Abstract
There are over 8 million species in this world that live in widely varying environments, from hot thermal fissures to cold arctic settings. These species have evolved over millions of years and vary markedly in how they have adapted to their environments. In the last decades, studies of how species have succeeded in surviving in different environments and with different resources have been recognized to provide not only insights into disease but also novel means for developing treatments. Here we provide an overview of two related and overlapping approaches (biomimetics and zoobiquity), which are turning to the natural world for insights to better understand, treat, and prevent human “burden of life style” pathologies from heart disease and cancer to degeneration and premature aging. We suggest that expanding biomedical investigation beyond its decades old conventional practices to new approaches based on a broad awareness of the diversity of animal life and comparative physiology can accelerate innovations in health care under the motto “Nature knows best”.
Keywords: evolution, environment, biomimetics, hypoxia, NRF2
Graphical Abstract

Look deep into nature, and then you will understand everything better
Albert Einstein
Biomimetics is the study of nature and natural phenomenon geared toward creating similar structures, devices, materials or processes for the benefit of humans. It is based on several principles; at first, all biology represents evolved adaptations, secondly, within the multitudes of these adaptations in animals and plants may be “solutions” to challenges facing human beings and third, by studying the natural world, humans can leverage these evolved solutions to innovate and solve problems. Biomimetics has the potential to accelerate innovation in many fields including engineering, architecture, synthetic chemistry and medicine. Although the past 10 years has witnessed a surge of interest in biomimicry (”copy nature”) and biomimetics (”inspiration by nature”) in many disciplines, such as robotics, tissue engineering, architecture and material design, its development in the biomedical sciences has been limited (1). Recent developments within the biomimetic field in industry (2) includes innovations, such as Velcro inspired by the tendency of the burr of the fruit to stick to dogs hair, novel architectural designs with improved thermoregulation inspired by termite mounds (3), stable building structures inspired by the backbone of turban shells, bionic cars inspired by box fish, fluid-drag reduction swimsuits inspired by the structure of shark’s skin, design of airplanes inspired by birds, design of the Shinkansen train inspired by Kingsfisher birds, explosive chemical jet inspired by Bombardier beetle (4) and robotics inspired by motor mechanisms of insects (5). Unlike engineering, material sciences and product design, the field of medicine is only beginning to leverage the power of biomimetics to accelerate biomedical innovation and find novel approaches to fight human diseases. To enable the coexistence of humans, animals and plants on a planet that face many problems such as global warming, pollution and shortage of water (6, 7), we believe biomimetics will be a field increasingly in demand since it has the potential to provide many answers.
Evolution and selection of survival benefits
The enormous diversity of species and the diverse biology contained within each of these, is one of the most striking aspects of life on our planet. With about 8.7 million species (6.5 million species on land and 2.2 million in oceans) in the world (8), medical scientists have yet only scratched the surface of inspirations that can be provided from nature. In fact, it has been suggested that 91% of species in the ocean and around 86% of existing species on Earth still await description (8). Animal life emerged at 650 million years ago (9). Every individual since that time has survived, or not, based largely on whether their adaptations have been beneficial or not. At least five major mass extinction events have occurred over the last 450 million years, such as global cooling, volcanic activity (i.e. massive Siberian volcanic traps 250 million years ago), asteroids and changes in atmospheric gases (10). In the aftermaths of mass extinctions, ecosystems were restructured and long-term evolutionary trajectories were set that often accelerated the evolutionary process, often resulting in “ingenious” adaptations facilitating survival in animals. During evolution, the genes responsible for adaptations that were successful are passed on to subsequent generations. Those that don’t work are not successful. Failure to evolve adaptations which aid survival may lead to extinction of species. Humans are responsible of the ongoing 6th mass extinction, driven by climate change, overexploitation and habitat loss (11). To survive well in a rapidly changing world, adaptation would be very slow, hence it requires us to make use of biomimetic tools to maintain our health. The multiple essential physiologic challenges of life on the planet - oxidation, respiration, temperature control, obtaining sufficient food and water, metabolism, reproduction, infection, predation, trauma – are common to all species. In nature multitudes of adaptive strategies to these common challenges have evolved. Contained within these countless adaptations, evolved over hundreds of millions of years may be strategies with salience for human health. Our bodies possess limited physiologic responses to short periods of hypoxia, dehydration, starvation, infections and trauma (6). However, among the millions of other species on Earth facing the same challenges we find countless other strategies (adaptations). Some of these may be far more effective than those that has evolved in Homo Sapiens. Thus, the magnitude of opportunity is staggering.
Animals have evolved unique mechanisms for organ regeneration and tissue repair, for pathogen resistance, for protection from UV rays, for protection of hypoxia and from senescence. Furthermore, each species has evolved unique adaptive mechanisms to these challenges. This means that, contained within the natural world, aren’t just a handful of solutions, but likely countless strategies we might adapt to solve our physiologic needs or treat pathophysiologic diseases. To state it simply, the concept that “Nature knows best” is based on the fact that over evolutionary time natural selection has acted on countless species resulting in the emergence of life forms possessing a wide range of adaptations to the challenges of life. These provide alternative, and potentially superior strategies to those human bodies naturally possess, and inspire therapeutic approaches with greater efficacy than those used today.
The massive release of oxygen from photosynthetic cyanobacteria in oceans to the atmosphere about 2.3 billion years ago altered the course of evolution completely and was the fundamental driver for the development of complex multicellular life and aerobic respiration. The genes required for photosynthesis were acquired rather late in the evolution of cyanobacteria (12). Exposure to oxygen drove divergence of the transcription factor, nuclear factor-erythroid related factor 2 (Nrf2) (13), which promote the transcription of a battery of hundreds of anti-inflammatory and anti-oxidative genes. The evolution of cysteine-rich kelch-like ECH-associated protein 1 (KEAP1) provided mammals with a more sophisticated way to adjust Nrf2 activity in higher organisms (14). The activity of Nrf2-KEAP1 may have major implications for a cluster of burden of life style diseases that we today encounter (15).
Biomimetics - a novel strategy to conduct medical research
The need for a novel strategy for identifying models relevant to human health is staggering. The classical approach to solving medical problems is to use a combination of basic and clinical research into a translational approach. However, the basic science is often delegated to cell culture and murine and rat models, using an approach that may bridge molecular biology and physiology. There is a need to broaden our approaches to solving medical diseases and Benner et al (16) suggested that we should implement a ‘planetary biology’ approach that includes evolution and history. Biomimetics have emerged as a powerful tool for innovation as scientists recognize how powerful evolution’s massive “trial and error” has been in optimizing structures, substances and physiologies. As nature never cheats, the quality of solutions identified in nature are sustainable, offering enduring long-term outcomes exceeding those developed through any artificial means. Animals have evolved their genome over millions of years. Moreover, rapid epigenetic changes occurring in settings where environments are extreme, such as living in hot thermal vents or in Arctic regions, can provide important lessons. Today, much basic medical research relies on studies of a small number of primitive model organisms including invertebrates that lack stem cells, such as the worm Caenorhabditis Elegans and fruit fly Drosophila melanogaster, and established vertebrate animal models, such as mice (mus musculus) and rats (rattus rattus). This traditional approach neglects the enormous diversity of adaptations that have evolved in nature (Figure 1). Moreover, since the health of laboratory animals is poor and standard control mice and rats are metabolically morbid, these animal models may be inadequate for preclinical studies relevant to humans (17). This may in part explain why translation of findings from laboratory animals to humans often fail. With the advent of novel techniques that target genes, such as such as CRISPR gene editing, DNA repair and regenerative medicine it will be easier to explore a larger diversity of species and not only rely on a few established animal models.
