Abstract
Background
Nocturnal hypertension, defined by a mean asleep systolic blood pressure (SBP)/diastolic blood pressure (BP) ≥120/70 mm Hg, and nondipping SBP, defined by an awake‐to‐asleep decline in SBP <10%, are each associated with increased risk for cardiovascular disease.
Methods and Results
We developed predictive equations to identify adults with a high probability of having nocturnal hypertension or nondipping SBP using data from the CARDIA (Coronary Artery Risk Development in Young Adults) study (n=787), JHS (Jackson Heart Study) (n=1063), IDH (Improving the Detection of Hypertension) study (n=395), and MHT (Masked Hypertension) study (n=772) who underwent 24‐hour ambulatory BP monitoring. Participants were randomized to derivation (n=2511) or validation (n=506) data sets. The prevalence rates of nocturnal hypertension and nondipping SBP were 39.7% and 44.9% in the derivation data set, respectively, and 36.6% and 44.5% in the validation data set, respectively. The predictive equation for nocturnal hypertension included age, race/ethnicity, smoking status, neck circumference, height, high‐density lipoprotein cholesterol, albumin/creatinine ratio, and clinic SBP and diastolic BP. The predictive equation for nondipping SBP included age, sex, race/ethnicity, waist circumference, height, alcohol use, high‐density lipoprotein cholesterol, and albumin/creatinine ratio. Concordance statistics (95% CI) for nocturnal hypertension and nondipping SBP predictive equations in the validation data set were 0.84 (0.80–0.87) and 0.73 (0.69–0.78), respectively. Compared with reference models including antihypertensive medication use and clinic SBP and diastolic BP as predictors, the continuous net reclassification improvement (95% CI) values for the nocturnal hypertension and nondipping SBP predictive equations were 0.52 (0.35–0.69) and 0.51 (0.34–0.69), respectively.
Conclusions
These predictive equations can direct ambulatory BP monitoring toward adults with high probability of having nocturnal hypertension and nondipping SBP.
Keywords: ambulatory, blood pressure, nocturnal hypertension, nondipping, predictive equation, validation
Subject Categories: High Blood Pressure, Cost-Effectiveness, Cardiovascular Disease
Clinical Perspective
What Is New?
Nocturnal hypertension and nondipping systolic blood pressure (BP) can be identified using ambulatory BP monitoring, but it is not practical to screen all adults for these phenotypes.
We developed predictive equations to identify adults with a high probability of having nocturnal hypertension or nondipping systolic BP.
What Are the Clinical Implications?
Compared with screening methods based on clinic BP and antihypertensive medication use, the predictive equations we developed exhibited superior classification characteristics in a validation data set.
The equations developed in the current analysis may direct ambulatory BP monitoring to adults with a high probability of having nocturnal hypertension and nondipping systolic BP.
Blood pressure (BP) in humans varies over a 24‐hour period, with the lowest levels typically occurring during sleep.1 Nocturnal hypertension and nondipping systolic BP (SBP) have each been associated with an increased risk for cardiovascular disease events, independent of SBP and diastolic BP (DBP) measured in the clinic setting.2, 3, 4, 5, 6 Clinicians and researchers may seek to screen adults for nocturnal hypertension and nondipping SBP. Clinicians may recommend lifestyle modification or drug therapy to their patients with nocturnal hypertension or nondipping BP.7 Researchers may seek to enroll a cohort of participants with nocturnal hypertension to test interventions that lower asleep BP.8 Ambulatory BP monitoring (ABPM) is the primary approach used to identify nocturnal hypertension and nondipping SBP. However, it is not practical to conduct ABPM in all adults to identify those with nocturnal hypertension and nondipping SBP.9, 10 A more feasible approach is to conduct ABPM screening among adults with a high probability of having these BP phenotypes. Therefore, we developed predictive equations to identify adults with a high probability of having nocturnal hypertension or nondipping SBP.
Methods
We pooled data from participants in the JHS (Jackson Heart Study) (n=1063), the CARDIA (Coronary Artery Risk Development in Young Adults) study (n=787), the IDH (Improving the Detection of Hypertension) study (n=395), and the MHT (Masked Hypertension) study (n=772) study who underwent 24‐hour ABPM and had ≥10 SBP and DBP readings while awake and ≥5 SBP and DBP readings while asleep (Figure S1).11, 12, 13, 14, 15 Additional details on each study are available in Data S1. All studies were approved by institutional review boards, and all participants provided written informed consent.
Requests to access JHS and CARDIA study data from qualified researchers trained in human subject confidentiality protocols may be submitted to BioLINCC, the National Heart, Lung, and Blood Institute repository (https://biolincc.nhlbi.nih.gov/home/). Alternatively, investigators may submit manuscript proposals to the CARDIA study or the JHS at https://www.cardia.dopm.uab.edu and https://www.jacksonheartstudy.org, respectively. Deidentified data from the IDH and MHT studies for the purpose of replicating this analysis may be made available on request to Dr Joseph Schwartz (E‐mail: JES222@cumc.columbia.edu).
Ambulatory BP Monitoring
The JHS and IDH and MHT studies used a SpaceLabs model 90207 monitor (Snoqualmie, WA) to conduct 24‐hour ABPM. The CARDIA study used a SpaceLabs OnTrak model 90227 monitor.16, 17 SBP and DBP were measured every 20 minutes (JHS), 28 to 30 minutes (MHT study), or 30 minutes (CARDIA and IDH studies).18 In the current analysis, BP measurements outside of preset limits (SBP 70–250 mm Hg and DBP 40–150 mm Hg while awake; SBP ≥60 mm Hg and DBP ≥30 mm Hg while asleep) were excluded. In the CARDIA, IDH, and MHT studies, actigraphy data and sleep diaries were used to determine awake and asleep periods. JHS participants were only asked to complete a sleep diary. We identified 123 JHS participants who did not provide a valid sleep diary but did record ≥10 and ≥5 BP readings during daytime (10 am–8 pm) and nighttime (12 am–6 am) hours, respectively. For these participants, we computed mean awake and asleep BP during daytime and nighttime hours, respectively.
Nocturnal Hypertension and Nondipping SBP
Awake and asleep BP levels were computed as the mean of all readings during each period. Nocturnal hypertension was defined as an asleep SBP/DBP ≥120/70 mm Hg. Nondipping SBP was defined as a decline in SBP from wakefulness to sleep <10% (ie, ratio of mean asleep SBP/mean awake SBP >0.90).
