Abstract
Non-healing chronic ulcers are a serious complication of diabetes and are a major healthcare problem. While a host of treatments have been explored to heal or prevent these ulcers from forming, these treatments have not been found to be consistently effective in clinical trials. An understanding of the changes in gene expression in the skin of diabetic patients may provide insight into the processes and mechanisms that precede the formation of non-healing ulcers. In this study, we investigated genome wide changes in gene expression in skin between patients with type 2 diabetes and non-diabetic patients using next generation sequencing. We compared the gene expression in skin samples taken from 27 patients (13 with type 2 diabetes and 14 non-diabetic). This information may be useful in identifying the causal factors and potential therapeutic targets for the prevention and treatment of diabetic related diseases.
Introduction
Type 2 diabetes affects 29 million people in the U.S., and 170 million people in the world[1]. This condition can often lead to the disturbance of the blood vessel wall through promotion of vascular inflammation and endothelial cell dysfunction[2, 3]. These abnormalities increase the severity of vascular disease in diabetic patients[4]. A major complication of diabetes is the formation of non-healing ulcers. Patients with type 2 diabetes are prone to the development of non-healing ulcers, particularly on the lower limbs[5]. These non-healing ulcers are a major factor in the cost of treating diabetes. One estimate suggested that non-healing ulcers add 9–13 billion dollars to the overall annual cost of treating diabetes in the United States alone[6]. A wide variety of treatments have been explored to heal or prevent these ulcers from forming[7]. However, the majority of these treatments have been found to either be ineffective in clinical trials or have limited benefits in a subset of patients[8].
Our group has recently identified that patients with diabetes have a reduction in cell surface proteoglycans in their skin, including syndecan-4 and glypican-1[7, 9, 10]. These proteoglycans serve as co-receptors for growth factor signaling and their absence would suggest that diabetic tissues would be resistant to growth factor therapies for enhancing angiogenesis and wound healing. Delivery of syndecan-4 and glypican-1 in a proteoliposomal formulation enhanced the effectiveness of growth factor therapies in diabetic animals in models of limb ischemia and wound healing[7, 10]. These studies demonstrated that an increased understanding into the biology of non-healing ulcers and the changes that occur in skin with type 2 diabetes could identify potentially treatable deficits in healing and angiogenesis that may improve their treatment.
In this work, we used RNA sequencing (RNAseq) to examine the changes in gene expression in the skin between patients with type 2 diabetes and non-diabetic patients. These findings provide a window into the changes that occur in diabetic patients that may predispose them to the formation of non-healing ulcers and could provide pathways for further investigation into the mechanisms of poor healing in diabetic wounds.
Methods and materials
Human samples
Human skin samples were obtained from the Glasgow Caledonian University Skin Research Tissue Bank, Glasgow UK. The tissue bank has NHS research ethics approval to supply human skin for research (REC REF: 16/ES/0069). All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by the NHS East of Scotland Research Ethics Service. Informed consent was obtained from all subjects (no patients were under 18 years of age). All the patients were Caucasian and from western Scotland. Patient with diabetes were on treatment with metformin. Samples were formalin fixed and embedded in paraffin following standard procedures prior to sectioning. Control skin samples were taken from either clinical cases of breast reduction surgery or lower limb interventions for peripheral arterial disease. Skin sample for diabetic patients were taken from during limb amputation surgeries. Metadata for the patients are listed in Table 1.
Table 1. Patient metadata.
| Sex | Age | Diabetic State | Sample Location |
|---|---|---|---|
| M | 59 | Normal | Lower Limb |
| F | 49 | Normal | Lower Limb |
| F | 49 | Normal | Lower Limb |
| M | 69 | Normal | Lower Limb |
| M | 69 | Normal | Lower Limb |
| F | 80 | Normal | Lower Limb |
| F | 39 | Normal | Breast |
| M | 62 | Normal | Lower Limb |
| F | 19 | Normal | Breast |
| F | 34 | Normal | Breast |
| F | 60 | Normal | Breast |
| F | 27 | Normal | Breast |
| F | 69 | Normal | Breast |
| M | 73 | Type II Diabetic | Lower Limb |
| F | 47 | Type II Diabetic | Lower Limb |
| M | 80 | Type II Diabetic | Lower Limb |
| M | 81 | Type II Diabetic | Lower Limb |
| M | 53 | Type II Diabetic | Lower Limb |
| M | 77 | Type II Diabetic | Lower Limb |
| M | 61 | Type II Diabetic | Lower Limb |
| M | 79 | Type II Diabetic | Lower Limb |
| M | 61 | Type II Diabetic | Lower Limb |
| M | 49 | Type II Diabetic | Lower Limb |
| M | 58 | Type II Diabetic | Lower Limb |
| F | 72 | Type II Diabetic | Lower Limb |
| F | 59 | Type II Diabetic | Lower Limb |
| F | 59 | Type II Diabetic | Lower Limb |
RNA sequencing and analysis
RNA was isolated from formalin-fixed, paraffin-embedded tissue sections using the RNeasy FFPE kit (Qiagen). The mRNA was sequenced using an Illumina HiSeq 4000 at the Genomic Sequencing and Analysis Facility at UT Austin. Single reads of 50 base pairs were performed after poly-A mRNA capture used Ambion Poly(A) Tailing Kit and NEBNext Ultra II Directional RNA Library Prep Kit to isolate mRNA and dUTP directional preparation of the mRNA library. Gene expression analysis was performed using DESeq2 and R software. Plots were created using Prism GraphPad and Microsoft Excel.
Statistical analysis
Gene abundances were then normalized using DESeq2 normalization and log2 transformed using variance-stabilizing transformation (VST). Differential expression testing was performed based on the DESeq2 negative binomial distribution by comparing type II diabetic samples to normal samples, while controlling for sex differences. This was done by including sex as a covariate to control for in the DESeq2 design matrix along with the factor of interest, diabetic state. Genes that met the adjusted p-value cutoffs of 0.05 or lower and an absolute fold change of 2 or higher.
Results and discussion
Overall changes in gene expression in the skin in type 2 diabetes
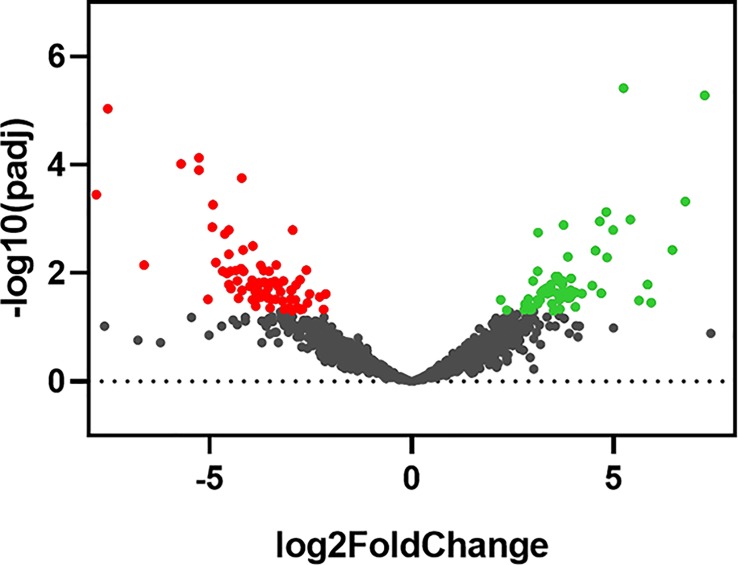
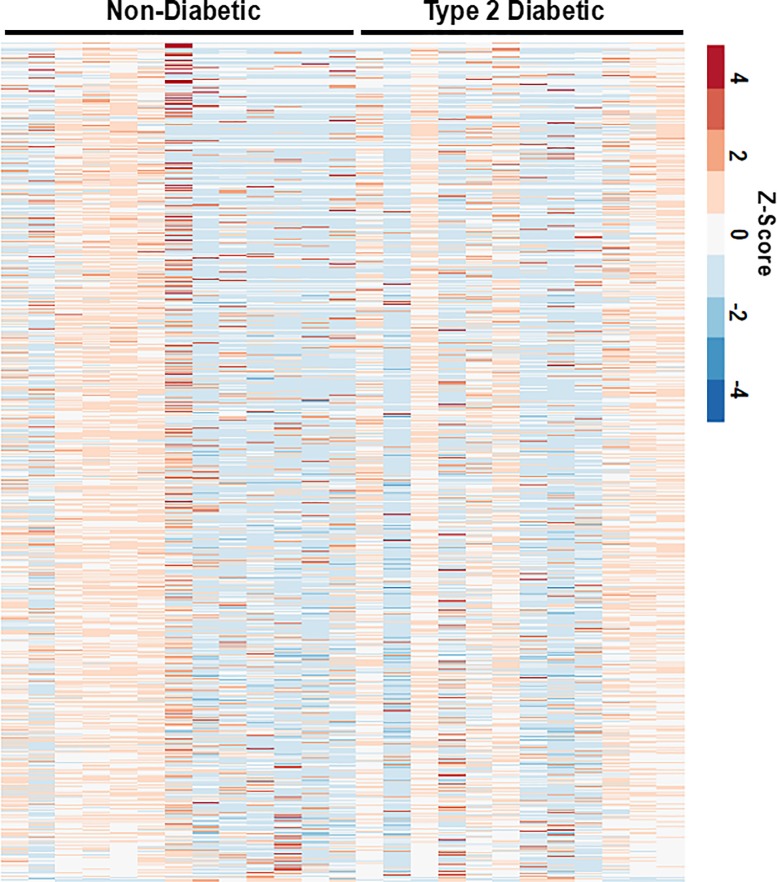
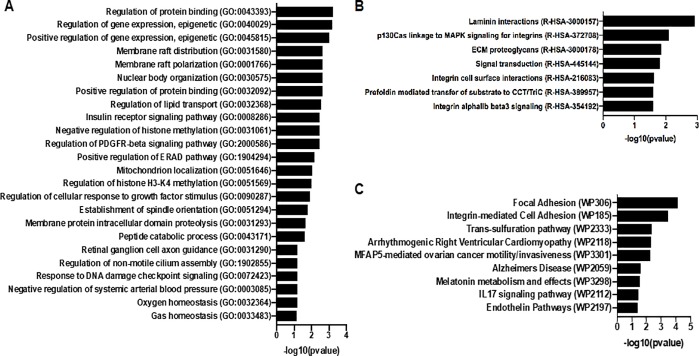
Skin tissues were obtained from 13 non-diabetic and 14 type 2 diabetes donors. We examined expression changes of 58,037 transcribed genes. Differential expression testing was performed based on the DESeq2 negative binomial distribution by comparing type 2 diabetic samples to normal samples. We found 64 significantly upregulated genes and 120 downregulated genes (Fig 1; S1 Table). There was significant patient-to-patient variability in the samples (Fig 2). Overall the five most upregulated genes when comparing skin samples from diabetic patients to non-diabetic patients included three non-coding RNA (lncRNA) genes (LINC01118, RP11-545I5.3 and an unknown lncRNA), an enzyme involved in lipid metabolism (ABHD16A) and a potassium channel that is important in smooth muscle tone and nerve function (KCNMA1). Conversely, the most downregulated genes included multiple pseudogenes and lncRNA (LINC01060, HPRT1P2, and CCNYL3) as well as the transcription factor NKX2_1 and a member of the TPD52 protein family associated with proliferation and vesicle trafficking (TPD52L3). Pathway analysis of the significantly altered genes revealed significant alterations in genes relating to epigenetic regulation of gene expression, lipid membrane rafts, growth factor signaling/PDGFRβ signaling, and proteolysis (Fig 3A). Gene ontology analysis of the biological processes most regulated in the genes and the functional pathways being regulated supported significant regulation of genes relating to integrin-based adhesion, focal adhesion formation and ECM proteoglycans (Fig 3B and 3C).