Figure 1:
The classical approach to solving medical problems is to use a combination of basic and clinical research into a translational approach. However, the basic science is often delegated to cell culture and/or murine and rat models using an intervention, such as knock-out, to better understand pathogenetic mechanisms. A problem with this approach is that translation of findings from laboratory animals to humans often fail. By using the biomimetic approach, we instead identify species that during million years of evolution have adapted mechanisms to protect themselves during extreme environments. Such species include extreme ageing, such as ocean quahog, naked mole rat and Greenland shark or species that are protected against burden of life style diseases, such as hibernating bears, colibris, elephants and elephant seals. Other species, such as blue finned tuna have evolved mechanisms to adapt to extreme metabolic demands. An alternative or complementary approach is to identify species that are more susceptible to disease, such as the Turquoise killifish and Tasmanian devil. As nature never cheats, the quality of solutions identified in nature are likely to be sustainable, offering enduring long-term outcomes exceeding those developed through than any artificial means.
We trust that a biomimetic approach has a vast potential value for biomedical investigation but remains tremendously underleveraged in medicine (1). We propose/challenge the field of medicine to turn to the natural world specifically focusing on the evolved physiologic adaptations of non-human animals living in the natural world. This may improve our understanding of, and approach to high impact burden of life style diseases. Using examples from a wide range of burden of life style diseases that accumulate with age and link with persistent low-grade inflammation, such as dementia, coronary heart disease, cancer, congestive heart disease, chronic kidney disease, obesity, type-2 diabetes and non-alcoholic fatty liver disease, we offer a roadmap for leveraging the natural world’s multitudes of solutions to benefit human health (15). To fully understand the underlying causes of the cluster of burden of life style diseases, medical researchers should take advantage the possibility to learn from nature and the dramatic changes that occurred during evolution. As an example, two mutations (the blockade of vitamin C synthesis and silencing of the uricase gene) that occurred during periods of mass extinction may have had major effects on the current panorama of burden of life style diseases since these mutations enhanced the activity of fructose to generate fat and, thus, predispose modern sedentary man to fat mass accumulation and metabolic diseases (6).
The Zoobiquity concept – medical doctors can learn from veterinarian medicine and zoology
Embedded within biological diversity of life on Earth are many insights with salience for human health. However, these insights remain largely undiscovered and their impact unrealized. Several factors contribute to this (1). First, physicians have insufficient knowledge of the non-human animal life and the diversity of physiologies found in nature. Training in human medicine focuses on Homo sapiens and a few model species used for investigation. Second, opportunities to interact with experts in veterinary medicine and wildlife biology have been limited, decreasing opportunities for collaboration and innovation. Finally, a systematic methodology for identifying evolved adaptations, studying them and finding translational opportunities does not yet exist. Nevertheless, there has been some progress in the medically-focused biomimetics. Bio-inspired products intended for medical application are being developed including tenacious biological glues with anti-proliferative, anti-inflammatory and anti-microbial activity based on sticky proteins (natures glue) secreted by the bivalve mussel species (18) microprobes for brain surgery based on mosquito skin penetration (19), the red sweat of the hippopotamus that have antibiotic and sunscreen activity (20), and development of anti-microbial surfaces modelled after the diamond-like architecture of shark skin (21).
However, biomimetic efforts focused on complex human pathophysiology have been limited. In some cases, the identification of natural animal models has been more serendipitous than strategic. A shift to a more strategic and proactive approach to identifying these models and creating environments in which experts from the worlds of human and veterinary medicine, zoology and wildlife biology can collaborate is clearly needed. To counter the lack of communication and collaboration between the fields of human and veterinary medicine and to accelerate biomedical innovation, the Zoobiquity conferences were launched in 2011. Zoobiquity - from the Greek word “zoe” (animal) to the Latin word ubiquitare (everywhere) - describes the continuity of animal and human life including our shared physiology, vulnerability to pathology, and common challenges (22). These research-focused collaborations between veterinarians and physicians have been an early step in bringing physician-investigator communities into contact with experts in animal physiology and the natural world - the source of insights for bio-inspired medicine. Since that time yearly conferences in the US and internationally are helping to expand awareness of the opportunities presented by bio-inspired medicine and the necessity for transdisciplinary engagement and collaboration. In response to global warming’s threats to human health, Zoobiquity conferences, along with One Health (23) (24), a global movement connecting the health of the environment to the health of humans and animals, have increasingly focused on learning from animals that have already developed means for surviving in hostile, heated environments where water resources are limited. Other approaches include emerging collaborations between physicians, basic scientists, biologists, ecologists, zoologists and veterinarians that identify and together study species whose physiology may contain insights for human health (25). Below we discuss some specific areas in which bio-inspired medicine with lessons from the animal kingdom may benefit medical research and help us to better understand how we can combat “burden of life style” diseases (Figure 2).
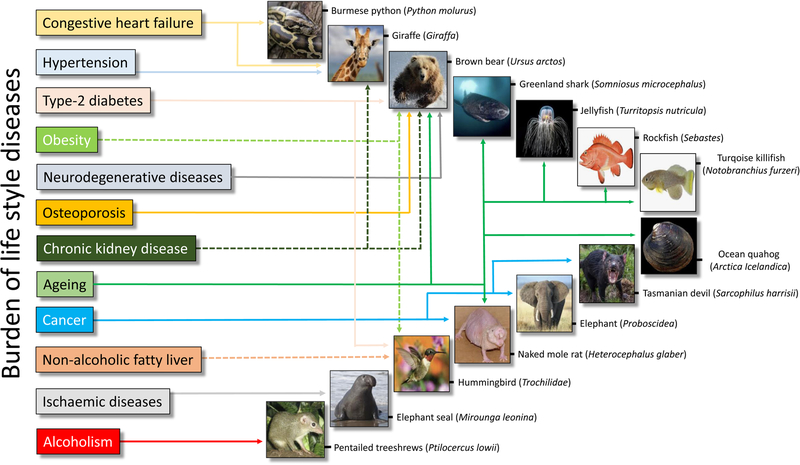
Figure 2:
Examples of species that already have been identified as being of interest for finding novel targets for “burden of life style” diseases that accumulate as we get older. Among a number diseases that often tend to cluster (diseasome), studies of some species in nature have already provided some clues for better treatment of congestive heart failure, hypertension, type-2 diabetes, obesity, neurodegenerative diseases, osteoporosis, chronic kidney disease, ageing, cancer, non-alcoholic fatty liver, ischaemic diseases (such as acute kidney injury) and alcoholism.
Longevity in the animal kingdom - novel insights benefit gerontology research
Ageing is by far the biggest risk factor for human disease and as we become older “burden of life style” diseases increase, possibly as a consequence of common features that cluster in a “diseasome”, including persistent inflammation, oxidative stress, mitochondrial dysfunction, metabolic imbalances, tissue hypoxia and senescence (15), (26). As the prevalence of “burden of life-style” diseases increase with age, and the proportion of the world population >60 years old will rise from 20% to 40% by 2050, the gap between life span and health span will expand and place a tremendous pressure on health care costs, which in USA are expected to reach a total of 47 trillion USD over 2010–2030 (27). Our life expectancy at birth has increased markedly since the emergence of Homo sapiens five hundred thousand years ago. Since industrialisation started at about 1800, lifespans have doubled, largely due to improvements in nutrition, public health (such as vaccinations and improved hygiene), and directed medical treatments (such as antibiotics) that have minimized mortality at young ages. For the first time in human history four generations are likely to co-exist.
