Abstract
Currently, antibiotic resistance and cancer are two of the most important public health problems killing more than ∼1.5 million people annually, showing that antibiotics and current chemotherapeutics are not as effective as they were in the past. Nanotechnology is presented here as a potential solution. However, current protocols for the traditional physicochemical synthesis of nanomaterials are not free of environmental and social drawbacks, often involving the use of toxic catalysts. This article shows the production of pure naked selenium nanoparticles (SeNPs) by a novel green process called pulsed laser ablation in liquids (PLAL). After the first set of irradiations, another set was performed to reduce the size below 100 nm, which resulted in a colloidal solution of spherical SeNPs with two main populations having sizes around ∼80 and ∼10 nm. The particles after the second set of irradiations also showed higher colloidal stability. SeNPs showed a dose-dependent antibacterial effect toward both standard and antibiotic-resistant phenotypes of Gram-negative and Gram-positive bacteria at a range of concentrations between 0.05 and 25 ppm. Besides, the SeNPs showed a low cytotoxic effect when cultured with human dermal fibroblasts cells at a range of concentrations up to 1 ppm while showing an anticancer effect toward human melanoma and glioblastoma cells at the same concentration range. This article therefore introduces the possibility of using totally naked SeNPs synthesized by a new PLAL protocol as a novel and efficient nanoparticle fabrication process for biomedical applications.
Introduction
Over the past several decades, there has been some significant growing concerns related to bacteria and cancer that have been threatening our global healthcare system.1 The overuse and misuse of antibiotics have brought society to the post-antibiotic era, making these drugs no longer as effective as they used to be, consequently leading to a continuous rise of antimicrobial resistance (AMR) cases.2,3 On the other hand, cancer is the second leading cause of death worldwide.4,5 The use of current treatments, such as radiotherapy, chemotherapy, or the combination of both, present plenty of reported side effects having a high impact on a patient’s life.6 Moreover, recent studies show that, in a similar way that bacteria have adapted to conventional drug treatment, tumors are able to develop resistance toward chemotherapy drugs presenting a new class of chemotherapeutic-resistant cancer cells.7 Therefore, AMR and cancer are craving for an immediate solution far away from current and old-fashioned traditional treatments for which we have outgrown.
Nanotechnology is presented here as a suitable solution,8 with a deep focus on materials with at least one dimension below 100 nm, as a field, which has seen rising interest since its beginning around the 1960s.9 The impact of nanotechnology into medicine comes from size-dependent material properties10 that materials offer when confined within nanoscale dimensions and also from the ability of the nanostructures to interact efficiently with biological materials due to their large surface-to-volume ratio, making them extremely reactive.11,12 Since its inception, a large variety of nanomaterials, including metallic, polymeric, or biomolecule-based nanomaterials (from nanoparticles (NPs) and nanowires (NWs) to nanotubes (NTs) or nanocomposites), have been reported as useful in the fight toward both bacterial infections and cancer.13 The ability of nanomaterials to show biomedical properties has been related to different mechanisms. One of the most reported processes involves the production of reactive oxygen species (ROS), a group of oxygen-containing compounds including radicals and nonradicals (like superoxide (O2·–) or hydrogen peroxide (H2O2), respectively).14 Therefore, the contact between nanoparticles and biological membranes in cells trigger the production of ROS, affecting the survival of the living organisms and producing different reponses that may lead to antibacterial or anticancer effects.15,16 For instance, selenium (Se), either pure or capped with chitosan, was efficiently tested against bacteria, that is, Escherichia coli, Staphylococcus aureus,17 and fungi, that is, Candida albicans.18
Nevertheless, the beneficial impact of nanotechnology on human health is not always accompanied by a similar beneficial effect in the environment. As a matter of fact, the growing problem of chemical contamination from nanoparticle synthesis has demanded the need to find eco-friendly alternatives to all known chemical and physical processes. Consequently, nanotechnology has found a suitable answer in the incorporation of green chemistry principles, giving rise to what is called green nanotechnology, which pursues nanoparticle reactions that are environmentally friendly, cost-effective, and safe for both the environment and the patients.19,20 Therefore, different raw materials have been used for the generation of nanomaterials, from living organisms (such as plants,21 bacteria,22 or fungi23) to biocompounds (sourced from edibles24 or food waste25).
From all over the periodic table, several elements, such Ag or Au, present antimicrobial and anticancer activity with a low associated cytotoxicity.26,27 However, some researchers have reported resistance of bacterial populations to some of these nanostructures, most notably, AgNPs.28 Therefore, alternative formulations have been used showing identical or even better effects. For instance, selenium (Se), a chalcogen element that is present in trace amounts in the Earth’s core, and due to its scarity, has been classified by the American Physical Society and the Materials Research Socity as a critical element.29 Indeed, selenium is widely known as an essential element for life, found in amino acids and proteins.30−33 Therefore, it is crucial to find a synthesis protocol that could use selenium in a very efficient way to allow for its use in various applications, especially medical34 and solar cells.35
Physical methods have also been redesigned and adapted to these green principles, producing extremely cost-effective techniques without the production of toxic by-products. One of these techniques is pulsed laser ablation in liquids (PLAL), which is capable of producing NPs with sizes between 5 and 120 nm in an environmentally-friendly fashion. PLAL uses an electrical charge at the surface of the nanoparticle to promote electrostatic repulsion in order to prevent NPs from agglomerating. As reported in the literature, this technique is capable of creating nanostructures with biomedical applications.
In this research, SeNPs were produced by a clean, environmentally friendly, and cost-effective PLAL approach. The protocol was optimized to produce a large amount of SeNPs within 10 min of irradiation (first set of irradiation, 5 min; second set of irradiation, 5 min). The structures were characterized in terms of morphology, size, and composition, utilizing a variety of techniques, and their antibacterial properties were tested on antibiotic-resistant bacteria (such as multidrug-resistant E. coli (MDR) and methicillin-resistant S. aureus (MRSA)) and regular bacteria (Pseudomonas aeruginosa (PA) and Staphylococcus epidermitis (SE)) strains. Furthermore, SeNP anticancer properties were determined on human malignant melanoma and glioblastoma cells showing a promising decrease on cell proliferation while remaining biocompatible toward human dermal fibroblasts (HDF). Consequently, PLAL-synthesized SeNPs are presented here as a suitable biomedical tool whose production can overcome the surface purity limitation of the traditional wet-chemical synthesis of nanomaterials.
