Abstract
The detrimental impact of air pollution as a result of frequent exposure to fine particles posed a global public health risk mainly to the pulmonary disorders in pediatric and geriatric population. Here, we reviewed the current literature regarding the role of ginseng and/or its components as antimicrobials, especially against pathogens that cause respiratory infections in animal and in vitro models. Some of the possible mechanisms for ginseng-mediated viral inhibition suggested are improvements in systemic and mucosa-specific antibody responses, serum hemagglutinin inhibition, lymphocyte proliferation, cell survival rate, and viral clearance in the lungs. In addition, ginseng reduces the expression levels of proinflammatory cytokines (IFN-γ, TNF-α, IL-2, IL-4, IL-5, IL-6, IL-8) and chemokines produced by airway epithelial cells and macrophages, thus preventing weight loss. In case of bacterial infections, ginseng acts by alleviating inflammatory cytokine production, increasing survival rates, and activating phagocytes and natural killer cells. In addition, ginseng inhibits biofilm formation and induces the dispersion and dissolution of mature biofilms. Most clinical trials revealed that ginseng, at various dosages, is a safe and effective method of seasonal prophylaxis, relieving the symptoms and reducing the risk and duration of colds and flu. Taken together, these findings support the efficacy of ginseng as a therapeutic and prophylactic agent for respiratory infections.
Keywords: Bacteria, Clinical trials, Ginseng, Respiratory tract infections, Virus
Abbreviations: ARI, acute respiratory illness; COPD, chronic obstructive pulmonary disease; GSLS, ginseng stem–leaf saponins; HRV, human rhinovirus; IFN, interferon; IL, interleukin; IgA, immunoglobulin A; PD, protopanaxadiol; PT, protopanaxatriol; RSV, respiratory syncytial virus; RTIs, respiratory tract infections; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-alpha
1. Introduction
Environmental exposure to fine particles in polluted air is the leading risk factor for pulmonary dysfunction that can alter the development of both immune function and lung mechanics, including substantial adverse impact on human health mainly in growing children and immunocompromised patients [1,2]. Polluted air contains a mixture of multiple substances, including gaseous pollutants and particulate matter (PM) [3], both of which affect the respiratory system and cause pulmonary disorders and adverse health effects [4]. PM is primarily emitted from combustion engines or formed by chemical transformation. It is classified based on particle diameter; aerosolized particles with a diameter of 10 μm or less are denoted as PM10 and those with a diameter of 2.5 μm or less as PM2.5. The World Health Organization provides air quality guidelines that reference the daily and annual concentrations of PM2.5 and PM10 (World Health Organization) [5]. Recent global multicenter studies demonstrated that short-term exposure to PM2.5 and PM10 is associated with increased respiratory and cardiovascular mortality rates [6]. Moreover, air pollution increases the risk of respiratory infections in growing children and people with immune deficiencies [2,7].
Respiratory tract infections (RTIs) are illnesses that spread through either direct or indirect contact, such as sneezing or coughing, and most RTIs are the result of viral or bacterial infections, with bacterial infections being less common causes. These microbial infections can interfere with and damage the normal physiology of the respiratory tract, resulting in the deterioration of the lungs and airways [8]. These infections occur most commonly in the fall and winter include the common cold, influenza, chronic obstructive pulmonary disease (COPD), asthma, and pneumonia. Because of the atypical presentations of influenza infections, which make effective control difficult, a self-healing approach is taken for the management of common cold [8]. However, seasonal prophylactic treatment with antiviral drugs and adjunct vaccine therapies still remain an attractive strategy for preventing and controlling seasonal infections [9]. COPD is likely to become the third leading cause of death worldwide by 2020 [10,11]. Currently, the prevalence of COPD in the adult population (aged ≥40 years) is estimated to be around 10 % [12]. Although it is a preventable infection, increasing air pollution may result in an increase in COPD prevalence, making it a priority for individuals and health-care providers [13]. People with COPD commonly experience symptoms, including dyspnea, wheezing, and coughing and have an increased susceptibility to microbial infections and complications that damage lung function [14]. COPD also increases vulnerability to adverse events, including bruising, oropharyngeal candidiasis, and pneumonia. As there is no cure for COPD, improving quality of life, reducing complications, and managing symptoms are the primary objectives for novel COPD treatment strategies [15]. Asthma is an inflammatory disease leading to mucus hyperproduction, airway hyperresponsiveness, and airway wall remodeling and affects more than 300 million people worldwide. Asthma is a typical Th2 inflammatory disease that causes increased IgE levels in the airway [16]. Similar to asthma, pneumonia, commonly caused by Streptococcus pneumoniae, is an inflammatory disorder of the lungs primarily affecting the air sacs and resulting in chest pain, dry or productive coughing, fever, and difficulty in breathing. Pneumonia is most damaging in infants and older people as a result of their reduced immune function [17].
1.1. Pathogenicity of microbial infections
Infectious diseases are the leading cause of morbidity and mortality worldwide. They are primarily caused by either bacterial or viral infections [18] and often experience treatment failure https://www.sciencedirect.com/science/article/pii/S0165587606000358?via%3Dihub [18]. Viruses are the most common cause of respiratory infections and facilitate secondary bacterial infections by aiding bacterial adherence, colonization, and translocation through the epithelial barrier of respiratory cells https://www.sciencedirect.com/science/article/pii/S0165587606000358?via%3Dihub [19]. Clinical features do not reliably distinguish bacterial from viral infection; however, their management and treatment are different [20]. Both management and treatment of bacterial and viral infections can be complicated, if the patient is exposed to air pollutants, which have recently emerged as one of the greatest environmental health risks worldwide as a result of rapid industrial development and urbanization [1,2,4]. Although, viral infection areas more common in the pediatric and geriatric populations than bacterial infections to cause respiratory symptoms, yet viral infections may induce bacterial infection, and this condition complicates investigation of the role of each microorganism in the pathogenesis and clinical outcomes [21,22]. Because majority of viral infections is self-resolving; however, rapid molecular diagnostics tests have increased our understanding to identify bacterial and viral pathogens [23]. The diagnostic yield is influenced by antibiotic therapy, specimen collection, transport, rapid processing, and correct use of cytological criteria [24]. Regarding viral and atypical pathogens, conventional culture of bacteria from normally sterile sites remains the ‘gold standard’ for confirming bacterial infection; however, it may take several days and are frequently negative when infection resides in inaccessible sites or when antibiotics have been previously administered [25]. In addition, presence of viral epidemics in the community, patient's age, rapid onset of disease, symptoms, radiographic changes, and response to treatment can help differentiate viral from bacterial pneumonia [26]; however, detection of a virus in the specimen does not rule out bacterial infection and is of little help in decisions on whether to administer antibiotics. Clarifying the differences and dynamics of respiratory infections can elucidate pathogenesis of viral–bacterial interactions and provide a basis for developing novel approaches for the prevention, treatment, or management of acute respiratory infection.
1.2. Ginseng as an immune modulator
Although there are a number of ginseng species, Korean ginseng (Panax ginseng C. A. Meyer), American ginseng (Panax quinquefolius L.), and Chinese ginseng (Panax notoginseng) are the most popular experimental models and have received significant attention for their potential use to alleviate disease symptoms and improve health conditions. These species of ginseng have also been included in health-care products and food additives worldwide [27,28]. P. ginseng is abundant in North Asian countries, especially Korea, the eastern regions of China, Japan, and Russia. P. notoginseng is cultivated mainly in China [29,30]. P. quinquefolium is found in the United States and Canada and has been used by Americans for several years [31]. Ginseng is known to possess immunomodulatory activities with a wide array of therapeutic applications against microbial infections. Contradictory data regarding the immunomodulatory properties of ginseng are most likely a result of differences in the extraction method, origin and source of ginseng, and laboratory practices [32]. This is because it contains numerous pharmacologically active ingredients, including ginsenosides, saponins, carbohydrates, phytosterols, polyacetylenes, polyphenolic compounds, sugars, acidic polysaccharides, organic acids, amino acids, vitamins, nitrogenous substances, and minerals, each of which can play a significant role in protection from and treatment of many diseases [[33], [34], [35]]. In common practices, P. ginseng has been shown to promote physical performance, improve vitality, increase resistance to stress and aging [36]. Recently, approximately 200 active compounds, such as ginsenosides, polyacetylenes, polysaccharides, amino acids, and peptides have been validated from Korean ginseng [37], whereas P. quinquefolium commonly known as a popular nutritional supplement and herbal remedy [33,34] has approximately more than 100 substances isolated from American ginseng. Chemically, several differences exist among Korean ginseng and American ginseng which make them different in terms of their mechanism of action [38]. Ginsenosides, including Rb1, Rd, Re, Rg1, Rg2, Rg3, Rh1, and Rh2, are the major active ingredients in ginseng known to possess enhanced therapeutic activity and stability due to change in its chemical constituents [39,40]. An important parameter used for differentiation is the presence of the ginsenoside Rf in Korean ginseng and notoginseng, the pseudoginsenoside F11 in American ginseng [38], and the notoginsenoside R1 in Korean ginseng. In addition, the ratio of Rg1/Rb1 has been widely used to differentiate between these ginsengs. Ratios less than 0.4 are indicative of American ginseng, whereas a high value ratio is characteristic of Korean ginseng [41]. Apart from ginsenoside, saponins are a heterogeneous group of triterpene glycosides and sterols [42] and increase cellular and humoral immune responses when used as adjuvants during vaccination [43].
