Abstract
Background:
Current exercise guidelines for clinical populations recommend an exercise therapy (ET) prescription of fixed intensity (moderate), duration (40–50 mins/session), and volume (120–160 mins/week). A critical overarching element of exercise programming that has received minimal attention is dose scheduling. We investigated tolerability and efficacy of two exercise training dose regimens on cardiorespiratory fitness and patient reported outcomes (PROs) in patients with post-treatment primary breast cancer.
Methods:
Using a parallel-group randomized trial, 174 postmenopausal patients (2.8 years post adjuvant therapy) with impaired peak oxygen consumption (VO2peak) were randomly allocated to one of two supervised exercise training interventions delivered using a standard linear (LET) (fixed dose-intensity / session for 160 mins/wk) or non-linear (NLET) (variable dose-intensity / session for ~120 mins/wk) schedule in comparison with a stretching attention-control (AC) group for 16 consecutive weeks. Stretching was matched to exercise dosing arms on the basis of location, frequency, duration, and treatment length. The primary end point was change in VO2peak (ml O2·kg−1·min−1) from baseline to post-intervention. Secondary end points were PROs, tolerability, and safety.
Results:
No serious adverse events were observed. Mean attendance was 64%, 75%, and 80% for AC, LET, and NLET, respectively. In intention-to-treat analysis VO2peak (ml O2·kg−1·min−1) increased 0.6 (± 1.7) ml O2·kg−1·min−1 (p=0.05) and 0.8 (± 1.8) ml O2·kg−1·min−1 (p=0.07) in LET and NLET respectively, compared to AC. Change in VO2peak ranged from −2.7 to 4.1 ml O2·kg−1·min−1 and −3.6 to 5.1 ml O2·kg−1·min−1 in LET and NLET, respectively. Approximately 40% of patients in both exercise dosing regimens were classified as VO2peak “responders” (i.e., Δ ≥1.32 ml O2·kg−1·min−1). NLET improved all PROs compared with AC.
Conclusion:
Short-term exercise training, independent of dosing schedule, is associated with modest improvements in cardiorespiratory fitness in patients previously treated for early-stage breast cancer.
Clinical Trial Registration:
Clinicaltrials.gov Identifier:
Keywords: Exercise, breast cancer, cardiorespiratory fitness
INTRODUCTION
Post-treatment patients with early-stage breast cancer have marked and significant impairments in cardiorespiratory fitness (CRF1) as a consequence of adverse direct as well as the indirect (i.e., lifestyle perturbations) effects of adjuvant therapy on pulmonary, cardiac, blood-vascular, and skeletal muscle function.2 On average, patients with breast cancer reach a predicted CRF for a particular age group approximately 20 to 30 years earlier than apparently healthy women without a history of breast cancer.1 Collectively, these data indicate that breast cancer is a model of accelerated physiological aging.3, 4 Impaired CRF likely predisposes to the excess noncancer competing morbidity and mortality observed in early-stage breast cancer survivors,5, 6 as well as its attendant symptom burden (e.g., poor quality of life, fatigue).3
In a recent meta-analysis of 27 exercise-oncology trials (1,774 total patients) in post-treatment patients with adult-onset cancer, exercise training was associated with significant improvements in CRF compared to control.7 However, several important methodological limitations were apparent including but not limited to small trial sample sizes (mean, n<75), lack of eligibility criteria related to pre-treatment CRF, suboptimal monitoring and reporting of safety and tolerability / feasibility, and investigation of exercise doses of approximately 100 to 135 minutes per week (i.e., three times weekly for 30 to 45 minutes per session),7 which is not consistent with current exercise-oncology recommendations.8–10 Finally, virtually all exercise-oncology trials to date have investigated exercise doses prescribed at a fixed amount (per week), duration (per session) and intensity (per session) for the entire intervention period, known as a linear prescription. The tolerability and efficacy of alternative non-linear exercise dose scheduling approaches is unclear.
Accordingly, we conducted a randomized trial to evaluate the tolerability and efficacy of two exercise dosing regimens in patients following completion of adjuvant therapy for primary breast cancer. The primary objective was to evaluate changes in CRF. Secondary objectives were changes in patient-reported outcomes (PROs), safety, and tolerability.
METHODS
Trial Design and Patients
We conducted a parallel-group, dual-center, randomized trial to evaluate treatment with standard linear exercise training (LET) or non-linear exercise training (NLET), relative to attention control (AC), in postmenopausal women with primary breast cancer. Eligible patients were ≥ 1 year to <5 years after completion of primary adjuvant therapy, a CRF [peak oxygen consumption (VO2peak)] below age-sex-matched active levels,11 and acceptable pre-randomization cardiopulmonary exercise test (CPET) with cardiology ECG clearance as per established practice guidelines.12 Patients were randomly assigned in a 1:1:1 ratio to receive either one of the two exercise dosing regimens (LET or NLET) or AC. The random allocation sequence was generated and implemented using REDCap with a random permuted block design. No stratification factors were employed. The protocol, with full eligibility criteria, is available in the Supplement. All study procedures were reviewed and approved by Duke University Medical Center and Memorial Sloan Kettering Cancer Center institutional review boards. All patients provided written informed consent. Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Interventions
Full intervention details are provided in the Supplement. Exercise training in both dosing regimens consisted of 61 individualized (one-on-one), supervised treadmill walking (Jog Excite 700, Technogym, Inc.) sessions, 3 to 4 times-weekly for 16 consecutive weeks. Exercise training intensity for each session in each dosing arm was monitored using a combination of heart rate (continuous), blood pressure (every 10 minutes), oxygen saturation (every 5 minutes) and rate of perceived exertion (every 15 minutes). In LET, following a 4-week progressive increase in duration and/or intensity all sessions were conducted at ~70% of the prerandomization VO2peak for 40 minutes/session (planned amount: 160 mins/week) (Figure 1A) consistent with current guidelines.10, 13 The intensity of each session was individually prescribed to each patient on the basis of workload (the speed and incline) measured during the pre-randomization CPET. The corresponding heart rates and blood pressures measured at each workload during the CPET were then used in each training session to verify correct intensity (for each patient). In NLET, intensity alternated between five different dose intensities (i.e., 55%, 65%, 75%, 80%, and >95%) of VO2peak measured during the pre-randomization or midpoint (week 8) CPET, for 20 to 45 minutes/session (planned mean amount: 120 mins/wk); interval sessions consisted of 60 seconds to 120 seconds at peak VO2peak followed by 120 to 180 seconds of active recovery for 8 to 12 intervals. Intensity was individualized to each patient on the basis of workload (i.e., treadmill speed / grade) corresponding to a specific percent of ventilatory thresholds measured during the pre-randomization or midpoint (week 8) CPET. The planned dose was continually altered and progressed consistent with non-linear (periodized) dose scheduling (Figure 1B).14 The NLET was designed to improve VO2peak consistent with exercise principle of specificity.15
Figure 1.
