Abstract
In the present study, febuxostat (FBX)-loaded PEG-coated PLGA nanoparticles (FBX–PLGA–PEG) were developed and its anticancer activity on lung cancer cells was evaluated. FBX–PLGA–PEG were prepared by nanoprecipitation technique and characterized for particle size, size distribution, entrapment efficiency, and in vitro drug release study. The optimized formulations were used to evaluate cell viability, apoptosis, cell cycle, and caspase activity in A549 lung cancer cells. The optimized formulation showed spherical particle with average particle size of 180 ± 4.72 nm, particle-size distribution 0.223 ± 0.003, entrapment efficiency (70 ± 2.56%), and drug release (99.1 ± 2.33%) at 12 h. MTT cytotoxicity assay showed better cytotoxic potential of FBX-NPs than FBX solution against NSCLC A549 cells. The lower IC50 of FBX-NP (52.62 ± 2.52 µg/mL) compared to FBX (68.0 ± 4.12 µg/mL) are suggestive of a potential cytotoxic effect of nano-formulation compared to the drug itself. Furthermore, flow cytometry analysis showed significantly higher percentage of total apoptotic cells in FBX-NPs (10.38 ± 1.57%) as compared to FBX solution (2.76 ± 0.17%) showed strong proapoptotic potential of FBX nano-formulation. The increased caspase activity and percent of cells at G2/M phase of cell cycle increased for FBX nanoparticles were more effective than FBX solution in increasing caspase activity and percent of cells at G2/M phase of cell cycle. Our studies with FBX nanoparticles exhibited promising outcome which could be used as a strategies to combat lung cancer.
Keywords: Lung cancer, Febuxostat, Nanoparticles, Apoptosis, PLGA
Introduction
Nanomedicine has opened opportunities to overcome various obstacles in cancer therapy, though not able to implement in clinical settings as expected (Bor et al. 2019). Nanoparticles are extensively studied systems for a large number of therapeutic agents (Hauser-Kawaguchi and Luyt 2015). The nanostructures can significantly improve the therapeutic action of otherwise administered chemotherapeutic agents. Tumor-targeted nanoparticles are further advanced systems to avoid toxicity to normal tissues and improve treatment efficacy (Swain et al. 2016). The fabrication of nanoparticles depends upon the type of cancer and whether active or passive targeting (Torchilin 2010). Moreover, the formulation also warrants considerations on acidic tumor microenvironment, drug resistance, and efflux system (Fernandes et al. 2018). A variety of nanoparticles are available for tumor-targeted delivery such as polymeric, inorganic, micelles, polyplexes, drug–protein or drug–DNA conjugates, dendrimers, liposomes, and polymersomes (Swain et al. 2016). Out of these, polymeric systems are extensively studied and are well promising (Masood and Masood 2016). Poly(lactic-coglycolic acid) popularly known as PLGA is a widely employed copolymer which is biocompatible and biodegradable. These polymers are successful in encapsulation and targeting of gene and drug to tumors (Danhier et al. 2012). PLGA nanoparticles provide a plethora of opportunities to tune for a successful tumor-targeted delivery system. Unfortunately, PLGA polymers also suffer some disadvantages. It degrades to acidic species and the induction of local tissue damage can occur (Makadia and Siegel 2011; Dailey et al. 2006). The hydrophobicity of the PLGA polymer is another issue when we consider the drug loading efficiency, particularly in the case of hydrophilic drugs (Hill et al. 2019). At the same time, the advantage of polyethylene glycol (PEG)-based stealth coatings on nanoparticles was identified (Hadjesfandiari and Parambath 2018; Suk et al. 2016). The coatings provided long circulation time by avoiding opsonization. Subsequently, PEG-coated nanoparticulate systems were under highlight (Suk et al. 2016; Mishra et al. 2016; Pelaz et al. 2015). Immediately, immense studies were reported with amphiphilic block copolymers comprising of both the hydrophilic and hydrophilic molecules. PEG-PLGA diblock copolymer is such an example. The use of PEG-PLGA polymer is expected to provide a better drug loading along with prolonged circulation time. Both these properties could be owed to the presence of surface PEG molecules (Almoustafa et al. 2017; Wilkosz et al. 2018). Besides, nanoparticles with PEG-PLGA polymer shows enhanced drug release compared to nanoparticles with PLGA polymer alone (Rafiei and Haddadi 2017).
Lung cancers are one of the major contributors to cancer-caused deaths. Among them, non-small cell lung cancer (NSCLC) is the common one (Zappa et al. 2016; Zhao et al. 2018). Therefore, evaluation of anticancer activity against NSCLC cells has gained importance in drug discovery and screening process. In this scenario, NSCLC cells are nowadays used widely. Among the NSCLC cells, A549 is the most employed cell line (Gazdar et al. 2010). Febuxostat (FBX) is a hyperuricemic agent used for the treatment of gout and has been formulated as tablets, solid dispersion, and emulsions (Bisht and Bist 2011; Asif et al. 2016; Song et al. 2014). Recently, it has been a trend to evaluate the already approved molecules for another indication. This repurposing of existing drugs avoids the major hurdles in the drug approval process such as toxicity studies (Pushpakom et al. 2018). Febuxostat is reported for its actions such as prophylaxis for tumor lysis syndrome (Tamura et al. 2016). Thus, establishing the anti-tumor activity of febuxostat would be beneficial in repurposing its use. Here, for the first time, we reported the anti-tumor activity of febuxostat in A549 NSCLC cells from a PEG-PLGA nanoparticulate system. Therefore, in the present study, FBX-loaded PEG-coated PLGA nanoparticles were developed and characterized in terms of their particle size, particle-size distribution, and in vitro drug release study. Furthermore, prepared formulations were evaluated for cell viability, apoptosis, cell cycle arrest, and caspase activity in A549 NSCLC lung cancer cells.
