Abstract
Background
Wolbachia has been reported to suppress a variety of pathogen infections in mosquitoes, but the mechanism is undefined. Two possibilities have been proposed. One is that Wolbachia activates host immune responses, and the other one is that Wolbachia competes with pathogens for limited nutrients.
Methodology/Principal findings
In this study, we compared host immune responses and the densities of two different strains of Wolbachia in naturally occurring parental and artificially created hybrid host genetic backgrounds. No significant difference in Wolbachia density was found between these hosts. We found that Wolbachia could activate host innate immune responses when the host genetic profile was different from that of its natural host. When these hosts were challenged with pathogenic bacteria, mosquitoes in new host-Wolbachia symbioses had a higher survival rate than in old host-Wolbachia symbioses.
Conclusions/Significance
The presence of Wolbachia per se does not necessarily affect pathogen infections, suggesting that a competition for limited nutrients is not the main reason for Wolbachia-mediated pathogen suppression. Instead, host immune responses are responsible for it. The elucidation of an immunity nature of PI is important to guide future practice: Wolbachia may be genetically engineered to be more immunogenic, it is desired to search and isolate more strains of Wolbachia, and test more host-Wolbachia symbioses for future applications. Our results also suggest Wolbachia-based PI may be applied to naturally Wolbachia-infected mosquito populations, and extend to the control of a broader range of mosquito-borne diseases.
Introduction
Mosquito-borne diseases are one of the major public health problems. With increasing globalization, urbanization and global warming, the threat of mosquito-borne diseases is growing. Traditional and emerging mosquito-borne diseases, such as malaria, dengue, West Nile fever, Japanese encephalitis, chikungunya fever and Zika, have seriously affected human health and economic development [1, 2]. However, lack of effective vaccines and specific drugs for mosquito-borne diseases (such as dengue), as well as the development of resistance to therapeutic drugs in some pathogens (such as malaria), have contributed to this situation. Therefore, one of the main measures for the prevention and control of mosquito-borne diseases is still mosquito control. Chemical control has been the main method in mosquito control programs. However, continuous and large-scale insecticide usage has led to the emergence and development of resistance in mosquito vectors [3], and the negative effects of insecticides on human health and the environment should not be ignored [4, 5]. Recently, several biological approaches were called upon for the control of mosquito populations, including the introduction of Wolbachia [6–8].
The endosymbiotic bacterium Wolbachia is maternally inherited, infecting >65% of all insect species and ~28% of the surveyed mosquito species [9, 10]. Wolbachia can regulate the host's reproductive processes. For example, cytoplasmic incompatibility (CI) interferes with the normal development of a zygote formed by a sperm of Wolbachia-infected mosquito and egg of an uninfected or an incompatible strain of Wolbachia-infected mosquito [11]. CI provides a reproductive advantage to infected females over uninfected females, resulting in the invasion of Wolbachia into a population. Wolbachia can also inhibit pathogen infection of the host via pathogen interference (PI) phenomenon[12]. Studies have shown that Aedes aegypti mosquitoes artificially infected with Wolbachia have increased resistance to dengue virus, Zika virus, chikungunya virus, yellow fever virus, Plasmodium gallinaceum, filaria and certain bacteria [7, 13–16]. After transient somatic infections of Wolbachia, Anopheles gambiae has significantly reduced infection intensity of Plasmodium berghei [17]. Bian et al. established a stable Wolbachia infection in Anopheles stephensi which conferred resistance in the mosquito to Plasmodium falciparum [18]. Micieli et al. reported that Wolbachia infection of Cx. quinquefasciatus laboratory strain increased host resistance to West Nile virues infection [19].
Currently, Wolbachia-infected Ae. Aegypti mosquitoes have been released in dengue-endemic area as a population replacement strategy. For example, in northern Australia and central Vietnam such mosquitoes were released to replace the local Wolbachia-negative Ae. aegypti population and reduce dengue virues(DENV)-transmission capacity [20]. A mathematical model predicts that establishment of wMelPop-infected Ae. aegypti at high frequency in a dengue endemic setting would result in complete abatement of DENV [21]. However, the long-term effects of artificial release of Wolbachia-infected mosquitoes remain to be assessed, such as whether the Wolbachia will still be capable of inhibiting the virus after repeated vertical transmission in the mosquitoes, whether the pathogens will gradually adapt to Wolbachia-infected host through mutations, or changes in mosquito itself can increase vectorial capacity despite of the presence of Wolbachia infection. Host, Wolbachia, and virus genetic evolution could all influence the long-term success of Wolbachia programs [22].
Elucidating the mechanisms of PI phenomenon will be of great importance in maximizing the effects of Wolbachia-based mosquito-control strategies, extending the sustainability of this method, quickly understanding and correctly solving problems that may arise in the future. Till now, the mechanism underlying PI is still not completely understood. Currently two major explanations have been proposed. One is that Wolbachia activates the mosquito innate immune responses, and thus-primed immune system helps the host to fight subsequent pathogen infections [14, 17, 23]. The other one is that Wolbachia competes with pathogens for nutrients such as lipids [24, 25].
Although existing studies suggest that the innate immune response may play a leading role in Wolbachia induced PI in mosquitoes, we should also notice that those studies were all based on artificially or naturally Wolbachia-infected host, using uninfected host as a control. Compared with the control group, the presence of Wolbachia in the infected host may both up-regulate host immune response and compete for nutrients. The effects of these two concomitant processes on the replication of pathogens are indistinguishable.
Alternatively, a comparison between infected populations may help to elucidate the role of immune responses in PI. To that end, we choose Culex mosquitoes in which Wolbachia is prevalent. Culex mosquitoes are an important vector of lymphatic filariasis and several viral pathogens, including West Nile virus [26]. The most prevalent Culex species in China is Cx. pipiens pallens. Our previous study [27] revealed that the bi-directional incompatibilities between naturally existent populations from different geographic locations were dependent on the presence of Wolbachia, i.e. they were Wolbachia-induced CI. For example, Nanjing (NJ) and Tangkou (TK) populations were naturally infected with bi-directionally incompatible Wolbachia. Based on the fact that Wolbachia is maternally inherited, in this study, we propose to cross preexisting host-Wolbachia symbioses obtained in Nanjing and Tangkou to create new host-Wolbachia symbioses. Comparing the transcriptomes in the old and new host-Wolbachia symbiotic combinations in which nutrient competition is constantly present, we aim to delineate the contribution of innate immune responses to PI in Wolbachia-infected mosquitoes.
Materials and methods
Mosquitoes
The Cx. pipiens pallens larvae were collected from Nanjing (NJ), Jiangsu Province(32°3'30.11"N, 118°47'47.28"E), and Tangkou (TK), Shandong Province(34°52'34.97"N, 117°22'53.69"E) from July to August in 2017. All collection was done on public land. After morphology identification, the larvae were then maintained in an insectary. Mosquitoes were kept at 28°C, 75% relative humidity and a photoperiod of 14h light: 10h darkness. Adult mosquitoes were fed 10% (w/v) glucose solution prior to blood meals [27].
Tetracycline treatment
Tetracycline treatment to eliminate Wolbachia from Culex populations was carried out according to published methods [28]. Tetracycline (Amresco) at a concentration of 0.05 mg/ml was used for the treatment through both larval and pupal stages. Eggs were placed on tetracycline water solution to hatch. Surviving larvae were transferred to fresh tetracycline solution every 24 hours. A normal infusion was prepared in parallel and fed to larvae in tetracycline solution. After continuous tetracycline treatment for 6 generations, Wolbachia-negative Culex populations were established.
Establishment of new host-Wolbachia symbioses
To separate virgin females and males, pupae from each population were put into 15 ml tubes with water for individual emergence. Then, male and female adults were raised in 30.5×30.5×30.5 cm cages. Females 1 day post-eclosion and males 2 days post-eclosion were used in crossing experiments. Each set of crossings included combination groups of Wolbachia-negative virgin males from TK with Wolbachia-positive virgin females from NJ(NJ♀×TKtet♂), Wolbachia-negative virgin males from NJ with Wolbachia-positive virgin females from TK(TK♀×NJ tet♂). While combinations of virgin males and females from the same populations as controls (NJ♀×NJ♂ and TK♀×TK♂). Females and males placed in the same cages were given 2 days to mate. Females were blood fed after mating, then the egg rafts were given 48 hours after oviposition to hatch. Females of the first filial generation of these crossings, namely NJ♀×TKtet♂, TK♀×NJ tet♂, NJ and TK were collected 2 days post-eclosion for RNA extraction and sequencing.
