Abstract
Introduction
The role for steroids in acute spinal cord injury (ASCI) remains unclear; while some studies have demonstrated the risks of steroids outweigh the benefits,a meta-analyses conducted on heterogeneous patient populations have shown significant motor improvement at short-term but not at long-term follow-up. Given the heterogeneity of the patient population in previous meta-analyses and the publication of a recent trial not included in these meta-analyses, we sought to re-assess and update the safety and short-term and long-term efficacy of steroid treatment following ASCI in a more homogeneous patient population.
Materials and methods
A literature search was conducted on PubMed, EMBASE and Cochrane Library through June 2019 for studies evaluating the utility of steroids within the first 8 h following ASCI. Neurological and safety outcomes were extracted for patients treated and not treated with steroids. Pooled effect estimates were calculated using the random-effects model.
Results
Twelve studies, including five randomized controlled trials (RCTs) and seven observational studies (OBSs), were meta-analyzed. Overall, methylprednisolone was not associated with significant short-term or long-term improvements in motor or neurological scores based on RCTs or OBSs. An increased risk of hyperglycemia was shown in both RCTs (RR: 13.7; 95% CI: 1.93, 97.4; 1 study) and OBSs (RR: 2.9; 95% CI: 1.55, 5.41; 1 study). Risk for pneumonia was increased with steroids; while this increase was not statistically significant in the RCTs (pooled RR: 1.16; 95% C.I: 0.59, 2.29; 3 studies), it reached statistical significance in the OBSs (pooled RR: 2.00; 95% C.I: 1.32, 3.02; 6 studies). There was no statistically significant increased risk of gastrointestinal bleeding, decubitus ulcers, surgical site infections, sepsis, atelectasis, venous thromboembolism, urinary tract infections, or mortality among steroid-treated ASCI patients compared to untreated controls in either RCTs or OBSs.
Conclusions
Methylprednisolone therapy within the first 8 h following ASCI failed to show a statistically significant short-term or long-term improvement in patients' overall motor or neurological scores compared to controls who were not administered steroids. For the same comparison, there was an increased risk of pneumonia and hyperglycemia compared to controls. Routine use of methylprednisone following ASCI should be carefully considered in the context of these results.
Keywords: Neuroscience, Neurology, Neurosurgery, Trauma, Intensive care medicine, Endocrine system, Steroids, Methylprednisolone, Adverse effects, Acute spinal cord injury, Spinal cord injury, Pneumonia, Hyperglycemia
Neuroscience; Neurology; Neurosurgery; Trauma; Intensive care medicine; Endocrine system; Steroids; Methylprednisolone; Adverse effects; Acute spinal cord injury; Spinal cord injury; Pneumonia; Hyperglycemia.
1. Introduction
Acute spinal cord injury (ASCI) is a devastating condition that affects approximately 1.3 million people in North America each year [1]. Despite its prevalence, there is no recognized effective treatment for ASCI. While rehabilitation may allow for improved mobility in ASCI patients, it generally has little to no impact on the neurological function of these patients [2]. With respect to pharmacologic treatment options, based on the National Acute Spinal Cord Injury Study (NASCIS) II and III trials, methylprednisolone is currently recommended for the management of ASCI [3, 4, 5, 6]. These trials demonstrated effectiveness of methylprednisone in improving motor scores in ASCI patients when compared to placebo [3].
In 2016, a meta-analysis [7] demonstrated that although methylprednisolone was associated with a significant short-term motor improvement, it failed to improve the long-term motor outcomes in patients with ASCI. Furthermore, methylprednisone use in this setting has also been associated with an increased risk of gastrointestinal (GI) bleeding [7]. Contrarily, a recent meta-analysis in 2019 [26] showed no motor or ASIA score improvement at short-term or long-term follow-up; however, these previous meta-analyses have examined heterogeneous patient populations in regards to type, age, and number of patients, making their conclusions challenging to apply and limiting their overall utility in practice. Overall, given the low quality of evidence for all reported outcomes in the available analyses, there are presently no strong recommendations regarding the use of methylprednisone following ASCI. As an additional study [8] evaluating the role of steroids in ASCI patients has become available since the publication of the aforementioned meta-analyses, the objective of our study was to re-assess the safety and potential short-term and long-term efficacy of steroids following ASCI as compared with controls not treated with steroids among a homogeneous ASCI patient population.
2. Materials & methods
2.1. Literature search
This meta-analysis was done in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. PubMed, EMBASE and Cochrane Library were searched through June 30th, 2019 for studies evaluating the efficacy of steroids in ASCI. The search strategy combined various terms for ASCI (e.g. spinal cord laceration, spinal cord contusion) and dexamethasone or methylprednisolone. The detailed search items are included in Appendix 1.
2.2. Eligibility criteria and study selection
Studies were considered for inclusion in the current meta-analysis if they met the following criteria: (1) Study Design: clinical studies, randomized controlled trials (RCT), observational comparative studies, including cohort or case control studies assessing the use of methylprednisolone or dexamethasone in patients with ASCI; (2) Population: patients (13 years and above) with ASCI being treated with steroids within eight hours of injury; (3) Intervention/Exposure: methylprednisolone or dexamethasone alone; (4) Control: placebo or any other, non-steroid therapeutic agent; (5) Sample size: studies including at least 5 patients; (6) Measurable Outcome: motor and/or neurological function assessments and/or adverse events associated with steroid use (e.g. infections, ulcers, gastrointestinal bleeding, among others).
Studies were excluded if they met any of the following criteria: steroids were not administered within 8 h of ASCI; injury involved only the nerve root or cauda equina; patients had any other life-threatening morbidity; patients had a history of narcotic addiction; patients had a history of malignancy; patients were pregnant; and ASCI occurred in the setting of a gunshot wound. Review articles, case reports, clinical guidelines, case series, conference papers, editorials, animal studies, studies that used steroids (methylprednisolone and dexamethasone) in combination with non-steroidal agents, and studies written in languages other than English were also excluded.
