Abstract
Recent work shows that gut microbial dysbiosis contributes to the risk of obesity in children whose mothers consume a high-fat diet (HFD) during both gestation and lactation or during gestation alone. Obesity predisposes children to developing precocious puberty. However, to date, no study has examined how maternal HFD (MHFD) during lactation regulates the gut microbiota (GM), pubertal timing, and fertility of offspring. Here, we found that MHFD during lactation markedly altered the GM of offspring. The pups developed juvenile obesity, early puberty, irregular estrous cycles, and signs of disrupted glucose metabolism. Remarkably, permitting coprophagia between MHFD and maternal normal chow offspring successfully reversed the GM changes as well as early puberty and insulin insensitivity. Our data suggest that microbial reconstitution may prevent or treat early puberty associated with insulin resistance.
Keywords: maternal high-fat diet, lactation, gut microbiota, metabolism, puberty
Puberty marks the passage to sexual maturity and adulthood. Precocious puberty, or early puberty, can cause short stature (1) and social and emotional problems (2), while increasing the risk of diabetes (3), cardiovascular disease (4), breast cancer (5), and all-cause mortality (6). Worldwide, precocious puberty affects 20 out of 10 000 girls per year (7–9). Childhood obesity increases the rate of early puberty, especially in girls (10–12). In the obese state, insulin signaling increases gonadotropin and sexual hormone secretion (13), which may lead to precocious puberty and infertility. Maternal nutrition may also determine the development of obesity (14, 15) and the age of menarche in offspring (16, 17).
During the past decade, increasing attention has focused on the effects of the gut microbiota (GM) on energy homeostasis and obesity (18–21). Emerging evidence shows maternal high-fat diet (MHFD) shifts the gut microbial ecology in mothers, promoting maternal obesity and gut microbial dysbiosis in offspring (22, 23). Although multiple factors can influence the GM in children, such as mode of delivery, breastfeeding or formula feeding, use of antibiotics, introduction of solid foods, and cessation of nursing (24–26), breastfeeding appears to play a dominant role in the maturation of the GM (27).
The GM of children develops in a dynamic process that reaches a stable state reflecting the adult GM shortly after weaning (28, 29). The stability of the GM of children influences their health in adulthood (30). Lower GM diversity may reduce circulating estrogens by decreasing estrogen deconjugation (31, 32). These findings imply that the GM in childhood may alter pubertal maturation. However, whether the GM mediates the link between obesity and early puberty is not clear. Therefore, our first objective was to study whether MHFD during lactation alters the GM in offspring and influences the risk of obesity and early pubertal development.
Mice are coprophagic, so co-housed family members share GM through the fecal–oral route (33). Co-housing can reveal a causal relationship between gut microbial dysbiosis and host health. For example, Ridaura and colleagues demonstrated that co-housing obese with lean mice restored a lean phenotype (33). Further, co-housing letrozole-induced polycystic ovary syndrome (PCOS) mice with placebo mice improved both reproductive and metabolic PCOS phenotypes (34); notably, these findings suggest that modifying GM may be an effective treatment for reproductive diseases. However, little is known about the effect of co-housing on puberty. Therefore, we also tested whether co-housing MHFD with normal control diet (NCD) offspring can reverse their reproductive and metabolic deficits.
Materials and Methods
Animals
All mice were on a C57BL/6 background and were obtained from Jackson Laboratories (#000664). Mice were housed in the University of Toledo College of Medicine and Life Sciences animal facility at 22°C to 24°C on a 12-h light/12-h dark cycle and had access to food and water ad libitum. At 8 weeks old, females were paired with C57BL/6 adult males to produce experimental offspring. On the day of delivery, females were put on either standard rodent chow (NCD) (2016 Teklad Global 16% Protein Rodent Diet, 12% fat by calories; Harlan Laboratories, Indianapolis, IN, US) or high-fat diet (HFD) consisting of 60% kcal from fat and 20% kcal from carbohydrates (Research Diets, #D12492). At the end of the study, all animals were sacrificed by CO2 asphyxiation or by cardiac puncture under 2% isoflurane anesthesia to draw blood. All procedures were approved by University of Toledo College of Medicine Animal Care and Use Committee.
Co-housing experiment
On postnatal day 21, pups were weaned, and all were fed with NCD. Then they were randomly assigned to NCD, MHFD, or co-housed (co-NCD and co-MHFD) groups. Each individual NCD or MHFD cage contained 4 NCD or MHFD offspring. Co-housed groups consisted of individual cages containing 2 co-MHFD offspring with 2 co-NCD offspring.
Metabolic phenotype assessment
Pup body weight was measured weekly from week 3 to 12. Body length, which is defined as the distance from the nose to the base of the tail, was measured from week 3 to 12 under 2% isoflurane anesthesia. Body composition was assessed monthly by nuclear magnetic resonance (minispec mq7.5; Bruker Optics, Billerica, MA, US) to determine the percentage of fat mass, as described previously (35). Glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) were performed as described previously (35). Briefly, after a 6-h fast, mice were injected with dextrose (2 g/kg ip). Tail blood glucose was measured using a veterinary glucometer (AlphaTRAK; Abbott Laboratories, Abbott Park, IL, US) before and 15, 30, 45, 60, 90, and 120 min after injection. For ITTs, after a 3-h fast, mice were injected with recombinant insulin (0.75 U/kg ip). Tail blood glucose was measured again at specified time points.
Puberty and reproductive phenotype assessment
Pubertal development was checked daily after weaning by looking for vaginal opening in the female mice. Vaginal cytology was examined by collecting vaginal lavages from female mice. First, estrus (E) age was determined by the occurrence of 2 consecutive days with keratinized cells after 2 previous days with leukocytes (36). Estrus stage can be divided into 4 stages: proestrus (P), E, metestrus (M), and diestrus (D). Stages were assessed based on vaginal cytology as described previously (36, 37). Male pubertal development was checked daily after weaning by manually retracting the prepuce with gentle pressure to verify balanopreputial separation (38).
At 3 to 4 months of age, we examined adult fertility. Each female mouse was paired with 1 fertile wild-type male for 8 nights while monitoring daily for copulatory plugs. The paired mice were separated after 8 nights, and pregnancy rate, litter size, and interval from mating to birth were recorded. The age of sexual maturation was estimated from the birth of the first litter minus average pregnancy duration for mice (21 days).
