Abstract
The intestinal barrier is complex and consists of multiple layers, and it provides a physical and functional barrier to the transport of luminal contents to systemic circulation. While the epithelial cell layer and the outer/inner mucin layer constitute the physical barrier and are often referred to as the intestinal barrier, intestinal alkaline phosphatase (IAP) produced by epithelial cells and antibacterial proteins secreted by Panneth cells represent the functional barrier. While antibacterial proteins play an important role in the host defense against gut microbes, IAP detoxifies bacterial endotoxin lipopolysaccharide (LPS) by catalyzing the dephosphorylation of the active/toxic Lipid A moiety, preventing local inflammation as well as the translocation of active LPS into systemic circulation. The causal relationship between circulating LPS levels and the development of multiple diseases underscores the importance of detailed examination of changes in the “layers” of the intestinal barrier associated with disease development and how this dysfunction can be attenuated by targeted interventions. To develop targeted therapies for improving intestinal barrier function, it is imperative to have a deeper understanding of the intestinal barrier itself, the mechanisms underlying the development of diseases due to barrier dysfunction (eg, high circulating LPS levels), the assessment of intestinal barrier function under diseased conditions, and of how individual layers of the intestinal barrier can be beneficially modulated to potentially attenuate the development of associated diseases. This review summarizes the current knowledge of the composition of the intestinal barrier and its assessment and modulation for the development of potential therapies for barrier dysfunction-associated diseases.
Keywords: layers of intestinal barrier, macrophage activation, chronic inflammation, metabolic diseases, diabetes, intestinal alkaline phosphatase
In addition to being the main organ involved in the uptake of nutrients and water, the intestine also constitutes an essential barrier against harmful/toxic substances from the external environment entering the body mainly in the form of daily diet. Skin may be perceived as the main organ protecting against the exposure to environmental factors but compared to the total surface area of the skin, which is ~2 m2, the area of the important internal membranes exposed to environmental factors is ~100 m2 and ~400 m2 for the lungs and intestines, respectively. Furthermore, the number of microorganisms inhabiting the intestine or the gastrointestinal (GI) tract has been estimated to exceed 1014, which encompasses ∼10 times more bacterial cells than the number of human cells and over 100 times the amount of genomic content (microbiome) as the human genome [1, 2]; a recently revised estimate suggests that the ratio of human:bacterial cells may be closer to 1:1 [3]. Under normal conditions, gut microbiota offer many benefits to the host, such as harvesting energy [4], protecting against pathogens [5], and regulating host immunity [6]. This beneficial relationship is likely to be disrupted as a result of an altered microbial composition, known as dysbiosis, and extensive research efforts are currently directed towards the evaluation of specific changes in bacterial composition during dysbiosis. However, for dysbiosis-dependent changes to influence host metabolism and/or pathology will require direct interaction with, and subsequent modulation of, the intestinal barrier, underscoring the importance of detailed understanding and characterization of the intestinal barrier, per se.
1. What is Intestinal Barrier? The 4 Layers of the Intestinal Barrier
The GI tract is subjected daily to thousands of microorganisms and nutrient components via the ingested diet. Maintenance of gut homeostasis therefore requires a complex system capable of performing several functions, such as the detoxification of bacteria-derived endotoxins, limiting direct contact/interaction with bacteria or pathogens, regulating the absorption of nutrients while restricting the transport of toxic substances or bacteria, and mounting an immune response or limiting the growth of pathogenic bacteria. Accordingly, the gut or intestinal barrier has evolved as a functional unit organized in a multilayer system (Fig. 1), providing a physical as well as a functional barrier. The multiple layers of this barrier, starting from intestinal lumen to systemic circulation, include: (1) luminal intestinal alkaline phosphatase (IAP) that dephosphorylates bacterial endotoxin lipopolysaccharide (LPS) to detoxify it; (2) the mucus layer that provides a physical barrier preventing interactions between gut bacteria and intestinal epithelial cells; (3) the tight junctions between the epithelial cells that limit the paracellular transport of bacteria and/or bacterial products to systemic circulation; and (4) the antibacterial proteins secreted by the specialized intestinal epithelial cells or the Paneth cells and IgA secreted by the immune cells present in lamina propria underlying the epithelial cell layer.
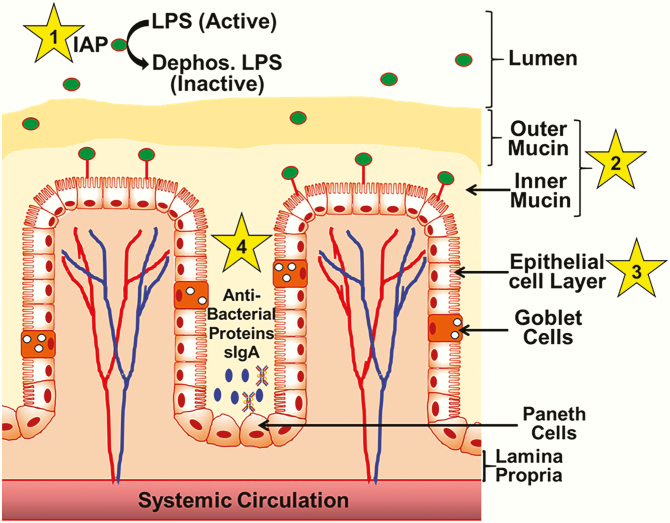
Figure 1.
The “layers” of the intestinal barrier. Functional intestinal barrier consists of four “layers” (shown by numbers 1–4) extending from the lumen that contains gut bacteria and bacterial endotoxin LPS. Layer 1, or intestinal alkaline phosphatase (IAP), released from the intestinal epithelial cells, dephosphorylates luminal LPS inactive, producing dephospho-LPS. Mucin layer (layer 2), consisting of a firmly attached inner layer and a loose outer layer provide the first physical barrier restricting the interaction between luminal bacteria and epithelial cells. A single layer of epithelial cells (layer 3) separates the lumen from systemic circulation. Specialized secretory cells of the epithelial layer, namely goblet and Paneth cells, contribute to the formation of the mucin layer and the production of antibacterial proteins, respectively. Panneth cell-derived antibacterial proteins/peptides, along with secreted IgA (sIgA) from plasma cells present in the lamina propria, restrict bacterial growth and represent the fourth layer (layer 4) of the intestinal barrier.
A. Layer 1, or the Intestinal Alkaline Phosphatase
Intestinal alkaline phosphatase is expressed and secreted by intestinal epithelial cells and remains active within the mucosal layer as well as the intestinal lumen. In addition to regulating bicarbonate secretion and duodenal surface pH and long chain fatty acid absorption, IAP removes phosphate groups from substrates such as bacterial endotoxin LPS and pathogen-associated molecular patterns (eg, flagellin, CpG DNA), and it reduces local intestinal inflammation [7]. In addition, it also dephosphorylates adenosine tri-phosphate (ATP) and adenosine diphosphate (ADP) in the lumen; high levels of luminal ATP inhibits the growth of commensal bacteria and disrupts bacterial homeostasis [8]. While all these functions of IAP are important to maintaining intestinal homeostasis, it is the ability of IAP to inactivate LPS that places IAP as the luminal first line of defense [7]. LPS, a constituent of the cell wall of gram-negative bacteria, is abundant in the GI tract and is responsible for causing systemic inflammation and septic shock. The toxicity of LPS resides in the Lipid-A moiety, which permits it to bind to toll-like receptor-4 (TLR4). Removal of 1 of the 2 phosphate groups on the Lipid-A moiety reduces LPS toxicity 100 fold [9]. Dephospho-LPS still binds to TLR4 but predominantly acts as a TLR4 antagonist [10]. This reduction in the toxicity of LPS inhibits downstream intracellular signaling [11], leading to a reduction in NF-κB activation and the release of proinflammatory cytokines.