Experimental Section
Preparation of Se NPs
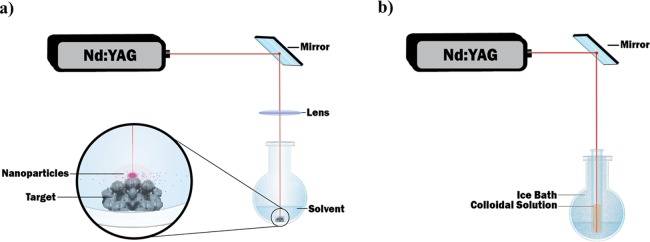
For the production of SeNPs, a pulsed laser ablation in liquids (PLAL) technique was used. The experimental setup employed is illustrated in Figure 1a. Briefly, a Q-switched Nd:YAG laser (Electro Scientific Industries) operating at a 1064 nm wavelength was used to irradiate the target, which consisted of bulk Se pellets (99.999% from Sigma Aldrich), ∼2 mm in diameter. The pellets were immersed in deionized (DI) water (5 mL), contained in a 50 mL rounded single-neck glass flask. Consequently, the height of the liquid above the surface of the target was set at 8 mm. The pulse repetition rate of the laser varied from 100 to 5000 Hz, and the pulse duration time varied from 70 to 200 ns depending on the repetition rate. The laser shined a pulsed beam with an energy per pulse around a 16.5 mJ pulse–1 at 1000 Hz. The beam was deflected by a flat mirror oriented at a 45-degree angle (with respect to the laser rail) in order to irradiate the target from the top and was then focused by using an 83 mm focal length lens. The spot size of the beam on the target was measured by scanning electron microscopy (SEM) to be around ∼45 μm. Therefore, the intensity of the laser was determined to be around ∼1 × 106 W cm–2. At 1000 Hz, the fluence was calculated to be ∼1 × 103 J cm–2. The target was finally irradiated for 5 min. After irradiation, the particles contained in the solution were irradiated again to reduce the size while being contained in a test tube, which was submerged in an ice bath in order to reduce agglomeration due to melting during the second irradiation, see Figure 1b. The colloidal solution containing the nanoparticles produced by the laser ablation of the target was then stored in a black Eppendorf microtube in order to be protected from ambient light.
Figure 1.
Setup for synthesis of Se NP by PLAL (a) initial irradiation and (b) ice bath post irradiation to control size and agglomeration.
Physico-Chemical Characterization of Se NPs
After synthesis, the samples were characterized by UV–visible spectroscopy (Cary 5000 from Agilent), atomic emission spectroscopy (4210 MP-AES from Agilent), dynamic light scattering (NanoBrook 90Plus from Brookhaven Instruments Corporation), Raman spectroscopy (EZRaman-N from Enwave Optronics, Inc.), scanning electron microscopy (JEOL JSM–7000F SEM, equipped with a field emission gun and operating at 30 kV), transmission electron microscopy (JEOL 2100-F TEM operating at 80 kV), and atomic force microscopy (Bruker Icon AFM). To perform SEM, AFM, and TEM analyses, a droplet of the colloidal solution was deposited onto a silicon wafer (SEM, AFM) and copper grid (TEM). Both substrates were then dried in an environmentally controlled glove box. AFM studies were performed in tapping mode using a silicon AFM probe from Ted Pella, Inc. [prod no. TAP300-G-10] with a resonant frequency of 300 kHz and a force constant of 40 N/m.
Antimicrobial Characterization of SeNPs
A total of four different strains of bacteria were tested for antimicrobial properties using the SeNPs: two Gram-negative bacteria (multidrug-resistant E. coli (MDR-EC) (ATCC BAA-2471; ATCC, Manassas, VA) and P. aeruginosa (PA) (ATCC 27853, ATCC, Manassas, VA)) and two Gram-Positive strains (methicillin-resistant S. aureus (MRSA) (ATCC 4330; ATCC, Manassas, VA) and Staphylococcus epidermidis (SE) (ATCC 35984; ATCC, Manassas, VA)) were utilized for the antibacterial tests. The cultures were kept on agar plates at 4 °C.
Colony counting unit assays were completed by seeding the bacteria in a 96-well plate mixed with different concentrations of SeNPs. The plates were incubated at 37 °C for 8 h; after that period of time, they were removed from the incubator and diluted with PBS in a series of vials by ×104 ×105 and ×106. Three drops of 10 μL were taken of each dilution and deposited on an LB agar plate. The plates were deposited inside an incubator at 37 °C until the colonies grew enough without reaching confluency. Afterward, the numbers of colonies formed were counted, and the data was processed.
In Vitro Cytotoxicity Characterization of SeNPs
Cytotoxicity assays were performed with primary human dermal fibroblasts (TCC PCS-201-012TM, Manassas, VA), melanoma cells (ATCC CRL-1619, Manassas, VA), and glioblastoma (T98G [T98-G] (ATCC CRL-1690) cells. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Thermo Fisher Scientific, Waltham, MA), supplemented with 10% fetal bovine serum (FBS; ATCC 30–2020, American Type Culture Collection, Manassas, VA) and 1% penicillin/streptomycin (Thermo Fisher Scientific, Waltham, MA). MTS assays (CellTiter 96 AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI) were carried out to assess cytotoxicity. Cells were seeded onto tissue culture-treated 96-well plates (Thermo Fisher Scientific, Waltham, MA) at a final concentration of 5000 cells per well in 100 μL of cell medium. After an incubation period of 24 h at 37 °C in a humidified incubator with 5% carbon dioxide (CO2), the culture medium was replaced with 100 μL of fresh cell medium containing different concentrations of SeNPs.
Cells were cultured for another 24 and 72 h for the 1 and 3 days of experiments, respectively, at the same incubation conditions. The media was then removed, and cells were washed twice with PBS. A total of 100 μL of the MTS solution (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium solution), prepared using a mixing ratio of 1:5 of MTS:medium, was then added. After the addition of the solution, the 96-well plate was incubated for 4 h in the incubator to allow for a color change. Then, the absorbance was measured at 490 nm on an absorbance plate reader (SpectraMAX M3, Molecular Devices) for cell viability after exposure to the SeNPs. Cell viability was calculated by dividing the average absorbance obtained for each sample by the one achieved by the control sample and then multiplied by 100. Optical density was then converted to cells mL–1 using appropriate cell line standardization curves. Controls containing cells and media and only media, were also included in the 96-well plate to identify the normal growth of cells without nanoparticles and to determine the absorbance of the media itself.
