Abstract
Background:
In 2017, measles elimination was verified in Bhutan, and the country appears to have sufficiently high vaccination coverage to achieve rubella elimination. However, a measles and rubella serosurvey was conducted to find if any hidden immunity gaps existed that could threaten Bhutan’s elimination status.
Methods:
A nationwide, three-stage, cluster seroprevalence survey was conducted among individuals aged 1–4, 5–17, and >20 years in 2017. Demographic information and children’s vaccination history were collected, and a blood specimen was drawn. Serum was tested for measles and rubella immunoglobulin G (IgG). Frequencies, weighted proportions, and prevalence ratios for measles and rubella seropositivity were calculated by demographic and vaccination history, taking into account the study design.
Results:
Of the 1325 individuals tested, 1045 (81%, 95% CI 78%–85%) were measles IgG seropositive, and 1290 (97%, 95% CI 95%–99%) were rubella IgG seropositive. Rubella IgG seropositivity was high in all three age strata, but only 47% of those aged 5–17 years were measles IgG seropositive. Additionally, only 41% of those aged 5–17 years who had documented receipt of two doses of measles– or measles-rubella–containing vaccine were seropositive for measles IgG, but almost all these children were rubella IgG seropositive.
Conclusions:
An unexpected measles immunity gap was identified among children 5–17 years of age. It is unclear why this immunity gap exists; however, it could have led to a large outbreak and threatened sustaining of measles elimination in Bhutan. Based on this finding, a mass vaccination campaign was conducted to close the immunity gap.
Keywords: Measles, Rubella, Seroprevalence, Bhutan
1. Introduction
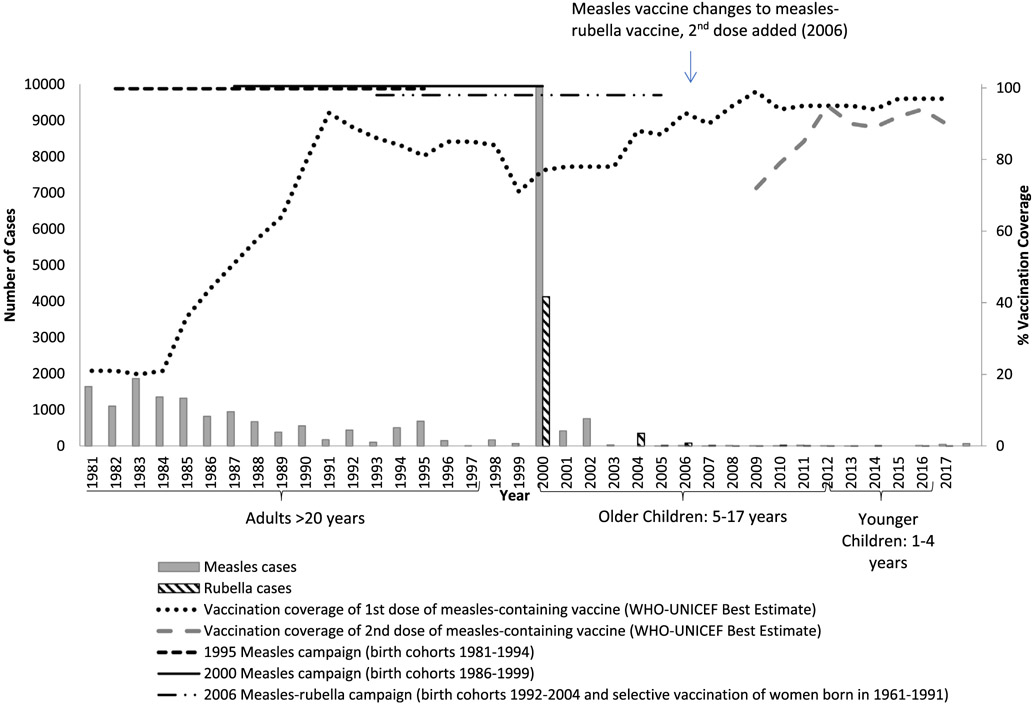
The Global Vaccine Action Plan calls for elimination of measles and rubella in five World Health Organization (WHO) Regions by 2020 [1]. The elimination of measles and rubella is defined as the absence of endemic cases of measles or rubella for at least 12 months in the presence of a high-quality surveillance system [2]. In 2017, the Kingdom of Bhutan, a country with a population of approximately 800,000, was verified as having eliminated measles; and in 2018, it was verified as having controlled rubella (an intermediary step toward rubella elimination) [3,4]. Bhutan has achieved this success because of a strong immunization program that provides free vaccinations. In 1979, 1-dose measles vaccination was introduced into the routine immunization schedule for those 9 months of age. In 2006, rubella vaccination was introduced into the routine immunization schedule in combination with measles vaccine, and a 2-dose measles rubella (MR) vaccine schedule was established for children aged 9 and 24 months. To help close immunity gaps, nationwide measles campaigns were conducted in 1995 and 2000, and a nationwide measles-rubella campaign was conducted in 2006. Coverage with the first and second doses of MR vaccine (MR1 and MR2, respectively) has been high since 2011; and the country’s efforts have resulted in a dramatic decrease in measles and rubella cases, with recent measles cases attributed to importation from other countries (Fig. 1).