RNA sequencing and analysis
Total RNA of 15 female mosquitoes of each group (NJ♀×NJ♂, NJ♀×TKtet♂, TK♀×TK♂ and TK♀×NJtet♂) was extracted using TRIzol reagent (Thermo Fisher Scientific, USA) following the manufacturer’s protocol. cDNA library construction and sequencing were performed according to standard procedures by Beijing Genomics Institute (BGI-Shenzhen, China) using BGISEQ-500 platform. At least 60 Mb clean reads of sequencing were obtained for each sample. Since no genomic sequence in any database was available for Cx. pipiens pallens, Trinity [29] was used to perform de novo assembly with clean reads, then Tgicl [30] was used on cluster transcripts to remove abundance and retain Unigenes. After assembly, Unigenes functional annotation was performed with 7 functional databases (NR, NT, GO, KOG, KEGG, SwissProt and InterPro), then all the clean reads of each sample were mapped to the Unigenes with Bowtie2 [31] software and the gene expression levels were calculated with RSEM [32]. Based on the gene expression levels, the DEGs (differential expression genes) between samples or groups were identified with PossionDis [33] (Fold Change > = 2.00 and FDR< = 0.001). The DEGs were classified based on the GO annotation results and official classification. Pathway analysis was performed to provide further information on the DEGs’ biological functions. The DEGs were also subjected to KEGG pathway classification and functional enrichment. As a biological replicate of this experiment, total RNA of another 15 female mosquitoes from each group was extracted for cDNA library construction and sequencing. A total of eight libraries were sequenced and analyzed.
Validation of immunity-related DEGs by real-time quantitative PCR
Each total RNA template was obtained from a pool of 5 female mosquitoes and extracted as described above. We generated three biological replicates for each group. For each biological replicate three independent total RNA templates were obtained. Totally, we have 3×3 total RNA templates for each group. The cDNA was synthesized with PrimeScript RT reagent kit (Takara, Otsu, Shiga, Japan) according to the manufacturer's protocol. PCR was performed on the LightCycler 96 Real-Time PCR System (Roche, Switzerland) using SYBR Green Master Mix kit (Roche, Switzerland). Primers specific for real-time quantitative PCR are listed in Table 1. We amplified 23 different genes from each template. Each gene amplication was carried out in triplicate. For each reaction, 10 μl of SYBR Green Master Mix was used, 1.0 μl of each primer solution at 10 μM and 8 μl of diluted cDNA were added. PCR cycling protocol was as follows: initial 50°C for 2 min, denaturation for 10 min at 95°C, followed by 40 cycles of 95°C for 15 s, 60°C for 1 min. The housekeeping gene Rps6 was used as an internal control and the data were analyzed with LightCycler 96 Software v1.1 (Roche, Switzerland). Quantitation of relative mRNA expression was calculated using 2−ΔΔCt method [34]. Significance was determined based on comparison of the ΔCT of each gene in old and new host-Wolbachia symbioses using Student’s t-tests. *P<0.05; **P<0.01.Immunity-related DEGs were further analyzed with PathVisio software 3.3.0. obtained from wikipathways (WP3830_92694) which is based on the Toll and Imd Pathways in Drosophila melanogaster.
Table 1. Primer sequences used in real-time quantitative PCR.
| Primer Name | Primer Sequence (5'→3') | |
|---|---|---|
| RPS6 | Sense | TGATTCGCTGTTGTATCGTGGA |
| Antisense | GATGTTATTCGCACGCTTCG | |
| WSP | Sense | TGCAAACAGTGTGGCAGCAT |
| Antisense | ACCAACACCAACACCAACGTA | |
| MODSP | Sense | AGAATTCCGCTTCTGCGACA |
| Antisense | ACTCCGGATACACGATGGGA | |
| GRASS |
Sense | ACATCAATGGGTACACGCGG |
| Antisense | GGAGTCGGTTCTCAAGGTCG | |
| SPIRIT |
Sense | GAGTCGATCGTGCTGCAAAA |
| Antisense | GCAAACTCCCGCCACATTTC | |
| SPZ1B |
Sense | ATCGGCAAGGATTTTGACGC |
| Antisense | GCGTTGCCATTTCCCTTCAG | |
| TOLL | Sense | CCAATGAATGTGGTGGCGTT |
| Antisense | TCCCAACATTCTGTGGCATCA | |
| DIF | Sense | ACGGTCGAGATCAACAGTGC |
| Antisense | GCTTGGCGTGACTGTAACCA | |
| DEFA | Sense | TGGATTCGGCGTCAACGATA |
| Antisense | CACACGCAAACCTTCTTGCC | |
| EFFETE | Sense | GATTTGCTCACTTCCGGTCG |
| Antisense | GACCTCCAGTATCCGCTTCC | |
| IAP2 | Sense | CTGGCCACCTTCGTCAACTG |
| Antisense | GACCTCCCACTGGCCGATAA | |
| TAK1 | Sense | TCCCTTAACATTTCCAACGCC |
| Antisense | CCAGGATGCTGTTGAGGGAT | |
| JNK | Sense | GCGGATGTTTGGACTGTTCC |
| Antisense | CCGATCATGGTCCAACTCCA | |
| RELISH | Sense | CCGTACTACGACGACGGAAG |
| Antisense | CGAAAGCGGAACTTGTCCAC | |
| PSH | Sense | TTCATCCGGAGTACGACCCT |
| Antisense | AAGCCCAACCACCTTGGAAA | |
| SPE | Sense | CTGGACGTTGGAGTGGAAGA |
| Antisense | CAGGCAAAGCGGAGAGATGA | |
| GNBPB3 | Sense | GTTGGCTGGCAATACGAACTG |
| Antisense | AACGGCTCACCGAACTCCTC | |
| GNBPB2 | Sense | GACAGATGTACCGACGAGCC |
| Antisense | ATTCAGAATTCGGGACGGGG | |
| PGRP-LC | Sense | GTGGTCACAGCACGGAGTTTATT |
| Antisense | TTCAGCTCATTTCCCTTGTCTATCT | |
| IMD | Sense | GCTCGCTGAGCAAATTTACCAATTT |
| Antisense | CGTTCCTTCCACAGCACCTTC | |
| CECA | Sense | GCTGTTCGTCATCGTCCTG |
| Antisense | CCCGTTTGCCAACTCCTT | |
| CECB | Sense | ATTGTCATTCTGGCAGCCCT |
| Antisense | CACTCGCTTGCCAGCTTTTT | |
| NEC | Sense | ACCAAGCGTGAACTCTCCAA |
| Antisense | AGTTGCCGTTCTCCTTCGTT |
Microbial challenge and survival experiments
Microbial challenge and survival experiments were performed in the same way as described in [35]. In brief, an acupuncture needle (0.20×25mm) was dipped into a concentrated overnight bacterial culture of Gram-negative (Escherichia coli) or Gram-positive (Micrococcus luteus) bacteria or sterile LB culture (negative control) and pricked mosquitoes (female 2 days post eclosion) in the rear part of the abdomen. For each mosquito population, three parallel groups with each group consisting of 15–20 adult females were inoculated per bacterial species [36]. A total of three biological replicates of the infection experiment were performed. Survival curves are significantly different between mosquitoes in old and new host-Wolbachia symbioses (compared using log-rank test).
Statistical analysis
All statistical analyses were carried out using SPSS Statistics 17.0.