Reactive Oxygen Species Analysis
For reactive oxygen species (ROS) quantification, 2,7-dichlorodihydrofluorescein diacetate (H2DCFDA) was used, as well as human melanoma cells. The cells were seeded in a 96-well plate at a concentration of 5 × 104 cells mL–1 in the presence of different concentrations of the SeNPs as well as in the control without any nanoparticles. The cells were cultured under standard culture conditions at 37 °C in a humidified incubator with a 5% carbon dioxide (CO2) atmosphere for 24 h before the experiment. Briefly, the ROS indicator was reconstituted in anhydrous dimethylsulfoxide (DMSO) to make a concentrated stock solution. Then, the media were carefully removed, and a fixed volume of the indicator in PBS was added to each one of the wells at a final concentration of 10 μM. The cells were incubated for 30 min at the optimal temperature, and the loading buffer was removed thereafter. Fresh media were added, and cells were allowed to recover. The baseline for fluorescence intensity of a sample for the loaded cell period of exposure was determined. Besides, positive controls were tested, stimulating the oxidative activity with hydrogen peroxide to a final concentration of 50 μM. The intensity of fluorescence was then observed by flow cytometry. Measurements were taken by an increase in fluorescence at 530 nm when the sample was excited at 485 nm. Fluorescence was also determined in the negative control, untreated sample loaded with dyed cells maintained in a buffer.
Statistical Analysis
All biological experiments (see Antimicrobial Characterization of SeNPs, In Vitro Cytotoxicity Characterization of SeNPs, and Reactive Oxygen Species Analysis) were repeated in triplicate (n = 3) to ensure the reliability of the results. Statistical significance was assessed using Student’s t tests, with a p < 0.05 being statistically significant. Results are displayed as mean ± standard deviation.
Results and Discussion
Physico-Chemical Characterization of Se NPs
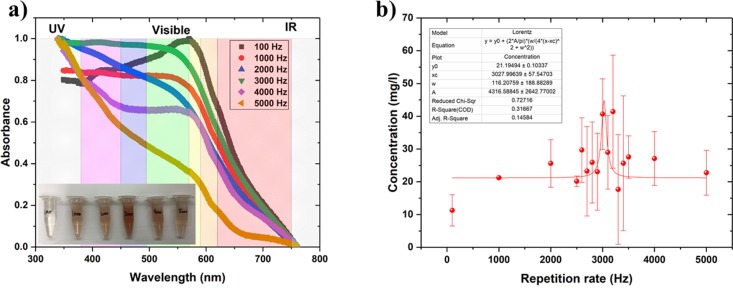
A colloidal solution of the SeNPs was generated by pulsed laser ablation in DI water by varying the laser repetition rate from 100 Hz to 5 kHz (Figure 2a). A series of samples were produced at 100, 1000, 2000, 3000, 4000, and 5000 Hz. The irradiation time was set to 5 min for all samples. The volume of DI water used as the solvent was 5 mL for each sample. From the series, it was possible to report that the maximal production of NPs reached around 3000 Hz. Indeed, most samples (1000–5000 Hz) displayed an orange coloration (due to the production of SeNPs) with the most intense coloration obtained at 3000 Hz. No coloration was noticed for the sample synthesized at 100 Hz (Figure 2a).
Figure 2.
(a) UV–visible spectra of the colloidal solutions shown in the photo, which is the inset. Inset: photo of colloidal solutions from 100 to 5000 Hz from left to right (credit: Tina Hesabizadeh). (b) Various concentrations of selenium in the colloidal solutions synthesized by PLAL at various repetition rates. The maximum was reached at 3028 ± 58 Hz. The irradiation time was set to 5 min for all samples.
UV–visible spectroscopy performed on all the samples demonstrated that different aliquots mainly absorbed in the violet-blue-green region of the visible spectrum. Hence, the samples exhibited a complementary color located in the orange-red region of the spectrum (Figure 2a).
With the aim to complete a quantitative analysis of the samples, the concentration of Se in each colloidal solution was measured by AES. A Lorentz curve was applied to fit the data displayed in Figure 2b. The optimal repetition rate was reached at 3028 ± 58 Hz. As the production decreased after passing this repetition rate, it can be hypothesized that the cavitation bubble was being hit. Indeed, the interaction between the pulsed beam and the target created a high-temperature plasma. This energy was transferred to the surrounding liquid causing it to vaporize creating a cavitation bubble containing the NPs. The lifetime of the cavitation bubble varied from microseconds to milliseconds, depending on the laser pulse parameters.36 In the present case, the cavitation bubble lifetime was estimated to be around 0.330 ± 0.06 ms, the value obtained by taking the reciprocal value of the repetition rate at the maximal production. Indeed, when the production started decreasing when the frequency increased beyond 3000 Hz due to the pulsed beam hitting the cavitation bubble, the formation of more nanoparticles was prevented. When the cavitation bubble finally collapsed, the nanoparticles were released into the solvent. Therefore, to bypass the cavitation bubble temporally and maximize the production of nanoparticles, the repetition rate was chosen at 3000 Hz close to the maximal value determined to be at 3028 ± 58 Hz by AES. The concentration reached at 3000 Hz was around 40 ppm, which is the concentration required for antibacterial applications to remove ∼30% of E. coli and ∼50% of S. aureus.(17)
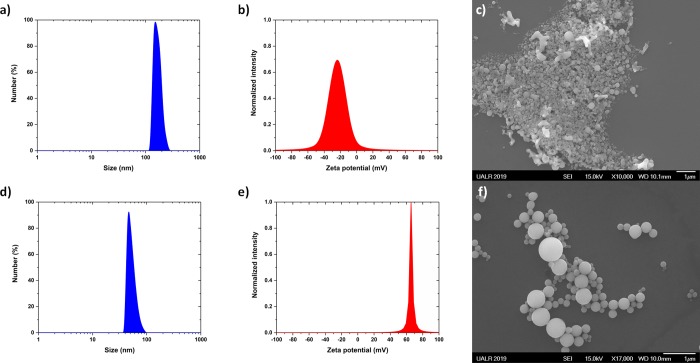
As can be seen in Figure 3a, the size distribution of the SeNPs was centered at 144 ± 46 nm after the first set of irradiation. Furthermore, the nanoparticles were not stable because the zeta potential was smaller than 30 mV (Figure 3b), which means that they easily agglomerated (Figure 3c). The morphology of these selenium nanoparticles synthesized at 3000 Hz was not perfectly spherical as is also shown in Figure 3c. In order to improve the morphology of those nanoparticles, the second set of irradiation (without focusing the beam) was performed for 5 min within a test tube to reshape the nanoparticles and decrease their size distribution to smaller sizes (Figure 3d). The test tube was kept in a refrigerated ice bath to prevent the colloidal solution from boiling, see Figure 1b for illustration of new setup. The size and zeta potential were determined to be 43 ± 20 nm and 66 ± 3 mV, respectively (Figure 3d,e). When this value is more significant than 30 mV, the colloidal solution can be considered stable, that is, there is no agglomeration or flocculation.37 This is likely the reason why the nanoparticle dispersion was observed to be highly stable for at least 3 months. From the SEM observations (Figure 3f), the shape of the Se NPs were spherical, which is in agreement with what other groups observed when the synthesis was performed in DI water.38−41
Figure 3.
