Significance
Triplet repeat expansion causes multiple neurological disorders, but the mechanisms of triplet repeat expansion are not well understood. Growing evidence indicates that DNA loops, MutSβ (MSH2–MSH3 heterodimer), and MutLγ (MLH1–MLH3 heterodimer) play important roles in triplet repeat expansion. We demonstrate here that human MutLγ is an endonuclease that nicks DNA in a MutSβ- and loop-dependent manner. Incision of loop-containing DNA by MutLγ endonuclease initiates events that lead to DNA expansion. Surprisingly, cleavage of loop-containing DNAs by MutSβ-dependent endonuclease activity of MutLγ is strongly biased to the strand that lacks the loop. These findings document an endonuclease activity and mechanism that may be important for triplet repeat expansion.
Keywords: DNA repair, genome instability, MLH3, endonuclease, triplet repeat expansion diseases
Abstract
MutL proteins are ubiquitous and play important roles in DNA metabolism. MutLγ (MLH1–MLH3 heterodimer) is a poorly understood member of the eukaryotic family of MutL proteins that has been implicated in triplet repeat expansion, but its action in this deleterious process has remained unknown. In humans, triplet repeat expansion is the molecular basis for ∼40 neurological disorders. In addition to MutLγ, triplet repeat expansion involves the mismatch recognition factor MutSβ (MSH2–MSH3 heterodimer). We show here that human MutLγ is an endonuclease that nicks DNA. Strikingly, incision of covalently closed, relaxed loop-containing DNA by human MutLγ is promoted by MutSβ and targeted to the strand opposite the loop. The resulting strand break licenses downstream events that lead to a DNA expansion event in human cell extracts. Our data imply that the mammalian MutLγ is a unique endonuclease that can initiate triplet repeat DNA expansions.
MutL proteins were first discovered in bacteria where they are involved in postreplicative DNA mismatch repair (MMR) and the control of genetic recombination (1–4). We now know that MutL proteins are ubiquitous and participate in other pathways of DNA metabolism (5–7). In eukaryotes, MutL proteins function as heterodimers, and mammalian cells contain three: MutLα (MLH1–PMS2 heterodimer), MutLβ (MLH1–PMS1), and MutLγ (MLH1–MLH3) (8–10). MutLα is an endonuclease that is required for MMR (11–13). The endonuclease activity of MutLα introduces 5′ and 3′ nicks into the daughter strand in a manner that depends on MutSα (MSH2–MSH6 heterodimer) or MutSβ (MSH2–MSH3), PCNA, RFC, ATP, a mismatch, and a DNA strand break. A 5′ nick produced in this manner activates downstream steps that lead to the mismatch removal (11, 12, 14, 15). Compared to MutLα, MutLγ and MutLβ are poorly understood. Recent progress in the field has implicated mammalian MutLα (16–18) and MutLγ (19–23) proteins in triplet repeat DNA expansion. Furthermore, MutLγ has been known to have an essential function in mammalian meiotic recombination (10, 24). Mouse Mlh3−/− spermatocytes display chromosome missegregation and undergo apoptosis, and fertilized Mlh3−/− oocytes fail to complete meiosis (24). In addition, MutLγ has a minor role in MMR (5, 15, 25, 26).
Expansion of simple DNA repeats is involved in the initiation of ∼40 human inherited disorders (27–30). These disorders have diverse clinical presentation and molecular characteristics, and some of them cause neuronal loss and ataxia (28). Effective treatments that prevent or block repeat expansion have not been developed (31). Most repeat expansion diseases are associated with expansion of triplet repeats, and the others are linked to expansion of tetranucleotide, pentanucleotide, or dodecanucleotide repeats (27, 28). Many of these diseases share a common feature, anticipation, which is defined as the tendency to have an earlier age of onset and increasing severity in successive generations (16, 28, 31). Normal alleles of the disease-associated genes carry a relatively small number of the repeat units and are relatively stable (32). In contrast, disease-associated alleles have a larger number of the repeat units and are prone to further expansion (32, 33). It is also known that the longer disease-associated alleles cause more severe symptoms (33, 34). Repeat expansions occur in both germ line and somatic tissues (33, 35–37). Whereas germ line expansions are responsible for the phenomenon of anticipation, somatic expansions contribute to the tissue specificity and the progressive nature of the repeat expansion diseases.
Our understanding of the mechanisms responsible for repeat expansion is at an early stage. DNA expansions are believed to occur in both replication-dependent and independent contexts (27, 38). The work described here will focus on the latter type of event. A recent study provided strong evidence that triplet repeat expansion in patients with myotonic dystrophy type 1 is the cumulative result of many expansion and contraction events (39). DNA loops are likely to be the key premutagenic intermediates in triplet repeat expansion and have been observed in triplet repeat-containing DNA in vitro and in vivo (19, 30, 38, 40, 41). Because triplet repeat expansion has been described in postmitotic neurons (42, 43), it presumably can occur in the absence of replication. Genome-wide association studies with human patients have suggested that MMR system genes MSH3, MLH1, MLH3, and PMS2 function as genetic modifiers of the age of onset of symptoms of Huntington’s disease and spinocerebellar ataxias (18, 22, 23, 44–46). Moreover, genetic analyses in mouse models and cultured human cells have demonstrated that the MMR system factors MutSβ, MutLα, and MutLγ are involved in triplet repeat expansion (16, 19, 21, 29, 37, 47–49). Further research has shown that MutSβ promotes triplet repeat expansion as a DNA loop recognition factor and MutLα as an endonuclease (17). However, the action of MutLγ in expansion remains unknown.
The MutLγ homolog MutLα contains the conserved DQHA(X)2E(X)4E motif that is part of its endonuclease active site (11, 12, 50). This motif and three other MutLα endonuclease motifs are present in the MLH3 subunits of yeast and mammalian MutLγ proteins (11, 51). Consistent with the presence of the endonuclease motifs in its MLH3 subunit, yeast MutLγ has been shown to possess an endonuclease activity that nicks DNA (52–54). However, it remains unknown how yeast MutLγ endonuclease activity contributes to the action of this protein in DNA metabolism. Furthermore, it has not been known whether mammalian MutLγ proteins have endonuclease activity. Here we show that human MutLγ has a unique MutSβ-dependent endonuclease activity that incises loop-containing DNAs in the strand that does not have the loop. The resulting nick is used by downstream activities to promote a DNA expansion event.
Results
Human MutLγ Is an Endonuclease.
We began this study to advance our understanding of the action of MutLγ in mammalian cells. Because the DQHA(X)2E(X)4E endonuclease motif is preserved in human and several other mammalian MLH3 proteins (11, 51) (SI Appendix, Fig. S1A), we decided to investigate whether human MutLγ had an endonuclease activity. We first purified human MutLγ and its mutant derivative, MutLγ-D1223N, which were produced in insect Sf9 cells (SI Appendix, Fig. S1 B and C). The MutLγ-D1223N variant contains a D-to-N change in the DQHA(X)2E(X)4E endonuclease motif (SI Appendix, Fig. S1A). The corresponding substitution inactivates the endonuclease function of human MutLα (11). To facilitate purification, a FLAG tag was placed at the N termini of the MLH3 and MLH3-D1223N subunits. The purity of the proteins obtained at the final purification step was ≥95%. During purification the MutLγ-D1223N variant behaved like wild-type protein, which suggested that the D1223N amino acid substitution did not cause a significant change in protein structure.