Titles and abstracts were screened and potentially relevant articles were selected for full text evaluation. This evaluation was performed by six independent investigators (I.S., D.P., L.G., J.A., S.J., I.T.). Discrepancies were resolved in consultation with two physicians (H.Z., L.A.). The quality of the randomized controlled trials was evaluated using the risk of bias tool from the Cochrane collaboration [9]. Each trial was evaluated based on 7 domains, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Each domain was rated as low risk, high risk, or unclear. Overall, studies were assessed as having a high risk of bias if at least one key domain was high risk; a low risk of bias if all key domains were low risk; and an unclear risk of bias if all key domains had low or unclear biases. The quality of the cohort and case control studies was assessed using the Newcastle Ottawa Scale (NOS) [10]. A star was awarded for high quality in each of the following three criteria: selection, comparability, and ascertainment of the exposure or the outcome. A maximum of 9 points could be obtained.
2.3. Data extraction
For each identified article, the following information was extracted whenever possible: study characteristics (publication year, country of origin, sample size, study design, and follow-up), participant characteristics (sex, age, inclusion/exclusion criteria, and diagnosis), intervention administered, intervention characteristics, motor score, and number and type of adverse events. If the mean age was not provided, it was calculated from the range, sample size, and median using the WAN formula [11]. Data extraction was conducted independently by 7 investigators in groups of 2 with any controversies resolved by a third investigator (D.P., I.S., L.G., J.A., J.D., S.J., I.T.).
2.4. Data analysis
Data analysis was performed using Comprehensive Meta-Analysis Version 3 (Biostat, Inc., Englewood, NJ, USA). Analysis of neurological improvement and adverse events for ASCI patients who received steroids was conducted using the random-effects model, which incorporates both within and between study variation using the method of DerSimonian and Laird [12]. Forest plots were used to visualize important estimates of the studies. The Cochran's Q test (p < 0.1) was used to determine heterogeneity among studies, with I2 values greater than 50% indicating high heterogeneity [13]. For outcomes that had at least 5 studies per design (RCTs or OBSs), each of the groups of RCTs and OBSs was further evaluated for potential sources of heterogeneity from other trial-level covariates, including mean age (continuous), study duration (continuous), follow-up (categorical: short term of ≤2 months; long-term > 2 months), and study quality (continuous). For outcomes with ≥10 studies, publication bias using the funnel plot, Begg's rank correlation test [14], and Egger's linear regression [15] was assessed if the number of studies for individual outcomes was at least 10.
3. Results
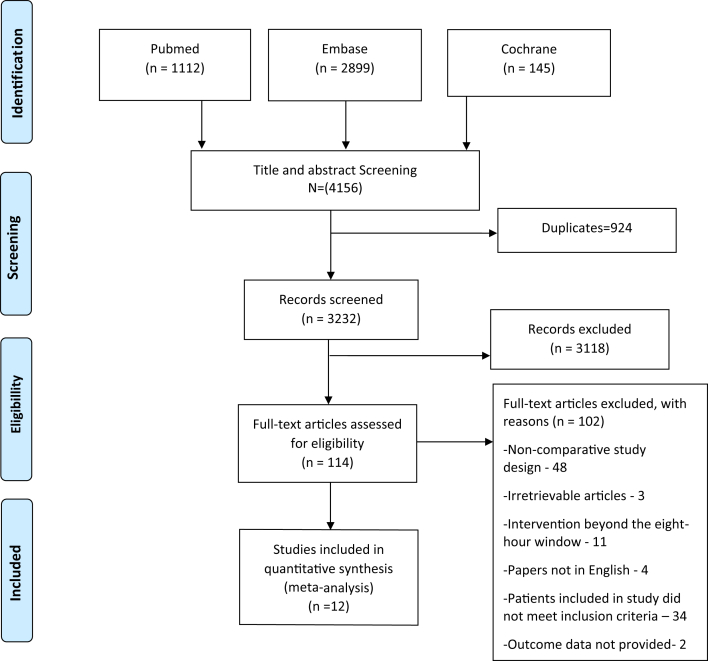
There were 1112 articles identified through the search strategy from PubMed; 2899 articles from Embase; and 145 articles from the Cochrane Library (Figure 1). After removal of duplicates and articles that did not meet the inclusion criteria following title and abstract screening, 114 articles remained for full-text review, of which 102 were further excluded with reasons listed in Figure 1. A total of 12 studies met the inclusion criteria and were incorporated into the meta-analysis. These included five randomized controlled trials (RCTs) [4, 8, 16, 17, 18] and seven observational studies (OBSs) [19, 20, 21, 22, 23, 24, 25]. The characteristics of the twelve studies are shown in Table 1. The intervention or exposed group in all studies incorporated the Second National Acute Spinal Cord Injury (NASCIS-II) protocol of administering a 30 mg/kg bolus dose of methylprednisolone over 1 h followed by a continuous methylprednisolone infusion of 5.4 mg/kg/h for 23 h. In RCTs, the mean age of patients ranged from 30.7 to 60.6 years and in OBSs, the mean age ranged from 32.6 to 60 years. The percentage of male participants ranged from 82.1% to 94.7% in RCTs and from 75.9% to 86.5% in OBSs. The included studies were conducted in Asia, North America, and Europe. Assessment of the quality of the randomized trials using the risk of bias assessment tool yielded three studies with low bias [4, 16, 18] and two studies with high bias (Table 1) [8, 17]. A total of four studies (2 RCTs and 2 OBSs) had short term follow-up [8, 17, 19, 24]. Assessment of study quality of the OBSs using the NOS score yielded an average of 6.86 ranging from 6 to 9.
Figure 1.
