Abstract
Blood pressure (BP) control is the most established practice for preventing the progression and complications of chronic kidney disease (CKD). We examined the influence of BP patterns on target organ damage in hypertensive patients with CKD by using long-term follow-up data of the APrODiTe-2 study.
We collected 5 years of data of APrODiTe-2 study (1 year longitudinal study) participants after the enrollment on the progression of estimated glomerular filtration (eGFR), renal outcomes (doubling of serum creatinine, 50% decrease of eGFR, maintenance dialysis, and kidney transplantation), cerebro-cardiovascular (CCV) accidents, and all-cause mortality (n=378) to evaluate the long-term influence of BP patterns on target organ damages.
Initially, more than 2/3 of patients showed masked (50.0%) and sustained uncontrolled (30.6%) BP control states as well as non- (31.3%) and reverse-dipping (35.0%) states. Only 18.8% and 20.8% of participants showed a better change in BP control patterns and a dipping pattern change to dippers over 1 year, respectively. Composite of new CCV accidents occurred in 43 patients (11.4%), and no BP patterns were associated with the occurrence of new CCV accidents. A worse change in BP control categories over 1 year was associated with increased occurrence of composites of renal outcomes after adjustment for age, sex, and the cause of CKD (HR 5.997 [1.454–24.742], P = .013 and HR 4.331 [1.347–13.927], P = .014, respectively). Patients with a worse initial BP control category, a worse change in BP control categories over 1 year, and higher clinic systolic BP and pulse pressure (PP) (> median level) were more likely to have faster eGFR progression (absolute eGFR and eGFR ratio).
Higher BP burden (a worse change in BP control categories, higher initial clinic systolic BP and PP) was associated with faster eGFR progression and increased occurrence of renal outcomes.
Keywords: blood pressure, chronic kidney disease, long-term, renal complication, target organ damage
1. Introduction
Hypertension is a main cause of chronic kidney disease (CKD), and CKD itself can aggravate hypertension-related complications.[1–4] Therefore, appropriate control of blood pressure (BP) is important, especially in CKD patients. However, the target BP in CKD patients remains a matter of debate. The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guidelines[5] and 2014 BP guidelines from the Eighth Joint National Committee (JNC 8)[6] generally recommend a target of < 140/90 mm Hg (<130/80 mm Hg in patients with proteinuria at > 300 mg/g Cr). However, 2 recent publications from the Systolic Blood Pressure Intervention Trial (SPRINT)[7] and the American College of Cardiology/American Heart Association (ACC/AHA) 2017 BP guidelines[8] changed the BP goals in CKD patients. ACA/AHA now recommends a BP goal of < 130/80 mm Hg in CKD patients regardless of proteinuria.[8] Unfortunately, achievement of the target BP in CKD patients has been poor. More than half of Korean CKD patients had uncontrolled BP and abnormal nocturnal dipping patterns.[9,10]
CKD is associated with high ambulatory BP and a lack of nocturnal dipping.[11,12] High ambulatory BP and/or an abnormal dipping pattern (ie, non-/reverse-dipper) better predict combined cardiovascular and renal outcomes than clinic BP values in patients with CKD.[13–17] Therefore, the assessment of out-of-clinic BP is important in CKD patients, and ambulatory BP monitoring (ABPM) is considered to be the gold standard in assessing the true BP status in CKD patients. Home BP measurements are also superior to clinic BP measurements in terms of assessing BP status[18] as well as predicting CKD-associated complications.[12] Home BP monitoring may be more practical for the day-to-day management of CKD patients because of its simplicity,[19] although this method cannot tell circadian BP patterns. In addition, we must consider the method of clinic BP measurements. Measurement by an automatic BP device was associated with 5.4 mm Hg lower clinic systolic BP (SBP) or 15/8 mm Hg lower clinic BP than by manual measurement.[20,21]
The Assessment of Blood Pressure Control and Target Organ Damage in Patients with CKD and Hypertension -2 (APrODiTe-2) study showed that a large majority of Korean hypertensive CKD patients had uncontrolled BP and abnormal dipping patterns. Additionally, a better change in BP control categories and dipping change to dipper over 1 year were associated with more stable estimated glomerular filtration rate (eGFR) and proteinuria changes as well as less cerebro-cardiovascular (CCV) damage.[10]
In this study, we evaluated the long-term influence of BP patterns on target organ damages in patients who participated in the APrODiTe-2 study.