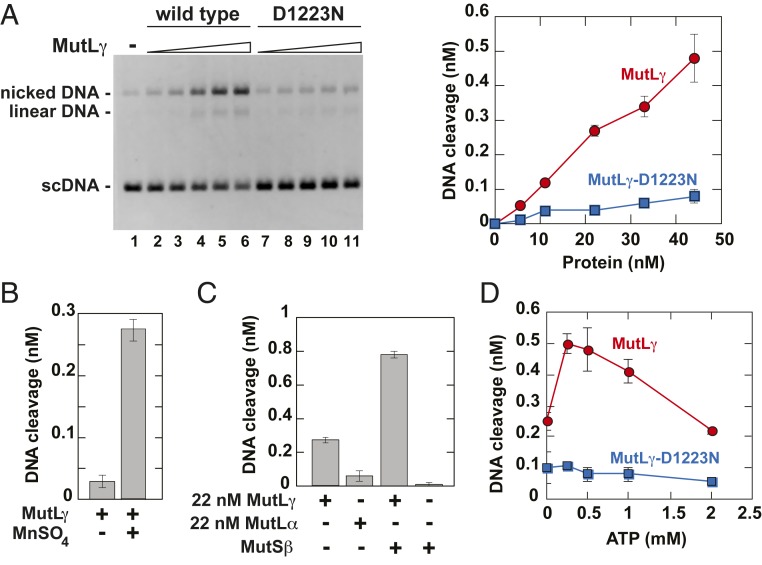
We next examined whether the purified human MutLγ possessed an endonuclease activity. Because we previously observed that human and yeast MutLα endonucleases can be gratuitously activated on supercoiled DNA under low-salt conditions in the presence of Mn2+ (11, 12), we tested whether human MutLγ displayed a similar endonuclease activity. The data demonstrated that the purified human MutLγ had a Mn2+-dependent endonuclease activity that nicked supercoiled homoduplex DNA (Fig. 1 A and B). Control experiments revealed that MutLγ-D1223N is defective in supporting this endonuclease reaction (Fig. 1A). We then compared the levels of the Mn2+-dependent endonuclease activities of human MutLγ and MutLα. The results showed that under the tested conditions, the specific Mn2+-dependent endonuclease activity of human MutLγ was ∼3 times higher than that of human MutLα (Fig. 1C and SI Appendix, Fig. S2). Together these findings demonstrated that human MutLγ is a metal-dependent endonuclease.
Fig. 1.
Human MutLγ is an ATP-stimulated endonuclease that nicks double-stranded DNA. (A) Endonuclease activity of human MutLγ on supercoiled homoduplex DNA. The Mn2+-dependent endonuclease reactions that occurred in the presence of the indicated concentrations of MutLγ and MutLγ-D1223N were carried out and analyzed as described in Materials and Methods. Data presented in the graph were obtained by quantification of images like the one shown in Left. During quantification, DNA cleavage values were corrected for nicked/relaxed species present in the preparation of the substrate DNA (lane 1). (B) Human MutLγ is a divalent metal-dependent endonuclease. Reactions were performed in the presence of 22 nM MutLγ as described in A. (C) Comparison of Mn2+-dependent endonuclease activities of human MutLγ and MutLα and the effect of MutSβ on the Mn2+-dependent endonuclease activity of human MutLγ. Reactions were carried out in the presence of indicated proteins as described in A. (D) Mn2+-dependent endonuclease activities of MutLγ and MutLγ-D1223N as functions of ATP concentration. Reactions were carried out in the presence of 44 nM MutLγ or 44 nM MutLγ-D1223N as described in A except that ATP concentration varied as indicated. The data shown in A–D are averages ±1 SD (n ≥ 2).
The MLH1 and MLH3 subunits of human MutLγ contain conserved motifs that are required for ATP binding and hydrolysis by the members of the GHKL family (55, 56). We analyzed whether the preparations of human MutLγ and MutLγ-D1223N were able to hydrolyze ATP. The data showed that the human MutLγ and MutLγ-D1223N preparations hydrolyzed ATP at similar rates, ∼0.27 mol of ATP hydrolyzed per min per mol of the MutL protein at an initial ATP concentration of 0.5 mM (SI Appendix, Fig. S3). This finding suggested that human MutLγ and MutLγ-D1223N had ATPase activities.
Next, we examined the impact of ATP on the endonuclease activity of human MutLγ. We established that the endonuclease activity of human MutLγ was stimulated by 0.25 to 1 mM ATP (Fig. 1D). This implied that ATP is a cofactor for human MutLγ endonuclease. However, higher ATP concentrations were not stimulatory, likely due to chelation of Mn2+ by ATP, rendering the cation unavailable for activation of the MutLγ endonuclease. We also established that dATP stimulated the Mn2+-dependent endonuclease activity of human MutLγ, but CTP, UTP, and GTP did not (SI Appendix, Fig. S4). This suggests that the enzyme can utilize dATP as a cofactor.
In addition to Mn2+, Mg2+ and Co2+ activate yeast MutLγ endonuclease to nick supercoiled DNA (53). We tested whether human MutLγ endonuclease activity was promoted by Mg2+, Co2+, Ca2+, Ni2+, or Zn2+. The results showed that human MutLγ endonuclease was activated by Mg2+ but not by Co2+, Ca2+, Ni2+, or Zn2+ (SI Appendix, Fig. S5). The observation that Co2+, a cation that activates yeast MutLγ endonuclease (53), did not promote human MutLγ endonuclease activity suggests that there may be a significant difference between the active sites of human and yeast MutLγ endonucleases.
MutLγ and the mismatch recognition factor MutSβ have been linked to triplet repeat expansion (19–21, 30, 37, 49). We studied whether human MutLγ and MutSβ interacted with each other. We established that the Mn2+-dependent endonuclease activity of human MutLγ on supercoiled homoduplex DNA was strongly promoted by human MutSβ (Fig. 1C). These findings demonstrated that human MutSβ directly interacted with human MutLγ endonuclease.
Human MutLγ Has a MutSβ-Dependent Endonuclease Activity That Cleaves a Loop-Containing DNA in the Strand That Lacks the Loop.
