Abstract
Background
Pediatric hypertension is recognized as an emerging global health concern. Although new guidelines are developed for facilitating clinical management, the reasons for the prevalence of hypertension in children remain unknown. Genetics and environmental factors do not fully account for the growing incidence of pediatric hypertension. Because stable bacterial flora in early life are linked with health outcomes later in life, we hypothesized that reshaping of gut microbiota in early life affects blood pressure (BP) of pediatric subjects.
Methods and Results
To test this hypothesis, we administered amoxicillin, the most commonly prescribed pediatric antibiotic, to alter gut microbiota of young, genetically hypertensive rats (study 1) and dams during gestation and lactation (study 2) and recorded their BP. Reshaping of microbiota with reductions in Firmicutes/Bacteriodetes ratio were observed. Amoxicillin treated rats had lower BP compared with untreated rats. In young rats treated with amoxicillin, the lowering effect on BP persisted even after antibiotics were discontinued. Similarly, offspring from dams treated with amoxicillin showed lower systolic BP compared with control rats. Remarkably, in all cases, a decrease in BP was associated with lowering of Veillonellaceae, which are succinate‐producing bacteria. Elevated plasma succinate is reported in hypertension. Accordingly, serum succinate was measured and found lower in animals treated with amoxicillin.
Conclusions
Our results demonstrate a direct correlation between succinate‐producing gut microbiota and early development of hypertension and indicate that reshaping gut microbiota, especially by depleting succinate‐producing microbiota early in life, may have long‐term benefits for hypertension‐prone individuals.
Keywords: gut microbiota, maternal, metabolite, pediatric hypertension, Veillonellaceae
Subject Categories: Animal Models of Human Disease, Basic Science Research, Mechanisms, Hypertension, High Blood Pressure
Clinical Perspective
What Is New?
By reshaping gut microbiota of the dams with a widely prescribed antibiotic, amoxicillin, blood pressure of offspring was reduced.
Amoxicillin administration in young animals was also similarly associated with lower blood pressure.
Amoxicillin administration was associated with lower circulating levels of the prohypertensive metabolite, succinate.
What Are the Clinical Implications?
Use of antibiotics during pregnancy or lactation may have lasting effects on blood pressure of offspring.
Amoxicillin, which is a widely prescribed antibiotic for pediatric subjects, may, by reshaping their gut microbiome, lower their susceptibility to develop hypertension.
Reshaping microbiota in developmental stages of life is demonstrated to influence complex traits later in life.1, 2, 3 There are reports of such reprogramming being beneficial or detrimental depending on the circumstances. For example, gut microbiota in early developmental stages of life is beneficial for host metabolism later in life,4, 5, 6, 7, 8 whereas gut flora of neonates affected by a cesarean procedure is associated with development of type 1 diabetes mellitus, celiac disease, and obesity.9, 10, 11 Reports of perturbation of gut microbiota during pregnancy and the lactational period also affect the health of offspring.12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 Intrapartum administration of antibiotics affects development of gut microbiota in infants, and the reshaped microbiota persist even after a year following birth.29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 Whether such a reshaping of microbiota during early developmental stages impacts blood pressure (BP) remains unknown.
Antibiotics were developed to eradicate infectious pathogens, which have had a large and positive impact on the well‐being of human lives. The enamored use of antibiotics is, however, curtailed in the modern era because of developing resistance to antibiotics.40 However, a problem of equal or larger concern for using antibiotics stems from the more‐recent understanding of the important role of commensal bacteria to maintain normal physiology.41 Earlier research indicates that when bacteria are completely depleted in germ‐free mice, cardiac inflammation, fibrosis, and systolic function are attenuated.42 It is also reported that antibiotic exposure perturbed host microbiota and had a long‐lasting effect on the gut.43, 44 This begs the question of how antibiotics impact the internal balance of the host with its commensal bacteria and what consequences they have on human health. As particularly pertinent to antibiotic mediated cardiovascular health, in previous work, we demonstrated that 3 different antibiotics had disparate effects on hemodynamic regulation in hypertensive model organisms.45 However, all these studies and other studies conducted to demonstrate links between microbiota and hypertension are conducted in adult humans, rats, or mice,46, 47, 48, 49 whereby our current understanding of how exposure of antibiotics at various developmental stages affects BP is limited. We therefore hypothesized that similar to adults, reshaping gut microbiota with an antibiotic during early life affects BP. The aim of this study was to test this hypothesis by following the BP of young rats treated with amoxicillin. Amoxicillin was chosen for the study because of its prolific usage in pediatric subjects and administered to rats to reshape their gut microbiota in 2 separate developmental stages. The first stage was comprised of the pre‐ and perinatal stages (representing gestational and lactational periods: study 2), and the second was during the postweaning stage (representing adolescence: study 1).
Methods
Data Availability
All data and materials have been made publicly available at the Mendeley Data and can be accessed at Chakraborty and Saroj (2019), “Reshaping commensal gut microbiota in early life with amoxicillin presents with lower blood pressure,” Mendeley Data, v1 (https://doi.org/10.17632/tr9kfmc5zv.1).
Animals and Diet
All animal procedures and protocols used were approved by the University of Toledo Institutional Animal Care and Use Committee (IACUC approval numbers 104573, 108390). Experiments were conducted in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The inbred Dahl salt‐sensitive (SS/Jr, aka S) rat strain used for all the studies was from the colony maintained in the vivarium at the University of Toledo College of Medicine and Life Sciences (Toledo, OH). Rats were maintained on a low‐salt diet (0.3% NaCl; Envigo Teklad diet TD 7034; Envigo, Madison, WI). The Envigo Teklad diet (TD94217) was used for experiments involving a high‐salt regimen (2% NaCl).
BP Measurements by Radiotelemetry and Diet
All rats were weaned at 30 days of age. In study 1, a week after weaning, male S rats were surgically implanted with radiotelemetry transmitters (C10; Data Science International, St Paul, MN), as previously described by our laboratory.50 After surgery, they were switched to a high‐salt diet (2% NaCl). In study 2, male rats underwent surgical implantation of radiotelemetry transmitters (C10) a week after weaning, but were maintained on a low‐salt diet (0.3% NaCl). After 19 days, rats were then switched to the high‐salt diet. Rats were individually housed and allowed to recover from surgery for 3 days before recording BP. All rats were the same age at time of surgery.
Antibiotic administration and fecal collection
Study 1: administration to adolescent and young adult rats
After the 3‐day recovery period from the radiotelemetry surgery described above, BP was recorded and the rats were assigned to experimental groups such that their initial systolic BP (SBP) readings were not statistically different between the 2 groups (left panel on Figure 1). They received either normal drinking water or water supplemented with amoxicillin (50 mg/kg per day; Sigma‐Aldrich, St. Louis, MO) a week after weaning. After 21 days of weaning, antibiotic administration was stopped, and BP monitoring was continued to day 40. Rats were euthanized on day 40. At various time points, including a week after weaning, the last day of antibiotic administration, and at euthanization, fresh fecal samples were collected from the anus of rats directly into sterile tubes, snap‐frozen on dry ice, and stored at −80°C (Figure 1). Fecal samples were collected at 3 time points from the day of weaning: day 7 (1 week after antibiotic); day 21 (last day of antibiotic), and day 40 (n=6/group, and collections were from the same animals at all the time points; Figures 2, 3, 4 through 5).
Figure 1.
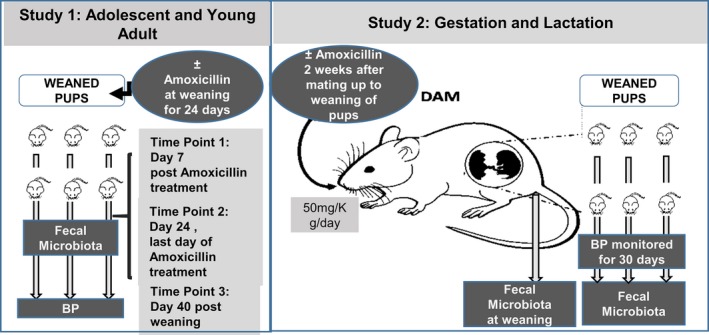
Overall study design. This schematic represents the overview of the 2 studies described in the Methods section. BP, blood pressure.
Figure 2.