B. Layer 2, or the Mucus Layer
In contrast to IAP representing a “functional” barrier, the intestinal mucosal layer (or the second layer of the intestinal barrier) is the first “physical” barrier encountered by bacteria in the GI tract. It is composed of 2 layers: an inner layer that is firmly attached to the epithelial cells and an outer layer that is thicker but much looser and less adherent. The inner mucosal layer does not allow bacteria to penetrate, keeping the surface of epithelial cells free of direct contact with bacteria. In contrast, the outer mucosal layer harbors commensal bacteria that prevent the entry of pathogenic bacteria into the outer as well as inner mucus layer. Consistently, an increase in the number of mucus-residing commensal bacteria by pro- or prebiotic interventions is thought to improve the barrier function of the mucosal layer [12]. These 2 mucosal layers consist of water (~95%) and glycoproteins (1–10%), as well as electrolytes, antibodies, and nucleic acids [13]. Highly glycated protein mucin (MUC2) is the major glycoprotein secreted by specialized epithelial cells called goblet cells. After secretion, MUC2 organizes in a hydrated and expanded network with other secreted proteins, forming an organized mucous layer. The amount and composition of the mucus layer reflects a balance between mucus secretion and its erosion and degradation by bacteria [14]. Depletion of the mucus layer either by increased degradation or deficient synthesis will, therefore, have a profound effect on this layer of the intestinal barrier. Consistently, Muc2-/- mice have increased bacterial adherence to the intestinal epithelium, enhanced susceptibility to colitis, and disrupted intestinal barrier function [15].
C. Layer 3, or the Epithelium
The intestinal epithelium is a single layer of cells that acts as a highly selective barrier preventing the passage of harmful luminal contents such as foreign antigens, microorganisms, and their toxins while allowing the translocation of essential dietary nutrients, electrolytes, and water from the intestinal lumen into the systemic circulation. This selectivity of transport is mediated by the regulation of 2 major mechanisms, namely the transepithelial/transcellular and paracellular transport pathways. In addition, this epithelial layer is also considered nonpermissive for endocytic uptake by any mechanism, largely due to unusually high amounts of glycolipids that are organized in lipid raft microdomains [16] stabilized by divalent galectin-4 [17]. Nutrients such as amino acids [18], electrolytes [19], short chain fatty acids, and sugars [20] are routinely transported via the transcellular pathway through the epithelial cells, and this uptake is predominantly regulated by selective transporters. Transport through the space between epithelial cells, or paracellular transport, is regulated by intercellular complexes localized at the apical-lateral membrane junction and along the lateral membrane [21, 22]. These intercellular complexes include desmosomes, adherens junctions, and tight junctions [23]. Adhesive junctional complexes and desmosomes are important in the mechanical linkage of adjacent cells and consist of transmembrane proteins that link adjacent cells to the actin cytoskeleton via cytoplasmic scaffolding proteins [24]. The tight junctions, on the other hand, are responsible for sealing the intercellular space and thereby regulating paracellular transport [25]. Tight junctions are formed by proteins such as occludins and members of the claudin family that cross the plasma membrane to interact with proteins from the adjoining cell. On the intracellular side of the membrane, the carboxyl terminal ends of these proteins interact with other tight junction proteins such as ZO-1, ZO-2, and ZO-3. Intracellularly, these proteins associate with a ring of actin microfilaments (for a detailed review of the organization of tight junction proteins, see [21, 26]). Consequently, the integrity of the epithelial cell layer and tight junction proteins is often referred to as the “intestinal barrier” despite the important contributions of the other three “layers.”
D. Layer 4, or the Antibacterial Peptides
In addition to providing a single cell layer physical barrier, the specialized secretory cells of the intestinal epithelium or the Paneth cells [27] located at the base of small intestinal crypts, act as important effectors of innate immunity though the secretion of antimicrobial peptides that play an important role in the host defense against gut microbes [28]. Thus, secreted antibacterial peptides constitute the “fourth” layer of the intestinal barrier. The most abundant antimicrobial peptide in the human intestine is α-defensin, a member of the defensing family of peptides. Both α- and β-defensins are bacteriocidal, with activity against Gram-negative as well as Gram-positive bacteria. Numerous studies have suggested that the disruption of genes involved in the expression and secretion of these antimicrobial peptides increases the susceptibility to inflammatory bowel disease, for example, X-box binding protein (Xbp-1) [29], autophagy-related 16-like 1 (Atg16l1) [30], nucleotide binding oligomerization domain-containing 2 (Nod2) [31], and transcription factor 4 (Tcf4) [32]. Consistently, Crohn’s disease patients not only have a reduced number of healthy Paneth cells but also a decreased expression of β-defensins in the areas of acute inflammation [33]. Recent studies have also implicated the important role of amino acids in regulating the expression of antimicrobial peptides [34]. For example, aberrations in tryptophan metabolism causes intestinal inflammation via changes in the expression of antibacterial proteins [35], and a glutamine-mediated increase in the expression of these proteins underlies the reduced gut inflammation observed in animal models [36–38]. Collectively, these studies provide a direct link between disruptions of this layer of intestinal barrier function to disease development.
In addition to the antibacterial proteins secreted by Paneth cells, IgA secreted (sIgA) by plasma immune cells present in lamina propria also plays a significant role in limiting direct interactions between pathogens and the epithelial cell monolayer. The primary mechanism of sIgA-mediated protection is immune exclusion wherein direct sIgA binding to microorganisms or toxins prevents colonization or toxicity/damage to the epithelial cells [39]. Within the lamina propria itself, IgA binds to the various immune complexes and facilitates their removal and attenuates systemic inflammatory responses [40]. Humans secrete an estimated 3 g of sIgA into the intestinal lumen every day, reflecting its important role in protecting the mucosal surface. B-cell deficient mice or mice lacking the immunoglobulin receptor required for sIgA transport to the lumen display enhanced stimulation of innate responses in gut epithelial cells, further demonstrating the role of adaptive immune responses in regulating intestinal inflammation [41].
