ABSTRACT
Seasonally breeding animals undergo shifts in physiology and behavior in response to changes in photoperiod (day length). Interestingly, some species, such as Siberian hamsters (Phodopus sungorus), are more aggressive during the short-day photoperiods of the non-breeding season, despite gonadal regression. While our previous data suggest that Siberian hamsters employ a ‘seasonal switch’ from gonadal to adrenal regulation of aggression during short-day photoperiods, there is emerging evidence that the gut microbiome, an environment of symbiotic bacteria within the gastrointestinal tract, may also change seasonally and modulate social behaviors. The goal of this study was to compare seasonal shifts in the gut microbiome, circulating levels of adrenal dehydroepiandrosterone (DHEA) and aggression in male and female Siberian hamsters. Hamsters were housed in either long-day (LD) or short-day (SD) photoperiods for 9 weeks. Fecal samples were collected and behaviors were recorded following 3, 6 and 9 weeks of housing, and circulating DHEA was measured at week 9. SD females that were responsive to changes in photoperiod (SD-R), but not SD-R males, displayed increased aggression following 9 weeks of treatment. SD-R males and females also exhibited distinct changes in the relative abundance of gut bacterial phyla and families, yet showed no change in circulating DHEA. The relative abundance of some bacterial families (e.g. Anaeroplasmataceae in females) was associated with aggression in SD-R but not LD or SD non-responder (SD-NR) hamsters after 9 weeks of treatment. Collectively, this study provides insight into the complex role of the microbiome in regulating social behavior in seasonally breeding species.
KEY WORDS: Aggression, Dehydroepiandrosterone, Gut–brain axis, Microbiota, Day length, Seasonality
Summary: Sex-specific changes in the gut microbiome are associated with aggression during the non-breeding season in Siberian hamsters, suggesting a potential role for the microbiome in regulating seasonal aggression.
INTRODUCTION
The gut microbiota is the collection of bacteria, archaea, fungi, viruses and protists that live in the gastrointestinal tract (reviewed in Shreiner et al., 2015). These microbes are not only responsible for metabolic functions but they can also influence the brain and behavior, the immune system and numerous other physiological systems (reviewed in Cani, 2018; Shreiner et al., 2015; see also Sylvia et al., 2017). Disruption of the gut microbiome has been linked to a variety of diseases, including irritable bowel syndrome, inflammatory bowel disease, obesity, diabetes, multiple sclerosis, autism, allergic disease, depression and anxiety (reviewed in Ghaisas et al., 2016; see also O'Mahony et al., 2009).
The gut microbiome, the genes encoding microbial communities, has been predominately studied in model systems, such as germ-free (GF) mice, but these models do not allow us to fully understand the role of these microbiota in the natural environment. Further, because behavior in GF mice is often observed in isolated tests, there is limited information about the role of the microbiome in modulating social behavior (Clarke et al., 2014; Desbonnet et al., 2014; Nguyen et al., 2015; Sylvia et al., 2017, 2018; reviewed in Collins et al., 2012). The relationship between the gut microbiome and social behavior (e.g. aggression) remains relatively understudied, especially in non-traditional systems, such as Siberian hamsters. Studying the gut microbiome in a non-traditional model organism that naturally changes behavior in response to changes in season allows us to understand the mechanisms underlying the relationships among the microbiome, physiology and behavior.
Siberian hamsters exhibit pronounced shifts in reproductive physiology and its associated behaviors in response to seasonal changes in photoperiod (i.e. day length) (Bartness and Wade, 1985). Photoperiod is the primary cue that Siberian hamsters use to anticipate changes in season, and shifts in day length are physiologically encoded by changes in the pattern and duration of melatonin secretion (Bartness et al., 1993; Goldman, 2001; reviewed in Walton et al., 2011). In response to this signal, hamsters will display specific characteristics during long-day (LD; characteristic of the breeding season) or short-day (SD; characteristic of the non-breeding season) photoperiods. During long, ‘summer-like’ days, hamsters have a brown pelage and functional gonads (Jasnow et al., 2000; Rendon et al., 2016). In contrast, hamsters housed in short, ‘winter-like’ days develop lighter pelage, exhibit gonadal regression, decrease body mass and show a pronounced increase in aggression (Jasnow et al., 2000; Rendon et al., 2016). Unlike these short-day responders (SD-R), a subset of animals housed in SDs does not respond to seasonal changes in photoperiod; these short-day non-responders (SD-NRs) typically exhibit a LD-like behavioral and physiological phenotype (Freeman and Goldman, 1997).
Previous work from our lab has examined the mechanisms underlying SD increases in aggressive behavior in Siberian hamsters. Classic neuroendocrine studies focus on the role of circulating gonadal steroids, such as testosterone and estradiol (E2), in directly regulating aggression in birds and rodents (reviewed in Soma, 2006; Wingfield, 1984). However, some species utilize alternative neuroendocrine pathways independent of circulating gonadal steroids to maintain or increase aggression during the non-breeding season, despite gonadal regression (reviewed in Soma et al., 2008; Wingfield and Soma, 2002). We have shown that both male and female Siberian hamsters increase circulating levels of the adrenal hormone dehydroepiandrosterone (DHEA) during SDs (Rendon et al., 2015; Scotti et al., 2008). DHEA is an androgen that can pass through the blood–brain barrier and be converted to biologically active androgens and estrogens within brain regions that possess the appropriate steroidogenic enzymes (Pradhan et al., 2010; reviewed in Soma et al., 2015). Thus, region-specific metabolism of DHEA to testosterone and/or E2 likely allows these animals to regulate the neural circuits relevant to aggression during the non-breeding season (reviewed in Munley et al., 2018; Rendon and Demas, 2016). Taken together, these studies suggest that Siberian hamsters employ a ‘seasonal switch’ from gonadal regulation of aggression during LDs to adrenal regulation of aggression during SDs.
Because gut microbes produce numerous byproducts that may be involved in physiological and behavioral changes (Foley et al., 2014; Hanstock et al., 2004; reviewed in MacFabe, 2015; Sylvia and Demas, 2018b), the microbiome may play an important role in mediating this ‘seasonal switch’ in neuroendocrine mechanisms. Previous work suggests that the gut microbiome may change seasonally and is related to aggressive behavior. Specifically, Siberian hamsters treated with broad-spectrum antibiotics show changes in gut microbial communities that are associated with decreased aggression (Sylvia et al., 2017). The gut microbial composition is also different between dogs classified as aggressive and non-aggressive (Kirchoff et al., 2019). In addition, other studies suggest that the gut microbiome changes on a seasonal basis. For example, chickens and mice exhibit seasonal shifts in the gut microbiome (Cui et al., 2016; Hieke et al., 2019; Wang et al., 2018). Further, body mass correlates with the relative abundance of Proteobacteria, Citrobacter and Firmicutes in LD male Siberian hamsters (Bailey et al., 2010). While these studies suggest that seasonal shifts in the gut microbiome may modulate social behavior, the specific mechanisms underlying this relationship have yet to be explored.
Moreover, recent work suggests that the gut microbiome can vary substantially between males and females (Sylvia and Demas, 2018a; Sylvia et al., 2017, 2018; Vemuri et al., 2018). For example, castrated male mice, which are incapable of producing gonadal steroids, exhibit a gut microbiome that more closely resembles that of female than male mice (Yurkovetskiy et al., 2013). In Siberian hamsters, antibiotic treatment is associated with more robust changes in the gut microbiome of males than females; however, repeated antibiotic treatment decreases aggression in a single treatment for females rather than two treatments for males, and aggression returns to baseline levels during the recovery period for males, but not for females (Sylvia et al., 2017). These findings suggest that studying both sexes is valuable and necessary when investigating the role of the gut microbiome in mediating social behavior.
The goal of the current study was to examine how seasonal changes in photoperiod affect the gut microbiome, circulating DHEA and social behavior of both male and female Siberian hamsters. We hypothesized that 9 weeks of photoperiodic treatment would be sufficient to elicit significant changes in the gut microbial composition of SD-R hamsters compared with LD and SD-NR hamsters. Furthermore, we predicted that seasonal shifts in the gut microbiome would be correlated with changes in physiology (e.g. body mass and serum DHEA levels) and aggressive behavior in a sex-specific manner. Collectively, this study aimed to enhance our understanding of the links among the often separately researched gut microbiome, circulating hormones, photoperiod and behavior across the sexes in a non-traditional system.
MATERIALS AND METHODS
Animal housing and photoperiodic treatment
Male and female adult (2–7 months of age) Siberian hamsters, Phodopus sungorus (Pallas 1773), were group-housed in polycarbonate cages (28×17×12 cm) and raised in LD conditions (light:dark, 16 h:8 h) in a colony maintained at Indiana University. Experimental animals (N=26 males, N=26 females) were moved to a new holding room under LD conditions, individually housed and allowed to acclimate for 1 week. Following the 1 week acclimation period, male and female hamsters were randomly assigned to either the LD (N=9 males, N=9 females; light:dark, 16 h:8 h as above) or SD (N=17 males and N=17 females; light:dark, 8 h:16 h) group and housed for 9 weeks, as outlined in previous studies for this species (Rendon et al., 2015). For all conditions, relative humidity was 55±5%, ambient temperature was 20±2°C, and hamsters had ad libitum access to purified tap water and standard laboratory rodent chow (Lab Diet 5001, PMI Nutrition). All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Bloomington Institutional Animal Care and Use Committee (BIACUC) at Indiana University (protocol no. 16-025).
