Abstract
Decidual macrophages secrete proteases that activate protease-activated receptor 1 (PAR-1). We hypothesized that activation of the inflammatory response by bacteria is amplified by proteases, initiating labor. In addition, we hypothesized that commensal bacteria trigger an inflammatory response by activating NF-κB and TET methylcytosine dioxygenase 2 (TET2), a DNA de-methylase, via a protease amplified PAR-1, RhoA kinase (ROCK) pathway. To evaluate these hypotheses, we compared responses of mononuclear cells with Lactobacillus crispatus, prevalent in the vaginal microbiome of women of European ancestry, with L. iners and Fusobacterium nucleatum, which are more prevalent in vaginal samples collected from African-American women. Decidual tissue was collected at term not-in-labor (TNL), term labor (TL), spontaneous preterm labor (sPTL), and infected preterm labor (iPTL) and immunostained for PAR-1, TET2, and CD14. Mononuclear cells and THP-1 macrophage cells were treated with bacteria and elastase, a known activator of PAR-1. The inflammatory response was monitored by confocal microscopy of TET2 and the p65 subunit of NF-κB, as well as IL-8 production. Decidual staining for PAR-1, TET2, and CD14 increased TNL < TL < sPTL < iPTL. All treatments stimulated translocation of TET2 and p65 from the cytosol to the nucleus and increased IL-8, but L. iners and F. nucleatum caused more robust responses than L. crispatus. Inhibition of PAR-1 or ROCK prevented TET2 and p65 nuclear translocalization and increases in IL-8. Our findings demonstrate that proteases amplify the inflammatory response to commensal bacteria. The more robust response to bacteria prevalent in African-American women may contribute to racial disparities in preterm birth.
Keywords: Lactobacillus iners, Lactobacillus crispatus, Fusobacterium nucleatum, Macrophages, Protease-activated receptor 1, TET methylcytosine dioxygenase 2, Preterm labor, African-American
Introduction
Preterm birth (PTB), which is defined as delivery before 37 weeks of completed gestation, is the leading cause of neonatal morbidity and mortality worldwide accounting for as many as 75% of perinatal deaths [1–3]. In the USA, 12.5% of births are preterm with the highest rate for non-Hispanic African-Americans at 17.8%. The annual economic burden associated with PTB in the USA is approximately $26.5 billion [1]. Both term and preterm labor are associated with inflammation and marked infiltration of leukocytes into intrauterine tissues [4–6]. Most bacteria found in intrauterine tissues in association with PTB are of vaginal origin, and it is thought the most common pathway of intrauterine infection is the ascending route from the vagina. However, bacterial DNA from the oral cavity has also been reported to be present [7–14]. Some studies suggest that both term and preterm intrauterine tissues can have bacteria present without overt infection [7, 9–11], so “sub-clinical” colonization by commensal bacteria may convert decidual macrophages into a proinflammatory state, triggering the onset of labor.
The strongest risk factors for PTB are a history of a previous PTB, family history of PTB, and African-American race [1, 15–18], consistent with a genetic component. Although a number of genetic variants have been associated with PTB, they can only explain a small portion of preterm births. Our group recently reported that the higher incidence of PTB in African-Americans is associated with a greater environmental contribution than in women of European ancestry [19]. Environmental contributions could be amplified by environmental gene interactions or produce their effects by epigenetic mechanisms [20–24].
The microbiome can be considered an environmental contributor to health, and our group has shown that the vaginal microbiome differs significantly between African-American women and women of European ancestry [25]. The microbiome of African-Americans is significantly more diverse with a prevalence of bacterial taxa that are associated with microbial invasion and Lactobacillus species that are not protective of the vaginal milieu. L. iners, the most common species in the vaginal microbiome of African-Americans, allows for a higher diversity of pathologic species because of its low production of lactic acid, which does not maintain a low enough pH to be protective [26, 27]. L. iners allows for colonization of species, such as Fusobacterium nucleatum, a gram-negative pathologic species associated with infection and preterm premature rupture of the membranes (PPROM) [7, 28]. L. crispatus, which is the most common species in women of European ancestry, is stable, maintains a low pH, and protects the vagina from being colonized by pathologic species [25–27].
Macrophages express protease-activated receptor 1 (PAR-1) [29–31] and release proteases, such as matrix metalloprotease-1 (MMP-1) and MMP-13 [32–34], that activate PAR-1. Macrophage release of proteases could amplify the bacterial response by activating PAR-1 [35, 36] on adjacent macrophages. We considered the possibility that PAR-1 activation could induce inflammation via the recently discovered TET (Ten-Eleven Translocation) proteins (aka, TET methylcytosine dioxygenases), which enzymatically de-methylate DNA to increased gene expression [37–41]. TET2 is the main TET protein expressed in leukocytes [42–44]. Nuclear factor-kappa B (NF-κB) is a transcription factor that regulates inflammatory response and is activated by protein kinase C (PKC) phosphorylation [45]. RhoA kinase (ROCK) is a down-stream effector of PAR-1 [46, 47], and ROCK is in the PKC family of serine/threonine phosphorylases, so activation of TET2 may involve PKC phosphorylation similar to NF-κB because TET2 contains several PKC phosphorylation sites, as well as a nuclear localization signal (PROSITE, prosite.expasy.org).
We developed a model system to test these concepts using mononuclear cells isolated from pregnant women and THP-1 cells, an established cell line used to assess macrophage function. We tested the hypothesis that commensal bacteria initiate an inflammatory response by activating TET2 and NF-κB via a protease amplified PAR-1, ROCK pathway. To evaluate racial disparity, we compared bacterial responses of L. crispatus with L. iners and F. nucleatum.
Materials and Methods
Study Subjects
Term not-in-labor (TNL) placentas were collected from women who underwent non-emergency caesarian sections between 38 and 39 weeks of gestation. Term labor (TL) placentas were collected from women with normal pregnancies who gave vaginal birth between 38 and 40 weeks of gestation. Spontaneous preterm labor (sPTL) placentas were collected from pregnant women who gave birth prior to 37 weeks of gestation and had no clinical signs of infection. Infected preterm labor (iPTL) placentas were collected from pregnant women who gave birth prior to 37 weeks of completed gestation with PPROM and clinical signs of infection. Infection was defined as fever ≥ 100.4 °F, uterine tenderness, malodorous vaginal discharge, maternal or fetal tachycardia. Exclusion criteria were smokers, HIV/AIDS, drug/alcohol users, pregnancies with stillborn babies, multiple fetuses, preeclampsia, lupus, congenital abnormalities, and hemorrhage. Demographic patient data are given in Table 1.
Table 1.
Clinical characteristics of patient groups for immunohistochemistry
| Variable | TNL n = 6 |
TL n = 5 |
sPTL n = 4 |
iPTL n = 3 |
|---|---|---|---|---|
| Maternal age (years) | 30.0 ± 5.9 | 26.8 ± 10.6 | 27.3 ± 8.1 | 23.3 ± 1.7 |
| Pre-pregnancy BMI (kg/m2) | 26.9 ± 5.7 | 25.1 ± 5.8 | 21.6 ± 3.6 | 23.7 ± 1.5 |
| BMI at sample collection (kg/m2) | 32.0 ± 5.9 | 30.2 ± 6.0 | 26.3 ± 3.4 | 27.3 ± 0.6 |
| Race | ||||
| White | 2 | 1 | ||
| Black | 3 | 3 | 2 | 3 |
| Asian | 1 | |||
| Hispanic | ||||
| Other | 1 | 2 | ||
| Primiparous | 3 | 1 | ||
| Multiparous | 6 | 2 | 3 | 3 |
| Gestational age (weeks) | 38.8 ± 0.4 | 39.2 ± 0.8 | 33.3 ± 1.7* | 30.3 ± 6.7** |
| Infant birth weight (g) | 3341 ± 508 | 3139 ± 506 | 2346 ± 496 | 1723 ± 1092* |
| Delivery method | ||||
| Vaginal | 3 | 2 | 3 | |
| C-Section | 6 | 2 | 2 | |
Values are mean ± SD
*P < 0.05, **P < 0.01 compared with TNL and TL
Immunohistochemistry
For immunohistochemistry, a rectangular section of the fetal membranes (approximately 6 cm × 4 cm) including amnion, chorion, and decidua was removed with surgical scissors. A roll with the decidua oriented towards the interior was prepared. Decidual tissue roles were formalin-fixed, paraffin-embedded, and cut into 7-μm sections with a microtome. Immunohistochemistry was performed as previously described [48–50]. To quench endogenous tissue peroxidase activity, slides were incubated in 3% hydrogen peroxide in methanol for 30 min. For antigen retrieval, slides were heat treated in 10 mM citrate buffer for 5 min with a pressure cooker. Slides were blocked for 1 h in 10% goat serum. Tissues were immunostained with rabbit polyclonal antibody to TET2 (1:50, Proteintech, Rosemont, IL, Cat. No. 21207-1-AP), rabbit polyclonal antibody to PAR-1 (1:50, Proteintech, Cat. No. 15607-1-AP), rabbit polyclonal antibody to CD14 (1:200, Proteintech, Cat. No. 17000-1-AP), or rabbit IgG isotype negative control pre-diluted in phosphate buffered saline (Invitrogen, Cat. No. 086199) using Vector ABC Kit (Thermo Fisher Scientific, Waltham, MA, Cat. No. PK-6101) and ImmPACT DAB (Thermo Fisher Scientific, Cat. No. SK-4105). Slides were counterstained with 1:5 dilution of Hematoxylin QS (Vector Laboratories, Burlingame, CA). The staining protocol was the same for all samples with regard to processing, incubation times, and temperature. Slides were analyzed with an Olympus BH-2 microscope (Olympus, Center Valley, PA) attached to a digital camera (Olympus QColor5) using image analysis software (cellSens, Olympus). Relative differences in staining were identified by highlighting specific stained areas with yellow overlay using the “Measuring Images” tool. Data were quantified and reported as area stained (μm2).
