Abstract
OBJECTIVE
This study investigates two-phase growth patterns in early life and their association with development of islet autoimmunity (IA) and type 1 diabetes (T1D).
RESEARCH DESIGN AND METHODS
The Environmental Determinants of Diabetes in the Young (TEDDY) study followed 7,522 genetically high-risk children in Sweden, Finland, Germany, and the U.S. from birth for a median of 9.0 years (interquartile range 5.7–10.6) with available growth data. Of these, 761 (10.1%) children developed IA and 290 (3.9%) children were diagnosed with T1D. Bayesian two-phase piecewise linear mixed models with a random change point were used to estimate children’s individual growth trajectories. Cox proportional hazards models were used to assess the effects of associated growth parameters on the risks of IA and progression to T1D.
RESULTS
A higher rate of weight gain in infancy was associated with increased IA risk (hazard ratio [HR] 1.09 [95% CI 1.02, 1.17] per 1 kg/year). A height growth pattern with a lower rate in infancy (HR 0.79 [95% CI 0.70, 0.90] per 1 cm/year), higher rate in early childhood (HR 1.48 [95% CI 1.22, 1.79] per 1 cm/year), and younger age at the phase transition (HR 0.76 [95% CI 0.58, 0.99] per 1 month) was associated with increased risk of progression from IA to T1D. A higher rate of weight gain in early childhood was associated with increased risk of progression from IA to T1D (HR 2.57 [95% CI 1.34, 4.91] per 1 kg/year) in children with first-appearing GAD autoantibody only.
CONCLUSIONS
Growth patterns in early life better clarify how specific growth phases are associated with the development of T1D.
Introduction
Type 1 diabetes (T1D) is a common pediatric chronic disease and is preceded by a preclinical period of islet autoimmunity (IA) in the presence of islet autoantibodies against GAD (GADA), IA2 (IA2A), and/or insulin (IAA). IA commonly develops as early as infancy, with a peak incidence at ∼1 to 2 years of age (1–3). The Environmental Determinants of Diabetes in the Young (TEDDY) study reported that HLA-DR-DQ and age are strongly associated with the specific islet autoantibody that appears at the initial seroconversion. The majority of children who seroconvert develop either IAA or GADA as a single first-appearing autoantibody. Over time, these children may remain single autoantibody positive, develop other autoantibody(s), or revert to autoantibody negative. IAA generally appears at an earlier age than GADA, and the order of appearance of the autoantibodies is related to HLA-DR-DQ genotypes (1). Factors in early life likely play an important role in the initiation of IA, and excess growth is a potential candidate, as proposed by the accelerator hypothesis (4) and the overload hypothesis (5). The accelerator hypothesis proposes that excess weight gain and associated insulin resistance accelerate β-cell apoptosis and thereby drive autoimmunity in genetically susceptible children. The overload hypothesis suggests that metabolic overload of the β-cell including insulin resistance might be due to a high growth rate.
Several retrospective studies have reported associations between increased linear growth and weight gain in childhood, particularly in the first 2 years of life, and later onset of T1D (6–8). Longitudinal growth data collected from birth in prospective studies provide an opportunity to study the role of growth in the initiation of IA and the progression to T1D. Several prospective studies reported associations between growth in early life and the risk of IA (9–12). The Australian Baby Diab Study reported that weight SD score (SDS) at 2 years was associated with the risk of IA (9). The U.S. Diabetes Autoimmunity Study in the Young (DAISY) of children aged >2 years showed that height growth velocity, but not weight or BMI growth velocity, was associated with the risks of IA and progression to T1D (10). Moreover, the German BABYDIAB and BABYDIET studies reported that a younger age at infant BMI peak was associated with the risk of IA (13). Previously, the TEDDY study investigated weight and height SDSs at several specific ages during the first 4 years of age and found that the weight SDS at 1 year of age was associated with the risk of IA (12). These findings give evidence that weight gain in very early life and height growth velocity in childhood are associated with the risk of IA. However, there is still no clear picture of whether or how the whole growth trajectory in early life is associated with the development of IA/T1D. This is mainly due to the lack of growth data in infancy and the lack of complex modeling techniques for longitudinal growth data in early life. By modeling of the entire growth trajectory, the growth patterns can be easily identified and help pinpoint the timing of a potential disturbance or exposure.