Data accessibility
The data supporting the results of this article have been submitted to NCBI Sequence Read Archive (SRA) repository (Accession number: SRP155507). The materials and methods part has been submitted to protocols.io. (DOI:http://dx.doi.org/10.17504/protocols.io.xcafise)
Results
No significant change of Wolbachia density in hosts with hybrid genetic profiles
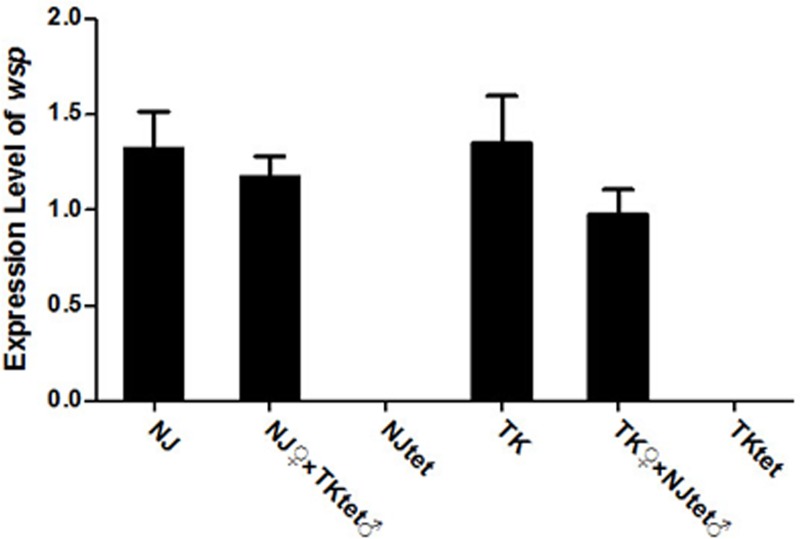
With continuous tetracycline treatment for 6 generations, we established Wolbachia-negative Culex populations TKtet and NJtet. To confirm the absence of Wolbachia, we conducted real-time quantitative PCR to quantify any residual Wolbachia-specific DNA. As shown in Fig 1, compared to that in TK and NJ populations, wsp (Wolbachia major surface protein) gene abundance decreased to an undetectable level in TKtet (t = 5.424, df = 4, P = 0.0056)and NJtet populations(t = 6.749, df = 4, P = 0.0025). Regular PCR amplification of wsp using total DNA from TKtet and NJtet as template also gave a negative result (data now shown). Then we conducted crossing experiments using Wolbachia-negative males and Wolbachia-positive females, and acquired NJ♀×TKtet♂ and TK♀×NJtet♂ populations which represent novel host-Wolbachia symbioses (NJ Wolbachia in TK-NJ hybrid host or TK Wolbachia in TK-NJ hybrid host) compared to the original NJ and TK populations (NJ Wolbachia in NJ host or TK Wolbachia in TK host). Real-time quantitative PCR results showed that Wolbachia densities were not significantly changed in hosts with hybrid genetic profiles (for NJ♀ and NJ♀×TKtet♂ groups: t = 0.6536, df = 4, P = 0.5491, for TK♀ and TK♀×NJtet♂ groups: t = 1.317, df = 4, P = 0.2581). (Fig 1)
Fig 1. No significant change of Wolbachia density in hosts with hybrid genetic profiles.
The expression of wsp gene was measured by real-time quantitative PCR in NJ♀×TKtet♂, TK♀×NJtet♂, NJtet, TKtet, NJ and TK virgin females at 2 days post-eclosion. wsp gene expression data were normalized with RPS6. The primer sequences are shown in Table 1. The bars indicate standard error. 2−ΔΔCT method was used to calculate the expression level. Significance was determined based on comparison of the ΔCT using Student’s t-tests.
Comparative transcriptome analysis of the original and newly created host-Wolbachia symbioses
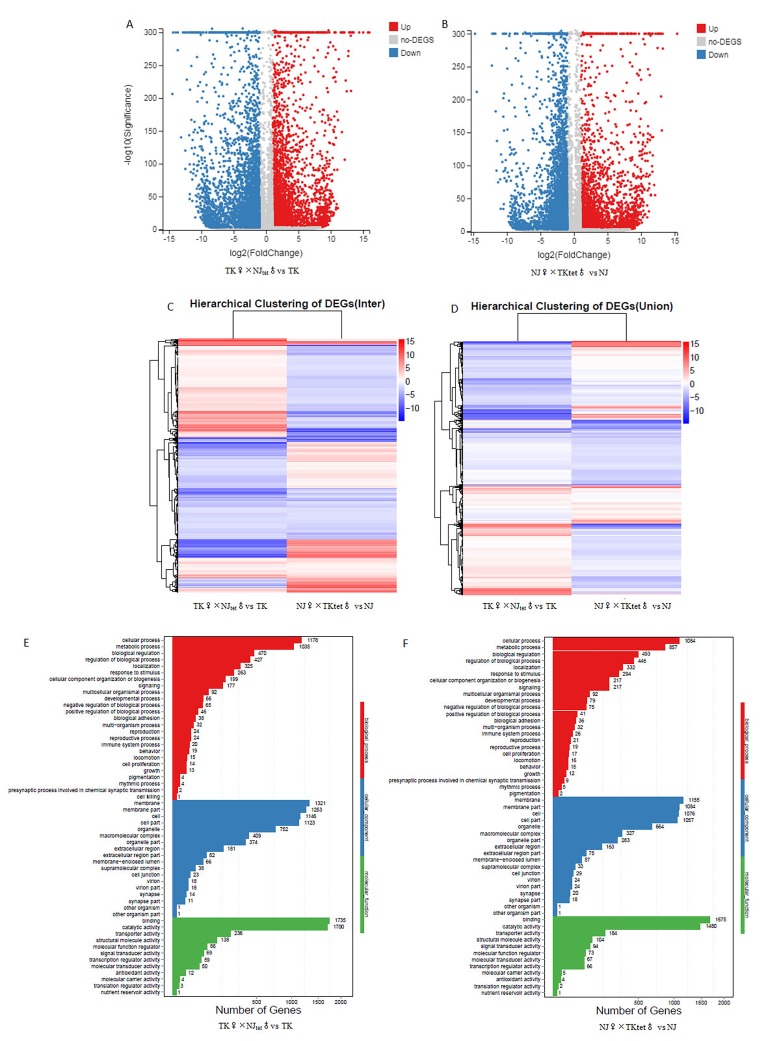
cDNA libraries were sequenced from the original and newly created mosquito host-Wolbachia symbioses. 24,659 (NJ♀×TKtet♂), 26,777 (TK♀×NJ tet♂), 27,513 (NJ♀×NJ♂) and 28,123 (TK♀×TK♂) unigenes were generated. A total of 24,970 unigenes were annotated against the NCBI NR protein database, 15,598 in GO function categories, and 19,396 unigenes were mapped onto the canonical pathways in KEGG. The unigene expression in new host-Wolbachia symbioses was compared with original mosquito host based on the fragments per kilobase of transcript per million mapped reads (FPKM) value. TK♀×NJtet♂ had 4,148 up-regulated unigenes and 5,036 down-regulated unigenes in comparison to the control TK♀×TK♂, and NJ♀×TKtet♂ had 2,712 up-regulated unigenes and 5,747 down-regulated unigenes in comparison to the control NJ♀×NJ♂ (Fig 2A and 2B). The intersection and union of the DEG heat map for the original and new host-Wolbachia symbioses are shown in Fig 2C and 2D. The identified DEGs were then assigned to the three standard subcategories of “molecular biological function”, “cellular component” and “biological process” in GO enrichment analysis (Fig 2E and 2F). In parallel, the unigenes were mapped onto the canonical pathways in KEGG to identify possible active biological pathways that contain DEGs. Twenty most significant DEGs in new vs. old host-Wolbachia symbioses are shown in Fig 3. RNA-seq data analysis of a biological replicate are presented in supplementary materials and shown in S1 and S2 Figs.
Fig 2.
(A and B) Volcano plot of DEGs. The unigenes up- or down-regulated more than two-fold when compared between old and new host-Wolbachia symbioses are displayed in red or blue, respectively. Y axis represents -log10 transformed significance. X axis represents log2 transformed fold change. Red points represent up-regulated DEGs. Blue points represent down-regulated DEGs. Gray points represent non-DEGs. (C and D) Heatmap of hierarchical clustering of DEGs. X axis represents comparison for clustering analysis. Coloring indicates fold change (high: red, low: blue). (E and F) GO classification of DEGs. X axis represents number of DEG. Y axis represents GO term.
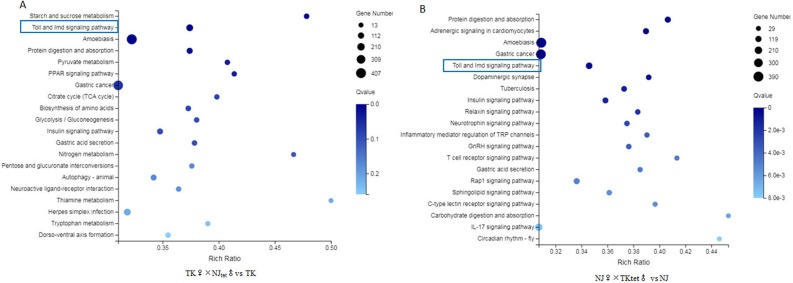
Fig 3. Up-regulation of Toll and IMD pathway genes in new host-Wolbachia symbioses.
