Abstract
Helminth infections are known to influence T- and B- cell responses in latent Mycobacterium tuberculosis infection (LTBI). Whether helminth infections also modulate monocyte responses in helminth-LTBI co-infection has not been fully explored. To this end, we examined the activation, polarization and function of human monocytes isolated from individuals with LTBI with (n=25) or without (n=25) co-incident Strongyloides stercoralis infection (Ss+ and Ss− respectively). Our data reveal that the presence of Ss infection is associated with lower frequencies of monocytes expressing CD54, CD80, CD86 at baseline (absence of stimulation) and in response to mycobacterial-antigen stimulation than monocytes from Ss−individuals. In contrast, Ss infection was associated with higher frequencies of M2-like monocytes, as determined by expression of CD206 and CD163. Monocytes from Ss+ individuals had a reduced capacity to phagocytose or exhibit respiratory burst activity following mycobacterial-antigen or LPS stimulation and were less capable of expression of IL-1β, TNFα, IL-6 and IL-12 at baseline and/or following antigen stimulation compared to those without Ss infection. In addition, definitive treatment of Ss infection resulted in a significant reversal of the altered monocyte function 6 months after anthelmintic therapy. Finally, T cells from Ss+ individuals exhibited significantly lower activation at baseline or following mycobacterial-antigen-stimulation. Therefore, our data highlights the induction of dampened monocyte activation, enhanced M2 polarization and impaired monocyte function in helminth-LTBI coinfection. Our data also reveal a different mechanism by which helminth infection modulates immune function in LTBI.
INTRODUCTION
Monocytes are critical players in the immunity to Mycobacterium tuberculosis (M.tb) infection and disease. Monocytes and macrophages are the primary target of M.tb and their innate capacity to control M.tb defines the early progression of infection (1). Although M.tb has traditionally been thought to survive and replicate in macrophages, recent work has revealed that M.tb infects multiple subsets of mononuclear phagocytes, including recruited monocytes in vivo and in vitro (2). Monocytes are also involved in transporting bacteria from the lungs to the local lymph nodes and presenting antigens for activation of CD4+ T cells (3-5). Finally, blood monocytes are the main sources of recruited monocytes to the lung during M.tb infection (5). Monocytes are typically elevated in active tuberculosis and are polarized to the M1 phenotype (upregulating iNOS to produce nitric oxide) (6, 7).
Helminths are considered to be a risk factor for the development of active TB in latently infected (LTBI) individuals (8-10). This is due to the ability of helminth infection to modulate both innate and adaptive immune responses in LTBI, with major effects on T and B cells. With regards to helminth-TB coinfection, very few studies have examined the role of helminth infections in modulating monocyte function in the context of M.tb coinfection (11, 12). Helminth infections are known to induce arginase-1 in macrophages which promotes lung inflammation and disease severity in TB (13) and to dampen phago-lysosomal maturation in macrophages (14). Helminth infections can modulate TLR expression and function on monocytes (15) and induce alternative activation of macrophages in the setting of M.tb coinfection (16). However, very little is known is about the effect of helminth infections and subsequent anthelmintic treatment on monocyte function, in particular activation (as determined by expression of CD154, CD80 and CD86), M2 polarization (as determined by CD206 and CD163 expression) and function (as determined by phagocytosis, respiratory burst response and cytokine response) in LTBI.
Therefore, we sought to examine the activation, polarization and function of monocytes in a helminth-LTBI co-infection. To this end, we studied the monocyte populations in LTBI individuals with or without Strongyloides stercoralis (Ss), a common helminth infection, affecting about 30-100 million people worldwide. Our data show that Ss infection is associated with dampened monocyte activation, enhanced M2 polarization, impaired monocyte function and finally decreased T cell activation in LTBI individuals and that this altered function can be at least partially reversed by anthelmintic chemotherapy.
Material and Methods
Ethics statement
All participants were examined as part of a natural history study protocol (12-I-073) approved by Institutional Review Boards of the National Institute of Allergy and Infectious Diseases (USA) and the National Institute for Research in Tuberculosis (India). Informed written consent was obtained from all participants.
Study population
We recruited a group of 50 individuals with LTBI, 25 with Ss infection - (hereafter Ss+), 25 without Ss infection (hereafter Ss−). 15 individuals in each group were used for the in vitro stimulation of monocytes for activation marker and cytokine expression and T cell activation. 10 individuals in each group were used for the functional assay experiments and 12 individuals were used for qPCR experiments. This study was conducted in a rural village in the suburbs of Chennai. All enrolled individuals were between 18 and 65 years old. None had previous anthelmintic treatment, a known history of helminth infections or HIV. LTBI was diagnosed based on positive results of Tuberculin skin test (TST) and Quantiferon (QFT) TB Gold in tube, with no symptoms or signs of active TB, no history of previous TB and normal chest radiographs. TST was performed using 2 tuberculin units of Tuberculin PPD RT 23 SSI (Serum Statens Institute). A positive skin test was defined as an induration of at least 12 mm diameter, based on the previously determined cut off norms for South India (17). QFT was performed according to the manufacturer’s instructions (Qiagen).
Parasitological examination and anthelmintic treatment
Ss infection was diagnosed by the presence of IgG antibodies to the recombinant NIE antigen as described previously (18, 19). This was further confirmed by stool microscopy using nutrient agar plates. Stool microscopy was performed to rule out the presence of other intestinal parasites. Filarial infection was excluded in all study participants by virtue of being negative in tests for circulating filarial antigen. All INF individuals were treated with single dose of ivermectin (12mg) and albendazole (400 mg) and follow – up blood draws were obtained six months later. Follow up examination was done by stool microscopy, which was negative for Ss infection. In addition, serology showed a significant decrease in the IgG titers to NIE antigen, as described previously (20).
Hematology
Hematological parameters were measured from fresh venous EDTA blood samples on all individuals using an ACT 5 Diff. hematology analyzer (Beckman Coulter, Brea, CA, USA).
Isolation of peripheral blood mononuclear cells and in vitro culture
PBMCs (peripheral blood mononuclear cells) were isolated from the whole blood samples using density gradient centrifugation method, as described previously (21). The buffy coat cells were washed with 1× PBS to remove platelet‐rich plasma fraction. Then the cells were incubated with ACK lysing solution (to remove excess erythrocytes). Finally, the isolated cells were washed and resuspended with 1× PBS and checked for viability and the cells were suspended in freezing medium, and stored at −80°C.
Immunophenotyping
PBMC were thawed and washed with PBS first and PBS/1% BSA later. The cultures were then stimulated with purified protein derivative (PPD), M.tb whole cell lysate (WCL), PMA/ionomycin (P/I) or media alone at 37°C for 6 h. After 6 hours of stimulation, the cells were harvested and stained with surface antibodies for 30–60 min. Surface antibodies used were CD45-PerCP (BD Biosciences), CD56 PerCP eFluor710 (eBiosciences), CD14-Pacific Blue (Biolegend), HLA-DR-PE-Cy7 (BD Biosciences) and CD16-APC- Cy7 (BD Biosciences), CD54 FITC (BD Biosciences), CD80 PerCP (BD Biosciences) and CD86 PE (BD Biosciences) chemokine receptors such as CCR2 PE (BD Biosciences) and CX3CR1 APC (BD Biosciences), CD69 FITC (BD Biosciences) and HLA-DR PerCP (BD Biosciences). The cells were washed and permeabilized with BD Perm/Wash buffer (BD Biosciences) and stained for intracellular cytokines IL-1α FΙΤC, IL-1β FΙΤC, TNF-α PE, IL-6 PE, IL-10 APC and IL-12 APC (all antibodies from Biolegend and BD Biosciences) for an additional 30 min before washing and acquisition. T cell activation was assessed by examining CD4+ and CD8+ T cells for expression of CD69 and HLA-DR. Eight- color flow cytometry was performed on a FACS Canto II flow cytometer with FACSDIVA software, version 6 (Becton Dickinson). The gating was set by forward and side scatter, and 100 000 gated events were acquired. Data were collected and analyzed using FLOW JO software version 10.4 (TreeStar, Ashland, OR). Compensation and gating boundaries were adjusted using unstained, single stained, and Fluorescence Minus One (FMO) controls. FMO controls for each marker were used to calculate fluorescence intensity for the population. Baseline values following stimulation with medium are depicted as baseline frequency, while frequencies following stimulation with antigens are depicted as net frequencies (with baseline values subtracted).