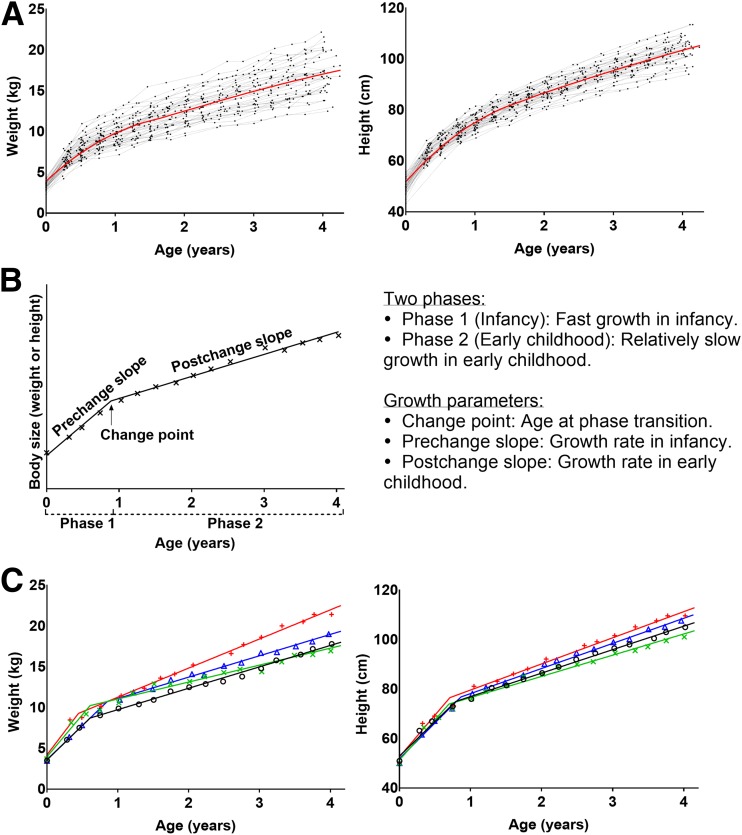
In this study, we investigated detailed growth data in early life and assessed their relationship with the development of IA and the progression to T1D in the TEDDY study. We modeled growth trajectories of weight and height in early life in two phases: fast growth in infancy and relatively slow growth subsequently in early childhood (Fig. 1). We used Bayesian two-phase piecewise linear mixed models with a random change point (14) to estimate a child’s individual-level random effects (prechange slope, postchange slope, and change point), which correspond to the individual-specific growth parameters (growth rate in infancy, growth rate in early childhood, and age at the phase transition) (Fig. 1B). The estimated change point is the point or time period in which the child transitioned from the phase of rapid infancy growth to another phase of slower childhood growth. Then, we assessed whether these growth parameters were associated with the risk of IA and the risk of progression from IA to T1D.
Figure 1.
Growth in early life. A: Trajectories of body size measurements of 50 randomly selected TEDDY subjects and the mean (loess) curves for weight and height. B: Description of the two-phase piecewise linear modeling of growth in early life. C: Fitted growth curves of weight and height from the Bayesian two-phase piecewise linear modeling for four randomly selected subjects.
Research Design and Methods
Participants
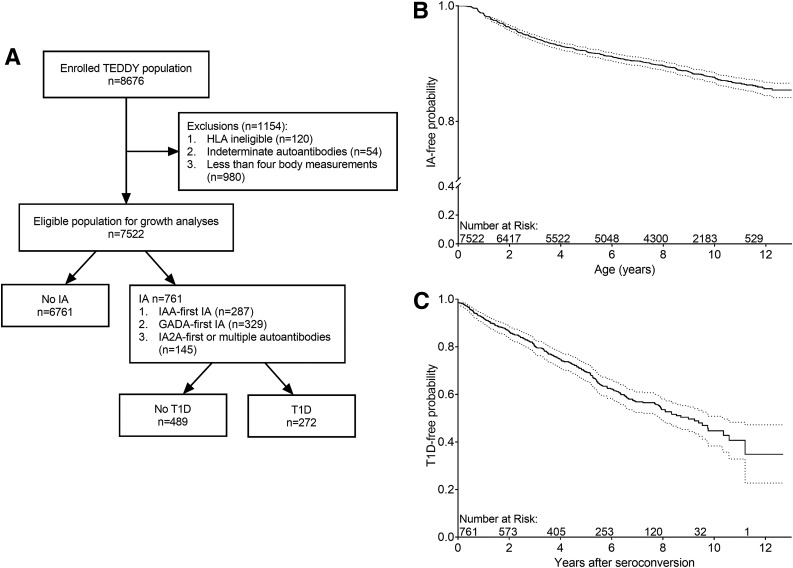
TEDDY is a prospective cohort study with the aim to identify environmental causes of T1D. The study included six clinical research centers: three in the U.S. (Colorado, Georgia/Florida, and Washington) and three in Europe (Finland, Germany, and Sweden). Written informed consents were obtained for all study participants from a parent or a primary caretaker, separately, for genetic screening and participation in the prospective follow-up, beginning at birth. The high-risk genotypes selected for inclusion for participants screened from the general population were as follows: DRB1*04-DQA1*03-DQB1*03:02/DRB1*03-DQA1*05-DQB1*02:01 (DR3/4), DRB1*04-DQA1*03-DQB1*03:02/DRB1*04-DQA1*03-DQB1*03:02 (DR4/4), DRB1*04-DQA1*03-DQB1*03:02/DRB1*08-DQA1*04-DQB1*04:02 (DR4/8), and DRB1*03-DQA1*05-DQB1*02:01/DRB1*03-DQA1*05-DQB1*02:01 (DR3/3). Additional study inclusion genotypes for first-degree relatives of a subject with T1D were: DRB1*04-DQA1*03-DQB1*03:02/DRB1*04-DQA1*03-DQB1*02:02 (DR4/4b), DRB1*04-DQA1*03-DQB1*03:02/DRB1*01-DQA1*01-DQB1*05:01 (DR4/1), DRB1*04-DQA1*03-DQB1*03:02/DRB1*13-DQA1*01-DQB1*06:04 (DR4/13), DRB1*04-DQA1*03-DQB1*03:02/DRB1*09-DQA1*03-DQB1*03:03 (DR4/9), and DRB1*03-DQA1*05-DQB1*02:01/DRB1*09-DQA1*03-DQB1*03:03 (DR3/9) (15). The HLA-DR-DQ genotype abbreviations shown in parentheses will be used throughout this article. A total of 8,676 children were enrolled and followed prospectively from 3 months to 15 years for measurements of weight and height (length before 2 and standing height after 2 years of age), and blood samples were drawn for measurements of islet autoantibodies with study visits every 3 months until 4 years of age and every 3 or 6 months thereafter, depending on autoantibody positivity. All children who were persistently positive for any autoantibody were followed every 3 months until 15 years of age or onset of T1D. In the analyses, 7,522 children were included after exclusion of 120 children whose HLA eligibility could not be confirmed at a repeated genotyping at 9 months of age, 54 children whose islet autoantibody results were indeterminate, and 980 children with <4 growth measurements in the first 4 years of life. The children were followed from birth for a median of 9.0 years (interquartile range [IQR] 5.7–10.6) (Fig. 2A).
