Abstract
Ligninolytic enzymes play a key role in degradation and detoxification of lignocellulosic waste in environment. The major ligninolytic enzymes are laccase, lignin peroxidase, manganese peroxidase, and versatile peroxidase. The activities of these enzymes are enhanced by various mediators as well as some other enzymes (feruloyl esterase, aryl-alcohol oxidase, quinone reductases, lipases, catechol 2, 3-dioxygenase) to facilitate the process for degradation and detoxification of lignocellulosic waste in environment. The structurally laccase is isoenzymes with monomeric or dimeric and glycosylation levels (10–45%). This contains four copper ions of three different types. The enzyme catalyzes the overall reaction: 4 benzenediol + O2 to 4 benzosemiquinone + 2H2O. While, lignin peroxidase is a glycoprotein molecular mass of 38–46 kDa containing one mole of iron protoporphyrin IX per one mol of protein, catalyzes the H2O2 dependent oxidative depolymerization of lignin. The manganese peroxidase is a glycosylated heme protein with molecular mass of 40–50kDa. It depolymerizes the lignin molecule in the presence of manganese ion. The versatile peroxidase has broad range substrate sharing typical features of the manganese and lignin peroxidase families. Although ligninolytic enzymes have broad range of industrial application specially the degradation and detoxification of lignocellulosic waste discharged from various industrial activities, its large scale application is still limited due to lack of limited production. Further, the extremophilic properties of ligninolytic enzymes indicated their broad prospects in varied environmental conditions. Therefore it needs more extensive research for understanding its structure and mechanisms for broad range commercial applications.
Keywords: Laccase, Lignin peroxidase, Manganese peroxidase, Versatile peroxidase, Lignocellulosic waste, Degradation and detoxification, Environmental science, Microbiology
Laccase; Lignin peroxidase; Manganese peroxidase; Versatile peroxidase; Lignocellulosic waste; Degradation and detoxification, Environmental science, Microbiology.
1. Introduction
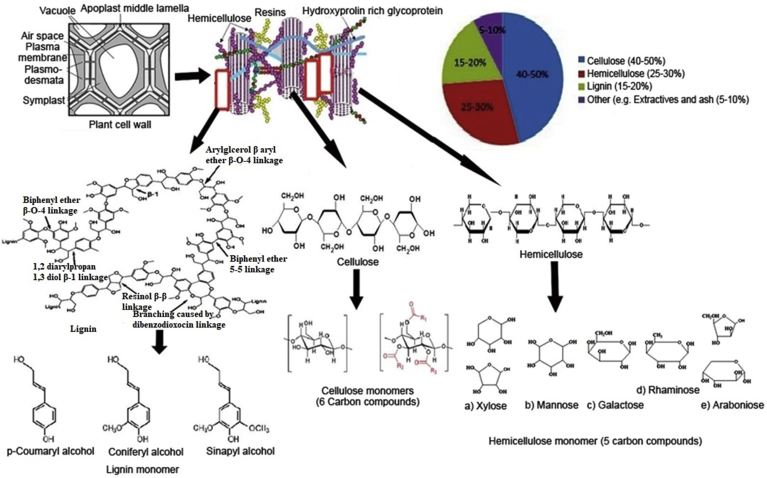
Lignocellulosic waste is considered as a chief component of renewable biomass on the Earth (Saini et al., 2015). This consists of three major components such as cellulose (40%–50%), hemicelluloses (25%–30%) and lignin (15%–20%) (Chaurasia, 2019). Cellulose is the primary polysaccharide as constituent of lignocellulosic materials that consist of hundred to over ten thousands β-1, 4 linked D-glucose units in unbranched linear chains. This is known as microfibrils with 3–5nm wide and many micrometers in length (Horn et al., 2012). Each cellulose chain-linked side by side through hydrogen bonding and van der waals interactions into microfibrils arrangement which are reported to consist 24 to 36 chains based on x-ray scattering data and the information available regarding the cellulose synthesis (Anwesha et al., 2011; Horn et al., 2012). Each cellulose microfibrils is synthesized from the spontaneous bundling and crystallization with dozens of the linked β-D glucan chains, each of which is made by a cellulose synthase (CESA) protein (Cosgrove, 2005). While hemicellulose is arranged in forms of strips tightly linked with cellulose and lignin in plant cell walls, it contains branched polysaccharide mainly xylans and mannans. It contains branched heterogenous polysaccharides of pentose (xylose and arabinose), hexoses (glucose, mannose, and galactose) and sugar acids (e.g. acetic, galacturonic and glucuronic) (Dimarogona et al., 2012). There is the presence of five monomeric sugars namely D-glucose, D-mannose, D-galactose, D-xylose and L-arabinose linked by β-1, 4-glycosidic bonds, that consist of both liners and branched heteropolymers. The major hemicelluloses in hardwoods are xylans (15%–30% dry weight), a polysaccharide having β-1,4 linked D-xylose units, which can be substituted with other monosaccharide units. However, softwood contains hemicelluloses mainly in the form of glactoglucomannan (15%–20% dry weight), a polysaccharide having β,1–4 linked D-glucose, and D-galactose units (Bugg et al., 2011). Similarly, lignin is the most abundant and complex biopolymer in nature because of its low biodegradability, due to complex chemical bonding between its monomers resulted into recalcitrant compound of lignocellulosic materials (Wong, 2009). This is composed of phenylpropane units, which is synthesized by radical polymerization of guaiacyl units (G), syringyl units (S) and p-hydroxyphenyl units (H) from precursor coniferyl, sinapyl and p-coumaryl alcohol as shown Figure 1 (Horn et al., 2012). Lignin consists of high molecular weight aromatic polymer, containing numerous biologically stable ether or ester linkages. Thus cellulose microfibrils are embedded in a complex matrix involving hemicellulose and lignin that hamper the way to cellulases and hemicellulases (Dimarogona et al., 2012). Due to its complexity, the degradation of lignocellulosic waste is a great challenge for sustainable development. The generation of lignocellulosic waste from various agro-based industries indicated the magnitude of problems including pulp and paper mill (effluent 150–200m3/ton and solid 160–450kg/ton), sugarcane molasses-based distilleries (effluent 15lit/1 lit alcohol production and 7.5 million tons/year), agricultural waste (200 billion tons/year), and food industry (1.3 billion ton/year) (Chandra et al., 2011; Gavrilescu, 2008; Kharayat, 2010; Kadam et al., 2013; Taha et al., 2016; Ravindran and Jaiswal, 2015). Due to their slow degradability and binding properties with other cationic molecules, they form complex compounds as environmental pollutants (Chandra et al., 2012; Dashtban et al., 2010). Therefore the lignocellulosic waste containing industrial waste is major source of environmental pollution (Sadh et al., 2018). The detail aquatic toxicity caused by pulp paper mill effluent has been also reported recently (Singh and Chandra, 2019). Besides, the adverse effects of lignocellulose containing wastewater have been reported for the reproductive system in fish i.e.masculinization, lower sex hormone, lower female vitellogenin, reduced gonad size, delayed maturity, circulation of sex hormones, fecundity, and modifications in secondary sexual characteristics to aquatic life (Chiang et al., 2015).
Figure 1.
Composition of lignocellulosic waste and structure of primary monomer cellulose, hemicelluloses and lignin structure and primary monomers: the most frequent bonds are indicated.
In nature, efficient lignocellulosic waste degradation during process of wood decay becomes interesting due to characterization of potential fungi and bacteria that are reported by various researchers (Janusz et al., 2017). These lignocellulytic fungi and bacteria have own capabilities to produce several ligninolytic enzymes for the degradation of lignocellulosic waste (Sanchez, 2009,Renugadevi et al., 2011). The degradation of cellulose and hemicellulose are complex in nature as it involves various enzymatic pathways and some of them also present in the form of insoluble crystalline fibers (Aarti et al., 2015). Degradation of crystalline cellulose is an enzymatic process including β–glucosidases, cellobiohydrolases, and β1-4-glucanases (Aarti et al., 2015). The degradation of cellulose and hemicellulose by various aerobic and anaerobic microorganisms has been reported with the production of carbon dioxide and glucose (Dimarogona et al., 2012). But the degradation of hemicelluloses requires different enzymes system (acetyl xylan, esterases, α-glucuronidases, β-D-xylosidases, and Endo-1,4-β-xylanases) (Vazquez et al., 2007). In general lignin-degrading enzymes have been divided into two major groups i.e. lignin-degrading auxiliary enzymes and lignin modifying enzymes. Lignin degrading auxiliary enzymes are unable to degrade lignin on their own functions which need additional enzyme involvement for complete degradation (Janusz et al., 2017). Lignin degrading auxiliary enzymes enables the process of lignin degradation through the sequential action of several proteins that may include oxidative H2O2 (Janusz et al., 2017). This group includes cellobiose dehydrogenase, aryl alcohol oxidases, glyoxal oxidase, glucose oxidase, and pyranose 2-oxidase (Janusz et al., 2017). Which the lignin modifying enzymes produced by various microorganisms that are grouped as laccase and heme-containing peroxidase i.e. lignin (LiP), manganese (MnP), versatile peroxidase (VP) and feruloyl esterase (Wong, 2009; Ozer et al., 2019). These lignin modifying enzymes also collectively called ligninolytic enzymes. These enzymes have gained great attention towards biological agents for the degradation of lignocellulosic waste containing compounds and other organic pollutants. It is also reported that the ligninolytic enzymes are effective in the treatment of industrial waste and other xenobiotic compounds through the biodegradation and decolorization process (Shi et al., 2013).
Several researchers have been reported numerous fungal sp. (Termetes versicolor, Pleurotous ostreatous, and Phanerochaete chrysosporium) for the degradation of lignocellulosic waste (Marquez et al., 2007). Three potential bacterial strains of Panibacillus sp., Aneurinibacillus aneurinilyticus, and Bacillus sp. were also found the potential for degradation and decolorization of synthetic lignin isolated from pulp paper mill sludge and characterized their metabolic products also by GC–MS (Chandra et al., 2012). Moreover, several other researchers have also reported that the Paenibacillus sp., Bacillus sp., and Streptomyces sp. were reported to be involved in lignin degradation process (Woo et al. (2014); Niladevi and Prema, 2005). Interestingly, microbes are well recorded in their capacity to degrade aromatic compounds such as lignin building blocks (Fuchs et al., 2011). Though the prevalence of ligninolytic enzyme has been reported in fungus, but due to their growth limitations restricted large scale application. In contrast the bacterial open an ample opportunity for the industrial application due to the versatile nature for their nutrients and environmental adaptions. Recently functions of ligninolytic enzymes in extremophilic environment also have been reported, in which conventional proteins are completely denatured (Chandra et al., 2017). The extremophilic activity of these enzymes is regulated due to presence of specific genes, various ion pairs, hydrophobic interaction, salt bridge, disulfide bridge and hydrogen bond between amino acids to maintain their stability for the catalytic function which indicated its broad range industrial application in diverse environment (Pace et al., 2014). But the complete information regarding ligninolytic enzyme properties, their mechanism of action and their purification process are fragmentary, which restricts its commercial application. Therefore it needs more extensive research for understanding its production and broad range industrial application. Hence, this review has been focused on the ligninolytic enzymes structure, reaction mechanisms, and prospect of their application for sustainable development.
2. Lignocellulosic waste degrading microorganisms
Numerous microorganisms such as bacteria, fungi, actinomycetes, and cyanobacteria have been reported which are capable to degrade lignocellulosic waste and other wood containing fibers Chandra, 2015. There is a growing concern among researchers to isolate bacteria and fungi directly from lignocellulosic waste contaminated sites due to acclimatized microbial genome pool with degrading enzyme-producing capabilities. The bacterial community of lignin degraders described till date comes under three classes such as actinomycetes, α- -proteobacteria and γ-proteobacteria (Bugg et al., 2011). Study carried out by several researchers, they evaluated that the bacterial strain such as Bacillus sp., Pseudomonas sp., Citrobacter sp., Klebsiella pneumonia, Serratia marcescens produced an extracellular peroxidases for the degradation of lignin (Anwar et al., 2014; Chandra et al., 2011; Chandra et al., 2012; Mathews et al., 2014; Raj et al., 2007a, b; Singh et al., 2009; Shi et al., 2013a; Yadav et al., 2014). In environment, lignin is probably hydrolyzed by the group of microorganisms shown in Table 1. The studies on bacterial enzymology for the degradation of lignocellulosic waste are fewer than those of fungi due to wood degrading bacteria that can tolerate a wide range of abiotic factors than fungi. Fungi are widespread in nature; especially include genera of ascomycetes and basidiomycetes phyla. More than 14,000 of fungal species expressing ligninolytic enzymes (laccase, LiP, MnP) in the environment (Dashtban et al., 2010). Most fungal species producing several synergistically active lingninolytic enzymes into the environment considerably contributing to the reduction of lignocellulosic waste (Howard et al., 2003). For instance, the most common fungal strains Trametes versicolor, Ganoderma lucidum, T. reesei, A. niger, P. chrysosporium, Penicillium brefeldianum, Trichoderma longibrachiatum, Aspergillus nidulans are more efficient towards cellulose, hemicellulose, and lignin degradation in a selective manner for the process of wood decay (Kersten and Cullen, 2007,Arora and Sharma, 2009; Dashtban et al., 2010).
Table 1.
Various ligninolytic bacterial strains isolated from lignocellulosic waste containing sites.
| Bacterial Strain | Source of isolation | Lignocellulose substrates supporting Growth | Relevant characteristics | Growth conditions | References |
|---|---|---|---|---|---|
| Psudomonas sp. | Soil | Effluents | Enhanced degradation of benzene and p-xylene in the presence of toluene | aerobic | Alvarez and Vogel (1991) |
| Aneurinibacillusaneurinilyticus (AY856831) | Pulp paper mill effluent | Kraft lignin | Decolorize kraft lignin, produce low-molecular-weight compounds | Facultative anaerobe/microaerophilic |
Chandra et al. (2007); Raj et al. (2007a) |
| Azotobacter | Soil | Decolorize and solubilize lignin | Aerobic | Morii et al. (1995) | |
| Bacillus cereus | Pulp paper mill effluent | Phenol (with glucose) | Degrade phenol and pentacholorophenol pollutants | Aerobic | Singh et al. (2009) |
| Bacillus megaterium | Soil | Decolorize and solubilize lignin | Morii et al. (1995) | ||
| Bacillus sp. (AY952465) | Pulp and paper sludge | Kraft lignin | Decolorize kraft lignin, produce low-molecular-weight compounds | Facultative anaerobe, 10 % NaCl |
Chandra et al. (2007); Raj et al. (2007a) |
| Citrobacterfreundii | Pulp paper mill effluent | 10 % Black liquor | Decolorize lignin | Microaerophilic | Chandra and Abhishek (2011) |
| Citrobacter sp. | Rayon grade pulp black liquor | 10 % Black liquor | Requires oxygen | Chandra et al. (2011) | |
| Enterobacter | Soil | Lignin model compounds | Oxidative ligninolytic enzymes | Requires ABTS | Yadav et al. (2014) |
| Escherichia coli | Soil | Lignin model compounds | Oxidative ligninolytic enzymes | Requires ABTS | Yadav et al. (2014) |
| Klebsiella pneumonia | Rayon grade pulp black liquor | 10 % Black liquor | Requires oxygen | Chandra et al. (2011) | |
| Paenibacillus sp. (AY952466) | Pulp and paper sludge | Kraft lignin, phenol (with glucose) | Decolorize kraft lignin produce low-molecular-weight compounds | Facultative anaerobe, 3 % NaCl |
Chandra et al. (2007); Raj et al. (2007a) |
| Pantoea sp. | Pulp paper mill effluent | Decolorize, reduce COD and BOD,degrade lignin and chlorophenol, ligninolytic enzymes | Aerobic, pH 7 | Chandra et al. (2012) | |
| Pseudochrobactrum glaciale | Pulp paper mill effluent | Decolorize, reduce COD and BOD, degrade lignin and chlorophenol, ligninolytic enzymes | Aerobic, pH 9 | Chandra et al. (2012) | |
| Pseudomonas putida | Pulp paper mill effluent | Effluent | Remove color, phenolics, and sulfide | Aerobic | Chandra et al. (2001) |
| Serratia marcescens | Soil; rayon grade pulp black Liquor | 10 % Black liquor | Decolorize and solubilize lignin | Requires oxygen |
Morri et al. (1995); Chandra et al. (2011) |
A wide range of lignocellulosic waste is produced all over the world, including whole plants, plant parts (e.g. seeds, roots, and stems) and processing by-products of several industrial wastes (pulp paper, distillery, timber, and food). Paper mill effluent can induce chronic toxic effects and endocrine disruption caused by largely unknown compounds that are released into the environment (Yadav and Chandra, 2018). Due to the high chemical diversity of the organic pollutants and solvents are present in pulp and paper mill effluent, different toxic effects on crops and aquatic communities have been observed in recipient watercourses (Kumar and Chopra, 2014; Yadav and Chandra, 2018). Therefore, the effluent discharged from various industries after secondary treatment does not meet the requirement for the environmental safety guidelines. There is a need to search the more potential organisms and reveals the mechanisms of its degradation process along with their inhibitory compounds.
