Abstract
Background:
For patients with chronic, non-cancer pain, traditional pain-relieving medications include opioids, which have shown benefits but are associated with increased risks of addiction and adverse effects. Medical cannabis has emerged as a treatment alternative for managing these patients and there has been a rise in the number of randomized clinical trials in recent years; therefore, a systematic review of the evidence was warranted.
Objective:
To analyze the evidence surrounding the benefits and harms of medical cannabinoids in the treatment of chronic, non-cancer-related pain.
Design:
Systematic review with meta-analysis.
Data sources:
Medline, Embase, CINAHL, SCOPUS, Google Scholar, and Cochrane Databases.
Eligibility criteria:
English language randomized clinical trials of cannabinoids for the treatment of chronic, non-cancer-related pain.
Data extraction and synthesis:
Study quality was assessed using the Cochrane risk of bias tool. All stages were conducted independently by a team of 6 reviewers. Data were pooled through meta-analysis with different durations of treatment (2 weeks, 2 months, 6 months) and stratified by route of administration (smoked, oromucosal, oral), conditions, and type of cannabinoids.
Main outcomes and measures:
Patient-reported pain and adverse events (AEs).
Results:
Thirty-six trials (4006 participants) were included, examining smoked cannabis (4 trials), oromucosal cannabis sprays (14 trials), and oral cannabinoids (18 trials). Compared with placebo, cannabinoids showed a significant reduction in pain which was greatest with treatment duration of 2 to 8 weeks (weighted mean difference on a 0-10 pain visual analogue scale −0.68, 95% confidence interval [CI], −0.96 to −0.40, I2 = 8%, P < .00001; n = 16 trials). When stratified by route of administration, pain condition, and type of cannabinoids, oral cannabinoids had a larger reduction in pain compared with placebo relative to oromucosal and smoked formulations but the difference was not significant (P[interaction] > .05 in all the 3 durations of treatment); cannabinoids had a smaller reduction in pain due to multiple sclerosis compared with placebo relative to other neuropathic pain (P[interaction] = .05) within 2 weeks and the difference was not significant relative to pain due to rheumatic arthritis; nabilone had a greater reduction in pain compared with placebo relative to other types of cannabinoids longer than 2 weeks of treatment but the difference was not significant (P[interaction] > .05). Serious AEs were rare, and similar across the cannabinoid (74 out of 2176, 3.4%) and placebo groups (53 out of 1640, 3.2%). There was an increased risk of non-serious AEs with cannabinoids compared with placebo.
Conclusions:
There was moderate evidence to support cannabinoids in treating chronic, non-cancer pain at 2 weeks. Similar results were observed at later time points, but the confidence in effect is low. There is little evidence that cannabinoids increase the risk of experiencing serious AEs, although non-serious AEs may be common in the short-term period following use.
Keywords: Cannabinoids, chronic pain, multiple sclerosis
Introduction
The opioid epidemic is arguably the greatest public health challenge currently facing health providers, policy makers, and most importantly, patients. This epidemic continues to dominate headlines as opioid-related hospitalizations and emergency department visits in Canada have ballooned by more than 50% during the last decade, most of which occurred over the last 3 years.1 Even more staggering is the 500% increase seen in opioid-related deaths across North America over the last year, with more than 50 000 reported fatalities, over a third of which were related to prescription medications.2,3
Acute and chronic musculoskeletal pain remains one of the leading reasons for opioid prescribing in North America. While the initiation of this class of analgesics may often be done to treat severe injuries or intractable pain, the highly addictive potential and narrow toxic range make it a high-risk medication. Canadian and American recommendations that are responsible for opiate prescribing discourage use of opioids for chronic, non-cancer pain.4-6 However, opiates remain the default choice for most orthopedic providers across North America, with deeply ingrained practice patterns leading to routine prescription of opioids following a fracture, surgery, or worsening degenerative bone and joint disease.7-9
On October 17, 2018, Canada became the second country to legalize both recreational and medical use of Cannabis. Surrounding this decision, the spotlight was on the evidence for efficacy and harms associated with use of cannabinoids across an array of medical indications. Chronic musculoskeletal pain may be a key driver of use; 65% of Canadians authorized to possess medicinal cannabis use it for “severe arthritis,” and in 1 US pain clinic up to 80% of cannabis users report myofascial pain as their primary diagnosis.10
Limited clinical evidence supports the use of cannabinoids for chronic, non-cancer-related pain; however studies continue to emerge at a rapid pace. Several systematic reviews have examined the role of cannabinoids across multiple indications. Two reviews presented an overall summary of the evidence using scoping or qualitative methods, and indicated that cannabinoids may be comparable with currently used analgesics11,12 Martin-Sanchez et al13 went on to perform a meta-analysis of 18 trials looking at the use of cannabinoids in chronic pain. They found cannabinoids to be moderately efficacious; however any benefits were offset by reported harms.13 Conversely, Whiting et al14 analyzed 8 trials in patients with chronic pain, and Lynch and Campbell15 analyzed 15 trials in patients with non-cancer pain, each finding it to be safe and effective. Given the contradictory findings among reviews over the last decade, as well as the large number of recently available trials, a thorough updated meta-analysis in this area was urgently required. The aim of this systematic review was therefore to analyze the best evidence surrounding the benefits and harms of medical cannabinoids in the treatment of chronic, non-cancer-related pain.
Method
In accordance with the PRISMA guidelines for reviews of health care interventions,16 we conducted a systematic review on the efficacy and adverse events (AEs) associated with using cannabinoids for the treatment of chronic, non-cancer-related pain.
Literature search
We performed a systematic search of the Medline, Embase, CINAHL, SCOPUS, Google Scholar, and Cochrane databases from inception to December 15, 2018. Structured search strategies were developed using keywords related to “cannabis,” “marijuana,” or “cannabinoids” AND “chronic, non-cancer pain,” using Medical Subject Heading (MeSH) terms wherever possible. We did not restrict our search by date of publication. Database-specific search strategies were developed and an example can be found in e-Table 1. Recently completed or ongoing studies were identified using online trial registries (clinicaltrials.gov, TrialsCentral.org). We further searched the references lists of included studies and previously performed related reviews for additional eligible articles.
Study eligibility
Randomized controlled trials (RCTs) comparing cannabinoids with placebo for patients with chronic, non-cancer pain were eligible for inclusion. We defined chronic pain as persistent or recurrent pain lasting beyond 3 months. If duration of pain was not stated, the article was still considered for inclusion if the study population had an established diagnosis for a chronic condition associated with pain (ie, multiple sclerosis, Parkinson disease, rheumatoid arthritis). Pain outcomes of interest included any validated scale dedicated to measuring pain, such as the visual analogue scale (VAS), numeric rating scale (NRS), neuropathic pain scale (NPS), or McGill pain questionnaire. We excluded (1) non-human or preclinical trials; (2) non-English language trials; (3) trials reporting on acute or cancer-related pain; (4) trials with less than 24-hours follow-up; (5) trials only reported as abstracts or posters, with no available full-text article; (6) pilot trials where patients overlapped with those in a subsequent full trial report; and (7) trials with incomplete pain outcome and AE reporting for analysis. Two authors independently screened search results in duplicate, with disagreements resulting in automatic inclusion at the title and abstract stage, and resolution through discussion with involvement of a third reviewer as necessary at the full-text stage. For full-text articles deemed ineligible, the reason(s) for exclusion were recorded.
