Abstract
Background
Maintenance of remission is a major issue in inflammatory bowel disease. In ulcerative colitis, the evidence for the effectiveness of azathioprine and 6‐mercaptopurine for the maintenance of remission is still controversial.
Objectives
To assess the effectiveness and safety of azathioprine and 6‐mercaptopurine for maintaining remission of ulcerative colitis.
Search methods
The MEDLINE, EMBASE and Cochrane Library databases were searched from inception to 30 July 2015. Both full randomized controlled trials and associated abstracts were included.
Selection criteria
Randomized controlled trials of at least 12 months duration that compared azathioprine or 6‐mercaptopurine with placebo or standard maintenance therapy (e.g. mesalazine) were included.
Data collection and analysis
Two authors independently extracted data using standard forms. Disagreements were solved by consensus including a third author. Study quality was assessed using the Cochrane risk of bias tool. The primary outcome was failure to maintain clinical or endoscopic remission. Secondary outcomes included adverse events and withdrawal due to adverse events. Analyses were performed separately by type of control (placebo, or active comparator). Pooled risk ratios were calculated based on the fixed‐effect model unless heterogeneity was shown. The GRADE approach was used to assess the overall quality of evidence for pooled outcomes.
Main results
Seven studies including 302 patients with ulcerative colitis were included in the review. The risk of bias was high in three of the studies due to lack of blinding. Azathioprine was shown to be significantly superior to placebo for maintenance of remission. Fourty‐four per cent (51/115) of azathioprine patients failed to maintain remission compared to 65% (76/117) of placebo patients (4 studies, 232 patients; RR 0.68, 95% CI 0.54 to 0.86). A GRADE analysis rated the overall quality of the evidence for this outcome as low due to risk of bias and imprecision (sparse data). Two trials that compared 6‐mercaptopurine to mesalazine, or azathioprine to sulfasalazine showed significant heterogeneity and thus were not pooled. Fifty per cent (7/14) of 6‐mercaptopurine patients failed to maintain remission compared to 100% (8/8) of mesalazine patients (1 study, 22 patients; RR 0.53, 95% CI 0.31 to 0.90). Fifty‐eight per cent (7/12) of azathioprine patients failed to maintain remission compared to 38% (5/13) of sulfasalazine patients (1 study, 25 patients; RR 1.52, 95% CI 0.66 to 3.50). One small study found that 6‐mercaptopurine was superior to methotrexate for maintenance of remission. In the study, 50% (7/14) of 6‐mercaptopurine patients and 92% (11/12) of methotrexate patients failed to maintain remission (1 study, 26 patients; RR 0.55, 95% CI 0.31 to 0.95). One very small study compared azathioprine with cyclosporin and found that there was no significant difference between patients failing remission on azathioprine (50%, 4/8) or cyclosporin (62.5%, 5/8) (1 study, 16 patients, RR 0.80 95% CI 0.33 to 1.92). When placebo‐controlled studies were pooled with aminosalicylate‐comparator studies to assess adverse events, there was no statistically significant difference between azathioprine and control in the incidence of adverse events. Nine per cent (11/127) of azathioprine patients experienced at least one adverse event compared to 2% (3/130) of placebo patients (5 studies, 257 patients; RR 2.82, 95% CI 0.99 to 8.01). Patients receiving azathioprine were at significantly increased risk of withdrawing due to adverse events. Eight per cent (8/101) of azathioprine patients withdrew due to adverse events compared to 0% (0/98) of control patients (5 studies, 199 patients; RR 5.43, 95% CI 1.02 to 28.75). Adverse events related to study medication included acute pancreatitis (3 cases, plus 1 case on cyclosporin) and significant bone marrow suppression (5 cases). Deaths, opportunistic infection or neoplasia were not reported.
Authors' conclusions
Azathioprine therapy appears to be more effective than placebo for maintenance of remission in ulcerative colitis. Azathioprine or 6‐mercaptopurine may be effective as maintenance therapy for patients who have failed or cannot tolerate mesalazine or sulfasalazine and for patients who require repeated courses of steroids. More research is needed to evaluate superiority over standard maintenance therapy, especially in the light of a potential for adverse events from azathioprine. This review updates the existing review of azathioprine and 6‐mercaptopurine for maintenance of remission in ulcerative colitis which was published in the Cochrane Library (September 2012).
Plain language summary
Azathioprine and 6‐mercaptopurine for maintenance of remission in ulcerative colitis
Studies of azathioprine and 6‐mercaptopurine for maintenance treatment of ulcerative colitis. Seven studies were reviewed and provide the best evidence we have. Study quality was mostly poor. The studies tested 302 people over the age of eighteen who had ulcerative colitis. The subjects received oral azathioprine or 6‐mercaptopurine, placebo (fake pills) or standard maintenance treatment (mesalazine or sulfasalazine). The studies lasted for at least 12 months.
What is ulcerative colitis and could azathioprine and 6‐mercaptopurine work? Ulcerative colitis is a chronic inflammatory disorder of the colon. The most common symptoms of ulcerative colitis are bloody diarrhoea and abdominal pain. Azathioprine and 6‐mercaptopurine are thought to reduce inflammation by blocking the immune system.
What did the studies show? The studies showed that azathioprine was better than placebo for maintenance treatment (i.e. preventing the disease from coming back once the patient has responded to treatment). Fifty‐six per cent of patients treated with azathioprine were disease free after one year of treatment compared to 35% of patients who received placebo.
How safe are azathioprine and 6‐mercaptopurine? The drugs were generally well tolerated and side effects occurred infrequently. However, serious side effects such as acute pancreatitis (inflammation of the pancreas that causes severe abdominal pain ‐ a 2% risk) and bone marrow suppression (failure to make normal blood cells ‐ a 4% risk) can occur. Patients taking these drugs should be regularly monitored for evidence of effectiveness and side effects.
What is the bottom line? Azathioprine may be an effective maintenance treatment for patients who have failed or cannot tolerate mesalazine or sulfasalazine and for patients who require repeated courses of steroids.
Summary of findings
Background
Maintenance of remission is a major issue in inflammatory bowel disease. An ideal maintenance therapy would be effective in reducing the occurrence of relapse, be free of adverse events, inexpensive and easy to use (Campieri 2003). Currently, sulfasalazine or mesalazine (5‐ASA) are the standard therapy for quiescent ulcerative colitis with good evidence to support their use (Wang 2016). In distal disease, topical preparations are also effective and safe (Marshall 2012). However, there are patients with relapsing disease despite standard maintenance therapy. In addition, some patients do not tolerate 5‐ASA or sulfasalazine, or therapy with these drugs is ineffective (Freeman 2012). Azathioprine or 6‐mercaptopurine are commonly used in these settings.
The antimetabolites, azathioprine and 6‐mercaptopurine, are purine analogues that interfere with nucleic acid metabolism by acting as substrate competitive antagonists, resulting in immunosuppression and reduced cell proliferation (Dubinsky 2004). 6‐Mercaptopurine was first synthesized in 1951 and initially used to treat leukaemia. Azathioprine, its S‐substituted precursor, was synthesized in 1957. Azathioprine has a longer half life and a different spectrum and perhaps lower level of adverse events than 6‐mercaptopurine (Dubinsky 2004), but there are no comparative trials in humans. Onset of action is delayed for up to 3 to 4 months of treatment (Su 2004). Toxicity, the risk for severe bone marrow suppression in particular, is increased in patients with thiopurine‐S‐methyltransferase (TPMT) deficiency, which occurs in 0.3% (homozygosity, low or absent levels) and 11% (heterozygosity, intermediate levels) of the general population respectively (Colombel 2000; Weinshilboum 1980).
The use of azathioprine for the treatment of quiescent ulcerative colitis was first reported in 1966 (Bowen 1966). A survey conducted by Hilsden 2003 showed that 12% of the patient members of the Crohn's and Colitis Foundation of Canada who are diagnosed with ulcerative colitis are treated with azathioprine or 6‐mercaptopurine. Other surveys have shown that 77% of gastroenterologists in Europe and North America, and up to 93% of British consultant gastroenterologists use azathioprine for the treatment of ulcerative colitis (Meuwissen 2000; Stack 1999). The common practice of using azathioprine or 6‐mercaptopurine for maintenance of remission in ulcerative colitis, however, is based on limited data (Bressler 2015). Although evidence exists to support the use of azathioprine and 6‐mercaptopurine for maintenance of remission in Crohn's disease (Chande 2015), the use of these drugs for maintenance of remission in ulcerative colitis remains controversial.
The first randomized double blind placebo controlled trial on azathioprine for maintenance treatment in ulcerative colitis was reported by Jewell 1974. There have been few studies since, most of them small, with inconsistent results. Evaluating the evidence concerning the safety and efficacy of purine antimetabolites is important, as azathioprine maintenance is usually considered long term treatment (Meuwissen 2000). Major adverse events may occur necessitating regular monitoring (Chouchana 2012). It is estimated that 9 to 25% of patients on azathioprine discontinue treatment due to adverse events (Gearry 2004; Gearry 2005). These include potentially serious adverse events including bone marrow suppression, pancreatitis, hepatotoxicity, lymphoma, and skin cancer (Kotlyar 2015; Long 2012; Present 1989; Wallace 2001). This systematic review is an update of a previously published Cochrane review (Timmer 2012).
Objectives
The primary objective of the review was to assess the efficacy and safety of azathioprine and 6‐mercaptopurine for the maintenance of remission in ulcerative colitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials of at least 12 months duration were considered for review. Comparison treatments included placebo, or other active maintenance therapies. Open label studies were also considered.