Cell Culture for Confocal Immunofluorescence
Mononuclear cells isolated from blood collected from normal pregnant women before 37 weeks’ gestation and THP-1 cells (ATCC, Manassas, VA), a widely used monocytic cell line that can be differentiated into a macrophage cell type, were used to assess inflammatory response to bacteria and elastase and to study the mechanism whereby proteases might amplify the inflammatory response via PAR-1. Both mononuclear cells and macrophages express PAR-1 [29–31]. Inflammatory response was assessed by the translocation of TET2 and NF-κB from the cytosol to the nucleus using immunofluorescence staining and confocal microscopy. Mononuclear cells composed of monocytes and lymphocytes, both of which infiltrate the decidua, were separated from granulocytes by Histopaque (1077/1119) density gradient centrifugation according to the manufacture’s protocol (Sigma, St. Louis, MO) and as previously described by our lab [51–55]. Mononuclear cells and THP-1 cells were cultured in RPMI-1640 medium supplemented with 2 mM l-glutamine and 1% antibiotics and antimycotics (100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin B) at 37 °C in a humidified 5% CO2 atmosphere. For the THP-1 cells, 50 μM 2-mercaptoethanol and 10% fetal bovine serum were added. Cells were seeded at 250,000 cells per ml in 4-chambered slides. THP-1 cells were treated with phorbol 12-myristate 13-acetate (PMA, Abcam, Cambridge, MA), 16 nM, for 24 h to differentiate them into a macrophage cell type and then clean media for 48 h. Mononuclear cells were used fresh. The following treatments were added for 1 h: (1) control media, and heat-killed bacteria (100:1 bacteria to cells): (2) L. crispatus; (3) L. iners; (4) F. nucleatum; and (5) elastase (0.033 U/ml, Sigma, Cat. No. E7885). Bacteria were grown in one of our laboratories (KKJ) and characterized by DNA sequencing as to the species. Treatment for 1 h was chosen because nuclear translocation of NF-κB is maximal at this time [56] and time course experiments for TET2 showed nuclear localization as early as 15 min. For inhibitor treatments, they were added 30 min before activating treatments: (1) PAR-1 inhibitor (2 μM, SCH-79797, Tocris, Minneapolis, MN); (2) ROCK inhibitor (20 μM Y-27632, Tocris). Media were aspirated, cells were washed with PBS, and fixed with 4% formalin for 1 h. Cells were washed, then blocked and permeabilized for 1 h in 10% goat serum + 3% BSA + 0.2% Triton X-100. Cells were incubated overnight at 4 °C with primary rabbit antibody specific for TET2 (1:250, Proteintech) or the p65 subunit of NF-κB (1:50, Proteintech). The next morning, cells were washed and incubated with anti-rabbit IgG secondary antibody green fluorescence dye (Alexa Fluor 488, 1:3000, Jackson ImmunoResearch, Burlingame, CA) for TET2 or red fluorescence dye (Cy3, 1:2000, Jackson ImmunoResearch) for p65 at room temperature for 1 h. Cells were washed and mounted with VectaMount containing DAPI (Vector Laboratories) for nuclear DNA staining. Images were taken using a confocal microscope (Zeiss LSM 710). Percentage of cells with nuclear localization of TET2 or NF-κB (i.e., negative vs. positive) were determined in 1–2 fields (× 63 lens) for each treatment. Intensity of fluorescence in cytosol vs. nucleus was quantified with ImageJ software (NIH) using the Freehand tool and Integrated Density measurement. The density of the nucleus was taken as a percentage of the density for the whole cell. Percentage of cells activated was quantified by counting cells with brighter fluorescence in the nucleus than in the cytosol and taking that as a percentage of the total cells. An average of 27 cells were analyzed in each field for mononuclear cells and an average of 20 cells in each field for THP-1 cells.
Cell Culture for Interleukin-8
To further evaluate inflammatory response, media concentrations of interleukin (IL-8), an NF-κB-regulated gene, were determined. Pregnancy mononuclear cells were isolated by Histopaque density gradient centrifugation as above. Cells were seeded at 250,000 cells per well in 1 ml of RPMI 1640 media in 24 well plates and cultured for 2 h with L. crispatus, L. iners, or F. nucleatum (50:1, bacteria to cells) or L. iners (50:1) plus inhibitors of PAR-1 (2 μM, SCH-79797) or ROCK (20 μM Y-27632). We used the THP-1 macrophage cell line to evaluate dose responses for bacteria and elastase. THP-1 cells were cultured as above and incubated for 18 h with bacteria (10:1, 50:1, 100:1, bacteria to cells) and elastase (0.0033, 0.033, and 0.33 U/ml). Media were collected and frozen at – 20 °C until assay. Media concentrations of IL-8 were measured by ELISA (R&D Systems, Minneapolis, MN).
Statistical Analysis
Data were analyzed by one-way ANOVA with Bonferroni multiple comparisons test. Demographic data are presented as mean ± SD. Quantitative data are presented as mean ± SE. A probability level of P < 0.05 was considered significant. A statistical software application was used (Prism 6.0 for Macintosh, GraphPad Software, Inc., San Diego, CA).
Results
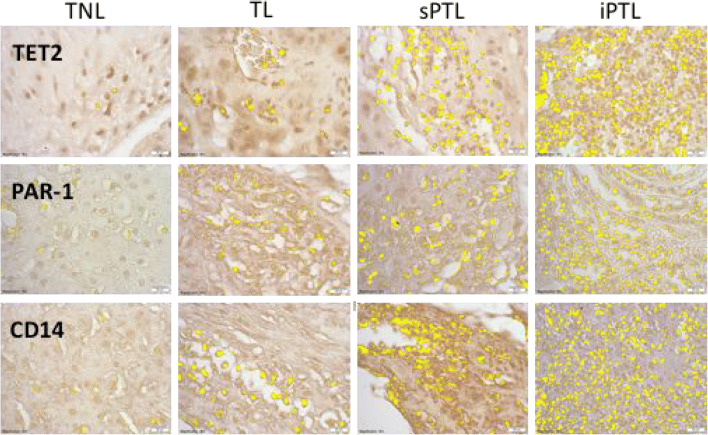
Figure 1 shows representative images for the area of staining highlighted in yellow for TET2, PAR-1, and CD14 in decidual tissue. TET2 and PAR-1 showed similar patterns of staining. There was little expression of either TET2 or PAR-1 in decidual tissue of TNL women. The amount expressed increased in women with TL but was markedly increased in women with sPTL and even more so in women with iPTL. Staining for TET2 and PAR-1 paralleled increases in the infiltration of macrophages as evidenced by staining for CD14. Staining for TET2 and PAR-1 was localized primarily to leukocytes in the tissue and was not present in decidual cells.
Fig. 1.