3. Molecular structure and mechanisms of ligninolytic enzymes
The most common lignocellulosic waste degrading enzymes are Laccase (EC 1.10.3.2), Lignin peroxidase (EC 1.11.1.14), Manganese peroxidase (EC 1.11.1.13) and Versatile peroxidase (EC 1.11.1.16). Recently, researches have been revealed the indirect role of several other enzymes such as feruloyl esterase (EC 3.1.1.73), aryl-alcohol oxidase (EC 1.1.3.7), quinone reductases (EC 1.6.5.5), lipases (EC 3.1.1.3), xylanase (EC 3.2.1.8) and catechol 2, 3-dioxygenase (EC 1.13.11.2) which facilitate the ligninolytic enzyme process for degradation of environmental pollutants (Ozer et al., 2019; Pothiraj et al., 2006; Guillen et al., 1997). Hydroxycinnamic acid, which is replaced by feruloyl esterase during lignin degradation, acts as a mediator for laccase enzyme (Ozer et al., 2019). Aryl-alcohol oxidase and quinone reductases are involved in lignin degradation by various bacteria and fungi (Pothiraj et al., 2006). It was proposed to act on the reduction of quinones, which can use by ligninolytic enzymes or in support of a peroxidase reaction (Guillen et al., 1997). Similarly, lipase enzymes can be used as first-rate biocatalysts for in-situ peracid formation in nonaqueous media, preliminary from carboxylic acids with diluted hydrogen peroxidase. Moreover, immobilized lipase was effectively used in a per acid-mediated lignocellulosic delignification method in a non-aqueous solvent: dimethyl carbonate was used for lipase-mediated oxidation both as a solvent and as an acyl-donor reagent (Wiermans et al., 2013; Pothiraj et al., 2006). Xylanase and catechol 2, 3-dioxygenase enzyme did not release significant amounts of lignin; however, the enzyme facilitated the extraction of the lignin fraction because breaking down monocyclic aromatic linking between xylan and lignin (Varnai et al., 2011; Eltis and Bolin, 1996). The ligninolytic enzymes are found in different molecular weights on the basis of amino acid sequence and co-factor composition.
3.1. Laccase: structure and properties
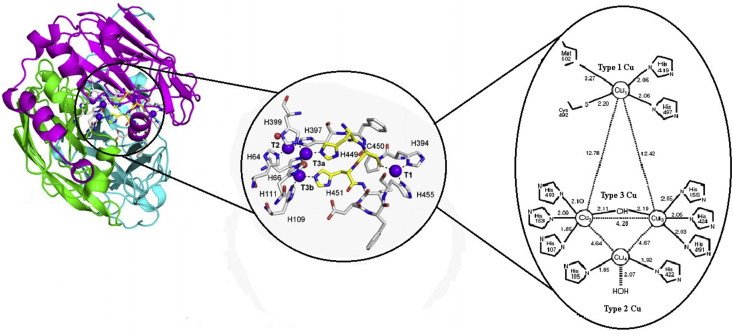
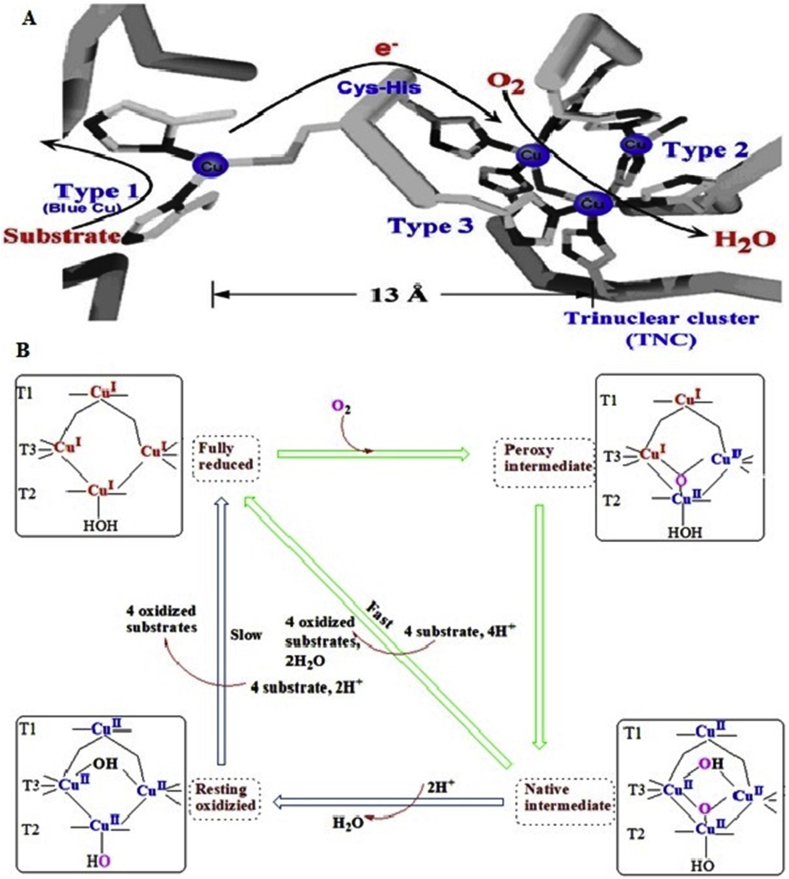
Laccase (EC 1.10.3.2) is copper-containing extracellular enzyme that consists of monomeric, dimeric and tetrameric glycoproteins. This is predominantly present in microorganisms i.e. bacteria, fungi, and actinomycetes (Janusz et al., 2017). Several workers have been reported different isoenzymes having average molecular weight of 50–300 kD (Chandra and Chowdhary, 2015). Molecular characterization of laccase enzyme contains approximately 500 amino acid residues, consecutive three domains, with a greek key β barrel topology that is circulated in a single molecule (Matera et al., 2008). The first domain contains initial 150 amino acids, second domain possesses between the 150 and 300 residues and the third domain contains 300 to 500 amino acids (Chandra and Chowdhary, 2015). The structure is stabilized generally into two disulfide bridges bond localized between domains I to II and domains I to III are also present (Matera et al., 2008; Ferraroni et al., 2007; Bertrand et al., 2002). While some laccase is also present in three disulfide bridges bond. Melanocarpus albomyces has disulfide bridges inside domain I, another between domain I and domain III, and the last one between domain II and III (Hakulinen et al., 2002). Laccase has three types of copper atoms present in variations linked with coordinate with each other's and maintain the active site of the amino acids. The first domain (T1 Cu) is also called substrate reducing site shows a diverse triangular planar coordination similarly to other multicopper oxidases (Dwivedi et al., 2011). They displayed active site contains a sequence of the amino acid such as one cysteine (Cys) and two histidines (His) as equatorial ligands. In contrast, other multicopper oxidases enzyme contains an additional axial ligand with methionine (Matera et al., 2008; Ferraroni et al., 2007; Bertrand et al., 2002; Hakulinen et al., 2002). In another way, T2 Cu exhibits coordination with two His amino acid and a water molecule. While, in T3 Cu, the two Cu molecules with six His are present in two groups of three active sites to share oxygen molecule that reduced into a water molecule and split out (Matera et al., 2008; Ferraroni et al., 2007; Bertrand et al., 2002) (Figure 2). Laccase is considered to be an ideal “green catalysts” because of its oxidizing property is a vast variety of compounds using O2 and liberating H2O as the only by-product (Alcalde et al., 2006, 2007; Giardina et al., 2010). In Table 2, the detail of bacterial species strain/laccase-like protein and their functions are mentioned.
Figure 2.
Molecular structure and active site of Laccase.
Table 2.
Bacterial strain/laccase-like protein and their function.
| Strains name | Protein | Functions | References |
|---|---|---|---|
| Aquifexaeolicus | SufI-2983586 | Work as cell division protein | Deckert et al. (1998) |
| Bacillus SF | Particularly stable at high temp. and pH if still bound to spore, Total decolorization of several phenolic dyes | Held et al. (2005) | |
| Streptomyces griseus | EpoA | Morphogenesis | Endo et al. (2002), Endo et al. (2003) |
| Streptomyces lavendulae | Thermostable broad substrate specificity | Suzuki et al. (2003) | |
| Bordetella pertussis | Contig-449e | ||
| Campylobacter jejuni | Contig-1e | ||
| Caulobactercrescentus | Contig-122e | ||
| Escherichia coli | PcoA-1073341 | ||
| Escherichia coli | YacK-2506227 | Cu2+ oxidation of phenolate-siderophores ferrooxidase activity | Kim et al. (2001); Roberts et al. (2003) |
| Escherichia coli | CueO | ||
| Mycobacterium avium | Contig-982e | ||
| Mycobacterium tuberculosum | Rv0846c-2916905 | ||
| Pseudomonas putida | CumA-4580028 | Mn2+ oxidation, Decolorization of violacein and azodyes | Brouwers et al. (1999); Senan and Abraham (2004) |
| Pseudomonas syringae | CopA -116921 | Cu2+ resistance activity | Cha and Cooksey (1991) |
| Pseudomonas aeruginosa | Contig-52e | ||
| Rhodobactercapsulatus | 3128288 | ||
| Xanthomonascampestris | CopA-1073083 | Cu2+ resistance | Lee et al. (1994) |
| Yersinia pestis | Contig-768e | ||
| Bacillus subtilis, | Cot A | Pigmentation of spores, UV and H2O2 resistance, Oxidation of substituted phenols | Hullo et al. (2001) |
| S.cerevisiae | RAD9 | ||
| Marinomonasmediterranea | PpoA | Pigmentation | Sanchez-Amat and Solano (1997), 2001) |
3.1.1. Natural mediators and synthetic mediators
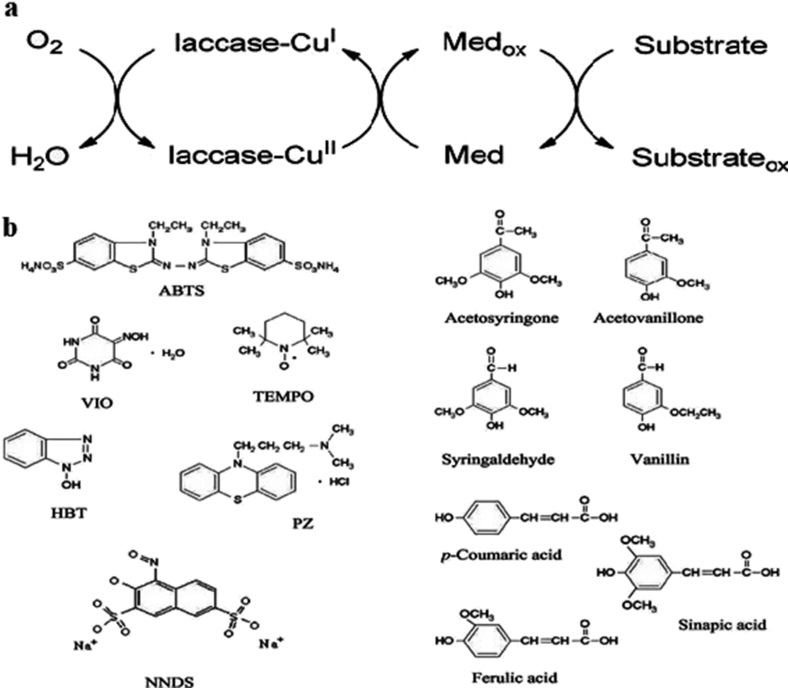
The low redox potential of laccase hinders them from directly taking part in catalysis of the complex compounds. Due to the uses of several natural and synthetic mediators should be possessing properties to cyclic bring efficient, low-cost, non-toxic, stable, oxidizing and reducing forms that enzyme reaction should not be inhibited (Morozova et al., 2007,Call and Mucke, 1997,Chandra, 2015). However, the redox mediators should be able to constantly control and conserve the cyclic redox process of conversion. A mediator is a small chemical compound that is continuously oxidized by the enzyme and then reduced by the substrate. As the compound especially lignocellulose complex, enzyme active site cannot penetrate, due to low affinity of enzyme. Due to this enzyme cannot directly oxidized substrate, for uses several mediators of the oxidizing substrate (Li et al., 1999; Christopher et al., 2014). However, mediators for laccase induction are a component of lignin and it can be removed by several enzymes i.e. feruloyl esterase. This enzyme acts as a mediator and also helps in dissolving lignin polysaccharide complexes (Ozer et al., 2019). The laccase enzyme activities are decreased with increasing the substrate size, this substrate accessibility is overcome through use of suitable laccase mediators. In the first step of the reaction, mediators are oxidized for unstable intermediates within high redox potential through laccase and followed controlled reaction kinetics. The oxidized mediator diffuses away from the enzyme active site, and its small size is able to penetrate into the pores of the plant cell walls to reach the target substrate. On the other hand, the vast range of substrate oxidized through laccase and it can be further accelerated by a mediator. In some cases, mediator is very reactive unstable cationic radicals which can oxidize more complex substrate before returning into their original state. Some ideal mediators like TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) and its analogs favor the ionic way and mediators such as HBT (1-hydroxybenztriazole) and ABTS [2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid)] react via radical paths (Wells and Teria, 2006; Fabbrini et al., 2002) shown in Figure 3.
Figure 3.
Laccase-mediator system b Structures of some representative artificial (ABTS, HBT, violuric acid -VIO-, TEMPO, promazine -PZ- and 1-nitroso-naphthol- 3,6-disulfonic acid -NNDS-) and natural mediators (AS, SA, vanillin, acetovanillone, p-coumaric acid, ferulic acid, and sinapic acid) (Kunamneni et al., 2008a, b).
Enzyme inhibitors are small, low molecular weight molecule that binds to an enzyme and inhibiting activity by blocking the active site. However, decreasing the enzyme's activity can kill a pathogen or correct a difference in metabolic functions (Fabbrini et al., 2002). Several drugs may also consider as enzyme inhibitors. Some most common inhibitors are vanillin, catechol, 4-methyl catechol, vanillic acid, 4-Hydroxybenzaldehyde, 4-Hydroxybenzoic acid, syringic acid, ferulic acid, 2-furaldehyde, formic and acetic acid.