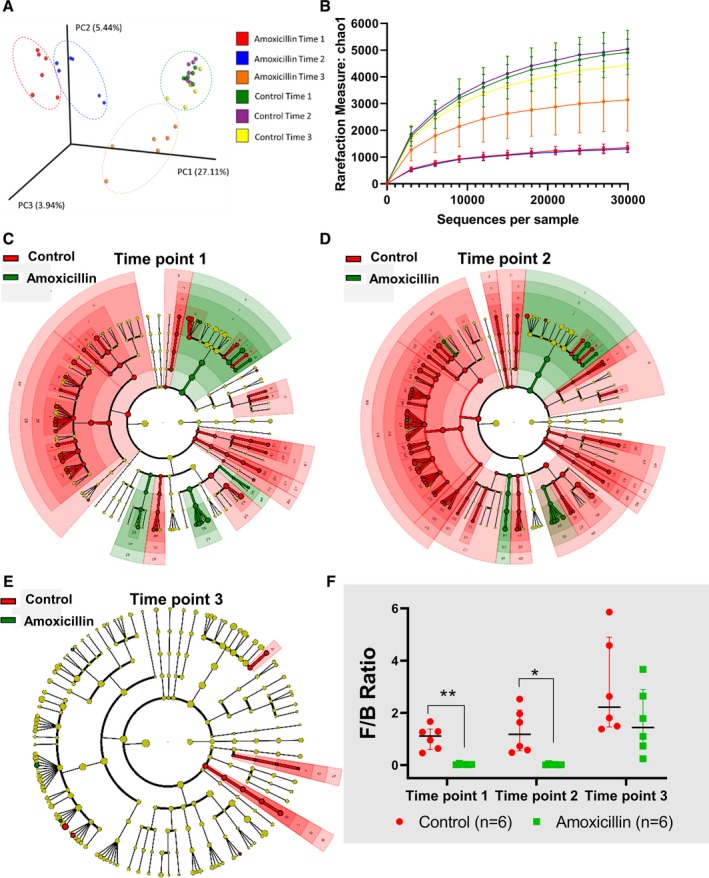
Study 1: gut microbiota of young rats on amoxicillin compared with control rats. Male Dahl S rats (n=6/group) were individually housed, and fresh fecal samples were collected from 3 time points. A, Unweighted‐beta diversity of all 3 time points of amoxicillin or control rats, (B) alpha‐diversity/chao1 measurement of all 3 time points of amoxicillin or control rat, (C) cladogram of bacterial community at time point 1, (D) cladogram of bacterial community at time point 2, (E) cladogram of bacterial community at time point 3, and (F) F/B ratio of all 3 time points of amoxicillin or control rats. *P<0.05; **P<0.01. Note: Larger versions of each of the cladograms and further details on the bacterial taxa are presented in Figures S1 through S3. Data are presented as median and interquartile range. PC indicates principle component. *p<0.05, **p<0.01.
Figure 3.
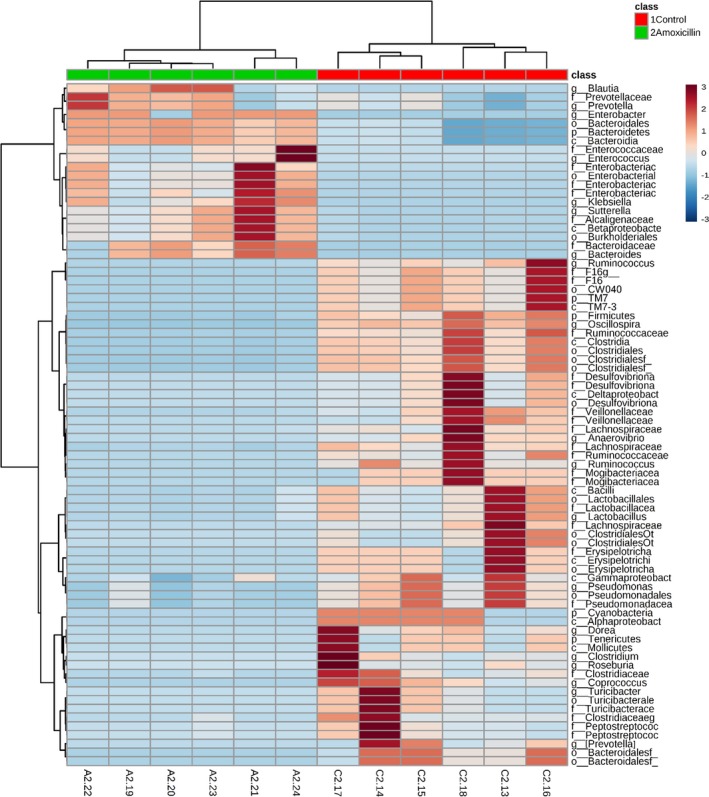
Differential clusters of bacterial communities from amoxicillin and control rats. Relative abundance data obtained from 16s RNA sequencing of fecal samples from adolescent rats in study 1 were subjected to unbiased hierarchical clustering. Data were autoscaled based on the column, and Ward's clustering method was used. Blue, lower abundance; maroon, greater abundance.
Figure 4.
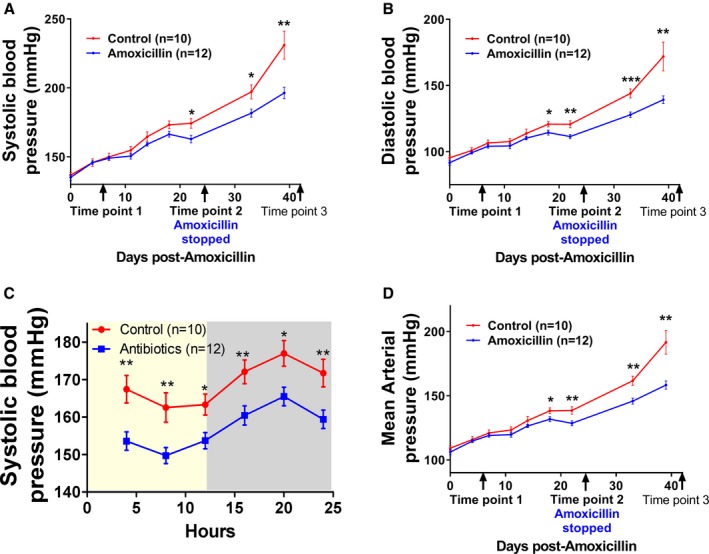
Rats treated with amoxicillin had significantly lower (A‐B) systolic, (C) diastolic, and (D) mean arterial pressure. These data are from rats in study 1 as described under Methods. Graphs are plotted from BP data obtained by BP radiotelemetry in these rats implanted with transmitters. Control, rats without amoxicillin; amoxicillin, rats given amoxicillin in drinking water. Time points are described in detail under the Methods section. Data presented as mean±SEM. *p<0.05, **p<0.01, ***p<0.001. BP indicates blood pressure.
Figure 5.
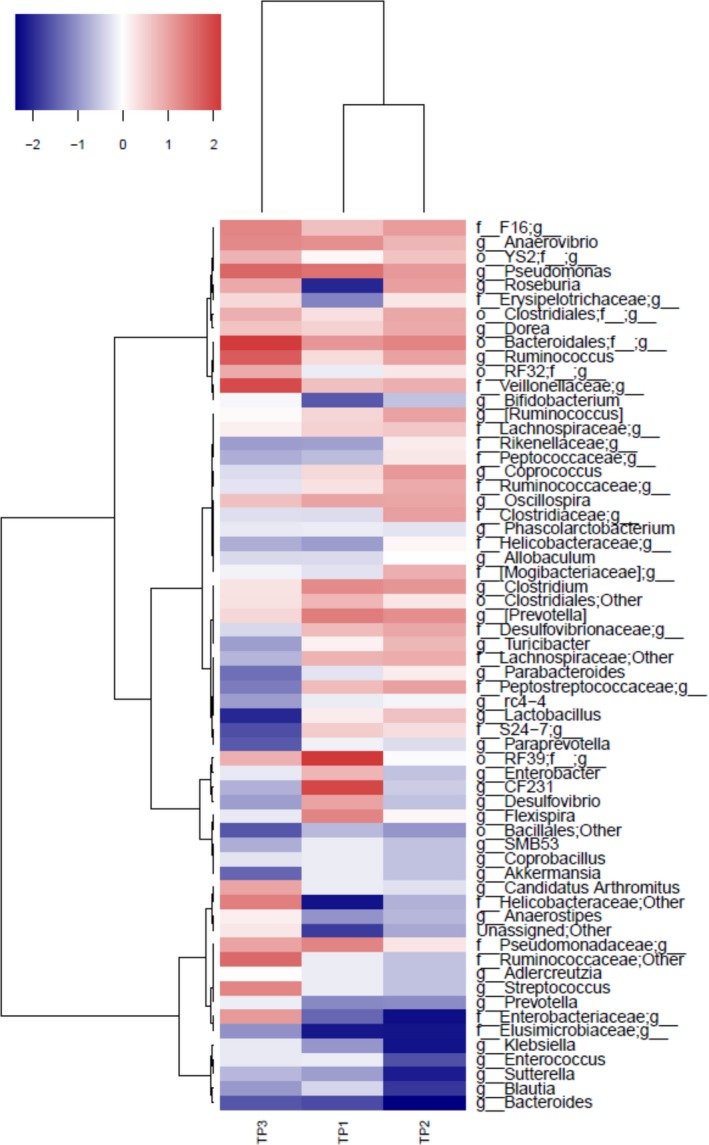
Correlation between microbiota and systolic BP in adolescent rats. Systolic BP data obtained from adolescent rats in study 1 were plotted against bacterial taxa of rats obtained by 16S RNA sequencing, and Spearman's correlation was determined. Spearman's correlation score was plotted using a hierarchical clustering approach in a red‐blue gradient scale; blue color indicates negative correlation, and red color indicates positive correlation. BP indicates blood pressure.