2. Consequences of Intestinal Barrier Dysfunction: Local and Systemic Inflammation
Intestinal barrier function is critical for normal homeostasis of the gut, and the breakdown or dysfunction of this barrier is associated with local as well as systemic consequences largely related to direct contact of bacteria/bacterial products with the epithelial cells, and translocation of these to the systemic circulation (Fig. 2). Direct contact with bacteria/bacterial products leads to the activation of immune cells via TLR4/MyD88-dependent signaling pathways in the lamina propria by interaction with gut bacteria–derived LPS, resulting in the secretion of proinflammatory mediators that perpetuate local inflammation. Consistently, intraperitoneal administration of the LPS-TLR4 signaling inhibitor TAK-242 attenuates these consequences of intestinal barrier disruption [42]. Local intestinal inflammation underlies the development of a number of gastrointestinal diseases, such as inflammatory bowel disease [43], Crohn’s disease [44], and ulcerative colitis (extensively reviewed recently in [45]).
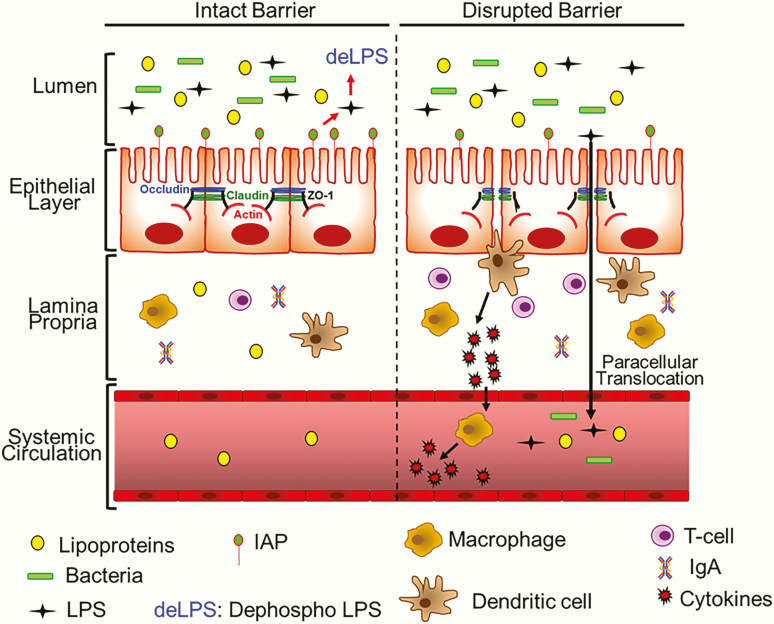
Figure 2.
Disruption of the intestinal barrier function. Under normal conditions with an intact barrier, while intestinal epithelial cells facilitate the transcellular movement of ions and nutrients, paracellular transport of bacteria/bacterial products such as LPS is restricted. Cells within the epithelial layer are sealed by tight junction proteins such as Occludin, Claudin, and ZO-1, preventing paracellular transport. In addition, appropriate/homeostatic expression of intestinal alkaline phosphatase (IAP) continuously dephosphorylates and detoxifies LPS in the luminal space. Lamina propria, below the epithelial layer, contains immune cells, both of the innate immune system (eg, macrophages, dendritic cells) and the adaptive immune system (eg, T-cells and IgA-producing plasma celsl [not shown here]). When the intestinal barrier is disrupted (eg, by a Western diet, pathogenic bacteria, LPS due to inadequate detoxification by reduced IAP levels, etc.), the tight junctions are disordered, allowing for paracellular transport of LPS as well as luminal bacteria. In response to these stimuli, dendritic cells and/or macrophages are activated to produce proinflammatory cytokines that not only enhance further infiltration of immune cells into the lamia propria but also activate macrophages in circulation. Paracellularly transported bacteria and LPS also enter systemic circulation, resulting in increased systemic inflammation.
However, the major consequence of a disruption in the intestinal barrier is the increased paracellular transport of LPS into systemic circulation. In blood, LPS is carried bound to either LPS binding protein (LBP) or lipoproteins and interacts with surface receptors (eg, TLR4) on immune cells initiating an inflammatory response (Fig. 3). TLR4 by itself cannot bind LPS but requires CD14 as a cofactor, which facilitates the transfer of LPS to TLR4 and MD2 that modulates LPS recognition. LPS binding protein shuttles LPS to CD14. The association of these auxiliary molecules triggers the signal resulting in the homodimerization of TLR4 and the consequent intracellular signaling via MyD88 [11, 46]. This cascade then leads to the activation of NF-κB that results in increased transcription of proinflammatory cytokines such as TNFα, IL-1β, and IL-6 [47]. Infiltration of activated macrophages or direct activation of resident macrophages in peripheral tissues by circulating LPS results in tissue inflammation. Further infiltration of immune cells (including neutrophils and monocytes) in response to this proinflammatory milieu perpetuates this inflammation, perturbing tissue homeostasis. For example, in the liver, increased inflammation leads to increased insulin resistance and lipogenesis resulting in fatty liver disease. Increased adipose tissue or skeletal muscle inflammation/insulin resistance underlies the development of diabetes. Increased infiltration of activated macrophages into the artery wall initiates atherogenesis.
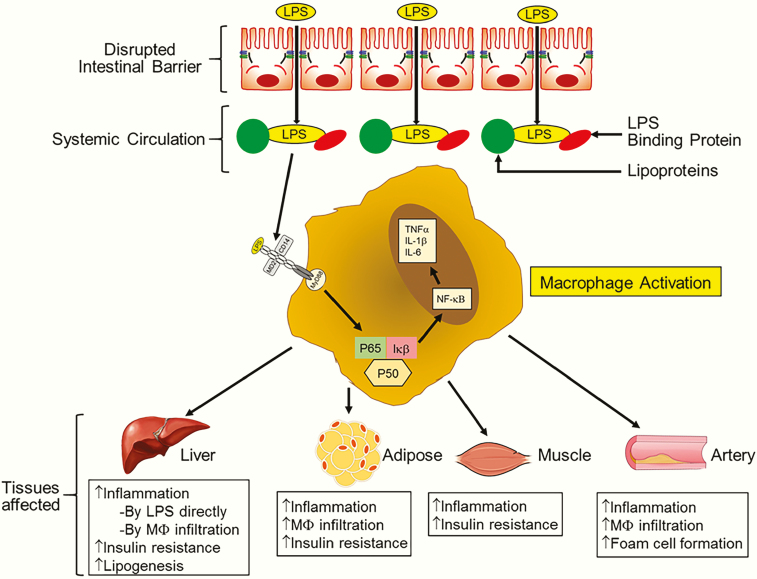
Figure 3.
Consequences of disrupted intestinal barrier. Increased LPS in systemic circulation is causally linked to the development of multiple diseases. LPS associates with circulating lipoproteins and also interacts with LPS binding protein (LBP). LPS acts as a trigger for macrophage activation via LBP-dependent binding to TLR4. In co-ordination with MD2 and CD14, this results in the homodimerization of TLR4 and the initiation of intracellular signaling. Activation and nuclear translocation of proinflammatory transcription factor NF-κB leads to the eventual production of proinflammatory cytokines (eg, TNFα, IL-1β, IL-6), resulting in increased tissue inflammation. In the liver, LPS reaching through portal blood also activates resident macrophages or Kupffer cells, and increased inflammation underlies increased hepatic insulin resistance and lipogenesis. Activated macrophages infiltrate adipose tissue and the resulting inflamed adipose tissue is also insulin resistant and underlies the development of diabetes. Increased inflammation also leads to insulin resistance in skeletal muscles. Increased infiltration of LPS-activated macrophages into the artery wall initiates foam cell formation, resulting in atherosclerotic plaque development.