(a) Size distribution obtained by DLS for the selenium nanoparticles synthesized at 3000 Hz according to the synthesis protocol shown in Figure 1a. The size distribution is centered at 144 ± 46 nm. (b) Zeta potential was measured to be −24 ± 16 mV, meaning that the colloidal solution was not stable with time. (c) SEM image of the selenium nanoparticles synthesized at 3000 Hz according to the synthesis protocol as shown in Figure 1a. The spherical shape is not well-defined after the first 5 min set of irradiations. (d) Size distribution obtained by DLS for the selenium nanoparticles synthesized at 3000 Hz; the size distribution is centered at 43 ± 20 nm. (e) Zeta potential was measured to be 66 ± 3 mV, meaning that the colloidal solution is going to be stable with time. (f) SEM image of the selenium nanoparticles synthesized after two sets of irradiations at 3000 Hz (first set of irradiation performed within a rounded flask cuvette, second set of irradiation performed within a test tube surrounded with ice). The irradiation time was kept to 5 min for both sets of irradiations.
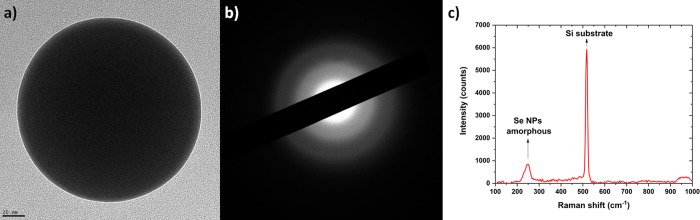
From the TEM observations, the sphericity of the nanoparticles was also confirmed (Figure 4a), and the amorphous structure was determined by electron diffraction (Figure 4b). The Raman spectra (Figure 4c) demonstrated that amorphous selenium nanoparticles were synthesized39,41,42 and also proved that no selenium oxides were synthesized.43 This is in excellent agreement with ref (18) where larger nanoparticles are amorphous and smaller ones are crystalline. The size, where the amorphous–crystalline transition occurs, depends strongly on the solvent temperature during synthesis.
Figure 4.
(a) TEM image of a representative selenium nanoparticle with its size ∼85 nm, and (b) its corresponding diffraction pattern revealing the amorphous structure of the selenium nanoparticle. (c) Raman spectra performed on selenium nanoparticles deposited on a silicon wafer confirming the amorphous structure of the selenium nanoparticles.
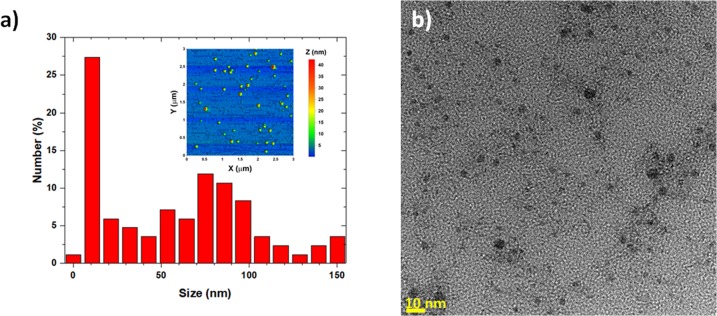
In order to more accurately determine the size distribution of the particles contained in the colloidal solution, AFM and high-magnification TEM studies were completed additionally. In Figure 5a, we see the sample after being drop-casted onto silicon and dried overnight, and it reaveals a very broad size distribution ranging from ∼1 to ∼150 nm, which is common by the PLAL technique.17,18,38,39,41 The AFM studies confirmed what was previously observed by SEM and DLS, but it also revealed a higher percentage of particles around and below 10 nm. The reason why the smallest population of nanoparticles was not observable with the DLS is because the intensity of the DLS signal is proportional to the sixth power of the diameter’s particle d6, which heavily biases the signal toward the largest particles in the solution.37,44 From the TEM images, we also observed smaller-sized particles, which were not observed by SEM or DLS. Some of the SeNPs sizes are below the exciton Bohr radius of Se, which is ∼5 nm40 (Figure 5b). This population of Se quantum dots represents ∼1% of all the Se NPs present in the colloidal solution.
Figure 5.
(a) Size histogram of selenium nanoparticles analyzed by AFM (Inset: AFM image, which is insetted into the size distribution on the x–y scale) and (b) by high magnification TEM (scale bar is 10 nm) showing some selenium quantum dots.