Study 2: antibiotic administration to dams for studying gestational and lactational effects
S rats were bred at 8 weeks of age. Two weeks after pairing, males and females were separated and females were either maintained on normal drinking water (control, n=3) or given water supplemented with amoxicillin (50 mg/kg per day, n=3; Sigma‐Aldrich; right panel on Figure 1). Amoxicillin was continued for dams until the pups were weaned (males). On the day of weaning, fecal pellets were collected from the dams. After weaning, all of the pups were maintained on normal drinking water. Fecal samples of offspring were collected at the time of euthanization (n=5/per group; Figures 6, 7, 8, 9 through 10).
Figure 6.
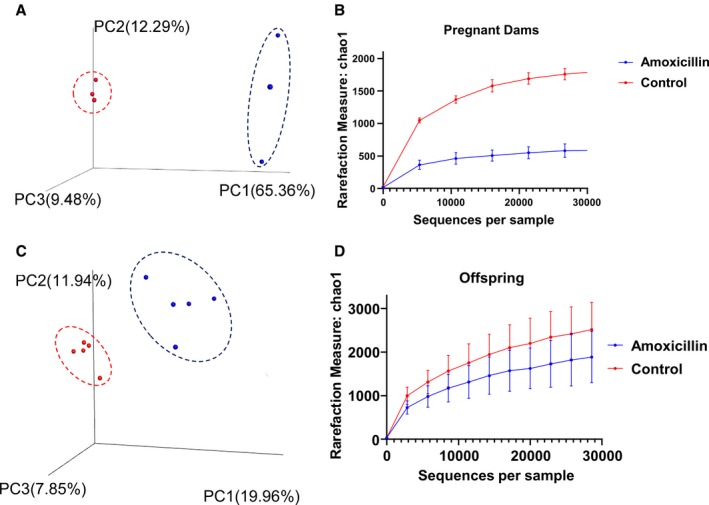
Beta‐diversity and alpha‐diversity are different with antibiotic treatment in dams and offspring. Data plotted are from animals (n=5/group) in study 2 obtained by 16S RNA sequencing of fecal samples from the dams and their offspring. A, Unweighted beta‐diversity of dams, red dots=control group, blue dots=amoxicillin group. (B) Alpha‐diversity: chao1 of dams. (C) Unweighted beta‐diversity of offspring, red dot=control group, blue dot=amoxicillin group. (D) Alpha‐diversity: chao1 of offspring. Data presented as mean±SEM. PC indicates principle component.
Figure 7.
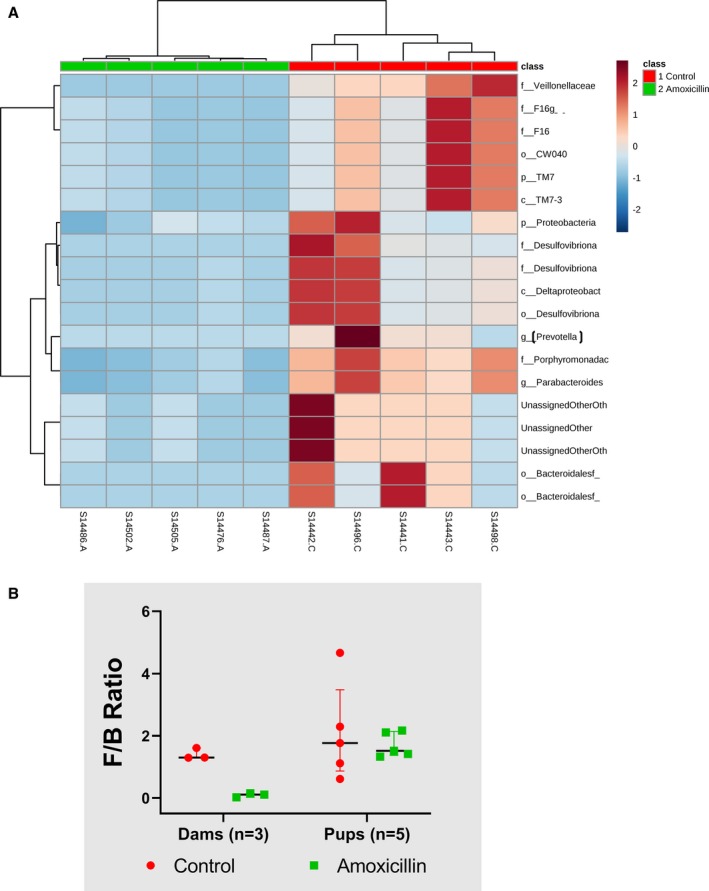
Microbiota of offspring was altered with amoxicillin administration in dams. A, Fecal microbiota data from samples A1 to A5 of offspring of amoxicillin‐treated dams and samples C1 to C5 from offspring of control dams without amoxicillin, clustered distinctly when subjected to an unbiased hierarchical clustering approach. Blue, less abundance; maroon, greater abundance. (B) Firmicutes/Bacteroidetes (F/B) ratio: dams and offspring of dams. Control: without amoxicillin. Data presented as median and interquartile range.
Figure 8.
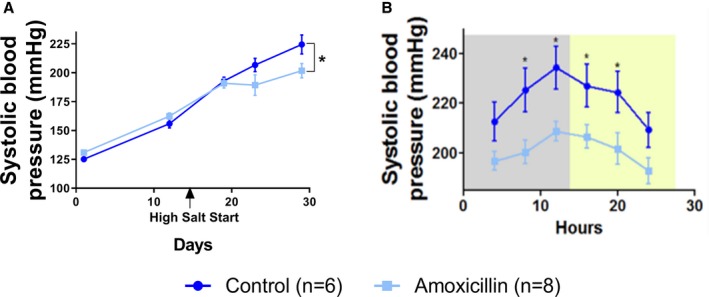
Systolic BP of offspring is significantly reduced with amoxicillin treatment in dams. Offspring in study 2 were implanted with radiotelemetry transmitters for recording their BP. All data are from male rats. Control, offspring of dams not receiving amoxicillin; amoxicillin, offspring of dams receiving amoxicillin. A, Systolic BP over the 30 days of BP measurement. B, Twenty‐four‐hour BP of day 29 plotted with 4‐hour moving averages to record diurnal rhythm of systolic BP. Gray, dark cycle; yellow: light cycle. Data presented as mean±SEM. BP indicates blood pressure. *p<0.05.
Figure 9.