2. Effect of ginseng on respiratory virus infections
2.1. Influenza virus
Belonging to the Orthomyxoviridae family is the most common human respiratory pathogen and the main causative agent of seasonal influenza [44], which is a serious respiratory disease estimated to cause around three to five million cases of severe illness and half a million deaths per annum [45]. Influenza has three main types, A, B, and C, with influenza A being the most virulent in humans, other mammals, and birds, and, thus, the best understood of the three. The H1N1 virus, commonly known as swine flu caused a lethal pandemic in 2009, affecting more than 74 countries [46]. Several studies have demonstrated the antiviral activity of Red Ginseng extract (RGE) and its purified components on influenza A infection both in vivo and in vitro as shown in Table 1. In vitro studies suggest that ginsenoside components, particularly Rb1, have the ability to interact with viral hemagglutinin proteins, preventing the attachment of the virus to α 2-3′ sialic acid receptors on the host cell surface, and thereby minimizing pandemic H1N1 viral entry into host cells [47]. Fermented RGEs containing ginsenosides (protopanaxatriol [PT], protopanaxadiol [PD], compound K, and Rh2) showed antiviral activity against several influenza subtypes (H1N1, H3N2, H5N1, H7N9) in mouse models [48]. PT-type ginsenoside Re protects human umbilical vein endothelial cells from avian H9N2/G1-induced cell death by inhibiting virus-induced IFN-inducible protein 10 production https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052424/ [49]. Yin et al., [50] showed that treatment with ginseng polysaccharide followed by treatment with RGE and saponin was effective in alleviating the symptoms of influenza viral infection. Intranasal coadministration of Korean RGE (200 mg/kg body weight) with inactivated influenza virus A (PR8) increased the specific antibody levels in treated animals and induced improved protective immunity when compared with immunization with PR8 alone (25 mg). Mice-fed ginseng polysaccharide fractions, total extracts, saponin fractions, or phosphate-buffered saline for 14 d before influenza A challenge had a 78%, 67%, 56%, and 17% survival rate, respectively. In addition, RGE as a mucosal adjuvant also played a protective role against influenza virus A/PR8 viral infection https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052424/ [51].
Table 1.
Effect of ginseng on respiratory virus infections
| Ginseng type | Virus type | model | Dosage | Results | Ref |
|---|---|---|---|---|---|
| Polysaccharide, saponin, and total extract | Influenza (H1N1 subtype) | BALB/c mice | Orally 250 μg/kg/d for two weeks before virus challenge | Reduced TNF-α, iNOS-producing dendritic cells (tipDCs) in the lungs and body weight loss after treatment with the polysaccharide fraction. | [50] |
| P. ginseng aqueous extract powdered capsules | Inactivated influenza virus A PR8 (25 mg) | Female inbred BALB/c mice | 200 mg/kg for 14 days intranasally | Increased levels of IgA and cytokines (IFN-g, IL-2, IL-4, IL-5, IL-6). Elevated CD69 expressing leukocytes, inducing protective immunity. | [51] |
| Ginsenoside Re + inactivated H3N2 | Inactivated influenza virus (H3N2) | Female ICR mice | 25, 50, or 100 μg Re for 21 days subcutaneously | Co-administration amplified IgG isotype responses, HI titers, lymphocyte proliferation, IL-5, and IFN-γ secretion. | [53] |
| RG extract or RG saponin | Influenza A/PR/8/34 | Balb/c mice | 0.25 mg/kg/d orally for 14 d prior to the primary immunization and 21 days until secondary immunization | Elevated serum IgG titers and survival rates. Increased cell-mediated immunity associated with tissue repair and healing. | [56] |
| P. ginseng | H1N1, and H3N2 (A/Philippines/82) | BALB/c mice | 10 to 100 mg/kg for 14 days by oral route | Lower levels of lung viral titers and IL-6, but higher levels of IFN-γ | [58] |
| RGE | HP H5N1 influenza virus | Female mice | 50 mg/kg body weight) for up to 80 days | Antiviral cytokines INF-α and -γ were induced | [59] |
| Polysaccharide | H1N1 (A/PR8) and H3N2 viruses | BALB/c mice | 25 mg/kg or IV 10 g/kg doses by i.n route | Enhanced survival rate and lower levels of lung viral titers and IL-6. | [60] |
| GSLS | Inactivated ND and AI vaccines | Hy-Line White layer chickens (male) | 2.5, 5, 10, and 20 mg/kg of BW for 7 days | Enhanced the HI response against inactivated ND and AI vaccines in chickens | [61] |
| GSLS | Newcastle disease | Hy-Line White layer chickens (male) | 2.5, 5, 10, and 20 mg/kg by oral route 7 days | GSLS increased serum HI titer, lymphocyte proliferation, intestinal mucosal IgA + cells and iIELs. | [62] |
| GSLS | Inactive ND and AI | Specific pathogen–free (SPF) chickens | 2.5, 5, and 10 mg/kg body weight by oral route for 7 days | Improved splenocyte proliferation induced by ConA, LPS and IgA + cells and iIELs. Improved systemic and mucosal specific antibody responses | [63] |
| RGE | influenza A H1N1 (A/PR8) and A/WSN/1933 viruses | BALB/c mice | 25 mg/kg/day by oral route for 30 days (500 μg/mL) | RGE reduced influenza A virus-induced CPE formation and blocked the induction of influenza A virus pro-inflammatory gene expression. Anti-oxidative and immunomodulatory effects | [72] |
| P. ginseng | RSV A2 strain | BALB/c, C57BL/6 mice (MHCII KO) HEp2 cells | 25 mg/kg/day for 80 or 130 days by oral route | Increased IgG2a isotype antibody response. Increased IFN-γ with modulation of CD3 T-cell populations. Decreased IL-4 production and diminished weight loss. | [73] |
| RGE | RSV | HEp2 cells | Direct mixing in medium | Partially protected HEp2 cells from RSV-induced cell death and viral replication. Inhibited TNF-α in murine dendritic and macrophage-like cells. | [75] |
| RGE | RSV | Female BALB/c mice | 500 μg/mL | Partial inhibition of viral replication, preventing body weight loss, improving cell survival, viral clearance, and modulation of TNF-α, and producing IFN-γ in bronchoalveolar lavage cells. Increased CD8+ T and CD11c + populations in dendritic cells. | [75] |
| P. ginseng | RSV A2 strain | Female BALB/c mice | Oral administration of 25 mg/kg/day for 60 days (250 and 500 μg/ml) | Modulation of host cellular phenotypes, producing cytokines and ROS and improving cell survival. Suppressed IL-6 and IL-8. | [76] |
| P. ginseng | RSV A2 strain | Human lung epithelial cells (A549 cell) | KRGE-mediated inhibition of RSV replication, lowering lung viral loads, enhancing level of IFN-γ, reducing ROS and proinflammatory cytokine production, and improving cellular survival. | [76] | |
| Ginsenosides Re, Ref and Rg2 | HRV3 | Human cervix epithelial cell line (HeLa, CCL-2) | Direct mixing in medium as 0.1, 1, 10, and 100 mg/mL | PT-type ginsenosides (Rf and Rg2) increased cell viability and decreased susceptibility to viral infection. | [83] |
Abbreviations: AI, Avian Influenza; BW, Body weight; CPE, Cell Viability and Cytopathogenic Effect; Cy, cyclophosphamide; GSLS, Ginseng Stem-Leaf Saponins; HEp2, Human Epithelial; IFN-γ, Interferon-Gamma; IgA + cells, Immunoglobulin A-secreting cells; iIELs, Intestinal Intraepithelial Lymphocytes; MHCII KO, Major Histocompatibility Class II Knockout mice; ND, Newcastle Disease; ROS, Reactive Oxygen Species; RSV, Respiratory Syncytial Virus; sIgA, Secretory Immunoglobulin A; TNF-α, Tumor Necrosis Factor-alpha; LPS, lipopolysaccharide; HRV3, human rhinovirus 3; RGE, Red Ginseng extract; BALB, Bagg Albino (inbred research mouse strain); ICR, Institute of Cancer research.
Ginseng modulates both T-helper type 1 and 2 (Th1) and (Th2) immune responses via antibody production, inhibiting the invasion and replication of influenza virus and aiding the detachment of the virus from mucosa. Cellular immunity mediated by Th1 and Th2 responses is associated with tissue remodeling and healing and is required to improve cell viability and viral clearance [52]. Coadministration of ginsenoside Re (25, 50, or 100 μg) with inactivated influenza virus (H3N2) antigen equivalent to 10 or 100 ng of hemagglutinin in mice immunized subcutaneously produced increased serum specific IgG, IgG isotype responses, hemagglutinin inhibition, lymphocyte proliferation, as well as IFN-γ and IL-5 secretion [53]. In addition, immunized mice presented with higher levels of influenza virus–specific antibodies with amplified neutralizing activities in mucosal secretions and blood. IgA antibodies in the lung were particularly amenable to this effect, suggesting that both Th1 and Th2 immune responses were activated in splenocytes https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052424/ [53]. Immune responses to respiratory illness are typically characterized by a classical Th1 immune response in healthy individuals [54], mediated by Th1 cells and enriched production of antibodies, such as IgG2a, IgG2b, and IgG3. Moreover, a Th1 immune response is mandatory for cytotoxic T lymphocyte production https://www.sciencedirect.com/science/article/pii/S0264410X06006797?via%3Dihub [55]. Dietary intake of both RGE and RG saponin for 14 d before primary immunization showed two times more anti-influenza A serum IgG titers with enhanced survival rates after influenza A infection in mice. Mice receiving RG saponin and RG extract had a total survival rate of 63% and 56%, respectively, whereas animals who received only the vaccine had a 38% survival rate [56]. Mice treated with ginsenoside Re also experienced increased hemagglutination inhibition and serum-specific IgG1 and IgG2a levels, which was mirrored in the results of in vitro activation of splenocytes, which produced elevated levels of Th1 and Th2 cytokines. Moreover, dietary intake of Korean Red Ginseng saponin, and RGE has also been implicated in increasing H1N1 vaccine efficacy by improving anti-influenza A–specific IgG titers and thus, survival rates https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052424/ [56]. The Th2 response induces IL-4, IL-5, IL-10, and IL-13, which contribute to mucus production, goblet cell formation, eosinophil recruitment in the lungs, and airway hyperresponsiveness [57]. Contrarily, the Th1 response is effective against intracellular infectious agents, providing protective immunity https://www.sciencedirect.com/science/article/pii/S0264410X06006797?via%3Dihub [55]. Oral administration of RGE (with a daily dose ranging from 10 to 100 mg/kg), with vaccination before infection, provided enhanced cross-protection against swine-origin influenza A viruses, including H1N1, antigenically distinct H1N1 subtype A/PR/8/34, and H3N2 subtype A/Philippines/82 [58]. Naive mice infected with virus mixed with Korean Red Ginseng extract (KRGE) showed higher levels of IFN-γ but lower levels of inflammatory cytokine IL-6 and lung viral titers when compared with control mice infected with virus without RGE [58]. The inhibitory effects of polysaccharides on viral replication were also tested in Madin–Darby canine kidney cells using the H1N1 virus https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052424/ [58]. In addition, RGE, ginseng saponin, and ginseng polysaccharides were evaluated for their antiviral effect after oral administration in chickens or mice. These studies measured antibody titer response, body weight, cytokine production, histopathology, lung viral titers, and survival rate https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052424/ [58,59], showing that the ginseng polysaccharide was able to prevent H1N1 and H3N2 infection https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052424/ [58] and that saponin had an antiviral effect against H1N1 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052424/ [50]. Moreover, P. ginseng polysaccharide administration (25 mg/kg) intranasally (i.n.) to mice 6 h before influenza infection exerted a protective effect in the lungs, as it increased survival rates and diminished viral titers and IL-6 expression [60].