Material and methods
Materials
The active ingredient, febuxostat (FBX), was obtained from (SPIMACO. Al-Qassem, Saudi Arabia) as gift sample. TPGS and dialysis membrane of 12 kDa cut-off were purchased from Sigma-Aldrich. PEG–PLGA, PEG Mn 2,000, PLGA Mn 5000 (lactide:glycolide 50:50), acetone, and other chemicals used were purchased from the Sigma-Aldrich Co. (St Louis, MO, USA). Rest of the chemicals used in this current experiment were of analytical grade.
Preparation of nanoparticles
The preparation of PEG-PLGA nanoparticles was executed by a process adapted from reported procedures (Donghai et al. 2013; Durán-Lobato et al. 2015). The preparation of febuxostat-loaded PEG-PLGA (FBX–PLGA–PEG) nanoparticles was carried out by nanoprecipitation. Briefly, 100 mg of PEG-PLGA copolymer was added to 8 mL of acetone and dissolved. The polymer solution was then added dropwise under magnetic stirring to 100 mL of aqueous Tween 80 solution (0.2% w/v). The resultant sample was stirred further for around 2 h at 60 °C. The resultant nanoparticle suspension was then centrifuged at 12000 rpm for 20 min. The obtained FBX–PLGA–PEG nanoparticles were then subjected to dialysis against water to remove any residual solvent. The purified nanoparticles were then freeze-dried and stored until further studies. Similarly, nanoparticles were also prepared using 150 and 200 mg copolymer.
Characterization of FBX–PLGA–PEGnanoparticles
Characterization of FBX–PLGA–PEGnanoparticles was done by particle-size analysis and transmission electron microscopy (TEM). Particle size was carried out after dispersion in deionized water. The particle size and polydispersity index (PDI) were determined by Zetatrac® (Microtrac Inc., Montgomeryville, PA, USA) using dynamic light scattering mode. TEM was carried out in a JEOL 2100F electron microscope (JEOL JEM1010, Tokyo, Japan). The images were obtained after placing a drop of the aqueous dispersion of nanoparticles over a copper-coated grid and dried overnight at room temperature and viewed under TEM at an operating voltage of 80 kV.
Entrapment efficiency
For determination of entrapment efficiency (EE), an accurate quantity of freeze-dried nanoparticles was allowed to dissolve in a predetermined volume of acetone. The sample was centrifuged and subsequently centrifuged at 3000 rpm for 15 min. The resultant supernatant was then analyzed for FBX content. EE was determined as the percent ratio of drug added initially to the amount present in the nanoparticle (Nassir et al. 2018):
In vitro drug release study
The drug release was studied by the dialysis bag method (Nassir et al. 2018; Singh et al. 2016). The freeze-dried FBX–PLGA–PEGnanoparticles were weighed and introduced into an activated dialysis bag (Molecular weight cut-off—12,000 Da) and sealed. The loaded dialysis bag was introduced into 100 mL of the phosphate-buffer saline (PBS) pH 7.4 medium. Aliquots were withdrawn, with subsequent replenishment with fresh medium, from the medium to maintain sink condition at predetermined time points (3, 4, 6, 8, 24, 48, and 72 h). Samples were centrifuged at 13,500 rpm for 15 min and analyzed by validated HPLC method. All the formulations were subjected to in vitro release in a set of three.
In vitro cell culture experiment
Adenocarcinomic human alveolar basal epithelial cells (A549) (ATCC, USA) was obtained from King Fahd Medical Centre, Jeddah, Saudi Arabia, and maintained in mammalian cell culture media (RPMI 1640, Life Technologies (Carlsbad, CA) which was supplemented with 10 U/mL penicillin and 10 µg/mL streptomycin, 10% (v/v) fetal bovine serum, and 0.25 µg/mL of amphotericin B in a humidified environment of 37 °C, 5% CO2. Rests of the chemicals used in this current experiment were of analytical grade.
Cell viability using MTT assay
The cell viability assay of the prepared FBX–PLGA–PEGnanoparticles was carried out in A549 non-small cell lung cancer cells (ATCC, USA). Briefly, approximately 1 × 105 mL−1 cells were seeded in a 96-well plate and incubated for 24 h at 37ºC in a 5% CO2 incubator. The cells were then exposed to the FBX–PLGA–PEGnanoparticles, FBX solution and placebo NPs for 24 h. The plates were incubated at 37 °C in 5% CO2 for 24 h followed by 5 h incubation after adding 20 µL MTT solution (5 mg/mL) per well. The supernatant MTT solution was removed from the wells and DMSO was added (200 µL) to each well. The medium was removed by centrifugation and the optical density of the supernatant was determined at 570 nm (Nassir et al. 2018; Zhao et al. 2017).
Apoptosis assay by flow cytometry using AnnexinV FITC-PI
The apoptotic effect of FBX–PLGA–PEGnanoparticles and FBX solution was determined using dual staining technique in A549 cells. A reported method was adopted for the study (Hsiao et al. 2016). A549 lung cancer cells were seeded in a 6-well plate at a density of 1 × 105 cells per well and incubated for 24 h. Cells were then treated with FBX-NPs and FBX solution for 24 h. Successively, by the addition of trypsin, the cells were harvested and centrifuged for 5 min at 1500 rpm. The cells were then dispersed in 500 μl binding buffer. The cells were stained using AnnexinV FITC-PI (BD Bioscience, CA, USA) as per the manufacturer’s instruction and quantification was carried out by flow cytometry (FACS Calibur, BD Bioscience, USA) and obtained data were evaluated via Multicycle software (Phoenix Flow Systems, San Diego, CA).