Representative sections of decidual tissue immunostained for TET2, PAR-1, and CD14 from women who were at term not-in-labor (TNL), women who were at term in labor (TL), women who delivered spontaneously preterm with no clinical signs of infection (sPTL), and women who delivered preterm with PPROM and clinical signs of infection (iPTL). Specific antigen staining was highlighted in yellow using the Measuring Images tool in the cellSens software. There was a little staining for TET2, PAR-1, or CD14 in women with TNL. Staining increased slightly for TL but was markedly increased in sPTL and iPTL. The increasing pattern of staining for TET2 and PAR-1 correlated with the macrophage marker, CD14. Decidual cells did not expression TET2 or PAR-1. All pictures and analyses were done with a × 40 lens
Figure 2 shows the area of staining quantified for statistical analysis. Although there was more area stained in TL versus TNL, the increases were not statistically significant. However, the areas of staining for CD14, TET2, and PAR-1 were significantly increased in sPTL as compared with TL and the areas of staining were significantly higher in iPTL as compared with sPTL.
Fig. 2.
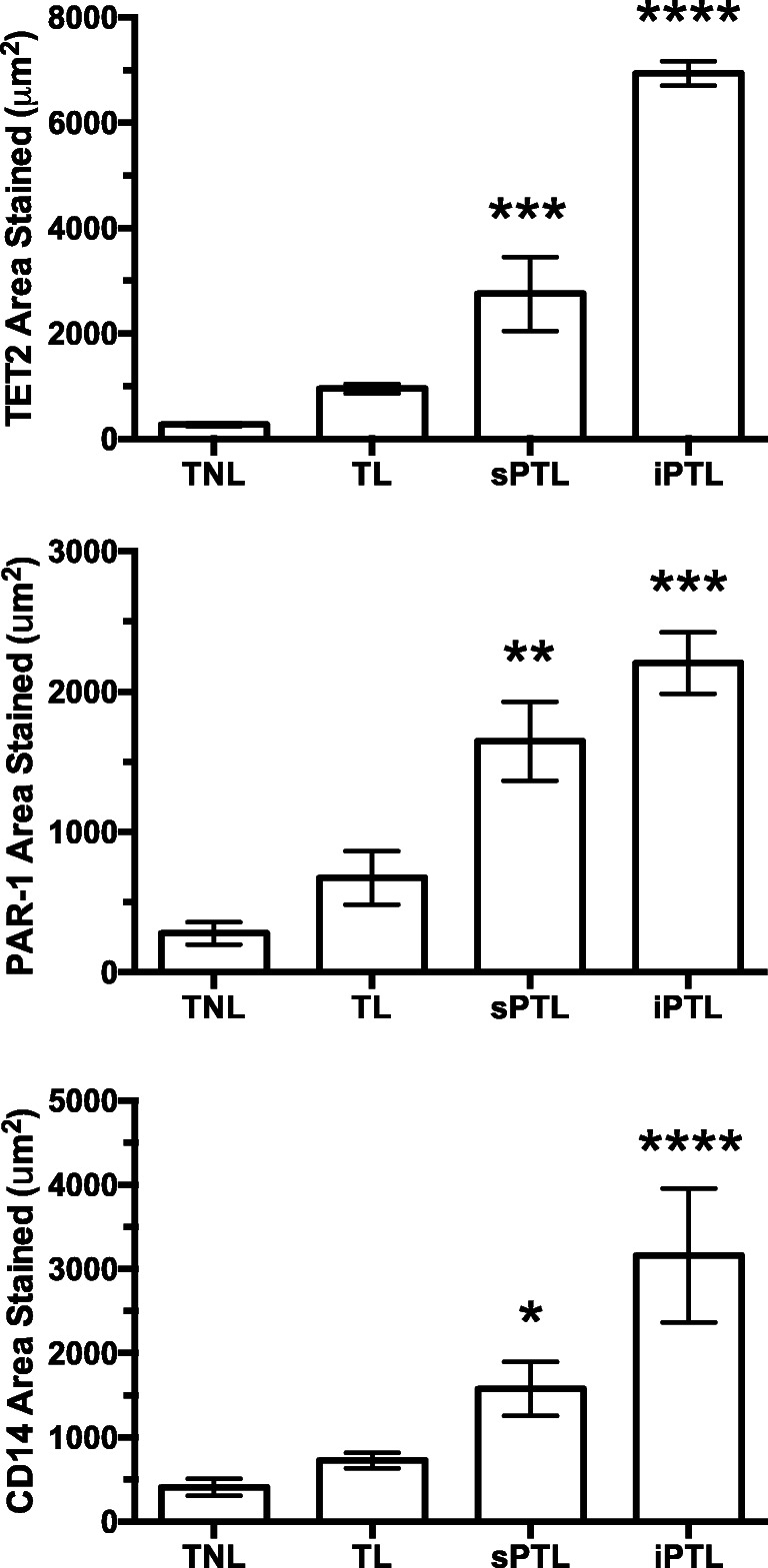
Area of staining in decidual tissue for women who were TNL (n = 6), women who were TL (n = 5), women who were sPTL (n = 4), and women who were iPTL (n = 3). The areas of staining for TET2 and PAR-1 were small in women with TNL or TL. However, there was a marked and significant increase in the areas stained for both TET2 and PAR-1 in women with sPTL and an even greater increase in women with iPTL. The increase in areas stained for TET2 and PAR-1 correlated with the staining for macrophages, CD14. The Measuring Images tool in the cellSens software was used to determine the area of staining for statistical analysis. Data represent mean ± SE, *P < 0.05, **P < 0.01, ***P < 0.001, ****P<0.0001
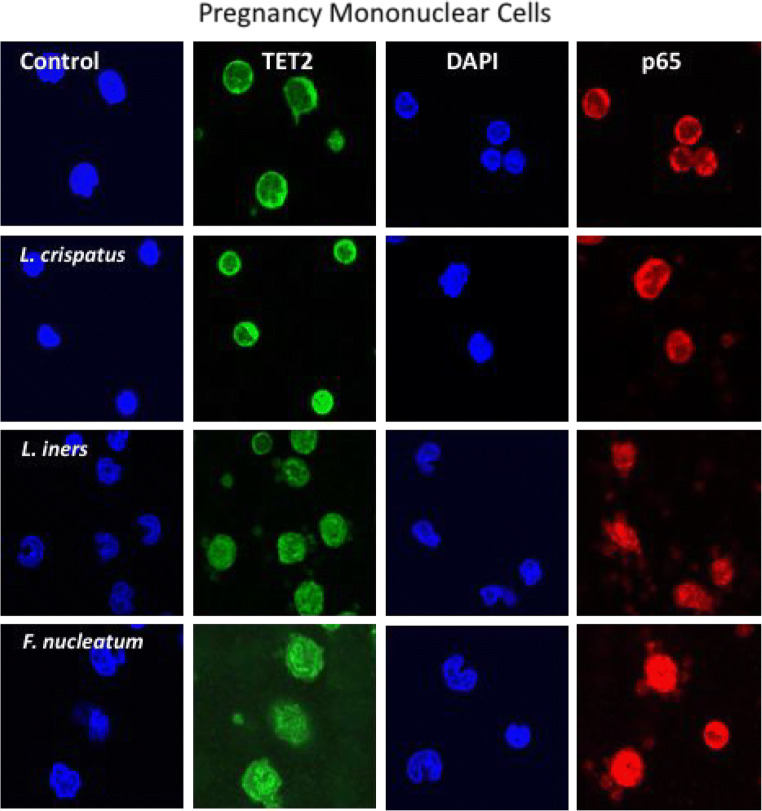
Figure 3 shows representative images for nuclear localization of TET2 (green) and the p65 subunit of NF-κB (red) induced by bacterial treatments in mononuclear cells isolated from normal pregnant women. DAPI staining (blue) identifies the nucleus. In untreated cells, both TET2 and p65 were primarily localized to the cytosol. L. crispatus, L. iners, and F. nucleatum all increased the translocation of TET2 and p65 from the cytosol to the nucleus. F. nucleatum caused the most intense nuclear localization.
Fig. 3.