Figure 2.
Study cohort. A: Flow chart of the TEDDY cohort and the study cohort. B: Survival curve of the development of IA. C: Survival curve of progression to T1D from initial seroconversion.
Islet Autoantibodies and T1D
Autoantibodies to IAA, GADA, or IA2A were measured in two laboratories by radiobinding assays (16,17). In the U.S., all sera were assayed at the Barbara Davis Center for Childhood Diabetes at the University of Colorado; in Europe, all sera were assayed at the University of Bristol (Bristol, U.K.). Both laboratories demonstrated high sensitivity and specificity as well as concordance (18). All positive and 5% of negative samples were retested in the other reference laboratory and deemed confirmed, if concordant. IA was defined as confirmed positive autoantibodies to IAA, GADA, or IA2A in at least two consecutive samples by both laboratories. T1D was diagnosed using American Diabetes Association criteria (19).
Statistical Analyses
The children’s weight and height trajectories were analyzed using Bayesian two-phase piecewise linear mixed models with a random change point (14). The models were estimated for girls and boys separately. The estimated individual-level random effects (prechange slope, postchange slope, and change point) (Fig. 1B) correspond to the individual-specific growth parameters (growth rate in infancy, growth rate in early childhood, and age at the phase transition).
Time-to-event analyses using multiple Cox proportional hazards (PH) models were performed to examine the growth parameters (weight and height separately) related to the risk of IA, IAA, or GADA as the first-appearing autoantibody and progression to T1D. The age at the development of IA was the age at the initial of two or more consecutive positive tests. The analyses of progression to T1D included children who developed persistent IA before the onset of T1D. Duration was calculated from the time at first IA positivity to the diagnosis date of T1D or date of last contact to assess T1D status for those who did not develop T1D. The magnitude of the associations was described by hazard ratios (HRs) with 95% CIs. Gestational age–adjusted birth measurement (birth weight in weight models and birth length in height models), HLA-DR-DQ genotype, family history of T1D, duration of exclusive breastfeeding, sex, and country of residence were adjusted for in the Cox PH models. The gestational age–adjusted birth measurements (birth weight or birth length) were the residuals calculated by linear regression of birth measurements on gestational ages for boys and girls separately. In addition, age at IA and type of first-appearing autoantibody (IAA, GADA, or multiple autoantibodies) were adjusted for in the analysis of progression to T1D, in which a small number of children with only IA2A autoantibody were grouped with children with multiple autoantibodies at initial seroconversion. Subgroup analyses stratified by the type of first-appearing autoantibody were also performed.
Sensitivity analyses with additional adjustments for previously identified dietary and genetic risk factors in the TEDDY study (20–24) were performed. Specifically, two dietary factors (early probiotic use and infant formula type in the first 3 months of life), eight T1D-associated non-HLA single nucleotide polymorphisms (SNPs) (rs1004446 in INS, rs10517086, rs2476601 in PTPN22, rs2816316 in RGS1, rs2292239 in ERBB3, rs3184504 in SH2B3, rs4948088 in COBL, and rs12708716 in CLEC16A), and two SNPs in complement genes (rs1143678 and rs4597342 in ITGAM) were adjusted for in the analysis of IA, while four SNPs (rs1004446 in INS, rs10517086, rs1534422, and rs2327832 in TNFAIP3) were adjusted for in the analysis of progression to T1D.
The Bayesian two-phase piecewise linear mixed models with a random change point were estimated using the WinBUGS 1.4.3 software (25). All of the other analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC).
Results
As of February 2018, the median (IQR) follow-up time of the last clinic visit for the 7,522 children was 9.0 (5.7–10.6) years. A total of 761 (10.1%) children developed one or more persistent autoantibodies (GADA, IAA, or IA2A) at the median age of 3.0 years (IQR 1.5–6.0), and 3.9% (290/7,522) developed T1D at the median age of 5.5 years (IQR 3.0–8.2) (Fig. 2B). Out of the 761 children with IA, 287 (37.7%) children had IAA only (IAA-first), 329 (43%) children had GADA only (GADA-first), 20 (2.6%) children had IA2A only, and 125 (16.4%) children had multiple autoantibodies at initial seroconversion. Of these children, 35.7% (272 out of 761) progressed to T1D (Fig. 2C). The characteristics of the children by the status of IA (IA negative, any IA, IAA-first IA, and GADA-first IA) and the children diagnosed with T1D are presented in Supplementary Table 1.