Monocyte Phagocytosis Assay
At 6 hours after incubation with media alone or WCL or LPS, monocytes were assessed for phagocytic activity using the Phagocytosis Assay Kit (Cayman Chemical, Ann Arbor, MI). Briefly, latex beads with rabbit IgG-FITC conjugates (1:100) were incubated with cells for 6 hours followed by a 1minute incubation with trypan blue to quench non-phagocytosed bead fluorescence. Cells were removed from the culture plate in which they were cultured by gentle scraping. The cells were transferred to tubes for with anti-human CD14, incubated on ice for 20 minutes, then washed once with assay buffer and read on a flow cytometer.
Monocyte Respiratory Burst Assay
The monocyte respiratory burst was assessed using the Monocyte Respiratory burst assay kit according to the manufacturer’s instructions (Cayman Chemical) in which 1× 106 cells/ml were incubated with WCL, LPS or PMA for 45 min at 37°C. Next, 20 μL of 1,2,3 dihydrorhodamine (1,2,3DHR) was then added for a further 20 min at 37°C, and the oxidation to rhodamine within CD14+ monocytes was measured by flow cytometry.
ELISA
PBMC culture supernatant levels of IL-1α, IL-1β, TNF-α, IL-6, IL-10 and IL-12 (R&D Systems, Minneapolis, MN, USA) were measured using ELISA kits, according to the manufacturer’s instructions.
qPCR of Arginase-1 and iNOS expression
PBMCs were lysed using the reagents of a commercial kit (QIAshredder; Qiagen, Valencia, CA, USA). Total RNA was extracted according to the manufacturer’s protocol (RNeasy mini kit; Qiagen), and RNA was dissolved in 50 μl of RNase-free water. The cDNA synthesis was performed with 1 ug RNA using prime script 1st strand cDNA synthesis kit according to the manufacturer’s instructions (RNeasy mini kit; Qiagen). The cDNA obtained from cells were quantified by quantitative real-time PCR and the levels of arginase-1(ARG1) and nitric oxide synthase (iNOS) and an endogenous 18S ribosomal RNA control were measured using an ABI 7500 sequence detection system ((Applied Biosystems, Inc., Fullerton, CA, USA), using Applied Biosystems, TaqMan Assays-on-Demand reagents. The relative transcript numbers were calculated using the formula 0.5CT of target − CT of control, where CT is the threshold cycle during the exponential phase of amplification.
Statistical analysis
Data analyses were performed using GraphPad PRISM (GraphPad Software, Inc., San Diego, CA, USA). Geometric means (GM) were used for measurements of central tendency. Statistically significant differences were assessed using the nonparametric Mann-Whitney U test or the Wilcoxon signed rank test for paired analyses. Multiple comparisons were corrected using the Holm’s correction. Analyses were performed using Graph-Pad PRISM Version 8.1.2.0 (GraphPad, San Diego, CA).
RESULTS
Study population characteristics
The baseline hematological characteristics of the study populations are shown in Table I. As can be seen, Ss+ individuals had significantly higher absolute eosinophil counts and significantly lower monocyte counts than Ss− individuals. No significant differences in the other hematological parameters were observed. In addition, there was no correlation between total monocyte counts and the parameters measured in the study indicating that variations in monocyte counts was not a confounding variable in the study results.
Table I.
Baseline hematological parameters of study population
| Hematology profile | Ss+ (n=25) | Ss− (n=25) | p value |
|---|---|---|---|
| Red blood cell count, x106/ml | 4.06 (3.7-6.08) |
4.08 (2.12-5.86) |
NS |
| White blood cell count, x103cells/ml | 9500 (5700-16900) |
8600 (5400-15500) |
NS |
| Lymphocyte count, cells/ml | 2516 (1554-3712) |
2464 (1487-4412) |
NS |
| Neutrophil count, cells/ml | 4804 (3112-11069) |
4998 (2563-9934) |
NS |
| Monocyte count, cells/ml | 465 (379-1000) |
658 (337-1100) |
p=0.0186 |
| Eosinophil count, cells/ml | 822 (503-3519) |
265 (74-433) |
P=0.0078 |
| Basophil count, cells/ml | 89 (13-306) |
86 (32-387) |
NS |
LTBI/Ss coinfection is associated with decreased monocyte activation and increased M2 polarization
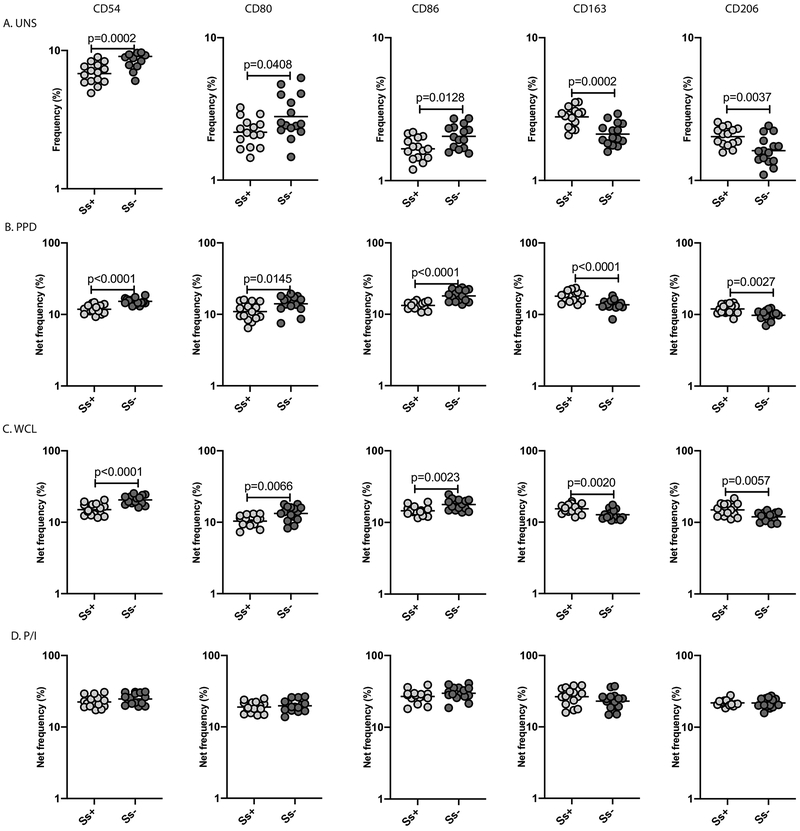
To determine the baseline and antigen -stimulated monocyte activation and M2 polarization in helminth-LTBI coinfection, we measured the frequency of monocytes expressing CD54, CD80 and CD86 (known activation markers) and CD163 and CD206 (M2-specific markers). A representative dot plot showing the expression of activation and M2 markers in an Ss+ and an Ss− individual is shown in S. Figure 1. As shown in Figure 1, we demonstrate that the baseline frequency of CD54+, CD80+ or CD86+ monocytes was significantly lower in Ss+ compared to Ss−individuals. Similarly, following stimulation with purified protein derivative (PPD) and M.tb whole cell lysate (WCL), the net frequency of monocytes expressing CD54, CD80 and CD86 was significantly lower in Ss+ compared to Ss− individuals (Figure 1B, C). There were no differences in the net frequency of monocytes expressing the above markers following PMA/ionomycin (P/I) stimulation (Figure 1D).
Figure.1. LTBI/Ss coinfection is associated with decreased monocyte activation and increased M2 polarization.
Baseline and antigen-stimulated frequencies of monocytes expressing activation and M2 markers were determined by flow cytometry in Ss+ (light grey n =15) and Ss− (dark grey n =15) individuals. The (A) baseline (unstimulated) as well as (B) PPD, (C) WCL and (D) P/I stimulated frequencies of monocytes expressing activation markers (CD54, CD80, CD86) and M2 markers (CD163 and CD206) are shown. Each circle represents a single individual and the bars represent the GM values. Net frequencies were calculated by subtracting baseline frequencies from the antigen-induced frequencies for each individual. The p values were calculated using the Mann–Whitney U test.