Data extraction
Data were abstracted independently by a team of 6 reviewers using the OrthoEvidence (OE) online platform. Data extraction forms were pilot-tested across the reviewers, and all abstracted data were confirmed in duplicate. Any discrepancies were resolved through discussion. We collected study and patient demographic information (author, year of publication, country, funding, study design, length of follow-up, sample size, patient population, condition(s) studied), as well as information regarding each of the treatment arms (type of cannabinoid/control, dose, route of administration). The outcomes of interest were pain and AEs. The type of pain scale used was recorded, as well as the mean score and standard deviation (SD) at baseline, follow-up, and/or change from baseline. Results from the between-group analyses reported by each study (mean difference, 95% confidence intervals [CIs], and P values) were also extracted. Adverse events were recorded by overall incidence for each study, and classified as serious or non-serious if reported, or by applying accepted criteria.17
Study appraisal
Methodological quality for each of the included RCTs was assessed in duplicate by a team of 6 independent reviewers for each outcome using a modified Cochrane Risk of Bias tool18 through the OE online platform. The following items were assessed: sequence generation; allocation sequence concealment; blinding of participants, providers, and outcome assessors; incomplete outcome data (loss to follow-up); selective outcome reporting; and other biases. Risk of bias for each item was determined to be “low risk” (+), “high risk” (−), or “unclear risk” (?). If all domains were judged as low, the trial was considered at low risk of bias. If 2 or more of the domains were rated as high or unclear, the trial was considered at high risk of bias. Otherwise, the trial was considered as having moderate risk of bias. Any discrepancies were resolved through discussion between the reviewers. No studies were excluded from the analysis due to high risk of bias.
Data analysis
For pain, we reported the mean difference in change from baseline, along with 95% CIs. We transformed all scores to the scale of an index instrument, the VAS, which resulted in scores that could range from 0 to 10, where higher scores reflect a worse outcome (more pain). If missing, SDs were calculated from other reported data (standard error, CIs, P values) or imputed using median values across similar study characteristics (intervention, population, follow-up duration). For AE data, we summarized number of patients and calculated proportions of specific AEs. To avoid double counting for studies with more than 2 treatment arms, we divided the control arms by the number of comparator arms for the meta-analysis. Similarly, for crossover studies, the overall sample size was divided by the number of treatment arms. For the analysis of harms, data regarding any Severe AE reported was summarized and pooled for analysis.
Heterogeneity was investigated visually through inspection of the forest plots, and objectively with the I2 statistic (I2 < 40%, low heterogeneity; I2 ⩾ 75%, substantial heterogeneity). We present pooled results of pain reduction with 3 durations of treatment and follow-up (1-14 days, 2-8 weeks, and 2-6 months). We conducted 3 stratified analyses independently, to investigate whether effects varied by route of administration (oral, oromucosal, or inhaled), by patients’ conditions (pain other than multiple sclerosis, multiple sclerosis pain, or rheumatoid arthritis pain), and by types of cannabinoids (Ajulemic acid, tetrahydrocannabinol [THC], cannabidiol [CBD], combination of THC and CBD, nabilone, or dronabinol). All analyses were performed using STATA 14.0 (STATA Corporation, College Station, TX, USA) and Review Manager 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration, 2014, Copenhagen, Denmark).
The Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach was used to evaluate confidence in the pooled effect estimates.19,20 According to GRADE, data from RCTs are considered high-quality evidence but can be rated down due to risk of bias, publication bias, imprecision, inconsistency, indirectness, and magnitude of effect.20 The quality of the evidence was graded as high, moderate, low, or very low, and applied to each outcome of interest separately.21
Results
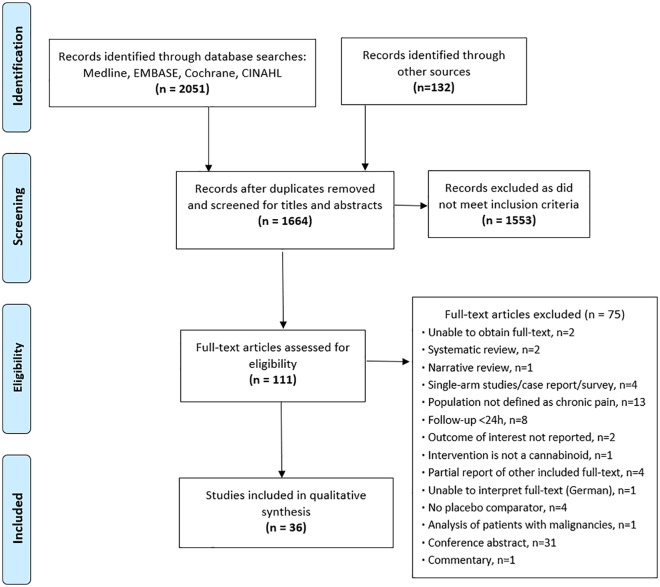
Following the removal of duplicates, we identified 1664 potential eligible studies for review through our database and gray literature searches. Of these, 111 were considered potentially relevant based on title and abstract screening, and full-text articles were reviewed. A total of 36 studies published between 2002 and 2018 (4006 participants) were eligible for final inclusion for both pain and AE outcomes (Figure 1).22-57
Figure 1.
Study flow diagram—Depiction of the number of studies at each stage of the review, and reasons for exclusion for full texts.
Study characteristics
Most of the trials included in the meta-analysis were conducted in the United Kingdom (16 out of 36, 44%),23-27,34,38-41,43,44,50,51,56,57 followed by Canada (7 out of 36, 19%),34,44,45,47,48,52,55 the Czech Republic (5 out of 36, 14%),27,34,37,39,44 the United States (4 out of 36, 11%),22,28,30,36 the Netherlands (3 out of 36, 8%),29,33,49 Austria (2 out of 36, 6%),37,54 Belgium (2 out of 36, 6%),30,44 Spain (2 out of 36, 6%),34,39 Switzerland (2 out of 36, 6%),31,53 Italy (2 out of 36, 6%),35,39 and Germany (2 out of 36, 6%),32,42 with the remaining countries (Denmark,46 France,34 Poland,39 and Romania)44 contributing participants to 1 study each. Of the included studies, 6 trials recruited patients from more than 1 country (range: 2-5 countries).27,34,37,39,40,44
Twenty-two of the included studies were parallel-group trials (3633 participants),22,23,25,27,29,31,34,36,37,39-45,47-49,51,56,57 and 14 were crossover trials (373 participants).24,26,28,30,32,33,35,38,46,50,52-55 All the studies compared a variety of cannabinoid-based interventions with a placebo control (Table 1), with 5 studies having more than 1 cannabinoid treatment arm.24,33,38,50,56 This led to 43 direct comparisons of a cannabinoid with a placebo control across the individual 36 trials, the details of which can be found in e-Table 2.
Table 1.