Antimicrobial Characterization of SeNPs
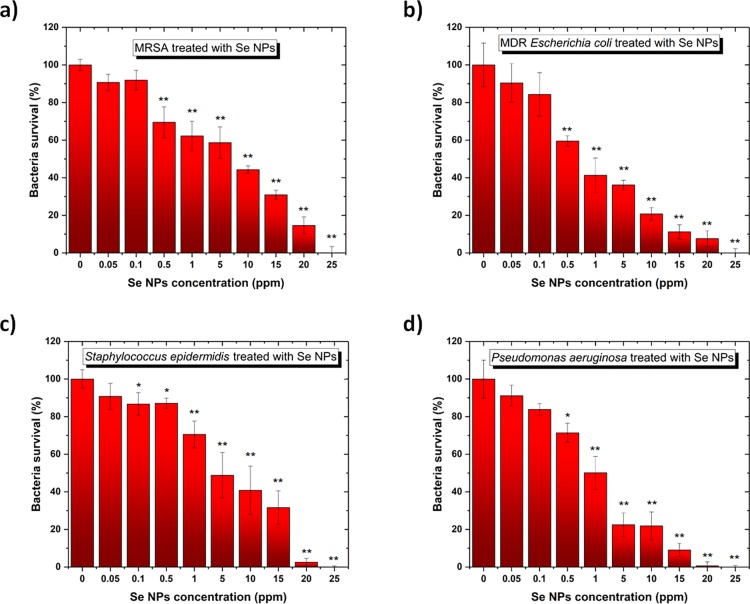
In order to determine the antibacterial activity of the SeNPs, colony counting unit assays were conducted in the presence of four different bacteria with standard and antibiotic-resistant phenotypes: two Gram-negative (PA and MDR-EC) and two Gram-positive (SE and MRSA). The results showed different inhibition trends of the bacterial species with a higher impact when the SeNPs were presented in the cell cultures of Gram-negative bacteria. MDR-EC showed an evident dose-dependent inhibition when the SeNPs were used. Besides, it is also possible to extract that a 1 ppm concentration was enough to cause inhibition in bacteria proliferation, with a clear significance compared to smaller concentrations. Further analysis was conducted using colony counting unit assays, with results plotted in Figure 6a–d. The experiments conducted with MRSA (Figure 6a), MDR-EC (Figure 6b), SE (Figure 6c), and PA (Figure 6d) showed a dose-dependent inhibition of bacterial growth when exposed to different concentrations of SeNPs. The nanostructures were active toward both the Gram-negative and positive bacteria at a range of concentrations between 0.5 and 25 ppm,. Therefore, SeNPs showed an effective bacterial inhibition at concentrations ∼25 ppm.
Figure 6.
Colony counting assay of (a) MRSA, (b) MDR E. coli, (c) S. epidermidis, and (d) P. aeruginosa for 8 h in the presence of different concentrations of SeNPs. All values represent the mean ± standard deviation. *p < 0.05, **p < 0.01(compared to controls).
The minimum inhibitory concentration (MIC) values were calculated for the four investigated bacteria as an extension of the antibacterial behavior (Table 1). These values differ from others found in the literature, showing either a decrease or similarity to the MIC values. For instance, different chemically synthesized SeNPs showed MIC values around 100 ppm when cultured with P. aeruginosa,45 while MIC values of 125 ppm were found when SeNPS were used to inhibit the proliferation of both S. epidermidis and S. aureus.(46) Besides, for E. coli, MIC values of 15 ppm were reported by Muthu et al. when SeNPs were used as antimicrobial agents.47 The lower MIC values calculated in the present work demonstrate that Se NPs synthesized by PLAL are more efficient to kill bacteria than their counterparts synthesized by wet chemistry. This may be attributed to the naked surface of the Se NPs being directly in contact with the bacteria’s surface.
Table 1. MIC Values for Different Nanoparticles against MDR E. coli, P. aeruginosa, S. epidermidis, and MRSA.
| bacteria | MIC values (ppm) |
|---|---|
| MDR-Escherichia coli | 2.35 |
| Pseudomonas aeruginosa | 4.45 |
| Staphylococcus epidermidis | 12.77 |
| MRSA | 14.26 |
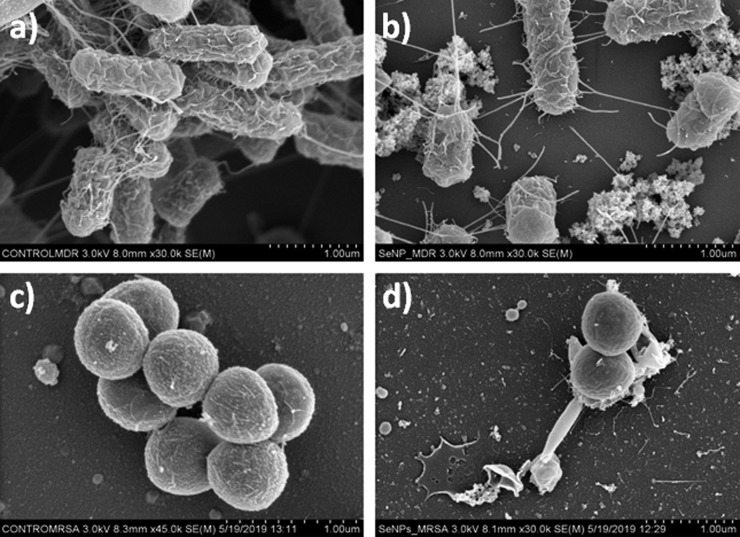
SEM micrographs of control MDR-EC and MRSA (Figure 7a,c) and bacteria after treatment with a selected concentration of SeNPs (Figure 7b,d) are shown. The characterization indicated that the treatment with the nanostructures induced changes of both bacterial strains, such as the disruption of the outer cell membrane. Furthermore, cell analysis can be easily seen after treatment with SeNPs. As a consequence, clear cell damage was observed with an abundant presence of cracks all over the cell membrane as well as bacterial deformation and collapse. Cell membrane damage is commonly found to be a cause of the action of ROS (as determined in the following section). Nevertheless, other mechanisms can also be inferred as the direct damage of the cells due to the morphology of the nanostructures.14,48
Figure 7.
SEM micrographs of (a, c) control MDR E. coli and MRSA and (b, d) bacteria after treatment with SeNPs.
In the light of the results, medium and higher concentrations of SeNPs might be useful for coating of external medical devices or surfaces that need to be sterilized.
In Vitro Cytotoxicity Characterization of Se NPs
To determine the cytotoxicity of the SeNPs on mammalian cells, in vitro MTS assays were performed with human dermal fibroblasts (HDF) and human melanoma and human glioblastoma cells using SeNPs concentrations ranging from 0.05 to 1 ppm for between 24 and 72 h (Figure 8). The SeNP range was restricted to 1 ppm for two reasons: no higher concentrations are expected to be introduced in the body, and a significant cell proliferation inhibition was found beyond 1 ppm. As shown in Figure 8a, a nanoparticle concentration ranging between 0.05 and 1 ppm showed no significant cytotoxicity toward HDF cells over 72 h. Therefore, the SeNPs can be considered biocompatible at a range of concentrations up to 1 ppm. Moreover, a slight cell proliferation decay was found when the nanoparticles were cultured with melanoma cells at the concentration of 1 ppm at a time of up to 72 h (Figure 8b). In order to further confirm the potential anticancer of the SeNPs, experiments with glioblastoma cells were done, showing a significant dose-dependent inhibition of cell proliferation at SeNP concentrations up to 1 ppm (Figure 8c). Thus, the SeNPs could be considered anticancer at a concentration of 1 ppm for 3-day treatment for melanoma cells while inducing a smaller anticancer effect at low concentrations for experiments at 1 and 3 days for brain tumors. However, further studies must be completed to completely support this hypothesis.