2. Methods
2.1. Study design
The APrODiTe-2 study was a longitudinal study conducted at 4 centers between May 2013 and October 2015 (n = 378). All clinic BP measurements were acquired using an oscillometric OMRON MX-3 automatic BP device (IntelliSenseTM, Omron Corporation, Kyoto, Japan). The BP reading was taken as the mean of the last 2 readings among 3 consecutive seated BP readings at intervals of 1 to 2 minutes. Twenty-four-hour ABPMs were collected with an oscillometric TM-2430 monitor (A&D Co. Ltd, Seoul, Korea). Controlled clinic BP was defined as the level of < 140/90 mm Hg. We used the ambulatory blood pressure (ABP) definitions proposed by the European Society of Hypertension. ABP was considered normal if the daytime value was < 135/85 mm Hg and the nighttime value was < 120/70 mm Hg.
True controlled hypertension was defined as a controlled clinic and ambulatory (daytime and nighttime) BP. Masked hypertension was defined as controlled clinic BP and elevated daytime/nighttime ABP. Sustained uncontrolled BP was defined as uncontrolled clinic and ambulatory BP. The nocturnal dipping pattern was defined as a ratio of the mean nighttime SBP to the mean daytime SBP. The patients were classified as extreme -dippers, if the ratio was less than 0.8; dippers, if the ratio was between 0.8 and 0.9; non-dippers, if the ratio was between 0.9 and 1.0; and reverse -dippers, if the ratio was greater than 1.0. The BP control category changes over 1 year were divided into 2 groups, ie, to true controlled and white-coat hypertension (better change) and to masked and sustained uncontrolled hypertension (worse change), while the dipping pattern changes over 1 year were divided into 2 groups, ie, to dippers and to other dipping patterns.[10]
The protocol was approved by the institutional review boards of the participating centers. And this work was performed in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. We collected 5 years of data after the enrollment on medical histories, including coronary artery disease, cerebrovascular accidents, peripheral artery occlusive disease/ interventions, initiation of maintenance dialysis, and kidney transplantation as well as annual laboratory data (window period 2 months), including serum creatinine (SCr), eGFR, serum uric acid, and random urine protein-to-creatinine ratio.
2.2. Statistical analysis
Continuous variables are expressed as the means ± standard deviations (median), and categorical variables are expressed as n (%). The baseline characteristics, each eGFR, serum uric acid level, and random urine protein-to-creatinine ratio between groups were compared using χ2 tests or Student t test/ Mann-Whitney U test, and ANOVA/Kruskal-Wallis tests, as appropriate. Hazards about renal outcomes, CCV accidents, and mortality were analyzed using Kaplan-Meier survival (log-rank P) and Cox-proportional hazard regression analysis. The P values were 2-sided and were considered significant at P < .05. Time course changes in eGFR, serum uric acid level, and proteinuria from baseline through the 5-year follow-up were analyzed according to a linear mixed model analysis. The structure of compound symmetry was presumed as the diagonal structure. P values for the linear mixed model were considered significant at P < .0083 (eGFR, serum uric acid level, and spot urine protein/Cr ratio) or P < .01 (delta eGFR and eGFR ratio).
3. Results
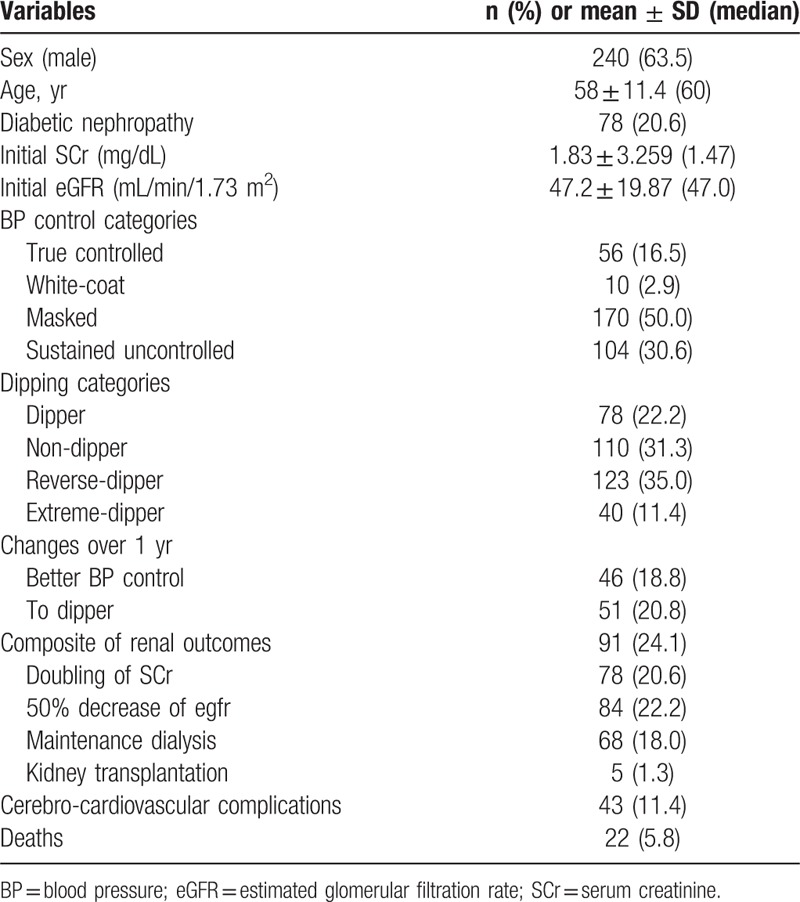