16s rRNA gene sequencing
Fecal samples were collected from offspring at week 3 and week 10. Fecal samples were frozen immediately after collection and stored at –80°C. Fecal deoxyribonucleic acid (DNA) was extracted using QIAamp PowerFecal DNA Kit (Qiagen) following the manufacture’s protocol. For the elution step, 50 µL of low TE buffer (0.1 mM ethylenediaminetetraacetic acid, Tris-HCl buffer, 10 mM, pH 8.5) was used instead of the AE buffer in the kit. DNA concentration was measured by NanoDrop spectrophotometer (ThermoFisher Scientific, Waltham, MA, US), and then diluted to be 5 ng/µL in low TE buffer for PCR library preparation. We followed the Illumina User Guide “16S Metagenomic Sequencing Library Preparation-Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System” (Illumina, San Diego, CA, US Part # 15044223 Rev. B; https://support.illumina.com/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf). The 16S ribosomal RNA gene (16S rRNA) targeting the variable V3 and V4 regions was amplified using the primers: 5’ TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG and 5’ GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT. For index PCR, Nextera XT index Kit (Illumina) was used to attach dual indices and sequencing adapters. Platinum™ Taq DNA Polymerase (ThermoFisher Scientific) was used in a 25 µL polymerase chain reaction (PCR) volume. Each 2.5 µL of 5ng/µL DNA was added on first PCR (target PCR), and 2.5µl of purified first PCR product was added on second PCR (index PCR). T100TM thermal cycler (BioRad, Hercules, CA, US) was used, and cycling conditions followed the 16S rRNA gene Metagenomic Sequencing User Guide. Each concentration of purified index PCR products was measured using Qubit 3.0 fluorometer (Invitrogen, Carlsbad, CA, US). Measured concentration was converted into nM and each diluted 4 nM amplicon was pooled equally. Then the pooled library was checked on a 2100 Bioanalyzer (Agilent, Santa Clara, CA, US) to verify the size, concentration and absence of primer dimers in the final library. Following the Illumina MiSeq System User Guide, a 10 pM denatured and diluted library was mixed with a 10 pM PhiX control spike-in to be 15% PhiX. Then, it was loaded in the Illumina MiSeq with the V3 flow cell kit for 2X300 cycles.
Raw 16S sequencing data were processed and analyzed using a bioinformatics pipeline using multiple software packages including USEARCH (39), Quantitative Insights Into microbial Ecology (QIIME) software package (version 1.9.1) (40), and linear discriminant analysis effect size (41). Raw paired-end reads were merged to create consensus sequences and then quality filtered using USEARCH (version 9). Chimeric sequences were identified and filtered using QIIME combined with the USEARCH (version 6) algorithm. Open reference operational taxonomic units (OTUs) were subsequently picked using QIIME combined with the USEARCH (version 6) algorithm, and taxonomy assignment was performed using Greengenes (42) as the reference database. Using a series of QIIME pipelines, alpha- and beta-diversity analyses were performed. Observed OTUs and Faith’s phylogenetic diversity were used to indicate the alpha diversity. The analysis of similarities statistical method was used to calculate the P-value of the beta diversity. Mann–Whitney U test was performed to compare the alpha diversity and relative abundance of species between 2 groups. Multiple group comparisons were made using a Kruskal–Wallis test with Bonferroni correction. Correlations between the relative abundance of microbiota species and phenotypes were assessed by Pearson’s correlation tests. P < .05 was used as statistical significance. The P-values obtained were adjusted for multiple comparisons by the false discovery rate method with a corresponding q-value threshold of 0.05. Tests were performed using SPSS 23 and GraphPad Prism.
Statistical analysis
Data are presented as means ± standard error of the mean. Comparison of 2 independent groups was performed with independent sample t tests. One-way analysis of variance (ANOVA) was used as the main statistical method to compare 4 groups, followed by the Tukey multiple comparison test. For GTT and ITT, 2-way ANOVA was used to compare changes over time between 4 groups. Bonferroni multiple comparison tests were then performed to compare differences among groups. Chi-square was used to analyze pregnancy rates. A value of P < .05 was considered to be significant.
Results
MHFD during lactation causes juvenile obesity and alters the GM in offspring at week 3
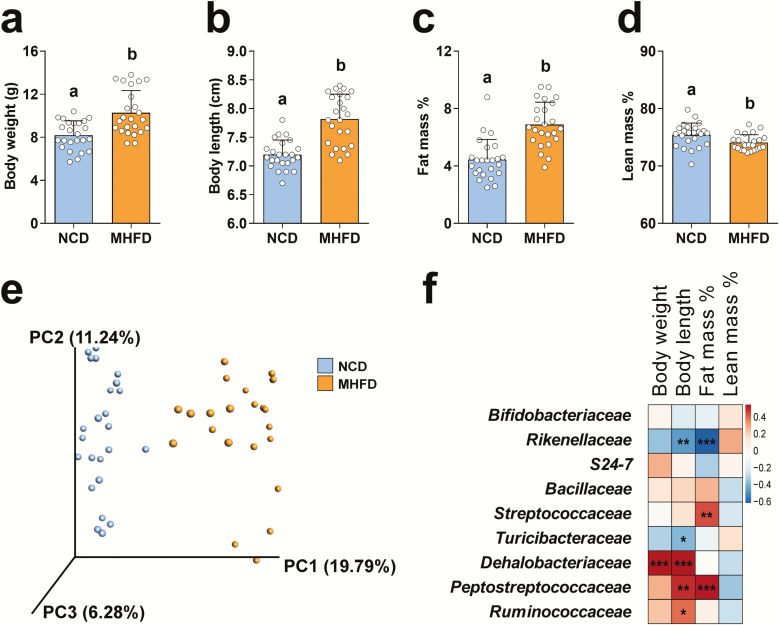
To determine whether MHFD during lactation causes metabolic abnormalities in female offspring, we fed new mothers either a HFD (60% calories from fat) or NCD (12% calories from fat) from the day of delivery to weaning (week 3 [W3]) and evaluated metabolic functions in the offspring at W3. As expected, MHFD female offspring showed significantly increased body weight (Fig. 1A) and body length (Fig. 1B) when compared to NCD offspring. The obese phenotype resulted from an increase in fat mass percentage (Fig. 1C) and a decrease in lean mass percentage (Fig. 1D). The total number of pups and the offspring sex ratio did not differ between MHFD and NCD groups (data not shown).
Figure 1.