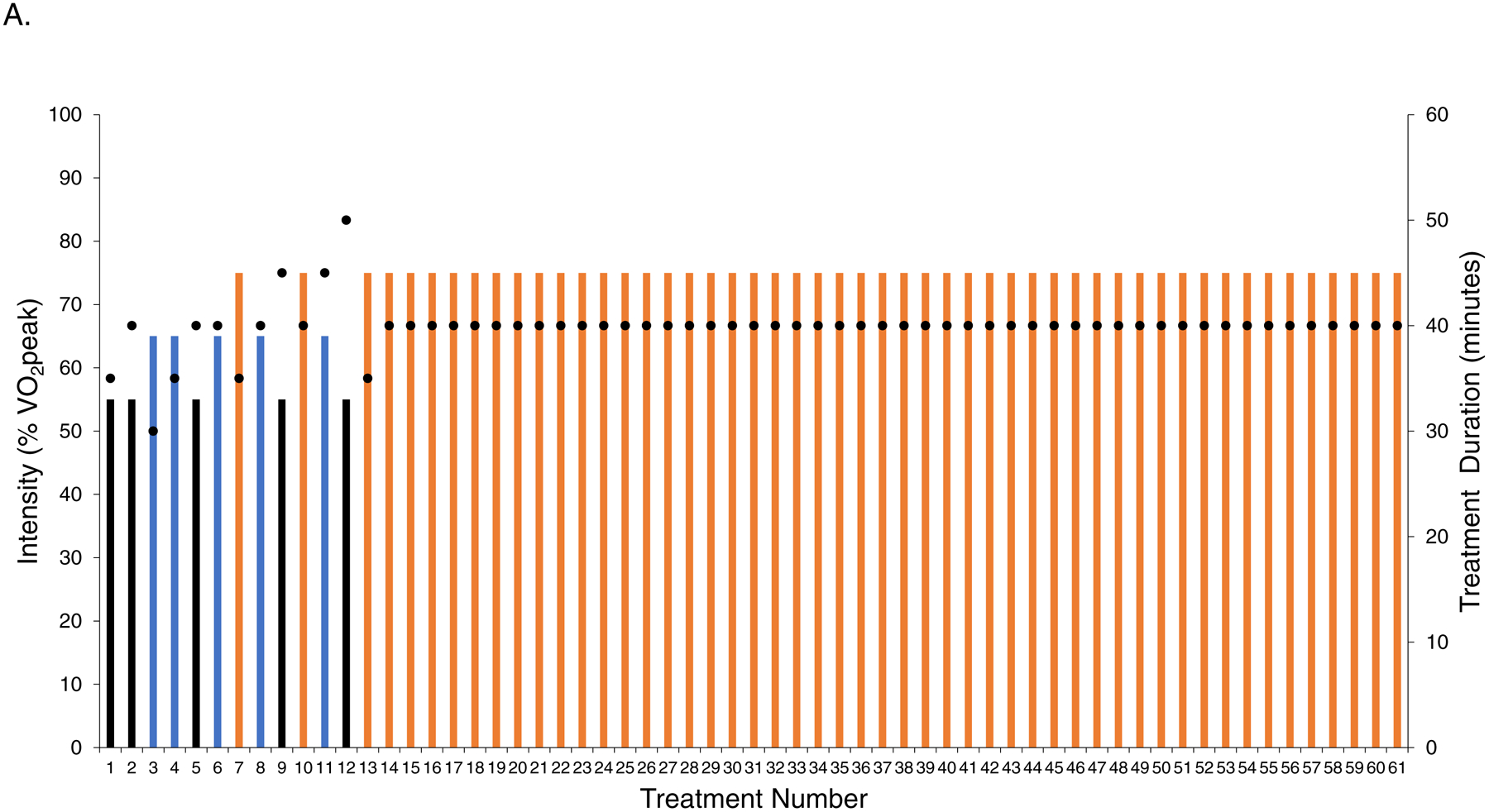
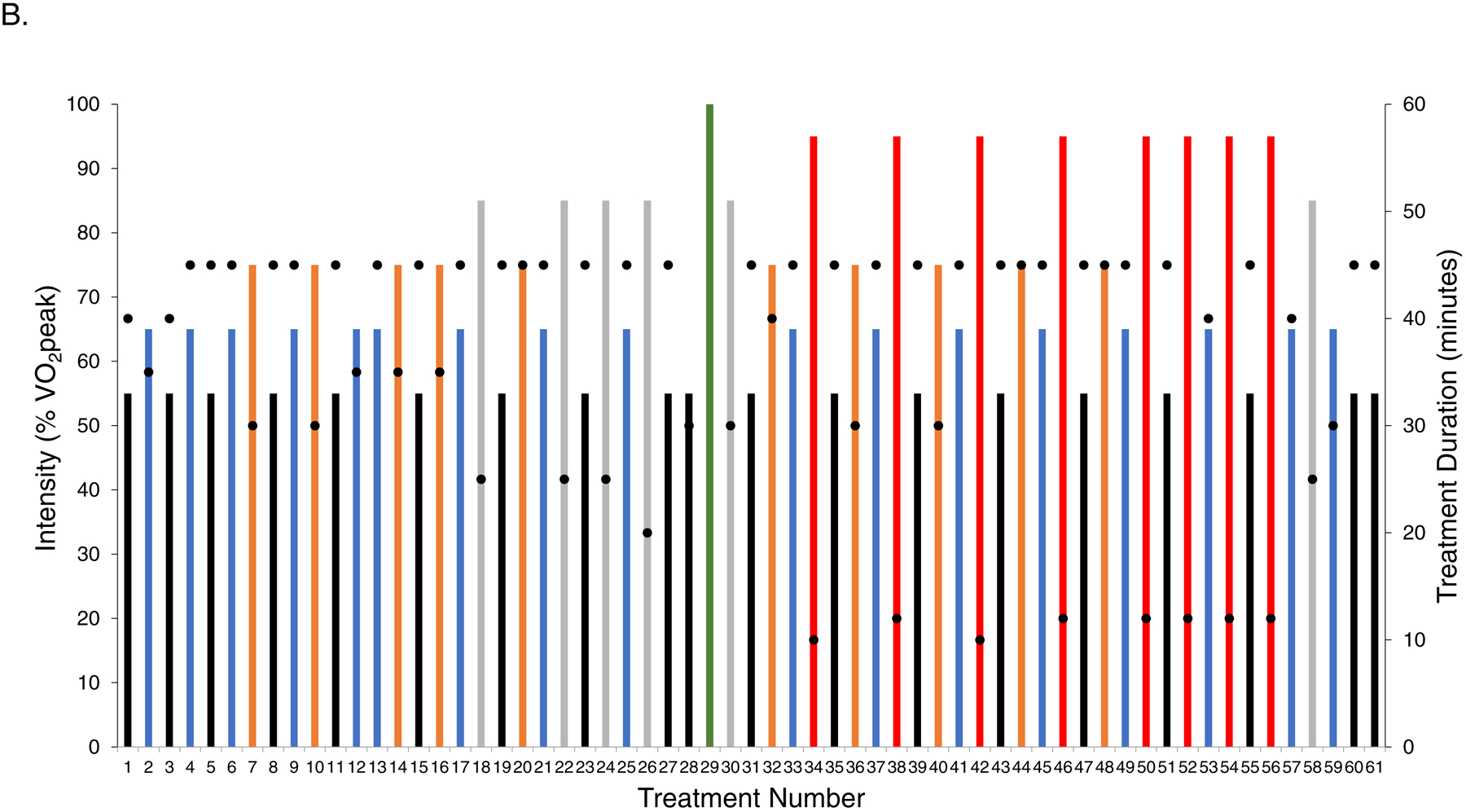
Planned intensity and duration of each individual session (i.e., dose) as well as the schedule of treatment dose across the study intervention period for (A) linear dosing and (B) non-linear dosing. The planned prescription depicts the intensity, duration, and scheduling of sessions under the assumption that no sessions were missed or dose modified, as per protocol. All doses were individualized to each patient on the basis of the pre-randomization cardiopulmonary exercise test (CPET). In the non-linear arm, at the end of week 8 the CPET was repeated to re-prescribe exercise intensity (green bar). The intensity of each session depicted by the colored bars as a percentage of VO2peak: (1) black – 55%, (2) blue – 65%, (3) orange – 70% to 75%, (4) grey – 85%, and (5) red – >95%. Black dots depict the planned duration of each session (mins), ranging from a minimum of 20 mins/session to a maximum of 45 mins/session. VO2peak, peak oxygen consumption.