Seasonal phenotypes
Seasonal phenotypes were determined following 9 weeks of photoperiodic treatment based on a priori criteria for male and female Siberian hamsters (Jasnow et al., 2000; Rendon et al., 2015; Scotti et al., 2007). Starting with the acclimation week, all hamsters and total food intake were weighed weekly, and reproductive tissue mass was recorded at the conclusion of the study. LD hamsters (N=18; N=9 males, N=9 females) maintained body and reproductive tissue mass and exhibited brown pelage. Of the hamsters placed in SD conditions (N=34; N=17 males, N=17 females), 53% of both males and females physiologically responded to changes in photoperiod (SD-R; N=9 males, N=9 females). SD-R hamsters were classified as animals that exhibited ≥5% loss of body mass and gonadal regression (compared with the average testes or ovaries mass of LD animals at week 9). A white pelage was used to help confirm each classification. Animals that did not meet these criteria were classified as SD-NR hamsters. Approximately 47% of males and 47% of females failed to respond to changes in photoperiod (SD-NR; N=8 males, N=8 females). The proportion of SD-NR hamsters in this study is within expectations based on past studies, which found that approximately 30% of Siberian hamsters and other rodents fail to respond to SD conditions (Goldman, 2001; Gorman and Zucker, 1995; Lynch et al., 1989; Rendon et al., 2017).
Fecal sampling and microbiome analysis
Fecal samples were obtained from each animal following 0 (pre-treatment), 3, 6 and 9 weeks of photoperiodic housing. Hamsters were removed from their cages and held over a sterile container to collect fecal samples. Hamsters were then returned to their cages. Fecal samples were stored at −80°C until further processing.
DNA was extracted from fecal samples (males: N=6 per treatment group; females: N=6 per treatment group) using a commercially available kit (Maxwell RSC Tissue DNA Kit, Promega, Madison, WI, USA) (Sylvia and Demas, 2018a; Sylvia et al., 2017, 2018); 80 μl of TE buffer, 20 μl of RNase A solution and 300 μl of lysis buffer were added to each sample. Samples were then homogenized and centrifuged at 4°C for 5 min at 1200 rpm. The supernatant was used for automated extraction (Maxwell Rapid Sample Concentrator Instrument, Promega). In addition to experimental samples, two negative controls were simultaneously extracted to indicate any contamination (elution buffer only; and TE buffer, RNase A solution and elution buffer all together). The purity and quality of DNA were verified with the Take3 microvolume plate (BioTek, Winooski, VT, USA) and 4200 TapeStation system (Agilent, Santa Clara, CA, USA).
Following Maxwell processing, samples were sent to the Indiana University Center for Genomics and Bioinformatics (Bloomington, IN, USA), where multiplexed amplicon libraries spanning the V4 hypervariable domain of the microbial 16S ribosomal RNA (rRNA) gene were prepared using NEXTflex 16S V4 Amplicon-Seq Library Prep Kit 2.0 (catalog number: NOVA-4203-01, Bioo Scientific, Austin, TX, USA; Earth Microbiome primers 515F-806R). Agencourt AMPure XP Magnetic Beads were used to clean the samples, PCR primers targeting the V4 domain amplified the samples, and the Illumina MiSeq v3 (600 cycle) platform was used to determine sequence information. Operational taxonomic units (OTUs) were determined through Swarm and matched against the Silva database, as described in previous studies (Armanhi et al., 2016; Mahé et al., 2014; Sylvia et al., 2018).
Behavioral testing and analyses
Behavioral videos were recorded following 0 (pre-treatment), 3, 6 and 9 weeks of photoperiodic treatment and were analyzed for same-sex aggression, investigation, grooming and scent-marking behaviors using previously outlined methods (Jasnow et al., 2000; Rendon et al., 2016). Specifically, within the first 2 h of the dark phase, an unfamiliar same-sex intruder (N=10 males, N=10 females) was placed into the home cage of the experimental (i.e. resident) hamster (N=26 males, N=26 females), and the animals were allowed to interact for 5 min. Intruders were housed in LD conditions in groups of 2, and intruders were of approximately the same age and mass (±10%) as the resident animals with which they were paired. All trials were recorded (Sony HandyCam Digital Camcorder HDR-SR7) under low-illumination red lights.
We scored aggression (latency to first attack and frequency and duration of attacks and chases), investigation (frequency and duration of anogenital and nose-to-nose investigation), grooming (frequency and duration of self-grooming) and scent-marking behaviors (frequency and duration of scent depositing) with ODLog (Macropod Software, Eden Prairie, MN, USA) using previously outlined methods (Jasnow et al., 2000; Rendon et al., 2015, 2016; Sylvia et al., 2017).
Tissue collection and blood sampling
All animals were anesthetized with isoflurane vapor following behavioral testing at week 9 to collect a terminal blood sample from the retro-orbital sinus (Sylvia et al., 2018). Hamsters were then euthanized using a lethal intraperitoneal injection of ketamine and xylazine mixture in 0.9% saline. Testes (males), ovaries (females), uterine horns (females), epididymal white adipose tissue (males, EWAT; pads surrounding testes and likely metabolically supporting reproductive capabilities) and parametrial white adipose tissue (females, PWAT; pads surrounding ovaries and likely metabolically supporting reproductive capabilities) were removed and weighed (Bailey et al., 2017; Carlton and Demas, 2015; Jaubert et al., 1995). Blood samples were clotted for 1 h at room temperature, the clots were removed, and the samples were centrifuged at 4°C for 30 min at 2500 rpm. Serum was stored at −20°C until further processing.
Serum DHEA quantification
Serum DHEA concentration was measured using a commercially available enzyme immunoassay kit (DHEA ELISA kit ADI-901-093, Enzo Life Sciences, Farmingdale, NY, USA; assay sensitivity 2.90 pg ml−1). The validity of this assay was determined by comparing male and female Siberian hamster serum samples of varying dilutions with a standard curve generated using reference standards provided by the kit. This assay has some cross-reactivity with sulfated DHEA (30%) and low cross-reactivity with androstenedione (0.73%), androsterone (0.29%), pregnenolone (0.28%) and testosterone (0.10%). All serum samples were run neat or diluted 1:2 or 1:4 with assay buffer to ensure 20–80% binding on a 4-parameter logistic standard curve (Microplate Manager 6 version 6.2, Bio-Rad Laboratories, Hercules, CA, USA). Samples were run in duplicate according to the manufacturer's instructions. Samples from animals of different treatment groups were counterbalanced across two plates from the same kit lot number (05071801). Samples with a coefficient of variability (CV) greater than 20% and a maximum binding less than 20% or greater than 80% were re-analyzed. The inter-assay CV was 12.8% and the average intra-assay CV was 10.9%.
Statistical analyses
All statistical analyses were performed in R v.1.1.383 (http://www.R-project.org/), and we attributed statistical significance at P<0.05 after controlling for false discovery rate in the case of multiple comparisons (Verhoeven et al., 2005). Females and males displayed significantly different quantities of several behaviors, including duration and frequency of attacks. Therefore, females and males were separated during statistical analysis. For behavioral analyses, mixed model ANOVA were used to compare the duration or frequency of aggression, investigation, grooming and scent-marking behaviors across treatment groups and time points. If a significant result was found between the interaction of treatment and time, pair-wise relationships were explored using Tukey's honest significant difference post hoc tests. For organ mass, body mass and serum DHEA analyses, variance and normality were assessed using Levene's tests and Shapiro–Wilk tests, respectively. If data had both equal variances and a normal distribution, one-way ANOVA were used to compare organ and body mass across treatment groups and time points (male and female body mass, male EWAT mass and paired testes mass) and to compare serum DHEA levels across treatment groups at the week 9 time point. Log transformations were used to transform some data to attain equal variances and normality. If data exhibited a normal distribution, but did not have equal variances, Welch's ANOVA were used (female PWAT mass and uterine horns mass).
Principal coordinates analyses (PCoA) were performed to visualize differences in microbial communities between treatment groups and across time points, and multi-variate non-parametric ANOVA of dissimilarities (PERMANOVA) were run to determine whether microbial communities were affected by treatment, time or an interaction of treatment and time based on Bray–Curtis distance (Sze et al., 2014). The Shannon–Wiener index was calculated to determine alpha diversity, and two-way ANOVA were used to determine statistically significant differences in alpha diversity between treatment groups and across time points (Hill, 1973; Jost, 2006). Bray–Curtis dissimilarity scores were calculated across treatments and were converted to percentage differences between groups for a more clear comparison (Maziarz et al., 2018). Mixed model ANOVA were used to compare the relative abundance of each phylum and family across treatments and time points. If a significant result was found between the interaction of treatment and time, pair-wise relationships were explored using Tukey's honest significant difference post hoc tests. In addition, Spearman's rank correlations were used to assess potential relationships between the relative abundance of bacterial phyla and families, aggressive and non-aggressive social behaviors, serum DHEA levels and body mass for each treatment group and sex at the week 9 time point alone or across all time points.