Fig. 1.
Measles and rubella cases and measles and measles-rubella vaccination coverage — Bhutan, 1981–2017 [20].
High administrative vaccination coverage estimates might be misleading, however, in assessing the number of susceptible persons in the population. Administrative coverage estimates might be inaccurate because of incorrect numerators (e.g., counting doses given to older-aged children outside the target age group) as well as denominators (i.e., the target population) [5]. The development of immunity after vaccination involves many factors, including the person’s age at vaccination, underlying genetics/immune system, and vaccine failure due to storage/handling conditions [6,7]. Serosurveys can identify susceptibles in a population and provide an estimate of population immunity; for measles and rubella, serosurveys reflect exposure either to vaccination or disease [8].
Bhutan is surrounded by India and China, countries that are endemic for measles and rubella, and thus it is at high risk for importations of virus. Bhutan has experienced measles outbreaks caused by importation in recent years, despite high vaccination coverage. However, these outbreaks were small, and it was unclear if there were significant immunity gaps in the population, thus making it challenging to target vaccination efforts to prevent future outbreaks [9]. The Bhutan Ministry of Health, in collaboration with the World Health Organization conducted a measles-rubella serosurvey (combined with a hepatitis B and hepatitis C serosurvey) to identify any remaining immunity gaps that needed to be addressed to sustain measles elimination and to achieve rubella elimination.
2. Methods
In April 2017, a nationwide, cross-sectional, 3-stage cluster survey was conducted to estimate the prevalence of measles and rubella immunoglobulin G (IgG) antibodies and biomarkers for hepatitis B and C infections among persons residing in Bhutan. This report provides the measles and rubella seroprevalence results; findings from the hepatitis serosurvey are reported elsewhere (under review). Three age strata were targeted based on the history of the hepatitis B vaccination program and risk for chronic hepatitis B acquisition: 1–4 years of age (birth years 2012–2016, referred to as younger children); 5–17 years of age (birth years 2000–2012, referred to as older children); and >20 years (born before 1997, referred to as adults). Those born from 1997 to 1999 were excluded because they were born during the start of the hepatitis B vaccination program, and hepatitis B serosurvey data from these cohorts would have been difficult to interpret, given variable implementation of hepatitis B vaccination and consequent low coverage. It was assumed that their measles and rubella seroprevalence would not differ significantly from those of a similar age who were included in the survey.
2.1. Sample size
For younger children, the sample size was calculated based on the estimated measles IgG seroprevalence; a minimum effective sample size of 123 was calculated assuming 95% seroprevalence, a one-side confidence interval of −5%, and α = 0.05. Accounting for the cluster design, a target of 8 young children per primary sampling unit, an intraclass correlation = 0.1, and a 15% nonresponse rate, the sample size was increased to 245 young children. Among the older children and adults, the sample size was calculated based on the expected prevalence of chronic hepatitis B infection, because this calculation would result in a larger sample size than the size needed for the MR serosurvey (245 persons in each age group), based on an expected 95% seroprevalence of measles and rubella in each group. In brief, a sample size of 657 was calculated for older children, assuming 1% seroprevalence of chronic hepatitis B, precision of ±1%, a design effect of 1.5, and α = 0.05, with a 13% non-response rate. For adults, a sample size of 785 was calculated assuming a chronic hepatitis B prevalence of 5%, precision of ±2%, a design effect of 1.5, and α = 0.05, with a 13% non-response rate. To achieve the targeted sample sizes, it was estimated that 30 clusters of 29 households (870 households in total) would have to be sampled, based on the 2005 census, to enroll the minimum sample of younger children.
2.2. Sampling
Based on a 2005 national census, 30 enumeration areas were selected by probability proportional to estimated size with replacement for the first stage of sampling. For the second stage, 29 households were randomly selected from an updated list of households. For the third stage, all eligible persons in the selected household, defined as those in one of the three age strata and living in the household for at least 6 months, were enumerated. One person per age stratum per household was randomly selected using a smartphone application. If the selected individual was absent, the household was revisited up to three times on two different days. If the selected person remained absent, the person was marked as absent and not replaced.