Dose modification was permitted and performed using standardized criteria (Supplemental Table 1) in both dosing arms. “Planned” dose of all sessions was quantified as metabolic equivalent task (MET)-hours/session. The “planned” intensity of each session was multiplied by the corresponding session duration (20–60 mins) to calculate MET/session; all sessions were summed to derive total “planned” cumulative MET-hours (MET·hrs)/patient.16 For context, 40 minutes at 2.7 miles per hour (mph) and grade of 5% is equivalent to 4.1 MET·hrs/session; 25 minutes at 2.9 mph and a grade of 14% is equivalent to 3.4 MET·hrs /session.
AC consisted of 3 to 4 supervised stretching sessions per week, of 12 to 20 different positions for 20 to 45 secs/stretch following a standardized progressive approach for a total of 20 – 45 min/session.17
Endpoints
The primary endpoint was change in VO2peak, ml O2·kg−1·min−1 evaluated by a symptom-limited CPET on an electronic motorized treadmill with breath-by-breath expired gas analysis (ParvoMedics, TrueOne 2400, USA) with 12-lead ECG monitoring (Mac® 5000, GE Healthcare).18 Given intrapatient variability (e.g., learning effects) all patients performed two pre-randomization CPETs (≤7 days of each other) under identical laboratory conditions (Supplemental Figure 1). Quality or acceptability of each CPET was evaluated using American Thoracic Society guidelines.19 If both CPETs were deemed acceptable, data from the second CPET were used in analyses. Secondary endpoints were other CPET variables, PROs including quality of life (Functional Assessment of Cancer Therapy-Breast (FACT-B),20 and fatigue (FACIT-fatigue),21 tolerability and safety. Tolerability was assessed by rates of lost to follow up (LTF), attendance, permanent discontinuation, and dose modification.16 “Planned” and “completed” dose in all exercise sessions was quantified as metabolic expenditure/session, with relative dose intensity (RDI) defined as the ratio of total “completed” to total “planned” cumulative dose.16 Safety was evaluated by the type and prevalence of serious (e.g., important medical events) and non-serious adverse events (AEs) during exercise therapy sessions only. Non-protocol exercise was assessed using the Godin-Leisure Time Exercise Questionnaire.22 All endpoints were evaluated at pre-randomization and repeated ≤14 days after the final intervention session (Week 17). Tolerability and safety were evaluated over the entire intervention period.
Statistical Analysis
Power calculations were based on change in primary trial endpoint (VO2peak, ml O2·kg−1·min−1) for an overall F-test comparison and on the basis of two specified pairwise comparisons (AC with each exercise dosing regimen), with Bonferroni adjustment for two comparisons (α= 0.05/2 = 0.025). Comparison of LET and NLET was for exploratory purposes only. Power estimates were calculated using the following expected changes in mean VO2peak: LET: 1.5 ml O2·kg−1·min−1; NLET: 4.0 ml O2·kg−1·min−1; and AC: 0.0 ml O2·kg−1·min−1, assuming a standard deviation of 4.0 ml O2·kg−1·min−1 obtained from prior work among post therapy breast cancer patients.23, 24 With 174 patients (n=58/arm), >80% power was provided to detect ≥2.5 ml O2·kg−1·min−1 difference for comparison of exercise dosing arms with AC. Analyses were conducted under the intention-to-treat (ITT) principle.
Missing values for the primary endpoint were imputed with both multiple imputation using a Monte Carlo Markov single-chain method assuming a multivariate normal distribution and initial mean and covariance estimates derived using the expectation–maximization algorithm, and last observation carried forward (LOCF). Results of both approaches were similar thus only data from LOCF analyses are reported. LOCF was also used for missing values in secondary end points. Changes between baseline and week 17 were estimated for each patient individually; the mean change within each treatment group was used to estimate group differences. Given that outcome data was skewed, nonparametric tests were conducted in lieu of the originally planned F-tests. Wilcoxon signed rank tests were used to test for within-group and between-group differences in the mean change from baseline to week 17. The proportion of patients in each exercise dosing arm with a VO2peak improvement greater than the technical error (TE) was evaluated as previously described.25 In this trial, TE was calculated as 1.32 ml O2·kg−1·min−1. In unplanned subgroup analyses, Wilcoxon Signed Rank tests were used to evaluate whether the effects of each exercise dosing regimen (on VO2peak) within subgroups of select medical characteristics. Spearman correlation was used to evaluate the correlation between RDI with change in VO2peak (ml O2·kg−1·min−1) for LET and NLET. A two-sided significance level of 0.025 was used for the analysis of the primary endpoint. No adjustments were made for multiple comparisons. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
A total of 174 postmenopausal patients (mean 2.8 years post primary adjuvant therapy) were allocated to LET (n=58), NLET (n=59), or AC (n=57) (Figure 2). The study was conducted at Duke University Medical Center between November, 2010 and December, 2013, continuing at Memorial Sloan Kettering Cancer Center from May, 2016 to September, 2018 (for a total accrual period of five years), with final post-intervention testing conducted in September, 2018. Baseline characteristics were balanced between arms (Table 1). For the entire cohort, mean pre-randomization VO2peak was 21.9 ± 4.1 ml O2·kg−1·min−1, the equivalent of 5% and 28% below age-matched healthy sedentary or active women, respectively.11 No patient had evidence of systolic dysfunction (LVEF <53%26) at baseline. One (0.5%) and 14 (8%) patients at baseline and follow-up, respectively did not meet acceptable CPET criteria. Self-reported non-protocol exercise significantly increased in all study arms with differences between groups being not significant (p’s>0.05).
Figure 2.