RESULTS
Reproductive phenotypes differ across photoperiod
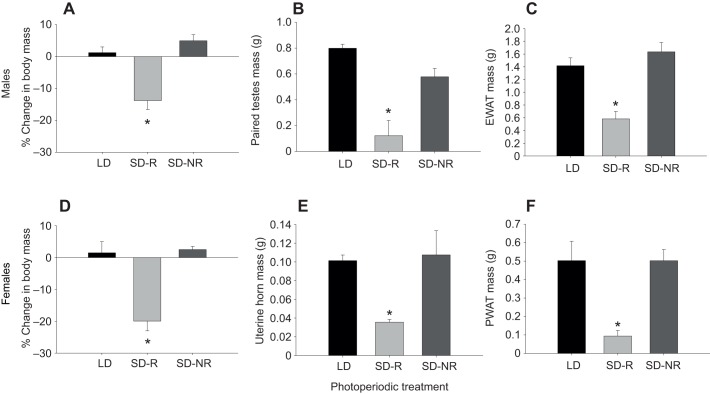
After 9 weeks of photoperiodic treatment, SD-R males had significantly lower body mass (P<0.001; Fig. 1A), paired testes mass (P<0.001; Fig. 1B) and EWAT mass (P<0.001; Fig. 1C) compared with both LD and SD-NR males. Similarly, SD-R females also had significantly decreased body mass (P<0.001; Fig. 1D), uterine horn mass (P<0.001; Fig. 1E) and PWAT mass (P<0.001; Fig. 1F) after 9 weeks of treatment compared with both LD and SD-NR females.
Fig. 1.
Percentage change in body mass, reproductive tissue mass, and epididymal and parametrial white adipose tissue (EWAT/PWAT) mass following 9 weeks of photoperiodic treatment. Percentage change in (A,D) body mass; (B,E) reproductive tissue mass; and (C,F) EWAT/PWAT mass in male (A–C) and female (D–F) long-day (LD) hamsters, and short-day responsive (SD-R) and non-responsive (SD-NR) hamsters. Bars represent means±s.e.m. (LD: N=9, SD-R: N=9, SD-NR: N=8, for both males and females). An asterisk indicates a statistically significant difference between group means (P<0.001; male and female percentage change in body mass, male paired testes mass and EWAT mass: one-way ANOVA; female uterine horns mass and PWAT mass: Welch's ANOVA).
Photoperiod significantly increases aggression in females, but not in males
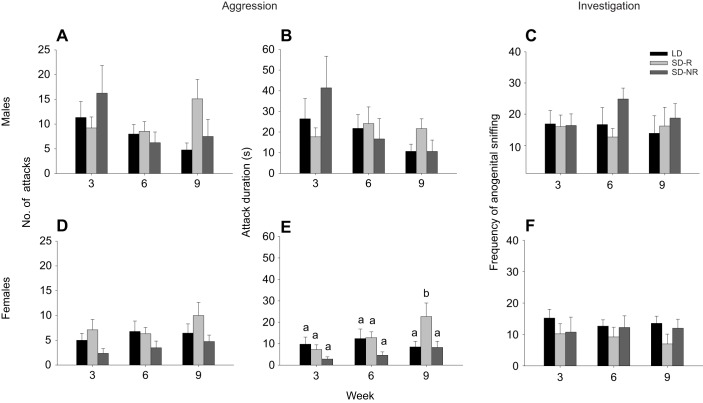
The duration of attacks (F11,52.4=1.546, P=0.144), number of attacks (F11,52.8=1.464, P=0.1735) and latency to first attack (F11,51.8=1.142, P=0.097) in SD-R males were not significantly different from those for SD-NR males and LD males across time (Fig. 2A,B; Table S1).
Fig. 2.
Aggressive and non-aggressive social behaviors in male and female hamsters following 3, 6 or 9 weeks of photoperiodic treatment. (A,D) Number of attacks; (B,E) attack duration; and (C,F) frequency of anogenital sniffing in male (A–C) and female (D–F) LD hamsters, SD-R hamsters and SD-NR hamsters. Bars represent means±s.e.m. (LD: N=9, SD-R: N=9, SD-NR: N=8, for both males and females). Bars with different letters represent statistically different group means (P<0.05; mixed model ANOVA).
In comparison, attack duration (F11,50.5=3.107, P=0.003) and number of attacks (F11,51.1=2.986, P=0.004) in SD-R females were significantly different from those for SD-NR and LD females across time (Fig. 2D,E; Table S2). Specifically, at week 9, SD-R females exhibited a longer attack duration than LD and SD-NR females (Z=2.852, P=0.041; Fig. 2E). SD-R females at week 0 also displayed a significantly higher number of attacks than SD-NR females at week 0 (P=0.008) and all other groups at later weeks (P<0.05; Fig. 2D). Overall, SD-R females had a significantly shorter latency to first attack than SD-NR females (F2,22.6=3.997, P=0.033; Table S2).
Photoperiod does not affect non-aggressive behaviors
The frequency of anogenital sniffing was similar across photoperiodic groups and time in males (treatment: F2,23=0.608, P=0.553; time: F3,75=0.473, P=0.702; interaction: F11,50.8=0.676, P=0.754; Fig. 2C) and females (treatment: F2,23=0.955, P=0.399; time: F4,76.1=2.223, P=0.074; interaction: F11,50.5=1.228, P=0.293; Fig. 2F). Other investigative, grooming and scent-marking behaviors were not different across photoperiodic treatments (data not shown). However, there were differences across time and in the interaction between time and treatment. Specifically, in males and females, the duration and frequency of nose-to-nose sniffing were affected by time (data not shown) and the interaction of time and treatment (Tables S1 and S2). The duration and frequency of nose-to-nose sniffing was higher in SD-NR males, SD-R males, LD females and SD-NR females at baseline (data not shown). However, overall, SD-R males and females tended to remain relatively stable in investigative, scent-marking and grooming behaviors.
Photoperiod affects microbial diversity in a sex-dependent manner
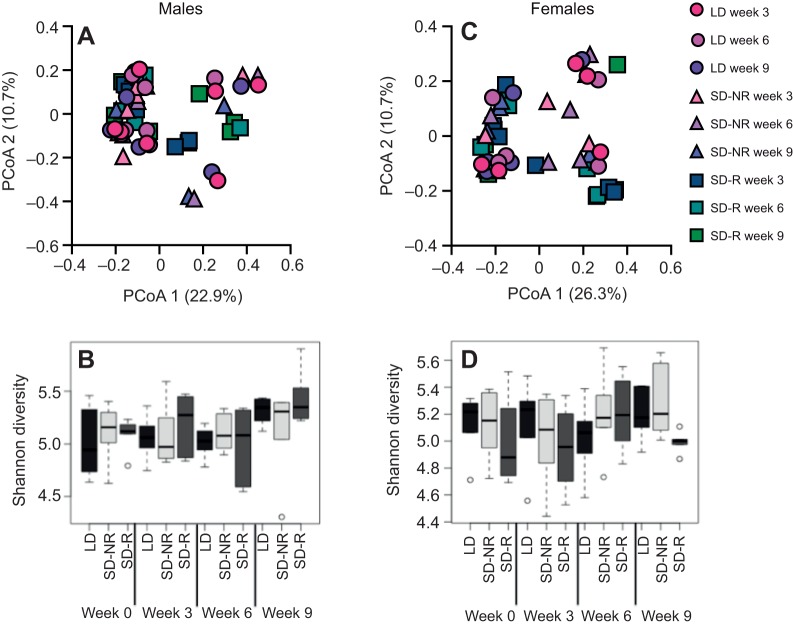
Based on PERMANOVA analyses, gut microbial communities were not significantly different between the sexes (P=0.114). However, all analyses were completed separately for each sex because behavior was significantly different between the sexes. In males, gut microbial communities were not significantly different across photoperiodic groups (P=0.182), time points (P=0.546) or the interaction between photoperiodic treatment and time (P=0.219; Fig. 3A). Similarly, female gut microbial communities were not different across photoperiodic groups (P=0.285), time points (P=0.340) or the interaction between photoperiodic treatment and time (P=0.708; Fig. 3C).
Fig. 3.
Overview of gut microbial communities in male and female hamsters. (A,C) Principal coordinates analyses (PCoAs) of the microbiome in male (A) and female (C) hamsters (LD: N=6, SD-R: N=6, SD-NR: N=6, for both males and females). Data are shown for LD hamsters (circles), SD-NR hamsters (triangles) and SD-R hamsters (squares) following 3, 6 and 9 weeks of treatment (see key). (B,D) Box and whisker plots of Shannon–Wiener diversity across photoperiodic treatment groups (LD, SD-NR and SD-R) and time (weeks 3, 6 and 9) in male (B) and female (D) hamsters. Lines represent the median; boxes represent quartiles; whiskers represent the minimum and maximum Shannon–Wiener diversity values. Outliers are represented as single open circles.
Alpha diversity between males and females was not significantly different (P=0.623; Fig. 3B,D). However, time affected alpha diversity in females, but not males. Specifically, all females at week 9 exhibited higher alpha diversity, regardless of photoperiodic treatment (P=0.043; Fig. 3D), but there was no effect of time on alpha diversity in males (P=0.244; Fig. 3B).
