Abstract
Axon degeneration is a prominent component of many neurological disorders. Identifying cellular pathways that contribute to axon vulnerability may identify new therapeutic strategies for maintenance of neural circuits. Dual Leucine Zipper Kinase (DLK) is an axonal stress response MAP3K that is chronically activated in several neurodegenerative diseases. Activated DLK transmits an axon injury signal to the neuronal cell body to provoke transcriptional adaptations. However, the consequence of enhanced DLK signaling to axon vulnerability is unknown. We find that stimulating DLK activity predisposes axons to SARM1-dependent degeneration. Activating DLK reduces levels of the axon survival factors NMNAT2 and SCG10, accelerating their loss from severed axons. Moreover, mitochondrial dysfunction independently decreases the levels of NMNAT2 and SCG10 in axons, and in conjunction with DLK activation leads to a dramatic loss of axonal NMNAT2 and SCG10 and evokes spontaneous axon degeneration. Hence, enhanced DLK activity reduces axon survival factor abundance and renders axons more susceptible to trauma and metabolic insult.
Keywords: DLK, NMNAT2, axon, SARM1, mitochondria, SCG10
Introduction:
Neurodegenerative disorders are among the most daunting maladies impacting human health. Considerable attention is invested in dissecting cellular pathways responsible for death of the neuronal cell body. However, functional connectivity in the nervous system requires not only survival of the neuronal soma, but also maintenance of axonal connections with their targets. Axons can extend for over a meter in humans; this great distance between the neuronal cell body and the axon it supports imposes unique challenges for axonal health and renders nerves vulnerable to disease. In response to injury or pathological damage, axons initiate a self-destruction pathway that is mechanistically distinct from classically studied death programs such as apoptosis [1, 2], The dissection of these intrinsic axonal death pathways and the signaling networks that regulate axon susceptibility to degeneration may provide additional avenues for treating neurodegenerative disorders.
In human postmortem tissue and mouse models of Alzheimer’s disease or Amytrophic Lateral Sclerosis, Le Pichon et al observed elevated signaling through Dual Leucine Zipper Kinase (DLK). Moreover, genetic or pharmacological inactivation of DLK conferred partial neuroprotection in these models [3], DLK is a component of an axonal stress response network that senses axon injury and signals through MKK4/MKK7 and JNK in a retrograde complex to elicit transcriptional changes in the soma [4, 5], Interestingly, DLK signaling can evoke seemingly opposite transcriptional outputs [6, 7], On the one hand, transection of peripheral axons activates the DLK-dependent expression of regeneration associated genes that promote axon regrowth after injury [7–11], This response can be stimulated in the absence of physical injury by elevating cAMP which promotes PKA-mediated activation of DLK [12, 13]. Conversely, axotomy of retinal ganglion cells evokes DLK-dependent, apoptotic cell death [6, 14, 15], DLK also promotes cellular death in response to trophic factor deprivation and excitotoxicity [16, 17] suggesting acute DLK activation contributes to neurodegeneration by stimulating apoptotic death of the soma. Though much attention is focused on DLK-dependent transcriptional changes in the soma, the consequences of activated DLK on the local health and vulnerability of distal axons requires further investigation.
Axon injury from blunt trauma or exposure to toxins stimulates an intrinsic axon dismantling program that is distinct from classic apoptosis and relies on the NADase SARM1 [18–20]. SARM1 knockout neurons are resistant to axonopathy triggered by traumatic injury, chemotherapeutic agents, and mitochondrial dysfunction [21–27], Healthy axons restrain SARM1-dependent degeneration, at least in part, by constant anterograde delivery of the axon survival factors NMNAT2 and SCG10 (STMN2). NMNAT2 is an NAD+ biosynthetic enzyme which inhibits SARM1 activation [28, 29], Furthermore, loss of function mutations in human NMNAT2 are linked to juvenile-onset peripheral neuropathy and fatal impairments in the developing fetus [30, 31]. The neuronal stathmin SCG10 regulates microtubule dynamics and is dramatically downregulated in the context of ALS-linked mutations in TDP-43 [32, 33], SCG10 protects axons from injury-induced degeneration, SCG10 depletion accelerates axon loss after transection [34], and co-overexpression with NMNAT2 confers additive axon protection [35].
Importantly, the regulated degradation of both NMNAT2 and SCG10 is partially tuned by constitutive DLK-MKK4/7-JNK signaling [35, 36] and inhibiting this constitutive activity suppresses axon degeneration [36–38], A highly related MAP3K called LZK shares some overlapping function with DLK. Though loss of LZK alone has marginal effects on axon protection, the combined inactivation of both kinases confers additive neuroprotection [36, 39], While consititutive DLK signaling regulates NMNAT2 degradation, it is not known whether activated DLK also tunes NMNAT2 and impacts axonal susceptibility to degeneration. Since this DLK stress response pathway is elevated in neurodegenerative disorders the therapeutic potential of targeting DLK could depend on the role of local DLK activity on axon health and degeneration.
In this study we investigate the effects of elevating DLK activity on axon susceptibility to injury-induced degeneration. Activating DLK through cAMP-PKA signaling accelerates axon dismantling during Wallerian degeneration. Deletion of the prodegenerative factor SARM1 suppresses DLK- accelerated degeneration. Acute stimulation of DLK reduces axonal levels of NMNAT2 and SCG10 and quickens their loss from severed axons. Inhibiting mitochondrial function also decreases NMNAT2/SCG10 levels, yet this effect is independent of DLK. Mitochondrial dysfunction synergizes with this MAPK stress pathway to induce spontaneous SARM1-dependent axon degeneration. Collectively, these studies demonstrate that elevated DLK signaling enhances the vulnerability of axons to SARM1-dependent degeneration.
Results:
Acute DLK activation accelerates SARM1-dependent axon degeneration.