Pathway functional enrichment of DEGs. X axis represents enrichment factor. Y axis represents pathway name. The color indicates q value (high: white, low: blue), a lower q value indicates a more significant enrichment. Point size indicates DEG number (A bigger dot refers to a larger amount). Rich Factor refers to the value of enrichment factor, which is the quotient of foreground value (the number of DEGs) and background value (total Gene amount). A larger Rich Factor value indicates a higher level of enrichment.
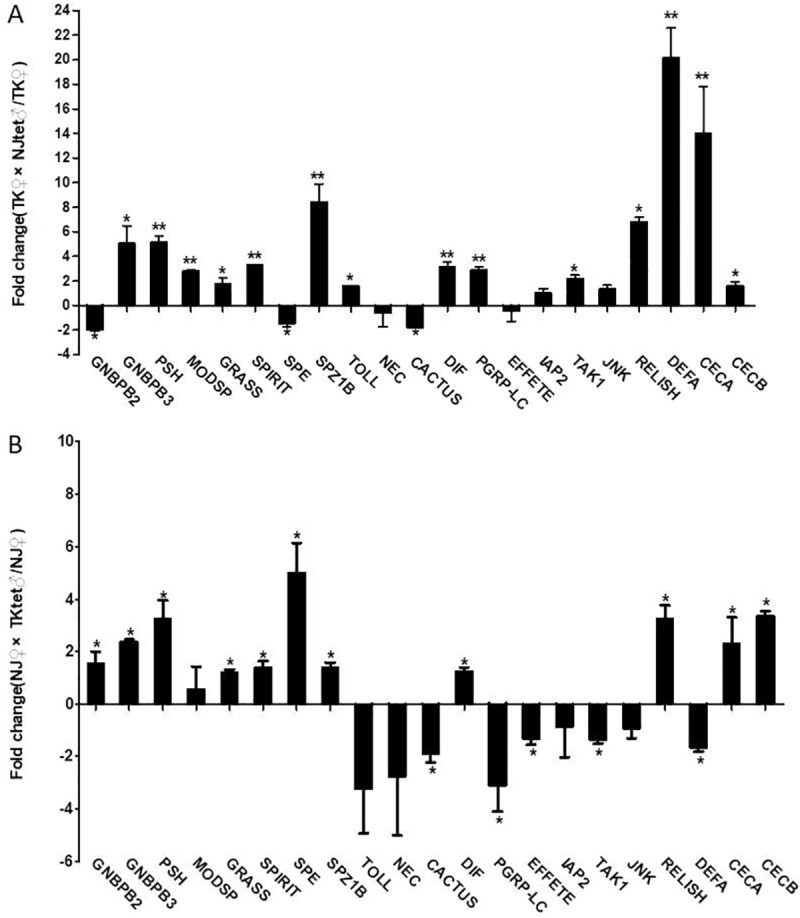
Innate immune responses are elevated in hosts with hybrid genetic profiles
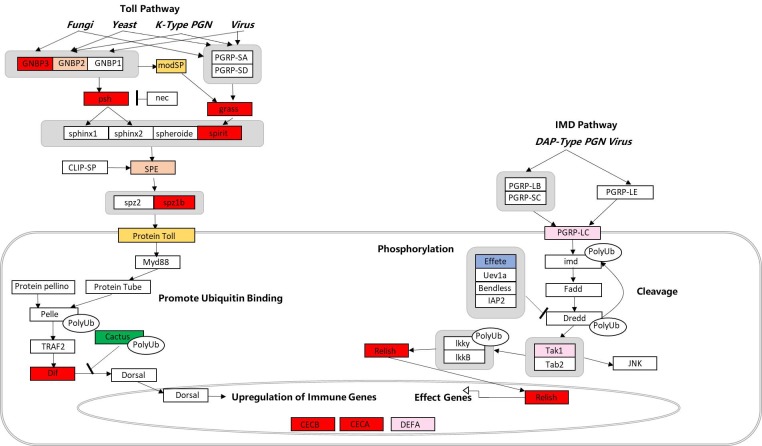
Based on the transcriptome assays, we compared mosquito innate immune responses in the original and new host-Wolbachia symbioses. As shown in Fig 3 and S1 Table, Genes in Toll and the immune deficiency (Imd) signaling pathway were up-regulated in both TK♀×NJtet♂ (compare to TK) and NJ♀×TKtet♂ (compare to NJ) groups. The differential activations of immune responses in hosts of different genetic profiles to the same Wolbachia were confirmed by real-time PCR quantification of genes in the Toll and Imd pathways (Fig 4). Our results showed that Toll pathway genes, such as Gram-negative binding protein B3 (GNBPB3, for NJ♀ and NJ♀×TKtet♂ groups: t = 2.136, df = 4, P = 0.0497, for TK♀ and TK♀×NJtet♂ groups: t = 3.214, df = 4, P = 0.0162), serine protease persephone (PSH, for NJ♀ and NJ♀×TKtet♂ groups: t = 3.192, df = 4, P = 0.0166, for TK♀ and TK♀×NJtet♂ groups: t = 8.187, df = 4, P = 0.0019), gram-positive specific serine protease (GRASS, for NJ♀ and NJ♀×TKtet♂ groups: t = 2.924, df = 4, P = 0.0215, for TK♀ and TK♀×NJtet♂ groups: t = 2.560, df = 4, P = 0.0416), serine protease immune response integrator (SPIRIT, for NJ♀ and NJ♀×TKtet♂ groups: t = 2.224, df = 4, P = 0.0451, for TK♀ and TK♀×NJtet♂ groups: t = 5.411, df = 4, P = 0.0028), Spaetzle-like cytokine 1B (SPZ1B, for NJ♀ and NJ♀×TKtet♂ groups: t = 2.628, df = 4, P = 0.0292, for TK♀ and TK♀×NJtet♂ groups: t = 8.305, df = 4, P = 0.0018), and Dorsal-related immunity factor (DIF, for NJ♀ and NJ♀×TKtet♂ groups: t = 2.933, df = 4, P = 0.0213, for TK♀ and TK♀×NJtet♂ groups: t = 6.436, df = 4, P = 0.0038) were up-regulated in the new host-Wolbachia symbioses. Developmental protein cactus (CACTUS) was down-regulated in the new host-Wolbachia symbioses(for NJ♀ and NJ♀×TKtet♂ groups: t = 2.550, df = 4, P = 0.0316, for TK♀ and TK♀×NJtet♂ groups: t = 2.685, df = 4, P = 0.00275). We also observed that genes representing the Imd pathway, such as nuclear factor NF-κB p105 subunit (RELISH) were up-regulated in the new host-Wolbachia symbioses(for NJ♀ and NJ♀×TKtet♂ groups: t = 2.649, df = 4, P = 0.0285, for TK♀ and TK♀×NJtet♂ groups: t = 3.562, df = 4, P = 0.0189). In addition to the majority genes of Toll and Imd pathways that were up-regulated in new host-Wolbachia symbioses, some genes did not show consistent upregulation. For example, Gram-negative binding protein B2 (GNBPB2) and Spaetzle-processing enzyme (SPE) were up-regulated in NJ♀×TKtet♂ while down-regulated in TK♀×NJtet♂(GNBPB2:for NJ♀ and NJ♀×TKtet♂ groups: t = 2.893, df = 4, P = 0.0222, for TK♀ and TK♀×NJtet♂ groups: t = 3.209, df = 4, P = 0.0163; SPE: for NJ♀ and NJ♀×TKtet♂ groups: t = 2.289, df = 4, P = 0.0420, for TK♀ and TK♀×NJtet♂ groups: t = 3.443, df = 4, P = 0.0131). Peptidoglycan recognition protein-lc (PGRP-LC) and TGF-Beta-Activated Kinase-1 (TAK1) were up-regulated in TK♀×NJtet♂ while down-regulated in NJ♀×TKtet♂(PGRP-LC:for NJ♀ and NJ♀×TKtet♂ groups: t = 3.141, df = 4, P = 0.0174, for TK♀ and TK♀×NJtet♂ groups: t = 7.730, df = 4, P = 0.0023; TAK1: for NJ♀ and NJ♀×TKtet♂ groups: t = 2.888, df = 4, P = 0.0223, for TK♀ and TK♀×NJtet♂ groups: t = 2.569, df = 4, P = 0.0403). Proteins Toll and modular serine protease (MODSP) were up-regulated only in TK♀×NJtet♂(t = 9.633, df = 4, P = 0.0012). Effete (EFFETE) was down-regulated only in NJ♀×TKtet♂(t = 3.254, df = 4, P = 0.0156). Transcripts of the antimicrobial peptide genes (effector genes) cecropin A (CECA) and cecropin B (CECB) were up-regulated in the new host-Wolbachia symbioses(CECA:for NJ♀ and NJ♀×TKtet♂ groups: t = 3.561, df = 4, P = 0.0118, for TK♀ and TK♀×NJtet♂ groups: t = 8.849, df = 4, P = 0.0015; CECB: for NJ♀ and NJ♀×TKtet♂ groups: t = 3.490, df = 4, P = 0.0251, for TK♀ and TK♀×NJtet♂ groups: t = 3.785, df = 4, P = 0.0323). While defensin A (DEFA) was up-regulated in TK♀×NJtet♂(t = 7.137, df = 4, P = 0.0028), it was down-regulated in NJ♀×TKtet♂(t = 3.323, df = 4, P = 0.0146). An analysis of the expression changes of Toll and Imd signaling pathway-related unigenes within new and old host-Wolbachia symbioses is given in Fig 5.
