Abstract
Commensal microbiota within a holobiont contribute to the overall health of the host via mutualistic symbiosis. Disturbances in such symbiosis is prominently correlated with a variety of diseases affecting the modern society of humans including cardiovascular diseases, which are the number one contributors to human mortality. Given that a hallmark of all cardiovascular diseases is changes in vascular function, we hypothesized that depleting microbiota from a holobiont would induce vascular dysfunction. To test this hypothesis, young mice of both sexes raised in germ-free conditions were examined vascular contractility and structure. Here we observed that male and female germ-free mice presented a decrease in contraction of resistance arteries. These changes were more pronounced in germ-free males than in germ-free females mice. Furthermore, there was a distinct change in vascular remodeling between males and females germ-free mice. Resistance arteries from male germ-free mice demonstrated increased vascular stiffness, as shown by the leftward shift in the stress-strain curve and inward hypotrophic remodeling, a characteristic of chronic reduction in blood flow. On the other hand, resistance arteries from germ-free female mice were similar in the stress-strain curves to that of conventionally raised mice, but were distinctly different and showed outward hypertrophic remodeling, a characteristic seen in aging. Interestingly, we observed that reactive oxygen species (ROS) generation from bone marrow derived neutrophils is blunted in female germ-free mice, but it is exacerbated in male germ-free mice. In conclusion, these observations indicate that commensal microbiota of a holobiont are central to maintain proper vascular function and structure homeostasis, especially in males.
Keywords: Germ-free mice, vascular contractility, sex differences
Graphical Abstract
Introduction
In 1991, Dr. Lynn Margulis introduced the concept of the “holobiont” [1]. This term is defined as the association of the host and its entire microbial community, including transient and stable members [1]. The human microbiome is the assemblage of all microbiota that resides in tissues and biofluids, including the saliva, oral mucosa, skin, mammary glands, uterus, placenta, seminal fluid, ovarian follicles, airways, and gastrointestinal tract. It was often proposed that in the human body, bacteria outnumber human cells by a ratio of at least 10:1 [2]. However, in 2016, the ratio was revised and now it is estimated to be closer to 1:1 [2,3]. The vast majority of commensal bacteria reside in the gastrointestinal tract, followed by the skin [3]. Within the gastrointestinal tract, the cecum and the colon are the dominant contributors to the total bacterial population. As a result, the microbiota of the gastrointestinal tract and its interplay with systemic health is the most extensively documented [2–13].
Gut commensal microbiota are required for host health [4–12]. However, gut dysbiosis is deleterious to health, for which there is limited, albeit definitive and emerging evidence in the literature pertaining to several human diseases including cancer [10], obesity [14] and hypertension [4–8, 11–13]. Alterations in gut bacteria as a result of either the genomic make-up of the host, environmental factors that the host is exposed to, or a combination thereof, constitute testable hypotheses as factors responsible for the etiology of cardiovascular diseases. Indeed, a pioneering study from Dr. Bina Joe’s group showed that gut microbial composition affects genetic models of hypertension [7]. 16S rRNA genes obtained from cecal samples of Dahl salt-sensitive (hypertensive) and Dahl salt-resistant (normotensive) rats were sequenced and it was observed that bacteria of the phylum Bacteroidetes were higher in the hypertensive rats compared with the normotensive rats [7]. Furthermore, the family S24–7 of the phylum Bacteroidetes and the family Veillonellaceae of the phylum Firmicutes were higher in the hypertensive rats compared with the normotensive rats [7]. Surprisingly, when hypertensive and normotensive animals were given high salt diet, it was observed that hypertensive animals, treated with cecal content from normotensive animals, presented with shorter lifespan and increased blood pressure [7]. Other groups have corroborating evidence from both animal models and in humans [12, 15]. Further, several studies also pointed to microbiotal reshaping disruption of the gut barrier, increased permeability and inflammation [12,16]. A study performed in humans demonstrated that intestinal fatty acid binding protein (I-FABP), lipopolysaccharide (LPS), and augmented gut-targeting proinflammatory T helper 17 (Th17) cells are increased in plasma of hypertensive patients, suggesting intestinal inflammation and increased permeability [17]. Subsequently, other studies emerged in supported of the notion that imbalance of gut microbiota with the host could indeed be associated not only with hypertension, but also with several cardiovascular diseases such as atherosclerosis, heart failure, chronic kidney disease, obesity, and type 2 diabetes mellitus [18].
One of the major pathophysiological characteristic of cardiovascular diseases is the dysfunction and remodeling of resistance artery [19–22]. Dr. Ernesto Schiffrin’s group has shown that in humans, small artery remodeling may be the first manifestation of target organ damage in hypertension [21]. Accordingly, they observed that ~100% of stage I hypertensive subjects present small artery remodeling. In cardiovascular diseases, dysfunction and remodeling of resistance arteries results from complex, yet incompletely understood interactions between genetic factors, sex, intrinsic adaptation of vascular wall to altered mechanical conditions, neurohumoral and local trophic influences [22]. However, it is unclear if the association of the host and its entire microbial community, or in other words “the holobiont”, would be associated or have a direct cause-effect on resistance artery physiology. Given that the core of almost all cardiovascular diseases is associated with changes in resistance artery contractility and remodeling, in the present study we questioned whether microbiota are central for the physiology of these small arteries.
Gnotobiotic or germ-free (GF) mice represent an important approach to explore the effects of the gut microbiota on physiology and disease. Based on the prior research showing the importance of gut microbiota on cardiovascular health, we designing this study using GF mice to test the hypothesis that total depletion of microbiota would induce dysfunction of the resistance arteries including changes in contractility and remodeling in mice. Further, we examined if sex-differences would play a role in GF-induced vascular dysfunction. Here, we observed that male and female GF mice presented with a remarkable decrease in contraction in resistance arteries. Although there were no sex-differences in the reduced contraction, there was a distinct difference in the type of vascular remodeling that occurred between male and female GF mice. In line, resistance arteries from male GF mice present increased vascular stiffness, as shown by the leftward shift in the stress-strain curve and inward hypotrophic remodeling, a characteristic of chronic reduction in blood flow. On the other hand, resistance arteries from female mice did not show changes in the stress-strain curve, but demonstrated outward hypertrophic remodeling, a characteristic seen in aging.
Methods
Ethical Approval
All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH) [23] and were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Toledo College of Medicine and Life Sciences, Toledo, OH.
Animals
Male and Female, 7–8-weeks-old C57BL6 genetic background (conventional, Conv) and GF mice were used. GF mice were obtained from The University of North Carolina at Chapel Hill Center for Gastrointestinal Biology and Disease Gnotobiotic Core. The animals were shipped overnight in a GF Shipper (Figure 1) purchased from Taconic Biosciences, Inc, USA. For this, the GF shipper was first sent from Taconic to the National Gnotobiotic Rodent Resource Center for inspection, one week prior the GF shipping to the University of Toledo. Conv C57BL6 mice were bred and maintained in University of Toledo. This study presented 2 groups of Conv (n=6/group) and GF (n=6/group) mice, but for sex-differences analyses, the groups were subdivided in 4 groups: Conv male or female (n=3/group) and GF male or female (n=3/group).