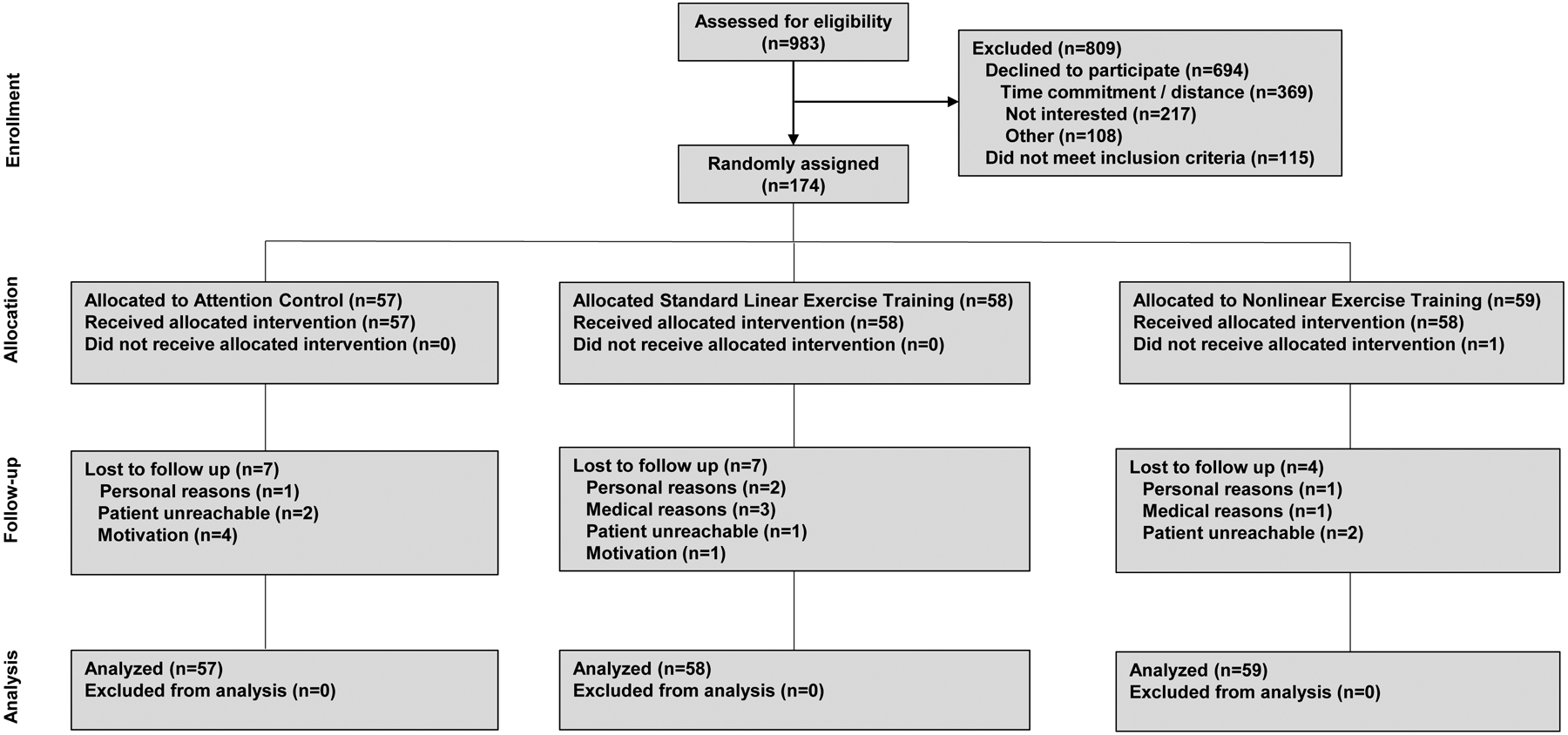
CONSORT Flow for Non-Pharmacological Trials.
Table 1.
Characteristics of the Participants
| Characteristic | All (n=174) | AC (n=57) | LET (n=58) | NLET (n=59) |
|---|---|---|---|---|
| Time (yrs) from completion of primary cancer treatment to enrollment – mean (SD) | 2.8 (1.3) | 2.7 (1.2) | 2.9 (1.1) | 2.8 (1.5) |
| Age (yrs) – mean (SD) | 58 (9) | 58 (9) | 59 (9) | 58 (9) |
| BMI (kg/m2) – mean (SD) | 29.5 (5.6) | 29.1 (5.2) | 30.1 (6.0) | 29.2 (5.6) |
| Exercise behavior (minutes/week)* – mean (SD) | 52.0 (103.6) | 43.6 (101.3) | 46.4 (113.4) | 65.6 (96.0) |
| Left ventricular ejection fraction (%) – mean (SD) | 62 (5) | 63 (5) | 63 (5) | 62 (4) |
| Study Site – no. (%) | ||||
| DUMC | 115 (66) | 38 (67) | 38 (66) | 39 (66) |
| MSK | 59 (34) | 19 (33) | 20 (35) | 20 (34) |
| Race – no. (%) | ||||
| Non-Hispanic white | 109 (63) | 35 (61) | 36 (62) | 38 (64) |
| Other group | 65 (37) | 22 (39) | 22 (38) | 21 (36) |
| Smoking – no. (%) | ||||
| Never | 106 (61) | 31 (54) | 37 (64) | 38 (64) |
| Former | 58 (33) | 22 (39) | 18 (31) | 18 (31) |
| Current | 10 (6) | 4 (7) | 3 (5) | 3 (5) |
| Disease stage – no. (%) | ||||
| I | 99 (57) | 32 (56) | 32 (55) | 35 (59) |
| II | 60 (35) | 22 (39) | 19 (33) | 19 (32) |
| III | 14 (8) | 3 (5) | 6 (10) | 5 (9) |
| Clinical subtype – no. (%) | ||||
| ER+/PR+ | 109 (63) | 40 (70) | 31 (53) | 38 (64) |
| HER2+ | 34 (20) | 10 (18) | 14 (24) | 10 (17) |
| ER−/PR−/HER2− | 30 (17) | 6 (12) | 13 (22) | 11 (19) |
| Adjuvant therapy – no. (%) | ||||
| Surgery | ||||
| Lumpectomy | 87 (50) | 30 (53) | 26 (45) | 31 (53) |
| Mastectomy | 87 (50) | 27 (47) | 32 (55) | 28 (48) |
| Chemotherapy– no. (%) | 102 (59) | 34 (60) | 33 (57) | 35 (59) |
| Anthracycline-containing* | 66 (65) | 21 (62) | 19 (58) | 26 (74) |
| Herceptin* | 24 (24) | 6 (18) | 10 (30) | 8 (23) |
| Radiotherapy – no. (%) | 123 (71) | 40 (70) | 38 (66) | 45 (76) |
| Left-sided | 65 (53) | 20 (50) | 22 (58) | 23 (51) |
| Endocrine therapy – no. (%) | 123 (71) | 39 (68) | 40 (69) | 44 (75) |
| Tamoxifen | 46 (37) | 12 (31) | 18 (45) | 16 (36) |
| Aromatase inhibitor | 85 (69) | 30 (77) | 25 (63) | 30 (68) |
| Current Medications | ||||
| Beta-blockers | 26 (15) | 8 (14) | 12 (21) | 6 (10) |
| ACE inhibitors | 22 (13) | 7 (12) | 8 (14) | 7 (12) |
| Angiotensin receptor blockers | 18 (10) | 8 (14) | 4 (7) | 6 (10) |
| Diuretic | 39 (22) | 11 (19) | 19 (33) | 9 (15) |
| Aspirin/Anti-Platelet | 38 (22) | 14 (25) | 13 (22) | 11 (19) |
| Statins | 40 (23) | 12 (21) | 13 (22) | 15 (25) |
| Calcium Channel Blocker | 16 (9) | 5 (9) | 7 (12) | 4 (7) |
| Pre-existing (controlled) cardiovascular conditions – no. (%) | ||||
| Coronary artery disease | 4 (2) | 1 (2) | 1 (2) | 2 (3) |
| Osteoporosis | 16 (9) | 2 (4) | 7 (12) | 7 (12) |
| Arthritis | 34 (20) | 14 (25) | 12 (21) | 8 (14) |
| Type II diabetes | 23 (13) | 10 (18) | 5 (9) | 8 (14) |
| Hyperlipidemia | 29 (17) | 9 (16) | 8 (14) | 12 (20) |
| Hypertension | 76 (44) | 27 (47) | 28 (48) | 21 (36) |
| Any | 102 (59) | 35 (61) | 37 (64) | 30 (51) |
All comparisons p>0.05
Exercise behavior defined as the total minutes of self-reported moderate/vigorous exercise per week.
Chemotherapy, radiation, and endocrine therapy rates include only those patients receiving each treatment.
Abbreviations: AC, attention control; LET, standard linear exercise training; NLET, non-linear exercise training; SD, standard deviation; DUMC, Duke University Medical Center; MSK, Memorial Sloan Kettering; BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor; ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blockers.