Cell cycle analysis by flow cytometry
This study was carried out by employing reported methods (Hsiao et al. 2016; Pumiputavon et al. 2017). For the purpose of studying cell cycle analysis, A549 cells were cultured for 24 h in the presence of FBX–PLGA–PEGnanoparticles. After incubation, the cells were separated by centrifugation and fixed with cold ethanol (70%). The cells were once again separated by centrifugation and washed with PBS buffer. Staining was carried out for 15 min in PBS containing propidium iodide (PI) and RNase staining buffer at room temperature. Flow cytometry was carried out for the final analysis of the sample.
Caspase activity
For studying the caspase activity, the procedure was carried out as per the directions mentioned on the assay kit (BD Biosciences, CA, USA). The human cervical cancer cells (5 × 104 cells per well) were treated with the FBX–PLGA–PEGnanoparticles for 24 h (Nassir et al. 2018; Baharara et al. 2016). The caspase activity was determined after measuring the absorbance of the cell lysate at 405 nm in a plate reader.
Statistical analysis
The results are represented as average ± SD, and data were analyzed using Student's t test and one-way ANOVA followed by Tukey's post hoc test (n = 3) for assessing statistical significance (p < 0.05) using SPSS software.
Results and discussion
Preparation of nanoparticles
PEG-PLGA nanoparticles were prepared using three different quantities of PEG-PLGA. The employed nanoprecipitation method was simple and no high-shear process was involved. The formed PEG-PLGA nanoparticles were expected of a typical structure formed by accumulated polymeric chains. Most importantly, they can be presumed under non-equilibrium thermodynamics (Zhu 2013). The formed nanoparticle structure can be imagined as a core consisting of PEG, PLGA, and drug molecules. Thus, the excessive presence of PEG molecules near the surface of the nanoparticles could be justified owing to its hydrophilicity (Almoustafa et al. 2017).
Characterization of nanoparticles using zetasizer and TEM
The observed particle size and polydispersity index of the nanoparticles are provided in Table 1. The mean particle size ranged from 180 to 275 nm when PEG concentration was varied from 200 to 100 mg. A linear, but an inverse relationship was observed between particle size and amount of PEG-PLGA used in the preparation. This observation was in contradiction to existing knowledge on the influence of PLGA concentration on particle size. The concentration of PLGA is reported to have a direct relationship with the particle size of their nanoparticles (Sahin et al. 2017). The major reason proposed for this observation is the inability of the entrapped solvent to move out of formed nanoparticles. From our observations, what we suggest is that the hydrophilic PEG part of the diblock copolymer, PEG-PLGA, would have facilitated a better escape of solvent molecules from the nanoparticles (Sahin et al. 2017; Singh et al. 2012). Moreover, high PEG-PLGA concentrations would provide more surface molecules of PEG which could result in the better escape of solvent molecules, thus enabling a better shrinking of nanoparticles and resulting in lower particle size (Sahin et al. 2017). Interestingly, we would also another mechanism for this observation based on higher hydrophilicity of PEG molecules. The better hydrophilicity might have resulted in less number of PEG-PLGA molecules per nanoparticle, thus resulting in lower particle size (Sahin et al. 2017). We could also assume that the above two mechanisms complement each other, with all processes favoring lower particle size with higher polymer concentration. However, one could not forget about the influence of solvent volume and stabilizer concentrations for a conclusion of this result. However, fortunately, we have kept these factors fixed, gaining a piece of more conclusive evidence for our assumptions. The polydispersity index (PDI) is used to indicate the particle-size distribution within the sample. The PDI value obtained by zetasizer (0.223 ± 0.003) which is lower than 0.5 indicates the unimodal and narrow particle-size distribution and particles are homogenously and uniformly distributed (Kalam and Alshamsan 2017; Singh et al. 2018; Tzeyung et al. 2019).
Table 1.
Composition, particle size, PDI, and EE % of FBX-loaded PLGA NPs’ formulations
| Formulation | Febuxostat (mg) | PEG-PLGA (mg) | PS ± SD (nm) | PDI ± SD | EE ± SD (%) |
|---|---|---|---|---|---|
| F1 | 50 | 100 | 275 ± 5.78 | 0.345 ± 0.005 | 42 ± 1.39 |
| F2 | 50 | 150 | 215 ± 4.22 | 0.287 ± 0.004 | 55 ± 1.88 |
| F3 | 50 | 200 | 180 ± 4.72 | 0.223 ± 0.003 | 70 ± 2.56 |
The particle size of formulation F3 was near to 200 nm for the formulations (Fig. 1a). The low PDI values were suggestive of the uniform particle-size distribution of nanoparticles (Fig. 1a). This was further confirmed from the TEM image of the formulation (Fig. 1b). The particle size measured through TEM was smaller as compared to zetasizer due to the dehydration of NPs during sample preparation leads to smaller particles (Singh et al. 2018; Tzeyung et al. 2019). The particles were almost spherical, of course with slight distortion in shape, pointing towards a lesser rigidness to the nanoparticle structure. A density difference towards the surface of the nanoparticles could be identified. The presence of surface PEG molecules is the major contributor to this observation. More precisely, a dense brush confirmation would have resulted from surface PEG. Similar results were previously reported for PEG-PLGA nanoparticles (Xu et al. 2015).
