Abstract
In this paper we summarize the state of the science of measurements of dry deposition of reactive nitrogen (Nr) compounds in North America, beginning with current understanding of the importance of dry deposition at the U.S. continental scale followed by a review of micrometeorological flux measurement methods. We then summarize measurements of Nr air-surface exchange in natural ecosystems of North America focusing on the U.S. and Canada. Drawing on thissynthesis, we identify research needed to address the incompleteness of dry deposition budgets, more fully characterize temporal and geographical variability of fluxes, and better understand air-surface exchange processes.
Our review points to several data and knowledge gaps that must be addressed to advance air-surface exchange modeling for North American ecosystems. For example, recent studies of particulate (NO3−) and gaseous (NOx, HONO, peroxy nitrates) oxidized N fluxes challenge the fundamental framework of unidirectional flux from the atmosphere to the surface employed in most deposition models. Measurements in forest ecosystems document the importance of in-canopy chemical processes in regulating the net flux between the atmosphere and biosphere, which can result in net loss from the canopy. These results emphasize the need for studies to quantify within- and near-canopy sources and sinks of the full suite of components of the Nr chemical system under study (e.g., NOy or HNO3-NH3-NH4NO3). With respect to specific ecosystems and geographical locations, additional flux measurements are needed particularly in agricultural regions (NH3), coastal zones (NO3− and organic N), and arid ecosystems and along urban to rural gradients (NO2). Measurements that investigate non-stomatal exchange processes (e.g., surface wetness) and the biogeochemical drivers of bidirectional exchange (e.g., NH3) are considered high priority. Establishment of long-term sites for process level measurements of reactive chemical fluxes should be viewed as a high priority long-term endeavor of the atmospheric chemistry and ecological communities.
Introduction
Atmospheric deposition is an important component of the nitrogen cascade (Galloway et al., 2003) that can contribute to eutrophication and acidification, reduced biodiversity, decreased resilience to climate variability and other effects in terrestrial and aquatic ecosystems (U.S. EPA, 2008). Deposition of reactive nitrogen (Nr) in excess of the ecosystem critical load (Nilsson and Grennfelt, 1988) can therefore negatively impact the services that ecosystems provide, such as clean water, climate regulation, food, recreational opportunities, and cultural and spiritual value (Compton et al., 2011; Cooter et al., 2013; Munns et al., 2016). A fundamental aspect of characterizing ecosystem risk from Nr over-enrichment is quantification of the amount of Nr entering the ecosystem via wet and dry deposition. Total (wet + dry) budgets of Nr deposition are needed to quantify critical load exceedances (Clark et al., 2018) and to relate air concentrations to deposition rates to assess the secondary U.S. National Ambient Air Quality Standards (NAAQS, U.S. EPA, 2012), which protect public welfare (e.g., soils, water, vegetation).
North American monitoring networks that are used for deposition assessments include sites at which wet deposition is measured directly and sites where atmospheric concentrations of gases and particulate matter (PM) are monitored. The key networks measuring wet deposition and precipitation chemistry in North America are the National Atmospheric Deposition Program (NADP)/National Trends Network (NTN) and the Canadian Air and Precipitation Monitoring Network (CAPMoN). The NADP/NTN network (http://nadp.slh.wisc.edu/NTN/) spans the continental U.S. (CONUS), extending into Canada, Puerto Rico, Mexico, and Alaska, and currently operates 257 sites at which precipitation chemistry and wet deposition are measured on a weekly basis. CAPMoN (http://data.ec.gc.ca/data/air/monitor/networks-and-studies/canadian-air-and-precipitation-monitoring-network-capmon/) currently operates 30 sites at which precipitation chemistry and wet deposition are measured on a daily basis. With respect to wet deposition of Nr, NTN and CAPMoN provide measurements of ammonium (NH4+) and nitrate (NO3−). The Clean Air Status and Trends Network (CASTNET) (https://www.epa.gov/castnet) and CAPMoN monitor gas and particulate air concentrations of nitric acid (HNO3), NH4+aerosol, and NO3− aerosol, on weekly and daily schedules, respectively. CASTNET currently operates 95 rural sites across the CONUS while CAPMoN operates 18 ambient monitoring sites across Canada. Additionally, the NADP Ammonia Monitoring Network (AMoN) provides bi-weekly measurements of ammonia (NH3) air concentrations at approximately 100 sites in the U.S. and Canada, approximately 70 of which are collocated with CASTNET and CAPMoN. Other ambient monitoring networks that measure Nr compounds include the Interagency Monitoring of Protected Visual Environments (IMPROVE, http://vista.cira.colostate.edu/Improve/), several networks that collectively feed data into the U.S. Environmental Protection Agency Air Quality System (AQS, https://www.epa.gov/aqs; https://www.epa.gov/amtic/amtic-ambient-air-monitoring-networks) and the Canadian National Air Pollution Surveillance Program (NAPS; https://www.canada.ca/en/environment-climate-change/services/air-pollution/monitoring-networks-data/national-air-pollution-program.html).
While wet deposition is well characterized by NADP and CAPMoN, the magnitude and patterns of dry deposition are less understood due to a lack of observations. Ambient monitoring networks provide important information on the spatial patterns and trends in air concentrations of inorganic Nr, to which the patterns of dry deposition are related. However, these networks do not provide measurements of dry deposition. Rather, the measured air concentrations are used to estimate dry deposition using inferential modeling approaches (Clarke et al., 1997; Sickles and Shadwick, 2015; Zhang et al., 2009; Li et al., 2016) or by applying deposition velocities output from chemical transport models (CTMs) (Bowker et al., 2011; Schwede and Lear, 2014). These networks also do not provide information on organic forms of N (ON), which contribute significantly to total Nr deposition (Jickells et al., 2013). Enhanced monitoring of additional N species (nitric oxide (NO), nitrogen dioxide (NO2), total oxides of nitrogen (NOy), peroxyacetyl nitrate (PAN), other ON) is, however, periodically conducted at select sites within CASTNET and CAPMoN for deposition and atmospheric chemistry assessments (Zhang et al., 2009).
Most direct measurements (i.e., using micrometeorological flux methods) of air-surface exchange (i.e., dry deposition and bidirectional exchange) span periods of a few weeks to months, failing to capture the range of atmospheric, biogeochemical, and phenological conditions that drive annual scale fluxes. Such measurements are typically conducted to characterize exchange processes rather than to develop annual speciated dry deposition budgets. Inferential models or CTMs are therefore commonly used to estimate the dry component (Fenn et al., 2010; Schwede and Lear, 2014; Nanus et al., 2017; McDonnell et al., 2018; U.S. EPA, 2019) of total deposition for North American ecosystem assessments. Though estimates vary depending on the time period of the model simulation and the particular model that is used, dry deposition of Nr generally contributes 50% or more of CTM derived total N deposition budgets across the CONUS (Zhang et al., 2012a; Dennis et al., 2013; Zhang et al., 2018). While the importance of dry deposition is well established, dry deposition models used at the field scale and employed in CTMs can exhibit large uncertainty. Assessments in Europe (Flechard et al., 2011) and the U.S. (Schwede et al., 2011; Li et al., 2016; Wu et al., 2011, 2012) show that fluxes of Nr species from commonly used models can differ substantially, up to a factor of 3 or more, even when using the same meteorological inputs and surface parameters. Variability among models reflects differences in parameterizations of atmospheric and surface resistances and continued lack of understanding of the underlying processes driving net canopy-atmosphere exchange.
Additional measurements of air-surface exchange and associated measurements of surface chemical, physical, and biological characteristics are needed to improve site-specific deposition budgets and air-surface exchange algorithms used in field scale deposition models and gridded CTMs. Assessment of existing datasets is needed to inform these measurement needs with respect to chemical species, ecosystems and geographical locations, and air-surface exchange processes. In this paper, we summarize the state of the science of measurements of dry deposition of Nr compounds in North America, beginning with the current understanding of the importance of dry deposition to total deposition at the U.S. continental scale, followed by a review of the micrometeorological and analytical methods used for direct measurements of air-surface exchange. We then summarize the existing measurements of Nr air-surface exchange measurements in natural ecosystems in North America, focusing on the U.S. and Canada. Based on this data synthesis, we outline research needed to address data gaps from the perspective of developing more complete deposition budgets and improving current understanding of air-surface exchange processes. We acknowledge that because the scope of this review is constrained to North America, the large body of Nr flux work performed in Europe and elsewhere, which established the basis for much of the work in the U.S., is not covered in detail.
State of the science
Deposition budget
To provide context for the discussion of dry deposition in the following sections, the total and dry components of the deposition budget for 2015 for the CONUS are shown in Figure 1. The budget was developed using version 5.2.1 of the Community Multi-scale Air Quality Model (CMAQ, www.epa.gov/CMAQ). The depositing species are categorized by wet or dry deposition and oxidized (labeled with ‘OXN’) versus reduced (labeled with ‘REDN’) forms of N. Reduced N comprises gaseous NH3 and particulate NH4+. OXN_NOX includes NO and NO2; OXN_PANT represents total peroxy nitrates (PNs) in the gas phase; OXN_ORGN represents other gas phase ON species such as isoprene nitrates; OXN_OTHR represents nitrogen pentoxide (N2O5) and HONO; NO3− and NH4+ represent particulate components; TNO3 represents total gaseous HNO3 + particulate NO3−; and REDN_ToT represents total gaseous NH3 + particulate NH4+. Relevant to the total budget, it is important to note that reduced forms of ON are not considered in either the dry or wet components, nor is the treatment of oxidized ON comprehensive. Thus, from a completeness standpoint the budget will be biased low with respect to total N deposition. With this caveat, the budgets shown in Figure 1 reflect the state of the science of deposition modeling as represented by a widely used regional CTM and, as such, are used here to illustrate the relative importance of the dry deposited fraction of Nr and the contribution of individual species or groups of compounds to the dry deposition budget for the U.S.
Figure 1.
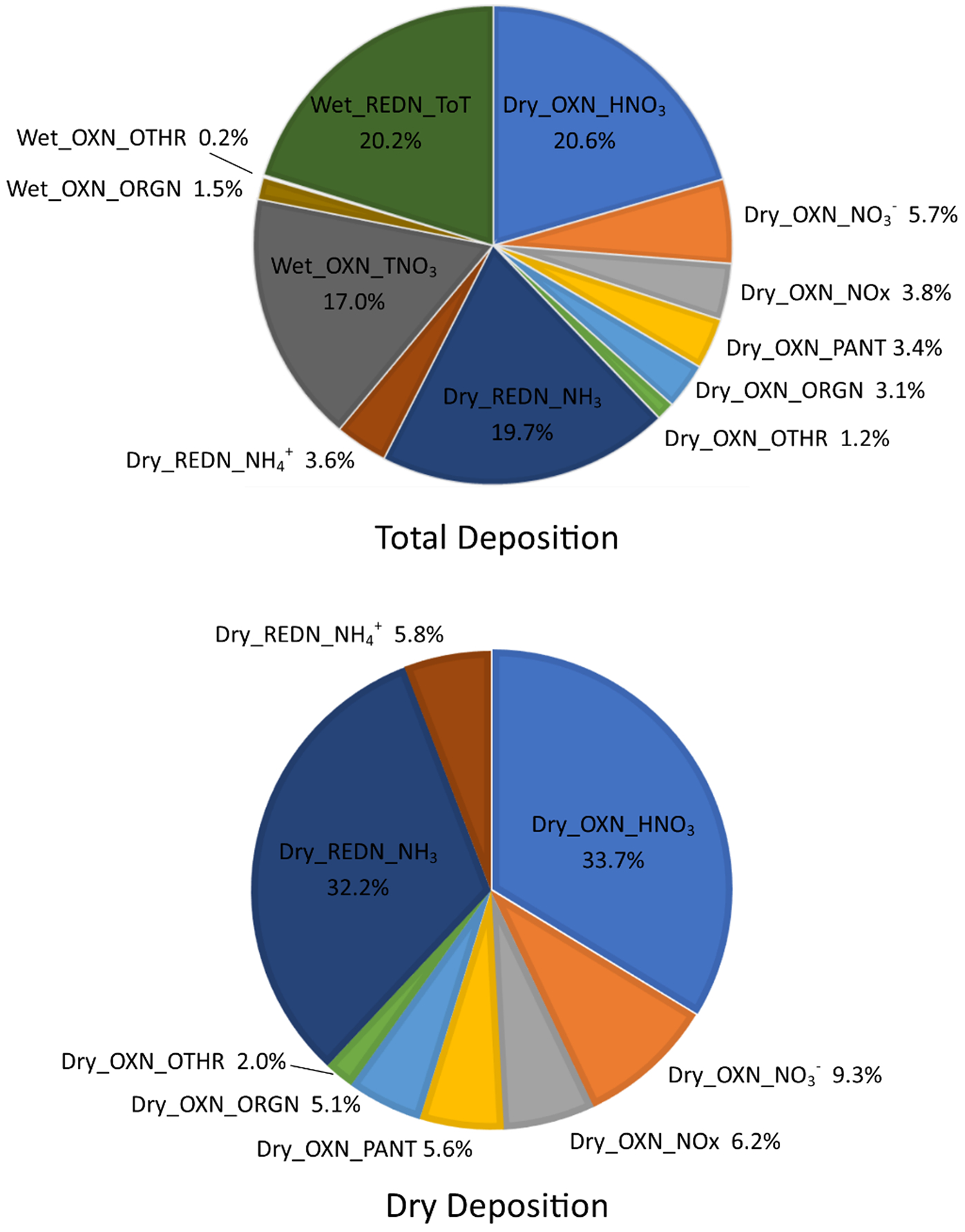
Nr deposition budget for the continental U.S. for 2015 (CMAQ V5.2.1). The top pie is the total Nr budget. Bottom pie is dry deposition only. The depositing species are categorized by wet versus dry deposition and oxidized (OXN) versus reduced (REDN) forms of nitrogen.
Nr deposition budgets have been previously developed for the U.S. and Canada using inferential modeling (Zhang et al., 2009), other versions of CTMs (Dennis et al., 2013; Zhang et al., 2012a; Zhang et al., 2018), and measurement-model fusion approaches (Schwede and Lear, 2014). Additionally, inferential approaches are now incorporating satellite observations to estimate dry deposition of a number of reactive nitrogen species, including NO2 (Cheng et al., 2013; Lu et al., 2013; Nowlan et al., 2014; Jia et al., 2016; Kharol et al., 2018), total nitrate (HNO3 + NO3−) and NH4+ (Jia et al., 2016), NOy (Geddes and Martin, 2017), and NH3 (Jia et al., 2016; Kharol et al., 2018).
The CMAQ simulation summarized in Figure 1 shows that dry deposition dominates the Nr budget at the continental scale, contributing 61% of total deposition compared to 39% from wet deposition. Oxidized and reduced forms of nitrogen account for 57% and 43% of total N deposition and 62% and 38% of dry deposition, respectively. The primary forms of dry deposition are HNO3 (33.7%) and NH3 (32.2%), which together account for approximately 2/3 of dry N deposition. We note that in this simulation NH3 fluxes are modeled using a bidirectional flux framework (Pleim et al., 2013; Bash et al., 2013). Deposition of NO3− aerosol (OXN_NO3−) contributes 9.3% of dry deposition. OXN_NOX, which due to atmospheric processing is essentially all NO2, contributes 6.2% of the dry budget, followed in importance by dry deposition of NH4+ aerosol (5.8%). Dry deposition of gas phase organics (OXN_PANT = 5.6%; OXN_ORG = 5.1%) together account for 10.7% of the dry budget while OXN_OTHR (N2O5 and HONO) contribute 2.0%. The domain-wide mass balance was not assessed in the CMAQ runs presented above; however, CTM predictions from earlier studies show that wet + dry deposition generally account for ~ 65% of oxidized Nr and ~ 75% of reduced Nr emissions, overall, at the U.S. continental scale (Dentener et al., 2006; Dennis et al., 2013). Acknowledging that the mass balance will be influenced by relationships between air concentrations and deposition as emissions change over time, these earlier studies illustrate the importance of deposition in the context of long-range transport.