Fig 1. Volcano plot of statistical significance against fold change between diabetic and non-diabetic skin.
Fig 2. Heat map of the z-score for the top 30% varying genes for diabetic and non-diabetic patients.
Fig 3. Gene and pathway ontology analysis of significantly regulated genes between diabetic and non-diabetic patient skin samples.
(A) Most altered pathways between diabetic and non-diabetic skin using the reactome database analysis. (B) Significantly alter pathways for the gene ontology analysis for biological process when comparing diabetic and non-diabetic patients. (C) Pathways significantly altered using the wikipathways analysis tool comparing diabetic and non-diabetic patients.
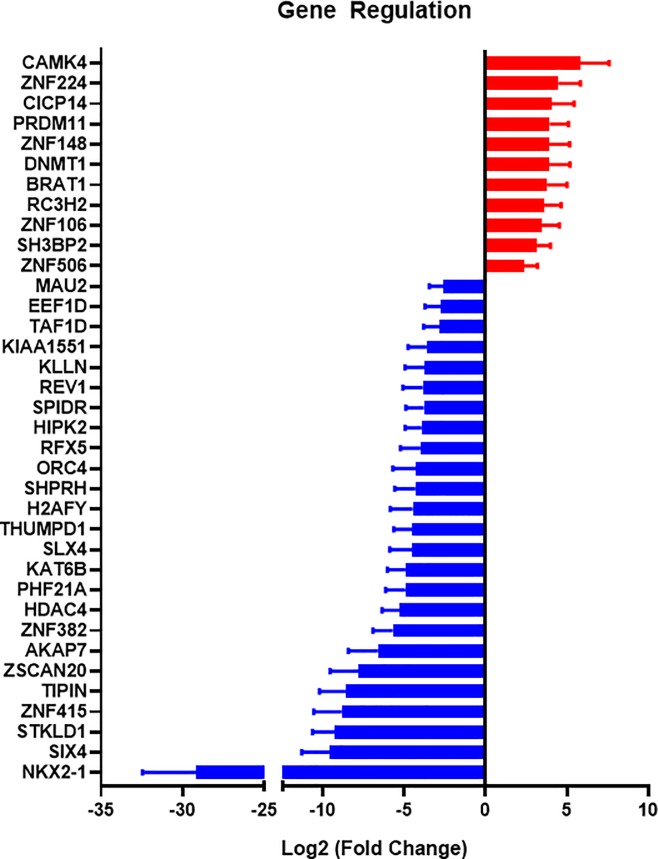
Regulation of genes related to transcription and gene regulation
The largest category of genes with significant regulation was that involved in gene regulation (Fig 4). Among the 11 upregulated genes, five genes (CAMK4, ZNF106, RC3H2, DMNT1, ZNF148) showed some relationship with type 2 diabetes in the previous studies[11–15]. One of the significantly upregulated genes was for calcium/calmodulin-dependent protein kinase (CAMK4). This protein phosphorylates transcription factors and can thereby regulate gene expression. Its target transcription factors include high-mobility group protein 1 (HMGB1), a proinflammatory mediator of chronic pain development[16]. Increased phosphorylation of CAMK4 is seen in a rat dorsal root ganglion after STZ-induced diabetes. [17].
Fig 4. Transcription- and regulation-related genes that were significantly regulated in type 2 diabetic patient skin samples.
Another notable gene that was upregulated in patients with diabetes was the transcriptional repressor ZNF224. Overexpression of ZNF224 is linked to the reduction of mitochondrial citrate carrier (CIC)[18, 19], a key enzyme in fatty acid and cholesterol synthesis[19]. Rats with STZ-induced diabetes had a reduced CIC activity[20], consistent with our findings.
Among the downregulated genes in our study, seven of these genes were found to also be associated with diabetes in previous studies[21–28]. The most downregulated gene in the gene regulation category was NKX2-1, also known as thyroid transcription factor 1 (TTF1). This transcription factor is involved in differentiation of the thyroid, lung and brain[29] and is linked to neuronal disorders[30, 31]. Mutation of NKX2-1 is also related to the reduction in mitochondrial respiratory chain complex activity[32]. Notably, reduced mitochondrial activity is one of the characteristics of diabetes[33]. Another highly downregulated transcription factor was SIX4, a key transcription factor in the development of sensory neurons and myogenesis[34–36]. Peripheral neuropathy is a common finding in diabetic patients and underlies alterations in biomechanics that leads to increased risk of ulcer formation[37]. In addition, diabetes can cause muscle weakness by altering muscle progenitor cells and other mechanisms[38, 39]. Thus, further investigation of these genes in the context of reduced mitochondrial activity and deficits in neural and muscular healing in diabetes would be merited.
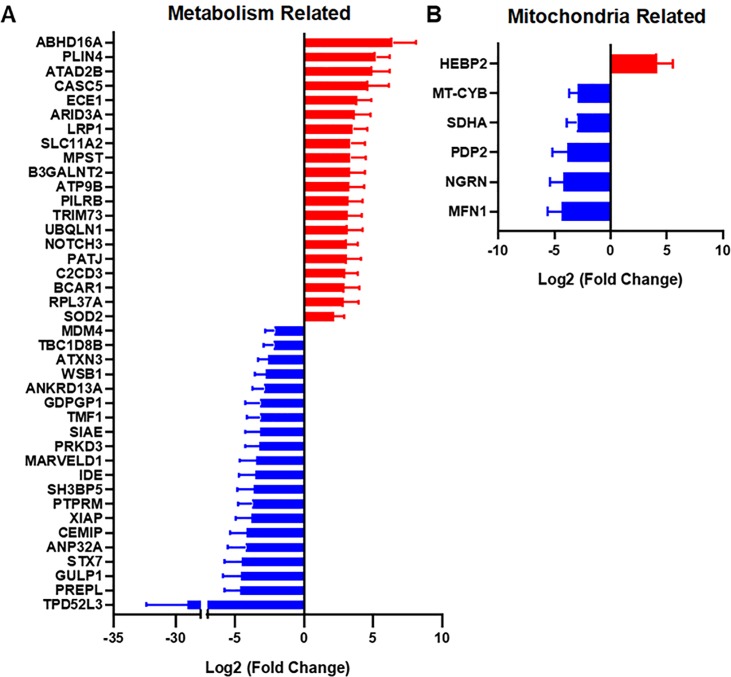
Regulation of metabolism and mitochondrial related genes
We observed significant regulation in a number of genes relating to metabolism (Fig 5A). The most upregulated gene in this category was α/β hydrolase domain containing 16A (ABHD16A), which is a member of the α/β hydrolase domain-containing (ABHD) protein family and is expressed in dendritic cells, macrophages, lymphocytes, and lymphoid organs. Interplay between ABHD16A and ABHD12 dynamically regulates immunomodulatory lysophosphatidylserines and can alter the release of proinflammatory cytokines from macrophages[40]. Other studies found several associations between ABHD16A and the genetic predisposition to coronary artery aneurysm and Kawasaki disease[41].
Fig 5. Metabolism genes and mitochondrial genes are significantly upregulated or downregulated in patients with diabetes.
(A) Alterations in metabolism-related genes when comparing patients with T2D and non-diabetic patients. (B) Mitochondrial genes that were significantly altered between the patient groups.
