Abstract
Until recently, the progress in the diagnosis and management of cancer has not been matched by similar progress in the assessment of the increasing numbers of older and more complex patients with cancer. Dr. Arti Hurria identified this gap at the outset of her career, which she dedicated toward studying the geriatric assessment (GA) as an improvement over traditional methods used in oncology to assess vulnerability in older patients with cancer. This review documents the progress of the GA and its integration into oncology. First, we detail the GA’s origins in the field of geriatrics. Next, we chronicle the early rise of geriatric oncology, highlighting the calls of early thought-leaders to meet the demands of the rapidly aging cancer population. We describe Dr. Hurria’s early efforts toward meeting these calls though the implementation of the GA in oncology research. We then summarize some of the seminal studies constituting the evidence base supporting GA’s implementation. Finally, we lay out the evolution of cancer-focused guidelines recommending the GA, concluding with future needs to advance the next steps toward more widespread implementation in routine cancer care. Throughout, we describe Dr. Hurria’s vision and its execution in driving progress of the GA in oncology, from her fellowship training to her co-authored guidelines recommending GA for all older adults with cancer—published in the year of her untimely death.
Keywords: Geriatric Assessment, Arti Hurria, Geriatric Oncology, Supportive Care, Vulnerability, Risk Stratification
Introduction
The American Society of Clinical Oncology (ASCO) now recommends a geriatric assessment (GA) for all older adults with cancer.1 The senior author of these guidelines, Dr. Arti Hurria, devoted her career to investigating and implementing the GA in oncology practice. This devotion stemmed from her passion to improve the care of her older patients with cancer. As a National Institute on Aging (NIA) Beeson Scholar and Board Member of ASCO, Dr. Hurria achieved the highest U.S. professional recognitions in both geriatrics and oncology while bridging the two fields. Although her career was tragically cut short with her death in 2018, she led many of the advancements in the care of older adults with cancer. This narrative review documents the progress of the GA in oncology, emphasizing how Dr. Hurria’s light illuminated the world of geriatric oncology.
History of GA in Geriatrics and its need in Oncology
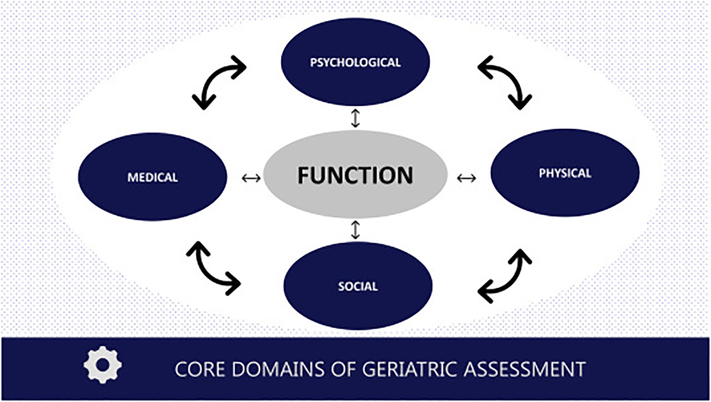
Before discussing the introduction and growth of the GA in oncology and Dr. Hurria’s contributions toward this end, one must ask the question: what is the GA and where did it come from? The GA has its origins in the field of gerontology and geriatrics, which focuses on the science of aging and clinical care of older adults. The history of the field is chronicled elsewhere,2 but its birth and maturation were driven by the need to consider the physiologic changes of aging and how they affect and are affected by the chronic conditions that accumulate in late life. Such an approach requires a more holistic view to health and care rather than a narrow focus on a single disease. Instead of viewing single diagnoses such as ischemic heart disease or osteoarthritis, geriatrics defines broad domains important in older adult health: physical health (including multimorbidity, mobility, nutrition, senses), psychological health (including cognition and mood), social well-being, and functional status (Figure 1).3
Figure 1:
Core geriatric assessment (GA) domains and their interdependency. Figure based on seminal papers of GA and Dr. Hurria’s early works as cited in this review. See American Society of Clinical Oncology guideline for examples of validated tools to measure each of these domains.
Formal evaluation of these domains is the essence of the GA, with the goal of optimizing the health, well-being, and functioning of older adults.4 The domains are interdependent. An older adult with cognitive impairment can continue to live in their home if they have a strong social support network; however, if socially isolated, they will be at high risk for institutionalization (the importance of cognition and social support is highlighted in two separate reviews published in the same Special Issue that includes our review).5,6 An older patient with multiple chronic conditions can stave off frailty by remaining physically active, but if one condition compromises their mobility, then a progression of frailty might ensue.7 Therefore, the more comprehensive the assessment of these domains, the better; indeed, there is debate about what differentiates a “comprehensive GA” from a “GA”.8,9 For the purposes of this review, we will use the more encompassing definition of GA that includes all age-related assessments centered on a domain-based rather than a disease-based approach.
The evidence base underlying the GA began to grow rapidly in the 1970s-80s with some of the first randomized clinical trials (RCT) evaluating clinical models of care for older adults that were becoming more widespread in the US and Europe.3 One of the first meta-analyses evaluating the GA was published in 1993, combining data from 28 RCTS across different GA-guided care models in inpatient and outpatient settings.10 Many other studies followed as the implementation of the GA spread, finding improvements in a range of outcomes—including better detection of age-related conditions, improved functional status, decreased rates of hospitalization, increased rates of living at home during follow-up, and decreased mortality.11–17 Although the effect size estimates vary across the heterogeneity of study designs, GA-guided care processes have been shown to be most effective when: (1) conducted with longitudinal follow-up; (2) paired with interventions aimed at modifying the deficits uncovered (versus assessment alone); and (3) analyzed with sensitive outcome measures.3,10,18 Ultimately, GA became the core of geriatric medicine, and in multiple iterations has been recommended by leading medicine and aging-focused societies to guide the care of older adults.19,20
While the GA was being studied and further implemented across care settings, cancer diagnostics and treatments rapidly expanded in the 1980s and 1990s.21,22 Enhanced imaging technology and innovation in laboratory and molecular analyses allowed for earlier and more accurate diagnosis and staging. Novel treatments in surgery, medical, and radiation therapies provided more effective elimination of malignant cells with greater preservation of normal tissue.23,24 However, this progress in the management of cancer was not matched by similar progress in the assessment of the patient with the cancer—of particular concern given that patients with cancer were growing older, frailer, and more complex.25 Closing this gap became the focus of Dr. Hurria’s career as she graduated from geriatrics fellowship at Harvard and entered training in oncology at Memorial Sloan-Kettering in 1999. She recognized a need to expand the disease-based model of oncology to incorporate the domain-based model of geriatrics. It was this need that drove her central question, posed to her mentor Harvey Cohen, which fueled the forthcoming years of research and clinical innovation: “Could we implement the GA in oncology for all older adults with cancer?”
Early Stages of GA in Oncology
Dr. Hurria often quoted the African proverb: “If you want to go fast, go alone, but if you want to go far, go together.” She knew that to make a meaningful difference in the lives of older adults with cancer, she needed to build a collaborative community—to create an environment where all, with her vision and leadership, could flourish.