Summary of interventions (43 cannabinoid arms) by included studies (36 trials).
| Intervention | Route | Dose/Specific Preparation Studied | Indication Studied | # of Studies |
|---|---|---|---|---|
| Cannabis (flower) | Inhaled (smoked) | Cannabis (3.56% THC) | HIV sensory neuropathy | 122 |
| Cannabis (4.0% THC) | Multiple sclerosis (spasticity) | 128 | ||
| Cannabis (1%-8% THC) | HIV sensory neuropathy | 130 | ||
| Cannabis (9.4% THC) | Postsurgical/posttraumatic neuropathic pain | 152 | ||
| Cannabis extract | Oral (capsule) | THC 2.5 mg/capsule, 5-25 mg THC/d | Multiple sclerosis (muscle stiffness, spasticity) | 333,56,57 |
| Parkinson dyskinesia | 126 | |||
| THC/CBD spray | Oromucosal | Nabiximols/THC 27 mg/mL: CBD 25 mg/mL (~0.1 mL/spray) | Neuropathic pain ± allodynia (general; diabetic; secondary to brachial plexus avulsion) | 524,40,43,44,50 |
| Rheumatoid arthritis | 125 | |||
| Multiple sclerosis (spasticity, central neuropathic pain) | 727,34,35,37,39,41,51 | |||
| Chronic pain | 138 | |||
| THC only spray | Oromucosal | THC 27 mg/mL (~0.1 mL/spray) | Neuropathic pain (general; secondary to brachial plexus avulsion) | 224,50 |
| Chronic pain | 138 | |||
| CBD only spray | Oromucosal | CBD 2.5 mg (~0.1 mL/spray) | Neuropathic pain | 150 |
| Chronic pain | 138 | |||
| Synthetic THC(delta-9 THC) | Oral | Dronabinol/marinol (2.5-15 mg/d) | Functional chest pain | 136 |
| Multiple sclerosis (spasticity, central neuropathic pain) | 523,33,42,46,56 | |||
| Amyotropic lateral sclerosis (cramps) | 153 | |||
| Idiopathic cervical dystonia | 155 | |||
| Spinal cord injury | 131 | |||
| Nabilone (0.5-4 mg/d) | Fibromyalgia | 145 | ||
| Neuropathic pain (diabetic) | 147 | |||
| Multiple sclerosis (spasticity, central neuropathic pain) | 248,54 | |||
| Namisol (9-24 mg/d) | Postsurgical/pancreatitis-related abdominal pain | 129 | ||
| Multiple sclerosis (spasticity) | 149 | |||
| Synthetic THC (THC-11) | Oral | Ajulemic acid/CT3 (40-80 mg/d) | Neuropathic pain (with hyperalgesia/allodynia) | 132 |
Abbreviations: CBD, cannabidiol; THC, tetrahydrocannabinol.
Synthetic THC capsules were the most frequently studied cannabinoid intervention (16 trials). Fifteen of these trials examined synthetic ∆9-tetrahydrocannabinol (THC) capsules known as Dronabinol,23,31,33,36,42,46,53,55,56 Nabilone,45,47,48,54 or Namisol,29,49 and a single study assessed ajuvenic acid capsules (THC-11, CT3).32 The next most commonly studied cannabinoids were in the form of oromucosal sprays (14 trials).24,25,27,34,35,37-41,43,44,50,51 The oromucosal sprays typically deliver 0.1 mL per spray, and were either a THC (27 mg/mL): CBD (25 mg/mL) formulation known as nabiximols, a THC only (27 mg/mL), or CBD only (25 mg/mL) spray. Four of the studies evaluated smoked, rolled cannabis (flower), from plants of varying THC potency (1%-9% THC),22,28,30,52 compared with identical placebos where the active THC components had been extracted out. There were also 4 studies that analyzed an herbal cannabis extract oil, containing THC (2.5 mg/mL) and CBD (1.25 mg/mL), which was administered orally as a capsule.26,32,56,57 The medical conditions associated with the chronic pain varied between studies and are summarized in Table 1, along with a breakdown of the interventional arms across all studies.
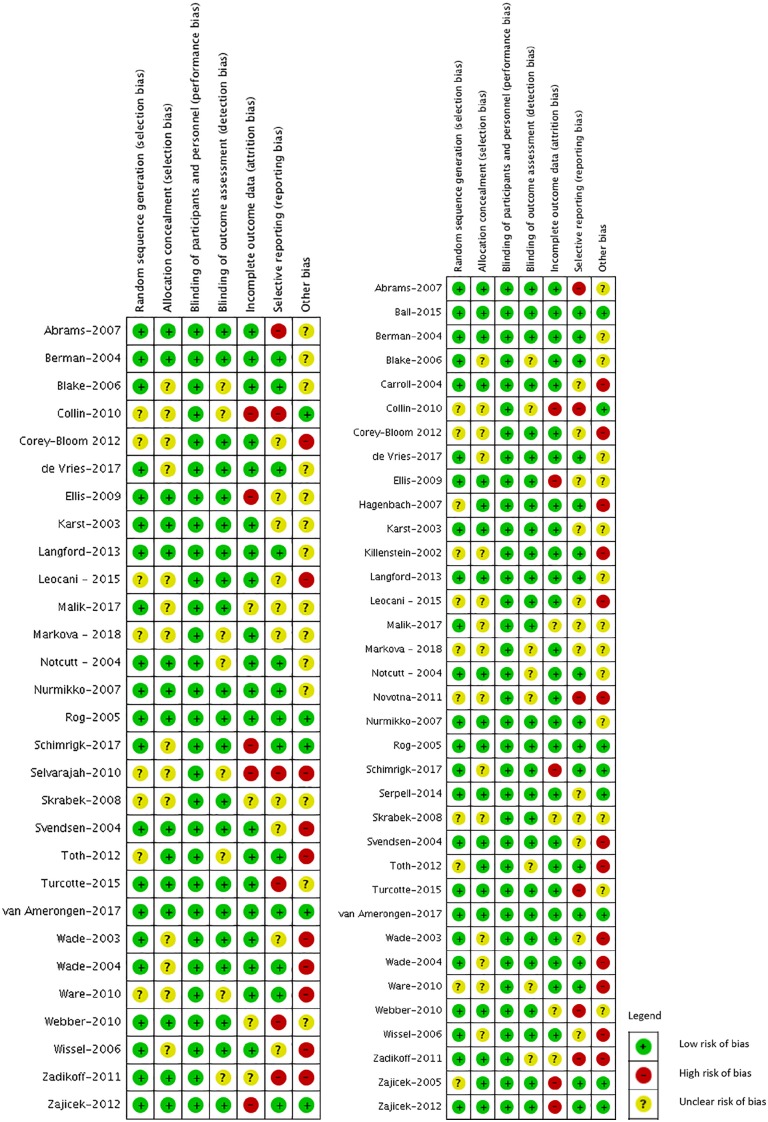
Risk of bias
Across all outcomes, 3 (8%) of the trials were judged to be at low risk of bias,23,41,49 5 (14%) were judged at moderate risk of bias,24,34,40,44,57 and 28 (77.8%) at high risk of bias (Figure 2A and B).22,25-33,35-39,42,43,45-48,50-56 The major sources of bias in the trials were inadequate sample size to determine efficacy (mean of 50 patients per study arm) and selective outcome reporting; the latter requiring imputation and additional calculations in 21 out of 34 (61.7%) of pain outcome comparisons for meta-analysis. As each of the studies had a placebo control, patients were adequately blinded in all 36 of the included studies, decreasing bias for each of the outcomes reviewed.
Figure 2.
Risk of bias summary—Review authors’ judgments about each risk of bias item for each outcome: (A) pain, 29 trials and (B) adverse events, 35 trials.
Pain
Across the 29 trials (34 comparisons) that had reported on pain outcomes, there was a significant treatment effect favoring the use of cannabinoids over placebo (−0.63, 95% CI, −0.85 to −0.42, I2 = 16%, P < .00001; low-quality evidence). When stratified by follow-up period, we found that within the first 2 weeks, cannabinoids had a greater reduction in pain compared with placebo (−0.54, 95% CI, −0.76 to −0.31, I2 = 0%; n = 13 trials; moderate-quality evidence). This difference remained at 2 months (−0.68, 95% CI, −0.96 to −0.40, I2 = 8%; n = 13 trials; low-quality evidence), however decreased by 6 months (−0.43, 95% CI, −0.75 to −0.10, I2 = 30%; n = 8 trials; low-quality evidence), yet still remained significant (Table 2, e-Figures 1-4).