Candidate Predictor Variables
We reviewed a list of variables measured under similar conditions and protocols in each study and selected a subset as candidate predictor variables for the nocturnal hypertension and nondipping SBP prediction equations. Candidate predictors were selected on the basis of routine availability, clinical knowledge, and variables associated with asleep BP in prior studies.19, 20 Variables selected as candidate predictors were age (years), sex (men/women), race/ethnicity (white/black/Asian or Pacific Islander/other), smoking (current/former/never), alcohol consumption (yes/no), sleep duration (hours), height (centimeters), weight (kg), body mass index (kg/m2), neck and waist circumference (centimeters), urinary albumin (mg/dL), urinary creatinine (g/dL), log‐transformed urinary albumin/creatinine ratio (ACR; no units), estimated glomerular filtration rate <60 mL/min per 1.73 m2 (yes/no), fasting blood glucose (mg/dL), diabetes mellitus (yes/no), high‐ and low‐density lipoprotein cholesterol (mg/dL), clinic‐measured SBP and DBP (mm Hg), and antihypertensive medication use (yes/no).21 Additional details about these variables are provided in Table S1.
Statistical Analyses
Derivation and validation data sets
All analyses were conducted using R version ≥3.6.0. Participants in the pooled JHS and CARDIA, IDH, and MHT study data were randomized to derivation (n=2511) or validation (n=506) data sets. Pooling all data sets versus keeping one out was applied to maximize the diversity of the derivation data set and, in turn, the generalizability of the predictive equations.22 Summary statistics for characteristics were calculated for participants in the derivation and validation data sets, separately.
Development of the predictive equations
We developed a set of prediction equations using the derivation data and subsequently validated those equations in the validation data set (Figure 1). We compared 7 candidate modeling algorithms to create a predictive equation for nocturnal hypertension and nondipping SBP, separately, using a 5‐step resampling process to internally validate predictive equations using the derivation data set (Figure 2 and Data S1).23, 24 Each candidate modeling algorithm was ranked by its discrimination, calibration, and overall goodness of fit using a concordance statistic (C‐statistic), the Hosmer and Lemeshow χ2 statistic, and the scaled Brier score, respectively.25 The candidate modeling algorithm with the highest mean ranking was selected to create the predictive equations using the full set of derivation data. We applied bootstrap resampling to estimate the probability of inclusion into each predictive equation for each candidate predictor variable. To compare the selected predictive equations with a less complex predictive equation, we fit reference models to the derivation data set for nocturnal hypertension and nondipping SBP, separately, using logistic regression. Each reference model included clinic‐measured SBP and DBP and antihypertensive medication use as predictors. Each reference model was formally compared with the selected predictive equations in the validation data set to determine whether the predictive equations outperformed a simpler set of equations outside of the derivation data set.
Figure 1.
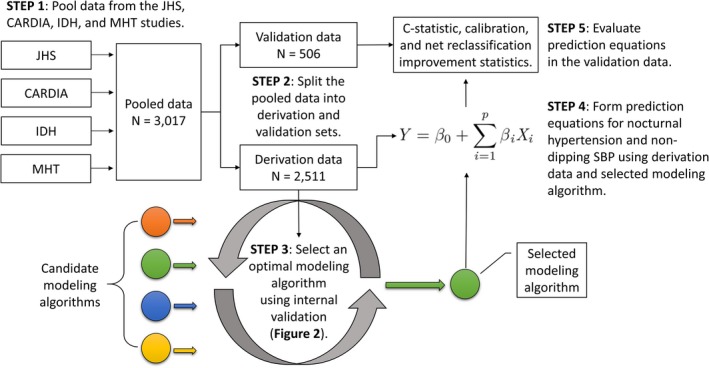
Description of the main steps taken to complete the current analysis. Candidate modeling algorithms comprise the sequence of steps taken to develop a prediction equation and were evaluated in step 3. CARDIA indicates Coronary Artery Risk Development in Young Adults; C‐statistic, concordance statistic; IDH, Improving the Detection of Hypertension; JHS, Jackson Heart Study; MHT, Masked Hypertension; SBP, systolic blood pressure.
Figure 2.
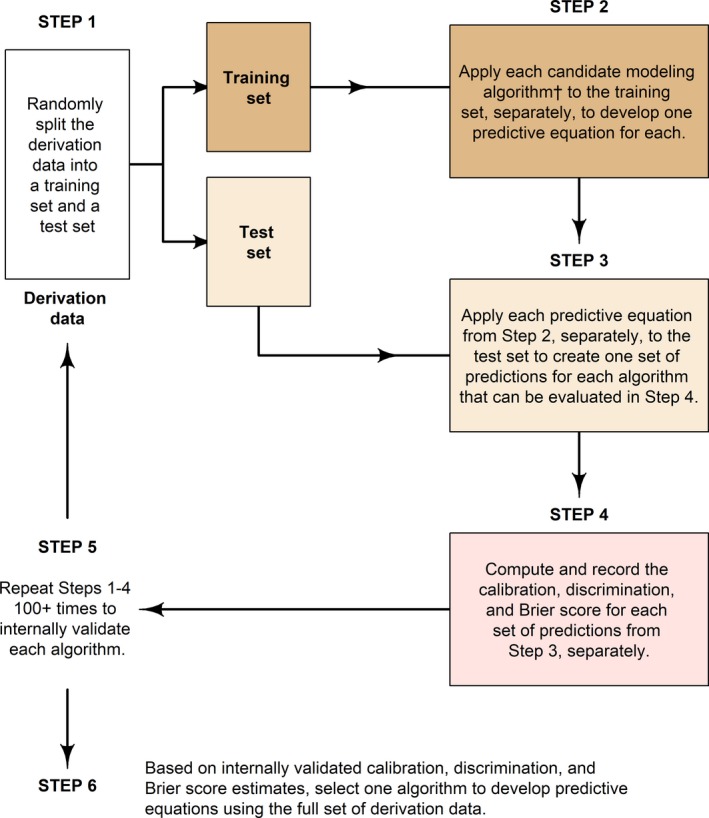
Five steps for the development and internal validation of candidate modeling algorithm. †A modeling algorithm is the collection of steps that translate data into a predictive equation. This process only uses the derivation data set. The validation data set is not used until a final modeling algorithm is selected and applied to the full derivation data set. Candidate modeling algorithms for the current analysis were as follows: (1) logistic regression using forward variable selection, (2) logistic regression using backwards variable selection, (3) generalized additive logistic regression using forward variable selection, (4) penalized logistic regression with a lasso penalty, (5) penalized logistic regression with a ridge penalty, (6) random forests, and (7) gradient boosted decision trees.
After developing predictive equations, we identified 4 cut points for categorizing participants as having a high probability of nocturnal hypertension and nondipping SBP, separately, that provided the following: (1) the closest number of predicted and observed cases (ie, maximizing calibration), (2) the maximum specificity with a sensitivity ≥0.80, (3) the maximum negative predictive value with a positive predictive value ≥0.80, and (4) the maximum Youden index (ie, sensitivity+specificity). The closest number of predicted and observed cases occurs when we chose a cut point that provided the same proportion of participants with the outcome as are defined as testing positive on the basis of the predictive equations.