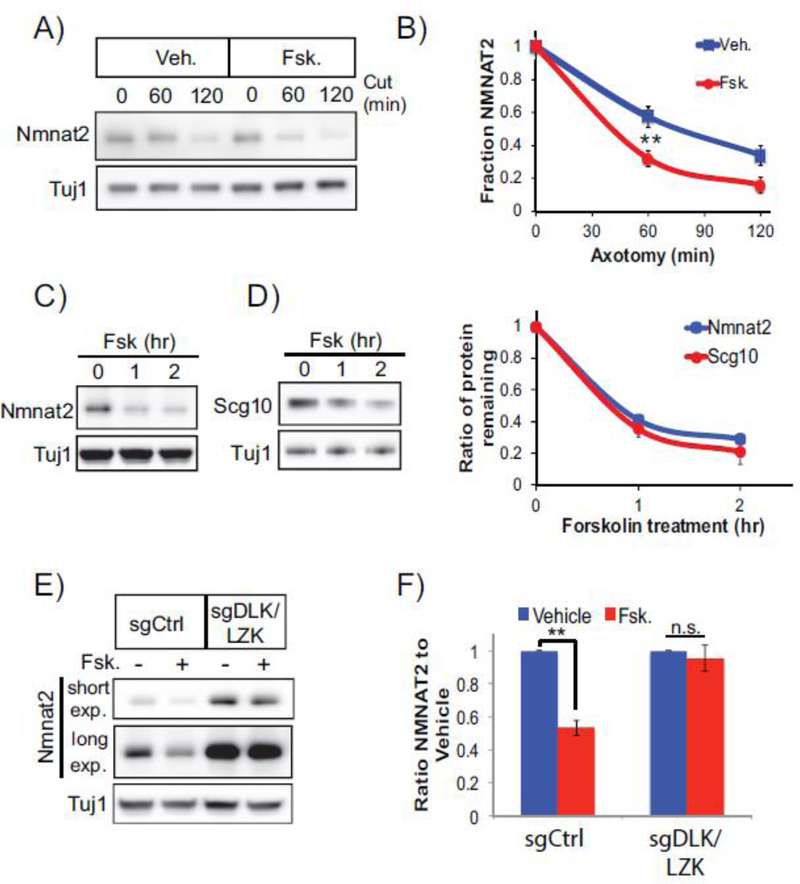
Elevating cAMP stimulates PKA-dependent phosphorylation of DLK and enhances axon regeneration after injury [12]. However, the consequence of cAMP-evoked DLK activation on the local axon segment was unknown. To investigate this question, Dorsal Root Ganglion (DRG) sensory neurons were treated with forskolin to elevate cAMP and axons were transected 30 minutes later. Axon transection stimulates Wallerian degeneration of the disconnected axon segment, a self-destruction pathway that depends on the pro-degenerative factor SARM1 and is independent of the soma. Immediately following axotomy there is a commitment phase when axons are morphologically intact and inactivating SARM1 will suppress axon degeneration [40], Axonal blebbing is apparent approximately four to six hours after axotomy and axonal fragmentation becomes increasingly prominent within eight to ten hours. We found that forskolin alone does not evoke spontaneous axon degeneration in the absence of an injury suggesting cAMP elevation is not inherently toxic to sensory neurons. However, in the context of axotomy, forskolin pretreatment leads to an acceleration in axon fragmentation with profound axonal blebbing as early as 3 hours after axotomy (Figure 1A,B). As a complementary approach, we pre-incubated sensory neurons with a cell-permeable cAMP analog (8-cpt-cAMP). As observed with forskolin, 8-cpt-cAMP accelerates axon degeneration after injury (Figure 1C). To completely exclude contributions from the soma and test whether forskolin-enhanced degeneration is restricted to the severed axon segment, forskoiin was added immediately after axon transection. Post-axotomy forskolin treatment also accelerates axon degeneration, demonstrating that elevated cAMP is acting locally within the axon to accelerate degeneration after injury (Figure 1D).
Figure 1. Acute DLK stimulation accelerates injury-induced axon degeneration.
(A) Phase contrast images of neurons treated as indicated. Scale bar = 5μm. (B) Quantification of axon degeneration at indicated time points after injury (N=4; p<0.0001, 2-way ANOVA for repeated measures). Forskolin pretreatment (30μM) accelerates axon degeneration after axotomy. (C) Neurons pre-treated with the cell permeable cAMP analog (8-cpt-cAMP; 250μM) show accelerated axon degeneration after axotomy (N=4, p=0.003, 2-way ANOVA for repeated measures). (D) Forskolin application 5 min after axotomy accelerates axon degeneration (N=3, p=0.0012, 2-way ANOVA for repeated measures). (E) Cas9-expressing neurons were transduced with sgRNAs to DLK and LZK or negative control (sgCtrl). Loss of DLK/LZK suppresses forskolin-enhanced axon degeneration (N=3, p<0.0001, 2-way ANOVA for repeated measures). (F) Axon degeneration is suppressed in SARM1−/− neurons pre-treated with forskolin (N=3, p<0.0001, 2-way ANOVA for repeated measures). Error bars represents +/-1 SEM.
We tested whether DLK is necessary for forskolin-accelerated degeneration using Cas9/CRISPR to inactivate DLK signaling. CRISPR inactivation of DLK alone preserves axons for over 12 hours after axotomy [36], We found however that forskolin treatment still induced partial axon degeneration in the absence of DLK (Supplementary Figure 1A), suggesting additional proteins could be involved. DLK shares partially redundant functions with the closely related MAP3K LZK [15, 36], Therefore, we targeted both MAP3Ks with previously validated sgRNAs [36], Indeed, loss of both MAP3Ks suppressed forskolin-enhanced axon degeneration (Figure 1E). As a complementary approach to inhibiting this pathway, we knocked down MKK4 and MKK7 with shRNAs [35] and observed strong suppression of axon degeneration in the presence of forskolin (Supplementary Figure 1B) confirming the role of this MAPK signal cascade downstream of forskolin-enhanced degeneration. Finally, we predicted that forskolin pretreatment accelerated axon degeneration through SARM1. Forskolin did not promote axon degeneration following axotomy in SARM1−/− neurons confirming that SARM1 is required for degeneration in the presence of elevated cAMP (Figure 1F). Thus, acute activation of the DLK pathway via cAMP-PKA signaling accelerates axon degeneration following a traumatic injury.
DLK activation accelerates degradation of axon survival factors.
We next investigated the mechanism by which forskolin accelerates SARM1-dependent axon degeneration. SARM1 activity is restrained by NMNAT2, a labile axon survival factor whose levels are a key determinant of the kinetics of axon degeneration [41]. We previously found that constitutive activity of DLK or the downstream kinases MKK4/7 or JNK regulates turnover of NMNAT2 as well as a second labile axon survival factor, SCG10/STMN2 [35, 36], Therefore, we examined whether acute DLK activation with forskolin influences the levels of these survival factors in severed axons. Forskolin was applied to DRG neurons and then axons were immediately severed. NMNAT2 and SCG10 are lost more rapidly from severed axons following forskolin treatment (Figure 2A,B). Indeed, acute forskolin treatment decreases steady state axonal levels of NMNAT2 and SCG10 within 2 hours of application (Figure 2C,D). We tested the requirement for DLK/LZK in forskolin-induced NMNAT2 loss using CRISPR-mediated gene inactivation. While there is an increase in NMNAT2 levels in neurons lacking DLK/LZK, the forskolin-mediated reduction in NMNAT2 levels observed in wildtype neurons no longer occurs in the absence of these kinases (Figure 2E,F). These studies collectively demonstrate that forskolin-enhanced DLK/LZK activity shortens the half-life of NMNAT2/SCG10 in axons, leading to the more rapid loss of these survival factors in severed axons.
Figure 2. Forskolin treatment promotes loss of NMNAT2 from DRG sensory axons.
(A,B) Forskolin treatment accelerates loss of NMNAT2 from severed axons. Forskolin (30 μM) was applied to DRG cultures five min prior to axon transection. Western blots are from axon-only extracts collected at the indicated time post axotomy and quantification shown in (B) (N=4 p=0.0093; unpaired t-test). Forskolin treatment stimulates a reduction in axonal steady-state levels of (C) NMNAT2 and (D) SCG10 levels with quantification of protein loss shown on the right (N=3). (E,F) DLK/LZK were inactivated in DRG neurons by CRISPR (sgDLK/LZK). DRG cultures were treated with forskolin for 2 hr and NMNAT2 levels detected from axon-only extracts. In (E), a short and long exposure (exp.) are shown from a representative western blot. (F) Quantification of endogenous, axonal NMNAT2 in the presence of control sgRNA (left panel) or sgDLK/LZK (right panel) after 2 hour treatment with forskolin (N=3; p=0.009 unpaired T-test, n.s. not significant). Since steady state levels of NMNAT2 are elevated in the absence of DLK/LZK the levels NMNAT2 are normalized internally in the left and right panels. Error bars represent +/- 1 SEM.