2.3. Data collection
Consent was requested from adults and from parents or caregivers of children before participation in the serosurvey; assent was also requested from those 10–17 years of age. A specific questionnaire was developed for each age group. If the person consented, the age-appropriate questionnaire was administered by trained field workers. The questionnaire included demographic and background data and, for younger and older children, measles and rubella vaccination history. If documented vaccination history was not available for younger children, vaccination history was obtained based on recall; for older children, only documented vaccination history was collected. The documented vaccination history reflected vaccine doses administered via routine immunization services, not via supplemental immunization activeities (SIAs).
2.4. Specimen collection and laboratory testing
Approximately 10 ml of blood were collected by venipuncture from older children and adults and approximately 5 ml of blood from younger children. Serum was separated in the field, transported at 2° to 8 °C to the Royal Centre for Disease Control, and subsequently stored at −20 °C until testing. All samples were tested using Siemens Enzygnost® Anti-measles Virus/IgG and Anti-rubella Virus/IgG ELISA kits (Siemens, Healthcare Diagnostics Products, GmbH Marburg, Germany). Test results were interpreted according to manufacturer’s instructions, with titers of <150 mIU/ml considered negative for measles and <4 mIU/ml considered negative for rubella. A random sample of 10% of specimens was sent to the WHO South-East Asia Regional Reference Laboratory for Measles and Rubella at the National Institute of Health (NIH) in Bangkok, Thailand, for quality assurance. Less than 10% of samples tested at the NIH Thailand were discordant with results obtained in Bhutan, so no additional retesting was done.
2.5. Additional testing
Based on unexpected findings of low IgG seroprevalence, a subset of serum specimens were sent to the US Centers for Disease Control and Prevention for plaque reduction neutralization test (PRNT), regarded as the gold standard for measuring measles immunity because it measures functional neutralizing antibody, which is considered a correlate of protection [10]. Titers of <120 mIU/ml were considered true negatives.
2.6. Data management/analysis
The data were double-entered into an Epi-Info7 database (Atlanta, GA). Data were analyzed with STATA® SE 14.1 (College Station, TX, USA) and SAS v9.4 (Cary, NC, USA). Measles and rubella vaccination rates were calculated by age group. All specimens with measles or rubella IgG titers above the aforementioned ELISA cutoffs were considered seropositive. PRNT results are presented for the subset of specimens tested by PRNT, but findings were not used to define seropositive and seronegative for the study. Frequencies, weighted proportions, and 95% logit confidence intervals (CI) using the Taylor series method were calculated for measles IgG and rubella IgG positivity by age group, sex, education level, and vaccination status. Weights were applied, factoring in the survey design, sampling probabilities, nonresponse rate, and the population distribution of Bhutan. Prevalence ratios were calculated to identify factors related to measles and rubella seropositivity. χ2 p-values were calculated for all remaining analyses. When evaluating the discordance between measles and rubella seropositivity, the differences in the percentage discordant (% measles negative among rubella positive minus % rubella negative among measles positive) and corresponding 95% CI were calculated. A 95% CI in the difference that did not include zero was considered statistically significant. For descriptive analyses conducted among small subgroup populations, unweighted proportions are presented.
2.7. Human Subjects’ Rights and Ethics
The researchers obtained informed consent from participants or caregivers before testing. The study protocol was approved by the Research and Ethical Board of Health, Bhutan, and the WHO Research Ethics Review Committee. This activity was reviewed in accordance with CDC human research protections procedures and was determined to be human subject research, but CDC involvement did not constitute direct engagement in human subject research.