Figure 8.
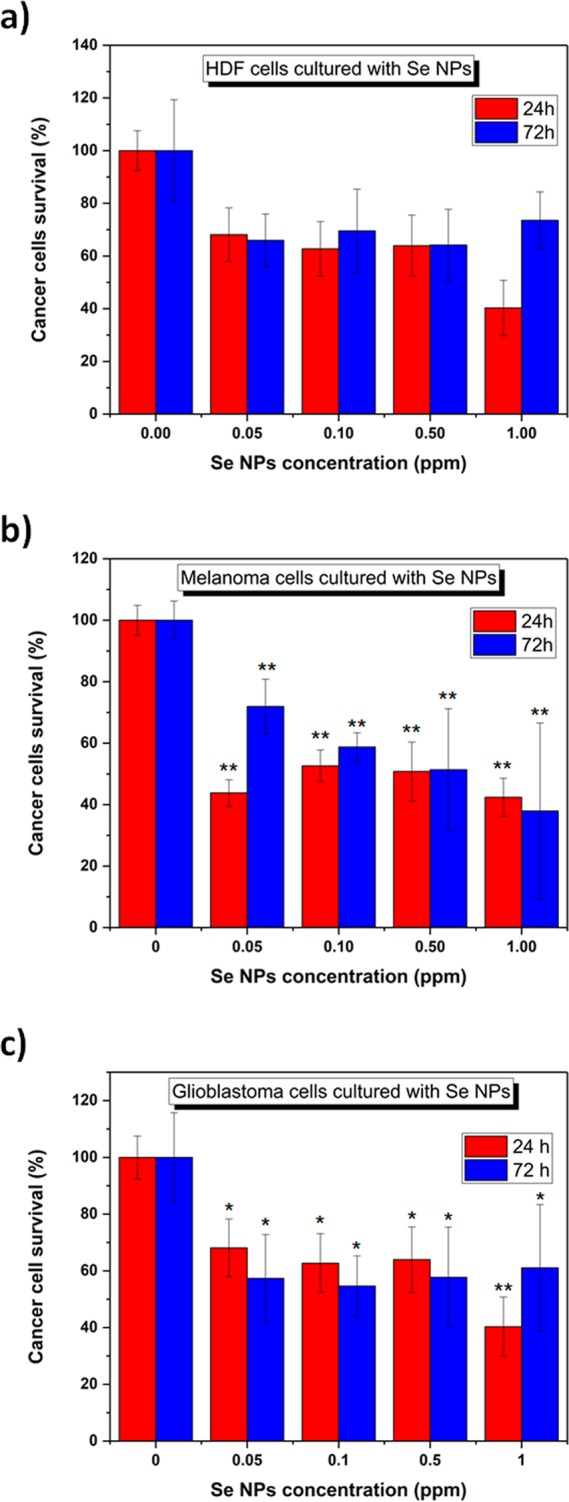
(a) HDF, (b) melanoma and (c) glioblastoma cells in the presence of SeNPs at concentrations ranging from 0.05–1.00 ppm. n = 3. All values represent the mean ± standard deviation. *p < 0.05, **p < 0.01(compared to controls).
Reactive Oxygen Species Analysis
The analysis of ROS allowed for the evaluation of toxicity towards human cells. The analysis was performed by exposing different concentrations (from 0.05 to 1 ppm) of the SeNPs to melanoma cells. After a 24 h treatment, the ROS could be successfully quantified in the cell media. An increase in the production of ROS (Figure 9) was observed when the nanoparticles interacted with the melanoma cells, with a dose-dependent effect. As ROS are species containing oxygen that are highly reactive, the intracellular mechanisms of defense have evolved to cope with this undesired chemical with the aim to avoid damaging the cell. However, under high levels of stress, the levels of ROS can dramatically increase. Their generation is one of the focal nanomaterials’ mechanisms of action that triggers the inhibition of both bacterial growth and cancer cells development.16,49 For the proposed SeNPs, it is well-known that after gaining cellular internalization, they stimulate the production of ROS. Therefore, the anticancer behavior previously observed in the presence of the nanoparticles (Figure 8b) could be easily related to an increase in ROS. In cancer cells, ROS levels are increased due to both environmental and internal mechanisms, leading to a high balance of these molecules. The mechanisms to cope with ROS are compromised and deteriorate within the cancer population, which contribute to the negative final impact on cancer biology.50
Figure 9.
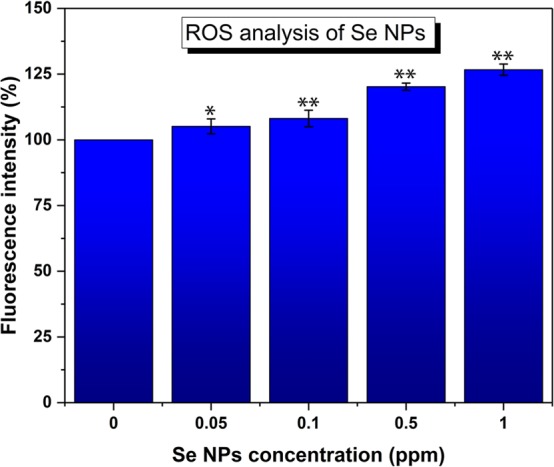
ROS study of SeNPs analysis. n = 3. Data is represented as mean ± SD; *p < 0.05, **p < 0.01(compared to controls).
Conclusions
In conclusion, selenium is a rare element on Earth but essential to living organisms on this planet. Therefore, an environmentally friendly PLAL approach was followed for a sustainable, efficient, and cost-effective production of SeNPs. The lifetime of the cavitation bubble induced by the irradiation of a Se target immersed in DI water by a pulsed laser has been determined to be around 0.330 ± 0.06 ms. By irradiating the target at such high repetition rates (kHz range), it was necessary to cool down the colloidal solution during the irradiation in order to prohibit the solvent from boiling. Beyond optimizing the synthesis conditions, the selenium nanoparticles were tested as biomedical agents. The SeNPs exhibited antibacterial properties in a range of concentrations between 0.5 to 1 ppm, triggering no significant cytotoxicity toward human healthy cells over the same period. Furthermore, the nanoparticles were found to be an anticancer toward human melanoma and glioblastoma cells at the concentration of 1 ppm for 72 h of treatment.