DLK activity sensitizes axons to degeneration after mitochondrial dysfunction
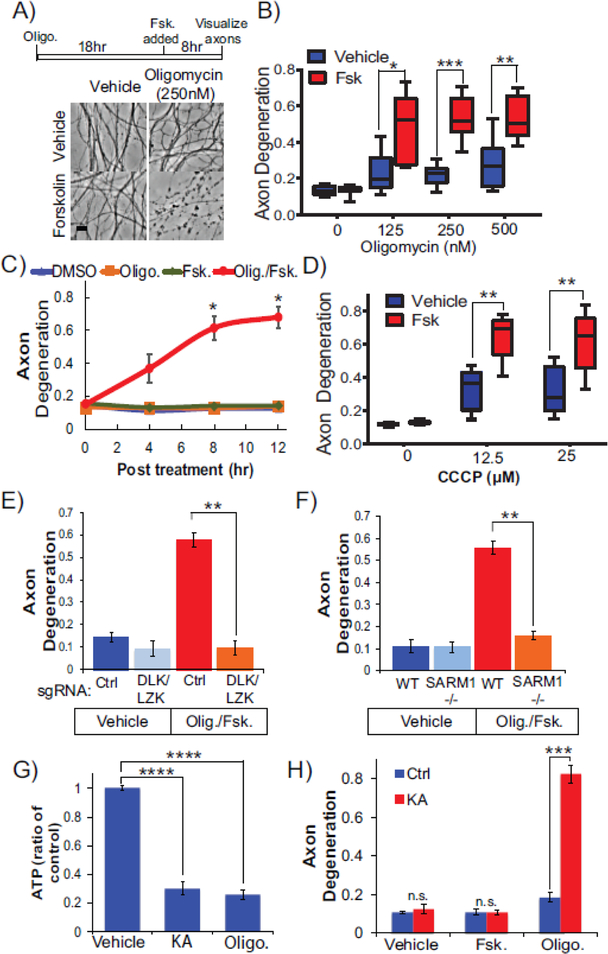
The above studies examine how stimulating DLK activity affects axon degeneration after transection. Acute DLK stimulation reduces steady state NMNAT2 levels yet does not evoke spontaneous degeneration. We therefore postulated that increases in DLK activity would sensitize axons to a second stressor. Defects in mitochondrial function are linked to many neurological disorders including most axonopathies [42]. We investigated whether activating DLK predisposes axons to degeneration mediated by mitochondrial dysfunction. To induce mitochondrial distress, we applied pharmacological inhibitors to the electron transport chain at doses that elicit mitochondrial stress without inducing axon degeneration. DRG sensory neurons were incubated with nanomolar doses of oligomycin for 18 hr to induce prolonged mitochondrial dysfunction while maintaining axon integrity. The addition of forskolin to these neurons undergoing chronic oligomycin treatment provoked axon fragmentation within 8 hr after forskoiin application (Figure 3A,B). Simultaneous application of oligomycin and forskolin induced spontaneous axon fragmentation reminiscent of axon dismantling observed in severed axons (Figure 3C).
Figure 3. Activating DLK sensitizes axons to degeneration in response to mitochondrial dysfunction.
(A) Experimental timeline and phase contrast images of DRGs neurons after the indicated treatment. DRG neurons were treated with oligomycin (250nM) for 18 hr, at which point Forskolin (30μM) was added to the media and then distal axons were imaged 8hr later. (A) Phase contrast images and (B) quantification of axon degeneration from DRG neurons pretreated with indicated doses of oligomycin followed by treatment with vehicle or forsklin (N=6. *p=0.0207, ***p=0.0007, and **p=0.0087, unpaired T-test within dosage).(C) Forskolin and oligomycin (500 nM) were applied to DRGs simultaneously and axon degeneration was monitored in distal axon segments (N=4, 8hr, *p=0.0434, 12hr, *p=0.0149; 2-way ANOVA repeated measures with post-hoc Bonferroni correction). (D) Neurons were treated with indicated concentration of CCCP. Axon degeneration was measured 8 hr post forskolin treatment (N=5, **p=0.0011 and p=0.003 for unpaired T-tests within doses 12.5μM and 50μM respectively). (E) Axon degeneration was measured in neurons treated with forskolin and oligomycin and expressing Cas9 with sgRNAs to DLK and LZK or scrambled sgRNA control (N=4 p=0.0025 unpaired T-test). (F) WT or SARM1−/− neurons were treated with vehicle, forskolin, oligomycin, or a combination of forksolin and oligomycin. Axon degeneration was quantified 8 hr later (N=3 p=0.0011 unpaired T-test). Scale Bar = 5μm. Error bars represent +/-1 SEM. (G) ATP is depleted from axons of DRG neurons 8 hr after treatment with koningic acid (KA) (5μM) or oligomycin (500 nM) (N=4, ****p<0.0001 unpaired T-test). (H) Neurons were pretreated with KA for 18 hr then forskolin or oligomycin was added. Treatment with KA and forskolin does not elicit degeneration. However, combined treatment with KA and oligomycin induces axon degeneration. Axon degeneration was measured 8 hr later (N=3, ***p=0.0003; unpaired T-test).
To determine if this effect is specific to inhibiting ATP synthase, we treated DRG neurons with rotenone, a commonly used pesticide that inhibits Complex I of the electron transport chain and induces Parkinsonian symptoms [43]. At non-toxic doses of rotenone the application of forskolin again stimulates axon degeneration (Supplementary Figure 2A,B). Finally, we triggered mitochondrial dysfunction by a third approach; inducing mitochondrial depolarization with the protonophore carbonyl cyanide m-chlorophenyl hydrazine (CCCP). At non-toxic doses, co-applying CCCP with forskolin induces axon degeneration as observed with oligomycin and rotenone (Figure 3D).
We suspected that axon degeneration evoked by oligomycin/forskolin acted through DLK/LZK and SARM1. Indeed, CRISPR knockout of DLK and LZK protects axons from degeneration induced by oligomycin and forskolin (Figure 3E). Co-applying a small molecule inhibitor to DLK with forskolin also suppressed axon degeneration in the presence of oligomycin (Supplementary Figure 2C). Furthermore, axon degeneration in response to these pharmacological agents is blocked in SARM1−/− sensory neurons (Figure 3F and Supplementary Figure 2B). These studies collectively demonstrate that the SARM1 pathway activated after mechanical injury to promote axon degeneration is also activated by mitochondrial dysfunction and increased DLK activity.
