Abstract
Ras analog in brain (Rab) proteins are small guanosine triphosphatases (GTPases) that belong to the Ras-like GTPase superfamily, and they can regulate vesicle trafficking. Rab proteins alternate between an activated (GTP-bound) state and an inactivated (GDP-bound) state. Early endosome marker Rab5 GTPase, a key member of the Rab family, plays a crucial role in endocytosis and membrane transport. The activated-state Rab5 recruits its effectors and regulates the internalization and trafficking of membrane receptors by regulating vesicle fusion and receptor sorting in the early endosomes. In this review, we summarize the role of small Rab GTPases Rab5 in membrane receptor trafficking and the activation of signaling pathways, such as Ras/MAPK and PI3K/Akt, which ultimately affect cell growth, apoptosis, tumorigenesis, and tumor development. This review may provide some insights for our future research and novel therapeutic targets for diseases.
1. Introduction
Ras analog in brain (Rab) proteins, belonging to the largest family of Ras superfamily, are small guanosine diphosphate (GTP)- bound proteins that regulate intracellular trafficking pathways [1]. There are more than 60 distinct proteins in humans, which constitute 41 functional subfamilies with tissue specificity. Rab proteins are similar to Ras and other GTP-bound proteins in their structures. They are composed of approximately 200 amino acids, and contain five highly conserved regions necessary for binding GTP and hydrolysis. Rab proteins are present in monomeric forms, and the amino acid sequence similarity of Rab family members ranges from 35% to 80% [2]. Rab proteins with more than 75% of sequence similarity can be identified as the same protein.
Rab5 is one of the most crucial members of the Rab family, whose functions and mechanisms are well studied. Rab5 transforms between the activated form, GTP-bound Rab5 (GTP-Rab5), and the inactivated form, guanosine diphosphate (GDP)- bound Rab5 (GDP-Rab5) [3]. Activated Rab5 interacts with its effectors and involves in vesicular transport, membrane trafficking, and signaling pathways [4].
In this review, we discussed the structure and activation of Rab5 and highlighted the recent advancements in the Rab5 regulating membrane receptor trafficking and signaling pathways, which will finally affect the occurrence and development of diseases.
2. The Rab GTPase Proteins
The Rab GTPase proteins were first studied in yeast S. cerevisiae by Novick. It was found a series of genes are necessary for the yeast secretion, which were named SEC (SEC1, SEC2, etc.) [5]. Subsequently, Gallwitz's group found the genes encoding the Ras-related YPT1 protein in yeast S. cerevisiae [6]. Further studies showed that the mutants of both SEC4 and YPT1 could encode small GTP-bound proteins, and the structural and functional analogues of SEC and YPT were cloned from a rat brain library and named Rab [7].
Rab proteins share similar structures, generally containing two cysteine residues at the carboxyl terminus generally, which appear in the form of -CC, -CXC, -CCXX, -CXXX, or -CCXXX (X represents any amino acid) and act as the membrane localization signal [8]. The key structures of Rab GTPase proteins contain a highly conserved G domain that comprises six β sheets (β1–β6), five α helixes (α1–α5), and five polypeptide rings; N- and C-terminals; and the molecular switches I and II [2]. The N-terminus may be involved in isoprene modification of the C-terminal cysteine. Molecular switches I and II, and N- and C-terminals determine the function of Rab GTPase proteins together. Highly related Rab GTPase proteins may be expressed in the same organelle, but exert different functions.