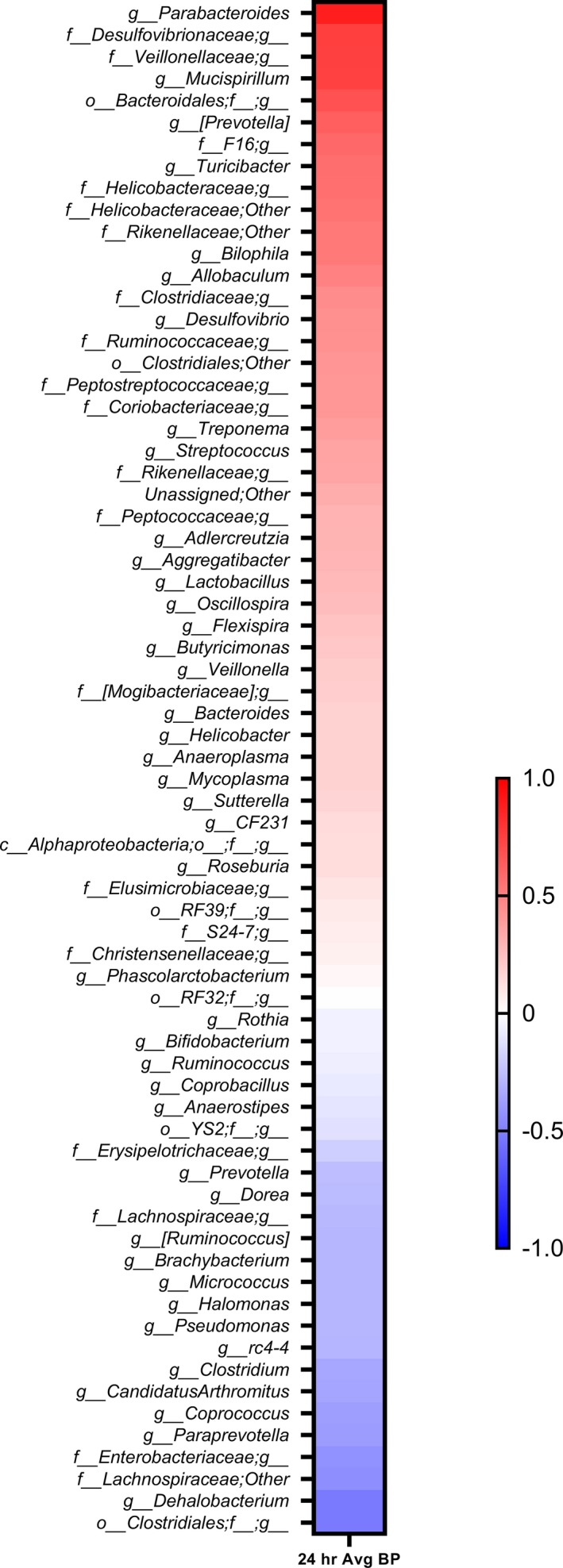
Correlation between microbiota and systolic BP in the offspring of study 2. Bacterial abundance data obtained from fecal samples of offspring from dams raised with or without amoxicillin were plotted against their 24‐hour systolic BP data. The resultant Spearman correlations are arranged in a red‐blue gradient scale; blue color indicates negative correlation, red color indicates positive correlation, and white color indicates no correlation with BP. A red‐blue gradient scale; blue color indicates negative correlation, and red color indicates positive correlation. BP indicates blood pressure.
Figure 10.
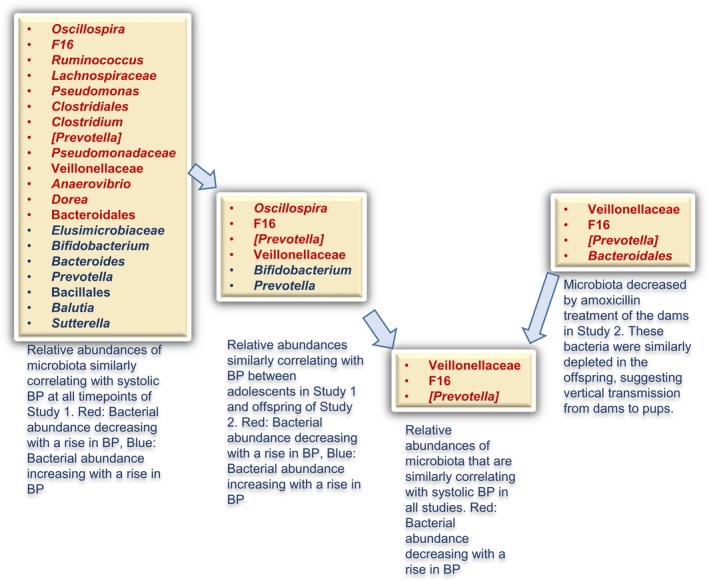
Summary of microbiota responsive to amoxicillin associated with BP. Microbiotal data obtained from offspring of studies 1 and 2 on the left and dams in study 2 on the right side were prioritized based on the descriptions in blue underneath each rectangle. The resultant convergence of microbiotal taxa, by associations of amoxicillin and BP on the left to acquisition by inheritance from the dams to the right, is evident toward the middle where the arrows point to only 3 taxa within the rectangle. BP indicates blood pressure.
Genomic DNA Isolation, 16S rRNA Gene Sequencing, and Analysis of Microbiotal Composition
Fecal DNA was extracted from 1 fecal pellet (≈0.2 g) as described in a previous work.45 PCR library preparation, 16S rRNA gene sequencing, and analysis were performed as previously described.45 Briefly, the V3 to V4 regions of the 16S rRNA gene were amplified following the Illumina User Guide: 16S rRNA gene Metagenomic Sequencing Library Preparation‐Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System (Illumina, Part # 15044223 Rev. B; Illumina, San Diego, CA). The 10‐pmol/L denatured and diluted library with 15% PhiX was loaded on an Illumina MiSeq V3 flow cell kit with 2×300 cycles. Raw 16S sequencing data were processed and analyzed using a bioinformatics pipeline comprising multiple software programs, including USEARCH (version 6),51 Quantitative Insights Into Microbial Ecology (QIIME) software package (version 1.9.1),52 and linear discriminant analysis effect size (LEfSe).53 Raw paired‐end reads were merged to create consensus sequences and then quality filtered using USEARCH51 (version 9). Chimeric sequences were identified and filtered using the Quantitative Insights Into microbial Ecology (QIIME)52 software package (version 1.9.1) combined with the USEARCH (version 6) algorithm. Open reference operational taxonomic units were subsequently picked using QIIME combined with the USEARCH (version 6) algorithm, and taxonomy assignment was performed using Greengenes54 as a reference database. Alpha‐diversity (Chao1) and beta‐diversity (unweighted and weighted UniFrac metrics) were calculated using the QIIME package. Comparison of group differences in microbiota within and between strains was performed using the Adonis function for permutational multivariate analysis of variance in the R package (R Foundation for Statistical Computing, Vienna, Austria), vegan (Figures S1 through S6).
Serum Succinate Measurement
Serum levels of succinate were monitored in a separate cohort of rats raised from weaning with or without amoxicillin (50 mg/kg body weight) until euthanization at time point 2. Animals were euthanized by CO2 inhalation. Blood was collected through heart puncture and serum was separated and stored at −80°C. Stored serum was thawed and diluted 1:4 times with distilled water and quantitated for succinate using the Succinic Acid Assay Kit from Megazyme (catalog no.: K‐Succ; Megazyme Ltd, Bray, Ireland; Figure 11).
Figure 11.
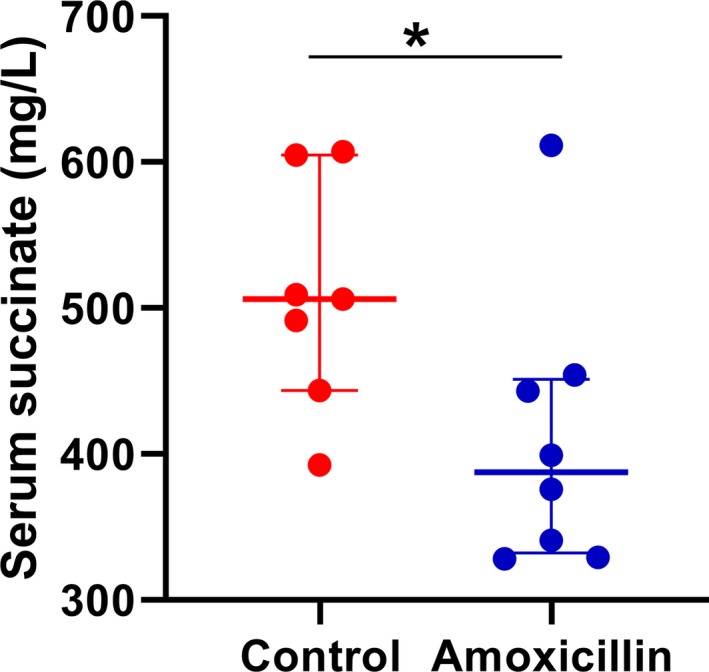
Lower levels of serum succinate in amoxicillin‐treated rats. Serum succinate concentration was measured as described under the Methods section. Control, rats on drinking water without amoxicillin (n=7); amoxicillin, rats on drinking water with amoxicillin (50 mg/kg body weight ad libitum; n=8); *P=0.05. Data presented as median and interquartile range.
Data Visualization
In Figures 3 and 7A and Figure S5, a clustered heatmap was created using Metaboanalyst's statistical analysis function.55 A correlation heatmap (Figure 5) was created using R (v3.5.2; R Foundation for Statistical Computing), heatmap3 function, and the Ward clustering method.