Ginseng stem–leaf saponins (GSLS) possess multiple biological functions, mainly immunomodulatory activities [61,62]. Thus, when the effect of orally administered GSLS (2.5, 5, 10, and 20 mg/kg) on immune responses in chickens vaccinated with live Newcastle disease vaccines was evaluated, GSLS enhanced hemagglutination inhibition and researchers determined its optimal dose at 5 mg/kg. In addition, administration of GSLS in drinking water at 5 mg/kg for 7 days before vaccination markedly improved intestinal intraepithelial lymphocyte proliferation and mucosal IgA+ cells in chickens immunized with a live Newcastle disease vaccine [62]. Similarly, the effect of administering GSLS at concentrations of 2.5, 5, and 10 mg/kg for seven days on the immune response after administration of a bivalent inactive vaccine of avian influenza in chickens was examined. Data showed that the spleen lymphocyte proliferation induced by Concanavalin A and lipopolysaccharides, and the numbers of IgA-secreting (IgA+) cells and intraepithelial lymphocytes in the duodenum were recovered in chickens treated with GSLS before immunization. GSLS also proved to be a potential vaccine candidate, as it enhanced serum antibody responses in immunosuppressed chickens after cyclophosphamide treatment at 100 mg/kg for 3 days [63].
2.2. Respiratory syncytial virus
Respiratory syncytial virus (RSV) is a negative nonsegmented single-stranded RNA virus [64], belonging to the Paramyxoviridae family. It is the primary cause of lower RTIs [65,66], generally exhibiting acute and indistinct symptoms, including bronchiolitis, common cold, and pneumonia, in infants and immunocompromised patients https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052424/ [67]. During primary RSV infection, alveolar macrophages and lung epithelia in the respiratory tract are likely to be infected [68], ultimately leading to higher expression of cytokines, including IL-6, IL-8, IL-10, and tumor necrosis factor-alpha (TNF-α); chemokines, including CCL5, CXCL10, IL-8, and IFN-inducible protein 10; growth-regulated proteins; and reactive oxygen species in different in vitro models [[69], [70], [71]], resulting in type 1 and 2 cytokine imbalance [72]. KRGE orally administered to mice (25 mg/kg/day) in conjunction with formalin-inactivated RSV showed beneficial effects and improved outcomes, including a 2-fold increase in IgG2a antibodies as well as IFN-γ production accompanied by lower levels of IL-4 mitigating weight loss, after RSV infection https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052424/ [73]. In addition, ginseng-treated groups also lowered pulmonary inflammatory responses and CD3 T-cell infiltration into the bronchoalveolar lavage (BAL) cell populations [73]. RGE has been shown to mitigate Th2 responses by improving the Th1 response in formalin-inactivated respiratory syncytial virus immunized mice, which have an intrinsic Th2-dominant immune response https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052424/ [73]. It has been suggested that excess type 2 and/or deficient type 1 immune responses play a critical role in the pathogenesis of RSV infection [74]. RGE, at a concentration of 500 μg/mL, suppressed the production of RSV-induced TNF-α in murine dendritic and macrophage-like cell lines, thereby protecting human epithelial cells from RSV-induced apoptosis and viral replication. In addition, RGE treatment increased the population of CD8+ T cells and CD11c+ dendritic cells in BAL fluids after RSV infection in mice [75]. Oral administration of KRGE in mice significantly inhibited the expression of RSV-induced genes (IL-6 and IL-8) and, subsequently, lowered viral titers and reduced reactive oxygen species production, thereby improving the survival of human lung epithelial A549 cells upon RSV infection [76]. The antioxidant activity of RGE might be linked to the inhibition of RSV-induced apoptosis [77].
Ginsenosides suppress the induction of the proinflammatory cytokines IL-6, IL-8, and TNF-α and proteases, such as MMP-9, and inhibit oxidative stress (superoxide, NO and iNOS) [27]. A recent study showed that ginsenosides isolated from P. ginseng significantly increased proinflammatory IFN-γ levels in an ovalbumin-induced murine asthmatic model but lowered IL-4 production [78].
2.3. Rhinoviruses
Rhinoviruses (RVs) are positive-sense, single-stranded RNA viruses belonging to the Picornaviridae family and Enterovirus genus. Although generally mild and self-limiting, RVs have been implicated in the induction of upper RTIs, including asthma, bronchiolitis, and common colds, in infants and the deterioration of airways in elderly people and immunocompromised patients [79,80]. In many RV strains, antigenic diversity and phenotype characteristics can affect disease severity and are significant obstacles to vaccine development [81]. Human rhinovirus (HRV) represents one of the most significant etiological agents of upper respiratory illnesses, particularly common cold. Although mild to indistinguishable, HRV infection can lead to severe health complications and severe exacerbation of asthma [82]. When the antiviral activities of PT-type ginsenosides (Re, Rf, and Rg2) and PD-type ginsenosides (Rb1, Rb2, Rc, and Rd) on RV infection were examined, PT-type ginsenosides protected HeLa cells from HRV3-induced apoptosis. However, PD-type ginsenosides activated HRV3-induced apoptosis and did not show any preventive effect, suggesting a structure-dependent effect of ginsenosides on HRV3 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052424/ [83]. Furthermore, PT-type ginsenosides showed noticeable antiviral effects against coxsackievirus B3 and HRV3 when administered at 100 mg/mL for 48 h. Among the PT-type ginsenosides, Rg2 exhibited significant antienterovirus 71 effects, with no cytotoxic activity at 100 mg/mL. However, PD-type ginsenosides did not display any antiviral effect against coxsackievirus B3, enterovirus 71, and HRV3, and showed cytotoxic activity in virus-infected cells [83].
Intranasal coadministration of 25 mg of inactivated influenza A PR8 and ginseng aqueous extract (200 mg/kg) in mice twice a day (day 0 and 14) significantly increased IgA titers in the intestines and lungs when compared with those in the intestines and lungs of mice that received only the inactivated virus. Although the levels of all cytokines (IFN-γ, IL-2, IL-4, IL-5) increased after ginseng treatment, IL-4 and IL-5 were more significantly upregulated, indicating a Th2 type response [51].
Altogether, these studies suggest that ginseng possesses antiviral activities and exerts immunomodulatory effects against viral infections through multiple mechanisms as shown in Table 1 and Fig. 1. Moreover, antiviral effects of ginseng, including blockade of viral attachment, membrane penetration, and inhibition of replication inside the cell, are associated with the host immune response (Fig. 1).
Fig. 1.
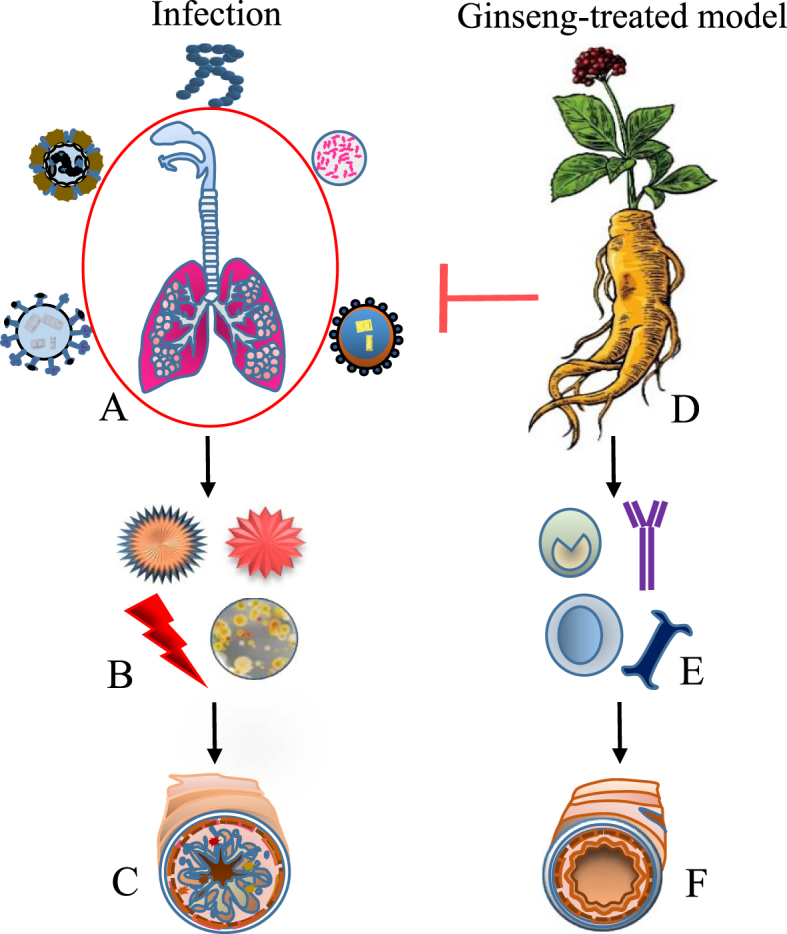
Effect of ginseng on respiratory tract infection. (A) Environmental and seasonal fluctuations influenced by rapid urbanization and drastic increase in air pollution increase the incidence rate and severity of respiratory tract infections causing respiratory outbreaks by microbial pathogens in host models. (B) Upon entry, contagious pathogens cause a decline in the immune status and triggers cytokines production and produce stress in the microenvironment, leading to increasing viral load and oxidative stress levels. (C) Thereby assisting bacterial and viral infections to break down the tissue defence barriers causing epithelial and cellular changes residing inside host cell known to contribute to eosinophil recruitment to the lung, mucosal damage, mucus production, and airway hyperresponsiveness. (D) Ginseng is believed to interrupt through multiple mechanism. (E) Activation of B cell causes production of antibody by triggering Th2 response to enhance systemic and mucosal specific antibody titer response serum hemagglutinin inhibition titer response, lymphocyte proliferative response. (F) In addition to possess anti-inflammatory and antioxidative properties, ginseng also improved cell-mediated immunity associated with tissue repair and healing and thus used to alleviate the symptoms and reduce the incidence rate and frequency of respiratory tract infections.