Figure 1.
Picture shows the Taconic GF Shipper upon arrival at the University of Toledo. The Taconic GF shipper is a large, flexible vinyl sleeve with filtered openings and an accompanying case. The sleeve measures approximately 12 inches in diameter and 3 ½ feet in length. Three mouse cages (11 ½” x 7 ½” x 5”) can fitted comfortably within the sleeve.
Upon arrival at the University of Toledo, the male mice were immediately used for the experiments. Female mice were maintained for 24 hours on a 12:12 hour light-dark cycle with both sterile standard chow and water ad libitum. The GF shipper was kept inside a sterile laminar flow hood.
GF status
A comprehensive health report from the GF mice is included in a table 1. The absence of germs were confirmed via PCR evaluation performed by Dr. Hayes from the University of North Carolina-Chapel Hill (Table 1).
Table 1:
Report shows the absence of germs in the GF mice used in the present study. The presence or absence of the germs was evaluated via PCR.
| Specie’s name | Status (in feces) |
|---|---|
| CAR bacillus | negative |
| Ectromelia | negative |
| EDIM | negative |
| Helicobacter spp. | negative |
| Helicobacter bilis | negative |
| Helicobacter ganmani | negative |
| Helicobacter hepaticus | negative |
| Helicobacter mastomyrinus | negative |
| Helicobacter rodentium | negative |
| Helicobacter typhlonius | negative |
| LCMV | negative |
| MAV1 | negative |
| MAV2 | negative |
| mCMV | negative |
| MHV | negative |
| MNV | negative |
| Mycoplasma pulmonis | negative |
| MVM | negative |
| MPV | negative |
| Pasteurella pneumotropica biotype Jawetz | negative |
| Pasteurella pneumotropica biotype Heyl | negative |
| Polyoma | negative |
| PVM | negative |
| REO3 | negative |
| Sendai | negative |
| TMEV | negative |
| Aspiculuris tetraptera | negative |
| Syphacia muris | negative |
| Syphacia obvelata | negative |
| Corynebacterium bovis | negative |
| Myocoptes | negative |
| Radfordia/Myobia | negative |
Equipment, surgical tools and supplies
For scientific rigor, all surgical tools and supplies used for tissue harvesting and clean for both strains (Conv and GF mice) were sterilized. Further, equipment, such as pressure culture and wire myograph were disinfected and kept in 70% ethanol. Just prior to the use of the mygraphs, ethanol was washed using sterile water.
Morphological parameters of the heart and cecum
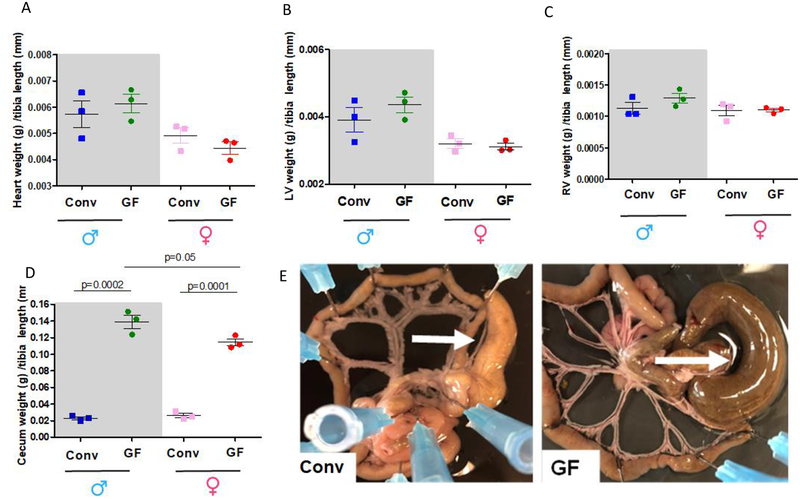
The animals were killed by quick excision of the hearts under continued deep anesthesia (5% isoflurane). After rapid excision and careful removal of non-cardiac tissue, total heart, left (LV) and right (RV) ventricles weights were measured and normalized by tibia length. Subsequently, the mesenteric bed was removed and the cecum was excised to verify its enlargement and confirm GF status. For this, the cecum was weighted and normalized by tibia length. GF animals have ceca which, with their contents, weigh about six times that of Conv animals [24].
Vascular function and structural measurements
Isometric measurements
The mesenteric arcade were carefully removed. Second and third-order branches from mesenteric arteries were removed and cleaned of surrounding tissue in cold Krebs-Henseleit solution (KHS, in mmol/l): NaCl 118; KCl 4.7; NaHCO3 25; CaCl2.2H2O 2.5; KH2PO4 1.2; MgSO4.7H2O 1.2; EDTA 0.01; glucose 11. Segments 2 mm in length, were mounted in a wire myograph chambers (Danish Myo Tech, model 610M; JP-Trading I/S), for isometric tension recordings, as described previously [25,26]. Briefly, for resistance arteries two steel wires (40 μm diameter) were introduced through the lumen of the segments and mounted according to the method described by Mulvany and Halpern [27]. After a 15-min equilibration period in oxygenated KHS at 37°C and pH 7.4, segments were stretched to their optimal lumen diameter for active tension development. This was determined based on the internal circumference/wall tension ratio of the segments by setting the internal circumference, L0, to 90% of what the vessels would have if they were exposed to a tension equivalent to that produced by a transmural pressure of 100 mmHg (L100) [25,26]. The diameter (I1) was determined according to the equation I1 = L1/π, using specific software for normalization of resistance arteries (DMT Normalization Module; ADInstruments). Segments were washed with KHS and left to equilibrate for 20 min. Vessel contractility and endothelium viability were then tested by an initial exposure to a high-K+ (120 mmol/L) solution and by using acetylcholine (3 μmol/L) in vessels contracted with phenylephrine (30 μmol/L) respectively. The endothelium was considered intact if the arteries relaxed more than 80% to acetylcholine for the Conv groups. Given that the function of the endothelium was unknown in arteries from the GF groups and all arteries from animals were performed in the same day, we used all segments for the experiments independent of endothelium viability. However, the outliers were excluded during analyzes, if it indicated experimental error. Arteries then rested for 30 min, after which they were subjected to each of the following three protocols: 1) concentration-response curves to phenylephrine (1 nmol/L to 100 μmol/L); 2) concentration-response curves to acetylcholine (1 nmol/L to 10 μmol/L); and 3) concentration-response curves to sodium nitroprusside (1 nmol/L to 10 μmol/L). For concentration-response curves using phenylephrine or sodium nitroprusside the endothelium was intact.