Types of participants
Patients in whom azathioprine or 6‐mercaptopurine were used to treat ulcerative colitis in remission, with or without a preceding period of induction of remission were considered for inclusion. Remission of ulcerative colitis was defined as mild or absent symptoms with complete discontinuation of corticosteroids, irrespective of the use of prophylactic medication, and continuing evidence on sigmoidoscopy of an uninflamed or grade 1 mucosa (Baron 1964). Patients with chronic active disease were not considered for inclusion.
Types of interventions
Randomized controlled trials of oral azathioprine or 6‐mercaptopurine used for the treatment of patients with ulcerative colitis in remission were considered for inclusion. This included trials in which these drugs were added to the treatment of patients in remission, withdrawal studies and studies in which there was more than one phase (e.g. active followed by maintenance).
Types of outcome measures
The primary outcome was defined as failure to maintain clinical or endoscopic remission at 12 months from randomization or later, i.e. clinical or endoscopic relapse, or early withdrawal from the study as defined by the investigators. For studies where life table analysis was used the estimated probability of relapse over time was to be examined. Patients failing to achieve clinical remission during an induction phase were considered treatment failures to maintenance therapy in an intention‐to‐treat approach. Separate analyses were performed excluding these cases.
Secondary outcomes included the occurrence of any adverse event (particularly opportunistic infection, pancreatitis, bone marrow suppression, cancer and death) and withdrawal due to adverse events. Data were extracted to investigate the influence of the dose of azathioprine or 6‐mercaptopurine treatment, the duration of previous azathioprine or 6‐mercaptopurine treatment (for withdrawal trials), and the effect of other concurrent therapies.
Search methods for identification of studies
We searched MEDLINE (Ovid), EMBASE (Ovid), and the Cochrane Library from inception to 30 July 2015 to identify relevant studies. Conference proceedings and references were also searched to identify additional studies. The electronic search strategies are reported in Appendix 1.
Data collection and analysis
Evaluation of Included Studies
Trials identified by the search strategy were independently assessed for inclusion by two authors (PP and JKM). Two authors (PP and JKM) independently extracted data from included studies. A third author (NC) was involved for problematic issues. Methodological criteria as well as the results of each study were recorded on standard data forms. All results were tabulated on an intention‐to‐treat basis. Results excluding treatment failures during an induction period, if present, were also tabulated. The methodological quality of the studies included in this review was assessed using the Cochrane risk of bias tool (Higgins 2011), which involves examining the following study characteristics:
Randomization sequence generation;
Patient allocation concealment;
Blinding;
Incomplete outcome data;
Selective reporting; and
Other sources of bias.
Studies were then judged as being at high, low or unclear risk of bias based on the evidence provided in the available publications (Higgins 2011). Any disagreement between the authors was resolved by consensus.
The GRADE approach was used to assess the overall quality of evidence supporting the outcomes in this review (Guyatt 2008; Schünemann 2011). This approach classifies outcomes as high, moderate, low or very low quality. Data from randomized trials start as high quality evidence. Evidence may be downgraded based on the following criteria:
Risk of bias from the studies;
Indirect evidence;
Inconsistency (i.e. unexplained heterogeneity);
Imprecision; and
Publication bias.
The overall quality of evidence behind each outcome was determined after considering each of these elements, and categorized as high quality (i.e. further research is very unlikely to change our confidence in the estimate of effect); moderate quality (i.e. further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate); low quality (i.e. further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate); and very low quality (i.e. we are very uncertain about the estimate) (Guyatt 2008; Schünemann 2011). Any disagreements between the authors on the GRADE analysis were resolved by consensus.
Statistical methods:
For each individual study a risk ratio (RR) for the primary outcome (relapse during the study period) along with 95% confidence intervals (95% CI) was calculated based on two by two tables for comparable periods of follow‐up (12 months). All patients randomized were included following an intention‐to‐treat approach, but additional analysis were performed excluding primary treatment failures from studies including an induction period. The presence of heterogeneity among studies was assessed using the chi2 test, a P value of 0.10 was regarded as statistically significant. The I2 statistic was used to quantify inconsistency (Higgins 2003). This statistic describes the percentage of the variability in effect estimates that are due to heterogeneity rather than sampling error. A value greater than 50% was considered evidence of substantial heterogeneity. In the presence of significant heterogeneity, pooled analysis was not performed. If homogeneity was likely (I2 < 0.03), pooled RR with 95% confidence intervals were calculated using a fixed‐effect model based on the method by Mantel and Haenszel as a primary analysis.
Sensitivity analyses were to be performed to examine the effects of drug dose (corrected for the differential potency of azathioprine and 6‐mercaptopurine), type of control intervention (active control, placebo) and study quality (concealed allocation, blinding) on the results of the analysis. Planned analyses also included calculating RR separately for withdrawal studies, studies including an induction period, and studies adding azathioprine to patients already in stable remission.
Results
Description of studies
A literature search conducted on July 30, 2015 identified 1082 records. After duplicates were removed, a total of 840 trials remained for review of titles and abstracts. Two authors (PP and JKM) independently reviewed the titles and abstracts of these trials and 24 studies were selected for full text review (see Figure 1). Fourteen of these studies were excluded (See: Characteristics of excluded studies). Ten reports of 7 studies, including 302 patients, were identified which examined the efficacy of purine antimetabolites compared to placebo or active maintenance therapy in ulcerative colitis (Hawthorne 1992; Jewell 1974; Mate‐Jimenez 2000; Paraskeva 2000; Sood 2000; Sood 2002; Sood 2003). The studies used azathioprine in different doses: 2.0 mg/kg/d throughout (Sood 2000), 2.5 mg/kg/d for three months followed by a reduction to 1.5 to 2.0 mg/kg/d (Jewell 1974), 2.5 mg/kg/d throughout (Paraskeva 2000; Sood 2002; Sood 2003) or at variable dosing (Hawthorne 1992). There was only one study examining 6‐mercaptopurine at a dose of 1.5 mg/kg/d (Mate‐Jimenez 2000). Four studies were placebo controlled (Hawthorne 1992; Jewell 1974; Sood 2000; Sood 2002), three used active comparators (mesalazine and methotrexate ‐ Mate‐Jimenez 2000; cyclosporin ‐ Paraskeva 2000; sulfasalazine ‐ Sood 2003). In the placebo controlled study by Sood 2000, all patients received co‐medication with 6 g/day sulfasalazine. Co‐medication with sulfasalazine or mesalazine was allowed in the study by Hawthorne 1992. In Paraskeva 2000, all patients were allowed tapering steroids and maintenance therapy with 5‐ASA.
1.
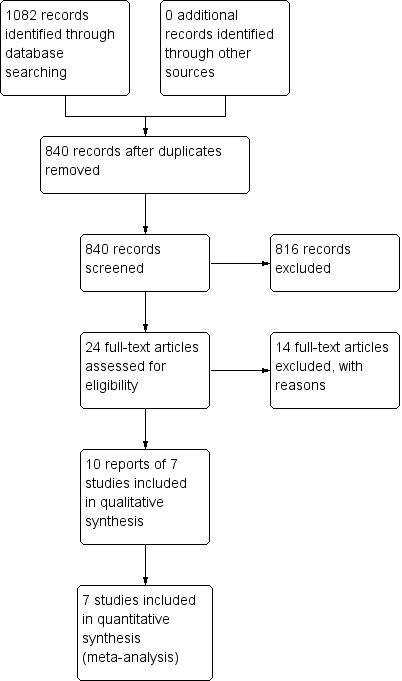
Study flow diagram.
In all but two of the trials (Hawthorne 1992; Paraskeva 2000), patients were randomized during active disease, and maintenance was preceded by an induction period. A steroid tapering scheme was used at the beginning of all of these trials. In addition, antibiotics were given during the induction period in two studies (Sood 2000; Sood 2002). In contrast, the study by Hawthorne 1992 used a withdrawal design where patients who had been in remission on azathioprine for 6 months or longer before being randomized to withdrawal (i.e. placebo) or maintenance on azathioprine. The study by Paraskeva 2000 used a 6 month treatment phase with either azathioprine or cyclosporin coupled with tapering steroids and 5‐ASA treatment before discontinuing all medications.
The time of follow up varied from 12 months to 76 weeks. All studies reported the proportion of patients relapsing, or remaining in remission. In addition there was information on reduction of clinical scores, safety data or number of relapses during the study period from single studies. There were no survival methods applied to evaluate the time to relapse.
The included studies are described in detail in the 'Characteristics of included studies' table" (Characteristics of included studies).
Risk of bias in included studies
The methodological quality of the studies was unsatisfactory in four of seven studies (See Figure 2). Four studies (Jewell 1974; Sood 2000; Sood 2002; Sood 2003), described adequate methods for sequence generation and were rated as low risk for this item. Three studies (Hawthorne 1992; Mate‐Jimenez 2000; Paraskeva 2000) were rated as unclear risk because the methods used for randomization were not described. Appropriate methods for allocation concealment were reported or supplied by the authors for two of the seven studies (Hawthorne 1992; Jewell 1974), and these studies were rated as low risk of bias for this item. The other five studies (Mate‐Jimenez 2000;Paraskeva 2000; Sood 2000; Sood 2002; Sood 2003), were rated as unclear for allocation concealment. Two of the three studies using active comparators were open label (Mate‐Jimenez 2000; Sood 2003), and therefore at high risk of bias for lack of blinding. Paraskeva 2000 did not describe blinding in the abstract. The study by Sood 2000 was single blind (patients only) and was rated as high risk of bias for blinding. Six studies were rated as low risk for incomplete outcome data (Hawthorne 1992; Jewell 1974; Mate‐Jimenez 2000; Paraskeva 2000; Sood 2002; Sood 2003). Three of the studies were rated at an unclear risk of bias for selective reporting because of missing outcomes (Sood 2003), or potential post hoc outcomes (Mate‐Jimenez 2000; Sood 2002). The other four studies were rated as low risk for this item. Furthermore, it is of note that all studies were small (maximum number of patients: 80 (Jewell 1974)).