PRISMA 2009 flow diagram.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Author, year, (ref. #) | Country, Study Design | Mean Age (years) | Follow-up | Single or multicenter | Sample size | % male participants | Study duration (years) | Control Type | Risk of Biasbor NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials (RCTs) | |||||||||
| Bracken M et al., 1997 [4] | USA; RCT | 40a | Long term | Multicenter | 332 | 86.14 | 4 | Tirizalad Mesylate | Risk of Bias (Low) |
| Costa et al, 2015 [16] | Italy; RCT | 38.5 | Long term | Multicenter | 19 | 94.7 | - | Erythropoietin | Risk of Bias (Low) |
| Matsumoto et al, 2001 [17] | Japan; RCT | 60.60 | Short term | Single | 46 | 91.30 | 6 | Placebo | Risk of Bias (High) |
| Pointillart et al, 2000 [18] | France; RCT | 30.07 | Long term | Single | 100 | 90.00 | 5 | Nimodipine | Risk of Bias (Low) |
| Wang et al, 2019 [8] |
+9China; RCT |
47 |
Short term |
Single |
78 |
82.1 |
2 |
Surgery |
Risk of Bias (High) |
| Observational studies (OBSs) | |||||||||
| Chikuda et al, 2014 [19] | Japan, Retrospective Cohort Study | 60.00 | Short term | Multicenter | 3508 | 78.3 | 2 | Non methylprednisolonec | NOS (6) |
| Evaniew N et al, 2016 [20] | Canada; Prospective Cohort Study | 45.45 | Long term | Multicenter | 88 | 87.50 | 10 | Non-steroidsc | NOS (9) |
| Gerndt S J et al, 1997 [21] | USA; Retrospective Cohort Study | 32.66 | Long term | Multicenter | 140 | 76.98 | 8 | Non-steroidsc | NOS (6) |
| Ito Y et al., 2009 [22] | Japan; Prospective Cohort Study | 57.50 | Long term | Single | 79 | 79.74 | Non methylprednisolonec | NOS (7) | |
| Khan M et al, 2014 [23] | USA/Quwait; Retrospective Case Control | 43.76 | NR | Single | 350 | 75.71 | 13 | Non-steroidsc | NOS (7) |
| Suberviola et al, 2008 [24] | Spain; Retrospective Cohort Study | 40.00 | Short term | Single | 82 | 84.00 | 11 | Non methylprednisolonec | NOS (6) |
| Tsutsumi et al, 2006 [25] | Japan; Retrospective Cohort Study | 50.81 | Long term | Single | 70 | 88.57 | 5 | Non methylprednisolonec | NOS (7) |
Abbreviations: NOS: Newcastle Ottawa Scale; RCT: Randomized Controlled Trial, NR: Not reported.
The mean age for Bracken M et al., 1997 was estimated using the WAN formula 1.
Each trial was evaluated based on 7 domains including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Each domain was rated as low risk, high risk, or unclear (see Appendix 2).
Control group patients were reported to not have received methylprednisolone/steroids, without any specification as to whether another treatment was given.
3.1. Neurologic improvement
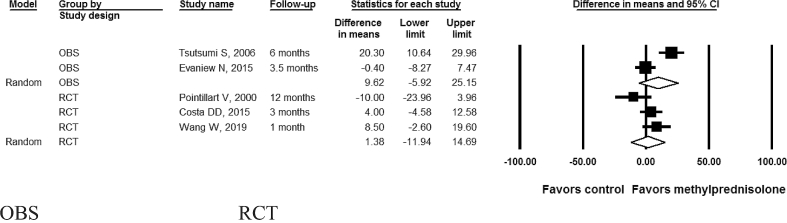
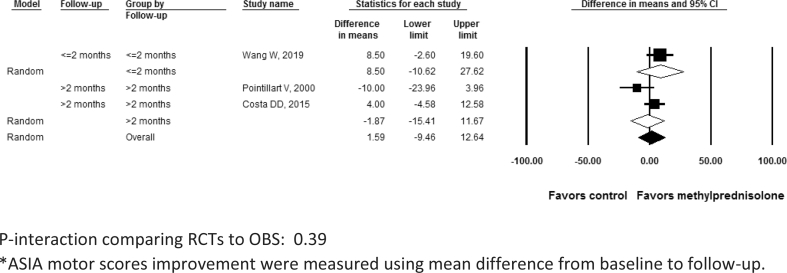
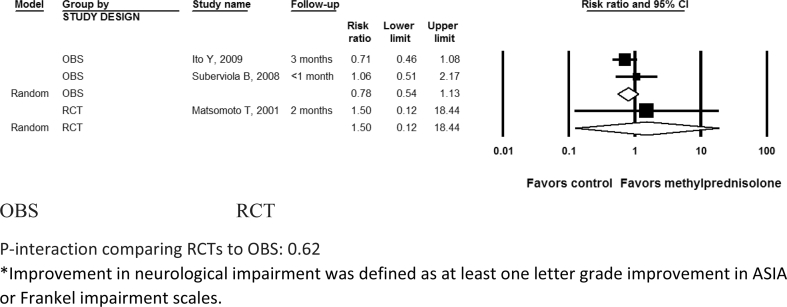
Neurological improvement in the included studies was measured using ASIA scores or Frankel scale. The analysis indicated methylprednisolone was not associated with a significant motor score improvement (P > 0.05) in either RCTs (difference in means 1.38; 95% CI: -11.9, 14.7; I2 = 53.6%; P- heterogeneity = 0.12; n = 3 studies) or OBSs (difference in means 9.62; 95% confidence interval [CI]: -5.92, 25.1; I2 = 90.6%; P- heterogeneity<0.01; 2 studies) (P-interaction comparing RCTs to OBS: 0.43) (Table 2; Figure 2). Motor score improvement results were consistently not significant after further subgrouping the RCTs by follow-up duration (≤2 months: difference in means 8.5; 95% CI: -10.6, 27.6; 1 study; vs. > 2 months: difference in means -1.8; 95% CI: -15.4, 11.6; 2 studies; P-interaction comparing the 2 subgroups: 0.39) (Figure 3). Similarly, neurological improvement by one letter grade or more in ASIA or Frankel impairment scale was not significant in RCTs (RR: 1.50; 95% CI: 0.12, 18.4; 1 study) or in OBSs (pooled RR: 0.78; 95% CI: 0.54, 1.13; I2 = 0%; P- heterogeneity = 0.34; 2 studies) (Table 2, Figure 4) (P-interaction comparing RCTs to OBS: 0.62). Further subgroup group analysis of neurological improvement for each of the RCTs or OBS by follow-up was not possible in this case due to the paucity of studies in each category; nevertheless, when qualitatively assessing results from individual studies, short-term improvement appeared more promising (RR > 1) than long-term improvement (RR < 1) but these results lacked statistical significance.
Table 2.
Summary of pooled estimate of efficacy outcomes stratified by study design, heterogeneity among studies, and P-interaction between study designs.
| Outcome | Study design (number of studies) | Pooled effect estimates | Heterogeneity between studies | P-interaction between study types (RCT and OBS) |
|---|---|---|---|---|
| ASIA motor score improvement | RCT (3 studies) | Difference in means: 1.38; 95% CI: -11.9, 14.7; | I2 = 53.6%; P-heterogeneity = 0.12 |
P = 0.43 |
| OBS (2 studies) | Difference in means: 9.62; 95% CI: -5.92, 25.1; | I2 = 90.6%; P-heterogeneity<0.01 |
||
| Neurological improvementa | RCT (1 study) | RR: 1.50; 95% CI: 0.12, 18.4; |
NA (1 study) | P = 0.62 |
| OBS (2 studies) | RR: 0.78; 95% CI: 0.54, 1.13; |
I2 = 0%; P-heterogeneity = 0.34 |
Abbreviations: OBS: Observational study; RCT = Randomized controlled trial.