Interestingly, females and males had comparable beta diversity, according to Bray–Curtis dissimilarity scores. In males, SD-R hamsters were 42.1% different from LD hamsters, SD-R hamsters were 45.3% different from SD-NR hamsters, and LD hamsters were 43.1% different from SD-NR hamsters across all time points. In females, SD-R hamsters were 50.3% different from LD hamsters, SD-R hamsters were 49.9% different from SD-NR hamsters, and LD hamsters were 42.8% different from SD-NR hamsters across all time points. However, Bray–Curtis dissimilarity scores increased over time, especially for SD-NR males and SD-R females. Specifically, SD-NR males at week 3 were 41.3% different from baseline; SD-NR males at week 6 were 45.5% different from baseline; and SD-NR males at week 9 were 60.6% different from baseline. Similarly, SD-R females at week 3 were 42.2% different from baseline; SD-R females at week 6 were 44.9% different from baseline; and SD-R females at week 9 were 74.9% different from baseline.
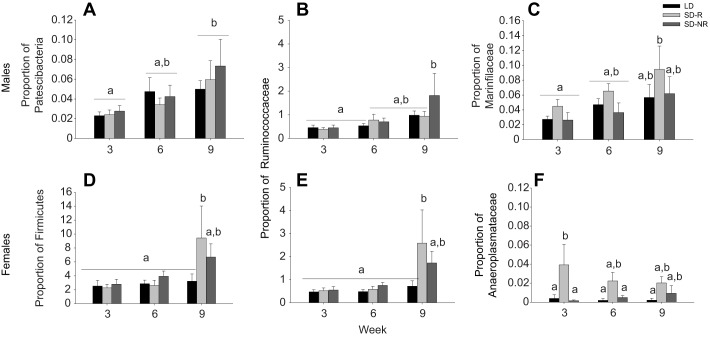
Photoperiod and time affect the relative abundance of gut microbes in males and females
Phyla and families in the male and female gut microbiome were similar across photoperiodic treatment groups at week 0 (P>0.10). However, specific differences in the relative abundance of gut microbes across time were not consistent within each sex. For example, at week 9, males had a significantly greater relative abundance of Patescibacteria (P≤0.028) than week 0 or week 3 males (Fig. 4A), whereas SD-R females at week 9 had a greater relative abundance of the phylum Firmicutes (P<0.05) than LD females at week 9 and all treatment groups at weeks 0, 3 and 6 (Fig. 4D). Further, SD-NR males at week 9 had a greater relative abundance of Ruminococcaceae than all other treatment groups at weeks 0 and 3 (P<0.05, Fig. 4B). In contrast, SD-R females at week 9 had a higher relative abundance of Ruminococcaceae (P<0.05) than LD females at week 9 and all treatment groups at weeks 0, 3 and 6 (Fig. 4E). SD-R males at week 9 had a higher relative abundance of Marinfilaceae than all treatment groups at week 0 and 3 (P<0.05), and there was a gradual increase in the relative abundance of Marinfilaceae over time in SD-R males compared with LD and SD-NR males (Fig. 4C). The same changes in Marinfilaceae were not seen in females (Table S4).
Fig. 4.
Relative abundance of bacterial phyla and families that differed in response to photoperiodic treatment and/or time in male and female hamsters. The proportion of bacteria from the phylum Patescibacteria (A), family Ruminococcaceae (B) and family Marinfilaceae (C) showed significant differences across treatments and/or time in male hamsters. The proportion of bacteria from the phylum Firmicutes (D), family Ruminococcaceae (E) and family Anaeroplasmataceae (F) showed significant differences across treatments and/or time in female hamsters. Bars represent means±s.e.m. (LD: N=6, SD-R: N=6, SD-NR: N=6 for both males and females). Bars with different letters represent statistically different group means (P<0.05; mixed model ANOVA).
The bacterial family Anaeroplasmataceae showed the most pronounced response to photoperiodic treatment in females (P=0.007; Fig. 4F). Specifically, SD-R females had a higher proportion of Anaeroplasmataceae following 3 weeks of treatment than LD and SD-NR females (SD-R and LD: P=0.028, SD-R and SD-NR: P=0.028; Fig. 4F). At week 9, the relative abundance of Anaeroplasmataceae in SD-R males also appeared to increase, though not significantly, compared with earlier time points (P=0.225; Table S3).
Finally, the following bacterial phyla were significantly different (P<0.05) across time points in males, regardless of photoperiodic treatment: Bacteroidetes, Cyanobacteria, Firmicutes, Patescibacteria, Proteobacteria, Spirochaetes and Tenericutes (Table S3). In addition, the following bacterial phyla were significantly different (P<0.05) across time points in females, regardless of photoperiodic treatment: Firmicutes and Spirochaetes (Table S4).
The gut microbiome is correlated with behavior and physiology in a sex-specific manner
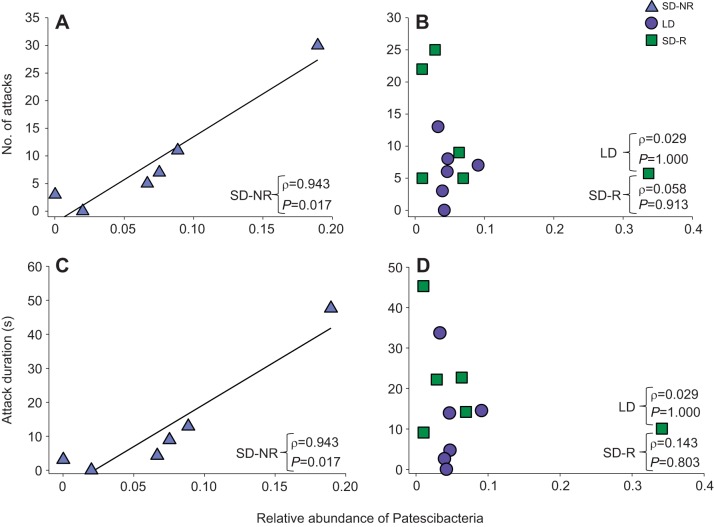
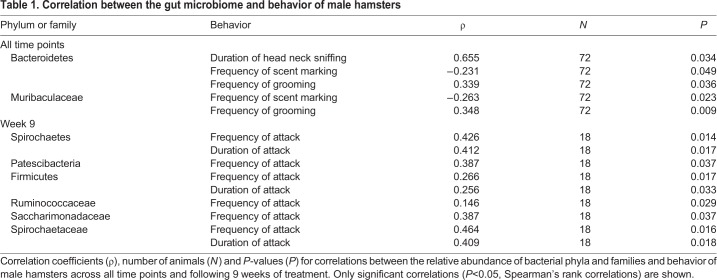
In SD-NR males, but not LD or SD-R males at week 9, the relative abundance of Patescibacteria was positively associated with number of attacks (ρ=0.943, N=6, P=0.017; Fig. 5A,B; Table 1) and attack duration (ρ=0.943, N=6, P=0.017; Fig. 5C,D). There was no association between number of attacks and the relative abundance of Patescibacteria at week 9 in females (ρ=0.350, N=18, P=0.142).
Fig. 5.
Correlation between relative abundance of the bacterial phylum Patescibacteria and aggressive behavior in male hamsters after 9 weeks of photoperiodic treatment. (A,B) The relative abundance of Patescibacteria was significantly correlated with the number of attacks in SD-NR males (A), but not in LD or SD-R males (B). (C,D) The relative abundance of Patescibacteria was significantly correlated with attack duration in SD-NR males (C), but not in LD or SD-R males (D). For each treatment group (LD: N=6, SD-R: N=6, SD-NR: N=6), correlation coefficients (ρ) and P-values are shown (Spearman's rank correlations).
Table 1.