3. Disease Associated with Intestinal Barrier Dysfunction and LPS Translocation
Fiddian-Green first described the role of enhanced translocation of bacterial toxins or bacteria during cardiac surgery, contributing to morbidity and mortality, and indicated that a reduction in this breach of the intestinal barrier would improve outcomes [48]. Severe endotoxemia is also seen during resuscitation after cardiac arrest, and ischemic injury to the intestine is considered to be the underlying trigger. In recent years the role of exogenous or dietary triggers in initiating barrier dysfunction is increasingly being recognized [49, 50]. High fat, high cholesterol–containing Western-type diets that are responsible for the obesity epidemic and metabolic syndromes have been shown to increase intestinal permeability, resulting in the release of LPS into systemic circulation, leading to metabolic endotoxemia [51–53]. Consistently, a continuous infusion of low-dose LPS to mimic metabolic endotoxemia leads to the development of Type 2 Diabetes Mellitus (T2DM) and atherosclerosis, emphasizing the causal relationship between intestinal barrier dysfunction and the development of metabolic diseases [54]. Based on the observed changes in the intestinal mucosa, including a decreased number of goblet cells, diminished mucus production, reduced levels of secretory IgA, and increased translocation of bacteria/bacterial products to pancreatic lymph nodes, Miranda et al suggests that a disruption of the intestinal barrier function precedes the onset of Type 1 Diabetes Mellitus (T1DM) [55]. Even in humans, obese phenotype is associated with endotoxemia, establishing the critical role of gut-derived LPS in the development of metabolic diseases [56, 57].
In addition to diet-induced metabolic diseases,the presence of LPS in systemic circulation is also identified as a causal or complicating factor in diverse diseases such as autism [58], Alzheimer’s disease [59], Parkinson’s disease [60], arthritis [61], obesity-induced osteoarthritis [62], asthma [63], and several autoimmune diseases [64]. People with multiple sclerosis also have altered biomarkers of intestinal barrier integrity [65]. A chronic inflammatory state [66] as well as endotoxin tolerance leading to a compensatory hypoinflammatory state is thought to underlie the immune-escape of cancer cells, directly linking circulating LPS to the likely development of multiple cancers [67]. High LPS levels in the portal vein are also linked to the development and progression of hepatocellular carcinoma [68]. Pasini et al showed that the improvement in glycemic control in T2DM subjects with chronic exercise was due to attenuation of intestinal barrier dysfunction [69]. Changes in gut permeability are also involved in the pathogenesis of T1DM [70]. These diverse reports establish the causal relationship between gut-derived LPS and the development of diseases, and they underscore the importance of the maintenance of the intestinal barrier function as well as targeted modulation as a novel therapeutic strategy.
4. Potential Therapies for Modulation Intestinal Barrier Function
Although targeted restoration of the intestinal barrier dysfunction seems to be a logical step in the modulation of 1 or more intestinal or systemic diseases, currently no therapies exist for clinical use. However, increased understanding of the multiple “layers” that constitute the overall intestinal barrier is likely to provide novel approaches for targeted modulation. Strategies examined in preclinical or clinical studies are discussed below in the context of the layers of the barrier. It needs to be emphasized that direct manipulation of the gut microbiome as a strategy to subsequently modulate intestinal inflammation is actively pursued and extensively reviewed [71, 72] and is not the focus of this review.
A. Modulation of Layer 1, or IAP
Preclinical studies have established the role of exogenous IAP administration on the improvement of intestinal barrier function and the subsequent attenuation of endotoxemia-mediated diseases. Kallannan et al demonstrated that orally supplemented IAP inhibited the absorption of LPS and prevented, as well as reversed, diet-induced metabolic syndrome in mice [73]. Oral supplementation of IAP was also effective in ameliorating alcohol-induced hepatic steatosis in mice [74] and, based on the IAP-mediated reversal of barrier dysfunction, Hamarneh et al suggested that enteral IAP supplementation may represent a novel approach to maintain the integrity of the intestinal barrier in critically ill patients with decreased IAP in ileal fluid samples [75]. We recently reported the development of intestine-specific IAP transgenic mice over-expressing human chimeric IAP and demonstrated attenuation of a high fat, high cholesterol–containing Western-type diet-induced intestinal barrier dysfunction as well as improved glucose intolerance [76], further establishing the validity of an IAP-based approach for improving barrier function. With concerns related to the partial degradation or inactivation of orally administered IAP during transit through a low gastric pH environment, intraduodenal administration of bovine IAP in 21 severe ulcerative colitis patients showed short-term improvement in disease activity scores and reductions in plasma C-reactive protein and stool calprotectin. More importantly, IAP treatment was well tolerated and was nonimmunogenic [77] in human subjects. Extensive research is underway to identify dietary components with the potential to increase endogenous IAP expression/activity (reviewed in [78]). We identified an increase in IAP as one of the mechanisms by which oral supplementation with curcumin protects against a high fat, high cholesterol–containing Western-type diet-induced barrier dysfunction and the subsequent development of glucose intolerance and atherosclerosis [51]. It is noteworthy that curcumin is not absorbed and yet attenuates several diseases, and its gut-specific action now provides a mechanistic insight [79].
B. Modulation of Layer 2, or the Mucin Layer
In the absence of adequate dietary fiber, colonic bacteria turn to the alternate energy source, the mucin-2 (MUC-2) glycoprotein-rich mucus layer [80], leading to the erosion of the mucin layer and the disruption of the intestinal barrier [81]. Therefore, fiber supplementation of western-type diets is a logical intervention to prevent or reverse the disruption of the mucin layer of the intestinal barrier. Okazaki et al demonstrated an increase in mucin by dietary fiber in rats that were fed a diet containing 30% lard [82]. Similarly, a diet supplemented with homogeneous Dendrobium huoshanense polysaccharide increased the expression of Muc-2 in mice [83]. Increased mucin expression is also noted in experimental animals with other dietary fiber supplementations, such as pea fiber [84], fermented rice bran [85], and fructo-oligosaccharides [86], and it is associated with an improved intestinal barrier and a decreased bacterial translocation, leading to improved disease (eg, glucose intolerance, colitis, or steatohepatitis) status. Studies from our laboratory have demonstrated that the supplementation of a high fat, high cholesterol–containing Western-type diet with galactooligosaccharide fiber increased Muc-2 expression and maintained the continuity of the mucin layer. Despite there being no changes in plasma cholesterol levels, improved barrier function and reduced plasma LPS levels led to improved glucose tolerance and decreased atherosclerosis [87].
