with EDV and a positive association between general obesity and EDV (Table 2). The direction of these associations persisted for Models 2 and 3 [Model 3; VAT: β −1.74 (−1.15 to −2.33), P < 0.001; SAT: β 1.01 (0.72–1.30), P < 0.001; BF%: β 0.37 (0.23–0.51), P < 0.001] (Figure 2A). Per 1 L change in VAT, EDV changed with −1.74 meaning that in an overt obese with 2SD more VAT compared to the average, EDV is on average 7.31 mL lower. Only for BF%, there was a significant difference between men and women (P for interaction < 0.001), where only women showed a positive association with EDV [women: β 0.54 (0.38–0.70), P < 0.001; men: β 0.03 (−0.26 to 0.32), P = 0.84]. A complete overview of the gender-specific associations is shown in Supplementary data online, Table S1.
Table 2.
Associations of obesity measures with cardiovascular function and geometry
| LV end-diastolic volume (mL) |
LV end-systolic volume (mL) |
LV ejection fraction (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P-value | β | 95% CI | P-value | β | 95% CI | P-value | |
| VAT (L) | |||||||||
| Crude | 3.74 | 3.29 to 4.19 | <0.001 | 2.46 | 2.19 to 2.73 | <0.001 | −0.49 | −0.57 to −0.41 | <0.001 |
| Model 1 | −0.25 | −0.72 to 0.22 | 0.29 | 0.23 | −0.04 to 0.50 | 0.12 | −0.14 | −0.24 to −0.04 | 0.005 |
| Model 2 | −0.37 | −0.86 to 0.12 | 0.13 | 0.10 | −0.19 to 0.39 | 0.52 | −0.13 | −0.23 to −0.03 | 0.01 |
| Model 3 | −1.74 | −2.33 to −1.15 | <0.001 | −0.33 | −0.70 to 0.04 | 0.08 | −0.24 | −0.36 to −0.12 | <0.001 |
| SAT (L) | |||||||||
| Crude | −0.93 | −1.24 to −0.62 | <0.001 | −0.62 | −0.82 to −0.42 | <0.001 | 0.17 | 0.11 to 0.23 | <0.001 |
| Model 1 | 0.98 | 0.71 to 1.25 | <0.001 | 0.48 | 0.30 to 0.66 | <0.001 | 0.001 | −0.06 to 0.06 | 0.98 |
| Model 2 | 1.01 | 0.72 to 1.30 | <0.001 | 0.45 | 0.27 to 0.63 | <0.001 | 0.02 | −0.04 to 0.08 | 0.55 |
| BF% (%) | |||||||||
| Crude | −1.36 | −1.48 to −1.24 | <0.001 | −0.77 | −0.83 to −0.71 | <0.001 | 0.12 | 0.10 to 0.14 | <0.001 |
| Model 1 | 0.33 | 0.19 to 0.47 | <0.001 | 0.16 | 0.08 to 0.24 | <0.001 | 0.01 | −0.01 to 0.03 | 0.58 |
| Model 2 | 0.37 | 0.23 to 0.51 | <0.001 | 0.16 | 0.06 to 0.26 | <0.001 | 0.02 | −0.02 to 0.06 | 0.31 |
Model 1 is adjusted for age and sex. Model 2 is adjusted for Model 1 plus ethnicity, socioeconomic status, alcohol consumption, smoking, hypertension, and diabetes. Model 3 is adjusted for Model 2 covariates plus BF%. BF%, body fat percentage; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Figure 2.
(A–F) Associations (β with 95% confidence interval) of obesity measures with: (A) LV EDV, (B) LV ESV, (C) cardiac output, (D) cardiac index, (E) LV EF, and (F) augmentation index. BF%, body fat percentage; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Visceral obesity was positively associated with ESV in the crude model, whereas general obesity showed a negative association with ESV. In adjustment Models 1–3, visceral obesity was not significantly associated with ESV (Table 2). General obesity was associated with larger ESV in correction Models 1 and 2 [Model 2; SAT: β 0.45 (0.27–0.63), P < 0.001; BF%: β 0.16 (0.06; 0.26), <0.001] (Figure 2B). No significant differences were found between men and women in the associations of obesity measures with ESV. Similar associations were observed between obesity measures and indexed EDV and ESV to height2.7 (Supplementary data online, Table S3).