The mitochondrial inhibitors described above impair ATP synthesis and thereby induce energetic stress. We wondered if general energetic stress sensitizes axons to forskolin-evoked degeneration. To test this hypothesis, we inhibited glycolysis rather than mitochondrial respiration. We treated neurons with the GAPDH inhibitor koningic acid and observed a reduction in steady-state ATP levels comparable to what we observe in oligomycin-treated neurons (Figure 3G). Yet in sharp contrast to our results with mitochondrial dysfunction, inhibition of glycolysis in the presence of forskolin does not induce axon degeneration (Figure 3H). We do observe axon degeneration with combined inhibition of glycolysis and mitochondrial dysfunction indicating robust inhibition and a necessity for both metabolic pathways [22], We conclude that the synergistic effects of mitochondrial dysfunction and DLK activation on axon degeneration are not due to ATP depletion alone.
Oligomycin and forskolin provoke local axon degeneration.
Prolonged mitochondrial dysfunction can activate cellular death programs including caspase-dependent apoptosis [44], Therefore, we investigated whether co-applying oligomycin and forskolin stimulate cellular death. Oligomycin/forskolin treatment for 8 hr elicits strong axon degeneration but does not induce neuronal death as assayed by somal uptake of ethidium homodimer, an indicator of diminished membrane permeability and cell death (Figure 4A). Furthermore, we do not observe the appearance of cleaved caspase 3 after co-application of forskolin and oligomycin (Figure 4B).
Figure 4. Local activation of DLK and mitochondrial dysfunction stimulates axon degeneration.
Assessment of cell death in DRG neurons in response to oligomycin and forskolin treatment. DRG neurons were first treated with oligomycin (500nM) or vehicle for 18 hr. Then, forskolin (30μM) was applied for eight hours and neuronal cell death was measured using (A) uptake of ethidium homodimer to assess membrane permeability or (B) generation of cleaved caspase 3. As a positive control, neurons were deprived of nerve growth factor for a 24-hour period to induce cell death (Membrane permeability: N=3 p<0.0001; Cleaved Caspase 3: N=3 ***p=0.001; Single Factor ANOVA with posthoc Turkey T-test). (C,D) DRG neurons were seeded in microfluidic chambers. Using the same conditions described above, oligomycin and forskolin were applied to chambers containing axons. DRG neurons were fixed and axon integrity assessed after immunostaining for TUJ1. (E) Quantification of degeneration was performed on TUJ1-stained axons to assess fragmentation in axon compartment (N=4 **p=0.0016 Single Factor ANOVA). Scale bar = 5μm. Error bars represent +/-1 SEM.
In the above studies, pharmacological agents were applied to the entire DRG sensory neuron, thus somal vs. axonal contributions could not be determined. To test whether inducing local mitochondrial dysfunction and DLK activation exclusively in axons is sufficient to trigger degeneration, we cultured neurons in microfluidic chambers. We exposed chambers containing only axons to forskolin, oligomycin, or both agents and examined axon integrity using β3-tubulin immunostaining. Treatment of axons with forskolin or oligomycin individually did not grossly alter microtubule integrity; however, axonal exposure to both agents for 8 hr induced tubulin fragmentation (Figure 4C–E). Thus, local dysreguiation of mitochondrial function and stimulation of DLK within the axon alone is sufficient to drive axon dismantling.
Oligomycin promotes loss of axonal survival factors
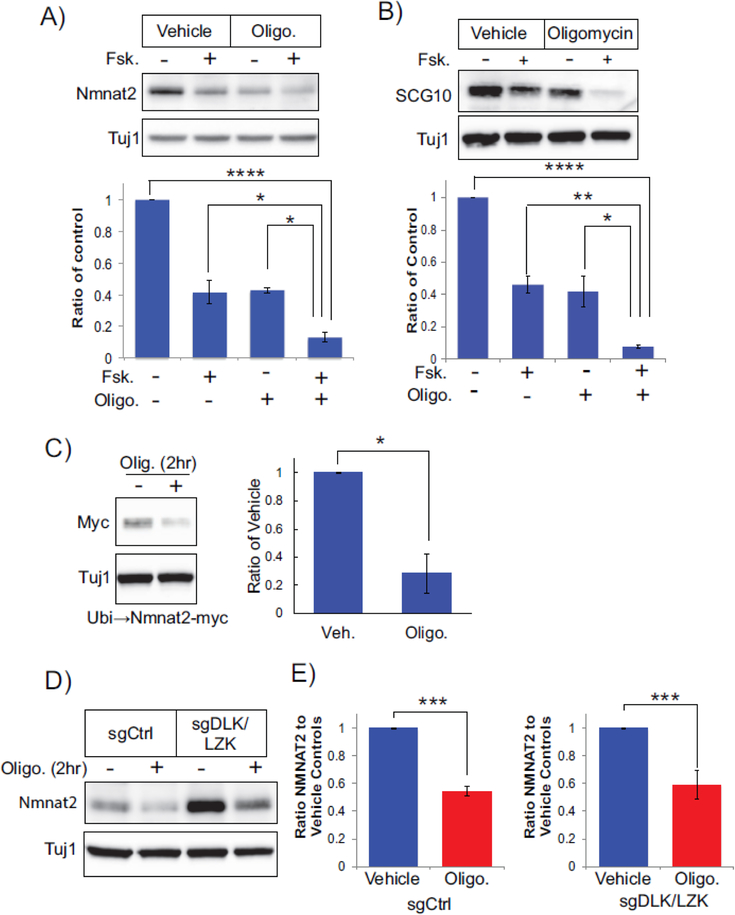
Finally, we sought to understand how mitochondrial dysfunction and forskolin induce spontaneous axon degeneration. Axon transection deprives the severed segment of axon survival factors delivered from the cell body, such as NMNAT2, and this leads to activation of SARM1. We show above that increasing cAMP to stimulate DLK enhances NMNAT2 loss in axons, however forskolin application alone does not induce axon degeneration. These results suggest there is a threshold level of NMNAT2 that must be breached before SARM1 becomes activated. To test whether this is the case for mitochondrial dysfunction, we monitored axonal levels of NMNAT2 in neurons treated with both oligomycin and/or forskolin (Figure 5A). Interestingly, NMNAT2 levels are reduced in axons treated with either oligomycin or forskolin by 50–60% within 2 hr, whereas NMNAT2 levels are reduced by 90% in neurons treated with both agents for 2 hr. Moreover, we see an analogous pattern with axonal SCG10 in which oligomycin or forskolin partially reduce levels and combined treatment strongly depletes axons of this survival factor (Figure 5B). The rapid loss of axonal NMNAT2 is similar to that observed in transected axons and suggests that inducing mitochondrial dysfunction in the setting of acute DLK activation stimulates axon degeneration by driving NMNAT2 levels below a critical threshold.
Figure 5. Oligomycin and forskolin promote loss of NMNAT2 from axons.
(A) Neurons were treated with forskolin, oligomycin, or a combination of forksolin and oligomycin for 2 hr and axonal NMNAT2 levels were assessed. Shown below is quantification of NMNAT2 levels as a ratio of vehicle control (N=4, ****p<0.0001, *p=.0147, 0.103 respectively with single factor ANOVA with Bonferroni post hoc T-test correction). (B) Neurons treated as in (A) were also assessed for axonal SCG10 levels with quantification below (N=3, ****p<0.0001, **p= 0.0076, and *p=0.0158 from single factor ANOVA with Bonferroni post hoc T-test correction). (C) Oligomycin reduces axonal NMNAT2-myc protein expressed via lentivirus from ubiquitin promoter. Neurons were treated with vehicle or oligomycin (500nM) for two hours. Quantification of NMNAT2-myc levels is shown to the right (N=4 *p=0.0144 unpaired t-test). (D, E) Axonal NMNAT2 protein is reduced by oligomycin in the presence of Cas9 and sgRNAs to DLK/ LZK (N=5, ***p=0.0011 and 0.0064 for unpaired T-tests within sgCtrl and sgDLK/LZK respectively). Error bars represent +/-1 SEM.