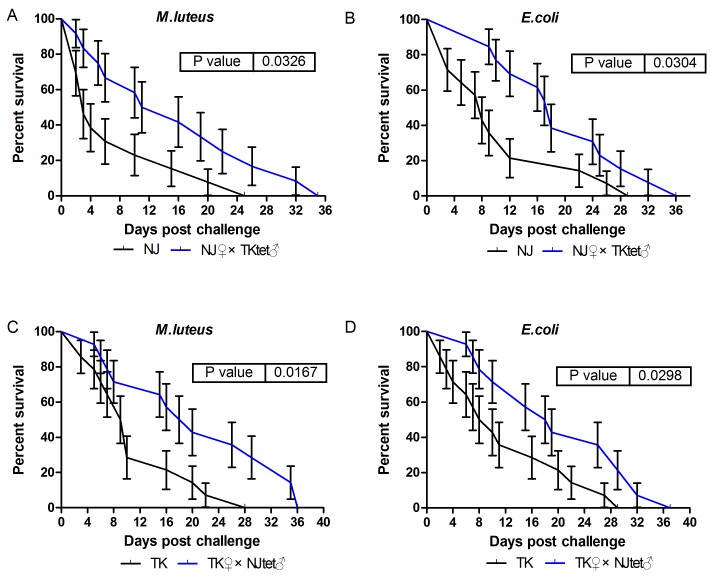
Fig 4.
Survival curves of the mosquitoes post-challenge with M. luteus (a, c) or E. coli (b, d). For each mosquito population, three parallel groups of 15-20 adult females each were inoculated per bacterial species. A total of three biological replicates of the infection experiment were performed. Error bars indicate the standard error. Survival curves are significantly different between mosquitoes in old and new host-Wolbachia symbioses (compared using log-rank test).
Fig 5. Regulation of putative Toll and Imd signaling pathway genes in hosts with hybrid genetic profiles.
Red color indicates Toll and Imd signaling pathway genes up-regulated in both TK♀×NJtet♂ (compare to TK♀) and NJ♀×TKtet♂ (compare to NJ♀) groups, pink color indicates up-regulated in TK♀×NJtet♂ but down-regulated in NJ♀×TKtet♂ group, orange color indicates up-regulated in NJ♀×TKtet♂ but down-regulated in TK♀×NJtet♂ group, green color indicates down-regulated in both TK♀×NJtet♂ and NJ♀×TKtet♂ groups, yellow color indicates up-regulated only in TK♀×NJtet group, blue color indicates down-regulated only in NJ♀×TKtet♂ group, white indicates unfound. The pathway was built with PathVisio software 3.3.0 based on the Toll and IMD Pathways of Drosophila melanogaster downloaded from wikipathways (WP3830_92694).
Microbial challenge and survival experiments
Toll and Imd pathways are expected to protect mosquitoes from Gram-positive and Gram-negative bacterial infections respectively. Our results showed that both Toll and Imd pathways were up-regulated in new host-Wolbachia symbioses. To test if the up-regulation of these pathways can help mosquitoes to fight pathogen infections, we challenged mosquitoes in old and new host-Wolbachia symbioses with Gram-negative bacteria (Escherichia coli) and Gram-positive bacteria (Micrococcus luteus). Results showed that mosquitoes in new host-Wolbachia symbioses had higher survival rate than in old host-Wolbachia symbioses when challenged with either E. coli (P<0.05, for NJ♀ and NJ♀×TKtet♂ groups: chi square = 4.685, df = 1, P = 0.0304, for TK♀ and TK♀×NJtet♂ groups: chi square = 4.395, df = 1, P = 0.0298) or M. luteus (P<0.05, for NJ♀ and NJ♀×TKtet♂ groups: chi square = 4.565, df = 1, P = 0.0326, for TK♀ and TK♀×NJtet♂ groups: chi square = 5.730, df = 1, P = 0.0167) (Fig 6).
Fig 6.
Survival curves of the mosquitoes post-challenge with E. coli (a) or M. luteus (b). For each mosquito population, three parallel groups of 15–20 adult females each were inoculated per bacterial species. A total of three biological replicates of the infection experiment were performed. Error bars indicate the standard error. Survival curves are significantly different between mosquitoes in old and new host-Wolbachia symbioses (compared using log-rank test).
Discussion
Population replacement aimed at Wolbachia-mediated PI is moving from benchtop to the field. Elucidation of PI mechanism may help to augment the efficacy of Wolbachia-based vector control, prolong its usage, and expedite comprehension of and solutions to unexpected problems in future practice. Insects have established a highly efficient innate immune system to distinguish between self and non-self molecules and resist infections. Host innate immune system recognizes pathogen-associated molecular patterns (PAMPs) via pattern-recognition receptors (PRRs) and initiates a cascade of responses [37]. PRR signaling is thought to be critical for the host to fight pathogens [38]. Studies in Drosophila melanogaster have shown that two main PRRs, Toll and Imd, are involved in arthropod immune responses. Gram-positive bacteria trigger the Toll pathway, fungi and Gram-negative bacteria trigger the Imd pathway, mediating innate immune responses and resulting in the production of antimicrobial peptides (AMPs) [39].
In mosquitoes, PI has been most thoroughly characterized in Aedes aegypti. Xi et al. propose that Wolbachia infection activates the innate immune response of Ae. aegypti by up-regulating the level of Toll pathway genes and the expression of antimicrobial peptides such as defensins, which enables mosquitoes to resist DENV. They found that when the Toll pathway inhibitor cactus gene was silenced, the extent of dengue infection in mosquitoes was reduced by 4.0-fold. When the Toll pathway was inactivated by silencing myd88, the virus load in mosquitoes increased 2.7 times compared to the control group [14, 23]. They also found that the elevation of reactive oxygen species (ROS) was a result of Wolbachia infection and was involved in the activation of the Toll pathway. Toll activation leads to the expression of antioxidants to alleviate oxidative stress and, as a “side-effect”, increases antimicrobial peptide production resulting in an enhanced resistance to pathogen infections [40]. Kambris et al. observed up-regulated immune genes in Anopheles gambiae somatically infected with Wolbachia and highly significant reductions in Plasmodium infection intensity. This effect was diminished after knockdown of TEP1 gene [17]. A different explanation of PI is that both Wolbachia and viruses such as DENV are heavily dependent on host lipids and other resources for survival, and a potential competitive effect could contribute to PI [24, 25]. Schultz et al. found that infection with Wolbachia inhibited the replication of ZIKV in mosquito cell lines, and increased supply of cholesterol moderately restored the replication of ZIKV [41]. However, there lacks reported research that extends this finding to adult mosquitoes.