Tolerability and Safety
Tolerability is presented in Supplemental Table 2. LTF was 12% in LET and AC compared with 7% in NLET, whereas mean attendance was 64%, 75%, and 80% for AC, LET, and NLET, respectively (attendance range, 0% to 100% in all arms). The most common reasons for missed sessions were health related (e.g., fatigue) and non-health related (e.g., time constraints). Rates of permanent discontinuation and dose interruption were 19% and 57%, and 10% and 49% in LET and NLET, respectively. Mean RDI was 73% and 80% in LET and NLET, respectively. No serious AEs were observed. A total of 35 of 58 patients (60%) in LET, 36 of 59 (61%) in NLET, and 9 of 58 (15%) in AC experienced at least one non-serious AE (Supplemental Table 3).
Efficacy
VO2peak (ml O2·kg−1·min−1) increased 0.6 (± 1.7) ml O2·kg−1·min−1 (p=0.05) and 0.8 (± 1.8) ml O2·kg−1·min−1 (p=0.07) in LET and NLET, compared to AC (Table 2). Change in VO2peak ranged from −2.7 to 4.1 ml O2·kg−1·min−1 and −3.6 to 5.1 ml O2·kg−1·min−1 in LET and NLET, respectively (Figure 3). The proportion VO2peak ‘responders’ (Δ ≥1.32 ml O2·kg−1·min−1) was 36% and 40% in LET and NLET, respectively (p=0.25). Significant within arm improvements were observed for several secondary cardiorespiratory fitness end points in both exercise dosing regimens, with few significant in comparison to AC (Table 2). FACT-B increased 1.7 (± 10.4) (p=0.22) points and 3.7 (± 7.7) (p=0.005) points in LET and NLET, respectively (Table 2) compared to AC. Significant improvements in fatigue were observed in both dosing regimens compared to AC (Table 2). In general, no differences between exercise dosing regimens was observed for any study endpoints (Supplemental Table 4).
Table 2.
Effects on Primary and Secondary End Points (Intention-to-Treat Analysis).
| AC | LET | NET | Mean Difference Between Arms | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Baseline | Week 17 | P† | Baseline | Week 17 | P† | Baseline | Week 17 | P† | LET vs. AC | P§ | NLET vs. AC | P§ | ||||
| Primary End Point | |||||||||||||||||
| VO2peak, ml O2·kg−1·min−1 | 21.8 (3.8) | 21.8 (3.7) | 0.89 | 21.5 (4.4) | 22.2 (4.6) | 0.003 | 22.2 (4.3) | 23.1 (4.8) | 0.002 | 0.6 (1.7) | 0.05 | 0.8 (1.8) | 0.07 | ||||
| Secondary End Points | |||||||||||||||||
| Resting Cardiopulmonary Function | |||||||||||||||||
| Heart rate, beats·min−1 | 77.9 (13.3) | 76.6 (11.6) | 0.34 | 75.8 (12.1) | 71.8 (9.2) | 0.001 | 75.0 (11.6) | 73.2 (12.1) | 0.06 | −2.6 (8.5) | 0.15 | −0.5 (8.6) | 0.55 | ||||
| Systolic blood pressure, mmHg | 123.4 (15.0) | 120.9 (14.7) | 0.13 | 123.6 (13.5) | 120.3 (14.2) | 0.04 | 119.5 (14.0) | 116.0 (14.6) | 0.06 | −0.7 (11.4) | 0.55 | −1.0 (12.3) | 0.52 | ||||
| Diastolic blood pressure, mmHg | 75.4 (8.1) | 73.2 (8.7) | 0.04 | 74.1 (9.7) | 72.0 (7.8) | 0.05 | 74.7 (8.2) | 71.1 (8.9) | <0.001 | 0.2 (8.5) | 0.85 | −1.4 (7.9) | 0.25 | ||||
| Peak Cardiopulmonary Function | |||||||||||||||||
| VO2peak, ml O2·min−1 | 1684.6 (299.5) | 1693.3 (314.7) | 0.66 | 1714.6 (330.0) | 1762.1 (369.2) | 0.005 | 1735.9 (377.7) | 1778.9 (378.8) | 0.05 | 38.0 (129.0) | 0.13 | 34.2 (154.0) | 0.27 | ||||
| RER | 1.1 (0.1) | 1.1 (0.1) | 0.82 | 1.1 (0.1) | 1.1 (0.1) | 0.12 | 1.1 (0.1) | 1.1 (0.1) | 0.88 | 0.0 (0.1) | 0.29 | 0.0 (0.1) | 0.85 | ||||
| Ventilation, L O2·min−1 | 62.8 (14.2) | 62.5 (12.7) | 0.60 | 64.1 (14.5) | 63.5 (14.0) | 0.41 | 65.4 (14.1) | 65.9 (13.7) | 0.68 | −0.3 (6.4) | 0.61 | 0.7 (7.1) | 0.57 | ||||
| Heart rate, beats·min−1 | 162.2 (17.8) | 160.9 (18.9) | 0.35 | 161.5 (15.0) | 156.6 (16.0) | <0.001 | 165.2 (16.3) | 163.9 (17.1) | 0.20 | −3.5 (10.1) | 0.06 | 0.0 (8.5) | 0.59 | ||||
| Systolic blood pressure, mmHg | 174.4 (22.3) | 176.6 (22.4) | 0.51 | 175.5 (18.5) | 175.5 (22.3) | 0.81 | 172.9 (22.1) | 176.2 (23.6) | 0.39 | −1.5 (12.4) | 0.92 | 0.7 (13.8) | 0.65 | ||||
| Diastolic blood pressure, mmHg | 68.2 (10.1) | 70.9 (10.7) | 0.07 | 70.7 (11.7) | 69.8 (13.0) | 0.57 | 70.8 (11.3) | 71.9 (11.0) | 0.66 | −2.3 (7.5) | 0.04 | −1.1 (8.0) | 0.12 | ||||
| FACT-B Total (0–140) | 107.6 (16.3) | 108.9 (16.2) | 0.31 | 104.8 (17.2) | 107.8 (20.7) | 0.05 | 111.6 (14.1) | 116.7 (14.0) | <0.001 | 1.7 (10.4) | 0.22 | 3.7 (7.7) | 0.005 | ||||
| FACT-G Total (0–108) | 87.6 (13.5) | 88.4 (13.6) | 0.49 | 85.3 (13.6) | 87.1 (16.8) | 0.11 | 90.33 (11.6) | 93.8 (11.3) | <0.001 | 1.0 (9.1) | 0.27 | 2.6 (6.7) | 0.02 | ||||
| FACIT-Fatigue (0–52) | 39.6 (10.9) | 39.9 (10.7) | 0.36 | 36.7 (11.9) | 39.5 (12.2) | 0.002 | 42.8 (8.9) | 44.8 (9.0) | 0.01 | 2.5 (7.8) | 0.001 | 1.7 (6.5) | 0.01 | ||||
Data presented as mean (SD)
Paired Wilcoxon Signed Rank p-value for change within group from baseline to week 17
Wilcoxon Rank Sum p-value for delta change between groups from baseline to week 17.
Abbreviations: AC, attention control; LET, standard linear exercise training; NLET, non-linear exercise training; VO2peak, peak oxygen consumption; FACT, Functional Assessment of Cancer Therapy, FACIT, Functional Assessment of Chronic Illness Therapy.
Figure 3.