MHFD offspring demonstrated increased body weight and fat mass, which were associated with alterations in the gut microbiota composition. (A and B) Body weight and body length in MHFD (n = 24) or NCD (n = 24) offspring at W3. (C and D) Nuclear magnetic resonance analysis showing body composition changes in MHFD (n = 24) or NCD (n = 24) offspring at W3. Data presented as means ± S.E with individual data points. Levels of statistical significance for two groups comparison were analyzed by independent sample t test. Differences in letters between bars (eg, a, b) indicate statistically significant differences between groups (P < .05). (E) Unweighted UniFrac principal coordinates analysis plot representing gut microbiota composition changes in MHFD (n = 24) and NCD (n = 23) offspring at W3. (F) Heatmap of the association between the abundance of the differential enriched families and body composition at W3 (total n = 40–42). Red denotes a positive correlation; blue, a negative correlation; and white, no association. Levels of statistical significance were analyzed by Pearson’s r correlation; *P < .05, **P < .01, and ***P < .001 with false discovery rate correction (also see Supplemental Data) (43).
To investigate how MHFD during lactation contributes to establishing the GM of offspring, we collected fecal samples from offspring at W3 and performed targeted amplification and sequencing of the 16S rRNA gene. Surprisingly, the GM richness as indicated by the observed OTUs and Faith’s phylogenetic diversity did not differ between NCD and MHFD offspring (Supplemental Figs. 1A and 1B) (43). However, the GM β-diversity of MHFD offspring diverged from NCD offspring (Fig. 1E). At a family level, we identified 9 bacteria showing significant differences between NCD and MHFD offspring (Supplemental Fig. 1C) (43). MHFD offspring showed decreased levels of Bifidobacteriaceae, Rikenellaceae, S24-7, and Turicibacteraceae and increased levels of Bacillaceae, Streptococcaceae, Dehalobacteriaceae, Peptostreptococcaceae, and Ruminococcaceae, compared to NCD offspring.
Interestingly, by Pearson correlation analysis (Fig. 1F and Supplemental Table 1) (43), we found that Dehalobacteriaceae strongly correlated with body weight and body length. In addition, Peptostreptococcaceae and Ruminococcaceae positively correlated with body length, whereas Rikenellaceae and Turicibacteraceae negatively correlated with body length. Two families, Streptococcaceae and Peptostreptococcaceae, positively correlated with fat mass percentage, whereas Rikenellaceae negatively correlated with fat mass percentage. These data suggest that MHFD during lactation altered the GM and led to early obesity in offspring.
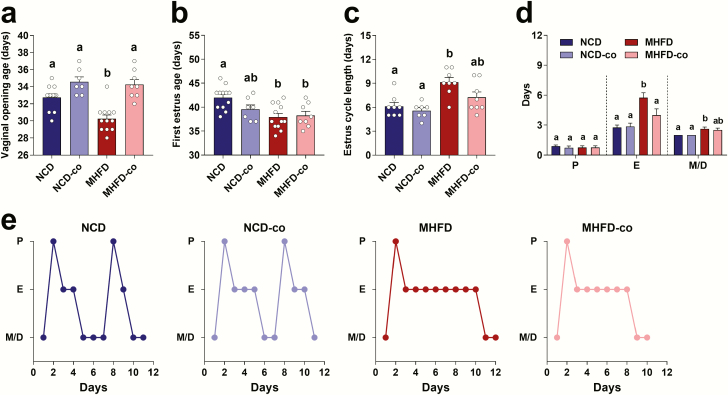
Co-housing reversed early puberty induced by MHFD during lactation
MHFD male offspring did not show advanced puberty as measured by date of balanopreputial separation (data not shown). To determine whether MHFD during lactation caused early puberty in female offspring, we examined pubertal timing in both MHFD and NCD pups. We used age of vaginal opening as an indicator of the onset of puberty. We found that vaginal opening age in MHFD offspring occurred 3 to 4 days earlier than in NCD offspring (Fig. 2A). We then evaluated age of first E of the mice by vaginal lavage. MHFD offspring had an earlier first E age compared to NCD offspring (Fig. 2B). MHFD offspring had significantly longer estrous cycles than NCD offspring (Fig. 2C), because of a longer time spent in E and M/D (Fig. 2D). Fig. 2E shows representative estrous cycles in NCD, co-housed NCD (NCD-co), MHFD and co-housed MHFD (MHFD-co) offspring. These data suggest that MHFD during lactation caused early puberty and irregular estrous cycles in offspring. To test whether MHFD during lactation also caused impaired fertility, we performed fertility testing in mice at 3- to 4-month-old age. The pregnancy rate, litter size, plug number, and interval from paring to birth did not differ between NCD and MHFD offspring (Supplemental Fig. 2A to 2D) (43).
Figure 2.
Co-housing MHFD with NCD offspring successfully reversed early puberty in co-housed MHFD offspring. (A–D) Vaginal opening age, first E age, and E cycle length and time spent in each E stage were evaluated in NCD (n = 12), NCD-co (n = 7), MHFD (n = 12), and MHFD-co (n = 8) offspring. Data presented as means ± standard error of the mean with individual data points. Levels of statistical significance were analyzed by 1-way ANOVA with post-hoc Tukey test; Differences in letters between bars (eg, a, b, c) indicate statistically significant differences between groups (P < .05). (E) Representative examples of E cycles in each individual mouse of NCD, NCD-co, MHFD, and MHFD-co offspring groups were shown. NCD offspring displayed regular 4- to 5-day cycles (2 E cycles) while MHFD offspring spent longer time in E and M/D-like cytology (only 1 cycle). The y axis represents E stage. Abbreviations: D, diestrus; E, estrus; M, metestrus; P, proestrus.
To test if GM reconstitution could reverse the early puberty and irregular estrous cycles, we co-housed MHFD (MHFD-co) with NCD (NCD-co) offspring at W3. Remarkably, co-housing MHFD with NCD offspring restored the normal age of vaginal opening (34.13 ± 1.81 days in MHFD-co offspring compared to 35.50 ± 2.98 days in NCD-co offspring) (Fig. 2A). Co-housing did not change the timing of the first E (Fig. 2B). While we did not see any effect of co-housing on estrous cycle length (Fig. 2C), the time exhibiting E-like cytology was shortened (4.00 ± 1.73 days in MHFD-co compared to 5.75 ± 1.39 days in MHFD offspring) (Fig. 2D).