3. Results
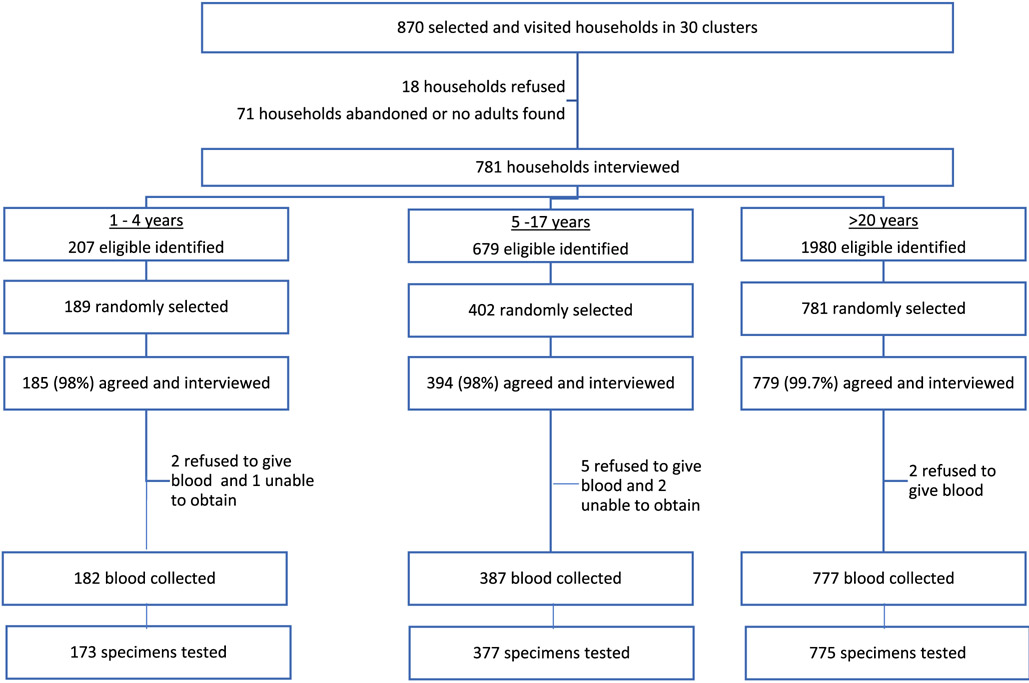
The serosurvey was conducted during March–April 2017. Of 870 preselected households, 781 (90%) agreed to participate in the survey, 18 (2%) refused to participate, and 71 (8%) were ineligible (non-residential, abandoned, or no adults available) (Fig. 2). From these 781 households, 2866 age-eligible persons were identified, and 1372 age-eligible persons were selected. Fourteen (1%) refused, 33 (2%) responded to the questionnaire but did not have blood drawn, and 1325 (97%) were enrolled, interviewed, and had their blood tested. Serosurvey participants (n = 1325) and non-participants (n = 33) were similar with respect to country of birth, education/maternal education, and vaccination history (among children) (Table 1). However, more children were nonparticipants than adults.
Fig. 2.
Participation and enrollment in the measles and rubella serosurvey — Bhutan, 2017.
Table 1.
Characteristics of measles-rubella serosurvey participants and nonparticipants – Bhutan, 2017.
| Characteristics | Non-participants | Participants in serosurvey | ||||||
|---|---|---|---|---|---|---|---|---|
| n | N | % | n | N | % | |||
| Total | 33 | 1325 | ||||||
| Female | 20 | 33 | 61% | 695 | 1325 | 52% | ||
| Age | ||||||||
| 1–4 years | 12 | 33 | 36% | 173 | 1325 | 13% | ||
| 5–17 years | 17 | 33 | 52% | 377 | 1325 | 28% | ||
| >20 years | 4 | 33 | 12% | 775 | 1325 | 58% | ||
| Highest education level (or maternal education level for children) | ||||||||
| No education | 17 | 33 | 52% | 774 | 1325 | 58% | ||
| Primary/Monastic | 10 | 33 | 30% | 284 | 1325 | 21% | ||
| Secondary/Tertiary | 6 | 33 | 18% | 267 | 1325 | 20% | ||
| Card available to review vaccination data (among 1- to 17-year-olds) | 16 | 29 | 55% | 381 | 550 | 69% | ||
| Number of measles or measles-rubella–containing doses delivered by routine immunization | ||||||||
| 0 doses | 2 | 29 | 7% | 14 | 550 | 3% | ||
| 1 dose | 7 | 29 | 24% | 128 | 550 | 23% | ||
| 2+ doses | 11 | 29 | 38% | 254 | 550 | 46% | ||
| Unknown | 9 | 29 | 31% | 154 | 550 | 28% | ||
3.1. Vaccination
Among 550 participating children, 396 (64%) submitted information on measles and rubella vaccination provided via routine immunization services. Vaccination data were available by vaccination card for 226 (54%) older children and for 155 (85%) younger children. An additional 15 (13%) younger children had vaccination data provided by recall. Among 173 younger children, 123 (72%) had received at least two doses of measles- and rubella-containing-vaccine (MRCV). Among 243 5- to 11-year-olds, 128 (47%, 95% CI 38% to 57%) had received at least two doses of MRCV; among 132 12- to 17-year-olds, 3 (1%, 95% CI 0% to 3%) had received at least two doses of measles vaccine.