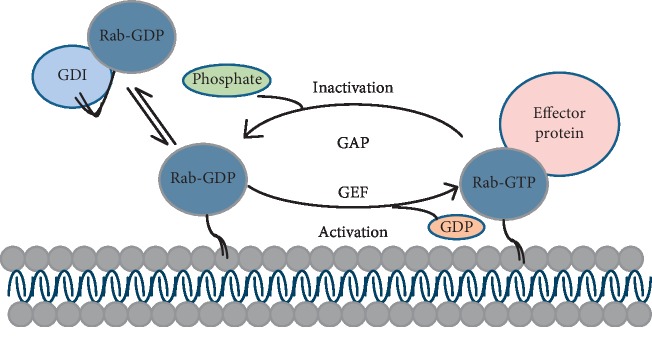
Rab GTPases can transform between the GTP-bound activated form and GDP-bound inactivated form [8]. The GTP-Rab is located on the plasma membrane, and GDP-Rab is located in the cytoplasm. The transformation between the activated and inactivated forms requires three crucial regulators: GDP dissociation inhibitor (GDI), guanine nucleotide exchange factor (GEF), and GTPase activating protein (GAP). As shown in Figure 1, GDI as a circulating factor that regulates the binding and unloading of Rab GTPases on the plasma membrane. After being released by GDI, Rab is activated by GEF, which catalyzes the conversion of GDP to GTP. Then, Rab-GTP may perform its roles by recruiting the downstream effectors. The inactivation of Rab GTPases involves the following steps: GAP inactivates Rab GTPases by catalyzing the hydrolysis of GTP. GDI binds with inactivated Rab-GDP to form a complex, impeding the interaction between Rab proteins and their effectors. Then, inactivated Rab proteins are transferred from the plasma membrane into the cytoplasm to start a new cycle [9, 10]. Although with similar structure, Rab family proteins perform different functions in membrane receptor trafficking and signaling pathways because they bind to different effectors [4]. Rab GTPases play their roles in organelles connection at different stages of vesicular transport, including budding, transport, tethering, docking, and fusion stages [11].
Figure 1.
Transformation of Rab proteins between the activated and inactivated forms. GAP catalyzes the hydrolysis of GTP and inactivates the Rab proteins. GDI stabilizes GDP-Rab. GEF removes GDP via guanine exchange, allowing Rab binding to GTP and further interacting with downstream effectors.
3. The Basic Information of Rab5
Rab5 is a key member of the Rab family, and Rab5A is its most important subtype, with well-identified functions and mechanisms. Rab5 is mentioned as Rab5A in most studies. Rab5A is located at 3p24.3 and is composed of 215 amino acids with a molecular weight of 23.658 kD [12]. The protein structure of Rab5 is nearly spherical: β sheets and α helixes are folded at the N terminus, and -CCXX structure and p-loop structure are at the C-terminal. The -CCXX structure is often modified by prenylation, contributing to the location of Rab5 in the plasma membrane. P-loop consists of three parts: (1) 27–34 residues induce the hydrolysis, binding, and dissociation of GTP in Rab5, (2) 49–51 residues act as switch I, and (3) 79–81 residues act as switch II [13].
The present studies on the mutants of Rab5 focus on S34N, Q79L, A30P, G78I, N125I, N133I, D136N, and C-terminal and N-terminal truncations. Wherein, Rab5-S34N, a persistently inactive form of Rab5, is a guanylate-bound deficient mutant, and preferable to bind GDP. Overexpression of the dominant negative Rab5-S34N inhibits fusion of early endosomes and endocytosis of transferring [14]. Rab5-Q79L is a GTP enzyme-deficient mutant and can impede GTP hydrolysis, sustaining the activation of Rab5. Overexpression of Rab5-Q79L induces the fusion and expansion of endosomes and suppresses lysosome generation [15].
Rab5 transforms between the activated form GTP-Rab5 and inactivated form of GDP-Rab5. The activation of Rab5 is regulated by GEFs, and the inactivation is regulated by GAPs [16]. GEFs contain the conserved Vps9 domains [17], which may catalyze the transformation of Rab5 between GDP-Rab5 and GTP-Rab5, such as Rabex-5 [18], RME-6 [19, 20], RIN1 [21], and p85 [22–24]. GAPs regulate the activated state of Rab5, such as Rab-GAP5 [25], tuberin [26], and Armus/TBC-2 [27] (Table 1).
Table 1.