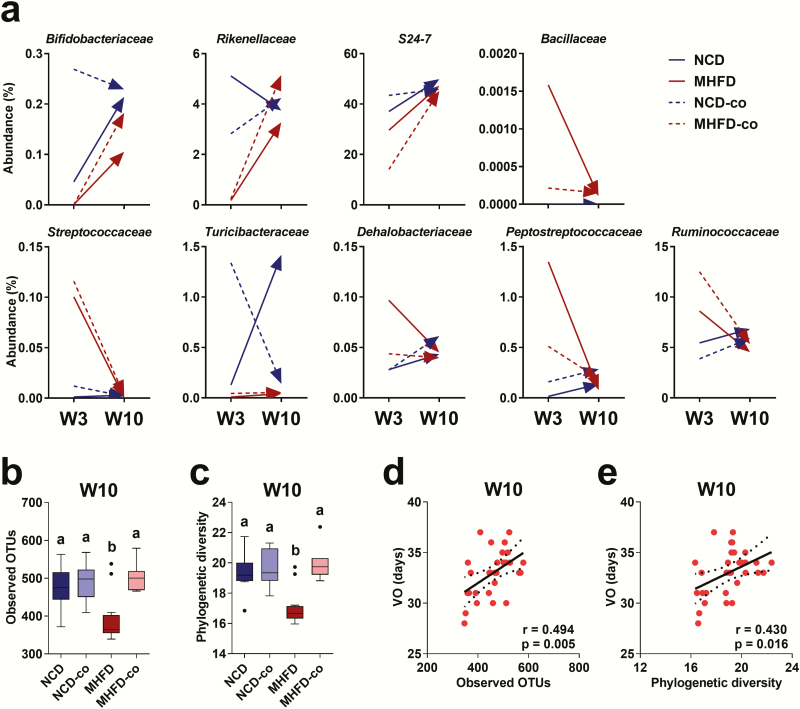
Co-housing reversed early puberty via increasing GM richness
We next analyzed the fecal samples of offspring from W3 to week 10 (W10) to evaluate the long-term effect of MHFD during lactation on offspring GM. The GM α-diversity in all four groups, NCD, NCD-co, MHFD, and MHFD-co, did not differ before co-housing at W3 (Supplemental Figs. 3A and 3B) (43). The unweighted UniFrac principal coordinates analysis showed dynamic changes of GM in offspring during the co-housing experiment from W3 to W10 (Supplemental Fig. 3C) (43). Moreover, we found that the relative abundances of nine bacteria were differentially influenced by MHFD during lactation at W3 but were comparable at W10 (Fig. 3A). This result suggests that the maternal influence was a critical factor in the development of offspring GM. Interestingly, MHFD offspring at W10 had significantly lower GM richness compared to NCD offspring (Figs. 3B and3C). Remarkably, after co-housing with NCD offspring, the GM richness increased in MHFD offspring, as indicated by the observed OTUs and Faith’s phylogenetic diversity (Figs. 3B and 3C). These results indicate that altered GM richness likely led to the phenotypic changes and effect of co-housing.
Figure 3.
Dynamic change of gut microbiota in offspring and co-housing reversed early puberty and positively correlated with bacterial richness. (A) Change in the abundance of 9 families with significant differences in NCD (n = 12), NCD-co (n = 8), MHFD (n = 13), and MHFD-co (n = 8) offspring from W3 to W10. Values are the mean of each group. (B and C) Boxplots showing alpha diversity comparisons in fecal samples of NCD (n = 12), NCD-co (n = 8), MHFD (n = 13), and MHFD-co (n = 8) offspring at W10 based on observed OTUs and phylogenetic richness as determined by 16s rRNA sequencing analysis. Differences in letters between bars (eg, a, b) indicate statistically significant differences between groups (Kruskal–Wallis test with Bonferroni correction; P < .05). (D) Scatter plots of correlations between vaginal opening age (VO) and alpha diversity as measured by observed OTUs and phylogenetic richness at W10 (total n = 31).
We next asked whether co-housing reversed early puberty by regulating GM richness. Specifically, we wanted to know whether lower GM richness led to an earlier age of vaginal opening. We performed a correlation study between vaginal opening age and GM α-diversity. Indeed, vaginal opening age was strongly and positively correlated with GM richness as indicated by observed OTUs and phylogenetic diversity at W10 (Figs. 3D and 3E), but not at W3 (Supplemental Figs. 3D and 3E) (43). However, we did not see any GM composition changes contributing to the regulatory effects of co-housing at W10 (data not shown). Overall, these data demonstrate that the decreased GM richness induced by MHFD during lactation was associated with early pubertal onset. Moreover, GM reconstitution by co-housing successfully reversed early puberty by increasing GM richness.
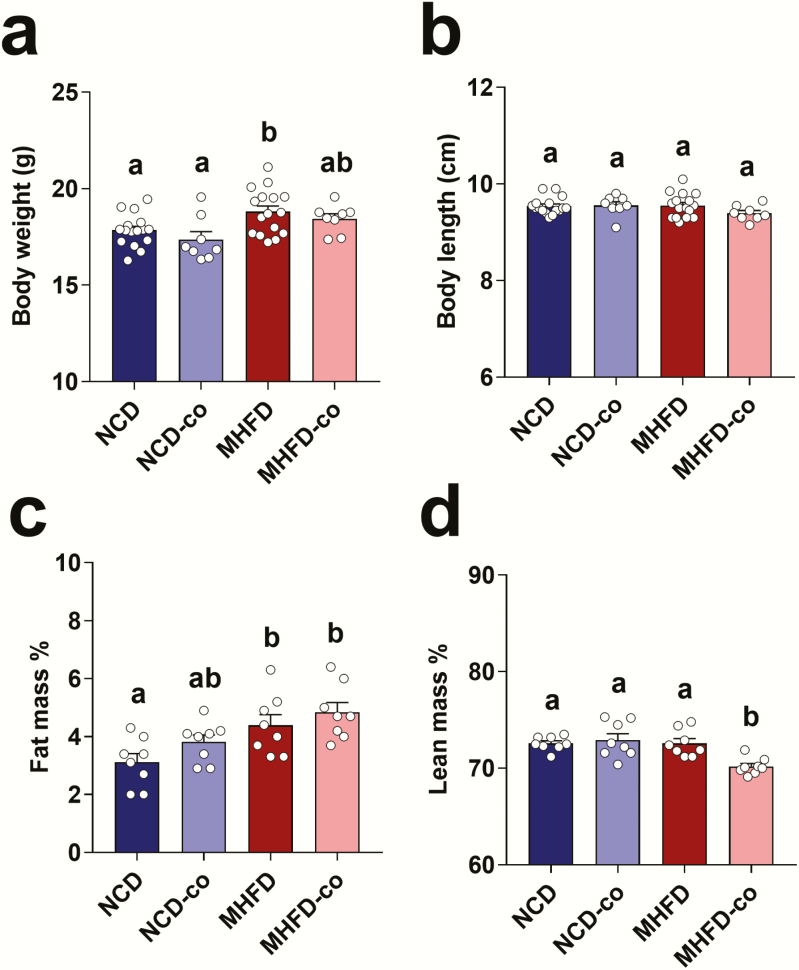
Co-housing reversed insulin insensitivity in offspring induced by MHFD during lactation
MHFD during lactation had long-term effects on the regulation of metabolic functions; body weight (Fig. 4A) and fat mass percentage (Fig. 4C) were increased in MHFD offspring at W10 compared to control offspring. However, the effect of MHFD on body length and lean mass percentage diminished by W10 (Figs. 4B and 4D). Co-housing MHFD with NCD offspring did not reverse body weight or fat mass percentage in MHFD offspring (Figs. 4A and 4C).