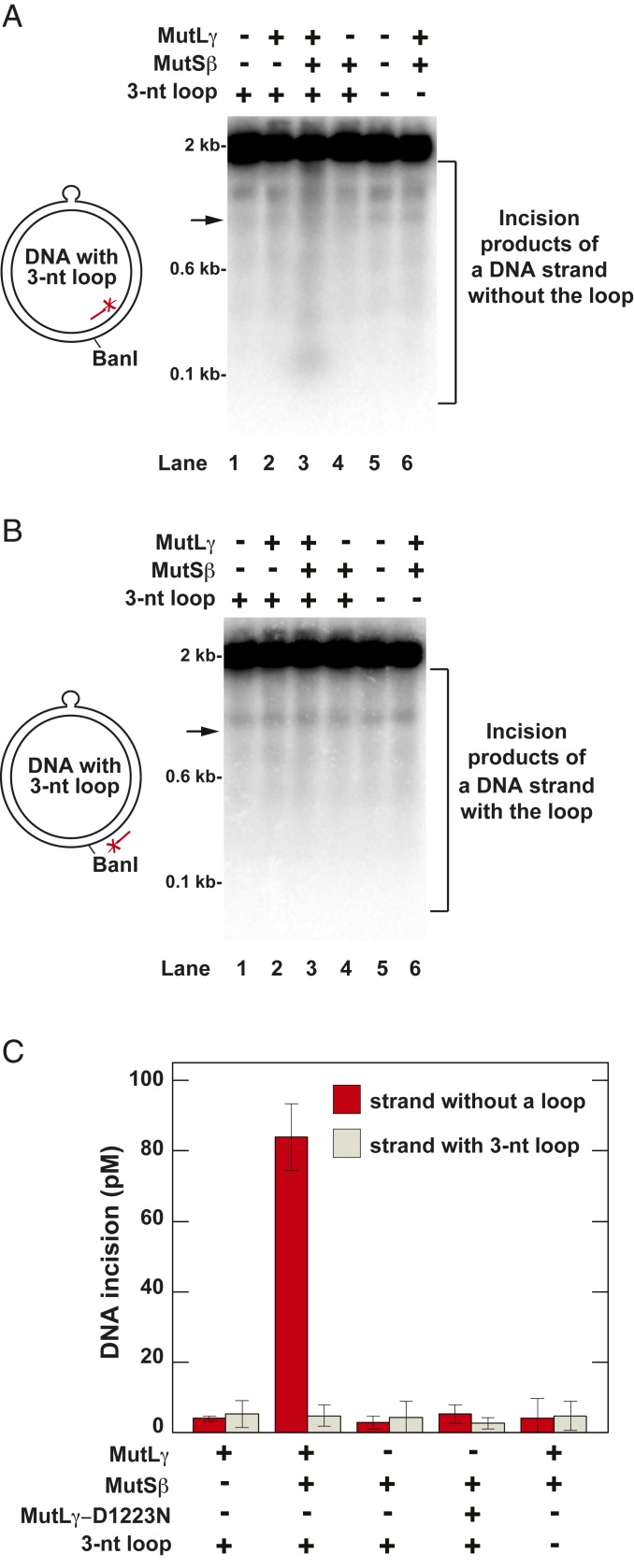
We next investigated whether in the presence of ATP and Mg2+ human MutLγ and MutSβ formed a two-protein system that incised a relaxed covalently closed DNA (ccDNA) containing a 3-nt (5′-AGC) loop in the top strand (Fig. 2). These experiments were done at physiological salt concentration to suppress nonspecific incision, and products were visualized by Southern hybridization after BanI cleavage and separation on a denaturing agarose gel. The data showed that the two-protein system incised the loop-lacking strand, but not the loop-containing strand, of the heteroduplex ccDNA (Fig. 2 A and B, lane 3). Inspection of the cleavage pattern of the heteroduplex ccDNA (Fig. 2A, lane 3) indicated that MutLγ endonuclease cleaved the loop-lacking strand at multiple sites. The omission of MutLγ or MutSβ or replacement of MutLγ with MutLγ-D1223N abolished the incision of the loop-lacking strand of the heteroduplex ccDNA (Fig. 2A, lanes 2 and 4, and Fig. 2C). Importantly, at the physiological salt concentration used, the two-protein system did not cause significant cleavage of either strand of the control homoduplex ccDNA (Fig. 2 A and B, lane 6, and Fig. 2C). These experiments revealed that human MutLγ has a MutSβ-dependent endonuclease activity that incises a 3-nt loop-containing ccDNA at multiple sites that are located on the loop-lacking strand.
Fig. 2.
Human MutLγ has a MutSβ-dependent endonuclease activity that incises a 3-nt loop-containing DNA in the strand that does not carry the loop. The defined reaction mixtures contained Mg2+ and the indicated proteins and DNAs. Reactions were carried out and analyzed as detailed in Materials and Methods. The products of the indicated defined reactions were cleaved with BanI, separated in 1.4% denaturing agarose gels, and visualized by Southern hybridizations with 32P-labeled oligonucleotides 5′-GACAGTTACCAATGCTTAATCAGTG-3′(A) and 5′-CACTGATTAAGCATTGGTAACTGTC-3′ (B). (A and B) Incision of the loop-lacking (A) and 3-nt loop-containing (B) strands of the heteroduplex DNA in the indicated reactions. The arrows indicate locations of DNA products that were formed by cleavage of the ccDNA at or near the loop site. The diagrams on the left illustrate the 3-nt loop-containing ccDNA substrate and show the relative positions of the 3-nt loop, the BanI site, and the 32P-labeled probes. (C) Quantification of the incision of the loop-lacking and 3-nt loop-containing strands of the heteroduplex DNA in the indicated reactions. The incision values were determined from phosphorimager data and are presented as averages ±1 SD (n ≥ 3).
Human MutLγ Endonuclease Promotes DNA Expansions.
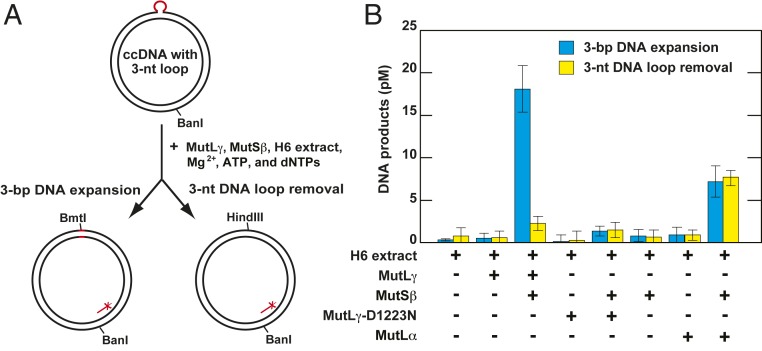
Small loops are formed in triplet repeat DNA in vivo and in vitro and are likely to be the structures that initiate triplet repeat expansion (17, 30, 41). We determined whether human MutLγ endonuclease and MutSβ promoted DNA expansion in the 3-nt loop-containing ccDNA in a reconstituted cell extract system that included ATP, the four dNTPs, and Mg2+ (Fig. 3). In these experiments, we utilized a cell-free extract that was prepared from human MLH1−/− MSH3−/− H6 cells (8, 11, 57). If the loop-containing bottom strand of this relaxed ccDNA is incised and then subject to repair DNA synthesis using the top strand as a template, a 3-bp expansion takes place (Fig. 3A). However, if the top strand is incised and then repaired using the bottom strand as a template, the 3-nt loop is removed. The two events, the 3-bp DNA expansion and 3-nt loop removal, can be differentiated from each other by diagnostic cleavages of the reaction products with the restriction endonucleases BmtI and HindIII (Fig. 3A). Supplementation of the MLH1−/− MSH3−/− H6 cell extract-containing reaction mixture with purified human MutLγ and MutSβ triggered repair to a 3-bp DNA expansion (Fig. 3B and SI Appendix, Fig. S6). Although the fraction of DNA subject to expansion in these experiments was small (∼3%), it is similar to the yield of expanded DNA in a MutLα endonuclease-dependent reaction in a human nuclear extract system (17), as well as the extent of cyclobutane thymine dimer repair that occurs in a CHO cell extract system (58).
Fig. 3.
Human MutLγ endonuclease promotes DNA expansion in a MutSβ-dependent process. (A) Outline of the experiments. (B) The 3-bp DNA expansion and 3-nt loop removal in human MLH1−/− MSH3−/− H6 cytosolic cell extracts supplemented with MutLγ, MutSβ, and Mg2+. Reactions were conducted and analyzed by Southern blot as detailed in Materials and Methods. The relative position of the 32P-labeled probe is shown in A. The data that are shown in B are averages ±1 SD (n = 3). Raw data for this type of experiment are shown in SI Appendix, Fig. S6.