Isobaric measurements
The fourth-order branches from mesenteric resistance arteries were carefully removed. The structural and mechanical properties were evaluated using pressure myograph as previously described [26]. The 2 mm segments were bathed in filtered KHS and cannulated at both ends in an arteriography (Culture Pressure myograph system, DMT, USA) using a glass microcannula (100–120 μm diameter, A-M Systems) and then knotted with surgical nylon suture. Subsequently, vessel length was adjusted to maintain the vessel walls parallel at increasing pressures. Intraluminal flow in the vessel was generated by gravity and superfusion using a peristaltic pump. Intraluminal pressure was then raised to 120 mmHg, and again the vessel length was adjusted. The segment was set to a pressure of 60 mmHg (no flow) and allowed to equilibrate for 20 min at 37°C in KHS, gassed with a mixture of 95% O2 and 5% CO2. Afterward, intraluminal pressure was decreased to 3 mmHg. A pressure-diameter curve was obtained by increasing intraluminal pressure in 20 mmHg steps from 3 to 140 mmHg. The same procedure was repeated in Ca2+ free-KHS + EGTA [26,28].
Arterial diameter was measured and recorded continuously using a video monitoring system (DMT, USA). Internal and external diameters were continuously measured under active and passive conditions (Di0Ca2+, De0Ca2+) for 2 min at each intraluminal pressure. The final value used was the mean of the two regions of interest, taken during the last 30s when the lumen diameter had reached a steady state [26,28]. Data analyses were performed using VasoTracker analyses software (VasoTracker Open source Pressure Myography, Scotland, UK).
Calculation of Myogenic tone, Structural and Mechanical Parameters
Myogenic tone and Structural parameters were calculated or normalized by passive conditions, as previously described:
Myogenic tone (%) = ((Di0Ca2+ −Di)/Di0Ca2+) x 100)
Diameter = (Di/Di0Ca2+60mmHg) x 100
Wall thickness (WT) = (De0Ca2+ − Di0Ca2+)/2
Wall:lumen = (De0Ca2+ − Di0Ca2+)/2Di0Ca2+
Cross-sectional area = (π/4) X (De0Ca2+ 2 − Di0Ca2+ 2)
Incremental distensibility represents the percentage of change in the arterial internal diameter for each mmHg change in intraluminal pressure:
Incremental Distensibility = (ΔDi0Ca2+ /(Di0Ca2+ × ΔP) × 100)
Circumferential wall strain (ε) = (Di0Ca2+ - D00Ca2+)/D00Ca2+, where D00Ca2+ is the internal diameter at 3 mmHg.
Circumferential wall stress (σ) = (P × Di0Ca2+)/(2WT), where P is the intraluminal pressure (1 mmHg = 1.334 × 103 dynes·cm-2) and WT is wall thickness at each intraluminal pressure in 0Ca2+-KHS.
Arterial stiffness is determined by the Young’s elastic modulus (E=stress/strain) [28]. The stress-strain relationship is non-linear; therefore, it is important to obtain a tangential or incremental elastic modulus (Einc) by determining the slope of the stress-strain curve (Einc =δσ/δε) [28] Einc was obtained by fitting the stress-strain data from each animal to an exponential curve using the equation: σ =σorigeβε, where σorig is the stress at the original diameter. Taking derivatives on the equation presented earlier, we see that Einc=βσ. For a given σ-value, Einc is directly proportional to β. An increase in β implies an increase in Einc, which means an increase in stiffness [28].
ROS generation in bone marrow derived neutrophils (BMDNs)
Neutrophils were isolated from bone marrow from Conv and GF mice by differential Histopaque gradient. The yield and purity was confirmed by flow cytometry (CD11b+ Ly6G+, BD Bioscience). Freshly isolated neutrophils (1X106 cells/well) were incubated with Phorbol myristate acetate (PMA, 50 nM) for 3 h at 37°C. Cells were washed with phosphate buffered saline (PBS), stained with 5.0 μM CellROX® Deep Red dye (Molecular Probes) for 30 min at 37°C in the dark and washed twice with PBS. Fluorescence was measured by flow cytometry (Accuri c6, BD Biosciences) and analyzed using the Accuri c6 software (Becton Dickinson). Intracellular ROS was expressed as fold-change of mean fluorescence intensity (MFI) normalized to the controls.
Pharmacological agents
Stock solutions of phenylephrine, acetylcholine and sodium nitroprusside were prepared freshly in sterile and distilled water. All drugs were purchased from Millipore-Sigma (Saint Louis, USA).
Statistical analyses
The statistical procedures used included Student’s unpaired t-tests and one or two-way analysis of variance (ANOVA). All analyses were performed using data analysis software GraphPad Prism 5.0 (USA, CA). Number (n) of the animals used is indicated in the graphs. Statistical significance was set at p<0.05. The data are presented as mean ± standard error of the mean (SEM). For concentration-response curves, maximum response (efficacy or Emax) was calculated to show the highest point on the curve. Half maximal effective concentration (EC50) was calculate to evaluate potency of the drug in case no differences were observed in Emax between curves.
Results
GF status induced cecum enlargement, but it did not affect heart weight
Total heart, LV and RV ventricles weights were measured and normalized by tibia length. As expected, total heart weight, LV and RV were higher in male when compared to female. No differences were observed between GF and Conv groups.
GF status of mice was confirmed by PCR showing in Table 1. Another gold standard to confirm GF status in animals is the presence of cecum enlargement. As expected, we observed that GF animals presented enlargement of the cecum in both sexes (Figure 2 D and E). Surprisingly, enlargement of the GF cecum was more pronounced in male mice (6.1-fold vs. Conv male) when compared to female mice (4.4-fold vs. Conv female) (Figure 2 D and E).
Figure 2:
Total heart (A), left (LV, B) and right (RV, C) ventricles and cecum (D) weights were measured and normalized by tibia length from 7–8 weeks old male and female Conv or GF mice. Number of animals used per group are = 3. Values are mean ± SEM. One-Way ANOVA and t-test. The images are representative pictures from the mesenteric bed of the male Conv and GF mice (E). Arrows show the cecum. Please note that the mesenteric bed from the GF mice presents an enlargement of the cecum. This is a typical characteristic for GF animals.
Microbiota are a central requirement for vascular physiology, as depletion of microbiota decreased contraction in response to potassium chloride (KCl) and phenylephrine
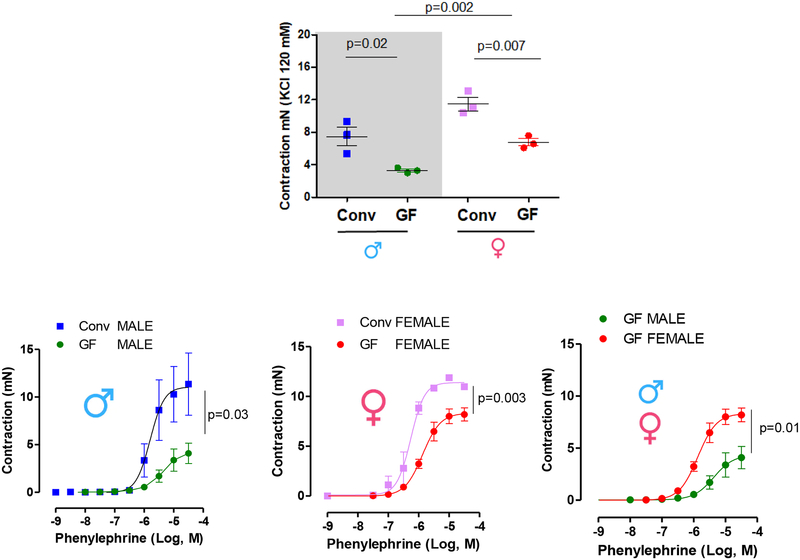
To determine if host gut microbiota is required for proper arterial function, isometric tension was measured in mesenteric resistance arteries (diameter < 300 μm) from Conv and GF mice. The absence of microbiota decreased both KCl (120 mM) and phenylephrine-induced contraction (Figure 3, table 2). However, the reduction in the phenylephrine-induced contraction was more evident in GF male mice (Figure 3 D, Table 2), suggesting that the absence of microbiota affects vascular function to a greater degree in male mice.