While the budget presented above reflects the overall pattern at the continental scale, the contributions of individual species are strongly dependent on location, varying with proximity to sources, meteorological patterns, and atmospheric chemistry. Furthermore, the deposition budget contains uncertainty and biases related to model treatment of emissions, chemistry, deposition and other processes (Walker et al., 2019). Comparing CMAQ V5.2.1 to weekly NADP/NTN wet deposition for 2015 across the CONUS, the model tends to underestimate NH4+ wet deposition (normalized mean bias = −29.9%) while NO3− wet deposition is only slightly biased at the continental scale (normalized mean bias = −7.6%). Model underestimation of wet deposition of NH4+ likely reflects uncertainty in NH3 emissions inventories and bidirectional air-surface exchange (Kelly et al., 2014; Butler et al., 2015; Battye et al., 2016; Zhang et al., 2018). As previously mentioned, incomplete representation of model organic N chemistry and deposition is an important source of uncertainty in total wet deposition of Nr. Lack of observations precludes an assessment of model biases in dry deposition at the continental scale and large differences in dry deposition models for the more common inorganic Nr species (HNO3, NH3, NO2, NO3−, NH4+) have been previously noted (Flechard et al., 2011; Schwede et al., 2011; Li et al., 2016). While PAN has received some attention (Wu et al., 2012; Wolfe et al., 2011), dry deposition schemes for organic Nr compounds have been less extensively evaluated (Nguyen et al., 2015). Parameterizations of non-stomatal deposition processes and NH3 compensation points are key sources of uncertainty in dry deposition estimates.
Flux measurement methods
Commonly used micrometeorological methods for direct measurement of Nr air-surface exchange include eddy covariance (EC), gradient methods, and relaxed eddy accumulation (Baldocchi et al., 1988; Moncrieff et al., 1997; Fowler et al., 2001; Zhang et al., 2010).
Eddy covariance
The most direct approach is the EC technique (Foken et al., 2012), in which the vertical flux (F) of mass through a horizontal plane in the atmosphere, such as above a forest canopy, is quantified as the covariance of the fluctuating components of the vertical wind velocity (w) and the concentration of the chemical species of interest (c) as:
| (1) |
The overbar in equation (1) represents time-averaging, usually 30 minutes, and the primes represent deviations from the mean as illustrated in equation (2):
| (2) |
The primary requirements for standard EC (e.g., ignoring advection and storage) are flat, homogeneous terrain over a sufficient area surrounding the measurement location, well-developed turbulence, and chemical and meteorological instruments of sufficient time response and precision to capture the range of eddy motions driving the air surface exchange. Time response requirements are typically between 1 and 10 measurements per second (hz) depending on the surface characteristics and corresponding sensor height.
Gradient methods
Gradient approaches involve measuring the vertical concentration profile at two or more heights above the exchange surface and applying the measured vertical concentration gradient to the measured eddy diffusivity for momentum, heat, or mass (Foken, 2008). The typical calculation (e.g., Thomas et al., 2009; Wolff et al., 2010; Rumsey and Walker, 2016; Ramsay et al., 2018) for F of chemical species c using concentration measurements at two heights takes the form:
| (3) |
where u* is friction velocity, calculated from the momentum flux measured by EC, ψH is the integrated stability function for sensible heat, z1 and z2 are the measurement heights above ground between which the concentration gradient (ΔC) is measured, L is the Monin-Obukhov length typically calculated from the EC derived sensible heat flux, k is the von Karman constant (k = 0.41), and d is the zero plane displacement height. A variant of this method is the modified Bowen-ratio (MBR) (Meyers et al., 1996) in which the turbulent diffusivity is assumed to be equivalent to the turbulent diffusivity for heat such that the flux may be calculated as:
| (4) |
Here is the kinematic heat flux measured by EC, and and are co-located mean concentration and air temperature differences between heights z1 and z2 above the canopy. The ratio of the heat flux to the temperature gradient is also known as the eddy diffusivity for heat (Kh). Gradient methods also require flat, homogeneous terrain and well-developed turbulence. Drawbacks relative to EC include the need to correct profiles for atmospheric stability (aerodynamic method), increased uncertainty during transition periods when heat fluxes (or other scalars on which the eddy diffusivity is based) become small (MBR method), difficulty in estimating the flux footprint, and the need to correct for effects of sampling within the roughness sublayer in the case of tall vegetation. The advantage of gradient methods relative to EC is that they can be employed to measure fluxes of compounds for which fast sensors are not available. Fluxes are typically determined from gradients of concentrations integrated over 30 minutes to an hour.
Relaxed eddy accumulation
Relaxed eddy accumulation (REA; Businger and Oncley, 1990) is an alternative technique that allows for measurement of the flux at a single height but without the time response requirements of EC for concentration measurements. Measurement of the flux at a single height avoids the need for stability corrections and uncertainty in flux footprint estimation associated with gradient techniques. The REA approach employs fast switching based on measurement of the vertical wind speed to sample air concentrations in upward versus downward moving eddies from which the flux is determined as:
| (5) |
Here c↑ and c↓ are the average air concentrations in the up- and down-drafts, respectively, σw is the standard deviation of the vertical wind velocity (measured at 10 Hz), and β is an empirical dimensionless parameter that can be estimated from EC measurements of temperature and other scalars (Katul et al., 1996). REA systems can employ continuous or time-integrated measurements of atmospheric concentrations, with averaging periods from 30 minutes to a few hours.
Flux datasets
Published datasets of Nr micrometeorological flux measurements for natural and semi-natural ecosystems in North America are summarized in Table 1. Data are categorized by flux measurement method and analytical (i.e., for online measurements) or sampling method (i.e., for time integrated approaches) is also indicated. Where possible, fluxes and deposition velocities (Vd) are reported with negative fluxes indicating deposition. Our review is limited to studies employing micrometeorological flux measurement techniques over natural ecosystems and focusses on studies in the U.S. and Canada. Earlier flux measurements conducted in North America and elsewhere have been previously summarized by Zhang et al. (2002).
Table 1.
Published datasets of Nr micrometeorological flux measurements for natural ecosystems in North America
| Oxidized inorganic N | ||||||||||
| Gas | NOy | Deciduous forest | Central Massachusetts, Harvard Forest (deciduous) | 1990 – 1994 | Average midday fluxes were −13.2 and −12.4 in summer and winter, respectively. | Reported as Vd NOy-NOx. Average hourly values ranged from ≈ 0.25 to 1.75. | CL | EC | Munger et al., 1996 | |
| Gas | NOy | Coniferous forest | Schefferville, Quebec (coniferous) | Summer 1990 | Average midday flux of −1.9. | Reported as Vd NOy-NOx. Average hourly values rand from ≈ 0.5 to 2.0. | CL | EC | Munger et al., 1996 | |
| Gas | NOy | Deciduous forest | Central Massachusetts, Harvard Forest | June-November, 2000 | Median midday flux ≈ −58.0 in summer and - 39.0 in fall with southwesterly winds | Not reported | CL | EC | Horii et al., 2005 | |
| Gas | NOy | Coniferous forest | Chapel Hill, NC, Duke Forest Blackwood Division | July, 2003 | Midday hourly average NOy fluxes of −15 to −20 | Midday hourly average values ranged from 0.5 to 0.7. | CL | EC | Turnipseed et al., 2006 | CELTICg study |
| Gas | NOy | Deciduous forest | Central Ontario, Halliburton Forest | July 20 - October 11, 2011 | Average of −1.6 | Average midday value of 0.2 ± 0.25 | CL | EC | Geddes et al., 2014 | |
| Gas | NOy | Deciduous forest | Northern Michigan, UMBSi PROPHETj site | July 24 - August 14, 2012 | Average of −3.9 | Average midday value of 0.67±0.1.24 | CL | EC | Geddes et al., 2014 | |
| Gas | HNO3 | Grassland | Champaign, Illinois | June, 1982 | Average of −14.4 | 2.5±0.9 | FP | Gradient | Huebert and Robert, 1985 | |
| Gas | HNO3 | Deciduous forest | Oak Ridge, Tennessee | September 9–18, 1982 | Average of −33.0±22.2 | Average of 3.7±3.0 for modified Bowen ratio method | FP | Gradient | Meyers et al., 1989 | |
| Gas | HNO3 | Grassland | Boulder Atmospheric Observatory, Colorado | Summer, 1983 | Not reported | Average of 0.68 | FP | Gradient | Huebert et al., 1988 | Evidence of NH4NO3 evaporation |
| Gas | HNO3 | Bare ground | Hawaii, Mauna Loa Observatory | August 14 - September 5, 1991 | Not reported | Ranged from 0.27 to 3.45 | FP | Gradient | Lee et al., 1993 | High elevation (>3000 m) |
| Gas | HNO3 | Grassland | Sand Mountain, Alabama | April 15 - June 13, 1995 | Average of −24.4±11.1 | Average of 3.2±1.3 | FP | Gradient | Meyers et al., 1998 | Fluxes to soybean and corn also reported. |
| Gas | HNO3 | Alpine tundra | Niwot Ridge, Colorado | August - September, 1998 | Average of −0.12±0.05 | Average of 1.3±0.6 | FP | REA | Rattray and Sievering, 2002 | High elevation (>3000 m) |
| Gas | HNO3 | Coniferous forest | Niwot Ridge, Colorado | July – August, 1999 | Average of −3.5 to −4.0 | Average of 7.6 | FP | Gradient | Sievering et al., 2001 | High elevation (>3000 m) |
| Gas | HNO3 | Deciduous forest | Southern Indiana, Morgan-Monroe State Forest | July 15 - August 6, 2001 | Average of −22.4±61.6 reported for REA | Average of 3.0 reported for REA. | MD | REA and gradient | Pryor et al., 2002 | |
| Gas | HNO3 | Grassland | Sydney, Florida | May, 2002 | Average of −12.7±12.0 | Average of 3.63±5.5 | MD | REA | Myles et al., 2007 | BRACEe study. Site is tertiary sprayfield for wastewater treatment facility. Coastal site. |
| Gas | HNO3 | Coniferous forest | California, Sierra Nevada Mountains, Blodgett Forest Research Station | June-November, 2003; May, 2004 - June, 2005 | ≈ −2.8 during the afternoon to ≈ −1.4 at night during winter | Median of 2.5 during winter afternoons. 80% of observations between −1.2 and 8.2. | TD-LIF | EC | Farmer et al., 2006 | Analysis and discussion focus on winter results (January - March, 2005). |
| Gas | HNO3 | Grassland | Chapel Hill, NC, Duke Forest Blackwood Division | Fall, 2012 | Average of −1.0±1.4 | Not reported | WRD-OIC | Gradient | Rumsey and Walker, 2016 | |
| Gas | HNO3 | Deciduous forest | Southern Indiana, Morgan-Monroe State Forest | Late summer and fall, 2013 | Midday average of −40.0±20 | Not reported | MD | REA | Hansen et al., 2015 | |
| Gas | HNO3 | Mixed deciduous-coniferous forest | Brent, Alabama, Centreville (SEARCHk) site | June, 2013 | Range from ≈ 0 to −14.0 | 3.8±1.3 | CIMS | EC | Nguyen et al, 2015 | SOASf study. Vd reported as daytime (10:00 – 15:00) average. |
| Gas | NOx (NO+NO2) | Grassland | Boulder Atmospheric Observatory, Colorado | June - July, 1983 | Daytime fluxes of NO and NO2 range from ≈ −55.0 to 115.0 and −115.0 to 230.0, respectively. | Vd for NO estimated as 0.35 | CL | EC | Delany et al., 1986 | |
| Gas | NOx (NO+NO2) | Deciduous forest | Central Massachusetts, Harvard Forest | April-November, 2000 | Downward fluxes of NO2 at night (median hourly flux up to ≈ −3.9) and upward fluxes during the day (median hourly flux up to ≈ 11.7). NO fluxes near zero at night and downward during the day (median hourly flux up to ≈ −3.9) | Derived NO2 deposition velocity of 0.2 | CL (NO), TD-LAS (NO2) | EC | Horii et al., 2004 | |
| Gas | NOx (NO+NO2) | Coniferous forest | California, Sierra Nevada Mountains, Blodgett Forest Research Station | June-November, 2003; May, 2004 - June, 2005 | Fluxes near zero to ≈ 3.0 during the afternoon in winter. | Fluxes primarily upward | TD-LIF | EC | Farmer et al., 2006 | Analysis and discussion focus on winter results (January - March, 2005). |
| Gas | NOx (NO+NO2) | Coniferous forest | California, Sierra Nevada Mountains, Blodgett Forest Research Station | June 15 - July 31, 2009 | Upward median midday fluxes of 0.18±0.15 and 0.38±0.12 for NO and NO2 respectively. | Not reported | CL (NO), TD-LIF (NO2) | EC | Min et al., 2014 | BEARPEXh |