Correlation between the gut microbiome and behavior of male hamsters
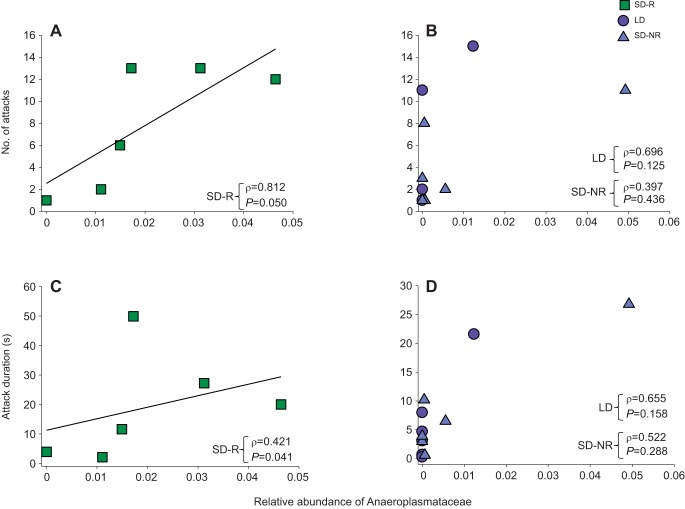
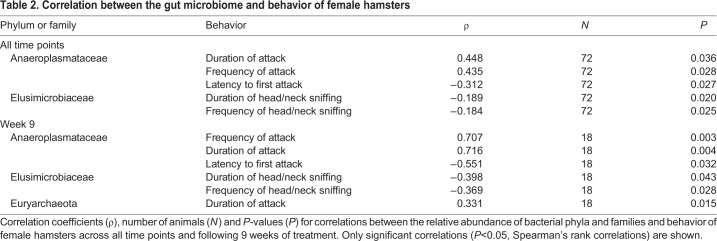
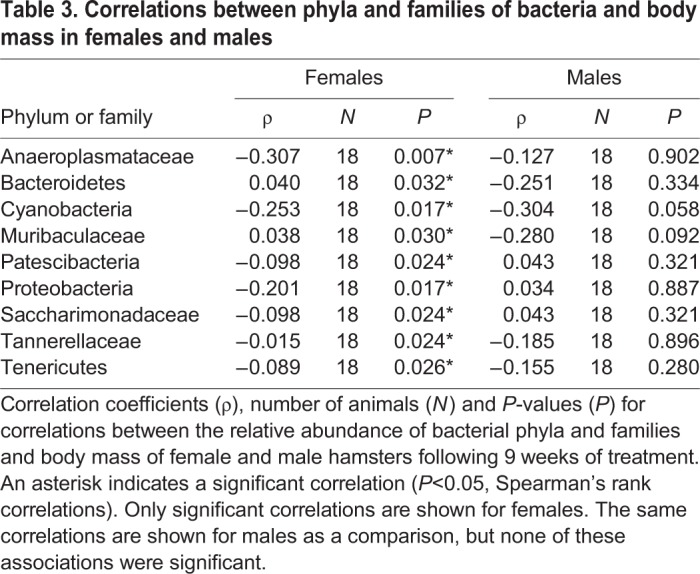
In SD-R females, but not in LD and SD-NR females at week 9, the relative abundance of Anaeroplasmataceae was positively correlated with number of attacks (ρ=0.812, N=6, P=0.050; Fig. 6A,B; Table 2) and attack duration (ρ=0.421, N=6, P=0.041; Fig. 6C,D; Table 2). At week 9, males showed no statistically significant correlation between the relative abundance of Anaeroplasmataceae and number of attacks (ρ=−0.048, N=18, P=0.510) or duration of attacks (ρ=0.126, N=18, P=0.776). Additionally, the relative abundance of Anaeroplasmataceae was negatively associated with body mass across all time points in females (ρ=−0.307, N=18, P=0.007; Table 3).
Fig. 6.
Correlation between relative abundance of the bacterial family Anaeroplasmataceae and aggressive behavior in female hamsters after 9 weeks of photoperiodic treatment. (A,B) The relative abundance of Anaeroplasmataceae was significantly correlated with the number of attacks in SD-R females (A), but not in LD or SD-NR females (B). (C,D) The relative abundance of Anaeroplasmataceae was significantly correlated with attack duration in SD-R females (C), but not in LD or SD-NR females (D). For each treatment group (LD: N=6, SD-R: N=6, SD-NR: N=6), correlation coefficients (ρ) and P-values are shown (Spearman's rank correlations).
Table 2.
Correlation between the gut microbiome and behavior of female hamsters
Table 3.
Correlations between phyla and families of bacteria and body mass in females and males
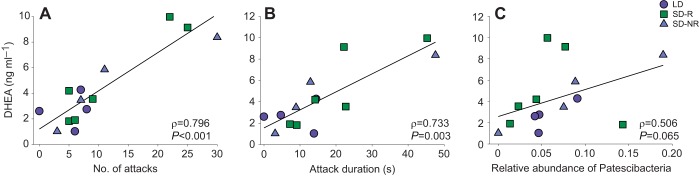
Serum DHEA levels in males and females were similar across groups after 9 weeks of photoperiodic treatment (males, P=0.692; females, P=0.463; Table S5). However, serum DHEA levels were significantly correlated with number of attacks (ρ=0.796, N=14, P<0.001; Fig. 7A), duration of attacks (ρ=0.733, N=14, P=0.003; Fig. 7B) and relative abundance of Patescibacteria (ρ=0.506, N=14, P<0.001; Fig. 7C) in males at week 9. Similar associations were not found in females at week 9 (Table S6).
Fig. 7.
Correlation between serum DHEA concentration and the gut microbiome and aggressive behavior in male hamsters following 9 weeks of photoperiodic treatment. (A,B) Serum DHEA concentration was significantly correlated with the number of attacks (A) and attack duration (B) in males. (C) The correlation between serum DHEA concentration and the relative abundance of Patescibacteria in males tended towards significance. For each treatment group (LD: N=4, SD-R: N=6, SD-NR: N=4), correlation coefficients (ρ) and P-values are shown (Spearman's rank correlations).
DISCUSSION
Previous work in our laboratory has demonstrated that seasonal changes in photoperiod cause a reduction in body mass, gonadal regression and increased aggression in SD-R Siberian hamsters. The ‘seasonal switch’ hypothesis, which suggests that Siberian hamsters switch from gonadal to adrenal regulation of aggression during SD conditions, helps to explain how SD-R hamsters are physiologically capable of increasing aggression during the non-breeding season. While it is unclear whether the gut microbiome plays a role in regulating this pathway, seasonal changes in the gut microbiome have been linked to decreases in body mass in SD-R male hamsters (Bailey et al., 2010). Several studies have provided evidence that the gut microbiome is sexually dimorphic, but the effect of photoperiod on the gut microbiome has yet to be studied in female hamsters. Here, we tested the hypothesis that photoperiodic changes in the gut microbiome affect circulating hormones and aggressive behavior in sex-specific ways.
We found that aggression increased in SD-R females, but not SD-R males at week 9. While photoperiod did not have an effect on overall gut microbial communities or beta diversity in either sex, it altered the relative abundance of specific bacterial phyla and families in the gut microbiome in a sex-specific manner. In males, the relative abundance of the phylum Patescibacteria increased over time, independent of treatment group; the relative abundance of the family Ruminococcaceae increased in SD-NR hamsters over time; and the relative abundance of the family Marinfilaceae increased in SD-R hamsters over time. In females, the relative abundance of the phylum Firmicutes and the family Ruminococcaceae increased in SD-R hamsters over time, and the relative abundance of the family Anaeroplasmataceae increased in SD-R hamsters following 3 weeks of treatment. Interestingly, the relative abundance of Patescibacteria was correlated with aggression at week 9 in SD-NR males, but not in LD males, SD-R males or females. Additionally, the relative abundance of Anaeroplasmataceae was correlated with aggression at week 9 in SD-R females, but not in LD females, SD-NR females or males. Finally, while SD-R males and females showed no changes in serum DHEA levels, DHEA levels at week 9 were correlated with aggression in males but not females. Collectively, these findings suggest that the gut microbiome interacts with the brain and/or periphery to modulate increased non-breeding aggression in a sex-specific manner. Further studies are necessary, however, to investigate the potential mechanisms underlying seasonal changes in the gut microbiome, circulating hormones and aggressive behavior.
Effects of photoperiod on reproductive physiology and behavior
Consistent with previous studies, we found that exposure to SD photoperiods resulted in reductions in body mass and reproductive tissue mass in male and female SD-R hamsters relative to SD-NR and LD hamsters (Bailey et al., 2010; Jasnow et al., 2000; Navara et al., 2007; Rendon et al., 2015). In addition, we found that SD-R males and females exhibited increased aggression in response to photoperiodic treatment, though some of these changes were not statistically significant (Rendon et al., 2015; Scotti et al., 2007). It is possible we did not observe differences in some measures of aggression between treatment groups because of our smaller sample sizes compared with past studies (Bedrosian et al., 2012; Rendon et al., 2016).
Moreover, we did not find any effect of photoperiod on post-behavior serum DHEA levels following 9 weeks of treatment. While the ‘seasonal switch’ hypothesis proposes that SDs upregulate the hypothalamic–pituitary–adrenal (HPA) axis and increase serum DHEA, we have also shown that aggressive behavior alone can decrease DHEA levels in SD animals (presumably due to conversion to other biologically active steroids; Rendon and Demas, 2016). Therefore, it is possible that SD-R hamsters had elevated DHEA concentrations prior to behavioral testing, and that engaging in aggressive behaviors decreased circulating DHEA in these animals relative to the other treatment groups. This physiological response may explain why serum DHEA levels appear unchanged after behavioral testing. Although basal DHEA levels were not measured in this study, the observed changes in post-behavior serum DHEA match those of previous studies (Rendon and Demas, 2016; Scotti et al., 2009). Collectively, these results suggest that DHEA acts as a precursor to increase circulating testosterone and E2 in SD-R hamsters following an aggressive interaction. Furthermore, changes in serum DHEA concentration before and after behavioral testing suggest that there are rapid effects of steroid hormones on aggression (reviewed in Heimovics et al., 2015; Navara et al., 2007).