Statistical Analyses
All statistical analyses were performed using a nonparametric Mann–Whitney U test (GraphPad Prism 8.02; GraphPad Software Inc, La Jolla, CA). A P value of <0.05 was considered as a significant difference. To determine the significant alterations in microbiota, Metaboanalyst statistical analysis was used. A P value of <0.05 used as a cutoff for significance using the nonparametric Wilcoxon rank‐sum test. Correlation analysis was done using IBM SPSS software (statistics v25; IBM Corp., Armonk, NY) with bivariate Spearman correlation.
Results
Study 1: Reshaped Gut Microbiota in Young Rats Treated With Amoxicillin
Fecal samples were collected from rats in study 1, which were groups of young rats (just‐weaned rats) implanted with radiotelemetry transmitters. Fecal samples were collected at 3 different time points: 1 week after starting amoxicillin, the last day of amoxicillin treatment, and at euthanization. These are denoted as time 1, 2, and 3, respectively (Figure 2 and Figures S1 through S3). As evident from the unweighted Unifrac principal coordinate analysis plot shown in Figure 2A, microbiota from all time points of the control group were superimposed and thereby clustered whereas the microbiotal communities of amoxicillin‐treated animals were not clustered with that of the control groups at any time point (Figure 2A). This indicates vast differences in beta‐diversity in gut microbiota between the control and amoxicillin‐treated groups. The cluster of dots within the red circle (Figure 2A), which represented time point 1, or 1 week after starting amoxicillin, showed the farthest separation from the control group. This reshaping of microbiota persisted during the 2 subsequent time points represented by the dots within the blue and orange circles. At time point 3, however, which is after cessation of amoxicillin, microbiota of the antibiotic group appeared to revert, as noted by the proximity of the dots within the orange‐colored circle representing time point 3 to that of the cluster of dots within the green‐colored circle representing the control groups (Figure 2A).
Alpha‐diversity was also significantly lower in the antibiotic‐treated groups compared with the control groups (Figure 2B). Whereas the first 2 time points are almost identical in the antibiotic treatment groups, the third time point showed that bacterial diversity tended to revert toward the control groups.
Each of the time points demonstrated specific alterations in bacterial communities (Figure 2C through 2E and Supplemental data). Overall, a prominent feature noted at all time points of amoxicillin treatment was the large reduction in Firmicutes with an accompanying increase in Bacteroidetes.
Amoxicillin Treatment Reshapes Specific Gut Microbiota
In order to determine whether specific bacteria were altered with amoxicillin treatment, fecal samples from time point 2 were examined for microbiota. As seen in the top left and bottom right quadrants of Figure 3, in the amoxicillin‐treated group distinct clusters of several groups of bacteria were different from that of controls. Bacteria belonging to the following phylogenetic groups were decreased with amoxicillin treatment: phylum: Firmicutes, TM7, and Tenericutes; Class‐TM7‐3, Clostridia, Delta Proteobacteria, Bacilli, Erysipelotrichia, Gamma Proteobacteria, and Mollicutes; order: Cw040, Clostridiales, Desulfovibrionales, Lactobacillales, Erysipelotrichales, Pseudomonadales, Turicibacterales, and Bacteroidales; family: F16, Rumincoccaceae, Clostridiales‐F, Desulphovibrionaceae, Veillonellaceae, Lacnosphiraceae, Mogibacteriaceae, Lactobacillaceae, Erysipelotrichaceae, Pseudomonadaceae, Clostridiaceae, Turicibacteraceae, and Peptostreptococcaceae; and genus: Ruminococcus, Oscillospira, Anaerovibrio, [Ruminococcus], Lactobacillus, Pseudomonas, Coprococcus, Dorea, Clostridium, Roseburia, Turicibacter, and [Prevotella] (from Paraprevotellaceae family). Similarly, bacteria belonging to the following phylogenetic groups were increased with amoxicillin treatment: phylum: Bacteroidetes; class: Bacteroidia, Beta Proteobacteria; order: Bacteroidales, Enterobacteriales, and Burkholderiales; family: Prevotellaceae, Enterococcaceae, Enterobacteriaceae, Alcaligenaceae, and Bacteroidaceae; and genus: Blautia, Prevotella, Enterobacter, Enterococcus, Klebsiella, Sutterella, and Bacteroides.
Amoxicillin Administration lowers BP in Young Rats
To assess the effect of reshaping gut microbiota by amoxicillin administration on hemodynamics, BP was temporally monitored. Compared with controls, rats administered with amoxicillin, which had already reshaped gut microbiota at day 7, demonstrated a trend toward lowering their SBP as early as day 10 after administration of the antibiotic, with a statistically significant lowering effect noted beginning from day 22 (Figure 4A). These data suggested that reshaping of gut microbiota occurred before the BP effect and not vice versa. SBP was consistently lower during the light and dark cycles (Figure 4B). SBP of the 2 groups continued to diverge significantly and, interestingly, persisted even after cessation of amoxicillin administration (Figure 4A). Similar trends were additionally noted with diastolic BP and mean arterial pressure (Figure 4C and 4D). Heart rates were not different between the 2 groups (data not shown).
Temporal Correlation Between BP‐Lowering Effects and Bacterial Populations
Next, we examined the correlation between BP effect and specific bacterial abundance at each time point. Hierarchical cluster analysis of correlation plots showed that time points 1 and 2 clustered together and were further away from time point 3 (Figure 5). We next looked for bacterial taxa whose abundance oscillated synchronously with BP. The bacterial taxa whose relative abundances positively correlated with BP clustered toward the top rows of the heatmap (Figure 5). These included genuses Oscillospira, Ruminococcus, Prevotella (from family Paraprevotellaceae), Pseudomonas, Anaerovibrio, Clostridium, and Dorea and families F16, Veillonellaceae, Pseudomonadaceae, and Lachnospiraceae. Similarly, the bacterial taxa whose relative abundances negatively correlated with BP clustered toward the bottom rows of the heatmap (Figure 5). These included the families Elusimicrobiaceae and genuses Bifidobacterium, Sutterella, Blautia, and Prevotella (from family Prevotellaceae).
Study 2: Amoxicillin Treatment During Pregnancy and Lactation Alters Maternal Gut Microbiota
To test the effects of reshaping gut microbiota early in life, pregnant and nursing dams were orally administered with amoxicillin. Before examining the microbiota of offspring, we first confirmed that the maternal microbiota was reshaped in response to amoxicillin. The principal coordinate analysis plots for beta‐diversity as well as alpha‐diversity of the maternal gut microbiota were significantly reshaped and lowered, respectively, with amoxicillin treatment (Figure 6A and 6B). Vast differences were noted in various groups of gut bacteria between amoxicillin‐treated and untreated dams (Figures S5 and S6). This was consistent with what was noted in study 1, wherein amoxicillin treatment reshaped gut bacteria of young rats (Figure 2 and Figures S2 through S4). The Firmicutes/Bacteroidetes (F/B) ratio of dams maintained on amoxicillin was also trending lower compared with controls (Figure 7B).
Amoxicillin Exposure In Utero and During Lactation Reshaped Gut Microbiota of Offspring
To determine whether alterations in maternal microbiota during the gestation and lactational periods affected gut microbiota of offspring, we analyzed fecal samples collected from offspring as described under study 2 methods. Consistent with the previous findings, we found that amoxicillin treatment altered beta‐diversity and lowered alpha‐diversity of offspring (Figure 6C and 6D). Similar to study 1, pups had a trend of reduced F/B ratio compared with controls (Figure 7B). Interestingly, they also displayed a reduction in Proteobacteria, which was not noted in study 1 (Figure 7A).
Hierarchical cluster analysis showed that bacterial groups belonging to various phylogenetic levels were significantly lowered in the amoxicillin‐treated group (Figure 7A). These include the phyla Proteobacteria and TM‐7; classes Delta Proteobacteria and TM7_3; orders Cw040 and Bacteriodales; families Veillonellaceae, F16, Desulfovibrionellaceae, and Porphyromonadaceae; and genus Parabacteroides (Figure 7A). However, when compared with their maternal microbiota, amoxicillin consistently reduced a few groups of bacteria in offspring. F16, Cw040, TM7_3, Veillonellaceae, and Bacteriodales remained consistently reduced in offspring.
Amoxicillin Treatment During Gestation and Lactation Significantly Lowered BP in Offspring
To assess the effect of the reshaped microbiota of dams on the hemodynamic health of pups, we implanted radiotelemetry transmitters into offspring soon after weaning and monitored their BP. Lower SBP was observed in offspring of dams given amoxicillin (Figure 8A). This BP‐lowering effect was noted day 29 postweaning and was consistently observed in both the dark and light phases (Figure 8B).