The Rise of Geriatric Oncology
In 1983, Dr. Rosemary Yancik organized a symposium on the aging cancer population sponsored by the National Cancer Institute (NCI) and NIA entitled “Perspectives on Prevention and Treatment of Cancer in the Elderly”.26–28 This conference called for increasing the knowledge base in geriatric oncology, and set the tone for further discussion and research in this emerging area. Shortly thereafter, in his 1988 ASCO Presidential Address, Dr. B. J. Kennedy—one of the founders of geriatric oncology—encouraged medical oncologists to better understand and study aging and cancer.29,30
In the subsequent years, ASCO played a pivotal role in promoting the field of geriatric oncology. ASCO sponsored a variety of educational sessions, presentations and publications.29,31 Leveraging these efforts in 1992, Dr. Lodovico Balducci published the first textbook in the field, Geriatric Oncology, outlining the unique aspects of cancer and aging.31,32 In 1995, the Cancer in the Elderly Working group formed within the Cancer and Leukemia Group B to spearhead the design of clinical studies focused on older adults.33 In 1997, the John A. Hartford Foundation sponsored a retreat in the United States to bring leaders from geriatrics and oncology together and set the agenda for practice, research, and training initiatives to address the gap in assessment and management of older adults with cancer.34 By 2000, an organization committed to fostering the development of geriatric oncology was founded in the International Society of Geriatric Oncology (SIOG), and has since established task forces to evaluate the geriatric oncology literature and make treatment recommendations.35
The Cancer and Aging Research Group
From 2001–2006, the Hartford Foundation partnered with ASCO to create joint training programs in geriatrics and oncology. Twenty-eight geriatric oncology trainees participated in the combined fellowship program at 10 institutions.30 This dual training provided trainees with the skills to design and develop research in the field of geriatric oncology. However, these investigators were geographically dispersed and often alone at their institutions in focusing on cancer and aging issues. These challenges limited their ability to successfully compete for grant funding and to develop and accrue patients for clinical trials.36
To address these needs, Dr. Hurria, with a grant from the John A. Hartford Foundation, hosted a meeting that aimed to bring together junior investigators in geriatric oncology with a senior mentoring team. This seminal meeting in April 2007 led to the formation of the Cancer and Aging Research Group (CARG, mycarg.org),36 whose mission was two-fold: (1) to join geriatric oncology researchers across institutions in a collaborative effort to design and implement clinical trials in older adults, and (2) to promote the development and mentorship of geriatric oncologists.
In collaboration with her long-standing colleagues and friends, Drs. William Dale (City of Hope) and Supriya Mohile (University of Rochester), Dr. Hurria led a five-year U13 cooperative conference grant “Geriatric Oncology Research to Improve Clinical Care” http://www.mycarg.org/carg_grants. The goal of this conference series was to provide a forum for a multidisciplinary team of investigators in geriatrics and oncology to review the current evidence in geriatric oncology, identify high priority research areas, and develop research approaches that, within the next 10 years, would improve clinical care for older adults with cancer.37 These meetings proved to be effective in defining the research agenda for the field and providing impactful mentorship to junior investigators.37–40 For instance, the last conference yielded 9 manuscripts each lead-authored by a junior investigator mentored by a senior investigator-author in the group.41–50 It was through CARG, her grants, and the U13 conferences that Dr. Hurria developed tools for feasible implementation of the GA in oncology practice.
Development of a GA for Older Patients with Cancer
Dr. Hurria knew that major barriers preventing the implementation of GA in oncology practice were limitations in time and geriatrics expertise. Some experts have suggested the use of a short screening tool to identify vulnerable patients for whom a more comprehensive GA could potentially optimize their cancer treatment.51,52 Multiple approaches have been made to develop a ‘simplified GA’, including the abbreviated CGA,53 the Vulnerable Elders Survey-13 (VES-13),54–57 the French Geriatric-8 (G8),58 and the Flemish version of the Triage Risk Screening Tool (fTRST).59 Although these screening tools require less time and response burden, they do not capture the full range of vulnerability as does a broader GA.60
Dr. Hurria therefore sought to develop a tool she called the cancer-specific GA that maintained a more comprehensive domain-based evaluation while still reducing the time and expertise required for administration. This reduction in administration burden was accomplished by complementing provider-completed items with patient-completed items—together measuring all GA domains including functional status, cognition, comorbidities, medications, psychologic health, social support, and nutritional status. She piloted this cancer-specific GA and demonstrated its feasibility, showing high completion rate without requiring excessive time.61 A subsequent larger prospective study confirmed the cancer-specific GA’s feasibility, demonstrating that the assessment took a median of 22 minutes to complete, with 87% of patients completing their portion without assistance.62 All health care professionals completed their portion.62,63 The evidence supporting the integration of GA into routine oncology practice has strengthened considerably over the past two decades, with multiple studies confirming its feasibility in practice and research.61,62,64,65
Building the Evidence Base to Support GA in Oncology
With the foundations of the GA in oncology now laid, Dr. Hurria faced several questions regarding the widespread integration of GA into oncology practice. Does the enhanced assessment add value to traditional performance status scales (e.g., Eastern Cooperative Oncology Group [ECOG] and Karnofsky Performance Status [KPS])? Does the GA predict important outcomes alongside established oncology prognostic factors? What other benefits could the GA provide to oncologists and their patients? To address these questions, Dr. Hurria led efforts to build the evidence base supporting implementation of the GA.
GA Detects Age-Related Vulnerabilities
GA has been found to add substantial information to the standard oncological assessment. By its very nature, GA evaluates domains important in older adult health—such as cognition and function—going beyond traditional performance status scales used in oncology that focus on disease-based activity limitations.66 For example, in patients considered to have a good ECOG-PS of 0–1, 38% required assistance with instrumental activities of daily living (IADL).67 In addition, GA detects many vulnerabilities that are frequently under-recognised or inadequately addressed in older adults with cancer. In a systematic review of 73 studies, GA detected multiple age-related vulnerabilities in a large portion of patients including falls (18–28%, range dependent on study population and setting), unintentional weight loss (34–48%), cognitive impairment (8–50%), and depressive symptoms (8–47%).68 Dr. Hurria knew that the diagnostic value of GA—proven in the general geriatrics literature—would extend to older adults with cancer.
GA Improves Prediction of Outcomes
The GA and its domains have been associated with important clinical outcomes, including mortality, treatment-related complications, treatment completion, hospitalization, and admission to long-term care.69–78 These associations have been found in older patients with both solid and hematologic malignancies, including patients undergoing sugery.79–83 Multiple studies have demonstrated the superiority of GA in identifying vulnerable older patients at risk of adverse outcomes in comparison with clinical judgement or traditional tools used in oncology. In a study of over 250 patients, a modified GA was able to distinguish vulnerable older patients from fit patients without geriatric deficits better than oncologist clinical judgement alone; this GA was independently predictive of survival.84 At least four systematic reviews have shown the prognostic value of specific GA domains for overall survival, especially physical function and nutritional status.64,71,73,85,86 Other studies have demonstrated that the addition of GA domains alongside traditional disease-based prognostic variables improves the ability to discriminate risk of death.87–91
The integration of GA in predicting chemotherapy toxicity has been one of Dr. Hurria’s pivotal achievements in building support for the GA. In a prospective CARG study of over 500 patients participating across seven institutions, she led the development of a chemotherapy toxicity tool based on key demographic, disease, and GA assessment domains (function, comorbidity, cognition, psychological state, social activity/support and nutritional status). This tool was derived from a predictive model that demonstrated good calibration (Hosmer-Lemeshow test, p=0.85) and discrimination (area under the curve [AUC]=0.72) for predicting grade 3–5 chemotherapy toxicity (as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events), performing better than a separate model containing physician-rated KPS (AUC=0.53).70 The results from this study were identified by ASCO as one of the Clinical Cancer Advances in 2012. Dr. Hurria led CARG in externally validating the toxicity risk score in 2016,92 and several other scores encompassing domains of the GA have also been shown to predict chemotherapy toxicity.93–96 Dr. Hurria additionally advocated for the use of digital technology in implementing the GA, and the CARG chemotherapy toxicity prediction tool is now widely available in multiple languages to clinicians via the internet (http://www.mycarg.org/SelectQuestionnaire).97
The ASCO guideline for geriatric oncology also recommends the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH), and other predictive tools exist.1 No consensus exists regarding specific cutoffs of these risk scores to direct changes in therapy, or regarding the optimum number and type of GA domains that should be included in a risk score for chemotherapy toxicity or other outcomes important in older adults with cancer. However, validated risk calculators supported by guidelines generally contain items representing multiple GA domains (in contrast to items limited to one GA domain), with measures of comorbidity, physical function/mobility, and functional status demonstrating consistent inclusion.1,98
GA Leads to Changes in Management
Age-associated vulnerabilities identified by GA can direct practical interventions to optimize outcomes for older adults with cancer. In a recently updated systemic review, GA resulted in recommendations for non-oncologic interventions in 72% of patients (range 26–100%), with the most common interventions directed towards social issues (39%), nutritional status (32%), polypharmacy (31%), mobility (20%), comorbidity (19%), psychological issues (19%) and cognitive deficits (14%).99 The provision of psychosocial support, transportation, and addressing geriatric syndromes may be critical for some patients to receive adequate cancer treatment and avoid toxicity of treatment. Indeed, several studies suggest that patients undergoing GA have a higher rate of cancer treatment completion.96,99,100 Dr. Mohile, Dr. Hurria, and colleagues generated expert-consensus recommendations for GA-guided interventions to address deficits uncovered by the GA.101 These recommendations, reproduced in ASCO’s guideline for geriatric oncology, provide within each GA domain specific follow-up assessment, management, and referral options appropriate for particular deficits (e.g., assessing patient decision-making capacity if a patient screened positive on a validated cognitive screening tool and referring to physical and/or occupational therapy if falls or functional dependency are detected).