Figure 4.
Co-housing MHFD with NCD offspring didn’t alter body weight in co-housed MHFD offspring. (A and B) Body weight and length in NCD (n = 16), NCD-co (n = 8), MHFD (n = 16), and MHFD-co (n = 8) offspring at W10. (C and D) Nuclear magnetic resonance analysis showing body composition changes in NCD (n = 16), NCD-co (n = 8), MHFD (n = 16), and MHFD-co (n = 8) offspring at W10. Data presented as means ± standard error of the means with individual data points. Levels of statistical significance were analyzed by 1-way ANOVA with post-hoc Tukey test; differences in letters between bars (eg, a, b, c) indicate statistically significant differences between groups (P < .05).
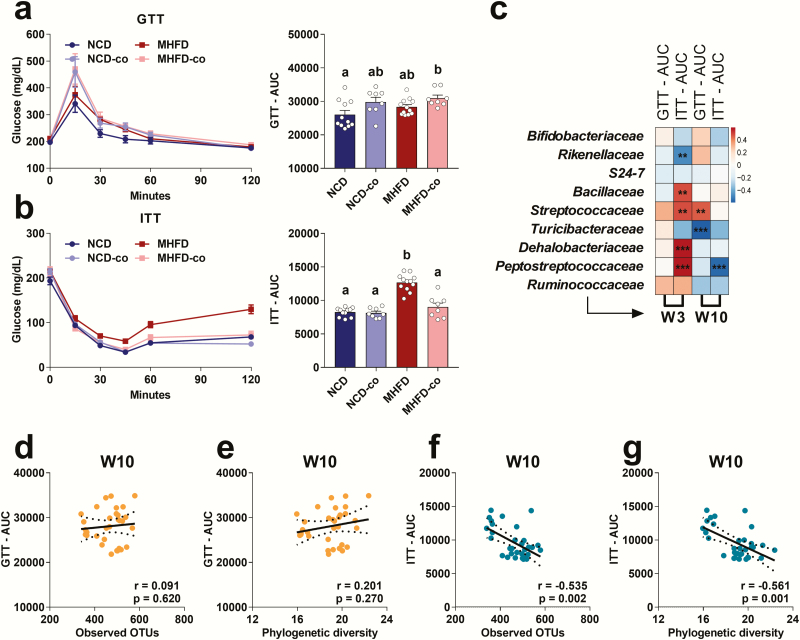
We next evaluated the long-term effect of MHFD during lactation on glucose metabolism. MHFD during lactation caused insulin insensitivity (Fig. 5B) in adult MHFD offspring when compared to NCD offspring. Surprisingly, insulin sensitivity in MHFD offspring improved when we co-housed them with NCD offspring (Fig. 5B). Therefore, MHFD during lactation disrupted glucose homeostasis in offspring and that co-housing with NCD offspring reversed insulin insensitivity.
Figure 5.
Co-housing MHFD with NCD offspring improved insulin insensitivity in co-housed MHFD offspring. (A) GTT and (B) ITT in NCD (n = 11), NCD-co (n = 8), MHFD (n = 12), MHFD-co (n = 8) offspring at W10. AUC for GTT and ITT were calculated and presented as means ± standard error of the means with individual data points. Levels of statistical significance were analyzed by 1-way ANOVA with post-hoc Tukey test; differences in letters between bars (eg, a, b) indicate statistically significant differences between groups (P < .05). (C) Heatmap of the association between the abundance of the differential enriched families and AUC of GTT and ITT at W3 and W10 (total n = 32). Red denotes a positive correlation; blue, a negative correlation; and white, no association. Levels of statistical significance were analyzed by Pearson’s r correlation; *P < .05, **P < .01, and ***P < .001 with false discovery rate correction (also see Supplemental Data) (43). (D and E) Scatter plots of correlations between AUC of GTT and alpha diversity as measured by observed OTUs and phylogenetic richness (total n = 39). (F and G) Scatter plots of correlations between AUC of ITT and alpha diversity as measured by observed OTUs and phylogenetic richness (total n = 39).
Pearson correlation analysis revealed that 4 families, Bacillaceae, Streptococcaceae, Dehalobacteriaceae, and Peptostreptococcaceae, strongly positively correlated with the area under the curve during ITT (ITT AUC), and 1 family, Rikenellaceae, negatively correlated with ITT AUC at W3 (Fig. 5C). These findings suggest that these altered bacterial families may disrupt insulin sensitivity in offspring at a young age. In addition, we also found Streptococcaceae and Turicibacteraceae correlated with GTT AUC at W10. Interestingly, we found that GM richness strongly and negatively correlated with ITT AUC (Figs. 5F and 5G) but did not correlate with GTT AUC (Figs. 5D and 5E), suggesting a critical role of GM richness in insulin sensitivity. Overall, these data demonstrate that the alteration in both composition and richness of GM was associated with insulin sensitivity. Moreover, GM reconstitution by co-housing successfully reversed insulin insensitivity in MHFD offspring, most likely by increasing GM richness.
Discussion
Treatment of precocious puberty is expensive and can be distressing for children. Currently, the primary agent to treat central precocious puberty is leuprolide acetate, a gonadotropin-releasing hormone analog, which must be administered via intramuscular injection every 4 weeks (44). Identifying a novel form of treatment for this condition would have a substantial clinical impact. The current study shows MHFD during lactation in dams substantially influenced metabolic and reproductive development in offspring. MHFD during lactation also altered the offspring’s GM composition and significantly decreased GM richness, promoting obesity, adiposity, and early onset of puberty. Moreover, we found co-housing MHFD with NCD offspring increased GM richness, reversed early puberty, and improved insulin sensitivity in MHFD-co offspring. These results raise the possibility that reshaping GM may offer a novel approach to management of early pubertal onset in obese and/or insulin resistant patients.