In total, 378 patients were enrolled. The age was 58 ± 11.4 (60) years, and 63.5% of the patients were men. Diabetic nephropathy was reported in 78 (20.6%) of the patients. The initial SCr and eGFR was 1.83 ± 3.259 (1.47) mg/dL and 47 ± 19.87 (47) mL/min/1.73 m2, respectively. The initial serum uric acid level and random urine protein/ creatinine ratio was 6.5 ± 1.74 (6.6) mg/dL and 905 ± 1530.9 (275) mg/g Cr, respectively. The levels of initial clinic SBP/ diastolic BP (DBP), PP, and pulse pressure (PP)/ SBP ratio were 132 ± 18.9 (130), 80 ± 11.1 (80), 52 ± 16.8 (49) mm Hg, and 0.388 ± 0.087 (0.378), respectively. Masked hypertension was encountered most frequently (n = 170, 50.0%), followed by sustained uncontrolled hypertension (n = 104, 30.6%), true controlled hypertension (n = 56, 16.5%), and white-coat hypertension (n = 10, 2.9%). The prevalence of reverse-dippers was the highest (n = 123, 35.0%), followed by nondippers (n = 110, 31.3%), dippers (n = 78, 22.2%), and extreme-dippers (n = 40, 11.4%). BP control category change over 1 year to a better condition was encountered in 46 (18.8%) patients, while only 51 (20.8%) patients changed to dipper category (Table 1).
Table 1.
Baseline characteristics of the APrODiTe-2 study participants and overall outcomes.
3.1. Renal outcomes, CCV complications, and mortality
Composite of doubling of SCr, 50% decrease of eGFR, initiation of maintenance dialysis, and kidney transplantation occurred in 91 (24.1%) patients [doubling of SCr, 78 (20.6%); 50% decrease of eGFR, 84 (22.2%); initiation of maintenance dialysis, 68 (18.0%); kidney transplantation, 5 (1.3%)]. Renal outcomes 1 and 2 are equal to the composite of the former 2 outcomes and the composite of all outcomes mentioned above, respectively. Composite of new CCV complications, including coronary artery disease, coronary artery intervention, cerebrovascular accidents, and peripheral artery occlusive diseases occurred in 43 patients (11.4%). Finally, 22 patients (5.8%) died during the follow-up period (Table 1).
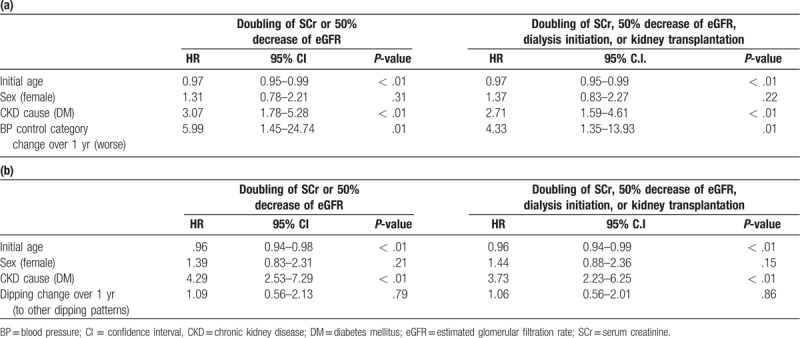
Kaplan-Meier survival analysis showed that the initial clinic SBP, initial BP control category, and changes in BP control categories over 1 year were associated with the occurrence of renal outcomes 1 (log-rank P = .02, .02, and < .01, respectively) and 2 (log-rank P = .03, .02, and < .01, respectively). Cox-proportional hazard regression analysis revealed that a worse change in BP control category over 1 year was associated with increased occurrence of renal outcomes 1 and 2 after adjustment for age, sex, and the cause of CKD (HR 5.99 (1.45–24.74), P = .01 and HR 4.33 (1.35–13.93), P = .01, respectively) (Table 2).
Table 2.
Cox-regression analysis of BP pattern changes [BP control category change (a) and dipping change (b)] over 1 year and renal outcomes.
No BP patterns were associated with the occurrence of new CCV accidents. Only initial clinic PP was associated with mortality (log-rank P = .01). No BP components or patterns showed statistically significant associations with the occurrence of new CCV accidents or mortality after adjustment for confounding factors.
3.2. Renal dysfunction progression
Progression of eGFR according to the initial BP control categories, initial dipping patterns, initial clinic BP subsets, and changes over 1 year are presented in Figures 1 to 3, respectively. Better initial BP control category (true controlled and white-coat hypertension); lower initial clinic SBP, PP, and PP/SBP ratio (< median); as well as better change in BP control categories over 1 year showed higher eGFR than each corresponding BP pattern.