Unfortunately, the extension in health span (i.e. years of healthy living) has not kept pace of the increase in life span (Figure 3). Lifestyle factors have a major influence on our health span. During industrialization humans have been exposed to a number of new environmental and life style risk factors, such as smoking, environmental toxins, air pollution, chemicals, water shortage, sugar rich-western diets, excessive calories and salt, processed food products and sedentary life style; factors to which our genome never had the chance to adapt to during evolution. Since we lack robust solutions to prevent the whole diseasome of burden of life style diseases (15), studies of nature give us an opportunity to identify mammals with limited senescence (28).
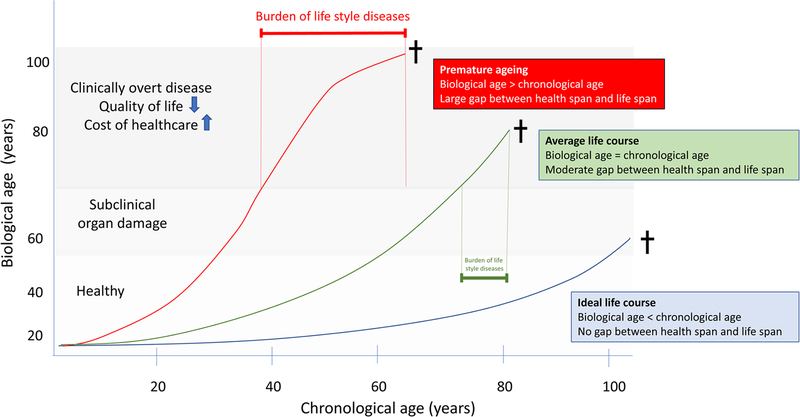
Figure 3:
The extension in health span (i.e. years of healthy living) has not kept pace of the increase in life span. Lifestyle factors, such as smoking, environmental toxins, air pollution, chemicals, water shortage, sugar rich-western diets, excessive calories and salt, processed food products and sedentary life style have a major influence on our health span and promote a gap between chronological and biological age. Three trajectories are depicted; 1) ideal life course when there is no long gap between life and health span and patients die at an old age without significant debilitating chronic diseases, 2) the average life course with a period of chronic disease before death and 3) premature ageing with an exceedingly long period of one or several chronic “burden of lifestyle” diseases(s) decrease the quality of life for the patient and associate with high healthcare costs. Studies of nature provide us with novel approaches to target the whole cluster of burden of life style diseases that often is associated with persistent inflammation.
Humans have the longest life span among primates; for example, great apes rarely age beyond 50 years. Like our foraging-farming forefathers with no access to modern medicine, infections is the major killer in chimpanzees in the wild (29). Chimpanzees have learned to identify herbs with anti-parasitic activity that they use for self-medication when they suffer from parasitic infections (30). Since chimpanzees often tend to select the bitter leaves of the Vernonia family for chewing this may decrease their risk of non-parasitic diseases. A survey of 109 plants of the Vernonia family (Asteraceae) revealed that Vernonia amygdalina holds promise for development into a nutraceutical against diabetes and malaria and Vernonia cinerea has potential against inflammatory conditions and cancer (31). Self-medication against parasite infections is also observed in animals without learning abilities, such as ants, fruit flies and moths; thus, this behaviour seems innate and adapted to the environment (30). Since chimpanzees in captivity have a lower incidence of ischemic heart disease, cancer, and neurodegeneration than humans (29) the gap between life span and health span is lower compared to humans who commonly suffer from burden of life style diseases for prolonged periods before death.
An extraordinary diversity in the life span is seen across different taxa (32), ranging from 1–2 days (mayfly) to >500 years (ocean quahog). Thus, comparative studies provide a golden opportunity to increase our understanding of causal mechanisms that drive senescence (28). A problem in animal ageing research is that most of the existing longevity data for animals consist of single observations of maximum lifespan. However, life expectancy estimates for 330 animal species exist, based on data from North American Zoos and aquariums (33). Although the size of the animal has been considered a main factor that determine longevity a number of other factors, such as telomere shortening rate (34), resting heart rate (35), DNA repair capacity (36), protein methionine tissue content (37) and serum phosphate levels (25) correlate to species life span. Studies of animal species that exhibit a high longevity quotient (actual longevity/expected longevity), negligible senescence and superior resistance to age-related diseases, such as naked mole rats (Heterocephalus glaber) (38), little brown bats (Myotis brandtii) (39), ocean quahog (Arctica Icelandica) (40), rockfish (Sebastes) (41), bowheads whale (Balaena mysticetus) (42) and Greenland shark (Somniosus microcephalus) (43) are of special interest for biomimetic studies since they have adapted beneficial changes during evolution that make them exceptionally long-lived (28). Detailed molecular studies of a jellyfish (Turritopsis nutricula) - the only documented immortal organism in the animal kingdom - may shed further light on ageing biology and this knowledge could applied to biomedical sciences especially foraging-related disorders (44).
So far, experimental gerontology research in vertebrate models has been limited by the relatively short life span of mice (3–4 years) and zebrafish (5 years). Thus, studies of the turquoise killifish (Notobranchius furzeri) with an exceptionally rapid timescale of aging (30–40 days) have provided a novel and complete genotype-to-phenotype platform that can be used for rapid exploration of ageing mechanisms (45). Studies in killifish show that key ageing genes are under positive selection and that these genes are clustered on sex chromosomes suggesting that differences in lifespan are genetically linked to sex (46). Indeed, it has been suggested that communication between the gonad and brain is an accomplished pathway to explore mechanisms that promote longevity (47).
Specific characteristics in phenotype and molecular adaptations that have been documented in selected long- or short-lived species (Table 1) provide clues for human ageing mechanisms. Although multiple metabolic alterations contribute to animal longevity it is evident that outstanding stress resistance linked to robust mitochondrial functions and maintenance of protein homeostasis are major pro-longevity factors. Accumulating data support the mitochondrial theory of ageing; i.e. biochemical damage in mitochondria play an important role in ageing and that treatments that target the transcription factor Nrf2, the ability to withstand protein modification, and upregulation of matrix antioxidants may decrease the gap between life and health span. Since down-regulated Nrf2 expression is a feature of Hutchinson Guilford Progeria syndrome (48) – a rare progeroid syndrome - and a cluster of inflammation-related “burden of life style” diseases (15) human data support a role of depressed Nrf2 in premature ageing. As both Nrf2 deficiency (49) and hypoxia (50) exacerbates senescence the interrelationships between hypoxia, repressed Nrf2 activity and senescence deserves attention in burden of life style diseases. Better understanding of the biology of exceptionally long-lived animals with negligible senescence may contribute to better understanding of ageing processes and lead to novel interventions that extend human health span.