3. Effects of ginseng on bacterial infections
3.1. Pseudomonas aeruginosa
Pseudomonas aeruginosa is a complex gram-negative opportunistic facultative anaerobe that causes nosocomial infections and pneumonias, leading to COPD, thermal injury, and urinary tract infections in infants and immunocompromised patients [84]. P. aeruginosa disrupts airway homeostasis in the lung by damaging the epithelium and evading innate and adaptive immune responses [[84], [85]]. Biofilm-associated chronic P. aeruginosa lung infections in patients with cystic fibrosis are difficult to manage with antibiotics due to the high tolerance of biofilm-associated bacteria to medical intervention and host defense mechanisms [86]. An aqueous extract of P. ginseng administered at a concentration of 25 mg/kg/day for 14 d in a rat model of chronic P. aeruginosa pneumonia mimicking cystic fibrosis significantly increased polymorphonuclear leukocyte chemiluminescence and serum IgG2a levels while decreasing IgG1 levels when compared with the control [87]. This suggests that P. ginseng could be a useful adjunct therapy in cystic fibrosis able to alleviate bacterial infections and reduce biofilm formation.
Aqueous extracts of P. quinquefolius exert an antibacterial activity in vitro against all P. aeruginosa strains, including the O1 strain, as they attenuate pyocyanin, pyoverdine, swarming and swimming motility, lipase concentrations, and stimulated twitching. In addition, P. quinquefolius extracts also successfully eliminated 6-day-old mature biofilms (5% w/v), and fluorescence microscopy indicated a reduction of live cells and biofilm complexes in treated biofilms compared with nontreated biofilms [88].
P. ginseng extracts did not inhibit the growth of P. aeruginosa PAO1 and its isogenic mucoid variant (PAOmucA22 or PDO300), but it did enhance extracellular protein production and stimulated the production of alginate. In addition, ginseng repressed the production of LasA and LasB and downregulated the synthesis of the N-acylated homoserine lactone factors [89].
P. ginseng aqueous extract used for treatment at a concentration of 0.5% for 24 h destroyed most 7-day-old mature biofilms formed by both mucoid and nonmucoid P. aeruginosa strains. Ginseng increased bacterial motility in biofilm-like alginate beads, resulting in the release of bacteria from the biofilm and loss of the protective effects of the polymeric matrix, suggesting that ginseng treatment might help to eradicate biofilm-associated chronic infections caused by P. aeruginosa. In addition, P. ginseng significantly inhibited bacterial biofilm formation in both mucoid and nonmucoid P. aeruginosa strains in vitro [90]. In a study wherein P. ginseng (250 mg/kg/day) was subcutaneously injected into a mouse model challenged with alginate beads containing mucoid P. aeruginosa (PAO 579), ginseng treatment resulted in higher levels of IFN-γ and TNF-α but lower levels of IL-4 in lung cells and splenocytes after 6 and/or 24 h of incubation compared with the control [91]. All these data suggest that ginseng facilitates bacterial clearance from the lungs through multiple mechanisms and has a negative effect on the quorum-sensing system of P. aeruginosa, resulting in a milder lung pathology. In addition, these findings suggest that the immunomodulatory functions of ginseng are most likely associated with the activation of a Th1 type cellular immune response and downregulation of the humoral immune response, reducing the formation of immune complexes [87].
3.2. Streptococcus pneumoniae
Streptococcus pneumoniae is a gram-positive facultative anaerobic bacterium causing pneumococcal diseases, particularly pneumonia, meningitis, and sepsis, with a high mortality rate in children (≤5 years) [92]. Mice pretreated with Korean Red Ginseng (KRG) at a dose of 100 mg/kg showed significantly increased survival rates and alleviated bacterial burden in the blood, lungs, and spleen at 24 and 48 h after infection and, thus, diminished inflammatory response in the lungs, compared with the control group. Moreover, KRG administration (100 mg/kg) prevented pneumococcal sepsis by inhibiting the production of cytokines, including IL-1β and TNF-α, neutrophil infiltration, and nitric oxide production 48 h after infection in vivo. Furthermore, aqueous extracts of KRG significantly reduced S. pneumoniae–induced TLR2, TLR4, and NF-κB expression in RAW264.7 macrophages [93].
4. Human trials evaluating the effect of ginseng on respiratory pathogens
COLD-fX (CVT-E002), a poly-furanosyl-pyranosyl-saccharide isolated from the root of American ginseng, is believed to be safe, effective, and widely accepted as a therapeutic regimen against respiratory pathogens, reducing viral and bacterial load in patients of all ages, especially in patients who are more vulnerable to seasonal influenza-like illness [83]. COLD-fX has been investigated as an immunomodulatory compound, acting through toll-like receptors. This activation leads to an increased number and function of cells within both the adaptive and innate immune systems [94,95].
Ginseng efficacy in the treatment of common cold, flu, and upper and lower RTIs has been investigated in several randomized controlled clinical trials with varying inclusion and exclusion criteria. These trials have shown ginseng effects on severity, duration, and frequency of overall symptoms with a positive trend for preventing the development of respiratory infections, as shown in Table 3. KRGE at a dose of 9 capsules per day for a period of 3 months in 100 patients effectively suppressed the incidence of influenza-like illness against placebo-treated group [96]. The efficacy of COLD-fX in preventing acute respiratory illness (ARI) was evaluated in a randomized, double-blinded trial comprising 43 adults (≥65 years) taking 2 capsules/day (200 mg/capsule) of either COLD-fX or placebo for a period of 4 months after one month of influenza vaccine. Evidence indicates that COLD-fX significantly increased lymphocyte proliferation and cytokine production (IL-1, IL-6, TNF-α, and nitric oxide) from peritoneal macrophages in vitro. IL-2 and IFN-γ are the major T cell and NK cytokines associated with virus-elicited adaptive immunity [97]. In addition, when COLD-fX as a prophylactic treatment reduced the duration of respiratory symptoms and relative risk by 55% and 48%, respectively in immunocompromised aged patients during the influenza season [97]. COLD-fX, as a seasonal prophylactic was evaluated in 783 seniors (≥65 years) at a daily dose of 400 or 800 mg/day for 6 months. This study showed that COLD-fX was safe, with a tendency to reduce the incidence and severity of upper RTIs [98].
Table 3.