In contrast, we demonstrate that the baseline frequency of CD163+ and CD206+ monocytes was significantly higher in Ss+ compared to Ss− individuals (Figure 1). Similarly, following stimulation with PPD and WCL, the net frequency of monocytes expressing CD163 and CD206 was significantly higher in Ss+ compared to Ss− individuals. Finally, there were no differences in the net frequency of monocytes expressing M2 markers upon P/I stimulation. To additionally verify the enhanced M2 polarization in monocytes in the Ss+ group, we also performed qPCR analysis of Arginase-1 (M2 marker) and iNOS expression (M1 marker) in baseline PBMC samples from Ss+ and Ss− individuals. As shown in S. Figure 4, Arginase-1 expression 1.4-fold higher in Ss+ compared to Ss− individuals while iNOS expression was 1.4-fold lower. Thus, LTBI/Ss coinfection is associated with diminished monocyte activation and enhanced M2 polarization.
LTBI/Ss coinfection is associated with diminished monocyte phagocytosis and respiratory burst activity
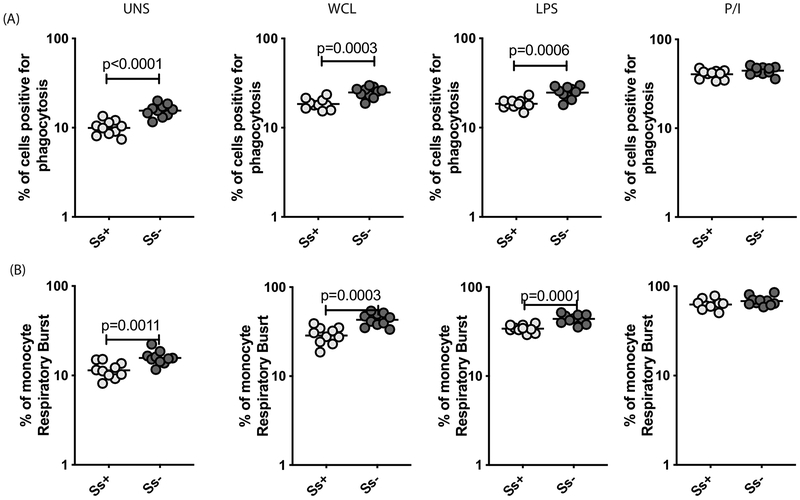
To understand the influence of Ss infection on monocyte function in LTBI, we examined the frequency of monocytes undergoing phagocytosis or respiratory burst activity in Ss+ and Ss− individuals with LTBI. A representative dot plot showing the gating strategy and the measurement of phagocytosis and respiratory burst activity at baseline and following antigen – stimulation in an Ss+ individual is shown in S. Figure 2A and 2B, respectively. As shown in Figure 2A, the baseline as well as WCL or LPS but not P/I stimulated frequency of monocytes undergoing phagocytosis was significantly lower in Ss+ compared to Ss− individuals. Similarly, as shown in Figure 2B, the baseline as well as WCL or LPS but not P/I stimulated frequency of monocytes undergoing respiratory burst activity was significantly lower in Ss+ compared to Ss−individuals. Thus, LTBI/Ss coinfection is associated with diminished respiratory burst activity in monocytes reduction in their ability to phagocytose.
Figure.2. LTBI/Ss coinfection is associated with diminished monocyte phagocytosis and respiratory burst activity.
Baseline and antigen stimulated frequencies of monocytes were assessed for phagocytic activity and respiratory burst activity in Ss+ (light grey n =10) and Ss− (dark grey n =10) individuals. (A) PBMC were stimulated with WCL, LPS and P/I for 6 h and the frequencies of monocytes undergoing phagocytosis was measured. (B) PBMC were stimulated with WCL, LPS and P/I and the frequencies of monocytes undergoing respiratory burst was measured. Each circle represents a single individual and the bars represent the GM values. Net frequencies were calculated by subtracting baseline frequencies from the antigen-induced frequencies for each individual. The p values were calculated using the Mann–Whitney U test.
LTBI/Ss coinfection is associated with diminished monocyte cytokine production
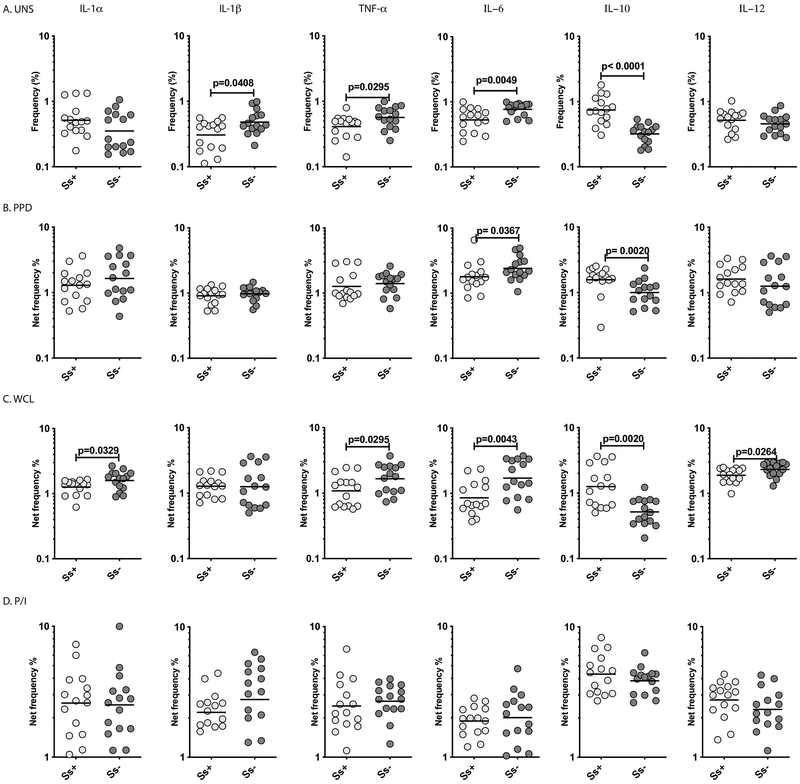
To characterize monocyte cytokine production in LTBI/Ss co-infection, we examined the frequency of monocytes expressing cytokines in Ss+ and Ss− individuals with LTBI. A representative dot plot showing the gating strategy and expression of cytokines in monocytes in an Ss+ individual is shown in S. Figure 3. As shown in Figure 3A, the baseline frequencies of monocytes expressing IL-1β, TNFα and IL-6 were significantly lower in Ss+ compared to Ss−individuals. In contrast, the baseline frequencies of monocytes expressing IL-10 were significantly higher in Ss+ individuals. In addition, following stimulation with PPD and WCL, the frequencies of monocytes expressing IL-6 (for PPD) and IL-1α, TNFα, IL-6 and IL-12 (for WCL) were significantly lower in Ss+ compared to Ss− individuals (Figure 3B, C). In contrast, the antigen stimulated frequency of IL-10 expressing monocytes was significantly higher in Ss+ individuals. Finally, as shown in Figure 3D, there was no difference in the frequency of monocytes expressing the above cytokines following P/I stimulation. Thus, LTBI/Ss coinfection is associated with diminished monocyte cytokine production at baseline and following M.tb-antigen stimulation.
Figure.3. LTBI/Ss coinfection is associated with diminished monocyte cytokine production.
Baseline and antigen-stimulated frequencies of monocytes expressing cytokines were determined by flow cytometry in Ss+ (light grey n =15) and Ss− (dark grey n =15) individuals. The (A) baseline (unstimulated) as well as (B) PPD, (C) WCL and (D) P/I stimulated frequencies of monocytes expressing cytokines (IL-1α, IL-1β, TNF-α, IL-6, IL-10 and IL-12) are shown. Each circle represents a single individual and the bars represent the GM values. Net frequencies were calculated by subtracting baseline frequencies from the antigen-induced frequencies for each individual. The p values were calculated using the Mann–Whitney U test.