We also found an 11.8 fold increase in gene expression for LDL Receptor Related Protein 1 (LRP1) in skin from diabetic patients in comparison to control patients. LRP1 interacts with many ligands including lipoproteins, extracellular matrix proteins, protease, cytokines and growth factors. Biological roles of LRP1 covers broad ranges of activities such as lipid metabolism, cell growth, migration and tissue invasion, but most notable role of LRP1 in terms of wound healing should be extra cellular matrix (ECM) remodeling. A recent study found that LRP1 markedly inhibited fibronectin remodeling by regulating cell-surface urokinase receptor and plasminogen activation[42]. This process may be relevant to reduced healing of wounds and altered ECM remodeling observed in diabetic patients. Another study found upregulation of LRP1 in the epicardial fat tissue from patients with type 2 diabetes[43]. Interestingly, GULP1, a ligand that binds to LRP1, was significantly decreased in patients with type 2 diabetes in our study. GULP1 is an adapter protein necessary for the engulfment of apoptotic cells by phagocytes[44] and also functions as a modulator of glycosphingolipid and cholesterol transport[45].
We also found an 8.7 fold increase in NOTCH3 expression in skin from patients with type 2 diabetes. The Notch3 protein plays a key role in the function and survival of vascular smooth muscle cells, and is essential for the maintenance of blood vessels[46]. Recently, several studies found that NOTCH3 polymorphism seems to be a risk factor for both ischemic disease and diabetes. For example, NOTCH3 381C>T and 1735T>C polymorphisms found in the peripheral blood were associated with ischemic stroke and to be the risk factors for ischemic stroke[47]. The C381T (rs3815188) variants in exon 3 and A684G (rs1043994) variants in exon 4 of the NOTCH3 gene in the peripheral blood were also strongly associated with type 2 diabetes[48].
Superoxide dismutase 2 (SOD2) was increased 4.6-fold in patients with type 2 diabetes in our study. This gene encodes a mitochondrial protein, also known as manganese superoxide dismutase (MnSOD), that is a manganese-dependent enzyme that acts on superoxide produced as a byproduct of oxidative phosphorylation. SOD2 converts superoxide into hydrogen peroxide, which can be further detoxified by other enzymes. This enzyme is induced by oxidative stress and is increased in chronic ischemic wound models[49, 50]. In addition to its role in protecting oxidative damage, SOD2 also regulates signaling through reactive oxygen species (ROS)[51]. Low levels of SOD2 impair wound healing and enhancing SOD2 expression/activity enhanced wound repair[52, 53]. Polymorphisms in the SOD2 gene are associated with type 2 diabetes in the Japanese American population. The A16V polymorphism of SOD2 was seen frequently in the peripheral blood of type 2 diabetes patients, and study confirmed that this polymorphism decreases SOD2 activity, which successively will increase oxidative stress[54]. The C47T polymorphism in SOD2 gene has also been associated with a protective effect against diabetic microvascular complications[55].
The most downregulated gene in the metabolism category was tumor protein D52-like family of proteins (TPD52L3; Fig 3A). This gene has not been linked to type 2 diabetes or wound healing yet, but a study found that exogenous expression of human TPD52 in cultured cells resulted in significantly increased numbers of lipid droplet.[56] Lipid droplets store excess fatty acids within adipocytes. Thus, TPD52L3 seems to be associated with excess lipid storage in adipose cell. We hypothesize that lowering of TPD52L3 in type 2 diabetes, may lead to alterations in lipid storage and altered function of adipocytes.
Previous studies have linked diabetes to the development of mitochondrial dysfunction[57]. For example, point mutations resulting in amino acid substitutions in MT-CYB (D214N) showed defective mitochondrial ATP production[58–60]. Therefore, it is imperative to examine how diabetes affects the expression of mitochondrial related genes. The only significantly upregulated mitochondrial gene was HEBP2, which codes for the Heme Binding Protein 2 (Fig 5B). The protein encoded by this gene plays a role in the loss of mitochondrial membrane potential prior to cell death in necrosis. Methylation on this gene has been found in diabetic patients[61, 62]. The most strongly downregulated mitochondrial gene was MFN1, which codes for the mitofusin-1 protein. This protein is expressed on the mitochondrial membrane and is involved in the regulation of mitochondrial fusion. A study showed that type 2 diabetes was related to mitochondrial network fragmentation in myocardium and a large decrease in MFN1 expression[63]. Interestingly, mice with MFN1 knockout displayed a higher preference for lipid use as energy substrate[64]. Among five downregulated genes, three genes were downregulated in previous studies, including PDP2, NGRN, and MFN1[63, 65].
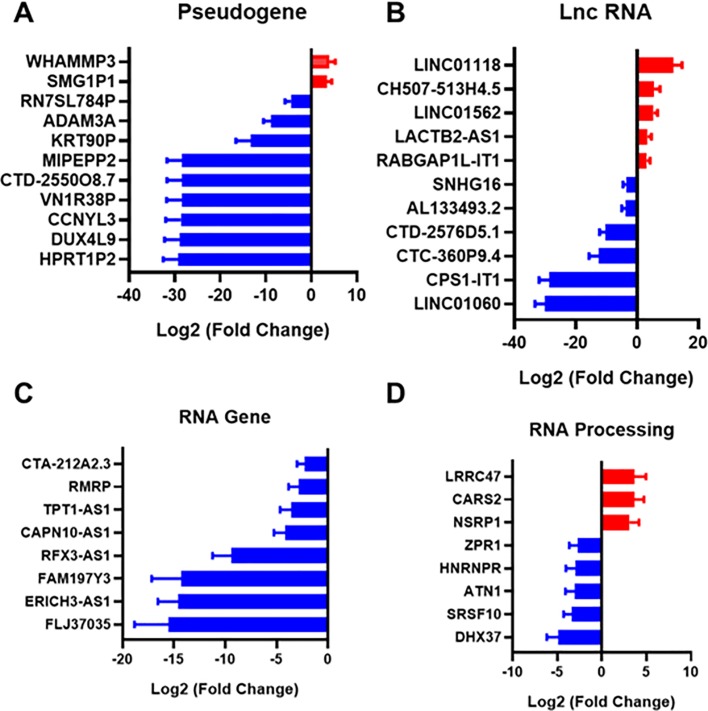
Alterations in pseudogene, lncRNA, RNA gene, RNA processing genes
A pseudogene is a DNA sequence that is an imperfect copy of a functional gene. Pseudogenes were once thought to be junk DNA, but it has since been recognized that some pseudogenes play essential roles in gene regulation of their parent genes[66]. We found two genes upregulated and nine genes downregulated in the pseudogene category (Fig 6A). Putative Cyclin-Y-Like Protein 3 is a pseudogene for cyclin-dependent protein serine/threonine kinase. The function of CCNYL3 is not yet fully understood, but overall it is expected to relate to the regulation of cell proliferation. Double Homeobox 4 Like 9 (DUX4L9), also known as DUX4c, is a pseudogene that has similarity to DUX4 transcription factor that was suggested to be a risk gene to cause facioscapulohumeral muscular dystrophy[67]. This gene rapidly downregulates the transcription factor Myogenic Differentiation 1 (MyoD), resulting in impaired myogenic differentiation and muscle regeneration[68]. Low expression of DUX4 impairs myogenesis through a reduction in myogenic gene expression[69]. DUX4L9 expression impairs myofibrillogenesis and potentially has a role in controlling muscle cell differentiation through association with type III intermediate filament protein desmin[70, 71].
Fig 6. Pseudogene, lncRNA, RNA gene, and RNA processing genes are significantly upregulated or downregulated in patients with diabetes.
(A) Pseudogenes that were altered inin skin from patients with type 2 diabetes. (B) Long non-coding RNAs (lncRNAs) that were altered in the skin of patients with type 2 diabetes (C) RNA genes with significant regulation in comparing the two patient groups. (D) Genes involved in RNA processing that were significantly changed between diabetic and non-diabetic patient groups.
Recent studies showed that deregulation of lncRNAs is associated with various diseases such as cancer, Alzheimer’s disease, and heart disease[72–76]. We found five upregulated genes and six downregulated genes under the long non-coding RNA (lncRNA) category (Fig 6B). Within the lncRNA category, CPS1-IT1 seems to be involved in type 2 diabetes. In the previous study, an impairment in neovascularization results from a high glucose induced defect in transactivation of hypoxia-inducible factor-1α (HIF-1α), and HIF-1α is controlled by CPS-IT1[77, 78]. The lncRNA LINC01118 was the most upregulated lncRNA in patients with type 2 diabetes in our study. LINC01118 has been associated with chemoresistance and increased cell migration in cancer[79]. The lncRNA LINC01060 was strongly decreased in our study in patients with type 2 diabetes. This lncRNA has been associated with poor prognosis in pancreatic cancer and inhibited pancreatic cancer proliferation and invasion[80]. Thus could also potentially contribute to poor wound healing prognosis.
In the RNA gene category, we found eight downregulated genes (Fig 6C). One of the downregulated RNA genes was Calcium-Activated Neutral Proteinase 10 Antisense 1 (CAPN10-AS1). In a previous study, CAPN10 expression was elevated in a pancreatic islet from type 2 diabetes[81]. It is generally known that antisense interferes the transcription of paired RNA (sense RNA)[82]. We also found the RNA gene ERICH3-AS1 to be highly downregulated in patients with type 2 diabetes. Consistent with our findings, this gene is downregulated in a sciatic nerve in diabetic mice[83]. Little is known about the function of ERICH3, however it is highly expressed in airway cilia and has been associated with major depressive disorder[84, 85].