Representative images of mononuclear cells isolated from normal pregnant women treated with commensal bacteria. Green fluorescence identifies TET2, red identifies the p65 subunit of NF-κB, and DAPI blue identifies the nucleus. In untreated cells, both TET2 and p65 were primarily localized to the cytosol. L. crispatus, L. iners, and F. nucleatum all increased the translocation of TET2 and p65 from the cytosol to the nucleus, with F. nucleatum causing the most intense nuclear localization. The dose for bacteria was 100:1 (bacteria to cells)
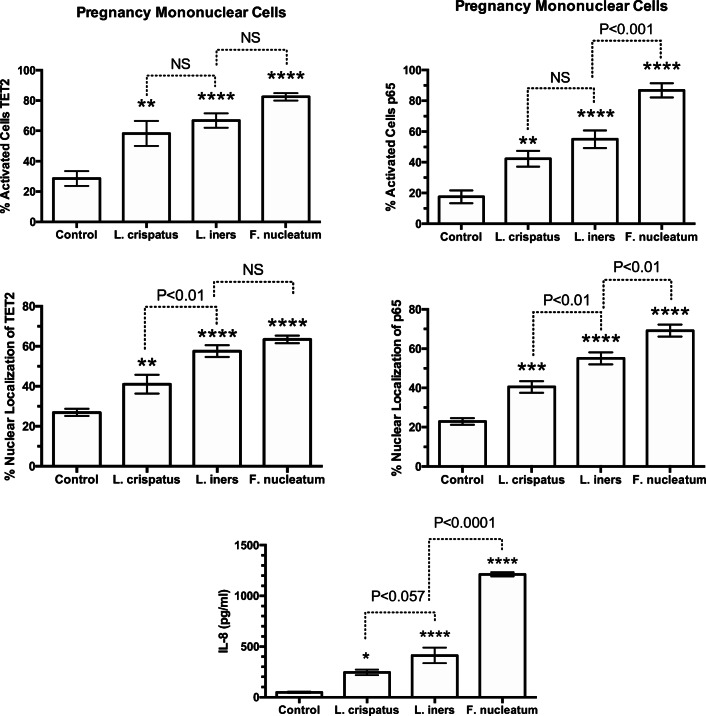
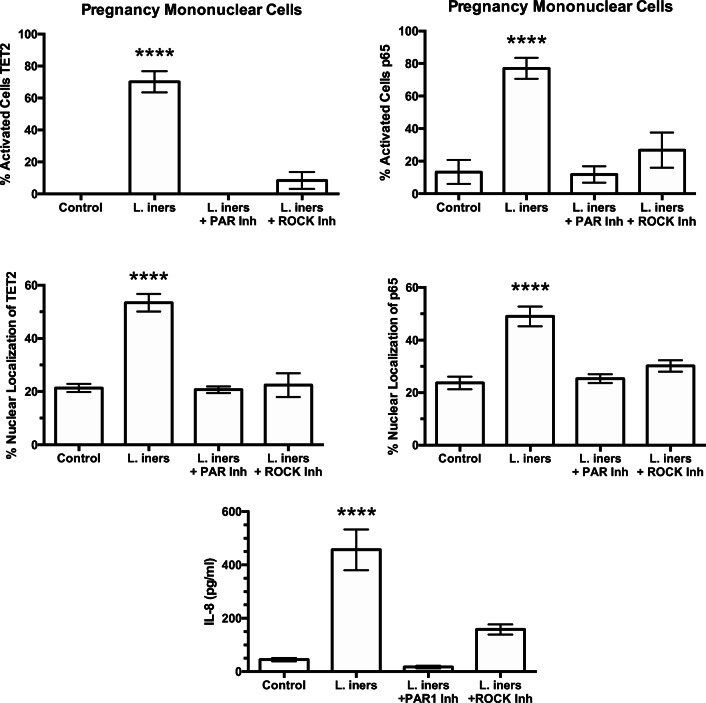
Figure 4 summarizes the results for percentage of activated cells and percentage of nuclear localization for TET2 and p65 in response to bacteria, as well as the IL-8 response. All three bacterial species increased mononuclear cell activation and the percentage of nuclear localization of TET2 and p65 with a parallel increase in media concentrations of IL-8. In general, the response for L. iners was greater than for L. crispatus with F. nucleatum the most potent.
Fig. 4.
Percent of pregnancy mononuclear cells activated, % nuclear localization for TET2 and p65, and media concentrations of IL-8 in response to treatments with bacteria. Nuclear localization was quantitated by the density of immunofluorescence in the area of the nucleus as a percentage of the density of the entire cell using ImageJ software. Percentage of cells activated was quantified by counting the number of cells with brighter fluorescence in the nucleus than in the cytosol and taking that as a % of the total cells. The average number of cells counted per field was 27. L. crispatus, L. iners, and F. nucleatum all activated the cells causing translocation of TET2 and p65 from the cytosol to the nucleus with a parallel increase in IL-8 in a general order of L. crispatus < L. iners < F. nucleatum. (n = 8 for TET2, n = 10 for p65, n = 11 for IL-8, data represent mean ± SE, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 as compared with control. P values are for comparisons between bacterial treatments. NS, non-significant)
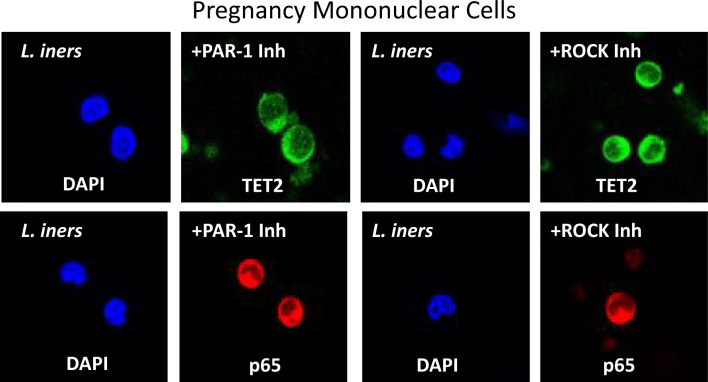
To evaluate the pathway of protease amplification to bacterial stimulation, mononuclear cells were stimulated with L. iners in the presence of inhibitors of PAR-1 or ROCK. L. iners was used because it caused intermediate activation between L. crispatus and F. nucleatum. Representative pictures are shown in Fig. 5. Inhibition of either PAR-1 or ROCK prevented the ability of L. iners to translocate TET2 and p65 from the cytosol to the nucleus as evidenced by the area of the nuclei being relatively absent of green or red fluorescence in cells treated with L. iners plus inhibitors.
Fig. 5.
Representative images of the cellular localization of TET2 (green) and p65 (red) in pregnancy mononuclear cells after treatment with L. iners plus inhibitors of PAR-1 or ROCK. Co-treatment with either PAR-1 inhibitor or ROCK inhibitor prevented the ability of L. iners to move TET2 or p65 from the cytosol into the nucleus
Figure 6 summarizes results for treatments with L. iners plus inhibitors of PAR-1 or ROCK. The significant increases in the percentage of cells activated and the percentage of nuclear localization for TET2 and p65 induced by L. iners were prevented when cells were co-treated with PAR-1 or ROCK inhibitors. Similarly, the increase in IL-8 induced by L. iners was significantly attenuated by inhibition of PAR-1 or ROCK. These findings demonstrate that the PAR-1, ROCK pathway amplifies bacterial inflammatory response.
Fig. 6.