Growth Parameters and T1D
The details of the children’s growth parameters by the status of IA (IA negative, any IA, IAA-first IA, and GADA-first IA) and T1D are presented in Supplementary Table 1, and additional details are provided in Supplementary Figs. 1–4. The fitted growth curves and measures for four randomly selected subjects are plotted in Fig. 1C.
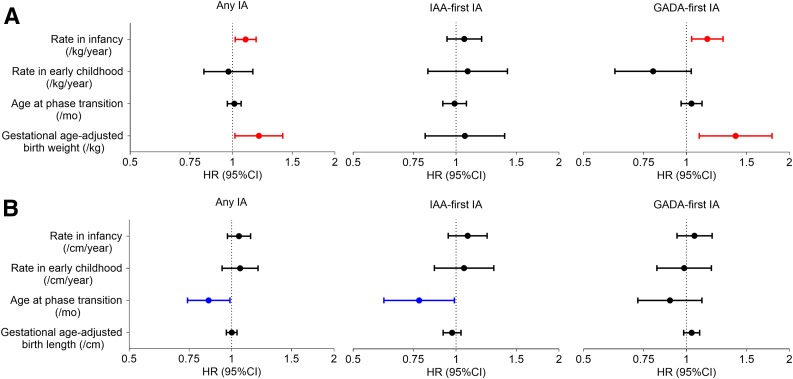
A higher rate of weight gain in infancy was associated with an increased IA risk adjusted for gestational age–adjusted birth weight, country of residence, sex, duration of exclusive breastfeeding, family history of T1D, and HLA DR-DQ genotype (HR 1.09 [95% CI 1.02, 1.17] per 1 kg/year increase; P = 0.015) (Fig. 3A and Supplementary Table 2). The effect of weight growth rate in infancy appeared to be most associated with the risk of GADA-first IA (HR 1.15 [95% CI 1.04, 1.28] per 1 kg/year increase; P = 0.009). In addition, gestational age–adjusted birth weight was also positively associated with the risk of IA (HR 1.19 [95% CI 1.02, 1.41] per 1 kg increase; P = 0.031) and the risk of GADA-first IA (HR 1.39 [95% CI 1.09, 1.78] per 1 kg increase; P = 0.008) (Fig. 3A and Supplementary Table 2).
Figure 3.
Forest plots of HR and 95% CI from multiple Cox PH regression analyses of effect of weight (A) and height (B) growth parameters, including gestational age–adjusted birth measurement, on the risk of IA, IAA-first IA, and GADA-first IA. Duration of exclusive breastfeeding, HLA-DR-DQ genotype, family history of T1D, sex, and country of residence were included in the Cox models. mo, months.
Height, but not weight, growth parameters were strongly associated with the risk of progression from initial IA seroconversion to T1D, adjusted for birth measurement, country, sex, family history of T1D, HLA DR-DQ genotype, age at IA, and type of first-appearing autoantibody (Fig. 4 and Supplementary Table 3). A height growth pattern with a lower rate in infancy (HR 0.79 [95% CI 0.70, 0.90] per 1 cm/year increase; P < 0.001), a higher rate in early childhood (HR 1.48 [95% CI 1.22, 1.79] per 1 cm/year increase; P < 0.001), and a younger age at the phase transition (HR 0.76 [95% CI 0.58, 0.99] per month increase; P = 0.044) were associated with an increased risk of progression from IA to T1D (Fig. 4B and Supplementary Table 3).
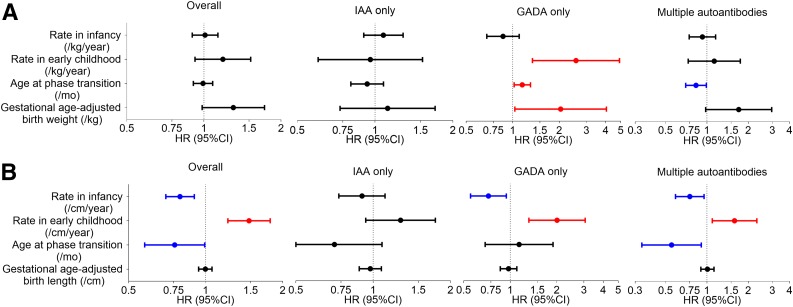
Figure 4.
Forest plots of HR and 95% CI from multiple Cox PH regression analyses of effect of weight (A) and height (B) growth parameters, including gestational age–adjusted birth measurement, on the risk of progression to T1D from IA and from subgroup analysis stratified by the type of first-appearing autoantibody (IAA only, GADA only, or multiple autoantibodies). Duration of exclusive breastfeeding, age at the development of IA, type of first-appearing autoantibody (if applicable), HLA-DR-DQ genotype, family history of T1D, sex, and country of residence were included in the Cox models. mo, months.