The rapid decrease in axonal NMNAT2 observed in neurons treated with oligomycin was unanticipated. Depolarizing mitochondria with CCCP also reduced NMNAT2 and SCG10 levels in axons suggesting that general mitochondrial stress decreases levels of these axon survival factors (Supplementary Figure 4). To further examine this phenomenon, we tested whether this was mediated directly at the protein level. We expressed a myc-tagged human NMNAT2 by lentivirus and examined its expression in oligomycin-treated neurons. We found that exogenously expressed NMNAT2 was also depleted after oligomycin treatment (Figure 5C) in a similar fashion to the endogenous NMNAT2 protein. These results are reminiscent of our findings with forskolin, so we wondered whether mitochondrial dysfunction also triggers NMNAT2 loss via DLK/LZK-mediated pathways. To test this hypothesis, we inactivated DLK/LZK with CRISPR and treated DRG neurons with oligomycin. In striking contrast to our studies with forskolin, oligomycin treatment reduced NMNAT2 protein levels in the presence of sgRNAs targeting DLK/LZK (Figure 5D,E). These results indicate that mitochondrial dysfunction depletes NMNAT2 via a DLK/LZK-independent pathway. Moreover, acute DLK activation provokes an additive loss of NMNAT2 from axonal segments that is then sufficient to trigger axon degeneration.
Discussion
DLK is an important component of an axon stress response network that is chronically upregulated in numerous neurodegenerative disorders including Alzheimer’s disease and ALS [3]. Considerable attention is focused on DLK signaling in the soma for promotion of apoptotic or regenerative responses. However, DLK is locally activated within axon segments and we previously demonstrated that inhibiting constitutive DLK signaling protects injured axons. Here, we investigate whether DLK activation influences the vulnerability of axons to pathological degeneration. We find that acute DLK activation drives the loss of axon survival factors. These axons are more susceptible to a secondary trauma which evokes axon dismantling through the executioner factor SARM1. In chronic disease, enhanced DLK signaling could intersect with multiple independent pathways to deplete axon survival factors and promote axon loss. For example, mRNA levels of NMNAT2 are reduced in models of tauopathy [45, 46] and ALS-linked mutations in TDP-43 reduce mRNA levels of SCG10 (STMN2) [32, 33], DLK signaling is elevated in these disorders which would further reduce the local abundance of axon survival factors and exacerbate neurodegeneration. These axonal effects may interact with DLK-dependent retrograde signals to cell body as well as surrounding glia and support cells that also influence axonal health [16, 47].
The mechanism by which DLK activity is increased in neurodegenerative disorders and/or under conditions of chronic stress are unclear. Axonal DLK is activated in response to defects in vesicular transport and microtubule dynamics [48,49], both of which are very prominent in diverse neurodegenerative diseases. Further, binding of apolipoprotein stimulates DLK in human IPSC-derived neurons and Alzheimer’s-linked alleles in apoliproprotein enhance this activation [50]. In this study, we show that increases in cAMP signaling reduce axonal levels of NMNAT2 and SCG10. While we cannot rule out additional roles for cAMP, in our study we suppress the effects of forskolin on axon degeneration via inhibiting either DLK orSARMI, supporting the model that forskolin treatment accelerates axon degeneration via the canonical SARM1 pathway. Furthermore, we observe enhanced axon degeneration when forskolin is applied after axon transection, highlighting the local function of this pathway in the axon segment. While we demonstrate that short-term forskolin treatment promotes the loss of NMNAT2 protein, chronic forskolin treatment enhances NMNAT2 transcription via CREB response elements in the NMNAT2 promoter [46, 51]. CREB-mediated NMNAT2 induction might be an adaptive response to transient DLK activation in order to replenish axonal NMNAT2 protein. Since cAMP-dependent pathways are often stimulated by G-protein coupled receptor (GPCR) signaling, we speculate that GPCR signaling may regulate axonal DLK activity and, hence, the vulnerability of axons in neurological disease. Identifying the GPCRs and ligands that activate axonal DLK signaling could represent new targets for treating axonopathies.
Stimulating DLK reduces axonal NMNAT2 and SCG10 protein levels, however activation of this MAP3K was not sufficient to induce axon degeneration. Instead, activating DLK renders axons more susceptible to a second insult, such as mitochondrial stress, that further depletes NMNAT2/SCG10 and activates SARM1-dependent degeneration. This degeneration pathway is entirely localized in the axon segment as demonstrated by our studies in microfluidic chambers. To our surprise, oligomycin treatment alone reduces axonal NMNAT2/SCG10 protein levels. The mechanism by which inhibiting the electron transport chain affects NMNAT2 protein homeostasis is unknown, although it is independent of DLK/LZK signaling. Our findings demonstrate that axons can tolerate acute fluctuations in the abundance of axon survival factors such as NMNAT2, however dramatic depletion triggers SARM1-dependent axon degeneration. Mitochondrial dysfunction occurs in many neurodegenerative diseases, and we postulate that chronic mitochondrial failure may synergize with DLK activation to drive loss of axon survival factors and induce axon degeneration. Targeting these axonal pathways could provide substantial therapeutic benefit for many neurodegenerative disorders..
Materials and Methods
Reagents:
The following chemicals were from Sigma Aldrich (St. Louis, MO): carbonyl cyanide m-chlorophenyl hydrazine (CCCP), forskolin, cycloheximide, and rotenone. Oligomycin, koningic acid, and GNE-3511 were from Cayman Chemicals (Ann Arbor, Ml). Hoescht 33342 was from ThermoFisher (Waltham, MA) and ethidiuim homodimer was from Biotium (Fremont, Ca). Antibodies used in this study are as follows: Anti-TUJ1 (BioLegend San Diego, CA; RRID: AB_10063408; WB: 1:5000; IF: 1:500), Anti-SCG10 (Shin et al., 2012b WB: 1:1000), anti-cleaved caspase 3 (Cell Signaling Technologies Danvers, MA RRID: AB_2341188; IF: 1:500). Nmnat2 was detected with a rabbit polyclonal antibody generated to a peptide corresponding to amino acids 232–253 from mouse NMNAT2 (YenZym Antibodies, South San Francisico, CA). Rabbit antiserum was purified on a sulfolink resin column coupled with the above-mentioned peptide (Thermo Fisher Scientific, Waltham MA). The glycine-eluted antibody was dialyzed in PBS and the specificity of the antibody confirmed by western immunoblotting using lysate DRGs lacking endogenous NMNAT2 upon CAS9/CRISPR knockout.