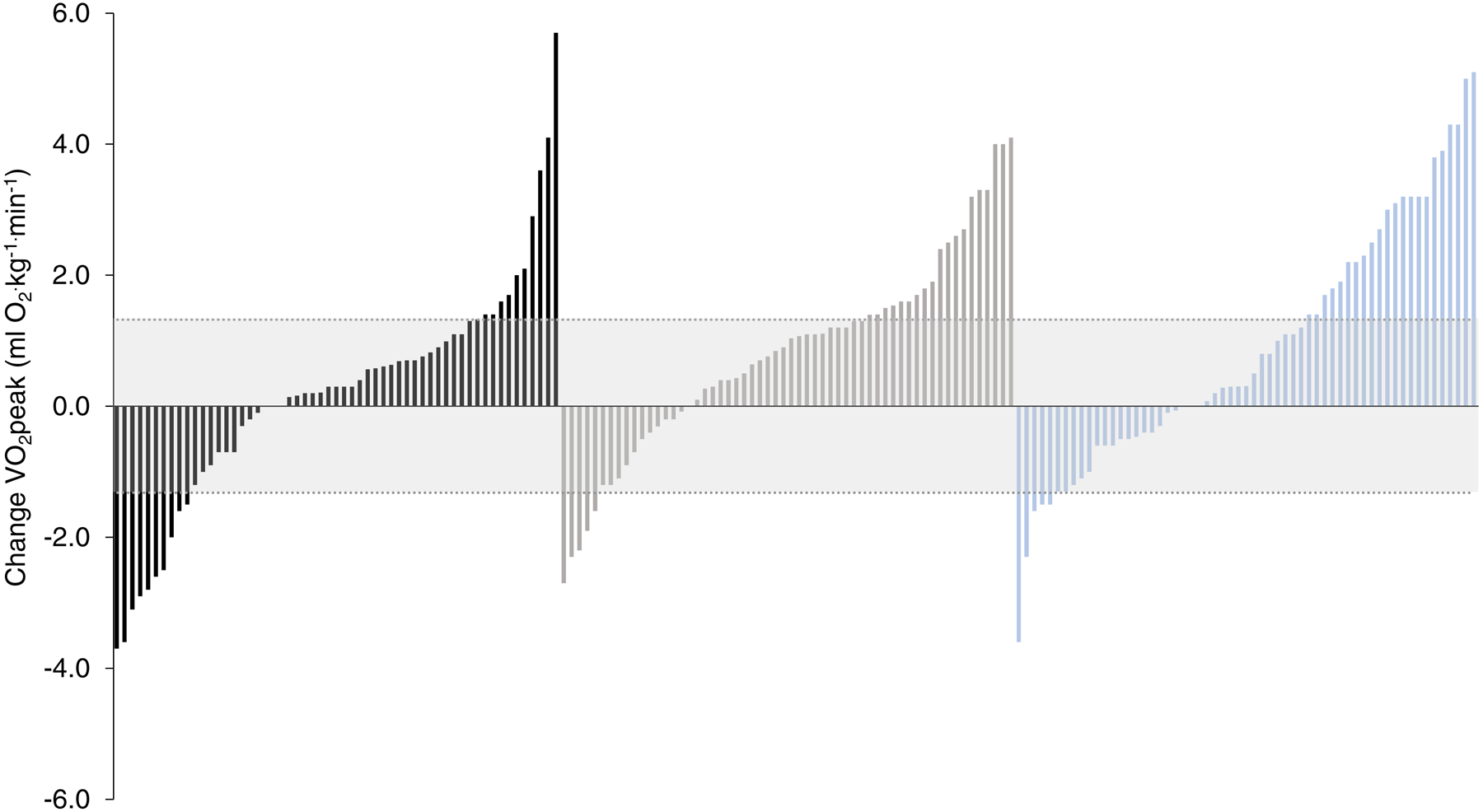
Waterfall plots for change in VO2peak. The technical error (TE) for VO2peak (Δ1.32 mL O2·kg−1·min−1) is illustrated by the shaded area. Patients with a change in VO2peak greater than TE classified as responders. Black bars: attention control; grey bars: standard linear exercise training; blue bars: non-linear exercise training. VO2peak, peak oxygen consumption.
In stratified analyses, in comparison to AC, improvement in VO2peak in LET was superior among younger patients and those not previously treated with anthracyclines or receiving current endocrine therapy. For NLET, improvement in VO2peak was superior for younger patients, lower BMI at baseline, and those not previously treated with anthracyclines or left-sided radiation or receiving current endocrine therapy (Supplemental Table 5). RDI did not predict change in VO2peak in either exercise dosing arm (Spearman correlation p=0.33 and p=0.61 for LET and NLET, respectively).
DISCUSSION
Findings of the present trial demonstrated that exercise training using either a linear (standard) or non-linear dosing scheduling approach is safe and tolerable but associated with only modest improvements in CRF and PROs among post-treatment primary breast cancer patients. Our findings are in stark contrast with previously reported smaller trials evaluating the efficacy of structured exercise training in postmenopausal patients with primary breast cancer as well as those in other post-treatment cancer populations. For instance, in the Rehabilitation Exercise for Health After Breast Cancer (REHAB) trial,27 VO2peak, evaluated by CPET, increased by 2.7 ml O2·kg−1·min−1 (~14% improvement) following 15 weeks of linear dosed aerobic training (i.e., cycle ergometry) compared with a 0.6 O2·kg−1·min−1 decrease in the non-intervention arm among 53 patients with primary breast cancer. These findings were corroborated in a recent meta-analysis among post-treatment cancer patients reporting exercise training (the majority of which followed a standard linear dosing schedule) increased VO2peak, on average, by 2.45 ml O2·kg−1·min−1 (~10% improvement) compared to a non-intervention control arm.7 The magnitude of exercise training-induced improvements in VO2peak observed in post-treatment cancer patients is also similar to that reported in other chronic diseases, with, in general, ~10% to 15% improvements in VO2peak following standard dosing regimens.28–30
There are important distinct differences between our study and previous trials of exercise training in clinical populations that may contribute to the observed discrepant findings. First, our trial carefully adhered to the ITT principle, with all randomized patients included in the analyses regardless of LTF or adherence to the intervention. Second, our trial only recruited patients with impaired CRF; physiological response to exercise training is likely distinct in patients with cardiovascular impairment, especially in those pre-treated with anticancer therapy.31 Third, several prior studies, especially those conducted in cancer, measured CRF using estimated VO2peak calculated from submaximal (indirect) methods as opposed direct measurement using CPET procedures; estimated methodologies are less reliable and may overestimate treatment effects.32 Finally, and perhaps most important, consistent with guidelines19 we conducted two pre-randomization CPETs to minimize the confounding impact of learning effects / variability on exercise treatment response. Specifically, in our trial, we observed that VO2peak increased on average ~0.9 ml O2·kg−1·min−1 (~4%) from the first ‘familiarization’ CPET to the second CPET. These findings indicate that learning effects may contribute to the observed VO2peak benefit reported in previously reported trials.
Another noteworthy finding was the considerable heterogeneity in VO2peak response observed among primary breast cancer patients in response to two uniformly controlled and tightly implemented exercise dosing regimens. Most prior exercise-oncology RCTs have focused on the overall treatment effect for the entire study population, adhering to underlying assumption that all patients within a given population respond equally to exercise training – i.e., a one-size-fits-all approach.31 Nevertheless, several recent trials outside of oncology report variability in exercise response. For instance, in the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION), mean change in VO2peak was 0.6 ml O2·kg−1·min−1 following 12 weeks of standard linear-dosed aerobic training whereas individual response ranged from −12 ml O2·kg−1·min−1 (−83%) to 14 ml O2·kg−1·min−1 (+97%); similar to our findings, an improvement in VO2peak above TE was observed in ~40% of patients.33 In our prior work, mean change in VO2peak was ~9% among men with clinically localized prostate cancer following a non-linear dosing regimen whereas individual response ranged from −18% to +32%.34 Hence, cardiovascular response to standard as well as non-linear dosing schedules following the conventional amount (~150 minutes/week) and length (12 to 16 weeks) not only displays considerable heterogeneity but also more importantly, appears suboptimal for the majority of patients following treatment for primary breast cancer.