While these previous studies on PI mechanism provided insightful information, they fell short of pinpointing the causes. In these studies, at least two coexistent factors were confounding each other, i.e., induction of innate immunity and competition for nutrients could both be effected by the presence of Wolbachia that was artificially introduced. It was difficult to rule out one of the two plausible explanations. In this study, we used preexisting host-Wolbachia symbioses (NJ Wolbachia—NJ mosquito & TK Wolbachia—TK mosquito) obtained in Nanjing and Tangkou to create mosquito populations representing new host-Wolbachia symbioses NJ♀×TKtet♂ and TK♀×NJtet♂. In the new and original mosquito populations, Wolbachia was always existent, so that nutrient competition was constantly present. Our results showed that Wolbachia densities in the new mosquito populations did not change significantly. Thus, comparing the new and old host-Wolbachia symbioses, we can exclude nutrient competition factor and focus on the contribution of innate immunity. To find out if host immune system was activated by Wolbachia in altered host genetic background, we compared the transcriptomes in the old and new mosquito populations. Our results showed that both genes in Toll and those in Imd signaling pathways were up-regulated in new host-Wolbachia symbioses, indicating that Wolbachia may induce stronger immune responses in a new host than in the original host.
As initially reported in D. melanogaster, Toll does not directly recognize PAMPs in insects. Instead, PAMPs are detected by PGRPs (peptidoglycan-recognition proteins) and GNBPs (Gram negative-binding proteins) which activate proteolytic enzymes, leading to the cleavage thus activation of cytokine Spaetzle. Spaetzle binding crosslinks the ectodomains of Toll, and activates Toll receptor. Through the adaptor proteins MYD88, Tube and Pelle, Toll can then activate NF-kB protein DIF in immune-responsive tissues by dissociating DIF from the ankyrin-repeat inhibitory protein Cactus, leading to the production of AMPs [42]. Our transcriptome results showed that cecropin B was up-regulated in both TK♀×NJtet♂ and NJ♀×TKtet♂, while cecropin A and defensin A were up-regulated in TK♀×NJtet♂ but down-regulated in NJ♀×TKtet♂ (S1 Table). When further tested with real-time RT-PCR, only defensin A was consistently up-regulated in TK♀×NJtet♂ and down-regulated in NJ♀×TKtet♂, both cecropin A and B were up-regulated in new host-Wolbachia symbioses (Fig 4). Although post-translational regulations (e.g. nuclear translocation) of upstream factors may be sufficient to induce the transcription of AMPs, both transcriptome and real-time RT-PCR results showed that GNBPB3, PSH, GRASS, SPIRIT, SPZ1B and DIF were all up-regulated in the new host-Wolbachia symbioses. Toll pathway inhibitor CACTUS was down-regulated in the new host-Wolbachia symbioses.
Imd pathway can be triggered by ligand binding to PGRP-LC [43]. The activation signal is transduced through intracellular adaptor IMD protein into two downstream branches. One branch has TAK1 acting as the downstream factor of Imd/FADD, which in turn activates IKK-β and IKK-γ homologues and directs phosphorylation of NF-kB transcription factor Relish. Activated Relish then translocates to the nucleus and promotes the transcription of AMPs [44]. The other branch activates the transcription factor AP-1 via JNK signaling [45, 46]. As some factors in Toll pathway, RELISH in Imd pathway was up-regulated in the new host-Wolbachia symbioses.
As a result of Toll and Imd pathway activation, cecropin A and B were consistently up-regulated as the host was replaced with a different genetic background. Cecropin A and B up-regulation are correlated with improved protection against challenge infections of bacteria. In contrast, defensin A was only up-regulated in TK♀×NJtet♂ and not in NJ♀×TKtet♂. One possible explanation is that an interplay between Toll and Imd pathways with participation of other factors results in the change in defensin A expression. Different Wolbachia may activate these pathways differentially and the balance between them determines if defensin A is up-regulated or down-regulated. It is also possible that different strains of Wolbachia have different sensitivities to defensin A, and those strains such as the one from Nanjing may have evolved more effective means to selectively down-regulate defensin A to assure their survival. This would be consistent with previous findings that not all strains of Wolbachia are equally susceptible to host immune responses [47]. Our results also showed, unlike cecropin A, defensin A was not correlated with protection against bacterial infections. These results are consistent with previous studies. For example, in a report by Pan et al., Ae. Aegypti infected with wAlbB showed defensin A up-regulation in the midgut but down-regulation in the rest of carcass [48].
In this study, the up-regulation of both Toll and Imd signaling pathways was not sufficient to significantly reduce the density of Wolbachia observed in the new hosts. It is unknown if this reflects a lack of enough genetic differences between the mosquitoes and between the Wolbachia strains. It is also unknown if an elevated overall immune response is able to suppress Wolbachia activity without altering its density. Nevertheless, the presence of Wolbachia helps to maintain the nonsterilizing immunity. Because the downstream effectors are not target-specific, the activated immune responses can also affect some pathogens. This has been tested in our challenge bacterial infections. When artificially infected with Gram-positive and Gram-negative bacteria, mosquitoes in new host-Wolbachia symbioses have significantly higher survival rates than the mosquitoes in original host-Wolbachia symbioses. Whether a similar effect can be observed in viral infections remains to be answered. There have been a number of reports on the contribution of innate immunity to the blocking of viral replications in insects [23, 40, 49]. Xi et al. reported that Toll pathway in Aedes aegypti controls dengue infection [23]. In a Drosophila model, Rancès et al. demonstrated that Toll pathway has an inhibitory effect on dengue in the presence or absence of Wolbachia, although neither Toll nor Imd pathway is necessary for Wolbachia-induced inhibition [47]. Because a host deficient in both Toll and Imd has not been tested, and other pathways such as JAK-STAT have been reported to suppress dengue replication, it remains possible that at least one of the Toll and Imd pathways has to be in place in order for Wolbachia to inhibit the viral replication [50]. In our study, both Toll and Imd were up-regulated by Wolbachia in new host genetic backgrounds. Whether these up-regulations will result in enhanced resistance to viral infections warrants future investigation.
In our study, Wolbachia was constantly present, so a competition for nutrients was also constitutive. In addition, Wolbachia densities in the original and new hosts were comparable, so the levels of nutrient deprivation would be comparable. It was unlikely that nutrient competition caused the difference in inhibition of pathogen proliferation and improvement of host survival. Instead, the elevated immune responses, likely induced by a “mismatch” between host and Wolbachia hence stronger antigen recognition, were responsible for the protection against subsequent infections. An immunity-mediated PI can also better explain the fact that naturally Wolbachia-infected insects retain their vectorial capacity. For example, Aedes albopictus is naturally infected with Wolbachia, but it can still transmit a variety of pathogens including dengue. In these hosts, native Wolbachia may have been recognized as self as a result of co-evolution. After all, immune responses induced by Wolbachia cause stress in the host and may deem undesirable in the absence of more pathogenic infections. An alternative explanation for natural Wolbachia infection not inducing PI is a reduced density and a more restricted tropism in the native hosts such as Aedes fluviatilis[7].
While the observed difference in resistance to bacteria is most likely caused by immunity, a contribution from nutritional factors to PI cannot be ruled out. It is possible that nutrient competition results in certain level of inhibition in all the mosquito hosts, and immune responses provide a further enhancement in those new hosts. In Drosophila melanogaster, Wolbachia has been found to cause virus interference without inducing overt up-regulation of immunity [51, 52]. At least for viral infections, Wolbachia can assert inhibition by depriving the host cells of essential nutrients.
By comparing mosquitoes that are all infected with Wolbachia, our study demonstrates the contribution of host innate immunity to PI phenomenon. Similar studies may be carried out using other genera of mosquitoes that are medically more important, such as Anopheles and Aedes. The elucidation of an immunity nature of PI is important to guide future practice. For example, Wolbachia may be genetically engineered to be more immunogenic. In current vector population replacement measures, it is difficult to predict how long the released insects will remain refractory to pathogen infections. In the event that these insects do acquire increased vectorial capacity, a possible solution may be to re-introduce a new strain of Wolbachia. Perhaps it is desired to search and isolate more strains of Wolbachia, and test more host-Wolbachia symbioses for future applications. Our results also suggest Wolbachia-based PI may be applied to naturally Wolbachia-infected mosquito populations, and extend to the control of a broader range of mosquito-borne diseases. A competition for nutrient may still be effected by Wolbachia, but this does not negate the potential of immunity-based strategies. Future practice may even forego the use of Wolbachia and focus on the introduction of non-self antigens into the genome of vector insects using transgenic techniques. Potential advantages of transgenic modification of host genome may include less technical difficulty and increased stability. For some insects, a stable Wolbachia infection may be difficult to achieve, such as in Anopheles gambiae. An immunogen-expressing transgene in vector genome may also be more stable since it is not subject to elimination due to chemical exposure.