Validation of the predictive equations
Using the validation data set, we assessed the predictive equations’ discrimination using a C‐statistic. C‐statistics were also computed for the reference models for nocturnal hypertension and nondipping SBP. We applied bootstrap resampling to test the null hypothesis of equivalence between the C‐statistics of each predictive equation and the reference model. We assessed the calibration of the predictive equations using a calibration slope curve, the Hosmer and Lemeshow goodness‐of‐fit test, and the Harrell unreliability test.26, 27 We computed C‐statistics and conducted Hosmer and Lemeshow goodness‐of‐fit tests in subgroups based on age, race, sex, medication use, and high school graduation status for each predictive equation. For each of the 4 probability cut points identified using the derivation data, we computed the sensitivity, specificity, and positive and negative predictive values of the predictive equations in the validation data. These test characteristics were also calculated for 4 alternative methods that may be used to identify suitable candidates for ABPM screening: (I) clinic‐measured SBP/DBP ≥120/70 mm Hg, (II) clinic‐measured SBP/DBP ≥130/80 mm Hg, (III) clinic‐measured SBP/DBP ≥140/90 mm Hg, or (IV) antihypertensive medication use. Categorical net reclassification improvement (NRI) was computed by initially classifying participants as having a low or high probability for nocturnal hypertension or nondipping SBP using screening methods (I–IV) listed above, separately, and then reclassifying participants on the basis of probability cut point 4 (ie, the cut point maximizing the Youden index) of the corresponding predictive equation.28, 29, 30, 31 Cut point 4 was chosen on the basis of the assumption that it would provide better overall classification characteristics than the other 3 cut points. Continuous NRI and integrated discrimination improvement index were computed by comparing predicted probabilities from the predictive equations versus reference models for nocturnal hypertension and nondipping SBP. Additional details on validation and the NRI are provided in Data S1.
Missing data
Albuminuria and neck circumference had the highest missing rates (9.9% and 5.0%, respectively). All other candidate predictors had <5.0% missing rates. Random forests were applied to impute missing values in the derivation and validation data sets, separately.32
Exploratory analyses
Prior studies that examined nocturnal BP patterns have focused on SBP versus DBP nondipping. We conducted exploratory analyses developing and evaluating predictive equations for nondipping DBP.
Results
Characteristics of Participants
There was minimal evidence of a difference between the characteristics of participants in the derivation versus the validation data sets (Table 1; 2 P<0.05 in 26 comparisons). The prevalence rates of nocturnal hypertension and nondipping SBP were 39.7% and 44.9% in the derivation data set, respectively, and 36.6% and 44.5% in the validation data set, respectively. Participants from the CARDIA study who were included in the current study were more likely to be women and have prevalent diabetes mellitus compared with their counterparts in the CARDIA study who were not included (Table S2). Participants from the JHS who were included in the current study were older and more likely to have albuminuria compared with their counterparts in the JHS who were not included (Table S3). Participants in the CARDIA study exhibited a more narrow age range compared with participants in the JHS and the IDH and MHT studies (Table S4).
Table 1.
Participant Characteristics Stratified by Assignment Into the Derivation or Validation Data Set
| Characteristicsa | Data Setb | P Value | |
|---|---|---|---|
| Derivation (n=2511) | Validation (n=506) | ||
| Study cohort, % | 0.977 | ||
| CARDIA study | 25.9 | 26.9 | |
| JHS | 35.3 | 35.0 | |
| IDH study | 13.1 | 12.8 | |
| MHT study | 25.6 | 25.3 | |
| Age, y | 51.9 (11.8) | 51.6 (12.5) | 0.587 |
| Men, % | 37.4 | 37.7 | 0.908 |
| Race/ethnicity, % | 0.598 | ||
| White | 34.9 | 37.2 | |
| Black | 56.7 | 55.9 | |
| Asian or Pacific Islander | 2.15 | 1.58 | |
| Other | 6.25 | 5.34 | |
| High school graduate, % | 90.3 | 91.2 | 0.568 |
| Smoking habits, % | 0.125 | ||
| Current | 11.0 | 8.38 | |
| Former | 20.7 | 23.4 | |
| Never | 68.3 | 68.3 | |
| Alcohol use, % | 65.2 | 69.5 | 0.073 |
| Sleep duration, h | 7.53 (1.68) | 7.56 (1.59) | 0.662 |
| Neck circumference, cm | 37.2 (4.27) | 37.1 (4.16) | 0.663 |
| Waist circumference, cm | 95.2 (15.8) | 94.5 (15.9) | 0.346 |
| Weight, kg | 84.8 (19.9) | 84.14 (20.3) | 0.510 |
| Height, cm | 168.3 (9.41) | 168.4 (9.89) | 0.883 |
| Body mass index, kg/m2 | 29.9 (6.46) | 29.6 (6.28) | 0.341 |
| Albumin/creatinine ratio, mg/g | 2.00 (0.94) | 1.93 (0.84) | 0.120 |
| Albuminuria, %b | 6.82 | 5.90 | 0.535 |
| eGFR <60 mL/min per 1.73 m2, % | 3.10 | 4.62 | 0.113 |
| Blood glucose, mg/dL | 98.5 (32.1) | 95.6 (27.0) | 0.037 |
| Diabetes mellitus, % | 16.3 | 15.5 | 0.672 |
| High‐density lipoprotein cholesterol, mg/dL | 56.1 (16.6) | 57.5 (16.0) | 0.071 |
| Low‐density lipoprotein cholesterol, mg/dL | 117.9 (35.5) | 113.9 (33.2) | 0.019 |
| Total cholesterol, mg/dL | 195.1 (39.6) | 191.8 (37.1) | 0.079 |
| Heart rate while awake, beats/min | 78.3 (10.4) | 77.3 (10.4) | 0.055 |
| Antihypertensive medication use, % | 31.7 | 31.3 | 0.899 |
| Conventional hypertensionc | 37.4 | 37.5 | 36.7 |
| Systolic blood pressure, mm Hg | |||
| Clinic | 121.8 (16.4) | 121.6 (15.7) | 0.832 |
| Sleep | 113.8 (15.3) | 112.6 (15.2) | 0.129 |
| Diastolic blood pressure, mm Hg | |||
| Clinic | 75.1 (9.55) | 75.4 (9.75) | 0.451 |
| Sleep | 65.9 (9.23) | 65.6 (9.54) | 0.648 |
| Nocturnal hypertension, %d | 39.7 | 36.6 | 0.209 |
| Nondipping systolic blood pressure, %e | 44.9 | 44.5 | 0.902 |
CARDIA indicates Coronary Artery Risk Development in Young Adults; eGFR, estimated glomerular filtration rate; IDH, Improving the Detection of Hypertension; JHS, Jackson Heart Study; MHT, Masked Hypertension.
Table values are mean (SD) and percentage for continuous and categorical variables, respectively.
Albuminuria was defined as a urinary albumin/urinary creatinine ratio ≥30 mg/g.
Conventional hypertension was defined as having a systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or currently taking antihypertensive medication.
Nocturnal hypertension was defined as having a mean systolic blood pressure ≥120 mm Hg or mean diastolic blood pressure ≥70 mm Hg while asleep.
Nondipping systolic blood pressure was defined as decline in mean systolic blood pressure from wakefulness to asleep <10%.