3.1.2. Oxidation mechanism of laccase: direct and in-direct
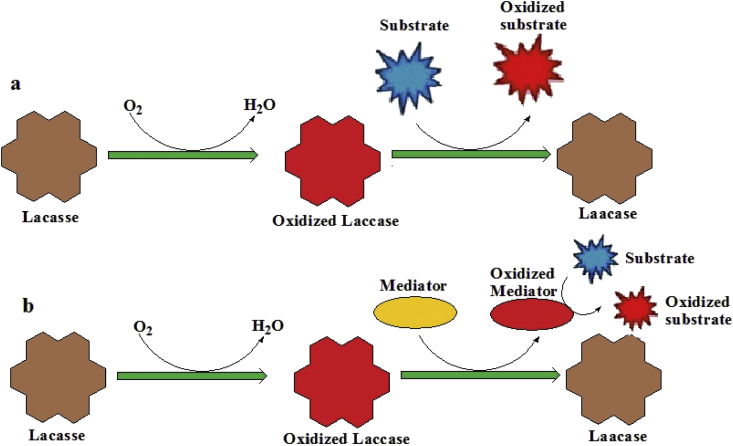
Laccase catalyzed reactions by two types: one is direct and second is indirect substrate oxidation. The direct oxidation contains the oxidation of substrate to the similar radical as a result of direct contact that occurs with copper cluster (Matera et al., 2008) shown as Figure 4. In some reactions, direct oxidation is not possible because T1 copper ion contains low redox potential (Morozova et al., 2007). However, in some cases limitation of enzyme does not directly catalyze the substrate and proceeded by using several mediators which, two-step process: the first enzyme catalyzes the mediator and then mediator oxidized the substrate known as indirect substrate oxidation process shown in Figure 4. In order to occurrence of reaction without any barrier, certain characteristics should be shown by mediators. These include a good substrate for laccase both in its oxidizing and reducing forms, do not inhibit the enzymatic reaction, and conversion must be cyclic in nature (Johannes and Majcherczyk, 2000; Komal et al., 2018).
Figure 4.
Catalyzed cycle by laccase; direct oxidation: the substrate is oxidized and b in-direct oxidation: the substrate is oxidized in the presence of a mediator (Komal et al., 2018).
3.1.3. Catalytic mechanisms of laccase
Laccase enzyme broadly divided into three categories based on its functions: (1) ring cleavage of organic compounds, (2) degradation of biopolymers and (3) cross-linking structure of monomers (Kawai et al., 1988; Dwivedi et al., 2011), depends upon the Cu atoms dispersion among the three altered binding sites. These Cu atoms play a crucial role in the enzyme catalytic mechanisms. Laccase active site is well conserved it contains three copper sites namely type 1 (T1, one Cu atom), type 2 (T2, one Cu atom) and type 3 (T3, two Cu atoms) per molecule of laccase (Chandra and Chowdhary, 2015). T1Cu has been showed electro-paramagnetic resonance (EPR) and blue color protein absorbance at about 600nm. T2Cu does not show any color but it can be detectable through electro-paramagnetic resonance (EPR) spectroscopy. And T3Cu are binuclear contains a pair of Cu atom that gives a weak absorbance (330nm) and cannot be detected by EPR spectroscopy (Chandra and Chowdhary, 2015). T1Cu is the substrate-binding site while T2 and T3 Cu bind the inducer/inhibitor and oxygen binding site. The O2 molecule binds to both Type 2 and Type 3 Cu active site that consists of a trinuclear cluster for the asymmetric activation. Molecular oxygen acts as an electron acceptor that represents one catalytic cycle of substrate oxidation. The electrons are transferred internally from T1Cu site to a trinuclear cluster made up of Type 2 and Type 3 Cu site, which O2 is involved in the enzyme catalytic mechanism (Chandra and Chowdhary, 2015). Hydrogen peroxidase is not detected outside of laccase during steady-state laccase catalysis which represented that a four-electron reduction of oxygen performed splitting of water molecule (Gianfreda et al., 1999). Laccase enzyme has the ability to operate the array of reducing molecule oxygen-storing electrons from separate substrate oxidation to reduce the reaction of molecular oxygen shown in Figure 5. There unique broad range substrate specificity acidic to alkaline conditions (Dwivedi et al., 2011).
Figure.5.
(A) Laccase active site with arrows marking the flow of substrates, electrons (e-), and O2, (B) mechanism of bacterial laccase (Solomon et al., 2008).
3.1.4. Laccase oxidation of phenolic and nonphenolic compounds
Laccase has capability to act on lignocellulosic substances containing phenolic and non-phenolic as well as on extremely residual compounds for detoxification and degradation. They considered as efficient tool for bioremediation. Laccase reduces one electron from hydroxyl groups of phenolic lignin-containing compounds, such as vanillyl glycol, 4,6-di (t-butyl) guaiacol, and syringaldehyde to generate phenoxy radicals by coupling undergo polymerization (Kawai et al., 1999a). The degradation of phenolic compound such as β-1 lignin model (1-(3,5-dirnethoxy-4-hydroxyphenyl)-2 (3,5-dimethoxy-4-ethoxyphenyl)propane-1,3-diol) occurs through the generation of phenoxy radicals (1-(3,5-dirnethoxy-4-hydroxyphenyl)-2 (3,5-dimethoxy-4-ethoxyphenyl)-3hydroxypropanone, 2,6-dimethoxy-p-benzoquinone, 2,6-dimethoxy-p-hydroquinone, 2-(3,5-dimethoxy-4-ethoxyphenyl)-3-hydroxy-propanal) that leads to aromatic ring cleavage, alkyl-aryl cleavage, Cα oxidation, Cα-Cβ cleavage (Kawai et al., 1999a, 1999b; Zoppellaro et al., 2000; Wong, 2009).
Laccase play a vital role in depolymerization of several compounds such as lignin and its derivatives for delignification of oxidizes β-O-4 lignin dimers are non-phenolic model compounds in the presence of a mediator (Bourbonnais and Paice, 1990). Four types of reactions, Cα-oxidation, Cα-Cβ, cleavage, β-ether cleavage, and aromatic ring cleavage, are catalyzed by laccase- (Butylated hydroxytoluene) BHT coupled system (Wong, 2009). The degradation of β-O-4 lignin model compounds, 1-(4-ethoxy-3-mthoxyphenyl)-1, 3-dihydroxy-2- (2, 6-dimethoxyphenoxy) propane, the enzyme system association and catalyzes 1e− oxidation of the substrate to formed β-aryl radical that is cation or benzylic (Cα) in nature (Kawai et al., 2002, 2004). However, if these reactions occurring through cleavage of the aromatic ring, they must be formed aryl cation radicals from degraded products (Umezawa, 1988). Recently, β-ether cleavage of a non-phenolic β-O-4 dilignol through fungus species (Trametes villosa laccase)–1-HBT associated with mediator interface for the delignification activities of pulp and kraft paper waste has been evaluated (Bourbonnais et al., 1998; Pfaller et al., 1998; Srebotnik et al., 1998).
| β-O-4 dilignol + Laccase + HBT → Aryl cation radicals |
3.2. Properties of lignin peroxidase
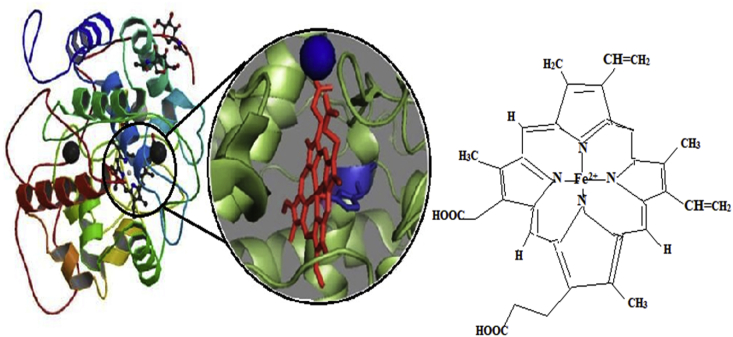
Lignin peroxidases (EC 1.11.1.14) belong to family oxidoreductase, which degrades lignin and its derivatives in the presence of H2O2 (Edwards et al., 1993). These are heme-containing enzymes secreted mainly by higher fungi and some bacteria, which degrade the polymer via an oxidative process (Pothiraj et al., 2006). In Addition, some insects have used this enzyme to digest higher woody debris such as Reticulitermes flavipes and eastern subterranean termite. Chemical structure of lignin peroxidases (LiPs) are monomeric glycosylated containing enzyme that has molecular weight (40–68 kDa) with four carbohydrates, 370 water molecules, 343 amino acids residues, two calcium ions, and heme group (Choinowski et al., 1999). On the other hand, LiP is helicoidal in nature contains major and minor eight helixes, two anti-parallel beta-sheets and two domains at both sides of the heminic group. The heme; includes 40 residues, that connects to protein by hydrogen bridges. This group supplements the protein but has two small channels for solvent accessibility (Choinowski et al., 1999). Furthermore, the heme iron associated with His amino acid and high redox potential of the enzyme. Distance between each heme group and His amino acid increases enzymatic redox potential and there creates an electronic deficiency in the porphyrin ring of the iron (Choinowski et al., 1999; Hammel and Cullen, 2008). In addition, molecular structure of lignin peroxidase producing fungi (Phlebia radiata) have molecular weight 38439.0 and contains 361 amino acids, theoretical isoelectric point is 4.29, formula C1692H2604N456O535S17, negatively charged remains (Asp + Glu) 48, positively charged remains (Arg + Lys) 19, index of computed instability (II) 44.32, index of aliphatic 76.32 and grand average of hydropathy -0.047 (Swapnil et al., 2016).
3.2.1. Catalytic mechanisms of lignin peroxidase
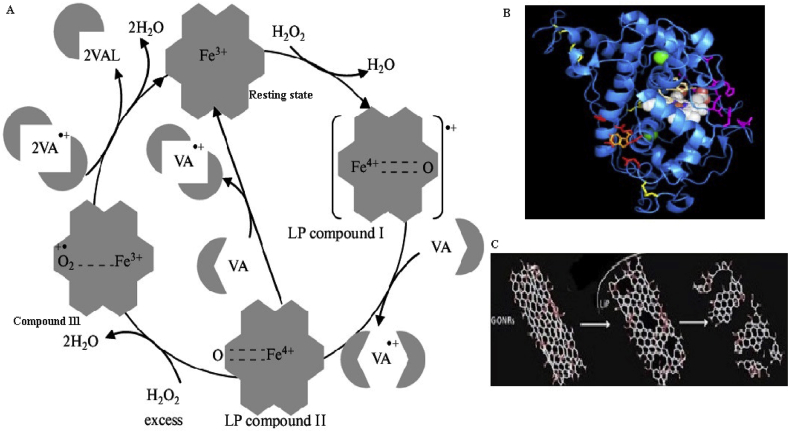
LiP enzymes are produced by several ligninocellulytic microorganisms that utilized in the efficient degradation of lignocellulosic waste. The overall LiP catalyzed mechanism is a two-step reaction involving the native enzyme of the ferric resting state, (1) the radical cation oxoferryl unstable intermediate compound I and (2) the impartial oxoferryl intermediate compound II (Castro et al., 2016). These enzymes also can oxidize substrates through electron transfer in the multi-step process (Wong, 2009). LiP is capable of oxidizing various aromatic organic compounds with high redox potentials than 1.4V by one electron abstraction, but this redox mechanism is not fully understood till yet (Piontek et al., 2001). LiPs are also degrading various phenolic compounds as well as lignin in the presence of co-substrate of the H2O2 and mediators like veratryl alcohol (VA). The reducing veratryl alcohol is formed [Fe(IV) = O, LiP-II] compound II and a VA radical cation (VA.+) and enzyme then return native state through 1e-reduction followed by finalizing the catalytic cycle (Pollegioni et al., 2015). The reaction of LiP–I with a reducing substrate to form LiP-II is pH-dependent and the rate decreased with increasing pH. Certainly, the pH dependence of reducing LiP–I to LiP-II rather than forming LiP–I determines the unusual low pH and optimized for the enzyme. The porphyrin π-cation radical first accepts an electron from the substrate in the first step of decrease and concomitant with a proton transfer to the distal His, LiP-II thus formed together with porphyrin field by the donor substrate is one-electron oxidation equal above the native LiP (Wong, 2009). In some cases, LiP–I can also return to the native state of the enzyme through a direct two-electron reduction (Castro et al., 2016) shown in Figure 6.
Figure 6.
a) Catalytic mechanisms of lignin peroxidase (Kulikova et al., 2011) b) LiP molecular structure c) LiP degradation of the compound structure.
3.2.2. Lignin peroxidase for the oxidation of phenolic and nonphenolic compounds
Lignin peroxidase enzyme has been used for oxidation of various organic compounds with the assistance of co-substrate (H2O2) as a mediator. These enzymes play a key role in the degradation of lignin-containing compounds β-O-4-linked and several non-phenolic lignin derivatives to homologous ketones or aldehydes. They are also involved in hydroxylation of benzylic methylene groups of aromatic ring cleavages (Archibald, 1992; Ikehata et al., 2004). It catalyzes oxidation of phenolic compounds such as vanillyl alcohol, catechol, acetosyringone, syringic acid, and guaiacol, etc. preferentially at a much faster rate compared to non-phenolic compounds. LiP is a key initiator for enzymes that are responsible for the breakdown of lignocellulosic-containing substance. This enzyme has the capability to oxidize phenolic compounds (1,2-bis (3,4-dimethoxyphenyl) propane-1,3-diol) results in Cα-Cβ cleavage to yield phenoxy radicals (1-(3,4-dimethoxyphenyl) ethane-1,2-diol, 3,4-dimethoxy benzaldehyde) (Wong, 2009). Oxidation of lignin monomer such as veratryl alcohol to veratraldehyde is the best-suited reaction for the standard assay of lignin peroxidases Ten Have et al., 1999.
| Veratryl alcohol + LiP + H2O2→ Veratraldehyde + H2O |
Lignin peroxidase has been also oxidized various non-phenolic compounds such as diarylpropane and β-O-4 lignin dimers to formed radical cation by 1e− oxidation through cleavage of side-chain for demethylation, methylation hydroxylation, intermolecular and dimerization (Christian et al., 2005; Baciocchi et al., 2001). The degradation of the non-phenolic compound such as 1-(3,4-diethoxyphenyl-1,3- dihydroxy, 4-methoxy-phenyl)-propane (diarylpropane lignin model compound) in the presence of lignin peroxidase and H2O2 during 1e-reduction to generated the cation radicals diethoxybenzaldehyde, 1-(4-methoxyphenyl) 1,2-dihydroxyethane and anisaldehyde (Lundell et al., 1993). The main route of oxidation is the ring cleavage Cα-Cβ (Lundell et al., 1993). In the mechanism of enzyme-catalysis, there is formation of radical cation whereas; other few reactions are non-enzymatic of the substrate. The decay of the cation radical depends on the groups that are present with aromatic rings (Harvey et al., 1985; Wariishi et al., 1991). Some alkoxy groups are donating electron to the aromatic ring formed and stabilized the cation aryl radical (Wong, 2009; Walling et al., 1984).