Seasonal changes and sexual dimorphism in the gut microbiome
Past studies suggest that there may be sex differences in the gut microbiome's response to photoperiod, though to varying degrees (Bailey et al., 2010; Davenport et al., 2014; Hieke et al., 2019; Wang et al., 2018). In the current study, photoperiod differentially affected bacterial diversity and the relative abundance of bacteria in the male and female gut, further suggesting that the gut microbiome may play a role in sex-specific seasonal changes. Specifically, at week 9, female but not male hamsters exhibited increased gut microbial diversity compared with earlier time points; and at week 3, the relative abundance of Anaeroplasmataceae was significantly higher in SD-R females than in LD and SD-NR females, but no differences were found in males. Interestingly, young female and male mice have similar gut microbial compositions, with the exception of the bacterial family Anaeroplasmataceae (Leclercq et al., 2017), suggesting the potential for a conserved sex-specific importance of Anaeroplasmataceae in the gut microbiome. Previous work suggests that bacteria from the family Anaeroplasmataceae aid in carbohydrate, purine and pyrimidine metabolism in some mammals (Petzel et al., 1989).
Moreover, the observed sex-specific response to photoperiodic treatment may be, in part, due to differences in sensitivity to environmental changes and stress. In CF-1 mice subjected to stress from restraint and forced swim sessions, Ruminococcus gnavus increased in females, but the opposite effects were found in males (Tsilimigras et al., 2018), suggesting that even short-term stressors can alter the profile of gut microbial communities in sex-specific ways (Bharwani et al., 2016; Desbonnet et al., 2015; Foster et al., 2017; Galley et al., 2014). In the current study, we found differences in the gut microbiome that were unrelated to changes in photoperiod (e.g. differences in the microbiome over time). Because differences in the gut microbiome over time were observed in all photoperiodic treatment groups, these results suggest that stress may have impacted the gut microbiome. Though previous work in our lab has shown that general handling and manipulation does not significantly impact the gut microbiome (Sylvia et al., 2017), future work should further investigate how the potential stress of photoperiodic treatment and repeated handling may affect the gut microbiome.
Not only does the microbiome change in response to photoperiod in SD-R males but also SD-NR males exhibit differences in the microbiome at week 9 (e.g. higher relative abundance of Ruminococacceae). This finding suggests that SD-R and SD-NR males likely have distinct microbiota, and future work should investigate the mechanisms by which the microbiome of SD responders and non-responders differ over time.
Potential mechanisms mediating relationships among seasonal changes in photoperiod, the gut microbiome and aggression
In the current study, we found that the relative abundance of bacteria (e.g. Anaeroplasmataceae, Ruminococcaceae and Patescibacteria) was positively associated with aggression in a sex-specific manner. Specifically, we found that in females, the relative abundance of Anaeroplasmataceae, a family in the phylum Tenericutes, was positively associated with aggression in SD-R hamsters and negatively associated with body mass, but this same relationship was not found in males. Further, the relative abundance of Ruminococcaceae and Patescibacteria were positively associated with aggression in male but not female hamsters. These data suggest that Anaeroplasmataceae, Ruminococcaceae and Patescibacteria may play sex-specific roles in regulating seasonal changes in body mass and behavior.
Little is known, however, about the precise role of many bacteria in regulating behavior. For example, Patescibacteria are presumed to be either symbiotic or parasitic, suggesting the need for further investigation into the function of these specific microbes (Cui et al., 2019; Frey et al., 2016; Lopez-Fernandez et al., 2018; Sánchez-Osuna et al., 2017). We have previously shown that the relative abundance of Tenericutes is associated with increased aggression in male and female Siberian hamsters treated with a broad-spectrum antibiotic (Sylvia et al., 2017), suggesting that shifts in the relative abundance of these families and phyla (induced by either photoperiodic changes or antibiotics) are likely linked to aggression. Other Tenericutes (e.g. Anaeroplasma) have been associated with increased levels of immunoglobulin A (Beller et al., 2019), suggesting that the immune system may also play a role in the mechanisms regulating aggression. Interestingly, Ruminococcaceae, a family in the phylum Firmicutes, has also been associated with immunoglobulin A production and anti-inflammatory properties (Dowhaniuk et al., 2019; Ingham et al., 2019). However, Ruminococcaceae is best known for its role in producing butyrate, a short-chain fatty acid (SCFA) that serves as the main energy source of colonocytes, reduces inflammation and regulates gene expression (Koh et al., 2016; Zhuang et al., 2019). Increased SCFA levels from a high carbohydrate diet increase aggressive and anxiety-like behaviors in rats, suggesting that microbial metabolites may also contribute to aggressive behavior (Hanstock et al., 2004; reviewed in MacFabe, 2015). The link between microbial metabolites, immune mediators and behavior is further supported by Bacteroides fragilis, a species within the phylum Bacteroidetes (associated with investigation and grooming in males in the current study) that alters gut metabolites, targets intestinal junctions through cytokines and modulates anxiety-like, locomotor and social behavior (Desbonnet et al., 2014; Hsiao et al., 2013; Sharon et al., 2014). Finally, because melatonin plays an important role in modulating seasonal changes in physiology and behavior (Jasnow et al., 2000; Rendon et al., 2015) and melatonin has been shown to influence the gut lining (Sommansson et al., 2013), it too may mediate seasonal shifts in the gut microbiome. Collectively, these findings suggest that seasonal changes in the gut microbiome may modulate aggression via peripheral and/or central neuroendocrine mechanisms, and further investigation into the function of specific microbes is needed.
Conclusions
The current study provides evidence that photoperiod is related to sex-specific changes in the gut microbiome and aggression and that the gut microbiome may be one component of the ‘seasonal switch’ hypothesis. In addition, our data support the idea that several interconnected systems contribute to seasonal changes in aggression, including the brain, immune mediators (e.g. cytokines), gut hormones and microbial metabolites. We are just beginning to understand the potential benefits of microbiome-targeted human therapies, and continued study of the mechanisms underlying aggressive behavior will likely improve such therapeutic treatments. Taken together, our research sheds important light on an area of research that is critical for understanding the basic mechanisms regulating seasonal shifts in physiology and behavior.
Supplementary Material
Acknowledgements
The authors thank C. G. Logan, E. A. Morrison and E. A. St John for assistance in behavioral filming, fecal sampling, necropsies and general animal procedures; and K. Hu and W. Forrester for their helpful discussions and suggestions on a previous version of the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: C.C.R., K.E.S., J.E.D., G.E.D.; Methodology: C.C.R., K.E.S., J.E.D., G.E.D.; Software: C.C.R., K.E.S., K.M.M.; Validation: C.C.R., K.E.S., K.M.M.; Formal analysis: C.C.R., K.E.S., K.M.M.; Investigation: C.C.R., K.E.S., K.M.M., J.E.D., S.G.H., M.P.V.; Resources: K.E.S., K.M.M., J.E.D., G.E.D.; Data curation: C.C.R.; Writing - original draft: C.C.R.; Writing - review & editing: C.C.R., K.E.S., K.M.M., J.E.D., S.G.H., M.P.V., G.E.D.; Visualization: C.C.R., K.E.S., K.M.M.; Supervision: K.E.S., K.M.M., J.E.D., G.E.D.; Project administration: C.C.R., K.E.S., J.E.D., G.E.D.; Funding acquisition: C.C.R., K.E.S., K.M.M., G.E.D.