Table 2.
GRADE evidence profile summary table for pain.
| Outcome Time Frame | Number of Studies (Number of Patients) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Magnitude of Effect (95% CI) | Overall Quality |
|---|---|---|---|---|---|---|---|---|
| Pain (1 day to 6 months) | Data from 2345 patients in 29 studies, 34 comparisons | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | Serious (publication bias) | Mean difference 0.63 less (0.85 less to 0.42 less) | Low |
| Pain (1 day to 2 weeks) | Data from 1252 patients in 13 studies, 18 comparisons | Seriousb | No serious inconsistency | No serious indirectness | No serious imprecision | None | Mean difference 0.54 less (0.76 less to 0.31 less) | Moderate |
| Pain (2-8 weeks) | Data from 1362 patients in 16 studies, 16 comparisons | Seriousc | No serious inconsistency | No serious indirectness | No serious imprecision | Serious (publication bias) | Mean difference 0.68 less (0.96 less to 0.40 less) | Low |
| Pain (2-6 months) | Data from 1399 patients in 9 studies, 9 comparisons | Seriousd | Seriouse | No serious indirectness | No serious imprecision | None | Mean difference 0.43 less (0.75 less to 0.10 less) | Low |
Abbreviations: CI, confidence interval; GRADE, Grades of Recommendation, Assessment, Development, and Evaluation.
Of the 29 trials, 8 had moderate risk of bias for randomization sequence generation, 16 had moderate risk of bias for allocation concealment, 10 had moderate risk of bias for blinding of outcome assessment, 5 had high risk of bias for incomplete outcome data, 6 had high risk of bias for selective reporting, and 12 had moderate risk of bias for selective reporting.
Of the 13 trials, 2 had moderate risk of bias for randomization sequence generation, 4 had moderate risk of bias for allocation concealment, 2 had moderate risk of bias for blinding of outcome assessment, 2 had high risk of bias for incomplete outcome data, 2 had high risk of bias for selective reporting, and 4 had moderate risk of bias for selective reporting.
Of the 16 trials, 2 had moderate risk of bias for randomization sequence generation, 8 had moderate risk of bias for allocation concealment, 2 had moderate risk of bias for blinding of outcome assessment, 2 had high risk of bias for incomplete outcome data, 2 had high risk of bias for selective reporting, and 5 had moderate risk of bias for selective reporting.
Of the 9 trials, 5 had moderate risk of bias for randomization sequence generation, 5 had moderate risk of bias for allocation concealment, 4 had moderate risk of bias for blinding of outcome assessment, 4 had high risk of bias for incomplete outcome data, 3 had high risk of bias for selective reporting and 2 had moderate risk of bias for selective reporting.
Heterogeneity I2 = 30%.
Across all time points, oral formulations demonstrated a superior effect compared with oromucosal and inhaled routes of administration. Results regarding pain outcomes are summarized in Tables 2 and 3, with additional forest and funnel plots available in the online supplementary materials (e-Figures 5-10).
Table 3.
Pain outcomes stratified by follow-up duration and route of administration.
| Cannabis vs Placebo by Route of Administration | Duration of Treatment | N of Comparisons | Weighted Mean Difference on 0-10 VAS Pain | Notes |
|---|---|---|---|---|
| Oral | 1-14 days | 3 | −1.07 (−2.11 to −0.02) | Difference > 1 point |
| 2-8 weeks | 10 | −0.81 (−1.17 to −0.45) | Mild difference | |
| 2-6 months | 5 | −0.48 (−0.91 to −0.05) | Mild difference | |
| Oromucosal spray | 1-14 days | 11 | −0.43 (−0.74 to −0.12) | Mild difference |
| 2-8 weeks | 6 | −0.46 (−1.04 to 0.11) | NS | |
| 2-6 months | 4 | −0.39 (−0.93 to 0.15) | NS | |
| Smoked | 1-14 days | 4 | −0.42 (−0.92 to 0.09) | NS |
Abbreviations: NS, not significant; VAS, visual analogue scale.
Small effects of cannabinoids in pain reduction were found in patients with neuropathic pain related to multiple sclerosis and those with other chronic neuropathic pain conditions, including HIV sensory neuropathy, postsurgical or posttraumatic pain, diabetes, functional chest pain, pancreatitis-related abdominal pain, amyotrophic lateral sclerosis, fibromyalgia, hyperalgesia, allodynia, and cervical dystonia. No statistically significant difference was found for patients with rheumatoid arthritis, which had only available data from 1 trial (Table 4, e-Figures 11-16).
Table 4.
Pain outcomes stratified by follow-up duration and pain condition.
| Cannabis vs Placebo by Route of Administration | Duration of Treatment | N of Comparisons | Weighted Mean Difference on 0-10 VAS Pain | Notes |
|---|---|---|---|---|
| Neuropathic or chronic pain other than multiple sclerosis | 1-14 days | 14 | −0.82 (−1.18 to −0.46) | Mild difference |
| 2-8 weeks | 5 | −1.19 (−1.79 to −0.60) | Difference > 1 point | |
| 2-6 months | 3 | −0.92 (−1.80 to −0.03) | Mild difference | |
| Multiple sclerosis pain | 1-14 days | 4 | −0.35 (−0.64 to −0.06) | Mild difference |
| 2-8 weeks | 10 | −0.57 (−0.94 to −0.19) | Mild difference | |
| 2-6 months | 6 | −0.36 (−0.71 to −0.02) | Mild difference | |
| Rheumatoid arthritis pain | 2-8 weeks | 1 | −0.50 (−1.85 to 0.85) | NS |
Abbreviations: NS, not significant; VAS, visual analogue scale.
Greater than 1 point differences favoring cannabinoids over placebo were found with ajulemic acid within 2 weeks and with nabilone beyond 2 weeks. Mild differences were found at shorter durations with the combination of THC and CBD, THC alone and dronabinol. No statistically significant differences were found for combination of THC and CBD after 2 weeks or for CBD alone within 2 weeks (Table 5, e-Figures 17-22).
Table 5.
Pain outcomes stratified by follow-up duration and type of cannabinoids.
| Cannabis vs Placebo by Type of Drugs | Duration of Treatment | N of Comparisons | Weighted Mean Difference on 0-10 VAS Pain | Notes |
|---|---|---|---|---|
| Ajulemic acid | 1-14 days | 1 | −1.90 (−2.78 to −1.02) | Difference > 1 point |
| THC and CBD | 1-14 days | 6 | −0.40 (−0.73 to −0.07) | Mild difference |
| 2-8 weeks | 6 | −0.46 (−1.04 to 0.11) | NS | |
| 2-6 months | 4 | −0.39 (−0.93 to 0.15) | NS | |
| THC | 1-14 days | 7 | −0.47 (−0.92 to −0.03) | Mild difference |
| 2-8 weeks | 3 | −0.65 (−1.25 to −0.05) | Mild difference | |
| 2-6 months | 1 | −0.60 (−1.30 to 0.10) | NS | |
| CBD | 1-14 days | 2 | −0.45 (−2.79 to 1.88) | NS |
| Nabilone | 2-8 weeks | 4 | −1.48 (−2.54 to −0.42) | Difference > 1 point |
| 2-6 months | 3 | −1.23 (−2.19 to −0.28) | Difference > 1 point | |
| Dronabinol | 1-14 days | 2 | −0.50 (−1.01 to 0.02) | NS |
| 2-8 weeks | 4 | −0.79 (−1.33 to −0.26) | Mild difference | |
| 2-6 months | 1 | −0.11 (−0.61 to 0.39) | NS |
Abbreviations: CBD, cannabidiol; NS, not significant; THC, tetrahydrocannabinol; VAS, visual analogue scale.