3.2. Seroprevalence
Of 1325 persons tested, 1045 (81%, 95% CI 78% to 85%) were measles IgG positive and 1290 (97%, 95% CI 95% to 99%) were rubella IgG positive (Table 2). Rubella IgG seroprevalence was >95% in all three age strata. However, measles IgG seroprevalence varied by stratum; 150 (85%, 95% CI 77% to 91%) of 173 young children were measles IgG seropositive compared with 179 (47%, 95% CI 39% to 56%) of 377 older children and 716 (92%, 95% CI 89% to 95%) of 775 adults. To evaluate if there was a difference within the 5–17-year-old stratum, additional analysis was performed. Among 243 children 5–11 years of age, 120 (52%, 95% CI 42% to 62%) were measles IgG seropositive; among 132 children 12–17 years of age, 59 (42%, 95% CI 31% to 54%) were measles seropositive. Neither measles nor rubella IgG seropositivity varied significantly by sex or education status. When measles IgG seropositivity was evaluated by vaccination status, those who had had two doses of measles vaccine or MRCV were significantly more likely to be measles IgG seropositive than those who had received no doses [prevalence ratio 2.8 (95% CI 1.03–7.77)], but vaccination status was not associated with higher rubella IgG seropositivity (Table 2).
Table 2.
Measles and Rubella immunoglobulin G (IgG) seropositivity (as determined by ELISA) by participant characteristics, Bhutan, 2017.*
| Number of samples tested |
Measles |
Rubella |
|||||
|---|---|---|---|---|---|---|---|
| # IgG positive | % (95% CI) | Prevalence Ratio | # IgG positive | % (95% CI) | Prevalence Ratio | ||
| Total | 1325 | 1045 | 81(78–85) | – | 1290 | 97(95–99) | – |
| Age | |||||||
| 1–4 years | 173 | 150 | 85 (77–91) | REF | 173 | 100 (−) | REF |
| 5–17 years | 377 | 179 | 47 (39–56) | 0.56 (0.44–0.69) | 362 | 96 (92–98) | 0.96 (0.92–0.99) |
| >20 years | 775 | 716 | 92 (89–95) | 1.08 (0.98–1.20) | 755 | 97 (95–99) | 0.97 (0.96–0.99) |
| Sex | |||||||
| Male | 629 | 470 | 79 (75–83) | REF | 609 | 97 (94–99) | REF |
| Female | 695 | 574 | 83 (78–88) | 1.06 (1.0–1.1) | 680 | 98 (95–99) | 1.01 (0.99–1.04) |
| Highest education level (or highest maternal education level for children) | |||||||
| No education | 774 | 617 | 81 (76–85) | REF | 749 | 97 (94–98) | REF |
| Primary/Monastic | 284 | 218 | 82 (76–87) | 1.01 (0.93–1.09) | 278 | 98 (94–99) | 1.01 (0.98–1.04) |
| Secondary/Tertiary | 267 | 210 | 81 (74–87) | 1.00 (0.91–1.09) | 263 | 98 (95–100) | 1.02 (0.99–1.04) |
| Measles/Measles-rubella containing vaccination by card (1–4 and 5–17 years), or recall (1–4 years) | |||||||
| 0 doses | 14 | 5 | 25 (8–57) | REF | 11 | 82 (57–94) | REF |
| 1 dose | 128 | 73 | 51 (41–62) | 2.04 (0.70–5.91) | 127 | 99 (96–100) | 1.21 (0.97–1.52) |
| ≥2 doses | 254 | 183 | 71 (64–78) | 2.83 (1.03–7.77) | 253 | 99 (93–100) | 1.21 (0.97–1.51) |
| Unknown | 154 | 68 | 45 (35–54) | 1.77 (0.60–5.18) | 144 | 94 (88–97) | 1.15 (0.93–1.42) |
REF = referent group.
Bold prevalence ratios highlight significant results.
Among the 1325 participants, 1028 (80%, 95% CI 76% to 84%) were both measles and rubella IgG seropositive (Table 3). The 5- to 17-year-old stratum had the smallest proportion (47%) who were both measles and rubella seropositive (Table 3). This finding was similar when the data were further analyzed by 5- to 11-year-old and 12- to 17-year-old subgroups (data not shown). Similarly, children aged 5–17 years had the highest discordance in measles and rubella IgG seropositivity, 49% (95% CI 40% to 57%), compared with 15% (95% CI 8% to 22%) among 1- to 4-year-olds and 5% (95% CI 2% to 8%) among adults.
Table 3.