Culture of Primary DRG Sensory Neurons:
Mouse embryonic sensory neurons dorsal root ganglia (DRG) were isolated from embryonic day 13.5 mouse pups (equal number of both sexes). DRGs were collected and trypsined, then the resulting suspension was seeded as droplets into plastic dishes precoated with poly-d-lysine (PDL) and laminin. Slight variations in plate-type and seeding method are described with the pertinent experiment. DRG neurons were grown in Neurobasal Medium (Life Technologies) supplemented with 2mM glutamine (ThermoFisher), penicillin/streptomycin (10U/mL-ThermoFisher), B27 supplement (0.5X-Life Technologies), nerve growth factor (50ng/mL-Envigo Bioproducts), uridine (1μM-Sigma) and 5-fluoro-2′-deoxyuridine (1μM-Sigma) to prevent proliferation of dividing cells. For studies using CRISPR/CAS9, embryonic DRGs were collected from a Cas9 knock-in mouse [52], All experiments are performed on DIV7 or DIV8. Mouse procedures in compliance with protocols approved by the Institutional Animal Care and Use Committee at Washington University School of Medicine.
Lentiviral plasmids and transduction of DRG neurons:
To produce lentiviral particles, HEK293T cells were transfected with plasmids expressing vesicular stomatitis viral G protein, the lentivral packaging plasmid psPAX2, a lentiviral expression plasmid derived from the FUGW backbone (ubiquitin promoter) or pLentiX (U6 promoter for CRISPR). Media containing lentiviral particles was collected, briefly centrifuged to remove debris, and supernatant applied to DRG neurons. For CRISPR studies, lentivirus containing sgRNAs were applied on days in vitro zero (DIV0, same day as spotting). For knockdown studies, shRNAs from the RNAi consortium were from at Sigma (MKK4: TRCN0000345130, MKK7: TRCN000012609) and previously validated in Walker et al 2017. For CRISPR/Cas9 studies, sgRNA sequences are as follows (scrambled #1: CGTCGCCGGCGAATTGACGG; scrambled #2 CGCGGCAGCCGGTAGCTATG; DLK #1: GTCGTATGTGATTAGCATGC, DLK #2: AGAGAGGGAATTGCTCAGGT, DLK #3: GGATTTTTTTAGGCGAGAGC, LZK #1 GGTCACGGTGTATAATCTTG LZK #2 TTCCCAATGATATTCCACAC). Loss of target protein is described in Summers et al 2018. Lentivirus for each sgRNA were prepared and added to neuron culture. SgRNAs targeting the same gene (or scrambled control) were added together.
Measurement of axon degeneration:
Axon degeneration was measured as previously described [53], For axotomy studies, DRG neurons were seeded in 96-well plates. On DIV 7 or 8, axons were manually severed with a razor blade and the degeneration of disconnected axon segments was monitored. For studies with pharmacological agents (vincristine, oligomycin, etc), the degeneration of distal axons was monitored after drug application. Brightfield images of injured axons were acquired with an Operetta automated imager (PerkinElmer, Waltham MA). For an experimental replicate, at least three wells were assessed per condition. Within each well, at least six independent fields were collected. For timecourse studies, the same axon fields were imaged during the experimental timeseries. Axon degeneration is quantified from an ImageJ macro [53] that scores the appearance of fragmented/circular axon segments within each individual image. For example images shown in figures, DRG neurons are seeded in a 48-well plate and otherwise treated in an identical manner as studies in 96-well plates. Distal axons were imaged under phase contrast at the indicated time point.
Biochemical analysis of axon-only protein levels:
For analysis of axon-only fractions, a concentrated suspension of DRG sensory neurons was generated as described above and seeded in a small spot in a 12-well plate coated with PDL/Laminin. DRG sensory neurons are treated as described above however fresh media is exchanged every two days to maintain culture health. Sensory neuron cell bodies typically remain in a spot at the center of the well. If cell bodies disperse beyond this spot, then the culture is not used for axon-only fractionation. Biochemical extraction is performed on DIV8. Cells are washed in cold PBS and a razor blade is used to cut around the spot of cell bodies. The spot of cells bodies is removed with a pipette. After thoroughly removing PBS from the well, cold lysis buffer is applied to the axon fraction that remains in the dish. Lysis buffer is composed of 150mM NaCl, 50mM Tris-HCl pH7.4, 1mM EDTA, 0.5% sodium deoxycholate, 0.1% SDS, 1% Triton-X, 1mM phenylmethylsulfonyl fluoride (added fresh), 1x protease inhibitor cocktail (added fresh-Pierce), 1x phosphatase inhibitor cocktail (added fresh -Sigma), The axon extract is removed and stored on ice. Extracts are stored for at least an hour in a −80 freezer to facilitate thorough lysis. After thawing, the extracts are vortexed briefly, cell debris is cleared by centrifugation (at 4°C) at 2,500xg for 5min. The supernatant is collected and added to sample buffer (62.5mM Tris-HCl pH 6.8, 2% SDS, 10% glycerol, 8% beta-mercaptoethanol, 0.025% bromophenol blue) for storage until analysis by SDS-PAGE and western immunoblotting. Western blots shown in figures are representative of at least three independent trials.
Isolation of axons in microfluidic devices:
To perform degeneration studies in isolated axon compartments, DRG sensory neurons were cultured in AXIS™ isolation devices (AX1510TC Millipore). A higher volume of neurobasal media prepared as above was maintained in the compartment containing cell bodies. On DIV8, the axon compartment was treated with DMSO, forskolin, oligomycin, or a combination of forksolin and oligomycin. Eight hours after treatment, cells and axons were fixed in 4% paraformaldehyde for 2 hours. Fixed cells/axons were stored in cold phosphate buffered saline. For immunostaining, cells/axons were permeabilized and blocked for 1 hour in PBS-T-GS (1xPBS, 0.1% TritonX, 3% goat serum). Cells/axons were incubated with anti-TUJ1 antibody (1:1000 in PBS-T-GS) overnight, washed in PBS-T, and incubated for 1 hour with Alexa488-conjugated secondary antibody (goat anti-mouse, 1:1000 in PBS-T-GS). Secondary antibody was washed in PBS-T and cells/axons stored in PBS during visualization with a CKX41 inverted Olympus microscope and images collected with a Nikon DS-Qi1Mc camera. For identification of nuclei, Hoescht 33342 (1μg/mL) was applied to the chambers. Axon degeneration was quantified as described above except that axon fields were inverted in ImageJ during analysis.
Analysis of axonal metabolites:
DRG sensory neurons were cultured as described above in 12-well plates for biochemical analysis of protein extracts. Immediately prior to extraction, media was removed and cold PBS added to the DRG neurons. A razor blade was used to sever axons surrounding the cell body which was subsequently removed with a pipet leaving on the axons in the dish. Axons were incubated with perchloric acid followed by sodium carbonate to extract metabolites. Extracts were analyzed by HPLC and ATP levels quantified using an ATP standard curve generated in parallel as a reference.