Adverse events
Data regarding AEs were reported in 35 studies, although reporting was inconsistent, precluding pooled analysis across the individual events reported. Compared with placebo, cannabinoids were associated with a similar risk of serious AE; however there were a greater number of non-serious treatment-related AEs reported for cannabinoids, due largely to events such as dizziness, throat discomfort, asthenia, fatigue, drowsiness, dry mouth, increased appetite, hallucinations, nausea, and refractory spasticity (Table 6). No studies evaluating the long-term AEs of cannabinoids were identified, even when searches were extended to lower levels of evidence, including non-randomized trials and retrospective cohort studies. Overall, 225 out of 3816 (5.9%) patients reported a serious AE, requiring either medical intervention or withdrawal from the trial. Of these, 74 out of 2176 (3.4%) occurred in patients receiving cannabinoids, and 53 out of 1640 (3.2%) occurred in patients receiving placebo, indicating little overall difference between the 2 treatment groups. All of the remaining AEs described in the included studies were classified as either moderate or minor. Among those receiving cannabinoids, 1046 out of 2176 (48%) described experiencing a moderate or minor AE, compared with 648 out of 1640 (40%) of those receiving placebo. Overall, 4561 individual AEs were reported (cannabinoid = 3280, placebo = 1281), with a further breakdown of the 20 most frequently reported events in the intervention group summarized in Table 6.
Table 6.
Adverse events for cannabinoid treatment arms.
| Adverse Events | All Cannabinoids | Cannabinoid Group |
|||||
|---|---|---|---|---|---|---|---|
| Inhaled |
Oromucosal |
Oral |
|||||
| Cannabis (Flower) | THC/CBD Sprawy (Nabiximols) | THC Only Spray | CBD Only Spray | Synthetic delta-9 THC (Dronabinol, Nabilone, Namisol) | Cannabis Extract | ||
| Dizziness | 356/1156 (31%) |
13/80 (16%) |
166/612 (27%) |
12/71 (17%) |
0/24 (0%) |
76/225 (34%) |
89/144 (62%) |
| Application site discomfort | 24/137 (18%) |
3/23 (13%) |
21/114 (18%) |
— | — | — | — |
| Asthenia | 53/334 (16%) |
2/23 (9%) |
26/167 (16%) |
— | — | — | 25/144 (17%) |
| Fatigue | 124/823 (15%) |
8/53 (15%) |
86/535 (16%) |
0/24 (0%) |
0/24 (0%) |
5/43 (12%) |
25/144 (17%) |
| Increased appetite | 10/65 (15%) |
2/23 (9%) |
— | — | — | 8/42 (19%) |
— |
| Dry mouth | 114/826 (14%) |
1/23 (4%) |
55/462 (12%) |
— | — | 24/197 (12%) |
34/144 (24%) |
| Drowsiness | 109/765 (14%) |
0/23 (0%) |
62/588 (11%) |
6/47 (13%) |
41/107 (38%) |
— | — |
| Nausea | 115/920 (13%) |
5/53 (9%) |
94/612 (15%) |
6/71 (8%) |
1/24 (4%) |
9/160 (6%) |
— |
| Hallucination | 22/164 (13%) |
— | — | — | — | — | 22/144 (15%) |
| Muscle spasticity | 20/179 (11%) |
— | 17/167 (10%) |
— | — | 3/12 (25%) |
— |
| Headache | 75/725 (10%) |
11/53 (21%) |
23/399 (6%) |
3/24 (13%) |
1/24 (4%) |
37/225 (16%) |
— |
| Vertigo | 39/400 (10%) |
— | 20/247 (8%) |
— | — | 19/153 (12%) |
— |
| Euphoria/euphoric mood | 11/113 (10%) |
1/23 (4%) |
2/34 (6%) |
— | — | 8/56 (14%) |
— |
| Dysgeusia (bad taste) | 25/322 (8%) |
— | — | — | — | — | — |
| Fall | 20/293 (7%) |
0/23 (0%) | 6/89 (7%) |
1/24 (4%) |
1/24 (4%) |
12/133 (9%) |
— |
| Feeling abnormal (drunk/high) | 31/546 (6%) |
2/53 (4%) |
21/390 (5%) |
4/47 (9%) |
— | 4/56 (7%) |
— |
| Attention disturbance | 23/458 (5%) |
— | 18/368 (5%) |
1/24 (4%) |
0/24 (0%) |
4/42 (10%) |
— |
| Vomiting | 16/390 (4%) |
0/23 (0%) |
15/319 (5%) |
1/24 (4%) |
0/24 (0%) |
4/42 (10%) |
— |
| Balance disorder or difficulty | 13/316 (4%) |
1/23 (4%) | 6/191 (3%) |
1/24 (4 %) |
0/24 (0%) |
5/54 (9%) |
— |
| Dysphagia/sore throat | 7/267 (3%) |
4/53 (8%) |
3/201 (1%) |
— | — | 0/13 (0%) |
— |
Abbreviations: CBD, cannabidiol; THC, tetrahydrocannabinol.
Discussion
We conducted an extensive systematic review of the benefits and harms associated with medical cannabinoids for chronic, non-cancer-related pain. We included 36 RCTs (4006 participants) and found that cannabinoids are an effective form of pain control in this patient population, with a particularly strong effect among those cannabinoids that are orally administered. Compared with the findings in a systematic review that concluded opioids were effective in chronic pain reduction versus placebo (weighted mean difference and 95% CI, −0.69 [−0.82 to −0.56]) on a 10-cm VAS between 3 and 6 months,58 the effect of cannabinoids versus placebo between 2 and 6 months in our current study was smaller and less precise (weighted mean difference and 95% CI, −0.43 [−0.75 to −0.10]); however, their CIs overlap and without more high-quality evidence directly comparing medical cannabis with opioids, and considerations of cost and AEs, it is difficult to assess if any differences between these 2 forms of therapy are statistically significant or cost-effective relative to one another.
Over the past 5 years, the political and cultural backdrop surrounding cannabis has undergone a major shift, leading to wider societal acceptance and use. Currently, the recreational and medical use of cannabis is legal across Canada and in 10 US states, with an additional 23 US states providing legal medical access only.59 Overall, the greater access to cannabis in North America has led to rapid growth in interest around the possible benefits and harms surrounding its use. Furthermore, the movement away from opiates as an analgesic has fueled an increased interest in applications for cannabinoids in the treatment of chronic, non-cancer-related pain.