Figure 1.
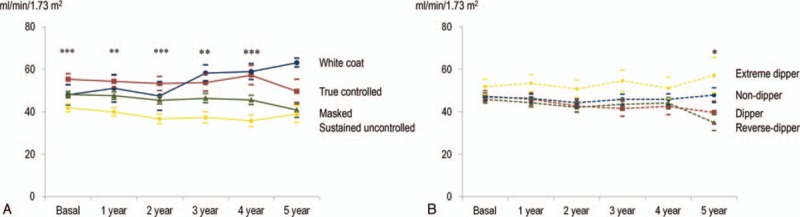
The progression of glomerular filtration rate according to initial BP control and dipping categories. Better initial BP control category (true controlled and white-coat hypertension) showed higher eGFR than each corresponding BP pattern. mean ± S.E. ∗P < .05, ∗∗P < .01 ∗∗∗P < .001 (Kruskal-Wallis test). (A) Initial BP control category (Ptime = .89, PBP control category < .01, Ptime X BP control category = .45). Solid blue: white-coat, red: true controlled, green: masked, and yellow: sustained uncontrolled hypertension. (B) Initial dipping category (Ptime = .16, Pdipping control category = .29, Ptime X dipping control category = .48). Dashed blue: non-dipper, red: dipper, green: reverse-dipper, and yellow: extreme-dipper. BP = blood pressure, eGFR = estimated glomerular filtration rate.
Figure 3.
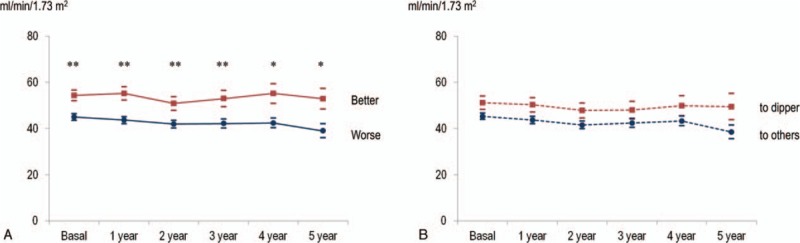
The progression of glomerular filtration rate according to BP control category and dipping category change over 1 year. Better change in BP control categories, not dipping pattern changes over 1 year, showed higher eGFR than each corresponding BP pattern. mean ± S.E. ∗P < .05, ∗∗P < .01 ∗∗∗P < .001 (Mann-Whitney U test). (A) BP control category change over 1 year (Ptime = .89, PBP control category change < .01, Ptime X BP control category change = .45). Solid red: better change to white-coat and true controlled, blue: worse change to masked and sustained uncontrolled. (B) Dipping category change over 1 year (Ptime = .16, Pdipping category change = .29, Ptime X dipping category change = .48). Dashed red: change to dipper, blue: change to other dipping patterns. BP = blood pressure.
Figure 2.
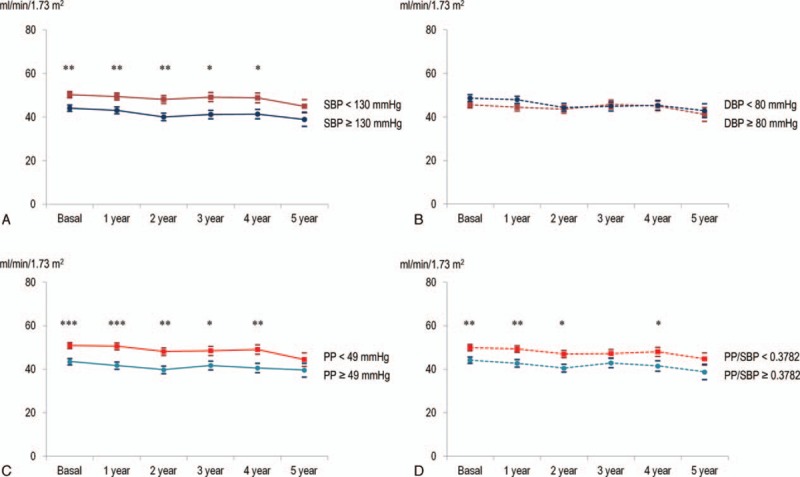
The progression of glomerular filtration rate according to each subset of initial clinic BP. Lower initial clinic SBP, PP, and PP/SBP ratio (< median) showed higher eGFR than each corresponding BP pattern. mean ± S.E. ∗P < .05, ∗∗P < .01 ∗∗∗P < .001 (Mann-Whitney U test). (A) Initial clinic SBP group (Ptime = .02, PSBP group < .01, Ptime X SBP group = .68). Solid red: SBP < 130 mm Hg, blue: SBP ≥130 mm Hg. (B) Initial clinic DBP group (Ptime = .04, PDBP group = .06, Ptime X DBP group = .28). Dashed red: DBP < 80 mm Hg, blue: DBP ≥80 mm Hg. (C) Initial clinic PP group (Ptime = .02, PPP group < .01, Ptime X PP group = .81). Solid red: PP < 49 mm Hg, blue: PP ≥ 49 mm Hg. (D) Initial clinic PP/SBP ratio group (Ptime = .02, PPP/SBP ratio group < .01, Ptime X PP/SBP ratio group = .98). Dashed red: PP/SBP ratio < 0.3782, blue: PP/SBP ratio ≥ 0.3782. BP = blood pressure, eGFR = estimated glomerular filtration rate, PP = pulse pressure, SBP = systolic blood pressure.