Percent of pregnancy mononuclear cells activated in response to treatment with L. iners or L. iners plus inhibitors of PAR-1 or ROCK. Inhibition of PAR-1 or ROCK prevented L. iners from causing nuclear translocation of TET2 and p65 and increasing IL-8 concentrations. (n = 7, mean ± SE, ****P < 0.0001)
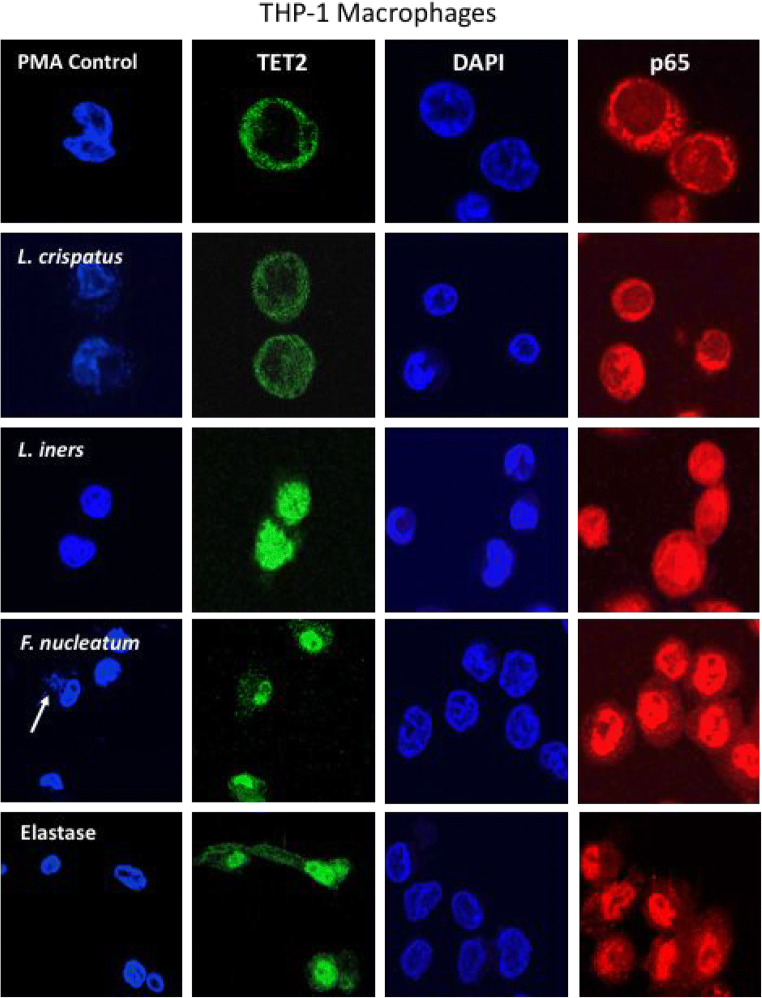
For the initial studies to test our hypotheses, we used primary cultures of mononuclear cells isolated from pregnant women. Mononuclear cells are composed of both monocytes and lymphocytes, both of which infiltrate the decidua during pregnancy. We were particularly interested in macrophages as first responders to bacterial invasion, and because of their extensive infiltration into the decidua of women with sPTL and iPTL. To determine if the responses observed for mononuclear cells were also present in macrophages, we repeated the experiments using THP-1 cells, a monocytic cell line that can be differentiated into a macrophage cell phenotype. Figure 7 shows representative pictures for nuclear localization of TET2 and p65. Results were similar to those for mononuclear cells. TET2 and p65 were primarily localized to the cytosol in control cells. L. crispatus slightly increased nuclear localization, whereas L. iners and F. nucleatum caused strong localization of TET2 and p65 to the nucleus with little remaining in the cytosol. To demonstrate protease activation, THP-1 cells were treated with elastase, a known activator of PAR-1. Similar to L. iners and F. nucleatum, elastase caused strong nuclear localization of TET2 and p65.
Fig. 7.
Representative images of the nuclear translocation of TET2 (green) and the p65 subunit of NF-κB (red) in THP-1 macrophage cells in response to bacteria (100:1, bacteria to cells) or elastase (0.033 U/ml). DAPI blue indicates the location of the nucleus. In control cells, TET2 and p65 were primarily localized to the cytosol indicating TET2 and NF-κB were not activated in most cells. In cells treated with L. crispatus, there were some movements of TET2 and p65 from the cytosol to the nucleus; however, in cells treated with L. iners, F. nucleatum, or elastase, TET2 and p65 were primarily localized to the nucleus in most cells. White arrow for F. nucleatum points to nuclei of bacteria engulfed by a THP-1 macrophage
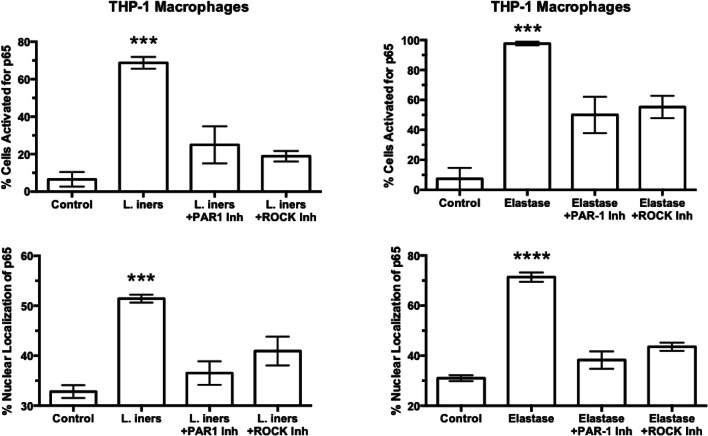
To quantify the inflammatory response of THP-1 macrophages, the p65 subunit of NF-κB, an accepted hallmark of inflammation [45, 57], was used (Fig. 8). In control cells, few cells were activated with p65 primarily localized to the cytosol. All bacterial treatments activated THP-1 macrophages with translocation of p65 from the cytosol to the nucleus indicating activation of NF-κB. L. crispatus activated the fewest cells with 15% of cells activated; however, L. iners activated over 60% of cells and F. nucleatum activated almost all cells similar to protease treatment with elastase.
Fig. 8.
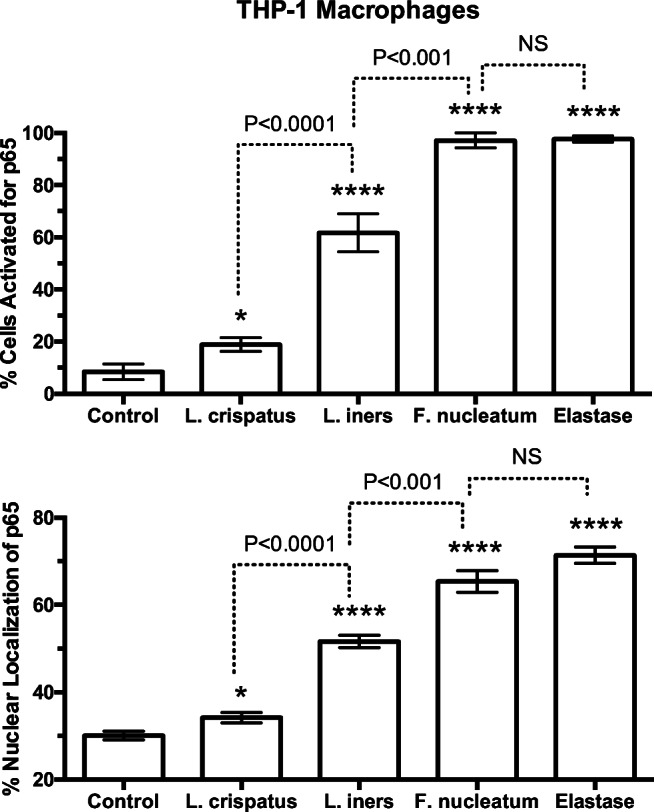
Percent of cells activated and % of nuclear fluorescence for the p65 subunit of NF-κB in THP-1 cells in response to treatments with bacteria or elastase. Percent of cells activated and % nuclear localization was quantitated as described for Fig. 4. The average number of cells counted per field was 20. L. crispatus activated 15% of cells; however, L. iners activated over 60% of cells and F. nucleatum activated almost all cells similar to protease treatment with elastase. (n = 5 for bacteria, n = 3 for elastase, mean ± SE, *P < 0.05, ****P < 0.0001 as compared with control. P values are for comparisons between bacterial treatments. NS, non-significant)
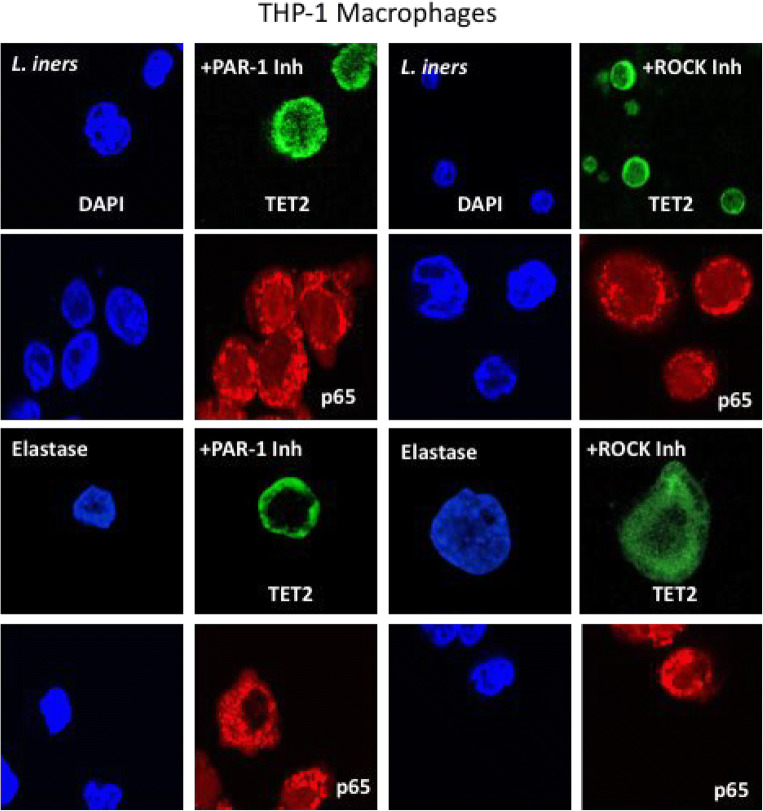
To evaluate protease amplification and the PAR-1, ROCK pathway in THP-1 macrophages, L. iners was again tested as a representative commensal bacteria and elastase was tested as a known activator of PAR-1. Figure 9 shows representative images. Inhibition of PAR-1 or inhibition of ROCK prevented the translocation of TET2 and p65 from the cytosol to the nucleus. In cells co-treated with L. iners or elastase plus inhibitors, TET2 and p65 were primarily localized to the cytosol.