Measles and Rubella immunoglobulin G seropositivity (as determined by ELISA) by age strata, Bhutan, 2017.
|
1–4 years of
age |
5–17 years of
age |
>20 years of
age |
||||
|---|---|---|---|---|---|---|
| Rubella seropositive (number, %, 95% CI) |
Rubella seronegative (number, %, 95% CI) |
Rubella
seropositive (number, %, 95% CI) |
Rubella seronegative (number, %, 95% CI) |
Rubella seropositive (number, %, 95% CI) |
Rubella seronegative (number, %, 95% CI) |
|
| Measles seropositive | 150 (85%, 95% CI 77–91%) | 0 | 177(47%, 95% CI 41–57%) | 2 (0.3%, 95% CI 0–2%) | 701 (91%, 95% CI 86%–94%) | 15 (2%, 95% CI 1%–3%) |
| Measles seronegative | 23 (15%, 95% CI 9–23%) | 0 | 185 (49%, 95% CI 41–57%) | 13 (4%, 95% CI 2–8%) | 54 (7%, 95% CI 5–10%) | 5 (0.8%, 95% CI 0–2%) |
| Difference of discordant proportions (%, 95% CI) | 15% (8–22%) | 49% (40–57%) | 5% (2–8%) | |||
3.3. Vaccination status and seroprevalence by age group
When measles IgG seropositivity was evaluated by age group and number of vaccination doses received, 110 (88%) of 123 1- to 4-year-olds who received two doses of MRCV were IgG seropositive, compared with 70 (58%) of 128 5- to 11-year-olds (p < 0.0001). All three 12- to 17-year-old children who received two doses of measles vaccine were IgG seropositive (Table 4). Given this large difference, vaccination details were reviewed. There was no clustering of documented two-dose measles or MRCV recipients who were measles IgG seronegative by geographic location, year of birth, or year of first or second dose vaccine receipt. Among the 73 children aged 5–17 years who received two measles or MRCV dose and were measles IgG seronegative, 68 (93%) received the first dose at or after 9 months of age, and 64 (88%) received the second dose at or after 24 months of age. Vaccination timing was similar among children who were seropositive and seronegative.
Table 4.
Descriptive analysis (unweighted) of measles seropositivity by vaccination status of doses administered through routine immunization among children – Bhutan, 2017.*
|
1–4
years |
5–11
years |
12–17
years |
Total |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # of measles-rubella containing vaccine
doses received by card (all ages) or recall (1–4 years) by routine immunization** |
Total | # measles IgG positive |
% | Total | # measles IgG positive |
% | Total | # measles IgG positive |
% | Total | # measles IgG positive |
% |
| 0 | 3 | 3 | 100 | 6 | 0 | 0 | 5 | 2 | 44 | 14 | 5 | 36 |
| 1 | 44 | 34 | 74 | 34 | 15 | 46 | 50 | 24 | 42 | 128 | 73 | 57 |
| ≥2 | 123 | 110 | 88 | 128 | 70 | 58 | 3 | 3 | 100 | 254 | 183 | 72 |
| Unknown | 3 | 3 | 100 | 75 | 35 | 50 | 74 | 30 | 41 | 152 | 68 | 45 |
Two children who were reported to be 5–17 years of age did not provide their date of birth, so we could not subcategorize them as belonging to the 5- to 11- or 12- to 17-year-old groups.
Among 1–4-year-olds, vaccination history is by documentation or recall. Among 5–17-year-olds, vaccination history is by documentation only. Those 12–17 years of age were not eligible for rubella vaccination through routine immunization.
Given the unexpectedly low seropositivity among 2-dose vaccine recipients, 58 measles IgG seronegative samples by ELISA testing from 5- to 11-year-olds who had documented receipt of two doses of MRCV vaccine were further tested for measles IgG by PRNT at US-CDC. Fifty-seven (98%) of the 58 samples were rubella IgG positive by ELISA testing at the Royal Centre for Disease Control. In contrast, 46 of 58 (79%, the negative predictive value) were <120 mIU/ml for measles IgG by PRNT and thus true measles negatives. Six (10%) had IgG titers from 120 to 150 mIU/ml, below the detectable limit of ELISA used in this serosurvey; these were classified as seronegative by ELISA but were true positives. Six (10%) had titers > 150 mIU/ml by PRNT; these were true positives, erroneously classified as seronegative by ELISA.
4. Discussion
Measles elimination has been verified in Bhutan and the country is on track to eliminate rubella, but immunity to measles in this study was only 81%, which is below the critical threshold of 95% recommended to achieve and maintain elimination [11]. This unexpected immunity gap to measles, confirmed by PRNT in a subset of children, was identified primarily among children aged 5–17 years and could have resulted in a large outbreak and re-establishment of measles endemicity. In contrast, rubella immunity was high among the three age strata studied and is sufficient for achieving rubella elimination.