In the RNA processing category (Fig 6D), we found a downregulation of Zinc Finger Protein 1 (ZPR1). The ZPR1 binds to the promoter of peroxisome proliferator-activated receptor gamma (PPARG) proteins 1 and 2, which play a key role in insulin sensitivity and obesity[86, 87]. A previous study reported the evidence linking the genetic susceptibility of common variants in ZPR1 to type 2 diabetes with the levels of fasting plasma glucose and blood hemoglobin A1c, suggesting ZPR1 might be involved in abnormal glucose metabolism[88]. However, the expression tendency seems to be different in skin tissue and others. ZPR1 mRNA in the brain is upregulated in mice fed a high-fat diet[89], whereas our result showed downregulation in the skin.
Several other notable genes were regulated in the RNA processing category including cysteinyl-tRNA synthetase 2 (CARS2), Heterogeneous Nuclear Ribonucleoprotein R (HNRNPR), and DEAH-box helicase 37 (DHX37). CARS2 plays a critical role in protein biosynthesis by charging tRNAs with their cognate amino acids. In a previous study, CARS2 was upregulated in a muscle tissue of patients with type 2 diabetes[90]. Heterogeneous Nuclear Ribonucleoproteins are RNA binding proteins and associated with pre-mRNAs in the nucleus. HNRNPR appears to influence pre-mRNA processing and other aspects of mRNA metabolism and transport. HNRNPR was downregulated in the islet-specific CD4 + T cells in type 1 diabetes -susceptible NOD mice[91]. DHX37 encodes a DEAD box protein. DEAD box proteins are characterized by the conserved motif Asp-Glu-Ala-Asp (DEAD), and implicated in alteration of RNA secondary structure such as translation initiation, nuclear and mitochondrial splicing, and ribosome and spliceosome assembly. In the previous study, DHX37 was downregulated in rat corneal epithelia in type 1 diabetes[27, 92]. Our result also showed a significant downregulation of DHX37, implying the malfunction in constructing RNA secondary structures may occur in the diabetic skin or corneal epithelia.
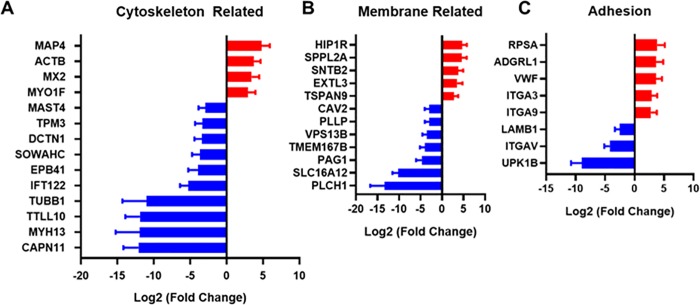
Cytoskeleton, membrane, and adhesion related genes
Elevated glucose levels can alter the mRNA and protein expression of contractile smooth muscle markers[93]. Fig 7A shows significantly up and downregulated cytoskeleton related genes in type 2 diabetic skin. We found β-actin (ACTB) to be significantly upregulated in the skin of type 2 diabetic patients. We also found increases in the Myosin-IF (MYO1F) gene. MYO1F exacerbates atherogenesis and is reported to be a biomarker candidate for patients with obstructive coronary artery disease or advanced atherosclerosis[94]. Among downregulated genes, Tropomyosin (TPM3) was downregulated ten-fold in the type 2 diabetic test group. This gene is translated into α-tropomyosin, which controls contraction in type I skeletal muscle fibers. Consistent with our findings, a previous study showed the downregulation of TPM3 in rats with alloxan-induced diabetes[95].
Fig 7. Cytoskeleton, membrane and adhesion genes are significantly upregulated or downregulated in patients with diabetes.
(A) Cytoskeletal genes altered in the skin in patients with type 2 diabetes. (B) Membrane related genes that were significantly different between diabetic and non-diabetic patients. (C) Cell adhesion related genes that were altered in type 2 diabetes.
We found 12 genes in this category that were significantly altered in the skin of diabetic patients in comparison to control patients (Fig 7B). Cell membrane lipid composition can alter the effectiveness of glucose transporters and has been implicated in the microvascular pathophysiology in diabetes[96]. The most upregulated gene in our study was the huntingtin interacting protein 1 related protein (HIP1R). This protein serves an important role in clathrin mediated endocytosis[97]. A recent study revealed its function in β-cell survival and glucose-stimulated insulin secretion, and HIP1R is downregulated in type 1 diabetes[98]. On the other hand, HIP1R expression is increased in the pancreas in type 2 diabetes[99].
We found a significant decrease in plasmolipin (PLLP) in the skin of patients with type 2 diabetes. A recent study showed that Notch signaling is mediated by PLLP and STX7 in epithelial cells: silencing STX7 impairs activation of Notch through PLLP[100, 101]. In our study, both of STX7 and PLLP were significantly downregulated in diabetic patient skin. The Notch1 pathway is a key regulator of wound healing[102]. Thus, defects in this pathway may be potential targets for improving healing in diabetes.
Tissue repair or wound healing process requires the regulation of cell adhesion molecules to control proliferation or migration of cells. The most upregulated gene in this category was RPSA, which codes for the Laminin Receptor 1 (Fig 7C). This gene is differentially expressed in wound margins at different sites on the body and has a role in adhesion and migration in the intestinal epithelia[103, 104]. We also observed increases in the genes for integrin α3 and α9, as well as a decrease in gene expression for integrin αv. Integrin α3β1 is suppressed by α9β1 1 during the resolution wound angiogenesis[105]. In one study, integrin α3β1 also inhibited re-epithelialization in wound healing[106]. Another study demonstrated that ITGA3 knockout mice had inhibited wound healing and that integrin α3β1 served as an inhibitor of Smad7 during the wound healing process[107]. Integrin αv is overexpressed in endothelial cells during the formation of new blood vessels and is a key adhesion receptor in many steps of angiogenesis[108]. In addition, αv integrins have a role in wound healing through the regulation of TGF-β and regulation of cell migration[109]. We also found an increase in von Willebrand factor (vWF) gene expression in patients with type 2 diabetes. The protein is involved in platelet adhesion and has a role in vascular inflammation and thrombosis[110]. There have been previous studies showing that high levels of VWF are associated with type 2 diabetes[111–113]. Our study adds to these findings in showing a significant upregulation in skin from diabetic patients.
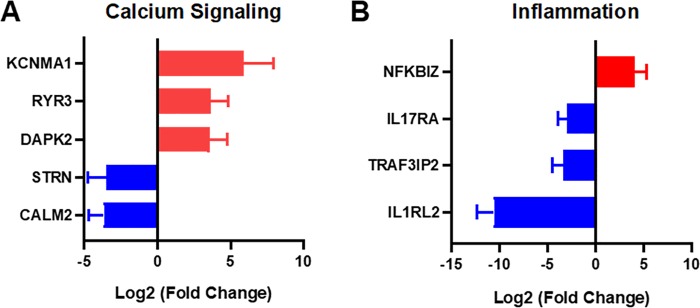
Calcium signaling and inflammation related genes
In the calcium-signaling gene category, we found a large increase in expression of KCNMA1 in patients with type 2 diabetes (Fig 8A). This gene encodes the calcium- and voltage-dependent potassium channel KCa1.1. This channel has been implicated in rheumatoid arthritis, the regulation of integrins, and myoblast function[114–116]. We also found decreases in expression for the genes for striatin (STRN) and calmodulin 2 (CALM2). Calmodulin 2 is involved in regulating a large number of proteins through calcium-mediated mechanisms and striatin is a calmodulin binding protein.
Fig 8. Inflammation and calcium signaling related genes that are significantly upregulated or downregulated in patients with diabetes.
(A) Calcium signaling related genes that were significantly altered between the patient groups. (B) Inflammation-related genes that were significantly different between the non-diabetic and type 2 diabetic groups.
Among the significantly regulated genes to inflammation, we found a significant increase in the gene expression NF-κB inhibitor zeta (NFKBIZ) in patients with type 2 diabetes (Fig 8B). The protein encoded by this gene inhibits NF-κB, a key transcription factor in inflammation. We also found three significantly downregulated inflammatory genes including a receptor for IL-17 family members (IL17RA), TRAF3 Interacting Protein 2 (TRAF3IP2) and Interleukin 1 Receptor Like 2 (IL1RL2; IL-36 receptor). Interleukin-17A is a proinflammatory cytokine that promotes the recruitment of neutrophils during wound healing. In wound healing, it likely delays wound healing while enhancing an inflammatory response that would promote the removal of microbes[117]. TRAF3IP2 alters keratinocyte differentiation in the skin and inhibits response to IL-17[118]. Both IL-17A and tumor necrosis factor–α (TNF-α) are induced by IL-36 cytokines in keratinocytes[119]. The cytokine IL-36γ promotes wound closure through a mechanism involving toll-like receptor 3 (TLR3), TIR-domain-containing adapter-inducing IFN-β (TRIF) and the transcription factor SLUG[120]. Thus, our findings suggest that IL17/IL36 mediated inflammatory pathways may be altered in diabetic skin. Indeed, the IL17/IL36 signaling axis is associated with psoriasis and targeted therapies are showing success. Psoriatic skin has hallmarks to some aspects of the hyperproliferative status of chronic non-healing wounds and comparisons between these established pathways are worthy of further investigation. We have summarized a comparison of our results to others in previous studies in Table 2[121–123].