Figure 3.
(A) KCl-induced contraction (120 mM) and (B), (C) and (D) represent phenylephrine-induced contraction in mesenteric resistance arteries from 7–8 weeks old male and female Conv or GF mice. Samples were examined in duplicate (two separate vascular tissue samples). Values are mean ± SEM. Number of animals used per group are = 3. P values represent the differences observed in KCl (120 mM)-induced contraction (A) or in Emax in the concentration-response curves (table 2) using Student’s t-test.
Table 2:
Emax to phenylephrine in resistance arteries of Conv and GF mice
| Phenylephrine: | Emax (mN) | ±SEM | (n) |
|---|---|---|---|
| Conv male | 11.76 | ± 2.890 | n=3 |
| Conv female | 11.88 | ± 0.2785 | n=3 |
| GF male | 4.087* | ± 1.068 | n=3 |
| GF female | 8.193*# | ± 0.6567 | n=3 |
Values are mean ± SEM. Samples were examined in duplicate. Number of animals us ed are included in the 4rd column. Student’s t test:
vs. CONV;
vs. GF male.
GF mice did not present changes in endothelium dependent and independent-relaxation
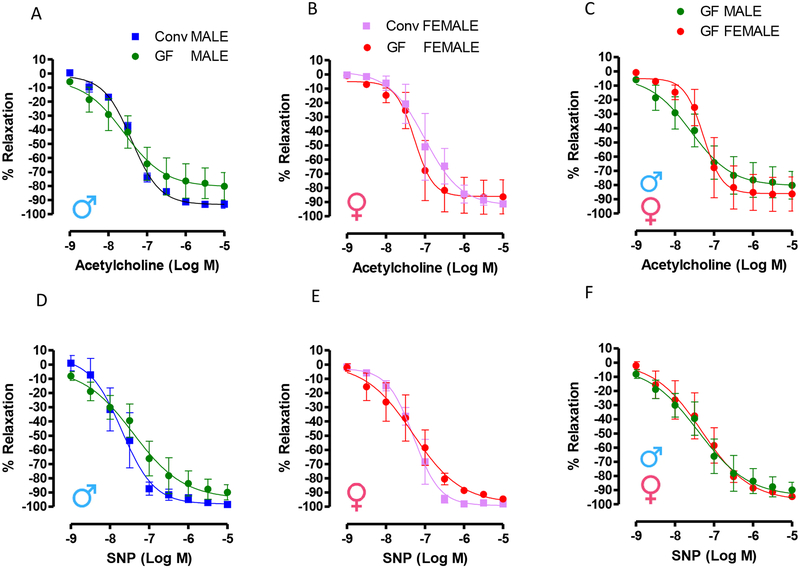
No changes were observed in the acetylcholine-induced relaxation or sodium nitroprusside-induced relaxation (Figure 4) in mesenteric resistance arteries from male and female Conv and GF mice.
Figure 4.
(A) (B) and (C) show concentration-response curves to acetylcholine and (D), (E) and (F) represent concentration-response curves to sodium nitroprusside (SNP) in mesenteric resistance arteries from 7–8 weeks old male and female Conv or GF mice. All arteries were initially contracted with phenylephrine (10 μM/L). Number of animals used per group are = 3. Samples were examined in duplicate. Values are mean ± SEM. No differences were observed in EC50 and Emax (Student’s t test, p >0.05) or variation between groups (Two-way ANOVA, p >0.05).
Microbiota are important for vascular mechanical properties
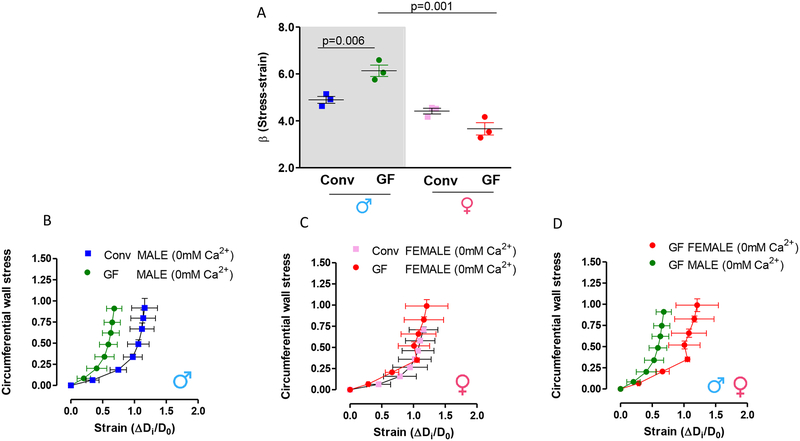
Here we observed for the first time that resistance arteries from male GF mice present increased vascular stiffness, as shown by the leftward shift in the stress-strain curve (Figure 5 B) and an increase in the elastic modulus β (Figure 5 A). Arterial stiffness is determined by the Young’s elastic modulus (E=stress/strain) [26,28]. The stress-strain relationship is non-linear; therefore, it is important to obtain a tangential or incremental elastic modulus (Einc) by determining the slope of the stress-strain curve. Einc was obtained by fitting the stress-strain data from each animal to an exponential curve. Taking derivatives on the equation presented in the method section, we see that Einc=βσ. For a given σ-value, Einc is directly proportional to β. An increase in β implies an increase in Einc, which means an increase in stiffness. On the other hand, no changes in vascular stiffness were observed in arteries from female mice (Figure 5 A, C and D).
Figure 5.
(A) represents β-values from slopes of the stress-strain relationships. An increase in β implies an increase in stiffness. (B), (C) and (D) represent stress-strain curves, which were calculated during incremental in intraluminal pressure (mmHg) in mesenteric resistance arteries from 7–8 weeks old male and female Conv and GF mice. The experiments were performed in a Ca2+-free solution. Statistical test for (A): One-way ANOVA.