The efficacy of cannabinoids on chronic, non-cancer-related pain varied by route of administration, with cannabinoids taken orally having the largest effect size, followed by oromucosal sprays and inhaled (smoked) cannabis although the interaction effect was not significant. The differences in efficacy are likely related to differences in cannabinoid absorption, metabolism, and distribution across the routes of administration. The effects of cannabinoids occur through interactions with the endogenous cannabinoid system (ECS), a complex network of receptors and transmitters that has been implicated in a number of physiological functions, both in the central and peripheral nervous systems as well as peripheral organs.60 The ECS is comprised of 2 main receptors (cannabinoid receptor type-1 [CB1] and type-2 [CB2]), endogenous ligands that bind to and activate these receptors (primarily N-arachidonylethanolamide [AEA] and 2-arachidonyl glycerol [2-AG]), and the enzymes responsible for their metabolism (fatty acid amide hydrolase and monoacyl glycerol lipase for AEA and 2-AG, respectively).61 Although found throughout the body, including the brain, endothelium, gastrointestinal lining, lungs, bone, and muscle,62 there is considerable variation in the expression of ECS components throughout the body.63 The differences in ECS distribution and in the bioavailability of cannabinoids across routes of administration likely underscore variation in drug efficacy of the different cannabinoid forms. The ECS is a highly dynamic system that is substantially altered in chronic pain states.64 Some effects of cannabinoids may be mediated through G protein-coupled receptor 55.65 Cannabidiol may interact with the serotonin 1A receptor66 and voltage gated sodium channels.67
We found statistically significant effects in favor of medical cannabis for patients with multiple sclerosis and those with neuropathic or chronic pain other than multiple sclerosis across all durations of treatment. Interestingly, of note, although medical cannabis has been prescribed for patients with arthritis, we only found 1 trial on patients with rheumatoid arthritis (over a treatment period of 2-8 weeks, which was not statistically significant) and none on patients with osteoarthritis; therefore, generalizing our results to patients with these arthritic conditions may be problematic.
We did not find a significant difference for pain reduction after stratifying by types of cannabinoids except for the analysis at 1 to 14 days duration (P value for interaction = .04), which was most likely due to the data from 1 study that evaluated ajulemic acid (e-Figure 17). Ajulemic acid is an orally taken cannabinoid and, when pooling the effects with the other 2 studies that evaluated oral cannabinoids (THC), the subgroup difference by route of administration was not significant (e-Figure 5). Although the study on ajulemic acid has a relatively low risk of bias (Figure 2B), the sample size was small with only 19 patients in total.32 The mechanism of a possibly larger pain reduction with ajulemic acid relative to other types of cannabinoids is not known.
The rate of absorption and rapidity of effects of cannabinoids on the endocannabinoid system will be largely influenced by the route of administration and specific drug formulation. Smoking, the traditional method of cannabis administration, provides rapid cannabinoid delivery, with THC being detectable in the blood immediately after the first puff of a cannabis cigarette, and reaching peak blood concentrations within 10 min, at a bioavailability of nearly 30%.68,69 However, large inter-subject differences have been shown in controlled laboratory and clinical experiments due to variability in number of puffs, length of inhalation, hold time, time between puffs, and depth of inhalation, despite using formulations with similar THC concentrations.68 In addition, the speed of delivery and ability to titrate dosing is offset by the substantial short- and long-term harmful effects of smoking, making it a non-preferred route for medical applications. Among the AEs recorded in the included studies, smoking appeared to have higher rates of dysphagia and sore throat, as well as headache over the study follow-up periods, with long-term risk of cancers and interstitial lung disease remaining unknown. To avoid many of the negative side effects of smoking, oral cannabinoids emerged as a therapeutic delivery alternative. Absorption is slower when cannabinoids are ingested orally compared with inhalation, with a more delayed time to reach peak THC blood concentrations that are typically lower.69,70 Dose and vehicle of delivery also play a role in circulating cannabinoid concentrations, along with other patient-related factors such as gastrointestinal content and motility.68,71 In addition, degradation of cannabinoids in the stomach and substantial first-pass metabolism lead to the oral bioavailability of cannabinoids only ranging from 4% to 20%, and reaching peak blood concentrations over 1 to 5 hours.71,72 Furthermore, first-pass hepatic metabolism of cannabis and cannabinoids results in the conversion of ∆9-THC to 11-OH-THC, a potent psychoactive metabolite that readily crosses the blood-brain-barrier, and thus likely contributes to the effects observed after oral ingestion.73 Oromucosal administration, on the contrary, uses absorption via the mucous membranes, avoiding the first-pass effect, yet still exhibiting bioavailability and pharmacodynamics similar to that of oral dosing, as demonstrated in an investigation of 10 patients by Karschner et al,74 where both formulations were administered. In a similar study where 17 volunteers had blood-concentration volumes measured after taking a single synthetic ∆9-THC capsule (10 mg), initial peak cannabinoid concentrations were reached within 1 to 2 hours of ingestion, with a second peak frequently being observed several hours later due to enterohepatic circulation, which was not present in those using oromucosal dosing.74 Overall, delayed absorption after oral ingestion leads to an extended period over which the effects were experienced, and a prolonged time to return to baseline concentrations.68 Thus, differences in cannabinoid absorption, metabolism, and interactions with the ECS across the different routes of administration may explain why those studies examining oral cannabinoid formulations demonstrated a greater improvement in pain over placebo, relative to both smoked and oromucosal formulations in our meta-analysis.
Strengths and limitations
Our article followed recommendations for systematic reviews, using a standardized, structured and extensive search strategy and multiple independent reviewers for study selection, data abstraction, and risk of bias evaluation.16,18 This strategy allowed us to complete a rigorous review and meta-analysis of the highest level of evidence available, including recent RCTs not previously assessed, supplemented by interpretation following the guidelines laid out by the GRADE working group.19-21 Potential heterogeneity through the inclusion of multiple different cannabinoids assessed over varying durations of follow-up was addressed through the use of stratified analysis. Additional methodological steps were taken to avoid double counting of studies with multiple treatment arms, and to limit the impact of missing data through the use of imputation. Despite these strengths, we were limited by the overall quality of the trials available, which were largely underpowered and selective and inconsistent in their reporting. These limitations affect our ability to collect and examine AE outcomes through meta-analysis, allowing us only to pool and present the data across those studies that had reported similar individual events by each treatment arm. Furthermore, a recent review suggested that elderly patients, who have a high prevalence of arthritis, which is a chronic non-cancer pain (CNCP) condition, may experience greater and more serious neuropsychiatric AEs (eg, dizziness, cognitive dysfunction, etc) associated with cannabinoid uptake.75 It would be valuable if we had sufficient evidence to determine whether or not the rate of neuropsychiatric AEs among seniors is indeed higher than in younger individuals; however, no data from the included trials in our study were available for such an analysis.
Conclusions
There was a moderate-quality evidence of small effect for the use of cannabinoids in treating chronic, non-cancer-related pain at all time points studied up to 6 months. There is little evidence that cannabinoids increase the risk of experiencing serious AEs, although mild and moderate AEs may be common in the short-term period following use. Of note, many conditions can be classified as “chronic, non-cancer pain” and the evidence base on this topic is represented by certain conditions more so than others. For example, there was very limited evidence on non-neuropathic chronic pain conditions. As such, large, high-quality clinical trials examining oral cannabinoids would help better establish the efficacy among this patient population, with particular attention to the reporting of AEs to better characterize the safety profile of this emerging analgesic class of medications.
Supplemental Material
Supplemental material, Suppl_fig_and_table for Cannabinoids in Chronic Non-Cancer Pain: A Systematic Review and Meta-Analysis by Herman Johal, Tahira Devji, Yaping Chang, Jonathan Simone, Christopher Vannabouathong and Mohit Bhandari in Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: TD and MB contributed to the design of the study. HJ, TD, YC, and CV contributed to the acquisition and interpretation of data. HJ drafted the manuscript. All the other authors reviewed and revised the manuscript prior to submission. All authors approved the final version of the manuscript.
ORCID iDs: Yaping Chang  https://orcid.org/0000-0002-0549-5087
https://orcid.org/0000-0002-0549-5087
Christopher Vannabouathong  https://orcid.org/0000-0002-9694-6364
https://orcid.org/0000-0002-9694-6364
Supplemental material: Supplemental material for this article is available online.