The cause of the low measles IgG seroprevalence among children aged 5–17 years is unknown. Children aged 5–11 years were eligible to receive two doses of MRCV in the routine immunization schedule. Almost all those aged 12–17 years (birth year 2000–2005) were eligible for one dose of monovalent measles vaccine, but they were also eligible for at least one additional dose of measles-rubella vaccine given during the 2006 nationwide SIA; those born between June 2005-December 2005 were only eligible for one dose of monovalent measles vaccine. The routine immunization data evaluated in this study showed that very few 12 to 17-year-olds received a second dose of measles vaccine. Despite these differences in opportunities for vaccination, there was no statistical difference in measles IgG seroprevalence between those aged 5–11 years and those 12–17 years of age. There was no clear-cut relationship identified between receipt of measles or rubella vaccine and seropositivity, but this relationship is challenging to evaluate because natural and vaccine-derived immunity are indistinguishable by testing. Additionally, data were only collected for doses in the routine immunization schedule and not for doses provided by SIAs because the latter were not documented.
It is also unclear why many 5- to 17-year-olds were measles IgG seronegative but rubella IgG seropositive. One potential hypothesis is that some 5- to 17-year-old children were naturally infected with rubella virus but never exposed to natural measles virus infection nor were vaccinated. This hypothesis, however, does not explain the low measles IgG seropositivity among 2-dose vaccine recipients. Another hypothesis is that those 5- to 17-year-old children with documented vaccination were immunized against rubella but were not immunized against measles; one study has shown that the measles component of the vaccine is much more sensitive to excursions outside of cold chain as compared with the rubella component [12]. However, this hypothesis seems unlikely because this would have to have been ongoing for 12 years (2000–2012). Another hypothesis is that immunity to measles vaccination has waned over time, whereas rubella immunity has not. Globally, there is little evidence that this is a significant problem because most measles cases are among unimmunized individuals or those too young to vaccinate [13]. However, some countries have experienced what appears to be waning immunity to measles vaccination [14-18]; the reason for waning immunity is unclear and potentially could be caused by mishandling of vaccine at the time of administration, resulting in the administration of a less potent dose.
This study has a few notable limitations. First, this is a nationally representative survey, and subnational variations could exist that could not be identified here. This study excluded those aged 18–20 years because of the objectives of the concomitant hepatitis B serosurvey; it is unclear if they have a measles immunity gap similar to that among 5- to 17-year-olds or those >20 years of age. Measles and rubella IgG testing is not 100% correlated with immunity. PRNT was done on a subset of 2-dose documented vaccine recipients who were IgG negative, and most of the PRNT results substantiate that those who were IgG negative were not immune; thus, this limitation is unlikely to significantly impact the results. Vaccination data were collected by card where available, but by recall among those 1–4 years of age, when a card was not available, which is subject to recall bias [19], although only a small (13%) proportion of children aged 1–4 years provided vaccination information by recall. However, vaccination history was unknown for 28% of children. Finally, although there was a high participation rate, the target sample size was not reached because it is hard to predict how many individuals per household would be eligible a priori.
Bhutan was fortunate that the measles immunity gap identified in this survey had not yet resulted in a large outbreak. Based on the measles immunity gap identified in this survey, two rounds of a nationwide measles-rubella SIA were conducted during 2017–2018, with the goal of achieving 95% measles immunity in the population to prevent an impending measles outbreak. In the first round, persons aged 9 months to 40 years were targeted in high-risk areas based on the measles epidemiology; in the second round, those aged 6–24 years were targeted nationwide. Continued high-coverage with two doses of MRCV for all eligible children will be needed to maintain measles and rubella elimination.