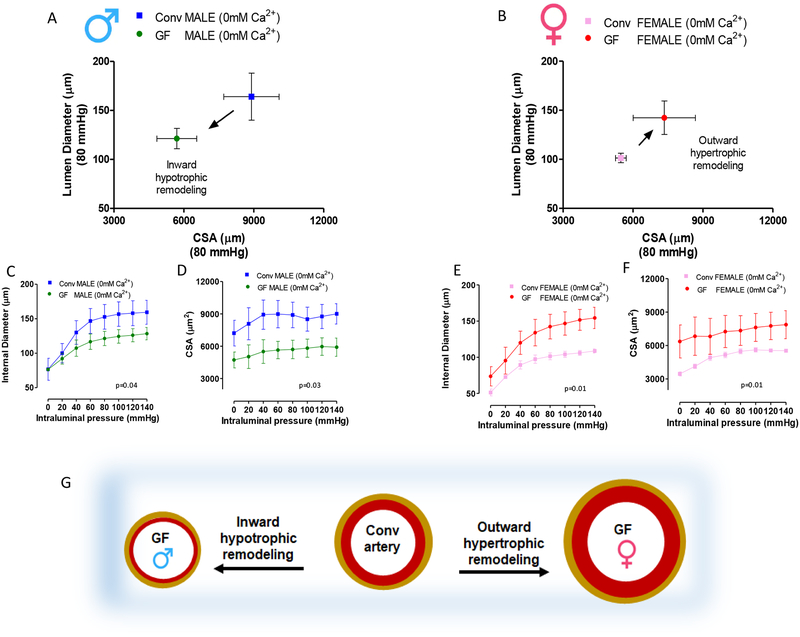
Interestingly, both male and female GF mice present vascular remodeling in resistance arteries (Figure 6 A and B) compared to Conv. However, there was a distinct change in the type of remodeling between sexes. Arteries from male GF mice presented a decrease in cross-sectional area and luminal diameter which results in an inward hypotrophic remodeling (Figure 6 A, C, D and G), a characteristic for chronic reduction in blood flow. On the other hand, arteries from female GF mice present an increase in cross-sectional area and luminal diameter (Figure 6 B, E, F and G), resulting in an outward hypertrophic remodeling, a characteristic seen in aging.
Figure 6.
(A) and (B) represent lumen diameter and cross-sectional area (CSA) measured in mesenteric resistance arteries at 80 mmHg intraluminal pressure in male and female mice, respectively. (C) and (E) show internal diameter and (D) and (F) cross-sectional area (CSA) measured during incremental in intraluminal pressure in mesenteric resistance arteries from 7–8 weeks old male and female Conv and GF mice. All structural measurements were performed in arteries submerged in a Ca2+-free Krebs. (G) Schematic representation of vascular remodeling in arteries from GF mice when compared to Conv. Number of animals used per group are = 3. Values are mean ± SEM. Two-way ANOVA.
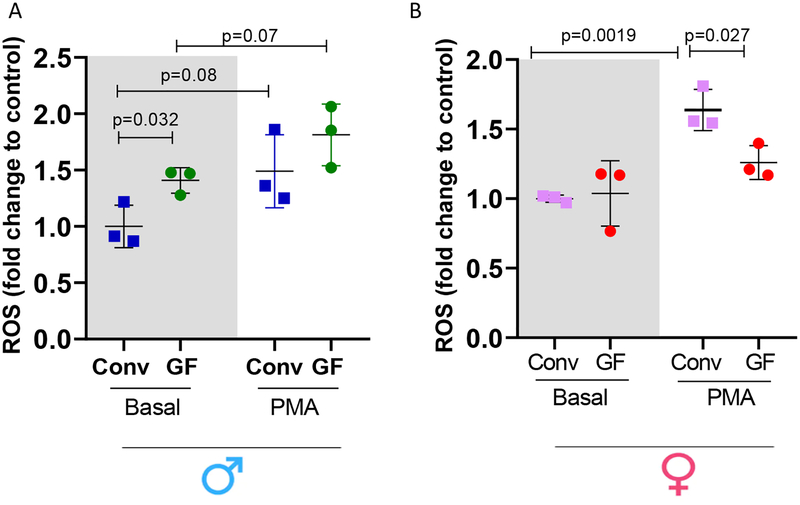
Bone marrow derived neutrophils (BMDNs) from a male GF mice are more prone to generate ROS. Female GF mice presented decreased ROS generation
ROS generation was measured in unstimulated (basal levels) or in stimulated BMDNs. Interestingly, ROS generation was increased at the basal level, but not after PMN stimulation, in BMDNs from male GF mice when compared to male Conv mice (Figure 7 A). On the other hand, no changes were observed in ROS generation at the basal level in BMDNs from female GF and Conv mice (Figure 7B). Strikingly, PMA challenge was unable to increase ROS generation in BMDNs from female GF mice (Figure 7B).
Figure 7.
Intracellular ROS generation in BMDNs from male (A) and female (B). ROS generation was normalized with respect to control. Each point represents data from one biological sample average from three technical replicates. Results are expressed as means SEM. One-Way ANOVA p<0.05. P values represent the differences observed in between groups using Student’s t-test.
Discussion
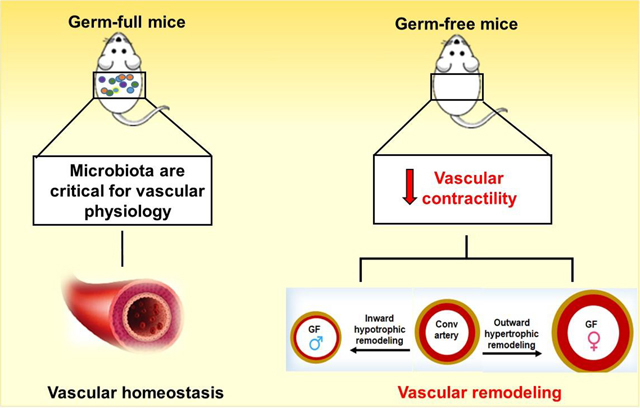
Over the last decade, our understanding of gut microbiota and its products in a variety of diseases such as inflammatory bowel disease and metabolic disease has increased, and more recent reports have revealed the gut microbiota contribute to cardiovascular and renal diseases [4–7, 29,30]. In the contemporary era, given the rise of antibiotic-resistant microorganisms, increased number of immune-compromised patients, and lack of new antimicrobial medications, researching pathogenic microbes remains highly relevant. Furthermore, recent research strongly suggests that commensal gut microbiota colonizing the human body also substantially influence host physiology and the risk for common diseases. Indeed, in the present study our observations revealed that the commensal microbiota colonizing the mice body substantially affect vascular physiology. Specifically, our findings demonstrated that the interaction between host and microbiota, or the holobiont, is required for vascular homeostasis. Also, our findings point to a holobiont-vascular network that is sex-specific. These data provide a new insight into how the holobiont and cardiovascular system have a crosstalk affecting vascular dynamics. Further, our results suggest that disturbances in microbiota synergy into the host is one of the major risks for common diseases and may perhaps lead to new mechanistic discoveries.
Commensal gut microbiota perform a variety of functions that are important to the host. Perhaps one of the most obvious functions of gut microbiota is to help the host to digest plant derived complex carbohydrates and generate energy in the form of short chain fatty acids [5, 31]. Gut microbiota are also important for source of biotin, vitamins K and B12, and essential amino acids [5,6]. All these elements are essential for well-being of the host. Therefore, based on these premises we questioned whether the lack of microbiota would disrupt the host vascular homeostasis, specifically if it would induce changes in vascular function in young mice relative to Conv mice, and if these changes, if any, would be in a sex-specific manner.