Associations between obesity measures and LV systolic function
In the unadjusted analysis, a negative association was observed between visceral obesity and EF [β −0.49 (−0.41 to −0.57), P < 0.001], where general obesity showed a mild positive association [SAT: β 0.17 (0.11–0.23), P < 0.001; BF%: β 0.12 (0.10–0.14), P < 0.001]. In the adjusted analysis, the negative association of visceral obesity with EF remained significant after adjustment for Model 1, 2, and 3 [Model 3: β −0.24 (−0.12; −0.36), P < 0.001] (Table 2 and Figure 2C). In the gender-specific analysis, there was a significant negative association for men between visceral obesity and EF, which remained significant after adjustment [Model 3: β −0.35 (−0.19 to −0.51), P < 0.001 for men vs β −0.01 (−0.23 to 0.21), P = 0.95 for women, P for interaction = 0.02]. No significant associations were observed between general obesity and EF in the gender-specific analysis.
In crude analysis of general obesity measures, SAT was associated with higher CO and BF% with lower CO. After adjustment for confounders both obesity measures were positively associated with CO (Figure 2D). In the unadjusted analysis, visceral obesity was positively associated with CO, however, after correction for general obesity (Model 3), visceral obesity alone had no significant effect on CO [β 0.01 (−0.01 to 0.03), P = 0.51] (Table 3). Visceral obesity showed a stronger negative association with CI compared to general obesity in the crude association, which remained so in the adjusted models [VAT: β −0.05 (−0.04 to 0.06), P < 0.001; SAT: β −0.02 (−0.03; −0.01), P < 0.001; BF%: β −0.01 (−0.007 to −0.013), P < 0.001] (Figure 2E). No significant differences were found between men and women in the associations of obesity measures with CO and CI. Sensitivity analysis, where participants with diabetes and cardiovascular disease were excluded, showed similar results compared to our main findings for the associations between obesity measures and LV function and geometry (Supplementary data online, Table S2).
Table 3.
Associations of obesity measures with cardiovascular function and geometry
| Cardiac output (L/min) |
Cardiac index (L/min/m²) |
Augmentation index (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P-value | β | 95% CI | P-value | β | 95% CI | P-value | |
| VAT (L) | |||||||||
| Crude | 0.12 | 0.11 to 0.13 | <0.001 | −0.03 | −0.04 to −0.02 | <0.001 | 0.13 | 0.01 to 0.25 | 0.03 |
| Model 1 | 0.06 | 0.04 to 0.08 | <0.001 | −0.04 | −0.05 to −0.03 | <0.001 | 0.64 | 0.50 to 0.78 | <0.001 |
| Model 2 | 0.05 | 0.03 to 0.07 | <0.001 | −0.04 | −0.05 to −0.03 | <0.001 | 0.63 | 0.49 to 0.77 | <0.001 |
| Model 3 | 0.01 | −0.01 to 0.03 | 0.51 | −0.05 | −0.06 to −0.04 | <0.001 | 0.71 | 0.53 to 0.89 | <0.001 |
| SAT (L) | |||||||||
| Crude | 0.02 | 0.01 to 0.03 | 0.002 | −0.02 | −0.03 to −0.01 | <0.001 | 0.47 | 0.39 to 0.55 | <0.001 |
| Model 1 | 0.06 | 0.05 to 0.07 | <0.001 | −0.02 | −0.03 to −0.01 | <0.001 | 0.33 | 0.25 to 0.41 | <0.001 |
| Model 2 | 0.06 | 0.05 to 0.07 | <0.001 | −0.02 | −0.03 to −0.01 | <0.001 | 0.37 | 0.29 to 0.45 | <0.001 |
| BF% (%) | |||||||||
| Crude | −0.02 | −0.024 to −0.016 | <0.001 | −0.01 | −0.012 to −0.008 | <0.001 | 0.26 | 0.22 to 0.30 | <0.001 |
| Model 1 | 0.02 | 0.01 to 0.03 | <0.001 | −0.01 | −0.012 to −0.008 | <0.001 | 0.15 | 0.11 to 0.19 | <0.001 |
| Model 2 | 0.02 | 0.01 to 0.03 | <0.001 | −0.01 | −0.013 to−0.007 | <0.001 | 0.15 | 0.11 to 0.19 | <0.001 |
Model 1 is adjusted for age and sex. Model 2 is adjusted for Model 1 plus ethnicity, socioeconomic status, alcohol consumption, smoking, hypertension, and diabetes. Model 3 is adjusted for Model 2 covariates plus BF%. The augmentation index was additionally adjusted for height, heart rate, and mean arterial pressure in Models 2 and 3.