2.
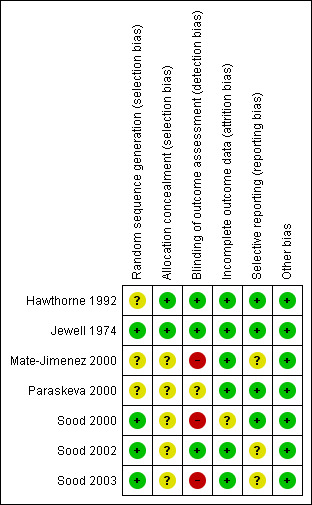
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Summary of findings for the main comparison. Azathioprine versus placebo for maintenance of remission in ulcerative colitis.
| Azathioprine versus placebo for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis Settings: Outpatient Intervention: Azathioprine or 6‐Mercaptopurine versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | AZA versus PBO | |||||
| Failure to maintain remission | 650 per 10001 |
442 per 1000 (351 to 559) |
RR 0.68 (0.54 to 0.86) |
232 (4 studies) | ⊕⊕⊝⊝ low2,3 | |
| Any adverse events | 26 per 10001 |
65 per 1000 (21 to 201) |
RR 2.51 (0.82 to 7.74) |
232 (4 studies) | ⊕⊝⊝⊝ very low2,4 | |
| Withdrawal due to adverse event | 0 per 10001 |
0 per 1000 (0 to 0) |
RR 7.00 (0.38 to 128.87) |
152 (3 studies) | ⊕⊝⊝⊝ very low2,5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; AZA: azathioprine; PBO: placebo | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to high risk of bias in one study in the pooled analysis (single‐blind). 3 Downgraded one level due to sparse data (127 events). 4 Downgraded two levels due to very sparse data (12 events). 5 Downgraded two levels due to very sparse data (3 events).
Summary of findings 2. Azathioprine versus sulfasalazine for maintenance of remission in ulcerative colitis.
| Azathioprine (AZA) versus sulfasalazine for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis Settings: Outpatients Intervention: Azathioprine versus sulfasalazine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | AZA versus Sulfasalazine | |||||
| Failure to maintain remission | 385 per 10001 | 585 per 1000 (254 to 1348) | RR 1.52 (0.66 to 3.50) | 25 (1 study) | ⊕⊝⊝⊝ very low2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of included study. 2 Downgraded one level due to high risk of bias (open label design). 3 Downgraded two levels due to very sparse data (12 events) and wide confidence interval.
Summary of findings 3. 6‐Mercaptopurine versus 5‐ASA for maintenance of remission in ulcerative colitis.
| 6‐Mercaptopurine versus 5‐ASA for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis Settings: Outpatients Intervention: 6‐Mercaptopurine versus 5‐ASA | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | 6‐MP versus 5‐ASA | |||||
| Failure to maintain remission | 1000 per 10001 | 530 per 1000 (310 to 900) | RR 0.53 (0.31 to 0.90) | 22 (1 study) | ⊕⊝⊝⊝ very low2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; 6‐MP: 6‐mercaptopurine | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of included study. 2 Down‐graded one level due to high risk of bias (open label design). 3 Down‐graded two levels due to very sparse data (15 events).
Summary of findings 4. 6‐Mercaptopurine versus methotrexate for maintenance of remission in ulcerative colitis.
| 6‐Mercaptopurine versus methotrexate for maintenance of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis Settings: Outpatients Intervention: 6‐Mercaptopurine versus methotrexate | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | 6‐MP versus MTX | |||||
| Failure to maintain remission | 917 per 10001 | 504 per 1000 (284 to 871) | RR 0.55 (0.31 to 0.95) | 26 (1 study) | ⊕⊕⊝⊝ very low2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; 6‐MP: 6 mercaptopurine; MTX: methotrexate | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of included study. 2 Downgraded one level due to high risk of bias (open label design). 3 Downgraded two levels due to very sparse data (18 events).
Summary of findings 5. Azathioprine (AZA) versus cyclosporin of remission in ulcerative colitis.
| Azathioprine (AZA) versus cyclosporin of remission in ulcerative colitis | ||||||
| Patient or population: Patients with quiescent ulcerative colitis Setting: Outpatients Intervention: Azathioprine Comparison: cyclosporin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with cyclosporin | Risk with Azathioprine | |||||
| Failure to maintain remission | 625 per 10001 | 500 per 1000 (206 to 1000) | RR 0.80 (0.33 to 1.92) | 16 (1 study) | ⊕⊝⊝⊝ very low2,3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Control group risk estimates come from control arm of included study. 2 Downgraded one level due to unclear risk of bias for random sequence generation, allocation concealment, and blinding. 3 Downgraded two levels due to very sparse data (9 events).
Summary of findings 6. Adverse events across placebo‐controlled and aminosalicylate‐comparator trials.
| Adverse events across placebo‐controlled and aminosalicylate‐comparator trials | ||||||
| Patient or population: Patients with quiescent ulcerative colitis Setting: Outpatient Intervention: Azathioprine and 6‐mercaptopurine Comparison: placebo/active therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Adverse events ‐ all trials | |||||
| Any adverse event | 23 per 10001 | 65 per 1000 (23 to 185) | RR 2.82 (0.99 to 8.01) | 257 (5 studies) | ⊕⊝⊝⊝ very low2,3 | |
| Withdrawal due to adverse event | 0 per 10001 | 0 per 1000 (0 to 0) | RR 5.43 (1.02 to 28.75) | 199 (5 studies) | ⊕⊝⊝⊝ very low2,4 | |
| Acute pancreatitis | 0 per 10001 | 0 per 1000 (0 to 0) | RR 4.13 (0.48 to 35.48) | 279 (6 RCTs) | ⊕⊝⊝⊝ very low2,5 | |
| Bone marrow suppression | 8 per 10001 | 24 per 1000 (5 to 114) | RR 3.09 (0.64 to 14.83) | 257 (5 RCTs) | ⊕⊝⊝⊝ very low2,6 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to high risk of bias in the pooled studies (blinding). 3 Downgraded two levels due to very sparse data (14 events). 4 Downgraded two levels due to very sparse data (8 events). 5 Downgraded two levels due to very sparse data (3 events). 6 Downgraded two levels due to very sparse data (6 events).
Azathioprine versus Placebo
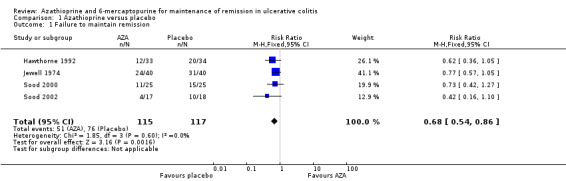
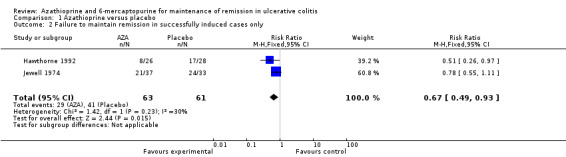
Four studies (Hawthorne 1992; Jewell 1974; Sood 2000; Sood 2002), including 232 patients, compared azathioprine to placebo. A pooled analysis showed azathioprine was significantly superior to placebo for maintenance of remission. Forty‐four per cent (51/115) of patients in the azathioprine group failed to maintain remission compared to 65% (76/117) of patients receiving placebo (RR 0.68, 95% CI 0.54 to 0.86). No heterogeneity was detected for this comparison (P = 0.60; I² = 0%). A GRADE analysis showed that the overall quality of the evidence supporting this outcome was low due to a high risk of bias in one study in the pooled analysis (Sood 2000), and imprecision due to sparse data (127 events; See Table 1). The results were similar when analyses were restricted to patients after successful induction of remission; however, data on primary treatment failures were only available for two studies. Forty‐six percent of patients in the azathioprine group failed to maintain remission compared to 67% of placebo patients (2 studies, 123 patients; RR 0.67, 95% CI 0.49 to 0.93). There was no obvious evidence for an effect by dose of azathioprine or use of co‐medication in these studies, but the treatment schedules were too varied for formal testing. Also, a difference between azathioprine and 6‐mercaptopurine could not be examined, as the only 6‐mercaptopurine study was open label and did not use a placebo control (Mate‐Jimenez 2000).
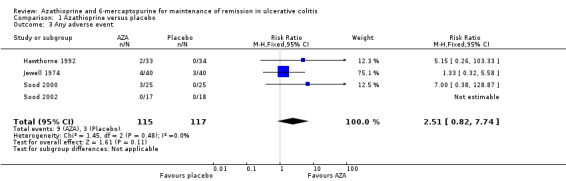
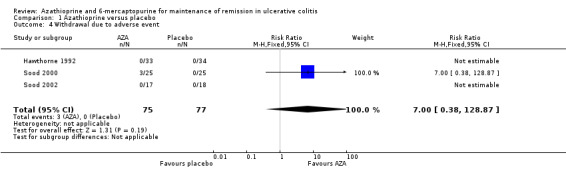
The four placebo‐controlled studies also reported adverse event rates (Hawthorne 1992; Jewell 1974; Sood 2000; Sood 2002). Overall, azathioprine and placebo were not significantly different with regard to the risk of adverse events. Eight percent (9/115) of azathioprine patients and 3% (3/117) of placebo patients experienced at least one adverse event (232 patients, RR 2.51, 95% CI 0.82 to 7.74). No heterogeneity was detected for this comparison (P = 0.48; I² = 0%). A GRADE analysis showed that the overall quality of the evidence supporting this outcome was very low due to risk of bias and serious imprecision due to sparse data (12 events; See Table 1). Withdrawals due to adverse events were reported in three studies (Hawthorne 1992; Sood 2000; Sood 2002). A pooled analysis revealed no statistically significant difference in withdrawals due to adverse events. Four percent (3/75) of azathioprine patients withdrew due to adverse events compared to and 0% (0/77) of placebo patients (152 patients, RR 7.00, 95% CI 0.38 to 128.87). However, this result should be interpreted with caution due to the small sample size and a GRADE rating of very low (risk of bias, very sparse data and very wide confidence intervals; See Table 1).