Effect of ginseng on respiratory infections in human clinical trials
| Study site | Disease state | Parameters | Ginseng (type) | Dosage | Results | Ref |
|---|---|---|---|---|---|---|
| Republic of Korea NCT01478009 |
Influenza-like illness (ILI) | (30-70 years years) N = 100 |
KRGE | 9 capsules/day for 3 months | Reduced ILI incidence | [96] |
| Alberta, Canada | Acute Respiratory infection | (≥65 years) N = 43 |
COLD-fX, | Freeze-dried extract for 4 months using 2 capsules/day (200 mg/capsule) | Reduced risk and duration of colds and flu. | [97] |
| Edmonton, Alberta, Canada | Upper respiratory infections | (≥65) N = 783 |
COLD-fX | Oral extract for 6 months | Safe, well tolerated, and effective as seasonal prophylaxis in duration of Jackson-confirmed URIs in healthy adults | [98] |
| Edmonton, Alberta, Canada | Common colds | (≥18 years) N = 747 | (P. quinquefolius or P. ginseng) root extract | Reduced the total number and shortened the duration of ARIs by 25% and 6.2 days respectively. | [99] | |
| Edmonton, Alberta, Canada. | Laboratory-confirmed ARI | (≥60) N = 198 |
COLD-fX | 200 mg capsule Oral extract twice-daily for 12 weeks | LCII and RSV illness was more severe in placebo than COLD-fX-treated patients. | [100] |
| Edmonton, Alberta, Canada |
Cold prevention | (18 - 65 years) N = 323 |
American ginseng | 2 capsules/day (200 mg/capsule) as freeze-dried extract capsules for 4 months | Effective in reducing the absolute risk of recurrent colds and the mean number of colds per person. | [101] |
| University of Alberta/Capital Health, Canada NCT00255307 |
Upper respiratory tract Infection (URTI) | (3 - 12 Years) N = 75 |
COLD-fX, | Oral aqueous solution for 6 months, adjusted to child weight | Well tolerated and appropriate for short-term use in children for URTI treatment | [102] |
| China (ACTRN: 12613000382774). | COPD | (57-73 years) N = 14 |
Panax ginseng (Ren shen) | 200 mg twice daily for four weeks | COPD exacerbations or adverse events | [104] |
| Edmonton, Alberta, Canada NCT00965822 | Upper Respiratory Tract Infection | (3-11 Years) N = 293 |
COLD-fX | 3 day, once daily, dosing by oral route for 14 days | No results | |
| Edmonton, Alberta, Canada NCT00726401 |
Seasonal Allergic Rhinitis (hay fever) | (12 - 75 years) N = 200 |
COLD-fX | 200 mg twice daily for 4 weeks | No results | |
| China, Hong Kong, Two General Outpatient Clinics | Acute Upper Respiratory Tract Infection | (≥18 Years), N = 327 | CHM 1. Wind-cold syndrome received treatment of Jing Fang Bai Du san 2. Wind-heat syndrome received treatment of Ying Qiao san |
Sachets granules, Oral route at day 7 | Jing Fang Bai Du san relieved and effectively cleared up the pathogenic cold. Ying Qiao san effectively cleared up the pathogenic heat. | |
| Wake Forest University, USA NCT00752895 |
ARI + chronic lymphocytic leukemia (CLL) | (≥18 years) N = 293 | COLD-fX | Oral extract for 3 months twice a day | Profound reduction in rates of moderate-severe ARI and sore throat, suggesting increased rates of seroconversion. Enhanced antibody responses | [109] |
| Italy | Influenza | 48 years, Mean 58% males, N = 227 | Standardized extract of ginseng root Ginsana G 115 (114) | Oral daily capsule contains 100 mg for 12 weeks | NK increased two fold in the G115 group and is able to protect against common cold and influenza. | [110] |
| Republic of Korea NCT01478009 |
Acute Respiratory Illness (ARI) | (30-70 years) N = 100 |
Korean Red Ginseng Extract | 3 times/day, 9 capsules/day, (3g/day) for 12 weeks | Effective in reducing duration and scores of ARI symptoms. | [111] |
| Republic of Korea NCT03028077 | Acute Respiratory infection | (39-65 years) N = 45 |
1. GS-3K8 (ultra-filtered red ginseng extract) 2. GINst15 (hydrolyzed ginseng extract) |
6 cap/day, 500 mg/cap for 8 weeks | Reduced the symptoms and duration of ARI. | [112] |
ARI, acute respiratory illness; CHM, Chinese herbal medicine; COPD, chronic obstructive pulmonary disease; LCCUs, laboratory-confirmed clinical upper respiratory infections; LCII, laboratory-confirmed influenza illness; NCT, clinical trial number; URTI, upper respiratory tract infection; KRGE, Korean Red Ginseng extract; RSV, respiratory syncytial virus
To assess the safety and efficacy margins in relation to preventing common colds, a randomized controlled clinical trial comparing American and Korean ginseng root extracts in healthy adults was conducted. This study conducted by Seida JK et al [99] in 747 patients showed that preventative ingestion of ginseng extracts for 8–16 weeks, significantly reduced the incidence and duration of common colds by 6.2 days when compared with the placebo control. McElhaney JE et al, [100] proposed that oral administration (2 times/day) of COLD-fX, 200 mg as a prophylactic treatment could reduce the incidence rate of acute respiratory illness (ARI) during the influenza season. Data obtained for laboratory-confirmed influenza and RSV infections showed that COLD-fX was safe and effective in preventing ARI symptoms. Ingestion of 2 capsules/day of COLD-fX–rich extract for 4 months in 323 patients between 18–65 years of age reduced the relative risk, duration, and the mean number of colds per person [101].
To document the safety and tolerance of American ginseng root extract, a randomized, phase II clinical trial in 3- to 12-year-old children was conducted. No serious adverse events for the oral consumption of ginsenosides were reported in either animal or in vitro studies when using the Canadian Acute Respiratory Infection Flu Scale [102]. A double-blinded clinical trial with 168 participants was conducted in Melbourne (Australia), wherein they administered P. ginseng capsules (100 mg) twice a day for 24 weeks [Clinical Trials Register (ANZCTR): ACTRN12610000768099]; it suggested that this treatment was safe and of therapeutic value, resulting in symptomatic relief in patients with COPD [103]. In a double-blind, pilot trial involving nine participants with COPD and 14 control participants (57–73 years old), researchers compared P. ginseng (200 mg twice daily for four weeks) with a placebo in Guangdong Province, China, ANZCTR (ACTRN: 12613000382774), and they found that the ginseng was well tolerated with no adverse events [104].
Gross et al. [105] showed that taking P. ginseng (G115, 100 mg) twice daily for 12 weeks enhanced respiratory endurance and improved pulmonary function in 92 moderate patients with COPD. In a study of 40 chronic bronchitis patients, P. ginseng (100 mg) taken twice daily for 8 weeks significantly decreased the number of alveolar macrophages in BAL fluid when compared with the placebo control [106]. When researchers evaluated P. ginseng (G115, 100 mg for 9 days) in combination with antibiotics in 75 patients with chronic bronchitis, they were able to show a reduced bacterial count compared with antibiotics alone [107]. In addition, P. ginseng treatment did not show any adverse effects [108]. Although well tolerated, these studies did not evaluate and measure appropriate COPD outcomes to evaluate ginseng's effect on the maintenance of health-related quality of life indicators [103].
A randomized, double-blind, trial comprising 293 participants showed that COLD-fX, although well-tolerated, yet did not effectively reduce the length of ARI or antibiotic use in patients with ARI infected with chronic lymphocytic leukemia [109]. Antibody titers and natural killer cell activities in 227 volunteers were significantly elevated in individuals who received P. ginseng standardized extract (G115, 100 mg) once a day via the oral route for 12 weeks when compared with the placebo group [110]. KRG effectively suppressed the duration and severity of ARI symptoms in 100 participants [111]. A placebo-controlled, pilot study divided 45 participants randomly and administered a capsule (500 mg/cap and 3000 mg/day) of GS-3K8, GINST, or placebo. GS-3K8 and GINST prevented the development of ARI with no adverse drug reactions during the intervention and reduced symptom duration, with no drug intolerance [112].
Taken together, in vivo and in vitro studies summarized in Table 1, Table 2, Table 3 and Fig. 1 reveal that ginseng and/or its related components exert immunomodulatory effects that reduce the level of proinflammatory cytokines and oxidative stress and, thus, alleviate the symptoms and reduce the incidence rate and frequency of RTIss.
Table 2.
Effect of ginseng on bacterial infections of the respiratory tract
| Extract | Pathogen | Dosage | Animal model | Anti-microbial effect with suggested mechanism | Ref |
|---|---|---|---|---|---|
| P. ginseng | P. aeruginosa PAO 579 | 25 mg/kg of body weight sc/day for 14 days | Female Lewis rats | Increased PMN and chemiluminescence, activated endotoxin-primed neutrophils. Faster bacterial clearance induced Th1 response and better phagocytosis. | [87] |
| P. quinquefolius | Nonmucoid P. aeruginosa strain (PAO1) | 1.25 g, 2.5 g and 5% | Inhibited bacterial growth and biofilm complexes and modulated the motility, adherence, and production of virulence factors. | [88] | |
| P. ginseng | P. aeruginosa, planktonic | 1.25, 2.5, and 5% | Upregulated extracellular protein and alginate production. Suppressed production of LasA and LasB and downregulated synthesis of AHL molecules. Bacterial clearance in vivo is linked to negative effects on the QS system | [89] | |
| P. ginseng | P. aeruginosa PAO1 strain | 0.25 g and 0.5% oral | Female Balb/c mice | Increased bacterial motility and phagocytosis rates, inhibited biofilm formation, induced dispersion and dissolution of mature biofilms, recovered polymeric matrices | [90] |
| P. ginseng | P. aeruginosa | 250 mg/kg for 7 days SC | Female CBA/J mice | Increased IFN-γ and TNF-α, but decreased IL-4. Activated phagocytes and NK cells to clear the bacterial infection and downregulate the antibody response | [91] |
| P. ginseng | S. pneumoniae | 25, 50, or 100 mg/kg for 15 days | Male ICR mice | Serotype-independent attenuation of high morbidity, alleviated bacterial burden in the blood, lungs, and spleen. Increased survival rates and diminished inflammatory cytokine production. | [93] |
AHL, acylated homoserine lactone; NK, natural killer; PMN, polymorphonuclear neutrophil; QS, quorum sensing; SC, subcutaneous; BAL, bronchoalveolar lavage; Th1, T-helper type 1
5. Concluding remarks
Although a plethora of articles demonstrate that ginseng is devoid of adverse events and is well tolerated and effective as a seasonal prophylaxis for preventing respiratory infections and reducing their duration irrespective of age, it is still necessary to evaluate and understand its molecular mechanisms. Studies should focus on the application of various molecular biology techniques, including pathway analysis and proteomics techniques, to identify the primary cellular targets that trigger the anti-inflammatory and antioxidative stress effects characteristic of ginseng that are responsible for the alleviation of the symptoms of RTIs. This would definitely help in determining factors that may result in adverse events due to frequent or prolonged ingestion of standardized ginseng extracts.
6. Future perspectives
Air pollution continues to be a leading risk factor and a major public health concern with a worldwide mortality rate each year, affecting nine of ten individuals living in urban areas. The majority of epidemiological studies linked to detrimental effect of air pollution rely on hospital-acquired data and mortality rates as health outcomes presenting serious morbidity are the ultimate consequence of severe air pollution. Respiratory diseases require significant medical intervention in the primary health-care setting, irrespective of gender and age. In addition to the reported immunomodulatory behavior, adverse events related to ginseng and drug interactions should also be taken into consideration when applying it as an adjunct to therapy. Future studies should include detailed investigations of ginseng and its related components in terms of pharmacokinetics and toxicity, mechanisms of action, specificity, and therapeutic efficacy in in vitro, animal, and human models to establish the critical parameters for their application in a clinical setting.
Funding sources
This work was supported by the National Research Foundation grant NRF-2018R1A2A1A05078102. The funding body did not play any role in the manuscript preparation, data collection and analysis, and decision to publish.