BF%, body fat percentage; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Associations between obesity measures and vascular stiffness
All obesity measures were significantly associated with higher AIx in the unadjusted analysis. The associations remained significant after adjustment for Model 1, 2, and 3 [Model 3: VAT: β 0.71 (0.53–0.89), P < 0.001; SAT: β 0.37 (0.29–0.45), P < 0.001 and BF%: β 0.15 (0.11–0.19), P < 0.001] (Table 3). Higher positive effect estimates were found for visceral obesity and vascular stiffness in women compared to men, as is shown in Figure 2F [β 1.27 (0.94–1.60), P < 0.001 vs β 0.46 (0.24–0.68), P < 0.001, respectively, P for interaction < 0.001]. Similar positive associations were found for general obesity and vascular stiffness in women and men.
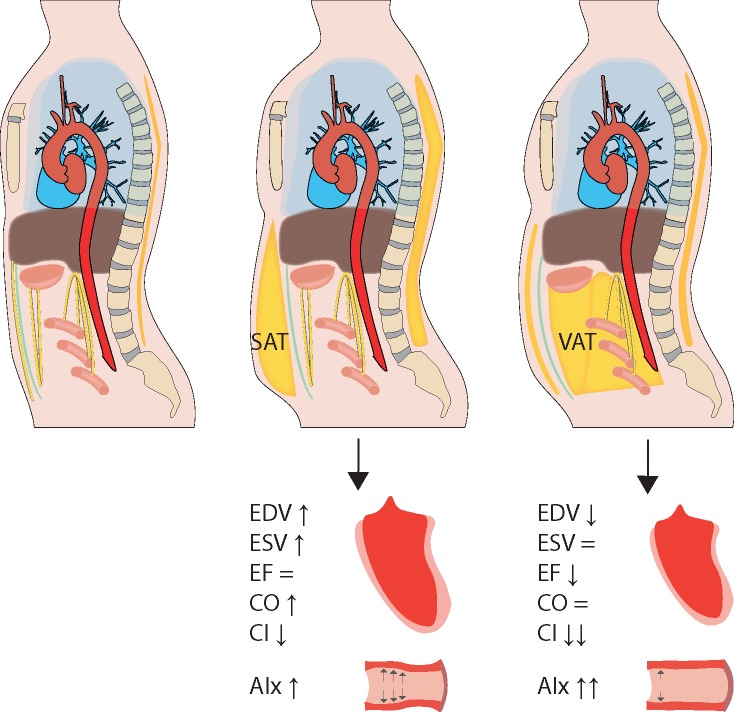
An overview of all results is summarized in Figure 3.
Figure 3.
The impact of fat distribution on cardiovascular function and geometry. Visceral obesity (assessed by VAT volume) and general obesity (assessed by SAT volume and BF%) exhibit different associations with structural and functional cardiovascular properties. Where general obesity is associated with larger LV volumes and greater CO, visceral obesity is associated with a smaller end-diastolic volume and lower LV systolic function. All measures of obesity are associated with increased vascular stiffness, with the strongest association for visceral obesity. AIx, augmentation index; CI, cardiac index; CO, cardiac output; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Discussion
In this population-based imaging study, we demonstrated the importance of body fat distribution in the association with cardiovascular function and geometry, where visceral obesity was associated with a smaller EDV, lower systolic cardiac function particularly in men and greater vascular stiffness in women.
Obesity measures and LV geometry
The positive association of general obesity with LV end-diastolic dimensions and CO is consistent with a theory postulated in 1986, which provides a potential pathophysiological process for obesity cardiomyopathy.16 This process has been described as an increase in perfusable tissue, expanding intravascular volume and by that demanding greater CO. Since heart rate is not influenced by obesity, CO is augmented through stroke volume. The expanded intravascular volume causes an increase in LV pressure, inducing a shift in the Frank–Starling curve and thereby initiating the chamber dilatation necessary for increased stroke volume. This theory recently found support, where through Mendelian Randomization, indications for a causal relation were found between BMI and CO driven by stroke volume.17 In contrast to general obesity, visceral obesity was associated with a smaller EDV. A possible contributing factor is the increased risk of diabetes and insulin resistance that is associated with visceral obesity, factors linked to smaller LV dimensions.18 However, in our regression models, we corrected for diabetes and results remained similar after excluding diabetics and patients with cardiovascular disease from the analysis. Additionally, allometric scaling did not change the associations observed between obesity and LV volumes.