Neurological improvement was measured by one letter grade improvement from baseline in ASIA impairment scale or Frankel impairment scale.
Figure 2.
Motor score improvement* after acute spinal cord injury with methylprednisolone compared with non-steroid therapy stratified by study design 2 observational and 3 randomized controlled studies. OBS: Observational studies; RCT= Randomized controlled trial. In the above forest plot, horizontal lines denote 95% CIs; solid squares represent the point estimate of each study and the diamond represents the pooled estimate of the intervention effect, for each of the subgroups (OBS and RCTs). The size of the solid squares is proportional to the weight of the study. P-interaction comparing RCTs to OBS: 0.43. *ASIA motor scores improvement were measured using mean difference from baseline to follow-up.
Figure 3.
Motor score improvement* after acute spinal cord injury with methylprednisolone compared with non-steroid therapy from 3 randomized controlled studies stratified by short-term (≤ 2 months) and long-term (> 2 months) follow-up. P-interaction comparing RCTs to OBS: 0.39. *ASIA motor scores improvement were measured using mean difference from baseline to follow-up.
Figure 4.
Neurological improvement* at follow-up after acute spinal cord injury with methylprednisolone compared with non-steroid therapy from 2 observational and 1 randomized controlled study. OBS: Observational studies; RCT= Randomized controlled trial. P-interaction comparing RCTs to OBS: 0.62. *Improvement in neurological impairment was defined as at least one letter grade improvement in ASIA or Frankel impairment scales.
3.2. Adverse events
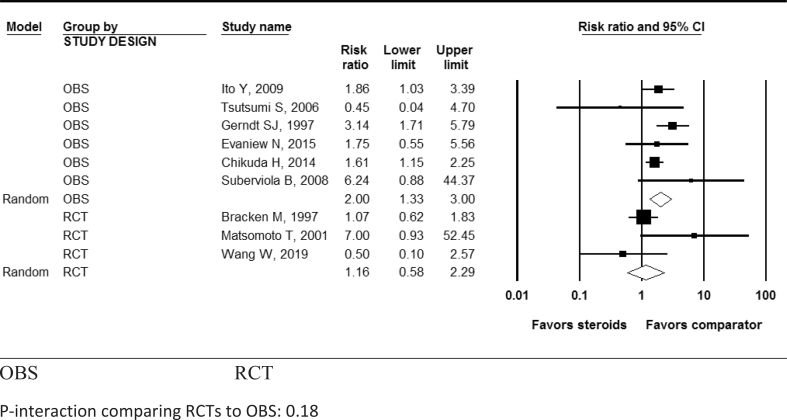
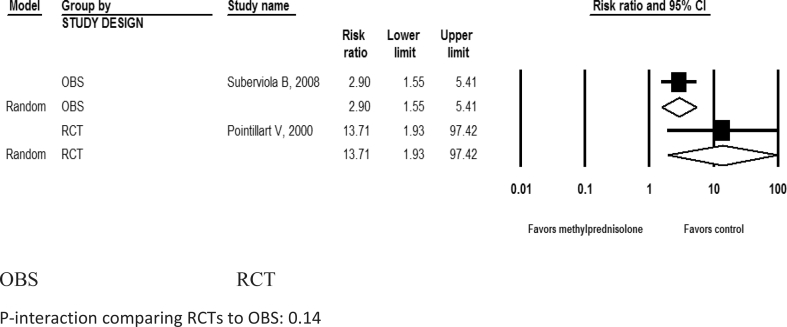
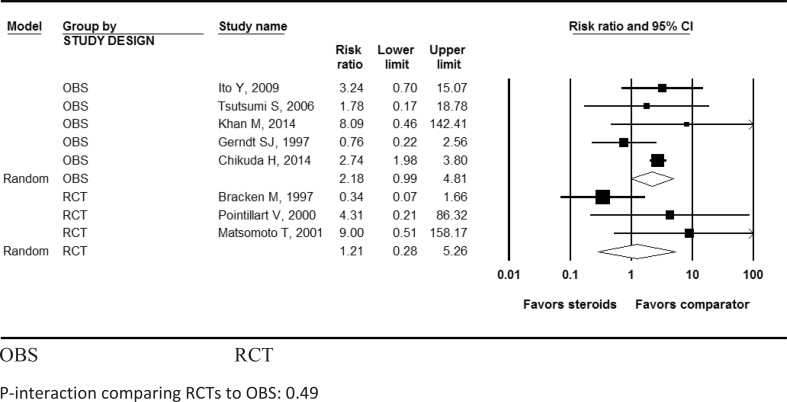
While the increased risk of pneumonia in patients with methylprednisolone was not statistically significant in RCTs [4, 8, 17] (pooled RR: 1.16; 95% CI: 0.59, 2.29; I2 = 57.1%; P-heterogeneity = 0.13; n = 3 studies), it was statistically significant in OBSs [19, 20, 21, 22, 24, 25] (pooled RR: 2.00; 95% CI: 1.33, 3.00; I2 = 22.5%; P- heterogeneity = 0.27; n = 6 studies) (Table 3, Figure 5). Methylprednisolone also showed a statistically significant increased risk of hyperglycemia in both RCTs (pooled RR: 13.7; 95% CI: 1.93, 97.4; n = 1) and OBSs (pooled RR: 2.9; 95% CI: 1.55, 5.41; n = 1 study) (Table 3, Figure 6). Methylprednisolone was not shown to be significantly associated with increased risk for gastrointestinal bleeding (Table 3, Figure 7), urinary tract infections, surgical site infections, development of decubitus ulcers, sepsis, atelectasis, venous thromboembolism, or mortality in either RCTs or OBSs (Table 3). There was no significant interaction between study types (RCTs vs. OBSs) for any adverse effect (all P-interaction >0.05; Table 3).
Table 3.