Conflicts of interest
The authors have no conflict of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2019.12.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Heroux M.E., Anderson H.R., Atkinson R., Brunekreef B., Cohen A., Forastiere F., Hurley F., Katsouyanni K., Krewski D., Krzyzanowski M. Quantifying the health impacts of ambient air pollutants: recommendations of a WHO/Europe project. Int J Public Health. 2015;60(5):619–627. doi: 10.1007/s00038-015-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martino D., Prescott S. Epigenetics and prenatal influences on asthma and allergic airways disease. Chest. 2011;139(3):640–647. doi: 10.1378/chest.10-1800. [DOI] [PubMed] [Google Scholar]
- 3.Schwela D. Air pollution and health in urban areas. Rev Environ Health. 2000;15(1–2):13–42. doi: 10.1515/reveh.2000.15.1-2.13. [DOI] [PubMed] [Google Scholar]
- 4.Kunzli N., Kaiser R., Medina S., Studnicka M., Chanel O., Filliger P., Herry M., Horak F., Jr., Puybonnieux-Texier V., Quenel P. Public-health impact of outdoor and traffic-related air pollution: a European assessment. Lancet. 2000;356(9232):795–801. doi: 10.1016/S0140-6736(00)02653-2. [DOI] [PubMed] [Google Scholar]
- 5.WHO air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide: global update 2005: summary of risk assessment. World Health Organization; Geneva: 2006. [PubMed] [Google Scholar]
- 6.Liu C., Chen R., Sera F., Vicedo-Cabrera A.M., Guo Y., Tong S., Coelho M.S.Z.S., Saldiva P.H.N., Lavigne E., Matus P. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med. 2019;381(8):705–715. doi: 10.1056/NEJMoa1817364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brugha R., Grigg J. Urban air pollution and respiratory infections. Paediatr Respir Rev. 2014;15(2):194–199. doi: 10.1016/j.prrv.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Heikkinen T., Jarvinen A. The common cold. Lancet. 2003;361(9351):51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nichol K.L. Improving influenza vaccination rates among adults. Cleve Clin J Med. 2006;73(11):1009–1015. doi: 10.3949/ccjm.73.11.1009. [DOI] [PubMed] [Google Scholar]
- 10.Chapman K.R., Mannino D.M., Soriano J.B., Vermeire P.A., Buist A.S., Thun M.J., Connell C., Jemal A., Lee T.A., Miravitlles M. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(1):188–207. doi: 10.1183/09031936.06.00024505. [DOI] [PubMed] [Google Scholar]
- 11.Murray C.J., Lopez A.D. Alternative projections of mortality and disability by cause 1990-2020: global burden of disease study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 12.Halbert R.J., Natoli J.L., Gano A., Badamgarav E., Buist A.S., Mannino D.M. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 13.Price D., Freeman D., Cleland J., Kaplan A., Cerasoli F. Earlier diagnosis and earlier treatment of COPD in primary care. Prim Care Respir J. 2011;20(1):15–22. doi: 10.4104/pcrj.2010.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organisation Burden of COPD. 2014. http://www.who.int/respiratory/copd/burden/en/ Available from:
- 15.Abramson M., Crockett A.J., Frith P.A., Glasgow N., Jenkins S., McDonald C.F., McKenzie D.K., Wood-Baker R. 2009. The COPDX plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease 2009.https://pdfs.semanticscholar.org/f4d3/23c5e688345b42916dc4b279fd2d6a598e9b.pdf [DOI] [PubMed] [Google Scholar]
- 16.Holgate S.T., Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8(3):218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 17.Lynch J.P., Zhanel G.G. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med. 2009;30(2):189–209. doi: 10.1055/s-0029-1202938. [DOI] [PubMed] [Google Scholar]
- 18.Fonkwo P.N. Pricing infectious disease. The economic and health implications of infectious diseases. EMBO Rep. 2008;9(Suppl 1):S13–S17. doi: 10.1038/embor.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hament J.M., Kimpen J.L., Fleer A., Wolfs T.F. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunol Med Microbiol. 1999;26(3–4):189–195. doi: 10.1111/j.1574-695X.1999.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 20.Kohanski M.A., Dwyer D.J., Collins J.J. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol. 2010;8(6):423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iroh Tam P.Y., Bernstein E., Ma X., Ferrieri P. Blood culture in evaluation of pediatric community-acquired pneumonia: a Systematic Review and Meta-analysis. Hosp Pediatr. 2015;5(6):324–336. doi: 10.1542/hpeds.2014-0138. [DOI] [PubMed] [Google Scholar]
- 22.Martin N.G., Sadarangani M., Pollard A.J., Goldacre M.J. Hospital admission rates for meningitis and septicaemia caused by Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae in children in England over five decades: a population-based observational study. Lancet Infect Dis. 2014;14(5):397–405. doi: 10.1016/S1473-3099(14)70027-1. [DOI] [PubMed] [Google Scholar]
- 23.Warhurst G., Dunn G., Chadwick P., Blackwood B., McAuley D., Perkins G.D., McMullan R., Gates S., Bentley A., Young D. Rapid detection of health-care-associated bloodstream infection in critical care using multipathogen real-time polymerase chain reaction technology: a diagnostic accuracy study and systematic review. Health Technol Assess. 2015;19(35):1–142. doi: 10.3310/hta19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandell L.A., Wunderink R.G., Anzueto A., Bartlett J.G., Campbell G.D., Dean N.C., Dowell S.F., File T.M., Jr., Musher D.M., Niederman M.S. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyashita N., Saito A., Kohno S., Yamaguchi K., Watanabe A., Oda H., Kazuyama Y., Matsushima T., CAP Study Group Multiplex PCR for the simultaneous detection of Chlamydia pneumoniae, Mycoplasma pneumoniae and Legionella pneumophila in community-acquired pneumonia. Respir Med. 2004;98(6):542–550. doi: 10.1016/j.rmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377(9773):1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu C., Wang C.Z., Zhou C.J., Wang B., Han L., Zhang C.F., Wu X.H., Yuan C.S. Adulteration and cultivation region identification of American ginseng using HPLC coupled with multivariate analysis. J Pharm Biomed Anal. 2014;99:8–15. doi: 10.1016/j.jpba.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L., Yu Q.T., Ge Y.Z., Zhang W.S., Fan Y., Ma C.W., Liu Q., Qi L.W. Distinct urine metabolome after Asian ginseng and American ginseng intervention based on GC-MS metabolomics approach. Sci Rep. 2016;6:39045. doi: 10.1038/srep39045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee O.R., Nguyen N.Q., Lee K.H., Kim Y.C., Seo J. Cytohistological study of the leaf structures of Panax ginseng Meyer and Panax quinquefolius L. J Ginseng Res. 2017;41(4):463–468. doi: 10.1016/j.jgr.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan Y., Wang X., Sun G., Li F., Gong X. Application of RAD sequencing for evaluating the genetic diversity of domesticated Panax notoginseng (Araliaceae) PLoS ONE. 2016;11(11) doi: 10.1371/journal.pone.0166419. e0166419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borchers A.T., Keen C.L., Stern J.S., Gershwin M.E. Inflammation and Native American medicine: the role of botanicals. Am J Clin Nutr. 2000;72(2):339–347. doi: 10.1093/ajcn/72.2.339. [DOI] [PubMed] [Google Scholar]
- 32.Shergis J.L., Di Y.M., Zhang A.L., Vlahos R., Helliwell R., Ye J.M., Xue C.C. Therapeutic potential of Panax ginseng and ginsenosides in the treatment of chronic obstructive pulmonary disease. Complement Ther Med. 2014;22(5):944–953. doi: 10.1016/j.ctim.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Cui L., Wu S.Q., Zhao C.A., Yin C.R. Microbial conversion of major ginsenosides in ginseng total saponins by Platycodon grandiflorum endophytes. J Ginseng Res. 2016;40(4):366–374. doi: 10.1016/j.jgr.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen N.H., Nguyen C.T. Pharmacological effects of ginseng on infectious diseases. Inflammopharmacology. 2019 doi: 10.1007/s10787-019-00630-4. [DOI] [PubMed] [Google Scholar]
- 35.Park J.D., Rhee D.K., Lee Y.H. Biological activities and chemistry of saponins from Panax ginseng C. A. Meyer., Phytochemistry Reviews. 2005;4:159–175. [Google Scholar]
- 36.Scaglione F., Ferrara F., Dugnani S., Falchi M., Santoro G., Fraschini F. Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. Drugs Exp Clin Res. 1990;16(10):537–542. [PubMed] [Google Scholar]
- 37.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58(11):1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 38.Choi K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29(9):1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 39.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9(3):259–274. [PubMed] [Google Scholar]
- 40.Kim W.Y., Kim J.M., Han S.B., Lee S.K., Kim N.D., Park M.K., Kim C.K., Park J.H. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 2000;63(12):1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- 41.Yuan C.S., Wang C.Z., Wicks S.M., Qi L.W. Chemical and pharmacological studies of saponins with a focus on American ginseng. J Ginseng Res. 2010;34(3):160–167. doi: 10.5142/jgr.2010.34.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song X., Hu S. Adjuvant activities of saponins from traditional Chinese medicinal herbs. Vaccine. 2009;27(36):4883–4890. doi: 10.1016/j.vaccine.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 43.Rivera E., Daggfeldt A., Hu S. Ginseng extract in aluminium hydroxide adjuvanted vaccines improves the antibody response of pigs to porcine parvovirus and Erysipelothrix rhusiopathiae. Vet Immunol Immunopathol. 2003;91(1):19–27. doi: 10.1016/s0165-2427(02)00269-6. [DOI] [PubMed] [Google Scholar]