Development of the Predictive Equations
On the basis of the concordance error, the Hosmer‐Lemeshow χ2 statistic, and scaled Brier score, generalized additive logistic regression with forward variable selection was chosen to develop predictive equations for nocturnal hypertension and nondipping SBP (Table S5). Variables included in the predictive equation for nocturnal hypertension were age, race/ethnicity, smoking status, neck circumference, height, high‐density lipoprotein cholesterol, ACR, and clinic SBP and DBP (Table 2; middle column). Variables included in the predictive equation for nondipping SBP were age, sex, race/ethnicity, waist circumference, height, alcohol use, high‐density lipoprotein cholesterol, and ACR (Table 2; right column). Predictors based on race, age, and ACR were selected in >85% bootstrapped replicates of the derivation data (Tables S6 and S7). Height and clinic‐measured SBP and DBP were selected as nonlinear predictors for nocturnal hypertension (Figure S2; top panel). Age, height, and ACR were selected as nonlinear predictors for nondipping SBP (Figure S2; bottom panel). The probability cut points associated with closest number of predicted and observed cases, maximum specificity with sensitivity ≥0.80, and maximum negative predictive value with positive predictive value ≥0.80 and to maximize Youden index were 0.46, 0.37, 0.65, and 0.34, respectively, for the nocturnal hypertension predictive equation and 0.48, 0.35, 0.71, and 0.43, respectively, for the nondipping SBP predictive equation (Figure 3).
Table 2.
Odds Ratios for Variables Selected for Inclusion in the Predictive Equations for Nocturnal Hypertension and Nondipping SBP
| Variable | Odds Ratio (95% CI) | |
|---|---|---|
| Nocturnal Hypertension | Nondipping Systolic Blood Pressure | |
| Age, 12 y | 1.47 (1.29–1.67) | 1.74 (1.40–2.00)a |
| Men | ··· | 0.60 (0.47–0.78) |
| Race | ||
| White | 1 (Reference) | 1 (Reference) |
| Black | 2.64 (2.09–3.34) | 3.08 (2.50–3.78) |
| Asian | 1.26 (0.50–3.16) | 1.22 (0.63–2.38) |
| Other race/ethnicity | 2.28 (1.45–3.58) | 1.37 (0.93–2.01) |
| Smoking status | ||
| Current | 1 (Reference) | ··· |
| Former | 0.72 (0.50–1.03) | ··· |
| Never | 0.68 (0.50–0.93) | ··· |
| Neck circumference, 4 cm | 1.16 (1.03–1.32) | ··· |
| Waist circumference, 16 cm | ··· | 1.19 (1.07–1.32) |
| Height, 10 cm | 1.20 (1.06–1.35)a | 1.10 (0.96–1.26)a |
| Alcohol use | ··· | 0.64 (0.53–0.78) |
| HDL cholesterol, 17 mg/dL | 0.87 (0.77–0.98) | 0.81 (0.73–0.91) |
| Log(ACR), 1 log g/24 h | 1.44 (1.28–1.63) | 1.18 (1.03–1.40)a |
| Clinic SBP, 16 mm Hg | 2.48 (2.15–2.87)a | ··· |
| Clinic DBP, 10 mm Hg | 1.26 (1.12–1.45)a | ··· |
Table values were computed using the derivation data. The odds ratios for the following predictor variables are presented for a 1‐SD higher level of the exposure value: age, neck circumference, waist circumference, height, high‐density lipoprotein cholesterol, clinic SBP, and clinic DBP. ACR indicates albumin/creatinine ratio; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; SBP, systolic blood pressure; ···, a variable was not selected for inclusion in the corresponding equation.
This is a nonlinear variable in the predictive equation. The odds ratio is presented using the mean as a reference value.
Figure 3.
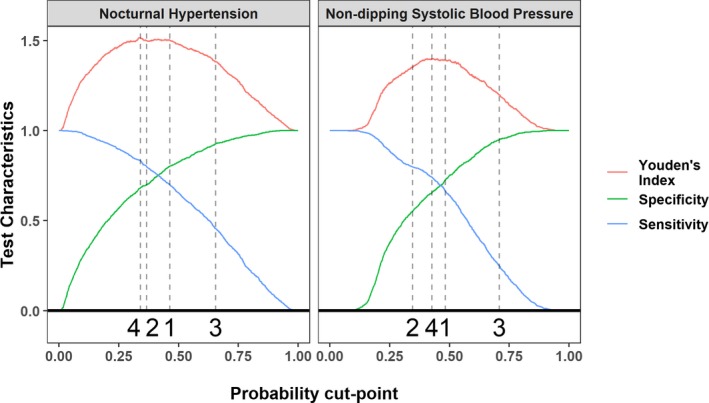
Sensitivity, specificity, and Youden index of the nocturnal hypertension and nondipping systolic blood pressure predictive equations. Results are based on the derivation data. Probability cut points selected for validation (bottom of each panel): (1) Closest number of predicted and observed cases with nocturnal hypertension and nondipping systolic blood pressure. (2) The maximum specificity with a sensitivity ≥0.80. (3) The maximum negative predictive value with a positive predictive value ≥0.80. (4) The maximum sum of sensitivity and specificity.
Validation of the Predictive Equations
For nocturnal hypertension, the predictive equation had a C‐statistic of 0.84 (95% CI, 0.80–0.87) versus the reference model C‐statistic of 0.82 (95% CI, 0.78–0.86; P value for nonzero difference=0.089). For nondipping SBP, the predictive equation's C‐statistic was 0.73 (95% CI, 0.69–0.78) compared with the reference model's C‐statistic of 0.65 (95% CI, 0.60–0.70) (P value for nonzero difference <0.001). There was no evidence of miscalibration for the nocturnal hypertension or nondipping SBP equations overall (Figure 4) or in subgroups based on age, race, sex, medication use, and education (Table S8).
Figure 4.
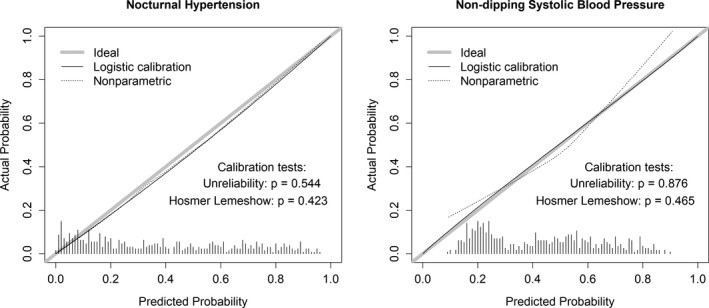
Calibration slope plots for nocturnal hypertension and nondipping systolic blood pressure. Results are based on the validation data. The ideal calibration curve shows the slope of a perfectly calibrated model. Histograms at the base of the panels show the distribution of predicted probabilities in the validation data. The logistic and nonparametric calibration slopes estimate the calibration of a predicted equation by fitting a logistic model and a locally estimated scatterplot smoothing model, with predicted probability and observed status playing the role of independent and dependent variables, respectively.