| Gas | NOx (NO+NO2) | Deciduous forest | Central Ontario, Halliburton Forest | July 20 - October 11, 2011 | Upward fluxes of NO2 peaking during midmorning at 1.5. Downward fluxes of NO peaking during midmorning at −1.2. | Not reported | CL | EC | Geddes et al., 2014 | |
| Gas | NOx (NO+NO2) | Deciduous forest | Northern Michigan, UMBSi PROPHETj site | July 24 - August 14, 2012 | Upward fluxes of NO2 peaking during midmorning at 2.8. Downward fluxes of NO peaking during midmorning at −2.9. | Not reported | CL | EC | Geddes et al., 2014 | |
| Gas | HONO | Deciduous forest | northern Michigan, UMBSi PROPHETj site | July 15 - August 10, 2008 | Average upward flux of 1.4 | Not reported | AP | REA | Zhou et al., 2011 | |
| Gas | HONO | Coniferous forest | California, Sierra Nevada Mountains, Blodgett Forest Research Station | July 4 – 30, 2009 | Positive and negative fluxes observed. Average daytime flux of −0.06±0.34. | Not reported | AP | REA | Ren et al., 2011 | |
| Gas | N2O5 | Snow | Fairbanks, Alaska | November 5 – 18, 2009 | Not reported | Average of 0.59±0.47 | CRDS | Gradient | Huff et al., 2011 | |
| Particulate | NO3- | Grassland | Boulder Atmospheric Observatory, Colorado | Summer, 1983 | Not reported | Average of 0.89 | FP | Gradient | Huebert et al., 1988 | Evidence of NH4NO3 evaporation |
| Particulate | NO3- | Alpine tundra | Niwot Ridge, Colorado | August - September, 1998 | Average of −0.06±0.02 | Average of 2.5±1.1 | FP | REA | Rattray and Sievering, 2001 | High elevation (>3000 m) |
| Particulate | NO3- | Mixed deciduous/coniferous forest | Ontario, Canada, Borden Forest Research Station | July 19 - August 2, 2006 | Average flux of −0.8±1.5 ng N m−2 s−1 | Average of 0.48 | AMS | EC | Gordon et al., 2011 | |
| Particulate | NO3- | Coniferous forest | California, Sierra Nevada Mountains, Blodgett Forest Research Station | August 10 - October 3, 2008 | Not reported | 0.14±0.1 between 8:00 and 13:00. | AMS | EC | Farmer et al., 2011; 2013 | BEARPEXh |
| Particulate | NO3- | Grassland | Chapel Hill, NC, Duke Forest Blackwood Division | Fall, 2012 | Average of −0.7±1.0 | Not reported | WRD-OIC | Gradient | Rumsey and Walker, 2016 | |
| Reduced inorganic N | ||||||||||
| Gas | NH3 | Grassland and deciduous forest | Northeastern Colorado, Pawnee National grasslands; Oak Ridge, Tennessee, Walker Branch site (deciduous) | June and July, 1988, grassland; June - September, 1989, grassland; September, 1988, deciduous forest | Bidirectional fluxes ranging from ≈ −82.0 at night to 165.0 during the afternoon with small average net deposition of −17.0 over grassland. Bidirectional fluxes ranging from ≈ −1.2 at night to 0.8 during the afternoon with small average net deposition of < 0.8 over deciduous forest. | Not reported | MD | Gradient | Langford et al., 1992 | Summary of multiple studies. |
| Gas | NH3 | Grassland | West Jefferson, Ohio, Battelle research facility | June 6 – 14, 1995 | Single observation of 119.0 | Not reported | CIMS | EC | Shaw et al., 1998 | |
| Gas | NH3 | Deciduous forest | Southern Indiana, Morgan-Monroe State Forest | spring, 1998 and 1999; winter 1999 and 2000 | Bidirectional flux observed. Forest generally a net sink for NH3 with a “typical” flux of −18.0 reported. | Not reported | WEDD-OF | Gradient | Pryor et al., 2001 | |
| Gas | NH3 | Alpine tundra | Niwot Ridge, Colorado | August - September, 1998 | Average of −0.44±0.17 | Average of 1.3±0.6 | FP | Gradient | Rattray and Sievering, 2001 | High elevation (>3000 m) |
| Gas | NH3 | Grassland | Sydney, Florida | May 2002 | Average of −30.0±192.0 | Average of 1.27±15.9 | MD | REA | Myles et al., 2007 | BRACEe study. Site is tertiary sprayfield for wastewater treatment facility. Coastal site. |
| Gas | NH3 | Grassland | Raleigh, North Carolina | Fall, 2001 - Summer, 2002 | Seasonal daily average fluxes were −91.0±115.0, −91.0±124.0, −115.0±157.0, and −17.0±25.0 in summer, spring, fall, and winter, respectively. | Seasonal daytime averages were 3.94±2.79, 2.85±2.01, and 2.82±1.98, and 2.41±1.92 in summer, spring, fall, and winter, respectively. | CL | Gradient | Phillips et al., 2004 | Site near a small swine facility at North Carolina State University Research Farm Units. |
| Gas | NH3 | Grassland | Chapel Hill, NC, Duke Forest Blackwood Division | Fall, 2012 | Average of 7.5±9.6 | Not reported | WRD-OIC | Gradient | Rumsey and Walker, 2016 | |
| Gas | NH3 | Deciduous forest | southern Indiana, Morgan-Monroe State Forest | Late summer and fall, 2014 | Primarily upward fluxes with a maximum of 110.0 | Not reported | WEDD-OF | REA | Hansen et al., 2015 | |
| Particulate | NH4+ | Alpine tundra | Niwot Ridge, Colorado | August - September, 1998 | Average of −0.8±0.3 | Average of 2.3±0.9 | FP | Gradient | Rattray and Sievering, 2001 | High elevation (>3000m) |
| Particulate | NH4+ | Coniferous forest | California, Sierra Nevada Mountains, Blodgett Forest Research Station | August 10 - October 3, 2007 | Hourly average ≈ −0.08 to −1.0 during midday. | 0.18 ± 0.08 between 0800 to 1300. | AMS | EC | Farmer et al., 2011; 2013 | BEARPEXh |
| Particulate | NH4+ | Grassland | Chapel Hill, NC, Duke Forest Blackwood Division | Fall, 2012 | Average of −0.8±1.6 | Not reported | WRD-OIC | Gradient | Rumsey and Walker, 2016 | |
| Organic N | ||||||||||
| Gas | Peroxy nitrates | Coniferous forest | California, Sierra Nevada Mountains, Blodgett Forest Research Station | June-November, 2003; May, 2004 - June, 2005 | Downward fluxes from midday to midnight (≈ −1.4 to −4.3) and upward fluxes from midnight to midday (≈ 0 to 1.1) | Median of 0.84 during winter afternoons. 80% of observations between −0.57 and 6.3. | TD-LIF | EC | Farmer et al., 2006 | Analysis and discussion focus on winter results (January - March, 2005). |
| Gas | Peroxy nitrates | Coniferous forest | Chapel Hill, NC, Duke Forest Blackwood Division | July, 2003 | Midday hourly average fluxes ≈ −0.25, −1.0, and −4.0 for PPN, MPAN, and PAN, respectively. | Midday hourly average values of 1.0 – 1.6 for PPN and MPAN and 0.8 – 1.2 for PAN. | TD-CIMS | EC | Turnipseed et al., 2006 | CELTICg study |
| Gas | Peroxy nitrates | Grassland | northern Illinois, Argonne National Laboratory | July-October | Downward fluxes | 0.13 ± 13 | MB-GC | Gradient | Doskey et al., 2004 | |
| Gas | Peroxy nitrates | Coniferous forest | California, Sierra Nevada Mountains, Blodgett Forest Research Station | August 24 - October 8, 2007 | Midday hourly average fluxes ≈ −0.15, −0.05, and −0.65 for PPN, MPAN, and PAN, respectively. | Midday hourly average values of 1.2 – 1.4 for PPN and 0.4 – 0.6 for PAN and MPAN. | TD-CIMS | EC | Wolfe et al., 2009 | BEARPEXhh |
| Gas | Peroxy nitrates |
Coniferous forest | California, Sierra Nevada Mountains, Blodgett Forest Research Station | June 15 - July 31, 2009 | Hourly average flux of ΣPNs and APNs ranged from near zero at night to ≈ −1.2 and −2.3, respectively, during midday. | Not reported | TD-LIF (ΣPNs); TD-CIMS (APNs) | EC | Min et al, 2012 | BEARPEXh; APNs include PAN, PPN, and MPAN. Inferred net upward flux of XPNs (XPNs = ΣPNs - APNs) |
| Particulate | Total Alkyl-nitrates (gas + particle) | Coniferous forest | California, Sierra Nevada Mountains, Blodgett Forest Research Station | June-November, 2003; May, 2004 - June, 2005 | Downward fluxes overnight through midafternoon (≈ 0 to −1.4) and upward fluxes from midafternoon through evening (≈ 1.4) | Median of 2.1 during winter afternoons. 80% of observations between 1.3 and 18.0. | TD-LIF | EC | Farmer et al., 2006 | BEARPEXh; Analysis and discussion focus on winter results (January - March, 2005). |
| Gas | HCN | Mixed deciduous/coniferous forest | Brent, Alabama, Centreville SEARCHk site | June, 2013 | Range from ≈ 0 to −0.28. | 0.3±0.1 | CIMS | EC | Nguyen et al., 2015 | SOASf study |
| Gas | INP | Mixed deciduous/coniferous forest | Brent, Alabama, Centreville SEARCHk site | June, 2013 | Range from ≈ 0 to −0.56 | 1.3±0.6 | CIMS | EC | Nguyen et al., 2015 | SOASf study |
| Gas | ISOPN | Mixed deciduous/coniferous forest | Brent, Alabama, Centreville SEARCHk site | June, 2013 | Range from ≈ 0 to −0.98 | 1.5±0.6 | CIMS | EC | Nguyen et al., 2015 | SOASf study |
| Gas | MACN+MVKN | Mixed deciduous/coniferous forest | Brent, Alabama, Centreville SEARCHk site | June, 2013 | Range from ≈ 0 to −0.56 | 1.5±0.5 | CIMS | EC | Nguyen et al., 2015 | SOASf study |
| Gas | MTNP | Mixed deciduous/coniferous forest | Brent, Alabama, Centreville SEARCHk site | June, 2013 | Range from ≈ 0 to −0.11 | 0.8±0.4 | CIMS | EC | Nguyen et al., 2015 | SOASf study |
| Gas | PROPNN | Mixed deciduous/coniferous forest | Brent, Alabama, Centreville SEARCHk site | June, 2013 | Fluxes range from ≈ 0 to −0.56. | 1.7±0.6 | CIMS | EC | Nguyen et al., 2015 | SOASf study |
Nr species
INP: isoprene nitrooxy hydroperoxide
ISOPN: isoprene hydroxy nitrate
MACN+MVKN: hydroxy nitrates with carbon backbones of methacrolein and methylvinylketone
MTNP: monoterpene nitrooxy hydroperoxide
PROPNN: propanone or propanal nitrate
Fluxes
Negative fluxes indicate deposition. Concentration conversions assume 25 °C and 1 atmosphere where applicable.
Analytical/sampling methods
AMS: aerosol mass spectrometer
AP: absorption photometer
CIMS: chemical ionization mass spectrometer
CL: chemiluminescence
CRDS : cavity ring-down spectroscopy
FP: filter pack
MD: manual denuder
MB-GC: manual bag-gas chromatography
TD-CIMS: thermal desorption-chemical ionization mass spectrometer
TD-LAS: tunable diode-laser absorption spectrometer
TD-LIF: thermal dissociation-laser induced fluorescence
WEDD-OF: wet effluent diffusion denuder-online fluorescence
WRD-OIC: wet rotating denuder-online ion chromatography
Flux techniques
EC: eddy covariance
REA: relaxed eddy accumulation
Field Campaigns and Sites
BRACE: Bay Region Atmospheric Chemistry Experiment
SOAS: Southern Oxidant and Aerosol Study
CELTIC: Chemical Emission, Loss, Transformation and Interactions with Canopies Study
BEARPEX: Biosphere Effects on Aerosol and Photochemistry Experiment
UMBS: University of Michigan Biological Station
PROPHET: Program for Research on Oxidants: PHotochemistry, Emissions, and Transport
SEARCH: SouthEastern Aerosol Research and CHaracterization Network
Oxidized inorganic N
In addition to understanding the total deposition budget of Nr and categorizing the deposition processes to both wet and dry fractions, it is necessary to understand the composition of Nr deposition in order to identify portions that are subject to regulatory control. In the U.S., this is limited to the anthropogenic fraction of oxidized inorganic Nr, which primarily originates from fossil fuel combustion. The oxidized Nr chemical system is summarized in Figure 2, illustrating the diversity of inorganic and organic species in both the particle and gas phase that make up the NOy budget. The flux of total oxidized Nr can be quantified by measuring the total NOy flux, which is dominated by inorganics but may contain a significant organic fraction, both in gaseous and particulate forms (e.g., organic nitrates). Daytime NOy fluxes summarized in Table 1 range from ≈ −1.5 to −60.0 ng N m−2 s−1.
Figure 2.
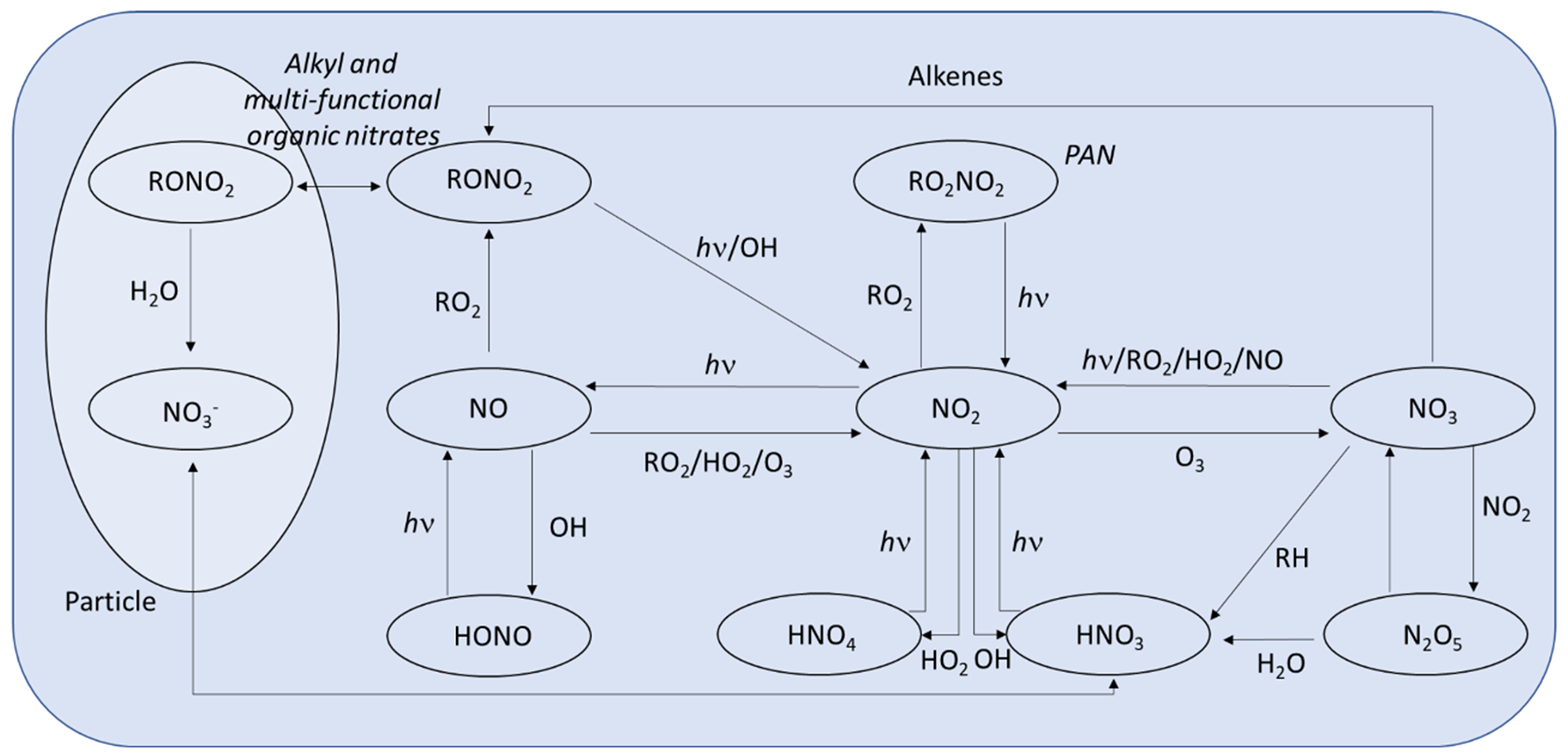
Schematic of the NOy system. Adapted from Seinfeld and Pandis, 1998.
Fluxes of NOy are typically measured by EC using a 3-D sonic anemometer for the micrometeorological parameters and the thermal conversion of all oxidized Nr to NO on a heated catalyst followed by detection of NO by chemiluminescence. While this approach is relatively straightforward compared to other techniques for specific compounds, existing North American datasets are limited to a few mixed deciduous forest sites (Haliburton Forest, central Ontario, Geddes et al., 2014; University of Michigan Biological Station (UMBS), Program for Research on Oxidants: PHotochemistry, Emissions, and Transport (PROPHET site), northern Michigan, Geddes et al., 2014; Harvard Forest, central Massachusetts, Munger et al., 1996; Horii et al., 2005) and coniferous forests in central North Carolina (Turnipseed et al., 2006) and Quebec (Munger et al., 1996). We note that the work of Munger and colleagues at Harvard Forest represents the only continuous multi-year long-term dataset of directly measured Nr dry deposition in North America. Because NOy is a bulk measurement of multiple species, it is most useful for budget development rather than process analysis and is most beneficial when conducted over temporal scales that allow for calculation of seasonal or annual deposition budgets.