3.3. Manganese peroxidase: structure and properties
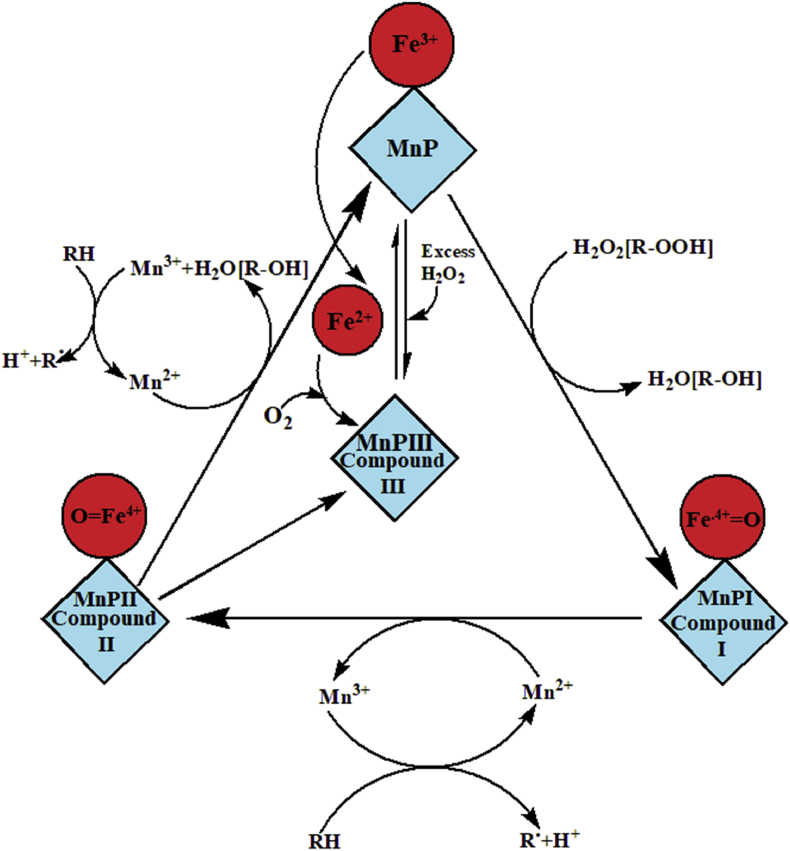
Manganese peroxidase (MnP) is a heme-containing enzyme that belongs to the oxidoreductases family (Kuwahara et al., 1984). Ligninolytic microorganisms secrete MnP enzyme in solid and liquid state into their microenvironments (Hatakka, 1994, 2003). The production of MnP isozymes averages molecular mass of 40–50kDa has reported in several bacteria, basidiomycetous fungi, and algae that utilize various genes after coding and regulating (Wong, 2009). MnP has been commonly observed as crucial enzymes for lignin degradation. The molecular structure contains MnP enzyme has two Ca2+ ions and five disulfide-bridging elements, that are responsible for maintaining the active site structure of the enzyme (Sutherland et al., 1997; Carmona-Ribeiro et al., 2015) as shown in Figure 7. The MnP enzyme active site comprises various amino acids such as proximal histidine ligand (His), H-bonded to an aspartic acid residue (Asp) and a distal side peroxidase-binding pocket containing catalytic His and arginine (Arg) residues. MnP enzyme is the crystal structure of the substrate-binding site and their mutants reveal that there is only one Mn2+ binding site, and consists of two water molecules, three acidic ligands and a heme propionate (Sundaramoorthy et al., 1997; Hofrichter, 2002). Ligninolytic microorganisms such as basidiomycetes, white-rot fungi, and bacteria oxidized Mn2+ to Mn3+ in a multistep process. The Mn2+ accelerates and triggers the functions and production of the substrate by MnP enzymes. Subsequently, the Mn3+ has been generated through MnP enzyme, acts as a mediator in the process of oxidation for several phenolic and non-phenolic compounds. The Mn3+ chelates oxalate is very small in size to diffuse into the active site of the enzyme. Certain case analogous structures of lignin, such as recalcitrant compounds buried deep within the soil, which is not inevitable for the enzymes (Ten Have and Teunissen, 2001). MnP enzymes possess specific, that act like an oxidase and peroxidase (Singh et al., 2011). Moreover, MnP not only catalyzes lignin and its derivative compounds but also catalyze the various non-phenolic compounds such as polycyclic aromatic hydrocarbons (PAH) through oxidation in the presence of H2O2 as an oxidant into the Mn2+ to Mn3+ (Steffen et al., 2003; Shin et al., 2005).
Figure 7.
Molecular structure and active site of MnP (Carmona-Ribeiro et al., 2015).
3.3.1. Catalytic mechanisms of MnP
The MnP catalytic cycle is started by H2O2 through binding to the resting ferric enzyme form an iron-peroxide complex (Hofrichter, 2002). Cleavage of peroxide enzyme O–O bond needs a two-electron transfer from the heme-porphyrin that leads to the formation of unstable intermediate MnP Compound I, (Fe4+ oxo-porphyrin radical cation). Consequently, dioxygen bond is cleaved heterolytically and one water molecule is spelled out (Hofrichter, 2002). A mono chelated Mn2+ ion is oxidized to Mn3+ and acts as one electron donor for this porphyrin to form unstable intermediate Compound II. Reduction of this intermediate Compound II proceeds in a similar way that another Mn3+ is formed from Mn2+ of leading to return of its resting state enzyme and second water molecule has been spelled out (Hofrichter, 2002) shown in Figure 8.
Figure 8.
Catalytic cycle of MnP (Hofrichter, 2002).
3.3.1.1. MnP compound I
In addition, the equivalent of hydrogen peroxidase in native MnP enzyme develops the electronic absorption spectrum. A Soret band at 407 nm (maximum) with decreased intensity correlates with resting-state of the enzyme at 558–650 nm (extra maxima), it seems to formed compound I. The spectral features of compound I are maximum similar to HRP (horseradish peroxidases) compound I of enzyme (Wariishi et al., 1988).
3.3.1.2. MnP compound II
Compound I has reduced one-electron of HRP compound by a substrate peroxidase and resulting in an unstable intermediate MnP compound II complex. This MnP compound II is red-shifted to 420 nm and the visible maxima are shown at 528 and 555 nm. The production of MnP compound II, under the condition having only H2O2 without any substrate, then probably H2O2 reduces compound I to compound II and it further oxidized into HO2/O2. (Wariishi et al., 1988).
3.3.1.3. MnP compound III
Addition of extra H2O2 can alter the compound II into unstable intermediate compound III (Dunford and Stillman, 1976). The absorption maxima for HRP compound III is 413, 546, 583 nm. The absorption spectrum of the ferrous-oxy species of HRP bent through the addition of oxygen to ferrous HRP (Yamazaki and Yokota, 1965) is very similar to that of HRP compound III. The HRP compound III encloses for oxidizing equivalents over the enzyme in the ferrous state (Dunford and Stillman, 1976). The excess of H2O2 in native MnP enzymes produces an intermediate complex with absorption maxima at 417, 545, and 579 nm. MnP compound III can also be formed with ferrous enzyme via additions of oxygen (Wariishi et al., 1988).
3.3.2. Manganese peroxidase enzyme oxidized lignocellulose-containing phenolic and nonphenolic compounds
MnP enzymes generated Mn (III) through oxidation of phenolic compounds such as phenol containing dyes, amines, and lignin derivatives. The Mn (III) is a chelator, their limited physiological conditions lead to the oxidation of phenolic lignin structures (Hammel et al., 1993). For phenolic compounds were 1e− oxidation from the compound (1-(3,5-dimethoxy-4-hydroxypheneyl)-2-(4-(hydroxymethyl)-2-methoxyphenoxy)1,3dihydroxypropane) into substrate Mn (III) chelator complex. It acts as a diffusible oxidant and generate intermediate phenoxy radicals, proceeds further to non-enzymatic degradation, bond cleavage and rearrangement to yield various products (1-(3,5-dimethoxy-4-hydroxypheneyl)-2-(4-(hydroxymethyl)-2-ethoxyphenoxyl)-1-oxo3-hydroxy-propane, 2,6-dimethoxy 1,4-benzoquinone, 2,6-dimethoxy 1,4-dihydroxybenzene) (Tuor et al., 1992).
Oxidation of several non-phenolic lignin derivative compounds via Mn (III) catalyzed the reaction in the presence of the second mediator. It utilized electron through the aromatic ring and form reactive radicals/phenoxy radical cation (Reddy et al., 2003). Mn (III) is present in thiols, such as glutathione, mediates the oxidation of replaced diarylpropane and benzyl alcohol structures into their respective ketones and aldehydes (Reddy et al., 2003; Wariishi et al., 1989c,Wariishi et al., 1989b). In these reactions, Mn (III) is oxidized thiols to generate thiyl radicals, therefor, benzylic radical substrate is formed. The enzyme contains Mn (III) also couples with peroxidation of lipids to catalyze β-aryl ether cleavage, Cα-Cβ cleavage non-phenolic diarylpropane, and, β-O-4 lignin, and its derivatives respectively (Reddy et al., 2003; Bao et al., 1994; Kapich et al., 2005).
3.4. Versatile peroxidase or hybrid peroxidase
Versatile peroxidase (VP) also known as hybrid peroxidase (manganese-lignin peroxidase) that contains glycoproteins and belongs to oxidoreductase family. The production of peroxidase shown by various fungal species such as Bjerkandera, Lipista, Pleurotus, and some bacterial sp. (Mester and Field, 1998; Zorn et al., 2003; Ruiz- Duenas et al., 1999; Sarkar et al., 1997; Camarero et al., 1996). The genetic studies of fungal genome have been revealed that they contain two families of enzymes such as LiP and MnP genes both in a ‘‘hybrid peroxidase’’ (Yadav et al., 2014). VP are attractive wild-type enzyme has dual oxidative capability with low to high redox potential, phenols dyes substituted and Mn2+ (Heinfling et al., 1998). These groups of enzymes are capable for oxidation of phenolic, non-phenolic and lignin derivatives in the absence of manganese. They did not require any mediator for oxidation of compounds.
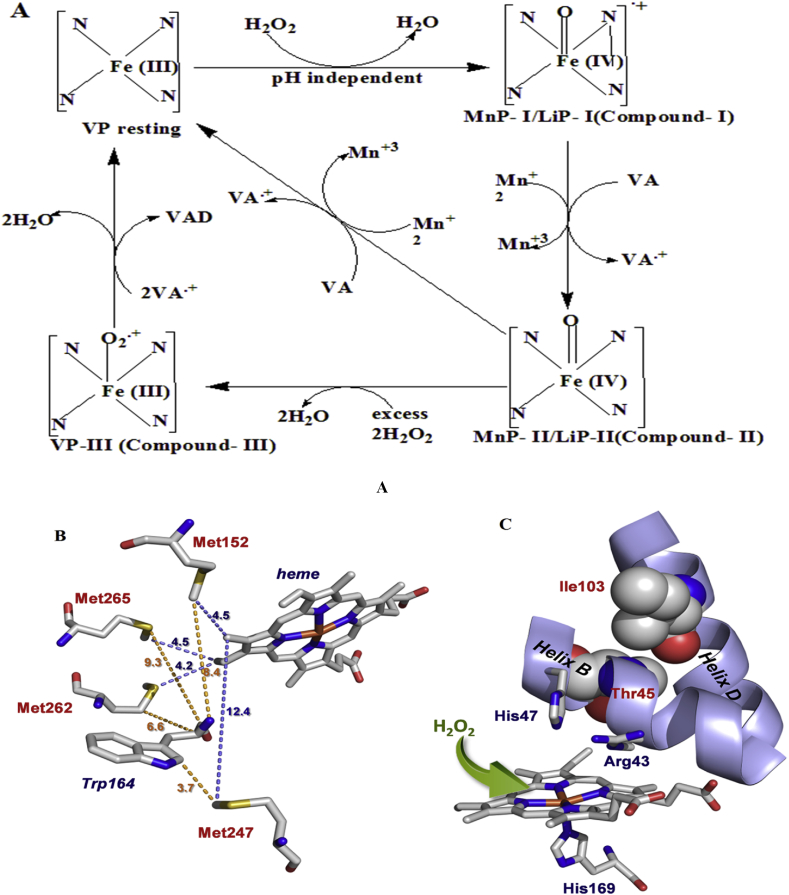
Molecular characterization of these versatile peroxidase enzymes seen as LiP and MnP isozymes that contain four amino acids (methionines) at positions 152, 247, 262 and 265 (Jimenez et al., 2015). They are suppressed within the molecular structures of enzyme, close to compose the heme cofactor and the catalytic Trp164 (Jimenez et al., 2015). Methionine amino acid substitution is an strategy intended to build the alteration of distal histidine amino acids (His47) in environment by mutations at Thr45 and Ile103. From this, the area occupied by two amino acids are placed straight to His47 (Ile103in helix D Thr45 and in helix B). It involves together with Arg43 in the two-electron initiation of the native enzymes through hydrogen peroxidase to form compound I (Jimenez et al., 2015). As the oxidation is probably associated with VP enzyme are inactivated substituted with less oxidizable amino acids by single-site- mutagenesis in directed (M247F, M152V, M265L, and M262F), dual (M265L/M262F) and several (/M265L/M152F/M262F and M262F/M265L/M152F/M247F) through variants (Jimenez et al., 2015). The P. eryngii produced VP that contains the molecular structure of 12 helices and 4 disulfide bonds, a heme pocket having features proximal His169, distal His47, and two structural Mn II as well as Ca+ binding site (Wong, 2009). This Mn (II) structure binding site having acidic in nature and different amino acids as shown by Glu36, Glu40, and Asp175 that adjoining the heme inner propionate.
3.4.1. Catalytic mechanisms of versatile peroxidase
The catalytic cycle for LiP, MnP, and another heme peroxidase are similar to VP. In which enzyme catalyzes the two-electron transfer through formation of unstable intermediate compound I and compound II (Morales et al., 2012; Wong, 2009). This involves two oxidation process, one is ferryl-oxo iron (Fe4+ = O) and another delocalized as a porphyrin/tryptophanyl radical. Compound I catalyze the substrate direct oxidation in which one-electron interacts with heme through tryptophanyl radical and formed Compound II (Jimenez et al., 2015). This intermediate stage, recollect the Fe4+ = O state and transfers the residual oxidation that is equivalent to the tryptophan in compound I. Intermediate compound II can also directly generate one-electron oxidation of substrates and shows interaction with the heme or tryptophanyl radical and returned to the enzymes original state (Jimenez et al., 2015). Compound I and Compound II both are unstable intermediates, very reactive compounds that lack a usual reducing substrate. Laterally in the excess of H2O2 concentration, formed compound III. Compound III is a superoxide anion (O2.-) having Fe3+ species (Wariishi and Gold, 1990). After the formation of compound III, it follows unlike pathways for the decomposition under superfluous of H2O2, producing reactive oxygen species have capability to oxidize side chains of porphyrin amino acid prior to inactivation of enzyme (Valderrama et al., 2002) shown in Figure 9.
Figure 9.
(a) Catalytic pathway and (b) molecular properties, of hybrid Versatile Peroxidase enzymes showing both MnP and LiP activities (Jimenez et al., 2015).