Correlation Between BP and Bacterial Communities
Next, we examined the correlation between BP and bacterial abundance in offspring. As shown in Figure 9, different groups of bacteria were both positively and negatively correlated with BP. Among these, several were consistent with data presented in Figure 5 from adolescent rats of study 1. These included positively correlated bacterial communities with BP, such as oscillospira, F16, Prevotella (from family Paraprevotellaceae), and Veillonellaceae, and negatively correlated genuses Bifidobacterium and Prevotella (from family Prevotellaceae). However, as compared with their own maternal microbiota, F16, Veillonellaceae, and Prevotella (from family Paraprevotellaceae) were the only 3 groups consistently cleared by amoxicillin administration in mothers and remained depleted in offspring and associated with their lower BP (right side of Figure 10). These data were then combined with results from study 1 using a prioritization scheme depicted in Figure 10. The data once again pointed to Veillonellaceae, F16, and Prevotella (from family Paraprevotellaceae) as the 3 microbiotal communities prominently cleared by amoxicillin at any age, and such a depletion was associated with lower BP in both studies.
Lower Levels of Serum Succinate in Amoxicillin‐Treated Rats
Veillonallaceae are succinate‐producing bacteria, and elevated succinate is associated with hypertension.50, 56, 57, 58, 59, 60 Therefore, to test whether depletion of Veillonalaceae in amoxicillin‐treated rats causes lowering of circulating succinate, we monitored the levels of this metabolite in rats with and without amoxicillin treatment. As shown in Figure 11, serum succinate was significantly lower in amoxicillin treated rats compared with rats without amoxicillin.
Discussion
This study was designed to examine the impact of altering microbiota during early developmental stages on both short‐ and long‐term BP regulation. The results indicate that reshaping of microbiota at any developmental stage in early life, beginning from prenatal to postnatal as well as adolescent stages, has a lasting impact on BP.
We found that in rats given amoxicillin immediately after weaning, there was a decrease in BP. Interestingly, this decreased BP persisted even after the cessation of antibiotics. In order to determine the role of microbiota in this change, we collected fecal samples at 3 time points and analyzed them for bacterial composition. The first time point was 7 days after amoxicillin was started. At this time point, there was no change in BP (Figure 4), but there were significant changes in bacterial diversity (Figure 2). The second time point selected was the last day of amoxicillin treatment. At this time point, BP was significantly lower (Figure 4), and there were still significant changes in bacterial diversity (Figure 2). Importantly, the bacterial communities in amoxicillin‐treated rats at these 2 time points were almost identical. This shows that amoxicillin reshaped microbiota before the observed change in BP, and that continued amoxicillin did not further change bacterial diversity. After this time point, amoxicillin was discontinued, and rats were given normal drinking water. Before euthanization, fecal samples were again collected and analyzed. At this point, BP remained significantly lower (Figure 4), but the bacterial community began to shift back toward the control microbiota (Figure 2). This shows that even though microbiota began to normalize, the BP change remained fixed. Therefore, these data, albeit collected from an animal model, suggest that temporarily reshaping the gut microbiota of pediatric subjects may have lasting effects on BP.
To take this even further, we asked whether reshaping microbiota during pre‐ and postnatal stages affect BP of an individual. To do this, we administered amoxicillin to pregnant rats and maintained them on amoxicillin until the offspring were weaned. This allowed exposure of offspring to the effects of amoxicillin administration to dams during development of the fetus, as well as very early in life during lactation, and a potential direct exposure to amoxicillin before weaning. The BP of such offspring were lower than those that did not receive amoxicillin, indicating that perturbing microbiota during early developmental stages of life affects BP.
Given that BP was lowered regardless of the developmental stage of the rat, we looked for commonalities in microbial communities across all our studies. The most notable feature was the lowering of the F/B ratio, which was consistent between adolescent rats (study 1) and in pregnant rats (study 2), both of which were directly given amoxicillin (Figures 2F and 7B). Amoxicillin reduced Firmicutes as well as increased Bacteriodetes in both groups, which correlated with the BP‐lowering effect by this antibiotic. However, in the offspring of the dams receiving amoxicillin, the F/B ratio was trending, but not statistically significantly lower than that of the ones not receiving amoxicillin (Figure 7B). Despite this, we noted a lower BP in offspring of dams receiving amoxicillin. Therefore, we concluded that the overall F/B ratio was insufficient to account for their BP effect. This led us to look for correlations deeper beyond F/B ratios for specific groups of bacteria that could account for the observed BP effects in all groups. First, we compared bacterial abundances correlating with BP in offspring of studies 1 and 2. Specifically, we examined the list of bacterial correlation with BP in Figure 9 and compared this list with that of adolescent rats in Figure 5. The data obtained were further compared with data in Figure S5, which are microbial data obtained from dams. F16, Veillonellaceae, and Prevotella (from family Paraprevotellaceae) were the only groups consistently lowered with amoxicillin treatment in both the studies, where the only difference was that offspring experienced the amoxicillin effect either directly (in study 1) or by maternal effects (in study 2; Figure 10).
Succinate is a tricarboxylic acid cycle intermediate produced by both host and microbial cells.61 Elevated levels of circulating succinate are associated with a higher relative abundance of succinate‐producing bacteria (ie, Veillonellaceae).57 Several studies link elevated succinate in the circulation to hypertension.56, 60 The Dahl hypertensive model is reported to have higher circulating levels of succinate as well as higher levels of Veillonellaceae.50, 58, 59 In the current study, amoxicillin is shown to deplete Veillonellaceae and lower BP. We therefore tested whether succinate was mediating the mechanism by which amoxicillin lowers BP. The observation that the hypertensinogenic metabolite succinate is lowered in amoxicillin‐treated rats indicates that succinate levels are mechanistically mediating the amoxicillin‐induced lowering of BP (Figure 11). Furthermore, succinate can initiate important protective mechanisms in response to metabolic stress or tissue damage, but in the context of other inflammatory stimuli, these responses could become dysregulated or inappropriately elaborated to contribute to inflammation.57 Local levels of succinate in the kidney also activate the renin‐angiotensin system and, together with succinate, may play a key role in exacerbating hypertension.56 In support, we observed that the elevated levels of the proinflammatory (retinoic acid receptor–related orphan receptor gamma‐t and NACHT, LRR, and PYD domains‐containing protein 3, in the gut and kidney of S rats, respectively, were lowered by the administration of amoxicillin; S. Galla et al, unpublished data, 2019). Our data are indeed supportive of earlier research using germ‐free mice wherein vascular inflammation was similarly observed to be lower under a condition of total bacterial depletion.42
In this study, we tested the stated hypothesis that reshaping of gut microbiota in early developmental stages of life affects BP and demonstrated that administration of amoxicillin during gestation or adolescence lowers BP, which is accompanied by the lowering of specific succinate‐producing bacteria such as Veillonellaceae. Although this is a novel and exciting finding, the study has some limitations. (1) The possibility that amoxicillin per se could have BP‐lowering effects by unknown mechanisms or side effects which are independent or in addition to its antimicrobial effects is not ruled out in our study. For example, antibiotics have direct effects on vascular function.62 (2) The observations presented in our study are attractive for indicating that amoxicillin is beneficial for lowering BP, but are only limited to testing in our rat model. Clearly, further studies are warranted in humans before this is translated into a clinical reality.
Sources of Funding
This study was funded by grant support (HL1430820) from the National Institutes of Health/National Heart, Lung, and Blood Institute to Joe.
Disclosures
None.
Supporting information
Figure S1. Cladogram generated from amoxicillin‐ vs control‐treated rat microbiota at time point 1.
Figure S2. Cladogram generated from amoxicillin‐ vs control‐treated rat microbiota at time point 2.
Figure S3. Cladogram generated from amoxicillin‐ vs control‐treated rat microbiota at time point 3.
Figure S4. Cladogram generated from microbiota of offspring of amoxicillin‐ vs control‐treated dams.
Figure S5. Heatmap representing amoxicillin‐fed and control dams’ bacterial communities.
Figure S6. Cladogram generated from microbiota of amoxicillin‐ vs control‐treated dams.
(J Am Heart Assoc. 2020;9:e014373 DOI: 10.1161/JAHA.119.014373.)