Interestingly, increasing the dose of exercise training (via amount or intensity) reduced the number of low VO2peak responders in sedentary obese adults compared with a conventional dose and amount of exercise.25 Whether such an approach will confer similar benefit in clinical populations such as post-treatment breast cancer patients is not known. However, comparison of the typical VO2peak response to standard exercise training in middle-aged apparently healthy women (~20% improvement) appears to indicate breast cancer patients have a blunted or abnormal physiological response to exercise training, possibly due to the cardiovascular toxic effects of anticancer therapy. Treatment with anthracyclines and left-sided radiation, both established determinants of cardiotoxicity,35, 36 as well as current endocrine therapy,37 were associated with a blunted VO2peak response in this trial. In addition to impairing central determinants of exercise tolerance,24, 38, 39 cytotoxic therapy may also impact peripheral mechanisms in skeletal muscle.40 Hence, a more personalized medicine paradigm may be required involving pretreatment evaluation of multiple patient characteristics permitting stratification of patients with a common but heterogeneous condition into homogeneous subgroups (phenogroups), with subsequent design and testing of phenogroup-targeted (personalized) exercise prescriptions that includes aerobic training and / or resistance training.31 Such a paradigm has not been tested in clinical exercise medicine; however ‘proof-of-concept’ has recently been demonstrated in nutritional science.41
On the basis of our prior work demonstrating the extent and clinical importance of impaired CRF in post-treatment breast cancer patients,1 the non-linear dosing regimen was designed specifically to target improvements in VO2peak; this is consistent with the fundamental exercise tenet of specificity.15 Nevertheless, as observed in the present trial even when exercise dosing regimens are targeted to a specific end points such as VO2peak, benefit is observed in other ‘non-targeted’ end points such as PROs even when the magnitude of VO2peak improvement was modest. This reflects the broad beneficial effects of exercise. However, the magnitude of exercise-induced improvements in PROs was again smaller than that reported in prior work.27, 42 These discrepancies may be explained by differences in the nature of the control groups used in these studies. Our trial utilized an AC condition matched to the exercise therapy arms for frequency and duration of social contact as well as setting – the majority of prior trials have employed non-intervention control conditions, with little interaction with other study participants or study personnel during the intervention period.7 Our findings suggest that social interaction likely contributes, at least in part, to the reported favorable effects of exercise on PROs. Second, the observed changes in study end points was, in general, equivalent between the two exercise therapy dosing regimens. NLET, however, had several important pragmatic advantages as characterized by lower rates of LTF, treatment discontinuation, and dose interruptions (resulting in a higher RDI). Further, completion of the NLET prescription required approximately 30 minutes per week less time compared to LET. The higher tolerability and quality of life efficacy of the NLET dosing regimen may be related to the variety of treatment sessions together with the overall shorter duration – such factors may be critical antecedents of generalizability and uptake in clinical practice. Finally, the standard approach in exercise trials of solely reporting attendance provides limited insight into the actual tolerability in a given clinical setting.16 Precise details such as RDI as well as pre- and during session dose modification underscores the importance of real-time monitoring of exercise sessions by qualified exercise personnel in clinical populations to maximize exercise benefit and safety. In totality therefore, our findings, together with prior work, support the recommendation of structured exercise therapy for post-treatment patients with primary breast cancer.8–10
Our trial limitations require consideration. First, our results are limited to less active and unfit breast cancer patients and do not generalize to those with normal CRF in which exercise training feasibility (tolerability) and response may be distinct. Second, we likely recruited a cohort of breast cancer patients highly motivated to voluntarily participate in a supervised lifestyle intervention that may impact the generalizability of our findings to all post-treatment breast cancer patients. The generalizability of our findings are also limited by the low participation rate (18% of eligible patients). Not surprisingly, the major reasons for non-participation related to inconvenience (i.e., time commitment, travel distance) indicating that integration of ‘site-less’ telemedicine solutions that enable exercise sessions and other study procedures to be conducted remotely may provide several distinct advantages to the conduct of exercise training investigations.4, 43 Third, our trial consisted of highly structured and monitored exercise therapy sessions with individualized supervision; thus, the feasibility, safety, and efficacy could differ under other conditions. Other limitations include the short length of treatment intervention and lack of long-term follow-up.
In conclusion, exercise therapy implemented using either a standard linear or alternative non-linear dosing schedule was tolerable and safe but associated with only modest improvements in CRF among post-treatment patients with primary breast cancer. These findings provide important information regarding the design and refinement of dosing regimens as well as clinical generalizability of exercise therapy in cancer as well as other clinical populations.
Supplementary Material
Clinical Perspective.
What is new?
Women with post-treatment primary breast cancer have significant impairments in global cardiovascular function that predisposes to increased risk of long-term cardiovascular disease.
We evaluated the efficacy of two different exercise dosing regimens (i.e., standard ‘fixed’ dosing versus non-linear dosing) compared to control on cardiorespiratory fitness in 174 post-treatment women with primary breast cancer.
Exercise training, independent of dosing schedule, was associated with only modest improvements in cardiorespiratory fitness for the overall cohort although considerable heterogeneity was observed.
What are the clinical implications?
Structured exercise training is a safe and tolerable intervention for post-treatment breast cancer patients associated with modest improvements in cardiorespiratory fitness and patient-reported outcomes.
Nevertheless, exercise training prescribed at the conventional amount (~150 minutes/week) and length (~16 weeks) is associated with suboptimal improvements in cardiorespiratory fitness for a high proportion of women following treatment for primary breast cancer.
Exercise programs of greater length or amount may be required to induce meaningful improvements in post-treatment patients with primary breast cancer.
Acknowledgment.
We would like to especially thank the study participants and their families.
Sources of Funding. This study was supported by a research grant from the National Cancer Institute (R01-CA142566) awarded to LWJ and grants from AKTIV Against Cancer and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Non-standard Abbreviations and Acronyms
- AC
Attention Control
- AE
Adverse Event
- CPET
Cardiopulmonary Exercise Test
- CRF
Cardiorespiratory Fitness
- ET
Exercise Therapy
- ITT
Intention-to-treat
- LET
Linear Exercise Therapy
- LOCF
Last Observation Carried Forward
- LTF
Lost to Follow Up
- NLET
Non-Linear Exercise Therapy
- MET
Metabolic Equivalent Task
- PROs
Patient Reported Outcomes
- RDI
Relative Dose Intensity
- TE
Technical Error
- VO2peak
Peak Oxygen Consumption
Footnotes
Disclosures. LWJ – stock ownership in Pacylex, Inc.