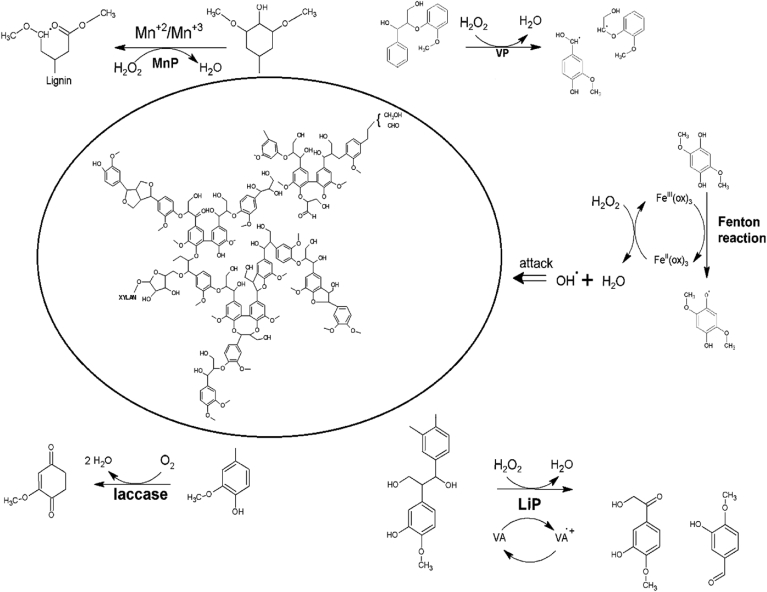
4. Role of ligninolytic enzymes in lignin degradation
Several microorganisms are producing ligninolytic enzymes that degrade lignocellulosic waste. These are major enzymes such as laccase and heme-containing peroxidases, for example, lignin, manganese, and multifunctional versatile peroxidase. In the last decade, there are several new approaches for delignification through enzyme activities have been reported (Figure 10). These enzymes have potential applications in biotechnology for biodegradation of lignin and other organic compounds.
Figure 10.
Comparison of lignin degradation by laccase and peroxidases manganese peroxidase (MnP), versatile peroxidase (VP), lignin peroxidase (LiP) (Janusz et al., 2017).
Recently, various residual lignocellulosic compounds are identified which are degraded by several bacteria and fungi through ligninolytic enzyme. The extracellular ligninolytic enzymes attack firstly on various types of bonds such as β-O-4 ether bond, biphenyl bond, O-demethylation enzyme systems, and aromatic organic pollutants performs one-electron oxidation to generate free cation radicals (Hammel, 1992). These cation radicals might suffer chemical reactions spontaneously such as hydroxylation or C–C bond cleavage resulting in hydrophilic products (Hammel and Moen, 1991). In lignin structure, several linkages are present such as β-O-4 aryl-ether linkage and biphenyl linkage is a fundamental part of lignin (Bugg et al., 2011). According to Masai et al. (1999), the bacterial species such as S. paucimobilis SYK-6 has potential to grow under the stress condition of lignin derivatives such as vanillate and syringate as the carbon source Sato et al., 2009. In this regard, vanillate and syringate have been altered into protocatechuate and 3-O-methylgallate through the O-demethylases LigM and DesA respectively (Kasai et al., 2005). Various fungal species are also metabolized those products that are taken up as a source of carbon to carbon dioxide (Hammel and Moen, 1991). The degradation mechanisms of ligninolytic enzymes are complex and connecting some cofactors low molecular weight compounds that can assist the redox mediators (Tunde and Ming, 2000). These are the multifaceted route i.e. hydroxylation, oxidation, reduction, and methylation for the degradation. On the contrary, the mechanisms of the ligninolytic enzymes for the degradation of lignin-containing compounds are not fully studied.
5. Improvement strategies for ligninolytic enzyme production
For the successful and attractive ligninolytic enzymes of high yields and excellent performance have significant importance towards industrial sector. There are several traditionally as well as modern technologies have been used for enhancing the degradation capacity of ligninolytic enzymes. The traditional technologies using ligninolytic enzymes have application in biotechnology. These are principally based on the screening of producing organisms and their optimization, purification of the enzyme and their biochemical characterization. However, modern technologies have been used for improved ligninolytic enzymes shown in Table 3: (1) firstly ligninolytic organisms used in genetic engineering, somewhere a heterologously produced molecule can be altered at the level of DNA. Secondary step for the developments in protein engineering to improve strategies for the specificity, stability, and economy for chemically modifying the wild type enzyme immobilization PEGylation, glycosylation and hydrophobization.
Table 3.
Improvement strategy for ligninolytic enzymes.
| Improvement strategy | Improved Characteristics | Reference |
|---|---|---|
| novel inducer with agitated submerged cultures | increase in enzyme production | Liebeskind et al. (1990) |
| Immobilization on electrodes | High electronic transfer | Gutierrez et al. (2012) |
| PEGylation | Improved thermal stability | Lopez et al. (2010) |
| Immobilization and orientation on MWCNT electrodes | Efficient electron transfer | Lalaoui et al. (2016) |
| Chemical modification of laccase amino acid residues with TDO and L-PME | Improved laccase activity and stability | Chen et al. (2016) |
| Substrate binding-pocket engineering | Improved catalytic efficiency | Gupta and Farinas (2009) |
| Heterologous expression | High redox potential laccase and thermal stability | Pinar et al. (2017) |
| Genetic engineering and purification | Enzymes orientated immobilization | Balland et al. (2008) |
| Codon optimization, heterologous expression, and computational analysis | Enhanced laccase production and substrate affinity | Rivera-Hoyos et al. (2013) |
| Heterologous expression, recombinant expression, and affinity chromatography | Improvement in production, time, and cost | Lambertz et al. (2016) |
| Immobilization and orientation on MWCNT electrodes | Efficient electron transfer | Lalaoui et al. (2016) |
| Solid-state and submerged fermentation | Production cost and optimization | Vantamuri and Kaliwal (2015) |
6. Industrial applications of ligninolytic enzymes
Extracellular ligninolytic enzymes have enormous potential for industrial applications in various areas including food processing, cosmetic, degradation and detoxification of pulp paper, textile, distillery effluent, and biosynthesis of various fine chemicals, biofuel production (Malherbe and Cloete, 2002; Maciel et al., 2010) shown in Table 4. Laccase-mediator system has been reported for depolymerization of lignin; delignify wood pulps, bleaching of kraft pulps as potential applications in pulp and paper industry (Widsten and Kandelbauer, 2008). Moreover, LiP and MnP participate in degradation of kraft and pulp paper mill effluents (Maijala et al., 2007). In food industry, laccase can be used to certain process that enhances the color appearance of food and beverages and eliminate the undesirable phenolic compounds. Besides, MnPs and LiPs have potential role in producing aromatic flavors (Barbosa et al., 2008). In the textile industry laccase, LiP and MnP are used for dye degradation and bleaching of waste generated from industries (Gomes et al., 2009; Kunamneni et al., 2008a; Shin, 2004). The major role of laccase, LiP, and MnP is in biodegradation of various polycyclic aromatic hydrocarbons (PAHs) and xenobiotics compounds (Anastasi et al., 2009; Wen et al., 2009; Robles-Hernandez et al., 2008). Its role has also been seen in manufacturing of medical, and pharmaceuticals utensils as well as polymers production, coupling of phenols and steroids, complex natural products synthesis, personal hygienic products, biosensors and bioreporters areas (Maciel et al., 2010; Ghindilis, 2000, Kuznetsov et al., 2001; Kunamneni et al., 2008a, b; Mikolasch and Schauer, 2009; Barbosa et al., 2008; Lee et al., 2006; Ponzoni et al., 2007). The most important has been reported in the conversion of lignocellulose waste material into value-added products such as animal feeds, composite, pulp and paper, biofuels, fine chemicals and several enzymes (Malherbe and Cloete, 2002).
Table 4.
Role of ligninolytic enzymes for Industrial applications.
| Enzyme | Substrate | Reaction | Applications |
|---|---|---|---|
| Laccase | Ortho and paradiphenols, aminophenols, polyphenols, polyamines, lignins, and aryldiamines | Oxidation, decarboxylation and demethylation of the substrate. | Paper and pulp industry, Food industry, textile industry, nanotechnology, synthetic chemistry, bioremediation, cosmetics, and so forth. |
| Lignin peroxidase | Halogenated phenolic compounds, polycyclic aromatic compounds and other aromatic compounds |
Oxidation of substrate in the presence of cosubstrate H2O2 and mediator like veratryl alcohol. |
Paper and pulp industry, Food industry, textile industry, pharmaceutical industry, bioremediation, and so forth. |
| Manganese peroxidase | Lignin and other phenolic compounds | In the presence of Mn2+ and H2O2 the co-substrate catalyzes oxidation of Mn2+ toMn3+ which results in anMn3+ chelate oxalate, which in turn oxidizes the phenolic substrates. | Paper and pulp industry, Food industry, textile industry, pharmaceutical industry, bioremediation, and so forth. |
| Versatile peroxidase | Methoxy benzenes and phenolic aromatic | The enzyme catalyzes the electron transfer from an oxidizable substrate, with the formation and reduction of compound I and compound II intermediates. |
Industrial biocatalyst, Bioremediation and so forth. |
| Cellulase | Cellulosic substance | Hydrolyzes the substrate to simple carbohydrates. | Paper and pulp industry, Textile manufacturing, detergent production, bioremediation, and so forth. |
7. Limitation of ligninolytic enzymes
The industrial application of ligninolytic enzymes for the biobleaching process in pulp paper industry faces the number of difficulties i.e. enzyme production of large scale, mediator cost, enzyme stability, transport limitations, accessibility, potential redox of the enzyme and substrate which restrict its commercialization. There is urgent to focus on extensive research, these specific parameters for their production and process optimization of technology. Moreover, the literature and knowledge available for the chemical properties of the ligninolytic enzyme and mechanism of action are fragmentary which also limits the interaction for its application. Literature showed several major studies on lignin degradation by fungi with ligninolytic enzyme but due to growth limitations their large scale utilization is not successful, and different methods suffer disadvantages due to the environmental conditions to which they are exposed. However, most of the fungi that have been reported so far in acidophilic conditions and require a semisolid fermentation process (Basu et al., 2015). Therefore, inefficient to grow on effluents containing medium of pulp and paper industries that remain alkaline.
The role of laccase has been found limited in biodegradation of lignin due to its low redox potential because laccase cannot have proper diffusion into pulp fiber. Often, mediators are used to overcoming these limitations that enhance the oxidation potential of laccase. According to Wong (2009), the role of mediators in biodegradation of lignin has been well covered. Several fungi (Pycnosporus cinnabarinus) does not produce either LiP or MnP and does produce the only laccase required for lignin breakdown by producing a metabolite that acts as a mediator for redox potential for the degradation of non-phenolic lignin-containing compounds by laccase (Eggert et al., 1996). Recent studies using the peroxidase family of enzymes, such as horseradish peroxidases (HRP) and myeloperoxidases (MPO), to degrade graphene oxide (Kotchey et al., 2011) and single and multi-walled carbon nanotubes (SWCNTs and MWCNTs, respectively) in vitro and in vivo (Russier et al., 2011; Allen et al., 2008; Zhao et al., 2011) have highlighted the importance of an eco-friendly enzymatic degradation strategy for carbon nanomaterials. However, there are several limitations to these methods, such as low degradation efficiency (Russier et al., 2011) and dependence on substrate chemistry, for instance, that decreased graphene oxide nanoribbons have not been degraded by horseradish peroxidase (Kotchey et al., 2011), hindering their practical use. Moreover, the reported single ligninolytic enzyme does not act on biodegradation, while some other enzymes i.e. feruloyl esterase, aryl-alcohol oxidase, quinone reductases and several mediators to help facilitate the process for degradation of lignocellulosic waste. Due to strong binding tendency of lignocellulosic waste also inhibited the enzyme activity, because of these limitations, improvements in the development of eco-friendly green approaches for the degradation of oxidized and reduced lignocellulosic materials remains an active area of research.
8. Future prospects
Lignocellulosic biomass is the single renewable resource on the earth which has broad prospective as biofuel material. Bioconversion of lignocellulosic materials has significant advantages over the other various value-added products i.e. bio-energy products such as bio-ethanol, 1-butanol, bio-methane, bio-hydrogen, organic acids including citric acid, succinic acid, and lactic acid, microbial polysaccharides, single-cell protein, and xylitol. They have potential to become future alternative energy sources because these biomaterials are the most abundant on the planet. Since the excess of lignocellulosic waste causes environmental problems, and concerns aimed at reducing the ambient pollution have boosted the search for “clean Technologies” to be used in the commodities of producing value-added bioproducts. Novel biotechnologies are vital for discovery, characterization of new ligninolytic enzymes for bioconversion process to degrade complex lignocellulosic waste in green biotechnology will be the main focus on future research.