Fig. 9.
Representative images of the cellular localization of TET2 (green) and p65 (red) in THP-1 macrophage cells after treatment with L. iners or elastase plus inhibitors of PAR-1 or ROCK. Co-treatment with either PAR-1 inhibitor or ROCK inhibitor prevented the ability of L. iners or elastase to move TET2 or p65 from the cytosol into the nucleus
Figure 10 shows summarized results for treatment with L. iners and elastase plus inhibitors of PAR-1 or ROCK for THP-1 macrophage cells. Inhibition of PAR-1 or ROCK significantly inhibited cell activation and nuclear translocation of TET2 and p65 induced by L. iners or elastase. These results confirm the findings with primary culture of mononuclear cells that proteases amplify the bacterial inflammatory response via a PAR-1, ROCK pathway.
Fig. 10.
Percent of THP-1 macrophage cells activated and % of nuclear fluorescence localization for p65 in response to treatments with L. iners or elastase plus inhibitors of PAR-1 or ROCK. Inhibition of PAR-1 or ROCK prevented the nuclear translocation of p65 induced by L. iners or elastase. (n = 5 for L. iners, n = 4 for elastase, mean ± SE, ***P < 0.001, ****P < 0.0001)
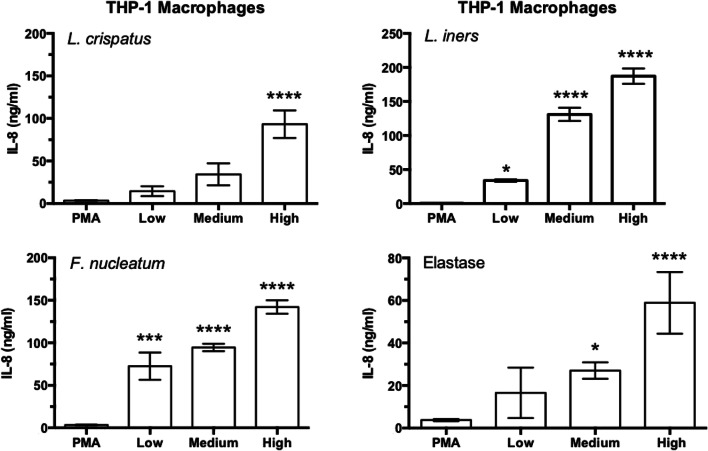
By using THP-1 cells, it was possible to grow a sufficient number of cells to test dose responses for IL-8 production. Figure 11 shows that bacteria and elastase induced dose-dependent increases in IL-8. All doses of L. iners and F. nucleatum significantly increased IL-8, whereas only the highest dose of L. crispatus did. The responses for L. iners and F. nucleatum were very robust with the lowest doses increasing IL-8 as much as the highest dose of L. crispatus. Elastase significantly increased IL-8 at the medium and high doses.
Fig. 11.
Dose response effect of bacteria and protease treatment on media concentrations of IL-8 in THP-1 macrophage cells. Doses for bacteria were low, 10:1; medium, 50:1; high, 100:1 bacteria to cells. Doses for elastase were low, 0.0033 U/ml; medium, 0.033 U/ml; high, 0.33 U/ml. L. iners and F. nucleatum significantly increased IL-8 at all doses as opposed to L. crispatus, which significantly increased IL-8 only at the highest dose. Elastase stimulated significant increases in IL-8 at the medium and highest doses. (n = 6 for bacteria, n = 4 for elastase, mean ± SE, *P < 0.05, ***P < 0.001 ****P < 0.0001)
Discussion
In this study, we provide evidence that proteases amplify the inflammatory response induced by commensal bacteria via a PAR-1, ROCK pathway. Treatment of pregnancy mononuclear cells or THP-1 macrophage cells with commensal bacteria or elastase, a protease known to activate PAR-1 [58], caused translocation of TET2 and the p65 subunit of NF-κB from the cytosol to the nucleus coincident with increased production of IL-8, an NF-κB-regulated gene. Inhibition of PAR-1 or ROCK prevented these changes. Immunohistochemical examination of decidual tissue obtained from women at delivery revealed a progressive increase in the expression of TET2 and PAR-1 that paralleled the infiltration of macrophages in the order TNL < TL < sPTL < iPTL. TET2 and PAR-1 staining was localized to leukocytes and not present in decidual cells.
The concurrent translocation of TET2 and NF-κB from the cytosol to the nucleus upon activation suggests enzymatic de-methylation may be necessary for inflammatory response. It makes sense that during pregnancy inflammatory genes would be silenced and a mechanism could be DNA methylation to block transcription factor binding sites. Upon activation, TET2 moves into the nucleus to erase methylation tags masking transcription factor binding sites, which allows NF-κB to bind and increase expression of inflammatory genes. Consistent with this idea is our recent finding that increased expression of Toll-like receptors is associated with reduced DNA methylation in sPTL [59].
We studied decidual tissue because it is in a key location to be first impacted by invasion of commensal microorganisms of vaginal origin and to, thus, act as a trigger of inflammatory response. As resident sentinels, macrophages are a first alert system to warn the body of danger. In vivo, protease amplification could occur as a consequence of release of proteases from activated macrophages, triggering PAR-1 signaling on adjacent macrophages, as well as other decidual leukocytes that express PAR-1, such as lymphocytes. Protease amplification may be particularly important in sPTL and iPTL because of the extensive infiltration of macrophages into the decidua and their close proximity to each other. Although bacteria produce proteases, we were not able to find any evidence that bacterial proteases activate PAR-1, whereas proteases released by macrophages do [32–34]. In our experiment, bacterial proteases cannot be responsible because the bacteria were heat-inactivated. To our knowledge, this is the first report that proteases amplify the inflammatory response to bacteria and is necessary for a robust response.
We were interested in how the vaginal microbiome might be implicated in the racial disparity of preterm birth of African-Americans. That is why we compared L. crispatus which is prevalent in European ancestry women with L. iners which is prevalent in African-American women. L. crispatus maintains a stable, healthy vaginal environment, whereas L. iners allows a higher diversity of pathologic species, such as F. nucleatum. Both Lactobacillus species initiated an inflammatory response, but L. iners initiated a more robust response than L. crispatus. The strongest response was induced by F. nucleatum. These results suggest that the greater prevalence of L. iners and F. nucleatum in the vaginal microbiome of African-American women might initiate an inflammatory response prematurely resulting in their higher incidence of PTB.
The results of this study raise an interesting question. If a beneficial bacterium like L. crispatus can cause an inflammatory response when in direct contact with immune cells, could vaginal bacteria be the initiating agents in term and preterm labor? This could come about as the cervix effaces and dilates and the fetal membranes are exposed to the vaginal microbiome. This would provide a route for vaginal bacteria to enter intrauterine tissues where they could initiate an inflammatory response to initiate the onset of labor.