Test characteristics
Using the predictive equations for nocturnal hypertension and nondipping SBP resulted in higher values of Youden's index compared with clinic‐measured SBP/DBP ≥120/70 mm Hg, ≥130/80 mm Hg, or ≥140/90 mm Hg, or antihypertensive medication use (Table 3).
Table 3.
Test Characteristics of the Predictive Equations and Alternative Screening Methods for Identifying Adults With a High Probability of Nocturnal Hypertension and Nondipping SBP
| Characteristics | Methods of Identifying Who Should Undergo 24‐h Ambulatory Blood Pressure Monitoring | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictive Equation Probability Cut Points | SBP/Diastolic Blood Pressure Cut Points, mm Hg | Current Use of Antihypertensive Medication | ||||||
| 1 | 2 | 3 | 4 | I | II | III | IV | |
| Nocturnal hypertension | ||||||||
| Classification cut points | ≥0.46 | ≥0.37 | ≥0.65 | ≥0.34 | ≥120/70 | ≥130/80 | ≥140/90 | Yes |
| Screened, % | 37.7 | 47.2 | 22.9 | 50.0 | 78.5 | 42.1 | 14.6 | 31.6 |
| Sensitivity | 0.69 | 0.79 | 0.50 | 0.83 | 0.95 | 0.68 | 0.32 | 0.49 |
| Specificity | 0.80 | 0.71 | 0.93 | 0.69 | 0.31 | 0.73 | 0.96 | 0.79 |
| Positive predictive value | 0.67 | 0.62 | 0.79 | 0.61 | 0.44 | 0.59 | 0.81 | 0.57 |
| Negative predictive value | 0.82 | 0.86 | 0.76 | 0.88 | 0.92 | 0.80 | 0.71 | 0.73 |
| Youden index | 1.50 | 1.51 | 1.42 | 1.52 | 1.26 | 1.40 | 1.28 | 1.28 |
| Nondipping systolic blood pressure | ||||||||
| Classification cut points | ≥0.48 | ≥0.35 | ≥0.71 | ≥0.43 | ≥120/70 | ≥130/80 | ≥140/90 | Yes |
| Screened, % | 43.5 | 58.9 | 12.8 | 50.6 | 78.5 | 42.1 | 14.6 | 31.6 |
| Sensitivity | 0.62 | 0.76 | 0.25 | 0.70 | 0.82 | 0.49 | 0.21 | 0.45 |
| Specificity | 0.72 | 0.55 | 0.97 | 0.65 | 0.24 | 0.63 | 0.90 | 0.79 |
| Positive predictive value | 0.64 | 0.58 | 0.88 | 0.62 | 0.46 | 0.52 | 0.64 | 0.64 |
| Negative predictive value | 0.70 | 0.75 | 0.62 | 0.73 | 0.62 | 0.61 | 0.59 | 0.64 |
| Youden index | 1.34 | 1.32 | 1.22 | 1.35 | 1.06 | 1.12 | 1.11 | 1.25 |
- Closest number of predicted and observed cases with nocturnal hypertension and nondipping systolic blood pressure.
- The maximum specificity with a sensitivity ≥0.80.
- The maximum negative predictive value with a positive predictive value ≥0.80.
- The maximum sum of sensitivity and specificity. SBP indicates systolic blood pressure.
Net reclassification improvement
Compared with screening methods based on clinic SBP and DBP or antihypertensive medication use, using the predictive equations resulted in overall categorical NRI values ranging from 0.11 (95% CI, 0.02–0.19) to 0.29 (95% CI, 0.20–0.40) (Table 4). Comparing the predictive equations with the reference models with the outcome of nocturnal hypertension and nondipping SBP resulted in continuous NRI values of 0.52 (95% CI, 0.35–0.69) and 0.51 (95% CI, 0.34–0.69), respectively, and integrated discrimination improvement indexes of 0.10 (95% CI, 0.07–0.12) and 0.07 (95% CI, 0.04–0.09), respectively.
Table 4.
NRI and Integrated Discriminative Improvement Using Predictive Equations From the Current Analysis Versus Screening Methods Based on Clinic Blood Pressure and Antihypertensive Medication Use
| Methods of Identifying Who Should Undergo 24‐h Ambulatory Blood Pressure Monitoring | Net Reclassification Index (95% CI) | |
|---|---|---|
| Nocturnal Hypertension | Nondipping Systolic Blood Pressure | |
| Overall categorical net reclassification index | ||
| Clinic SBP/DBP ≥120/70 mm Hg | 0.29 (0.20 to 0.40) | 0.26 (0.18 to 0.34) |
| Clinic SBP/DBP ≥130/80 mm Hg | 0.23 (0.12 to 0.34) | 0.12 (0.03 to 0.21) |
| Clinic SBP/DBP ≥140/90 mm Hg | 0.24 (0.14 to 0.34) | 0.24 (0.15 to 0.33) |
| Antihypertensive medication use | 0.11 (0.02 to 0.19) | 0.25 (0.16 to 0.34) |
| Negative categorical net reclassification index | ||
| Clinic SBP/DBP ≥120/70 mm Hg | 0.41 (0.34 to 0.48) | 0.38 (0.32 to 0.44) |
| Clinic SBP/DBP ≥130/80 mm Hg | 0.02 (−0.06 to 0.09) | −0.03 (−0.09 to 0.02) |
| Clinic SBP/DBP ≥140/90 mm Hg | −0.25 (−0.31 to −0.19) | −0.26 (−0.32 to −0.22) |
| Antihypertensive medication use | −0.14 (−0.20 to −0.10) | −0.09 (−0.15 to −0.05) |
| Positive categorical net reclassification index | ||
| Clinic SBP/DBP ≥120/70 mm Hg | −0.12 (−0.19 to −0.04) | −0.12 (−0.17 to −0.06) |
| Clinic SBP/DBP ≥130/80 mm Hg | 0.21 (0.12 to 0.30) | 0.16 (0.08 to 0.24) |
| Clinic SBP/DBP ≥140/90 mm Hg | 0.49 (0.42 to 0.57) | 0.51 (0.43 to 0.58) |
| Antihypertensive medication use | 0.25 (0.19 to 0.32) | 0.34 (0.27 to 0.42) |
| Continuous net reclassification index | ||
| Models using SBP, DBP, and antihypertensive medication usea | 0.52 (0.35 to 0.69) | 0.51 (0.34 to 0.69) |
| Integrated discriminative improvement index | ||
| Models using SBP, DBP, and antihypertensive medication use | 0.10 (0.07 to 0.12) | 0.07 (0.04 to 0.09) |
Table values were computed using the validation data. For categorical net reclassification indexes, the probability cut points maximizing the Youden index for the predictive equations (0.34 and 0.43 for nocturnal hypertension and nondipping systolic blood pressure, respectively) were used. These cut points were chosen assuming that they provide better overall classification characteristics than the other 3 cut points. DBP indicates diastolic blood pressure; NRI, net reclassification improvement; SBP, systolic blood pressure.