While childhood obesity results in an earlier age of onset of puberty in girls (10, 45), the mechanism involved is still unclear. Excess adiposity, a major contributor of obesity, is usually accompanied by insulin insensitivity and compensatory hyperinsulinemia (10). The subsequent reduction of hepatic sex hormone binding globulin may lead to increased bioavailable sex steroids and pubertal development (46). The importance of this pathway is supported by our finding that MHFD offspring showed increased adiposity and hyperinsulinemia at W3 and then early puberty. MHFD has been found led to precocious puberty in offspring in animal studies (47, 48), but little is known about the role of the GM in pubertal development. Recent studies showed that MHFD in dams had sex-specific effects on the offspring (49–51). Female offspring were more susceptible to metabolic abnormalities than male offspring in response to MHFD (49, 52). This is supported by our observation that MHFD male offspring did not showed advanced puberty. In humans, obesity is associated with early onset of puberty in girls, but the evidence for early puberty is inconsistent in boys (12).
Maternal obesity with altered GM increases the risk for childhood obesity (22). Mother-to-infant microbiota transmission can be influenced by prenatal factors (eg, placenta, maternal body weight), neonatal factors (eg, birth mode), and postnatal factors (eg, feeding type) (27, 53, 54). Applying an intervention during the lactation phase has potential because a recent human study showed that breastfeeding is the most significant factor influencing infant early life (months 3–14) GM structure (27). We observed that MHFD during lactation significantly altered offspring GM composition at W3 and significantly decreased GM richness at W10. These findings emphasize the importance of the lactation phase for normal GM development in offspring.
Breast milk provides essential nutrients and long-term beneficial effects for infants (55, 56). Given the fact that the breast milk microbiota represents an important factor contributing to the development of infant GM (57, 58), further studies are need to reveal the mechanism of how MHFD can influence breast milk microbiota and the transmission from mother to infant. Transmission of mother’s vaginal microbiota to the infant through vaginal delivery is another factor influencing infant GM. However, this route was not likely the cause of differences in GM between NCD and MHFD offspring in our study, since the MHFD intervention was initiated after delivery of the pups.
Previous animal studies showed that MHFD during lactation led to adiposity in offspring (59, 60). Consistent with these results, we found metabolic abnormalities induced by MHFD were linked to GM composition of offspring in our study. For example, the relative abundance of the family Streptococcaceae and Peptostreptococcaceae was increased in MHFD offspring and strongly correlated with adiposity. The relative abundance of Streptococcaceae had previously been found to be increased in the gut of mice fed with HFD (61, 62). Recent work also showed that the family Peptostreptococcaceae was increased in the colon of murine offspring from HFD fed dams (63). In addition, an intervention of tea reduced Peptostreptococcaceae and HFD induced obese in mice (64). Gut microbial dysbiosis associated inflammation likely contributed to the phenotypes we observed in offspring. Indeed, a recent study showed that MHFD altered GM composition in offspring, leading to an increased risk for early intestinal injury from lipopolysaccharide and platelet-activating factor exposure (65). Overall, these lives of evidence clearly demonstrate that MHFD not only alters GM composition but also increases the risk of metabolic abnormalities in offspring.
Emerging evidence shows that the GM, sex hormone levels, and reproductive diseases such as PCOS are interrelated (66, 67). In our study, we found that MHFD offspring showed lower GM richness compared to NCD offspring. Interestingly, co-housing MHFD with NCD offspring rescued the normal onset of puberty and increased GM richness. We did not see changes in estradiol levels between MHFD and NCD offspring at W3 and W10 (data not shown). Insulin sensitivity might be one possible mechanism linking low GM richness and early onset of puberty (12). Indeed, our previous study showed that insulin sensing in kisspeptin neurons is critical for pubertal timing (68). Several studies demonstrated that lower GM diversity is associated with insulin resistance (69, 70). In accord with those findings, we found that insulin sensitivity correlated with GM richness in MHFD offspring at W10. It is worth noting that increasing of GM richness via co-housing MHFD with NCD offspring improved insulin sensitivity and pubertal timing, but not obesity. Thus, insulin insensitivity, not obesity, contributed to early puberty through the modulation of GM.
In summary, our study demonstrates that lactation provides a critical window for the development of normal metabolic and reproductive function in offspring. MHFD during lactation influenced the maturation of offspring GM. MHFD during lactation also caused obesity and early puberty associated with altered GM. Co-housing MHFD with NCD offspring reversed early puberty and insulin insensitivity in MHFD-co offspring by increasing GM richness. Our results imply that GM-associated insulin resistance promoted MHFD-induced early puberty. Thus, the GM may provide novel therapeutic targets to treat metabolic and reproductive diseases.
Acknowledgments
We thank Alexandria Monet White for assistance with performing the experiments.
Financial Support: This study was supported by grant number P30DK020572 (MDRC) from the National Institute of Diabetes and Digestive and Kidney Diseases in the form of a Pilot/Feasibility award to JWH.
Author contributions: MW and JWH conceived and designed the project. MW and D.M. performed the experiments and collected samples. MW, YZ, XC, JY, and JWH analyzed and interpreted the data. MW drafted manuscript, and JWH made edits. All authors commented on the manuscript and approved the final version of manuscript.
Additional Information
Disclosure Summary: The authors have declared that no conflict of interest exists.