Importantly, we determined that the addition of the endonuclease mutant MutLγ-D1223N and MutSβ to the H6 extract-containing mixture did not affect the level of the DNA expansions. Strikingly, the addition of MutLγ and MutSβ to the H6 extract-containing reaction mixture caused no significant increase in the level of 3-nt loop removal. In contrast, the addition of MutLα and MutSβ to the H6 extract-containing reaction mixture increased both expansion and loop removal to similar degrees (Fig. 3B). A similar result was obtained in a recent study of human MutLα and MutSβ (17). The above findings demonstrated that MutLγ endonuclease and MutSβ act in the same pathway that specifically promotes DNA expansions.
Cleavage of (CTG)3/(CAG)1 and (CTG)1/(CAG)3 Heteroduplex DNAs by Activated Human MutLγ Endonuclease.
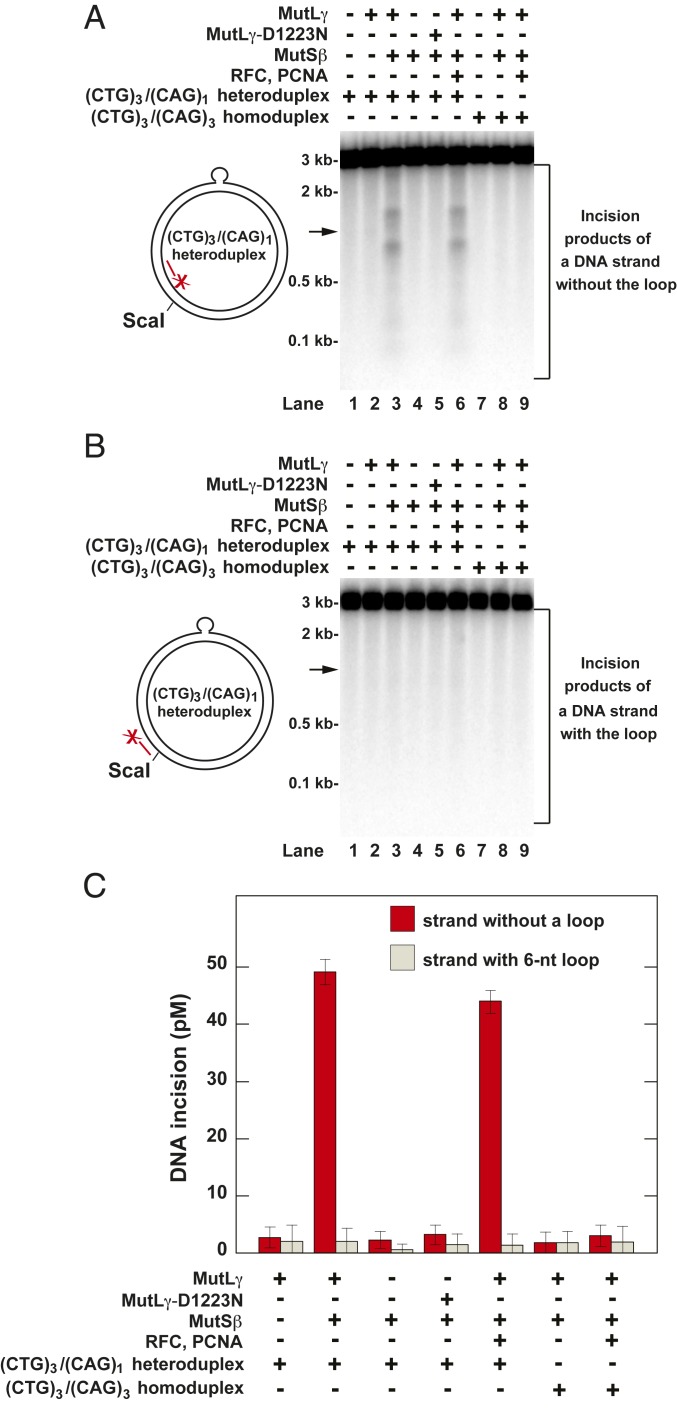
Expansion of CTG/CAG repeats in the human DMPK gene is an essential step in the process that causes myotonic dystrophy (59–61). In the next series of experiments, we studied whether the two-protein system cleaved a relaxed (CTG)3/(CAG)1 heteroduplex ccDNA in which a 6-nt loop was within the sequence context of the human DMPK gene (Fig. 4) (17). (Due to the surrounding sequence the 6-nt loop sequence in a (CTG)3/(CAG)1 heteroduplex molecule may be CTGCTG, GCTGCT, or TGCTGC.) The data demonstrated that in the presence of Mg2+ the two-protein system cleaved the loop-lacking strand of the (CTG)3/(CAG)1 heteroduplex ccDNA in a reaction that required the presence of both proteins and MutLγ endonuclease function (Fig. 4A, lanes 2 to 5, and Fig. 4C).
Fig. 4.
Human MutLγ incises the loop-lacking strand of a (CTG)3/(CAG)1 heteroduplex ccDNA in a reaction that displays a significant site specificity. Defined reactions that occurred in the presence of Mg2+ were carried out and analyzed as described under Materials and Methods. The products of the indicated reactions were cleaved with ScaI, separated in 1.2% denaturing agarose gels, and visualized by Southern hybridizations with 32P-labeled oligonucleotides 5′-GTGTATGCGGCGACCGAGTTGCTCTTG-3′(A) and 5′-CAAGAGCAATCGGTCGCCGCATACAC-3′ (B). (A) Incision of the loop-lacking strand of the (CTG)3/(CAG)1 heteroduplex ccDNA by the activated MutLγ endonuclease. (B) Lack of incision of the loop-containing strand of the (CTG)3/(CAG)1 heteroduplex ccDNA by the activated MutLγ endonuclease. The arrows mark locations of DNA products that were formed by cleavage of the ccDNA at the loop site. The diagrams on the left depict the (CTG)3/(CAG)1 heteroduplex ccDNA and show the relative positions of the 6-nt loop, the ScaI site, and the 32P-labeled probes. (C) Quantification of incision of the two strands of (CTG)3/(CAG)1 heteroduplex ccDNA by the activated MutLγ endonuclease. The incision values were determined from phosphorimager data and are presented as averages ±1 SD (n = 4).
Strikingly, the activated MutLγ preferred to incise the loop-lacking strand of the heteroduplex DNA at two regions located to either side of the lesion, each of which was ∼300 bp away from the loop (Fig. 4A, lane 3). We also found that the presence of PCNA and RFC did not increase the level of incision of the loop-lacking strand of the heteroduplex DNA. Consistent with the results shown in Fig. 2, we established that neither the control (CTG)3/(CAG)3 homoduplex DNA nor the loop-containing strand of the (CTG)3/(CAG)1 heteroduplex DNA was incised by the MutSβ-dependent endonuclease activity of MutLγ (Fig. 4 A and B, lanes 8 and 9; Fig. 4B, lanes 3 and 6; and Fig. 4C). The experiments summarized in Fig. 4 utilized a relaxed ccDNA that contained a 6-nt loop in the top strand. We also studied how human MutLγ endonuclease acted on a similar relaxed ccDNA, a (CTG)1/(CAG)3 heteroduplex, that carried a 6-nt loop in the bottom strand. The results showed that the endonuclease activity of human MutLγ incised the loop-lacking strand of (CTG)1/(CAG)3 heteroduplex ccDNA in a MutSβ- (SI Appendix, Fig. S7) and ATP-dependent reaction (SI Appendix, Fig. S8). Incision of the loop-lacking strand often took place in a region that encompassed the 6-nt loop but also occurred at several more distant minor sites (SI Appendix, Fig. S7B, lane 3, and SI Appendix, Fig. S8, lane 2). Collectively, the results of the above experiments (Figs. 2 and 4 and SI Appendix, Figs. S7 and S8) demonstrated that MutLγ has a unique MutSβ-dependent endonuclease activity that incises loop-containing ccDNAs in the strand that lacks the loop.