Obesity measures and LV systolic function
The association of visceral obesity with EF and CI was consistent across the unadjusted and adjusted models, suggesting that VAT is an independent risk factor for lower systolic LV function. Since men have larger volumes of VAT, the fact that only men had a negative association between VAT and EF supports the suggestion that VAT is an independent risk factor for cardiac dysfunction. Per 1 L change in VAT, LV EF changed with −0.35% in men, meaning that in an overt obese male with 2SD more VAT compared to the average, LVEF is on average 1.54% lower. While visceral obesity was associated with subclinical lower systolic LV function, general obesity was the predominant factor for the higher CO associated with obesity, where the association between VAT and CO diminished after correction for general obesity. Furthermore, an overall negative association of obesity with CI was found, with the strongest negative association for visceral obesity. We postulate that the mechanisms seen in general obesity leading to a state of high CO might be prevented in visceral obesity by a direct metabolic effect of VAT on LV structure and function resulting in smaller LV dimensions with a lower EF and CI. A process possibly guided by the proatherogenic and prohypertrophic adipokines released from VAT, recently demonstrated in the cardiac aging effect of VAT through myocardial fibrosis by secretion of osteopontin.19
Obesity measures and vascular stiffness
Overall, all obesity measures were positively associated with vascular stiffness, where visceral obesity in women showed the strongest association with vascular stiffness. The AIx is known to be higher in women compared with men, however, for general obesity, this did not result in higher AIx for women.13 It has been suggested that the increased inflammation status and adipokine secretion associated with visceral adiposity leads to endothelial dysfunction and vascular stiffness.20 The exact pathophysiological effect of (visceral) obesity on vascular stiffness and the differences between men and women herein are unclear and need further research. The early reflected pulse wave in vascular stiffness that augments systolic pressure increases afterload and is associated with increased LV concentricity, thereby possibly contributing to the association of visceral obesity with a smaller EDV.21
In context of current literature
Until recent, studies using anthropomorphic measures like BMI suggested that obesity was associated with larger LV volumes and increased CO but not with EF.17 With the knowledge that obesity increases the risk of heart failure without an apparent negative impact of obesity on systolic function, the effect of obesity on heart failure was contributed to diastolic dysfunction.5 More recent studies investigating the impact of body fat distribution on LV geometry, similar to our study, found that visceral adiposity was associated with smaller LV volumes.4
The effect of obesity on LV systolic function is complex, where a previous study showed complicated curvilinear associations between obesity measures and the LV.22 Many previous studies, using anthropomorphic obesity measures, observed no change or even an increase in EF with obesity. The MESA study, a population-based cohort, previously stated that LV systolic function was insensitive to myocardial changes associated with anthropomorphic obesity measures.23 However, a subsequent MESA study investigating fat distribution found that above-median VAT was associated with a significant lower EF.6 The Dallas Heart Study did not report EF, however they did show a similar positive association of SAT with CO.4 Unlike our results they found a negative association of VAT with CO, possibly due to their notably obese population (43.9% obese versus 16.6% in our population).
For vascular stiffness, in recent years few studies have investigated the influence of fat distribution beyond anthropomorphic measures on AIx. The Framingham Heart Study (n = 2735) is the only large cohort to investigate the association of VAT and AIx and found no significant association.7 This could be due to the lean population that was studied (mean VAT of 1.76 L compared to 3.5 L in our population). The Framingham study also did not find any gender differences, whereas in our study women showed a significantly stronger association between visceral obesity and vascular stiffness compared to men, an interesting finding which warrants further research.
Limitations
There are several limitations that need to be considered. First, this is a cross-sectional study, therefore, no causal relation between obesity measures and cardiovascular function can be determined. Second, although the adjustment models in our study corrected for many known confounders, we were unable to investigate the influences of medication use, blood serum data or physical activity because the data was unavailable or there were too many missing values. Despite the many LV parameters used in our analysis, data on LV mass and diastolic function were unavailable; consequently, the association of visceral obesity with hypertrophic remodelling and LV relaxation could not be assessed. The MRI scanner that was used has a maximum weight capacity of 250 kg, which could potentially be a source of selection bias. The UK population that was investigated consisted mainly of Caucasians aged 45–73 years, so the results may not be generalizable to other ethnic groups or ages. However, previous large scale studies investigating the impact of VAT on cardiovascular function were performed in the United States, with a notably obese population, and focussed on the USA specific ethnic groups.4,6 The current study is more representative for the European population.
Conclusion
In this large population-based imaging study, we showed that visceral and general obesity are associated with different structural and functional cardiovascular properties. Visceral obesity was associated with a smaller LV EDV and subclinical lower LV systolic function in men, suggesting that visceral obesity might play a more important role compared to general obesity in LV remodelling.