No BP patterns showed interactions with time in the linear mixed model analysis. Patients with a worse initial BP control category, worse change in BP control categories over 1 year, higher clinic SBP and PP (> median) were related to faster eGFR progression (absolute eGFR and eGFR ratio). P-values of each BP pattern were as follows: initial BP control category (Ptime = .89, PBP control category < .01, Ptime X BP control category = .45); initial dipping category (Ptime = .16, Pdipping control category = .29, Ptime X dipping control category = .48); initial clinic SBP group (Ptime = .02, PSBP group < .01, Ptime X SBP group = .68); initial clinic DBP group (Ptime = .04, PDBP group = .06, Ptime X DBP group = .28); initial clinic PP group (Ptime = .02, PPP group < .01, Ptime X PP group = .81); initial clinic PP/SBP ratio group (Ptime = .02, PPP/SBP ratio group < .01, Ptime X PP/SBP ratio group = .98); change in BP control categories over 1 year (Ptime = .24, Pchange in BP control categories < .01, Ptime X change in BP control categories = .59); change in dipping categories over 1 year (Ptime = .14, Pchange in dipping categories = .02, Ptime X change in dipping categories = .76).
3.3. Proteinuria and serum uric acid level
Lower initial clinic SBP, PP and PP/SBP ratio, better initial BP control category as well as better change in BP control categories over 1 year showed lower urinary protein excretion rates than each corresponding BP pattern during the early period of follow-up (initial and 1 year later). Furthermore, no BP patterns showed statistical significance with the repeated measurements of proteinuria.
Only the initial BP control category was significantly associated with lower serum uric acid levels at the initial, 1 year, 3 year, and 4 year follow-up. Patients with a better initial BP control category and better change in BP control categories over 1 year showed lower serum uric acid levels (PBP control category < .01 and Pchange in BP control categories over 1 year < .01, respectively).
3.4. Hospitalization and acute kidney injury
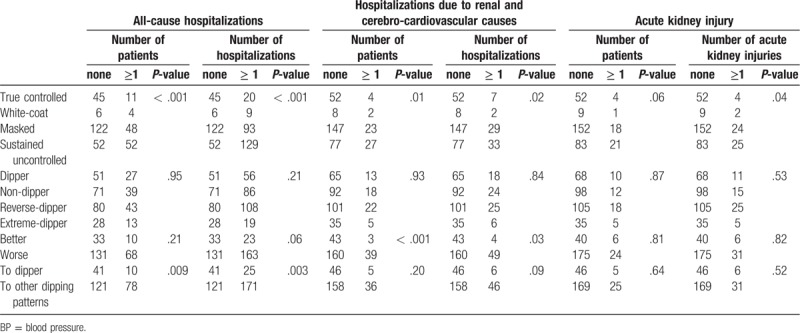
We also evaluated hospitalizations (excluding planned operations or interventions) and acute kidney injuries (Table 3). Overall, initial BP control categories were significantly associated with all of all-cause hospitalizations, hospitalizations due to renal and cerebro-cardioavscular (CCV) causes, and acute kidney injuries. Better change in BP control categories over 1 year also showed less occurrences of hospitalizations due to renal and CCV causes.
Table 3.
Comparison of hospitalizations and acute kidney injuries according to BP control categories and dipping patterns.
4. Discussion
In this study, we found that a worse change in BP control categories over 1 year was associated with increased occurrence of renal outcomes after adjustment for age, sex, and the cause of CKD. Patients with a worse initial BP control category, worse change in BP control categories over 1 year, and initial clinic SBP and PP (> median level) were related to faster eGFR progression (absolute eGFR and eGFR ratio). Furthermore, higher levels of initial clinic SBP, PP and PP/SBP ratio; worse initial BP control category; as well as a worse change in BP control categories over 1 year showed tendencies of higher urinary protein excretion rates and serum uric acid levels. In addition, less BP burden (good BP control categories and better change over 1 year) was associated with less occurrences of hospitalizations and acute kidney injuries, although we are not sure that those outcomes are directly associated with BP patterns.