C. Modulation of Layer 3, or the Epithelial Cell Layer
Strengthening this layer by reducing paracellular transport will require targeted regulation of intercellular junctional proteins. Increased immune activation of the epithelial cells leads to the increased production of TNFα and IL-13, which increase paracellular transport by interfering with the expression and/or organization of proteins within the tight junctions [88, 89]. Consistently, anti-TNFα antibodies reduce the severity of inflammatory bowel disease in patients with active Crohn’s disease by restoring barrier function in the setting of a dampened immune system [90, 91]. TNFα-induced loss of barrier function is also due to an increase in phosphorylation of myosin light chain (MLC) by MLC kinase, MLCK as demonstrated by in vitro studies using CaCo-2 monolayers [92]. Consistently, the pharmacological inhibition of MLCK improves barrier function and diarrhea in mice [93]. IL-13 induces the disruption of barrier function via upregulation of claudin-2 expression in cultured epithelial cell monolayers [94], and whether targeted inhibition of IL-13 or claudin-2 will be beneficial and without significant effects on gut function is yet to be examined [95]. Several phytochemicals improve intestinal permeability by targeting signaling pathways involved in the inflammation-mediated disruption of tight junction protein organization. For example, in addition to increasing IAP, curcumin also attenuates LPS or the IL-1β-induced disruption of tight junctions [96]. Berberine reduces systemic LPS levels and also antagonizes the effects of LPS-mediated signaling through the Wnt/beta-catenin pathway to affect intestinal permeability in a rat model of sepsis [97]. The hypoglycemic effects of berberine in Type 2 diabetic rats are related to the improvement in gut-derived hormones as well as the attenuation of intestinal barrier dysfunction [98]. The stress hormone, cortisol, decreases the expression of tight junction proteins by reducing the binding of the glucocorticoid receptor (GR) to the occludin promoter region and increases paracellular permeability. These effects are blocked by lubiprostone, both in rodents and in humans, indicating that lubriptostone prevents stress-induced visceral hyperalgesia by changes in intestinal barrier function [99]. In addition, lubiprostone supplementation also reduces atherosclerosis progression in ApoE-/- mice [100]. Recently, Xu et al demonstrated the amelioration of barrier dysfunction in vitro by a novel GR agonist, 16α-hydroxytrametenolic acid (from edible mushrooms), through a GR-mediated PI3K/Akt/NF-κB signaling pathway [101]. Hyperglycemia increases intestinal permeability via GLUT2-dependent transcriptional reprogramming of the intestinal cells, leading to the reduced expression of tight junction proteins, and in humans the systemic influx of intestinal bacteria-derived products correlates with individualized glycemic control [102]. Consistently, the glucose lowering drug metformin protects against intestinal barrier dysfunction by the inhibition of JNK activation via an AMPKα1-dependent signaling pathway as demonstrated by using in vitro cell culture systems as well as colitis mouse models [103].
Extensive research has also established the role of nutritional factors in improving overall intestinal permeability. These nutrients include antioxidants (Quercetin [104], Ginkgo biloba extract [105], N-acetyl cysteine [106]) as well as probiotics and prebiotics [107, 108]. Increasing knowledge of the specific role(s) of the various layers of the intestinal barrier is likely to provide opportunities for targeted improvement as needed to improve the efficacy of specific treatments. The assessment of intestinal barrier function and markers of disruption of one or more layers will be critical for the future development of these targeted strategies.
5. Assessment of Intestinal Barrier Dysfunction
Direct assessment of intestinal barrier function is challenging given the invasiveness of intestinal tissue sampling. However, a number of methods are available to indirectly assess intestinal barrier function, and it is noteworthy that all methods have their own advantages and disadvantages. While some methods directly assess the permeability function in vivo, others rely on the measurement of specific markers in blood/plasma or feces.
A. In Vivo Intestinal Permeability Assays: Determination of Urinary Lactulose/Mannitol Ratios
After the collection of a baseline or pretest urine sample, a sugar solution containing a mixture of lactulose and mannitol is orally administered. The larger size molecule (lactulose) can only cross the intestinal barrier by paracellular passage when the intestinal barrier is compromised. The smaller molecule (mannitol) crosses the epithelial barrier transcellularly and acts as a control for gastric emptying and dilution, transit time, and epithelial absorptive area, as well as systemic distribution and renal function. The urinary excretion ratio (lactulose:mannitol) is then used as a standardized assessment of intestinal permeability [109]. While the noninvasiveness of this method is certainly an advantage, the complicated and elaborate methods for the analyses of excreted products makes it difficult to adapt to the routine measurements needed for clinical evaluation.
B. Circulating Biomarkers
Zonulin, a prehaptoglobin, is one of the physiological modulators that alters intestinal permeability by modifying protein-protein interactions within the tight junctions. Disruption of the intestinal barrier is associated with an increase in circulating as well as fecal zonulin [110]. Caviglia et al demonstrated the suitability of serum zonulin levels in the evaluation of intestinal permeability in patients with inflammatory bowel disease [111]. Intestinal fatty acid binding protein (I-FABP) is a small 14 kDa cytosolic protein specific to mature enterocytes. Its appearance in circulation indicates a breakdown of the enterocyte membrane [112]. Diamine oxidase (DAO) is an intracellular enzyme present in the intestinal villi and is considered as a marker for the integrity of the intestinal epithelium. Circulating levels of DAO increase with damage and loss of intestinal barrier function [113]. Due to increased paracellular transport of LPS and other proinflammatory mediators, plasma levels of LPS, LBP, IL-6, and IL-8 are also considered as markers of increased barrier dysfunction [114–116].
C. Fecal Biomarkers
As stated above, increased levels of zonulin in the feces indicate a disruption of the intestinal barrier. Secretory immunoglobulin A (sIgA) is the main antibody found in the mucus membranes that are exposed to the environment, such as the nose, lungs, and the intestinal lining. Increase in fecal sIgA indicates intestinal inflammation and serves as a marker of barrier dysfunction. Increased neutrophil/eosinophil infiltration into the intestine is associated with damage to the gut lining. Therefore, calprotectin (produced by neutrophils) and eosinophil-derived neurotoxin are yet additional fecal markers of barrier disruption [117].
Although not on the list of routine clinical tests, plasma or fecal markers of intestinal barrier function and/or inflammation are increasingly being utilized and, with more evidence of the causal relationship between intestinal barrier function and the development of multiple diseases, the use of these noninvasive tests is expected to increase. Linking the appearance of these or other novel markers to individual layers of the barrier would be the next challenge in developing targeted therapies.
6. Summary and Conclusions
The causal relationship between chronic inflammation and the development of multiple diseases is increasingly being established, and the likely trigger for this low level, yet sustained systemic inflammation, is bacterial endotoxin LPS. While dietary components or intestinal injury (eg, by ischemia/reperfusion) may initiate the release of LPS from intestinal lumen to systemic circulation, the physical as well as the functional integrity of the intestinal barrier is the critical determinant of this translocation. This review summarizes the composition of the intestinal barrier, consequences of a dysfunctional barrier, and elaborates on the role of translocated LPS in the development of diseases. In addition, current knowledge on the successful manipulation of individual “layers” of the intestinal barrier is summarized. Discussion of the currently available tests emphasize the future use of the status of barrier function as a diagnostic parameter facilitating demonstration of causal relationship with pathogenesis of disease. The development of novel and more specific tests are expected to facilitate the identification of individual “layers” affected in a given disease process or person, leading to targeted interventions. For example, exogenous IAP supplementation or curcumin-mediated increase in IAP activity would be the therapeutic option where loss of IAP is identified. Galactooligosaccharide fiber supplementation would be the preferred option where mucosal layer disruption is evident. Phytochemicals and other nutritional supplements (eg, vitamin D) with demonstrated effects on improvement of tight junction protein expression/function are likely to be most beneficial where these disruptions are noted. Therefore, while correcting intestinal barrier dysfunction for modulation of multiple diseases can be envisioned as a viable therapeutic option, continuing progress in identifying the precise defect by use of specific biomarkers would facilitate targeted interventions.