Table 2. Comparison of study results with previous studies.
| Gene | This Study | Previous Studies | Model animal | Reference |
|---|---|---|---|---|
| CAMK4 | Increase in T2D | Increased phospho-CAMK4 | STZ-Induced Diabetes in Rat | 17 |
| ZNF224 | Increase in T2D | Reduced CIC activity | STZ-Induced Diabetes in Rat | 20 |
| ABHD16A | Increase in T2D | Genetic predisposition to coronary artery aneurysm and Kawasaki disease | Human | 41 |
| LRP1 | Increase in T2D | Upregulation in the epicardial fat tissue | T2D in Human | 43 |
| NOTCH3 | Increase in T2D | NOTCH3 polymorphism is a risk factor for ischemic disease and diabetes | Ischemic Stroke in Human | 47, 48 |
| SOD2 | Increase in T2D | SOD2 polymorphism is assocated with T2D | T2D in Human | 54 |
| HEBP2 | Increase in T2D | Methylation on this gene is found in T2D | T2D in Human | 61, 62 |
| CARS2 | Increase in T2D | Upregulated in muscle tissue | T2D in Human | 90 |
| MYO1F | Increase in T2D | MYO1F exaerbates atherogenesis | Coronary Artery Disease in Human | 94 |
| HIP1R | Increase in T2D | Downregulated in β-cell in T1D, but upregulated in the pancreas in T2D | T1D/T2D in Human | 98, 99 |
| vWF | Decrease in T2D | High levels of vWF | T2D in Human | 111–113 |
| NKX2-1 | Decrease in T2D | Mutation in NKX2-1 links to reduced mitochondrial activity | Human | 32 |
| MFN1 | Decrease in T2D | Downregulated | T2D in Human | 63 |
| PDP2 | Decrease in T2D | Decreased protein expression | T2D in Human | 65 |
| NGRN | Decrease in T2D | Downregulated | T2D in Human | 63 |
| CAPN10 | Decrease in T2D | Upregulated in pancreatic islet | T2D in Human | 81 |
| ERICH3-AS1 | Decrease in T2D | Downregulated in a sciatic nerve | Diabetic Mice | 83 |
| ZPR1 | Decrease in T2D | Upregulated in brain | High-Fat Diet Fed Mice | 89 |
| HNRNPR | Decrease in T2D | Downregulated in the islet-specific CD4 + T cells | T1D-Susceptible NOD Mice | 91 |
| DHX37 | Decrease in T2D | Downregulated in corneal epithelia | T1D Rat | 27, 92 |
| TPM3 | Decrease in T2D | Downregulated | Alloxan-Induced Diabetes in Rat | 95 |
Conclusions
We examined the gene expression in the skin from patients with type 2 diabetes and non-diabetic patients. Among the genes regulated there were many that had been identified as being modulated in diabetic animals and this study serves to provide confirmation that these changes also occur in the skin of human patients. This information could be useful in identifying the causal factors and potential therapeutic targets for the prevention and treatment of diabetic related diseases.
Supporting information
(TIF)
(XLSX)
Data Availability
Full RNAseq data will be deposited into the GEO datasets on pubmed (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE144441).
Funding Statement
The authors gratefully acknowledge funding through the American Heart Association (17IRG33410888), the DOD CDMRP (W81XWH-16-1-0580; W81XWH-16-1-0582) and the National Institutes of Health (1R21EB023551-01; 1R21EB024147-01A1; 1R01HL141761-01) to ABB. We thank Sebastian Greenhough for collection and processing of tissue via the GCU Skin Tissue bank, supported by a grant from Animal Free Research (AG) and who did not participate in any experiments involving animals or animal tissue. The URLs for the funders are follows: NIH (https://www.nih.gov/), DOD CDMRP (https://cdmrp.army.mil/), AHA (https://www.heart.org/), and Animal Free Research (https://www.animalfreeresearchuk.org/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.National Diabetes Statistics Report. Atlanta, GA: Centers for Disease Control and Prevention, Services USDoHaH; 2017. [Google Scholar]
- 2.Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, et al. The vascular endothelium and human diseases. International journal of biological sciences. 2013;9(10):1057–69. 10.7150/ijbs.7502 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP. Endothelial Dysfunction in Diabetes. The role of reparatory mechanisms. 2011;34(Supplement 2):S285–S90. 10.2337/dc11-s239 %JDiabetesCare [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiruvoipati T, Kielhorn CE, Armstrong EJ. Peripheral artery disease in patients with diabetes: Epidemiology, mechanisms, and outcomes. World journal of diabetes. 2015;6(7):961–9. Epub 2015/07/10. 10.4239/wjd.v6.i7.961 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. The Journal of clinical investigation. 2007;117(5):1219–22. 10.1172/JCI32169 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care. 2014;37(3):651–8. 10.2337/dc13-2176 . [DOI] [PubMed] [Google Scholar]
- 7.Das S, Baker AB. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Frontiers in bioengineering and biotechnology. 2016;4:82–. 10.3389/fbioe.2016.00082 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das S, Baker AB. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front Bioeng Biotechnol. 2016;4:82 10.3389/fbioe.2016.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2014;22(5):569–78. 10.1111/wrr.12205 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monteforte AJ, Lam B, Das S, Mukhopadhyay S, Wright CS, Martin PE, et al. Glypican-1 nanoliposomes for potentiating growth factor activity in therapeutic angiogenesis. Biomaterials. 2016;94:45–56. Epub 2016/04/11. 10.1016/j.biomaterials.2016.03.048 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebbar P, Elkum N, Alkayal F, John SE, Thanaraj TA, Alsmadi O. Genetic risk variants for metabolic traits in Arab populations. Scientific reports. 2017;7:40988–. 10.1038/srep40988 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bettahi I, Sun H, Gao N, Wang F, Mi X, Chen W, et al. Genome-wide transcriptional analysis of differentially expressed genes in diabetic, healing corneal epithelial cells: hyperglycemia-suppressed TGFβ3 expression contributes to the delay of epithelial wound healing in diabetic corneas. Diabetes. 2014;63(2):715–27. Epub 2014/01/16. 10.2337/db13-1260 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan J, Tie G, Wang S, Tutto A, DeMarco N, Khair L, et al. Diabetes impairs wound healing by Dnmt1-dependent dysregulation of hematopoietic stem cells differentiation towards macrophages. Nature Communications. 2018;9(1):33 10.1038/s41467-017-02425-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y-T, Liao J-W, Tsai Y-C, Tsai F-J. Inhibition of DNA methyltransferase 1 increases nuclear receptor subfamily 4 group A member 1 expression and decreases blood glucose in type 2 diabetes. Oncotarget. 2016;7(26):39162–70. 10.18632/oncotarget.10043 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thimmarayappa J, Sun JH, Schultz LE, Dejkhamron P, Lu CX, Giallongo A, et al. Inhibition of growth hormone receptor gene expression by saturated fatty acids: Role of Kruppel-like zinc finger factor, ZBP-89. Mol Endocrinol. 2006;20(11):2747–60. 10.1210/me.2006-0128 WOS:000241553100011. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Shen L, Xu L, Wang ZY, Ma C, Huang YG. Inhibition of CaMKIV relieves streptozotocin-induced diabetic neuropathic pain through regulation of HMGB1. Bmc Anesthesiol. 2016;16 ARTN 27 10.1186/s12871-016-0191-4 WOS:000376374500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao X, Shen L, Xu L, Wang Z, Ma C, Huang Y. Inhibition of CaMKIV relieves streptozotocin-induced diabetic neuropathic pain through regulation of HMGB1. Bmc Anesthesiol. 2016;16(1):27 10.1186/s12871-016-0191-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iacobazzi V, Infantino V, Convertini P, Vozza A, Agrimi G, Palmieri F. Transcription of the mitochondrial citrate carrier gene: Identification of a silencer and its binding protein ZNF224. Biochem Bioph Res Co. 2009;386(1):186–91. 10.1016/j.bbrc.2009.06.003 WOS:000267868800035. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan RS, Oliveira DL, Wilson GL. Streptozotocin-induced alterations in the levels of functional mitochondrial anion transport proteins. Arch Biochem Biophys. 1990;280(1):181–91. Epub 1990/07/01. 10.1016/0003-9861(90)90534-6 . [DOI] [PubMed] [Google Scholar]
- 20.Gnoni GV, Giudetti AM, Mercuri E, Damiano F, Stanca E, Priore P, et al. Reduced activity and expression of mitochondrial citrate carrier in streptozotocin-induced diabetic rats. Endocrinology. 2010;151(4):1551–9. 10.1210/en.2009-1352 . [DOI] [PubMed] [Google Scholar]
- 21.Meng Z-X, Wang L, Xiao Y, Lin JD. The Baf60c/Deptor Pathway Links Skeletal Muscle Inflammation to Glucose Homeostasis in Obesity. 2014;63(5):1533–45. 10.2337/db13-1061 %J Diabetes [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng W, Deshmukh HA, Donnelly LA, Torrance N, Colhoun HM, Palmer CNA, et al. A Genome-wide Association Study Provides Evidence of Sex-specific Involvement of Chr1p35.1 (ZSCAN20-TLR12P) and Chr8p23.1 (HMGB1P46) With Diabetic Neuropathic Pain. EBioMedicine. 2015;2(10):1386–93. 10.1016/j.ebiom.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suryavanshi SV, Jadhav SM, McConnell BK. Polymorphisms/Mutations in A-Kinase Anchoring Proteins (AKAPs): Role in the Cardiovascular System. Journal of cardiovascular development and disease. 2018;5(1):7 10.3390/jcdd5010007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Liu J, Zhen J, Zhang C, Wan Q, Liu G, et al. Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney International. 2014;86(4):712–25. 10.1038/ki.2014.111 [DOI] [PubMed] [Google Scholar]