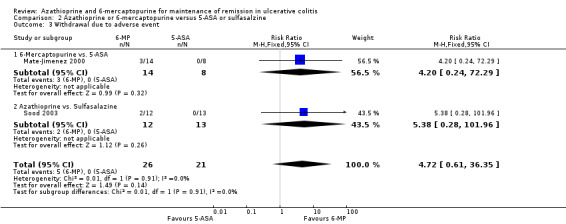
Azathioprine or 6‐Mercaptopurine versus 5‐Aminosalicylate or Sulfasalazine
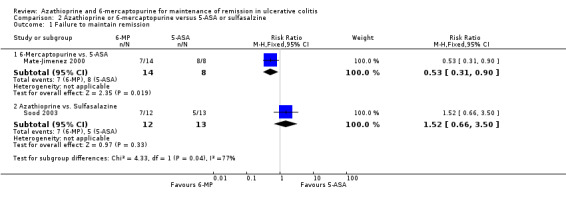
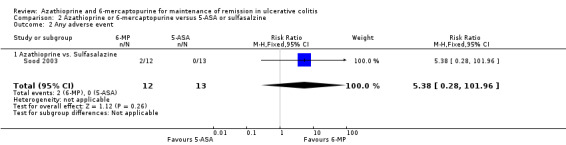
Sood 2003 compared azathioprine to sulfasalazine and Mate‐Jimenez 2000 compared 6‐mercaptopurine to 5‐ASA therapy. A pooled analysis of these studies to assess failure to maintain remission was not possible due to a high degree of heterogeneity (P = 0.03; I² = 79%). Sood 2003 reported that 58% (7/12) of azathioprine patients failed to maintain remission compared to 38% (5/13) of sulfasalazine patients (25 patients; RR 1.52, 95% CI 0.66 to 3.50). A GRADE analysis showed that the overall quality of the evidence supporting this outcome was very low due to a high risk of bias (open label study), and serious imprecision due to sparse data (12 events; See Table 2). Adverse events occurred in 17% (2/12) and 0% (0/13) patients administered azathioprine and sulfasalazine, respectively (RR 5.38, 95% CI 0.28 to 101.96). All of the patients who experienced adverse events withdrew. Mate‐Jimenez 2000 reported that 50% (7/14) of 6‐mercaptopurine patients failed to maintain remission compared to 100% (8/8) of 5‐aminosalicylate patients (22 patients; RR 0.53, 95% CI 0.31 to 0.90). A GRADE analysis showed that the overall quality of the evidence supporting this outcome was very low due to a high risk of bias (open label study), and serious imprecision due to sparse data (15 events; See Table 3). Withdrawals due to adverse events by week 30 occurred in 21% (3/14) patients receiving 6‐mercaptopurine compared to 0% (0/8) of patients receiving 5‐aminosalicylates (RR 4.20, 95% CI 0.24 to 72.29). These results should be interpreted with caution as both studies were unblinded and had small sample sizes (Mate‐Jimenez 2000; Sood 2003).
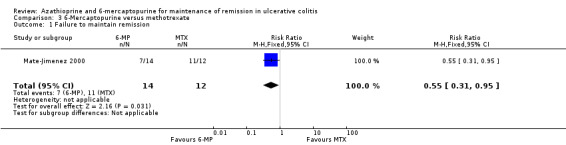
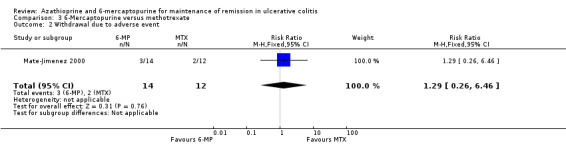
6‐Mercaptopurine versus Methotrexate
Mate‐Jimenez 2000 compared 6‐mercaptopurine (n = 14) to methotrexate (n = 12). Fifty percent (7/14) of 6‐mercaptopurine patients failed to maintain remission compared to 92% (11/12) of methotrexate patients (RR 0.55, 95% CI 0.31 to 0.95). A GRADE analysis showed that the overall quality of the evidence supporting this outcome was very low due to a high risk of bias (open label study), and serious imprecision due to sparse data (18 events; See Table 4). Twenty‐one percent (3/14) and 17% (2/12) patients receiving 6‐mercaptopurine and methotrexate, respectively, withdrew due to adverse events (RR 1.29, 95% CI 0.26 to 6.46). These results should be interpreted with caution as the study was open label and had a small sample size.
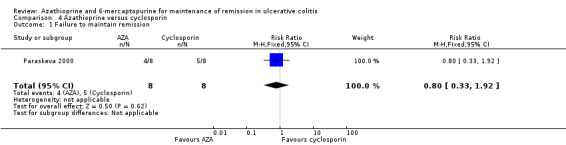
Azathioprine vs Cyclosporin
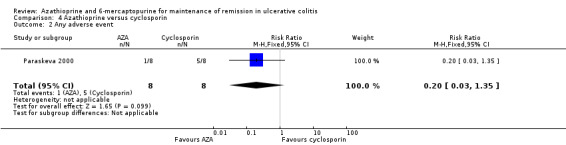
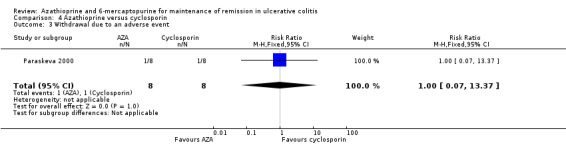
Paraskeva 2000 evaluated the effectiveness of oral azathioprine (2.5 mg/kg/day) (n = 8) against oral cyclosporin (4 mg/kg/day) (n = 8) at maintaining remission after 6 months of therapy, coupled with tapering steroids and 5‐ASA in patients with remissive UC, who had previously responded favourably to intravenous cyclosporin. The patients were followed for an additional 12 months to monitor for relapse after all medications were discontinued. At 18 moths, 50% (4/8) of patients receiving azathioprine had failed to maintain remission, compared to (62.5% (5/8) of those receiving cyclosporin (RR 0.80, 95% CI 0.33 to 1.92). A GRADE analysis showed that the overall quality of the evidence supporting this outcome was very low due to unclear risk of bias (abstract publication), and serious imprecision due to sparse data (9 events; See Table 5). One patient from each group withdrew due to adverse events (RR 1.00 95% CI 0.07 to 13.37), and 4/4 of those completing the 6 months of treatment in the cyclosporin group had experienced at least 1 adverse event (RR 0.20 95% CI 0.03 to 1.35). These results should be interpreted with caution due to the small sample size and a GRADE rating of very low.
Adverse Events Across Placebo‐controlled and Aminosalicylate‐comparator Trials
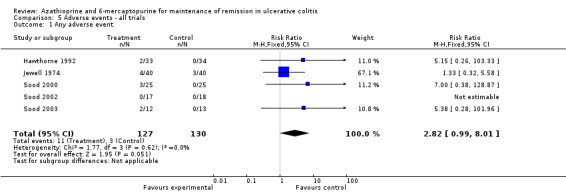
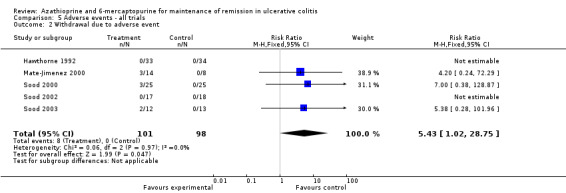
We decided to pool comparators with a known low adverse effect profile (i.e. aminosalicylates) with the placebo‐controlled studies to assess adverse effects. There was no statistically significant difference in the incidence of adverse events when these studies were pooled (Hawthorne 1992; Jewell 1974; Sood 2000; Sood 2002; Sood 2003). Nine per cent of azathioprine patients (11/127) experienced at least one adverse event compared to 2% (3/130) of control patients (RR 2.82, 95% CI 0.99 to 8.01). A GRADE analysis showed that the overall quality of the evidence supporting this outcome was very low due to high risk of bias (blinding), and serious imprecision due to sparse data (14 events; See Table 6). When studies that reported withdrawal due to adverse events were pooled, there was a statistically significant difference in withdrawals due to adverse events. Eight per cent of patients in the azathioprine group (8/101) withdrew due to adverse events compared to 0% of control patients (RR 5.43, 95% CI 1.02 to 28.75). A GRADE analysis showed that the overall quality of the evidence supporting this outcome was very low due to high risk of bias (blinding), and serious imprecision due to sparse data (8 events; See Table 6). Pancreatitis was reported in 3 of 141 patients receiving antimetabolites. Jaundice or hepatitis was reported in 1 of 127 patients receiving azathioprine. Bone marrow suppression was reported for 5 of 127 patients on azathioprine. One case of pancreatitis was reported in a cyclosporin patient. Deaths, opportunistic infections or neoplasia were not reported. Mate‐Jimenez 2000 reported several cases of bone marrow suppression (3 of 30 IBD patients receiving 6‐mercaptopurine) in patients with IBD, some of whom had ulcerative colitis. Mate‐Jimenez 2000 did not report separate the events by disease entity. Jewell 1974 did not formally withdraw patients with serious adverse events from the study, but paused therapy and restarted open label treatment. When studies that reported on acute pancreatitis were pooled, there was no statistically significant difference in the proportion of patients who developed pancreatitis. Two per cent of patients in the azathioprine/6‐mercaptopurine group (3/141) developed pancreatitis compared to 0% of control patients (RR 4.13, 95% CI 0.48 to 35.48). A GRADE analysis showed that the overall quality of the evidence supporting this outcome was very low due to high risk of bias (blinding), and serious imprecision due to sparse data (3 events; See Table 6). When studies that reported on bone marrow suppression were pooled, there was no statistically significant difference in the proportion of patients who developed bone marrow suppression. Four per cent of patients in the azathioprine group (5/127) developed bone marrow suppression compared to 0.8% (1/130) of control patients (RR 3.09, 95% CI 0.64 to 14.83). A GRADE analysis showed that the overall quality of the evidence supporting this outcome was very low due to high risk of bias (blinding), and serious imprecision due to sparse data (6 events; See Table 6).