Acknowledgments
Financial Support: Innovative Basic Science Award from the American Diabetes Association 1-16-IBS-105 to SG.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
References and Notes
- 1. Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. [DOI] [PubMed] [Google Scholar]
- 2. Gill SR, Pop M, Deboy RT, et al. . Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. Plos Biol. 2016;14(8):e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bäumler AJ, Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016;535(7610):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352(6285):539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lallès JP. Intestinal alkaline phosphatase: novel functions and protective effects. Nutr Rev. 2014;72(2):82–94. [DOI] [PubMed] [Google Scholar]
- 8. Malo MS, Moaven O, Muhammad N, et al. . Intestinal alkaline phosphatase promotes gut bacterial growth by reducing the concentration of luminal nucleotide triphosphates. Am J Physiol Gastrointest Liver Physiol. 2014;306(10):G826–G838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schromm AB, Brandenburg K, Loppnow H, et al. . The charge of endotoxin molecules influences their conformation and IL-6-inducing capacity. J Immunol. 1998;161(10):5464–5471. [PubMed] [Google Scholar]
- 10. Bentala H, Verweij WR, Huizinga-Van der Vlag A, van Loenen-Weemaes AM, Meijer DK, Poelstra K. Removal of phosphate from lipid A as a strategy to detoxify lipopolysaccharide. Shock. 2002;18(6):561–566. [DOI] [PubMed] [Google Scholar]
- 11. Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151. [DOI] [PubMed] [Google Scholar]
- 12. Everard A, Belzer C, Geurts L, et al. . Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Macfarlane S, Woodmansey EJ, Macfarlane GT. Colonization of mucin by human intestinal bacteria and establishment of biofilm communities in a two-stage continuous culture system. Appl Environ Microbiol. 2005;71(11):7483–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001;73(6):1131S–1141S. [DOI] [PubMed] [Google Scholar]
- 15. Kissoon-Singh V, Moreau F, Trusevych E, Chadee K. Entamoeba histolytica exacerbates epithelial tight junction permeability and proinflammatory responses in Muc2(-/-) mice. Am J Pathol. 2013;182(3):852–865. [DOI] [PubMed] [Google Scholar]
- 16. Danielsen EM, Hansen GH. Lipid raft organization and function in brush borders of epithelial cells. Mol Membr Biol. 2006;23(1):71–79. [DOI] [PubMed] [Google Scholar]
- 17. Hansen GH, Immerdal L, Thorsen E, et al. . Lipid rafts exist as stable cholesterol-independent microdomains in the brush border membrane of enterocytes. J Biol Chem. 2001;276(34):32338–32344. [DOI] [PubMed] [Google Scholar]
- 18. Bröer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008;88(1):249–286. [DOI] [PubMed] [Google Scholar]
- 19. Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82(1):245–289. [DOI] [PubMed] [Google Scholar]
- 20. Ferraris RP, Diamond J. Regulation of intestinal sugar transport. Physiol Rev. 1997;77(1):257–302. [DOI] [PubMed] [Google Scholar]
- 21. Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2(4):285–293. [DOI] [PubMed] [Google Scholar]
- 22. Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. [DOI] [PubMed] [Google Scholar]
- 23. Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778(3):660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992;262(6 Pt 1):L647–L661. [DOI] [PubMed] [Google Scholar]
- 26. Anderson JM. Molecular structure of tight junctions and their role in epithelial transport. News Physiol Sci. 2001;16:126–130. [DOI] [PubMed] [Google Scholar]
- 27. Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59(1):156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. [DOI] [PubMed] [Google Scholar]
- 29. Kaser A, Lee AH, Franke A, et al. . XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134(5):743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cadwell K, Liu JY, Brown SL, et al. . A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456(7219):259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kobayashi KS, Chamaillard M, Ogura Y, et al. . Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307(5710):731–734. [DOI] [PubMed] [Google Scholar]
- 32. Wehkamp J, Wang G, Kübler I, et al. . The Paneth cell alpha-defensin deficiency of ileal Crohn’s disease is linked to Wnt/Tcf-4. J Immunol. 2007;179(5):3109–3118. [DOI] [PubMed] [Google Scholar]
- 33. Wehkamp J, Salzman NH, Porter E, et al. . Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci U S A. 2005;102(50):18129–18134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hashimoto T, Perlot T, Rehman A, et al. . ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487(7408):477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nikolaus S, Schulte B, Al-Massad N, et al. . Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterol. 2017;153(6):1504–1516.e2. [DOI] [PubMed] [Google Scholar]
- 36. Ren W, Duan J, Yin J, et al. . Dietary L-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids. 2014;46(10):2403–2413. [DOI] [PubMed] [Google Scholar]
- 37. Vicario M, Amat C, Rivero M, Moretó M, Pelegrí C. Dietary glutamine affects mucosal functions in rats with mild DSS-induced colitis. J Nutr. 2007;137(8):1931–1937. [DOI] [PubMed] [Google Scholar]
- 38. Jeong SY, Im YN, Youm JY, et al. . L-glutamine attenuates DSS-induced colitis via induction of MAPK phosphatase-1. Nutrients. 2018;10(3):e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mestecky J, Russell MW, Elson CO. Intestinal IgA: novel views on its function in the defence of the largest mucosal surface. Gut. 1999;44(1):2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaetzel CS, Robinson JK, Chintalacharuvu KR, Vaerman JP, Lamm ME. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc Natl Acad Sci U S A. 1991;88(19):8796–8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Keilbaugh SA, Shin ME, Banchereau RF, et al. . Activation of RegIIIbeta/gamma and interferon gamma expression in the intestinal tract of SCID mice: an innate response to bacterial colonisation of the gut. Gut. 2005;54(5):623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Horioka K, Tanaka H, Isozaki S, et al. . Acute colchicine poisoning causes endotoxemia via the destruction of intestinal barrier function: the curative effect of endotoxin prevention in a murine model. Dig Dis Sci. 2019. doi: 10.1007/s10620-019-05729-w. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Michielan A, D’Incà R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015;2015:628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Teshima CW, Dieleman LA, Meddings JB. Abnormal intestinal permeability in Crohn’s disease pathogenesis. Ann N Y Acad Sci. 2012;1258:159–165. [DOI] [PubMed] [Google Scholar]
- 45. Martini E, Krug SM, Siegmund B, Neurath MF, Becker C. Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2017;4(1):33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pålsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunol. 2004;113(2):153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. [DOI] [PubMed] [Google Scholar]
- 48. Fiddian-Green RG. Gut mucosal ischemia during cardiac surgery. Semin Thorac Cardiovasc Surg. 1990;2(4):389–399. [PubMed] [Google Scholar]