9. Conclusions
Lignocellulosic waste being a major pollutant of the environment, containing the complex composition of cellulose, hemicellulose, and lignin along with plant resins and fatty acids. Due to its complex structure; lignin, resin and plant fatty acid they are not easily degradable by microbial communities. Therefore, these constituents of lignocellulosic waste have been detected has residual organic pollutants discharged from agro-waste industries. Hence, these pollutants require special attention for their complete degradation. Moreover, the lignocellulosic waste has strong binding tendency with cationic compounds i.e. heavy metals and salts, which aggravate their toxicity properties with the formation of complex structure. The natural capabilities of microorganisms to degrade lignocellulosic waste efficiently due to highly effective enzymatic systems are attractive as new strategies for the development of industrial processes. Therefore, the group of ligninolytic enzymes i.e. laccase, lignin peroxidase, manganese peroxidase, and versatile peroxidase involve in for their sequential degradation and transformation. Due to their structural (i.e. copper, iron, manganese and calcium ions) specificity (hydrophobic interaction, salt bridge, disulfide bridge, and hydrogen bond) it retains extremophilic property and stability. But the detailed mechanism is still unknown? In addition, Ligninolytic enzymes are potential applications for the degradation of various environmental pollutants including polycyclic aromatic hydrocarbons, synthetic dyes, pesticides, herbicides, polychlorinated biphenyls, xenobiotics, and several other lignocellulosic compounds. Ligninolytic enzymes are able to penetrate inside the structural unit of lignocellulosic waste for the degradation. This process makes use of another material that requires less energy, reduces the pollutants in environmental, as cost-effective and ecofriendly. Currently, several types of research across the planet worked on the biodegradation of industrial lignocellulosic waste using various microorganisms. The present study evaluated important information about the key role of ligninolytic enzymes for the biodegradation of lignocellulosic waste and their application in the field of biotechnology.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Ram Chandra was supported by DBT, New Delhi, India Letter No. BT/PR18896/BCE/8/1372/2016 dated 28-03-2018 and Adarsh Kumar was supported by University Grant Commission, New Delhi.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Aarti C., Mariadhas V.A., Paul A. Lignin degradation: a microbial approach. South Indian Journal of Biological Sciences. 2015;1(3):119–127. [Google Scholar]
- Alcalde M. Springer; New York: 2007. Industrial Enzymes: Structure, Functions and Applications; pp. 459–474. [Google Scholar]
- Alcalde M., Ferrer M., Plou F.J., Ballesteros A. Environmental biocatalysis: from remediation with enzymes to novel green processes. Trends Biotechnol. 2006;24:281–287. doi: 10.1016/j.tibtech.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Allen B.L., Kichambare P.D., Gou P., Vlasova I.I., Kapralov A.A., Konduru N. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 2008;8:3899–3903. doi: 10.1021/nl802315h. [DOI] [PubMed] [Google Scholar]
- Alvarez P.J.J., Anid P.J., Vogel T.M. Kinetics of aerobic biodegradation of benzene and toluene in sandy aquifer material. Biodegradation. 1991;2:43–51. doi: 10.1007/BF00122424. [DOI] [PubMed] [Google Scholar]
- Anastasi A., Coppola T., Prigione V., Varese G. Pyrene degradation and detoxification in soil by a consortium of basidiomycetes isolated from compost: role of laccases and peroxidases. J. Hazard Mater. 2009;165:1229–1233. doi: 10.1016/j.jhazmat.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Anwar F., Hussain S., Ramzan S., Hafeez F., Arshad M., Imran M., Maqbool Z., Abbas N. Characterization of reactive red-120 decolorizing bacterial strain Acinetobacter junii FA10 capable of simultaneous removal of azo dyes and hexavalent chromium. Water Air Soil Pollut. 2014;225:2017. [Google Scholar]
- Anwesha N.F., Lynne H.T., Clemens M.A., Philip C., Forsyth V.T., David C.A., Craig J.K., Michael C.J. Nanostructure of cellulose microfiberils in spruce wood. Proc. Natl. Acad. Sci. 2011;47:E1195–E1203. doi: 10.1073/pnas.1108942108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F.S. A new assay for lignin-type peroxidases employing the dye Azure B. Appl. Environ. Microbiol. 1992;58:3110–3116. doi: 10.1128/aem.58.9.3110-3116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora D.S., Sharma R.K. Enhancement in in vitro digestibility of wheat straw obtained from different geographical regions during solid state fermentation by white rot fungi. BioResour. 2009;4:909–920. [Google Scholar]
- Baciocchi E., Bietti M., Gerini M.F., Lanzalunga O., Mancinelli S. Oxidation of nonphenolic β-O-aryl-lignin model dimers catalyzed by lignin peroxidase, comparison with the oxidation induced by potassium 12-tungstocobalt (III)ate. J. Chem. Soc. 2001;41:506–1511. [Google Scholar]
- Bao W., Fukushima Y., Jensen K.A., Moen M.A., Hammel K.E. Oxidative degradation of non-phenolic lignin during lipid peroxidation by fungal manganese peroxidase. FEBS (Fed. Eur. Biochem. Soc.) Lett. 1994;354:297–300. doi: 10.1016/0014-5793(94)01146-x. [DOI] [PubMed] [Google Scholar]
- Balland V., Joseph P., Paul A. Modeling soil hydraulic properties for a wide range of soil conditions. Ecol. Model. 2008;219:300–316. [Google Scholar]
- Barbosa E.S., Perrone D., Vendramini A.L.A., Leite S.G.F. Vanillin production by Phanerochaete chrysosporium grown on green coconut agro-industrial husk in solid state fermentation. BioResources. 2008:1042–1050. [Google Scholar]
- Basu S., Bose C., Ojha N., Das N., Das J., Pal M., Khurana S. Evolution of bacterial and fungal growth media. Bioinformation. 2015;11(4):182–184. doi: 10.6026/97320630011182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand T., Jolivalt C., Briozzo P., Caminade E.J., Madzak C., Mougin C. Crystal structure of a four-copper laccase complexed with an Arylamine: insights into substrate recognition and correlation with kinetics. Biochemistry. 2002;41:7325–7333. doi: 10.1021/bi0201318. [DOI] [PubMed] [Google Scholar]
- Bourbonnais R., Paice M.G. Oxidation of non-phenolic substrates, an expanded role for laccase in lignin biodegradation. FEBS Lett. 1990;267:99–102. doi: 10.1016/0014-5793(90)80298-w. [DOI] [PubMed] [Google Scholar]
- Bourbonnais R., Paice M.G., Leech D., Rochefort D. Proceedings of the 7th International Conference on Biotechnology in the Pulp and Paper Industry. Vancouver; Canada: 1998. Laccase/mediator bleaching of kraft pulps: identification of mediator active species and their role in oxidation of residual lignin subunit; pp. A103–A106. [Google Scholar]
- Brouwers G.J., de Vrind J.P., Corstjens P.L., Cornelis P., Baysse C., Jong E.W. cumA, a gene encoding a multicopper oxidase, is involved in Mn2+ 469 oxidation in Pseudomonas putida GB-1. Appl. Environ. Microbiol. 1999;65:1762–1768. doi: 10.1128/aem.65.4.1762-1768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg T.D., Ahmad M., Hardiman E.M., Rahmanpour R. Pathways for degradation of lignin in bacteria and fungi. Nat. Prod. Rep. 2011;28:1883–1896. doi: 10.1039/c1np00042j. [DOI] [PubMed] [Google Scholar]
- Call H.P., Mucke I. History, overview and applications of mediated lignolytic systems, especially laccase-mediator-systems. Lignozym process. 1997;53 Issues 2–3 Pages 163–202. [Google Scholar]
- Camarero S., Bockle B., Martinez M.J., Martinez A.T. Manganese-mediated lignin degradation by Pleurotus pulmonarius. Appl. Environ. Microbiol. 1996;62:1070–1072. doi: 10.1128/aem.62.3.1070-1072.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Ribeiro A.M., Prieto T., Nantes I. Nanostructures for peroxidases. Front. Mol. Biosci. 2015;2:50. doi: 10.3389/fmolb.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro L., Crawford L., Mutengwa A., Gotze J.P., Michael B. Insights into structure and redox potential of lignin peroxidase from QM/MM calculations. Org. Biomol. Chem. 2016;14:2385–2389. doi: 10.1039/c6ob00037a. [DOI] [PubMed] [Google Scholar]
- Cha J.S., Cooksey D.A. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl.Acad. Sci. USA. 1991;88:8915–8919. doi: 10.1073/pnas.88.20.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra R. CRC Press, Taylor & Francis Group; Boca Raton/London/New York: 2015. Advances in Biodegradation and Bioremediation of Industrial Waste. [Google Scholar]
- Chandra R., Abhishek A. Bacterial decolorization of black liquor in axenic and mixed condition and characterization of metabolites. Biodegradation. 2011;22:603–611. doi: 10.1007/s10532-010-9433-1. [DOI] [PubMed] [Google Scholar]
- Chandra R., Abhishek A., Sankhwar M. Bacterial decolorization and detoxification of black liquor from rayon grade pulp manufacturing paper industry and detection of their metabolic products. Bioresour. Technol. 2011;102:6429–6436. doi: 10.1016/j.biortech.2011.03.048. [DOI] [PubMed] [Google Scholar]
- Chandra R., Raj A., Purohit H.J., Kapley A. Characterization and optimization of three potential aerobic bacterial strains for kraft lignin degradation from pulp paper waste. Chemosphere. 2007;67:839–846. doi: 10.1016/j.chemosphere.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Chandra R., Singh R., Yadav S. Effect of bacterial inoculum ratio in mixed culture for decolourization and detoxification of pulp paper mill effluent. J. Chem. Technol. Biotechnol. 2012;87:436–444. [Google Scholar]
- Chandra R., Chowdhary P. Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environ. Sci.: Processes Impacts. 2015;17:326–342. doi: 10.1039/c4em00627e. [DOI] [PubMed] [Google Scholar]
- Chandra R., Kumar V., Yadav S. Extremophilic ligninolytic enzymes. In: Sani R.K., Krishnaraj R.N., editors. Extremophilic Enzymatic Processing of Lignocellulosic Feedstocks to Bioenergy. Springer International Publishing; 2017. [Google Scholar]
- Chaurasia B. Biological pretreatment of lignocellulosic biomass (Water hyacinth) with different fungus for Enzymatic hydrolysis and Bio-ethanol production Resource: advantages, Future work and Prospects. Acta scientific agriculture. 2019 ISSN: 2581-365X. [Google Scholar]
- Chen Y., Stemple B., Kumar M., Wei N. Cell surface display fungal laccase as a renewable biocatalyst for degradation of persistent micropollutants bisphenol A and sulfamethoxazole. Environ. Sci. Technol. 2016;50:8799–8808. doi: 10.1021/acs.est.6b01641. [DOI] [PubMed] [Google Scholar]
- Chiang Gustavo, Barra Ricardo, Diaz-Jaramillo Mauricio, Rivas Meyling, Bahamonde Paulina, Munkittrick Kelly R. Estrogenicity and intersex in juvenile rainbow trout (Oncorhynchus mykiss) exposed to Pine/Eucalyptus pulp and paper production effluent in Chile. Aquat. Toxicol. 2015;164:126–134. doi: 10.1016/j.aquatox.2015.04.025. [DOI] [PubMed] [Google Scholar]
- Choinowski T., Blodig W., Winterhalter K.H., Piontek K. The crystal structure of lignin peroxidase at 1.70 Å resolution reveals a hydroxy group on the Cβ of tryptophan 171: a novel radical site formed during the redox cycle. J. Mol. Biol. 1999;286:809–827. doi: 10.1006/jmbi.1998.2507. [DOI] [PubMed] [Google Scholar]
- Christian V., Shrivastava R., Shukla D., Modi H.A., Vyas B.R. Degradation of xenobiotic compounds by lignin-degrading white-rot fungi: enzymology and mechanisms involved. Indian J. Exp. Biol. 2005;43(4):301–312. [PubMed] [Google Scholar]
- Christopher L.P., Yao B., Ji Y. Lignin biodegradation with laccase-mediator systems. Front. Energy Res. 2014 [Google Scholar]
- Cosgrove D.J. Growth of the plant cell wall. Molecular cell biology. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- Dashtban Mehdi, Schraft Heidi, Syed Tarannum A., Wensheng Qin. Fungal biodegradation and enzymatic modification of lignin. Int J Biochem Mol Biol. 2010;1(1):36–50. [PMC free article] [PubMed] [Google Scholar]
- Deckert G., Warren P.V., Gaasterland T., Young W.G., Lenox A.L., Graham D.E., Overbeek R., Snead M.A., Keller M., Aujay M., Huber R., Feldman R.A., Short J.M., Olsen G.J., Swanson R.V. The complete genome of the hyper thermophilic bacterium Aquifex aeolicus. Nature. 1998;392:353–358. doi: 10.1038/32831. [DOI] [PubMed] [Google Scholar]
- Dimarogona M., Topakas E., Christakopoulos P. Cellulose degradation by oxidative enzymes. Comput. Struct. Biotechnol. J. 2012;2 doi: 10.5936/csbj.201209015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunford H.B., Stillman J.S. On the function and mechanism of action of peroxidases. Coord. Chem. Rev. 1976;19:187–251. [Google Scholar]
- Dwivedi U.N., Singh P., Pandey V.P., KumarA Structure–function relationship among bacterial, fungal and plant laccases. J. Mol. Catal. B Enzym. 2011;68:117–128. [Google Scholar]
- Edwards S.L., Raag R., Wariishi H., Gold M.H., Poulos T.L. Crystal structure of lignin peroxidase. Proc. Natl. Acad. Sci. 1993;90:750–754. doi: 10.1073/pnas.90.2.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert C., Temp U., Eriksson K.E.L. The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl. Environ. Microbiol. 1996;62:1151–1158. doi: 10.1128/aem.62.4.1151-1158.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltis L.D., Bolin J.T. Evolutionary relationships among extradiol dioxygenases. J. Bacteriol. 1996;178:5930–5937. doi: 10.1128/jb.178.20.5930-5937.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo K., Hayashi Y., Hibi T., Hosono K., Beppu T., Ueda K. Enzymological characterization of EpoA, a laccase-like phenol oxidase produced by Streptomyces griseus. J. Biochem. 2003;133:671–677. doi: 10.1093/jb/mvg086. [DOI] [PubMed] [Google Scholar]
- Endo K., Hosono K., Beppu T., Ueda K. A novel extra cytoplasmatic phenol oxidase of Streptomyces: its possible involvement in the onset of morphogenesis. Microbiology. 2002;148:1767–1776. doi: 10.1099/00221287-148-6-1767. [DOI] [PubMed] [Google Scholar]
- Fabbrini M., Galli C., Gentili P. Comparing the catalytic efficiency of some mediators of laccase. J. Mol. Catal. B Enzym. 2002;16:231–240. [Google Scholar]
- Ferraroni M., Myasoedova N., Schmatchenko V., Leontievsky A., Golovleva L., Scozzafava A., Briganti F. Crystal structure of a blue laccase from Lentinus tigrinus evidences for intermediates in the molecular oxygen reductive splitting by multicopper oxidases. BMC Struct. Biol. 2007;7:60. doi: 10.1186/1472-6807-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs G., Boll M., Heider J. Microbial degradation of aromatic compounds -from one strategy to four. Nat. Rev. Microbiol. 2011;9:803–816. doi: 10.1038/nrmicro2652. [DOI] [PubMed] [Google Scholar]
- Gavrilescu D. Energy from biomass in pulp and paper mills. Environmental Engineering and Management Journal. 2008;7(5):537–546. [Google Scholar]
- Ghindilis A.L. Direct electron transfer catalysed by enzymes: application for biosensor development. Biochem. Soc. Trans. 2000;28:84–89. doi: 10.1042/bst0280084. [DOI] [PubMed] [Google Scholar]
- Gianfreda L., Xu F., Bollag J.M. Laccases: a useful group of oxidoreductive enzymes. Ann. Finance. 1999;3:1–25. [Google Scholar]