Discussion
Based on four trials (Hawthorne 1992; Jewell 1974; Sood 2000; Sood 2002), azathioprine was shown to be superior to placebo for the prevention of relapse in ulcerative colitis. The difference was statistically significant and appears to be clinically relevant. Relapse rates in the placebo groups ranged from 55% to 78%. Based on a mean failure rate of 65% in the combined placebo group, the pooled risk ratio of 0.68 would translate into a number needed to treat of 5 (absolute risk reduction 21%).
Several observations limit the reliability and clinical usefulness of this conclusion. Foremost, the quality of the trials was unsatisfactory for the majority of evaluated trials. Inadequate concealment of allocation has been shown to impact on effect sizes in clinical trials (Schulz 1995). Adequateness of concealment could not be assessed for five out of seven studies due to insufficient information (Mate‐Jimenez 2000; Paraskeva 2000; Sood 2000; Sood 2002; Sood 2003). All of the trials had small sample sizes. Information on the assessment of relapse or remission remained mostly obscure, although most reported Baron's criteria for inclusion. The number of eligible trials was too small to assess the occurrence of publication bias, or any bias arising from differences in methodological quality.
In addition to these threats to the internal validity of the results, several issues arose concerning the applicability of the results to clinical practice. There were no long term studies. Moreover, it is unclear from the results of the included studies when it is appropriate to use azathioprine in clinical practice. Clinicians can prescribe purine antimetabolites when 5‐ASA agents fail, or they can be used in addition to 5‐ASA agents or instead of 5‐ASA agents. The role of azathioprine as maintenance therapy in ulcerative colitis in the era of biologic agents is uncertain. These issues should be addressed in future studies.
Due to the limited number of trials and variable dosing of azathioprine we were unable to assess a dose‐response relationship, or differences by use of co‐medication or duration of treatment. Only two trials compared antimetabolites to current standard maintenance therapy with aminosalicylates (Mate‐Jimenez 2000; Sood 2003). Both were of insufficient quality due to lack of blinding. One of these open label trials was the only study available that evaluated 6‐mercaptopurine (Mate‐Jimenez 2000).
For four of the included studies (Jewell 1974; Sood 2000; Sood 2002; Sood 2003), patients were randomized while in active disease. Applying an intention‐to‐treat analysis approach, we decided to include all patients randomized into the main analysis, including those who failed to reach remission. Strictly speaking, these trials were not mere maintenance trials, even though the analysis excluding induction failures rendered very similar results. There was no difference in the pooled results if the single withdrawal study was excluded (Hawthorne 1992), but this may be due to the small sample size. None of the trials included patient related outcomes such as quality of life or hospitalizations.
Case numbers were too small to conclusively assess the frequency of adverse events. However, the incidence of major adverse events in the treatment group was in accordance with previous reports from the literature, including a 2% risk of pancreatitis, and 4% risk of bone marrow suppression.
Azathioprine is commonly used as a maintenance treatment for patients with ulcerative colitis, however the lack of high quality trials evaluated in this review is remarkable. Considering the well established efficacy and safety of aminosalicylates for the maintenance of remission in ulcerative colitis (Marshall 2012; Wang 2016), antimetabolites cannot be recommended for first line treatment for this purpose. There may be a place for purine antimetabolites in patients who do not tolerate aminosalicylates. Moreover, purine antimetabolites may be appropriate maintenance treatment for patients who fail aminosalicylates or require induction with steroid therapy. Future high quality trials should allow for separate analysis of these patients, or use active comparators.
Authors' conclusions
Implications for practice.
Azathioprine may be an effective treatment for maintaining remission in ulcerative colitis. It may be most useful in patients who have failed or cannot tolerate aminosalicylates, as well as patients who require steroid therapy to induce remission. However, there is insufficient evidence to assess superiority of azathioprine alone, or azathioprine in addition to standard maintenance, as compared to maintenance with aminosalicylates, methotrexate or cyclosporin. The effectiveness of azathioprine compared to biologic therapy is unknown. Overall, given the potential for serious adverse events, azathioprine may not be an ideal first line therapy in quiescent ulcerative colitis.
Implications for research.
More data from good quality clinical trials are needed to assess the efficacy and safety of azathioprine for the maintenance of remission in ulcerative colitis as compared to standard maintenance therapy or biologic therapy. The question of when it is appropriate to use purine antimetabolite therapy in clinical practice should be addressed in future studies.
What's new
| Date | Event | Description |
|---|---|---|
| 30 July 2015 | New citation required but conclusions have not changed | Updated review with new authors |
| 30 July 2015 | New search has been performed | New literature searches conducted on 30 July 2015. One new included study added |
Acknowledgements
This work was based on a previous protocol by Steinhart AH, Hawkey CJ, and Modigliani R, first published in Issue 2, 1996.
Funding for the IBD/FBD Review Group (September 1, 2010 ‐ August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105145) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III) and the Ontario Ministry of Health and Long Term Care (HLTC3968FL‐2010‐2235).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Appendices
Appendix 1. Electronic Search Strategy
EMBASE
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20. 18 not 19
21. (anti‐metabolite* or anti‐metabolite* or antimetabolite*).mp.
22. (AZA or azathioprine).mp.
23. (6‐mercaptopurine or mercaptopurine or 6‐MP or 6MP).mp.
24. azathioprine.mp. or exp azathioprine/
25. ulcerative colitis.mp. or exp ulcerative colitis/
26. inflammatory bowel disease*.mp.
27. IBD.mp.
28. or/21‐24
29. or/25‐27
30. 20 and 28 and 29
Results: 696
MEDLINE
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20. 18 not 19
21. (anti‐metabolite* or anti‐metabolite* or antimetabolite*).mp.
22. (AZA or azathioprine).mp.
23. (6‐mercaptopurine or mercaptopurine or 6‐MP or 6MP).mp.
24. azathioprine.mp. or exp azathioprine/
25. ulcerative colitis.mp. or exp ulcerative colitis/
26. inflammatory bowel disease*.mp.
27. IBD.mp.
28. or/21‐24
29. or/25‐27
30. 20 and 28 and 29
Results: 163
Cochrane Library search strategy:
#1 ulcerative colitis or UC #2 inflammatory bowel disease or IBD #3 MeSH descriptor Colitis, Ulcerative explode all trees #4 MeSH descriptor Inflammatory Bowel Diseases explode all trees #5 (#1 OR #2 OR #3 OR #4) #6 anti‐metabolite* or anti metabolite* or antimetabolite* #7 MeSH descriptor Antimetabolites explode all trees #8 6‐mercaptopurine or mercaptopurine or 6‐MP #9 MeSH descriptor 6‐Mercaptopurine explode all trees #10 aza or azathioprine #11 MeSH descriptor Azathioprine explode all trees #12 (#6 OR #7 OR #8 OR #9 OR #10 OR #11) #13 (#5 AND #12)
Results: 223
IBD Specialized Register
Title:(6‐mercaptopurine or mercaptopurine or 6‐MP or 6MP or aza or azathioprine) and (colitis or UC or inflammatory bowel disease or IBD)
Results: 0
Total: 1082
Data and analyses
Comparison 1. Azathioprine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to maintain remission | 4 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.54, 0.86] |
| 2 Failure to maintain remission in successfully induced cases only | 2 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.49, 0.93] |
| 3 Any adverse event | 4 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.82, 7.74] |
| 4 Withdrawal due to adverse event | 3 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 128.87] |
1.1. Analysis.
Comparison 1 Azathioprine versus placebo, Outcome 1 Failure to maintain remission.
1.2. Analysis.
Comparison 1 Azathioprine versus placebo, Outcome 2 Failure to maintain remission in successfully induced cases only.
1.3. Analysis.
Comparison 1 Azathioprine versus placebo, Outcome 3 Any adverse event.
1.4. Analysis.
Comparison 1 Azathioprine versus placebo, Outcome 4 Withdrawal due to adverse event.
Comparison 2. Azathioprine or 6‐mercaptopurine versus 5‐ASA or sulfasalzine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to maintain remission | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6‐Mercaptopurine vs. 5‐ASA | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.90] |
| 1.2 Azathioprine vs. Sulfasalazine | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.66, 3.50] |
| 2 Any adverse event | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.38 [0.28, 101.96] |
| 2.1 Azathioprine vs. Sulfasalazine | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.38 [0.28, 101.96] |
| 3 Withdrawal due to adverse event | 2 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.72 [0.61, 36.35] |
| 3.1 6‐Mercaptopurine vs. 5‐ASA | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.2 [0.24, 72.29] |
| 3.2 Azathioprine vs. Sulfasalazine | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.38 [0.28, 101.96] |
2.1. Analysis.
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐ASA or sulfasalzine, Outcome 1 Failure to maintain remission.