The reason for using low concentrations of Se NPs (below 1 ppm) was to ensure their nontoxicity with human cells when used internally in the human body. However, larger concentration of Se NPs (∼25 ppm) demonstrated strong inhibition and killing behavior on the four investigated bacteria; the Se NPs could therefore be used externally as a preventive coating for medical devices. Finally, those “naked” SeNPs can be used as biomedical agents with both antibacterial and anticancer properties at very low concentrations. More work is undergoing to determine if the antibacterial and anticancer properties can be further improved by changing the morphology of those naked SeNPs.
Acknowledgments
L.G., M.K., and G.G. would like to thank the Center for Integrative Nanotechnology Sciences (CINS) of UA Little Rock for the use of their UV–vis, AFM, SEM, and TEM. T.H. and P.T. would like to thank the Mc Nair Research Program for the financial support. D.M., A.V., A.A., J.C. and T.J.W. would like to thank Northeastern University for funding.
Author Contributions
§ L.G., T.H., and D.M. contributed equally to this work.
The authors declare no competing financial interest.
References
- Pang T.; Guindon G. E. Globalization and risks to health. EMBO Rep. 2004, 5, 11–16. 10.1038/sj.embor.7400226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestinaci F.; Pezzotti P.; Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog. Global Health 2015, 109, 309–318. 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola C. L. The antibiotic resistance crisis: part 1: causes and threats. Pharm. Ther. 2015, 40, 277–283. [PMC free article] [PubMed] [Google Scholar]
- Ma X.; Yu H. Global burden of cancer. Yale J. Biol. Med. 2006, 79, 85–94. [PMC free article] [PubMed] [Google Scholar]
- Thun M. J.; DeLancey J. O.; Center M. M.; Jemal A.; Ward E. M. The global burden of cancer: priorities for prevention. Carcinogenesis 2010, 31, 100–110. 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin E. J.; Denson L. A.; Whitford H. S. Cancer Treatment Side Effects: A Meta-analysis of the Relationship Between Response Expectancies and Experience. J. Pain Symptom Manage. 2017, 54, 245–258.e2. e2 10.1016/j.jpainsymman.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Housman G.; Byler S.; Heerboth S.; Lapinska K.; Longacre M.; Snyder N.; Sarkar S. Drug resistance in cancer: an overview. Cancers 2014, 6, 1769–1792. 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini R.; Saini S.; Sharma S. Nanotechnology: the future medicine. J. Cutan. Aesthet. Surg. 2010, 3, 32–33. 10.4103/0974-2077.63301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feynman R. P. There’s plenty of room at the bottom [data storage]. J. Microelectromech. Syst. 1992, 1, 60–66. 10.1109/84.128057. [DOI] [Google Scholar]
- Guisbiers G. Size-dependent materials properties toward a universal equation. Nanoscale Res. Lett. 2010, 5, 1132–1136. 10.1007/s11671-010-9614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulla J. E.; Sahu S. C.; Hayes A. W. Nanotechnology: History and future. Hum. Exp. Toxicol. 2015, 34, 1318–1321. 10.1177/0960327115603588. [DOI] [PubMed] [Google Scholar]
- Jeevanandam J.; Barhoum A.; Chan Y. S.; Dufresne A.; Danquah M. K. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salata O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. 10.1186/1477-3155-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdal Dayem A.; Hossain M. K.; Lee S. B.; Kim K.; Saha S. K.; Yang G. M.; Choi H. Y.; Cho S. G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. 10.3390/ijms18010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manke A.; Wang L.; Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin Y. N.; Asnis J.; Hafeli U. O.; Bach H. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. 10.1186/s12951-017-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisbiers G.; Wang Q.; Khachatryan E.; Mimun L. C.; Mendoza-Cruz R.; Larese-Casanova P.; Webster T. J.; Nash K. L. Inhibition of E. coli and S. aureus with selenium nanoparticles synthesized by pulsed laser ablation in deionized water. Int. J. Nanomed. 2016, 11, 3731–3736. 10.2147/IJN.S106289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara H. H.; Guisbiers G.; Mendoza J.; Mimun L. C.; Vincent B. A.; Lopez-Ribot J. L.; Nash K. L. Synergistic antifungal effect of chitosan-stabilized selenium nanoparticles synthesized by pulsed laser ablation in liquids against Candida albicans biofilms. Int. J. Nanomed. 2018, 13, 2697–2708. 10.2147/IJN.S151285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Guardia M. The challenges of green nanotechnology. BioImpacts 2014, 4, 1–2. 10.5681/bi.2014.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison J. E. Greener nanoscience: a proactive approach to advancing applications and reducing implications of nanotechnology. ACS Nano 2008, 2, 395–402. 10.1021/nn800131j. [DOI] [PubMed] [Google Scholar]
- Makarov V. V.; Love A. J.; Sinitsyna O. V.; Makarova S. S.; Yaminsky I. V.; Taliansky M. E.; Kalinina N. O. ″Green″ Nanotechnologies: Synthesis of Metal Nanoparticles Using Plants. Acta Naturae 2014, 6, 35–44. 10.32607/20758251-2014-6-1-35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Li Q.; Ma X.; Tian B.; Li T.; Yu J.; Dai S.; Weng Y.; Hua Y. Biosynthesis of gold nanoparticles by the extreme bacterium Deinococcus radiodurans and an evaluation of their antibacterial properties. Int. J. Nanomed. 2016, Volume 11, 5931–5944. 10.2147/IJN.S119618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.; He D.; Qian Y.; Guan B.; Gao S.; Cui Y.; Yokoyama K.; Wang L. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int. J. Mol. Sci. 2012, 13, 466–476. 10.3390/ijms13010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip D. Honey mediated green synthesis of gold nanoparticles. Spectrochim. Acta, Part A 2009, 73, 650–653. 10.1016/j.saa.2009.03.007. [DOI] [PubMed] [Google Scholar]