- 49. Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49(9):1359–1377. [DOI] [PubMed] [Google Scholar]
- 50. Korth U, Krieter H, Denz C, et al. . Intestinal ischaemia during cardiac arrest and resuscitation: comparative analysis of extracellular metabolites by microdialysis. Resuscitation. 2003;58(2):209–217. [DOI] [PubMed] [Google Scholar]
- 51. Ghosh SS, Bie J, Wang J, Ghosh S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR-/- mice–role of intestinal permeability and macrophage activation. Plos One. 2014;9(9):e108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moreira AP, Texeira TF, Ferreira AB, Peluzio Mdo C, Alfenas Rde C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012;108(5):801–809. [DOI] [PubMed] [Google Scholar]
- 53. Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterol. 2012;142(5):1100–1101.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cani PD, Amar J, Iglesias MA, et al. . Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. [DOI] [PubMed] [Google Scholar]
- 55. Miranda MCG, Oliveira RP, Torres L, et al. . Frontline Science: abnormalities in the gut mucosa of non-obese diabetic mice precede the onset of type 1 diabetes. J Leukoc Biol. 2019;106(3):513–529. [DOI] [PubMed] [Google Scholar]
- 56. Creely SJ, McTernan PG, Kusminski CM, et al. . Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292(3):E740–E747. [DOI] [PubMed] [Google Scholar]
- 57. Kallio KA, Hätönen KA, Lehto M, Salomaa V, Männistö S, Pussinen PJ. Endotoxemia, nutrition, and cardiometabolic disorders. Acta Diabetol. 2015;52(2):395–404. [DOI] [PubMed] [Google Scholar]
- 58. Emanuele E, Orsi P, Boso M, et al. . Low-grade endotoxemia in patients with severe autism. Neurosci Lett. 2010;471(3):162–165. [DOI] [PubMed] [Google Scholar]
- 59. Zhang R, Miller RG, Gascon R, et al. . Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol. 2009;206(1-2):121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hasegawa S, Goto S, Tsuji H, et al. . Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. Plos One. 2015;10(11):e0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fotis L, Shaikh N, Baszis KW, et al. . Serologic evidence of gut-driven systemic inflammation in juvenile idiopathic arthritis. J Rheumatol. 2017;44(11):1624–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Metcalfe D, Harte AL, Aletrari MO, et al. . Does endotoxaemia contribute to osteoarthritis in obese patients? Clin Sci (Lond). 2012;123(11):627–634. [DOI] [PubMed] [Google Scholar]
- 63. Dubin W, Martin TR, Swoveland P, et al. . Asthma and endotoxin: lipopolysaccharide-binding protein and soluble CD14 in bronchoalveolar compartment. Am J Physiol. 1996;270(5 Pt 1):L736–L744. [DOI] [PubMed] [Google Scholar]
- 64. Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42(1):71–78. [DOI] [PubMed] [Google Scholar]
- 65. Camara-Lemarroy CR, Silva C, Greenfield J, Liu WQ, Metz LM, Yong VW. Biomarkers of intestinal barrier function in multiple sclerosis are associated with disease activity. Mult Scler. 2019. doi: 10.1177/1352458519863133. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66. Djuric Z. Obesity-associated cancer risk: the role of intestinal microbiota in the etiology of the host proinflammatory state. Transl Res. 2017;179:155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wirthgen E, Hoeflich A. Endotoxin-induced tryptophan degradation along the kynurenine pathway: the role of indolamine 2,3-dioxygenase and aryl hydrocarbon receptor-mediated immunosuppressive effects in endotoxin tolerance and cancer and its implications for immunoparalysis. J Amino Acids. 2015;2015:973548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Roderburg C, Luedde T. The role of the gut microbiome in the development and progression of liver cirrhosis and hepatocellular carcinoma. Gut Microbes. 2014;5(4):441–445. [DOI] [PubMed] [Google Scholar]
- 69. Pasini E, Corsetti G, Assanelli D, et al. . Effects of chronic exercise on gut microbiota and intestinal barrier in human with type 2 diabetes. Minerva Med. 2019;110(1):3–11. [DOI] [PubMed] [Google Scholar]
- 70. Li X, Atkinson MA. The role for gut permeability in the pathogenesis of type 1 diabetes–a solid or leaky concept? Pediatr Diabetes. 2015;16(7):485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hills RD Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut microbiome: profound implications for diet and disease. Nutrients. 2019; 11(7):E1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Peters VBM, van de Steeg E, van Bilsen J, Meijerink M. Mechanisms and immunomodulatory properties of pre- and probiotics. Benef Microbes. 2019;10(3):225–236. [DOI] [PubMed] [Google Scholar]
- 73. Kaliannan K, Hamarneh SR, Economopoulos KP, et al. . Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2013;110(17):7003–7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hamarneh SR, Kim BM, Kaliannan K, et al. . Intestinal alkaline phosphatase attenuates alcohol-induced hepatosteatosis in mice. Dig Dis Sci. 2017;62(8):2021–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hamarneh SR, Mohamed MM, Economopoulos KP, et al. . A novel approach to maintain gut mucosal integrity using an oral enzyme supplement. Ann Surg. 2014;260(4):706–714; discussion 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ghosh SS, He H, Wang J, Korzun W, Yannie PJ, Ghosh S. Intestine-specific expression of human chimeric intestinal alkaline phosphatase attenuates Western diet-induced barrier dysfunction and glucose intolerance. Physiol Rep. 2018;6(14):e13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lukas M, Drastich P, Konecny M, et al. . Exogenous alkaline phosphatase for the treatment of patients with moderate to severe ulcerative colitis. Inflamm Bowel Dis. 2010;16(7):1180–1186. [DOI] [PubMed] [Google Scholar]
- 78. Lalle`s JP. Recent advances in intestinal alkaline phosphatase, inflammation, and nutrition. Nut Rev. 2019;77(10):710–724. [DOI] [PubMed] [Google Scholar]
- 79. Ghosh SS, He H, Wang J, Gehr TW, Ghosh S. Curcumin-mediated regulation of intestinal barrier function: the mechanism underlying its beneficial effects. Tissue Barriers. 2018;6(1):e1425085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Desai MS, Seekatz AM, Koropatkin NM, et al. . A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167(5):1339–1353.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Birchenough G, Schroeder BO, Bäckhed F, Hansson GC. Dietary destabilisation of the balance between the microbiota and the colonic mucus barrier. Gut Microbes. 2019;10(2):246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Okazaki Y, Katayama T. Consumption of non-digestible oligosaccharides elevates colonic alkaline phosphatase activity by up-regulating the expression of IAP-I, with increased mucins and microbial fermentation in rats fed a high-fat diet. Br J Nutr. 2019;121(2):146–154. [DOI] [PubMed] [Google Scholar]
- 83. Xie SZ, Liu B, Ye HY, et al. . Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr Polym. 2019;206:149–162. [DOI] [PubMed] [Google Scholar]