There is increasing evidence supporting how the results of a GA in older patients with cancer can change treatment decisions.102–105 In a recently updated systematic review, GA was found to alter the oncological treatment plan in a median of 28% (range 8–54%) of patients, with the majority of changes involving less intensive treatment.99 The percentage of patients receiving a more intensive treatment option varied from 2% to 28%. This demonstrates how a GA can alter treatment in both directions to match treatment intensity with patient vulnerability. In another study, the addition of geriatric domain impairments to age and clinical variables influenced hypothetical treatment decisions made by community oncologists, independently of prescriber characteristics.106
There is also evidence that a GA can facilitate improved communication between oncologists and their older patients. A randomized trial of 544 patients with advanced solid tumors or lymphoma evaluated the effect of providing oncologists with a summary from a GA.107 Provision of this summary led to significantly more discussions concerning age-related problems in comparison to usual care, two times more high-quality doctor-patient conversations, and higher patient satisfaction regarding communication with physicians. Dr. Hurria emphasized that “the best medical decisions are personalized,” and referred to details such as health status, personal values and goals, and health care preferences.
GA Across Disciplines
Dr. Hurria’s vision placed a high value on the multidisciplinary team and acknowledged the critical role that all team members play in optimizing the care for older adults with cancer.108 To achieve the best outcomes, the GA-guided care process relies on a core team consisting of a treating physician (e.g., oncologist, hematologist), a geriatrician, and a nurse, and—when appropriate (and feasible)—draws upon an extended team of social workers, physical and occupational therapists, dieticians, pharmacologists, psychologists, and primary care physicians. Implementation models vary widely by the available resources in each setting. Ideally, they include multiple members of the multidisciplinary team in specialized inpatient/outpatient settings, or specialized inpatient units with care provided by staff with both geriatrics and oncology expertise.109 In locations where access to geriatric oncology specialists is limited, nurse or nurse-practitioner (NP) led-clinics have been adopted as a strategy to enable access to high quality care for older adults with cancer and CGA where it may otherwise be impossible.110–113
Several studies provide international consensus on the importance of GA from the perspective of diverse geriatric oncology experts.114,115 In addition, research shows that nurses are often the most appropriate providers to perform the GA from an organizational, practical, and economic point of view.116 Following the GA, in-depth evaluations are performed by other multidisciplinary team members to develop tailored geriatric interventions for vulnerabilities detected by the GA.117,118 A recent study by Kenis and colleagues showed that the highest rate of adherence was among those referred to dieticians, geriatricians, social workers, physical and occupational therapists, and psychologists.118 Drawing on the expertise of each member of the multidisciplinary team most effectively addresses outstanding health issues across domains and serves to holistically support patients as they live with their cancer diagnosis.
Evolution of Cancer-Focused Guidelines to Incorporate GA
Following its formation in the early 2000s, SIOG published its first guideline on surgical management of older patients with cancer in 2004. Since then, 37 guidelines have been published focusing on different aspects related to the care for older patients with cancer, e.g., specific tumor types, specific treatments, quality of life (QoL).119
SIOG played a pivotal role in developing consensus guidelines to support GA utilization in oncology practice. In 2005, SIOG created a task force to review the early evidence on the use of GA in older patients with cancer, concluding that a GA with follow-up should be used for older adults with cancer given its value beyond historical assessments used in oncology.120 In 2015, with the valuable contributions of Dr. Hurria, an update of this SIOG guideline was released integrating the latest scientific evidence.109 In addition to the SIOG guideline on GA, a guideline was published with recommendations on the incorporation of screening tools to identify patients in need of further evaluation using GA.51 This guideline reviewed the evidence and performance of numerous tools—such as the G8 and the VES-13—suggesting that all tools have merit and that further research is needed to recommend one or more over others.
In parallel to SIOG’s efforts abroad, Dr. Hurria continued to push guideline development in the United States. Dr. Hurria was a member (2008–2018) and chair (2010–2016) of the National Comprehensive Cancer Network (NCCN) Older Adult Oncology Guidelines Panel.121 NCCN delivers several widely-used tumor-specific diagnostic and treatment guidelines, and Dr. Hurria used this avenue to incorporate the latest evidence supporting GA to guide treatment decision-making. Her tireless efforts culminated in the development of ASCO’s guideline for geriatric oncology recommending that all older adults with cancer undergoing chemotherapy receive a GA in place of or in addition to traditional performance status measures. She co-led this expert panel along with Drs. Mohile and Dale in synthesizing the evidence to recommend a minimum dataset of GA domains to help risk stratify vulnerable patients and guide non-oncologic interventions.1
Finally, shortly prior to her passing, Dr. Hurria led a task force at ASCO that is working on developing metrics for quality assessment based on the ASCO guidelines. She contributed significantly to defining the research priorities for advancing the next steps in research involving GA in older adults with cancer (Table 1). Ongoing efforts at ASCO include surveys of oncologists’ awareness and utilization of the GA. Additionally, there are several efforts to guide community oncologists’ implementation of the GA in their practices. Collectively, it was Dr. Hurria’s hope that these efforts and guidelines can inform new health policies to improve care delivery and outcomes of older patients with cancer.