Fig. 1.
Particle size, size distribution (a) and TEM image (b) of FBX-loaded PEG-PLGA nanoparticles
Entrapment efficiency
The entrapment efficiency obtained for the formulations is listed in Table 1. The drug, febuxostat, has poor water solubility. Thus, it is reasonable to assume that the drug will be placed near the hydrophobic region of the amphiphilic copolymer PEG-PLGA. However, it is known that hydrophobic drugs are not to properly utilize the hydrophobic are of PLGA molecules in the PEG-PLGA nanoparticles (Wilkosz et al. 2018). Thus, they are more likely to occupy near the surface of the nanoparticles. In our study, we could achieve a drastic increase in entrapment efficiency by employing a higher amount of PEG-PLGA polymer. As proposed under particle-size analysis, higher concentrations of PEG-PLGA polymer might have resulted in the better surface presentation of PEG molecules and better solvent movement out of the nanoparticles (Wilkosz et al. 2018; Kalam and Alshamsan 2017). The same could be presented as an underlying mechanism of better drug entrapment inside the nanoparticles. Also, PEG molecules could have some cosolvent action to solubilize the drug. Yet, another possibility is the larger surface area of the articles due to lower particle size at a higher amount of polymer (causing a higher polymer concentration). The large surface area promotes more surface incorporation of drug molecules, thus resulting in higher entrapment efficiency (Kalam and Alshamsan 2017). This mechanism might have more influence on entrapment efficiency when compared to the former two. Overall, all these might have resulted in a better entrapment efficiency at higher polymer concentrations.
In vitro drug release study
In a previously reported study, PEG has shown a definite effect on drug release from PLGA nanoparticles (Rafiei and Haddadi 2017). In their study, a distinct enhancement of drug release from the PEG-PLGA system was observed for the drug (Fig. 2). In our case also, PEG-PLGA system has caused a marked increase in the drug release of febuxostat. An initial burst release of febuxostat could be observed from the PEG-PLGA nanoparticles. This supported the assumption of better surface loading of drug in the nanoparticles. The burst release was observed for about 2 h (69.32 ± 1.67). Later on, there was a gradual slow release of the drug (99.1 ± 2.33%). This might be due to the diffusion of the entrapped drug into the dissolution medium. The release study confirms that the release of FBX from PEG–PLGA NPs is controlled by both erosion and following diffusion of the degradation products through the pores (Singh et al. 2012). A similar drug release was observed for febuxostat from tablets in a previous study (Asif et al. 2016). Unfortunately, a direct comparison of this drug from tablets and nanoparticles are not possible. Nevertheless, it can be seen that the dissolution behavior of the drug is similar.
Fig. 2.
In vitro drug release profile of free FBX and FBX NPs. Data are expressed as mean ± SD (n = 3)
Cytotoxic effect/s of FBX and FBX-NP
Evaluation of cytotoxic potential of a natural or synthetic drug against cancerous cells has been the most widely used assay (Bahuguna et al. 2017). Therefore, the effect of FBX and its nano-formulation (FBX-NP) was evaluated on a human non-small cell lung cancer cell line (NSCLC) A549. After 24 h of treatment, both FBX as well as FBX-NP showed a dose-dependent response, evident by the cell viability (Fig. 3). While the difference in cell death effects was not much significant at lower concentrations, however, the effect was more pronounced in FBX-NPs treated cells at higher concentrations (Fig. 3). Overall, the cytotoxicity of FBX-NPs was more pronounced and significant (p ≤ 0.05) compared to FBX alone (Fig. 3), suggestive of a better cytotoxic potential of Febuxostat when used as a nano-formulation. In concordance, the lower IC50 of FBX-NP (52.62 ± 2.52 µg/mL) compared to FBX (68.0 ± 4.12 µg/mL) are also suggestive of a potential cytotoxic effect of nano-formulation compared to the drug itself. The higher cytotoxic effect of FBX-NPs could be ascribed to the smaller overall dimension of the nano-formulation, thereby facilitating its passive transport into the cell compared to nascent FBX. Taken together, the results are indicative of a better cytotoxic potential of FBX-NP than FBX alone against NSCLC A549 cells.
Fig. 3.