Funding
This work was supported by National Science Foundation grant IOS-1656414 and National Institutes of Health (NIH) grant MH109942 (G.E.D.), NIH Training grant T32HD049336 (K.E.S. and K.M.M.), a Hutton Honors College Research Grant (C.C.R.), a Hutton Honors College Research Partnership Grant (C.C.R.), and Indiana University. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jeb.biologists.org/lookup/doi/10.1242/jeb.212548.supplemental
References
- Armanhi J. S. L., de Souza R. S. C., de Araújo L. M., Okura V. K., Mieczkowski P., Imperial J. and Arruda P. (2016). Multiplex amplicon sequencing for microbe identification in community-based culture collections. Sci. Rep. 6, 29543 10.1038/srep29543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M. T., Walton J. C., Dowd S. E., Weil Z. M. and Nelson R. J. (2010). Photoperiod modulates gut bacteria composition in male Siberian hamsters (Phodopus sungorus). Brain. Behav. Immun. 24, 577-584. 10.1016/j.bbi.2009.12.010 [DOI] [PubMed] [Google Scholar]
- Bailey A. M., Legan S. J., Meretsky V. J. and Demas G. E. (2017). Effects of exogenous leptin on seasonal reproductive responses to interacting environmental cues in female Siberian hamsters. Gen. Comp. Endocrinol. 250, 95-103. 10.1016/j.ygcen.2017.06.004 [DOI] [PubMed] [Google Scholar]
- Bartness T. J. and Wade G. N. (1985). Photoperiodic control of seasonal body weight cycles in hamsters. Neurosci. Biobehav. Rev. 9, 599-612. 10.1016/0149-7634(85)90006-5 [DOI] [PubMed] [Google Scholar]
- Bartness T. J., Powers J. B., Hastings M. H., Bittman E. L. and Goldman B. D. (1993). The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J. Pineal Res. 15, 161-190. 10.1111/j.1600-079X.1993.tb00903.x [DOI] [PubMed] [Google Scholar]
- Bedrosian T. A., Fonken L. K., Demas G. E. and Nelson R. J. (2012). Photoperiod-dependent effects of neuronal nitric oxide synthase inhibition on aggression in Siberian hamsters. Horm. Behav. 61, 176-180. 10.1016/j.yhbeh.2011.11.011 [DOI] [PubMed] [Google Scholar]
- Beller A., Kruglov A., Durek P., von Goetze V., Hoffmann U., Maier R., Heiking K., Siegmund B., Heinz G., Mashreghi M.-F. et al. (2019). P104 Anaeroplasma, a potential anti-inflammatory probiotic for the treatment of chronic intestinal inflammation. Ann. Rheum. Dis. 78, A45-A46. 10.1136/annrheumdis-2018-EWRR2019.92 [DOI] [Google Scholar]
- Bharwani A., Mian M. F., Foster J. A., Surette M. G., Bienenstock J. and Forsythe P. (2016). Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology 63, 217-227. 10.1016/j.psyneuen.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Cani P. D. (2018). Human gut microbiome: hopes, threats and promises. Gut 67, 1716-1725. 10.1136/gutjnl-2018-316723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton E. D. and Demas G. E. (2015). Body mass affects seasonal variation in sickness intensity in a seasonally breeding rodent. J. Exp. Biol. 218, 1667-1676. 10.1242/jeb.120576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G., O'Mahony S., Dinan T. G. and Cryan J. F. (2014). Priming for health: gut microbiota acquired in early life regulates physiology, brain and behaviour. Acta Paediatr. 103, 812-819. 10.1111/apa.12674 [DOI] [PubMed] [Google Scholar]
- Collins S. M., Surette M. and Bercik P. (2012). The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 10, 735-742. 10.1038/nrmicro2876 [DOI] [PubMed] [Google Scholar]
- Cui M., Xiao H., Luo D., Zhang X., Zhao S., Zheng Q., Li Y., Zhao Y., Dong J., Li H. et al. (2016). Circadian rhythm shapes the gut microbiota affecting host radiosensitivity. Int. J. Mol. Sci. 17, 1786 10.3390/ijms17111786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G., Li J., Gao Z. and Wang Y. (2019). Spatial variations of microbial communities in abyssal and hadal sediments across the Challenger Deep. PeerJ 7, e6961 10.7717/peerj.6961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport E. R., Mizrahi-Man O., Michelini K., Barreiro L. B., Ober C. and Gilad Y. (2014). Seasonal variation in human gut microbiome composition. PLoS ONE 9, e90731 10.1371/journal.pone.0090731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L., Clarke G., Shanahan F., Dinan T. G. and Cryan J. F. (2014). Microbiota is essential for social development in the mouse. Mol. Psychiatry 19, 146-148. 10.1038/mp.2013.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L., Clarke G., Traplin A., O'Sullivan O., Crispie F., Moloney R. D., Cotter P. D., Dinan T. G. and Cryan J. F. (2015). Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain. Behav. Immun. 48, 165-173. 10.1016/j.bbi.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Dowhaniuk J. K., Szamosi J., Chorlton S., Owens J., Mileski H., Clause R., Pernica J. M., Bowdish D. M. E., Surette M. G. and Ratcliffe E. M. (2019). Starving the gut: a deficit of butyrate in the intestinal ecosystem of children with intestinal failure. J. Parenter. Enteral Nutr. jpen.1715 10.1002/jpen.1715 [DOI] [PubMed] [Google Scholar]
- Foley K. A., MacFabe D. F., Vaz A., Ossenkopp K.-P. and Kavaliers M. (2014). Sexually dimorphic effects of prenatal exposure to propionic acid and lipopolysaccharide on social behavior in neonatal, adolescent, and adult rats: Implications for autism spectrum disorders. Int. J. Dev. Neurosci. 39, 68-78. 10.1016/j.ijdevneu.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Foster J. A., Rinaman L. and Cryan J. F. (2017). Stress & the gut-brain axis: regulation by the microbiome. Neurobiol. Stress 7, 124-136. 10.1016/j.ynstr.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D. A. and Goldman B. D. (1997). Photoperiod nonresponsive Siberian hamsters: effect of age on the probability of nonresponsiveness. J. Biol. Rhythms 12, 110-121. 10.1177/074873049701200203 [DOI] [PubMed] [Google Scholar]
- Frey B., Rime T., Phillips M., Stierli B., Hajdas I., Widmer F. and Hartmann M. (2016). Microbial diversity in European alpine permafrost and active layers. FEMS Microbiol. Ecol. 92, fiw018 10.1093/femsec/fiw018 [DOI] [PubMed] [Google Scholar]
- Galley J. D., Nelson M. C., Yu Z., Dowd S. E., Walter J., Kumar P. S., Lyte M. and Bailey M. T. (2014). Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 14, 189 10.1186/1471-2180-14-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaisas S., Maher J. and Kanthasamy A. (2016). Gut microbiome in health and disease: Linking the microbiome–gut–brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol. Ther. 158, 52-62. 10.1016/j.pharmthera.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman B. D. (2001). Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J. Biol. Rhythms 16, 283-301. 10.1177/074873001129001980 [DOI] [PubMed] [Google Scholar]
- Gorman M. R. and Zucker I. (1995). Testicular regression and recrudescence without subsequent photorefractoriness in Siberian hamsters. Am. J. Physiol. 269, R800-R806. 10.1152/ajpregu.1995.269.4.R800 [DOI] [PubMed] [Google Scholar]
- Hanstock T. L., Clayton E. H., Li K. M. and Mallet P. E. (2004). Anxiety and aggression associated with the fermentation of carbohydrates in the hindgut of rats. Physiol. Behav. 82, 357-368. 10.1016/j.physbeh.2004.04.002 [DOI] [PubMed] [Google Scholar]
- Heimovics S. A., Trainor B. C. and Soma K. K. (2015). Rapid Effects of estradiol on aggression in birds and mice: the fast and the furious. Integr. Comp. Biol. 55, 281-293. 10.1093/icb/icv048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieke A.-S. C., Hubert S. M. and Athrey G. (2019). Circadian disruption and divergent microbiota acquisition under extended photoperiod regimens in chicken. PeerJ 7, e6592 10.7717/peerj.6592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. O. (1973). Diversity and evenness: a unifying notation and its consequences. Ecology 54, 427-432. 10.2307/1934352 [DOI] [Google Scholar]
- Hsiao E. Y., McBride S. W., Hsien S., Sharon G., Hyde E. R., McCue T., Codelli J. A., Chow J., Reisman S. E., Petrosino J. F. et al. (2013). Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451-1463. 10.1016/j.cell.2013.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham A. C., Kielsen K., Cilieborg M. S., Lund O., Holmes S., Aarestrup F. M., Müller K. G. and Pamp S. J. (2019). Specific gut microbiome members are associated with distinct immune markers in pediatric allogeneic hematopoietic stem cell transplantation. Microbiome 7, 131 10.1186/s40168-019-0745-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow A. M., Huhman K. L., Bartness T. J. and Demas G. E. (2000). Short-day increases in aggression are inversely related to circulating testosterone concentrations in male Siberian hamsters (Phodopus sungorus). Horm. Behav. 38, 102-110. 10.1006/hbeh.2000.1604 [DOI] [PubMed] [Google Scholar]
- Jaubert A.-M., Pecquery R., Dieudonne M.-N. and Giudicelli Y. (1995). Estrogen binding sites in hamster white adipose tissue: sex- and site-related variations; modulation by testosterone. Gen. Comp. Endocrinol. 100, 179-187. 10.1006/gcen.1995.1147 [DOI] [PubMed] [Google Scholar]
- Jost L. (2006). Entropy and diversity. Oikos 113, 363-375. 10.1111/j.2006.0030-1299.14714.x [DOI] [Google Scholar]
- Kirchoff N. S., Udell M. A. R. and Sharpton T. J. (2019). The gut microbiome correlates with conspecific aggression in a small population of rescued dogs (Canis familiaris). PeerJ 7, e6103 10.7717/peerj.6103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A., De Vadder F., Kovatcheva-Datchary P. and Bäckhed F. (2016). From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332-1345. 10.1016/j.cell.2016.05.041 [DOI] [PubMed] [Google Scholar]