Table 1:
Recommendations for next steps in incorporating GA in research. Dr. Hurria significantly contributed to these recommendations
| ASCO 2015 Workshop | ASCO-FDA Workshop | ASCO 2019 Research Priorities |
|---|---|---|
| NCI, FDA, and other organizations should advocate for collection of GA domains in trials | Design more trials including elements of GA and endpoints important to older adults | Use GA as standard measure of aging physiology to reliably predict treatment side-effects |
| Research and clinical databases should include GA domains in their variables measured | GA should be routinely collected and documented in EHRs | Study the impact of cancer treatment on GA domains, such as cognition and function |
| Database developers should partner with EHR vendors to incorporate GA domains | Study the effects of cancer treatment on older adults with impaired GA domains | |
| Conduct trials studying effects of GA-guided care |
ASCO = American Society of Clinical Oncology; NCI = National Cancer Institute; FDA = Food and Drug Administration; EHR = Electronic Health Record
Next Steps: GA as Essential Element in Precision Oncology
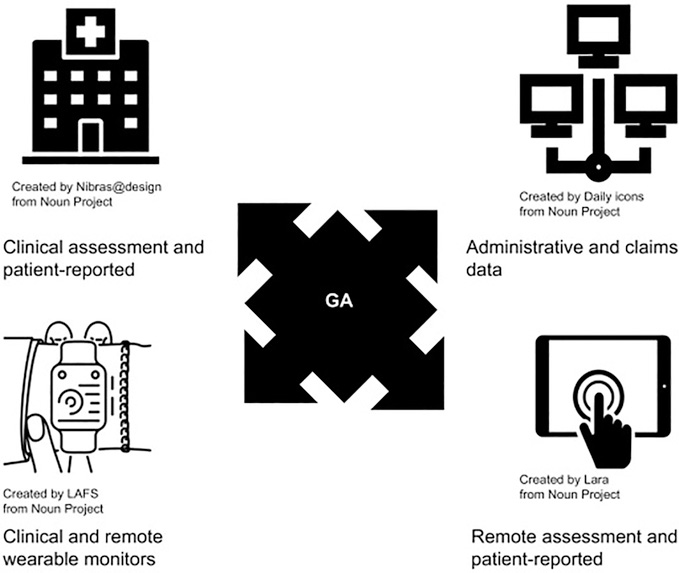
Significant strides have been made in the development and testing of GA with mounting evidence of its promising use in management of patients with cancer, but many gaps remain. Although the GA is now recommended for all older adults with cancer by leading cancer-focused organizations,1,122 in reality few of these assessments are occurring in routine clinical practice. Although Dr. Hurria pioneered translating the GA into more feasible self-assessment methods, this approach is not applicable to every setting. There is great promise in building on her foundation by using digital health technology to efficiently collect GA domains and derive estimates of risk (Figure 2), as well as using screening tools and summary measures based on GA domains.82,123 Data from the geriatric assessment can not only be measured via in-person clinical assessment and patient-reported measures, but can now also be measured using administrative and claims data, clinical and remote wearable monitors, and remote patient-reported assessments. The discussion of how these domains are collected, which tools to use, and what constitutes a full or comprehensive GA should not overshadow the fact all tools share a common foundation in the domains themselves (Table 2). The assessment of functional status, physical and psychological health, and social support were the defining feature that developed the field of geriatrics—borne of the need to expand on the disease-based model to improve care for older adult. This defining feature should continue to drive the innovation and individualization of care in geriatric oncology as Dr. Hurria envisioned.
Figure 2:
GA domains and different modalities of their measurement. Data from the geriatric assessment can not only be measured via in-person clinical assessment and patient-reported measures (top left), but can also be measured using administrative and claims data (top right), clinical and remote wearable monitors (bottom left), and remote patient-reported assessments (bottom right).
Table 2:
Core domains of the geriatric assessment (GA) as the common foundation underlying selected screening, prognostic, and assessment tools recommended for use in geriatric oncology
| Tool | Function | Physical | Psychological | Social |
|---|---|---|---|---|
| Lee Schonberg Index (ePrognosis) | • | • | ||
| VES-13 | • | • | ||
| G8 | • | • | • | |
| CARG Chemotox | • | • | • | |
| CRASH score | • | • | • | |
| DAFI | • | • | • | • |
| Cancer-specific GA | • | • | • | • |
VES-13 = Vulnerable Elder Survey-13; G8 = Geriatric 8 Screening Tool; CARG = Cancer and Aging Research Group Chemotoxicity Risk Calculator; CRASH = Chemotherapy Risk Assessment Scale for High-Age Patients; DAFI = deficit accumulation frailty index.
Moreover, how GA information should be used to modify treatment decisions remains unclear. Corre and colleagues evaluated in a randomized controlled trial the use of GA in determining chemotherapy allocation compared to usual care, and although they did not demonstrate an improvement in the primary outcome (treatment failure-free survival [TFFS]), older adults on the GA arm received less intense chemotherapy, had less toxicity and better QOL, all while maintaining similar overall survival.96,124 Although the trial was reported as negative based on the primary outcome, the secondary outcomes that were positive—decreased toxicity and increased QOL—are often valued by older adults more than surrogate endpoints or even survival.125–127 These results could be interpreted as the GA leading to better individualized care by matching appropriate treatment intensity commensurate with an older patient’s vulnerability (minimizing harms without sacrificing benefit).128 Other ongoing trials with this end in mind are testing whether frail patients determined by a GA receive similar benefit with reduced harms on modified-intensity regimens in comparison with standard full intensity regimens. Demonstrating no difference in survival together with lower toxicity and improved quality of life is being considered a “positive” result for these studies.129,130 Further research into understanding the role of GA in decision-making and additional data on specific sub-populations of older adults regarding important outcomes, such as severe chemotherapy-related toxicity and functional status, is needed in order to make the GA results more useful in clinical care.
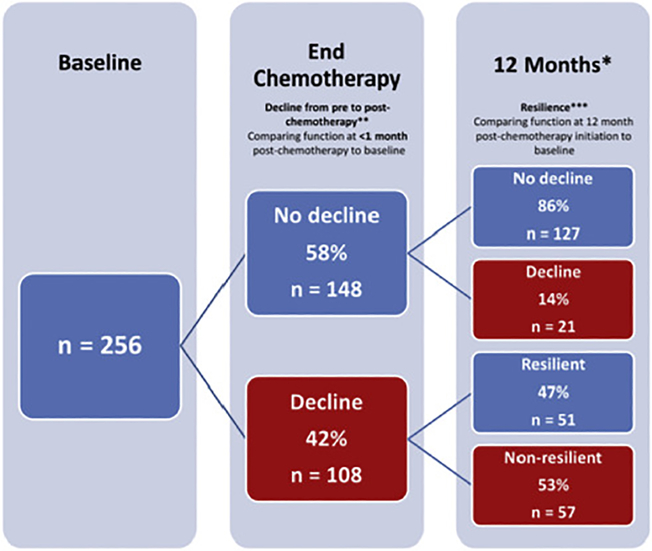
For example, Dr. Hurria’s recent study on refining the CARG chemotherapy toxicity tool for use in older adults with breast cancer undergoing adjuvant chemotherapy were presented at the San Antonio Breast Cancer Conference.68 This breast cancer-specific tool has greatly increased the relevance of the GA in the adjuvant breast cancer setting, and similar work is needed in other cancer populations. Moreover, GA domains should not only be evaluated as predictors, but also as outcomes important in and of themselves. Dr. Hurria was also an early leader in this effort. As an example, she evaluated functional changes in older women with breast cancer treated with adjuvant chemotherapy and found that many experienced a clinically meaningful functional decline post-treatment, with a significant proportion showing resilience and recovery of their function (Figure 3).40,131 Finally, there is a great need to understand how to implement interventions aimed at GA-identified impairments and how these interventions alter outcomes in older adults with cancer.46 Several ongoing studies are examining how GA-guided interventions modify outcomes, including a study led by Dr. Mohile examining whether GA interventions impact chemotherapy-related toxicity (); another was led by Dr. Hurria examining whether GA-guided interventions implemented using a multidisciplinary team reduces toxicity and improves other outcomes ().