- 25.Ung C, Sanchez AV, Shen L, Davoudi S, Ahmadi T, Navarro-Gomez D, et al. Whole exome sequencing identification of novel candidate genes in patients with proliferative diabetic retinopathy. Vision Research. 2017;139:168–76. 10.1016/j.visres.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson AA, Helmering J, Juan T, Li C-M, McCormick J, Graham M, et al. Pancreatic islet expression profiling in diabetes-prone C57BLKS/J mice reveals transcriptional differences contributed by DBA loci, including Plagl1 and Nnt. PathoGenetics. 2009;2(1):1–. 10.1186/1755-8417-2-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berry GJ, Frielle C, Brucklacher RM, Salzberg AC, Waldner H. Identifying type 1 diabetes candidate genes by DNA microarray analysis of islet-specific CD4 + T cells. Genomics data. 2015;5:184–8. 10.1016/j.gdata.2015.05.041 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldari S, Garufi A, Granato M, Cuomo L, Pistritto G, Cirone M, et al. Hyperglycemia triggers HIPK2 protein degradation. Oncotarget. 2016;8(1):1190–203. 10.18632/oncotarget.13595 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Small EM, Vokes SA, Garriock RJ, Li D, Krieg PA. Developmental expression of the Xenopus Nkx2-1 and Nkx2-4 genes. Mech Dev. 2000;96(2):259–62. 10.1016/s0925-4773(00)00400-7 . [DOI] [PubMed] [Google Scholar]
- 30.Lazzaro D, Price M, Defelice M, Dilauro R. The Transcription Factor-Ttf-1 Is Expressed at the Onset of Thyroid and Lung Morphogenesis and in Restricted Regions of the Fetal Brain. Development. 1991;113(4):1093–&. WOS:A1991GZ00100005. [DOI] [PubMed] [Google Scholar]
- 31.Malt EA, Juhasz K, Malt UF, Naumann T. A Role for the Transcription Factor Nk2 Homeobox 1 in Schizophrenia: Convergent Evidence from Animal and Human Studies. Frontiers in behavioral neuroscience. 2016;10:59 Epub 2016/04/12. 10.3389/fnbeh.2016.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coon EA, Ahlskog J, Patterson MC, Niu Z, Milone M. Expanding phenotypic spectrum of nkx2-1–related disorders—mitochondrial and immunologic dysfunction. JAMA Neurology. 2016;73(2):237–8. 10.1001/jamaneurol.2015.2976 [DOI] [PubMed] [Google Scholar]
- 33.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxidants & redox signaling. 2010;12(4):537–77. 10.1089/ars.2009.2531 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magli A, Baik J, Mills LJ, Kwak IY, Dillon BS, Mondragon Gonzalez R, et al. Time-dependent Pax3-mediated chromatin remodeling and cooperation with Six4 and Tead2 specify the skeletal myogenic lineage in developing mesoderm. PLoS Biol. 2019;17(2):e3000153 10.1371/journal.pbio.3000153 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakroun I, Yang D, Girgis J, Gunasekharan A, Phenix H, Kaern M, et al. Genome-wide association between Six4, MyoD, and the histone demethylase Utx during myogenesis. FASEB J. 2015;29(11):4738–55. 10.1096/fj.15-277053 . [DOI] [PubMed] [Google Scholar]
- 36.Konishi Y, Ikeda K, Iwakura Y, Kawakami K. Six1 and Six4 promote survival of sensory neurons during early trigeminal gangliogenesis. Brain Res. 2006;1116(1):93–102. 10.1016/j.brainres.2006.07.103 . [DOI] [PubMed] [Google Scholar]
- 37.Said G. Diabetic neuropathy—a review. Nat Clin Pract Neurol. 2007;3(6):331–40. 10.1038/ncpneuro0504 . [DOI] [PubMed] [Google Scholar]
- 38.D'Souza DM, Al-Sajee D, Hawke TJ. Diabetic myopathy: impact of diabetes mellitus on skeletal muscle progenitor cells. Front Physiol. 2013;4:379 10.3389/fphys.2013.00379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersen H, Schmitz O, Nielsen S. Decreased isometric muscle strength after acute hyperglycaemia in Type 1 diabetic patients. Diabet Med. 2005;22(10):1401–7. 10.1111/j.1464-5491.2005.01649.x . [DOI] [PubMed] [Google Scholar]
- 40.Xu J, Gu W, Ji K, Xu Z, Zhu H, Zheng W. Sequence analysis and structure prediction of ABHD16A and the roles of the ABHD family members in human disease. Open biology. 2018;8(5):180017 10.1098/rsob.180017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsieh Y-Y, Lin Y-J, Chang C-C, Chen D-Y, Hsu C-M, Lo M-M, et al. Human lymphocyte antigen B-associated transcript 2, 3, and 5 polymorphisms and haplotypes are associated with susceptibility of Kawasaki disease and coronary artery aneurysm. 2010;24(4):262–8. 10.1002/jcla.20409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaultier A, Hollister M, Reynolds I, Hsieh E-h, Gonias SL. LRP1 regulates remodeling of the extracellular matrix by fibroblasts. Matrix biology: journal of the International Society for Matrix Biology. 2010;29(1):22–30. Epub 2009/08/20. 10.1016/j.matbio.2009.08.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nasarre L, Juan-Babot O, Gastelurrutia P, Llucia-Valldeperas A, Badimon L, Bayes-Genis A, et al. Low density lipoprotein receptor–related protein 1 is upregulated in epicardial fat from type 2 diabetes mellitus patients and correlates with glucose and triglyceride plasma levels. Acta Diabetologica. 2014;51(1):23–30. 10.1007/s00592-012-0436-8 [DOI] [PubMed] [Google Scholar]
- 44.Park SY, Kim IS. Engulfment signals and the phagocytic machinery for apoptotic cell clearance. Exp Mol Med. 2017;49(5):e331 10.1038/emm.2017.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiss RS, Ma Z, Nakada-Tsukui K, Brugnera E, Vassiliou G, McBride HM, et al. The lipoprotein receptor-related protein-1 (LRP) adapter protein GULP mediates trafficking of the LRP ligand prosaposin, leading to sphingolipid and free cholesterol accumulation in late endosomes and impaired efflux. J Biol Chem. 2006;281(17):12081–92. 10.1074/jbc.M600621200 . [DOI] [PubMed] [Google Scholar]
- 46.Wang T, Baron M, Trump D. An overview of Notch3 function in vascular smooth muscle cells. Prog Biophys Mol Biol. 2008;96(1–3):499–509. 10.1016/j.pbiomolbio.2007.07.006 . [DOI] [PubMed] [Google Scholar]
- 47.Yuan X, Dong Z. The Association Between the Genetic Variants of the NOTCH3 Gene and Ischemic Stroke Risk. Medical science monitor: international medical journal of experimental and clinical research. 2016;22:3910–4. 10.12659/MSM.896297 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozbayer C, Degirmenci I, Kurt H, Kebapci MN, Colak E, Gunes HV. The rs1043994 and rs3815188 genetic variations of the NOTCH3 gene and risk of type 2 diabetes mellitus. Biotechnol Biotec Eq. 2017;31(3):563–7. 10.1080/13102818.2017.1294034 WOS:000399341600016. [DOI] [Google Scholar]
- 49.Roy S, Biswas S, Khanna S, Gordillo G, Bergdall V, Green J, et al. Characterization of a preclinical model of chronic ischemic wound. Physiol Genomics. 2009;37(3):211–24. 10.1152/physiolgenomics.90362.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong GH, Elwell JH, Oberley LW, Goeddel DV. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989;58(5):923–31. 10.1016/0092-8674(89)90944-6 . [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Branicky R, Noe A, Hekimi S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018;217(6):1915–28. 10.1083/jcb.201708007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellot GL, Dong X, Lahiri A, Sebastin SJ, Batinic-Haberle I, Pervaiz S, et al. MnSOD is implicated in accelerated wound healing upon Negative Pressure Wound Therapy (NPWT): A case in point for MnSOD mimetics as adjuvants for wound management. Redox Biol. 2019;20:307–20. 10.1016/j.redox.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujiwara T, Duscher D, Rustad KC, Kosaraju R, Rodrigues M, Whittam AJ, et al. Extracellular superoxide dismutase deficiency impairs wound healing in advanced age by reducing neovascularization and fibroblast function. Exp Dermatol. 2016;25(3):206–11. 10.1111/exd.12909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lawi ZKK. Investigation of SOD2 Gene Polymorphism in the Patients with Type Two Diabetes Disease in Babylon Province. Journal of Global Pharma Thechnology. 2018;10(06):70–5. [Google Scholar]
- 55.Tian C, Fang S, Du X, Jia C. Association of the C47T polymorphism in SOD2 with diabetes mellitus and diabetic microvascular complications: a meta-analysis. Diabetologia. 2011;54(4):803–11. 10.1007/s00125-010-2004-5 [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Frost S, Byrne JA. Dropping in on the lipid droplet- tumor protein D52 (TPD52) as a new regulator and resident protein. Adipocyte. 2016;5(3):326–32. 10.1080/21623945.2016.1148835 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect. 2015;4(1):R1–R15. 10.1530/EC-14-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sethumadhavan S, Vasquez-Vivar J, Migrino RQ, Harmann L, Jacob HJ, Lazar J. Mitochondrial DNA variant for complex I reveals a role in diabetic cardiac remodeling. The Journal of biological chemistry. 2012;287(26):22174–82. Epub 2012/04/27. 10.1074/jbc.M111.327866 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sinyov VV, Chicheva MM, Barinova VA, Ryzhkova AI, Zilinyi RI, Karagodin VP, et al. The heteroplasmy level of some mutations in gene MT-CYB among women with asymptomatic atherosclerosis. Russ J Genet+. 2016;52(8):847–52. 10.1134/S1022795416080123 WOS:000383338200009. [DOI] [PubMed] [Google Scholar]
- 60.Van Vranken JG, Bricker DK, Dephoure N, Gygi SP, Cox JE, Thummel CS, et al. SDHAF4 promotes mitochondrial succinate dehydrogenase activity and prevents neurodegeneration. Cell metabolism. 2014;20(2):241–52. Epub 2014/06/19. 10.1016/j.cmet.2014.05.012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nilsson E, Jansson PA, Perfilyev A, Volkov P, Pedersen M, Svensson MK, et al. Altered DNA Methylation and Differential Expression of Genes Influencing Metabolism and Inflammation in Adipose Tissue From Subjects With Type 2 Diabetes. 2014;63(9):2962–76. 10.2337/db13-1459 [DOI] [PubMed] [Google Scholar]