Summary of pooled effect estimates of safety outcomes and heterogeneity among studies.
| Outcomes | Pooled effect estimates from RCTs | Heterogeneity among RCTs (n: number of studies) |
Pooled effect estimates from observational studies | Heterogeneity among OBS (n: number of studies) |
P-interaction (RCT vs, OBS) |
|---|---|---|---|---|---|
| Atelectasis | RR: 0.63; 95% CI: 0.21, 1.91; |
I2 = 12.12%; P-hetero = 0.29; n = 2 |
RR: 0.30; 95% CI: 0.01, 7.94; |
NA (1 study); P-hetero = NA; n = 1 |
P = 0.67 |
| Decubitus ulcer | RR: 0.67; 95% CI: 0.40, 1.11; |
I2 = 0%; P-hetero = 0.66; n = 2 |
RR: 3.00; 95% CI: 0.64, 14.1; |
NA (1 study); P-hetero = NA; n = 1 |
P = 0.07 |
| Gastrointestinal bleeding | RR: 1.21; 95% CI: 0.28, 5.26; |
I2 = 59.6%; P-hetero = 0.84; n = 3 |
RR: 2.18; 95% CI: 0.99, 4.81; |
I2 = 17.0%; P-hetero = 0.31; n = 5 |
P = 0.49 |
| Hyperglycemia |
RR: 13.7; 95% CI: 1.93, 97.4*; |
NA (1 study); P-hetero = NA; n = 1 |
RR: 2.9; 95% CI: 1.55, 5.41*; |
NA (1 study); P-hetero = NA; n = 1 |
P = 0.14 |
| Mortality | RR: 0.77; 95% CI: 0.33, 1.76; |
NA (1 study); P-hetero = NA; n = 1 |
RR: 0.80; 95% CI: 0.53, 1.20; |
I2 = 0%; P-hetero = 0.75; n = 4 |
P = 0.93 |
| Pneumonia | RR: 1.16; 95% CI: 0.59, 2.29; |
I2 = 51.7%; P-hetero = 0.13; n = 3 |
RR: 2.00; 95% CI: 1.33, 3.00*; |
I2 = 22.5%; P-hetero = 0.27;n = 6 |
P = 0.18 |
| Sepsis | RR: 1.82; 95% CI: 0.70, 4.69; |
I2 = 0%; P-hetero = 0.74; n = 3 |
RR: 1.54; 95% CI: 0.79, 3.02; |
I2 = 0%; P-hetero = 0.95; n = 3 |
P = 0.79 |
| Surgical site infection | - | n = 0 | RR: 0.64; 95% CI: 0.32, 1.25; |
I2 = 0%; P-hetero = 0.55; n = 5 |
- |
| Urinary tract infection | RR: 1.11; 95% CI: 0.66, 1.87; |
I2 = 0%; P-hetero = 0.77; n = 4 |
RR: 0.98; 95% CI: 0.69, 1.41; |
I2 = 58.1%; P-hetero = 0.04; n = 6 |
P = 0.70 |
| Venous thromboembolism | RR: 0.51; 95% CI: 0.01, 21.2; |
NA (1 study); P-hetero = NA; n = 1 |
RR: 2.30; 95% CI: 0.25, 21.5; |
I2 = 76.5%; P-hetero = 0.01; n = 3 |
P = 0.50 |
Abbreviations: NA = Not applicable; OBS: Observational studies; P-hetero = P-heterogeneity; RCT = Randomized controlled trial;
*Values in bold are statistically significant.
Figure 5.
Pooled risk ratio for developing pneumonia after acute spinal cord injury with methylprednisolone compared with non-steroid therapy from 6 observational and 3 randomized controlled studies. OBS: Observational studies; RCT= Randomized controlled trial. P-interaction comparing RCTs to OBS: 0.18.
Figure 6.
Pooled risk ratio for developing hyperglycemia after acute spinal cord injury with methylprednisolone compared with non-steroid therapy from 1 observational and 1 randomized controlled study. OBS: Observational studies; RCT= Randomized controlled trial. P-interaction comparing RCTs to OBS: 0.14.
Figure 7.
Pooled risk ratio for developing gastrointestinal bleed after acute spinal cord injury with methylprednisolone compared with non-steroid therapy from 5 observational and 3 randomized controlled studies. OBS: Observational studies; RCT= Randomized controlled trial. P-interaction comparing RCTs to OBS: 0.49.
Due to the paucity of studies (<5) in the RCT category per individual outcome, a univariate meta-regression by different covariates was not feasible for these outcomes. Univariate meta-regression was conducted for OBSs on gastrointestinal bleeding (5 studies), pneumonia (6 studies), surgical site infection (5 studies), and urinary tract infections (6 studies) on three different trial-level covariates. Mean age and study duration were found to be significant effect modifiers for only urinary tract infection (Table 4), so that a higher risk of urinary tract infections was seen with increased age and shorter study durations when comparing patients exposed to methylprednisolone to those who were unexposed.
Table 4.
Regression coefficients (95% CI, P-interaction) of different trial level covariates resulting from univariate meta-regression using the random effect model for the observational studies.
| Outcome | Covariate | Slope (95% CI) from univariate meta-regression |
P-for interaction | Number of observational studies |
|---|---|---|---|---|
| Gastrointestinal bleeding | Mean age; Study duration; Study quality |
0.04 (-0.00, 0.09); -0.05 (-0.23; 0.14); -0.57 (-1.02; 2.17) |
0.07; 0.60; 0.48 |
5 |
| Pneumonia | Mean age; Study duration; Study quality |
-0.02 (-0.05, 0.00); 0.08 (-0.01, 0.17); -0.11 (-0.55, 0.33); |
0.05; 0.08; 0.61; |
6 |
| Surgical site infection | Mean age; Study duration; Study quality |
-0.06 (-0.14; 0.02); 0.15 (-0.03, 0.33); 0.34 (-0.30, 0.99); |
0.15; 0.10; 0.30; |
5 |
| Urinary tract infections | Mean age; Study duration; Study quality |
0.03 (0.01, 0.05)*; -0.06 (-0.12, -0.01)*; 0.06 (-0.35, 0.48) |
<0.01; 0.03; 0.76 |
6 |
*Values in bold are statistically significant.
Potential publication bias using the funnel plot, Begg's rank correlation test, and Egger's linear regression was not assessed as the number of studies for individual outcomes was fewer than 10.