- Giardina P., Faraco V., Pezzella C., Piscitelli A., Vanhulle S., Sannia G. Laccases: a never-ending story. Cell. Mol. Life Sci. 2010;67:369–385. doi: 10.1007/s00018-009-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes E., Aguiar A.P., Carvalho C.C., Bonfa M.R.B., Da Silva R., Boscolo M. Ligninases production by Basidiomycetes strains on lignocellulosic agricultural residues and their application in the decolorization of synthetic dyes. Braz. J. Microbiol. 2009:31–39. doi: 10.1590/S1517-83822009000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillen F., Martinez M.J., Munoz C., Martinez A.T. Quinone redox cycling in the ligninolytic fungus Pleurotus eryngii leading to extracellular production of superoxide anion radical. Arch. Biochem. Biophys. 1997;339:190–199. doi: 10.1006/abbi.1996.9834. [DOI] [PubMed] [Google Scholar]
- Gupta N., Farinas E.T. Narrowing laccase substrate specificity using active site saturation mutagenesis. Send to Comb Chem High Throughput Screen. 2009;12(3):269–274. doi: 10.2174/138620709787581675. [DOI] [PubMed] [Google Scholar]
- Gutierrez P., Rocha L., Reyes S.N., Paredes V., Mendieta Araica B. Ruminal degradation rate of Moringa oleifera foliage in Reyna cattle using in sacco technique. La Calera. 2012;12(18):37–44. [Google Scholar]
- Hakulinen N., Kiiskinen L., Kruus K., Saloheimo M., Paananen A., Koivula A., Rouvinen J. Crystal structure of a laccase from Melanocarpus albomyces with an intact trinuclear copper site. Nat. Struct. Mol. Biol. 2002;9:601–605. doi: 10.1038/nsb823. [DOI] [PubMed] [Google Scholar]
- Hammel K.E. Oxidation of aromatic pollutants by lignin-degrading fungi and their extracellular peroxidases. In: Siegel H., Siegel A., editors. Metal Ions in Biological Systems Vol 28 Degradation of Environmental Pollutants by Microorganisms and Their Metalloenzymes. Marcel Dekker; New York: 1992. pp. 41–60. [Google Scholar]
- Hammel K.E., Cullen D. Role of fungal peroxidases in biological ligninolysis. Curr. Opin. Plant Biol. 2008;11:349–355. doi: 10.1016/j.pbi.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Hammel K.E., Moen M.A. Depolymerization of a synthetic lignin in vitro by peroxidase. Enzym. Microb. Technol. 1991;13:15–18. [Google Scholar]
- Hammel K.E., Jensen Mozuch K.A.M.D., Jr., Landucci L.L., Tien M., Pease E.A. Ligninolysis by a purified lignin peroxidase. J. Biol. Chem. 1993;268:12274–12281. [PubMed] [Google Scholar]
- Harvey P.J., Schoemaker H.E., Bowen R.M., Palmer J.M. Single-electron transfer processes and the reaction mechanism of enzymic degradation of lignin. FEBS (Fed. Eur. Biochem. Soc.) Lett. 1985;183:13–16. [Google Scholar]
- Hatakka A. Lignin-modifying enzymes from selected white-rot fungi: production and role from in lignin degradation. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Rev. 1994;13:125–135. [Google Scholar]
- Hatakka A., Lundell T., Hofrichter M., Maijala P. Manganese peroxidase and its role in the degradation of wood lignin. In: Mansfield S.D., Saddler J.N., editors. Applications of Enzymes to Lignocellulosic. 2003. pp. 230–243. [Google Scholar]
- Heinfling A., Ruiz-Duenas F.J., Martinez M.J., Bergbauer M., Szewzyk U., Martinez A.T. A study on reducing substrates of manganese-oxidizing peroxidases from Pleurotus eryngii and Bjerkandera adusta. FEBS (Fed. Eur. Biochem. Soc.) Lett. 1998;428:141–146. doi: 10.1016/s0014-5793(98)00512-2. [DOI] [PubMed] [Google Scholar]
- Held C., Kandelbauer A., Schroeder M., Cavaco-Paulo A., Guebitz G.M. Biotransformation of phenolics with laccase-containing bacterial spores. Environ. Chem. Lett. 2005;3:74–77. [Google Scholar]
- Hofrichter M. Review: lignin conversion by manganese peroxidase (MnP) Enzym. Microb. Technol. 2002;30:454–466. [Google Scholar]
- Horn S.J., Vaaje-Kolstad G., Westereng B., Eijsink V.G.H. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels. 2012;5:45. doi: 10.1186/1754-6834-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R.L., Abotsi E., Jansen van Rensburg E.L., Howard S. Lignocellulose biotechnology: issues of bioconversion and enzyme production. Afr. J. Biotechnol. 2003:602–619. [Google Scholar]
- Hullo M.F., Moszer I., Danchin A., Martin-Verstraete I. CotA of Bacillus subtilis is a copper-dependent laccase. J. Bacteriol. 2001;183:5426–5430. doi: 10.1128/JB.183.18.5426-5430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehata K., Buchanan I., Smith D. Recent developments in the production of extracellular fungal peroxidases and laccases for waste treatment. J. Environ. Eng. Sci. 2004;3:1–19. [Google Scholar]
- Janusz G., Pawlik A., Sulej J., Burek U.S., Wilkołazka A.J., Paszczynski A. Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiology Reviews fux049. 2017;41:941–962. doi: 10.1093/femsre/fux049. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez-Jiménez V., Acebes S., Guallar V., Martínez A.T., Ruiz-Dueñas F.J. Improving the oxidative stability of a high redox potential fungal peroxidase by rational design. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0124750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes C., Majcherczyk A. Laccase activity tests and laccase inhibitors. J. Biotechnol. 2000;78(2):193–199. doi: 10.1016/s0168-1656(00)00208-x. [DOI] [PubMed] [Google Scholar]
- Kadam A.A., Kamatkar J.D., Khandare R.V., Jadhav J.P., Govindwar S.P. Solid-state fermentation: tool for bioremediation of adsorbed textile dyestuff on distillery industry waste-yeast biomass using isolated Bacillus cereus strain EBT1. Environ. Sci. Pollut. Res. 2013;20:1009–1020. doi: 10.1007/s11356-012-0929-6. [DOI] [PubMed] [Google Scholar]
- Kapich A.N., Steffen K.T., Hofrichte r M., Hatakk a A. Involvement of lipid oxidation in the degradation of a non-phenolic lignin model compound by manganese peroxidase of the litter decomposing fungus Stropharia coronilla. Biochem. Biophys. Res. Commun. 2005;330:371–377. doi: 10.1016/j.bbrc.2005.02.167. [DOI] [PubMed] [Google Scholar]
- Kasai D., Masai E., Miyauchi K., Katayama Y., Fukuda M. Characterization of the gallate dioxygenase gene: three distinct ring cleavage dioxygenases are involved in syringate degradation by Sphingomonas paucimobilis SYK-6. J. Bacteriol. 2005;187:5067–5074. doi: 10.1128/JB.187.15.5067-5074.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Iwatsuki M., Nakagawa M., Inagaki M., Hamabe A., Ohashi H. An alternative β-ether cleavage pathway for a non-phenolic β-O-4 lignin model dimer catalyzed by a laccase-mediator system. Enzym. Microb. Technol. 2004;35:154–160. [Google Scholar]
- Kawai S., Nakagawa M., Ohashi H. Degradation mechanisms of nonphenolic β-O-4 lignin model dimer by Trametes versicolor laccase in the presence of 1-hydroxybenzotriazole. Enzym. Microb. Technol. 2002;30:482–489. [Google Scholar]
- Kawai S., Umezawa T., Higuchi T. Degradation mechanisms of phenolic β-1 lignin substructure model compounds by laccase of Coriolus versicolor. Arch. Biochem. Biophys. 1999;262:99–110. doi: 10.1016/0003-9861(88)90172-5. [DOI] [PubMed] [Google Scholar]
- Kawai S., Umezawa T., Shimada M., Higuchi T. Aromatic ring cleavage of 4,6-di(tert) guaiacol, a phenolic lignin model compound, by laccase of Coriolus versicolor. FEBS (Fed. Eur. Biochem. Soc.) Lett. 1999;236:309–311. doi: 10.1016/0014-5793(88)80043-7. [DOI] [PubMed] [Google Scholar]
- Kawai S., Umezawa T., Shimada M., Higushi T. Aromatic ring cleavage of 4, 6-di(tert-butyl) guaiacol, a phenolic lignin model compound, by laccase of Coriolus versicolor. FEBS Lett. 1988;236:309–311. doi: 10.1016/0014-5793(88)80043-7. [DOI] [PubMed] [Google Scholar]
- Kersten P., Cullen D. Extracellular oxidative systems of the lignin-degrading Basidiomycete Phanerochaete chrysosporium. Forest Genet Biol. 2007;44:77–87. doi: 10.1016/j.fgb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Kharayat Y. Distillery wastewater: bioremediation approaches. J. Integr. Environ. Sci. 2010;9(2):69–91. [Google Scholar]
- Kim C., Lorenz W.W., Hoopes J.T., Dean J.F. Oxidation of phenolate siderophores by the multicopper oxidase encoded by the Escherichia coli yacK gene. J. Bacteriol. 2001;183:4866–4875. doi: 10.1128/JB.183.16.4866-4875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal Komal, Chaturvedi Venkatesh, Verma Pradeep. Fungal laccase discovered but yet undiscovered. Bioresources and Bioprocessing. 2018;5:4. [Google Scholar]
- Kotchey G.P., Allen B.L., Vedala H., Yanamala N., Kapralov A.A., Tyurina Y.Y. The enzymatic oxidation of graphene oxide. ACS Nano. 2011;5:2098–2108. doi: 10.1021/nn103265h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikova N.A., Kleina O.I., Stepanovaa E.V., Koroleva O.V. Use of basidiomycetes in industrial waste processing and utilization technologies: fundamental and applied aspects (review) Appl. Biochem. Microbiol. 2011;47(6):565–579. [PubMed] [Google Scholar]
- Kumar V., Chopra A.K. Ferti-irrigation effect of paper mill effluent on agronomical practices of Phaseolus vulgaris (L.) in two seasons. Journal Communications in Soil Science and Plant Analysis. 2014;45:2151–2170. [Google Scholar]
- Kunamneni A., Ghazi I., Camarero S., Ballesteros A., Plou F.J., Alcalde M. Decolorization of synthetic dyes by laccase immobilized on epoxy-activated carriers. Process Biochem. 2008:169–178. [Google Scholar]
- Kunamneni A., Camarero S., Garcia-Burgos C., Plou F.J., Ballesteros A., Alcalde M. Engineering and Applications of fungal laccases for organic synthesis. Microb. Cell Factories. 2008;7:32. doi: 10.1186/1475-2859-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara M., Glenn J.K., Morgan M.A., Gold M.H. Separation and characterization of two extracellular H2O2 dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS (Fed. Eur. Biochem. Soc.) Lett. 1984;169:247–250. [Google Scholar]
- Kuznetsov B.A., Shumakovich G.P., Koroleva O.V., Yaropolov A.I. On applicability of laccase as label in the mediated and mediatorless electroimmuno assay: effect of distance on the direct electron transfer between laccase and electrode. Biosens. Bioelectron. 2001;16:73–84. doi: 10.1016/s0956-5663(00)00135-4. [DOI] [PubMed] [Google Scholar]
- Lalaoui N., Pailley P.R., Robert V., Mekmouche Y., Villalonga R., Holzinger M., Cosnier S., Tron T., Goff A.L. Direct electron transfer between a site-specific pyrene-modified laccase and carbon nanotube/gold nanoparticle supramolecular assemblies for bioelectrocatalytic dioxygen reduction. ACS Catal. 2016;6(3):1894–1900. [Google Scholar]
- Lambertz Commandeur Progress and obstacles in the production and application of recombinant lignin-degrading peroxidases. Bioengineered. 2016;7:145–154. doi: 10.1080/21655979.2016.1191705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.W., Lee S.M., Hong E.J., Jeung E.B., Kang H.Y., Kim M.K., Choi I.G. Estrogenic reduction of styrene monomer degraded by Phanerochaete chrysosporium KFRI 20742. J. Microbiol. 2006;44:177–184. [PubMed] [Google Scholar]
- Lee Y.A., Hendson M., Panopoulos N.J., Schroth M.N. Molecular cloning, chromosomal mapping, and sequence analysis of copper resistance genes from Xanthomonas campestri sp. Homology with small blue copper proteins and multicopper oxidase. J. Bacteriol. 1994;176:173–188. doi: 10.1128/jb.176.1.173-188.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Xu F., Eriksson K.E.L. Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compound. Appl. Environ. Microbiol. 1999;65(6):2654–2660. doi: 10.1128/aem.65.6.2654-2660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebeskind M., Hocker H., Wandrey C., jager A.G. Strategies for improved lignin peroxidase production in agitated pellet cultures of Phanerochaete chrysosporium and the use of a novel inducer. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 1990;71:325–330. [Google Scholar]
- Lopez M., Loera O., Guerrero-Olazarán M. Cell growth and Trametes versicolor laccase production in transformed Pichia pastoris cultured by solid-state or submerged fermentations. J. Chem. Technol. Biotechnol. 2010;85(4):435–440. [Google Scholar]
- Lundell T., Wever R., Floris R., Harvey P., Hatakka A., Brunow G. Lignin peroxidase L3 from Phlebia radiate, Pre-steady-state and steady-state studies with veratryl alcohol and a non-phenolic lignin model compound 1-(3,4-dimethoxyphenyl)-2-(2-methoxyphenoxy) propane-1,3-diol. Eur. J. Biochem. 1993;211:391–402. doi: 10.1111/j.1432-1033.1993.tb17562.x. [DOI] [PubMed] [Google Scholar]
- Maciel M.J.M., Silva A.C., Ribeiro H.C.T. Industrial and biotechnological applications of ligninolytic enzymes of the basidiomycota: a review. Electron. J. Biotechnol. 2010 ISSN: 0717-3458. [Google Scholar]
- Maijala P., Mettala A., Kleen M., Westin C., Poppius-Levin K., Herranen K., Lehto J.H., Reponen P., Maentausta O., Hatakka A. 10th International Congress on Biotechnology in the Pulp and Paper Industry. Madison Wisconsin, Book of Abstracts. 2007. Treatment of softwood chips with enzymes may reduce refining energy consumption and increase surface charge of fibers; p. 65. [Google Scholar]
- Malherbe A., Cloete T.E. Lignocellulose biodegradation: fundamentals and applications. Rev. Environ. Sci. Biotechnol. 2002;1:105–114. [Google Scholar]
- Marquez A.T.A., Mendoza M.G.D., Gonzalez M.S.S. Actividad fibrolitica de enzymes produced as por Trametes sp. EUM1, Pleurotus ostreatus IE8, Aspergillus niger AD96.4 in fermentacion solida. Interciencia. 2007;32:780–785. [Google Scholar]
- Masai E., Katayama Y., Nishikawa S., Fukuda M. Characterization of Sphingomonas paucimobilis SYK-6 genes involved in degradation of lignin-related compounds. J. Ind. Microbiol. Biotechnol. 1999;23:364–373. doi: 10.1038/sj.jim.2900747. [DOI] [PubMed] [Google Scholar]
- Matera I., Gullotto A., Tilli S., Ferraroni M., Scozzafava A., Briganti F. Crystal structure of the blue multicopper oxidase from the white-rot fungus Trametes trogii complexed with p-toluate. Inorg. Chim. Acta. 2008;361:4129–4137. [Google Scholar]
- Mathews S.L., Pawlak J.J., Grunden A.M. Isolation of Paenibacillus glucanolyticus from pulp mill sources with potential to deconstruct pulping waste. Bioresour. Technol. 2014;164:100–105. doi: 10.1016/j.biortech.2014.04.093. [DOI] [PubMed] [Google Scholar]
- Mester T., Field J.A. Characterization of a novel manganese peroxidase–lignin peroxidase hybrid isozyme produced by Bjerkandera species strain BOS55 in the absence of manganese. J. Biol. Chem. 1998;273:15412–15417. doi: 10.1074/jbc.273.25.15412. [DOI] [PubMed] [Google Scholar]
- Mikolasch A., Schauer F. Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl. Microbiol. Biotechnol. 2009;82:605–624. doi: 10.1007/s00253-009-1869-z. [DOI] [PubMed] [Google Scholar]
- Morales M., Mate M.J., Romero A., Martinez M.J., Martinez A.T., Ruiz-Duenas F.J. Two oxidation sites for low redox-potential substrates: a directed mutagenesis, kinetic and crystallographic study on Pleurotus eryngii versatile peroxidase. J. Biol. Chem. 2012;287:41053–41067. doi: 10.1074/jbc.M112.405548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morii H., Nakamiya K., Kinoshita S. Isolation of a lignin decolorizing bacterium. J. Ferment. Bioeng. 1995;80(3):296–299. [Google Scholar]
- Morozova O.V., Shumakovich G.P., Gorbacheva M.A., Shleev S.V., Yaropolov A.I. “Blue” laccases. J. Biochem. 2007;72(10):1136–1150. doi: 10.1134/s0006297907100112. [DOI] [PubMed] [Google Scholar]