Assessments of neuronal cell death:
Cellular death was quantified by two methods. First, cell permeability was measured by influx of ethidium homodimer. DRG sensory neurons were seeded in 96-well plates as described for axotomy studies. On DIV6, DRGs were incubated with oligomycin (500nM) overnight (14hr) then forskolin (30μM) was applied and cells were imaged 6hr later. Within each experiment, nerve growth factor withdrawal was performed as a positive control for cellular death in both assays. For this control, neurobasal media containing NGF was removed and DRG neurons gently washed at least three times with neurobasal media prepared as described above except that it is lacking NGF. Finally, DRGs were incubated with neurobasal media lacking NGF and containing anti-NGF antibody (1:5000) for 24hr and analyzed simultaneously with oligomycin/forskolin studies. To visualize cell permeability, ethidium homodimer 2μM and Hoeschst 33342 (1μg/mL) were added to DRG neurons. After 15 minute incubation at 37°C, DRG neurons were visualized with an Operetta High Content imager as described above. The number of ethidium homodimer positive nuclei was counted as a ratio of total cells in the population. At least 100 cells from three wells were visualized within each experimental replicate. As a second measure of cell death, cleaved caspase 3 was detected by immunofluorescence. DRGs sensory neurons were seeded in Permanonx chamber slides (Lab-Tek) coated with poly-D-lysine and laminin and cultured as described above. On DIV7, DRGs were treated as described then fixed in 4% paraformaldehyde. Fixed neurons were washed in PBS then permeabilized in PBS with 0.2% Triton-X and 10% goat serum. Permeabilized neurons were incubated in anti-cleaved Caspase 3 (1:500) and anti-TUJ1 (1:500) overnight at 4°C. After washing in PBS (with 0.2% Triton-X) fluorescently-conjugated secondary antibodies and Hoeschst (1:5000) were applied and visualized on a Leica DMI 4000B confocal microscope using 20x or 40x objectives with Leica DFC7000T color fluorescence camera. Cleaved-caspase 3 positive cells were quantified as a percent of total cells in a field. Within an experiment, at least 100 cells were counted per condition. Three independent replicates were performed. Within each experiment, nerve growth factor withdrawal was performed as a positive control.
Statistics:
Data analysis was performed with Prism GraphPad. Statistical tests, p-values, and sample sizes are described in figure legends. For statistical analysis of western blots the individual timepoints from two different conditions are compared. For CRISPR studies and quantification of western blots, sgRNAs to DLK/LZK elevate steady state levels of NMNAT2 so statistical comparisons are made within this genotype (ex. vehicle vs forskolin). For axon degeneration timecourses, we performed two-way ANOVA for repeated measures to test whether there is an interaction between the indicated treatments and time post axotomy.
Supplementary Material
Acknowledgements:
We thank members of the DiAntonio and Milbrandt labs for constructive feedback in the generation of this manuscript. D.W.S is supported by a Development Grant from the Muscular Dystrophy Association (MDA344513). This work was also supported by funds from the National Institutes of Health (R01-NS65053 to A.D., RF1-AG013730 to J.M, R01-NS087632 to J.M and A.D., and R01-CA219866 to A.D. and J.M).
Footnotes
Competing Interests: A.D and J.M are co-founders of Disarm Therapeutics and members of the Scientific Advisory Board. The authors have no additional competing financial interests.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Gerdts J, Summers DW, Milbrandt J, DiAntonio A (2016) Axon Self-Destruction: New Links among SARM1, MAPKs, and NAD+ Metabolism. Neuron 89:449–460. 10.1016/j.neuron.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiAntonio A (2019) Axon degeneration: mechanistic insights lead to therapeutic opportunities for the prevention and treatment of peripheral neuropathy. Pain 160 Suppl 1 :S17–S22. 10.1097/j.pain.0000000000001528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Pichon CE, Meilandt WJ, Dominguez S, et al. (2017) Loss of dual leucine zipper kinase signaling is protective in animal models of neurodegenerative disease. Sci Transl Med 9:eaag0394 10.1126/scitranslmed.aag0394 [DOI] [PubMed] [Google Scholar]
- 4.Asghari Adib E, Smithson LJ, Collins CA (2018) An axonal stress response pathway: degenerative and regenerative signaling by DLK. Curr. Opin. Neurobiol 53:110–119. 10.1016/j.conb2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farley MM, Watkins TA (2018) Intrinsic Neuronal Stress Response Pathways in Injury and Disease. Annu Rev Pathol Mech Dis. 10.1146/annurev-pathol-012414-040354 [DOI] [PubMed] [Google Scholar]
- 6.Watkins TA, Wang B, Huntwork-Rodriguez S, et al. (2013) DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc Natl Acad Sci 110:4039–4044. 10.1073/pnas.1211074110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin JE, Ha H, Kim YK, et al. (2019) DLK regulates a distinctive transcriptional regeneration program after peripheral nerve injury. Neurobiol Dis 127:178–192. 10.1016/j.nbd.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin JE, Cho Y, Beirowski B, et al. (2012) Dual Leucine Zipper Kinase Is Required for Retrograde Injury Signaling and Axonal Regeneration. Neuron 74:1015–1022. 10.1016/j.neuron.2012.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammarlund M, Nix P, Hauth L, et al. (2009) Axon regeneration requires a conserved MAP kinase pathway. Science (80-) 323:802–806. 10.1126/science.1165527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan D, Wu Z, Chisholm AD, Jin Y (2009) The DLK-1 Kinase Promotes mRNA Stability and Local Translation in C. elegans Synapses and Axon Regeneration. Cell 138:1005–1018. 10.1016/j.ceii.2009.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong X, Wang X, Ewanek R, et al. (2010) Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol 191:211–223. 10.1083/jcb.201006039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao Y, Frey E, Yoon C, et al. (2016) An evolutionary conserved mechanism for cAMP elicited axonal regeneration involves direct activation of the dual leucine zipper kinase DLK. Elife 5:. 10.7554/eLife.14048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh-Roy A, Wu Z, Goncharov A, et al. (2010) Calcium and Cyclic AMP Promote Axonal Regeneration in Caenorhabditis elegans and Require DLK-1 Kinase. J Neurosci. 10.1523/jneurosci.5464-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes KA, Harder JM, John SW, et al. (2014) DLK-dependent signaling is important for somal but not axonal degeneration of retinal ganglion cells following axonal injury. Neurobiol Dis 69:108–116. 10.1016/j.nbd.2014.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welsbie DS, Yang Z, Ge Y, et al. (2013) Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc Natl Acad Sci 110:4045–4050. 10.1073/pnas.1211284110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh AS, Wang B, Pozniak CD, et al. (2011) DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. J Cell Biol 194:751–764. 10.1083/jcb.201103153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pozniak CD, Sengupta Ghosh A, Gogineni A, et al. (2013) Dual leucine zipper kinase is required for excitotoxicity-induced neuronal degeneration. J Exp Med 210:2553–2567. 10.1084/jem.20122832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osterloh JM, Yang J, Rooney TM, et al. (2012) dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science 337:481–4. 10.1126/science.1223899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerdts J, Summers DW, Sasaki Y, et al. (2013) Sarm1 -mediated axon degeneration requires both SAM and TIR interactions. J Neurosci 33:13569–80. 