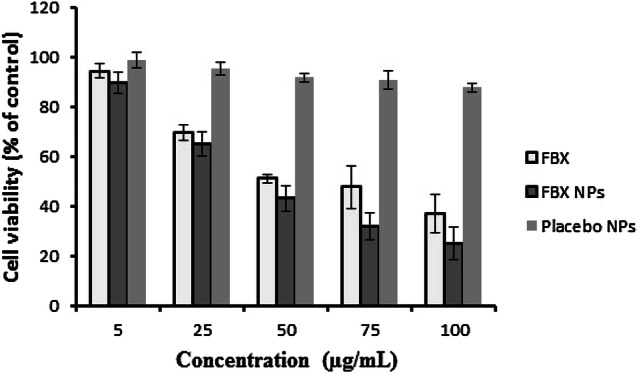
Cell viability of lung cancer A549 cells treated with different concentrations of free FBX, FBX-loaded nanoparticles (FBX NPs), and placebo NPs. Data are expressed as mean ± SD (n = 3)
Effect of FBX and FBX-NP on apoptosis induction
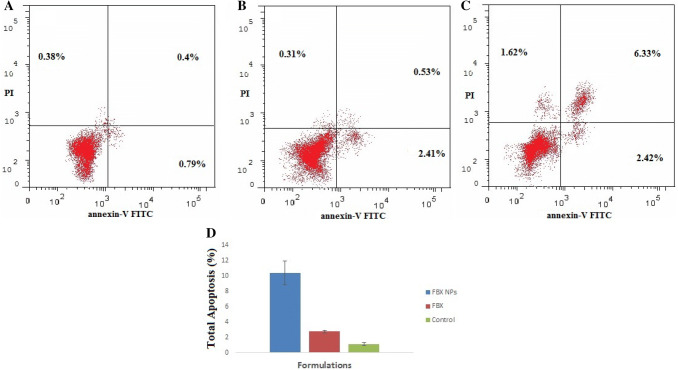
To study the underlying mechanism of cytotoxic induction by FBX and FBX-NP, effect on cell apoptosis was studied by employing Flow cytometry, using annexin V/FITC kit. It was observed that FBX (68.0 ± 4.12 µg/mL/24 h.) significantly induced cellular apoptosis compared to the control (Fig. 4); however, treatment with FBX-NP (52.62 ± 2.52 µg/mL/24 h.) significantly increased the proportion of apoptotic cells compared to either control or FBX-treated A549 cells. Looking at the results, FBX-NPs treated groups induced cell necrosis (1.62%) compared to FBX (0.31%) alone or control (0.38%) groups. Furthermore, the percentage of sum total of early and late apoptotic cells in FBX-NP (10.38 ± 1.57%) treatment group was significantly higher compared to either FBX (2.76 ± 0.17%) or control (1.06 ± 0.19%), suggestive of a strong pro apoptotic potential of FBX nano-formulation. Apoptosis is the programmed cell death phenomenon intended to remove the cells with extensive damage to DNA to avoid falling prey to various etiologies including cancer (Hanahan and Weinberg 2011). Apoptotic evasion being a well-known cancer hallmark has been a key target in cancer therapy (Kerr et al. 1972). After obtaining better cytotoxic results, we sought to find whether such inhibitory effects are associated with apoptosis induction in NSCLC cells. Previous reports about FBX as a therapeutic molecule against tumor lysis disease progression prompted us to evaluate anticancerous potential of Febuxostat and its nano-formulation in NSCLC cells. Apoptosis can be either intrinsic or extrinsic, both of which converge by the executioner caspases at the final pathway (Xu 2015). Annexin V (AV) is a Ca2+-dependent phospholipid-binding protein with higher affinity for phosphatidyl serine (PS) which is found towards the cytosolic side of the cell membrane. After staining with annexin V FITC/PI, four types of cells are detected—live, necrotic, early, and late apoptotic cells based on the staining results (Kim 2007). It can be speculated that both FBX and its nano-composite FBX-NPs activated the intrinsic apoptotic pathway which is supported by the fact that a well-marked induction of caspases-3 to initiate apoptosis (Cory and Adams 2002; Lin 2018; Green and Llambi 2015). Furthermore, according to some previous reports caspase 3 activation marks the irreversible commitment towards cell apoptosis (Thornberry and Lazebnik 1998), which also is in concordance with our results. Likely, this could be one aspect of the mechanism/s responsible for increased cell death and increased apoptosis by FBX or its nano-formulation FBX-NP; however, more studies are needed to explore this mechanism of action comprehensively.
Fig. 4.
Dot plots of FACS analysis using an annexin V-FITC probe showed induction of apoptotic cell death: a control group, b free FBX, and c FBX NPs. d Bar graphs of FACS analysis showing greater apoptotic cell death induced by FBX NPs treatment compared to free FBX. The data were analyzed using one-way ANOVA followed by Tukey's post hoc test. Percentage of cells in each group was compared with control; p < 0.05 indicates a significant difference
Effect of FBX and FBX-NP on cell cycle
To explore whether inducing cell apoptosis is related to cell cycle progression, FBX and FBX-NP-treated cells were subjected to FACS analysis, using Propidium Iodide staining. It was found that both FBX as well as FBX-NP-treated groups significantly induced cell cycle arrest compared to control group (Fig. 5). Though there was no significant effect on Go/G1 or G1/S phases, however, the proportion of cells at G2/M significantly increased post-treatment with either FBX (12.96 ± 1.38%) or FBX-NPs (21.07 ± 3.67) (Fig. 5). Furthermore, FBX (2.49 ± 0.73%) as well as FBX-NP (12.46 ± 1.83%) treated groups showed a tremendous rise in sub G1 populations; however, the extent was much more pronounced in FBX-NP compared to FBX alone, indicating that such effect could be due to cells undergoing apoptosis. Overall the results suggest that both FBX and FBX-NP had a well-pronounced effect on cell cycle arrest, and the presence of sub G1cells indicates a strong apoptotic effect owing to fragmented DNA.
Fig. 5.
Flow cytometric analysis showing the effect of a control group (untreated A549 cells), b free FBX, and c FBX NPs on A549 lung cancer cell lines at 24 h on cell cycle. d Bar graph represent each cell cycle phase, Sub G1 phase, G0/G1 phase, and G2/M and S phase, and results are represented as the mean ± SD (n = 3). Y-axis represents number of cell population (%) and X-axis represents FL2H flow cytometric analysis stained with propidium iodide (PI) histograms. The data were analyzed using one-way ANOVA followed by Tukey's post hoc test. Percentage of cells in each group was compared with control; p < 0.05 indicates a significant difference. The orange peak represents G1 phase in the cell cycle and the red peak represents G2/M phase in the cell cycle
Furthermore, we carried out caspase 3 activity assay to assess further if FBX and its nano-formulation induce caspases 3 activity, a well-known marker of cell apoptosis. It was observed that both FBX (323.6 ± 12 pg/mL) and FBX-NPs (495 ± 15.3 pg/mL) treatment for 24 significantly increased caspases 3 activity respectively by almost 10.91- and 7.13-fold compared to that of control treated (45.3 ± 9.5 pg/mL) cells (Fig. 6). At molecular level also, both FBX as well as FBX-NPs were more promising in inducing caspase 3 activity compared to control, though the induction was higher in FBX-NP than the FBX group (Fig. 6).