In our previous analysis of the defined two-protein system (Figs. 2 and 4 and SI Appendix, Figs. S7 and S8), we carried out the endonuclease reactions in the presence of Mg2+. We have also found that MutSβ- and loop-dependent endonuclease activity of human MutLγ on the (CTG)1/(CAG)3 heteroduplex ccDNA is also evident in the presence of Mn2+ provided that the salt concentration is elevated to suppress nonspecific MutLγ nuclease activity. As observed in the presence of Mg2+, the MutSβ-dependent endonuclease activity of human MutLγ incised the loop-lacking strand of the loop-containing ccDNA in the presence of 0.05 to 1 mM of Mn2+, but it was silent in the presence of 0.01 to 0.03 mM of Mn2+ (SI Appendix, Fig. S9), concentrations similar to those present in the mammalian cell (62).
Discussion
Despite their significant impact on DNA metabolism (19–21, 24), mammalian MutLγ proteins and the mechanisms of their action have been poorly understood. Since the discovery of the DQHA(X)2E(X)4E endonuclease motif in PMS2 and its homologs (11, 12), a key question has been whether a mammalian MutLγ possesses an endonuclease activity. Here we have shown that human MutLγ is a metal-dependent endonuclease. Our study has discovered that human MutLγ displays a distinct MutSβ-dependent endonuclease activity that incises loop-containing DNAs in the strand that does not carry the loop.
A recent report was the first to implicate endonuclease activity of a MutL protein in triplet repeat expansion (17). That study showed that human MutLα endonuclease promotes triplet repeat expansion by incising ccDNA in a loop-dependent manner. The incision of triplet repeat extrusion-containing ccDNA by human MutLα occurs in either strand and requires the presence of MutSβ, PCNA, and RFC (17). The current work has provided evidence for the involvement of endonuclease activity of another human MutL protein in loop-directed DNA expansion (Figs. 2–4 and SI Appendix, Figs. S6 and S7). Unlike MutLα, MutLγ incises triplet repeat extrusion-containing ccDNA in a reaction that does not depend on PCNA and RFC (Fig. 4).
Human MutLγ alone incises a supercoiled DNA in the presence of Mn2+ and 70 mM KCl + NaCl (Fig. 1C). However, human MutLγ alone does not cleave the (CTG)1/(CAG)3 heteroduplex ccDNA in the presence of Mn2+ at an ionic strength that approximates physiological conditions (140 mM KCl + NaCl) (SI Appendix, Fig. S9). We attribute this difference to suppression of Mn2+-dependent endonuclease activity at the higher salt concentration (SI Appendix, Fig. S10). Both MutLα and MutLγ endonucleases promote DNA expansion in a manner that requires the integrity of the DQHA(X)2E(X)4E motif (17) (Fig. 3). Nevertheless, there is a fundamental difference between the behaviors of the two proteins: whereas the activated MutLα endonuclease incises either strand of loop-containing ccDNA (17), the activated MutLγ only incises the loop-lacking strand (Figs. 2 and 4 and SI Appendix, Fig. S7). This difference in the behaviors of the two nucleases provides a simple explanation for the fact that in the H6 extract system the activated MutLγ exclusively promotes DNA expansion, whereas the activated MutLα endonuclease promotes both DNA expansion and loop removal (17) (Fig. 3).
The activated MutLγ endonuclease cleaves the 3-nt loop-containing ccDNA at numerous sites on the loop-lacking strand (Fig. 2A, lane 3). However, the pattern of incision of the (CTG)3/(CAG)1 and (CTG)1/(CAG)3 heteroduplex ccDNAs by the activated MutLγ endonuclease is different (Fig. 4A and SI Appendix, Fig. S7B). In the case of the (CTG)3/(CAG)1 heteroduplex ccDNA, activated MutLγ endonuclease often cleaves the loop-lacking strand within two regions on either side of the loop (Fig. 4A). For the (CTG)1/(CAG)3 heteroduplex ccDNA, the activated protein frequently cuts the loop-lacking strand at the region that encompasses the loop (SI Appendix, Fig. S7B), although cleavage does occur at several other regions as well. Similar to the activated MutLγ (Fig. 4), the activated MutLα often cleaves the (CTG)3/(CAG)1 heteroduplex at several preferred regions that are near the 6-nt loop (17). These different modes of endonuclease action on the several DNAs presumably reflect differences in the structural nature and/or lifetimes of the protein–DNA complexes involved.
Like its homolog MutLα (11, 12), human MutLγ has Mg2+- and Mn2+-dependent endonuclease activities that nick DNA (Figs. 1, 2, and 4 and SI Appendix, Figs. S5 and S7–S9). The Mn2+-dependent endonuclease activities of human MutLα and MutLγ are promoted by ATP (11, 12) (Fig. 1D). This supports the view that the two human MutL proteins act as ATP-dependent endonucleases. In contrast, the endonuclease activity of yeast MutLγ is not influenced by ATP (52, 53). The comparison of the specific Mn2+-dependent endonuclease activities of human MutLγ and MutLα revealed that they are in the same range (Fig. 1C), which indicates that the mechanisms of DNA nicking in the active sites of human MutLα and MutLγ endonucleases may be similar. An important question is, does the Mn2+-dependent endonuclease activity of MutLγ contribute to DNA expansion? Given that the intracellular concentration of Mn2+ is likely to be low (∼30 µM with only 0.7 µM free) (62), the Mn2+-dependent endonuclease activity of human MutLγ probably does not play a significant role in DNA expansion when the intracellular Mn2+ concentration is in the normal range (SI Appendix, Fig. S9).
MutSβ recognizes 1- to 12-nt loops (63, 64) and plays an important role in triplet repeat expansion (5, 30). Consistent with this, we have determined that human MutSβ activated the Mg2+-dependent endonuclease activity of human MutLγ to incise relaxed ccDNA in a loop-dependent manner (Figs. 2 and 4 and SI Appendix, Fig. S7). Furthermore, we have determined that human MutSβ promoted the Mn2+-dependent endonuclease activities of human MutLγ and MutLα (Fig. 1C and SI Appendix, Figs. S9 and S11). This is in agreement with a previous study that showed that yeast MutSβ stimulated the Mn2+-dependent endonuclease activity of yeast MutLγ on supercoiled homoduplex DNA (53). Thus, it is likely that the ability of MutSβ to interact with MutLγ and MutLα endonucleases has been conserved throughout evolution of eukaryotes. Because both MutLγ and MutLα contain MLH1 as a subunit, it is tempting to speculate that these endonucleases interact with MutSβ via MLH1. A prior study showed that MutSβ physically and functionally interacts with MutLα via a sequence element that overlaps its PCNA-binding motif, which is located near the N terminus of its MSH3 subunit (65). It would be important to study whether the PCNA-binding motif of MutSβ is also required for the interaction of this mismatch recognition factor with MutLγ endonuclease. Although MutSβ activates the Mn2+-dependent endonuclease of both yeast and human MutLγ on a supercoiled homoduplex, the basis of this activation is unclear, but it may involve recognition of non-B DNA structures, the formation of which is driven by superhelical free energy.