GF rodents have large ceca, in part because their cecum accumulates an unknown substance(s) that induces smooth muscles relaxation [32]. Cecal contents are also more fluidic in GF mice compared to Conv mice due, in part, to decreased sodium and chloride ion concentrations in the cecum contents and impaired water absorption in the colon [33]. In the present study, we confirm the previous findings showing that the absence of microbiota lead to an enlargement of the cecum in both sexes. However, we noticed that the cecum enlargement were more pronounced in male mice when compared to female mice. Admittedly, although novel, our observations do not offer mechanistic insights for the observed phenotypes in a sex-specific manner. Descriptive observations, which have equal relevance when compared to mechanistic studies, are still emerging regarding the pathophysiology of the holobiont, and consequently, studies showing cause-effect relationship between cardiovascular system and sex differences are too premature or do not exist. However, a few studies indeed support our findings that gut microbiota affects males and females in a distinct way. For example, the absence of microbiota attenuated liver sexual dimorphism and sex-specific rhythmicity [34]. The authors suggested that, the resulting feminization of male and masculinization of female GF animals is likely caused by altered sexual development and growth hormone secretion, associated with differential activation of xenobiotic receptors [34]. Similarly, it was observed that chronic angiotensin II (Ang II) infusion decreases at least 8 plasma metabolites, in Conv mice. However, some of these compounds were nearly undetected in GF mice implying that they are dependent on the gut microbiota and that gut microbial production is downregulated with Ang II treatment. Interestingly, the authors noted that several of the metabolites measured in Conv and GF animals were different between sexes [35]. Also, it was found that the gut microbiota from specific pathogen-free and non-obese diabetic male mice protected the immature female recipients from type 1 diabetes by altering gut microbiota, elevating testosterone, reducing islet inflammation and autoantibody production [36].
In 1971, Drs. Baez and Gordon [37] were the first to observe that the gut microbiota affects vascular physiology [37]. In line, they observed that GF rats present a profound hyporesponsiveness of the mesentery precapillary vessels to catecholamines. Corroborating these data, we observed that mesenteric resistance arteries from GF mice present a dramatic decrease in vascular contraction independent of the agonist and sex. For instance, in the intact body, the process of smooth muscle cell contraction is regulated mainly by receptor and mechanical (stretch) activation of the contractile proteins myosin and actin [38]. For contraction to occur, myosin light chain kinase phosphorylates the 20-kDa light chain of myosin, enabling the molecular interaction of myosin with actin [38]. Energy released from ATP by myosin ATPase activity results in the cycling of the myosin cross-bridges with actin for contraction. Thus contractile activity in smooth muscle is determined primarily by the phosphorylation state of the light chain of myosin. In some smooth muscle cells, the phosphorylation of the light chain of myosin is maintained at a low level in the absence of external stimuli (i.e., no receptor or mechanical activation). This activity results in what is known as smooth muscle tone and its intensity can be varied [38]. Viewing this from the context of host-microbiota interactions, the enlargement of the cecum was due, at least in part, to the cecum accumulating an unknown substance that induces smooth muscles to relax and the gut microbiota inhibits the accumulation of this unknown substance [37]. Apparently, this substance is synthesized continuously in the small bowel of all rodents but is inactivated in the cecum by the gut microbiota in Conv animals [37,39]. Given that vessels are also comprised of smooth muscle cells, it is possible to infer that the absence of gut microbiota would abolish the inhibitory effect on this unknown substance and, consequently, similar to what was observed in the cecum, the vessels would decrease their tone and subsequently, present a profound hyporesponsive state. Interestingly, we did not observed any change in the acetylcholine-induced endothelium dependent relaxation or sodium nitroprusside-induced endothelium independent-relaxation in mesenteric resistance arteries from male and female Conv and GF mice. These data suggest that the gut microbiota do not play a role in the endothelial function in the absence of a challenge (e.g. Ang II infusion; high-fat diet, etc) and the vascular hyporesponsivenes observed in GF mice could be, at least in part, due to a vascular remodeling. Also, the absence of microbiota did not change heart weight or morphology. Corroborating these data, Karbach et al. [29] did not observed differences in LV mass in Conv when compared to GF on a Swiss Webster and C57BL/6J background.
Vascular smooth muscle cell structural changes in cardiovascular diseases and aging are collectively termed vascular remodeling [40]. Vascular remodeling is observed when there is a change in diameter of a fully relaxed vessel that is not explained by a change in transmural pressure or compliance, and thus is structural in nature [40]. Remodeling can be increase (hypertrophic), no change (eutrophic) or decrease (hypotrophic) of the cross-sectional area. These forms of remodeling can be reduction (inward) or increase (outward) in the lumen diameter [20]. It is important to emphasize that, the total peripheral resistance is defined by Poiseuille’s law, in which three factors are the primary determinants of the resistance to blood flow within a vessel: lumen diameter, vessel length, and viscosity of the blood. The most significant by far is the lumen diameter, given that vessel resistance is inversely proportional to the radius to the fourth power. Therefore, a 50% reduction in radius should increase resistance sixteen-fold. Adjustments in total peripheral resistance are directly determined by alterations in the morphology and/or function of vascular smooth muscle cells. Therefore, in the present study we wanted to understand if the mechanical and structural proprieties of the arteries from GF mice would be altered. These experiments would clarify if the vascular hyporesponsiveness seen in GF animals occurs chronically. This means that after removal of the arteries from the mesenteric bed of the GF mice, any influence from hormones (e.g. cecum-derived relaxant factor) would not be the determinant factor for the mechanical proprieties. On the other hand, the hyporesponsiveness that we observed in experiments performed using wire myograph, which allow us to observe vascular function (e.g. phenylephrine-induced contraction), could be because the arteries may still be under influences of hormones (e.g. cecum-derived relaxant factor) following immediate mesenteric removal and not due to intrinsic changes of the vessels. If this is the case, changes in vascular function, such as decreased receptor and/or calcium sensitization, due to hormones influences, would mask the intrinsic changes of the vessels from GF mice. Therefore, using pressure-culture myography we were able to observe the most striking difference in resistance artery vasculature between GF and Conv mice. The arteries from male and female GF mice presented marked changes in vascular structure and mechanical properties, suggesting that the absence of the microbiota leads to vascular remodeling, further suggesting that the microbiota are important to ‘shape” the arteries or to the development of the arterial wall. Here we observed that, arteries from female GF mice present an outward hypertrophic remodeling, resulting from an increase in cross-sectional area associated with an increase in the lumen diameter. These observations are also seen in aging, implying that the absence of gut microbiota induces premature vascular aging. Briones et al. [28] have shown that arteries from old rats also present an outward hypertrophic remodeling due to wall and media thickness, greater number of smooth muscle cell layers and an increase in the internal diameter. They suggested that an increase in fibrosis contributes to the thickened media [28]. In the present study, in arteries from male GF mice, increased vascular stiffness was observed along with a decrease in cross-sectional area and internal diameter, which points to an inward hypotrophic remodeling. It is known that circumferential wall stress and wall shear stress are important driving forces during development of the arterial wall and in the adult [41,42]. Only few studies were able to observe inward hypotrophic remodeling [42]. Accordingly, it is known that reduced blood flow results in inward hypotrophic remodeling accompanied by hyporeactivity of the arterial smooth muscle [42]. In the present manuscript, we also observed a pronounced hyporeactivity to adrenergic receptor agonist in arteries from male GF mice. However, a causative relationship between inward hypotrophic remodeling, blood flow and hyporeactivity was not assessed in the present study, and will be the subject of future studies.