Table 1.
| Naked mole rats (Heterocephalus glaber) | |
|
|
| Ocean qua hog (Arctica Icelandica) | |
|
|
| Rockfish (Sebastes) | |
|
|
| Greenland shark (Somniosus microcephalus) | |
|
|
| Turquoise killifish (Notobranchius furzeri) | |
|
|
Biomimetic secrets found in deep oceans
The marine environment is a rich source of both biological and chemical diversity and plankton and microalgae are affluent in bioactivity. Since complex marine field samples can be used to investigate bioactivities from otherwise inaccessible sources (51) this open up for a novel source of drugs with the conceivable use in human medicine, such as for treatment of obesity (52). For example, marine-derived phoma (phytopathogens widely distributed in aquatic systems) possess anti-cancer, anti-microbial, radical scavenging and cytotoxic properties, has been regarded as a source of supply for novel bioactive compounds (53). Fish, the most diverse class of vertebrates with >20,000 existing species, provide a vast spectrum of species for bioinspired research. Fish exhibit an enormous range of senescence (54) ranging from rapid (such as eels, killifish and pacific salmon) to gradual (such as guppy and platyfish) and negligible (such as sturgeons and rockfish). Fish with minor signs of senescence could be an avenue to perform comprehensive studies of characteristics of slow ageing; i.e. muscle extracts from fish with negligible senescence have protective effects on senescence induced by oxidative stress (55). Since recent studies suggest that drugs targeting senescent cells may arrest human ageing processes (56) comparative studies of fish with rapid or negligible senescence would be of interest (54). In the animal kingdom, the levels of mitochondrial enzymes vary more than 100-fold, from low in fish white muscle to high in the flight muscles of birds (57). The Atlantic bluefin tuna (Thunnus thynnus) is a migratory fish with high metabolic demands due a remarkable swimming endurance, which stands out from other fish because they possess red muscle (due to an exceptionally high content of mitochondria). It was recently reported that the upstream open reading frame (uORF), which slows down production of the transcriptional coactivator peroxisome proliferator-activated receptor gamma co-activator 1 (PGC1a), was completely absent in the Atlantic blue fish tuna (58). This observation indicates that disruption of uORF may be an important evolutionary adaption in the animal kingdom that allow high metabolic demand. Despite the major influence of Nrf2-KEAP1 in regulating vertebrate antioxidant system, this transcription factor has been poorly investigated in marine species. However, since it was recently reported that UBV-absorbing metabolites typically found in seaweeds produced by cyanobacteria activate Nrf2-KEAP1 (59) and another study showed that it play a major role in protection of the early life stages of the Antarctic silverfish (Pleuragramma antarctica) toward the environmental changes of pro-oxidant pressure during ice melting (60), this cytoprotective system play a role also in the oceans.
Surviving hypoxia – clues provided in the animal kingdom
One important survival tool is the ability to live with limited oxygen supplies. This is a major issue for populations living at high altitudes. Systemic hypoxemia is common in the hospital setting; emerging evidence suggest that local tissue hypoxemia is an important feature of many “burden of life style” diseases linked to repressed Nrf2-KEAP1 expression and diminished mitochondrial biogenesis (15). One of the best studied animals that lives in hypoxic environment is the naked mole rat, which can survive in burrows with markedly limited oxygen availability. They survive by down-regulating their mitochondrial function and living in a glycolytic state, which allows them to reduce their oxygen demands. One of the ways this happens is by the production of fructose as its metabolism helps shift the animal to a glycolytic state (6) (61). Since hypoxia causes a oxidative stress, this rat handles this aspect by markedly increasing its antioxidant systems, based on upregulation of Nrf2-KEAP1 (38). Heat stress trigger accumulation of reactive oxygen species (ROS), reduced proliferation and apoptosis; i.e. effective activity of Nrf2-KEAP1 may be more important in the future due to global warming (62).
Another group of animals that survive periods of hypoxia are the deep diving mammals, such as the elephant seal (Phociade). They have developed an astonishing capacity to tolerate repeated cycles of ischemia and reperfusion to protect their organs during deep dives. In theory, reoxygenation of hypoxic tissues results after the dive may also generate ROS that overwhelm the antioxidant capacity and result in cell damage. In order to secure acceptable perfusion of the heart and brain during apnoea-induced hypoxemia in diving, there is an cardiovascular adjustment with heightened constriction of peripheral arterial beds in several organs, including the liver and kidneys (63). This uninterrupted vasoconstriction leads to almost total cessation of renal function and glomerular filtration rate decreases >90% (64). Thus, the seal kidney is considered extraordinary tolerant to repeated periods of ischemia (65) and due to their excellent antioxidant defence mechanisms generation of the superoxide radical does not translate into tissue damage (66). The situation seems similar to the protective effect of experimental preconditioning before graft transplantation (67). Elephant seals undergo prolonged periods of food and water deprivation. As prolonged natural periods of fasting in elephants seals activate Nrf2-KEAP1 (68), it can be hypothesized that up-regulation of this cytoprotective transcription mcontribute to seals’ amazing capacity to protect their kidneys and liver during deep sea dives. As we lack potent treatments to block effects of ischemia and hypoxia in medicine, mammalian models that help us to better understand tolerance against hypoxia-anoxia are meaningful. Brain ischemic preconditioning protects against ischemic injury via oxidative signalling and Nrf2-KEAP1 activation (69). Thus, nutrients and drugs that stimulate Nrf2-KEAP1 should be tested in conditions with tissue hypoxia (15). Indeed, many nutrients with rather potent Nrf2 stimulating effects, such as curcumin, carnosol and sulphoraphane, have consistently been reported to have beneficial effects in burden of life style diseases, such as type-2 diabetes (70).
Physiological magicians in the animal kingdom – implications for burden of lifestyle diseases
A good example of a magical metabolic feat is the capacity of the hummingbird (Sephanoides) to avoid diabetic complications. The hummingbird have a heart rate of 1200 beats/min and wings that flap 50 times/min, feeds on a sugar-based nectar (rich in energy) every day and regularly obtains blood glucose levels that would be consistent with severe diabetes, coupled with the development of fatty liver (50% fat) (71). Rapidly after feeding, extreme glucose levels was reported (40 mmol/L), which was accompanied by HbA1c levels of 4.5%, i.e. higher than measured in most birds but lower than in non-diabetic humans (72). Every night, hummingbirds will burn off the fat in the liver and its glucose levels return to the normal range and during 20 hours of flying across the Mexican Gulf they metabolize around 75% of the fat reserves (71). To support the high metabolic fuel requirements of hummingbirds it has been proposed that they possess the most biosynthetically active livers in nature (73). Moreover, they achieve the highest known mass-specific metabolic rates among vertebrates and their flight muscle is the most O2 demanding skeletal muscle per unit tissue mass known among vertebrates (74). Although they have remarkably high daily blood glucose levels, and a pearly white liver, the bird does not become diabetic in the sense of developing polyuria (glucosuria), polydipsia and polyphagia. Neither do they develop diabetic complications or progressive liver disease. How hummingbirds tolerate blood glucose levels that cause serious microvascular pathologies in diabetic patients remain unknown. Understanding their protective mechanisms could give key insights into the management of diabetes.
Increased and prolonged alcohol consumption can have devastating consequences in humans. Pentailed treeshrews (Ptilocercus lowii) living in the West Malaysian rainforest regularly consume fermented alcoholic nectar from flower buds of the Bertam palm (max alcohol concentration 3.8%) (75). As analysis of ethyl glucuronide (an alcohol metabolite) in their hair showed concentrations that in humans would indicate alcohol intoxication, biomimetic studies could resolve how they can obtain such a high metabolic tolerance for alcohol and mitigate the risk of continuous high blood alcohol concentrations without any seemingly adverse effects. Mimicking such a phenotype could eventually help combat human pathologies related to alcohol abuse.