The results of this study imply that lower BP burden for at least 1 year increases the probability of better renal outcomes over 5 years. However, the target BP in CKD patients remains a matter of debate. Which is more reasonable between ‘the lower, the better’ and ‘around 130 mm Hg of SBP’?
KDIGO 2012 and JNC 8 recommend different target BPs according to urinary protein excretion (<140/90 vs < 130/80 mm Hg).[5,6] However, the SPRINT trial and ACC/AHA 2017 guidelines recommend more aggressive control of BP (<130/80 mm Hg, regardless of proteinuria) in CKD patients.[7,8] The SPRINT trial, which included patients with increased cardiovascular risk without diabetes, revealed better outcomes in the intensive BP control group,[7] although CKD patients, who comprised approximately 28.4% of the study participants, only showed a borderline benefit of intensive BP control. The chronic renal insufficiency cohort reported that lower SBP was associated with fewer occurrences of incidental end stage renal disease in patients whose mean eGFR was about 45 mL/min/1.73 m2.[22]
In addition, eGFR changes of the intensive group in the SPRINT trial were most pronounced during the 6 months of treatment and were relatively similar during the study period after the acute phase. This suggested that eGFR changes may have been related with hemodynamic changes rather than intrinsic renal damages.[23] Subgroup analysis of the SPRINT trial, which analyzed the urine biomarkers of tubule function and repair, showed that eGFR declines in the intensive group reflected hemodynamic changes rather than intrinsic damage to renal tubule cells.[24] Furthermore, the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET) and Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) study indicated that maintaining good quality of BP control is important because the mean SBP more accurately predicted outcomes than accidental control of baseline or time-updated (last value before an event) SBP.[25] However, the achievement of a lower target BP in CKD patients is difficult, as seen from the APrODiTe and APrODiTe-2 studies as well as the SPRINT trial.[7,9,10,26] Only 50% of patients in the intensive group in the SPRINT trial achieved the target BP, and the majority of CKD patients in the APrODiTe-2 study showed masked hypertension (n = 170, 50.0%) and stained uncontrolled hypertension (n = 104, 30.6%).
On the other hand, data from the ONTARGET and TRANSCEND trials, which included high risk patients, aged over 55 years and with cardiovascular disease histories, showed that the mean achieved BP of 130/75 mmHg showed the lowest risk, suggesting that the lowest possible BP might not be the optimal target for high risk patients, although it could not rule out the reverse causality.[25] This J-shape phenomenon can be due to dysfunction of autoregulatory mechanisms in CKD patients, which are associated with aging or atherosclerosis and resultant vascular structure changes, leading to impaired renal adaptation to BP fluctuation.[27] The SHAPR (Study of Heart and Renal Protection) trial, which included patients with moderate to advanced CKD, showed a log-linear relationship between SBP and cardiovascular outcomes in the subgroup without cardiovascular diseases, although the relationship was U shaped among whole participants.[28] This suggested that the lack of a clear association between SBP and cardiovascular risk in CKD patients could stem from confounding factors. In this study, the initial clinic SBP, initial BP control category, and changes of BP control categories over 1 year were associated with the occurrence of renal outcomes. Notably, a worse change in BP control category over 1 year was associated with increased occurrence of renal outcomes after adjustment for age, sex, and the cause of CKD. These data suggest that SBP control under the target has a large effect on renal outcome, although the lower limit of the SBP could not be discussed.
The follow-up study of modification of diet in renal disease and African American Study of Kidney Disease reported that patients with greater proteinuria (>1 g/d) benefitted from intensive BP lowering in unadjusted analysis.[29] In addition, the action in diabetes and vascular disease: Preterax and Diamicron-MR Controlled Evaluation trial showed a greater reduction of cardiovascular disease absolute risk by treating with perindopril-indapamide in patients with CKD stage ≥3 and albuminuria.[30] Proteinuria could be appreciated as a cardiovascular risk factor, differentiating patients who could benefitted from intensive BP lowering.[31] However, the effect of each BP component on patients’ outcomes was not different according to the magnitude of proteinuria in this study.
PP is determined by stroke volume, aortic stiffness, and wave reflections. PP amplification has been associated with proteinuria, increased SCr, and aortic stiffness in many studies.[32] Findings about PP and PP/SBP ratio, which were good surrogate markers of hard renal outcomes and eGFR progression in this study, coincide with previous reports.
Target BP should be set according to the whole risk score, even though existing evidence supports around 130 mm Hg of SBP as appropriate to prevent renal and cardiovascular complications.[27] The risk of incidental CKD was much higher in patients with type 2 diabetes, even if the eGFR progression in the SPRINT and ACCORD trials was associated with hemodynamic change.[33] This implied that type 2 diabetes patients were more susceptible to hemodynamic changes. The whole risk for CCV and renal complications, including age, proteinuria, diabetes, and atherosclerosis, should be considered when determining the individual target BP.