In the present study, the sex differences were consistent and marked in the cardiovascular system. It is well known that sex difference plays a fundamental role on arterial function and cardiovascular physiology and pathophysiology [43]. The incidence of cardiovascular disease by sex suggested that premenopausal women had fewer cardiovascular and coronary events compared with men and also compared with postmenopausal women of the same age [43]. Interestingly, in preclinical studies, it has been shown that spontaneously hypertensive female rats (SHR) have lower blood pressures than males which is independent of the renal nerves control [44]. Further, it has been suggested that sex differences in the developmental programming of blood pressure may originate from innate sex-specific differences in expression of the renin angiotensin system that occur in response to adverse influences during early life [45]. Regarding vascular function, it was demonstrated that female but not male mice were sensitive to obesity-induced endothelial dysfunction, whereas endothelial function was impaired in obese hyperlipidemic males and females [46]. In males, obesity or hyperlipidemia decreased the nitric oxide component of vasodilation without altering superoxide production or endothelial nitric oxide synthase expression or phosphorylation. However, endothelial cell mineralocorticoid receptor deletion prevented endothelial dysfunction induced by risk factors only in females [46]. Therefore, based on our observations showing that the complete lack of microbiota has a higher impact in the enlargement of the cecum and vascular contractility in male mice, we can infer that male mice are more susceptible to the absence of microbiota or, in other words, microbiota would have a higher contribution to the vascular homeostasis of the males when compared to females.
In the vasculature, ROS play key roles in signal transduction related to contraction, relaxation, hypertrophy, proliferation, migration, and cell death, and the Nox (NADPH oxidase) isozymes are a major source of these ROS [47]. Also, ROS generated by Nox play an important role in antimicrobial host defense and inflammation. Host immune cells, such as neutrophils release enormous amounts of ROS at the site of infection following the activation of surface receptors [48]. Recent data have demonstrated that gut epithelia contacted by enteric commensal bacteria rapidly generate ROS [49]. Interaction(s) of intestinal epithelia with natural commensal bacterial strains (and formylated peptides) is associated with rapid accumulation of ROS, especially at the leading edge of the migrating cell monolayer [49]. However, it is unclear whether gut commensal bacteria would affect ROS generation by the host innate immune system. In the present study, the absence of microbiota increased ROS generation at the BMDNs from male GF mice. However, when BMDNs were stimulated with PMA, these differences disappeared. On the other hand, ROS generation was not different at the basal levels in BMDNs from female GF mice when compared to female Conv mice. Remarkably, when the BMDNs were stimulated with PMA, ROS generation was increased only in neutrophils from female Conv mice, suggesting that the BMDNs from female GF mice decreased the response to infection. Supporting these data, it has been shown that the immune response of GF mice is blunted, given that these animals produce less of the proinflammatory cytokine tumor necrosis factor α after splenocyte stimulation with lipopolysaccharide [50]. Together, these findings indicate that microbiota are necessary to mount a normal immune response, possibly because microbiota allow long-term exposure of bacteria to the innate immune system.
In the vasculature, ROS production is a precisely controlled process during homeostatic conditions, and disturbance of this mechanism could lead to excessive ROS production, oxidative stress, and irreversible injury to the vasculature. Here, we did not evaluated the ROS generation in the vasculature. However it is possible that the ROS system is also disrupted in the arteries, given that the VSMCs and endothelial cells present several innate immune receptors, such as formyl peptide receptors. Formyl peptide receptor-1 activation exerts a critical role for the dynamic plasticity of arteries via actin polymerization and ROS generation similar to the one observed in sentinel cells (e.g. neutrophils) of the innate immune system [26]. However, the connection between gut microbiota, ROS generation and sex differences, especially in disease conditions, still needs clarification.
In summary, this study establishes a novel role for the homeostasis of the gut microbiota and cardiovascular health showing that the commensal bacteria are essential for normal function and structure of the vascular system. However, it also revealed that the absence of microbiota has a potential link for worse vascular outcomes in males. Therapies targeting the pathophysiological contributions by gut microbiota and modalities by which the host innate immune system maintains surveillance of gut microbiota and its products/metabolites can be improved. Overall, our study constitutes the basis for the discovery of new therapeutic targets and/or mechanistic leads directed towards this new interface between man and microorganisms within the holobiont.
Acknowledgements
We would like to thank Josh Frost and Megan Brockett from National Gnotobiotic Rodent Resource Center (NGRRC) and Scott Bechaz from the University of Toledo College Of Medicine for their excellent technical handling of the germfree animals.