Figure 3:
Example of measuring GA domains as outcomes affected by cancer and its treatment. This study by Dr. Hurria and colleagues measured physical function at baseline, end of chemotherapy, and 12 months post-chemotherapy—tracking longitudinal change. Reproduced with permission from John Wiley and Sons, Inc., © 2018, Journal of the American Geriatrics Society, Hurria et al., Functional Decline and Resilience in Older Women Receiving Adjuvant Chemotherapy for Breast Cancer.
*12 months post-chemotherapy initiation
**Decline: ≥10 point decrease in European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC) physical function subscale
***Resilience: Return to within 10 points of pre-chemotherapy EORTC physical function subscale result at the 12 month post-chemotherapy initiation timepoint. Only patients with a decline in physical function from pre- to post-chemotherapy were included in the analysis
Over the last decade, there has been tremendous excitement regarding the potential to personalize oncologic care based on an individual’s tumor characteristics. The concept of “precision medicine” should extend beyond tumor-specific markers to also include “host” factors that are critical in developing a personalized and successful oncologic treatment plan.132 GA can be used as a standardized tool to assess the overall health status of older patients, and practicing oncologists’ need to recognize the importance of “staging the aging” as well as the cancer.133 Dr. Hurria’s mission and dream was that “all older adults with cancer will receive personalized, tailored care, utilizing evidence-based medicine with a multidisciplinary approach.” In order to turn this dream into reality, more infrastructure in the field of cancer and aging research is needed to overcome the barriers to implementation. Dr. Hurria, along with Drs. Mohile and Dale, received funding for a R21/R33 grant from NIA to provide additional infrastructure to the field. This funding, along with the existing Cancer and Aging Research Group (CARG), will provide more support to investigators to execute studies in older adults and provide additional guidance to junior investigators in the field of geriatric oncology. In addition, ASCO has developed an endowed young investigator award in honor of Dr. Arti Hurria to help provide early career support in this important area of research.
Conclusion
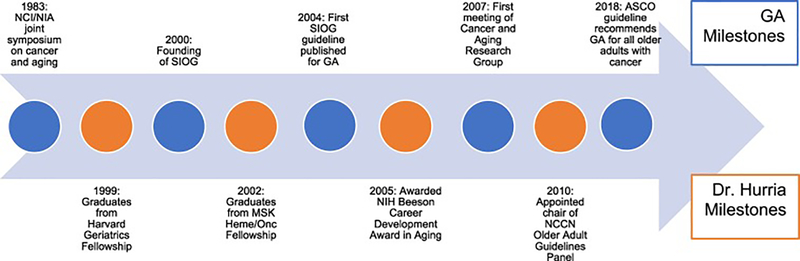
We provide this review as both evidence of the progress GA has made in oncology, as well as evidence of the significant impact that one individual can have in changing a field. Dr. Hurria’s life was cut far too short, but her accomplishments could have been the work of many lifetimes. Figure 4 depicts a timeline portraying selected milestones of the GA in oncology along with those of Dr. Hurria’s career. Her legacy will live on in the lives of her mentors, colleagues, collaborators, and mentees who she touched along the way. Her vision of delivering the GA for older adults with cancer will now be carried forth by this legacy.
Figure 4:
Timeline of selected milestones of GA in oncology and selected milestones of Dr. Hurria’s Career. NCI = National Cancer Institute; NIA = National Institute on Aging; SIOG = International Society of Geriatric Oncology; MSK = Memorial Sloan Kettering; GA = Geriatric Assessment; NIH = National Institutes of Health; NCCN = National Comprehensive Cancer Network
Acknowledgments
Icons used in Figure 2 are from the Noun Project and created by Nibras@design, Daily Icons, LAFS, and Lara.
Rebecca Silliman, MD, PhD and Jane Driver, MD, MPH for their review of the manuscript.
C DuMontier is supported by the Harvard Translational Research in Aging Training Program (National Institute on Aging of the National Institutes of Health: T32AG023480)
WK Soo is supported by a NHMRC Postgraduate Scholarship (APP1074676) and Eastern Health Clinical School Scholarship
G Williams is supported by the National Cancer Institute of the National Institutes of Health (K08CA234225)
KP Loh is supported by the Wilmot Research Fellowship.
W Dale shared with Dr. Hurria the following grants from the National Institutes of Health: U13AG038151 (also shared with Dr. Supriya Mohile); R21AG059206 (also shared with Dr. Supriya Mohile); 5K24AG055693-02; 5R25CA183723 (also shared with Matthew Loscalzo, LCSW, with Dr. Supriya Mohile as faculty member)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol. 2018;36(22):2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morley JE. A brief history of geriatrics. J Gerontol A Biol Sci Med Sci. 2004;59(11):1132–1152. [DOI] [PubMed] [Google Scholar]
- 3.Rubenstein LZ. Joseph T. Freeman award lecture: comprehensive geriatric assessment: from miracle to reality. J Gerontol A Biol Sci Med Sci. 2004;59(5):473–477. [DOI] [PubMed] [Google Scholar]
- 4.Gill T Assessment In: Harper GM, Lyons WL, Potter JF, eds. Geriatrics Review Syllabus: A Core Curriculum in Geriatric Medicine 10th ed. New York: American Geriatrics Society; 2019. [Google Scholar]
- 5.Holwerda TJ, Deeg DJ, Beekman AT, et al. Feelings of loneliness, but not social isolation, predict dementia onset: results from the Amsterdam Study of the Elderly (AMSTEL). J Neurol Neurosurg Psychiatry. 2014;85(2):135–142. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090–2097. [DOI] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 8.Puts MTE, Alibhai SMH. Fighting back against the dilution of the Comprehensive Geriatric Assessment. J Geriatr Oncol. 2018;9(1):3–5. [DOI] [PubMed] [Google Scholar]
- 9.Klepin HD, Wildes TM. Fighting for the integration of geriatric principles into oncology. J Geriatr Oncol. 2018;9(6):705–706. [DOI] [PubMed] [Google Scholar]
- 10.Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993;342(8878):1032–1036. [DOI] [PubMed] [Google Scholar]
- 11.Hendriksen C, Lund E, Stromgard E. Consequences of assessment and intervention among elderly people: a three year randomised controlled trial. Br Med J (Clin Res Ed). 1984;289(6457):1522–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuck AE, Egger M, Hammer A, Minder CE, Beck JC. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287(8):1022–1028. [DOI] [PubMed] [Google Scholar]
- 13.Ellis G, Gardner M, Tsiachristas A, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2017;9:CD006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reuben DB, Frank JC, Hirsch SH, McGuigan KA, Maly RC. A randomized clinical trial of outpatient comprehensive geriatric assessment coupled with an intervention to increase adherence to recommendations. J Am Geriatr Soc. 1999;47(3):269–276. [DOI] [PubMed] [Google Scholar]
- 15.Boult C, Boult LB, Morishita L, Dowd B, Kane RL, Urdangarin CF. A randomized clinical trial of outpatient geriatric evaluation and management. J Am Geriatr Soc. 2001;49(4):351–359. [DOI] [PubMed] [Google Scholar]
- 16.Applegate WB, Miller ST, Graney MJ, Elam JT, Burns R, Akins DE. A randomized, controlled trial of a geriatric assessment unit in a community rehabilitation hospital. N Engl J Med. 1990;322(22):1572–1578. [DOI] [PubMed] [Google Scholar]
- 17.Cohen HJ, Feussner JR, Weinberger M, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med. 2002;346(12):905–912. [DOI] [PubMed] [Google Scholar]