2.2. Analysis.
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐ASA or sulfasalzine, Outcome 2 Any adverse event.
2.3. Analysis.
Comparison 2 Azathioprine or 6‐mercaptopurine versus 5‐ASA or sulfasalzine, Outcome 3 Withdrawal due to adverse event.
Comparison 3. 6‐Mercaptopurine versus methotrexate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to maintain remission | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.31, 0.95] |
| 2 Withdrawal due to adverse event | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.26, 6.46] |
3.1. Analysis.
Comparison 3 6‐Mercaptopurine versus methotrexate, Outcome 1 Failure to maintain remission.
3.2. Analysis.
Comparison 3 6‐Mercaptopurine versus methotrexate, Outcome 2 Withdrawal due to adverse event.
Comparison 4. Azathioprine versus cyclosporin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to maintain remission | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.33, 1.92] |
| 2 Any adverse event | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.35] |
| 3 Withdrawal due to an adverse event | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 13.37] |
4.1. Analysis.
Comparison 4 Azathioprine versus cyclosporin, Outcome 1 Failure to maintain remission.
4.2. Analysis.
Comparison 4 Azathioprine versus cyclosporin, Outcome 2 Any adverse event.
4.3. Analysis.
Comparison 4 Azathioprine versus cyclosporin, Outcome 3 Withdrawal due to an adverse event.
Comparison 5. Adverse events ‐ all trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any adverse event | 5 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [0.99, 8.01] |
| 2 Withdrawal due to adverse event | 5 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.43 [1.02, 28.75] |
| 3 Acute pancreatitis | 6 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.13 [0.48, 35.48] |
| 4 Bone marrow suppression | 5 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.64, 14.83] |
5.1. Analysis.
Comparison 5 Adverse events ‐ all trials, Outcome 1 Any adverse event.
5.2. Analysis.
Comparison 5 Adverse events ‐ all trials, Outcome 2 Withdrawal due to adverse event.
5.3. Analysis.
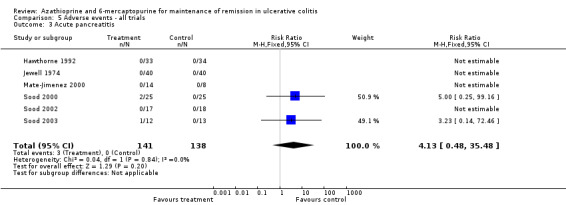
Comparison 5 Adverse events ‐ all trials, Outcome 3 Acute pancreatitis.
5.4. Analysis.
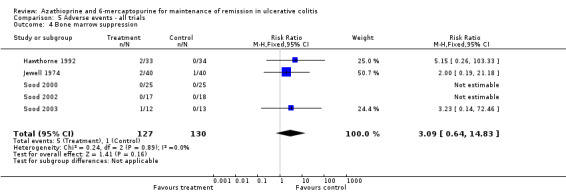
Comparison 5 Adverse events ‐ all trials, Outcome 4 Bone marrow suppression.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hawthorne 1992.
| Methods | Randomized controlled trial Double blind, placebo controlled Withdrawal study Sudy duration 1 year |
|
| Participants | Diagnosis of ulcerative colitis In remission (or chronic low grade activity or steroid dependent) On azathioprine for 6 months or longer Only patients in remission used for review: n = 67 | |
| Interventions | Azathioprine continued at variable dosing (median 100 mg/day), P.O.
Compared to azathioprine withdrawal ‐ change to placebo Co‐medication ‐ continued from pre‐trial medication: Sulfasalazine 1‐4 g/day in 39 patients (mean 2 g/day) Mesalazine 0.8 to 3.2 g/day in 28 patients (mean 1.2g/day) None in 12 patients |
|
| Outcomes | Main: (time to) clinical relapse, one year relapse rate Secondary: adverse events | |
| Notes | Additional information given by investigator, method of sequence generation was not remembered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not explicitly discussed |
| Allocation concealment (selection bias) | Low risk | Centralised pharmacy‐controlled randomisation Quote: "Randomisation was performed in hospital pharmacies" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind" Quote: "an equivalent number of placebo tablets of identical appearance" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals were noted and explained Intention‐to‐treat was followed |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Nothing of note |
Jewell 1974.
| Methods | Randomized controlled trial Double blind, placebo controlled Induction period included (steroid tapering) Study duration 1 year | |
| Participants | Diagnosis of ulcerative colitis Active disease n = 80 | |
| Interventions | Azathioprine 2.5 mg/kg/d for 3 months, then dose reduction to 1.5 to 2.0 mg/kg/d, P.O.
Compared to placebo Co‐medication induction period: Outpatients/mild attack: prednisolone 20 mg/day P.O. and prednisolone enema for 1 months, then tail off over 2 weeks Inpatient/severe attack: nil per mouth, i.v. steroids 40 mg, tetracycline, topical steroids, for 4 days, then prednisolone 40 mg P.O., taper off Co‐medication in maintenance: none |
|
| Outcomes | Main: Number of relapses during 1 year Maintenance of remission over 1 year Secondary: Failure to achieve remission Clinical severity of attacks, endoscopic and histological grading Adverse events Immunological changes | |
| Notes | Additional information on randomization/methods supplied by the author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Author correspondence |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation Quote: "trial treatment was prescribed as 'azathioprine special' and the hospital pharmacists worked from a master sheet indicating whether a particular patient was to be given real or dummy azathioprine" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, double‐dummy Quote: "dummy azathioprine" Quote: "trial treatment was prescribed as 'azathioprine special' and the hospital pharmacists worked from a master sheet indicating whether a particular patient was to be given real or dummy azathioprine" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 80 patients completed the trial |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Nothing of note |
Mate‐Jimenez 2000.
| Methods | Randomized controlled trial open label, active controls (3 treatment arms) Study duration: 30 weeks induction period, 76 weeks maintenance (numbers also available for 48 to 56 weeks) | |
| Participants | Diagnosis of ulcerative colitis (and Crohn's disease) Outpatients, steroid dependent (steroids > 20 mg or frequent relapses) Maintenance part: successful withdrawal of steroids Only ulcerative colitis used for review: n = 34 (20 in maintenance) | |
| Interventions | 6‐mp 1.5 mg/kg/day; decreased to 1 mg/kg/day once in remission
a) compared to methotrexate 15 mg/week; decreased to 10 mg once in remission
b) compared to 5‐ASA 3 g/day Co‐medication induction phase: Prednisolone continued from pre‐trial dosis, max. 1 mg/kg/day for 2 more weeks Followed by tapering by 8 mg/week, depending on clinical status Co‐medication maintenance phase: Antidiarrhoeals and folic acid as needed |
|
| Outcomes | Main: attaining remission (induction phase)
Maintaining remission (maintenance phase) Secondary: adverse events, compliance |
|
| Notes | Information received from author not sufficient to assess randomization /allocation procedure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not explicitly discussed |
| Allocation concealment (selection bias) | Unclear risk | Not described in published study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not explicitly discussed in the publish study, it was assumed to be open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24/72 patients withdrew in the first 30 weeks of the trial with reasons described. Worst outcome assumed in analysis |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were reported, as well as some post hoc comparisons |
| Other bias | Low risk | No other issues |
Paraskeva 2000.
| Methods | Randomized, active control trial | |
| Participants | Patients (N = 16), aged 18‐58 years, with acute, steroid resistant Ulcerative colitis, which had previously responded and tolerated IV cyclosporin (4 mg/kg/day). Pateints were in maintenance therapy on tapered steroids and 5‐ASA | |
| Interventions | Group A: n = 8, oral azathioprine (2.5 mg/kg/day) for 6 months Group B: n = 8, oral cyclosporin (4 mg/kg/day) for 6 months All patients were on tapering steroids and were on maintenance therapy with 5‐ASA Follow‐up after medication discontinuation for another 12 months |
|
| Outcomes | Primary outcome: Proportion of patients still in remission Secondary outcome: Safety data |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in abstract |
| Allocation concealment (selection bias) | Unclear risk | Not described in abstract |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in abstract |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing/withdrawn data was accounted for with explanation. Intention to treat analysis used |
| Selective reporting (reporting bias) | Low risk | No protocol was available, all outcomes were reported |
| Other bias | Low risk | The study appears not to have any other forms of bias |
Sood 2000.
| Methods | Randomized controlled trial Single (patient) blind placebo controlled Induction period included (steroid tapering) Study duration 12 months | |
| Participants | Diagnosis of ulcerative colitis Relapse within 2 months of steroid withdrawal following successful induction of remission n = 50 | |
| Interventions | Azathioprine 2.0 mg/kg/d for 1 year
Compared to placebo Co‐medication induction period: i.v. hydrocortisone 400 mg/day, sulfasalazine 6‐8 g/day, ciprofloxacin and metronidazole for 5 days Taper off of steroids to 1 mg/kg/day P.O., then by 10 mg every 10 days to 20 mg, then by 5 mg every 10 days Co‐medication maintenance: sulfasalazine 6 g/day |
|
| Outcomes | Main: Complete or partial remission at 1 year (after successful induction) Secondary: adverse events | |
| Notes | The author was approached for clarification of the randomization and allocation concealment; no response was received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "pseudorandom numbers ranging from 0‐1 generated by a scientific calculator" |
| Allocation concealment (selection bias) | Unclear risk | Not described in the published study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "single‐blind" Quote: "identical matched placebo drugs" Quote: "drugs were provided by a single coordinator (VK) to the patients in identical blister packs" Quote: "The treating physician was aware of the drug treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Five patients...were excluded because of noncompliance and violation of treatment protocol" It is difficult to determine how these were dealt with in the analysis |
| Selective reporting (reporting bias) | Low risk | All outcome were reported |
| Other bias | Low risk | Nothing of note |
Sood 2002.