As illustrated in Figure 1, HNO3 deposition is the largest contributor to the oxidized Nr dry deposition budget (33.7%), owing to its higher concentration relative to other compounds and large deposition velocity (Vd). Vd for HNO3 is generally thought to be limited only by atmospheric rather than surface resistances to deposition. For this reason, it is one of the most studied components of the NOy deposition budget across a range of North American ecosystems. As detailed in Table 1, fluxes of HNO3 have been quantified using gradient and REA methods employing a variety of analytical and batch sampling techniques. More recently, advancements in online chemical ionization mass spectrometry (CIMs, Nguyen et al., 2015) and spectroscopic methods (Farmer et al., 2006) have allowed the use of EC techniques. HNO3 fluxes are on the same order as NOy, with average values reported in the studies summarized in Table 1 ranging from < 1.0 to −40.0 ng m−2 s−1. The measured (Vd) for HNO3 is mostly in the range of 1.0 – 10.0 cm s−1 (Brook et al., 1999, Table 1). Vd values of 1.0 – 4.0 cm s−1 are generally recommended for modeling, with higher values more applicable to tall canopies and daytime conditions (Zhang et al., 2003).
While a number of datasets exist for model evaluation across a range of ecosystems, additional studies are needed to better understand the potential influences of surface characteristics and processes on the HNO3 canopy resistance (Pryor and Klemm, 2004). HNO3 has often been assumed to deposit according to Vdmax, calculated as 1/(Ra + Rb) where Ra is the aerodynamic resistance to turbulent transfer and Rb is the diffusive resistance at the leaf boundary layer, resulting in a surface or canopy resistance (Rc) of zero (Hicks et al., 1987; Meyers et al., 1989; Sievering et al., 2001). Accumulation of HNO3 during dry periods (Tarnay et al., 2002) and equilibrium between HNO3 and NO3− on foliage surfaces (Nemitz et al., 2004a) are examples of processes that may result in the presence of a “non-zero” Rc for HNO3. Additional field studies are needed to characterize these processes for North American conditions to validate HNO3 Rc parameterizations in current CTMs (e.g., Zhang et al., 2003).
New field studies are also needed to quantify sources of uncertainty in measured canopy scale fluxes resulting from gas-particle interconversion within the NH4NO3 + HNO3 + NH3 system (i.e. flux divergence; Brost et al., 1988). As summarized by Nemitz et al. (2004b) and references therein, perturbation of the NH4NO3 thermodynamic equilibrium within and above the canopy affects the vertical gradients NH4NO3 + HNO3 + NH3. NH4NO3 evaporation near the surface, for example, can result in apparent emissions of HNO3 and NH3 from the canopy (Huebert et al., 1988; Zhang et al., 1995; Pryor et al., 2001) and corresponding rates of NO3− and NH4+ deposition that are too large to be explained by deposition alone (Huebert et al., 1988; Brost et al., 1988; Wyers and Duyzer, 1997; Wolff et al., 2010; Aan de Brugh et al., 2013). This process can furthermore result in apparent emission of smaller particles from the canopy (Nemitz et al., 2004a).
At the continental scale, NOx (NO + NO2), which is primarily NO2, may contribute on the order of 6% of the Nr dry deposition budget (Figure 1), though larger contributions (>10%) have been estimated at Canadian CAPMoN sites using a combination of measured air concentrations and inferential modeling (Zhang et al., 2009). The NOx fraction of the oxidized inorganic Nr flux has been studied relatively extensively in the context of in-canopy and near surface chemical processing within the NOy system. Because the chemical timescale of the cycling of NOx between NO and NO2 is similar to the turbulence time scale, their canopy-scale fluxes will reflect a combination of emission from the soil, deposition to the canopy, and in-situ chemical processing. NO and NO2 fluxes are measured by EC using several approaches. NO fluxes are measured directly by fast chemiluminescence (e.g., Geddes et al., 2014) whereby NO2 fluxes may be determined directly by spectroscopic techniques (Horii et al., 2004; Farmer et al., 2006), or by fast NO chemiluminescence after conversion of NO2 by photolysis (Geddes et al., 2014). In North America, canopy scale NO and NO2 fluxes have been conducted in a few coniferous (Blodgett Forest, Sierra Nevada Mountains, California, Farmer et al., 2006; Min et al., 2014) and mixed deciduous forests (Harvard Forest, Massachusetts, Horii et al., 2004; Haliburton Forest, Ontario, Geddes et al., 2014; and the PROPHET site, Michigan, Geddes et al., 2014). Measurements at these sites exhibit patterns of upward and downward canopy-scale fluxes of NO and NO2, reflecting the net result of chemical processing within the canopy air-space and turbulent exchange. Geddes et al. (2014) noted that the fluxes at different times of day tended to offset, yielding a total NOx flux near zero, while net upward fluxes of NO and NO2 were observed at Blodgett Forest (Min et al., 2014). Net downward fluxes of NO2 consistent with the presence of a compensation point were observed at Harvard Forest (Horii et al., 2004). NO and NO2 fluxes have also been measured by EC from aircraft over the southeastern U.S. (Wolfe et al., 2015).
Downward NO2 fluxes reported in Table 1 are generally < 5.0 ng N m−2 s−1 except for the early work of Delany et al. (1986) where much higher concentrations (up to 40 ppb NO2) were observed. The measured (Vd) for NO2 is mostly in the range of <0.0 – 1.0 cm s−1 (Wesely and Hicks, 2000; Zhang et al., 2002; Table 1). For practical reasons, dry deposition of NO can be neglected in the deposition budget. Typical Vd values of 0.1 – 0.8 cm s−1 for NO2 are recommended for modeling over vegetated canopies and much smaller values over bare land and water surfaces (Zhang et al., 2003). As an example, Kharol et al. (2018) calculated the 10th – 90th percentiles of the annual average Vd (NO2) over North America to be 0.04 – 0.26 cm s−1 for 2013 using the dry deposition scheme of Zhang et al. (2003). Patterns of NO2 concentrations and deposition inferred from satellite measurements over North America (Nowlan et al., 2014; Kharol et al., 2018) highlight the need for additional NOx flux measurements in locations such as urban to rural gradients where NO2 contributes a more significant fraction of NOy than experienced in the more rural locations reflected in Table 1. The accuracy of modeled NO2 deposition rates is likely more critical in ecosystems experiencing higher exposure to NOx. Site differences in patterns of NOx air-surface exchange, and in the relative importance of in-situ chemical processing to the net canopy-scale flux, reinforce the need for measurements and models that explicitly quantify in-canopy and near-canopy sources and sinks as well as net-canopy scale exchange.
As shown in Figure 1, CMAQ simulations suggest that HONO and N2O5 (OXN_OTHR) likely make a relatively small contribution (2%) to the Nr deposition budget at the continental scale. Theoretically parameterized Vd values for HONO and N2O5 are as high as those for HNO3 (Wesely and Hicks 2000; Zhang et al., 2003), however, negative HONO Vd are often observed. Of the gas phase oxidized inorganic Nr compounds, HONO has received less attention than NOx in terms of canopy-scale fluxes in North American natural ecosystems. As noted in Table 1, only a few published studies were identified, which describe fluxes measured by REA at the PROPHET deciduous forest site in Michigan (Zhang et al., 2012b; Zhou et al., 2011) and the Blodgett Forest ponderosa pine site in California (Ren et al., 2011), both rural low-NOx environments. Net upward fluxes from the canopy to the atmosphere (i.e., negative Vd) were observed at both forest sites, with lower fluxes at Blodgett Forest corresponding to lower concentrations. Viewed in the context of the deposition budget shown in Figure 1, which reflects a model algorithm in which HONO fluxes are unidirectional toward the surface, these studies point to the need for a more detailed treatment of within- and near-canopy chemistry in order to accurately resolve the net canopy-scale flux of HONO. Additional measurements are also needed in natural ecosystems experiencing higher atmospheric concentrations of HONO than observed at these two rural forested sites.
Measurements of N2O5 fluxes are more limited. The study by Huff et al. (2011) over a snow-covered agricultural field is the only published measurements that could be found for a North American terrestrial ecosystem. Fluxes suggested that N2O5 deposition was likely limited by turbulent transfer (i.e., similar to HNO3), which is expected given its high solubility and is in agreement with N2O5 fluxes measured above the air-sea interface near San Diego, California (Kim et al., 2014). Additional measurements of N2O5 fluxes are needed, particularly in coastal ecosystems and downwind of urban areas (Thornton, et al., 2010).
NO3− aerosol is estimated to contribute ~ 9% of the dry N deposition budget at the continental scale (Figure 1). While the regional patterns and trends of atmospheric concentrations are relatively well characterized by national monitoring networks (e.g., CASTNET, IMPROVE), there exist relatively few published studies in which NO3− fluxes and deposition velocities have been directly measured in North America (Table 1). Earlier measurements (Huebert et al., 1988; Rattray and Sievering, 2001) employed filter packs to measure fluxes in a gradient mode, while online techniques employing steam aerosol collection and ion chromatography have been used more recently (Rumsey and Walker, 2016). Gradient studies have been conducted in grassland (Huebert et al., 1985; Rumsey and Walker, 2016) and alpine (Rattray and Sievering, 2001) environments. Recent advancement of online aerosol mass spectrometry has enabled the use of EC techniques for NO3− flux measurements at two North American forest sites (Blodgett Forest, Calilfornia, ponderosa pine, Farmer et al., 2011; 2013; Borden Forest, Ontario, mixed deciduous/coniferous, Gordon et al., 2011). NO3− fluxes measured in the studies summarized in Table 1 are typically < 1.0 ng N m−2 s−1.
In CTMs, size distributions of fine versus coarse mass fractions of NO3− need to be simulated reasonably well in order to estimate its dry deposition using size-resolved or modal-based dry deposition schemes. Typical Vd values are in the range 0.1–0.3 cm s−1 for the fine particle fraction and nearly double for coarse particle fraction over vegetated canopies (Zhang and He, 2014). Thus, Vd of NO3− should be mostly in the range of 0.1–0.5 cm s−1 over vegetated canopies, noting that it has a substantial coarse fraction especially in warm seasons, and is likely < 0.1 cm s−1 over smooth surfaces. With the exception of fluxes measured at the high elevation Niwot Ridge site (Rattray and Sievering, 2001), the Vd summarized in Table 1 are generally consistent with this range. Coastal environments, where deposition of coarse mode NO3− may contribute more significantly to the Nr deposition budget than fine mode NO3− at inland sites, represent an important geographical data gap where flux measurements are needed. As previously noted, additional observations of NO3− flux, coincident with HNO3 and NH3 fluxes, are needed to better understand potential sources of uncertainty in measured canopy-scale fluxes resulting from gas-particle interconversion within the NH4NO3 + HNO3 + NH3 system (i.e. flux divergence). Gordon et al. (2011) note flux divergence as a possible explanation for the observed NO3− fluxes from the canopy to atmosphere in their study at Borden Forest.
Reduced inorganic N
Reduced inorganic N (NHx = gaseous NH3 and particulate NH4+) in the atmosphere primarily originates from agricultural sources of NH3, including animal manure and fertilized soil (Reis et al., 2009). In contrast to oxidized N emissions, NH3 emissions are not regulated in the U.S. NH4+ aerosol is, however, relevant to the U.S. primary NAAQS as a component of PM2.5. As illustrated in Figure 1, deposition of gaseous NH3 is an important contributor to the continental scale Nr deposition budget (32.2% of dry N). Furthermore, the relative contribution of reduced forms of N to the atmospheric inorganic N budget is increasing over time as NOx emissions continue to decline (Li et al., 2016). Li et al. (2016) show that reduced N now dominates the inorganic Nr deposition budget in many areas of the U.S., with the contribution of NH3 dry deposition alone varying regionally from 19% (Northwest) to 63% (Southwest). Thus, knowledge of the role of NH3 and NH4+ in Nr deposition budgets is becoming more important for understanding ecological impacts and for developing approaches to maintain or reduce deposition rates below critical N loads in North American ecosystems (Pardo et al., 2011; Ellis et al., 2013).
NH3 is unique to other Nr compounds in that it is exchanged bi-directionally between the atmosphere and biosphere depending on the compensation point and emission potential of the underlying surface. NH3 may be emitted from or taken up at the leaf surface via stomatal and cuticular pathways and may emit from or deposit to soil and the overlying litter layer (see Massad et al., 2010 and Flechard et al., 2013). Bidirectional NH3 air-surface exchange algorithms used in North American deposition assessments, both at the field scale (Li et al., 2016) and within gridded CTMs (Zhang et al., 2010; Pleim et al., 2013; Bash et al., 2013; Zhu et al., 2015; Whaley et al., 2018), are largely based on parameterizations developed from European datasets (see Massad et al., 2010 and Flechard et al., 2013). Stomatal and soil exchange pathways are regulated by NH3 emission potentials that vary by vegetation and soil type along with other aspects of ecosystem biogeochemistry. Cuticular exchange processes are affected by surface wetness and the acidity of the exchange surface, which is influenced by the vegetation itself as well as the chemical composition of material deposited to the surface (Flechard et al., 1999; Burkhardt et al., 2009; Burkhardt and Hunsch, 2013; Wentworth et al., 2016). Because these properties are to some extent ecosystem specific and dependent on atmospheric chemistry, datasets are needed to assess seasonal and annual net fluxes of NH3 and to validate or revise current parameterizations for North American conditions.
While NH3 fluxes in natural ecosystems generally reflect bidirectional exchange, reported Vd are in the range of 0.1 to ~ 4.0 cm s−1 for semi-natural and natural terrestrial ecosystems, with highest Vd observed over coniferous forests (weighted average = 2.2 cm s−1) and lower Vd over deciduous forests (weighted average = 1.1 cm s−1) and grassland/heathland (weighted average = 0.9 cm s−1) (Schrader and Brummer, 2014). Although bi-directional exchange models are now commonly used in CTMs, big-leaf dry deposition models (i.e., Vd) are still used for generating long-term fluxes over large spatial scales, such as the application of satellite NH3 observations for deposition assessments (Kharol et al., 2018). Using the big-leaf deposition scheme of Zhang et al. (2003), Kharol et al. (2018) found 10th – 90th percentiles of the annual average Vd (NH3) of 0.28 – 1.01 cm s−1 over North America (Kharol et al., 2018). Where reported, average NH3 Vd summarized in Table 1 range from ≈ 1.3 to 4.0 cm s−1, with higher values observed over grassland in the vicinity of a swine production facility (Phillips et al., 2004).