Accession numbers
The GenBank (http://www.ncbi.nlm.nih.gov/Genbank) accession numbers for sequences mentioned in the paper are: RPS6(XM_001848257.1), WSP(JX050186.1), GNBPB2(XM_001845757.1), GNBPB3(XM_001845228.1), PSH (XM_001868422.1), MODSP(XM_001849027.1), GRASS(XM_001844187.1), SPIRIT(XM_001842673.1), SPE (XM_001848834.1), SPZ1B (XM_001848360.1), TOLL (XM_001847119.1), NEC (XM_001866644.1), CACTUS (XM_001846332.1), DIF (XM_001844026.1), PGRP-LC (XM_001848006.1), EFFETE (XM_001845858.1), IAP2 (XM_001869624.1), TAK1 (XM_001848067.1), JNK (XM_001842775.1), RELISH (XM_001862241.1), DEFA (XM_001842893.1), CECA (XM_001861705.1), CECB (XM_001846866.1)
Supporting information
As a biological replicate, total RNA of another 15 female mosquitoes of each group was extracted. cDNA library construction and sequencing were performed as the first time. At least 60 Mb clean reads of sequencing were obtained for each sample.35,236 (NJ♀×TKtet♂), 34,965 (TK♀×NJ tet♂), 34,845 (NJ♀×NJ♂) and 34,708 (TK♀×TK♂) unigenes were generated. A total of 28,476 unigenes were annotated against the NCBI NR protein database, 16,973 in GO function categories, and 21,332 unigenes were mapped onto the canonical pathways in KEGG. (A and B) Volcano plot of DEGs. The unigenes up- or down-regulated more than two-fold when compared between old and new host-Wolbachia symbioses are displayed in red or blue, respectively. Y axis represents -log10 transformed significance. X axis represents log2 transformed fold change. Red points represent up-regulated DEGs. Blue points represent down-regulated DEGs. Gray points represent non-DEGs. TK♀×NJtet♂ had 5,742 up-regulated unigenes and 4,143 down-regulated unigenes in comparison to the control TK♀×TK♂, and NJ♀×TKtet♂ had 4,226 up-regulated unigenes and 4,122 down-regulated unigenes in comparison to the control NJ♀×NJ♂. (C and D) The intersection and union of the DEG heat map for the original and new host-Wolbachia symbiosis. X axis represents comparison for clustering analysis. Coloring indicates fold change (high: red, low: blue). (E and F) The identified DEGs were then assigned to the three standard subcategories of “molecular biological function”, “cellular component” and “biological process” in GO enrichment analysis. X axis represents number of DEG. Y axis represents GO term.
(TIF)
In parallel, the unigenes were mapped onto the canonical pathways in KEGG to identify possible active biological pathways of DEGs in biological replicate of RNA sequencing experiment. Twenty most significant DEGs in new vs. old host-Wolbachia symbiosis are shown here. X axis represents enrichment factor. Y axis represents pathway name. The color indicates q value (high: white, low: blue), a lower q value indicates a more significant enrichment. Point size indicates DEG number (A bigger dot refers to a larger amount). Rich Factor refers to the value of enrichment factor, which is the quotient of foreground value (the number of DEGs) and background value (total Gene amount). A larger Rich Factor value indicates a higher level of enrichment.
(TIF)
(XLSX)
Acknowledgments
We thank Fei Chen, Yunqing Zhu and Jiahui Wang for experimental assistance, and Haifang Wang for his help with mosquito collection.
Abbreviations
- PI
pathogen interference
- CI
cytoplasmic incompatibility
- PCR
polymerase chain reaction
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Hoey J. West Nile fever in New York City. CMAJ: Canadian Medical Association journal = journal de l'Association medicale canadienne. 2000;162(7):1036 [PMC free article] [PubMed] [Google Scholar]
- 2.Brasil P, Calvet GA, Siqueira AM, Wakimoto M, de Sequeira PC, Nobre A, et al. Zika Virus Outbreak in Rio de Janeiro, Brazil: Clinical Characterization, Epidemiological and Virological Aspects. PLoS neglected tropical diseases. 2016;10(4):e0004636 10.1371/journal.pntd.0004636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–91. 10.1146/annurev.ento.45.1.371 . [DOI] [PubMed] [Google Scholar]
- 4.Wassie F, Spanoghe P, Tessema DA, Steurbaut W. Exposure and health risk assessment of applicators to DDT during indoor residual spraying in malaria vector control program. J Expo Sci Environ Epidemiol. 2012;22(6):549–58. 10.1038/jes.2012.45 . [DOI] [PubMed] [Google Scholar]
- 5.Martinez FD, Trejo-Acevedo A, Betanzos AF, Espinosa-Reyes G, Alegria-Torres JA, Maldonado IN. Assessment of DDT and DDE levels in soil, dust, and blood samples from Chihuahua, Mexico. Arch Environ Contam Toxicol. 2012;62(2):351–8. 10.1007/s00244-011-9700-0 . [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476(7361):454–7. 10.1038/nature10356 . [DOI] [PubMed] [Google Scholar]
- 7.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139(7):1268–78. 10.1016/j.cell.2009.11.042 . [DOI] [PubMed] [Google Scholar]
- 8.Sinkins SP. Wolbachia and cytoplasmic incompatibility in mosquitoes. Insect Biochem Mol Biol. 2004;34(7):723–9. 10.1016/j.ibmb.2004.03.025 . [DOI] [PubMed] [Google Scholar]
- 9.Ricci I, Cancrini G, Gabrielli S, D'Amelio S, Favi G. Searching for Wolbachia (Rickettsiales: Rickettsiaceae) in mosquitoes (Diptera: Culicidae): large polymerase chain reaction survey and new identifications. J Med Entomol. 2002;39(4):562–7. 10.1603/0022-2585-39.4.562 . [DOI] [PubMed] [Google Scholar]
- 10.Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiol Lett. 2008;281(2):215–20. 10.1111/j.1574-6968.2008.01110.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serbus LR, Casper-Lindley C, Landmann F, Sullivan W. The genetics and cell biology of Wolbachia-host interactions. Annu Rev Genet. 2008;42:683–707. 10.1146/annurev.genet.41.110306.130354 . [DOI] [PubMed] [Google Scholar]
- 12.Caragata EP, Dutra HL, Moreira LA. Exploiting Intimate Relationships: Controlling Mosquito-Transmitted Disease with Wolbachia. Trends Parasitol. 2016;32(3):207–18. 10.1016/j.pt.2015.10.011 . [DOI] [PubMed] [Google Scholar]
- 13.Kambris Z, Cook PE, Phuc HK, Sinkins SP. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science. 2009;326(5949):134–6. 10.1126/science.1177531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bian G, Xu Y, Lu P, Xie Y, Xi Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010;6(4):e1000833 10.1371/journal.ppat.1000833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Hurk AF, Hall-Mendelin S, Pyke AT, Frentiu FD, McElroy K, Day A, et al. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS neglected tropical diseases. 2012;6(11):e1892 10.1371/journal.pntd.0001892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dutra HL, Rocha MN, Dias FB, Mansur SB, Caragata EP, Moreira LA. Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host Microbe. 2016;19(6):771–4. 10.1016/j.chom.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kambris Z, Blagborough AM, Pinto SB, Blagrove MS, Godfray HC, Sinden RE, et al. Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathog. 2010;6(10):e1001143 10.1371/journal.ppat.1001143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bian G, Joshi D, Dong Y, Lu P, Zhou G, Pan X, et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science. 2013;340(6133):748–51. 10.1126/science.1236192 . [DOI] [PubMed] [Google Scholar]
- 19.Micieli MV, Glaser RL. Somatic Wolbachia (Rickettsiales: Rickettsiaceae) levels in Culex quinquefasciatus and Culex pipiens (Diptera: Culicidae) and resistance to West Nile virus infection. J Med Entomol. 2014;51(1):189–99. 10.1603/me13152 . [DOI] [PubMed] [Google Scholar]
- 20.Nguyen TH, Nguyen HL, Nguyen TY, Vu SN, Tran ND, Le TN, et al. Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasit Vectors. 2015;8:563 10.1186/s13071-015-1174-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson NM, Kien DT, Clapham H, Aguas R, Trung VT, Chau TN, et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med. 2015;7(279):279ra37 10.1126/scitranslmed.3010370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritchie SA, van den Hurk AF, Smout MJ, Staunton KM, Hoffmann AA. Mission Accomplished? We Need a Guide to the 'Post Release' World of Wolbachia for Aedes-borne Disease Control. Trends Parasitol. 2018;34(3):217–26. 10.1016/j.pt.2017.11.011 . [DOI] [PubMed] [Google Scholar]