The enormous intermittent meals, such as a pig or a goat, consumed by Burmese pythons (Python molurus) increase oxygen consumption markedly and place an extreme load not only on the cardiovascular system but also other organs, such as intestines, kidneys and liver (76). The extraordinarily rapid 40% increase in heart ventricular muscle mass just 48 hours after feeding was accompanied by increased gene expression of muscle-contractile proteins, such as myosin (77). Riquelme et al (78) reported that despite high postprandial levels of triglycerides (x 50) and fatty acids (x 3) there is no accumulation of triglycerides or fatty acids in the python heart. In contrast, they observed a robust activation of fatty acid transport pathways and oxidation combined with increased expression and activity of the cardioprotective enzyme superoxide dismutase. Since injection of this combination of three fatty acids promoted physiological heart growth in both mice and python it would be of interest to examine the effects of this specific fatty acid combination on the human heart. Increased expression of Nrf2-KEAP1 was shown to be important in for the extreme regenerative growth that occur after the massive meals (79). Since fasting increase expression of Nrf2-KEAP1 and antioxidant responses in mouse and humans (80) as well as in elephant seals (68), periods of intermittent fasting may be a prerequisite for survival in extreme conditions in nature. Interestingly, diurnal intermittent fasting during Ramadan increase expression of Nrf2 in patients with overweight and obesity (81).
From an evolutionary point of view, the absence of cardiac and renal damage in the giraffe (Giraffa) could have major implications for cardiovascular medicine. Their mean arterial pressure range from 210 to 325 mmHg to overcome the huge hydrostatic pressure needed to supply oxygen to the brain 2.5–3.0 meters above the heart. Although there are signs of arterial smooth hypertrophy and the weight of heart of giraffes is 2–4 times greater than in other ruminants there are no signs of functional, structural, renal nor cardiac damages that normally accompany cardiac hypertrophy (82). Their heart valves have evolved to adapt to an extreme-pressure system with compact collagen construction (83). A strong renal capsule supporting high renal interstitial hydrostatic pressure reduce the net filtration pressure and may protect giraffe kidneys against high blood pressure (84). The protective mechanisms in giraffes provide a conjectural framework for experimental explorations into mechanisms as well as treatment and prevention of hypertension, cardiovascular and renal disease in humans.
Hibernation is an ingenious survival strategy that evolved during evolution in certain species, such as bears, to conserve energy during long periods of nutrient-water shortage, coldness and hypoxia. Since hibernating bears are forced to maintain a careful balance between metabolic supply and demand they are used as a model to develop novel approaches to counteract burden of lifestyle diseases, such as chronic kidney disease (85), obesity- and sedentary-related diseases (86), organ preservation (87), ischemic brain damage (88) osteoporosis and muscle wasting (89). It is notable that many similarities exist between hibernation phenotypes and various long-lived animal models (90) i.e. hibernation seems to retard biological aging in proportion to time spent in the hibernating state (91). As Nrf2 (and FOXO) play major roles in the regulation of antioxidant defences in hibernating bat brains (92) upregulation of the Nrf2-KEAP1 system may be a prerequisite to avoid organ damage during hibernation (15). Models of hibernation have revealed considerable remodelling of synaptic connections (93); thus, studies of hibernating animals, such as bears and dormouse (Glis glis), may provide important information on how to cure neurodegenerative diseases, such as Alzheimer, in which a reduction in synapse number is a consistent early feature.
Cancer resistance - lessons learned from nature
Cancer, an unavoidable risk of ageing, is a major cause of morbidity and mortality also in the animal kingdom. In pet dogs, cancer closely recapitulate their human counterparts with respect to clinical presentation (94). As our understanding regarding the molecular drivers of canine cancers has become much better, unique opportunities have emerged to better guide cancer drug development so that we earlier eliminate therapies likely to fail and therapies with true potential are optimized prior to human studies. In contrast to common belief, sharks do get cancer (95); thus there are no justification for using shark cartilage as a means to prevent cancer (96). Among animals with high risk of cancer, the Tasmanian devil (Sarcophilus harrisii), is of much interest since they have become endangered due to a transmissible facial cancer expansion by direct transport of living cancer cells due to a distinct mutational process through biting (97). Lessons can also be learned from species in which risk of cancer is lower than expected, such as elephants and naked mole rats. As a greater number of cells and cell divisions increase the likelihood of mutations that transform into malignancies, Peto et al (98) hypothesized that the incidence of cancer does not increase as would be expected for larger body size and longer life span in the animal kingdom. When number of necropsies with tumours were plotted as a function of body mass × life span it was evident that whereas risk of cancer was higher in species, such as Tasmanian devil, cheetah and prairie dog, the risk of cancer (5%) was lower-than-expected in elephants (Proboscidea) (99). When the elephant genome was investigated, their cells demonstrated better apoptotic responses following DNA damage (compared to humans) and multiple copies of the tumour suppressor gene p53 (TP53). Whereas humans have one copy (two alleles) of the gene, elephants had multiple copies of TP53 (99). Although the exact role of p53 remains to be determined these findings represent an approach based on evolution to better understand mechanisms related to cancer suppression.
The inquisitive case of the naked mole rat represents another amazing example of cancer resistance in the animal kingdom. Out of 1000ś of necropsies only two cases of cancer has been reported in this long-lived rat (100). Although many of the mechanisms that promote longevity, such as protein resistance to unfolding, high levels of autophagy and high Nrf2-KEAP1 expression may also contribute to their cancer resistance, not all mechanisms involved in longevity contribute to cancer resistance (101). A low mutation rate, better protein homeostasis, improvement in telomere maintenance and resistance against oxidative stress could contribute to their cancer resistance. Surprisingly, high expression of hyaluronan in skin fibroblasts (102) may be another protective factor against cancer since naked mole rats lose their cancer resistance when hyaluronan synthase 2 is knock-downed. Thus, the thickness of this polymer not only provide skin elasticity needed for their life in burrows but may also control the cells mechanical strength and regulate their growth.
Conclusion
Using a comparative physiological approach based on species that have made successful adaptations in nature should definitely broaden the approach and likely lead to creative solutions to the medical problems of today, as well as for the future. Emerging evidence suggests that answers on how to better manage a cluster of “modern burden of lifestyle” diseases may be found in nature. It is evident that across taxa upregulation of the cytoprotective transcription factor Nrf2-KEAP1 and improved mitochondrial biogenesis by environmental factors, such as fasting, nutrients or hypoxia is associated with a survival advantage in species that survive extreme conditions. These features should be kept in mind when novel treatment strategies for “burden of life style” diseases are developed. With around 8.7 million described species, researchers pursuing biomimetic approaches have barely scratched the surface of biological inspiration. How many of these can be applied to the problems facing human enterprise and survival? For every “burden of lifestyle” disease we try to treat we should ask ourselves “How did nature solve this?”
Acknowledgements
Dr. Stenvinkels research is funded by CIMED, Heart and Lung Foundation and the “Strategic Research Programme in Diabetes at Karolinska Institutet (Swedish Research Council grant No 2009–1068)”. Dr Johnson is funded by the National Institute of Health in the USA.
Footnotes
Conflicts of interest: Dr. Stenvinkel is a member of REATAS scientific advisory board.