Fluxes of NH3 summarized in Table 1 reflect both emission and deposition in natural ecosystems, ranging in magnitude from <1.0 to >100.0 ng N m−2 s−1. The work of Langford et al. (1992) summarizes early studies of NH3 fluxes at grassland (Pawnee grasslands, northeast Colorado) and forest sites (Walker Branch, Oak Ridge, Tennessee) measured using batch collection techniques in a flux gradient configuration. More recent measurements in North America have employed a range of gradient approaches using batch collection of NH3 on acid coated filters (alpine tundra, Colorado, Rattray and Sievering, 2001); NH3 conversion to NO by heated catalyst/chemiluminescence in gradient mode (grass, North Carolina, Phillips et al., 2004) and continuously wetted denuder with online concentration measurement (Morgan-Monroe State Forest, deciduous, Indiana; Pryor et al., 2001; grass, North Carolina, Rumsey and Walker, 2016). REA has been used with batch NH3 collection by denuder (grass, Florida, Myles et al., 2007) and by wet effluent diffusion denuder with online concentration measurement above a forest (Morgan-Monroe State Forest, Indiana, deciduous; Hansen et al., 2015). Shaw et al. (1998) report fluxes measured by EC over a grass field using a tandem mass spectrometer.
While a number of studies have been conducted in grasslands and to a lesser extent forests, coastal ecosystems and wetlands represent geographical gaps where NH3 flux measurements are needed. Additionally, flux measurements are needed within and downwind of agricultural areas to better characterize rates of NH3 deposition to natural ecosystems experiencing elevated NH3 concentrations. It is also notable that the only published datasets for North American forests (Langford et al., 1992; Pryor et al., 2001; and Hansen et al., 2015) are for mixed-hardwood ecosystems; published studies in coniferous North American forests could not be identified. From a process standpoint, additional flux datasets are needed in deciduous and coniferous forest ecosystems targeting a range of atmospheric concentrations of NH3 and atmospheric acidity. Supporting datasets of surface wetness and biogeochemistry are also critical for interpreting fluxes within the context of surface emission potentials and cuticle chemistry.
From a technological standpoint, open-path measurement techniques suitable for EC NH3 fluxes are advancing (Sun et al., 2015) and show promise for application to flux measurements in natural ecosystems. Open-path technology has an obvious advantage in avoiding inlet NH3 effects which limit the effective response time of fast detectors such as quantum cascade (QCL), tunable diode laser (TDL), or CIMS systems in a “closed” configuration (Zöll et al., 2016; Famulari et al., 2004; Ellis et al., 2010; Sintermann et al., 2011; Ferrara et al., 2012).
Averaged over the CONUS, NH4+ aerosol makes a smaller contribution (5.8% of dry N) to the overall Nr deposition budget than NH3 (Figure 1). Fluxes of NH4+ aerosol summarized in Table 1 are typically < −1.0 ng N m−2 s−1. NH4+ is mostly in fine particles, thus typical Vd are similar to those of fine mode NO3− mentioned above. Where concentrations of NH3 are much lower than NH4+, contributions of NH3 and NH4+ may be similar. This pattern may be observed in locations distant from NH3 sources, as was found at several rural Canadian locations (Zhang et al., 2009). As with NO3−, the regional patterns and trends of atmospheric concentrations of NH4+ aerosol are relatively well characterized by national monitoring networks (e.g., CASTNET, IMPROVE, CAPMoN). However, published direct flux measurements for North American sites appear to be limited to three studies (Table 1). Rattray and Sievering (2001) employed batch collection with filter packs in a gradient configuration to measure fluxes above alpine tundra (Niwot Ridge, Colorado). Rumsey and Walker (2016) used a steam-jet aerosol collector with online ion-chromatography in gradient mode to measure fluxes over grass (Chapel Hill, North Carolina). Aerosol mass spectrometry was used to measure fluxes by EC at a single North American forest site (Blodgett Forest, California, ponderosa pine, Farmer et al., 2011; 2013). As with NO3−, additional studies of NH4+ deposition are needed to better understand potential sources of uncertainty in measured canopy scale fluxes resulting from gas-particle interconversion within the NH4NO3 + HNO3 + NH3 system (i.e. flux divergence).
Organic N
On a global basis, ON may contribute ~ 25% of the total N deposition in precipitation on average (Jickells et al., 2013). While both measurements (Jickells et al., 2013; Cape et al., 2011) and recent global modeling (Kanakidou et al., 2016) reflect the importance of ON to total N deposition, the composition, sources, and deposition processes for ON remain poorly characterized for all but a relatively few compounds or groups of compounds. ON comprises a wide range of gaseous and particulate forms whose sources include soil dust, biomass burning, agricultural, marine, and anthropogenic emissions. Classes of compounds include primary emissions and secondary reaction products such as amines and amino acids, urea, nitrophenols, alkyl amides, N-heterocyclic alkaloids, and organic nitrates (Jickells et al., 2013; Cape et al., 2011). Dry deposition of ON remains poorly characterized at the global scale, though technological advances in measurement techniques suitable for flux measurements have led to an increase in dry deposition studies in recent years. Here we provide a brief summary of published measurements of dry deposition and bi-directional air-surface exchange of ON compounds for North American natural ecosystems.
Oxidized organic N
With respect to air-surface exchange, the oxidized portion of ON in the atmosphere has been studied in the context of particulate and gas phase organic nitrates. When volatile organic compounds (VOCs) are present, NOx can react with organic peroxy radicals (RO2) to form peroxy nitrates (PNs, RO2NO2) and alkyl and multifunctional nitrates (RONO2) (Figure 2). PNs may account for 10–80% of total NOy in high NOx environments (Roberts, 1990; Roberts et al., 2004; Cleary et al., 2007; Murphy et al., 2006; Day et al., 2008), with PAN contributing the majority of the PN budget. PN species exist in the gas phase and are thermally unstable, with lifetimes ranging from a few hours to weeks depending on temperature. With respect to air-surface exchange, PNs are the most studied class of ON compounds in North America. Figure 1 shows that PNs may contribute ~ 6% of the total Nr dry deposition budget at the continental scale.
As summarized in Table 1, PNs are reported as speciated PAN, peroxypropionyl nitrate (PPN) and peroxymethacryloyl nitrate (MPAN), where total acyl peroxy nitrates (APN) = PAN + PPN + MPAN, or as total peroxy nitrates (ΣPN). With the exception of a single study employing the gradient method and offline analysis of bag samples (Doskey et al., 2004), fluxes are typically measured by EC using online CIMS (Turnipseed, 2006) or thermal dissociation to NO2 followed by laser induced fluorescence (TDLIF, Farmer et al., 2008). In North America, PN fluxes have been measured over grass (Illinois, Doskey et al., 2004); loblolly pine (Duke Forest, Chapel Hill, North Carolina, Turnipseed et al., 2006); ponderosa pine (Blodgett Forest, California, Farmer et al., 2006; Wolfe et al., 2009; Min et al., 2012); and by aircraft over the southeastern U.S. (Wolfe et al., 2015). Both upward and downward fluxes have been reported, with average fluxes in Table 1 generally < 5.0 ng N m−2 s−1.
PN fluxes have been studied extensively at the Blodgett Forest and observations spanning multiple years reflect the complexities of PN air-surface exchange. Farmer et al. (2008) report a net upward flux of ΣPN from the canopy in a 2004 study, driven by production within the canopy air-space. Wolfe et al. (2009) report net deposition of PAN, PPN, and MPAN during the 2007 Biosphere Effects on Aerosols and Photochemistry Experiment (BEARPEX) experiment, with the majority of deposition attributed to stomatal uptake and vertical gradients in PAN decomposition, leaving a small residual flux attributed to “non-stomatal” uptake. Min et al. (2012) also report net deposition of APN during BEARPEX-2009, but much smaller net fluxes of ΣPN attributed to in-canopy production and emission of PN species other than APNs. Differences across years may be attributed to differences in photochemical conditions and biogenic emissions of PN precursors. Wolfe et al. (2009) estimated an overall contribution of PN to the N deposition budget at their site of 4–19%. Across PN species, Wolfe et al. (2009) report larger average mid-day deposition velocities for PPN (1.2 – 1.4 cm s−1) than PAN and MPAN (0.4 – 0.6 cm s−1) while Turnipseed et al. (2006) report average mid-day deposition velocities of PPN and MPAN of 1.0 – 1.6 cm s−1 and slightly lower values for PAN (0.8 – 1.2 cm s−1). Turnipseed et al. (2006) also report that approximately half of daytime deposition could be explained by stomatal uptake and that night-time fluxes tended to be larger when the canopy was wet. Theoretically parameterized Vd values for PAN, PPN and MPAN are slightly smaller than those of NO2, which are typically in the range of 0.1 – 0.8 cm s−1 as mentioned above (Zhang et al., 2003).
Similar to studies of NOx and HONO, which show a combination of net emission and deposition to the canopy across sites and time periods, measurements of PAN fluxes reiterate the importance of quantifying the role of in-canopy chemistry in net canopy exchange with the atmosphere, processes which are not captured in the model algorithms employed in most CTMs including CMAQ. Deciduous forests represent a notable data gap for PN fluxes where additional measurements are needed.
Alkyl and multifunctional nitrates (ANs), which can exist in the gas or particle phase, can be the dominant chemical sink for NOx in high biogenic VOC (BVOC)/low NOx environments (Browne and Cohen, 2012; Paulot et al., 2012; Browne et al., 2014). Recent aircraft and ground-based observations combined with Goddard Earth Observing System global CTM (GEOS-CHEM) simulations show that 25–50% of surface RONO2 is contributed by gas-phase isoprene nitrates, 10% from gas phase monoterpene nitrates, and ~ 10% is in the particle phase (Fisher et al., 2016). CMAQ simulations suggest that the gas phase portion of these “other” organic nitrates may contribute ~ 5% of the Nr dry deposition budget (Figure 1, “OXN_ORGN”). While understanding of the importance of ANs to the NOx budget and the AN chemical system is expanding rapidly, the processes of AN air surface exchange are poorly known. Only four North American studies could be identified in which air-surface exchange of ANs was directly measured. In all cases, fluxes were measured by EC using TD-LIF (Farmer et al., 2006; Min et al., 2012) or CIMS (Wolfe et al., 2015; Nguyen et al., 2015). Measurements of total AN fluxes (gas + particulate) at Blodgett Forest during 2004 and 2005 showed net downward fluxes to the canopy, with a median Vd of 2.1 cm s−1 (Table 1) indicating the presence of a surface resistance when compared with the maximum Vd allowed by turbulence (Farmer et al., 2006). Total AN fluxes were generally < −2.0 ng N m−2 s−1 (Farmer et al., 2006; Table 1).
Published measurements of speciated AN fluxes at North American sites are also few (Table 1). EC fluxes of isoprene hydroxy nitrates (ISOPN) have been measured by CIMS (Wolfe et al., 2015; Nguyen et al., 2015). Wolfe et al. (2015) report an average Vd of 1.1 cm s−1 from spatially integrated aircraft flux measurements over the “isoprene volcano” region of the Ozark mountains, which agrees with the ground-based flux measurements (mean Vd of 1.5 cm s−1) of Nguyen et al. (2015, Table 1) over a mixed coniferous/deciduous forest in the southeastern U.S. (Centerville SouthEastern Aerosol Research and CHaracterization Network (SEARCH) site, Brent, Alabama). Nguyen et al. (2015, Table 1) also report CIMS EC fluxes of several other multifunctional gas phase organic nitrates, including methacrolein and methyl vinyl ketone hydroxy nitrates (MACN + MVKN), propanone nitrate (PROPNN), isoprene nitrooxy hydroperoxide (IPN), and monoterpene nitrooxy hydroperoxide (MTNP). With the exception of MTNP, average deposition velocities of these compounds are similar over the approximately 4-week period of study, ranging from 1.3 to 1.7 cm s−1. The Vd for MTNP was lower, averaging 0.8 cm s−1. All species deposited more slowly than allowable by purely turbulent exchange, indicating the presence of a surface resistance. These measurement-based Vd values are either not significantly different from or slightly higher than the theoretical values (Zhang et al., 2003), keeping in mind that existing dry deposition schemes could have large uncertainties even for well-studied species (Flechard et al., 2011). Fluxes of speciated ANs summarized in Table 1 are generally < −1.0 ng N m−2 s−1.
Reduced forms of organic N
While measurements of rainfall composition suggest that reduced N compounds may cumulatively make a significant contribution to the atmospheric ON budget (Neff et al., 2002; Altieri et al., 2012), the processes by which reduced ON compounds dry deposit are largely unknown. This component of the dry deposition budget is not represented in the budget shown in Figure 1. Fluxes of hydrogen cyanide (HCN) measured by Nguyen et al. (2015) are the only published dry deposition data for reduced ON compounds that could be identified in the literature for North America. HCN is of interest as a tracer for biomass burning (Rinsland et al., 1999). Nguyen et al. (2015) report a low average Vd = 0.3 cm s−1 (flux < −0.5 ng N m−2 s−1) over a mixed coniferous/deciduous forest (Brent, Alabama) during summer, likely resulting from low solubility and reactivity at the leaf surface. Measurements of air-surface exchange of other gas phase reduced ON compounds, such as amino acids, aliphatic amines, and N containing nitroso compounds in North America could not be identified.
Future research
The data and knowledge gaps summarized above motivate research needed to address the incompleteness of dry deposition budgets, more fully characterize temporal and geographical variability of fluxes, and better understand air-surface exchange processes to improve models used for deposition assessments.
Completeness of deposition budgets –
Model deposition budgets used for critical loads assessments do not include the full contribution of ON forms. Global measurements (Jickells et al., 2013) suggest that omission of the water soluble ON fraction may result in a low bias of the wet N deposition budget by 25% on average; total contribution of ON to the dry deposition fraction is unknown. In the near term, PM measurements conducted by CASTNET and CAPMoN could be expanded to include an analysis of total water-soluble N, which would allow for estimation of bulk ON in PM after subtraction of the inorganic components (NO3− and NH4+). Measurement of bulk water soluble ON in PM in a routine monitoring mode would represent a large step forward in understanding the contribution of ON to dry deposition in PM and its spatial and temporal patterns.
For dry deposition of gas phase reduced ON species, techniques which allow for direct measurement of the total reduced N flux (Brϋmmer et al., 2013) represent an important first step in accounting for this component of the dry deposition budget and could be implemented in the near term. For oxidized ON, application of bulk alkyl and peroxy nitrate converter methods (Farmer et al., 2006) to chemiluminescence detection for EC fluxes should be explored. Separation of aerosol and gas phase contributions to the total oxidized ON flux using coincident online aerosol and gas phase mass spectrometry methods should be a long-term goal. Following advances in measurements of oxidized ON forms (Nguyen et al., 2015), application of online mass spectrometry techniques to quantify speciated fluxes of amines and amides (You et al., 2014; Yao et al., 2016) may also be possible in the short term.
While direct measurements of dry deposition are critically needed, a combination of measurements and inferential modeling may be a useful first step in complementing current monitoring networks for missing ON species. For example, some dry algorithms may be developed for the missing flux fractions by conducting scoping studies in which concentrations of ON species are measured, along with micrometeorology and canopy characteristics, over representative seasons and locations (e.g., Zhang et al., 2009; Flechard et al., 2011) and/or by modeling studies (such as using CMAQ for simulating more N species). Considering the large uncertainties in estimating fluxes of the monitored N species, such an approach may be worthwhile for the missing N species in the short-term.
Temporal and geographical variability of fluxes –
With exception of the work of Munger et al. (1996) at Harvard Forest, most air-surface exchange data sets span periods of a few weeks to months, failing to capture the range of atmospheric, biogeochemical, and phenological conditions that drive annual scale fluxes. For this reason, establishment of long-term sites for process level measurements of reactive chemical fluxes should be viewed as a high priority long-term endeavor of the atmospheric chemistry and ecological communities. Because the expense of process-level measurements makes the establishment of a large number of sites unfeasible, use of low-cost approaches for direct flux measurements, such as the Conditional Time-Averaged Gradient (COTAG) technique (Famulari et al., 2010), should be considered. Such techniques could potentially be deployed in a routine monitoring mode within existing infrastructure (e.g., CASTNET, NADP, Ameriflux) to quantify dry deposition for seasonal and annual deposition budgets at a relatively large number of sites.