4. Discussion
The present study demonstrated no statistically significant improvement in motor or neurological recovery among patients treated with methylprednisolone in the first 8-hour period following ASCI as compared to patients not receiving steroids; however, methylprednisone utilization was associated with significantly increased risks of hyperglycemia and pneumonia. Although the increased risk for pneumonia reached statistical significance in OBSs only, the greater risk for hyperglycemia achieved statistical significance in both OBSs and RCTs. Moreover, although not statistically significant, both RCTs and OBSs suggested a direct association between methylprednisone use and risk for GI bleeding and sepsis and an inverse association with risk for atelectasis and mortality.
Two previously published clinical trials suggested a possible benefit with respect to neurological recovery if steroids were administered after ASCI. The Second National Acute Spinal Cord Injury (NASCIS-II) trial [3] assessed patients randomized to methylprednisolone when given within 8 or 12 h after ASCI. When assessing all patients receiving steroids within 12 h of ASCI, the authors found no significant neurological benefit for steroids; however, when assessing only patients receiving methylprednisone within 8 h of injury, they demonstrated improvements in neurological scores. These results are discordant with what we found in our meta-analysis, whereby we did not detect any significant neurological benefit among those receiving steroids within 8 h in either short- or long-term follow-up. Notably, we were not able to include NASCIS-II results in our meta-analysis because the authors did not provide numerical demographic or safety data on the specific patients receiving steroids within 8 h of ASCI. The third NASCI-III trial [4], which was included in our meta-analysis, also reported improved motor recovery in patients receiving methylprednisolone therapy within 3–8 h of ASCI, and specifically found this association to be present at 6 weeks (short-term follow-up) and 6 months (long-term follow-up) in patients receiving prolonged methylprednisone therapy (48 h) compared to those receiving a shorter course of therapy (24 h), once again, contrary to our pooled analysis. Although further study is needed, when considered in the context of this previous literature, it is conceivable that we may have failed to detect a neurological benefit with steroids due to exclusion of the NASCIS - II trial data from our pooled analysis; and hence, our lack of power. Another potential reason is that fact the therapy duration could have varied across the different studies; yet, not all studies provided this information and if they did, data were not stratified for each subgroup. While previous meta-analyses have also explored the association between steroid use after ASCI and patient outcomes, these studies have included more heterogeneous patient populations than the one we focused on in our review. For example, in 2012, the Cochrane Collaboration published a meta-analysis [2] evaluating 8 clinical trials and concluded that methylprednisolone was the only pharmacologic therapy that showed some efficacy in the management of ASCI. Specifically, they found an improvement in motor function in patients when methylprednisolone was administered within 8 h of injury, with a greater benefit if given for 48 h. While the authors of the Cochrane meta-analysis included patients with chronic SCI, we focused on acute SCI only. Given that acute and chronic injuries reflect distinct mechanisms of trauma and can be expected to result in different sequelae, our study focused on a more specific population than the Cochrane review. Hence, our focus on patients with acute SCI only may account for why our results, unlike the Cochrane study, failed to demonstrate a significant motor benefit following methylprednisone.
In 2016, another meta-analysis [7] suggested that steroids might lead to significant motor improvements at short-term follow-up after ASCI (unlike our findings), but not at long-term follow-up. However, it should be noted that the authors of this meta-analysis included studies regardless of the timing or regimen of methylprednisone administration, while we only included studies that administered steroids according to NASCIS –II guideline. While in 2019, another meta-analysis suggested no motor or ASIA score improvement at short-term or long-term follow-up and an increased risk of gastrointestinal bleeding and respiratory tract infections with steroids [26]. Our study findings were similar to the latter, as we also did not find significant short-term or long-term improvement associated with steroid use and instead found increased risks of hyperglycemia and pneumonia. Moreover, both published meta-analyses [7, 26] included gunshot wound victims, as well as pediatric patients. Due to the mechanism of injury of gunshot wounds, steroid administration is considered ineffective [27], so we decided to exclude these patients from our analysis. Additionally, we included a recent randomized control trial published this year [8], which was not included in none of the above-mentioned meta-analyses. Ultimately, we sought to build upon the two aforementioned meta-analyses by assessing the role of methylprednisone in a more homogeneous patient population and found that even among this particular patient population, there did not appear to be a significant benefit to steroid use, whether short-term or long-term.
While the previously conducted meta-analyses highlighted the increased risk of gastrointestinal bleeding and respiratory tract infections with steroids, we did not find the risk for GI bleeding to be statistically significant. However, we assessed several other potential risks. Overall, we identified significant associations between methylprednisolone treatment for ASCI and hyperglycemia and hospital-acquired pneumonia. Although not significant, we noted trends towards increased risks for urinary tract infections and sepsis, as well; it is possible that we were unable to detect a significant association in these latter complications due to the lower number of studies available for review and our limited power to detect a significant association even if present.
Our review highlights the need for clinicians to carefully consider the full and long-term implications of steroid-associated side effects. Steroid-induced hyperglycemia can precipitate severe complications such as nonketotic hyperosmolar state and diabetic ketoacidosis [28]. Particular patient populations, such as those with baseline diabetes or other metabolic disorders, may be particularly susceptible to these risks and represent groups for whom steroids should be initiated even more cautiously, if at all.
Similarly, hospital-acquired pneumonia is a potentially fatal condition associated with longer and costlier hospital stays, with an estimated attributable mortality of 13% [29, 30]. Thus, the value of methylprednisolone in the management of ASCI should be carefully weighed against this increased risk of developing hospital-acquired pneumonia. This risk warrants extra attention among patients with poor immunity with limited ability to fight infections or those with co-morbid lung conditions, such as asthma or chronic obstructive pulmonary disease. Further studies that assess the benefits and harms of steroids among patients with and without such conditions may serve to offer more specific guidance with respect to the safety of steroid use following ASCI and regarding which patients, if any, can be thought to benefit from its application in this setting.
It should be noted that despite inclusion of dexamethasone in the search criteria for this analysis, only studies evaluating methylprednisolone in the management of ASCI were identified and subsequently used for this meta-analysis. Pre-clinical studies have suggested benefits of using dexamethasone in ASCI models, including lower levels of autophagy and apoptosis, and decreased inflammation of nerve myelin sheaths [31, 32]. While anecdotal reports on the use of dexamethasone for the management of ASCI exist, no randomized control trials evaluating this approach have been published to date [33]. Outcomes on patients treated with dexamethasone following ASCI should be reported on by those institutions utilizing dexamethasone to allow for incorporation of these results in future meta-analyses. Such data will increase our understanding of the role of dexamethasone in the management of ASCI.