References
- 1.Snell-Rood E Interdisciplinarity: Bring biologists into biomimetics. Nature. 2016;529:277–8. [DOI] [PubMed] [Google Scholar]
- 2.Hwang J, Jeong Y, Park JP, Lee KH, Hong JW, Choi J. Biomimetics: forecasting the future of science, engineering and medicine. Int J Nanomedicine. 2015;10:5701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engineering Korb J.. Robots acting locally and building globally. Science. 2014;343:742–3. [DOI] [PubMed] [Google Scholar]
- 4.Arndt EM, Moore W, Lee WK, Ortiz C. Biomechanics. Mechanistic origins of bombardier beetle (Brachinini) explosion-induced defensive spray pulsation. Science. 2015;348:563–7. [DOI] [PubMed] [Google Scholar]
- 5.Werfel J, Petersen K, Nagpal R. Designing Collective Behavior in a Termite-Inspired Robot Construction Team. Science. 2014;343:754–8. [DOI] [PubMed] [Google Scholar]
- 6.Johnson RJ, Stenvinkel P, Andrews P, Sánchez-Lozada LG, Nakagawa T, Gaucher E, et al. Identification of a Common Evolutionary Pathway of Survival associated with climate change, food shortage and droughts. J Int Med 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson RJ, Stenvinkel P, Jensen T, Lanaspa MA, Roncal C, Song Z, et al. Metabolic and Kidney Diseases in the Setting of Climate Change, Water Shortage, and Survival Factors. J Am Soc Nephrol. 2016;27:2247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mora C, Tittensor DP, Adl S, Simpson AG, Worm B. How many species are there on Earth and in the ocean? PLoS Biol. 2011;8:e1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.https://www.nature.com/articles/s41559-018-0676-2 [
- 10.Hull P Life in the Aftermath of Mass Extinctions. Curr Biol. 2015;25:R941–52. [DOI] [PubMed] [Google Scholar]
- 11.Ceballos G, Ehrlich PR, Barnosky AD, García A, Pringle RM, Palmer TM. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Science Advances. 2015;1:e140025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soo RM, Hemp J, Parks DH, Fischer WW, Hugenholtz P. On the origins of oxygenic photosynthesis and aerobic respiration in Cyanobacteria. Science. 2017;355:1436–40. [DOI] [PubMed] [Google Scholar]
- 13.Gacesa R, Dunlap WC, Barlow DJ, Laskowski RA, Long PF. Rising levels of atmospheric oxygen and evolution of Nrf2. Sci Rep. 2016;6:27740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maher J, Yamamoto M. The rise of antioxidant signaling - the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol 2010;244:4–15. [DOI] [PubMed] [Google Scholar]
- 15.Stenvinkel P, Meyer CJ, Block GA, Chertow GM, Shiels PG. Understanding the role of the cytoprotective transcription factor NRF2 - Lessons from evolution, the animal kingdom and rare progeroid syndromes. Nephrol Dial Transpl. 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benner SA, Caraco MD, Thomson JM, Gaucher EA. Planetary biology--paleontological, geological, and molecular histories of life. Science. 2002;296(5569):864–8. [DOI] [PubMed] [Google Scholar]
- 17.Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci U S A 2010;107:6127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaushik NK, Kaushik N, Pardeshi S, Sharma JG, Lee SH, Choi EH. Biomedical and Clinical Importance of Mussel-Inspired Polymers and Materials. Mar Drugs. 2015;13:6792–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoffstall AJ, Srinivasan S, Willis M, Stiller AM, Ecker M, Voit WE, et al. A Mosquito Inspired Strategy to Implant Microprobes into the Brain . Sci Rep.8:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saikawa Y, Hashimoto K, Nakata M, Yoshihara M, Nagai K, Ida M, et al. Pigment chemistry: the red sweat of the hippopotamus. Nature. 2004;429:363. [DOI] [PubMed] [Google Scholar]
- 21.Dundar Arisoy F, Kolewe KW, Homyak B, Kurtz IS, Schiffman JD, Watkins JJ. Bioinspired Photocatalytic Shark-Skin Surfaces with Antibacterial and Antifouling Activity via Nanoimprint Lithography. ACS Appl Mater Interfaces. 2018;10:20055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.https://bnatterson-horowitz.com/zoobiquity-conference/ [
- 23.Zinsstag J, Schelling E, Wyss K, Mahamat MB. Potential of cooperation between human and animal health to strengthen health systems. Lancet. 2005;366:2142–5. [DOI] [PubMed] [Google Scholar]
- 24.Rock M, Buntain BJ, Hatfield JM, Hallgrimsson B. Animal–human connections, “one health,” and the syndemic approach to prevention. Social Science & Medicine. 2009;68:991–5. [DOI] [PubMed] [Google Scholar]
- 25.Stenvinkel P, Painer J, Kuro-O M, Lanaspa M, Arnold W, Ruf T, et al. Novel treatment strategies for chronic kidney disease: insights from the animal kingdom. Nat Rev Nephrol. 2018;14:265–84. [DOI] [PubMed] [Google Scholar]
- 26.Fougère B, Boulanger E, Nourhashémi F, Guyonnet S, Cesari M. Chronic Inflammation: Accelerator of Biological Aging. J Gerontol A Biol Sci Med Sci. 2017;72:1218–25. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, Kuhn M, Prettner K, Bloom DE. The macroeconomic burden of noncommunicable diseases in the United States: Estimates and projections. PLoS One 2018;13:e0206702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenvinkel P, Shiels PG. Long lives animals with negligible senescence - clues for ageing. Biochem Soc Trans. 2019;47:1157–64. [DOI] [PubMed] [Google Scholar]
- 29.Finch CE. Evolution in health and medicine Sackler colloquium: Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc Natl Acad Sci U S A. 2010;107 (Suppl 1):1718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Roode JC, Lefèvre T, Hunter MD. Ecology. Self-medication in animals. Science. 2013;340:150–1. [DOI] [PubMed] [Google Scholar]
- 31.Toyang NJ, Verpoorte R. A review of the medicinal potentials of plants of the genus Vernonia (Asteraceae). J Ethnopharmacol. 2013;146:681–723. [DOI] [PubMed] [Google Scholar]
- 32.Austad SN. Methusaleh’s Zoo: how nature provides us with clues for extending human health span. J Comp Pathol. 2010;142 (Suppl 1):S10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Che-Castaldo JP, Byrne A, Perišin K, Faust LJ. Sex-specific median life expectancies from ex situ populations for 330 animal species. Sci Data. 2019;6:190019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whittermore K, Vera E, Martinez-Nevado E, Sanpera C, Blasco MA. Telomere shortening rate predicts species life span. Proc Natl Acad Sci U S A. 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine HJ. Rest heart rate and life expectancy. J Am Coll Cardiol. 1997;30 1104–6. [DOI] [PubMed] [Google Scholar]
- 36.Hall KY, Hart RW, Benirschke AK, Walford RL. Correlation between ultraviolet-induced DNA repair in primate lymphocytes and fibroblasts and species maximum achievable life span. Mech Ageing Dev. 1984;24:163–73. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz MC, Ayala V, Portero-Otín M, Requena JR, Barja G, Pamplona R. Protein methionine content and MDA-lysine adducts are inversely related to maximum life span in the heart of mammals. Mech Ageing Dev. 2005;126:1106–14. [DOI] [PubMed] [Google Scholar]