- Leclercq S., Mian F. M., Stanisz A. M., Bindels L. B., Cambier E., Ben-Amram H., Koren O., Forsythe P. and Bienenstock J. (2017). Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun. 8, 15062 10.1038/ncomms15062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Fernandez M., Åström M., Bertilsson S. and Dopson M. (2018). Depth and dissolved organic carbon shape microbial communities in surface influenced but not ancient saline terrestrial aquifers. Front. Microbiol. 9, 2880 10.3389/fmicb.2018.02880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G. R., Lynch C. B. and Kliman R. M. (1989). Genetic analyses of photoresponsiveness in the Djungarian hamster,Phodopus sungorus. J. Comp. Physiol. A 164, 475-481. 10.1007/BF00610441 [DOI] [PubMed] [Google Scholar]
- MacFabe D. F. (2015). Enteric short-chain fatty acids: microbial messengers of metabolism, mitochondria, and mind: implications in autism spectrum disorders. Microb. Ecol. Health Dis. 26, 28177 10.3402/mehd.v26.28177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahé F., Rognes T., Quince C., de Vargas C. and Dunthorn M. (2014). Swarm: robust and fast clustering method for amplicon-based studies. PeerJ 2, e593 10.7717/peerj.593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziarz M., Pfeiffer R. M., Wan Y. and Gail M. H. (2018). Using standard microbiome reference groups to simplify beta-diversity analyses and facilitate independent validation. Bioinformatics 34, 3249-3257. 10.1093/bioinformatics/bty297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munley K. M., Rendon N. M. and Demas G. E. (2018). Neural androgen synthesis and aggression: insights from a seasonally breeding rodent. Front. Endocrinol. (Lausanne) 9, 136 10.3389/fendo.2018.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navara K. J., Trainor B. C. and Nelson R. J. (2007). Photoperiod alters macrophage responsiveness, but not expression of toll-like receptors in Siberian hamsters. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 148, 354-359. 10.1016/j.cbpa.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Nguyen T. L. A., Vieira-Silva S., Liston A. and Raes J. (2015). How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 8, 1-16. 10.1242/dmm.017400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony S. M., Marchesi J. R., Scully P., Codling C., Ceolho A.-M., Quigley E. M. M., Cryan J. F. and Dinan T. G. (2009). Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 65, 263-267. 10.1016/j.biopsych.2008.06.026 [DOI] [PubMed] [Google Scholar]
- Petzel J. P., McElwain M. C., DeSantis D., Manolukas J., Williams M. V., Hartman P. A., Allison M. J. and Pollack J. D. (1989). Enzymic activities of carbohydrate, purine, and pyrimidine metabolism in the Anaeroplasmataceae (class Mollicutes). Arch. Microbiol. 152, 309-316. 10.1007/BF00425166 [DOI] [PubMed] [Google Scholar]
- Pradhan D. S., Newman A. E. M., Wacker D. W., Wingfield J. C., Schlinger B. A. and Soma K. K. (2010). Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm. Behav. 57, 381-389. 10.1016/j.yhbeh.2010.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendon N. M. and Demas G. E. (2016). Bi-directional actions of dehydroepiandrosterone and aggression in female Siberian hamsters. J. Exp. Zool. A Ecol. Genet. Physiol. 325, 116-121. 10.1002/jez.2001 [DOI] [PubMed] [Google Scholar]
- Rendon N. M., Rudolph L. M., Sengelaub D. R. and Demas G. E. (2015). The agonistic adrenal: melatonin elicits female aggression via regulation of adrenal androgens. Proc. Biol. Sci. 282, 20152080 10.1098/rspb.2015.2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendon N. M., Soini H. A., Scotti M.-A. L., Weigel E. R., Novotny M. V. and Demas G. E. (2016). Photoperiod and aggression induce changes in ventral gland compounds exclusively in male Siberian hamsters. Horm. Behav. 81, 1-11. 10.1016/j.yhbeh.2016.02.005 [DOI] [PubMed] [Google Scholar]
- Rendon N. M., Amez A. C., Proffitt M. R., Bauserman E. R. and Demas G. E. (2017). Aggressive behaviours track transitions in seasonal phenotypes of female Siberian hamsters. Funct. Ecol. 31, 1071 10.1111/1365-2435.12816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Osuna M., Barbé J. and Erill I. (2017). Comparative genomics of the DNA damage-inducible network in the Patescibacteria. Environ. Microbiol. 19, 3465-3474. 10.1111/1462-2920.13826 [DOI] [PubMed] [Google Scholar]
- Scotti M.-A. L., Place N. J. and Demas G. E. (2007). Short-day increases in aggression are independent of circulating gonadal steroids in female Siberian hamsters (Phodopus sungorus). Horm. Behav. 52, 183-190. 10.1016/j.yhbeh.2007.03.029 [DOI] [PubMed] [Google Scholar]
- Scotti M.-A. L., Belén J., Jackson J. E. and Demas G. E. (2008). The role of androgens in the mediation of seasonal territorial aggression in male Siberian hamsters (Phodopus sungorus). Physiol. Behav. 95, 633-640. 10.1016/j.physbeh.2008.09.009 [DOI] [PubMed] [Google Scholar]
- Scotti M. A. L., Schmidt K. L., Newman A. E. M., Bonu T., Soma K. K. and Demas G. E. (2009). Aggressive encounters differentially affect serum dehydroepiandrosterone and testosterone concentrations in male Siberian hamsters (Phodopus sungorus). Horm. Behav. 56, 376-381. 10.1016/j.yhbeh.2009.07.004 [DOI] [PubMed] [Google Scholar]
- Sharon G., Garg N., Debelius J., Knight R., Dorrestein P. C. and Mazmanian S. K. (2014). Specialized metabolites from the microbiome in health and disease. Cell Metab. 20, 719-730. 10.1016/j.cmet.2014.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreiner A. B., Kao J. Y. and Young V. B. (2015). The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 31, 69-75. 10.1097/MOG.0000000000000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma K. K. (2006). Testosterone and aggression: berthold, birds and beyond. J. Neuroendocrinol. 18, 543-551. 10.1111/j.1365-2826.2006.01440.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma K. K., Scotti M.-A. L., Newman A. E. M., Charlier T. D. and Demas G. E. (2008). Novel mechanisms for neuroendocrine regulation of aggression. Front. Neuroendocrinol. 29, 476-489. 10.1016/j.yfrne.2007.12.003 [DOI] [PubMed] [Google Scholar]
- Soma K. K., Rendon N. M., Boonstra R., Albers H. E. and Demas G. E. (2015). DHEA effects on brain and behavior: Insights from comparative studies of aggression. J. Steroid Biochem. Mol. Biol. 145, 261-272. 10.1016/j.jsbmb.2014.05.011 [DOI] [PubMed] [Google Scholar]
- Sommansson A., Nylander O. and Sjöblom M. (2013). Melatonin decreases duodenal epithelial paracellular permeability via a nicotinic receptor-dependent pathway in rats in vivo. J. Pineal Res. 54, 282-291. 10.1111/jpi.12013 [DOI] [PubMed] [Google Scholar]
- Sylvia K. E. and Demas G. E. (2018a). Acute intraperitoneal lipopolysaccharide influences the immune system in the absence of gut dysbiosis. Physiol. Rep. 6, e13639 10.14814/phy2.13639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia K. E. and Demas G. E. (2018b). A gut feeling: microbiome-brain-immune interactions modulate social and affective behaviors. Horm. Behav. 99, 41-49. 10.1016/j.yhbeh.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvia K. E., Jewell C. P., Rendon N. M., St John E. A. and Demas G. E. (2017). Sex-specific modulation of the gut microbiome and behavior in Siberian hamsters. Brain Behav Immun. 60, 51-62. 10.1016/j.bbi.2016.10.023 [DOI] [PubMed] [Google Scholar]
- Sylvia K. E., Deyoe J. E. and Demas G. E. (2018). Early-life sickness may predispose Siberian hamsters to behavioral changes following alterations of the gut microbiome in adulthood. Brain. Behav. Immun. 73, 571-583. 10.1016/j.bbi.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze M. A., Tsuruta M., Yang S.-W. J., Oh Y., Man S. F. P., Hogg J. C. and Sin D. D. (2014). Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. PLoS ONE 9, e111228 10.1371/journal.pone.0111228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilimigras M. C. B., Gharaibeh R. Z., Sioda M., Gray L., Fodor A. A. and Lyte M. (2018). Interactions between stress and sex in microbial responses within the microbiota-gut-brain axis in a mouse model. Psychosom. Med. 80, 361-369. 10.1097/PSY.0000000000000572 [DOI] [PubMed] [Google Scholar]
- Vemuri R., Sylvia K. E., Klein S. L., Forster S. C., Plebanski M., Eri R. and Flanagan K. L. (2018). The microgenderome revealed : sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin. Immunopathol. 41, 265-275. 10.1007/s00281-018-0716-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven K. J. F., Simonsen K. L. and McIntyre L. M. (2005). Implementing false discovery rate control: increasing your power. Oikos 108, 643-647. 10.1111/j.0030-1299.2005.13727.x [DOI] [Google Scholar]
- Walton J. C., Weil Z. M. and Nelson R. J. (2011). Influence of photoperiod on hormones, behavior, and immune function. Front. Neuroendocrinol. 32, 303-319. 10.1016/j.yfrne.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Nesengani L. T., Gong Y., Yang Y. and Lu W. (2018). 16S rRNA gene sequencing reveals effects of photoperiod on cecal microbiota of broiler roosters. PeerJ 6, e4390 10.7717/peerj.4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield J. C. (1984). Environmental and endocrine control of reproduction in the song sparrow, Melospiza melodia. I. Temporal organization of the breeding cycle. Gen. Comp. Endocrinol. 56, 406-416. 10.1016/0016-6480(84)90083-2 [DOI] [PubMed] [Google Scholar]
- Wingfield J. C. and Soma K. K. (2002). Spring and autumn territoriality in song sparrows: same behavior, different mechanisms? Integr. Comp. Biol. 42, 11-20. 10.1093/icb/42.1.11 [DOI] [PubMed] [Google Scholar]
- Yurkovetskiy L., Burrows M., Khan A. A., Graham L., Volchkov P., Becker L., Antonopoulos D., Umesaki Y. and Chervonsky A. V. (2013). Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400-412. 10.1016/j.immuni.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang M., Shang W., Ma Q., Strappe P. and Zhou Z. (2019). Abundance of probiotics and butyrate-production microbiome manages constipation via short-chain fatty acids production and hormones secretion. Mol. Nutr. Food Res. 63, 1801187 10.1002/mnfr.201801187 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.