Diminished pro-inflammatory cytokine production and enhanced IL-10 production LTBI/Ss coinfection
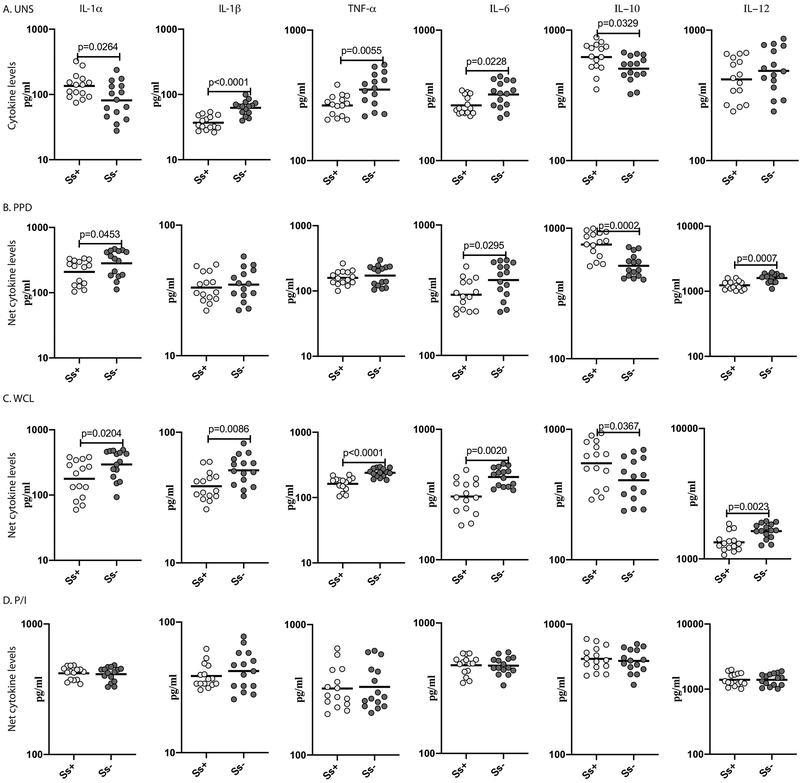
To additionally verify cytokine production in LTBI/Ss co-infection, we measured the levels of cytokines in baseline and stimulated PBMC culture supernatants of Ss+ and Ss− individuals with LTBI. As shown in Figure 4A, at baseline, IL-1α, IL-1β, TNFα and IL-6 levels were significantly diminished in Ss+ compared to Ss−individuals. In contrast, at baseline, IL-10 levels were significantly elevated in Ss+ individuals. Upon PPD stimulation, the levels of IL-1α, IL-6 and IL-12 levels were significantly diminished while following WCL stimulation, IL-1α, IL-1β, TNFα, IL-6 and IL-12 levels were significantly diminished in Ss+ compared to Ss− individuals (Figure 4B, C). In contrast, PPD and WCL antigen stimulated IL-10 levels were significantly elevated in Ss+ individuals. Finally, as shown in Figure 4D, there was no significant difference in the levels of the above cytokines following P/I stimulation. Thus, LTBI/Ss coinfection is associated with diminished cytokine production at baseline and following M.tb-antigen stimulation.
Figure.4. Diminished pro-inflammatory cytokine production and enhanced IL-10 production LTBI/Ss coinfection.
Baseline and antigen-stimulated levels of pro-inflammatory cytokines were measured by ELISA using PBMC culture supernatants of Ss+ (light grey n =15) and Ss− (dark grey n =15) individuals. The (A) baseline (unstimulated) as well as (B) PPD, (C) WCL and (D) P/I stimulated levels of cytokines (IL-1α, IL-1β, TNF-α, IL-6, IL-10 and IL-12) are shown. Each circle represents a single individual and the bars represent the GM values. Net cytokine levels were calculated by subtracting baseline cytokine levels from the antigen-stimulated cytokine levels for each individual. The p values were calculated using the Mann–Whitney U test.
Anthelmintic therapy significantly increases monocyte activation and decreases M2 polarization in LTBI/Ss coinfection
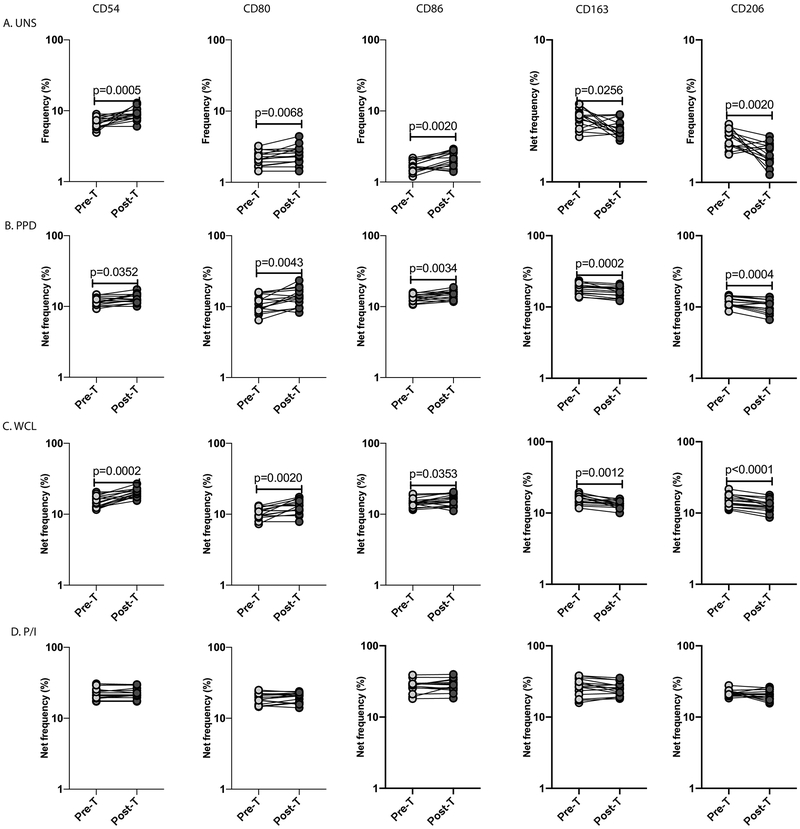
To examine whether the diminished monocyte activation and enhanced M2 polarization in LTBI/Ss co-infection is reversible following anthelmintic therapy, we examined the frequency of monocytes expressing activation markers (CD54, CD80 and CD86) and M2 markers (CD163 and CD206) in LTBI/Ss individuals six months following anthelmintic treatment. As shown in Figure 5A, the baseline frequency of monocytes expressing CD54, CD80 and CD86 was significantly increased, while the frequency of monocytes expressing CD163 and CD206 was significantly decreased in Ss+ individuals six months following anthelmintic treatment (Post-T) compared to the pre-treatment frequency (Pre-T). Similarly, as shown in Figure 5B and C, the PPD and WCL stimulated net frequencies of monocytes expressing CD54, CD80 and CD86 were significantly increased while the net frequencies of monocytes expressing CD163 and CD206 were significantly decreased at Post-T compared to Pre-T. Finally, as shown in Figure 5D, no significant changes in the net frequency of monocytes expressing the above markers was observed upon P/I stimulation at Post-T. Thus, anthelmintic therapy significantly reverses the diminished monocyte activation and enhanced M2 polarization in LTBI/Ss coinfection.
Figure.5. Anthelmintic therapy significantly increases monocyte activation and decreases M2 polarization in LTBI/Ss coinfection.
Baseline and antigen-stimulated frequencies of monocytes expressing activation and M2 markers were determined by flow cytometry in Ss+ (light grey n =15) individuals before and after treatment with a standard dose of ivermectin and albendazole. The (A) baseline (unstimulated) as well as (B) PPD Ag, (C) WCL and (D) P/I stimulated frequencies of monocyte expressing activation (CD54, CD80, CD86) and M2 (CCR2, CX3CR1) markers are shown. Each line represents a single individual. Net frequencies were calculated by subtracting baseline frequencies from the antigen-induced frequencies for each individual. The p values were calculated using the Wilcoxon signed rank test.