References
- 1. Carel JC, Lahlou N, Roger M, Chaussain JL. Precocious puberty and statural growth. Hum Reprod Update. 2004;10(2):135–147. [DOI] [PubMed] [Google Scholar]
- 2. Kim EY, Lee MI. Psychosocial aspects in girls with idiopathic precocious puberty. Psychiatry Investig. 2012;9(1):25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elks CE, Ong KK, Scott RA, et al. ; InterAct Consortium. Age at menarche and type 2 diabetes risk: the EPIC-InterAct study. Diabetes Care. 2013;36(11):3526–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prentice P, Viner RM. Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int J Obes (Lond). 2013;37(8):1036–1043. [DOI] [PubMed] [Google Scholar]
- 5. Bodicoat DH, Schoemaker MJ, Jones ME, et al. Timing of pubertal stages and breast cancer risk: the Breakthrough Generations Study. Breast Cancer Res. 2014;16(1):R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charalampopoulos D, McLoughlin A, Elks CE, Ong KK. Age at menarche and risks of all-cause and cardiovascular death: a systematic review and meta-analysis. Am J Epidemiol. 2014;180(1):29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soriano-Guillén L, Corripio R, Labarta JI, et al. Central precocious puberty in children living in Spain: incidence, prevalence, and influence of adoption and immigration. J Clin Endocrinol Metab. 2010;95(9):4305–4313. [DOI] [PubMed] [Google Scholar]
- 8. Teilmann G, Pedersen CB, Jensen TK, Skakkebaek NE, Juul A. Prevalence and incidence of precocious pubertal development in Denmark: an epidemiologic study based on national registries. Pediatrics. 2005;116(6):1323–1328. [DOI] [PubMed] [Google Scholar]
- 9. Partsch CJ, Sippell WG. Pathogenesis and epidemiology of precocious puberty. Effects of exogenous oestrogens. Hum Reprod Update. 2001;7(3):292–302. [DOI] [PubMed] [Google Scholar]
- 10. Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140(3):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li W, Liu Q, Deng X, Chen Y, Liu S, Story M. Association between obesity and puberty timing: a systematic review and meta-analysis. Int J Environ Res Public Health. 2017;14(10): E1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reinehr T, Roth CL. Is there a causal relationship between obesity and puberty? Lancet Child Adolesc Health. 2019;3(1):44–54. [DOI] [PubMed] [Google Scholar]
- 13. DiVall SA, Herrera D, Sklar B, et al. Insulin receptor signaling in the GnRH neuron plays a role in the abnormal GnRH pulsatility of obese female mice. PLoS ONE. 2015;10(3):e0119995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bartha JL, Marín-Segura P, González-González NL, Wagner F, Aguilar-Diosdado M, Hervias-Vivancos B. Ultrasound evaluation of visceral fat and metabolic risk factors during early pregnancy. Obesity (Silver Spring). 2007;15(9):2233–2239. [DOI] [PubMed] [Google Scholar]
- 15. Cnattingius S, Villamor E, Lagerros YT, Wikström AK, Granath F. High birth weight and obesity: a vicious circle across generations. Int J Obes (Lond). 2012;36(10):1320–1324. [DOI] [PubMed] [Google Scholar]
- 16. Lawn RB, Lawlor DA, Fraser A. Associations between maternal prepregnancy body mass index and gestational weight gain and daughter’s age at menarche: the avon longitudinal study of parents and children. Am J Epidemiol. 2018;187(4):677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deardorff J, Berry-Millett R, Rehkopf D, Luecke E, Lahiff M, Abrams B. Maternal pre-pregnancy BMI, gestational weight gain, and age at menarche in daughters. Matern Child Health J. 2013;17(8):1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baothman OA, Zamzami MA, Taher I, Abubaker J, Abu-Farha M. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids Health Dis. 2016;15:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cornejo-Pareja I, Muñoz-Garach A, Clemente-Postigo M, Tinahones FJ. Importance of gut microbiota in obesity. Eur J Clin Nutr. 2019;72(Suppl 1):26–37. [DOI] [PubMed] [Google Scholar]
- 20. Murugesan S, Nirmalkar K, Hoyo-Vadillo C, García-Espitia M, Ramírez-Sánchez D, García-Mena J. Gut microbiome production of short-chain fatty acids and obesity in children. Eur J Clin Microbiol Infect Dis. 2018;37(4):621–625. [DOI] [PubMed] [Google Scholar]
- 21. White ND. Gut Microbiota and obesity: potential therapeutic targets and probiotic treatment. Am J Lifestyle Med. 2016;10(2):104–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galley JD, Bailey M, Kamp Dush C, Schoppe-Sullivan S, Christian LM. Maternal obesity is associated with alterations in the gut microbiome in toddlers. PLoS ONE. 2014;9(11):e113026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma J, Prince AL, Bader D, et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun. 2014;5:3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin R, Makino H, Cetinyurek Yavuz A, et al. Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS ONE. 2016;11(6):e0158498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tanaka M, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int. 2017;66(4):515–522. [DOI] [PubMed] [Google Scholar]
- 26. Yasmin F, Tun HM, Konya TB, et al. ; CHILD Study Investigators . Cesarean section, formula feeding, and infant antibiotic exposure: separate and combined impacts on gut microbial changes in later infancy. Front Pediatr. 2017;5:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stewart CJ, Ajami NJ, O’Brien JL, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562(7728):583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mermer H, Güven M, Yılmaz MS, Karabay O, Kaymaz R, Yeniay M. [Postoperative use of antibiotics in septoplasty cases: is it really necessary?]. Kulak Burun Bogaz Ihtis Derg. 2014;24(1):17–20. [DOI] [PubMed] [Google Scholar]
- 29. Rodríguez JM, Murphy K, Stanton C, et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. 2015;26:26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee YY, Hassan SA, Ismail IH, et al. Gut microbiota in early life and its influence on health and disease: a position paper by the Malaysian Working Group on Gastrointestinal Health. J Paediatr Child Health. 2017;53(12):1152–1158. [DOI] [PubMed] [Google Scholar]
- 31. Flores R, Shi J, Fuhrman B, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang G, Xu J, Lefever DE, Glenn TC, Nagy T, Guo TL. Genistein prevention of hyperglycemia and improvement of glucose tolerance in adult non-obese diabetic mice are associated with alterations of gut microbiome and immune homeostasis. Toxicol Appl Pharmacol. 2017;332:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Torres PJ, Ho BS, Arroyo P, et al. Exposure to a healthy gut microbiome protects against reproductive and metabolic dysregulation in a PCOS mouse model. Endocrinology. 2019;160(5):1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hill JW, Xu Y, Preitner F, et al. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology. 2009;150(11):4874–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garcia-Galiano D, Borges BC, Donato J Jr, et al. PI3Kalpha inactivation in leptin receptor cells increases leptin sensitivity but disrupts growth and reproduction. JCI Insight. 2017;2(23):96728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Torsoni MA, Borges BC, Cote JL, et al. AMPKα2 in Kiss1 neurons is required for reproductive adaptations to acute metabolic challenges in adult female mice. Endocrinology. 2016;157(12):4803–4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17(2):298–303. [DOI] [PubMed] [Google Scholar]
- 39. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. [DOI] [PubMed] [Google Scholar]
- 40. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72(7):5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang M, Hill J. W. Supporting information_endocrinology.docx. figshare. Journal contribution. Deposited 17 December 2019. https://doi.org/10.6084/m9.figshare.11374605.v1.