Taken together, without a quantifiable benefit in motor or neurological recovery and the potential for multi-system, adverse reactions, especially in at-risk populations, methylprednisone should not be routinely used following ASCI. However, it should be noted that there may be specific instances such as acute spinal cord compression due to malignant processes in which high-dose steroids are known to have beneficial effects in preventing or ameliorating neurological injury. Steroids could therefore be beneficial in traumatic spinal cord injury in which mechanisms leading to neurological injury are similar to those observed in spinal cord injury due to a malignant process. For example, traumatic injury leading to an acute process causing progressive direct compression of the spine (e.g. an epidural spinal hematoma). While our meta-analysis did not demonstrate a benefit with steroids even if initiated in the acute phase following traumatic injury, we recognize that there are multiple factors that may influence a potential role for steroids. These include how soon after injury steroids are initiated (within 8 or 12 h), how long the steroids are administered for (24 vs. 48 hours), and over what period of time benefits are assessed (short-term vs. long-term). Future studies should aim to provide data on each of the above-mentioned factors when assessing the utility of steroids in ASCI. By focusing on homogenous patient populations and clearly delineating time and dose-specific variations in steroid utilization, future studies can help elucidate the scope of particular risks and benefits following ASCI.
4.1. Strengths and limitations
Limitations of the study include the diversity of the control group [4, 8, 16, 17, 19, 23], especially when assessing the motor score and neurological improvement outcomes, as well as the paucity of comparative studies reporting outcomes for a control population where a placebo was used. Nevertheless, the results of studies in which a non-steroid treatment was administrated were similar to results of studies that used a placebo or no treatment for the control group, increasing our confidence in including all such studies as controls. An additional limitation of our study is that we were unable to perform sub-group analyses on whether continuation of steroid treatment for 24 or 48 h was associated with any neurological benefit as there was a lack of data pertaining to this outcome to allow for pooling across studies. Despite these limitations, our study had several strengths; we focused on a more homogeneous patient population who were administered methylprednisolone within 8 h of injury, who did not present with ASCI from gunshot wounds, and who were above 12 years old. Moreover, we assessed the quality of the RCTs using the risk of bias tool from the Cochrane collaboration, discerned the results of RCTs from observational studies, and explored other potential sources of heterogeneity within each stratum when feasible. We also reported pooled analyses on several adverse events, which were not as comprehensively reported in previously published meta-analyses.
5. Conclusions
Methylprednisolone therapy within the first 8 h following an ASCI failed to result in a statistically significant short-term or long-term improvement in patients' overall motor or neurological scores compared to controls who did not receive steroids. Moreover, steroid use was significantly associated with an increased risk of hyperglycemia in both RCTs and OBSs and pneumonia based on data from OBSs. The risk-benefit ratio should be carefully considered before initiation of steroid therapy following ASCI. Current literature advocating for routine use of methylprednisone following ASCI should be reconsidered.
Declarations
Author contribution statement
Ihtisham Sultan, Nayan Lamba, Aaron Liew, Phoung Doung, Christian D. Cerecedo-Lopez, Stefania Papatheodorou, Ian Tafel: Analyzed and interpreted the data; Wrote the paper.
Ishaan Tewarie, James J. Amamoo, Laxmi Gannu, Shreya Chawla, Joanne Doucette: Performed the experiments.
Linda S. Aglio, Timothy R Smith, Hasan Zaidi: Conceived and designed the experiments.
Rania A Mekary: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ahuja C.S., Martin A.R., Fehlings M. Recent Advances in Managing a Spinal Cord Injury Secondary to Trauma. F1000Res. 2016;5 doi: 10.12688/f1000research.7586.1. F1000 Faculty Rev-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bracken M.B. Steroids for acute spinal cord injury. Cochrane Database Syst. Rev. 2012;1 doi: 10.1002/14651858.CD001046.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bracken M.B., Shepard M.J., Collins W.F., Jr., Holford T.R., Baskin D.S., Eisenberg H.M., Flamm E., Leo-Summers L., Maroon J.C., Marshall L.F. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J. Neurosurg. 1992;76(1):23–31. doi: 10.3171/jns.1992.76.1.0023. [DOI] [PubMed] [Google Scholar]
- 4.Bracken M.B., Shepard M.J., Holford T.R., Leo-Summers L., Aldrich E.F., Fazl M., Fehlings M., Herr D.L., Hitchon P.W., Marshall L.F., Nockels R.P., Pascale V., Perot P.L., Jr., Piepmeier J., Sonntag V.K., Wagner F., Wilberger J.E., Winn H.R., Young W. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the third national acute spinal cord injury randomized controlled trial. National acute spinal cord injury study. Jama. 1997;277(20):1597–1604. [PubMed] [Google Scholar]
- 5.Walters B.C., Hadley M.N., Hurlbert R.J., Aarabi B., Dhall S.S., Gelb D.E., Harrigan M.R., Rozelle C.J., Ryken T.C., Theodore N. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery. 2013;60(Suppl 1):82–91. doi: 10.1227/01.neu.0000430319.32247.7f. [DOI] [PubMed] [Google Scholar]
- 6.Fehlings M.G., Wilson J.R., Cho N. Methylprednisolone for the treatment of acute spinal cord injury: counterpoint. Neurosurgery. 2014;61(Suppl 1):36–42. doi: 10.1227/NEU.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 7.Evaniew N., Belley-Cote E.P., Fallah N., Noonan V.K., Rivers C.S., Dvorak M.F. Methylprednisolone for the treatment of patients with acute spinal cord injuries: a systematic review and meta-analysis. J. Neurotrauma. 2016;33(5):468–481. doi: 10.1089/neu.2015.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W., Zuo B., Liu H., Cui L. Intermittent injection of methylprednisolone sodium succinate in the treatment of cervical spinal cord injury complicated with incomplete paraplegia. Pak J Med Sci. 2019;35(1):141–145. doi: 10.12669/pjms.35.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins J.P., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells G., Shea B., O’Connell D., P. J, Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonradomised studies in meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 11.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials. 2015;45(Pt A):139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. PMID: 7786990. [PubMed] [Google Scholar]