Anthelmintic therapy reverses the diminished phagocytosis or respiratory burst activity in monocytes in LTBI/Ss coinfection
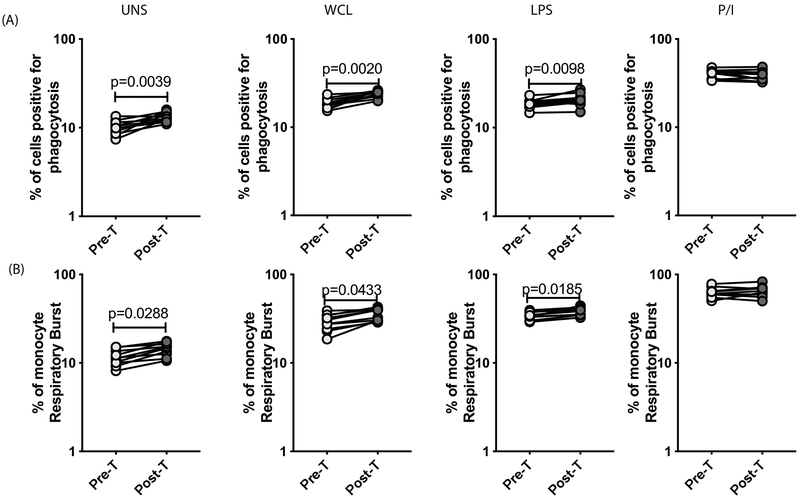
To examine whether the diminished monocyte function in LTBI/Ss coinfection is reversible following anthelmintic therapy, we examined the frequency of monocytes undergoing phagocytosis or respiratory burst activity in LTBI/Ss individuals six months following anthelmintic treatment. As shown in Figure 6A, the baseline as well as WCL or LPS but not P/I stimulated frequencies of monocytes undergoing phagocytosis were significantly increased in Ss+ individuals six months following anthelmintic treatment (Post-T) compared to the pre-treatment frequency (Pre-T). Similarly, as shown in Figure 6B, the baseline as well as WCL or LPS but not P/I stimulated frequencies of monocytes undergoing respiratory burst activity were significantly increased at Post-T compared to Pre-T. Thus, anthelmintic therapy significantly reverses the diminished monocyte phagocytosis and respiratory burst function in LTBI/Ss coinfection.
Figure.6. Anthelmintic therapy reverses the diminished phagocytosis or respiratory burst activity in monocytes in LTBI/Ss coinfection.
Baseline and antigen stimulated frequencies of monocytes were assessed for phagocytic activity and respiratory burst activity by flow cytometry in Ss+ (light grey n =10) individuals before and after treatment with a standard dose of ivermectin and albendazole (A) PBMC were stimulated at baseline (unstimulated), WCL, LPS and P/I for 6 h and the frequencies of monocytes undergoing phagocytosis was measured. (B) PBMC were stimulated at baseline (unstimulated), WCL, LPS and P/I for 6 h and the frequencies of monocytes undergoing respiratory burst activity was measured. Each line represents a single individual. Net frequencies were calculated by subtracting baseline frequencies from the antigen-induced frequencies for each individual. The p values were calculated using the Wilcoxon signed rank test.
Elevated levels of monocytes producing cytokines in LTBI/Ss co-infection following anthelmintic therapy
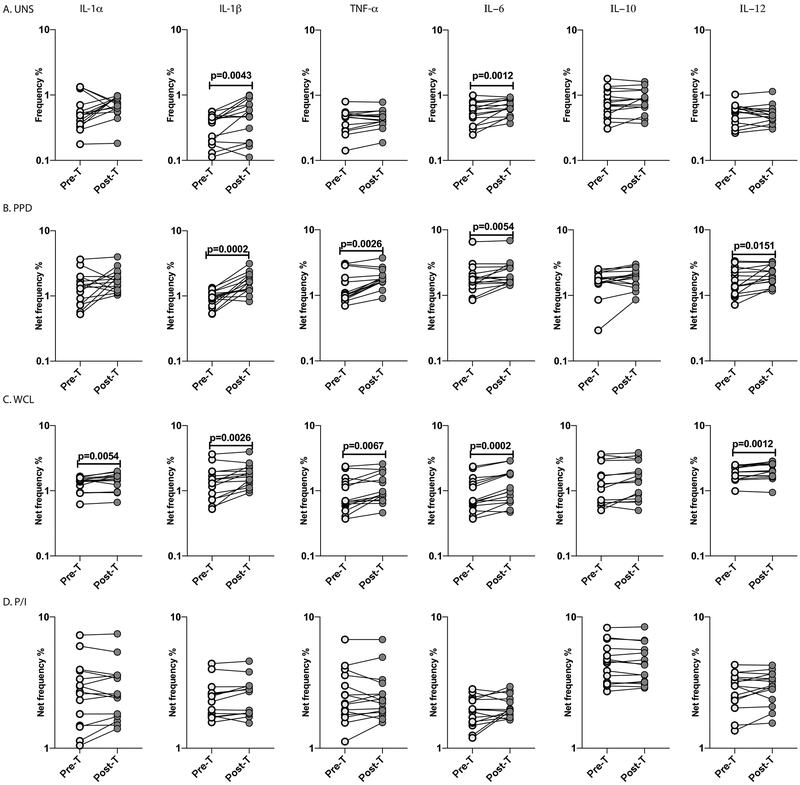
To examine whether the diminished monocyte expression of cytokines in LTBI/Ss co-infection is reversible following anthelmintic therapy, we examined the frequency of monocytes expressing cytokines in LTBI/Ss individuals six months following anthelmintic treatment. As shown in Figure 7A, the baseline frequency of monocytes expressing IL-1β and IL-6 was significantly increased in Ss+ individuals six months following anthelmintic treatment (Post-T) compared to the pre-treatment frequency (Pre-T). Similarly, as shown in Figure 7B and C, the PPD and WCL stimulated net frequencies of monocytes expressing IL1α (except PPD stimulation), IL-1β, TNFα, IL-6 and IL-12 were significantly increased at Post-T compared to Pre-T. Finally, as shown in Figure 7D, no significant changes in the net frequency of monocytes expressing the above cytokines was observed upon P/I stimulation at Post-T. Thus, anthelmintic therapy significantly reverses the diminished monocyte production of cytokines in LTBI/Ss coinfection.
Figure.7. Elevated levels of monocytes producing cytokines in LTBI/Ss co-infection following anthelmintic therapy.
Baseline and antigen-stimulated frequencies of monocytes expressing cytokines were determined by flow cytometry in Ss+ (light grey n =15) individuals before and after treatment with a standard dose of ivermectin and albendazole. The (A) baseline (unstimulated) as well as (B) PPD, (C) WCL and (D) P/I stimulated frequencies of monocytes expressing cytokines (IL-1α, IL-1β, TNF-α, IL-6, IL-10 and IL-12) were measured. Each line represents a single individual. Net frequencies were calculated by subtracting baseline frequencies from the antigen-induced frequencies for each individual. The p values were calculated using the Wilcoxon signed rank test.