- 18.Wieland D, Ferrucci L. Multidimensional geriatric assessment: back to the future. J Gerontol A Biol Sci Med Sci. 2008;63(3):272–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comprehensive geriatric assessment. AGS Public Policy Committee. J Am Geriatr Soc. 1989;37(5):473–474. [PubMed] [Google Scholar]
- 20.Comprehensive functional assessment for elderly patients. Health and Public Policy Committee, American College of Physicians. Ann Intern Med. 1988;109(1):70–72. [PubMed] [Google Scholar]
- 21.DeVita VT Jr., Chu E A history of cancer chemotherapy. Cancer Res. 2008;68(21):8643–8653. [DOI] [PubMed] [Google Scholar]
- 22.Sokolenko AP, Imyanitov EN. Molecular Diagnostics in Clinical Oncology. Front Mol Biosci. 2018;5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imaging Fass L. and cancer: a review. Mol Oncol. 2008;2(2):115–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urruticoechea A, Alemany R, Balart J, Villanueva A, Vinals F, Capella G. Recent advances in cancer therapy: an overview. Curr Pharm Des. 2010;16(1):3–10. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro CL. Cancer Survivorship. N Engl J Med. 2018;379(25):2438–2450. [DOI] [PubMed] [Google Scholar]
- 26.Yancik R Perspectives on Prevention and Treatment of Cancer in the Elderly. New York: Raven Press; 1983. [Google Scholar]
- 27.Yancik R, Ries LA. Aging and cancer in America. Demographic and epidemiologic perspectives. Hematol Oncol Clin North Am. 2000;14(1):17–23. [DOI] [PubMed] [Google Scholar]
- 28.Yancik R, Ries LA. Cancer in older persons: an international issue in an aging world. Semin Oncol. 2004;31(2):128–136. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy BJ. Aging and cancer. J Clin Oncol. 1988;6(12):1903–1911. [DOI] [PubMed] [Google Scholar]
- 30.Rao AV, Hurria A, Kimmick G, Pinheiro S, Seo PH. Geriatric oncology: past, present, future. J Oncol Pract. 2008;4(4):190–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lichtman SM, Balducci L, Aapro M. Geriatric Oncology: A Field Coming of Age. Journal of Clinical Oncology. 2007;25(14):1821–1823. [DOI] [PubMed] [Google Scholar]
- 32.Balducci L, Lyman GH, Ershler WB. Geriatric Oncology. New York, NY: J.B. Lippincott; 1992. [Google Scholar]
- 33.Cohen HJ, Muss HB. The cancer and leukemia group B cancer in the elderly committee: addressing a major cancer need. Clin Cancer Res. 2006;12(11 Pt 2):3606s–3611s. [DOI] [PubMed] [Google Scholar]
- 34.Calabresi P, Freeman H. Concerns of special populations: cancer and the aging population--a meeting of the President’s Cancer Panel, July 31, 1997. Cancer. 1997;80(7):1258–1260. [DOI] [PubMed] [Google Scholar]
- 35.Lichtman SM, Wildiers H, Chatelut E, et al. International Society of Geriatric Oncology Chemotherapy Taskforce: evaluation of chemotherapy in older patients--an analysis of the medical literature. J Clin Oncol. 2007;25(14):1832–1843. [DOI] [PubMed] [Google Scholar]
- 36.Hurria A, Balducci L, Naeim A, et al. Mentoring junior faculty in geriatric oncology: report from the Cancer and Aging Research Group. J Clin Oncol. 2008;26(19):3125–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arti H, Supriya Gupta M, William D. Research Priorities in Geriatric Oncology: Addressing the Needs of an Aging Population. J Natl Compr Canc Netw. 2012;10(2):286–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dale W, Mohile SG, Eldadah BA, et al. Biological, clinical, and psychosocial correlates at the interface of cancer and aging research. J Natl Cancer Inst. 2012;104(8):581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohile S, Dale W, Hurria A. Geriatric oncology research to improve clinical care. Nat Rev Clin Oncol. 2012;9(10):571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurria A, Dale W, Mooney M, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol. 2014;32(24):2587–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guerard EJ, Nightingale G, Bellizzi K, et al. Survivorship care for older adults with cancer: U13 conference report. Journal of Geriatric Oncology. 2016;7(4):305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nipp RD, Yao N, Lowenstein LM, et al. Pragmatic study designs for older adults with cancer: Report from the U13 conference. Journal of Geriatric Oncology. 2016;7(4):234–241. [DOI] [PubMed] [Google Scholar]
- 43.Kilari D, Soto-Perez-de-Celis E, Mohile SG, et al. Designing exercise clinical trials for older adults with cancer: Recommendations from 2015 Cancer and Aging Research Group NCI U13 Meeting. Journal of Geriatric Oncology. 2016;7(4):293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohile S, Hurria A, Dale W. Introduction to U13 supplement. Journal of Geriatric Oncology. 2016;7(4):223–224. [DOI] [PubMed] [Google Scholar]
- 45.Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magnuson A, Allore H, Cohen HJ, et al. Geriatric assessment with management in cancer care: Current evidence and potential mechanisms for future research. J Geriatr Oncol. 2016;7(4):242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flannery M, Mohile SG, Dale W, et al. Interventions to improve the quality of life and survivorship of older adults with cancer: The funding landscape at NIH, ACS and PCORI. J Geriatr Oncol. 2016;7(4):225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loh KP, Janelsins MC, Mohile SG, et al. Chemotherapy-related cognitive impairment in older patients with cancer. J Geriatr Oncol. 2016;7(4):270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Presley CJ, Dotan E, Soto-Perez-de-Celis E, et al. Gaps in nutritional research among older adults with cancer. J Geriatr Oncol. 2016;7(4):281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karuturi M, Wong ML, Hsu T, et al. Understanding cognition in older patients with cancer. J Geriatr Oncol. 2016;7(4):258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288–300. [DOI] [PubMed] [Google Scholar]
- 52.Valéro S, Migeot V, Bouche G, et al. Who needs a comprehensive geriatric assessment? A French Onco-Geriatric Screening tool (OGS). Journal of Geriatric Oncology. 2011;2(2):130–136. [Google Scholar]
- 53.Overcash JA, Beckstead J, Extermann M, Cobb S. The abbreviated comprehensive geriatric assessment (aCGA): a retrospective analysis. Crit Rev Oncol Hematol. 2005;54(2):129–136. [DOI] [PubMed] [Google Scholar]
- 54.Min LC, Elliott MN, Wenger NS, Saliba D. Higher vulnerable elders survey scores predict death and functional decline in vulnerable older people. J Am Geriatr Soc. 2006;54(3):507–511. [DOI] [PubMed] [Google Scholar]
- 55.Mohile SG, Bylow K, Dale W, et al. A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer. 2007;109(4):802–810. [DOI] [PubMed] [Google Scholar]
- 56.Luciani A, Ascione G, Bertuzzi C, et al. Detecting disabilities in older patients with cancer: comparison between comprehensive geriatric assessment and vulnerable elders survey-13. J Clin Oncol. 2010;28(12):2046–2050. [DOI] [PubMed] [Google Scholar]
- 57.Spyropoulou D, Pallis AG, Leotsinidis M, Kardamakis D. Completion of radiotherapy is associated with the Vulnerable Elders Survey-13 score in elderly patients with cancer. J Geriatr Oncol. 2014;5(1):20–25. [DOI] [PubMed] [Google Scholar]
- 58.Bellera CA, Rainfray M, Mathoulin-Pelissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23(8):2166–2172. [DOI] [PubMed] [Google Scholar]
- 59.Kenis C, Geeraerts A, Braes T. The Flemish version of the Triage Risk Screening Tool. Crit Rev Oncol Hematol. 2006;60(31). [Google Scholar]