Subgroup analysis stratified by the type of first-appearing autoantibody showed similar associations between the two height growth rates and the risk of progression to T1D in those who had GADA only or multiple autoantibodies at the time of initial seroconversion (Fig. 4B and Supplementary Table 3). In addition, gestational age–adjusted birth weight and the weight growth rate in early childhood were positively associated with the risk of progression from IA to T1D (HR 2.04 [95% CI 1.03, 4.04] per 1 kg increase, P = 0.040; HR 2.57 [95% CI 1.34, 4.91] per 1 kg/year increase, P = 0.004) in children who had GADA only at the time of initial seroconversion (Fig. 4A and Supplementary Table 3).
The results were not affected in the sensitivity analyses with the additional adjustments for previously identified risk factors in the TEDDY study (data not shown), in which two dietary factors (early probiotic use and infant formula type in the first 3 months of life), eight T1D-associated non-HLA SNPs (rs1004446 in INS, rs10517086, rs2476601 in PTPN22, rs2816316 in RGS1, rs2292239 in ERBB3, rs3184504 in SH2B3, rs4948088 in COBL, and rs12708716 in CLEC16A), and two SNPs in complement genes (rs1143678 and rs4597342 in ITGAM) were adjusted for in the analysis of IA, while four SNPs (rs1004446 in INS, rs10517086, rs1534422, and rs2327832 in TNFAIP3) were adjusted for in the analysis of progression to T1D. Additional analyses by dichotomizing the growth parameters using country- and sex-specific tertiles showed consistent conclusions on the reported associations (data no shown) with an additional association between weight growth rate in early childhood and the risk of progression to T1D overall.
Conclusions
In this large international multicountry cohort of 7,522 children at high genetic risk for T1D, we modeled growth in early life using two distinct phases. Growth transited from a fast phase (infancy) to a relatively slow phase (early childhood) at ∼7–10 months of age. The weight growth rate in infancy and gestational age–adjusted birth weight were positively associated with the risk of IA and appeared to associate most with the risk of GADA-first IA. Furthermore, the weight growth rate in early childhood and gestational age–adjusted birth weight were associated with the risk of progression from IA to T1D in children with GADA only at initial seroconversion. A lower height growth rate in infancy and a higher height growth rate in early childhood were associated with an increased risk of progression from IA to T1D. The two height growth rates appeared to be more associated with the progression from IA to T1D in children who had GADA only or multiple autoantibodies at initial seroconversion.
Our finding that birth weight and the weight growth rate in infancy, but not in early childhood, were positively associated with the risk of IA is consistent with the findings from our previous study (12) and the Australian Baby Diab Study (9), in which weight at a younger age predicted IA more than weight at an older age. Together with our previous finding of a weak association between weight SDS and the risk of IA (12) in the first 4 years of life, it suggests that birth weight, the weight growth rate in infancy, and the resulting weight over time influence the development of IA. Our finding that the height growth rate in early childhood was associated with the risk of progression to T1D is consistent with the U.S. DAISY study (10), though growth data before 2 years of age were not assessed in the DAISY study. However, we did not confirm the association reported in DAISY that the height growth rate in early childhood increased the risk of IA (10).
Growth in children is directly related to nutrient intake (26) and a marker of metabolic (dys)function. Increased growth is glucose dependent and requires greater insulin secretion. Historically, it has been suggested that an inverse relationship exists between birth weight and diabetes risk, with a period of early rapid postnatal weight gain (27). This has subsequently led to the suggestion that prenatal factors may influence risk of T1D. In this study, we found that both higher birth weight and increased weight gain in infancy were associated with the risk of IA and, in particular, with the risk of IA with GADA as a first-appearing autoantibody. Higher birth weight with increased weight gain in early childhood was also associated with an increased risk of progression to T1D in those who developed GADA first. This provides evidence for the overload hypothesis in which excess growth leads to IA and the progression to T1D (4). Such rapid growth in infancy and early childhood prior to IA or T1D may indicate a marker of metabolic dysregulation by providing an exposure window to help identify potential triggers, such as dietary feeding patterns, related to disease development. Alternatively, it could be an early indication or symptom of disease onset. GADA autoimmunity may cluster with growth dysregulation in those with a greater risk of progressing to clinical T1D. GADA positivity has been linked to reduced insulin secretory capacity, a marker of impaired β-cell function in adults without diabetes (28). However, in the Type 1 Diabetes Prediction and Prevention (DIPP) Study, insulin secretion as measured by first-phase insulin responses was equally compromised in children who had GADA, IAA, or IA2A as the first-appearing autoantibody as compared with those without these autoantibodies (29,30).