- 44.Neumann G., Chen H., Gao G.F., Shu Y., Kawaoka Y. H5N1 influenza viruses: outbreaks and biological properties. Cell Res. 2010;20(1):51–61. doi: 10.1038/cr.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization . World Health Organization; 2016. Fact sheets 211 influenza (seasonal) [Internet] Geneva.http://www.who.int/mediacentre/factsheets/fs211/en Available from: [Google Scholar]
- 46.Farooqui A., Lei Y., Wang P., Huang J., Lin J., Li G., Leon A.J., Zhao Z., Kelvin D.J. Genetic and clinical assessment of 2009 pandemic influenza in southern China. J Infect Dev Ctries. 2011;5(10):700–710. doi: 10.3855/jidc.2251. [DOI] [PubMed] [Google Scholar]
- 47.Dong W., Farooqui A., Leon A.J., Kelvin D.J. Inhibition of influenza A virus infection by ginsenosides. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171936. e0171936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y., Jung Y.J., Kim K.H., Kwon Y., Kim Y.J., Zhang Z., Kang H.S., Wang B.Z., Quan F.S., Kang S.M. Antiviral activity of fermented ginseng Extracts against a broad range of influenza viruses. Viruses. 2018;10(9) doi: 10.3390/v10090471. E471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan L.Y., Kwok H.H., Chan R.W., Peiris M.J., Mak N.K., Wong R.N., Chan M.C., Yue P.Y. Dual functions of ginsenosides in protecting human endothelial cells against influenza H9N2-induced inflammation and apoptosis. J Ethnopharmacol. 2011;137(3):1542–1546. doi: 10.1016/j.jep.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 50.Yin S.Y., Kim H.J., Kim H.J. A comparative study of the effects of whole red ginseng extract and polysaccharide and saponin fractions on influenza A (H1N1) virus infection. Biol Pharm Bull. 2013;36(6):1002–1007. doi: 10.1248/bpb.b13-00123. [DOI] [PubMed] [Google Scholar]
- 51.Quan F.S., Compans R.W., Cho Y.K., Kang S.M. Ginseng and Salviae herbs play a role as immune activators and modulate immune responses during influenza virus infection. Vaccine. 2007;25(2):272–282. doi: 10.1016/j.vaccine.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 52.Wu H., Hoiby N., Yang L., Givskov M., Song Z. Effects of radix ginseng on microbial infections: a narrative review. J Tradit Chin Med. 2014;34(2):227–233. doi: 10.1016/s0254-6272(14)60083-2. [DOI] [PubMed] [Google Scholar]
- 53.Song X., Chen J., Sakwiwatkul K., Li R., Hu S. Enhancement of immune responses to influenza vaccine (H3N2) by ginsenoside Re. Int Immunopharmacol. 2010;10(3):351–356. doi: 10.1016/j.intimp.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Ritchie A.I., Farne H.A., Singanayagam A., Jackson D.J., Mallia P., Johnston S.L. Pathogenesis of viral infection in exacerbations of airway disease. Ann Am Thorac Soc. 2015;12(Suppl 2):S115–S132. doi: 10.1513/AnnalsATS.201503-151AW. [DOI] [PubMed] [Google Scholar]
- 55.Cox J.C., Coulter A.R. Adjuvants--a classification and review of their modes of action. Vaccine. 1997;15(3):248–256. doi: 10.1016/s0264-410x(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 56.Xu M.L., Kim H.J., Choi Y.R., Kim H.J. Intake of Korean red ginseng extract and saponin enhances the protection conferred by vaccination with inactivated influenza a virus. J Ginseng Res. 2012;36(4):396–402. doi: 10.5142/jgr.2012.36.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwarze J., Mackenzie K.J. Novel insights into immune and inflammatory responses to respiratory viruses. Thorax. 2013;68(1):108–110. doi: 10.1136/thoraxjnl-2012-202291. [DOI] [PubMed] [Google Scholar]
- 58.Yoo D.G., Kim M.C., Park M.K., Song J.M., Quan F.S., Park K.M., Cho Y.K., Kang S.M. Protective effect of Korean red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice. J Med Food. 2012;15(10):855–862. doi: 10.1089/jmf.2012.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park E.H., Yum J., Ku K.B., Kim H.M., Kang Y.M., Kim J.C., Kim J.A., Kang Y.K., Seo S.H. Red Ginseng-containing diet helps to protect mice and ferrets from the lethal infection by highly pathogenic H5N1 influenza virus. J Ginseng Res. 2014;38(1):40–46. doi: 10.1016/j.jgr.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoo D.G., Kim M.C., Park M.K., Park K.M., Quan F.S., Song J.M., Wee J.J., Wang B.Z., Cho Y.K., Compans R.W. Protective effect of ginseng polysaccharides on influenza viral infection. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033678. e33678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhai L., Li Y., Wang W., Hu S. Enhancement of humoral immune responses to inactivated Newcastle disease and avian influenza vaccines by oral administration of ginseng stem-and-leaf saponins in chickens. Poult Sci. 2011;90(9):1955–1959. doi: 10.3382/ps.2011-01433. [DOI] [PubMed] [Google Scholar]
- 62.Zhai L., Li Y., Wang W., Wang Y., Hu S. Effect of oral administration of ginseng stem-and-leaf saponins (GSLS) on the immune responses to Newcastle disease vaccine in chickens. Vaccine. 2011;29(31):5007–5014. doi: 10.1016/j.vaccine.2011.04.097. [DOI] [PubMed] [Google Scholar]
- 63.Yu J., Shi F.S., Hu S. Improved immune responses to a bivalent vaccine of Newcastle disease and avian influenza in chickens by ginseng stem-leaf saponins. Vet Immunol Immunopathol. 2015;167(3–4):147–155. doi: 10.1016/j.vetimm.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 64.Graham B.S. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev. 2011;239(1):149–166. doi: 10.1111/j.1600-065X.2010.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murphy B.R., Prince G.A., Collins P.L., Van Wyke Coelingh K., Olmsted R.A., Spriggs M.K., Parrott R.H., Kim H.W., Brandt C.D., Chanock R.M. Current approaches to the development of vaccines effective against parainfluenza and respiratory syncytial viruses. Virus Res. 1988;11(1):1–15. doi: 10.1016/0168-1702(88)90063-9. [DOI] [PubMed] [Google Scholar]
- 66.Kim H.W., Canchola J.G., Brandt C.D., Pyles G., Chanock R.M., Jensen K., Parrott R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 67.Munoz F.M. Respiratory syncytial virus in infants: is maternal vaccination a realistic strategy? Curr Opin Infect Dis. 2015;28(3):221–224. doi: 10.1097/QCO.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 68.Legg J.P., Hussain I.R., Warner J.A., Johnston S.L., Warner J.O. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2003;168(6):633–639. doi: 10.1164/rccm.200210-1148OC. [DOI] [PubMed] [Google Scholar]
- 69.Garofalo R.P., Kolli D., Casola A. Respiratory syncytial virus infection: mechanisms of redox control and novel therapeutic opportunities. Antioxid Redox Signal. 2013;18(2):186–217. doi: 10.1089/ars.2011.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y., Luxon B.A., Casola A., Garofalo R.P., Jamaluddin M., Brasier A.R. Expression of respiratory syncytial virus-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. J Virol. 2001;75(19):9044–9058. doi: 10.1128/JVI.75.19.9044-9058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blanco J.C., Richardson J.Y., Darnell M.E., Rowzee A., Pletneva L., Porter D.D., Prince G.A. Cytokine and chemokine gene expression after primary and secondary respiratory syncytial virus infection in cotton rats. J Infect Dis. 2002;185(12):1780–1785. doi: 10.1086/340823. [DOI] [PubMed] [Google Scholar]
- 72.Lee J.S., Hwang H.S., Ko E.J., Lee Y.N., Kwon Y.M., Kim M.C., Kang S.M. Immunomodulatory activity of red ginseng against influenza A virus infection. Nutrients. 2014;6(2):517–529. doi: 10.3390/nu6020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee J.S., Cho M.K., Hwang H.S., Ko E.J., Lee Y.N., Kwon Y.M., Kim M.C., Kim K.H., Lee Y.T., Jung Y.J. Ginseng diminishes lung disease in mice immunized with formalin-inactivated respiratory syncytial virus after challenge by modulating host immune responses. J Interferon Cytokine Res. 2014;34(11):902–914. doi: 10.1089/jir.2013.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Becker S., Soukup J., Yankaskas J.R. Respiratory syncytial virus infection of human primary nasal and bronchial epithelial cell cultures and bronchoalveolar macrophages. Am J Respir Cell Mol Biol. 1992;6(4):369–374. doi: 10.1165/ajrcmb/6.4.369. [DOI] [PubMed] [Google Scholar]
- 75.Lee J.S., Lee Y.N., Lee Y.T., Hwang H.S., Kim K.H., Ko E.J., Kim M.C., Kang S.M. Ginseng protects against respiratory syncytial virus by modulating multiple immune cells and inhibiting viral replication. Nutrients. 2015;7(2):1021–1036. doi: 10.3390/nu7021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee J.S., Ko E.J., Hwang H.S., Lee Y.N., Kwon Y.M., Kim M.C., Kang S.M. Antiviral activity of ginseng extract against respiratory syncytial virus infection. Int J Mol Med. 2014;34(1):183–190. doi: 10.3892/ijmm.2014.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Q., Kou J.P., Yu B.Y. Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-κB activation. Neurochem Int. 2011;58(1):119–125. doi: 10.1016/j.neuint.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 78.Jung I.D., Kim H.Y., Park J.W., Lee C.M., Noh K.T., Kang H.K., Heo D.R., Lee S.J., Son K.H., Park H.J. RG-II from Panax ginseng C.A. Meyer suppresses asthmatic reaction. BMB Rep. 2012;45(2):79–84. doi: 10.5483/BMBRep.2012.45.2.79. [DOI] [PubMed] [Google Scholar]
- 79.Seemungal T., Harper-Owen R., Bhowmik A., Moric I., Sanderson G., Message S., Maccallum P., Meade T.W., Jeffries D.J., Johnston S.L. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 80.Jarjour N.N., Gern J.E., Kelly E.A., Swenson C.A., Dick C.R., Busse W.W. The effect of an experimental rhinovirus 16 infection on bronchial lavage neutrophils. J Allergy Clin Immunol. 2000;105(6 Pt 1):1169–1177. doi: 10.1067/mai.2000.106376. [DOI] [PubMed] [Google Scholar]