Anthelmintic therapy significantly increases pro-inflammatory cytokine production in LTBI/Ss co-infection
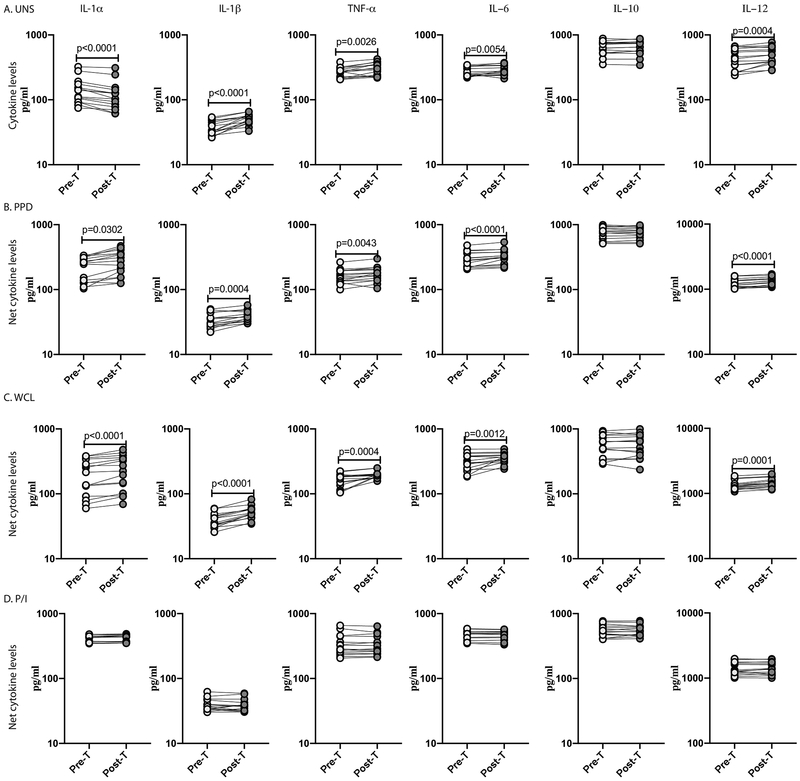
To examine whether the diminished production of cytokines in LTBI/Ss co-infection is reversible following anthelmintic therapy, we examined the levels of cytokines in LTBI/Ss individuals six months following anthelmintic treatment. As shown in Figure 8A, at baseline, IL-1α, IL-1β, TNFα and IL-6 levels were significantly elevated in Ss+ individuals six months following anthelmintic treatment (Post-T) compared to the pre-treatment frequency (Pre-T). Similarly, as shown in Figure 8B and C, the PPD and WCL stimulated net cytokine levels of IL1α, IL-1β, TNFα, IL-6 and IL-12 were significantly elevated at Post-T compared to Pre-T. Finally, as shown in Figure 8D, no significant changes in the net cytokine levels of the above cytokines was observed upon P/I stimulation at Post-T. Thus, anthelmintic therapy significantly reverses the diminished production of cytokines in LTBI/Ss coinfection.
Figure.8. Anthelmintic therapy significantly increases pro-inflammatory cytokine production in LTBI/Ss co-infection.
Baseline and antigen-stimulated levels of pro-inflammatory cytokines were determined by ELISA using PBMC culture supernatants of Ss+ (light grey n =15) individuals before and after treatment with a standard dose of ivermectin and albendazole. The (A) baseline (unstimulated) as well as (B) PPD, (C) WCL and (D) P/I stimulated levels of cytokines (IL-1α, IL-1β, TNF-α, IL-6, IL-10 and IL-12) were measured. Each line represents a single individual. Net cytokine levels were calculated by subtracting baseline cytokine levels from the antigen-induced cytokine levels for each individual. The p values were calculated using the Wilcoxon signed rank test.
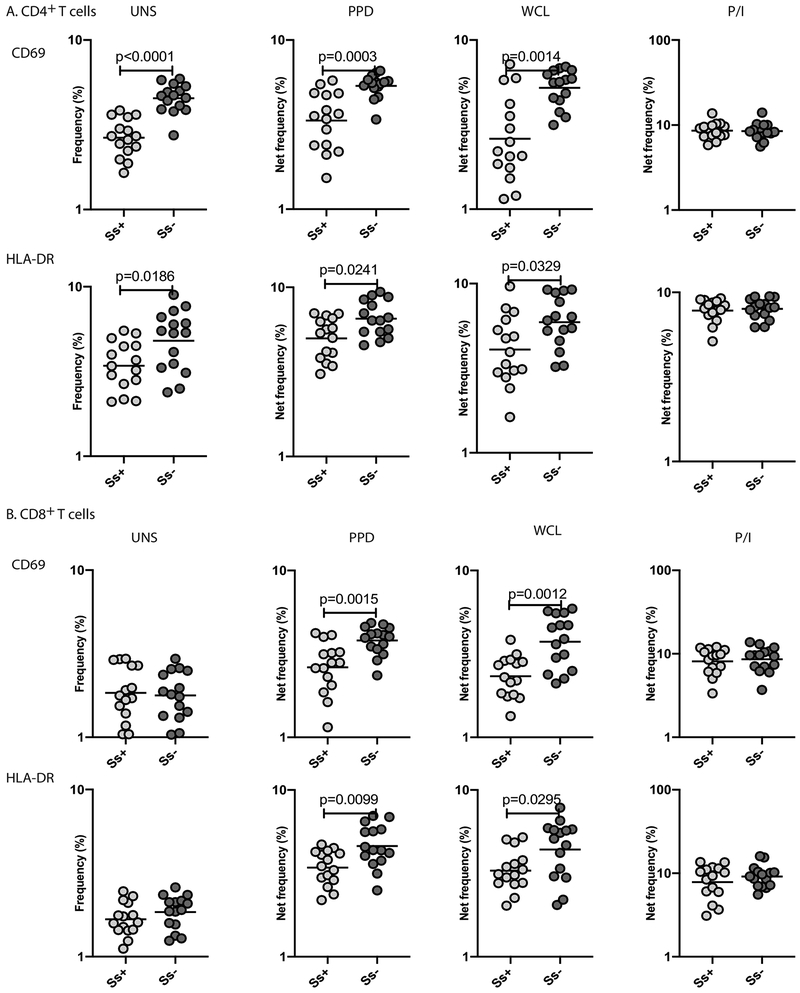
LTBI/Ss coinfection is associated with decreased T cell activation
To understand the implications of the Ss−induced monocyte dysfunction in LTBI/Ss coinfection, we measured the frequency of activated CD4+ and CD8+ T cells expressing CD69 and HLA-DR following stimulation with M.tb antigens. As shown in Figure 9A, we demonstrate that in the absence of stimulation, or following stimulation with PPD and WCL, the frequencies of CD4+ T cells expressing CD69 and HLA-DR were significantly lower in Ss+ compared to Ss−individuals. As shown in Figure 9B, we also demonstrate that PPD- and WCL-stimulated but not baseline or P/I-stimulated frequency of activated CD8+ T cells were significantly lower in Ss+ compared to Ss−individuals.
Figure.9. LTBI/Ss coinfection is associated with decreased T cell activation.
Baseline and antigen-stimulated frequencies of T cell activation markers were determined by flow cytometry in Ss+ (light grey n =15) and Ss− (dark grey n =15) individuals. (A) Baseline (unstimulated), PPD Ag, WCL and P/I stimulated frequencies of T cell activation markers (CD69 and HLA-DR) expressed by CD4+ T cells are shown. (B) Baseline (unstimulated), PPD Ag, WCL and P/I stimulated frequencies of T cell activation markers (CD69 and HLA-DR) expressed by CD8+ T cells are shown. Each circle represents a single individual and the bars represent the GM values. Net frequencies were calculated by subtracting baseline frequencies from the antigen-induced frequencies for each individual. The p values were calculated using the Mann–Whitney U test.
Discussion
Monocytes/macrophages are central players in immunity to helminth infections (22). Helminth interactions with macrophages typically induces a population of cells preferentially expressing arginine instead of nitric oxide and these macrophages are termed as alternatively activated macrophages (23). Certain helminth infections can also elicit an alternative activation phenotype in monocytes (24). Monocyte dysfunction in helminth infections is associated with decreased capacity to express costimulatory molecules and chemokine receptors, decreased functional activity including phagocytosis and decreased production of pro-inflammatory or protective cytokines (25-27). Whether any or all of these mechanisms play a role in helminth- latent TB interaction is not known. Our study explores the role of four different aspects of monocyte activation and function in helminth-TB interactions. First, our study examines monocyte activation in terms of the ability to express activation markers in coinfected individuals. Second, our study examines the M2 polarization potential of monocytes in coinfected individuals. Third, our study examines the function of monocytes in terms of phagocytosis and respiratory burst activity. Finally, our study examines the capacity of monocytes to produce protective cytokines in the context of coinfection. Our study innovates by not only examining each of these parameters at baseline and following M.tb-antigen stimulation but also by re-examining the same responses under the same conditions following anthelmintic treatment.