Fig. 6.
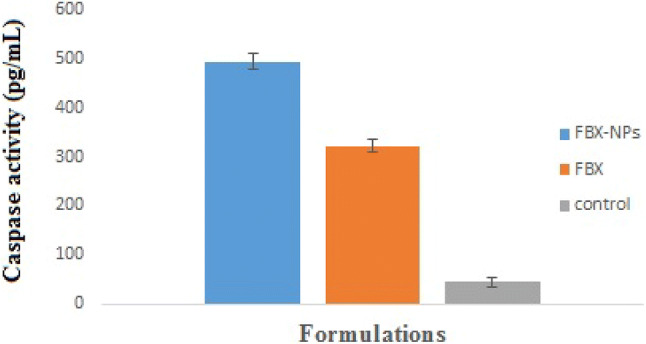
Caspase activity of free FBX and FBX NPs
Unrestrained cell proliferation characterized by aberrant cell cycle is one of the most conspicuous cancer hallmarks (Hanahan and Weinberg 2011), so tempting cell cycle arrest is a desirable attribute for most anticancer drugs. In a stimulated phase, cancerous cells incline to remain in a state of active growth and division characterized by a spontaneous transition between G1-S and G2-M phases of cell cycle. However, in normal cells, this progression is regulated by checkpoints—G1/S (in cells tending to enter synthesis phase) and G2/M (cells tending to enter active mitotic phase). The G1/S checkpoint is a limiting step, and if the cancerous cells are checked at this stage, the continued proliferative propensities could be abrogated efficiently. In the present study, we did not observe an increase/arrest of G0/G1 cells upon FBX or FBX-NP treatment. Also, there was no significant S phase arrest upon either FBX or FBX-NP treatment; however, a significant rise/increase at G2/M transition both FBX as well as FBX-NP groups is indicative of a potent failure of cells to enter mitosis, indicative of kind of cell death known as mitotic cell death or mitotic catastrophe (Skladanowski and Larsen 1997; Dou et al. 1995). Talking about cell cycle arrest, neither FBX nor FBX-NPs showed any significant change in cell cycle arrest compared to control.
Conclusions
In conclusion, febuxostat-loaded PEG-coated PLGA nanoparticles were developed, characterized, and evaluated for lung cancer cells. The prepared FBX NPs nanoparticles showed a satisfactory particle size, narrow PDI, spherical shape, high entrapment efficiency, and sustain drug release. The release study confirms that the release of FBX from PEG–PLGA NPs is controlled by both erosion and following diffusion of the degradation products through the pores. MTT cytotoxicity assay showed a better cytotoxic potential of FBX-NP than FBX solution against NSCLC A549 cells. The percentage of sum total apoptotic cells in FBX-NPs was significantly higher compared to FBX solution showed strong pro apoptotic potential of FBX nano-formulation. Cell cycle status and caspase activity showed that FBX NPs induced apoptosis in A549 cells. Based on these results, it can be concluded that the FBX NPs could be used as promising strategies for the treatment of lung cancer because of its high entrapment efficiency and satisfactory efficacy in cancer cell line. However, further animals and human studies are required to establish the benefit–risk ratio in using this approach for the treatment of lung cancer.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Research Group project under Grant Number (R.G.P.1/149/40).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s13205-024-04007-5
Change history
5/30/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s13205-024-04007-5
Contributor Information
Mohammad Y. Alfaifi, Email: alfaifi@kku.edu.sa
Shadab Md, Email: Shadabmd1982@gmail.com.
References
- Almoustafa HA, Alshawsh MA, Chik Z. Technical aspects of preparing PEG-PLGA nanoparticles as carrier for chemotherapeutic agents by nanoprecipitation method. Int J Pharm. 2017;533:275–284. doi: 10.1016/j.ijpharm.2017.09.054. [DOI] [PubMed] [Google Scholar]
- Asif U, Sherwani AK, Akhtar N, Shoaib MH, Hanif M, Qadir MI, Zaman M. Formulation development and optimization of febuxostat tablets by direct compression method. Adv Polym Technol. 2016;35:129–135. doi: 10.1002/adv.21536. [DOI] [Google Scholar]
- Baharara J, Ramezani T, Divsalar A, Mousavi M, Seyedarabi A. Induction of apoptosis by green synthesized gold nanoparticles through activation of caspase-3 and 9 in human cervical cancer cells. Avicenna J Med Biotechnol. 2016;8:75–83. [PMC free article] [PubMed] [Google Scholar]
- Bahuguna Ashutosh, Khan Imran, Bajpai Vivek K., Kang Sun Chul. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh Journal of Pharmacology. 2017;12(2):8. [Google Scholar]
- Bisht M, Bist SS. Febuxostat: a novel agent for management of hyperuricemia in gout. Indian J Pharm Sci. 2011;73:597–600. doi: 10.4103/0250-474X.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor G, Mat Azmi ID, Yaghmur A. Nanomedicines for cancer therapy: current status, challenges and future prospects. Ther Deliv. 2019;10:113–132. doi: 10.4155/tde-2018-0062. [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Dailey LA, Jekel N, Fink L, Gessler T, Schmehl T, Wittmar M, Kissel T, Seeger W. Investigation of the proinflammatory potential of biodegradable nanoparticle drug delivery systems in the lung. Toxicol Appl Pharmacol. 2006;215:100–108. doi: 10.1016/j.taap.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Danhier F, Ansorena E, Silva JM, Coco R, Breton AL, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- Donghai L, Li G, Qin L, Wen Z, Wang J, Sun X. Preparation, characterization and uptake of PEG-coated, muco-inert nanoparticles in HGC-27 cells, a mucin-producing, gastric-cancer cell line. J Biomed Nanotechnol. 2013;9:2017–2023. doi: 10.1166/jbn.2013.1708. [DOI] [PubMed] [Google Scholar]