As mentioned in the introduction, methods that measure BP affect the readings. Clinic BP by automatic devices was 15/8 mm Hg lower than clinic BP by manual measurement.[21] Furthermore, the clinic BP provides an incomplete or misleading assessment due to the loss of normal dipping in CKD patients. Therefore, a thorough assessment of BP via ambulatory BP, which is the recognized gold standard for the assessment of hypertension,[34] or home BP monitoring, which is more practical for the day-to-day management,[19] is earnestly needed to slow the progression of renal dysfunction and prevent CCV complications in CKD patients. Ryu et al reported that representative 24-hour BP measurements of CKD patients were taken at 7 am and 9:30 pm .[35]
This study has several limitations. First, we only collected BP data at the time of enrollment and 1 year after. As we can see from the ONTARGET and TRANSCEND data, maintaining good BP control is more accurate than BP values at the specific time-point, such as baseline and the last before an event. We cannot ensure that the quality of BP control during the early period of the study lasted before the occurrence of outcomes. Only 46.3% and 25.4% of patients with true controlled hypertension and normal dipping, respectively, maintained the same BP patterns 1 year after in the APrODiTe-2 study.[10] Therefore, we only evaluated the effect of BP patterns during the early period, and we did not collect data about annual clinic BP values as a result of the uncertainty of general BP control and dipping status due to skipping serial ABPM. Second, the number of study participants was small. Statistical significance might be determined by combinations of each BP component, such as high SBP/low PP and low SBP/ high PP. However, the small size of the study was a limitation, and this might have led to unclear results of the relationship between certain BP patterns and target organ diseases. A larger study is needed to draw conclusions about the relationship between each BP pattern and patients’ outcomes.
In conclusion, higher BP burden (worse change in BP patterns, higher clinic SBP and PP) was associated with faster eGFR progression and increased occurrence of renal outcomes. We should aim to thoroughly comprehend patients’ BP patterns via frequent home or ambulatory BP monitoring and should be more careful in maintaining good quality of BP control through appropriate prescription of medicines and life style modification encouragement, such as diet, exercise, and smoking cessation.
Author contributions
Conceptualization: Ran-hui Cha, Hajeong LEE, Jung Pyo LEE, Yon Su KIM, Sung Gyun KIM.
Data curation: Ran-hui Cha, Hajeong LEE, Jung Pyo LEE.
Formal analysis: Ran-hui Cha.
Investigation: Ran-hui Cha.
Methodology: Ran-hui Cha, Hajeong LEE, Jung Pyo LEE.
Validation: Ran-hui Cha, Hajeong LEE, Jung Pyo LEE, Yon Su KIM, Sung Gyun KIM.
Supervision: Yon Su KIM, Sung Gyun KIM.
Footnotes
Abbreviations: ABPM = ambulatory blood pressure monitoring, BP = blood pressure, CCV = cerebro-cardiovascular, CKD = chronic kidney disease, DBP = diastolic blood pressure, eGFR = estimated glomerular filtration rate, PP = pulse pressure, SBP = systolic blood pressure, SCr = serum creatinine.
How to cite this article: Cha Rh, Lee H, Lee JP, Kim YS, Kim SG. The influence of blood pressure patterns on renal outcomes in patients with chronic kidney disease: The long-term follow up result of the APrODiTe-2 study. Medicine. 2020;99:8(e19209).