We measured IL-8 production, which is encoded by an NF-κB-regulated gene. IL-8 has additional significance with regard to labor as it is a potent neutrophil chemokine, and neutrophils infiltrate reproductive tissues with the onset of labor [4–6]. We have shown that neutrophil proteases are potent stimulators of uterine contractility via PAR-1 [60], and neutrophil matrix metalloproteinases, such as MMP-1 and MMP-8, have been recognized as important contributors to labor because of their ability to degrade extracellular matrix and, as such, have been implicated in cervical ripening and preterm premature rupture of fetal membranes [4, 27, 61–63]. The significant increases we observed in IL-8 induced by vaginal bacteria lend support to the idea that commensal bacteria may be initiators of both term and preterm labor.
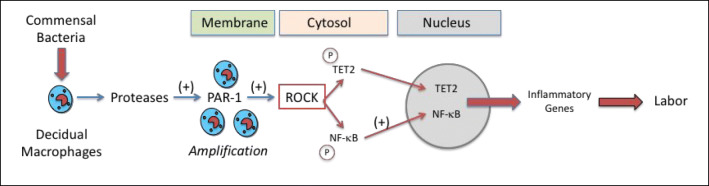
Figure 12 summarizes the implications of this study. Commensal bacterial stimulate decidual macrophages to release proteases that amplify the inflammatory response by activating PAR-1 on adjacent macrophages and other decidual leukocytes that express PAR-1. Activation of PAR-1 leads to downstream signaling whereby ROCK phosphorylates TET2 and NF-κB, causing their translocation from the cytosol to the nucleus. Enzymatic de-methylation of DNA by TET2 opens up transcription factor binding sites for NF-κB to increase inflammatory gene expression initiating labor. The more robust inflammatory responses initiated by L. iners and F. nucleatum, which are prevalent in the vaginal microbiome of African-American women, as compared with L. crispatus, which is prevalent in the vaginal microbiome of women of European ancestry, might explain the higher incidence of PTB in African-Americans. However, in both cases, commensal bacteria could play a role in the initiation of labor.
Fig. 12.
Proposed mechanism for protease amplification of the inflammatory response induced by commensal bacteria that could trigger the onset of labor. Commensal bacteria present in decidual tissue stimulate resident macrophages to release proteases that act in an autocrine manner to amplify the response by activating PAR-1 on adjacent macrophages and other decidual leukocytes that express PAR-1. Activation of PAR-1 leads to down-stream signaling to ROCK that phosphorylates TET2 and NF-κB causing their translocation from the cytosol to the nucleus. In the nucleus, TET2 enzymatically de-methylates DNA opening up transcription factor binding sites for NF-κB resulting in increased expression of inflammatory genes to initiate labor. Commensal bacteria tested were L. crispatus, which is prevalent in the vaginal microbiome of women of European ancestry, and L. iners and F. nucleatum, which are prevalent in the vaginal microbiome of African-American women. We observed a more robust inflammatory response for L. iners and F. nucleatum than for L. crispatus which might explain the higher incidence of PTB in African-Americans; however, commensal bacteria may be the initiators of labor in both term and preterm labor
Acknowledgments
Microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility, supported, in part, by funding from the NIH-NINDS Center Core Grant 5 P30 NS047463 and, in part, by funding from the NIH-NCI Cancer Center Support Grant P30 CA016059.
Funding Information
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article. This work was supported by grants from NHLBI, R01 HL069851 (SWW), NICHD 5R01 HD088386 (SWW), and NCMHD P60 MD002256 (JFS, KKJ, SWW).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Institute of Medicine . Preterm birth: causes, consequences, and prevention. 2010/07/30. Washington DC: National Academy of Sciences; 2007. [Google Scholar]
- 2.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88(1):31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16(2):206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 5.Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci. 2013;20(2):154–167. doi: 10.1177/1933719112446084. [DOI] [PubMed] [Google Scholar]
- 6.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14(1):229–236. [PubMed] [Google Scholar]
- 7.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. Br J Obstet Gynaecol. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 9.Jones HE, Harris KA, Azizia M, Bank L, Carpenter B, Hartley JC, Klein N, Peebles D. Differing prevalence and diversity of bacterial species in fetal membranes from very preterm and term labor. PLoS One. 2009;4(12):e8205. doi: 10.1371/journal.pone.0008205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steel JH, Malatos S, Kennea N, Edwards AD, Miles L, Duggan P, Reynolds PR, Feldman RG, Sullivan MH. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res. 2005;57(3):404–411. doi: 10.1203/01.PDR.0000153869.96337.90. [DOI] [PubMed] [Google Scholar]
- 11.Stout MJ, Conlon B, Landeau M, Lee I, Bower C, Zhao Q, Roehl KA, Nelson DM, Macones GA, Mysorekar IU. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol. 2013;208(3):226. doi: 10.1016/j.ajog.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J, et al. Sci Transl Med. 2014;6(237):237ra265. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prince AL, Antony KM, Chu DM, Aagaard KM. The microbiome, parturition, and timing of birth: more questions than answers. J Reprod Immunol. 2014;104-105:12–19. doi: 10.1016/j.jri.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141–147. doi: 10.1016/j.mib.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Pineles BL, Gotsch F, Mittal P, Than NG, Espinoza J, Hassan SS. Recurrent preterm birth. Semin Perinatol. 2007;31(3):142–158. doi: 10.1053/j.semperi.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meis PJ, Goldenberg RL, Mercer BM, Iams JD, Moawad AH, Miodovnik M, Menard MK, Caritis SN, Thurnau GR, Bottoms SF, Das A, Roberts JM, McNellis D. The preterm prediction study: risk factors for indicated preterm births. Maternal-Fetal Medicine Units Network of the National Institute of Child Health and Human Development. Am J Obstet Gynecol. 1998;178(3):562–567. doi: 10.1016/s0002-9378(98)70439-9. [DOI] [PubMed] [Google Scholar]
- 17.Mercer BM, Goldenberg RL, Moawad AH, Meis PJ, Iams JD, Das AF, Caritis SN, Miodovnik M, Menard MK, Thurnau GR, Dombrowski MP, Roberts JM, McNellis D. The preterm prediction study: effect of gestational age and cause of preterm birth on subsequent obstetric outcome. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1999;181(5 Pt 1):1216–1221. doi: 10.1016/s0002-9378(99)70111-0. [DOI] [PubMed] [Google Scholar]
- 18.Shen TT, DeFranco EA, Stamilio DM, Chang JJ, Muglia LJ. A population-based study of race-specific risk for preterm premature rupture of membranes. Am J Obstet Gynecol. 2008;199(4):373. doi: 10.1016/j.ajog.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 19.York Timothy P., Strauss Jerome F., Neale Michael C., Eaves Lindon J. Racial Differences in Genetic and Environmental Risk to Preterm Birth. PLoS ONE. 2010;5(8):e12391. doi: 10.1371/journal.pone.0012391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sassone-Corsi P. Physiology. When metabolism and epigenetics converge. Science. 2013;339(6116):148–150. doi: 10.1126/science.1233423. [DOI] [PubMed] [Google Scholar]
- 21.Fraga MF, Esteller M. Epigenetics and aging: the targets and the marks. Trends Genet. 2007;23(8):413–418. doi: 10.1016/j.tig.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Tost J. DNA methylation: an introduction to the biology and the disease-associated changes of a promising biomarker. Mol Biotechnol. 2010;44(1):71–81. doi: 10.1007/s12033-009-9216-2. [DOI] [PubMed] [Google Scholar]
- 23.Zaina S, Lindholm MW, Lund G. Nutrition and aberrant DNA methylation patterns in atherosclerosis: more than just hyperhomocysteinemia? J Nutr. 2005;135(1):5–8. doi: 10.1093/jn/135.1.5. [DOI] [PubMed] [Google Scholar]
- 24.Zaina S, Lund G. Epigenetics: a tool to understand diet-related cardiovascular risk? J Nutrigenet Nutrigenomics. 2011;4(5):261–274. doi: 10.1159/000334584. [DOI] [PubMed] [Google Scholar]