- Dou QP, An B, Will PL. Induction of a retinoblastoma phosphatase activity by anticancer drugs accompanies p53-independent G1 arrest and apoptosis. Proc Natl Acad Sci USA. 1995;92(20):9019–9023. doi: 10.1073/pnas.92.20.9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán-Lobato M, Martín-Banderas L, Gonçalves LMD, Fernández-Arévalo M, Almeida AJ. Comparative study of chitosan- and PEG-coated lipid and PLGA nanoparticles as oral delivery systems for cannabinoids. J Nanoparticle Res. 2015;17:61. doi: 10.1007/s11051-015-2875-y. [DOI] [Google Scholar]
- Fernandes C, Suares D, Yergeri MC. tumor microenvironment targeted nanotherapy. Front Pharmacol. 2018;9:1230. doi: 10.3389/fphar.2018.01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar AF, Girard L, Lockwood WW, Lam WL, Minna JD. Lung cancer cell lines as tools for biomedical discovery and research. J Natl Cancer Inst. 2010;102:1310–1321. doi: 10.1093/jnci/djq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Llambi F. Cell death signaling. Cold Spring Harbor Perspect Biol. 2015;7(12):a006080. doi: 10.1101/cshperspect.a006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjesfandiari Narges, Parambath Anilkumar. Engineering of Biomaterials for Drug Delivery Systems. 2018. Stealth coatings for nanoparticles; pp. 345–361. [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hauser-Kawaguchi Alexandra M. N., Luyt Leonard G. Cancer Metastasis - Biology and Treatment. Cham: Springer International Publishing; 2014. Nanomedicine—Nanoparticles in Cancer Imaging and Therapy; pp. 205–244. [Google Scholar]
- Hill M, Cunningham RN, Hathout RM, Johnston C, Hardy JG, Migaud ME. Formulation of antimicrobial tobramycin loaded PLGA nanoparticles via complexation with AOT. J Funct Biomater. 2019;10:26. doi: 10.3390/jfb10020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao KY, Wu Y-J, Liu ZN, Chuang CW, Huang HH, Kuo SM. Anticancer effects of sinulariolide-conjugated hyaluronan nanoparticles on lung adenocarcinoma cells. Molecules. 2016;21:297. doi: 10.3390/molecules21030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalam MA, Alshamsan A. Poly (d, l-lactide-co-glycolide) nanoparticles for sustained release of tacrolimus in rabbit eyes. Biomed Pharmacother. 2017;94:402–411. doi: 10.1016/j.biopha.2017.07.110. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, et al. Protein kinase C-ERK1/2 signal pathway switches glucose depletion-induced necrosis to apoptosis by regulating superoxide dismutases and suppressing reactive oxygen species production in A549 lung cancer cells. J Cell Physiol. 2007;211(2):371–385. doi: 10.1002/jcp.20941. [DOI] [PubMed] [Google Scholar]
- Lin W, et al. Glaucocalyxin A induces G2/M cell cycle arrest and apoptosis through the PI3K/Akt pathway in human bladder cancer cells. Int J Biol Sci. 2018;14(4):418. doi: 10.7150/ijbs.23602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel) 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater Sci Eng C. 2016;60:569–578. doi: 10.1016/j.msec.2015.11.067. [DOI] [PubMed] [Google Scholar]
- Mishra P, Nayak B, Dey RK. PEGylation in anti-cancer therapy: an overview. Asian J Pharm Sci. 2016;11:337–348. doi: 10.1016/j.ajps.2015.08.011. [DOI] [Google Scholar]
- Nassir AM, Shahzad N, Ibrahim IAA, Ahmad I, Md S, Ain MR. Resveratrol-loaded PLGA nanoparticles mediated programmed cell death in prostate cancer cells. Saudi Pharm J. 2018;26:876–885. doi: 10.1016/j.jsps.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaz B, del Pino P, Maffre P, Hartmann R, Gallego M, Rivera-Fernández S, de la Fuente JM, Nienhaus GU, Parak WJ. Surface functionalization of nanoparticles with polyethylene glycol: effects on protein adsorption and cellular uptake. ACS Nano. 2015;9:6996–7008. doi: 10.1021/acsnano.5b01326. [DOI] [PubMed] [Google Scholar]
- Pumiputavon K, Chaowasku T, Saenjum C, Osathanunkul M, Wungsintaweekul B, Chawansuntati K, Wipasa J, Lithanatudom P. Cell cycle arrest and apoptosis induction by methanolic leaves extracts of four Annonaceae plants. BMC Complement Altern Med. 2017;17:294. doi: 10.1186/s12906-017-1811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, Norris A, Sanseau P, Cavalla D, Pirmohamed M. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2018;18:41. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- Rafiei P, Haddadi A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: pharmacokinetics and biodistribution profile. Int J Nanomed. 2017;12:935–947. doi: 10.2147/IJN.S121881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin A, Esendagli G, Yerlikaya F, Caban-Toktas S, Yoyen-Ermis D, Horzum U, Aktas Y, Khan M, Couvreur P, Capan Y. A small variation in average particle size of PLGA nanoparticles prepared by nanoprecipitation leads to considerable change in nanoparticles’ characteristics and efficacy of intracellular delivery. Artif Cells Nanomed Biotechnol. 2017;45:1657–1664. doi: 10.1080/21691401.2016.1276924. [DOI] [PubMed] [Google Scholar]