- 62.Xie X, Yi Z, Sinha S, Madan M, Bowen BP, Langlais P, et al. Proteomics analyses of subcutaneous adipocytes reveal novel abnormalities in human insulin resistance. Obesity (Silver Spring, Md). 2016;24(7):1506–14. 10.1002/oby.21528 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rovira-Llopis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biology. 2017;11:637–45. 10.1016/j.redox.2017.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kulkarni SS, Joffraud M, Boutant M, Ratajczak J, Gao AW, Maclachlan C, et al. Mfn1 Deficiency in the Liver Protects Against Diet-Induced Insulin Resistance and Enhances the Hypoglycemic Effect of Metformin. 2016;65(12):3552–60. 10.2337/db15-1725 %J Diabetes [DOI] [PubMed] [Google Scholar]
- 65.Popov KM. Regulation of Energy Metabolism by PDP1 and PDP2 University of Alabama Birmingham: National Institutes of Health; 2010. [Google Scholar]
- 66.Pink RC, Wicks K, Caley DP, Punch EK, Jacobs L, Carter DR. Pseudogenes: pseudo-functional or key regulators in health and disease? RNA. 2011;17(5):792–8. 10.1261/rna.2658311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ansseau E, Eidahl JO, Lancelot C, Tassin A, Matteotti C, Yip C, et al. Homologous Transcription Factors DUX4 and DUX4c Associate with Cytoplasmic Proteins during Muscle Differentiation. PloS one. 2016;11(1):e0146893–e. 10.1371/journal.pone.0146893 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bosnakovski D, Daughters RS, Xu Z, Slack JMW, Kyba M. Biphasic myopathic phenotype of mouse DUX, an ORF within conserved FSHD-related repeats. PloS one. 2009;4(9):e7003–e. 10.1371/journal.pone.0007003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bosnakovski D, Gearhart MD, Toso EA, Ener ET, Choi SH, Kyba M. Low level DUX4 expression disrupts myogenesis through deregulation of myogenic gene expression. Sci Rep. 2018;8(1):16957 10.1038/s41598-018-35150-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vanderplanck C, Tassin A, Ansseau E, Charron S, Wauters A, Lancelot C, et al. Overexpression of the double homeodomain protein DUX4c interferes with myofibrillogenesis and induces clustering of myonuclei. Skelet Muscle. 2018;8(1):2 10.1186/s13395-017-0148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ansseau E, Eidahl JO, Lancelot C, Tassin A, Matteotti C, Yip C, et al. Homologous Transcription Factors DUX4 and DUX4c Associate with Cytoplasmic Proteins during Muscle Differentiation. PLoS One. 2016;11(1):e0146893 10.1371/journal.pone.0146893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y, Pan S, Liu L, Zhai X, Liu J, Wen J, et al. A genetic variant in long non-coding RNA HULC contributes to risk of HBV-related hepatocellular carcinoma in a Chinese population. PloS one. 2012;7(4):e35145–e. 10.1371/journal.pone.0035145 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298–307. 10.1016/j.cell.2013.02.012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schonrock N, Harvey RP, Mattick JS. Long Noncoding RNAs in Cardiac Development and Pathophysiology. 2012;111(10):1349–62. 10.1161/CIRCRESAHA.112.268953 [DOI] [PubMed] [Google Scholar]
- 75.Tan L, Yu J-T, Hu N, Tan L. Non-coding RNAs in Alzheimer's Disease. Molecular Neurobiology. 2013;47(1):382–93. 10.1007/s12035-012-8359-5 [DOI] [PubMed] [Google Scholar]
- 76.Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, et al. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase. Nature medicine. 2008;14(7):723–30. Epub 2008/06/29. 10.1038/nm1784 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang T-H, Yu C-C, Lin Y-S, Chen T-C, Yeh C-T, Liang K-H, et al. Long noncoding RNA CPS1-IT1 suppresses the metastasis of hepatocellular carcinoma by regulating HIF-1α activity and inhibiting epithelial-mesenchymal transition. Oncotarget. 2016;7(28):43588–603. 10.18632/oncotarget.9635 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vial IN, Grogan RH, Yao D, Shi Y, Januszyk M, Galiano RD, et al. HIF-1α dysfunction in diabetes AU—Thangarajah, Hariharan. Cell Cycle. 2010;9(1):75–9. 10.4161/cc.9.1.10371 [DOI] [PubMed] [Google Scholar]
- 79.Shi C, Wang M. LINC01118 Modulates Paclitaxel Resistance of Epithelial Ovarian Cancer by Regulating miR-134/ABCC1. Medical science monitor: international medical journal of experimental and clinical research. 2018;24:8831–9. 10.12659/MSM.910932 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi X, Guo X, Li X, Wang M, Qin R. Loss of Linc01060 induces pancreatic cancer progression through vinculin-mediated focal adhesion turnover. Cancer Lett. 2018;433:76–85. 10.1016/j.canlet.2018.06.015 . [DOI] [PubMed] [Google Scholar]
- 81.Ling C, Groop L, Guerra SD, Lupi R. Calpain-10 expression is elevated in pancreatic islets from patients with type 2 diabetes. PloS one. 2009;4(8):e6558–e. 10.1371/journal.pone.0006558 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wight M, Werner A. The functions of natural antisense transcripts. Essays in biochemistry. 2013;54:91–101. 10.1042/bse0540091 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gu Y, Qiu Z-L, Liu D-Z, Sun G-L, Guan Y-C, Hei Z-Q, et al. Differential gene expression profiling of the sciatic nerve in type 1 and type 2 diabetic mice. Biomedical reports. 2018;9(4):291–304. Epub 2018/07/26. 10.3892/br.2018.1135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blackburn K, Bustamante-Marin X, Yin W, Goshe MB, Ostrowski LE. Quantitative Proteomic Analysis of Human Airway Cilia Identifies Previously Uncharacterized Proteins of High Abundance. J Proteome Res. 2017;16(4):1579–92. 10.1021/acs.jproteome.6b00972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gupta M, Neavin D, Liu D, Biernacka J, Hall-Flavin D, Bobo WV, et al. TSPAN5, ERICH3 and selective serotonin reuptake inhibitors in major depressive disorder: pharmacometabolomics-informed pharmacogenomics. Mol Psychiatry. 2016;21(12):1717–25. 10.1038/mp.2016.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–9. 10.1016/0092-8674(95)90199-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Corton JC, Anderson SP, Stauber A. Central Role of Peroxisome Proliferator–Activated Receptors in the Actions of Peroxisome Proliferators. 2000;40(1):491–518. 10.1146/annurev.pharmtox.40.1.491 . [DOI] [PubMed] [Google Scholar]
- 88.Guan F, Niu Y, Zhang T, Liu S, Ma L, Qi T, et al. Two-stage association study to identify the genetic susceptibility of a novel common variant of rs2075290 in ZPR1 to type 2 diabetes. Scientific reports. 2016;6:29586–. 10.1038/srep29586 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshihito Nogusa NY, Naoki Sumiyoshi, Keiko Takeda, Norihisa Kato Expression of zinc finger protein ZPR1 mRNA in brain is up-regulated in mice fed a high-fat diet. International journal of molecular medicine. 2006;17(3):491–6. [PubMed] [Google Scholar]
- 90.Hansen JS, Zhao X, Irmler M, Liu X, Hoene M, Scheler M, et al. Type 2 diabetes alters metabolic and transcriptional signatures of glucose and amino acid metabolism during exercise and recovery. Diabetologia. 2015;58(8):1845–54. 10.1007/s00125-015-3584-x [DOI] [PubMed] [Google Scholar]
- 91.Berry GJ, Frielle C, Brucklacher RM, Salzberg AC, Waldner H. Identifying type 1 diabetes candidate genes by DNA microarray analysis of islet-specific CD4 + T cells. Genom Data. 2015;5:184–8. 10.1016/j.gdata.2015.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bettahi I, Sun H, Gao N, Wang F, Mi X, Chen W, et al. Genome-Wide Transcriptional Analysis of Differentially Expressed Genes in Diabetic, Healing Corneal Epithelial Cells: Hyperglycemia-Suppressed TGFβ3 Expression Contributes to the Delay of Epithelial Wound Healing in Diabetic Corneas. 2014;63(2):715–27. 10.2337/db13-1260 %J Diabetes [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hien TT, Turczyńska KM, Dahan D, Ekman M, Grossi M, Sjögren J, et al. Elevated Glucose Levels Promote Contractile and Cytoskeletal Gene Expression in Vascular Smooth Muscle via Rho/Protein Kinase C and Actin Polymerization. The Journal of biological chemistry. 2016;291(7):3552–68. Epub 2015/12/18. 10.1074/jbc.M115.654384 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai Y, Zeng C. GW29-e1533 MYO1F exacerbates atherogenesis and can be as a novel candidate biomarker for patients with obstructive coronary artery disease or advanced atherosclerosis. 2018;72(16 Supplement):C74 10.1016/j.jacc.2018.08.419 %J Journal of the American College of Cardiology [DOI] [Google Scholar]
- 95.Karthik D, Ilavenil S, Kaleeswaran B, Sunil S, Ravikumar S. Proteomic Analysis of Plasma Proteins in Diabetic Rats by 2D Electrophoresis and MALDI-TOF-MS. Applied Biochemistry and Biotechnology. 2012;166(6):1507–19. 10.1007/s12010-012-9544-8 [DOI] [PubMed] [Google Scholar]
- 96.Weijers RN. Lipid composition of cell membranes and its relevance in type 2 diabetes mellitus. Curr Diabetes Rev. 2012;8(5):390–400. 10.2174/157339912802083531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gottfried I, Ehrlich M, Ashery U. The Sla2p/HIP1/HIP1R family: similar structure, similar function in endocytosis? 2010;38(1):187–91. 10.1042/BST0380187 %J Biochemical Society Transactions [DOI] [PubMed] [Google Scholar]
- 98.Berchtold LA, Størling ZM, Ortis F, Lage K, Bang-Berthelsen C, Bergholdt R, et al. Huntingtin-interacting protein 14 is a type 1 diabetes candidate protein regulating insulin secretion and β-cell apoptosis. 2011;108(37):E681–E8. 10.1073/pnas.1104384108 %J Proceedings of the National Academy of Sciences [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.GEO Signatures of Differentially Expressed Genes for Diseases. Type 2 Diabetes Mellitus_Pancreas_GSE2470. Harmonizome.