10.1523/JNEUROSCI.1197-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Essuman K, Summers DW, Sasaki Y, et al. (2017) The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD+ Cleavage Activity that Promotes Pathological Axonal Degeneration. Neuron 93:1334–1343.e5. 10.1016/j.neuron.2017.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henninger N, Bouley J, Sikoglu EM, et al. (2016) Attenuated traumatic axonal injury and improved functional outcome after traumatic brain injury in mice lacking Sarml. Brain 139:1094–1105. 10.1093/brain/aww001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Summers DW, DiAntonio A, Milbrandt J (2014) Mitochondrial dysfunction induces sarm1 -dependent cell death in sensory neurons. J Neurosci 34:9338–50. 10.1523/JNEUROSCI.0877-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziogas NK, Koliatsos VE (2018) Primary traumatic axonopathy in mice subjected to impact acceleration: a reappraisal of pathology and mechanisms with high-resolution anatomical methods. J Neurosci 2343–17. 10.1523/JNEUROSCI.2343-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geisler S, Doan RA, Strickland A, et al. (2016) Prevention of vincristine-induced peripheral neuropathy by genetic deletion of SARM1 in mice. Brain 139:3092–3108. 10.1093/brain/aww251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turkiew E, Falconer D, Reed N, Höke A (2017) Deletion of Sarm1 gene is neuroprotective in two models of peripheral neuropathy. J Peripher Nerv Syst 22:162–171. 10.1111/jns.12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y, Zhou P, Qian L, et al. (2007) MyD88–5 links mitochondria, microtubules, and JNK3 in neurons and regulates neuronal survival. J Exp Med 204:2063–2074. 10.1084/jem.20070868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godzik K, Coleman MP (2015) The Axon-Protective WLDS Protein Partially Rescues Mitochondrial Respiration and Glycolysis After Axonal Injury. J Mol Neurosci 55:865–871. 10.1007/s12031-014-0440-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilley J, Orsomando G, Nascimento-Ferreira I, Coleman MP (2015) Absence of SARM1 rescues development and survival of NMNAT2-Deficient axons. Cell Rep 10:1975–1982. 10.1016/j-celrep.2015.02.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki Y, Nakagawa T, Mao X, et al. (2016) NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD(+) depletion. Elife 5:. 10.7554/eLife.19749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huppke P, Wegener E, Gilley J, et al. (2019) Homozygous NMNAT2 mutation in sisters with polyneuropathy and erythromelalgia. Exp Neurol 320:112958 10.1016/j.expneurol.2019.112958 [DOI] [PubMed] [Google Scholar]
- 31.Lukacs M, Gilley J, Zhu Y, et al. (2019) Severe biallelic loss-of-function mutations in nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2) in two fetuses with fetal akinesia deformation sequence. Exp Neurol 320:112961 10.1016/j.expneurol.2019.112961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klim JR, Williams LA, Limone F, et al. (2019) ALS-implicated protein TDP-43 sustains levels of STMN2, a mediator of motor neuron growth and repair. Nat Neurosci epub: 10.1038/s41593-018-0300-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melamed Z, López-Erauskin J, Baughn MW, et al. (2019) Premature polyadenylation-mediated loss of stathmin-2 is a hallmark of TDP-43-dependent neurodegeneration. Nat Neurosci. 10.1038/s41593-018-0293-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin JE, Miller BR, Babetto E, et al. (2012) SCG10 is a JNK target in the axonal degeneration pathway. Proc Natl Acad Sci U S A 109:E3696–705. 10.1073/pnas.1216204109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker LJ, Summers DW, Sasaki Y, et al. (2017) MAPK signaling promotes axonal degeneration by speeding the turnover of the axonal maintenance factor NMNAT2. Elife 6:. 10.7554/eLife.22540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Summers DW, Milbrandt J, DiAntonio A (2018) Palmitoylation enables MAPK-dependent proteostasis of axon survival factors. Proc Natl Acad Sci 115:E8746–E8754. 10.1073/pnas.1806933115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller BR, Press C, Daniels RW, et al. (2009) A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci 12:387–389. 10.1038/nn.2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J, Wu Z, Renier N, et al. (2015) Pathological axonal death through a Mapk cascade that triggers a local energy deficit. Cell 160:161–176. 10.1016/j.cell.2014.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welsbie DS, Mitchell KL, Jaskula-Ranga V, et al. (2017) Enhanced Functional Genomic Screening Identifies Novel Mediators of Dual Leucine Zipper Kinase-Dependent Injury Signaling in Neurons. Neuron 94:1142–1154. 10.1016/j.neuron.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerdts J, Brace EJ, Sasaki Y, et al. (2015) SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science 348:453–7. 10.1126/science.1258366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milde S, Gilley J, Coleman MP (2013) Subcellular Localization Determines the Stability and Axon Protective Capacity of Axon Survival Factor Nmnat2. PLoS Biol 11:. 10.1371/journal.pbio.1001539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Misgeld T, Schwarz TL (2017) Mitostasis in Neurons: Maintaining Mitochondria in an Extended Cellular Architecture. Neuron 96:651–666. 10.1016/j.neuron.2017.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betarbet R, Sherer TB, MacKenzie G, et al. (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3:1301–1306. 10.1038/81834 [DOI] [PubMed] [Google Scholar]
- 44.Green DR, Galluzzi L, Kroemer G (2014) Metabolic control of cell death. Science (80-) 345:1250256–1250256. 10.1126/science.1250256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ali YO, Allen HM, Yu L, et al. (2016) NMNAT2:HSP90 Complex Mediates Proteostasis in Proteinopathies. PLOS Biol 14:e1002472 10.1371/journal.pbio.1002472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ljungberg MC, Ali YO, Zhu J, et al. (2012) CREB-activity and NMNAT2 transcription are down-regulated prior to neurodegeneration, while NMNAT2 over-expression is neuroprotective, in a mouse model of human tauopathy. Hum Mol Genet 21:251–267. 10.1093/hmg/ddr492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiong X, Collins CA (2012) A Conditioning Lesion Protects Axons from Degeneration via the Wallenda/DLK MAP Kinase Signaling Cascade. J Neurosci 32:610–615. 10.1523/JNEUROSCI.3586-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valakh V, Frey E, Babetto E, et al. (2015) Cytoskeletal disruption activates the DLK/JNK pathway, which promotes axonal regeneration and mimics a preconditioning injury. Neurobiol Dis 77:13–25. 10.1016/j.nbd.2015.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Zhang YV, Adib EA, et al. (2017) Restraint of presynaptic protein levels by Wnd/DLK signaling mediates synaptic defects associated with the kinesin-3 motor Unc-104. Elife 6:. 10.7554/eLife.24271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang YWA, Zhou B, Wemig M, Südhof TC (2017) ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Aß Secretion. Cell 168:427–441.e21. 10.1016/j.cell.2016.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ali YO, Bradley G, Lu HC (2017) Screening with an NMNAT2-MSD platform identifies small molecules that modulate NMNAT2 levels in cortical neurons. Sci Rep 7:. 10.1038/srep43846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Platt RJ, Chen S, Zhou Y, et al. (2014) CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 159:440–455. 10.1016/j.cell.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerdts J, Sasaki Y, Vohra B, et al. (2011) Image-based Screening Identifies Novel Roles for l{kappa}B Kinase and Glycogen Synthase Kinase 3 in Axonal Degeneration. J Biol Chem 286:28011–28018. 10.1074/jbc.M111.250472 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.