TEDDY is a large prospective study covering 4 countries with longitudinal collection of growth data every 3 months from birth to 4 years of age, which provides a great opportunity to examine the role of growth in the development of IA and T1D using the entire growth trajectory in early life. Yet, there are limitations. The accelerator hypothesis suggests that excess weight gain and associated insulin resistance accelerate β-cell apoptosis. However, insulin resistance was not measured in the TEDDY study, and we could not test the accelerator hypothesis to examine whether insulin resistance was in the causal pathway. In the DIPP study, no difference was found in insulin sensitivity (as measured by HOMA index) between children with or without biochemical autoantibodies, even though insulin secretory capacity was lower in autoantibody-positive children (29). Growth hormone, IGF-1, and IGF-binding protein-3 (31,32) have been suggested to associate with T1D and possibly be in the causal pathway; however, these were not measured in TEDDY. Further studies to investigate insulin resistance, insulin secretory capacity, growth hormone, and IGFs at critical time points such as at birth, at 9 months, and at a later age regarding risk of IA and T1D are needed to clarify both the timing and the role that these factors play in the putative causal pathway. Another limitation of the study is that only weight and height were considered, but not BMI in the first 4 years of life because BMI is usually not calculated until 2 years of age. Insulin resistance as an accelerator for progression to T1D may have a greater impact in older children, and the growth pattern during puberty may be different. The children in this study are still young, with a median age of 10.4 years, and will be followed until 15 years of age. It has been reported that the growth trajectory has a childhood BMI rebound (13,14,33); thus, further analyses considering the BMI trajectory at an older age in relation to the clinical onset of T1D would be of great interest and evaluated as the data become available.
In this study, we modeled growth in early life using two distinct growth phases, which is novel and clarified that weight growth in infancy and height growth in early childhood associated with the development of IA and progression to T1D, respectively. Moreover, we assessed whether growth patterns were different depending on the first-appearing autoantibody of IAA or GADA. Our findings showed that overall weight and height are associated with IA and progression to T1D at different phases in early growth, and this effect was more pronounced in those who developed GADA as a first-appearing autoantibody. It is well known that growth is not linear, and during certain time periods in early life, it follows distinct patterns, such as the time from birth to 6 years. The pattern of infancy growth, phase transition, and childhood growth may serve as a surrogate of perinatal programming, similar to catch-up growth. Identifying the distinct growth patterns and age of transition from one growth phase to another may provide a rough time period to assess environmental triggers and other viable biomarkers associated with the T1D prodrome.
Supplementary Material
Article Information
Acknowledgments. The authors thank Sarah Austin-Gonzalez with the Health Informatics Institute at the University of South Florida (Tampa, FL) for assistance with preparing the figures.
Funding. The TEDDY study is funded by National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Environmental Health Sciences, JDRF, and Centers for Disease Control and Prevention grants U01-DK-63829, U01-DK-63861, U01-DK-63821, U01-DK-63865, U01-DK-63863, U01-DK-63836, U01-DK-63790, UC4-DK-63829, UC4-DK-63861, UC4-DK-63821, UC4-DK-63865, UC4-DK-63863, UC4-DK-63836, UC4-DK-95300, UC4-DK-100238, UC4-DK-106955, UC4-DK-112243, and UC4-DK-117483 and contract number HHSN267200700014C. This work is supported in part by the National Institutes of Health/National Center for Advancing Translational Sciences Clinical and Translational Science Awards to the University of Florida (UL1-TR-000064) and the University of Colorado (UL1-TR-001082).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors attest to meeting International Committee of Medical Journal Editors uniform requirements for authorship by making substantial contributions to conception and design of this manuscript; acquisition, analysis, and interpretation of the data; drafting or revising the manuscript for intellectual content; and giving final approval of the published version. X.L. proposed and performed the analysis, interpreted the results, and wrote the manuscript. K.V. provided input on the interpretation of the results and reviewed and edited the manuscript. Y.H. performed the analysis and reviewed and edited the manuscript. H.E.L. reviewed and edited the manuscript. J.T., A.G.Z., J.-X.S., M.R., W.A.H., B.A., and J.P.K. designed the study and reviewed and edited the manuscript. X.L. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
A complete list of the members of the TEDDY Study Group can be found in the Supplementary Data online.
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1670/-/DC1.
Contributor Information