- 60.Hamaker ME, Jonker JM, de Rooij SE, Vos AG, Smorenburg CH, van Munster BC. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol. 2012;13(10):e437–444. [DOI] [PubMed] [Google Scholar]
- 61.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104(9):1998–2005. [DOI] [PubMed] [Google Scholar]
- 62.Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011;29(10):1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hurria A, Lichtman SM, Gardes J, et al. Identifying vulnerable older adults with cancer: integrating geriatric assessment into oncology practice. J Am Geriatr Soc. 2007;55(10):1604–1608. [DOI] [PubMed] [Google Scholar]
- 64.Puts MT, Santos B, Hardt J, et al. An update on a systematic review of the use of geriatric assessment for older adults in oncology. Ann Oncol. 2014;25(2):307–315. [DOI] [PubMed] [Google Scholar]
- 65.Williams GR, Deal AM, Jolly TA, et al. Feasibility of geriatric assessment in community oncology clinics. J Geriatr Oncol. 2014;5(3):245–251. [DOI] [PubMed] [Google Scholar]
- 66.Kelly CM, Shahrokni A. Moving beyond Karnofsky and ECOG Performance Status Assessments with New Technologies. J Oncol. 2016;2016:6186543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20(2):494–502. [DOI] [PubMed] [Google Scholar]
- 68.Transferability to clinical practice of the results of controlled clinical trials: the case of antiemetic prophylactic treatment for cancer chemotherapy-induced nausea and vomiting. Italian Group for Antiemetic Research. Annals of oncology: official journal of the European Society for Medical Oncology. 1998;9(7):759–765. [PubMed] [Google Scholar]
- 69.Kenis C, Baitar A, Decoster L, et al. The added value of geriatric screening and assessment for predicting overall survival in older patients with cancer. Cancer. 2018;124(18):3753–3763. [DOI] [PubMed] [Google Scholar]
- 70.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bruijnen CP, van Harten-Krouwel DG, Koldenhof JJ, Emmelot-Vonk MH, Witteveen PO. Predictive value of each geriatric assessment domain for older patients with cancer: A systematic review. J Geriatr Oncol. 2019. [DOI] [PubMed] [Google Scholar]
- 72.Williams GR, Dunham L, Chang Y, et al. Geriatric Assessment Predicts Hospitalization Frequency and Long-Term Care Use in Older Adult Cancer Survivors. J Oncol Pract. 2019:JOP1800368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puts MT, Hardt J, Monette J, Girre V, Springall E, Alibhai SM. Use of geriatric assessment for older adults in the oncology setting: a systematic review. J Natl Cancer Inst. 2012;104(15):1133–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohen HJ, Smith D, Sun CL, et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016;122(24):3865–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rao AV, Hsieh F, Feussner JR, Cohen HJ. Geriatric evaluation and management units in the care of the frail elderly cancer patient. J Gerontol A Biol Sci Med Sci. 2005;60(6):798–803. [DOI] [PubMed] [Google Scholar]
- 76.Clough-Gorr KM, Stuck AE, Thwin SS, Silliman RA. Older breast cancer survivors: geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow-up. J Clin Oncol. 2010;28(3):380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol. 2012;30(15):1829–1834. [DOI] [PubMed] [Google Scholar]
- 78.Guerard EJ, Deal AM, Chang Y, et al. Frailty Index Developed From a Cancer-Specific Geriatric Assessment and the Association With Mortality Among Older Adults With Cancer. J Natl Compr Canc Netw. 2017;15(7):894–902. [DOI] [PubMed] [Google Scholar]
- 79.Abel GA, Klepin HD. Frailty and the management of hematologic malignancies. Blood. 2018;131(5):515–524. [DOI] [PubMed] [Google Scholar]
- 80.DuMontier C, Liu MA, Murillo A, et al. Function, Survival, and Care Utilization Among Older Adults With Hematologic Malignancies. J Am Geriatr Soc. 2019. [DOI] [PubMed] [Google Scholar]
- 81.Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121(21):4287–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shahrokni A, Tin A, Alexander K, et al. Development and Evaluation of a New Frailty Index for Older Surgical Patients With Cancer. JAMA Netw Open. 2019;2(5):e193545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahmed A, Deng W, Tew W, et al. Pre-operative assessment and post-operative outcomes of elderly women with gynecologic cancers, primary analysis of NRG CC-002: An NRG oncology group/gynecologic oncology group study. Gynecol Oncol. 2018;150(2):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirkhus L, Saltyte Benth J, Rostoft S, et al. Geriatric assessment is superior to oncologists’ clinical judgement in identifying frailty. Br J Cancer. 2017;117(4):470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hamaker ME, Vos AG, Smorenburg CH, de Rooij SE, van Munster BC. The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. The oncologist. 2012;17(11):1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramjaun A, Nassif MO, Krotneva S, Huang AR, Meguerditchian AN. Improved targeting of cancer care for older patients: a systematic review of the utility of comprehensive geriatric assessment. J Geriatr Oncol. 2013;4(3):271–281. [DOI] [PubMed] [Google Scholar]
- 87.Giantin V, Valentini E, Iasevoli M, et al. Does the Multidimensional Prognostic Index (MPI), based on a Comprehensive Geriatric Assessment (CGA), predict mortality in cancer patients? Results of a prospective observational trial. J Geriatr Oncol. 2013;4(3):208–217. [DOI] [PubMed] [Google Scholar]
- 88.DuMontier C, Clough-Gorr KM, Silliman RA, Stuck AE, Moser A. Health-Related Quality of Life in a Predictive Model for Mortality in Older Breast Cancer Survivors. J Am Geriatr Soc. 2018;66(6):1115–1122. [DOI] [PubMed] [Google Scholar]
- 89.Boulahssass R, Gonfrier S, Ferrero JM, et al. Predicting early death in older adults with cancer. Eur J Cancer. 2018;100:65–74. [DOI] [PubMed] [Google Scholar]
- 90.Bourdel-Marchasson I, Diallo A, Bellera C, et al. One-Year Mortality in Older Patients with Cancer: Development and External Validation of an MNA-Based Prognostic Score. PLoS One. 2016;11(2):e0148523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferrat E, Paillaud E, Caillet P, et al. Performance of Four Frailty Classifications in Older Patients With Cancer: Prospective Elderly Cancer Patients Cohort Study. J Clin Oncol. 2017;35(7):766–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hurria A, Mohile S, Gajra A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol. 2016;34(20):2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hamaker ME, Seynaeve C, Wymenga AN, et al. Baseline comprehensive geriatric assessment is associated with toxicity and survival in elderly metastatic breast cancer patients receiving single-agent chemotherapy: results from the OMEGA study of the Dutch breast cancer trialists’ group. Breast. 2014;23(1):81–87. [DOI] [PubMed] [Google Scholar]
- 94.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118(13):3377–3386. [DOI] [PubMed] [Google Scholar]
- 95.Freyer G, Geay JF, Touzet S, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: a GINECO study. Ann Oncol. 2005;16(11):1795–1800. [DOI] [PubMed] [Google Scholar]
- 96.Corre R, Greillier L, Le Caer H, et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients With Advanced Non-Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08–02 Study. J Clin Oncol. 2016;34(13):1476–1483. [DOI] [PubMed] [Google Scholar]