- 44. Carel JC, Eugster EA, Rogol A, et al. ; ESPE-LWPES GnRH Analogs Consensus Conference Group . Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009;123(4):e752–e762. [DOI] [PubMed] [Google Scholar]
- 45. Bau AM, Ernert A, Schenk L, et al. Is there a further acceleration in the age at onset of menarche? A cross-sectional study in 1840 school children focusing on age and bodyweight at the onset of menarche. Eur J Endocrinol. 2009;160(1):107–113. [DOI] [PubMed] [Google Scholar]
- 46. Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20(4):535–582. [DOI] [PubMed] [Google Scholar]
- 47. Takumi K, Shimada K, Iijima N, Ozawa H. Maternal high-fat diet during lactation increases Kiss1 mRNA expression in the arcuate nucleus at weaning and advances puberty onset in female rats. Neurosci Res. 2015;100:21–28. [DOI] [PubMed] [Google Scholar]
- 48. Ullah R, Su Y, Shen Y, et al. Postnatal feeding with high-fat diet induces obesity and precocious puberty in C57BL/6J mouse pups: a novel model of obesity and puberty. Front Med. 2017;11(2):266–276. [DOI] [PubMed] [Google Scholar]
- 49. Dudele A, Hougaard KS, Kjølby M, et al. Chronic maternal inflammation or high-fat-feeding programs offspring obesity in a sex-dependent manner. Int J Obes (Lond). 2017;41(9):1420–1426. [DOI] [PubMed] [Google Scholar]
- 50. McKee SE, Zhang S, Chen L, Rabinowitz JD, Reyes TM. Perinatal high fat diet and early life methyl donor supplementation alter one carbon metabolism and DNA methylation in the brain. J Neurochem. 2018;145(5):362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou L, Xiao X, Zhang Q, et al. Improved glucose and lipid metabolism in the early life of female offspring by maternal dietary genistein is associated with alterations in the gut microbiota. Front Endocrinol (Lausanne). 2018;9:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ribaroff GA, Wastnedge E, Drake AJ, Sharpe RM, Chambers TJG. Animal models of maternal high fat diet exposure and effects on metabolism in offspring: a meta-regression analysis. Obes Rev. 2017;18(6):673–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aagaard KM. Author response to comment on “the placenta harbors a unique microbiome.” Sci Transl Med. 2014;6(254):254lr3. [DOI] [PubMed] [Google Scholar]
- 54. Wang S, Ryan CA, Boyaval P, Dempsey EM, Ross RP, Stanton C. Maternal vertical transmission affecting early-life microbiota development. Trends Microbiol. 2020;28(1):28–45. [DOI] [PubMed] [Google Scholar]
- 55. Miliku K, Duan QL, Moraes TJ, et al. Human milk fatty acid composition is associated with dietary, genetic, sociodemographic, and environmental factors in the CHILD Cohort Study. Am J Clin Nutr. 2019;110(6):1370–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Le Huërou-Luron I, Blat S, Boudry G. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev. 2010;23(1):23–36. [DOI] [PubMed] [Google Scholar]
- 57. Warren MF, Hallowell HA, Higgins KV, Liles MR, Hood WR. Maternal dietary protein intake influences milk and offspring gut microbial diversity in a rat (Rattus norvegicus) model. Nutrients. 2019;11(9):E2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moossavi S, Sepehri S, Robertson B, et al. Composition and variation of the human milk microbiota are influenced by maternal and early-life factors. Cell Host Microbe. 2019;25(2):324–335.e4. [DOI] [PubMed] [Google Scholar]
- 59. Tsuduki T, Kitano Y, Honma T, Kijima R, Ikeda I. High dietary fat intake during lactation promotes development of diet-induced obesity in male offspring of mice. J Nutr Sci Vitaminol (Tokyo). 2013;59(5):384–392. [DOI] [PubMed] [Google Scholar]
- 60. Hafner H, Chang E, Carlson Z, et al. Lactational high-fat diet exposure programs metabolic inflammation and bone marrow adiposity in male offspring. Nutrients. 2019;11(6):E1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zeng H, Ishaq SL, Zhao FQ, Wright AG. Colonic inflammation accompanies an increase of β-catenin signaling and Lachnospiraceae/Streptococcaceae bacteria in the hind gut of high-fat diet-fed mice. J Nutr Biochem. 2016;35:30–36. [DOI] [PubMed] [Google Scholar]
- 62. Chiu CC, Ching YH, Li YP, et al. Nonalcoholic fatty liver disease is exacerbated in high-fat diet-fed gnotobiotic mice by colonization with the gut microbiota from patients with nonalcoholic steatohepatitis. Nutrients. 2017;9(11):E1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sanguinetti E, Guzzardi MA, Tripodi M, et al. Microbiota signatures relating to reduced memory and exploratory behaviour in the offspring of overweight mothers in a murine model. Sci Rep. 2019;9(1):12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu D, Huang J, Luo Y, et al. Fuzhuan brick tea attenuates high-fat diet-induced obesity and associated metabolic disorders by shaping gut microbiota. J Agric Food Chem. 2019;67(49):13589–13604. [DOI] [PubMed] [Google Scholar]
- 65. Babu ST, Niu X, Raetz M, Savani RC, Hooper LV, Mirpuri J. Maternal high-fat diet results in microbiota-dependent expansion of ILC3s in mice offspring. JCI Insight. 2018;3(19):99223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Charalampakis V, Tahrani AA, Helmy A, Gupta JK, Singhal R. Polycystic ovary syndrome and endometrial hyperplasia: an overview of the role of bariatric surgery in female fertility. Eur J Obstet Gynecol Reprod Biol. 2016;207:220–226. [DOI] [PubMed] [Google Scholar]
- 67. Lindheim L, Bashir M, Münzker J, et al. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with Polycystic Ovary Syndrome (PCOS): a pilot study. PLoS ONE. 2017;12(1):e0168390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Qiu X, Dao H, Wang M, et al. Insulin and leptin signaling interact in the mouse Kiss1 neuron during the peripubertal period. PLoS ONE. 2015;10(5):e0121974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Le Chatelier E, Nielsen T, Qin J, et al. ; MetaHIT consortium . Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. [DOI] [PubMed] [Google Scholar]
- 70. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]