| Methods | Randomized controlled trial Double blind placebo controlled Induction period included (steroid tapering) Study duration 12 months | |
| Participants | Newly diagnosed ulcerative colitis, severely active (SEO > 220) n = 35 | |
| Interventions | Azathioprine 2.5 mg/kg/d for 1 year
Compared to placebo Co‐medication induction period: i.v. hydrocortisone 400 mg/day, sulfasalazine 6‐8 g/day, ciprofloxacin and metronidazole for 7 days Taper off of steroids over 12‐16 weeks |
|
| Outcomes | Main: Maintenance of remission (after successful induction) Number of patients suffering clinical relapse within 1 year Secondary: Course of mean activity index over 1 year Induction period: time to remission Adverse events | |
| Notes | The author was approached for clarification of the randomization and allocation concealment; no response was received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "pseudo‐random numbers ranging from 0‐1, generated by a scientific calculator" |
| Allocation concealment (selection bias) | Unclear risk | Not described in published study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind" Quote: "identical matched placebo drugs" Quote: "The drugs were provided by a single coordinator (V.K.) to the patients in identical blister packs" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All the 35 patients completed the study period" |
| Selective reporting (reporting bias) | Unclear risk | All data was reported, but also hemoglobin and albumin levels which appear to post hoc |
| Other bias | Low risk | Nothing of note |
Sood 2003.
| Methods | Randomized controlled trial open label, active comparison (sulfasalazine) Induction period included (steroid tapering) Study duration 18 months | |
| Participants | Newly diagnosed ulcerative colitis, severely active (SEO > 220) n = 25 | |
| Interventions | Azathioprine 2.5 mg/kg/day for 18 months
Compared to sulfasalazine 6 g/day Co‐medication induction period: prednisolone 1 mg/kg/day; Taper off by 10 mg/day every fortnight until 20 mg/day, then 5 mg/day every 2 weeks Co‐medication maintenance: not reported |
|
| Outcomes | Main: maintenance of remission (after successful induction) for 18 months Treatment failure within 18 months Secondary: Failure to attain remission in induction phase Course of mean activity index Adverse events | |
| Notes | The author was approached for clarification of the randomization and allocation concealment; no response was received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "generating pseudo‐random numbers ranging from 0‐1 using a scientific calculator" |
| Allocation concealment (selection bias) | Unclear risk | Unclear, not described in the published study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two withdrawals due to side effects |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported, except endoscopic evaluation |
| Other bias | Low risk | Nothing of note |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ardizzone 1997 | Retrospective study of patients with either steroid resistant or steroid dependant ulcerative colitis receiving azathioprine |
| Ardizzone 2006 | This was a 6 month trial of patients with active steroid dependent ulcerative colitis. They were randomized to receive azathioprine or 5‐aminosalicylic acid |
| Cassinotti 2009 | Retrospective study of patients who discontinued azathioprine while in steroid free remission |
| Chebli 2010 | Prospective non‐randomized study of patients receiving azathioprine for steroid dependent ulcerative colitis |
| Cuffari 2004 | Prospective non‐randomized study of patients on azathioprine or 6‐mercaptopurine |
| Domenech 2002 | Retrospective chart review. |
| Fernandez‐Banares 1996 | Case series, not an RCT. |
| Hibi 2003 | Two different studies, one a retrospective study of 82 inflammatory bowel disease patients receiving azathioprine or 6‐mercaptopurine to assess the frequency of TMPT gene mutation. The other, a prospective non‐randomized trial of 22 patients receiving azathioprine |
| Holtmann 2006 | Retrospective chart review of 1176 patients with inflammatory bowel disease to evaluate the effect of azathioprine therapy |
| Kirk 1982 | Patients had chronic active ulcerative colitis |
| Mantzaris 2004 | All patients received azathioprine |
| Paoluzi 2002 | Not an RCT, no control group |
| Rosenberg 1975 | This was a randomized double‐blind trial involving patients with active ulcerative colitis. Patients received placebo or azathioprine (1.5 mg/kg/day) |
| Sakuraba 2012 | This was an open label pilot study examining granulocyte and monocyte apheresis against mercaptopurine therapy. Apheresis was not considered standard maintenance therapy |
Declarations of interest
Antje Timmer received grants (paid to institution) from Sanofi‐Aventis, Bayer, Takeda, Celgene, and Novartis for pharmacoepidemiological studies prior to 2014; and payment for lectures from Abbott, Ferring, The Falk Foundation, and MSD Sharp. All of these activities are outside the submitted work.
Petrease H Patton: None known.
Nilesh Chande has received fees for consultancy from Abbott/AbbVie , Ferring, and Actavis; fees for lectures from Abbott, travel expenses from Merck and has stock/stock options in Pfizer, Glaxo Smith Kline, Proctor and Gamble and Johnson and Johnson. All of these financial activities are outside the submitted work.
John WD McDonald: None known.
John K MacDonald: None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Hawthorne 1992 {published data only}
- Hawthorne AB, Logan RF, Hawkey CJ, Foster PN, Axon AT, Swarbrick ET, et al. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ 1992;305(6844):20‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jewell 1974 {published data only}
- Jewell DP, Truelove SC. Azathioprine in ulcerative colitis. Gut 1972;13(4):323. [PubMed] [Google Scholar]
- Jewell DP, Truelove SC. Azathioprine in ulcerative colitis: an interim report on a controlled therapeutic trial. British Medical Journal 1972;1(5802):709‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell DP, Truelove SC. Azathioprine in ulcerative colitis: final report on controlled therapeutic trial. British Medical Journal 1974;4(5945):627‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mate‐Jimenez 2000 {published data only}
- Hermida C, Cantero J, Moreno‐Otero R, Mate‐Jimenez J. Methotrexate and 6‐mercaptopurine in steroid‐dependent inflammatory bowel disease patients: A randomized controlled clinical trial.. Gut 1999;45(Suppl V):A132. [Google Scholar]
- Mate‐Jimenez J, Hermida C, Cantero‐Perona J, Moreno‐Otero R. 6‐mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid‐dependent inflammatory bowel disease. European Journal of Gastroenterology and Hepatology 2000;12(11):1227‐33. [DOI] [PubMed] [Google Scholar]
Paraskeva 2000 {published data only}
- Paraskeva KD, Karagiannis JA. Comparative effectiveness and safety of oral azathioprine and low‐dose oral cyclosporin in maintenance of the response in patients with acute steroid‐resistant ulcerative colitis.. Gut 2000;47(Suppl III A241):P932. [Google Scholar]
Sood 2000 {published data only}
- Sood A, Midha V, Sood N, Kaushal V. Role of azathioprine in severe ulcerative colitis: one‐year, placebo‐controlled, randomized trial. Indian Journal of Gastroenterology 2000;19(1):14‐6. [PubMed] [Google Scholar]
Sood 2002 {published data only}
- Sood A, Kaushal V, Midha V, Bhatia KL, Sood N, Malhotra V. The beneficial effect of azathioprine on maintenance of remission in severe ulcerative colitis. Journal of Gastroenterology 2002;37(4):270‐4. [DOI] [PubMed] [Google Scholar]
Sood 2003 {published data only}
- Sood A, Midha V, Sood N, Avasthi G. Azathioprine versus sulfasalazine in maintenance of remission in severe ulcerative colitis. Indian Journal of Gastroenterology 2003;22(3):79‐81. [PubMed] [Google Scholar]
References to studies excluded from this review
Ardizzone 1997 {published data only}
- Ardizzone S, Molteni P, Imbesi V, Bollani S, Bianchi Porro G. Azathioprine in steroid‐resistant and steroid‐dependent ulcerative colitis. Journal of Clinical Gastroenterology 1997;25(1):330‐3. [DOI] [PubMed] [Google Scholar]
Ardizzone 2006 {published data only}
- Ardizzone S, Maconi G, Russo A, Imbesi V, Colombo E, Bianchi Porro G. Randomised controlled trial of azathioprine and 5‐aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut 2006;55(1):47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cassinotti 2009 {published data only}
- Cassinotti A, Actis GC, Duca P, Massari A, Colombo E, Gai E, et al. Maintenance treatment with azathioprine in ulcerative colitis: outcome and predictive factors after drug withdrawal. American Journal of Gastroenterology 2009;104(11):2760‐7. [DOI] [PubMed] [Google Scholar]
Chebli 2010 {published data only}
- Chebli LA, Felga GG, Chaves LD, Pimentel FF, Guerra DM, Gaburri PD, et al. Early onset steroid‐dependent ulcerative colitis is a predictor of azathioprine response: a longitudinal 12‐month follow‐up study. Medical Science Monitor 2010;16(2):PI1‐6. [PubMed] [Google Scholar]
Cuffari 2004 {published data only}