Acknowledgements
We are indebted to the healthcare workers in the selected clusters for their assistance in facilitating field work. We also would like to thank all the EPI and NACP staff of the Ministry of Health and the Royal CDC, supervisors, field teams, and drivers for their efforts in the selection, recruitment, and enrollment of participants, data collection and blood specimen acquisition, and processing, transport and testing of specimens. We also thank the Thai-NIH for confirmatory testing and Sun Bae Sowers of CDC for PRNT testing. We are grateful to Yvan Hutin (WHO) and Dr. Guru Prasad Dhakal (Jigme Dorji Wangchuk National Referral Hospital, Thimphu, Bhutan) for assistance with protocol development and contributions to the hepatitis portion of the serosurvey.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Financial support
Funding for this study was provided by the Bill and Melinda Gates Foundation.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Global vaccine action plan 2011-2020. Geneva, Switzerland: World Health Organization; 2013. https://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/. Accessed: October 30, 2018. [Google Scholar]
- [2].World Health Organization. Guidance for evaluating progress towards elimination of measles and rubella. Wkly Epidemiol Rec 2018;93:544–52. [Google Scholar]
- [3].World health organization regional office for South-East Asia. Bhutan, Maldives eliminate measles. New Delhi; 2017. http://www.searo.who.int/mediacentre/releases/2017/1651/en/. Accessed: October 30, 2018. [Google Scholar]
- [4].World health organization regional office of South-East Asia. DPR Korea, Timor-Leste eliminate measles, six countries in WHO South-East Asia achieve rubella control. New Delhi; 2018. http://www.searo.who.int/mediacentre/releases/2018/1693/en/. Accessed: October 30, 2018. [Google Scholar]
- [5].Cutts FT, Hanson M. Seroepidemiology: an underused tool for designing and monitoring vaccination programmes in low-and middle-income countries. Trop Med Int Health 2016;21:1086–98. [DOI] [PubMed] [Google Scholar]
- [6].Measles vaccines: WHO position paper - April 2017. Wkly Epidemiol Rec 2017;92:205–27. [PubMed] [Google Scholar]
- [7].Fowotade A, Okonko IO, Nwabuisi C, Bakare RA, Fadeyi A, Adu FD. Measles vaccine potency and sero-conversion rates among infants receiving measles immunization in Ilorin, Kwara State, Nigeria. J Immunoassay Immunochem 2015;36:195–209. [DOI] [PubMed] [Google Scholar]
- [8].Durrheim DN, Orenstein WA, Schluter WW. Assessing population immunity for measles elimination - The promise and peril of serosurveys. Vaccine 2018;36:4001–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Durrheim DN. Measles elimination - using outbreaks to identify and close immunity gaps. N Engl J Med 2016;375:1392–3. [DOI] [PubMed] [Google Scholar]
- [10].Cohen BJ, Parry RP, Doblas D, Samuel D, Warrener L, Andrews N, et al. Measles immunity testing: comparison of two measles IgG ELISAs with plaque reduction neutralisation assay. J Virol Methods 2006;131:209–12. [DOI] [PubMed] [Google Scholar]
- [11].Durrheim DN, Crowcroft NS, Strebel PM. Measles - The epidemiology of elimination. Vaccine 2014;32:6880–3. [DOI] [PubMed] [Google Scholar]
- [12].Hachiya M, Miyano S, Mori Y, Vynnycky E, Keungsaneth P, Vongphrachanh P, et al. Evaluation of nationwide supplementary immunization in Lao People’s Democratic Republic: Population-based seroprevalence survey of anti-measles and anti-rubella IgG in children and adults, mathematical modelling and a stability testing of the vaccine. PLoS ONE 2018;13 e0194931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Patel MK, Orenstein WA. Classification of global measles cases in 2013–17 as due to policy or vaccination failure: a retrospective review of global surveillance data. Lancet Glob Health 2019;7:e313–20. [DOI] [PubMed] [Google Scholar]
- [14].Breakwell L, Moturi E, Helgenberger L, Gopalani SV, Hales C, Lam E, et al. Measles outbreak associated with vaccine failure in adults-federated states of Micronesia, February-August 2014. MMWR Morb Mortal Wkly Rep 2015;64:1088–92. [DOI] [PubMed] [Google Scholar]
- [15].Hales CM, Johnson E, Helgenberger L, Papania MJ, Larzelere M, Gopalani SV, et al. Measles outbreak associated with low vaccine effectiveness among adults in Pohnpei State, Federated States of Micronesia, 2014. Open Forum Infect Dis. 2016; 3: ofw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sa Machado R, Perez Duque M, Almeida S, Cruz I, Sottomayor A, Almeida I, et al. challenges in the post-elimination era. Euro Surveill 2018;2018:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kang HJ, Han YW, Kim SJ, Kim YJ, Kim AR, Kim JA, et al. An increasing, potentially measles-susceptible population over time after vaccination in Korea. Vaccine 2017;35:4126–32. [DOI] [PubMed] [Google Scholar]
- [18].Seventh meeting of the vaccine-preventable diseases laboratory networks in the Western Pacific Region Manila, Philippines: World Health Organization Regional Office for the Western Pacific; 2018. https://iris.wpro.who.int/bitstream/handle/10665.1/14072/RS-2017-GE-66-PHL-eng.pdf?ua=1. Accessed: May 6, 2019. [Google Scholar]
- [19].Miles M, Ryman TK, Dietz V, Zell E, Luman ET. Validity of vaccination cards and parental recall to estimate vaccination coverage: a systematic review of the literature. Vaccine 2013;31:1560–8. [DOI] [PubMed] [Google Scholar]
- [20].WHO vaccine-preventable diseases: monitoring system. 2018 global summary. Retrieved April 4, 2019, from http://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria%5Bcountry%5D%5B%5D=BTN.