- 100.Rodríguez-Fraticelli AE, Bagwell J, Bosch-Fortea M, Boncompain G, Reglero-Real N, García-León MJ, et al. Developmental regulation of apical endocytosis controls epithelial patterning in vertebrate tubular organs. Nature cell biology. 2015;17(3):241–50. Epub 2015/02/23. 10.1038/ncb3106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Le Guelte A, Macara IG. Plasmolipin—a new player in endocytosis and epithelial development. The EMBO journal. 2015;34(9):1147–8. Epub 2015/03/30. 10.15252/embj.201591448 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shu B, Yang RH. Notch1 Signaling Regulates Wound Healing via Changing the Characteristics of Epidermal Stem Cells. Journal of Stem Cell Research & Therapy. 2016;2016(7). 10.4172/2157-7633.1000348 pub.1023115511. [DOI] [Google Scholar]
- 103.Khalfaoui T, Groulx JF, Sabra G, GuezGuez A, Basora N, Vermette P, et al. Laminin receptor 37/67LR regulates adhesion and proliferation of normal human intestinal epithelial cells. PLoS One. 2013;8(8):e74337 10.1371/journal.pone.0074337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miragliotta V, Lussier JG, Theoret CL. Laminin receptor 1 is differentially expressed in thoracic and limb wounds in the horse. Vet Dermatol. 2009;20(1):27–34. 10.1111/j.1365-3164.2008.00718.x . [DOI] [PubMed] [Google Scholar]
- 105.Longmate WM, Lyons SP, Chittur SV, Pumiglia KM, Van De Water L, DiPersio CM. Suppression of integrin alpha3beta1 by alpha9beta1 in the epidermis controls the paracrine resolution of wound angiogenesis. J Cell Biol. 2017;216(5):1473–88. 10.1083/jcb.201510042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Margadant C, Raymond K, Kreft M, Sachs N, Janssen H, Sonnenberg A. Integrin alpha3beta1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci. 2009;122(Pt 2):278–88. 10.1242/jcs.029108 . [DOI] [PubMed] [Google Scholar]
- 107.Reynolds LE, Conti FJ, Silva R, Robinson SD, Iyer V, Rudling R, et al. alpha3beta1 integrin-controlled Smad7 regulates reepithelialization during wound healing in mice. J Clin Invest. 2008;118(3):965–74. 10.1172/JCI33538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weis SM, Cheresh DA. alphaV integrins in angiogenesis and cancer. Cold Spring Harb Perspect Med. 2011;1(1):a006478 10.1101/cshperspect.a006478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Longmate WM, Dipersio CM. Integrin Regulation of Epidermal Functions in Wound s. Adv Wound Care (New Rochelle). 2014;3(3):229–46. 10.1089/wound.2013.0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gragnano F, Sperlongano S, Golia E, Natale F, Bianchi R, Crisci M, et al. The Role of von Willebrand Factor in Vascular Inflammation: From Pathogenesis to Targeted Therapy. Mediators Inflamm. 2017;2017:5620314 10.1155/2017/5620314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frankel DS, Meigs JB, Massaro JM, Wilson PWF, O'Donnell CJ, D'Agostino RB, et al. Von Willebrand factor, type 2 diabetes mellitus, and risk of cardiovascular disease: the framingham offspring study. Circulation. 2008;118(24):2533–9. Epub 2008/11/24. 10.1161/CIRCULATIONAHA.108.792986 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Seligman BG, Biolo A, Polanczyk CA, Gross JL, Clausell N. Increased plasma levels of endothelin 1 and von Willebrand factor in patients with type 2 diabetes and dyslipidemia. 2000;23(9):1395–400. 10.2337/diacare.23.9.1395 %J Diabetes Care [DOI] [PubMed] [Google Scholar]
- 113.Mannucci PM. von Willebrand Factor. 1998;18(9):1359–62. 10.1161/01.ATV.18.9.1359 [DOI] [PubMed] [Google Scholar]
- 114.Tajhya RB, Hu X, Tanner MR, Huq R, Kongchan N, Neilson JR, et al. Functional KCa1.1 channels are crucial for regulating the proliferation, migration and differentiation of human primary skeletal myoblasts. Cell Death Dis. 2016;7(10):e2426 10.1038/cddis.2016.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tanner MR, Pennington MW, Chauhan SS, Laragione T, Gulko PS, Beeton C. KCa1.1 and Kv1.3 channels regulate the interactions between fibroblast-like synoviocytes and T lymphocytes during rheumatoid arthritis. Arthritis Res Ther. 2019;21(1):6 10.1186/s13075-018-1783-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tanner MR, Pennington MW, Laragione T, Gulko PS, Beeton C. KCa1.1 channels regulate beta1-integrin function and cell adhesion in rheumatoid arthritis fibroblast-like synoviocytes. FASEB J. 2017;31(8):3309–20. 10.1096/fj.201601097R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takagi N, Kawakami K, Kanno E, Tanno H, Takeda A, Ishii K, et al. IL-17A promotes neutrophilic inflammation and disturbs acute wound healing in skin. Exp Dermatol. 2017;26(2):137–44. 10.1111/exd.13115 . [DOI] [PubMed] [Google Scholar]
- 118.Lambert S, Swindell WR, Tsoi LC, Stoll SW, Elder JT. Dual Role of Act1 in Keratinocyte Differentiation and Host Defense: TRAF3IP2 Silencing Alters Keratinocyte Differentiation and Inhibits IL-17 Responses. J Invest Dermatol. 2017;137(7):1501–11. 10.1016/j.jid.2016.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carrier Y, Ma HL, Ramon HE, Napierata L, Small C, O'Toole M, et al. Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: implications in psoriasis pathogenesis. J Invest Dermatol. 2011;131(12):2428–37. 10.1038/jid.2011.234 . [DOI] [PubMed] [Google Scholar]
- 120.Jiang Z, Liu Y, Li C, Chang L, Wang W, Wang Z, et al. IL-36gamma Induced by the TLR3-SLUG-VDR Axis Promotes Wound Healing via REG3A. J Invest Dermatol. 2017;137(12):2620–9. 10.1016/j.jid.2017.07.820 . [DOI] [PubMed] [Google Scholar]
- 121.Wu C, Chen X, Shu J, Lee CT. Whole-genome expression analyses of type 2 diabetes in human skin reveal altered immune function and burden of infection. Oncotarget. 2017;8(21):34601–9. Epub 2017/04/22. 10.18632/oncotarget.16118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ramirez HA, Liang L, Pastar I, Rosa AM, Stojadinovic O, Zwick TG, et al. Comparative Genomic, MicroRNA, and Tissue Analyses Reveal Subtle Differences between Non-Diabetic and Diabetic Foot Skin. PLoS One. 2015;10(8):e0137133 Epub 2015/09/01. 10.1371/journal.pone.0137133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Theocharidis G, Bhasin SS, Kounas K, Bhasin MK, Veves A. Single Cell RNA-Seq Analyses of Healthy Lower Extremity Skin and Diabetic Foot Ulcers Uncover Distinct Immune Landscape of Diabetic Wound Healing. Diabetes. 2018;67(Supplement 1):647-P 10.2337/db18-647-P [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(XLSX)
Data Availability Statement
Full RNAseq data will be deposited into the GEO datasets on pubmed (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE144441).