Collaborators: TEDDY Study Group, Marian Rewers, Aaron Barbour, Kimberly Bautista, Judith Baxter, Daniel Felipe-Morales, Kimberly Driscoll, Brigitte I. Frohnert, Marisa Stahl, Patricia Gesualdo, Michelle Hoffman, Rachel Karban, Edwin Liu, Jill Norris, Stesha Peacock, Hanan Shorrosh, Andrea Steck, Megan Stern, Erica Villegas, Kathleen Waugh, Jorma Toppari, Olli G. Simell, Annika Adamsson, Suvi Ahonen, Mari Åkerlund, Leena Hakola, Anne Hekkala, Henna Holappa, Heikki Hyöty, Anni Ikonen, Jorma Ilonen, Sinikka Jäminki, Sanna Jokipuu, Leena Karlsson, Jukka Kero, Miia Kähönen, Mikael Knip, Minna-Liisa Koivikko, Merja Koskinen, Mirva Koreasalo, Kalle Kurppa, Jarita Kytölä, Tiina Latva-aho, Katri Lindfors, Maria Lönnrot, Elina Mäntymäki, Markus Mattila, Maija Miettinen, Katja Multasuo, Teija Mykkänen, Tiina Niininen, Sari Niinistö, Mia Nyblom, Sami Oikarinen, Paula Ollikainen, Zhian Othmani, Sirpa Pohjola, Petra Rajala, Jenna Rautanen, Anne Riikonen, Eija Riski, Miia Pekkola, Minna Romo, Satu Ruohonen, Satu Simell, Maija Sjöberg, Aino Stenius, Päivi Tossavainen, Mari Vähä-Mäkilä, Sini Vainionpää, Eeva Varjonen, Riitta Veijola, Irene Viinikangas, Suvi M. Virtanen, Jin-Xiong She, Desmond Schatz, Diane Hopkins, Leigh Steed, Jennifer Bryant, Katherine Silvis, Michael Haller, Melissa Gardiner, Richard McIndoe, Ashok Sharma, Stephen W. Anderson, Laura Jacobsen, John Marks, P.D. Towe, Anette G. Ziegler, Ezio Bonifacio, Anita Gavrisan, Cigdem Gezginci, Anja Heublein, Verena Hoffmann, Sandra Hummel, Andrea Keimer, Annette Knopff, Charlotte Koch, Sibylle Koletzko, Claudia Ramminger, Roswith Roth, Marlon Scholz, Joanna Stock, Katharina Warncke, Lorena Wendel, Christiane Winkler, Åke Lernmark, Daniel Agardh, Carin Andrén Aronsson, Maria Ask, Rasmus Bennet, Corrado Cilio, Helene Engqvist, Emelie Ericson-Hallström, Annika Fors, Lina Fransson, Thomas Gard, Monika Hansen, Hanna Jisser, Fredrik Johansen, Berglind Jonsdottir, Silvija Jovic, Helena Elding Larsson, Marielle Lindström, Markus Lundgren, Marlena Maziarz, Maria Månsson-Martinez, Maria Markan, Jessica Melin, Zeliha Mestan, Caroline Nilsson, Karin Ottosson, Kobra Rahmati, Anita Ramelius, Falastin Salami, Anette Sjöberg, Birgitta Sjöberg, Malin Svensson, Carina Törn, Anne Wallin, Åsa Wimar, Sofie Åberg, William A. Hagopian, Michael Killian, Claire Cowen Crouch, Jennifer Skidmore, Masumeh Chavoshi, Rachel Hervey, Rachel Lyons, Arlene Meyer, Denise Mulenga, Jared Radtke, Matei Romancik, Davey Schmitt, Sarah Zink, Dorothy Becker, Margaret Franciscus, MaryEllen Dalmagro-Elias Smith, Ashi Daftary, Mary Beth Klein, Chrystal Yates, Jeffrey P. Krischer, Sarah Austin-Gonzalez, Maryouri Avendano, Sandra Baethke, Rasheedah Brown, Brant Burkhardt, Martha Butterworth, Joanna Clasen, David Cuthbertson, Stephen Dankyi, Christopher Eberhard, Steven Fiske, Jennifer Garmeson, Veena Gowda, Kathleen Heyman, Belinda Hsiao, Christina Karges, Francisco Perez Laras, Hye-Seung Lee, Qian Li, Shu Liu, Xiang Liu, Kristian Lynch, Colleen Maguire, Jamie Malloy, Cristina McCarthy, Aubrie Merrell, Hemang Parikh, Ryan Quigley, Cassandra Remedios, Chris Shaffer, Laura Smith, Susan Smith, Noah Sulman, Roy Tamura, Dena Tewey, Michael Toth, Ulla Uusitalo, Kendra Vehik, Ponni Vijayakandipan, Keith Wood, Jimin Yang, Michael Abbondondolo, Lori Ballard, David Hadley, Wendy McLeod, Steven Meulemans, Liping Yu, Dongmei Miao, Polly Bingley, Alistair Williams, Kyla Chandler, Olivia Ball, Ilana Kelland, Sian Grace, William Hagopian, Masumeh Chavoshi, Jared Radtke, Sarah Zink, Henry Erlich, Steven J. Mack, Anna Lisa Fear, Sandra Ke, Niveen Mulholland, Stephen S. Rich, Wei-Min Chen, Suna Onengut-Gumuscu, Emily Farber, Rebecca Roche Pickin, Jonathan Davis, Jordan Davis, Dan Gallo, Jessica Bonnie, Paul Campolieto, Beena Akolkar, Kasia Bourcier, Thomas Briese, Suzanne Bennett Johnson, and Eric Triplett
References
- 1.Krischer JP, Lynch KF, Schatz DA, et al.; TEDDY Study Group . The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 2015;58:980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vehik K, Dabelea D. The changing epidemiology of type 1 diabetes: why is it going through the roof? Diabetes Metab Res Rev 2011;27:3–13 [DOI] [PubMed] [Google Scholar]
- 3.Knip M, Veijola R, Virtanen SM, Hyöty H, Vaarala O, Åkerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes 2005;54(Suppl. 2):S125–S136 [DOI] [PubMed] [Google Scholar]
- 4.Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between type I and type II diabetes. Diabetologia 2001;44:914–922 [DOI] [PubMed] [Google Scholar]
- 5.Dahlquist G. Can we slow the rising incidence of childhood-onset autoimmune diabetes? The overload hypothesis. Diabetologia 2006;49:20–24 [DOI] [PubMed] [Google Scholar]
- 6.Larsson HE, Hansson G, Carlsson A, et al.; DiPiS Study Group . Children developing type 1 diabetes before 6 years of age have increased linear growth independent of HLA genotypes. Diabetologia 2008;51:1623–1630 [DOI] [PubMed] [Google Scholar]
- 7.EURODIAB Substudy 2 Study Group Rapid early growth is associated with increased risk of childhood type 1 diabetes in various European populations. Diabetes Care 2002;25:1755–1760 [DOI] [PubMed] [Google Scholar]
- 8.Bruining GJ. Association between infant growth before onset of juvenile type-1 diabetes and autoantibodies to IA-2. Netherlands Kolibrie study group of childhood diabetes. Lancet 2000;356:655–656 [DOI] [PubMed] [Google Scholar]