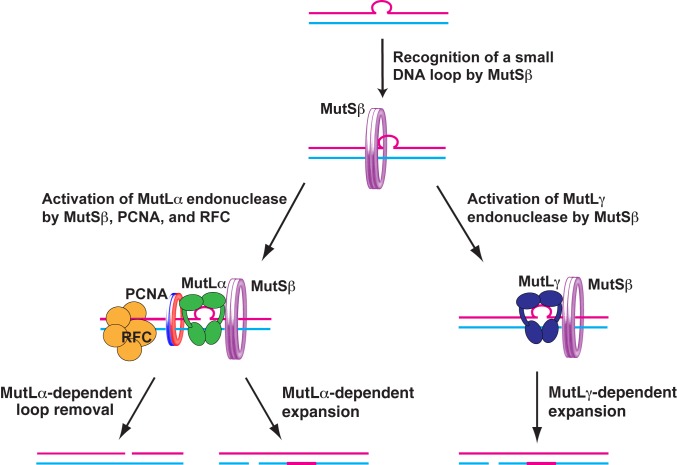
Previous research (17, 33) and our findings suggest a model for MMR system-dependent triplet repeat expansion events that may occur in nonreplicating DNA (Fig. 5). In this model, expansion is a net result of multiple events of two different classes. One class of event depends on MutSβ, MutLα, PCNA, and RFC, and the other class of event requires MutSβ and MutLγ. RFC contributes to the MutLα-dependent event by loading PCNA onto the loop-containing DNA (17). Both the MutLα-dependent and MutLγ-dependent events are initiated by recognition of a small loop in the triplet repeat DNA by MutSβ. After recognizing a small loop, MutSβ cooperates with loaded PCNA to activate MutLα or it acts alone to activate MutLγ. The activated MutLα or MutLγ incises the loop-containing DNA. The incision of the loop-containing DNA by the activated MutLα endonuclease occurs in the loop-containing or loop-lacking strand, whereas the cleavage of the loop-containing DNA by the activated MutLγ endonuclease only takes place in the loop-lacking strand. Pol β (66, 67) or another DNA polymerase utilizes the generated strand break to perform a DNA synthesis reaction that leads to triplet repeat expansion or contraction in the event that involves MutLα endonuclease and to triplet repeat expansion in the event that entails MutLγ endonuclease. After the DNA synthesis step, the nick is sealed by DNA ligase I or III (68). If this model is correct, it would be important to determine the relative contributions of the MutLα- and MutLγ-dependent events to triplet repeat expansion.
Fig. 5.
A model for MMR system-dependent triplet repeat expansion in nonreplicating DNA. This model suggests that two MMR system-dependent pathways promote triplet expansion in nonreplicating DNA. One of the pathways involves MutSβ, MutLα, PCNA, and RFC, and the other involves MutSβ and MutLγ. Please note that the MutLα-dependent pathway can also form contractions.
A consensus that has emerged from studies of triplet repeat instability indicates that triplet repeats can be expanded via MMR system-dependent and independent mechanisms (29, 33, 69, 70). Importantly, genetic studies in mice have linked the MMR system to both germ line expansion and somatic expansion/contraction events that involve a relatively small number of repeat units (16, 21, 30, 37, 71). Thus, our model for MMR system-dependent triplet repeat expansion (Fig. 5) might account for conversion of normal alleles, especially long normal alleles, into premutation alleles and for small-scale somatic expansions that take place in expanded alleles in Huntington’s disease. However, MMR-dependent events may be not necessary for production of large intergenerational expansions that have been observed in some repeat expansion diseases.
Materials and Methods
Proteins, H6 Cell-Free Extract, and DNAs.
Human MutLα, MutSβ, PCNA, and RFC were isolated in near homogeneous forms as previously described (11, 14, 65). Human MutLγ and MutLγ-D1223N proteins containing a FLAG tag at the N terminus of their MLH3 subunits were produced in insect Sf9 cells and then purified by chromatography on M2 anti-Flag beads (Sigma) and a MonoQ column (GE HealthCare). Human MLH1−/− MSH3−/− H6 cells were grown, and their cytosolic extracts were prepared as previously described (72).
The relaxed 3-nt loop-containing ccDNA and the relaxed control homoduplex ccDNA were prepared using the gapped form of the plasmid pAH1A (73) according to previously described procedures (17, 74). To prepare the 3-nt loop-containing ccDNA a phosphorylated 39-mer oligonucleotide with the sequence 5′-GCTACCGTCCTCGAAGCTAGCTCCGCATCGGAGTCGACG-3′ (the 3-nt loop sequence is underlined) was utilized. The control homoduplex ccDNA was prepared using a phosphorylated 36-mer oligonucleotide (5′-GCTACCGTCCTCGAAGCTTCCGCATCGGAGTCGACG-3′).
The relaxed (CTG)3/(CAG)1 and (CTG)1/(CAG)3 heteroduplex and (CTG)3/(CAG)3 homoduplex ccDNAs that carry a part of 3′ untranslated region of the human DMPK gene were prepared as previously described (17). The DNA sequence of the human DMPK gene 3′ untranslated region in the homoduplex DNA was 5′-CGTCCTTGTAGCCGGGATGCTGCTGCTGGGGGGATCACAGACCATTT-3′, and the DNA sequences of the human DMPK gene 3′ untranslated region in the loop-containing strands of the relaxed (CTG)3/(CAG)1 and (CTG)1/(CAG)3 heteroduplex ccDNAs were 5′-CGTCCTTGTAGCCGGGATGCTGCTGCTGGGGGGATCACAGACCATTT-3′ and 5′-AAATGGTCTGTGATCCCCCCAGCAGCAGCATCCCGGCTACAAGGACG -3′, respectively.
Endonuclease, ATPase, and DNA Expansion Assays.
Endonuclease, ATPase, and DNA expansion assays were based on previously developed methods (11, 12). Details of these assays are available in SI Appendix.
Data Availability.
Raw data associated with this paper have been deposited in Figshare (https://figshare.com/s/9ff51080eda218a64d7d).
Supplementary Material
Acknowledgments
This work was supported in part by NIH Grants GM095758 (to F.A.K.) and GM045190 (to P.L.M.). P.L.M. is an Investigator of the Howard Hughes Medical Institute. We thank Josef Jiricny for providing human MLH3 DNA and antibodies against human MLH3.
Footnotes
Competing interest statement: P.L.M. serves on the Scientific Advisory Board of Elysium Health.
Data deposition: Raw data associated with this paper have been deposited in Figshare (https://figshare.com/s/9ff51080eda218a64d7d).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1914718117/-/DCSupplemental.