- 15.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa D.D., Beghi E., Carignano P., Pagliacci C., Faccioli F., Pupillo E., Messina P., Gorio A., Redaelli T.J.N.S. Tolerability and efficacy of erythropoietin (EPO) treatment in traumatic spinal cord injury: a preliminary randomized comparative trial vs. methylprednisolone (MP) Neurol. Sci. 2015;36(9):1567–1574. doi: 10.1007/s10072-015-2182-5. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto T., Tamaki T., Kawakami M., Yoshida M., Ando M., Yamada H. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine (Phila Pa 1976) 2001;26(4):426–430. doi: 10.1097/00007632-200102150-00020. [DOI] [PubMed] [Google Scholar]
- 18.Pointillart V., Petitjean M.E., Wiart L., Vital J.M., Lassie P., Thicoipe M., Dabadie P. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord. 2000;38(2):71–76. doi: 10.1038/sj.sc.3100962. [DOI] [PubMed] [Google Scholar]
- 19.Chikuda H., Yasunaga H., Takeshita K., Horiguchi H., Kawaguchi H., Ohe K., Fushimi K., Tanaka S. Mortality and morbidity after high-dose methylprednisolone treatment in patients with acute cervical spinal cord injury: a propensity-matched analysis using a nationwide administrative database. Emerg. Med. J. 2014;31(3):201–206. doi: 10.1136/emermed-2012-202058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evaniew N., Noonan V.K., Fallah N., Kwon B.K., Rivers C.S., Ahn H., Bailey C.S., Christie S.D., Fourney D.R., Hurlbert R.J., Linassi A.G., Fehlings M.G., Dvorak M.F. Methylprednisolone for the treatment of patients with acute spinal cord injuries: a propensity score-matched cohort study from a Canadian multi-center spinal cord injury registry. J. Neurotrauma. 2015;32(21):1674–1683. doi: 10.1089/neu.2015.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerndt S.J., Rodriguez J.L., Pawlik J.W., Taheri P.A., Wahl W.L., Micheals A.J., Papadopoulos S.M. Consequences of high-dose steroid therapy for acute spinal cord injury. J. Trauma. 1997;42(2):279–284. doi: 10.1097/00005373-199702000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Ito Y., Sugimoto Y., Tomioka M., Kai N., Tanaka M. Does high dose methylprednisolone sodium succinate really improve neurological status in patient with acute cervical cord injury?: a prospective study about neurological recovery and early complications. Spine (Phila Pa 1976) 2009;34(20):2121–2124. doi: 10.1097/BRS.0b013e3181b613c7. [DOI] [PubMed] [Google Scholar]
- 23.Khan M.F., Burks S.S., Al-Khayat H., Levi A.D. The effect of steroids on the incidence of gastrointestinal hemorrhage after spinal cord injury: a case-controlled study. Spinal Cord. 2014;52(1):58–60. doi: 10.1038/sc.2013.122. [DOI] [PubMed] [Google Scholar]
- 24.Suberviola B., Gonzalez-Castro A., Llorca J., Ortiz-Melon F., Minambres E. Early complications of high-dose methylprednisolone in acute spinal cord injury patients. Injury. 2008;39(7):748–752. doi: 10.1016/j.injury.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Tsutsumi S., Ueta T., Shiba K., Yamamoto S., Takagishi K. Effects of the Second National Acute Spinal Cord Injury Study of high-dose methylprednisolone therapy on acute cervical spinal cord injury-results in spinal injuries center. Spine (Phila Pa 1976) 2006;31(26):2992–2996. doi: 10.1097/01.brs.0000250273.28483.5c. discussion 2997. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z., Yang Y., He L., Pang M., Luo C., Liu B., Rong L. High-dose methylprednisolone for acute traumatic spinal cord injury, A meta-analysis. Neurology. 2019;93(9):e841–e850. doi: 10.1212/WNL.0000000000007998. [DOI] [PubMed] [Google Scholar]
- 27.Heary R.F., Vaccaro A.R., Vaccaro A.R., Mesa J.J., Northrup B.E., Albert T.J., Albert T.J., Balderston R.A., Cotier J.M. Steroids and gunshot wounds to the spine. Neurosurgery. 1997;41(3):576–584. doi: 10.1097/00006123-199709000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Tamez-Perez H.E., Quintanilla-Flores D.L., Rodriguez-Gutierrez R., Gonzalez-Gonzalez J.G., Tamez-Pena A.L. Steroid hyperglycemia: prevalence, early detection and therapeutic recommendations: a narrative review. World J. Diabetes. 2015;6(8):1073–1081. doi: 10.4239/wjd.v6.i8.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalil A.C., Metersky M.L., Klompas M., Muscedere J., Sweeney D.A., Palmer L.B., Napolitano L.M., O'Grady N.P., Bartlett J.G., Carratala J., El Solh A.A., Ewig S., Fey P.D., File T.M., Jr., Restrepo M.I., Roberts J.A., Waterer G.W., Cruse P., Knight S.L., Brozek J.L. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American Thoracic Society. Clin. Infect. Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melsen W.G., Rovers M.M., Groenwold R.H., Bergmans D.C., Camus C., Bauer T.T., Hanisch E.W., Klarin B., Koeman M., Krueger W.A., Lacherade J.C., Lorente L., Memish Z.A., Morrow L.E., Nardi G., van Nieuwenhoven C.A., O'Keefe G.E., Nakos G., Scannapieco F.A., Seguin P., Staudinger T., Topeli A., Ferrer M., Bonten M.J. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 2013;13(8):665–671. doi: 10.1016/S1473-3099(13)70081-1. [DOI] [PubMed] [Google Scholar]
- 31.Kwiecien J., Jarosz B., Urdzikova L.M., Rola R., Dabrowski W. Subdural infusion of dexamethasone inhibits leukomyelitis after acute spinal cord injury in a rat model. Folia Neuropathol. 2015;53(1):41–51. doi: 10.5114/fn.2015.49973. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z., Zhou L., Zheng X., Liu W. Effects of dexamethasone on autophagy and apoptosis in acute spinal cord injury. Neuroreport. 2018;29(13):1084–1091. doi: 10.1097/WNR.0000000000001076. [DOI] [PubMed] [Google Scholar]
- 33.Kiwerski J.E. Application of dexamethasone in the treatment of acute spinal cord injury. Injury. 1993;24(7):457–460. doi: 10.1016/0020-1383(93)90149-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.