REFERENCES
- 1.Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, Hornsby WE, Coan AD, Herndon JE 2nd, Douglas PS, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koelwyn GJ, Jones LW and Moslehi J. Unravelling the causes of reduced peak oxygen consumption in patients with cancer: complex, timely, and necessary. J Am Coll Cardiol. 2014;64:1320–1322. [DOI] [PubMed] [Google Scholar]
- 3.Cupit-Link MC, Kirkland JL, Ness KK, Armstrong GT, Tchkonia T, LeBrasseur NK, Armenian SH, Ruddy KJ and Hashmi SK. Biology of premature ageing in survivors of cancer. ESMO Open. 2017;2:e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott JM, Dolan LB, Norton L, Charles JB and Jones LW. Multisystem Toxicity in Cancer: Lessons from NASA’s Countermeasures Program. Cell. 2019;179:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gernaat SAM, Ho PJ, Rijnberg N, Emaus MJ, Baak LM, Hartman M, Grobbee DE and Verkooijen HM. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res Treat. 2017;164:537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS and Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. [DOI] [PubMed] [Google Scholar]
- 7.Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, Moskowitz CS, Matsoukas K, Iyengar NM, Dang CT, et al. Efficacy of Exercise Therapy on Cardiorespiratory Fitness in Patients With Cancer: A Systematic Review and Meta-Analysis. J Clin Oncol. 2018;36:2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cormie P, Atkinson M, Bucci L, Cust A, Eakin E, Hayes S, McCarthy S, Murnane A, Patchell S and Adams D. Clinical Oncology Society of Australia position statement on exercise in cancer care. Med J Aust. 2018;209:184–187. [DOI] [PubMed] [Google Scholar]
- 9.Denlinger CS, Sanft T, Baker KS, Broderick G, Demark-Wahnefried W, Friedman DL, Goldman M, Hudson M, Khakpour N, King A, et al. Survivorship, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2018;16:1216–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, Zucker DS, Matthews CE, Ligibel JA, Gerber LH, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51:2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzgerald MD, Tanaka H, Tran ZV and Seals DR. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: a meta-analysis. J Appl Physiol. 1997;83:160–165. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic S and American College of Chest P. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. [DOI] [PubMed] [Google Scholar]
- 13.Denlinger CS, Sanft T, Baker KS, Baxi S, Broderick G, Demark-Wahnefried W, Friedman DL, Goldman M, Hudson M, Khakpour N, et al. Survivorship, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1140–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seiler KS and Kjerland GO. Quantifying training intensity distribution in elite endurance athletes: is there evidence for an “optimal” distribution? Scand J Med Sci Sports. 2006;16:49–56. [DOI] [PubMed] [Google Scholar]
- 15.Sasso JP, Eves ND, Christensen JF, Koelwyn GJ, Scott J and Jones LW. A framework for prescription in exercise-oncology research. J Cachexia Sarcopenia Muscle. 2015;6:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsen TS, Scott JM, Michalski M, Capaci C, Thomas S, Herndon JE 2nd, Sasso J, Eves ND and Jones LW. Novel Methods for Reporting of Exercise Dose and Adherence: An Exploratory Analysis. Med Sci Sports Exerc. 2018;50:1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott JM, Iyengar NM, Nilsen TS, Michalski M, Thomas SM, Herndon J 2nd, Sasso J, Yu A, Chandarlapaty S, Dang CT, et al. Feasibility, safety, and efficacy of aerobic training in pretreated patients with metastatic breast cancer: A randomized controlled trial. Cancer. 2018;124:2552–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. [DOI] [PubMed] [Google Scholar]
- 19.ATS/ACCP. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. [DOI] [PubMed] [Google Scholar]
- 20.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M and Shiomoto G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. [DOI] [PubMed] [Google Scholar]
- 21.Lai JS, Cella D, Chang CH, Bode RK and Heinemann AW. Item banking to improve, shorten and computerize self-reported fatigue: an illustration of steps to create a core item bank from the FACIT-Fatigue Scale. Qual Life Res. 2003;12:485–501. [DOI] [PubMed] [Google Scholar]
- 22.Godin G and Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 23.Jones LW, Haykowsky M, Peddle CJ, Joy AA, Pituskin EN, Tkachuk LM, Courneya KS, Slamon DJ and Mackey JR. Cardiovascular risk profile of patients with HER2/neu-positive breast cancer treated with anthracycline-taxane-containing adjuvant chemotherapy and/or trastuzumab. Cancer Epidemiol Biomarkers Prev. 2007;16:1026–1031. [DOI] [PubMed] [Google Scholar]
- 24.Jones LW, Haykowsky M, Pituskin EN, Jendzjowsky NG, Tomczak CR, Haennel RG and Mackey JR. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor--positive operable breast cancer. Oncologist. 2007;12:1156–1164. [DOI] [PubMed] [Google Scholar]
- 25.Ross R, de Lannoy L and Stotz PJ. Separate Effects of Intensity and Amount of Exercise on Interindividual Cardiorespiratory Fitness Response. Mayo Clin Proc. 2015;90:1506–1514. [DOI] [PubMed] [Google Scholar]
- 26.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. [DOI] [PubMed] [Google Scholar]
- 27.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ and Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21:1660–1668. [DOI] [PubMed] [Google Scholar]
- 28.Ellingsen O, Halle M, Conraads V, Stoylen A, Dalen H, Delagardelle C, Larsen AI, Hole T, Mezzani A, Van Craenenbroeck EM, et al. High-Intensity Interval Training in Patients With Heart Failure With Reduced Ejection Fraction. Circulation. 2017;135:839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haykowsky MJ, Timmons MP, Kruger C, McNeely M, Taylor DA and Clark AM. Meta-analysis of aerobic interval training on exercise capacity and systolic function in patients with heart failure and reduced ejection fractions. Am J Cardiol. 2013;111:1466–1469. [DOI] [PubMed] [Google Scholar]
- 30.Gomes-Neto M, Duraes AR, Reis H, Neves VR, Martinez BP and Carvalho VO. High-intensity interval training versus moderate-intensity continuous training on exercise capacity and quality of life in patients with coronary artery disease: A systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24:1696–1707. [DOI] [PubMed] [Google Scholar]
- 31.Scott JM, Nilsen TS, Gupta D and Jones LW. Exercise Therapy and Cardiovascular Toxicity in Cancer. Circulation. 2018;137:1176–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross R, Blair SN, Arena R, Church TS, Despres JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e653–e699. [DOI] [PubMed] [Google Scholar]
- 33.Leifer ES, Brawner CA, Fleg JL, Kraus WE, Whellan DJ, Pina IL and Keteyian SJ. Are there negative responders to exercise training among heart failure patients? Med Sci Sports Exerc. 2014;46:219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones LW, Hornsby WE, Freedland SJ, Lane A, West MJ, Moul JW, Ferrandino MN, Allen JD, Kenjale AA, Thomas SM, et al. Effects of nonlinear aerobic training on erectile dysfunction and cardiovascular function following radical prostatectomy for clinically localized prostate cancer. Eur Urol. 2014;65:852–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khouri MG, Douglas PS, Mackey JR, Martin M, Scott JM, Scherrer-Crosbie M and Jones LW. Cancer therapy-induced cardiac toxicity in early breast cancer: addressing the unresolved issues. Circulation. 2012;126:2749–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. [DOI] [PubMed] [Google Scholar]
- 37.Amir E, Seruga B, Niraula S, Carlsson L and Ocana A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103:1299–1309. [DOI] [PubMed] [Google Scholar]
- 38.Koelwyn GJ, Lewis NC, Ellard SL, Jones LW, Gelinas JC, Rolf JD, Melzer B, Thomas SM, Douglas PS, Khouri MG, et al. Ventricular-Arterial Coupling in Breast Cancer Patients After Treatment With Anthracycline-Containing Adjuvant Chemotherapy. Oncologist. 2016;21:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beaudry RI, Howden EJ, Foulkes S, Bigaran A, Claus P, Haykowsky MJ and Gerche A. Determinants of exercise intolerance in breast cancer patients prior to anthracycline chemotherapy. Physiol Rep. 2019;7:e13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilliam LA and St Clair DK. Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxid Redox Signal. 2011;15:2543–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell. 2015;163:1079–1094. [DOI] [PubMed] [Google Scholar]
- 42.Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Sweeney FC, Stewart C, Buchanan TA, Spicer D, Tripathy D, et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Res. 2018;20:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Topol EJ. A decade of digital medicine innovation. Sci Transl Med. 2019;11 DOI: 10.1126/scitranslmed.aaw7610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