- 97.Hurria A, Akiba C, Kim J, et al. Reliability, Validity, and Feasibility of a Computer-Based Geriatric Assessment for Older Adults With Cancer. J Oncol Pract. 2016;12(12):e1025–e1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Verduzco-Aguirre HC, Gomez-Moreno C, Chavarri-Guerra Y, Soto-Perez-de-Celis E. Predicting Life Expectancy for Older Adults with Cancer in Clinical Practice: Implications for Shared Decision-making. Curr Oncol Rep. 2019;21(8):68. [DOI] [PubMed] [Google Scholar]
- 99.Hamaker ME, Te Molder M, Thielen N, van Munster BC, Schiphorst AH, van Huis LH. The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients - A systematic review. J Geriatr Oncol. 2018;9(5):430–440. [DOI] [PubMed] [Google Scholar]
- 100.Kalsi T, Babic-Illman G, Ross PJ, et al. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. Br J Cancer. 2015;112(9):1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mohile SG, Velarde C, Hurria A, et al. Geriatric Assessment-Guided Care Processes for Older Adults: A Delphi Consensus of Geriatric Oncology Experts. J Natl Compr Canc Netw. 2015;13(9):1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Caillet P, Canoui-Poitrine F, Vouriot J, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol. 2011;29(27):3636–3642. [DOI] [PubMed] [Google Scholar]
- 103.Schulkes KJG, Souwer ETD, Hamaker ME, et al. The Effect of A Geriatric Assessment on Treatment Decisions for Patients with Lung Cancer. Lung. 2017;195(2):225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cudennec T, Gendry T, Labrune S, et al. Use of a simplified geriatric evaluation in thoracic oncology. Lung Cancer. 2010;67(2):232–236. [DOI] [PubMed] [Google Scholar]
- 105.Chaibi P, Magne N, Breton S, et al. Influence of geriatric consultation with comprehensive geriatric assessment on final therapeutic decision in elderly cancer patients. Crit Rev Oncol Hematol. 2011;79(3):302–307. [DOI] [PubMed] [Google Scholar]
- 106.Mohile SG, Magnuson A, Pandya C, et al. Community Oncologists’ Decision-Making for Treatment of Older Patients With Cancer. J Natl Compr Canc Netw. 2018;16(3):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mohile SG, Epstein RM, Hurria A, et al. Improving communication with older patients with cancer using geriatric assessment (GA): A University of Rochester NCI Community Oncology Research Program (NCORP) cluster randomized controlled trial (CRCT). Journal of Clinical Oncology. 2018;36(18_suppl):LBA10003–LBA10003. [Google Scholar]
- 108.Hurria A The facts and the need for a multidisciplinary approach. In. Journal of Geriatric Oncology 2014. [Google Scholar]
- 109.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McCorkle R, Strumpf N, Nuamah I, et al. A specialized home care intervention improves survival among older post-surgical cancer patients. Journal of American Geriatric Society 2000;48:6. [DOI] [PubMed] [Google Scholar]
- 111.Goodwin J, Satish S, Anderson E, Nattinger A, Freeman J. Effect of nurse case management on the treatment of older women with breast cancer. Journal of the American Geriatric Society. 2003:7. [DOI] [PubMed] [Google Scholar]
- 112.Burhenn PS, Perrin S, McCarthy AL. Models of Care in Geriatric Oncology Nursing. Semin Oncol Nurs. 2016;32(1):24–32. [DOI] [PubMed] [Google Scholar]
- 113.Mason H, DeRubeis MB, Foster JC, Taylor JM, Worden FP. Outcomes evaluation of a weekly nurse practitioner-managed symptom management clinic for patients with head and neck cancer treated with chemoradiotherapy. Oncol Nurs Forum. 2013;40(6):581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.O’Donovan A, Mohile SG, Leech M. Expert consensus panel guidelines on geriatric assessment in oncology. European journal of cancer care, 24(4), 574–589. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. Journal of the National Comprehensive Cancer Network,. 2015;13(9):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kenis C, Heeren P, Decoster L, et al. A Belgian Survey on Geriatric Assessment in Oncology Focusing on Large-Scale Implementation and Related Barriers and Facilitators. J Nutr Health Aging. 2016;20(1):60–70. [DOI] [PubMed] [Google Scholar]
- 117.Magnuson A, Lemelman T, Pandya C, et al. Geriatric assessment with management intervention in older adults with cancer: a randomized pilot study. Supportive Care in Cancer. 2018;26(2):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kenis C, Decoster L, Flamaing J, et al. Adherence to geriatric assessment-based recommendations in older patients with cancer: a multicenter prospective cohort study in Belgium. Ann Oncol. 2018;29(9):1987–1994. [DOI] [PubMed] [Google Scholar]
- 119.Dubianski R, Wildes TM, Wildiers H. SIOG guidelines- essential for good clinical practice in geriatric oncology. J Geriatr Oncol. 2019;10(2):196–198. [DOI] [PubMed] [Google Scholar]
- 120.Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol. 2005;55(3):241–252. [DOI] [PubMed] [Google Scholar]
- 121.Noam V, Reshma J, Efrat D, et al. NCCN Guidelines Insights: Older Adult Oncology, Version 2.2016. J Natl Compr Canc Netw. 2016;14(11):1357–1370. [DOI] [PubMed] [Google Scholar]
- 122.VanderWalde N, Jagsi R, Dotan E, et al. NCCN Guidelines Insights: Older Adult Oncology, Version 2.2016. J Natl Compr Canc Netw. 2016;14(11):1357–1370. [DOI] [PubMed] [Google Scholar]
- 123.Loh KP, McHugh C, Mohile SG, et al. Using Information Technology in the Assessment and Monitoring of Geriatric Oncology Patients. Curr Oncol Rep. 2018;20(3):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gajra A, Loh KP, Hurria A, et al. Comprehensive Geriatric Assessment-Guided Therapy Does Improve Outcomes of Older Patients With Advanced Lung Cancer. J Clin Oncol. 2016;34(33):4047–4048. [DOI] [PubMed] [Google Scholar]
- 125.Chen EY, Joshi SK, Tran A, Prasad V. Estimation of Study Time Reduction Using Surrogate End Points Rather Than Overall Survival in Oncology Clinical Trials. JAMA Intern Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061–1066. [DOI] [PubMed] [Google Scholar]
- 127.Loh KP, Mohile SG, Epstein RM, et al. Willingness to bear adversity and beliefs about the curability of advanced cancer in older adults. Cancer. 2019;125(14):2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nipp RD, Abel GA. Small Step for Geriatric Oncology That Could Have Been a Giant Leap. J Clin Oncol. 2016;34(33):4048–4049. [DOI] [PubMed] [Google Scholar]
- 129.Zweegman S, Engelhardt M, Larocca A, ‘Aging ESo, Hematology. Elderly patients with multiple myeloma: towards a frailty approach? Curr Opin Oncol. 2017;29(5):315–321. [DOI] [PubMed] [Google Scholar]
- 130.Hall P, Swinson D, Waters J, et al. Optimizing chemotherapy for frail and elderly patients (pts) with advanced gastroesophageal cancer (aGOAC): The G02 phase III trial. J Clin Oncol. 2019;37(suppl; abstr 4006). [Google Scholar]
- 131.Hurria A, Soto-Perez-de-Celis E, Allred JB, et al. Functional Decline and Resilience in Older Women Receiving Adjuvant Chemotherapy for Breast Cancer. J Am Geriatr Soc. 2019;67(5):920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Williams GR. Geriatric Assessment: Precision Medicine for Older Adults With Cancer. J Oncol Pract. 2018;14(2):97–98. [DOI] [PubMed] [Google Scholar]
- 133.Dale W “Staging the aging” when considering androgen deprivation therapy for older men with prostate cancer. J Clin Oncol. 2009;27(21):3420–3422. [DOI] [PubMed] [Google Scholar]