CD80 and CD86 are homologous costimulatory molecules expressed predominantly on antigen – presenting cells, including monocytes and interaction of these molecules with CD28 and CTLA-4 on T cells is a critical step in T cell activation (28). Previous work has shown that both CD80 and CD86 are equally important in host immunity to TB (29, 30). Additionally, decreased expression of CD80/CD86 is associated with impaired T cell activation in TB (29). Therefore, our data on reduced frequencies of monocytes expressing both CD80 and CD86 both at baseline and following M.tb-antigens stimulation implies a major negative effect on the activation of T cells in response to M.tb-antigens. CD54 or ICAM-1 is an adhesion molecule that is critical for the arrest and transmigration of leukocytes out of blood vessels and into tissues (31). The LFA-1/CD54 pathway is important for T cell proliferation (32). CD54 functions as an activation marker on monocytes and its expression is upregulated upon TB infection (33). Thus, diminished expression of these receptors either at baseline or following M.tb-antigen stimulation also implies a defect in the host innate immunity to TB in coinfected individuals. Indeed, we and others have previously reported compromised T cell activation and function in helminth – latent TB coinfection (11).
M2 polarization of monocytes/macrophages is associated with diminished resistance to mycobacterial infection (34-36). Since helminth infections are the predominant inducers of alternative activation (22), we examined and demonstrated that the expression of markers associated M2 polarization is significantly elevated in coinfected individuals. Interestingly, increased polarization is evident both at baseline and in response to M.tb antigens, indicating a trained immunity type of an immune response in coinfected individuals (37). It is also highly probable that this increased frequency of M2 monocytes potentially underlies the altered functional responses of monocytes to M.tb antigens. We have not addressed the mechanism underlying the shift in monocyte activation in Ss infection and this will be examined in future studies.
Phagocytosis and respiratory burst activity are important components of host innate immunity to M.tb (38-40). Processes involved in phagocytosis include binding of the bacterium to the host cell, internalization, and finally growth inhibition or killing (39, 40). After pathogenic bacteria are engulfed into phagosomes, they are subject to killing via a variety of mechanisms, including phagosome-lysosome fusion, generation of reactive oxygen intermediates, and generation of reactive nitrogen intermediates, particularly nitric oxide (41). Understanding of these initial monocyte defenses may lead to important insights into the development of clinically active disease, as evasion of these monocyte defense mechanisms is likely a key step in promoting the active disease process (38). Indeed, our data clearly show that monocyte defense mechanisms are compromised in the setting of helminth-coinfection. In particular, both baseline and M.tb -antigen (and LPS) induced monocyte phagocytosis and respiratory burst activity (reflecting generation of superoxide and other radicals) is significantly reduced in coinfected individuals. This suggests that the ability of monocytes to engulf and kill M.tb might be inefficient in the presence of coinfection. Indeed, our lab and others have shown that helminth infection is associated with higher bacterial burden in active TB (42, 43).
Monocyte production of IL-1α, IL-1β, TNFα, IL-6 and IL-12 also plays an important role in host defense against M.tb (38, 44, 45). While IL-1α and IL-1β play a protective role in resistance, TNFα contributes to host immunity by both promoting killing of M.tb and formation/maintenance of granulomas (46). IL-6 is an important for recruitment of immune cells and IL-12 is the key cytokine in the induction of Th1 responses, which are critical against M.tb (46, 47). Our study demonstrates a deficiency in the production of these cytokines in the presence of coinfection, suggesting that one major mechanism by which helminth infection modulate immunity to TB is by impairment of monocyte cytokine production. In contrast, monocyte production of IL-10 is enhanced in coinfection and since IL-10 is detrimental to host defense against M.tb (48), indicates another pathway for compromising host immunity. Our data on the ELISA measurements of culture supernatants either unstimulated or TB-antigen stimulated additionally verifies the alteration in the cytokine production in Ss infections and its reversibility following chemotherapy. Finally, our data also reveal that the impairment of monocyte activation and function appears to be associated with diminished T cell activation, suggesting that helminth infection impairs both the innate and adaptive arms of the immune system in the context of LTBI. This data is similar to our previous study demonstrating parasite – antigen specific down modulation of Th1 and Th17 responses in chronic Ss infection (49).
Our study also reveals an important facet of compromised immunity engendered by helminth infection in that most of these defects are at least partially reversed upon anthelmintic treatment. Thus, examination of coinfected individuals six months following administration of a single dose of ivermectin and albendazole is sufficient to restore (at least partially) immune homeostasis and the ability of monocytes to respond optimally to TB antigenic stimuli. This is an important finding since it suggests that prior administration of anthelmintic treatment, especially in endemic areas, would be a prudent strategy to restore immune responsiveness before the conduct of TB vaccine trials or host directed therapies. In summary, our study identifies yet another mechanism of hypo-responsiveness or immune dysfunction in host immunity to helminth-TB coinfection and this time reveals a compromise in the immune response of monocytes as a major component of this deficit.
Supplementary Material
Key points.
Helminth infections alter monocyte activation and function in latent TB
Helminth infections also alter T cell activation in latent TB
These alterations are for the most part reversed by anthelmintic chemotherapy
Acknowledgments:
We thank Dr. Satiswaran and Prabbu Balakrishnan for valuable assistance in collecting the clinical data for this study. We thank the staff of the Department of Epidemiology, NIRT for valuable assistance in recruiting the individuals for this study.
Grant Support: This work was supported by the Division of Intramural Research, NIAID, NIH. This project was also funded in part by Department of Science & Technology, Science and Engineering Research Board (SERB), Government of India, National Post-Doctoral Fellowship.
Abbreviations:
- LTBI
Latent tuberculosis infection
- M.tb
Mycobacterium tuberculosis
- PPD
Purified protein derivative
- P/I
PMA/Ionomycin
- Ss
Strongyloides stercoralis
- TB
tuberculosis
- WCL
whole cell lysate
REFERENCES
- 1.Scriba TJ, Coussens AK, and Fletcher HA. 2017. Human Immunology of Tuberculosis. Microbiol Spectr 5. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava S, Ernst JD, and Desvignes L. 2014. Beyond macrophages: the diversity of mononuclear cells in tuberculosis. Immunol Rev 262: 179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norris BA, and Ernst JD. 2018. Mononuclear cell dynamics in M. tuberculosis infection provide opportunities for therapeutic intervention. PLoS Pathog 14: e1007154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava S, and Ernst JD. 2014. Cell-to-cell transfer of M. tuberculosis antigens optimizes CD4 T cell priming. Cell Host Microbe 15: 741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samstein M, Schreiber HA, Leiner IM, Susac B, Glickman MS, and Pamer EG. 2013. Essential yet limited role for CCR2(+) inflammatory monocytes during Mycobacterium tuberculosis-specific T cell priming. Elife 2: e01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray PJ 2017. Macrophage Polarization. Annu Rev Physiol 79: 541–566. [DOI] [PubMed] [Google Scholar]