- 38.Lewis KN, Wason E, Edrey YH, Kristan DM, Nevo E, Buffenstein R. Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proc Natl Acad Sci U S A. 2015;112:3722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podlutsky AJ, Khritankov AM, Ovodov ND, Austad SN. A new field record for bat longevity. J Gerontol A Biol Sci Med Sci. 2005;60:1366–8. [DOI] [PubMed] [Google Scholar]
- 40.Sosnowska D, Richardson C, Sonntag WE, Csiszar A, Ungvari Z, Ridgway I. A heart that beats for 500 years: age-related changes in cardiac proteasome activity, oxidative protein damage and expression of heat shock proteins, inflammatory factors, and mitochondrial complexes in Arctica islandica, the longest-living noncolonial animal. J Gerontol A Biol Sci Med Sci. 2014;69:1448–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hua X, Cowman P, Warren D, Bromham L. Longevity Is Linked to Mitochondrial Mutation Rates in Rockfish: A Test Using Poisson Regression. Mol Biol Evol. 2015;32:2633–45. [DOI] [PubMed] [Google Scholar]
- 42.George JC, Bada J, Zeh J, Scott L, Brown SE, O’Hara T, et al. Age and growth estimates of bowhead whales (Balaena mysticetus) via aspartic acid racemization. Can J Zool. 1999;77:571–80. [Google Scholar]
- 43.Nielsen J, Hedeholm R, Heinemeier J, Bushnell PG, Christiansen JS, Olsen J, et al. Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus). Science. 2016;353:702–4. [DOI] [PubMed] [Google Scholar]
- 44.Devarapalli P, Kumavath RN, Barh D, Azevedo V. The conserved mitochondrial gene distribution in relatives of Turritopsis nutricula, an immortal jellyfish. Bioinformation. 2014;10:586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harel I, Benayoun BA, Machado B, Singh PP, Hu CK, Pech MF, et al. A platform for rapid exploration of aging and diseases in a naturally short-lived vertebrate. Cell. 2015;160:1013–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valenzano DR, Benayoun BA, Singh PP, Zhang E, Etter PD, Hu CK, et al. The African Turquoise Killifish Genome Provides Insights into Evolution and Genetic Architecture of Lifespan. Cell. 2015;163:1539–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Austad SN. Sex differences in health and aging: a dialog between the brain and gonad? Geroscience. 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kubben N, Zhang W, Wang L, Voss TC, Yang J, Qu J, et al. Repression of the Antioxidant NRF2 Pathway in Premature Aging. Cell. 2016;165:1361–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fulop G, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, et al. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Regina C, Panatta E, Candi E, Melino G, Amelio I, Balistreri CR, et al. Vascular ageing and endothelial cell senescence: Molecular mechanisms of physiology and diseases. Mech Ageing Dev. 2016;159:14–21. [DOI] [PubMed] [Google Scholar]
- 51.Ingebrigtsen RA, Hansen E, Andersen JH, Eilertsen HC. Field sampling marine plankton for biodiscovery. Sci Rep 2017;20:15863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin Q, Yu H, Li P. The Evaluation and Utilization of Marine-derived Bioactive Compounds with Anti-obesity Effect. Curr Med Chem. 2018;25:861–78. [DOI] [PubMed] [Google Scholar]
- 53.Rai M, Gade A, Zimowska B, Ingle AP, Ingle P. Marine-derived Phoma-the gold mine of bioactive compounds. Appl Microbiol Biotechnol. 2018;102:9053–66. [DOI] [PubMed] [Google Scholar]
- 54.Patnaik BK, Mahapatro N, Jena BS. Ageing in fishes. Gerontology. 19945;40:113–32. [DOI] [PubMed] [Google Scholar]
- 55.Mikhailova MV, Belyaeva NF, Kozlova NI, Zolotarev KV, Mikhailov AN, Berman AE, et al. Protective action of fish muscle extracts against cellular senescence induced by oxidative stress. Biomed Khim. 2017;63:351–5. [DOI] [PubMed] [Google Scholar]
- 56.Tchkonia T, Kirkland JL. Aging, Cell Senescence, and Chronic Disease: Emerging Therapeutic Strategies. JAMA. 2018;320:1319–20. [DOI] [PubMed] [Google Scholar]
- 57.Moyers CD. Controlling muscle mitochondrial content. J Exp Biol. 2003;206:4385–91. [DOI] [PubMed] [Google Scholar]
- 58.Dumesic PA, Egan DF, Gut P, Tran MT, Parisi A, Chatterjee N, et al. An Evolutionarily Conserved uORF Regulates PGC1α and Oxidative Metabolism in Mice, Flies, and Bluefin Tuna. Cell Metab 2019;30:190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gacesa R, Lawrence KP, Georgakopoulos ND, Yabe K, Dunlap WC, Barlow DJ, et al. The mycosporine-like amino acids porphyra-334 and shinorine are antioxidants and direct antagonists of Keap1-Nrf2 binding. Biochimie. 2018;154:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giuliani M, Benedetti M, Nigro M, Regoli F. Nrf2 and regulation of the antioxidant system in the Antarctic silverfish, Pleuragramma antarctica: Adaptation to environmental changes of pro-oxidant pressure. Mar Environ Res. 2017;129:1–13. [DOI] [PubMed] [Google Scholar]
- 61.Park TJ, Reznick J, Peterson BL, Blass G, Omerbasic D, Bennett NC, et al. Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science. 2017;356(6335):307–11. [DOI] [PubMed] [Google Scholar]
- 62.Alemu TW, Pandey HO, Salilew Wondim D, Gebremedhn S, Neuhof C, Tholen E, et al. Oxidative and endoplasmic reticulum stress defense mechanisms of bovine granulosa cells exposed to heat stress. Theriogenology 2018;110:130–41. [DOI] [PubMed] [Google Scholar]
- 63.Bron KM, Murdaugh HV Jr., Millen JE, Lenthall R, Raskin P, Robin ED. Arterial constrictor response in a diving mammal. Science. 1966;152(3721):540–3. [DOI] [PubMed] [Google Scholar]
- 64.Davis RW, Castellini MA, Kooyman GL, Maue R. Renal glomerular filtration rate and hepatic blood flow during voluntary diving in Weddell seals. Am J Physiol. 1983;245(5 Pt 1):R743–8. [DOI] [PubMed] [Google Scholar]
- 65.Halasz NA, Elsner R, Garvie RS, Grotke GT. Renal recovery from ischemia: a comparative study of harbor seal and dog kidneys. Am J Physiol. 1974;227(6):1331–5. [DOI] [PubMed] [Google Scholar]
- 66.Zenteno-Savin T, Clayton-Hernandez E, Elsner R. Diving seals: are they a model for coping with oxidative stress? Comp Biochem Physiol C Toxicol Pharmacol. 2002;133(4):527–36. [DOI] [PubMed] [Google Scholar]
- 67.Zarbock A, Kellum JA. Remote Ischemic Preconditioning and Protection of the Kidney--A Novel Therapeutic Option. Crit Care Med. 44;607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vázquez-Medina JP, Soñanez-Organis JG, Rodriguez R, Viscarra JA, Nishiyama A, Crocker DE, et al. Prolonged fasting activates Nrf2 in post-weaned elephant seals. J Exp Biol. 2013;216:2870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang T, Sun Y, Mao L, Zhang M, Li Q, Zhang L, et al. Brain ischemic preconditioning protects against ischemic injury and preserves the blood-brain barrier via oxidative signaling and Nrf2 activation. Redox Biol. 2019;17:323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Axelsson AS, Tubbs E, Mecham B, Chacko S, Nenonen HA, Tang Y, et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci Transl Med. 2017;9:pii: eaah4477. doi: 10.1126/scitranslmed.aah4477. [DOI] [PubMed] [Google Scholar]