- 84. Hashemi Z, Fouhse J, Im HS, Chan CB, Willing BP. Dietary pea fiber supplementation improves glycemia and induces changes in the composition of gut microbiota, serum short chain fatty acid profile and expression of mucins in glucose intolerant rats. Nutrients. 2017;9(11):E1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Islam J, Koseki T, Watanabe K, et al. . Dietary supplementation of fermented rice bran effectively alleviates dextran sodium sulfate-induced colitis in mice. Nutrients. 2017;9(7):E747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Matsumoto K, Ichimura M, Tsuneyama K, et al. . Fructo-oligosaccharides and intestinal barrier function in a methionine-choline-deficient mouse model of nonalcoholic steatohepatitis. Plos One. 2017;12(6):e0175406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ghosh SS, Wnag J, Yannie PJ, Sandhu YK, Korzun WJ, Ghosh S. Dietary supplementation with galactooligosaccharides attenuates high fat, high cholesterol diet-induced disruption of colonic mucin layer and improves glucose intolerance in C57BL/6 mice and reduces atherosclerosis in Ldlr-/- mice. J Nutr. 2019. [In Press] [DOI] [PubMed] [Google Scholar]
- 88. Mankertz J, Amasheh M, Krug SM, et al. . TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res. 2009;336(1):67–77. [DOI] [PubMed] [Google Scholar]
- 89. Heller F, Florian P, Bojarski C, et al. . Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterol. 2005;129(2):550–564. [DOI] [PubMed] [Google Scholar]
- 90. Suenaert P, Bulteel V, Lemmens L, et al. . Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am J Gastroenterol. 2002;97(8):2000–2004. [DOI] [PubMed] [Google Scholar]
- 91. Baert FJ, D’Haens GR, Peeters M, et al. . Tumor necrosis factor alpha antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn’s ileocolitis. Gastroenterol. 1999;116(1):22–28. [DOI] [PubMed] [Google Scholar]
- 92. Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166(2):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116(10):2682–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Weber CR, Raleigh DR, Su L, et al. . Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285(16):12037–12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14(1):9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang J, Ghosh SS, Ghosh S. Curcumin improves intestinal barrier function: modulation of intracellular signaling, and organization of tight junctions. Am J Physiol Cell Physiol. 2017;312(4):C438–C445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. He Y, Yuan X, Zuo H, Sun Y, Feng A. Berberine exerts a protective effect on gut-vascular barrier via the modulation of the Wnt/beta-catenin signaling pathway during sepsis. Cell Physiol Biochem. 2018;49(4):1342–1351. [DOI] [PubMed] [Google Scholar]
- 98. Gong J, Hu M, Huang Z, et al. . Berberine attenuates intestinal mucosal barrier dysfunction in type 2 diabetic rats. Front Pharmacol. 2017;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zong Y, Zhu S, Zhang S, Zheng G, Wiley JW, Hong S. Chronic stress and intestinal permeability: Lubiprostone regulates glucocorticoid receptor-mediated changes in colon epithelial tight junction proteins, barrier function, and visceral pain in the rodent and human. Neurogastroenterol Motil. 2019;31(2):e13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Arakawa K, Ishigami T, Nakai-Sugiyama M, et al. . Lubiprostone as a potential therapeutic agent to improve intestinal permeability and prevent the development of atherosclerosis in apolipoprotein E-deficient mice. Plos One. 2019;14(6):e0218096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Xu H, Wang Y, Jurutka PW, et al. . 16α-Hydroxytrametenolic acid from Poria cocos improves intestinal barrier function through glucocorticoid receptor mediated PI3K/Akt/NF-κB pathway. J Agric Food Chem. 2019. doi: 10.1021/acs.jafc.9b04613. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 102. Thaiss CA, Levy M, Grosheva I, et al. . Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359(6382):1376–1383. [DOI] [PubMed] [Google Scholar]
- 103. Deng J, Zeng L, Lai X, et al. . Metformin protects against intestinal barrier dysfunction via AMPKα1-dependent inhibition of JNK signalling activation. J Cell Mol Med. 2018;22(1):546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Suzuki T, Hara H. Quercetin enhances intestinal barrier function through the assembly of zonula [corrected] occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J Nutr. 2009;139(5):965–974. [DOI] [PubMed] [Google Scholar]
- 105. Mustafa A, El-Medany A, Hagar HH, El-Medany G. Ginkgo biloba attenuates mucosal damage in a rat model of ulcerative colitis. Pharmacol Res. 2006;53(4):324–330. [DOI] [PubMed] [Google Scholar]
- 106. Sun Z, Lasson A, Olanders K, Deng X, Andersson R. Gut barrier permeability, reticuloendothelial system function and protease inhibitor levels following intestinal ischaemia and reperfusion–effects of pretreatment with N-acetyl-L-cysteine and indomethacin. Dig Liver Dis. 2002;34(8):560–569. [DOI] [PubMed] [Google Scholar]
- 107. Chen CC, Walker WA. Probiotics and prebiotics: role in clinical disease states. Adv Pediatr. 2005;52:77–113. [DOI] [PubMed] [Google Scholar]
- 108. Fujimori S, Gudis K, Mitsui K, et al. . A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. Nutrition. 2009;25(5):520–525. [DOI] [PubMed] [Google Scholar]
- 109. van Wijck K, Verlinden TJ, van Eijk HM, et al. . Novel multi-sugar assay for site-specific gastrointestinal permeability analysis: a randomized controlled crossover trial. Clin Nutr. 2013;32(2):245–251. [DOI] [PubMed] [Google Scholar]
- 110. Vanuytsel T, Vermeire S, Cleynen I. The role of Haptoglobin and its related protein, Zonulin, in inflammatory bowel disease. Tissue Barriers. 2013;1(5):e27321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Caviglia GP, Dughera F, Ribaldone DG, et al. . Serum zonulin in patients with inflammatory bowel disease: a pilot study. Minerva Med. 2019;110(2):95–100. [DOI] [PubMed] [Google Scholar]
- 112. Pelsers MM, Hermens WT, Glatz JF. Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta. 2005;352(1-2):15–35. [DOI] [PubMed] [Google Scholar]
- 113. Honzawa Y, Nakase H, Matsuura M, Chiba T. Clinical significance of serum diamine oxidase activity in inflammatory bowel disease: importance of evaluation of small intestinal permeability. Inflamm Bowel Dis. 2011;17(2):E23–E25. [DOI] [PubMed] [Google Scholar]
- 114. Pastor Rojo O, López San Román A, Albéniz Arbizu E, de la Hera Martínez A, Ripoll Sevillano E, Albillos Martínez A. Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(3):269–277. [DOI] [PubMed] [Google Scholar]
- 115. Liebregts T, Adam B, Bredack C, et al. . Immune activation in patients with irritable bowel syndrome. Gastroenterol. 2007;132(3):913–920. [DOI] [PubMed] [Google Scholar]
- 116. Fujimoto M, Uemura M, Nakatani Y, et al. . Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 2000;24(4 Suppl):48S–54S. [PubMed] [Google Scholar]
- 117. Guardiola J, Lobatón T, Rodríguez-Alonso L, et al. . Fecal level of calprotectin identifies histologic inflammation in patients with ulcerative colitis in clinical and endoscopic remission. Clin Gastroenterol Hepatol. 2014;12(11):1865–1870. [DOI] [PubMed] [Google Scholar]