- 9.Couper JJ, Beresford S, Hirte C, et al. Weight gain in early life predicts risk of islet autoimmunity in children with a first-degree relative with type 1 diabetes. Diabetes Care 2009;32:94–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamb MM, Yin X, Zerbe GO, et al. Height growth velocity, islet autoimmunity and type 1 diabetes development: the Diabetes Autoimmunity Study in the Young. Diabetologia 2009;52:2064–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyerlein A, Liu X, Uusitalo UM, et al.; TEDDY Study Group . Dietary intake of soluble fiber and risk of islet autoimmunity by 5 y of age: results from the TEDDY study. Am J Clin Nutr 2015;102:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elding Larsson H, Vehik K, Haller MJ, et al.; TEDDY Study Group . Growth and risk for islet autoimmunity and progression to type 1 diabetes in early childhood: The Environmental Determinants of Diabetes in the Young study. Diabetes 2016;65:1988–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beyerlein A, Thiering E, Pflueger M, et al. Early infant growth is associated with the risk of islet autoimmunity in genetically susceptible children. Pediatr Diabetes 2014;15:534–542 [DOI] [PubMed] [Google Scholar]
- 14.Brilleman SL, Howe LD, Wolfe R, Tilling K. Bayesian piecewise linear mixed models with a random change point: an application to BMI rebound in childhood. Epidemiology 2017;28:827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagopian WA, Erlich H, Lernmark A, et al.; TEDDY Study Group . The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes 2011;12:733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes 2007;8:286–298 [DOI] [PubMed] [Google Scholar]
- 17.TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann N Y Acad Sci 2008;1150:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 2010;95:3360–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care 2014;37(Suppl. 1):S14–S80 [DOI] [PubMed] [Google Scholar]
- 20.Uusitalo U, Liu X, Yang J, et al.; TEDDY Study Group . Association of early exposure of probiotics and islet autoimmunity in the TEDDY study. JAMA Pediatr 2016;170:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Törn C, Hadley D, Lee H-S, et al.; TEDDY Study Group . Role of type 1 diabetes-associated SNPs on risk of autoantibody positivity in the TEDDY study. Diabetes 2015;64:1818–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Törn C, Liu X, Hagopian W, et al.; TEDDY Study Group . Complement gene variants in relation to autoantibodies to beta cell specific antigens and type 1 diabetes in the TEDDY Study. Sci Rep 2016;6:27887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krischer JP, Liu X, Lernmark Å, et al.; TEDDY Study Group . The influence of type 1 diabetes genetic susceptibility regions, age, sex, and family history on the progression from multiple autoantibodies to type 1 diabetes: a TEDDY study report. Diabetes 2017;66:3122–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hummel S, Beyerlein A, Tamura R, et al.; TEDDY Study Group . First infant formula type and risk of islet autoimmunity in The Environmental Determinants of Diabetes in the Young (TEDDY) study. Diabetes Care 2017;40:398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS - a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325–337 [Google Scholar]
- 26.Lifshitz F. Nutrition and growth. J Clin Res Pediatr Endocrinol 2009;1:157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabricius-Bjerre S, Jensen RB, Færch K, et al. Impact of birth weight and early infant weight gain on insulin resistance and associated cardiovascular risk factors in adolescence. PLoS One 2011;6:e20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendivil CO, Toloza FJ, Ricardo-Silgado ML, et al. Antibodies against glutamic acid decarboxylase and indices of insulin resistance and insulin secretion in nondiabetic adults: a cross-sectional study. Diabetes Metab Syndr Obes 2017;10:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koskinen MK, Helminen O, Matomäki J, et al. Reduced β-cell function in early preclinical type 1 diabetes. Eur J Endocrinol 2016;174:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koskinen MK, Lempainen J, Löyttyniemi E, et al. Class II HLA genotype association with first-phase insulin response is explained by islet autoantibodies. J Clin Endocrinol Metab 2018;103:2870–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W, Salojin KV, Mi Q-S, et al. Insulin-like growth factor (IGF)-I/IGF-binding protein-3 complex: therapeutic efficacy and mechanism of protection against type 1 diabetes. Endocrinology 2004;145:627–638 [DOI] [PubMed] [Google Scholar]
- 32.Kelley KM, Oh Y, Gargosky SE, et al. Insulin-like growth factor-binding proteins (IGFBPs) and their regulatory dynamics. Int J Biochem Cell Biol 1996;28:619–637 [DOI] [PubMed] [Google Scholar]
- 33.Wen X, Kleinman K, Gillman MW, Rifas-Shiman SL, Taveras EM. Childhood body mass index trajectories: modeling, characterizing, pairwise correlations and socio-demographic predictors of trajectory characteristics. BMC Med Res Methodol 2012;12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.