References
- 1.Jones M., Wagner R., N-Methyl-N'-nitro-N-nitrosoguanidine sensitivity of E. coli mutants deficient in DNA methylation and mismatch repair. Mol. Gen. Genet. 184, 562–563 (1981). [DOI] [PubMed] [Google Scholar]
- 2.Pang P. P., Tsen S.-D., Lundberg A. S., Walker G. C., The mutH, mutL, mutS, and uvrD genes of Salmonella typhimurium LT2. Cold Spring Harb. Symp. Quant. Biol. 49, 597–602 (1984). [DOI] [PubMed] [Google Scholar]
- 3.Grilley M., Welsh K. M., Su S.-S., Modrich P., Isolation and characterization of the Escherichia coli mutL gene product. J. Biol. Chem. 264, 1000–1004 (1989). [PubMed] [Google Scholar]
- 4.Rayssiguier C., Thaler D. S., Radman M., The barrier to recombination between Escherichia coli and Salmonella typhimurium is disrupted in mismatch-repair mutants. Nature 342, 396–401 (1989). [DOI] [PubMed] [Google Scholar]
- 5.Iyer R. R., Pluciennik A., Burdett V., Modrich P. L., DNA mismatch repair: Functions and mechanisms. Chem. Rev. 106, 302–323 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Kadyrova L. Y., Kadyrov F. A., Endonuclease activities of MutLα and its homologs in DNA mismatch repair. DNA Repair (Amst.) 38, 42–49 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manhart C. M., Alani E., Roles for mismatch repair family proteins in promoting meiotic crossing over. DNA Repair (Amst.) 38, 84–93 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li G.-M., Modrich P., Restoration of mismatch repair to nuclear extracts of H6 colorectal tumor cells by a heterodimer of human MutL homologs. Proc. Natl. Acad. Sci. U.S.A. 92, 1950–1954 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Räschle M., Marra G., Nyström-Lahti M., Schär P., Jiricny J., Identification of hMutLbeta, a heterodimer of hMLH1 and hPMS1. J. Biol. Chem. 274, 32368–32375 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Wang T. F., Kleckner N., Hunter N., Functional specificity of MutL homologs in yeast: Evidence for three Mlh1-based heterocomplexes with distinct roles during meiosis in recombination and mismatch correction. Proc. Natl. Acad. Sci. U.S.A. 96, 13914–13919 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadyrov F. A., Dzantiev L., Constantin N., Modrich P., Endonucleolytic function of MutLalpha in human mismatch repair. Cell 126, 297–308 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Kadyrov F. A., et al. , Saccharomyces cerevisiae MutLalpha is a mismatch repair endonuclease. J. Biol. Chem. 282, 37181–37190 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pluciennik A., et al. , PCNA function in the activation and strand direction of MutLα endonuclease in mismatch repair. Proc. Natl. Acad. Sci. U.S.A. 107, 16066–16071 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadyrov F. A., et al. , A possible mechanism for exonuclease 1-independent eukaryotic mismatch repair. Proc. Natl. Acad. Sci. U.S.A. 106, 8495–8500 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunkel T. A., Erie D. A., Eukaryotic mismatch repair in relation to DNA replication. Annu. Rev. Genet. 49, 291–313 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes-Pereira M., Fortune M. T., Ingram L., McAbney J. P., Monckton D. G., Pms2 is a genetic enhancer of trinucleotide CAG.CTG repeat somatic mosaicism: Implications for the mechanism of triplet repeat expansion. Hum. Mol. Genet. 13, 1815–1825 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Pluciennik A., et al. , Extrahelical (CAG)/(CTG) triplet repeat elements support proliferating cell nuclear antigen loading and MutLα endonuclease activation. Proc. Natl. Acad. Sci. U.S.A. 110, 12277–12282 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bettencourt C., et al. ; SPATAX Network , DNA repair pathways underlie a common genetic mechanism modulating onset in polyglutamine diseases. Ann. Neurol. 79, 983–990 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinto R. M., et al. , Mismatch repair genes Mlh1 and Mlh3 modify CAG instability in Huntington’s disease mice: Genome-wide and candidate approaches. PLoS Genet. 9, e1003930 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halabi A., Fuselier K. T. B., Grabczyk E., GAA•TTC repeat expansion in human cells is mediated by mismatch repair complex MutLγ and depends upon the endonuclease domain in MLH3 isoform one. Nucleic Acids Res. 46, 4022–4032 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X., Zhang Y., Wilkins K., Edelmann W., Usdin K., MutLγ promotes repeat expansion in a Fragile X mouse model while EXO1 is protective. PLoS Genet. 14, e1007719 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J. M., et al. ; Genetic Modifiers of Huntington’s Disease (GeM-HD) Consortium , CAG repeat not polyglutamine length determines timing of Huntington’s disease onset. Cell 178, 887–900.e14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciosi M., et al. ; TRACK-HD team; Enroll-HD team , A genetic association study of glutamine-encoding DNA sequence structures, somatic CAG expansion, and DNA repair gene variants, with Huntington disease clinical outcomes. EBioMedicine 48, 568–580 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipkin S. M., et al. , Meiotic arrest and aneuploidy in MLH3-deficient mice. Nat. Genet. 31, 385–390 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Flores-Rozas H., Kolodner R. D., The Saccharomyces cerevisiae MLH3 gene functions in MSH3-dependent suppression of frameshift mutations. Proc. Natl. Acad. Sci. U.S.A. 95, 12404–12409 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannavo E., et al. , Expression of the MutL homologue hMLH3 in human cells and its role in DNA mismatch repair. Cancer Res. 65, 10759–10766 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Mirkin S. M., Expandable DNA repeats and human disease. Nature 447, 932–940 (2007). [DOI] [PubMed] [Google Scholar]
- 28.La Spada A. R., Taylor J. P., Repeat expansion disease: Progress and puzzles in disease pathogenesis. Nat. Rev. Genet. 11, 247–258 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Usdin K., House N. C., Freudenreich C. H., Repeat instability during DNA repair: Insights from model systems. Crit. Rev. Biochem. Mol. Biol. 50, 142–167 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt M. H. M., Pearson C. E., Disease-associated repeat instability and mismatch repair. DNA Repair (Amst.) 38, 117–126 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Di Prospero N. A., Fischbeck K. H., Therapeutics development for triplet repeat expansion diseases. Nat. Rev. Genet. 6, 756–765 (2005). [DOI] [PubMed] [Google Scholar]
- 32.López Castel A., Cleary J. D., Pearson C. E., Repeat instability as the basis for human diseases and as a potential target for therapy. Nat. Rev. Mol. Cell Biol. 11, 165–170 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Gomes-Pereira M., Monckton D. G., Chemical modifiers of unstable expanded simple sequence repeats: What goes up, could come down. Mutat. Res. 598, 15–34 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Harper P. S., Harley H. G., Reardon W., Shaw D. J., Anticipation in myotonic dystrophy: New light on an old problem. Am. J. Hum. Genet. 51, 10–16 (1992). [PMC free article] [PubMed] [Google Scholar]
- 35.Duyao M., et al. , Trinucleotide repeat length instability and age of onset in Huntington’s disease. Nat. Genet. 4, 387–392 (1993). [DOI] [PubMed] [Google Scholar]
- 36.Telenius H., et al. , Somatic and gonadal mosaicism of the Huntington disease gene CAG repeat in brain and sperm. Nat. Genet. 6, 409–414 (1994). [DOI] [PubMed] [Google Scholar]