- Cuffari C, Dassopoulos T, Turnbough L, Thompson RE, Bayless TM. Thiopurine methyltransferase activity influences clinical response to azathioprine in inflammatory bowel disease. Clinical Gastroenterology and Hepatology 2004;2(5):410‐7. [DOI] [PubMed] [Google Scholar]
Domenech 2002 {published data only}
- Domenech E, Garcia‐Planella E, Bernal I, Rosinach M, Cabre E, Fluvia L, et al. Azathioprine without oral ciclosporin in the long‐term maintenance of remission induced by intravenous ciclosporin in severe, steroid‐refractory ulcerative colitis. Alimentary pharmacology & therapeutics 2002;16(12):2061‐5. [DOI] [PubMed] [Google Scholar]
Fernandez‐Banares 1996 {published data only}
- Fernandez‐Banares F, Bertran X, Esteve‐Comas M, Cabre E, Menacho M, Humbert P, et al. Azathioprine is useful in maintaining long‐term remission induced by intravenous cyclosporine in steroid‐refractory severe ulcerative colitis. The American journal of gastroenterology 1996;91(12):2498‐9. [PubMed] [Google Scholar]
Hibi 2003 {published data only}
- Hibi T, Naganuma M, Kitahora T, Kinjyo F, Shimoyama T. Low‐dose azathioprine is effective and safe for maintenance of remission in patients with ulcerative colitis. Journal of Gastroenterology 2003;38(8):740‐6. [DOI] [PubMed] [Google Scholar]
Holtmann 2006 {published data only}
- Holtmann MH, Krummenauer F, Claas C, Kremeyer K, Lorenz D, Rainer O, et al. Long‐term effectiveness of azathioprine in IBD beyond 4 years: a European multicenter study in 1176 patients. Digestive Diseases and Sciences 2006;51(9):1516‐24. [DOI] [PubMed] [Google Scholar]
Kirk 1982 {published data only}
- Kirk AP, Lennard‐Jones JE. Controlled trial of azathioprine in chronic ulcerative colitis. British Medical Journal 1982;284(6325):1291‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantzaris 2004 {published data only}
- Mantzaris GJ, Sfakianakis M, Archavlis E, Petraki K, Christidou A, Karagiannidis A, et al. A prospective randomized observer‐blind 2‐year trial of azathioprine monotherapy versus azathioprine and olsalazine for the maintenance of remission of steroid‐dependent ulcerative colitis. American Journal of Gastroenterology 2004;99(6):1122‐8. [DOI] [PubMed] [Google Scholar]
Paoluzi 2002 {published data only}
- Paoluzi OA, Pica R, Marcheggiano A, Crispino P, Iacopini F, Iannoni C, et al. Azathioprine or methotrexate in the treatment of patients with steroid‐dependent or steroid‐resistant ulcerative colitis: results of an open‐label study on efficacy and tolerability in inducing and maintaining remission. Alimentary pharmacology & therapeutics 2002;16(10):1751‐9. [PUBMED: 12269968] [DOI] [PubMed] [Google Scholar]
Rosenberg 1975 {published data only}
- Rosenberg JL, Wall AJ, Levin B, Binder HJ, Kirsner JB. A controlled trial of azathioprine in the management of chronic ulcerative colitis. Gastroenterology 1975;69(1):96‐9. [PubMed] [Google Scholar]
Sakuraba 2012 {published data only}
- Sakuraba A, Sato T, Morohoshi Y, Matsuoka K, Okamoto S, Inoue N, et al. Intermittent granulocyte and monocyte apheresis versus mercaptopurine for maintaining remission of ulcerative colitis: a pilot study. Therapeutic Apheresis and Dialysis 2012;16(3):213‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Baron 1964
- Baron JH, Connell AM, Lennard‐Jones JE. Variation between observers in describing mucosal appearances in proctocolitis. British Medical Journal 1964;1(5375):89‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowen 1966
- Bowen GE, Irons GV Jr, Rhodes JB, Kirsner JB. Early experiences with azathioprine in ulcerative colitis; a note of caution. JAMA 1966;195(6):460‐4. [PubMed] [Google Scholar]
Bressler 2015
- Bressler B, Marshall JK, Bernstein CN, Bitton A, Jones J, Leontiadis GI, Panaccione R, Steinhart AH, Tse F, Feagan B, Toronto Ulcerative Colitis Consensus Group. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology 2015;148(5):1035‐1058. [DOI: 10.1053/j.gastro.2015.03.001; PUBMED: 25747596] [DOI] [PubMed] [Google Scholar]
Campieri 2003
- Campieri M, Rizzello F, Gionchetti P. Maintenance of remission in ulcerative colitis. In: Satsangi J, Sutherland LR, Colombel JF, Fiocchi C, Lofberg R, Pemberton JH, et al. editor(s). Inflammatory Bowel Diseases. Edinburgh, Oxford: Churchill Livingstone, 2003:419‐30. [Google Scholar]
Chande 2015
- Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6‐mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD000067.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chouchana 2012
- Chouchana L, Narjoz C, Beaune P, Loriot MA, Roblin X. Review article: the benefits of pharmacogenetics for improving thiopurine therapy in inflammatory bowel disease. Aliment Pharmacol Ther 2012;35(1):15‐36. [DOI: 10.1111/j.1365-2036.2011.04905.x; PUBMED: 22050052 ] [DOI] [PubMed] [Google Scholar]
Colombel 2000
- Colombel JF, Ferrari N, Debuysere H, Marteau P, Gendre JP, Bonaz B, et al. Genotypic analysis of thiopurine S‐methyltransferase in patients with Crohn's disease and severe myelosuppression during azathioprine therapy. Gastroenterology 2000;118(6):1025‐30. [DOI] [PubMed] [Google Scholar]
Dubinsky 2004
- Dubinsky MC. Azathioprine, 6‐mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clinical Gastroenterology and Hepatology 2004;2(9):731‐43. [DOI] [PubMed] [Google Scholar]
Freeman 2012
- Freeman HJ. Medical management of ulcerative colitis with a specific focus on 5‐aminosalicylates. Clin Med Insights Gastroenterol 2012;Oct(5):77‐83. [DOI: 10.4137/CGast.S8673; PUBMED: 24833937 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gearry 2004
- Gearry RB, Barclay ML, Burt MJ, Collett JA, Chapman BA. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiology and Drug Safety 2004;13(8):563‐7. [DOI] [PubMed] [Google Scholar]
Gearry 2005
- Gearry RB, Barclay ML. Azathioprine and 6‐mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. Journal of Gastroenterology and Hepatology 2005;20(8):1149‐57. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Hilsden 2003
- Hilsden RJ, Verhoef MJ, Best A, Pocobelli G. A national survey on the patterns of treatment of inflammatory bowel disease in Canada. BMC Gastroenterology 2003;3(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotlyar 2015
- Kotlyar DS, Lewis JD, Beaugerie L, Tierney A, Brensinger CM, Gisbert JP, Loftus EV Jr, Peyrin‐Biroulet L, Blonski WC, Domselaar M, Chaparro M, Sandilya S, Bewtra M, Beigel F, Biancone L, Lichtenstein GR. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6‐mercaptopurine: a meta‐analysis. Clin Gastroenterol Hepatol 2015;13(5):847‐58. [DOI: 10.1016/j.cgh.2014.05.015; PUBMED: 24879926] [DOI] [PubMed] [Google Scholar]
Long 2012
- Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology 2012;143(2):390‐399. [DOI: 10.1053/j.gastro.2012.05.004; PUBMED: 22584081] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2012
- Marshall JK, Thabane M, Steinhart AH, Newman JR, Anand A, Irvine EJ. Rectal 5‐aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD004118.pub2; PUBMED: 23152224] [DOI] [PubMed] [Google Scholar]
Meuwissen 2000
- Meuwissen SG, Ewe K, Gassull MA, Geboes K, Jewell D, Pallone F, et al. IOIBD questionnaire on the clinical use of azathioprine, 6‐mercaptoptopurine, cyclosporin A and methotrexate in the treatment of inflammatory bowel diseases. European Journal of Gastroenterology and Hepatology 2000;12(1):13‐8. [DOI] [PubMed] [Google Scholar]
Present 1989
- Present DH, Meltzer SJ, Krumholz MP, Wolke A, Korelitz BI. 6‐Mercaptopurine in the management of inflammatory bowel disease: short‐ and long‐term toxicity. Annals of Internal Medicine 1989;111(8):641‐9. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Stack 1999
- Stack WA, Williams D, Stevenson M, Logan RF. Immunosuppressive therapy for ulcerative colitis: results of a nation‐wide survey among consultant physician members of the British Society of Gastroenterology. Alimentary Pharmacology and Therapeutics 1999;13(5):569‐75. [DOI] [PubMed] [Google Scholar]
Su 2004
- Su C, Lichtenstein GR. Treatment of inflammatory bowel disease with azathioprine and 6‐mercaptopurine. Gastroenterology Clinics of North America 2004;33(2):209‐34, viii. [DOI] [PubMed] [Google Scholar]
Wallace 2001
- Wallace TM, Veldhuyzen van Zanten SJ. Frequency of use and standards of care for the use of azathioprine and 6‐mercaptopurine in the treatment of inflammatory bowel disease: a systematic review of the literature and a survey of Canadian gastroenterologists. Canadian Journal of Gastroenterology 2001;15(1):21‐8. [DOI] [PubMed] [Google Scholar]
Wang 2016
- Wang Y, Parker CE, Feagan BG, MacDonald JK. Oral 5‐aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD000544.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinshilboum 1980
- Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. American Journal of Human Genetics 1980;32(5):651‐62. [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Timmer 2007
- Timmer A, McDonald JW, MacDonald JK. Azathioprine and 6‐mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD000478.pub2] [DOI] [PubMed] [Google Scholar]
Timmer 2012
- Timmer A, McDonald JW, Tsoulis DJ, Macdonald JK. Azathioprine and 6‐mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD000478.pub3; PUBMED: 22972046] [DOI] [PubMed] [Google Scholar]