References
- 1. Bai J, Hu Y, Bruner DW. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7‐18 years old children from the American Gut Project. Pediatr Obes. 2019;14:e12480. [DOI] [PubMed] [Google Scholar]
- 2. Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Vosa U, Mujagic Z, Masclee AAM, Jonkers D, Oosting M, Joosten LAB, Netea MG, Franke L, Zhernakova A, Fu J, Wijmenga C, McCarthy MI. Causal relationships among the gut microbiome, short‐chain fatty acids and metabolic diseases. Nat Genet. 2019;51:600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, Watanabe H, Masuda K, Nishimoto Y, Kubo M, Hosoda F, Rokutan H, Matsumoto M, Takamaru H, Yamada M, Matsuda T, Iwasaki M, Yamaji T, Yachida T, Soga T, Kurokawa K, Toyoda A, Ogura Y, Hayashi T, Hatakeyama M, Nakagama H, Saito Y, Fukuda S, Shibata T, Yamada T. Metagenomic and metabolomic analyses reveal distinct stage‐specific phenotypes of the gut microbiota in colorectal cancer. Nat Med. 2019;25:968–976. [DOI] [PubMed] [Google Scholar]
- 4. Backhed F. Programming of host metabolism by the gut microbiota. Ann Nutr Metab. 2011;58(suppl 2):44–52. [DOI] [PubMed] [Google Scholar]
- 5. Ilchmann‐Diounou H, Olier M, Lencina C, Riba A, Barretto S, Nankap M, Sommer C, Guillou H, Ellero‐Simatos S, Guzylack‐Piriou L, Theodorou V, Menard S. Early life stress induces type 2 diabetes‐like features in ageing mice. Brain Behav Immun. 2019;80:452–463. [DOI] [PubMed] [Google Scholar]
- 6. Yan F, Liu L, Cao H, Moore DJ, Washington MK, Wang B, Peek RM, Acra SA, Polk DB. Neonatal colonization of mice with LGG promotes intestinal development and decreases susceptibility to colitis in adulthood. Mucosal Immunol. 2017;10:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhong H, Penders J, Shi Z, Ren H, Cai K, Fang C, Ding Q, Thijs C, Blaak EE, Stehouwer CDA, Xu X, Yang H, Wang J, Wang J, Jonkers D, Masclee AAM, Brix S, Li J, Arts ICW, Kristiansen K. Impact of early events and lifestyle on the gut microbiota and metabolic phenotypes in young school‐age children. Microbiome. 2019;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu J, Kong Y, Yu J, Shao S, Mao M, Zhao M, Yue S. Consumption of drinking water N‐nitrosamines mixture alters gut microbiome and increases the obesity risk in young male rats. Environ Pollut. 2019;248:388–396. [DOI] [PubMed] [Google Scholar]
- 9. Decker E, Hornef M, Stockinger S. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Gut Microbes. 2011;2:91–98. [DOI] [PubMed] [Google Scholar]
- 10. Rodriguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, Avershina E, Rudi K, Narbad A, Jenmalm MC, Marchesi JR, Collado MC. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. 2015;26:26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang J, Zheng J, Shi W, Du N, Xu X, Zhang Y, Ji P, Zhang F, Jia Z, Wang Y, Zheng Z, Zhang H, Zhao F. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut. 2018;67:1614–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Astbury S, Song A, Zhou M, Nielsen B, Hoedl A, Willing BP, Symonds ME, Bell RC. High fructose intake during pregnancy in rats influences the maternal microbiome and gut development in the offspring. Front Genet. 2018;9:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chu DM, Antony KM, Ma J, Prince AL, Showalter L, Moller M, Aagaard KM. The early infant gut microbiome varies in association with a maternal high‐fat diet. Genome Med. 2016;8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gomez de Aguero M, Ganal‐Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, McCoy KD, Macpherson AJ. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351:1296–1302. [DOI] [PubMed] [Google Scholar]
- 15. Hansen CH, Krych L, Buschard K, Metzdorff SB, Nellemann C, Hansen LH, Nielsen DS, Frokiaer H, Skov S, Hansen AK. A maternal gluten‐free diet reduces inflammation and diabetes incidence in the offspring of NOD mice. Diabetes. 2014;63:2821–2832. [DOI] [PubMed] [Google Scholar]
- 16. Hsu CN, Lin YJ, Hou CY, Tain YL. Maternal administration of probiotic or prebiotic prevents male adult rat offspring against developmental programming of hypertension induced by high fructose consumption in pregnancy and lactation. Nutrients. 2018;10:E1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB, Huh JR. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Y, Dugyala SR, Ptacek TS, Gilmore JH, Frohlich F. Maternal immune activation alters adult behavior, gut microbiome and juvenile brain oscillations in ferrets. eNeuro. 2018;5:ENEURO.0313‐18.2018; 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mao Z, Li Y, Dong T, Zhang L, Zhang Y, Li S, Hu H, Sun C, Xia Y. Exposure to titanium dioxide nanoparticles during pregnancy changed maternal gut microbiota and increased blood glucose of rat. Nanoscale Res Lett. 2019;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paul HA, Collins KH, Bomhof MR, Vogel HJ, Reimer RA. Potential impact of metabolic and gut microbial response to pregnancy and lactation in lean and diet‐induced obese rats on offspring obesity risk. Mol Nutr Food Res. 2018;62 DOI: 10.1002/mnfr.201700820; 1–11. [DOI] [PubMed] [Google Scholar]
- 21. Priyadarshini M, Thomas A, Reisetter AC, Scholtens DM, Wolever TM, Josefson JL, Layden BT. Maternal short‐chain fatty acids are associated with metabolic parameters in mothers and newborns. Transl Res. 2014;164:153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schei K, Avershina E, Oien T, Rudi K, Follestad T, Salamati S, Odegard RA. Early gut mycobiota and mother‐offspring transfer. Microbiome. 2017;5:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sherman SB, Sarsour N, Salehi M, Schroering A, Mell B, Joe B, Hill JW. Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes. 2018;9:400–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sugino KY, Paneth N, Comstock SS. Michigan cohorts to determine associations of maternal pre‐pregnancy body mass index with pregnancy and infant gastrointestinal microbial communities: late pregnancy and early infancy. PLoS One. 2019;14:e0213733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tachibana K, Sakurai K, Watanabe M, Miyaso H, Mori C. Associations between changes in the maternal gut microbiome and differentially methylated regions of diabetes‐associated genes in fetuses: a pilot study from a birth cohort study. J Diabetes Investig. 2017;8:550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Torres J, Hu J, Seki A, Eisele C, Nair N, Huang R, Tarassishin L, Jharap B, Cote‐Daigneault J, Mao Q, Mogno I, Britton GJ, Uzzan M, Chen CL, Kornbluth A, George J, Legnani P, Maser E, Loudon H, Stone J, Dubinsky M, Faith JJ, Clemente JC, Mehandru S, Colombel JF, Peter I. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ‐free mice. Gut. 2020;69:42–51. [DOI] [PubMed] [Google Scholar]
- 27. Soderborg TK, Clark SE, Mulligan CE, Janssen RC, Babcock L, Ir D, Young B, Krebs N, Lemas DJ, Johnson LK, Weir T, Lenz LL, Frank DN, Hernandez TL, Kuhn KA, D'Alessandro A, Barbour LA, El Kasmi KC, Friedman JE. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun. 2018;9:4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fouhy F, Watkins C, Hill CJ, O'Shea CA, Nagle B, Dempsey EM, O'Toole PW, Ross RP, Ryan CA, Stanton C. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10:1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lynn MA, Tumes DJ, Choo JM, Sribnaia A, Blake SJ, Leong LEX, Young GP, Marshall HS, Wesselingh SL, Rogers GB, Lynn DJ. Early‐life antibiotic‐driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe. 2018;23:653–660.e5. [DOI] [PubMed] [Google Scholar]
- 30. Tormo‐Badia N, Hakansson A, Vasudevan K, Molin G, Ahrne S, Cilio CM. Antibiotic treatment of pregnant non‐obese diabetic mice leads to altered gut microbiota and intestinal immunological changes in the offspring. Scand J Immunol. 2014;80:250–260. [DOI] [PubMed] [Google Scholar]