- 23.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4(7):e1000098 10.1371/journal.ppat.1000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin M, Rikihisa Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect Immun. 2003;71(9):5324–31. 10.1128/IAI.71.9.5324-5331.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, Brownlie JC, et al. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol. 2004;2(3):E69 10.1371/journal.pbio.0020069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sudeep AB. Culex gelidus: an emerging mosquito vector with potential to transmit multiple virus infections. J Vector Borne Dis. 2014;51(4):251–8. . [PubMed] [Google Scholar]
- 27.Chen L, Zhu C, Zhang D. Naturally occurring incompatibilities between different Culex pipiens pallens populations as the basis of potential mosquito control measures. PLoS neglected tropical diseases. 2013;7(1):e2030 10.1371/journal.pntd.0002030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yen JH, Barr AR. The etiological agent of cytoplasmic incompatibility in Culex pipiens. J Invertebr Pathol. 1973;22(2):242–50. 10.1016/0022-2011(73)90141-9 . [DOI] [PubMed] [Google Scholar]
- 29.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–52. 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pertea G, Huang X, Liang F, Antonescu V, Sultana R, Karamycheva S, et al. TIGR Gene Indices clustering tools (TGICL): a software system for fast clustering of large EST datasets. Bioinformatics. 2003;19(5):651–2. 10.1093/bioinformatics/btg034 . [DOI] [PubMed] [Google Scholar]
- 31.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–9. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Audic S, Claverie JM. The significance of digital gene expression profiles. Genome Res. 1997;7(10):986–95. 10.1101/gr.7.10.986 . [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. 10.1006/meth.2001.1262 . [DOI] [PubMed] [Google Scholar]
- 35.Bian G, Shin SW, Cheon HM, Kokoza V, Raikhel AS. Transgenic alteration of Toll immune pathway in the female mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2005;102(38):13568–73. 10.1073/pnas.0502815102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin SW, Kokoza V, Lobkov I, Raikhel AS. Relish-mediated immune deficiency in the transgenic mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2003;100(5):2616–21. 10.1073/pnas.0537347100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janeway CA Jr., Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54 Pt 1:1–13. 10.1101/sqb.1989.054.01.003 . [DOI] [PubMed] [Google Scholar]
- 38.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. 10.1016/j.cell.2006.02.015 . [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann JA. The immune response of Drosophila. Nature. 2003;426(6962):33–8. 10.1038/nature02021 . [DOI] [PubMed] [Google Scholar]
- 40.Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, et al. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2012;109(1):E23–31. 10.1073/pnas.1116932108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultz MJ, Isern S, Michael SF, Corley RB, Connor JH, Frydman HM. Variable Inhibition of Zika Virus Replication by Different Wolbachia Strains in Mosquito Cell Cultures. J Virol. 2017;91(14). 10.1128/JVI.00339-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng X, Khanuja BS, Ip YT. Toll receptor-mediated Drosophila immune response requires Dif, an NF-kappaB factor. Genes Dev. 1999;13(7):792–7. 10.1101/gad.13.7.792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choe KM, Werner T, Stoven S, Hultmark D, Anderson KV. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science. 2002;296(5566):359–62. 10.1126/science.1070216 . [DOI] [PubMed] [Google Scholar]
- 44.Stoven S, Silverman N, Junell A, Hedengren-Olcott M, Erturk D, Engstrom Y, et al. Caspase-mediated processing of the Drosophila NF-kappaB factor Relish. Proc Natl Acad Sci U S A. 2003;100(10):5991–6. 10.1073/pnas.1035902100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleino A, Valanne S, Ulvila J, Kallio J, Myllymaki H, Enwald H, et al. Inhibitor of apoptosis 2 and TAK1-binding protein are components of the Drosophila Imd pathway. EMBO J. 2005;24(19):3423–34. 10.1038/sj.emboj.7600807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverman N, Zhou R, Erlich RL, Hunter M, Bernstein E, Schneider D, et al. Immune activation of NF-kappaB and JNK requires Drosophila TAK1. J Biol Chem. 2003;278(49):48928–34. 10.1074/jbc.M304802200 . [DOI] [PubMed] [Google Scholar]
- 47.Rances E, Johnson TK, Popovici J, Iturbe-Ormaetxe I, Zakir T, Warr CG, et al. The toll and Imd pathways are not required for wolbachia-mediated dengue virus interference. J Virol. 2013;87(21):11945–9. 10.1128/JVI.01522-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan X, Pike A, Joshi D, Bian G, McFadden MJ, Lu P, et al. The bacterium Wolbachia exploits host innate immunity to establish a symbiotic relationship with the dengue vector mosquito Aedes aegypti. ISME J. 2018;12(1):277–88. 10.1038/ismej.2017.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zambon RA, Nandakumar M, Vakharia VN, Wu LP. The Toll pathway is important for an antiviral response in Drosophila. Proc Natl Acad Sci U S A. 2005;102(20):7257–62. 10.1073/pnas.0409181102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci U S A. 2009;106(42):17841–6. Epub 2009/10/07. 10.1073/pnas.0905006106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rances E, Ye YH, Woolfit M, McGraw EA, O'Neill SL. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 2012;8(2):e1002548 10.1371/journal.ppat.1002548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bourtzis K, Pettigrew MM, O'Neill SL. Wolbachia neither induces nor suppresses transcripts encoding antimicrobial peptides. Insect Mol Biol. 2000;9(6):635–9. 10.1046/j.1365-2583.2000.00224.x . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a biological replicate, total RNA of another 15 female mosquitoes of each group was extracted. cDNA library construction and sequencing were performed as the first time. At least 60 Mb clean reads of sequencing were obtained for each sample.35,236 (NJ♀×TKtet♂), 34,965 (TK♀×NJ tet♂), 34,845 (NJ♀×NJ♂) and 34,708 (TK♀×TK♂) unigenes were generated. A total of 28,476 unigenes were annotated against the NCBI NR protein database, 16,973 in GO function categories, and 21,332 unigenes were mapped onto the canonical pathways in KEGG. (A and B) Volcano plot of DEGs. The unigenes up- or down-regulated more than two-fold when compared between old and new host-Wolbachia symbioses are displayed in red or blue, respectively. Y axis represents -log10 transformed significance. X axis represents log2 transformed fold change. Red points represent up-regulated DEGs. Blue points represent down-regulated DEGs. Gray points represent non-DEGs. TK♀×NJtet♂ had 5,742 up-regulated unigenes and 4,143 down-regulated unigenes in comparison to the control TK♀×TK♂, and NJ♀×TKtet♂ had 4,226 up-regulated unigenes and 4,122 down-regulated unigenes in comparison to the control NJ♀×NJ♂. (C and D) The intersection and union of the DEG heat map for the original and new host-Wolbachia symbiosis. X axis represents comparison for clustering analysis. Coloring indicates fold change (high: red, low: blue). (E and F) The identified DEGs were then assigned to the three standard subcategories of “molecular biological function”, “cellular component” and “biological process” in GO enrichment analysis. X axis represents number of DEG. Y axis represents GO term.
(TIF)
In parallel, the unigenes were mapped onto the canonical pathways in KEGG to identify possible active biological pathways of DEGs in biological replicate of RNA sequencing experiment. Twenty most significant DEGs in new vs. old host-Wolbachia symbiosis are shown here. X axis represents enrichment factor. Y axis represents pathway name. The color indicates q value (high: white, low: blue), a lower q value indicates a more significant enrichment. Point size indicates DEG number (A bigger dot refers to a larger amount). Rich Factor refers to the value of enrichment factor, which is the quotient of foreground value (the number of DEGs) and background value (total Gene amount). A larger Rich Factor value indicates a higher level of enrichment.
(TIF)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.
The data supporting the results of this article have been submitted to NCBI Sequence Read Archive (SRA) repository (Accession number: SRP155507). The materials and methods part has been submitted to protocols.io. (DOI:http://dx.doi.org/10.17504/protocols.io.xcafise)