- Singh V, Singh S, Das S, Kumar A, Self WT, Seal S. A facile synthesis of PLGA encapsulated cerium oxide nanoparticles: release kinetics and biological activity. Nanoscale. 2012;4(8):2597–2605. doi: 10.1039/c2nr12131j. [DOI] [PubMed] [Google Scholar]
- Singh A, Ahmad I, Ahmad S, Iqbal Z, Ahmad FJ. A novel monolithic controlled delivery system of resveratrol for enhanced hepatoprotection: nanoformulation development, pharmacokinetics and pharmacodynamics. Drug Dev Ind Pharm. 2016;42:1524–1536. doi: 10.3109/03639045.2016.1151032. [DOI] [PubMed] [Google Scholar]
- Singh S, Asal R, Bhagat S. Multifunctional antioxidant nanoliposome-mediated delivery of PTEN plasmids restore the expression of tumor suppressor protein and induce apoptosis in prostate cancer cells. J Biomed Mater Res Part A. 2018;106A:3152–3164. doi: 10.1002/jbm.a.36510. [DOI] [PubMed] [Google Scholar]
- Skladanowski A, Larsen AK. Expression of wild-type p53 increases etoposide cytotoxicity in M1 myeloid leukemia cells by facilitated G2 to M transition: implications for gene therapy. Cancer Res. 1997;57(5):818–823. [PubMed] [Google Scholar]
- Song W, Du R, Song P, Zhong T, Zhang W, Zhao Y, Wang C, Zhang X, Zhang Q. The preparation and characteristics of febuxostat SiO2 solid dispersions. J Chin Pharm Sci. 2014;23:463–470. doi: 10.5246/jcps.2014.07.061. [DOI] [Google Scholar]
- Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S, Sahu PK, Babu SM. Nanoparticles for cancer targeting: current and future directions. Curr Drug Deliv. 2016;13(8):1290–1302. doi: 10.2174/1567201813666160713121122. [DOI] [PubMed] [Google Scholar]
- Tamura K, Kawai Y, Kiguchi T, Okamoto M, Kaneko M, Maemondo M, Gemba K, Fujimaki K, Kirito K, Goto T, Fujisaki T, Takeda K, Nakajima A, Ueda T. Efficacy and safety of febuxostat for prevention of tumor lysis syndrome in patients with malignant tumors receiving chemotherapy: a phase III, randomized, multi-center trial comparing febuxostat and allopurinol. Int J Clin Oncol. 2016;21:996–1003. doi: 10.1007/s10147-016-0971-3. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281(5381):1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Torchilin Vladimir P. Drug Delivery. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009. Passive and Active Drug Targeting: Drug Delivery to Tumors as an Example; pp. 3–53. [DOI] [PubMed] [Google Scholar]
- Tzeyung S, Md S, Bhattamisra SK, Madheswaran T, Alhakamy NA, Aldawsari HM, Radhakrishnan AK. Fabrication, optimization, and evaluation of rotigotine-loaded chitosan nanoparticles for nose-to-brain delivery. Pharmaceutics. 2019;11(26):1–17. doi: 10.3390/pharmaceutics11010026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wilkosz N, Łazarski G, Kovacik L, Gargas P, Nowakowska M, Jamróz D, Kepczynski M. Molecular insight into drug-loading capacity of PEG–PLGA nanoparticles for itraconazole. J Phys Chem B. 2018;122:7080–7090. doi: 10.1021/acs.jpcb.8b03742. [DOI] [PubMed] [Google Scholar]
- Xu W, et al. Bax-PGAM5L-Drp1 complex is required for intrinsic apoptosis execution. Oncotarget. 2015;6(30):30017. doi: 10.18632/oncotarget.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Ensign LM, Boylan NJ, Schön A, Gong X, Yang J-C, Lamb NW, Cai S, Yu T, Freire E, Hanes J. Impact of surface polyethylene glycol (PEG) density on biodegradable nanoparticle transport in mucus ex vivo and distribution in vivo. ACS Nano. 2015;9:9217–9227. doi: 10.1021/acsnano.5b03876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5:288–300. doi: 10.21037/tlcr.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Hu L, Wang Z, Li Z, Wang A, Liu J. Resveratrol-loaded folic acid-grafted dextran stearate submicron particles exhibits enhanced antitumor efficacy in non-small cell lung cancers. Mater Sci Eng C. 2017;72:185–191. doi: 10.1016/j.msec.2016.10.077. [DOI] [PubMed] [Google Scholar]
- Zhao C, Li Y, Qiu W, He F, Zhang W, Zhao D, Zhang Z, Zhang E, Ma P, Liu Y, Ma L, Yang F, Wang Y, Shu Y. C5a induces A549 cell proliferation of non-small cell lung cancer via GDF15 gene activation mediated by GCN5-dependent KLF5 acetylation. Oncogene. 2018;37:4821–4837. doi: 10.1038/s41388-018-0298-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z. Effects of amphiphilic diblock copolymer on drug nanoparticle formation and stability. Biomaterials. 2013;34:10238–10248. doi: 10.1016/j.biomaterials.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]