- Niladevi K.N., Prema P. Mangrove Actinomycetes as the source of ligninolytic enzymes. Actinomycetologica. 2005;19:40–47. [Google Scholar]
- Ozer A., Sal F.A., Ali Belduz O., Kirci H., Canakci S. Use of feruloyl esterase as laccase-mediator system in paper bleaching. Appl. Biochem. Biotechnol. 2019:1–11. doi: 10.1007/s12010-019-03122-x. [DOI] [PubMed] [Google Scholar]
- Pace N., Scholtz J.M., Grimsley G.R. Forces stabilizing proteins. FEBS Lett. 2014;588(14):2177–2184. doi: 10.1016/j.febslet.2014.05.006. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller R., Amann M., Freudenreich J. Proceedings of the 7th International Conference on Biotechnology in the Pulp and Paper Industry. Vancouver; Canada: 1998. Analysis of laccase and mediator interactions in the LMS; pp. A99–A102. [Google Scholar]
- Pinar O., Tamerler C., Yazgankaratas A. Heterologous expression and characterization of a high redox potential laccase from Coriolopsis polyzona MUCL 38443. Turk. J. Biol. 2017;41:278–291. [Google Scholar]
- Piontek K., Smith A.T., Blodig W. Lignin peroxidase structure and function. Biochem. Soc. Trans. 2001;29(2):111–116. doi: 10.1042/0300-5127:0290111. [DOI] [PubMed] [Google Scholar]
- Pollegioni L., Tonin F., Rosini E. Lignin-degrading enzymes. FEBS J. 2015;282:1190–1213. doi: 10.1111/febs.13224. [DOI] [PubMed] [Google Scholar]
- Ponzoni C., Beneventi E., Cramarossa M.R., Raimondi S., Trevisi G., Pagnoni U.M., Riva S., Forti L. Laccase-catalyzed dimerization of hydroxystilbenes. Adv. Synth. Catal. 2007;349:1497–1506. [Google Scholar]
- Pothiraj C., Kanmani P., Balaji P. Bioconversion of lignocellulose materials. Mycobiology. 2006;34(4):159–165. doi: 10.4489/MYCO.2006.34.4.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A., Reddy K.M.M., Chandra R. Decolourisation and treatment of pulp and paper mill effluent by lignin-degrading Bacillus sp. J. Chem. Technol. Biotechnol. 2007;82:399–406. [Google Scholar]
- Raj A., Reddy K.M.M., Chandra R. Identification of low molecular weight aromatic compounds by gas chromatography-mass spectrometry (GC-MS) from kraft lignin degradation by three Bacillus sp. Int. Biodeterior. Biodegrad. 2007;59:292–296. [Google Scholar]
- Ravindran R., Jaiswal A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: challenges and opportunities. Bioresour. Technol. 2015;199:92–102. doi: 10.1016/j.biortech.2015.07.106. [DOI] [PubMed] [Google Scholar]
- Reddy G.V.B., Sridhar M., Gold M.H. Cleavage of nonphenolic β-1 diarylpropane lignin model dimers by manganese peroxidase from Phanerochaete chrysosporium. Eur. J. Biochem. 2003;270:284–292. doi: 10.1046/j.1432-1033.2003.03386.x. [DOI] [PubMed] [Google Scholar]
- Renugadevi R., Aryyappadas M.P., Preethy P.H., Savetha S. Isolation, screening and induction of mutation in strain for extracellular lignin peroxidase producing bacteria from soil and its partial purification. J. Res. Biol. 2011;4:312–318. [Google Scholar]
- Rivera-Hoyos C.M., Morales-Álvarez E.D., Poutou-Pinales R.A., Pedroza-Rodríguez A.M., Rodriguez-Vazquez R., Delgado-Boada J.M. Fungal laccases. Fungal Biol Rev. 2013;27:67–82. [Google Scholar]
- Roberts S.A., Wildner G.F., Grass G., Weichsel A., Ambrus A., Rensing C., Montfort W.R. A labile regulatory copper ion lies near the T1 copper site in the multicopper oxidase CueO. J. Biol. Chem. 2003;278:31958–31963. doi: 10.1074/jbc.M302963200. [DOI] [PubMed] [Google Scholar]
- Robles-Hernandez L., Gonzales-Franco A.C., Crawford D.L., Chun W.W.C. Review of environmental organopollutants degradation by white-rot basidiomycete mushrooms. Tecnociencia Chihuahua. 2008;2:32–39. [Google Scholar]
- Ruiz-Duenas F.J., Martinez M.J., Martinez A.T. Molecular characterization of a novel peroxidase isolated from the ligninolytic fungus Pleurotus eryngii. Mol. Microbiol. 1999;31:223–235. doi: 10.1046/j.1365-2958.1999.01164.x. [DOI] [PubMed] [Google Scholar]
- Russier J., Menard-Moyon C., Venturelli E., Gravel E., Marcolongo G., Meneghetti M. Oxidative biodegradation of single- and multi-walled carbon nanotubes. Nanoscale. 2011;3:893–896. doi: 10.1039/c0nr00779j. [DOI] [PubMed] [Google Scholar]
- Sadh P.K., Duhan S., Duhan J.S. Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour. Bioprocess. 2018;5:1. [Google Scholar]
- Saini J.K., Saini R., Tewari L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech. 2015;5:337–353. doi: 10.1007/s13205-014-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C. Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009;27:185–194. doi: 10.1016/j.biotechadv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Sanchez-Amat A., Solano F. A pluripotent polyphenol oxidase from the melanogenic marine Alteromonas sp. shares catalytic capabilities of tyrosinases and laccases. Biochem. Biophys. Res. Commun. 1997;240:787–792. doi: 10.1006/bbrc.1997.7748. [DOI] [PubMed] [Google Scholar]
- Sanchez-Amat A., Lucas-Elio P., Fernandez E., Garcia-Borron J.C., Solano F. Molecular cloning and functional characterization of a unique multipotent polyphenol oxidase from Marinomonas mediterranea. Biochim. Biophys. Acta. 2001;1547:104–116. doi: 10.1016/s0167-4838(01)00174-1. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Martinez A.T., Martinez M. Biochemical and molecular characterization of a manganese peroxidase isoenzyme from Pleurotus ostreatus. Biochim. Biophys. Acta. 1997;1339:23–30. doi: 10.1016/s0167-4838(96)00201-4. [DOI] [PubMed] [Google Scholar]
- Sato Y., Moriuchi H., Hishiyama S., Otsuka Y. Identification of three alcohol dehydrogenase genes involved in the stereospecific catabolism of arylglycerol-beta-aryl ether by Sphingobium sp strain SYK-6. Appl. Environ. Microbiol. 2009;75:5195–5201. doi: 10.1128/AEM.00880-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senan R., Abraham C.T.E. Bioremediation of textile azo dyes by aerobic bacterial consortium. Biodegradation. 2004;15:275–280. doi: 10.1023/b:biod.0000043000.18427.0a. [DOI] [PubMed] [Google Scholar]
- Shi Y., Chai L., Tang C. Characterization and genomic analysis of kraft lignin biodegradation by the beta-proteobacterium Cupriavidus basilensis. B-8 Biotechnology for Biofuels. 2013;6:1. doi: 10.1186/1754-6834-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K.S. The role of enzymes produced by white-rot fungus Irpex lacteus in the decolorization of the textile industry effluent. J. Microbiol. 2004;42:37–41. [PubMed] [Google Scholar]
- Shin K.S., Kim Y.H., Lim J.S. Purification and characterization of manganese peroxidase of the white-rot fungus Irpex lacteus. J. Microbiol. 2005;43:503–509. [PubMed] [Google Scholar]
- Singh A.K., Chandra R. Pollutants released from the pulp paper mill: aquatic toxicity and their health hazards. Aquat. Toxicol. 2019;211:202–216. doi: 10.1016/j.aquatox.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Singh S., Singh B.B., Chandra R. Biodegradation of phenol in batch culture by pure and mixed strains of Paenibacillus sp. and Bacillus cereus. Pol. J. Microbiol. 2009;58(4):319–325. [PubMed] [Google Scholar]
- Singh D., Zeng J., Chen S. Increasing manganese peroxidase productivity of Phanerochaete chrysosporium by optimizing carbon sources and supplementing small molecules. Lett. Appl. Microbiol. 2011;53:120–123. doi: 10.1111/j.1472-765X.2011.03070.x. [DOI] [PubMed] [Google Scholar]
- Solomon E.I., Anthony J., Augustine, Yoon J. O2 reduction to H2O by the multicopper oxidases. Dalton Trans. 2008;30:3921–3932. doi: 10.1039/b800799c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srebotnik E., Jensen K.A., Hammel K.E. Proceedings of the 7th International Conference on Biotechnology in the Pulp and Paper Industry. Vancouver; Canada: 1998. Cleavage of nonphenolic lignin structures by laccase in the presence of 1-hydroxybenzotriazole; pp. B195–B197. [Google Scholar]
- Steffen K.T., Hofrichte r M., Hatakka A. Purification and characterization of manganese peroxidases from the litter-decomposing basidiomycetes Agrocybe praecox and Stropharia coronilla. Enzym. Microb. Technol. 2003;30:550–555. [Google Scholar]
- Sundaramoorthy M., Kishi K., Gold M.H., Poulos T.L. Crystal structures of substrate binding site mutants of manganese peroxidase. J. Biol. Chem. 1997;272:17574–17580. doi: 10.1074/jbc.272.28.17574. [DOI] [PubMed] [Google Scholar]
- Sutherland G.R., Zapanta L.S., Tien M., Aust S.D. Role of calcium in maintaining the heme environment of manganese peroxidase. Biochemistry. 1997;36:3654–3662. doi: 10.1021/bi962195m. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Endo K., Ito M., Tsujibo H., Miyamoto K., Inamori Y. A thermostable laccase from Streptomyces lavendulae REN-7: purification, characterization, nucleotide sequence, and expression. Biosci. Biotechnol. Biochem. 2003:2167–2175. doi: 10.1271/bbb.67.2167. [DOI] [PubMed] [Google Scholar]
- Swapnil K., Kale Amit., Deshmukh G. 3D structure prediction of ligninolytic enzymes lignin peroxidase and manganese peroxidase based on homology modeling. J BioSci Biotechnol. 2016;5(1):1–11. [Google Scholar]
- Taha M., Foda M., Shahsavari E., Aburto-Medina A., Adetutu E., Ball A. Commercial feasibility of lignocellulose biodegradation: possibilities and challenges. Curr. Opin. Biotechnol. 2016;38:190–197. doi: 10.1016/j.copbio.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Ten Have R., Robber G. deT., Henk J.S., Jim A.F. Veratryl alcohol-mediated oxidation of isoeugenyl acetate by lignin peroxidase. Eur. J. Biochem. 1999;265:1008–1014. doi: 10.1046/j.1432-1327.1999.00808.x. [DOI] [PubMed] [Google Scholar]
- Ten Have R., Teunissen P.J.M. Oxidative mechanisms involved in lignin degradation by white-rot fungi. Chem. Rev. 2001;101(11):3397–3413. doi: 10.1021/cr000115l. [DOI] [PubMed] [Google Scholar]
- Tunde Mester., Ming Tien. Oxidation mechanism of ligninolytic enzymes involved in the degradation of environmental pollutants. Int. Biodeterior. Biodegrad. 2000;46:51–59. [Google Scholar]
- Tuor U., Wariishi H., Schoemaker H.E., Gold M.H. Oxidation of phenolic aryglycerol β- aryl ether lignin model compounds by manganese peroxidase from Phanerochaete chrysosporium: oxidative cleavage of an α-carbonyl model compound. Biochemistry. 1992;31:4986–4995. doi: 10.1021/bi00136a011. [DOI] [PubMed] [Google Scholar]
- Umezawa T. Mechanisms for chemical reactions involved in lignin biodegradation by Phanerochaete chrysosporium. Wood Res. 1988;75:21–79. Kyoto University. [Google Scholar]
- Valderrama B., Ayala M., Vazquez Duhalt R. Suicide inactivation of peroxidases and the challenge of engineering more robust enzymes. Chem. Biol. 2002;9:555–565. doi: 10.1016/s1074-5521(02)00149-7. [DOI] [PubMed] [Google Scholar]
- Vantamuri A.B., Kaliwal B.B. Isolation, screening and identification of Laccase producing fungi. Int. J. Pharma Bio Sci. 2015;6(3):242–250. B. [Google Scholar]
- Varnai A., Huikko L., Pere J., Siika-Aho M., Viikari L. Synergistic action of xylanase and mannanase improves the total hydrolysis of softwood. Biores Technol. 2011;102:9096–9104. doi: 10.1016/j.biortech.2011.06.059. [DOI] [PubMed] [Google Scholar]
- Vazquez M., Oliva M., Tellez-Luis S.J., Ramirez J.A. Hydrolysis of sorghum straw using phosphoric acid: evaluation of furfural production. Bioresour. Technol. 2007;98:3053–3060. doi: 10.1016/j.biortech.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Walling C., El-Talwi G.M., Amarnalth K. Oxidation of styrene derivatives by S2O8 2– CuII in acetic acid and acetonitrile, Reaction paths in oxidations via radical cations. J. Am. Chem. Soc. 1984;106:7373–7578. [Google Scholar]
- Wariishi H., Gold M.H. Lignin peroxidase compound III. Mechanism of formation and decomposition. J. Biol. Chem. 1990;265:2070–2077. [PubMed] [Google Scholar]
- Wariishi H., Huang J., Dunford B., Gold M.H. Reactions of lignin compounds I and II with veratryl alcohol. J. Biol. Chem. 1991;266:20694–20699. [PubMed] [Google Scholar]
- Wariishi H., Lakshmi A., Michael H. Gold. Manganese peroxidase from the basidiomycete Phanerochaete chrysosporium: spectral characterization of the oxidized states and the catalytic cycle. Biochemistry. 1988;27:5365–5370. doi: 10.1021/bi00414a061. [DOI] [PubMed] [Google Scholar]
- Wariishi H., Valli K., Renganathan V., Gold M.H. Oxidative cleavage of a phenolic diarylpropane lignin model dimer by manganese peroxidase from Phanerochaete chrysosporium. Biochemistry. 1989;28:6017–6023. [Google Scholar]
- Wariishi H., Valli K., Renganathan V., Gold M.H. Thiol-mediated oxidation of nonphenolic lignin model compounds by manganese peroxidase of Phanerochaete chrysosporium. J. Biol. Chem. 1989;264:14185–14191. [PubMed] [Google Scholar]
- Wells M., Teria T. Eve Green oxidations with laccase–mediator systems. Biochem. Soc. Trans. 2006;34(part 2) doi: 10.1042/BST20060304. [DOI] [PubMed] [Google Scholar]
- Wen X., Jia Y., Li J. Degradation of tetracycline and oxytetracycline by crude lignin peroxidase prepared from Phanerochaete chrysosporium-a white rot fungus. Chemosphere. 2009;75:1003–1007. doi: 10.1016/j.chemosphere.2009.01.052. [DOI] [PubMed] [Google Scholar]
- Widsten P., Kandelbauer A. Laccase applications in the forest products industry: a review. Enzym. Microb. Technol. 2008;42:293–307. [Google Scholar]
- Wiermans L., Perez-Sanchez M., Dominguez de., Maria P. Lipase-mediated oxidative delignification in non-aqueous media: formation of de-aromatized lignin-oil and cellulase-accessible polysaccharides. ChemSusChem. 2013;6:251–255. doi: 10.1002/cssc.201200704. [DOI] [PubMed] [Google Scholar]
- Wong W.S.D. Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 2009;157:174–209. doi: 10.1007/s12010-008-8279-z. [DOI] [PubMed] [Google Scholar]
- Woo H.L., Hazen T.C., Simmons B.A., DeAngelis K.M. Enzyme activities of aerobic lignocellulolytic bacteria isolated from wet tropical forest soils. Syst. Appl. Microbiol. 2014;37:60–67. doi: 10.1016/j.syapm.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Yadav S., Chandra R. Detection and assessment of the phytotoxicity of residual organic pollutants in sediment contaminated with pulp and paper mill effluent. Environ. Monit. Assess. 2018;190:581. doi: 10.1007/s10661-018-6947-1. [DOI] [PubMed] [Google Scholar]
- Yadav S., Gangwar N., Mittal P., Sharama S., Bhatnagar T. Isolation, screening and biochemical characterization of laccase producing bacteria for degradation of lignin. Int. J. Educ. Sci. Res. 2014;1(3):17–21. [Google Scholar]
- Yamazaki I., Yokota K. Conversion of ferrous peroxidase into compound III in the presence of NADH. Biochem. Biophys. Res. Commun. 1965;19:249–254. doi: 10.1016/0006-291x(65)90513-9. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Allen B.L., Star A. Enzymatic degradation of multiwalled carbon nanotubes. J. Phys. Chem. A. 2011;115:9536–9544. doi: 10.1021/jp112324d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoppellaro G., Huang H.W., Sakurai T. Kinetic studies on the reaction of the fully reduced laccase with dioxygen. Inorg. React. Mech. 2000;2:79–84. [Google Scholar]
- Zorn H., Langhoff S., Scheibner M., Nimtz M., Berger R.G. A peroxidase from Lepista irina cleaves b,b-carotene to flavor compounds. Biol. Chem. 2003;384:1049–1056. doi: 10.1515/BC.2003.117. [DOI] [PubMed] [Google Scholar]