Predicted probabilities were obtained from equations formed for nocturnal hypertension and nondipping systolic blood pressure, separately, using logistic regression in the derivation data set with clinic SBP and DBP and antihypertensive medication use as independent variables.
Deployment of the Predictive Equations
A website automating the application of predictive equations developed in this research is available at https://bcjaeger.shinyapps.io/DPE4NHTN_WebApp/. Source codes are available from the corresponding author's GitHub site (https://github.com/bcjaeger/DPE-for-NHTN-and-NDSBP). Written instructions to compute the predicted probability of nocturnal hypertension and nondipping SBP using the equations developed in the current study by hand are provided in Table S9.
Exploratory Analyses
Results from exploratory analyses are presented in Data S1 and Tables S10 through S13.
Discussion
In the current analysis, we developed predictive equations for nocturnal hypertension and nondipping SBP. For each equation, 4 probability cut points were selected on the basis of the equation's test characteristics in a derivation data set. Calibration of the predictive equations in a validation data set was acceptable, as indicated by a calibration slope plot, the Hosmer and Lemeshow goodness‐of‐fit test, and the Harrell unreliability test. The predictive equations demonstrated superior discrimination, as indicated by C‐statistics, the NRI, and the integrated discrimination improvement index in comparison to reference models using SBP and DBP measured in a clinic setting and antihypertensive medication use. In addition, using the 4 probability cut points from the derivation data, the predictive equations provided superior test characteristics in comparison to screening methods based on antihypertensive medication use and clinic‐measured SBP and DBP.
There were differences between participants in the JHS and CARDIA, IDH, and MHT studies with respect to age, race, and sex. These characteristics have been associated with nocturnal hypertension and nondipping SBP in prior studies.33, 34 The prediction equations developed in the current analysis account for these differences by incorporating these variables. Although sex is not included in the prediction equation for nocturnal hypertension, neck circumference and height, which have strong correlations with sex, are each included. Although the superior test characteristics of the prediction equations compared with screening methods based on SBP, DBP, and antihypertensive medication use may be attributed to the increased number of variables leveraged in the equations, the improved prognostic accuracy of the prediction equations suggests that their use in practice could substantially improve decisions related to ABPM screening.
High asleep BP and nondipping SBP have each been associated with an increased risk for cardiovascular disease events. In an analysis of the International Database of ABPM and Cardiovascular Outcomes, the hazard ratios for cardiovascular disease events associated with a 20–mm Hg increase in nighttime SBP and a 0.10 increase in night‐to‐day SBP ratio were 1.36 (95% CI, 1.30–1.43) and 1.14 (95% CI, 1.08–1.19), respectively, after multivariable adjustment.35
Recommendations on who to screen for nocturnal hypertension and nondipping SBP vary across guidelines. The 2018 European Society of Cardiology/European Society of Hypertension BP guideline recommends patients with sleep apnea, chronic kidney disease, diabetes mellitus, endocrine hypertension, or autonomic dysfunction undergo 24‐hour ABPM to screen for nocturnal hypertension and nondipping SBP. Results from the current study were consistent with these recommendations as the predictive equations we developed included variables related to chronic kidney disease (ie, log of 1 plus ACR). The 2017 American College of Cardiology/American Heart Association BP guideline does not provide specific recommendations on who to screen for nocturnal hypertension or nondipping SBP.36 However, the guideline mentions several areas of inquiry related to ABPM, including the importance of nocturnal hypertension.
The equations we developed can direct ABPM screening to patients who are most likely to have nocturnal hypertension and nondipping SBP, which can be helpful in both clinical and research settings. ABPM is recommended by the 2017 American College of Cardiology/American Heart Association BP guideline for >100 million US adults, but it is not widely implemented in the United States. Although home BP monitoring is an alternative to ABPM, it does not provide measurement of nocturnal BP. The equations developed in the current study could be used to identify nocturnal hypertension and nondipping SBP among patients using home BP monitoring. Also, these equations may be useful in research settings. Future studies may aim to enroll participants with nocturnal hypertension or nondipping SBP to evaluate interventions designed to lower nocturnal BP. Study investigators could use the predictive equations developed in the current analysis to identify participants with a high likelihood of having these phenotypes, and this in turn would reduce the cost and time needed for recruitment. As an illustrative example, 79% of participants in the validation data set with a predicted probability for nocturnal hypertension ≥0.65 had nocturnal hypertension, compared with 39.7% of all participants in this data set. If a study aimed to recruit 800 people with nocturnal hypertension from a population where the prevalence of nocturnal hypertension is 40% (rounded up from 39.7%), investigators would expect to conduct ABPM for ≈2000 adults (ie, 2000×0.4=800). However, if the investigators only conducted ABPM for adults with a predicted probability of nocturnal hypertension ≥0.65, they could expect to conduct ABPM on ≈1013 adults to identify 800 participants with nocturnal hypertension (ie, 1013×0.79≈800). These results illustrate one way in which the predictive equations developed in the current analysis could substantially increase the efficiency and decrease the cost of recruitment for future studies.
The current analysis has several strengths. The JHS and CARDIA, IDH, and MHT studies were conducted following standardized protocols that included rigorous procedures for data collection. The use of a validation data set provided an unbiased assessment of the predictive equations. The application of multiple performance metrics (eg, C‐statistic, Hosmer‐Lemeshow goodness‐of‐fit statistic, Brier scores, and categorical and continuous NRI) provided a comprehensive and robust analysis for the performance of the predictive equations. The development and deployment of a public website ensures that researchers can seamlessly incorporate these predictive equations into study recruitment protocols and validate the equations using an external data set. Results from the current analysis should be interpreted in the context of certain limitations. All ABPM data used in the current analysis were based on a single 24‐hour monitoring period. Although the reproducibility of nocturnal hypertension is moderately high, it is lower for nondipping SBP.37, 38, 39 Some variables that may be associated with nocturnal hypertension or nondipping (eg, glycated hemoglobin) were not measured in all of the studies and, therefore, were not considered as candidates for the predictive equations.
In conclusion, we developed predictive equations that can be used to identify who to screen for nocturnal hypertension and nondipping SBP. These equations outperformed screening methods based on antihypertensive medication use and SBP and DBP measured in a clinic setting. We have developed publicly available tools for the application of these predictive equations. Application of the predictive equations may increase the efficiency and decrease the cost of ABPM screening for nocturnal hypertension and nondipping SBP.
Sources of Funding
The JHS (Jackson Heart Study) is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSNs268201300048C, HHSN268201300049C, and HHSN268201300050C from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute on Minority Health and Health Disparities. The CARDIA (Coronary Artery Risk Development in Young Adults) study is conducted and supported by the NHLBI in collaboration with the University of Alabama at Birmingham (HHSN268201800005I and HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). The funding to conduct ambulatory blood pressure monitoring in the CARDIA study was provided by grant 15SFRN2390002 from the American Heart Association. This article has been reviewed by the CARDIA study for scientific content. The IDH (Improving the Detection of Hypertension) and the MHT (Masked Hypertension) studies were supported by a program project grant from the NHLBI (PO1‐HL047540). Drs Jaeger, Booth, Schwartz, Shimbo, Shikany, and Muntner receive support through 15SFRN2390002 from the American Heart Association. Dr Shimbo receives support from R01HL137818 and K24‐HL125704 from the NHLBI at the National Institutes of Health (NIH). Drs Edwards, Shimbo, and Muntner receive support from R01HL117323 from the NHLBI at the NIH. Drs Jaeger, Yano, and Muntner receive support from R01HL144773 from the NHLBI at the NIH.
Disclosures
Dr Muntner receives research grant support from Amgen Inc, unrelated to the current article. Dr Booth is currently employed by CTI Clinical Trials and Consulting Services, which followed the completion of this article. As a group, we have no other conflicts of interest to report.
Supporting information
Data S1.
Table S1. Description of Candidate Variables in the Jackson Heart, Coronary Artery Risk Development in Young Adults, Improving the Detection of Hypertension, and Masked Hypertension Studies
Table S2. Characteristics of Participants in the Coronary Artery Risk Development In Young Adults (CARDIA) Study Stratified by Inclusion in the Current Analysis
Table S3. Characteristics of Participants in the Jackson Heart Study (JHS) Stratified by Inclusion in the Current Analysis
Table S4. Age, Sex, and Prevalence of Nocturnal Blood Pressure Phenotypes Stratified by Study
Table S5. Bootstrapped Means of Performance Metrics and Overall Ranks of Competing Modeling Algorithms for Prediction of Nocturnal Hypertension and Non‐Dipping Systolic Blood Pressure
Table S6. Proportions of Bootstrap Replicates Where Candidate Variables Were Selected for Inclusion in Predictive Equations for Nocturnal Hypertension
Table S7. Proportions of Bootstrap Replicates Where Candidate Variables Were Selected for Inclusion in Predictive Equations for Non‐Dipping Systolic Blood Pressure
Table S8. Calibration and Discrimination of Predictive Equations for Nocturnal Hypertension and Non‐Dipping Systolic Blood Pressure Overall and in Sub‐Groups Determined by Race, Sex, and Antihypertensive Medication Use
Table S9. Predictive Equations for Nocturnal Hypertension and Non‐Dipping Systolic Blood Pressure
Table S10. Odds Ratios for Variables Selected for Inclusion in the Predictive Equations for Non‐Dipping Diastolic Blood Pressure
Table S11. Calibration and Discrimination of Predictive Equations for Non‐Dipping Diastolic Blood Pressure Overall and in Sub‐Groups Determined by Race, Sex, and Antihypertensive Medication Use
Table S12. Test Characteristics of the Predictive Equations for Non‐Dipping Diastolic Blood Pressure Versus Alternative Screening Methods for Identifying Adults With a High Probability of Non‐Dipping Diastolic Blood Pressure
Table S13. Net Reclassification Improvement and Integrated Discriminative Improvement Using a Predictive Equation for Non‐Dipping Diastolic Blood Pressure Versus Screening Methods Based on Clinic Blood Pressure and Antihypertensive Medication Use
Figure S1. Inclusion cascade of participants from four studies that contributed data to the current analysis.
Figure S2. Predicted probability of nocturnal hypertension (top panels) and non‐dipping systolic blood pressure (bottom panels) according to non‐linear variables in the predictive equations.
Acknowledgments
The authors would like to thank the participants in the CARDIA (Coronary Artery Risk Development in Young Adults) study, JHS (Jackson Heart Study), IDH (Improving the Detection of Hypertension) study, and MHT (Masked Hypertension) study who volunteered to undergo 24‐hour ambulatory blood pressure monitoring.
(J Am Heart Assoc. 2020;9:e013696 DOI: 10.1161/JAHA.119.013696.)
References
- 1. Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, Porcellati C. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990;81:528–536. [DOI] [PubMed] [Google Scholar]
- 2. O'Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, De La Sierra A, De Leeuw P, Dolan E. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. [DOI] [PubMed] [Google Scholar]
- 3. Friedman O, Logan AG. Can nocturnal hypertension predict cardiovascular risk? Integr Blood Press Control. 2009;2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fan H‐Q, Li Y, Thijs L, Hansen TW, Boggia J, Kikuya M, Björklund‐Bodegård K, Richart T, Ohkubo T, Jeppesen J. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J Hypertens. 2010;28:2036–2045. [DOI] [PubMed] [Google Scholar]
- 5. Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A, Santucci C, Reboldi G. Ambulatory blood pressure: an independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. [DOI] [PubMed] [Google Scholar]
- 6. Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Björklund‐Bodegård K, Richart T, Ohkubo T, Kuznetsova T, Torp‐Pedersen C, Lind L, Ibsen H, Imai Y, Wang J, Sandoya E, O'Brien E, Staessen JA; International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes (IDACO) Investigators . Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219–1229. [DOI] [PubMed] [Google Scholar]
- 7. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 8. Effect of intense vs. standard hypertension management on nighttime blood pressure—an ancillary study to SPRINT—full text view—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01835249. Accessed December 13, 2018.
- 9. Booth JN, Muntner P, Diaz KM, Viera AJ, Bello NA, Schwartz JE, Shimbo D. Evaluation of criteria to detect masked hypertension. J Clin Hypertens (Greenwich). 2016;18:1086–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kronish IM, Kent S, Moise N, Shimbo D, Safford MM, Kynerd RE, O'Beirne R, Sullivan A, Muntner P. Barriers to conducting ambulatory and home blood pressure monitoring during hypertension screening in the United States. J Am Soc Hypertens. 2017;11:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thijs L, Hansen TW, Kikuya M, Björklund‐Bodegård K, Li Y, Dolan E, Tikhonoff V, Seidlerová J, Kuznetsova T, Stolarz K, Bianchi M, Richart T, Casiglia E, Malyutina S, Filipovsky J, Kawecka‐Jaszcz K, Nikitin Y, Ohkubo T, Sandoya E, Wang J, Torp‐Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA, O'Brien E; IDACO Investigators . The International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit. 2007;12:255–262. [DOI] [PubMed] [Google Scholar]
- 12. Bromfield SG, Booth JN, Loop MS, Schwartz JE, Seals SR, Thomas SJ, Min Y‐I, Ogedegbe G, Shimbo D, Muntner P. Evaluating different criteria for defining a complete ambulatory blood pressure monitoring recording: data from the Jackson Heart Study. Blood Press Monit. 2018;23:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor HA Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15:S6‐4‐17. [PubMed] [Google Scholar]
- 14. Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz JE, Burg MM, Shimbo D, Broderick JE, Stone AA, Ishikawa J, Sloan R, Yurgel T, Grossman S, Pickering TG. Clinic blood pressure underestimates ambulatory blood pressure in an untreated employer‐based US population clinical perspective: results from the Masked Hypertension Study. Circulation. 2016;134:1794–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Brien E, Mee F, Atkins N, O'Malley K. Accuracy of the SpaceLabs 90207 determined by the British Hypertension Society protocol. J Hypertens. 1991;9:S25–S31. [DOI] [PubMed] [Google Scholar]
- 17. de Greef A, Shannan AH. Validation of Spacelabs 90227 OnTrak upper arm blood pressure monitor, for clinical use, according to the European Society of Hypertension International Protocol 2010 and the British Hypertension Society Protocol. Available at: http://www.dableducational.org/Publications/2014/ESH-IP%202010%20and%20BHS%20Validation%20of%20Spascelabs%2090227%20OnTrak.pdf. Accessed June 17, 2018.
- 18. Jackson Heart Study protocol, manual 4, blood pressure, visit 1 (version 1.0). 2001. https://www.jacksonheartstudy.org/Portals/0/pdf/manuals1/Blood_pressure_manual4_02-18-2001(1).pdf. Accessed February 5, 2018.
- 19. Profant J, Dimsdale JE. Race and diurnal blood pressure patterns: a review and meta‐analysis. Hypertension. 1999;33:1099–1104. [DOI] [PubMed] [Google Scholar]
- 20. Birkenhager AM, Van den Meiracker AH. Causes and consequences of a non‐dipping blood pressure profile. Neth J Med. 2007;65:127–131. [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yadlowsky S, Hayward RA, Sussman JB, McClelland RL, Min Y‐I, Basu S. Clinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Ann Intern Med. 2018;169:20–29. [DOI] [PubMed] [Google Scholar]
- 23. Friedman J, Hastie T, Tibshirani R. The Elements of Statistical Learning. New York, NY: Springer Series in Statistics; 2001. [Google Scholar]
- 24. Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35:1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 26. Harrell FE. Regression Modeling Strategies With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Second. New York, NY: Springer; 2015. [Google Scholar]
- 27. Harrell FE Jr. rms: Regression Modeling Strategies: R Package Version 4.0‐0. Comprehensive R Archive Network (CRAN). Available at: https://CRAN.R-project.org/package=rms, 2013. [Google Scholar]
- 28. Pencina MJ, D'Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. [DOI] [PubMed] [Google Scholar]
- 29. Pencina MJ, D'Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pencina MJ, D'Agostino RB Sr, Demler OV. Novel metrics for evaluating improvement in discrimination: net reclassification and integrated discrimination improvement for normal variables and nested models. Stat Med. 2012;31:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leening MJ, Vedder MM, Witteman JC, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician's guide. Ann Intern Med. 2014;160:122–131. [DOI] [PubMed] [Google Scholar]
- 32. Shah AD, Bartlett JW, Carpenter J, Nicholas O, Hemingway H. Comparison of random forest and parametric imputation models for imputing missing data using MICE: a CALIBER study. Am J Epidemiol. 2014;179:764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Booth JN, Anstey DE, Bello NA, Jaeger BC, Pugliese DN, Thomas SJ, Deng L, Shikany JM, Lloyd‐Jones D, Schwartz JE. Race and sex differences in asleep blood pressure: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Clin Hypertens (Greenwich). 2019;21:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kario K. Nocturnal hypertension: new technology and evidence. Hypertension. 2018;71:997–1009. [DOI] [PubMed] [Google Scholar]
- 35. Yang W‐Y, Melgarejo JD, Thijs L, Zhang Z‐Y, Boggia J, Wei F‐F, Hansen TW, Asayama K, Ohkubo T, Jeppesen J. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. 2019;322:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 37. Abdalla M, Goldsmith J, Muntner P, Diaz KM, Reynolds K, Schwartz JE, Shimbo D. Is isolated nocturnal hypertension a reproducible phenotype? Am J Hypertens. 2015;29:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cuspidi C, Meani S, Salerno M, Valerio C, Fusi V, Severgnini B, Lonati L, Magrini F, Zanchetti A. Reproducibility of nocturnal blood pressure fall in early phases of untreated essential hypertension: a prospective observational study. J Hum Hypertens. 2004;18:503. [DOI] [PubMed] [Google Scholar]
- 39. Palatini P, Mormino P, Canali C, Santonastaso M, De Venuto G, Zanata G, Pessina AC. Factors affecting ambulatory blood pressure reproducibility: results of the HARVEST Trial: Hypertension and Ambulatory Recording Venetia Study. Hypertension. 1994;23:211–216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Table S1. Description of Candidate Variables in the Jackson Heart, Coronary Artery Risk Development in Young Adults, Improving the Detection of Hypertension, and Masked Hypertension Studies
Table S2. Characteristics of Participants in the Coronary Artery Risk Development In Young Adults (CARDIA) Study Stratified by Inclusion in the Current Analysis
Table S3. Characteristics of Participants in the Jackson Heart Study (JHS) Stratified by Inclusion in the Current Analysis
Table S4. Age, Sex, and Prevalence of Nocturnal Blood Pressure Phenotypes Stratified by Study
Table S5. Bootstrapped Means of Performance Metrics and Overall Ranks of Competing Modeling Algorithms for Prediction of Nocturnal Hypertension and Non‐Dipping Systolic Blood Pressure
Table S6. Proportions of Bootstrap Replicates Where Candidate Variables Were Selected for Inclusion in Predictive Equations for Nocturnal Hypertension
Table S7. Proportions of Bootstrap Replicates Where Candidate Variables Were Selected for Inclusion in Predictive Equations for Non‐Dipping Systolic Blood Pressure
Table S8. Calibration and Discrimination of Predictive Equations for Nocturnal Hypertension and Non‐Dipping Systolic Blood Pressure Overall and in Sub‐Groups Determined by Race, Sex, and Antihypertensive Medication Use
Table S9. Predictive Equations for Nocturnal Hypertension and Non‐Dipping Systolic Blood Pressure
Table S10. Odds Ratios for Variables Selected for Inclusion in the Predictive Equations for Non‐Dipping Diastolic Blood Pressure
Table S11. Calibration and Discrimination of Predictive Equations for Non‐Dipping Diastolic Blood Pressure Overall and in Sub‐Groups Determined by Race, Sex, and Antihypertensive Medication Use
Table S12. Test Characteristics of the Predictive Equations for Non‐Dipping Diastolic Blood Pressure Versus Alternative Screening Methods for Identifying Adults With a High Probability of Non‐Dipping Diastolic Blood Pressure
Table S13. Net Reclassification Improvement and Integrated Discriminative Improvement Using a Predictive Equation for Non‐Dipping Diastolic Blood Pressure Versus Screening Methods Based on Clinic Blood Pressure and Antihypertensive Medication Use
Figure S1. Inclusion cascade of participants from four studies that contributed data to the current analysis.
Figure S2. Predicted probability of nocturnal hypertension (top panels) and non‐dipping systolic blood pressure (bottom panels) according to non‐linear variables in the predictive equations.