Short term flux measurements also miss potentially important deposition episodes. For example, large enhancements of Nr compounds have been observed in smoke plumes (Benedict et al., 2017; Prenni et al., 2014; Geddes et al., 2014). While these observations demonstrate that smoke plumes represent a significant source of site-specific temporal variability in atmospheric Nr, there remains a paucity of measurements sufficient to characterize the importance of biomass burning episodes to annual deposition budgets, which may be particularly important at remote sites where background Nr deposition is low. Characterization of Nr deposition associated with smoke plumes is a high priority but likely a longer-term, opportunistic effort.
With respect to Nr flux measurements in natural ecosystems, low elevation forests and grasslands have been studied most extensively. However, with the exception of HNO3, relatively few geographical locations have been characterized. As a general conclusion, more flux measurements are needed in forest ecosystems, particularly deciduous forests for oxidized N and coniferous forests for NH3. Other geographical and ecosystem specific gaps are summarized below.
High elevation and alpine environments are particularly sensitive to Nr inputs (Bowman et al., 2015). Only two studies (Rattray and Sievering, 2001; Sievering et al., 2001) in high elevation (>3000 m) and alpine environments could be identified for North America (excluding the work of Lee et al. (1993) over lava at Mauna Loa, Hawaii). Such environments are challenging due to non-ideal terrain and the generally low concentrations observed in these remote areas.
Urban-to-rural gradients represent areas where deposition of oxidized Nr forms to natural ecosystems is expected to be large and poorly understood species such as NO2 and HONO may make particularly important contributions. These areas are not characterized by the studies summarized in Table 1.
Agricultural regions represent areas where NH3 deposition is highly spatially variable. With the exception of Phillips et al. (2004), studies summarized here do not characterize NH3 fluxes to natural ecosystems at high concentrations typical of agricultural areas. These may also be areas where reduced ON forms (e.g., aliphatic amines) may be particularly important.
Coastal zones represent areas where coarse NO3− aerosol and ON compounds from marine sources may be particularly important components of the dry deposition budget. Table 1 contains a single study at a coastal site (Myles et al., 2007).
Dry deposition is the dominant pathway in arid ecosystems, which cover large areas of the western U.S., yet direct dry deposition measurements in these areas are lacking. Additional measurements that elucidate the processes of exchange with the soil surface are particularly needed in these ecosystems (Fenn et al., 20090; Padgett et al., 1999; 2001).
Experiments targeting these specific environments should be a long-term objective. We note, however, that this list is not comprehensive with respect to the need for direct measurements of deposition to sensitive ecosystems. For example, large areas of boreal forest are subject to deposition resulting from industrial emissions associated with extraction and processing of the Canadian oil sands (Proemse et al., 2013; Hsu et al., 2016; Makar et al., 2018).
Air-surface exchange processes –
While data are easily accessible for more routine measurements (e.g., wet deposition, air concentrations) collected within networks, direct measurements of air-surface exchange of particles and trace gases (i.e., dry deposition and bidirectional exchange) are typically conducted in intensive, shorter-term studies. These datasets are therefore often less visible and accessible to the user community. Establishment of a publicly available metadatabase for Nr flux measurements would serve the atmospheric science and ecological communities interested in better understanding the processes and drivers of land-atmosphere exchange of Nr and development of models to better simulate these processes.
Our review of existing North American flux datasets points to several data and knowledge gaps related to air-surface exchange processes that must be addressed in order to advance model algorithms. Recent studies of particulate and gas phase oxidized N fluxes (Farmer et al., 2006; Wolfe et al., 2009; Min et al., 2012; Gordon et al., 2011) in North American forest ecosystems document both emission and deposition at the canopy scale, challenging the unidirectional (i.e., deposition only) flux models employed in field scale and gridded CTMs. These studies highlight the importance of in-canopy chemical processes in regulating the net flux between the atmosphere and biosphere, which can result in net loss from the canopy. These results point to the need for studies to quantify within- and near-canopy sources and sinks of the components of the chemical system under study (e.g., NOy or HNO3-NH3-NH4NO3) such that models can be advanced to incorporate underlying biological, chemical, and physical processes. This is a high priority and represents a long-term effort.
Measurements to elucidate the role of surface wetness and cuticle chemistry in the “non-stomatal” canopy resistances for gas phase HNO3, NH3, and PNs are also seen as a high priority. Assessment of the volume and chemistry of dew (e.g., Wentworth et al., 2016) and guttation could be incorporated into flux experiments in the near term. For NH3, flux measurements should also be accompanied by measurements of soil and vegetation chemistry in order to constrain the emission potentials responsible for soil and stomatal compensation points. Such measurements could also be incorporated into flux experiments in the short term.
From a technological standpoint, further development of open-path techniques for NH3 flux measurements is a short-term, high priority objective. Extension of micrometeorological flux measurement techniques to complex terrain typical of Nr sensitive high-elevation environments is also a long-term objective.
Opportunities for collaboration –
This review also highlights the need for closer collaboration between the atmospheric chemistry and ecological communities with respect to advancement of Nr deposition budgets in North America. Coordinated multi-agency field studies, leveraging expertise and resources, can be a cost-effective approach to addressing the most urgent process-oriented research questions. Historically, large-scale atmospheric measurement campaigns in the U.S. have been developed and coordinated by the atmospheric chemistry community. While measurements of Nr and deposition are often components of these studies, the ecological community should engage with these efforts more directly where possible to advocate for science objectives that integrate atmospheric chemistry and ecological questions relevant to Nr deposition.
Footnotes
Publisher's Disclaimer: Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. EPA.
References
- Aan de Brugh JMJ, Ouwersloot HG, Vilà -Guerau de Arellano J, Krol MC (2013) A large-eddy simulation of the phase transition of ammonium nitrate in a convective boundary layer. J. Geophys. Res. Atmos, 118, 826–836. [Google Scholar]
- Altieri KE, Hastings MG, Peters AJ, Sigman DM (2012) Molecular characterization of water soluble organic nitrogen in marine rainwater by ultra-high resolution electrospray ionization mass spectrometry. Atmos. Chem. Phys, 12, 3557–3571. [Google Scholar]
- Baldocchi DD, Hicks BB, Meyers TP (1988) Measuring biosphere-atmosphere exchanges of biologically related gases with micrometeorological methods. Ecology, 69, 1331–1340. [Google Scholar]
- Bash JO, Cooter EJ, Dennis RL, Walker JT, Pliem JE (2013) Evaluation of a regional air-quality model with bidirectional NH3 exchange coupled to an agroecosystem model. Biogeosciences, 10, 1635–1645. [Google Scholar]
- Battye WH, Bray CD, Aneja VP, Tong D, Lee P, Tang Y (2016) Evaluating ammonia (NH3) predictions in the NOAA National Air Quality Forecast Capability (NAQFC) using in situ aircraft, ground-level, and satellite measurements from the DISCOVER-AQ Colorado campaign. Atmos. Environ, 140, 342–351. [Google Scholar]
- Benedict KB, Prenni AJ, Carrico CM, Sullivan AP, Schichtel BA, Collett JL Jr. (2017) Enhanced concentrations of reactive nitrogen species in wildfire smoke. Atmos. Environ, 148, 8–15. [Google Scholar]
- Bowker GE, Schwede DB, Lear GG, Warren-Hicks WJ, Finkelstein PL (2011) Quality Assurance Decisions with Air Models: A Case Study of Imputation of Missing Input Data Using EPA’s Multi-Layer Model. Water, Air, Soil Poll, 223, 391–402. [Google Scholar]
- Bowman WD, Nemergut DR, McKnight DM, Miller MP, Williams MW (2015) A slide down a slippery slope – alpine ecosystem responses to nitrogen deposition. Plant Ecol. Divers, 8, 727–738. [Google Scholar]
- Brook JR, Zhang L, Li Y, Johnson D (1999) Description and evaluation of a model of deposition velocities for routine estimates of air pollutant dry deposition over North America. Part II: Review of past measurements and model results. Atmos. Environ, 33, 5053–5070. [Google Scholar]
- Brost RA, Delany AC, Huebert BJ (1998) Numerical modeling of concentrations of fluxes of HNO3, NH3, and NH4NO3 near the surface. J. Geophys. Res. Atmos, 93, 7137–7152. [Google Scholar]
- Browne EC, Cohen RC (2012) Effects of biogenic nitrate chemistry on the NOx lifetime in remote continental regions. Atmos. Chem. Phys, 12, 11917–11932. [Google Scholar]
- Browne EC, Wooldridge PJ, Min K-E, Cohen RC (2014) On the role of monoterpene chemistry in the remote continental boundary layer. Atmos. Chem. Phys, 14, 1225–1238. [Google Scholar]
- Brümmer C, Marx O, Kutsch W, Ammann C, Wolff V, Flechard CR, Freibauer A (2013) Fluxes of total reactive atmospheric nitrogen (Nr) using eddy covariance above arable land. Tellus Ser B, 65, 19770. [Google Scholar]
- Burkhardt J, Flechard CR, Gresens F, Mattsson M, Jongejan PAC, Erisman JW, Weidinger T, Meszaros R, Nemitz E, Sutton MA (2009) Modelling the dynamic chemical interactions of atmospheric ammonia with leaf surface wetness in a managed grassland canopy. Biogeosci, 6, 67–84. [Google Scholar]
- Burkhardt J, Hunsche M (2013) “Breath figures” on leaf surfaces—formation and effects of microscopic leaf wetness. Front. Plant Sci, 4, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Businger JA, Oncley SP (1990) Flux Measurement with Conditional Sampling. J. Atmos. Ocean. Technol, 7, 349–352. [Google Scholar]
- Butler T, Marino R, Schwede D, Howarth R, Sparks J, Sparks K (2015) Atmospheric ammonia measurements at low concentration sites in the northeastern USA: implications for total nitrogen deposition and comparison with CMAQ estimates. Biogeochemistry, 122, 191–210. [Google Scholar]
- Cape JN, Cornell SE, Jickells TD, Nemitz E (2011) Organic nitrogen in the atmosphere – Where does it come from? A review of sources and methods. Atmos. Res, 102, 30–48. [Google Scholar]
- Cheng M, Jiang H, Guo Z, Zhang X, Lu X (2013) Estimating NO2 dry deposition using satellite data in eastern China. Int. J. Remote Sens, 34, 2548–2565, [Google Scholar]
- Clark CM, Phelan J, Doraiswamy P, Buckley J, Cajka JC, Dennis RL, Lynch J, Nolte CG, Spero TL (2018) Atmospheric deposition and exceedances of critical loads from 1800–2025 for the conterminous United States. Ecol. Appl, 28, 978–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JF, Edgerton ES, Martin BE (1997) Dry deposition calculations for the clean air status and trends network. Atmos. Environ, 31, 3667–3678. [Google Scholar]
- Cleary PA, Wooldridge PJ, Millet DB, McKay M, Goldstein AH, Cohen RC (2007) Observations of total peroxy nitrates and aldehydes: measurement interpretation and inference of OH radical concentrations. Atmos. Chem. Phys, 7, 1947–1960. [Google Scholar]
- Compton JE, Harrison JA, Dennis RL, Greaver T, Hill BH, Jordan SJ, Walker H, Campbell HV, (2011) Ecosystem services altered by changes in reactive nitrogen: a new perspective for U.S. decision making. Ecol. Lett, 14, 804–815. [DOI] [PubMed] [Google Scholar]
- Cooter E, Rea A, Dennis R, Bruins R, Schwede D (2013) The role of the atmosphere in the provision of ecosystem services. Sci. Total Environ, 448, 197–208. [DOI] [PubMed] [Google Scholar]
- Day DA, Wooldridge PJ, Cohen RC (2008) Observations of the effects of temperature on atmospheric HNO3, ΣANs, ΣPNs, and NOx: evidence for a temperature-dependent HOx source. Atmos. Chem. Phys, 8, 1867–1879. [Google Scholar]
- Delany AC, Fitzjarrald DR, Lenschow DH, Pearson R Jr., Wendel GJ, Woodruff B (1986) Direct measurements of nitrogen oxides and ozone fluxes over grassland. J. Atmos. Chem, 4, 429–444. [Google Scholar]
- Dennis RL, Schwede DB, Bash JO, Pleim JE, Walker JT, Foley KM (2013) Sensitivity of continental United States atmospheric budgets of oxidized and reduced nitrogen to dry deposition parameterizations. Philos. Tr. R. Soc. B, 368, 20130124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doskey PV, Kotamarthi RV, Fukui Y, Cook DR, Breitbeil III FW, Wesely ML (2004) Air-surface exchange of peroxyacetyl nitrate at a grassland site. J. Geophys. Res. Atm, 109, D10310. [Google Scholar]
- Ellis RA, Murphy JG, Pattey E, van Haarlem R, O’Brien JM, Herndon SC (2010) Characterizing a Quantum Cascade Tunable Infrared Laser Differential Absorption Spectrometer (QCTILDAS) for measurements of atmospheric ammonia. Atmos. Meas. Tech, 3, 397–406. [Google Scholar]
- Ellis RA, Jacob DJ, Sulprizio MP, Zhang L, Holmes CD, Schichtel BA, Blett T, Porter E, Pardo LH, Lynch JA (2013) Present and future nitrogen deposition to national parks in the United States: critical load exceedances. Atmos. Chem. Phys, 13, 9083–9095. [Google Scholar]