- 81.Makris S., Johnston S. vol. 7. 2018. Recent advances in understanding rhinovirus immunity. F1000Res. F1000 Faculty Rev-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu J., Message S.D., Qiu Y., Mallia P., Kebadze T., Contoli M., Ward C.K., Barnathan E.S., Mascelli M.A., Kon O.M. Airway inflammation and illness severity in response to experimental rhinovirus infection in asthma. Chest. 2014;145(6):1219–1229. doi: 10.1378/chest.13-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song J.H., Choi H.J., Song H.H., Hong E.H., Lee B.R., Oh S.R., Choi K., Yeo S.G., Lee Y.P., Cho S. Antiviral activity of ginsenosides against coxsackievirus B3, enterovirus 71, and human rhinovirus 3. J Ginseng Res. 2014;38(3):173–179. doi: 10.1016/j.jgr.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bucior I., Pielage J.F., Engel J.N. Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathog. 2012;8(4) doi: 10.1371/journal.ppat.1002616. e1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Curran C.S., Bolig T., Torabi-Parizi P. Mechanisms and targeted therapies for Pseudomonas aeruginosa lung infection. Am J Respir Crit Care Med. 2018;197(6):708–727. doi: 10.1164/rccm.201705-1043SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35(4):322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 87.Song Z., Kharazmi A., Wu H., Faber V., Moser C., Krogh H.K., Rygaard J., Hoiby N. Effects of ginseng treatment on neutrophil chemiluminescence and immunoglobulin G subclasses in a rat model of chronic Pseudomonas aeruginosa pneumonia. Clin Diagn Lab Immunol. 1998;5(6):882–887. doi: 10.1128/cdli.5.6.882-887.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alipour M., Omri A., Suntres Z.E. Ginseng aqueous extract attenuates the production of virulence factors, stimulates twitching and adhesion, and eradicates biofilms of Pseudomonas aeruginosa. Can J Physiol Pharmacol. 2011;89(6):419–427. doi: 10.1139/y11-057. [DOI] [PubMed] [Google Scholar]
- 89.Song Z., Kong K.F., Wu H., Maricic N., Ramalingam B., Priestap H., Schneper L., Quirke J.M., Hoiby N., Mathee K. Panax ginseng has anti-infective activity against opportunistic pathogen Pseudomonas aeruginosa by inhibiting quorum sensing, a bacterial communication process critical for establishing infection. Phytomedicine. 2010;17(13):1040–1046. doi: 10.1016/j.phymed.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu H., Lee B., Yang L., Wang H., Givskov M., Molin S., Høiby N., Song Z. Effects of ginseng on Pseudomonas aeruginosa motility and biofilm formation. FEMS Immunol Med Microbiol. 2011;62(1):49–56. doi: 10.1111/j.1574-695X.2011.00787.x. [DOI] [PubMed] [Google Scholar]
- 91.Song Z., Moser C., Wu H., Faber V., Kharazmi A., Hoiby N. Cytokine modulating effect of ginseng treatment in a mouse model of Pseudomonas aeruginosa lung infection. J Cyst Fibros. 2003;2(3):112–119. doi: 10.1016/S1569-1993(03)00065-1. [DOI] [PubMed] [Google Scholar]
- 92.Mook-Kanamori B.B., Geldhoff M., van der Poll T., van de Beek D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev. 2011;24(3):557–591. doi: 10.1128/CMR.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nguyen C.T., Luong T.T., Lee S.Y., Kim G.L., Kwon H., Lee H.G., Park C.K., Rhee D.K. Panax ginseng aqueous extract prevents pneumococcal sepsis in vivo by potentiating cell survival and diminishing inflammation. Phytomedicine. 2015;22(11):1055–1061. doi: 10.1016/j.phymed.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 94.Wang M., Guilbert L.J., Ling L., Li J., Wu Y., Xu S., Pang P., Shan J.J. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolium) J Pharm Pharmacol. 2001;53(11):1515–1523. doi: 10.1211/0022357011777882. [DOI] [PubMed] [Google Scholar]
- 95.Wang M., Guilbert L.J., Li J., Wu Y., Pang P., Basu T.K., Shan J.J. A proprietary extract from North American ginseng (Panax quinquefolium) enhances IL-2 and IFN-gamma productions in murine spleen cells induced by Con-A. Int Immunopharmacol. 2004;4(2):311–315. doi: 10.1016/j.intimp.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 96.Ha K.C., Kim M.G., Oh M.R., Choi E.K., Back H.I., Kim S.Y., Park E.O., Kwon D.Y., Yang H.J., Kim M.J. A placebo-controlled trial of Korean red ginseng extract for preventing influenza-like illness in healthy adults. BMC Complement Altern Med. 2012;12:10. doi: 10.1186/1472-6882-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McElhaney J.E., Goel V., Toane B., Hooten J., Shan J.J. Efficacy of COLD-fX in the prevention of respiratory symptoms in community-dwelling adults: a randomized, double-blinded, placebo controlled trial. J Altern Complement Med. 2006;12(2):153–157. doi: 10.1089/acm.2006.12.153. [DOI] [PubMed] [Google Scholar]
- 98.McElhaney J.E., Simor A.E., McNeil S., Predy G.N. Efficacy and safety of CVT-E002, a proprietary extract of Panax quinquefolius in the prevention of respiratory infections in influenza-vaccinated community-dwelling adults: a multicenter, randomized, double-blind, and placebo-controlled trial. Influenza Res Treat. 2011;2011:759051. doi: 10.1155/2011/759051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seida J.K., Durec T., Kuhle S. North American (Panax quinquefolius) and Asian ginseng (Panax ginseng) preparations for prevention of the common cold in healthy adults: a systematic review. Evid Based Complement Alternat Med. 2011;2011:282151. doi: 10.1093/ecam/nep068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McElhaney J.E., Gravenstein S., Cole S.K., Davidson E., O'neill D., Petitjean S., Rumble B., Shan J.J. A placebo-controlled trial of a proprietary extract of North American ginseng (CVT-E002) to prevent acute respiratory illness in institutionalized older adults. J Am Geriatr Soc. 2004;52(1):13–19. doi: 10.1111/j.1532-5415.2004.52004.x. [DOI] [PubMed] [Google Scholar]
- 101.Predy G.N., Goel V., Lovlin R., Donner A., Stitt L., Basu T.K. Efficacy of an extract of North American ginseng containing poly-furanosyl-pyranosyl-saccharides for preventing upper respiratory tract infections: a randomized controlled trial. CMAJ. 2005;173(9):1043–1048. doi: 10.1503/cmaj.1041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vohra S., Johnston B.C., Laycock K.L., Midodzi W.K., Dhunnoo I., Harris E., Baydala L. Safety and tolerability of North American ginseng extract in the treatment of pediatric upper respiratory tract infection: a phase II randomized, controlled trial of 2 dosing schedules. Pediatrics. 2008;122(2):e402–e410. doi: 10.1542/peds.2007-2186. [DOI] [PubMed] [Google Scholar]
- 103.Xue C.C., Shergis J.L., Zhang A.L., Worsnop C., Fong H., Story D., Da Costa C., Thien F.C. Panax ginseng C.A Meyer root extract for moderate chronic obstructive pulmonary disease (COPD): study protocol for a randomised controlled trial. Trials. 2011;12:164. doi: 10.1186/1745-6215-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu L., Zhang A.L., Di Y.M., Shergis J.L., Chen Y., Guo X., Wen Z., Thien F., Worsnop C., Lin L. Panax ginseng therapy for chronic obstructive pulmonary disease: a clinical trial protocol and pilot study. Chin Med. 2014;9:20. doi: 10.1186/1749-8546-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gross D., Krieger D., Efrat R. Ginseng extract G115® for the treatment of chronic respiratory diseases. Scweiz Z Ganzheits Med. 1995;1:29–33. [Google Scholar]
- 106.Scaglione F., Weiser K., Alessandria M. Effects of the standardised ginseng extract G115® in patients with chronic bronchitis: a nonblinded, randomised, comparative pilot study. Clin Drug Investig. 2001;21:41–45. [Google Scholar]
- 107.Scaglione F., Cogo R., Cocuzza C., Arcidiacono M., Beretta A. Immunomodulatory effects of Panax ginseng C.A. Meyer (G115) on alveolar macrophages from patients suffering with chronic bronchitis. Int J Immunother. 1994;10:21–24. [Google Scholar]
- 108.Coon J.T., Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf. 2002;25(5):323–344. doi: 10.2165/00002018-200225050-00003. [DOI] [PubMed] [Google Scholar]
- 109.High K.P., Case D., Hurd D., Powell B., Lesser G., Falsey A.R., Siegel R., Metzner-Sadurski J., Krauss J.C., Chinnasami B. A randomized, controlled trial of Panax quinquefolius extract (CVT-E002) to reduce respiratory infection in patients with chronic lymphocytic leukemia. J Support Oncol. 2012;10(5):195–201. doi: 10.1016/j.suponc.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scaglione F., Cattaneo G., Alessandria M., Cogo R. Efficacy and safety of the standardised ginseng extract G115 for potentiating vaccination against the influenza syndrome and protection against the common cold [corrected] Drugs Exp Clin Res. 1996;22(2):65–72. [PubMed] [Google Scholar]
- 111.Lee C.S., Lee J.H., Oh M., Choi K.M., Jeong M.R., Park J.D., Kwon D.Y., Ha K.C., Park E.O., Lee N. Preventive effect of Korean red ginseng for acute respiratory illness: a randomized and double-blind clinical trial. J Korean Med Sci. 2012;27(12):1472–1478. doi: 10.3346/jkms.2012.27.12.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hwang J.-H., Park S.-H., Choi E.-K., Jung S.-J., Pyo M.K., Chae S.-W. A randomized, double-blind, placebo-controlled pilot study to assess the effects of protopanaxadiol saponin–enriched ginseng extract and pectinase-processed ginseng extract on the prevention of acute respiratory illness in healthy people. J Ginseng Res. 2019 doi: 10.1016/j.jgr.2019.01.002. https://www.sciencedirect.com/science/article/pii/S1226845318301519 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