References
- 1. Canadian Institute for Health Information/Institut canadien d’information sur la santé. Opioid-related harms in Canada. https://www.cihi.ca/en/opioids-in-canada/2018/opioid-related-harms-in-canada. Published 2017.
- 2. Katz J. Drug deaths in America are rising faster than ever. The New York Times. June 5, 2017. https://www.nytimes.com/interactive/2017/06/05/upshot/opioid-epidemic-drug-overdose-deaths-are-rising-faster-than-ever.html.
- 3. Public Health Agency of Canada. Overview of national data on opioid-related harms and deaths. Ottawa, ON, Canada: Government of Canada; 2018. [Google Scholar]
- 4. Morris BJ, Mir HR. The opioid epidemic: Impact on orthopaedic surgery. J Am Acad Orthop Surg. 2015;23:267-271. https://www.ncbi.nlm.nih.gov/pubmed/25911660. [DOI] [PubMed] [Google Scholar]
- 5. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ. 2017;189: E659-E666. https://www.ncbi.nlm.nih.gov/pubmed/28483845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Canadian Orthopaedic Association/L’Association Canadienne d’Orthopédie. COA position statement: opioids and orthopaedic surgical practice. https://coa-aco.org/wp-content/uploads/2017/01/COA-Position-Statement-Opioids-and-Orthopaedic-Surgical-Practice-2018-June-ENG.pdf. Accessed August 1, 2019.
- 7. Attum B, Rodriguez-Buitrago A, Harrison N, et al. Opioid prescribing practices by orthopaedic trauma surgeons after isolated femur fractures. J Orthop Trauma. 2018;32:e106-e111. [DOI] [PubMed] [Google Scholar]
- 8. Bedard NA, Pugely AJ, Westermann RW, Duchman KR, Glass NA, Callaghan JJ. Opioid use after total knee arthroplasty: trends and risk factors for prolonged use. J Arthroplasty. 2017;32:2390-2394. [DOI] [PubMed] [Google Scholar]
- 9. Zarling BJ, Yokhana SS, Herzog DT, Markel DC. Preoperative and postoperative opiate use by the arthroplasty patient. J Arthroplasty. 2016;31:2081-2084. [DOI] [PubMed] [Google Scholar]
- 10. Aggarwal SK, Carter GT, Sullivan MD, ZumBrunnen C, Morrill R, Mayer JD. Characteristics of patients with chronic pain accessing treatment with medical cannabis in Washington state. J Opioid Manag. 2018;5:257-586. doi: 10.5055/jom.2009.0028. [DOI] [PubMed] [Google Scholar]
- 11. Madden K, van der Hoek N, Chona S, et al. Cannabinoids in the management of musculoskeletal pain: a critical review of the evidence. JBJS Rev. 2018;6:e7 https://www.ncbi.nlm.nih.gov/pubmed/29787450. [DOI] [PubMed] [Google Scholar]
- 12. Campbell FA, Tramer MR, Carroll D, Reynolds DJ, Moore RA, McQuay HJ. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ. 2001;323:13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martín-Sánchez E, Furukawa TA, Taylor J, Martin JLR. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med. 2009;10:1353-1368. https://academic.oup.com/painmedicine/article-lookup/doi/10.1111/j.1526-4637.2009.00703.x [DOI] [PubMed] [Google Scholar]
- 14. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use. JAMA. 2015;313:2456-2473. http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2015.6358 [DOI] [PubMed] [Google Scholar]
- 15. Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72:735-744. doi: 10.1111/j.1365-2044.2004.03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. https://www.ncbi.nlm.nih.gov/pubmed/19621070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255-1259. https://www.sciencedirect.com/science/article/pii/S0140673600027999. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. https://boris.unibe.ch/7356/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Welch VA, Akl EA, Guyatt G, et al. GRADE equity guidelines 1: considering health equity in GRADE guideline development: introduction and rationale. J Clin Epidemiol. 2017;90:59-67. https://www.sciencedirect.com/science/article/pii/S0895435617303396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401-406. https://www.clinicalkey.es/playcontent/1-s2.0-S089543561000332X. [DOI] [PubMed] [Google Scholar]
- 21. Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66:151-157. https://www.clinicalkey.es/playcontent/1-s2.0-S089543561200025X. [DOI] [PubMed] [Google Scholar]
- 22. Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=17296917&retmode=ref&cmd=prlinks [DOI] [PubMed] [Google Scholar]
- 23. Ball S, Vickery J, Hobart J, et al. The cannabinoid use in progressive inflammatory brain disease (CUPID) trial: a randomised double-blind placebo-controlled parallel-group multicentre trial and economic evaluation of cannabinoids to slow progression in multiple sclerosis. Health Technol Assess. 2015;19:vii-viii. https://www.ncbi.nlm.nih.gov/pubmed/25676540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004;112:299-306. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006396-200412000-00010 [DOI] [PubMed] [Google Scholar]
- 25. Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology. 2006;45:50-52. http://academic.oup.com/rheumatology/article/45/1/50/1788693/Preliminary-assessment-of-the-efficacy. [DOI] [PubMed] [Google Scholar]
- 26. Carroll CB, Bain PG, Teare L, et al. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology. 2004;63:1245-1250. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=15477546&retmode=ref&cmd=prlinks [DOI] [PubMed] [Google Scholar]
- 27. Collin C, Ehler E, Waberzinek G, et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010;32:451-459. http://www.tandfonline.com/doi/full/10.1179/016164109X12590518685660. [DOI] [PubMed] [Google Scholar]
- 28. Corey-Bloom J, Wolfson T, Gamst A, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ. 2012;184:1143-1150. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=22586334&retmode=ref&cmd=prlinks [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Vries M, van Rijckevorsel DCM, Vissers KCP, Wilder-Smith OHG, van Goor H; for Pain and Nociception Neuroscience Research Group. Tetrahydrocannabinol does not reduce pain in patients with chronic abdominal pain in a phase 2 placebo-controlled study. Clin Gastroenterol Hepatol. 2017;15:1079-1086.e4. doi: 10.1016/j.cgh.2016.09.147. [DOI] [PubMed] [Google Scholar]
- 30. Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680. http://www.nature.com/articles/npp2008120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hagenbach U, Luz S, Ghafoor N, et al. The treatment of spasticity with Δ9-tetrahydrocannabinol in persons with spinal cord injury. Spinal Cord. 2007;45:551-562. doi: 10.1038/sj.sc.3101982. [DOI] [PubMed] [Google Scholar]
- 32. Karst M, Salim K, Burstein S, Conrad I, Hoy L, Schneider U. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial. JAMA. 2003;290:1757-1762. doi: 10.1001/jama.290.13.1757. [DOI] [PubMed] [Google Scholar]
- 33. Killestein J, Hoogervorst E, Reif M, et al. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology. 2002;58:1404-1407. https://www.ncbi.nlm.nih.gov/pubmed/12011290. [DOI] [PubMed] [Google Scholar]
- 34. Langford RM, Mares J, Novotna A, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013;260:984-997. http://link.springer.com/10.1007/s00415-012-6739-4. [DOI] [PubMed] [Google Scholar]
- 35. Leocani L, Nuara A, Houdayer E, et al. Sativex® and clinical-neurophysiological measures of spasticity in progressive multiple sclerosis. J Neurol. 2015;262:2520-2527. https://www.ncbi.nlm.nih.gov/pubmed/26289497. [DOI] [PubMed] [Google Scholar]
- 36. Malik Z, Bayman L, Valestin J, Rizvi-Toner A, Hashmi S, Schey R. Dronabinol increases pain threshold in patients with functional chest pain: a pilot double-blind placebo-controlled trial. Dis Esophagus. 2017;30:1-8. https://academic.oup.com/dote/article-lookup/doi/10.1111/dote.12455. [DOI] [PubMed] [Google Scholar]
- 37. Markovà J, Essner U, Akmaz B, et al. Sativex® as add-on therapy vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: a double-blind, placebo-controlled randomised clinical trial. Int J Neurosci. 2019;129:119-128. https://www.ncbi.nlm.nih.gov/pubmed/29792372. [DOI] [PubMed] [Google Scholar]