- 71.Hargrove JL. Adipose energy stores, physical work, and the metabolic syndrome: lessons from hummingbirds. Nutr J. 2005;4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beuchat CA, Chong CR. Hyperglycemia in hummingbirds and its consequences for hemoglobin glycation. Comp Biochem Physiol A Mol Integr Physiol 1998;120:409–16. [DOI] [PubMed] [Google Scholar]
- 73.Suarez RK, Brownsey RW, Vogl W, Brown GS, Hochachka PW. Biosynthetic capacity of hummingbird liver. Am J Ohysiol. 1988;255:R699–702. [DOI] [PubMed] [Google Scholar]
- 74.Mathieu-Costello O, Suarez RK, Hochachka PW. Capillary-to-fiber geometry and mitochondrial density in hummingbird flight muscle. Respir Physiol. 1992;89:113–32. [DOI] [PubMed] [Google Scholar]
- 75.Wiens F, Zitzmann A, Lachance MA, Yegles M, Pragst F, Wurst FM, et al. Chronic intake of fermented floral nectar by wild treeshrews. Proc Natl Acad Sci U S A. 2008;105:10426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Secor SM DJ. Determinants of the postfeeding metabolic response of Burmese pythons, Python molurus. Physiol Zool. 1997;70:202–12. [DOI] [PubMed] [Google Scholar]
- 77.Andersen JB, Rourke BC, Caiozzo VJ, Bennett AF, Hicks JW. Physiology: postprandial cardiac hypertrophy in pythons. Nature. 2005;434:37–8. [DOI] [PubMed] [Google Scholar]
- 78.Riquelme CA, Magida JA, Harrison BC, Wall CE, Marr TG, Secor SM, et al. Fatty acids identified in the Burmese python promote beneficial cardiac growth. Science. 2011;334:528–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andrew AL, Perry BW, Card DC, Schield DR, Ruggiero RP, McGaugh SE, et al. Growth and stress response mechanisms underlying post-feeding regenerative organ growth in the Burmese python. BMC Genomics. 2017;18:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kulkarni SR, Donepudi AC, Xu J, Wei W, Cheng QC, Driscoll MV, et al. Fasting induces nuclear factor E2-related factor 2 and ATP-binding Cassette transporters via protein kinase A and Sirtuin-1 in mouse and human. Antioxid Redox Signal 2014;20:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Madkour MI, El-Serafi T, Jahrami HA, Sherif NM, Hassan RE, Awadallah S, et al. Ramadan diurnal intermittent fasting modulates SOD2, TFAM, Nrf2, and sirtuins (SIRT1, SIRT3) gene expressions in subjects with overweight and obesity. Diabetes Res Clin Pract. 2019;155:107801. [DOI] [PubMed] [Google Scholar]
- 82.Zhang QG. Hypertension and counter-hypertension mechanisms in giraffes. Cardiovasc Hematol Disord Drug Targets 2006;6:63–7. [DOI] [PubMed] [Google Scholar]
- 83.Amstrup Funder J, Christian Danielsen C, Baandrup U, Martin Bibby B, Carl Andelius T, Toft Brøndum E, et al. How Heart Valves Evolve to Adapt to an Extreme-Pressure System: Morphologic and Biomechanical Properties of Giraffe Heart Valves. J Heart Valve Dis. 2017;26:63–71. [PubMed] [Google Scholar]
- 84.Damkjaer M, Wang T, Brøndum E, Østergaard KH, Baandrup U, Hørlyck A, et al. The giraffe kidney tolerates high arterial blood pressure by high renal interstitial pressure and low glomerular filtration rate. Acta Physiol (Oxf). 2015;214:497–510. [DOI] [PubMed] [Google Scholar]
- 85.Stenvinkel P, Jani AH, Johnson RJ. Hibernating bears (ursidae): metabolic magicians of definite interest for the nephrologist. Kidney Int. 2013;83:207–12. [DOI] [PubMed] [Google Scholar]
- 86.Fröbert O, Frobert AM, Kindberg J, Arnemo JM, Overgaard MT. The brown bear as a translational model for sedentary lifestyle related diseases. J Int Med 2019;In press. [DOI] [PubMed] [Google Scholar]
- 87.Dugbartey GJ, Bouma HR, Saha MN, Lobb I, Henning RH, Sener A, Dugbartey GJ, Bouma HR, Saha MN, Lobb I, Henning RH, Sener A. Antioxid Redox Signal 2018;28:1503–15. [DOI] [PubMed] [Google Scholar]
- 88.Dave KR, Christian SL, Perez-Pinzon MA, Drew KL. Neuroprotection: lessons from hibernators. Comp Biochem Physiol B Biochem Mol Biol. 2012;162:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reilly BD, Franklin CE. Prevention of muscle wasting and osteoporosis: the value of examining novel animal models. J Exp Biol. 2016;219:2582–95. [DOI] [PubMed] [Google Scholar]
- 90.Wu CW, Storey KB. Life in the cold: links between mammalian hibernation and longevity. Biomol Concepts. 2016;7:41–52. [DOI] [PubMed] [Google Scholar]
- 91.Lyman CP, O’Brien RC, Greene GC, Papafrangos ED. Hibernation and longevity in the Turkish hamster Mesocricetus brandti. Science 1981;212:668–70. [DOI] [PubMed] [Google Scholar]
- 92.Yin Q, Ge H, Liao CC, Liu D, Zhang S, Pan YH. Antioxidant Defenses in the Brains of Bats during Hibernation. PLoS One. 2016;11:e0152135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peretti D, Bastide A, Radford H, Verity N, Molloy C, Martin MG, et al. RBM3 mediates structural plasticity and protective effects of cooling in neurodegeneration. Nature. 2015;518:236–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gardner H, Fenger JM, London CA. Dogs as a Model for Cancer. Annu Rev Anim Biosci. 2016;4:199–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robbins R, Bruce B, Fox A. First reports of proliferative lesions in the great white shark, Carcharodon carcharias L., and bronze whaler shark, Carcharhinus brachyurus Günther. J Fish Dis. 2014;37:997–1000. [DOI] [PubMed] [Google Scholar]
- 96.Ostrander GK, Cheng KC, Wolf JC, Wolfe MJ. Shark cartilage, cancer and the growing threat of pseudoscience. Cancer Res. 2004;64:8485–91. [DOI] [PubMed] [Google Scholar]
- 97.Murchison EP, Schulz-Trieglaff OB, Ning Z, Alexandrov LB, Bauer MJ, Fu B, et al. Genome sequencing and analysis of the Tasmanian devil and its transmissible cancer. Cell. 2012;148:780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peto R, Roe FJ, Lee PN, Levy L, Clack J. Cancer and ageing in mice and men. Br J Cancer. 1975;32:411–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abegglen LM, Caulin AF, Chan A, Lee K, Robinson R, Campbell MS, et al. Potential Mechanisms for Cancer Resistance in Elephants and Comparative Cellular Response to DNA Damage in Humans. JAMA. 2015;314:1850–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Delaney MA, Ward JM, Walsh TF, Chinnadurai SK, Kerns K, Kinsel MJ, et al. Initial Case Reports of Cancer in Naked Mole-rats (Heterocephalus glaber). Vet Pathol. 2016;53:691–6. [DOI] [PubMed] [Google Scholar]
- 101.Lagunas-Rangel FA, Chávez-Valencia V. Learning of nature: The curious case of the naked mole rat. Mech Ageing Develop. 2017;164:76–81. [DOI] [PubMed] [Google Scholar]
- 102.Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013;499:346–9. [DOI] [PMC free article] [PubMed] [Google Scholar]