- 7.Sampath P, Moideen K, Ranganathan UD, and Bethunaickan R. 2018. Monocyte Subsets: Phenotypes and Function in Tuberculosis Infection. Front Immunol 9: 1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perry S, Hussain R, and Parsonnet J. 2011. The impact of mucosal infections on acquisition and progression of tuberculosis. Mucosal Immunol 4: 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Resende Co T, Hirsch CS, Toossi Z, Dietze R, and Ribeiro-Rodrigues R. 2007. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exp Immunol 147: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taghipour A, Mosadegh M, Kheirollahzadeh F, Olfatifar M, Safari H, Nasiri MJ, Fathi A, Badri M, Piri Dogaheh H, and Azimi T. 2019. Are intestinal helminths playing a positive role in tuberculosis risk? A systematic review and meta-analysis. PLoS One 14: e0223722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babu S, and Nutman TB. 2016. Helminth-Tuberculosis Co-infection: An Immunologic Perspective. Trends Immunol 37: 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang R, and Schick J. 2017. Review: Impact of Helminth Infection on Antimycobacterial Immunity-A Focus on the Macrophage. Front Immunol 8: 1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monin L, Griffiths KL, Lam WY, Gopal R, Kang DD, Ahmed M, Rajamanickam A, Cruz-Lagunas A, Zuniga J, Babu S, Kolls JK, Mitreva M, Rosa BA, Ramos-Payan R, Morrison TE, Murray PJ, Rangel-Moreno J, Pearce EJ, and Khader SA. 2015. Helminth-induced arginase-1 exacerbates lung inflammation and disease severity in tuberculosis. J Clin Invest 125: 4699–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiNardo AR, Mace EM, Lesteberg K, Cirillo JD, Mandalakas AM, Graviss EA, Orange JS, and Makedonas G. 2016. Schistosome Soluble Egg Antigen Decreases Mycobacterium tuberculosis-Specific CD4+ T-Cell Effector Function With Concomitant Arrest of Macrophage Phago-Lysosome Maturation. J Infect Dis 214: 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babu S, Bhat SQ, Kumar NP, Anuradha R, Kumaran P, Gopi PG, Kolappan C, Kumaraswami V, and Nutman TB. 2009. Attenuation of toll-like receptor expression and function in latent tuberculosis by coexistent filarial infection with restoration following antifilarial chemotherapy. PLoS Negl Trop Dis 3: e489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aira N, Andersson AM, Singh SK, McKay DM, and Blomgran R. 2017. Species dependent impact of helminth-derived antigens on human macrophages infected with Mycobacterium tuberculosis: Direct effect on the innate anti-mycobacterial response. PLoS Negl Trop Dis 11: e0005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radhakrishna S, Frieden TR, and Subramani R. 2003. Association of initial tuberculin sensitivity, age and sex with the incidence of tuberculosis in south India: a 15-year follow-up. Int J Tuberc Lung Dis 7: 1083–1091. [PubMed] [Google Scholar]
- 18.Bisoffi Z, Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, Albonico M, Gobbo M, Bonafini S, Angheben A, Requena-Mendez A, Munoz J, and Nutman TB. 2014. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 8: e2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, Albonico M, Degani M, Tais S, Angheben A, Requena-Mendez A, Munoz J, Nutman TB, and Bisoffi Z. 2015. Accuracy of five serologic tests for the follow up of Strongyloides stercoralis infection. PLoS Negl Trop Dis 9: e0003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anuradha R, Munisankar S, Bhootra Y, Jagannathan J, Dolla C, Kumaran P, Shen K, Nutman TB, and Babu S. 2016. Systemic Cytokine Profiles in Strongyloides stercoralis Infection and Alterations following Treatment. Infect Immun 84: 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babu S, and Nutman TB. 2003. Proinflammatory cytokines dominate the early immune response to filarial parasites. J Immunol 171: 6723–6732. [DOI] [PubMed] [Google Scholar]
- 22.Allen JE, and Maizels RM. 2011. Diversity and dialogue in immunity to helminths. Nat Rev Immunol 11: 375–388. [DOI] [PubMed] [Google Scholar]
- 23.Maizels RM, and Yazdanbakhsh M. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol 3: 733–744. [DOI] [PubMed] [Google Scholar]
- 24.Babu S, Kumaraswami V, and Nutman TB. 2009. Alternatively Activated and Immunoregulatory Monocytes in Human Filarial Infections. J Infect Dis 199: 1827–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semnani RT 2013. The interaction between filarial parasites and human monocyte/macrophage populations. Adv Exp Med Biol 785: 49–56. [DOI] [PubMed] [Google Scholar]
- 26.Maizels RM, and Hewitson JP. 2016. Myeloid Cell Phenotypes in Susceptibility and Resistance to Helminth Parasite Infections. Microbiol Spectr 4. [DOI] [PubMed] [Google Scholar]
- 27.Talaat KR, Bonawitz RE, Domenech P, and Nutman TB. 2006. Preexposure to live Brugia malayi microfilariae alters the innate response of human dendritic cells to Mycobacterium tuberculosis. J Infect Dis 193: 196–204. [DOI] [PubMed] [Google Scholar]
- 28.Greenwald RJ, Freeman GJ, and Sharpe AH. 2005. The B7 family revisited. Annu Rev Immunol 23: 515–548. [DOI] [PubMed] [Google Scholar]
- 29.Bhatt K, Uzelac A, Mathur S, McBride A, Potian J, and Salgame P. 2009. B7 costimulation is critical for host control of chronic Mycobacterium tuberculosis infection. J Immunol 182: 3793–3800. [DOI] [PubMed] [Google Scholar]
- 30.Bhatt K, Kim A, Kim A, Mathur S, and Salgame P. 2013. Equivalent functions for B7.1 and B7.2 costimulation in mediating host resistance to Mycobacterium tuberculosis. Cell Immunol 285: 69–75. [DOI] [PubMed] [Google Scholar]
- 31.Pribila JT, Quale AC, Mueller KL, and Shimizu Y. 2004. Integrins and T cell-mediated immunity. Annu Rev Immunol 22: 157–180. [DOI] [PubMed] [Google Scholar]
- 32.Wingren AG, Parra E, Varga M, Kalland T, Sjogren HO, Hedlund G, and Dohlsten M. 2017. T Cell Activation Pathways: B7, LFA-3, and ICAM-1 Shape Unique T Cell Profiles. Crit Rev Immunol 37: 463–481. [DOI] [PubMed] [Google Scholar]
- 33.Lopez Ramirez GM, Rom WN, Ciotoli C, Talbot A, Martiniuk F, Cronstein B, and Reibman J. 1994. Mycobacterium tuberculosis alters expression of adhesion molecules on monocytic cells. Infect Immun 62: 2515–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahnert A, Seiler P, Stein M, Bandermann S, Hahnke K, Mollenkopf H, and Kaufmann SH. 2006. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol 36: 631–647. [DOI] [PubMed] [Google Scholar]
- 35.Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, and Salgame P. 2011. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J Exp Med 208: 1863–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatterjee S, Talaat KR, van Seventer EE, Feng CG, Scott AL, Jedlicka A, Dziedzic A, and Nutman TB. 2017. Mycobacteria induce TPL-2 mediated IL-10 in IL-4-generated alternatively activated macrophages. PLoS One 12: e0179701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O’Neill LA, and Xavier RJ. 2016. Trained immunity: A program of innate immune memory in health and disease. Science 352: aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serbina NV, Jia T, Hohl TM, and Pamer EG. 2008. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 26: 421–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlesinger LS 1996. Role of mononuclear phagocytes in M tuberculosis pathogenesis. J Investig Med 44: 312–323. [PubMed] [Google Scholar]
- 40.Schlesinger LS 1996. Entry of Mycobacterium tuberculosis into mononuclear phagocytes. Curr Top Microbiol Immunol 215: 71–96. [DOI] [PubMed] [Google Scholar]
- 41.Schluger NW, and Rom WN. 1998. The host immune response to tuberculosis. Am J Respir Crit Care Med 157: 679–691. [DOI] [PubMed] [Google Scholar]
- 42.Elias D, Mengistu G, Akuffo H, and Britton S. 2006. Are intestinal helminths risk factors for developing active tuberculosis? Trop Med Int Health 11: 551–558. [DOI] [PubMed] [Google Scholar]
- 43.Kathamuthu GR, Munisankar S, Sridhar R, Baskaran D, and Babu S. 2019. Helminth mediated modulation of the systemic and mycobacterial antigen - stimulated cytokine profiles in extra-pulmonary tuberculosis. PLoS Negl Trop Dis 13: e0007265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ernst JD 2012. The immunological life cycle of tuberculosis. Nat Rev Immunol 12: 581–591. [DOI] [PubMed] [Google Scholar]
- 45.Rajaram MV, Ni B, Dodd CE, and Schlesinger LS. 2014. Macrophage immunoregulatory pathways in tuberculosis. Semin Immunol 26: 471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayer-Barber KD, and Sher A. 2015. Cytokine and lipid mediator networks in tuberculosis. Immunol Rev 264: 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooper AM, and Khader SA. 2008. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev 226: 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, and Berry MP. 2013. The immune response in tuberculosis. Annu Rev Immunol 31: 475–527. [DOI] [PubMed] [Google Scholar]
- 49.Anuradha R, Munisankar S, Dolla C, Kumaran P, Nutman TB, and Babu S. 2015. Parasite Antigen-Specific Regulation of Th1, Th2, and Th17 Responses in Strongyloides stercoralis Infection. J Immunol 195: 2241–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.