Funding
This work was supported by National Institutes of Health (NIGMS: R00 GM118885 - C.F.W.; NHLBI: R01 HL143082 - B.J., and NCI: R01CA219144-01 – M.V.K.), American Heart Association (18POST34060003 – C.G.M.) and NSF (AGEP#1432878 – J.M.E.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None declared
References
- 1.Margulis L Symbiosis as a source of evolutionary innovation: speciation and morphogenesis. Cambridge, Mass: :MIT Press, c1991. [PubMed] [Google Scholar]
- 2.Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol 14(8): e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell, 164(2016),337–40. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty S, et al. , Salt-Responsive Metabolite, beta-Hydroxybutyrate, Attenuates Hypertension. Cell Rep, 25(3) (2018), 677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galla S, et al. , Disparate effects of antibiotics on hypertension. Physiol Genomics, 50(10) (2018), 837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galla S, et al. , Microbiotal-Host Interactions and Hypertension. Physiology (Bethesda), 32(3) (2017): 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mell B, et al. , Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics, 47(6) (2015), 187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padmanabhan S and Joe B. Towards Precision Medicine for Hypertension: A Review of Genomic, Epigenomic, and Microbiomic Effects on Blood Pressure in Experimental Rat Models and Humans. Physiol Rev, 97(4) (2017), 1469–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh V, et al. , Microbiota-Dependent Hepatic Lipogenesis Mediated by Stearoyl CoA Desaturase 1 (SCD1) Promotes Metabolic Syndrome in TLR5-Deficient Mice. Cell Metab, 22(6) (2015), 983–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh V, et al. , Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell, 175(3) (2018), 679–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waghulde H, et al. , Attenuation of Microbiotal Dysbiosis and Hypertension in a CRISPR/Cas9 Gene Ablation Rat Model of GPER1. Hypertension, 72(5) (2018): 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santisteban MM, et al. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ Res, 120(2) (2017), 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilck N, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature, 551(7682) (2017), 585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridaura VK1, Faith JJ, Rey FE, Cheng J, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science, 341(6150) (2013), 1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durgan DJ. Obstructive Sleep Apnea-Induced Hypertension: Role of the Gut Microbiota. Curr Hypertens Rep. 19(4) (2017), 35. [DOI] [PubMed] [Google Scholar]
- 16.Taylor WR, Takemiya K. Hypertension Opens the Flood Gates to the Gut Microbiota. Circ Res. 20; 120(2) (2017): 249–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, Goel R, Kumar A, Qi Y, Lobaton G, et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci (Lond). 132(6) (2018), 701–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. 16(3) (2019),137–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, et al. Vascular remodeling. Hypertension 28 (1996), 505–506. [PubMed] [Google Scholar]
- 20.Mulvany MJ. Small artery remodelling in hypertension: causes, consequences and therapeutic implications. Med. Biol. Eng. Comput 46 (2008), 461–467. [DOI] [PubMed] [Google Scholar]
- 21.Park JB, Schiffrin EL. Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension. J Hypertens 19(5) (2001), 921–30. [DOI] [PubMed] [Google Scholar]
- 22.Feihl F, Liaudet L, Levy BI, Waeber B (2008). Hypertension and microvascular remodeling. Cardiovasc Res. 1;78(2) (2008), 274–85. [DOI] [PubMed] [Google Scholar]
- 23.Grundy D Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol, 593 (2015), 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wostmann B, Brickner-Kardoss E. Development of cecal distention in germ-free baby rats. Am J Physiol, 197 (1959), 1345–6. [DOI] [PubMed] [Google Scholar]
- 25.Wenceslau CF, Rossoni LV. Rostafuroxin ameliorates endothelial dysfunction and oxidative stress in resistance arteries from deoxycorticosterone acetate-salt hypertensive rats: the role of Na+K+-ATPase/ cSRC pathway. J Hypertens, 32(3) 92014), 542–54. [DOI] [PubMed] [Google Scholar]
- 26.Wenceslau CF, McCarthy CG, Szasz T, Calmasini FB, Mamenko M, Webb RC. Formyl peptide receptor-1 activation exerts a critical role for the dynamic plasticity of arteries via actin polymerization. Pharmacol Res, 141(2019), 276–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels 1068 in spontaneously hypertensive and normotensive rats. Circ Res, 41 (1977),19–26. [DOI] [PubMed] [Google Scholar]
- 28.Briones AM, Salaices M, Vila E. Mechanisms underlying hypertrophic remodeling and increased stiffness of mesenteric resistance arteries from aged rats. J Gerontol A Biol Sci Med Sci, 62 (2007), 696–706. [DOI] [PubMed] [Google Scholar]
- 29.Karbach SH Schönfelder T Brandão I Wilms E, et al. Gut Microbiota Promote Angiotensin II-Induced Arterial Hypertension and Vascular Dysfunction. J Am Heart Assoc, 5(9) (2016), pii: e003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pluznick JL., Gut microbiota in renal physiology: focus on short-chain fatty acids and their receptors. Kidney Int, 90(6) (2016), 1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouwehand A, Isolauri E, Salminen S. The role of the intestinal microflora for the development of the immune system in early childhood. Eur J Nutr, 41 (2002), I32–7. [DOI] [PubMed] [Google Scholar]
- 32.Savage DC and McAllister JS. Cecal Enlargement and Microbial Flora in Suckling Mice Given Antibacterial Drugs. Infect Immun, 3(1971), 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grover M and Kashyap PC. Germ free mice as a model to study effect of gut microbiota on host physiology. Neurogastroenterol Motil, 26(6) (2014), 745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weger BD, Gobet C, Yeung J, Martin E, Jimenez S, et al. The Mouse Microbiome Is Required for Sex-Specific Diurnal Rhythms of Gene Expression and Metabolism. Cell Metab, 5;29(2) (2019), 362–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheema MU, Pluznick JL. Gut Microbiota Plays a Central Role to Modulate the Plasma and Fecal Metabolomes in Response to Angiotensin II. Hypertension, 74(2019), 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markle JG1, Frank DN, Mortin-Toth S, Robertson CE, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science, 339(2013), 1084–8. [DOI] [PubMed] [Google Scholar]
- 37.Baez S, Gordon HA. Tone and reactivity of vascular smooth muscle in germfree rat mesentery. J Exp Med. 134 (1971):846–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ, 27(2003), 201–6. [DOI] [PubMed] [Google Scholar]
- 39.Gordon HA Demonstration of a bioactive substance in the caecal contents of the germfree animals. Nature (London), 205 (1965), 571. [Google Scholar]
- 40.Martinez-Quinones P, McCarthy CG, Watts SW, Klee NS, et al. Hypertension Induced Morphological and Physiological Changes in Cells of the Arterial Wall. Am J Hypertens, 31(10) (2018), 1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubanyi GM, Freany AD, Kauser K, Johns A, and Harder DR. Mechanoreception by the endothelium: mediators and mechanisms of pressure- and flow-induced vascular response. Blood Vessels, 27(1990), 246–257. [DOI] [PubMed] [Google Scholar]
- 42.Pourageaud F and De Mey JGR. Structural properties of rat mesenteric small arteries after 4-wk exposure to elevated or reduced blood flow. Am. J. Physiol - Heart Circ. Physiol, 273 (1997), H1699–H1706. [DOI] [PubMed] [Google Scholar]
- 43.Green DJ, Hopkins ND, Jones H, et al. Sex differences in vascular endothelial function and health in humans: impacts of exercise. Exp Physiol, 101(2) (2016), 230–42. [DOI] [PubMed] [Google Scholar]
- 44.Iliescu R1, Yanes LL, Bell W, Dwyer T, et al. Role of the renal nerves in blood pressure in male and female SHR. Am J Physiol Regul Integr Comp Physiol, 290(2) (2006) R341–4. [DOI] [PubMed] [Google Scholar]
- 45.Ojeda NB, Intapad S, Alexander BT. Sex differences in the developmental programming of hypertension. Acta Physiol (Oxf), 210(2) (2014), 307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davel AP, Lu Q, Moss ME, Rao S, et al. Sex-Specific Mechanisms of Resistance Vessel Endothelial Dysfunction Induced by Cardiometabolic Risk Factors. J Am Heart Assoc, 16;7(4) (2018), pii: e007675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wenceslau CF, McCarthy CG, Webb RC. To Be, or Nox to Be, Endoplasmic Reticulum Stress in Hypertension. Hypertension, 72(1) (2018), 59–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen GT, Green ER, Mecsas J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front Cell Infect Microbiol, 7 (2017), 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones RM, Mercante JW, Neish AS. Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic implications. Curr Med Chem, 19(10) (2012),1519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clarke G, Grenham S, Scully P, Fitzgerald P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry, 18(6) (2013), 666–73. [DOI] [PubMed] [Google Scholar]