- 38. Notcutt W, Price M, Miller R, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: Results from 34 “N of 1” studies. Anaesthesia. 2004;59:440-452. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=15096238&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 39. Novotna A, Mares J, Ratcliffe S, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex®), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. European Journal of Neurology. 2011;18:1122-1131. http://doi.wiley.com/10.1111/j.1468-1331.2010.03328.x. [DOI] [PubMed] [Google Scholar]
- 40. Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain. 2007;133:210-220. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=17997224&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 41. Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65:812-819. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=16186518&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 42. Schimrigk S, Marziniak M, Neubauer C, Kugler EM, Werner G, Abramov-Sommariva D. Dronabinol is a safe long-term treatment option for neuropathic pain patients. Eur Neurol. 2017;78:320-329. doi: 10.1159/000481089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Selvarajah D, Gandhi R, Emery CJ, Tesfaye S. Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: depression is a major confounding factor. Diabetes Care. 2010;33:128-130. http://care.diabetesjournals.org/cgi/doi/10.2337/dc09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain. 2014;18:999-1012. http://doi.wiley.com/10.1002/j.1532-2149.2013.00445.x. [DOI] [PubMed] [Google Scholar]
- 45. Skrabek RQ, Galimova L, Ethans K, Perry D. Nabilone for the treatment of pain in fibromyalgia. J Pain. 2008;9:164-173. http://linkinghub.elsevier.com/retrieve/pii/S1526590007008735. [DOI] [PubMed] [Google Scholar]
- 46. Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ. 2004;329:253 http://www.bmj.com/lookup/doi/10.1136/bmj.38149.566979.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Toth C, Mawani S, Brady S, et al. An enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic pain. Pain. 2012;153:2073-2082. doi: 10.1016/j.pain.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 48. Turcotte D, Doupe M, Torabi M, et al. Nabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: a randomized controlled trial. Pain Med. 2015;16:149-159. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=25288189&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 49. van Amerongen G, Kanhai K, Baakman AC, et al. Effects on spasticity and neuropathic pain of an oral formulation of Δ9-tetrahydrocannabinol in patients with progressive multiple sclerosis. Clin Ther. 2018;40:1467-1482. doi: 10.1016/j.clinthera.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 50. Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003;17:21-29. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=12617376&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 51. Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10:434-441. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=15327042&retmode=ref&cmd=prlinks [DOI] [PubMed] [Google Scholar]
- 52. Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010;182:E694-E701. http://www.cmaj.ca/cgi/doi/10.1503/cmaj.091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weber M, Goldman B, Truniger S. Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatry. 2010;81:1135-1140. http://jnnp.bmj.com/cgi/doi/10.1136/jnnp.2009.200642. [DOI] [PubMed] [Google Scholar]
- 54. Wissel J, Haydn T, Muller J, et al. Low dose treatment with the synthetic cannabinoid nabilone significantly reduces spasticity-related pain: a double-blind placebo-controlled cross-over trial. J Neurol. 2006;253:1337-1341. http://link.springer.com/10.1007/s00415-006-0218-8. [DOI] [PubMed] [Google Scholar]
- 55. Zadikoff C, Wadia PM, Miyasaki J, et al. Cannabinoid, CB1 agonists in cervical dystonia: failure in a phase IIa randomized controlled trial. Basal Ganglia. 2011;1:91-95. doi: 10.1016/j.baga.2011.04.002. [DOI] [Google Scholar]
- 56. Zajicek JP, Sanders HP, Wright DE, et al. Cannabinoids in multiple sclerosis (CAMS) study: Safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry. 2005;76:1664-1669. https://www.ncbi.nlm.nih.gov/pubmed/16291891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry. 2012;83:1125-1132. http://jnnp.bmj.com/lookup/doi/10.1136/jnnp-2012-302468. [DOI] [PubMed] [Google Scholar]
- 58. Busse JW, Wang L, Kamaleldin M, et al. Opioids for chronic noncancer pain: a systematic review and meta-analysis. JAMA. 2018;320:2448-2460. doi: 10.1001/jama.2018.18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Berke J, Gould S. States where marijuana is legal. Business Insider. January 4, 2019. https://www.businessinsider.com/legal-marijuana-states-2018-1. Accessed January 5, 2019.
- 60. Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21-47. [DOI] [PubMed] [Google Scholar]
- 61. Di Marzo V. Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight. Nat Neurosci. 2011;14:9-15. [DOI] [PubMed] [Google Scholar]
- 62. Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68:619-631. [DOI] [PubMed] [Google Scholar]
- 63. Mackie K. Cannabinoid receptors: where are they and what do they do? J Neuroendocrinol. 2008;20:10-14. [DOI] [PubMed] [Google Scholar]
- 64. Sagar DR, Gaw AG, Okine BN, et al. Dynamic regulation of the endocannabinoid system: implications for analgesia. Mol Pain. 2009;5:59 https://molecularpain.biomedcentral.com/articles/10.1186/1744-8069-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Henstridge CM. Off-target cannabinoid effects mediated by GPR55. Pharmacology. 2012;89:179-187. doi: 10.1159/000336872. [DOI] [PubMed] [Google Scholar]
- 66. De Gregorio D, McLaughlin RJ, Posa L, et al. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 2019;160:136-150. doi: 10.1097/j.pain.0000000000001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Watkins AR. Cannabinoid interactions with ion channels and receptors. Channels (Austin). 2019;13:162-167. doi: 10.1080/19336950.2019.1615824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4:1770-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta 9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther. 1980;28:409-416. [DOI] [PubMed] [Google Scholar]
- 70. Law B, Mason PA, Moffat AC, Gleadle RI, King LJ. Forensic aspects of the metabolism and excretion of cannabinoids following oral ingestion of cannabis resin. J Pharm Pharmacol. 1984;36:289-294. [DOI] [PubMed] [Google Scholar]
- 71. Reyes MP, Lipton MA, Timmons MC, Wall ME, Brine DR, Davis KH. Pharmacology of orally administered 9-tetrahydrocannabinol. Clin Pharmacol Ther. 1973;14:48-55. [DOI] [PubMed] [Google Scholar]
- 72. Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M. Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther. 1983;34:352-363. [DOI] [PubMed] [Google Scholar]
- 73. Lemberger L, Crabtree RE, Rowe HM. 11-hydroxy-Δ9-tetrahydrocannabinol: pharmacology, disposition, and metabolism of a major metabolite of marihuana in man. Science. 1972;177:62-64. [DOI] [PubMed] [Google Scholar]
- 74. Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral Δ9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem. 2011;57:66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Minerbi A, Häuser W, Fitzcharles MA. Medical cannabis for older patients. Drugs Aging. 2019;36:39-51. doi: 10.1007/s40266-018-0616-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Suppl_fig_and_table for Cannabinoids in Chronic Non-Cancer Pain: A Systematic Review and Meta-Analysis by Herman Johal, Tahira Devji, Yaping Chang, Jonathan Simone, Christopher Vannabouathong and Mohit Bhandari in Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders