Abstract
Background
There is a paucity of contemporary data estimating the incidence of major adverse cardiovascular events (MACE) in patients with established atherosclerotic disease or multiple risk factors managed in routine practice. We estimated 1‐ and 4‐year incidences of MACE and the association between MACE and vascular beds affected in these patients.
Methods and Results
Using US IBM MarketScan data from January 1, 2013 to December 31, 2017, we identified patients ≥45 years old with established coronary artery disease, cerebrovascular disease, peripheral artery disease, or the presence of ≥3 risk factors for atherosclerosis during 2013 with a minimum of 4 years of follow‐up. We calculated 1‐ and 4‐year incidences of MACE (cardiovascular death or hospitalization for myocardial infarction or ischemic stroke). A Cox proportional hazards regression model adjusted for age and sex was used to evaluate the association between vascular bed number/location(s) affected and MACE. We identified 1 302 856 patients with established atherosclerotic disease or risk factors for atherosclerosis. Coronary artery disease was present in 16.9% of patients, cerebrovascular disease in 7.6%, peripheral artery disease in 13.6%, and risk factors for atherosclerosis only in 66.0%. The 1‐ and 4‐year incidences of MACE were 1.4% and 6.9%, respectively. At 4 years, MACE was more frequent in patients with atherosclerotic disease in a single (hazard ratio=1.51, 95% CI=1.48–1.55), 2‐(hazard ratio=2.35, 95% CI=2.27–2.44), or all 3 vascular beds (hazard ratio=3.30, 95% CI=2.97–3.68) compared with having risk factors for atherosclerosis.
Conclusions
Patients with established atherosclerotic disease or who have multiple risk factors and are treated in contemporary, routine practice carry a substantial risk for MACE at 1‐ and 4‐ years of follow‐up. MACE risk was shown to vary based on the number and location of vascular beds involved.
Keywords: cerebrovascular disease, coronary artery disease, established atherosclerotic disease, major adverse cardiovascular events, peripheral artery disease, risk factors
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors
Clinical Perspective
What Is New?
Incidences of major adverse cardiovascular events at 1 and 4 years were provided for a contemporary at‐risk population.
Major adverse cardiovascular events incidence increased considerably over time and when multiple vascular beds were involved.
What Are the Clinical Implications?
These findings may serve as a benchmark for both clinicians and other health professionals when making statements about major adverse cardiovascular events rates in a contemporary population, and when designing future clinical studies.
There is a need for continued improvement in the prevention and treatment of patients with or at risk of atherosclerosis.
Introduction
Cardiovascular disease is one of the leading causes of mortality in the United States.1 Patients with atherosclerosis are at an elevated risk of major adverse cardiovascular events (MACE). Understanding the incidence rates and comparative risk of MACE based on atherosclerotic disease characteristics may greatly aid efforts to better treat such patients.2
The REACH (Reduction of Atherothrombosis for Continued Health) registry provided much‐‐needed insight into clinical event rates of patients at risk of, or with, established atherosclerotic disease. Results from the 1‐ and 4‐year REACH analyses demonstrated that MACE incidence increased over time, with the proportion experiencing MACE surpassing 14% at 4 years of follow‐up.2, 3 Several predictors of future atherosclerotic events were also identified in REACH, including polyvascular disease.3 However, the REACH cohort was enrolled between 2003 and 2004 and followed patients up to 2008. Advances in treatment and prevention of atherosclerotic diseases and changes to evidence‐based guidelines over the past decade have had a positive impact on MACE risk.4 Furthermore, there was likely an important degree of selection bias in the enrollment of REACH patients resulting in a final study cohort not fully representative of the types of atherosclerotic disease seen in daily practice.3
This study aimed to provide benchmark estimates for the incidence of MACE in US patients at risk of, or with, established atherosclerotic disease in contemporary routine practice. We also sought to assess the association between the number and type of vascular beds affected and MACE risk.
Methods
Data used in this study were obtained from IBM MarketScan under a license to Bayer AG (and provided to the researchers under a third‐party agreement) and cannot be shared.
We performed a retrospective claims database analysis using US IBM MarketScan data from January 1, 2013 through December 31, 2017. IBM MarketScan combines 2 separate databases, a commercial and a Medicare supplemental database, to cover all age groups, and contains claims from 260 contributing employers, 40 health plans, and government and public organizations representing ≈240 million lives.5 IBM MarketScan captures enrollment records, demographics, International Classification of Diseases, Tenth Revision (ICD‐10) diagnosis codes (and cross‐walked ICD‐9 codes), procedure codes, admission and discharge dates, outpatient medical services data, and prescription dispensing records. All MarketScan data are de‐identified and are compliant with the Health Insurance Portability and Accountability Act of 1996. This study was determined by our institutional review board to not constitute research involving human subjects according to 45 Code of Federal Regulations 46.102(f) and was deemed exempt from board oversight.
We identified eligible patients according to similar criteria used in the REACH registry by examining claims data in calendar year 2013 (January 1, 2013 through December 31, 2013). All patients ≥45 years of age with established coronary artery disease (CAD) (ie, diagnosis codes suggesting a history of stable6 or unstable angina,7 percutaneous coronary intervention,8, 9 coronary artery bypass grafting,8, 10 or myocardial infarction10), cerebrovascular disease (CVD) (ie, diagnosis codes suggesting a history of ischemic stroke or transient ischemic attack11) or peripheral artery disease (PAD) (ie, diagnosis codes suggesting a history of peripheral arterial disease9, 12 or a prior intervention including angioplasty, stenting, atherectomy, peripheral arterial bypass grafting or amputations9, 10) or with 3 or more risk factors for atherosclerosis (ie, diagnosis codes suggesting a history of diabetes mellitus,13 diabetic nephropathy,9, 13 carotid stenosis,14 hypertension,13 hypercholesterolemia,13 smoking,14 or age ≥65 years for men or ≥70 years for women) were included (Table S1).
Our primary outcome measure was the incidence of MACE (composite of cardiovascular death, myocardial infarction, or ischemic stroke). Secondary outcomes include the incidence of individual MACE components. Myocardial infarction and ischemic stroke were defined as the occurrence of a hospitalization with the appropriate billing codes in the primary position. Cardiovascular death was defined as death occurring in the hospital within 14 days of a myocardial infarction, ischemic stroke, heart failure,13 acute coronary syndrome,7 coronary artery bypass grafting, or percutaneous coronary intervention. Baseline data included demographics, vascular disease status, atherothrombotic risk factors, and medications. Patient selection and identification of baseline characteristics were based on the presence of ICD‐10 (or cross‐walked ICD‐9) billing codes from medical and/or prescription claims. Starting on January 1, 2014, patients who met eligibility criteria during calendar year 2013 were followed for 1 and 4 years (patients with at least 9 months of follow‐up were included in the 1‐year analysis and with at least 3 years and 9 months of follow‐up were included in the 4‐year analysis) or until MACE occurrence.
Baseline characteristics were analyzed using descriptive statistics. Categorical data were reported as percentages and continuous data as medians with accompanying 25%, 75% ranges. Outcomes were reported as cumulative incidences (proportion of patients experiencing an event) and incidence rates (events/100 person‐years [PYs]). A multivariable Cox proportional hazards regression model adjusted for age and sex were utilized to evaluate the association between the number and different vascular bed locations and MACE rates (model #1). The proportional hazards assumption was tested based on Schoenfeld residuals and was found to be valid for all outcomes. An additional multivariable regression analysis in which we adjusted for age, sex as well as additional risk factors and medications was also performed (model #2). Associations were reported as adjusted hazard ratios with 95% CIs. We performed a sensitivity analysis in which we evaluated an alternative MACE end point that added hospitalization for vascular events or procedures (ie, heart failure, coronary artery bypass graft, percutaneous coronary intervention, acute coronary syndrome, and major adverse limb events). All data management and statistical analysis were performed using IBM SPSS Statistics version 25.0 (IBM Corp., Armonk, NY) and SAS 9.4 (SAS Institute Inc, Cary, NC).
Results
Baseline Characteristics
We identified 1 302 856 patients with established atherosclerotic disease or ≥3 risk factors, of whom 1 220 144 (94%) were eligible for inclusion in the 1‐year analysis and 625 951 (48%) were eligible for the 4‐year analysis (Figure 1). Eligible patients had a median (25%, 75% range) age of 69 years (59, 77) and 46.6% were women (Table 1). Patients with established disease comprised 34.0% of our total eligible population, with the remainder limited to having 3 or more risk factors for atherosclerosis only. Among patients with established atherosclerotic disease, 49.9% had CAD, 22.4% had CVD, and 40.0% had PAD involvement. Most patients had hypertension (95.7%) and hypercholesterolemia (83.0%) and diabetes mellitus was present in >50% of patients. Approximately two thirds of patients received an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, three quarters received a statin, ≈14% a P2Y12, and about half were taking a β‐blocker at baseline.
Figure 1.
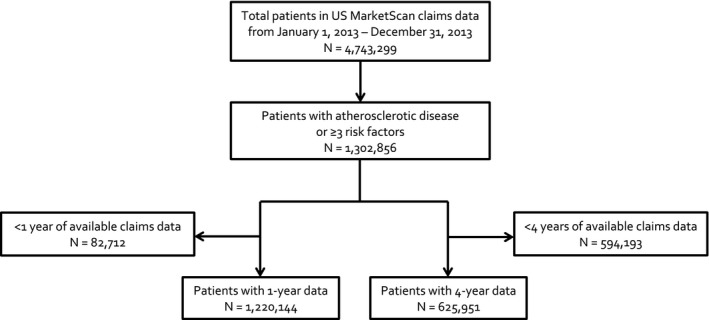
Flow diagram of patient selection.
Table 1.
Baseline Characteristics of Individuals Evaluated at 1 Year of Follow‐Up
| All | Total Established Disease | Any CADa | Any CVDa | Any PADa | Multiple Risk Factors Only | |
|---|---|---|---|---|---|---|
| N=1 220 144 | N=414 718 | N=206 804 | N=92 695 | N=165 967 | N=805 426 | |
| Demographics, n (%) | ||||||
| Age, y (median, 25%, 75% range) | 69 (59, 77) | 68 (58, 77) | 66 (57, 76) | 70 (60, 79) | 70 (60, 79) | 69 (59, 77) |
| 45–64 | 482 682 (39.6) | 177 817 (42.9) | 97 413 (47.1) | 33 921 (36.6) | 61 874 (37.3) | 304 865 (37.9) |
| 65–74 | 347 443 (28.5) | 102 711 (24.8) | 51 626 (25.0) | 22 772 (24.6) | 42 786 (25.8) | 244 732 (30.4) |
| 75–84 | 301 033 (24.7) | 98 639 (23.8) | 44 877 (21.7) | 25 978 (28.0) | 43 528 (26.2) | 202 394 (25.1) |
| ≥85 | 88 986 (7.3) | 35 551 (8.6) | 12 888 (6.2) | 10 024 (10.8) | 17 779 (10.7) | 53 435 (6.6) |
| Females | 568 449 (46.6) | 182 121 (43.9) | 75 582 (36.5) | 48 838 (52.7) | 78 008 (47.0) | 386 328 (48.0) |
| Vascular disease status, n (%) | ||||||
| CAD | 206 804 (16.9) | 206 804 (49.9) | 206 804 (100) | 14 372 (15.5) | 26 593 (16.0) | 0 (0) |
| CVD | 92 695 (7.6) | 92 695 (22.4) | 14 372 (6.9) | 92 695 (100) | 12 892 (7.8) | 0 (0) |
| PAD | 165 967 (13.6) | 165 967 (40.0) | 26 593 (12.9) | 12 892 (13.9) | 165 967 (100) | 0 (0) |
| Polyvascular disease | 47 639 (11.5) | 47 639 (11.5) | 37 856 (18.3) | 24 155 (26.1) | 36 376 (21.9) | 0 (0) |
| Risk factors only | 805 426 (66.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 805 426 (100) |
| CAD alone | 168 948 (13.8) | 168 948 (40.7) | 168 948 (81.7) | 0 (0) | 0 (0) | 0 (0) |
| CVD alone | 68 540 (5.6) | 68 540 (16.5) | 0 (0) | 68 540 (73.9) | 0 (0) | 0 (0) |
| PAD alone | 129 591 (10.6) | 129 591 (31.2) | 0 (0) | 0 (0) | 129 591 (78.1) | 0 (0) |
| CAD+CVD | 11 263 (0.9) | 11 263 (2.7) | 11 263 (5.4) | 11 263 (12.2) | 0 (0) | 0 (0) |
| CAD+PAD | 23 484 (1.9) | 23 484 (5.7) | 23 484 (11.4) | 0 (0) | 23 484 (14.1) | 0 (0) |
| CVD+PAD | 9783 (0.8) | 9783 (2.4) | 0 (0) | 9783 (10.6) | 9783 (5.9) | 0 (0) |
| CAD+CVD+PAD | 3109 (0.3) | 3109 (0.7) | 3109 (1.5) | 3109 (3.4) | 3109 (1.9) | 0 (0) |
| Risk factors, n (%) | ||||||
| Diabetes mellitus | 664 852 (54.5) | 158 985 (38.3) | 78 617 (38.0) | 34 435 (37.1) | 70 941 (42.7) | 505 867 (62.8) |
| Diabetic nephropathy | 35 461 (2.9) | 8745 (2.1) | 4353 (2.1) | 1847 (2.0) | 4258 (2.6) | 26 716 (3.3) |
| Carotid stenosis | 119 502 (9.8) | 61 549 (14.8) | 25 793 (12.5) | 22 576 (24.4) | 28 499 (17.2) | 57 953 (7.2) |
| Hypertension with treatment | 1 167 903 (95.7) | 372 716 (89.9) | 193 102 (93.4) | 83 534 (90.1) | 144 929 (87.3) | 795 187 (98.7) |
| Hypercholesterolemia with treatment | 1 012 856 (83.0) | 287 540 (69.3) | 159 801 (77.3) | 62 524 (67.5) | 104 989 (63.3) | 725 316 (90.1) |
| Smoker | 82 309 (6.7) | 30 172 (7.3) | 15 909 (7.7) | 6246 (6.7) | 13 131 (7.9) | 52 137 (6.5) |
| Age ≥70 y in females or ≥65 y in males | 692 709 (56.8) | 217 950 (52.6) | 101 416 (49.0) | 53 959 (58.2) | 95 744 (57.7) | 474 759 (58.9) |
| Medication use, n (%) | ||||||
| ACE/ARB | 819 305 (67.1) | 238 925 (57.6) | 126 245 (61.0) | 52 377 (56.5) | 92 405 (55.7) | 580 380 (72.1) |
| β‐blockers | 603 103 (49.4) | 229 397 (46.4) | 140 479 (67.9) | 45 618 (49.2) | 78 324 (47.2) | 373 706 (46.4) |
| Calcium channel blockers | 388 394 (31.8) | 125 793 (30.3) | 60 270 (29.1) | 31 832 (34.3) | 52 535 (31.7) | 262 601 (32.6) |
| Diuretics | 541 473 (44.4) | 167 789 (40.5) | 83 814 (40.5) | 37 480 (40.4) | 71 110 (42.8) | 373 684 (48.4) |
| Antidiabetic agents | 502 417 (41.2) | 112 629 (27.2) | 56 698 (27.4) | 22 981 (24.8) | 50 095 (30.2) | 389 788 (48.4) |
| Statin | 958 703 (78.6) | 275 428 (66.4) | 154 072 (74.5) | 59 954 (64.7) | 99 705 (60.1) | 683 275 (84.8) |
| P2Y12 inhibitors | 172 802 (14.2) | 111 640 (26.9) | 75 184 (36.4) | 24 181 (26.1) | 33 998 (20.5) | 61 162 (7.6) |
| Oral anticoagulants | 137 180 (11.2) | 58 432 (14.1) | 29 058 (14.1) | 17 521 (18.9) | 22 271 (13.4) | 78 748 (9.8) |
| NSAIDs including COX‐2 inhibitors | 261 459 (21.4) | 84 030 (20.3) | 41 413 (20.0) | 18 492 (19.9) | 33 427 (20.1) | 177 429 (22.0) |
ACE/ARB indicates angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker; CAD, coronary artery disease; COX‐2, cyclo‐oxygenase‐2; CVD, cerebrovascular disease; NSAIDs, nonsteroidal anti‐inflammatory drugs; PAD, peripheral artery disease.
These cohorts overlap each other.
1‐Year Outcomes
MACE occurred in 1.4% (1.47 events/100 PYs) of all patients at 1 year with 2.1% (2.12 events/100 PYs) in patients with established disease and 1.1% (1.14 events/100 PYs) in patients with multiple risk factors (Figure 2). MACE events were mainly driven by hospitalization for myocardial infarction (0.8%, 0.77 events/100 PYs), followed by ischemic stroke (0.6%, 0.64 events/100 PYs), and cardiovascular death (0.2%, 0.16 events/100 PYs). Among patients with established disease, MACE incidences were highest in patients with CVD (3.0%), followed by CAD (2.1%) and PAD (2.0%) involvement. Upon sensitivity analysis our alternative MACE end point found higher rates of MACE because of a 2.9% (2.99 events/100 PYs) incidence of hospitalization for additional vascular events or procedures (Figure 3). Cumulative incidence curves for each subgroup by vascular bed are shown in Figure 4.
Figure 2.
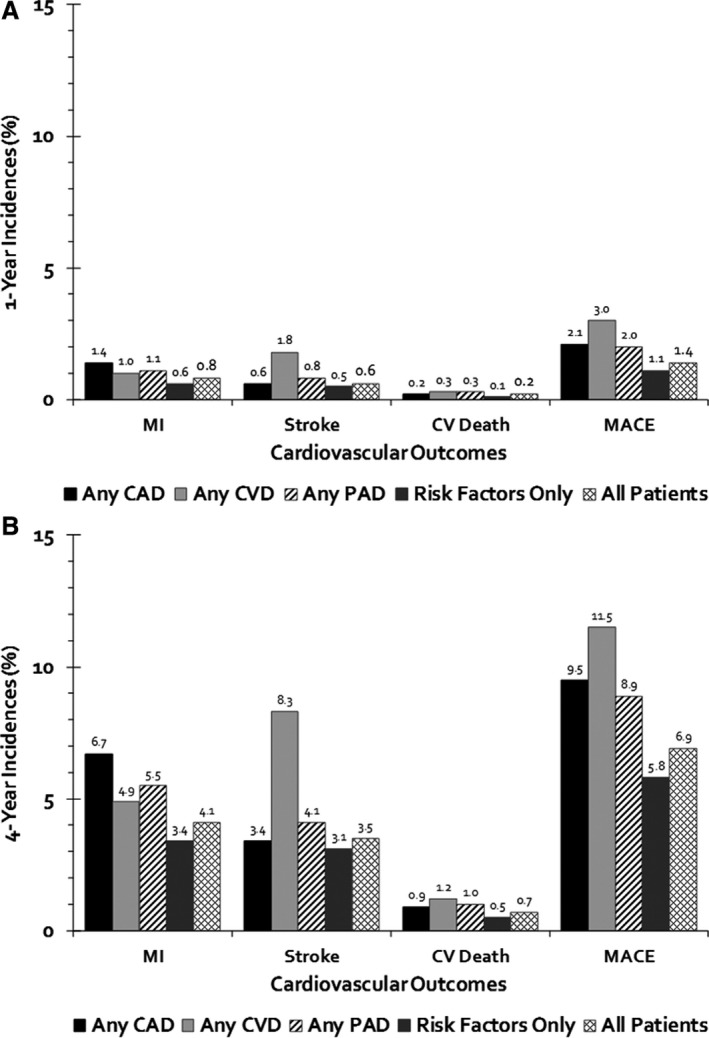
Incidence of MACE of individuals evaluated at 1 (A) and 4 years (B) of follow‐up. CAD indicates coronary artery disease; CV, cardiovascular; CVD, cerebrovascular disease; MACE, major adverse cardiovascular event; MI, myocardial infarction; PAD, peripheral artery disease.
Figure 3.
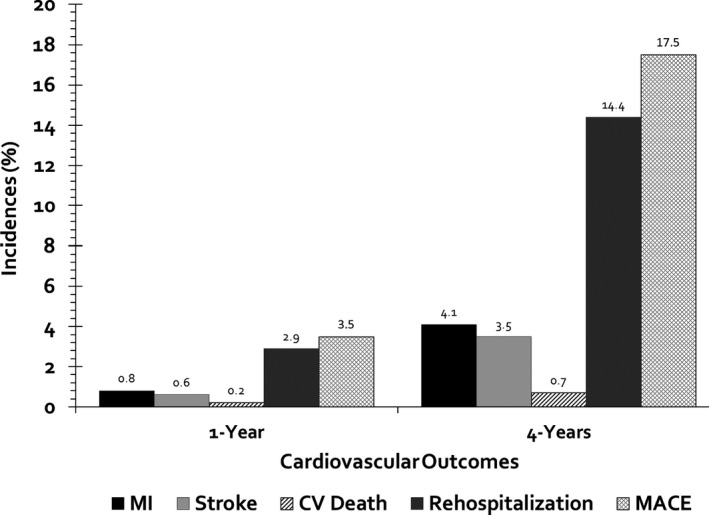
Incidence of major adverse cardiovascular events including hospitalization for vascular events or procedures at 1 and 4 years of follow‐up. CV indicates cardiovascular; MACE, major adverse cardiovascular event; MI, myocardial infarction.
Figure 4.
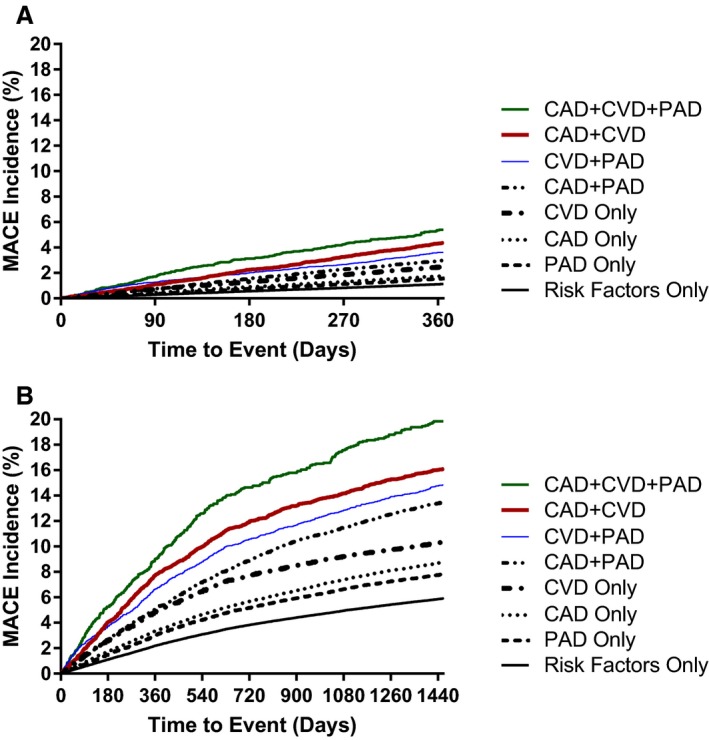
Cumulative incidence of MACE of individuals evaluated at 1 (A) and 4 years (B) of follow‐up by vascular beds. CAD indicates coronary artery disease; CVD, cerebrovascular disease; MACE, major adverse cardiovascular event; PAD, peripheral artery disease.
Multivariable Cox regression analyses adjusted for age and sex (model #1) suggested that compared with patients with risk factors only, established atherosclerotic disease in a single vascular bed was associated with a 68% increased risk of MACE (ranging from 38% for PAD to 121% for CVD) (Figure 5, Table 2). Results of multivariable regression adjusting for age, sex, and additional risk factors and medications (model #2) provided results similar to model #1. Involvement of 2 vascular beds was associated with an increased MACE risk of 186% (ranging from 152% for PAD+CAD to 256% for CAD+CVD). The presence of disease in all 3 vascular beds was associated with a 338% increased risk of MACE risk versus having ≥3 risk factors only. The types of MACE experienced in the CAD, CVD, PAD, and multiple risk factor cohorts are shown in Table 3.
Figure 5.
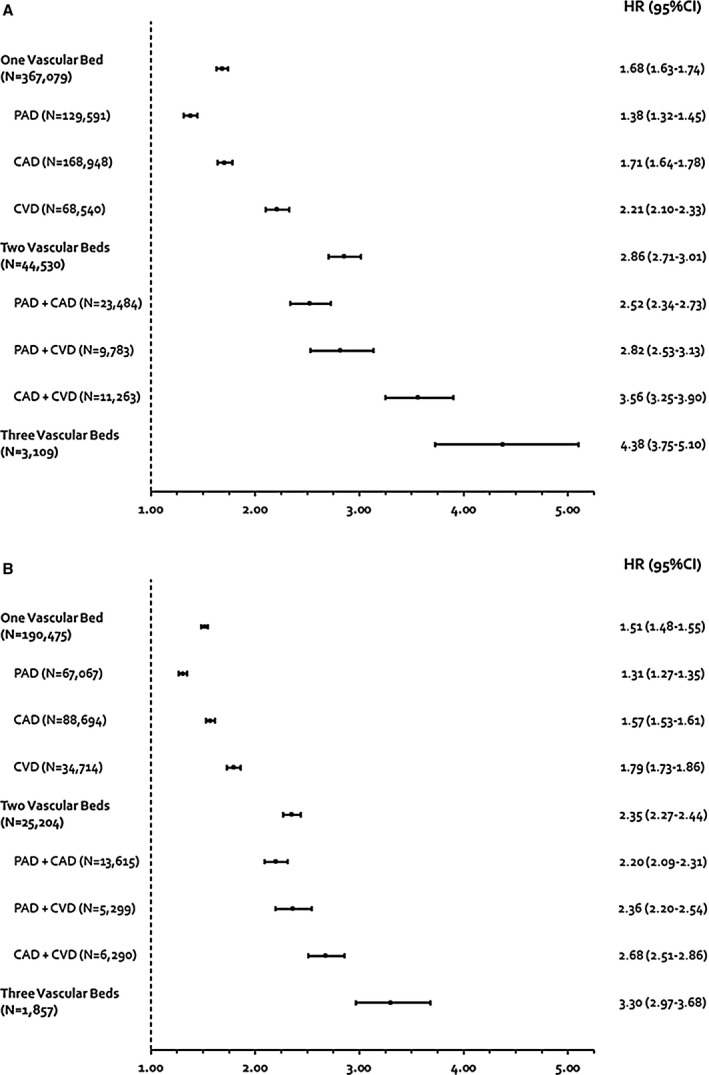
Relative hazard of MACE of individuals evaluated at 1 (A) and 4 years (B) of follow‐up by vascular beds. CAD indicates coronary artery disease; CVD, cerebrovascular disease; HR, hazard ratio; PAD, peripheral artery disease.
Table 2.
Cox Regression Models of MACE at 1‐ and 4 Years of Follow‐Up
| Variables | 1‐Year of Follow‐Up | 4 Years of Follow‐Up | ||
|---|---|---|---|---|
| Model #1 HR (95% CI) | Model #2 HR (95% CI) | Model #1 HR (95% CI) | Model #2 HR (95% CI) | |
| Age, y | ||||
| 45–64 | 1.44 (1.38–1.50) | 1.47 (1.41–1.54) | 1.32 (1.28–1.35) | 1.35 (1.31–1.38) |
| 75–84 | 2.17 (2.08–2.25) | 2.26 (2.16–2.35) | 2.15 (2.10–2.21) | 2.24 (2.18–2.30) |
| ≥85 | 3.50 (3.33–3.67) | 3.71 (3.53–3.91) | 3.71 (3.59–3.83) | 3.92 (3.79–4.06) |
| Female | 0.80 (0.78–0.83) | 0.81 (0.79–0.84) | 0.79 (0.78–0.81) | 0.80 (0.79–0.82) |
| Vascular beds (RFO as referent) | ||||
| CAD only | 1.71 (1.64–1.78) | 1.50 (1.43–1.57) | 1.57 (1.53–1.61) | 1.36 (1.33–1.40) |
| CVD only | 2.21 (2.10–2.33) | 2.05 (1.95–2.17) | 1.79 (1.73–1.85) | 1.66 (1.60–1.72) |
| PAD only | 1.38 (1.32–1.45) | 1.28 (1.22–1.35) | 1.31 (1.27–1.35) | 1.21 (1.17–1.24) |
| CAD+CVD | 3.56 (3.25–3.90) | 2.68 (2.44–2.94) | 2.68 (2.51–2.86) | 2.01 (1.88–2.15) |
| CAD+PAD | 2.52 (2.34–2.73) | 1.83 (1.69–1.98) | 2.20 (2.09–2.31) | 1.61 (1.53–1.70) |
| CVD+PAD | 2.82 (2.53–3.13) | 2.29 (2.05–2.55) | 2.36 (2.20–2.54) | 1.94 (1.80–2.09) |
| CAD+CVD+PAD | 4.38 (3.75–5.10) | 2.82 (2.41–3.29) | 3.30 (2.97–3.68) | 2.16 (1.94–2.41) |
| Risk factors | ||||
| Diabetes mellitus | ··· | 1.25 (1.20–1.30) | ··· | 1.17 (1.14–1.20) |
| Diabetic nephropathy | ··· | 1.27 (1.17–1.37) | ··· | 1.25 (1.19–1.31) |
| Carotid stenosis | ··· | 1.13 (1.08–1.18) | ··· | 1.09 (1.06–1.12) |
| Hypertension with treatment | ··· | 1.16 (1.06–1.26) | ··· | 1.16 (1.09–1.22) |
| Hypercholesterolemia | ··· | 0.88 (0.81–0.95) | ··· | 0.91 (0.87–0.96) |
| Smoker | ··· | 1.56 (1.47–1.65) | ··· | 1.47 (1.42–1.53) |
| Medications | ||||
| ACEI or ARB | ··· | 0.94 (0.91–0.97) | ··· | 0.93 (0.91–0.95) |
| β‐blocker | ··· | 1.29 (1.25–1.33) | ··· | 1.24 (1.22–1.27) |
| Calcium channel blocker | ··· | 1.13 (1.10–1.17) | ··· | 1.14 (1.12–1.17) |
| Diuretics | ··· | 1.07 (1.04–1.10) | ··· | 1.05 (1.03–1.08) |
| Statin | ··· | 0.87 (0.80–0.93) | ··· | 0.84 (0.80–0.88) |
| P2Y12 inhibitor | ··· | 1.49 (1.43–1.54) | ··· | 1.51 (1.48–1.55) |
| Oral anticoagulant | ··· | 1.04 (0.99–1.08) | ··· | 1.05 (1.02–1.08) |
| Metformin | ··· | 0.88 (0.84–0.91) | ··· | 0.91 (0.89–0.94) |
| α‐glucosidase inhibitor | ··· | 1.37 (1.06–1.79) | ··· | 1.10 (0.91–1.32) |
| DPP4 inhibitors | ··· | 0.97 (0.91–1.03) | ··· | 0.98 (0.94–1.02) |
| GLP1 agonists | ··· | 0.76 (0.68–0.85) | ··· | 0.80 (0.75–0.86) |
| SGLT2 inhibitors | ··· | 1.00 (0.78–1.28) | ··· | 0.95 (0.81–1.11) |
| Sulphonylureas or glinides | ··· | 1.24 (1.18–1.29) | ··· | 1.21 (1.18–1.25) |
| Thiazolidinediones | ··· | 0.89 (0.82–0.98) | ··· | 0.92 (0.87–0.98) |
| Insulin | ··· | 1.74 (1.67–1.82) | ··· | 1.76 (1.71–1.81) |
ACEI or ARB indicates angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker; CAD, coronary artery disease; CVD, cerebrovascular disease; DPP4, dipeptidyl peptidase IV; GLP‐1, glucagon‐like peptide‐1; HR, hazard ratio; MACE, major adverse cardiovascular event; PAD, peripheral artery disease; RFO, risk factors only; SGLT2, sodium∕glucose cotransporter member 2.
Table 3.
Incidences and Rates of MACE at 1‐Year of Follow‐Up
| All | Total Established Disease | Any CADa | Any CVDa | Any PADa | Multiple Risk Factors Only | |
|---|---|---|---|---|---|---|
| N=1 220 144 Events/100 PY (95% CI) n (%) | N=414 718 Events/100 PY (95% CI) n (%) | N=206 804 Events/100 PY (95% CI) n (%) | N=92 695 Events/100 PY (95% CI) n (%) | N=165 967 Events/100 PY (95% CI) n (%) | N=805 426 Events/100 PY (95% CI) n (%) | |
| MACE | 1.47 (1.45–1.49) | 2.12 (2.07–2.16) | 2.19 (2.12–2.25) | 3.04 (2.93–3.16) | 2.04 (1.97–2.11) | 1.14 (1.11–1.16) |
| 17 671 (1.4) | 8620 (2.1) | 4437 (2.1) | 2749 (3.0) | 3322 (2.0) | 9051 (1.1) | |
| Myocardial infarction | 0.77 (0.76–0.79) | 1.14 (1.11–1.17) | 1.44 (1.39–1.49) | 1.07 (1.00–1.13) | 1.11 (1.06–1.16) | 0.59 (0.57–0.60) |
| 9320 (0.8) | 4648 (1.1) | 2922 (1.4) | 971 (1) | 1812 (1.1) | 4672 (0.6) | |
| Ischemic stroke | 0.64 (0.62–0.65) | 0.88 (0.86–0.91) | 0.65 (0.61–0.68) | 1.89 (1.80–1.98) | 0.81 (0.77–0.86) | 0.51 (0.49–0.52) |
| 7669 (0.6) | 3616 (0.9) | 1325 (0.6) | 1713 (1.8) | 1333 (0.8) | 4053 (0.5) | |
| Cardiovascular death | 0.16 (0.15–0.17) | 0.23 (0.22–0.25) | 0.23 (0.21–0.25) | 0.31 (0.27–0.35) | 0.26 (0.24–0.29) | 0.12 (0.11–0.13) |
| 1914 (0.2) | 955 (0.2) | 475 (0.2) | 283 (0.3) | 426 (0.3) | 959 (0.1) |
CAD indicates coronary artery disease; CVD, cerebrovascular disease; MACE, major adverse cardiovascular event; PAD, peripheral artery disease; PY, person‐years.
These cohorts overlap each other.
4‐Year Outcomes
At 4 years, MACE was 6.9% (1.89 events/100 PYs) overall with 9.1% (2.58 events/100 PYs) in patients with established disease and 5.8% (1.54 events/100 PYs) in patients with multiple risk factors (Figure 2). Incidence rates for MACE outcomes increased at 4 years compared with that of 1 year. Events remained highest in CVD (11.5%), followed by CAD (9.5%) and PAD (8.9%). Myocardial infarction, ischemic stroke, and cardiovascular death occurred in 4.1% (1.11 events/100 PYs), 3.5% (0.95 events/100 PYs), and 0.7% (0.17 events/100 PYs) of all patients, respectively. Upon sensitivity analysis, our alternative MACE end point again found higher rates of MACE because of a 14.4% (4.02 events/100 PYs) incidence of hospitalization for additional vascular events or procedures.
Upon multivariable Cox regression adjusted for age and sex (model #1), there was a 51% increase in MACE risk in single vascular disease (ranging from 31% for PAD to 79% for CVD) compared with patients with risk factors only. Cox regression adjusting for age, sex, and additional risk factors and medications (model #2) yielded results that were overall consistent with model #1. Involvement of 2 vascular beds was associated with a 135% increase in risk (ranging from 120% for PAD+CAD to 168% for CAD+CVD). When 3 vascular beds were involved, MACE risk was increased by 230%. Rates for the overall and each MACE outcome separately are listed in Table 4.
Table 4.
Incidences and Rates of MACE at 4 Years of Follow‐Up
| All | Total Established Disease | Any CADa | Any CVDa | Any PADa | Multiple Risk Factors Only | |
|---|---|---|---|---|---|---|
| N=625 951 Events/100 PY (95% CI) n (%) | N=217 536 Events/100 PY (95% CI) n (%) | N=110 456 Events/100 PY (95% CI) n (%) | N=48 160 Events/100 PY (95% CI) n (%) | N=87 838 Events/100 PY (95% CI) n (%) | N=408 415 Events/100 PY (95% CI) n (%) | |
| MACE | 1.89 (1.87–1.91) | 2.58 (2.54–2.61) | 2.70 (2.65–2.76) | 3.34 (3.26–3.43) | 2.54 (2.49–2.60) | 1.54 (1.53–1.56) |
| 43 428 (6.9) | 19 859 (9.1) | 10 542 (9.5) | 5539 (11.5) | 7853 (8.9) | 23 569 (5.8) | |
| Myocardial infarction | 1.11 (1.10–1.13) | 1.54 (1.52–1.57) | 1.89 (1.84–1.93) | 1.37 (1.31–1.42) | 1.53 (1.49–1.58) | 0.90 (0.88–0.91) |
| 25 918 (4.1) | 12 088 (5.6) | 7443 (6.7) | 2337 (4.9) | 4810 (5.5) | 13 830 (3.4) | |
| Ischemic stroke | 0.95 (0.93–0.96) | 1.21 (1.18–1.23) | 0.95 (0.92–0.98) | 2.36 (2.28–2.43) | 1.15 (1.12–1.19) | 0.81 (0.80–0.83) |
| 22 051 (3.5) | 9487 (4.4) | 3800 (3.4) | 3975 (8.3) | 3631 (4.1) | 12 564 (3.1) | |
| Cardiovascular death | 0.17 (0.17–0.18) | 0.25 (0.24–0.26) | 0.23 (0.22–0.25) | 0.33 (0.31–0.36) | 0.28 (0.26–0.30) | 0.14 (0.13–0.14) |
| 4123 (0.7) | 1985 (0.9) | 949 (0.9) | 580 (1.2) | 904 (1.0) | 2138 (0.5) |
CAD indicates coronary artery disease; CVD, cerebrovascular disease; MACE, major adverse cardiovascular event; PAD, peripheral artery disease; PY, person‐years.
These cohorts overlap each other.
Discussion
In this large, contemporary real‐world study of patients with established atherosclerosis or at high risk for atherosclerotic complications, the proportion of patients experiencing MACE increased by nearly 5‐fold from year 1 to 4 of follow‐up. Sensitivity analysis in which hospitalizations for additional vascular events and procedures were included in the definition of MACE showed an even higher incidence of events that were driven largely by rehospitalizations. The development of MACE appeared to vary depending on the number and location of vascular bed(s) affected. Atherosclerotic disease in a single vascular bed increased risk for MACE by up to 68%, whereas disease in 3 beds was associated with an ≈4‐fold increase in MACE. Patients with any CVD involvement experienced the highest rates of MACE followed by patients with any CAD then any PAD, regardless of the number of vascular beds affected. Our findings may serve as a benchmark for both clinicians and other health professionals when making statements about MACE rates in a contemporary population, and when designing future clinical trials.
Although our inclusion criteria were similar to that of REACH and most of our findings were consistent with those observed in the prospective studies, there are key differences in findings worth mentioning. After 4 years of follow‐up, MACE risk in patients with established disease increased both in REACH and our study by a substantial degree (3.4‐fold in REACH and 4.3‐fold in our study). Absolute incidences for MACE were, however, different. At 1 year, 4.2% of patients in REACH experienced MACE versus only 1.4% in our evaluation. Similarly, the absolute risk of MACE varied when focusing solely on patients with established atherosclerotic disease (15.8% in REACH versus 9.1% in our study at 4 years).2, 3 These inconsistencies in absolute MACE risk evaluations are likely a consequence of important differences in patients’ relative involvement of various vascular beds. Selection of patients in the REACH registry may not have resulted in a cohort that is entirely representative of those with established or at high risk for atherosclerotic disease. In particular, this selection bias in REACH appears to have resulted in patients with established PAD or risk factors only being substantially under‐represented. The 2019 heart disease statistics report from the American Heart Association15 suggest that in contrast to the relative prevalence of established CAD, there should be about one third as many patients with CVD and nearly twice as many with PAD. In REACH, the ratio of CAD to PAD patients was only 1 to 0.22, while the ratio in our study (1–0.80) was closer to that reported by the American Heart Association. The investigators of REACH have acknowledged the limited external validity of their findings resulting from their non‐population‐based registry.3
Moreover, REACH patients with established PAD had a high MACE risk in comparison to patients with disease in other vascular beds. Findings from the 1‐year analysis of REACH concluded that PAD patients suffered from the highest MACE and cardiovascular death rates,3 whereas we found PAD patients had the lowest rates of MACE and second lowest rates of cardiovascular death among patients with atherosclerotic disease at both 1 and 4 years. Prior research16 suggests that fewer than 50% of patients with PAD are aware of the condition, while physicians are unaware of the presence of PAD in 70% of those with the condition. Classic claudication symptoms are found in only a minority of patients (11%),17 and the US Preventive Services Task Force has stated there is insufficient evidence to support the use of the ankle–brachial index in screening for PAD among asymptomatic individuals (likely impeding the diagnosis of patients with less severe disease).18 It is therefore possible that the patients with established PAD who were selected for enrollment into the REACH registry had more severe disease (presence of acute limb ischemia/Fontaine class 3 or higher) compared with PAD patients included in our analysis. Such differences in patient selection/inclusion could certainly explain some of the difference in MACE rates observed between the 2 studies.
Advances in the prevention and treatment of atherosclerotic disease have contributed substantially to reductions in the incidence of MACE.4 Since the REACH registry completed its patient follow‐up in 2008, notable changes have been made to standards of care for the management of atherosclerotic disease patients. These included several updates to American College of Cardiology/American Heart Association guidelines, which have recommended more intensive blood pressure control, increased use of high‐intensity statins, and an increased scope and duration of treatment with dual antiplatelet therapy.19, 20, 21 As a result, over the past decade, age‐adjusted death rates for cardiovascular disease patients dropped by 22.1% in the United States,15 with data suggesting that ≈47% of the decline in cardiovascular death was because of evidence‐based medical and surgical treatments, including secondary preventive therapies after myocardial infarction or revascularization (11%), initial treatments for acute myocardial infarction or unstable angina (10%), treatments for heart failure (9%), revascularization for chronic angina (5%), and other therapies (12%). Another 44% of the decline in cardiovascular death was attributed to greater reductions in key risk factors, such as total cholesterol (24%), systolic blood pressure (20%), smoking prevalence (12%), and physical inactivity (5%).4, 22 As our study cohort consisted of patients identified during calendar year 2013 and treated/followed through 2017, it provides data on patients with established or at high risk for atherosclerotic disease managed under these new standards of care. Our study suggests that patients with established or at high risk for atherosclerotic disease still have a substantial residual risk for MACE when treated in routine practice (and that patients with polyvascular disease have the highest residual risk) even when being treated in the era of updated guidelines that call for more aggressive medical management. This study therefore emphasizes the need for continued improvement in the prevention and treatment of patients with or at risk of atherosclerosis. Importantly, while our study can assess the proportion of patients using preventative strategies such as medications (statins, antihypertensives, antidiabetic agents), we could not assess whether the intensity of treatment prescribed was optimal.
Our study has limitations worth discussing. First, misclassification bias must always be considered in claims database analyses and, when present, can detrimentally impact a study's internal validity.23 Second, clinical adjudication of events was not possible within our claims database analysis. Of note, the REACH registry, while performed prospectively, also did not independently adjudicate outcomes. Third, our MarketScan claims data only allowed the capture of in‐hospital death outcomes and, therefore, absolute cardiovascular death rates in our study may be underestimated (though relative rates across atherosclerotic disease types are likely accurate). Fourth, because of the methods used to select our established atherosclerotic disease and risk‐factors‐only cohorts, some degree of selection bias was likely present in our study. As an example, to be included in the risk‐factor‐only cohort, patients had to have ≥3 risk factors for atherosclerotic disease including diabetes mellitus, diabetic nephropathy, hypercholesterolemia with treatment, hypertension with treatment, carotid stenosis, smoking, or advanced age (males ≥65 or females ≥70). The requirement for hypercholesterolemia to be “treated” for the risk‐factor‐only cohort, while patients with established disease could have untreated or no history of hypercholesterolemia likely explains why the risk‐factor‐only patients had the highest utilization of statins (88.4% versus 66.4% for established disease patients). Of note, a similar finding of higher statin use in the risk‐factor‐only cohort compared with many established disease groups was also seen in the REACH registry.24 Next, we used US commercial and Medicare supplemental plan claims data. As a consequence, our results are most generalizable to an insured US population with established or at high risk for atherosclerotic disease.5 Finally, while we highlight some similarities and contrasts between our findings and that of the REACH registry, because of differences in methodology and types of patients included, direct comparison of results is not recommended.
Conclusions
This large, contemporary study identified a substantial risk for MACE in patients with established atherosclerotic disease or with multiple risk factors treated in routine clinical practice. We also showed that the number and specific location of vascular bed involvement were associated with MACE risk. Our findings may serve as a benchmark for both clinicians and other health professionals when making statements about MACE rates in a contemporary population, and when designing future clinical trials. Moreover, they emphasize the need for continued improvement in the prevention and treatment of patients with or at risk of atherosclerosis.
Sources of Funding
Funding was provided by Bayer AG, Berlin, Germany and a Medical Staff Internal Grant from Hartford Hospital, Hartford, CT. MarketScan data used in this study were obtained from IBM under a third‐party license agreement with Bayer AG, Berlin, Germany. The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors have approved the final manuscript.
Disclosures
Coleman has received research funding and honoraria from Bayer AG (significant), Janssen Scientific Affairs LLC (significant), and Portola Pharmaceuticals, Inc (modest). Alberts reports consultancy or speaker fees and honoraria from Genentech (modest), Janssen Pharmaceuticals (significant), Boehringer Ingelheim (modest), Pfizer (significant), Bristol‐Myers Squibb (significant), Medscape (modest), Portola (significant), and patents/royalties from Duke University (modest). The remaining authors have no disclosures to report.
Supporting information
Table S1. List of Baseline and Outcome Variable Codes
(J Am Heart Assoc. 2020;9:e014402 DOI: 10.1161/JAHA.119.014402.)
References
- 1. Heron M. Deaths: leading causes for 2015. Natl Vital Stat Rep. 2017;66:1–76. [PubMed] [Google Scholar]
- 2. Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D'Agostino R, Liau CS, Mas JL, Röther J, Smith SC Jr, Salette G, Contant CF, Massaro JM, Steg PG; REACH Registry Investigators . Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. [DOI] [PubMed] [Google Scholar]
- 3. Steg PG, Bhatt DL, Wilson PW, D'Agostino R Sr, Ohman EM, Röther J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, Pencina MJ, Goto S; REACH Registry Investigators . One‐year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. [DOI] [PubMed] [Google Scholar]
- 4. Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, Mussolino ME, Hsu LL, Addou E, Engelgau MM, Gordon D. Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120:366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. IBM Watson Health . White Paper: IBM MarketScan Research Databases for Health Services Researchers. April 2019. Available at: https://www.ibm.com/downloads/cas/6KNYVVQ2. Accessed November 21, 2019.
- 6. Birman‐Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD‐9‐CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. [DOI] [PubMed] [Google Scholar]
- 7. Bezin J, Girodet PO, Rambelomanana S, Touya M, Ferreira P, Gilleron V, Robinson P, Moore N, Pariente A. Choice of ICD‐10 codes for the identification of acute coronary syndrome in the French hospitalization database. Fundam Clin Pharmacol. 2015;29:586–591. [DOI] [PubMed] [Google Scholar]
- 8. Davis LA, Mann A, Cannon GW, Mikuls TR, Reimold AM, Caplan L. Validation of diagnostic and procedural codes for identification of acute cardiovascular events in US veterans with rheumatoid arthritis. EGEMS (Wash DC). 2014;1:1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.“Clinical Classifications Software Refined (CCSR) for ICD‐10‐CM Diagnoses.” Healthcare Cost and Utilization Project, AHRQ, October 2019. Available at: https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp. Accessed November 21, 2019.
- 10. Henderson T, Shepheard J, Sundararajan V. Quality of diagnosis and procedure coding in ICD‐10 administrative data. Med Care. 2006;44:1011–1019. [DOI] [PubMed] [Google Scholar]
- 11. McCormick N, Bhole V, Lacaille D, Avina‐Zubieta JA. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PLoS One. 2015;10:e0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jaff MR, Cahill KE, Yu AP, Birnbaum HG, Engelhart LM. Clinical outcomes and medical care costs among Medicare beneficiaries receiving therapy for peripheral arterial disease. Ann Vasc Surg. 2010;24:577–587. [DOI] [PubMed] [Google Scholar]
- 13. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in‐hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55:698–705. [DOI] [PubMed] [Google Scholar]
- 14.“2019 ICD‐10 PCS.” Centers for Medicare and Medicaid Services September 18, 2018. Available at: https://www.cms.gov/Medicare/Coding/ICD10/2019-ICD-10-PCS. Accessed November 21, 2019.
- 15. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 16. Novo S. Classification, epidemiology, risk factors, and natural history of peripheral arterial disease. Diabetes Obes Metab. 2002;4(suppl 2):S1–S6. [DOI] [PubMed] [Google Scholar]
- 17. Hirsch AT, Criqui MH, Treat‐Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. [DOI] [PubMed] [Google Scholar]
- 18. US Preventive Services Task Force , Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Silverstein M, Simon MA, Tseng CW, Wong JB. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle‐brachial index: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:177–183. [DOI] [PubMed] [Google Scholar]
- 19. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O'Gara PT, Sabatine MS, Smith PK, Smith SC Jr. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines: an update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST‐Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non‐ST‐Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134:e123–e155. [DOI] [PubMed] [Google Scholar]
- 20. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 21. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 22. Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. [DOI] [PubMed] [Google Scholar]
- 23. Gandhi SK, Salmon W, Kong SX, Zhao SZ. Administrative databases and outcomes assessment: an overview of issues and potential utility. J Manag Care Spec Pharm. 1999;5:215–222. [Google Scholar]
- 24. Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Röther J, Wilson PW; REACH Registry Investigators . International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of Baseline and Outcome Variable Codes