This work has not been published previously in whole or in part, except in abstract form.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Mancia G, Backer GD, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension. The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007;28:1462–536. [DOI] [PubMed] [Google Scholar]
- [2].Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- [3].Hansoon L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomized trial. HOT Study Group Lancet 1998;351:1755–62. [DOI] [PubMed] [Google Scholar]
- [4].Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting=enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med 2000;342:145–53. [DOI] [PubMed] [Google Scholar]
- [5].Becker GJ, Wheeler DC. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012;2:337–414. [Google Scholar]
- [6].James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20. [DOI] [PubMed] [Google Scholar]
- [7].Research Group SPRINT, Wright JT, Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood –pressure control. N Engl J Med 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guideline. Hypertension 2018;71:1269–324. [DOI] [PubMed] [Google Scholar]
- [9].Cha RH, Kim S, Yoon SA, et al. Association between blood pressure and target organ damage in patients with chronic kidney disease and hypertension: results of the APrODiTe study. Hypertens Res 2014;37:172–8. [DOI] [PubMed] [Google Scholar]
- [10].Cha RH, Lee J, Lee JP, et al. Changes of blood pressure patterns and target organ damage in patients with chronic kidney disease: results of the APrODiTe-2 study. J Hypertens 2017;35:593–601. [DOI] [PubMed] [Google Scholar]
- [11].Terawaki H, Metoki H, Nakayama M, et al. Masked hypertension determined by self-measured blood pressure at home and chronic kidney disease in the Japanese general population: the Ohasama study. Hypertens Res 2008;31:2129–35. [DOI] [PubMed] [Google Scholar]
- [12].Portaluppi F, Montanari L, Massari M, et al. Loss of nocturnal decline of blood pressure in hypertension due to chronic renal failure. Am J Hypertens 1991;4:20–6. [DOI] [PubMed] [Google Scholar]
- [13].Lurbe E, Redon J, Kesani A, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 2002;347:797–805. [DOI] [PubMed] [Google Scholar]
- [14].Timio M, Venanzi S, Lolli S, et al. Non-dipper” hypertensive patients and progressive renal insufficiency: a 3-year longitudinal study. Clin Nephrol 1995;43:382–7. [PubMed] [Google Scholar]
- [15].Thomson AM, Pickering TG. The role of ambulatory blood pressure monitoring in chronic and end-stage renal disease. Kidney Int 2006;70:1000–7. [DOI] [PubMed] [Google Scholar]
- [16].Agarwal R, Andersen MJ. Correlates of systolic hypertension in patients with chronic kidney disease. Hypertension 2005;46:514–20. [DOI] [PubMed] [Google Scholar]
- [17].Liu M, Takahashi H, Morita Y, et al. Non-dipping is a potent predictor of cardiovascular mortality and is associated with autonomic dysfunction in haemodialysis patients. Nephrol Dial Transplant 2003;18:563–9. [DOI] [PubMed] [Google Scholar]
- [18].Andersen MJ, Khawandi W, Agarwal R. Home blood pressure monitoring in CKD. Am J Kidney Dis 2005;45:994–1001. [DOI] [PubMed] [Google Scholar]
- [19].Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension 2011;57:29–38. [DOI] [PubMed] [Google Scholar]
- [20].Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomized parallel design controlled trial. BMJ 2011;342:d286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mancia G, Kjeldsen SE. Adopting systolic pressure intervention trial (SPRINT)-like office blood pressure measurements in clinical practice. J Hypertens 2017;35:471–2. [DOI] [PubMed] [Google Scholar]
- [22].Anderson AH, Yang W, Townsend RR, et al. Time-updated systolic blood pressure and the progression of chronic kidney disease: a cohort study. Ann Intern Med 2015;162:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cheung AK, Rahman M, Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol 2017;28:2812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Malhotra R, Craven T, Ambrosius WT, et al. Effects of intensive blood pressure lowering on kidney tubule injury in CKD: A longitudinal soubgroup analysis in SPRINT. Am J Kidney Dis 2019;73:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Böhm M, Schumachr H, Teo KK, et al. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCENT trials. Lancet 2017;389:2226–37. [DOI] [PubMed] [Google Scholar]
- [26].Cha RH, Lee H, Lee JP, et al. Physician perceptions of blood pressure control in patients with chronic kidney disease and target blood pressure achievement rate. Kidney Res Clin Pract 2017;36:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Viazzi F, Leoncini G, Grassi G, et al. Antihypertensive treatment and renal protection: is there a J-curve relationship? J Clin Hypertens 2018;20:1560–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Herrington W, Staplin N, Judge PK, et al. Evidence for reverse causality in the association between blood pressure and cardiovascular risk in patients with chronic kidney disease. Hypertension 2017;69:314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ku E, Gassman J, Appel LJ, et al. BP control and long-term risk of ESRD and mortality. J Am Soc Nephrol 2017;28:671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Heerspink HJ, Ninomiya T, Perkovic V, et al. Effects of a fixed combination of perindopril and indapamide in patients with type 2 diabetes and chronic kidney disease. Eur Heart J 2010;31:2888–96. [DOI] [PubMed] [Google Scholar]
- [31].Chang AR, Appel LJ. Target blood pressure for cardiovascular disease prevention in patients with CKD. Clin J Am Soc Nephrol 2018;13:1572–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Safar ME, Plante GE, Mimran A. Arterial stiffness, pulse pressure, and the kidney. Am J Hypertens 2015;28:561–9. [DOI] [PubMed] [Google Scholar]
- [33].Beddhu S, Greene T, Boucher R, et al. Intensive systolic blood pressure control and incident chronic kidney disease in people with and without diabetes mellitus: secondary analyses of two randomized controlled trials. Lancet Diabetes Endocrinol 2018;6:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pickering TG, Shimbo D, Haas D. Ambulatory blood pressure monitoring. N Engl J Med 2006;354:2368–74. [DOI] [PubMed] [Google Scholar]
- [35].Ryu J, Cha RH, Kim DK, et al. Time points for obtaining representative values of 24-hour blood pressure in chronic kidney disease. Korean J Intern Med 2015;30:665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