- Famulari D, Fowler D, Hargreaves K, Milford C, Nemitz E, Sutton MA, Weston K (2004) Measuring eddy covariance fluxes of ammonia using tunable diode laser absorption spectroscopy. Water Air Soil Pollut, 4, 151–158. [Google Scholar]
- Famulari D, Fowler D, Nemitz E, Hargreaves KJ, Storeton-West RL, Rutherford G, Tang YS, Sutton MA, Weston KJ (2010) Development of a low-cost system for measuring conditional time-averaged gradients of SO2 and NH3. Environmental Monit. Assess, 161, 11–27. [DOI] [PubMed] [Google Scholar]
- Farmer DK, Wooldridge PJ, Cohen RC (2006) Application of thermal-dissociation laser induced fluorescence (TD-LIF) to measurement of HNO3, ∑alkyl nitrates, ∑peroxy nitrates, and NO2 fluxes using eddy covariance. Atmos. Chem. Phys, 6, 3471–3486. [Google Scholar]
- Farmer DK, Cohen RC (2008) Observations of HNO3, PP AN, PN and NO2 fluxes: evidence for rapid HOx chemistry within a pine forest canopy, Atmos. Chem. Phys, 8, 3899–3917. [Google Scholar]
- Farmer DK, Kimmel JR, Phillips G, Docherty KS, Worsnop DR, Sueper D, Nemitz E, Jimenez JL (2011) Eddy covariance measurements with high-resolution time-of-flight aerosol mass spectrometry: A new approach to chemically resolved aerosol fluxes. Atmos. Meas. Tech, 4, 1275–1289. [Google Scholar]
- Farmer DK, Chen Q, Kimmel JR, Docherty KS, Nemitz E, Artaxo PA, Cappa CD, Martin ST, Jimenez JL (2013) Chemically-resolved particle fluxes over tropical and temperate forests. Aerosol Sci. Tech, 47, 818–830. [Google Scholar]
- Fenn ME, Sickman JO, Bytnerowicz A, Clow DW, Molotch NP, Pleim JE, Tonnesen GS, Weathers KC, Padgett PE, Campbell DH (2009) Methods for measuring atmospheric nitrogen deposition inputs in arid and montane ecosystems of western North America In: Legge AH, ed. 2009. Developments in Environmental Science, Vol. 9: Air Quality and Ecological Impacts: Relating Sources to Effects; Elsevier, Amsterdam: pp.179–228. [Google Scholar]
- Fenn ME, Allen EB, Weiss SB, Jovan S, Geiser LH, Tonnesen GS, Johnson RF, Rao LE, Gimeno BS, Yuan F, Meixner T, Bytnerowicz A (2010) Nitrogen critical loads ad management alternatives for N-impacted ecosystems in California. J. Environ. Manage, 91, 2404–2423. [DOI] [PubMed] [Google Scholar]
- Ferrara RM, Loubet B, Di Tommasi P, Bertolini T, Magliulo V, Cellier P, Eugster W, Rana G (2012) Eddy covariance measurement of ammonia fluxes: Comparison of high frequency correction methodologies. Agr. Forest Meteorol, 158, 30–42. [Google Scholar]
- Fisher JA, Jacob DJ, Travis KR, Kim PS, Marais EA, et al. , (2016) Organic nitrate chemistry and its implications for nitrogen budgets in an isoprene- and monoterpene-rich atmosphere: constraints from aircraft (SEAC4RS) and ground-based (SOAS) observations in the Southeast US. Atmos. Chem. Phys, 16, 5969–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechard CR, Fowler D, Sutton MA, Cape JN (1999) A dynamic chemical model of bi-directional ammonia exchange between semi-natural vegetation and the atmosphere. Q. J. Roy. Meteor. Soc, 125, 2611–2641. [Google Scholar]
- Flechard CR, Nemitz E, Smith RI, Fowler D, Vermeulen AT, Bleeker A, Erisman JW, Simpson D, Zhang L, Tang YS, Sutton MA (2011) Dry deposition of reactive nitrogen to European ecosystems: a comparison of inferential models across the NitroEurope network. Atmos. Chem. Phys, 11, 2703–2728. [Google Scholar]
- Flechard CR, Massad R-S, Loubet B, Personne E, Simpson D, Bash JO, Cooter EJ, Nemitz E, & Sutton MA (2013) Advances in understanding, models and parameterizations of biosphere-atmosphere ammonia exchange. Biogeosciences, 10, 5183–5225. [Google Scholar]
- Foken T (2008) Micrometeorology, 2nd ed., Springer, Berlin, 290 pp. [Google Scholar]
- Foken T, Aubinet M, Leuning R (2012) The Eddy Covariance Method In: Aubinet M, Vesala T, Papale D (eds) Eddy Covariance. Springer Atmospheric Sciences; Springer, Dordrecht. [Google Scholar]
- Fowler D, Coyle M, Flechard C, Hargreaves K, Nemitz E, Storeton-West R, Sutton M, Erisman JW (2001) Advances in micrometeorological methods for the measurement and interpretation of gas and particle nitrogen fluxes. Plant and Soil, 228, 117–129. [Google Scholar]
- Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby J, (2003) The nitrogen cascade. Bioscience, 53, 341–356. [Google Scholar]
- Geddes JA, Murphy JG (2014) Observations of reactive nitrogen oxide fluxes by eddy covariance above two mid-latitude North American mixed hardwood forests. Atmos. Chem. Phys, 14, 2939–2957. [Google Scholar]
- Geddes JA, Martin RV (2017) Global deposition of total reactive nitrogen oxides from 1996 to 2014 constrained with satellite observations of NO2 columns. Atmos. Chem. Phys, 17, 10071–10091. [Google Scholar]
- Gordon M, Staebler RM, Liggio J, Vlasenko A, Li S-M, Hayden K (2011) Aerosol flux measurements above a mixed forest at Borden, Ontario. Atmos. Chem. Phys, 11, 6773–6786. [Google Scholar]
- Hansen K, Pryor SC, Boegh E, Hornsby KE, Sørensen LL (2015) Background concentrations and fluxes of atmospheric ammonia over a deciduous forest. Agric. For. Meteorol, 214-215, 380–392. [Google Scholar]
- Horii CV, Munger JW, Wofsy SC, Zahniser M, Nelson D, McManus JB (2004) Fluxes of nitrogen oxides over a temperate deciduous forest. J. Geophys. Res. Atmos, 109, D08305. doi: 10.1029/2003JD004326. [DOI] [Google Scholar]
- Horii CV, Munger JW, Wofsy SC, Zahniser M, Nelson D, McManus JB (2005) Atmospheric reactive nitrogen concentration and flux budgets at a Northeastern U.S. forest site. Agric. For. Meteorol, 133, 210–225. [Google Scholar]
- Hsu Y-M, Bytnerowicz A, Fenn ME, Percy KE, (2016) Atmospheric dry deposition of sulfu and nitrogen in the Athabasca oil sands region, Alberta, Canada. Sci. Total Environ, 15, 285–295. [DOI] [PubMed] [Google Scholar]
- Huebert BJ, Robert CH (1985) The dry deposition of nitric acid to grass. J. Geophys. Res. Atmos, 90, 2085–2090. [Google Scholar]
- Huebert BJ, Luke WT, Delany AC, Brost RA (1988) Measurements of concentrations and dry surface fluxes of atmospheric nitrates in the presence of ammonia. J. Geophys. Res. Atmos, 93, 7127–7136. [Google Scholar]
- Huff DM, Joyce PL, Fochesatto GJ, Simpson WR (2011) Deposition of dinitrogen pentoxide, N2O5, to the snowpack at high latitudes. Atmos. Chem. Phys, 11, 4929–4938. [Google Scholar]
- Jia Y, Yu G, Gao Y, He N, Wang Q, Jiao C, Zuo Y (2016) Global inorganic nitrogen dry deposition inferred from ground- and space-based measurements. Scientific Reports, 6, 19810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jickells T, Baker AR, Cape JN, Cornell SE, Nemitz E (2013) The cycling of organic nitrogen through the atmosphere. Philos. Tr. R. Soc. B, 368, 20130115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakidou M, Myriokefalitakis S, Daskalakis N, Fanourgakis G, Nenes A, Baker AR, et al. (2016) Past, present, and future atmospheric nitrogen deposition. J. Atmos. Sci, 73, 2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katul G, Finkelstein PL, Clarke JF, Ellestad TG (1996) An investigation of the conditional sampling methods used to estimate fluxes of active, reactive and passive scalars. J. Appl. Meteorol, 35, 1835–1845. [Google Scholar]
- Kelly JT, Baker KR, Nowak JB, Murphy JG, Markovic MZ, et al. (2014) Fine-scale simulation of ammonium and nitrate over the South coast air basin and San Joaquin Valley of California during CalNex-2010. J. Geophys. Res. – Atmos, 119, 3600–3614. [Google Scholar]
- Kharol SK, Shephard MW, McLinden CA, Zhang L, Sioris CE, O’Brien JM, Vet R, Cady-Pereira KE, Hare E, Siemons J, Krotkov NA (2018) Dry deposition of reactive nitrogen from satellite observations of ammonia and nitrogen dioxide over North America. Geophys. Res. Lett, 45, 1157–1166. [Google Scholar]
- Kim MJ, Farmer DK, Bertram TH (2014) A controlling role for the air-sea interface in the chemical processing of reactive nitrogen in the coastal marine boundary layer. P. Natl. Acad. Sci. USA, 111, 3943–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford A, Fehsenfeld F, Zachariassen J, Schimel D (1992) Gaseous ammonia fluxes and background concentrations in terrestrial ecosystems of the United States. Global Biogeochem. Cy, 6, 459–483. [Google Scholar]
- Lee G, Zhuang L, Huebert BJ, Meyers TP (1993) Concentration gradients and dry deposition of nitric acid vapor at the Mauna Loa Observatory, Hawaii. J. Geophys. Res. Atmos, 98, 12661–12671. [Google Scholar]
- Li Y, Schichtel B, Walker JT, Schwede DB, Chen XI, Lehmann C, Puchalski M, Gay D, Collett JL Jr. (2016) The Increasing Importance of Deposition of Reduced Nitrogen in the United States. P. Natl. Acad. Sci. USA, 113, 5874–5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Jiang H Zhang X, Liu J Zhang Z, Jin J, Wang Y, Xu J, Cheng M (2013) Estimated global nitrogen deposition using NO2 column density. Int. J. Remote Sens, 34, 8893–8906. [Google Scholar]
- Makar PA, Akingunola A, Aherne J, Cole AS, Aklilu Y-A, Zhang J, Wong I, Hayden K, Li S-M, Kirk J, Scott K, Moran MD, Robichaud A, Cathcart H, Baratzedah P, Pabla B, Cheung P, Zheng Q, and Jeffries DS (2018) Estimates of exceedances of critical loads for acidifying deposition in Alberta and Saskatchewan. Atmos. Chem. Phys, 18, 9897–9927. [Google Scholar]
- Massad R-S, Nemitz E, Sutton MA (2010) Review and parameterization of bi-directional ammonia exchange between vegetation and the atmosphere. Atmos. Chem. Phys, 10, 10359–10386. [Google Scholar]
- McDonnell TC, Reinds GJ, Sullivan TJ, Clark CM, Bonten LTC, Mol-Dijkstra JP, Wamelink GWW, Dovciak M (2018) Feasibility of coupled empirical and dynamic modeling to assess climate change and air pollution impacts on temperate forest vegetation of the eastern United States. Environ. Pollut, 234, 902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers TP, Huebert BJ, Hicks BB (1989) HNO3 deposition to a deciduous forest. Bound.-Lay. Meteorol, 49, 395–410. [Google Scholar]
- Meyers TP, Hall ME, Lindberg SE, Kim K, (1996) Use of the modified Bowen-ratio technique to measure fluxes of trace gases. Atmos. Environ, 30, 3321–3329. [Google Scholar]
- Meyers TP, Finkelstein P, Clarke J, Ellestad TG, Sims PF (1998) A multilayer model for inferring dry deposition using standard meteorological measurements. J. Geophys. Res. Atmos, 103, 22645–22661. [Google Scholar]
- Min K-E, Pusede SE, Browne EC, Lafranchi BW, Wooldridge PJ, Wolfe GM, Harrold SA, Thornton JA, Cohen RC (2012) Observations of atmosphere-biosphere exchange of total and speciated peroxynitrates: Nitrogen fluxes and biogenic sources of peroxynitrates, Atmos. Chem. Phys, 12, 9763–9773. [Google Scholar]
- Min K-E, Pusede SE, Browne EC, LaFranchi BW, Cohen RC (2014) Eddy covariance fluxes and vertical concentration gradient measurements of NO and NO2 over a ponderosa pine ecosystem: observational evidence for within-canopy chemical removal of NOx. Atmos. Chem. Phys, 14, 5495–5512. [Google Scholar]
- Moncrieff J, Valentini R, Greco S, Seufert G, Ciccioli P (1997) Trace gas exchange over terrestrial ecosystems: methods and perspectives in micrometeorology. J. Exp. Bot, 48, 1133–1142. [Google Scholar]
- Munger JW, Wofsy SC, Bakwin PS, Fan SM, Goulden ML, Daube BC, Goldstein AH, Moore KE, Fitzjarrald DR, (1996) Atmospheric deposition of reactive nitrogen oxides and ozone in a temperate deciduous forest and a subarctic woodland .1. Measurements and mechanisms. J. Geophys. Res. Atmos, 101, 12639–12657. [Google Scholar]
- Munns WR Jr., Poulsen V, Gala W, Marshall SJ, Rea AW, Sorensen M, von Stackelberg K (2016) Ecosystem services in risk assessment and management. Integr. Environ. Assess. Manag, 13, 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JG, Day DA, Cleary PA, Wooldridge PJ, Cohen RC (2006) Observations of the diurnal and seasonal trends in nitrogen oxides in the western Sierra Nevada. Atmos. Chem. Phys, 6, 5321–5338. [Google Scholar]
- Myles L, Meyers TP, Robinson L(2007) Relaxed eddy accumulation measurements of ammonia, nitric acid, sulfur dioxide and particulate sulfate dry deposition near Tampa, FL, USA. Environ. Res. Lett, 2, 034004. [Google Scholar]
- Nanus L, McMurray JA, Clow DW, Saros JE, Blett T, Gurdak JJ (2017) Spatial variation of atmospheric nitrogen deposition and critical loads for aquatic ecosystems in the Greater Yellowstone Area. Environ. Pollut, 223, 644–656. [DOI] [PubMed] [Google Scholar]
- Neff JC, Holland EA, Dentener FJ, McDowell WH, Russell KM (2002) The origin, composition and rates of organic nitrogen deposition: a missing piece of the nitrogen cycle? Biogeochem, 57, 99–136. [Google Scholar]
- Nemitz E, Sutton MA, Wyers GP, Jongejan PAC (2004a) Gas-particle interactions above a Dutch heathland: I. Surface exchange fluxes of NH3, SO2, HNO3 and HCl. Atmos. Chem. Phys, 4, 989–1005. [Google Scholar]
- Nemitz E, Sutton MA, Wyers GP, Otjes RP, Mennen MG, van Putten EM, Gallagher MW, (2004b) Gas-particle interactions above a Dutch heathland: II. Concentrations and surface exchange fluxes of atmospheric particles. Atmos. Chem. Phys, 4, 1007–1024. [Google Scholar]
- Nguyen TB, Crounse JD, Teng AP St. Clair JM, Paulot F, Wolfe GM, Wennberg PO (2015) Rapid deposition of oxidized biogenic compounds to a temperate forest. Proc. Nat. Acad. Sci. USA, 112, E392–E401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Grennfelt P (1988) Critical loads for sulphur and nitrogen Report from UNECE/Nordic Council workshop held in Skokloster, Sweden; March 19–24, Nordic Council of Ministers, Copenhagen, Denmark: (1988). [Google Scholar]
- Nowlan CR, Martin RV, Philip S, Lamsal LN, Krotkov NA, Marais EA, Wang S, Zhang Q (2014) Global dry deposition of nitrogen dioxide and sulfur dioxide inferred from space-based measurements. Global Biogeochem. Cycles, 28, 1025–1043. [Google Scholar]
- Padgett PE, Allen EB, Bytnerowicz A, Minich RA (1999) Changes in soil inorganic nitrogen as related to atmospheric nitrogenous pollutants in southern California. Atmos. Environ, 33, 769–781. [Google Scholar]