- 37.van den Broek W. J., et al. , Somatic expansion behaviour of the (CTG)n repeat in myotonic dystrophy knock-in mice is differentially affected by Msh3 and Msh6 mismatch-repair proteins. Hum. Mol. Genet. 11, 191–198 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Iyer R. R., Pluciennik A., Napierala M., Wells R. D., DNA triplet repeat expansion and mismatch repair. Annu. Rev. Biochem. 84, 199–226 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higham C. F., Monckton D. G., Modelling and inference reveal nonlinear length-dependent suppression of somatic instability for small disease associated alleles in myotonic dystrophy type 1 and Huntington disease. J. R. Soc. Interface 10, 20130605 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wells R. D., Molecular basis of genetic instability of triplet repeats. J. Biol. Chem. 271, 2875–2878 (1996). [DOI] [PubMed] [Google Scholar]
- 41.Axford M. M., et al. , Detection of slipped-DNAs at the trinucleotide repeats of the myotonic dystrophy type I disease locus in patient tissues. PLoS Genet. 9, e1003866 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonitel R., et al. , DNA instability in postmitotic neurons. Proc. Natl. Acad. Sci. U.S.A. 105, 3467–3472 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shelbourne P. F., et al. ; US-Venezuela Collaborative Research Group , Triplet repeat mutation length gains correlate with cell-type specific vulnerability in Huntington disease brain. Hum. Mol. Genet. 16, 1133–1142 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Lee J. M., et al. ; Genetic Modifiers of Huntington’s Disease (GeM-HD) Consortium , Identification of genetic factors that modify clinical onset of Huntington’s disease. Cell 162, 516–526 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee J. M., et al. , A modifier of Huntington’s disease onset at the MLH1 locus. Hum. Mol. Genet. 26, 3859–3867 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moss D. J. H., et al. ; TRACK-HD investigators; REGISTRY investigators , Identification of genetic variants associated with Huntington’s disease progression: A genome-wide association study. Lancet Neurol. 16, 701–711 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Manley K., Shirley T. L., Flaherty L., Messer A., Msh2 deficiency prevents in vivo somatic instability of the CAG repeat in Huntington disease transgenic mice. Nat. Genet. 23, 471–473 (1999). [DOI] [PubMed] [Google Scholar]
- 48.Wheeler V. C., et al. , Mismatch repair gene Msh2 modifies the timing of early disease in Hdh(Q111) striatum. Hum. Mol. Genet. 12, 273–281 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Zhao X. N., et al. , A MutSβ-dependent contribution of MutSα to repeat expansions in Fragile X premutation mice? PLoS Genet. 12, e1006190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gueneau E., et al. , Structure of the MutLα C-terminal domain reveals how Mlh1 contributes to Pms1 endonuclease site. Nat. Struct. Mol. Biol. 20, 461–468 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Kosinski J., Plotz G., Guarné A., Bujnicki J. M., Friedhoff P., The PMS2 subunit of human MutLalpha contains a metal ion binding domain of the iron-dependent repressor protein family. J. Mol. Biol. 382, 610–627 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Ranjha L., Anand R., Cejka P., The Saccharomyces cerevisiae Mlh1-Mlh3 heterodimer is an endonuclease that preferentially binds to Holliday junctions. J. Biol. Chem. 289, 5674–5686 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogacheva M. V., et al. , Mlh1-Mlh3, a meiotic crossover and DNA mismatch repair factor, is a Msh2-Msh3-stimulated endonuclease. J. Biol. Chem. 289, 5664–5673 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manhart C. M., et al. , The mismatch repair and meiotic recombination endonuclease Mlh1-Mlh3 is activated by polymer formation and can cleave DNA substrates in trans. PLoS Biol. 15, e2001164 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ban C., Yang W., Crystal structure and ATPase activity of MutL: Implications for DNA repair and mutagenesis. Cell 95, 541–552 (1998). [DOI] [PubMed] [Google Scholar]
- 56.Räschle M., Dufner P., Marra G., Jiricny J., Mutations within the hMLH1 and hPMS2 subunits of the human MutLalpha mismatch repair factor affect its ATPase activity, but not its ability to interact with hMutSalpha. J. Biol. Chem. 277, 21810–21820 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Parsons R., et al. , Hypermutability and mismatch repair deficiency in RER+ tumor cells. Cell 75, 1227–1236 (1993). [DOI] [PubMed] [Google Scholar]
- 58.Reardon J. T., Sancar A., Recognition and repair of the cyclobutane thymine dimer, a major cause of skin cancers, by the human excision nuclease. Genes Dev. 17, 2539–2551 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brook J. D., et al. , Molecular basis of myotonic dystrophy: Expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 68, 799–808 (1992). [DOI] [PubMed] [Google Scholar]
- 60.Fu Y.-H., et al. , An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 255, 1256–1258 (1992). [DOI] [PubMed] [Google Scholar]
- 61.Mahadevan M., et al. , Myotonic dystrophy mutation: An unstable CTG repeat in the 3′ untranslated region of the gene. Science 255, 1253–1255 (1992). [DOI] [PubMed] [Google Scholar]
- 62.Ash D. E., Schramm V. L., Determination of free and bound manganese(II) in hepatocytes from fed and fasted rats. J. Biol. Chem. 257, 9261–9264 (1982). [PubMed] [Google Scholar]
- 63.Palombo F., et al. , hMutSbeta, a heterodimer of hMSH2 and hMSH3, binds to insertion/deletion loops in DNA. Curr. Biol. 6, 1181–1184 (1996). [DOI] [PubMed] [Google Scholar]
- 64.Genschel J., Littman S. J., Drummond J. T., Modrich P., Isolation of MutSbeta from human cells and comparison of the mismatch repair specificities of MutSbeta and MutSalpha. J. Biol. Chem. 273, 19895–19901 (1998). [DOI] [PubMed] [Google Scholar]
- 65.Iyer R. R., et al. , MutLalpha and proliferating cell nuclear antigen share binding sites on MutSbeta. J. Biol. Chem. 285, 11730–11739 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lokanga R. A., Senejani A. G., Sweasy J. B., Usdin K., Heterozygosity for a hypomorphic Polβ mutation reduces the expansion frequency in a mouse model of the Fragile X-related disorders. PLoS Genet. 11, e1005181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lai Y., et al. , Crosstalk between MSH2-MSH3 and polβ promotes trinucleotide repeat expansion during base excision repair. Nat. Commun. 7, 12465 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Howes T. R., Tomkinson A. E., DNA ligase I, the replicative DNA ligase. Subcell. Biochem. 62, 327–341 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neil A. J., Kim J. C., Mirkin S. M., Precarious maintenance of simple DNA repeats in eukaryotes. BioEssays 39, 1700077 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kononenko A. V., Ebersole T., Vasquez K. M., Mirkin S. M., Mechanisms of genetic instability caused by (CGG)n repeats in an experimental mammalian system. Nat. Struct. Mol. Biol. 25, 669–676 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao X. N., et al. , Mutsβ generates both expansions and contractions in a mouse model of the Fragile X-associated disorders. Hum. Mol. Genet. 24, 7087–7096 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kadyrova L. Y., Blanko E. R., Kadyrov F. A., CAF-I-dependent control of degradation of the discontinuous strands during mismatch repair. Proc. Natl. Acad. Sci. U.S.A. 108, 2753–2758 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.York S. J., Modrich P., Mismatch repair-dependent iterative excision at irreparable O6-methylguanine lesions in human nuclear extracts. J. Biol. Chem. 281, 22674–22683 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kadyrova L. Y., Dahal B. K., Kadyrov F. A., The major replicative histone chaperone CAF-1 suppresses the activity of the DNA mismatch repair system in the cytotoxic response to a DNA-methylating agent. J. Biol. Chem. 291, 27298–27312 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data associated with this paper have been deposited in Figshare (https://figshare.com/s/9ff51080eda218a64d7d).