- de Barros C. H. N.; Cruz G. C. F.; Mayrink W.; Tasic L. Bio-based synthesis of silver nanoparticles from orange waste: effects of distinct biomolecule coatings on size, morphology, and antimicrobial activity. Nanotechnol., Sci. Appl. 2018, 11, 1–14. 10.2147/NSA.S156115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S.; Hirst D. G.; O’Sullivan J. M. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–113. 10.1259/bjr/59448833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ouay B.; Stellacci F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. 10.1016/j.nantod.2015.04.002. [DOI] [Google Scholar]
- Salas-Orozco M.; Niño-Martínez N.; Martínez-Castañón G.-A.; Méndez F. T.; Jasso M. E. C.; Ruiz F. Mechanisms of Resistance to Silver Nanoparticles in Endodontic Bacteria: A Literature Review. J. Nanomater. 2019, 2019, 7630316. 10.1155/2019/7630316. [DOI] [Google Scholar]
- Hurd A. J.; Kelley R. L.; Eggert R. G.; Lee M.-H. Energy-critical elements for sustainable development. MRS Bull. 2012, 37, 405–410. 10.1557/mrs.2012.54. [DOI] [Google Scholar]
- Guan B.; Yan R.; Li R.; Zhang X. Selenium as a pleiotropic agent for medical discovery and drug delivery. Int. J. Nanomed. 2018, 13, 7473–7490. 10.2147/IJN.S181343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalickova S.; Milosavljevic V.; Cihalova K.; Horky P.; Richtera L.; Adam V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. 10.1016/j.nut.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Tan V. L. C.; Hinchman A.; Williams R.; Tran P. A.; Fox K. Nanostructured biomedical selenium at the biological interface (Review). Biointerphases 2018, 13, 06D301 10.1116/1.5042693. [DOI] [PubMed] [Google Scholar]
- Weekley C. M.; Harris H. H. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8894. 10.1039/c3cs60272a. [DOI] [PubMed] [Google Scholar]
- Hosnedlova B.; Kepinska M.; Skalickova S.; Fernandez C.; Ruttkay-Nedecky B.; Peng Q.; Baron M.; Melcova M.; Opatrilova R.; Zidkova J.; Bjorklund G.; Sochor J.; Kizek R. Nano-selenium and its nanomedicine applications: a critical review. Int. J. Nanomed. 2018, 13, 2107–2128. 10.2147/IJN.S157541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferhati H.; Djeffal F.; Arar D. Above 14% efficiency earth-abundant selenium solar cells by introducing gold nanoparticles and Titanium sub-layer. Opt. Mater. 2018, 86, 24–31. 10.1016/j.optmat.2018.09.025. [DOI] [Google Scholar]
- Kusper M.; Guisbiers G. Synthesis of aluminum oxide nanoparticles by laser ablation in liquids. MRS Adv. 2018, 3, 3899–3903. 10.1557/adv.2018.626. [DOI] [Google Scholar]
- Bhattacharjee S. DLS and zeta potential - What they are and what they are not?. J. Controlled Release 2016, 235, 337–351. 10.1016/j.jconrel.2016.06.017. [DOI] [PubMed] [Google Scholar]
- Guisbiers G.; Wang Q.; Khachatryan E.; Arellano-Jimenez M. J.; Webster T. J.; Larese-Casanova P.; Nash K. L. Anti-bacterial selenium nanoparticles produced by UV/VIS/NIR pulsed nanosecond laser ablation in liquids. Laser Phys. Lett. 2015, 12, 016003 10.1088/1612-2011/12/1/016003. [DOI] [Google Scholar]
- Kuzmin P. G.; Shafeev G. A.; Voronov V. V.; Raspopov R. V.; Arianova E. A.; Trushina E. N.; Gmoshinskii I. V.; Khotimchenko S. A. Bioavailable nanoparticles obtained in laser ablation of a selenium target in water. Quantum Electron. 2012, 42, 1042–1044. 10.1070/QE2012v042n11ABEH014754. [DOI] [Google Scholar]
- Singh S. C.; Mishra S. K.; Srivastava R. K.; Gopal R. Optical Properties of Selenium Quantum Dots Produced with Laser Irradiation of Water Suspended Se Nanoparticles. J. Phys. Chem. C 2010, 114, 17374–17384. 10.1021/jp105037w. [DOI] [Google Scholar]
- Van Overschelde O.; Guisbiers G. Photo-fragmentation of selenium powder by Excimer laser ablation in liquids. Opt. Laser Technol. 2015, 73, 156–161. 10.1016/j.optlastec.2015.04.020. [DOI] [Google Scholar]
- Lucovsky G.; Mooradian A.; Taylor W.; Wright G. B.; Keezer R. C. Identification of the fundamental vibrational modes of trigonal, α - monoclinic and amorphous selenium. Solid State Commun. 1967, 5, 113–117. 10.1016/0038-1098(67)90006-3. [DOI] [Google Scholar]
- Anderson A.; Sanders A.; Smith W. Raman spectra of selenium dioxide at low temperatures. J. Raman Spectrosc. 2000, 31, 403–406. . [DOI] [Google Scholar]
- Pecora R. Dynamic Light Scattering Measurement of Nanometer Particles in Liquids. J. Nanopart. Res. 2000, 2, 123–131. 10.1023/A:1010067107182. [DOI] [Google Scholar]
- Cremonini E.; Zonaro E.; Donini M.; Lampis S.; Boaretti M.; Dusi S.; Melotti P.; Lleo M. M.; Vallini G. Biogenic selenium nanoparticles: characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb. Biotechnol. 2016, 9, 758–771. 10.1111/1751-7915.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroumand S.; Safari M.; Shaabani E.; Shirza M.; Faridi-Majidi R. Selenium nanoparticles: synthesis, characterization and study of their cytotoxicity, antioxidant and antibacterial activity. Mater. Res. Express 2019, 6, 0850d8 10.1088/2053-1591/ab2558. [DOI] [Google Scholar]
- Muthu S.; Raju V.; Gopal V. B.; Gunasekaran A.; Narayan K. S.; Malairaj S.; Lakshmikanthan M.; Duraisamy N.; Krishnan K.; Perumal P. A rapid synthesis and antibacterial property of selenium nanoparticles using egg white lysozyme as a stabilizing agent. SN Appl. Sci. 2019, 1, 1543. 10.1007/s42452-019-1509-x. [DOI] [Google Scholar]
- Kim K. S.; Lee D.; Song C. G.; Kang P. M. Reactive oxygen species-activated nanomaterials as theranostic agents. Nanomedicine 2015, 10, 2709–2723. 10.2217/nnm.15.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G.; Wu X.; Chen P.; Zhang L.; Yang C. S.; Zhang J. Selenium nanoparticles are more efficient than sodium selenite in producing reactive oxygen species and hyper-accumulation of selenium nanoparticles in cancer cells generates potent therapeutic effects. Free Radical Biol. Med. 2018, 126, 55–66. 10.1016/j.freeradbiomed.2018.07.017. [DOI] [PubMed] [Google Scholar]
- Yang H.; Villani R. M.; Wang H.; Simpson M. J.; Roberts M. S.; Tang M.; Liang X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. 10.1186/s13046-018-0909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]