- Padgett PE, Bytnerowicz A (2001) Deposition and adsorption of the air pollutant HNO3 vapor to soil surfaces. Atmos. Environ, 35, 2405–2415. [Google Scholar]
- Pardo LH, Fenn M, Goodale CL, Geiser LH, Driscoll CT, et al. (2011) Effects of nitrogen deposition and empirical nitrogen critical loads for ecoregions of the United States. Ecol. Appl, 21, 3049–3082. [Google Scholar]
- Paulot F, Henze DK, Wennberg PO (2012) Impact of the isoprene photochemical cascade on tropical ozone. Atmos. Chem. Phys, 12, 1307–1325. [Google Scholar]
- Phillips SB, Arya SP, Aneja VP, 2004. Ammonia flux and dry deposition velocity from near-surface concentration gradient measurements over a grass surface in North Carolina. Atmos. Environ, 38, 3469–3480. [Google Scholar]
- Pleim JE, Bash JO, Walker JT, Cooter E (2013) Development and evaluation of an ammonia bidirectional flux parameterization for air quality models. J. Geophys. Res. Atmos, 118, 3794–3806. [Google Scholar]
- Prenni AJ, Levin EJT, Benedict KB, Sullivan AP, Schurman MI, Gebhart KA, Day DE, Carrico CM, Malm WC, Schichtel BA, Collett JL, Kreidenweis SM (2014) Gas-phase reactive nitrogen near Grand Teton National Park: Impacts of transport, anthropogenic emissions, and biomass burning. Atmos. Environ 89, 749–756. [Google Scholar]
- Proemse BC, Mayer B, Fenn ME, Ross CS (2013) A multi-isotope approach for estimating industrial contributions to atmospheric nitrogen deposition in the Athabasca oil sands region in Alberta, Canada. Environ. Pollut, 80–91. [DOI] [PubMed] [Google Scholar]
- Pryor SC, Barthelmie RJ, Sørensen LL, Jensen B (2001) Ammonia concentrations and fluxes over a forest in the midwestern USA. Atmos. Environ, 35, 5645–5656. [Google Scholar]
- Pryor SC, Barthelmie RJ, Jensen B, Jensen NO and Sørensen LL (2002) HNO3 fluxes to a deciduous forest derived using gradient and REA methods. Atmos. Environ, 36, 5993–5999. [Google Scholar]
- Pryor SC, Klemm O, 2004. Experimentally derived estimates of nitric acid dry deposition velocity and viscous sub-layer resistance at a conifer forest. Atmos. Environ, 38, 2769–2777. [Google Scholar]
- Ramsay R, Di Marco CF, Heal MR, Twigg MM, Cowan N, et al. (2018) Surface-atmosphere exchange of inorganic water-soluble gases and associated ions in bulk aerosol above agricultural grassland pre- and postfertilization. Atmos. Chem. Phys, 18, 16953–16978. [Google Scholar]
- Rattray G and Sievering H (2001) Dry deposition of ammonia, nitric acid, ammonium, and nitrate to alpine tundra at Niwot Ridge, Colorado, Atmos. Environ, 35, 1105–1109. [Google Scholar]
- Reis S, Pinder R, Zhang M, Lijie G, Sutton M (2009) Reactive nitrogen in atmospheric emission inventories. Atmos. Chem. Phys, 9, 7657–7677. [Google Scholar]
- Ren X, Sanders JE, Rajendran A, Weber RJ, Goldstein AH, Pusede SE, Browne EC, Min K-E, Cohen RC (2011) A relaxed eddy accumulation system for measuring vertical fluxes of nitrous acid. Atmos. Meas. Tech, 4, 2093–2103. [Google Scholar]
- Rinsland CP, Goldman A, Murcray FJ, Stephen TM, Pougatetchev NS, Fishman J, David SJ, Blatherwick RD, Novelli PC, Jones NB, Conner BJ (1999) Infrared solar spectroscopic measurements of free tropospheric CO, C2H, and HCN above Mauna Loa, Hawaii: Seasonal variations and evidence for enhanced emissions from the Southeast Asian tropical fires of 1997–1998. J. Geophys. Res, 104, 18667–18680. [Google Scholar]
- Roberts JM (1990) The atmospheric chemistry of organic nitrates. Atmos. Environ, 24A, 243–287. [Google Scholar]
- Roberts JM, Flocke F, Chen G, de Gouw J, Holloway JS, Hubler G, Neuman JA, Nicks DK Jr., Nowak JB, Parrish DD, Ryerson TB, Sueper DT, Warneke C, Fehsenfeld FC (2004) Measurement of peroxycarboxylic nitric anhydrides (PANs) during the ITCT 2K2 aircraft intensive experiment. J. Geophys. Res. Atmos, 109, D23S21. [Google Scholar]
- Rumsey I, Walker JT (2016) Application of an online ion chromatography-based instrument for gradient flux measurements of speciated nitrogen and sulfur. Atmos. Meas. Tech, 9, 2581–2592. [Google Scholar]
- Schrader F, Brummer C (2014) Land use specific ammonia deposition velocities: a review of recent studies (2004 – 2013) Water Air Soil Pollut, 225, 2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwede D, Zhang L, Vet R, Lear G (2011) An intercomparison of the deposition models used in the CASTNET and CAPMoN networks. Atmos. Environ, 45, 1337–1346. [Google Scholar]
- Schwede DB, Lear GG (2014) A novel hybrid approach for estimating total deposition in the United States. Atmos. Environ, 92, 207–220. [Google Scholar]
- Seinfeld JH, Pandis SN (1998) Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, John Wiley and Sons. [Google Scholar]
- Shaw WJ, Spicer CW, Kenny DW (1998) Eddy correlation fluxes of trace gases using a tandem mass spectrometer. Atmos. Environ, 32, 2887–2898. [Google Scholar]
- Sickles II JE, Shadwick DS (2015) Air quality and atmospheric deposition in the eastern US: 20 years of change. Atmos. Chem. Phys, 15, 173–197. [Google Scholar]
- Sievering H, Kelly T, McConville G, Seibold C, Turnipseed A, (2001) Nitric acid dry deposition to conifer forests: Niwot Ridge spruce-fir-pine study. Atmos. Environ, 35, 3851–3859. [Google Scholar]
- Sintermann J, Spirig C, Jordan A, Kuhn U, Ammann C, Neftel A (2011) Eddy covariance flux measurements of ammonia by high temperature chemical ionisation mass spectrometry. Atmos. Meas. Tech, 4, 599–616. [Google Scholar]
- Sun K, Tao L, Miller DJ, Zondlo MA, Shonkwiler KB, Nash C, & Ham JM (2015) Open-path eddy covariance measurements of ammonia fluxes from a beef cattle feedlot. Agr. Forest Meteorol, 213, 193–202. [Google Scholar]
- Tarnay LW, Gertler A, Taylor GE Jr., 2002. The use of inferential models for estimating nitric acid vapor deposition to semi-arid coniferous forests. Atmos. Environ, 36, 3277–3287. [Google Scholar]
- Thom AS (1975) Momentum, mass and heat exchange of plant communities In Vegetation and Atmosphere. Ed. Monteith JL. pp 57–109. Academic Press, London. [Google Scholar]
- Thomas RM, Trebs I, Otjes R, Jongejan PAC, Brink H. t., Phillips G, Kortner M, Meixner FX, Nemitz E (2009) An automated analyzer to measure surface-atmosphere exchange fluxes of water soluble inorganic aerosol compounds and reactive trace gases. Environ. Sci. Technol, 43, 1412–1418. [DOI] [PubMed] [Google Scholar]
- Thornton JA, Kercher JP, Riedel TP, Wagner NL, Cozic J, Holloway JS, Dube WP, Wolfe GM, Quinn PK, Middlebrook AM, Alexander B, Brown SS (2010) A large atomic chlorine source inferred from mid-continental reactive nitrogen chemistry. Nature, 464, 271–274. [DOI] [PubMed] [Google Scholar]
- Turnipseed AA, Huey LG, Nemitz E, Stickel R, Higgs J, Tanner DJ, Slusher DL, Sparks JP, Flocke F, Guenther A (2006) Eddy covariance fluxes of peroxyacetyl nitrates (PANs) and NOy to a coniferous forest. J. Geophys. Res. Atmos, 111, D09304. [Google Scholar]
- U.S. EPA, 2008. U.S. Environmental Protection Agency. Integrated Science Assessment for Oxides of Nitrogen and Sulfur – Ecological Criteria. EPA/600/R-08/082F. EPA, Research Triangle Park, NC. [Google Scholar]
- U.S. EPA, 2012. U.S. Environmental Protection Agency. Secondary National Ambient Air Quality Standards for Oxides of Nitrogen and Oxides of Sulfur. Final Rule, 40 CFR part 50 Federal Register, 77(64), 20218–20272. Office of the Federal Register, National Archives and Records Administration, Washington, D.C. [Google Scholar]
- U.S. EPA, 2019. U.S. Environmental Protection Agency Critical Loads Mapper Tool https://www.epa.gov/air-research/critical-loads-mapper-tool [Google Scholar]
- Walker JT, Bell MD, Schwede D, Cole A, Beachley G, Lear G, Wu Z, 2019. Aspects of uncertainty in total reactive nitrogen deposition estimates for North American critical load applications. Sci. Tot. Environ, 690, 1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth GR, Murphy JG, Benedict KB, Bangs EJ, Collett JL (2016) The role of dew as a night-time reservoir and morning source for atmospheric ammonia. Atmos. Chem. Phys, 16, 7435–7449. [Google Scholar]
- Wesely ML, Hicks BB (2000) A review of the current status of knowledge in dry deposition. Atmos. Environ, 34, 2261–2282. [Google Scholar]
- Whaley CH, Makar PA, Shephard MW, Zhang L, Zhang J, Zheng Q, Akingunola A, Wentworth GR, Murphy JG, Kharol SK, Cady-Pereira KE (2018) Contributions of natural and anthropogenic sources to ambient ammonia in the Athabasca Oil Sands and north-western Canada. Atmos. Chem. Phys, 18, 2011–2034. [Google Scholar]
- Wolfe GM, Thornton JA, Yatavelli RLN, McKay M, Goldstein AH, LaFranchi B, Min K-E, Cohen RC (2009) Eddy covariance fluxes of acyl peroxy nitrates (PAN, PPN and MPAN) above a Ponderosa pine forest. Atmos. Chem. Phys, 9, 615–634. [Google Scholar]
- Wolfe GM, Thornton JA, Bouvier-Brown NC, Goldstein AH, Park J-H, et al. (2011) The Chemistry of Atmosphere-Forest Exchange (CAFE) Model – Part 2: Application to BEARPEX-2007 observations. Atmos. Chem. Phys, 11, 1269–1294. [Google Scholar]
- Wolfe GM, Hanisco TF, Arkinson HL, Bui TP, Crounse JD, et al. (2015) Quantifying sources and sinks of reactive gases in the lower atmosphere using airborne flux observations. Geophys. Res. Lett, 42, 8231–8240. [Google Scholar]
- Wolff V, Trebs I, Foken T, Meixner FV (2010) Exchange of reactive nitrogen compounds: Concentrations and fluxes of total ammonium and total nitrate above a spruce canopy. Biogeosciences, 7, 1729–1744. [Google Scholar]
- Wu Z, Wang X, Chen F, Turnipseed AA, Geunther AB, Niyogi D, Charusombat U, Xia B, Munger JW, Alapaty K (2011) Evaluating the calculated dry deposition velocities of reactive nitrogen oxides and ozone from two community models over a temperate deciduous forest. Atmos. Environ, 45, 2663–2674. [Google Scholar]
- Wu ZY, Wang X, Turnipseed AA, Chen F, Zhang L, Guenther AB, et al. (2012) Evaluation and improvements of two community models in simulating dry deposition velocities for peroxyacetyl nitrate (PAN) over a coniferous forest. J. Geophys. Res. – Atmos, 117, D04310. [Google Scholar]
- Wyers GP, Duyzer JH (1997) Micrometeorological measurement of the dry deposition flux of sulphate and nitrate aerosols to coniferous forest. Atmos. Environ, 31, 333–343. [Google Scholar]
- Yao L, Wang M-Y, Wang X-K, Liu Y-J, Chen H-F, Zheng J, Nie W, Ding A-J, Geng F-H, Wang D-F, Chen J-M, Worsnop DR, Wang L (2016) Detection of atmospheric gaseous amines and amides by a high-resolution time-of-flight chemical ionization mass spectrometer with protonated ethanol reagent ions. Atmos. Chem. Phys, 16, 14527–14543. [Google Scholar]
- You Y, Kanawade VP, de Gouw JA, Guenther AB, Madronich S, Sierra-Hernández MR, Lawler M, Smith JN, Takahama S, Ruggeri G, Koss A, Olson K, Baumann K, Weber RJ, Nenes A, Guo H, Edgerton ES, Porcelli L, Brune WH, Goldstein AH, Lee S-H (2014) Atmospheric amines and ammonia measured with a chemical ionization mass spectrometer (CIMS). Atmos. Chem. Phys, 14, 12181–12194. [Google Scholar]
- Zhang Y, ten Brink HM, Slanina J, Wyers GP (1995) The influence of ammonium nitrate equilibrium on the measurement of exchange fluxes of ammonia and nitric acid In Acid Rain Research: Do We Have Enough Answers?, edited by Heij GJ and Erisman JW, Elsevier Science B. V., 103–112. [Google Scholar]
- Zhang L, Moran M, Makar P, Brook J, and Gong S (2002) Modelling gaseous dry deposition in AURAMS A Unified Regional Air-quality Modelling System. Atmos. Environ, 36, 537–560. [Google Scholar]
- Zhang L, Brook JR, Vet R (2003) A revised parameterization for gaseous dry deposition in air‐quality models. Atmos. Chem. Phys, 3, 2067–2082. [Google Scholar]
- Zhang L, Vet R, O’Brien JM, Mihele C, Liang Z, Wiebe A, (2009) Dry deposition of individual nitrogen species at eight Canadian rural sites. J. Geophys. Res. Atmos, 114, D02301. [Google Scholar]
- Zhang L, Wright LP, Asman WAH (2010) Bi‐directional air‐surface exchange of atmospheric ammonia: A review of measurements and a development of a big‐leaf model for applications in regional‐scale air‐quality models. J. Geophys. Res. Atmos, 115, D20. [Google Scholar]
- Zhang L, Jacob DJ, Knipping EM, Kumar N, Munger JW, Carouge CC, van Donkelaar A, Wang YX, Chen D (2012a) Nitrogen deposition to the United States: Distribution, sources, and processes. Atmos. Chem. Phys, 12, 4539–4554. [Google Scholar]
- Zhang N, Zhou X, Bertman S, Tang D, Alaghmand M, Shepson PB, Carroll MA (2012b) Measurements of ambient HONO concentrations and vertical HONO flux above a northern Michigan forest canopy. Atmos. Chem. Phys, 12, 8285–8296. [Google Scholar]
- Zhang L, He Z, (2014) Technical Note: An empirical algorithm estimating dry deposition velocity of fine, coarse and giant particles. Atmos. Chem. Phys, 14, 3729–3737. [Google Scholar]
- Zhang Y, Mathur R, Bash JO, Hogrefe C, Xing J, Roselle SJ (2018) Long-term trends in total inorganic nitrogen and sulfur deposition in the US from 1990 to 2010, Atmos. Chem. Phys, 18, 9091–9106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Zhang N, TerAvest M, Tang D, Hou J, Bertman S, Alaghmand M, Shepson PB, Carroll MA, Griffith S, Dusanter S, Stevens PS (2011) Nitric acid photolysis on forest canopy surface as a source for tropospheric nitrous acid. Nat. Geosci, 4, 440–443. [Google Scholar]
- Zhu L, Henze D, Bash J, Jeong G-R, Cady-Pereira K, Shephard M, Luo M, Paulot F, Capps S (2015) Global evaluation of ammonia bidirectional exchange and livestock diurnal variation schemes. Atmos. Chem. Phys, 15, 12823–12843. [Google Scholar]
- Zöll U, Brümmer C, Schrader F, Ammann C, Ibrom A, Flechard CR, Nelson DD, Zahniser M, Kutsch W (2016) Surface-atmosphere exchange of ammonia over peatland using QCL-based eddy-covariance measurements and inferential modeling. Atmos. Chem. Phys, 16, 11283–11299. [Google Scholar]