- 31. Hu Y, Jin P, Peng J, Zhang X, Wong FS, Wen L. Different immunological responses to early‐life antibiotic exposure affecting autoimmune diabetes development in NOD mice. J Autoimmun. 2016;72:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Candon S, Perez‐Arroyo A, Marquet C, Valette F, Foray AP, Pelletier B, Milani C, Ventura M, Bach JF, Chatenoud L. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin‐dependent diabetes. PLoS One. 2015;10:e0125448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schulfer AF, Battaglia T, Alvarez Y, Bijnens L, Ruiz VE, Ho M, Robinson S, Ward T, Cox LM, Rogers AB, Knights D, Sartor RB, Blaser MJ. Intergenerational transfer of antibiotic‐perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol. 2018;3:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simioni J, Hutton EK, Gunn E, Holloway AC, Stearns JC, McDonald H, Mousseau A, Schertzer JD, Ratcliffe EM, Thabane L, Surette MG, Morrison KM. A comparison of intestinal microbiota in a population of low‐risk infants exposed and not exposed to intrapartum antibiotics: the Baby & Microbiota of the Intestine cohort study protocol. BMC Pediatr. 2016;16:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, A DL, Wu F, Perez‐Perez GI, Chen Y, Schweizer W, Zheng X, Contreras M, Dominguez‐Bello MG, Blaser MJ. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8:343ra382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coker MO, Hoen AG, Dade E, Lundgren S, Li Z, Wong AD, Zens MS, Palys TJ, Morrison HG, Sogin ML, Baker ER, Karagas MR, Madan JC. Specific class of intrapartum antibiotics relates to maturation of the infant gut microbiota: a prospective cohort study. BJOG. 2020;127:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zou ZH, Liu D, Li HD, Zhu DP, He Y, Hou T, Yu JL. Prenatal and postnatal antibiotic exposure influences the gut microbiota of preterm infants in neonatal intensive care units. Ann Clin Microbiol Antimicrob. 2018;17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nyangahu DD, Lennard KS, Brown BP, Darby MG, Wendoh JM, Havyarimana E, Smith P, Butcher J, Stintzi A, Mulder N, Horsnell W, Jaspan HB. Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome. 2018;6:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Azad MB, Moossavi S, Owora A, Sepehri S. Early‐life antibiotic exposure, gut microbiota development, and predisposition to obesity. Nestle Nutr Inst Workshop Ser. 2017;88:67–79. [DOI] [PubMed] [Google Scholar]
- 40. Chatterjee A, Modarai M, Naylor NR, Boyd SE, Atun R, Barlow J, Holmes AH, Johnson A, Robotham JV. Quantifying drivers of antibiotic resistance in humans: a systematic review. Lancet Infect Dis. 2018;18:e368–e378. [DOI] [PubMed] [Google Scholar]
- 41. Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host‐microbiota mutualism. Nat Rev Microbiol. 2011;9:233–243. [DOI] [PubMed] [Google Scholar]
- 42. Karbach SH, Schonfelder T, Brandao I, Wilms E, Hormann N, Jackel S, Schuler R, Finger S, Knorr M, Lagrange J, Brandt M, Waisman A, Kossmann S, Schafer K, Munzel T, Reinhardt C, Wenzel P. Gut microbiota promote angiotensin ii‐induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016;5:e003698 DOI: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Palleja A, Mikkelsen KH, Forslund SK, Kashani A, Allin KH, Nielsen T, Hansen TH, Liang S, Feng Q, Zhang C, Pyl PT, Coelho LP, Yang H, Wang J, Typas A, Nielsen MF, Nielsen HB, Bork P, Wang J, Vilsboll T, Hansen T, Knop FK, Arumugam M, Pedersen O. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat Microbiol. 2018;3:1255–1265. [DOI] [PubMed] [Google Scholar]
- 44. Suez J, Zmora N, Zilberman‐Schapira G, Mor U, Dori‐Bachash M, Bashiardes S, Zur M, Regev‐Lehavi D, Ben‐Zeev Brik R, Federici S, Horn M, Cohen Y, Moor AE, Zeevi D, Korem T, Kotler E, Harmelin A, Itzkovitz S, Maharshak N, Shibolet O, Pevsner‐Fischer M, Shapiro H, Sharon I, Halpern Z, Segal E, Elinav E. Post‐antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174:1406–1423.e16. [DOI] [PubMed] [Google Scholar]
- 45. Galla S, Chakraborty S, Cheng X, Yeo J, Mell B, Zhang H, Mathew AV, Vijay‐Kumar M, Joe B. Disparate effects of antibiotics on hypertension. Physiol Genomics. 2018;50:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mahler A, Balogh A, Marko L, Vvedenskaya O, Kleiner FH, Tsvetkov D, Klug L, Costea PI, Sunagawa S, Maier L, Rakova N, Schatz V, Neubert P, Fratzer C, Krannich A, Gollasch M, Grohme DA, Corte‐Real BF, Gerlach RG, Basic M, Typas A, Wu C, Titze JM, Jantsch J, Boschmann M, Dechend R, Kleinewietfeld M, Kempa S, Bork P, Linker RA, Alm EJ, Muller DN. Salt‐responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bartolomaeus H, Balogh A, Yakoub M, Homann S, Marko L, Hoges S, Tsvetkov D, Krannich A, Wundersitz S, Avery EG, Haase N, Kraker K, Hering L, Maase M, Kusche‐Vihrog K, Grandoch M, Fielitz J, Kempa S, Gollasch M, Zhumadilov Z, Kozhakhmetov S, Kushugulova A, Eckardt KU, Dechend R, Rump LC, Forslund SK, Muller DN, Stegbauer J, Wilck N. Short‐chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139:1407–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bartolomaeus H, Marko L, Wilck N, Luft FC, Forslund SK, Muller DN. Precarious symbiosis between host and microbiome in cardiovascular health. Hypertension. 2019;73:926–935. [DOI] [PubMed] [Google Scholar]
- 49. Sun S, Lulla A, Sioda M, Winglee K, Wu MC, Jacobs DR Jr, Shikany JM, Lloyd‐Jones DM, Launer LJ, Fodor AA, Meyer KA. Gut microbiota composition and blood pressure. Hypertension. 2019;73:998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay‐Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Edgar RC. Search and clustering orders of magnitude faster than blast. Bioinformatics. 2010;26:2460–2461. [DOI] [PubMed] [Google Scholar]
- 52. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. Qiime allows analysis of high‐throughput community sequencing data. Nat Methods. 2010;7:335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera‐checked 16s rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xia J, Psychogios N, Young N, Wishart DS. Metaboanalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009;37:W652–W660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Khamaysi A, Anbtawee‐Jomaa S, Fremder M, Eini‐Rider H, Shimshilashvili L, Aharon S, Aizenshtein E, Shlomi T, Noguchi A, Springer D, Moe OW, Shcheynikov N, Muallem S, Ohana E. Systemic succinate homeostasis and local succinate signaling affect blood pressure and modify risks for calcium oxalate lithogenesis. J Am Soc Nephrol. 2019;30:381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Serena C, Ceperuelo‐Mallafre V, Keiran N, Queipo‐Ortuno MI, Bernal R, Gomez‐Huelgas R, Urpi‐Sarda M, Sabater M, Perez‐Brocal V, Andres‐Lacueva C, Moya A, Tinahones FJ, Fernandez‐Real JM, Vendrell J, Fernandez‐Veledo S. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018;12:1642–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tian Z, Liu Y, Usa K, Mladinov D, Fang Y, Ding X, Greene AS, Cowley AW Jr, Liang M. Novel role of fumarate metabolism in Dahl‐salt sensitive hypertension. Hypertension. 2009;54:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang L, Hou E, Wang Z, Sun N, He L, Chen L, Liang M, Tian Z. Analysis of metabolites in plasma reveals distinct metabolic features between Dahl salt‐sensitive rats and consomic SS.13(BN) rats. Biochem Biophys Res Commun. 2014;450:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sadagopan N, Li W, Roberds SL, Major T, Preston GM, Yu Y, Tones MA. Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am J Hypertens. 2007;20:1209–1215. [DOI] [PubMed] [Google Scholar]
- 61. Connors J, Dawe N, Van Limbergen J. The role of succinate in the regulation of intestinal inflammation. Nutrients. 2018;11:E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bayer F, Ascher S, Pontarollo G, Reinhardt C. Antibiotic treatment protocols and germ‐free mouse models in vascular research. Front Immunol. 2019;10:2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Cladogram generated from amoxicillin‐ vs control‐treated rat microbiota at time point 1.
Figure S2. Cladogram generated from amoxicillin‐ vs control‐treated rat microbiota at time point 2.
Figure S3. Cladogram generated from amoxicillin‐ vs control‐treated rat microbiota at time point 3.
Figure S4. Cladogram generated from microbiota of offspring of amoxicillin‐ vs control‐treated dams.
Figure S5. Heatmap representing amoxicillin‐fed and control dams’ bacterial communities.
Figure S6. Cladogram generated from microbiota of amoxicillin‐ vs control‐treated dams.
Data Availability Statement
All data and materials have been made publicly available at the Mendeley Data and can be accessed at Chakraborty and Saroj (2019), “Reshaping commensal gut microbiota in early life with amoxicillin presents with lower blood pressure,” Mendeley Data, v1 (https://doi.org/10.17632/tr9kfmc5zv.1).