Supplementary Material
Acknowledgements
This research has been conducted using the UK Biobank Resource under Application Number ‘20666’.
Conflict of interest: none declared.
References
- 1. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A. et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giordano A, Frontini A, Cinti S.. Convertible visceral fat as a therapeutic target to curb obesity. Nat Rev Drug Discov 2016;15:405–24. [DOI] [PubMed] [Google Scholar]
- 3. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010;11:11–18. [DOI] [PubMed] [Google Scholar]
- 4. Neeland IJ, Gupta S, Ayers CR, Turer AT, Rame JE, Das SR. et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging 2013;6:800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Powell BD, Redfield MM, Bybee KA, Freeman WK, Rihal CS.. Association of obesity with left ventricular remodeling and diastolic dysfunction in patients without coronary artery disease. Am J Cardiol 2006;98:116–20. [DOI] [PubMed] [Google Scholar]
- 6. Abbasi SA, Hundley WG, Bluemke DA, Jerosch-Herold M, Blankstein R, Petersen SE. et al. Visceral adiposity and left ventricular remodeling: the Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis 2015;25:667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Britton KA, Wang N, Palmisano J, Corsini E, Schlett CL, Hoffmann U. et al. Thoracic periaortic and visceral adipose tissue and their cross-sectional associations with measures of vascular function. Obesity (Silver Spring) 2013;21:1496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee JY, Choi JW, Kim H.. Determination of body surface area and formulas to estimate body surface area using the alginate method. J Physiol Anthropol 2008;27:71–82. [DOI] [PubMed] [Google Scholar]
- 10. West J, Dahlqvist Leinhard O, Romu T, Collins R, Garratt S, Bell JD. et al. Feasibility of MR-based body composition analysis in large scale population studies. PLoS One 2016;11:e0163332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petersen SE, Matthews PM, Francis JM, Robson MD, Zemrak F, Boubertakh R. et al. UK Biobank's cardiovascular magnetic resonance protocol. J Cardiovasc Magn Reson 2015;18:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Rourke MF, Gallagher DE.. Pulse wave analysis. J Hypertens Suppl 1996;14:S147–57. [PubMed] [Google Scholar]
- 13. Hughes AD, Park C, Davies J, Francis D, Mc G, Mayet J. et al. Limitations of augmentation index in the assessment of wave reflection in normotensive healthy individuals. PLoS One 2013;8:e59371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seidell JC, Bouchard C.. Visceral fat in relation to health: is it a major culprit or simply an innocent bystander? Int J Obes 1997;21:626–31. [DOI] [PubMed] [Google Scholar]
- 15. Stoner L, Young JM, Fryer S.. Assessments of arterial stiffness and endothelial function using pulse wave analysis. Int J Vasc Med 2012;2012:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Messerli FH. Cardiopathy of obesity—a not-so-Victorian disease. N Engl J Med 1986;314:378–80. [DOI] [PubMed] [Google Scholar]
- 17. Wade K, Chiesa S, Hughes A, Chaturvedi N, Charakida M, Rapala A. et al. Assessing the causal role of body mass index on cardiovascular health in young adults: Mendelian randomization and recall-by-genotype analyses. Circulation 2018;138:2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoneyama K, Venkatesh BA, Wu CO, Mewton N, Gjesdal O, Kishi S. et al. Diabetes mellitus and insulin resistance associate with left ventricular shape and torsion by cardiovascular magnetic resonance imaging in asymptomatic individuals from the multi-ethnic study of atherosclerosis. J Cardiovasc Magn Reson 2018;20:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sawaki D, Czibik G, Pini M, Ternacle J, Suffee N, Mercedes R. et al. Visceral adipose tissue drives cardiac aging through modulation of fibroblast senescence by osteopontin production. Circulation 2018;138:809. [DOI] [PubMed] [Google Scholar]
- 20. Sun Z. Aging, arterial stiffness, and hypertension. Hypertension 2015;65:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gulsin GS, Swarbrick DJ, Hunt WH, Levelt E, Graham-Brown MPM, Parke KS. et al. Relation of aortic stiffness to left ventricular remodeling in younger adults with type 2 diabetes. Diabetes 2018;67:1395–400. [DOI] [PubMed] [Google Scholar]
- 22. Shah RV, Anderson A, Ding J, Budoff M, Rider O, Petersen SE. et al. Pericardial, but not hepatic, fat by CT is associated with CV outcomes and structure: the multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging 2017;10:1016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP. et al. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol Img 2010;3:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.