- 25.Fettweis JM, Brooks JP, Serrano MG, Sheth NU, Girerd PH, Edwards DJ, Strauss JF, 3rd, Jefferson KK, Buck GA. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology. 2014;160(Pt 10):2272–2282. doi: 10.1099/mic.0.081034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leizer J, Nasioudis D, Forney LJ, Schneider GM, Gliniewicz K, Boester A, Witkin SS. Properties of epithelial cells and vaginal secretions in pregnant women when Lactobacillus crispatus or Lactobacillus iners dominate the vaginal microbiome. Reprod Sci. 2018;25(6):854–860. doi: 10.1177/1933719117698583. [DOI] [PubMed] [Google Scholar]
- 27.Witkin SS, Mendes-Soares H, Linhares IM, Jayaram A, Ledger WJ, Forney LJ. Influence of vaginal bacteria and D- and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. mBio. 2013;4(4):e00460-13. [DOI] [PMC free article] [PubMed]
- 28.Prince AL, Antony KM, Ma J, Aagaard KM. The microbiome and development: a mother’s perspective. Semin Reprod Med. 2014;32(1):14–22. doi: 10.1055/s-0033-1361818. [DOI] [PubMed] [Google Scholar]
- 29.Bruges Gustavo, Crespo Gustavo, Salazar Victor, Deglesne Pierre-Antoine, Schneider Heike, Cabrera-Fuentes Hector, Schmitz M. Lienhard, Preissner Klaus, Lopéz Mercedes. Thrombin selectively induces transcription of genes in human monocytes involved in inflammation and wound healing. Thrombosis and Haemostasis. 2014;112(11):992–1001. doi: 10.1160/TH14-01-0034. [DOI] [PubMed] [Google Scholar]
- 30.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53(2):245–282. [PubMed] [Google Scholar]
- 31.Shpacovitch V, Feld M, Hollenberg MD, Luger TA, Steinhoff M. Role of protease-activated receptors in inflammatory responses, innate and adaptive immunity. J Leukoc Biol. 2008;83(6):1309–1322. doi: 10.1189/jlb.0108001. [DOI] [PubMed] [Google Scholar]
- 32.Austin KM, Covic L, Kuliopulos A. Matrix metalloproteases and PAR1 activation. Blood. 2013;121(3):431–439. doi: 10.1182/blood-2012-09-355958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Back M, Ketelhuth DF, Agewall S. Matrix metalloproteinases in atherothrombosis. Prog Cardiovasc Dis. 2010;52(5):410–428. doi: 10.1016/j.pcad.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90(3):251–262. [PubMed] [Google Scholar]
- 35.Ahn HS, Chackalamannil S, Boykow G, Graziano MP, Foster C. Development of proteinase-activated receptor 1 antagonists as therapeutic agents for thrombosis, restenosis and inflammatory diseases. Curr Pharm Des. 2003;9(28):2349–2365. doi: 10.2174/1381612033453884. [DOI] [PubMed] [Google Scholar]
- 36.Trivedi V, Boire A, Tchernychev B, Kaneider NC, Leger AJ, O’Callaghan K, Covic L, Kuliopulos A. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137(2):332–343. doi: 10.1016/j.cell.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, Sun Y, Li X, Dai Q, Song CX, Zhang K, He C, Xu GL. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333(6047):1303–1307. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333(6047):1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324(5929):930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin R, Mao SQ, Zhao B, Chong Z, Yang Y, Zhao C, Zhang D, Huang H, Gao J, Li Z, Jiao Y, Li C, Liu S, Wu D, Gu W, Yang YG, Xu GL, Wang H. Ascorbic Acid enhances tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J Am Chem Soc. 2013;135(28):10396–10403. doi: 10.1021/ja4028346. [DOI] [PubMed] [Google Scholar]
- 41.Nabel CS, Kohli RM. Molecular biology. Demystifying DNA demethylation. Science. 2011;333(6047):1229–1230. doi: 10.1126/science.1211917. [DOI] [PubMed] [Google Scholar]
- 42.Klug M, Schmidhofer S, Gebhard C, Andreesen R, Rehli M. 5-Hydroxymethylcytosine is an essential intermediate of active DNA demethylation processes in primary human monocytes. Genome Biol. 2013;14(5):R46. doi: 10.1186/gb-2013-14-5-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu H, Zhang Y. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes Dev. 2011;25(23):2436–2452. doi: 10.1101/gad.179184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klug M, Heinz S, Gebhard C, Schwarzfischer L, Krause SW, Andreesen R, Rehli M. Active DNA demethylation in human postmitotic cells correlates with activating histone modifications, but not transcription levels. Genome Biol. 2010;11(6):R63. doi: 10.1186/gb-2010-11-6-r63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes PJ, Karin M. Nuclear factor-kappa B: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 46.Coughlin SR. Protease-activated receptors in vascular biology. Thromb Haemost. 2001;86(1):298–307. [PubMed] [Google Scholar]
- 47.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3(8):1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 48.Leik CE, Walsh SW. Neutrophils infiltrate resistance-sized vessels of subcutaneous fat in women with preeclampsia. Hypertension. 2004;44(1):72–77. doi: 10.1161/01.HYP.0000130483.83154.37. [DOI] [PubMed] [Google Scholar]
- 49.Shah TJ, Leik CE, Walsh SW. Neutrophil infiltration and systemic vascular inflammation in obese women. Reprod Sci. 2010;17(2):116–124. doi: 10.1177/1933719109348252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah TJ, Walsh SW. Activation of NF-kappaB and expression of COX-2 in association with neutrophil infiltration in systemic vascular tissue of women with preeclampsia. Am J Obstet Gynecol. 2007;196(1):48.e41–48.e48. doi: 10.1016/j.ajog.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 51.Vaughan JE, Walsh SW. Neutrophils from pregnant women produce thromboxane and tumor necrosis factor-alpha in response to linoleic acid and oxidative stress. Am J Obstet Gynecol. 2005;193(3 Pt 1):830–835. doi: 10.1016/j.ajog.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 52.Vaughan JE, Walsh SW, Ford GD. Thromboxane mediates neutrophil superoxide production in pregnancy. Am J Obstet Gynecol. 2006;195(5):1415–1420. doi: 10.1016/j.ajog.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 53.Estrada-Gutierrez G, Cappello RE, Mishra N, Romero R, Strauss JF, 3rd, Walsh SW. Increased expression of matrix metalloproteinase-1 in systemic vessels of preeclamptic women a critical mediator of vascular dysfunction. Am J Pathol. 2011;178(1):451–460. doi: 10.1016/j.ajpath.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mishra N, Nugent WH, Mahavadi S, Walsh SW. Mechanisms of enhanced vascular reactivity in preeclampsia. Hypertension. 2011;58(5):867–873. doi: 10.1161/HYPERTENSIONAHA.111.176602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walsh SW. Plasma from preeclamptic women stimulates transendothelial migration of neutrophils. Reprod Sci. 2009;16(3):320–325. doi: 10.1177/1933719108327594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noursadeghi M, Tsang J, Haustein T, Miller RF, Chain BM, Katz DR. Quantitative imaging assay for NF-kappaB nuclear translocation in primary human macrophages. J Immunol Methods. 2008;329(1-2):194–200. doi: 10.1016/j.jim.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baldwin ASJ. The NF-kB and IkB proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki T, Moraes TJ, Vachon E, Ginzberg HH, Huang TT, Matthay MA, Hollenberg MD, Marshall J, McCulloch CA, Abreu MT, Chow CW, Downey GP. Proteinase-activated receptor-1 mediates elastase-induced apoptosis of human lung epithelial cells. Am J Respir Cell Mol Biol. 2005;33(3):231–247. doi: 10.1165/rcmb.2005-0109OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walsh SW, Chumble AA, Washington SL, Archer KJ, Sahingur SE, Strauss JF, 3rd, et al. J Reprod Immunol. 2017;121:35–41. doi: 10.1016/j.jri.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walsh SW, Nugent WH, Solotskaya AV, Anderson CD, Grider JR, Strauss JF., 3rd Matrix metalloprotease-1 and elastase are novel uterotonic agents acting through protease-activated receptor 1. Reprod Sci. 2018;25(7):1058–1066. doi: 10.1177/1933719117732162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, Yoon BH. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183(1):94–99. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Ogawa M, Wood JR, Bartolomei MS, Sammel MD, Kusanovic JP, Walsh SW, Romero R, Strauss JF., 3rd Genetic and epigenetic mechanisms combine to control MMP1 expression and its association with preterm premature rupture of membranes. Hum Mol Genet. 2008;17(8):1087–1096. doi: 10.1093/hmg/ddm381. [DOI] [PubMed] [Google Scholar]
- 63.Strauss JF., 3rd Extracellular matrix dynamics and fetal membrane rupture. Reprod Sci. 2013;20(2):140–153. doi: 10.1177/1933719111424454. [DOI] [PMC free article] [PubMed] [Google Scholar]