Summary of Rab5 regulator and effectors.
| Key structures | Functions | References | |||
|---|---|---|---|---|---|
| Regulators | GEFs | Rabex-5 | Ubiquitin-binding domain, E3 ubiquitin ligase domain | Activation of Rab5 GTPases during endocytosis | [18] |
| RME-6 | Vps9-domain | Regulation of clathrin-coated vesicle uncoating and delivery of endocytic cargo to early endosomes | [19, 20] | ||
| RIN1 | Proline-rich domain, tyrosine 36 | Internalization, trafficking and degradation of activated receptors, cytoskeleton remodeling | [21] | ||
| p85 | C-terminal and N-terminal domains | Activation of Rab5 GTPases during endocytosis, migration of cancer cells | [22–24] | ||
| GAPs | Rab-GAP5 | Tre2/Bub2/Cdc16 domain | Inactivation of Rab5 GTPases during endocytosis and trafficking | [25] | |
| Tuberin | C-terminal domain | Inactivation of Rab5 GTPases during endocytosis and trafficking | [26] | ||
| Armus/TBC-2 | PH domain | Inactivation of Rab5 to promote Rab5 to Rab7 conversion during endosome maturation | [27] | ||
|
| |||||
| Effectors | EEA1 | C-terminal and N-terminal domains, C2H2 zinc finger domain | Fusion, docking and sorting of the early endosome | [28, 29] | |
| Rabaptin-5 | C-terminal domain | Fusion, docking and sorting of the early endosome | [30, 31] | ||
| Rabenosyn-5 | N-terminal domain, C2H2 zinc finger domain, and FYVE finger domain | Regulation of macropinocytosis, initiation of tubular endocytosis and surface flattening | [32, 33] | ||
| APPL1/2 | PH domain, PTB domain, and leucine zipper motif | Stable cargo-sorting compartments, membrane traffic/signaling, cell proliferation | [33, 34] | ||
| ZFYVE21 | FYVE-finger domain | Phosphoinositide remodeling of early endosome membranes to mediate signal activation and tissue inflammation | [35] | ||
| Rabankyrin-5 | FYVE finger domain, ankyrin repeats | Formation of endosomes and remodeling of the apical plasma membrane | [36] | ||
3.1. The Effectors of Rab5
Rab5 recruits the effector proteins via their GTP-dependent switch I and II to distinct subcellular compartments to regulate membrane trafficking events. The crucial effectors of Rab5 are early endosome antigen-1 (EEA1) [28, 29], rabaptin-5 [30, 31, 37], rabenosyn-5 [32, 38], APPL1/2 [33, 34], and ZFYVE21 [35] (Table 1).
EEA1, a key effector of Rab5 with a molecular weight of 162 kD, is a biomarker for early endosomes and has a parallel coiled-coil homodimer structure. EEA1 contains two binding sites for Rab5 : N-terminal C2H2 zinc finger structure and C-terminal domain [29], which can form complexes with Rab5 and specifically binds to phosphatidylinositol 3-phosphate. Phosphatidylinositol 3-phosphate further enhances the stability of GTP-Rab5, ensuring the recruitment of EEA1 to early endosomes [39]. Then, Rab5 competes with soluble NSF attachment protein receptors (SNAREs) [40] and fuses with the C-terminal of EEA1, mediating the docking of Rab5 on the membrane and regulating early endosome transport [41].
Rabaptin-5 is another Rab5 effector that plays a crucial role in membrane docking [30]. Rab5 interacts with the C-terminus of rabaptin-5 to form a complex, with its binding affinity reflecting the Rab5 activation level. The remaining structures of rabaptin-5 interact with other molecules, such as Rab4 and Rab11, to regulate the recirculation of receptors [37]. Knockdown of rabaptin-5 promotes the formation of extracellular circulating vesicles, and overexpression of rabaptin-5 exerts inhibitory effects, which reveals that rabaptin-5 maintains the balance of the receptors on the plasma membrane [31, 42].
There is close interaction among GEFs, Rab5, and Rab5 effectors. For example, after activation by Rabex-5, Rab5 recruits its effector Vps15 to interact with phosphatidylinositol 3-kinase (PI3K). Then, PI3K generates phosphatidylinositol 3-phosphate, which further recruit more effectors to interact with Rab5. Moreover, activated Rab5 interacts with its effector rabaptin-5 to form a complex. Rabaptin-5 further promotes the activity of Rabex-5 to facilitate the positive feedback from GTP-Rab5 and the binding of Rab5 to its downstream effectors [43].
4. The Function of Rab5 in Membrane Receptor Trafficking and Signal Transduction
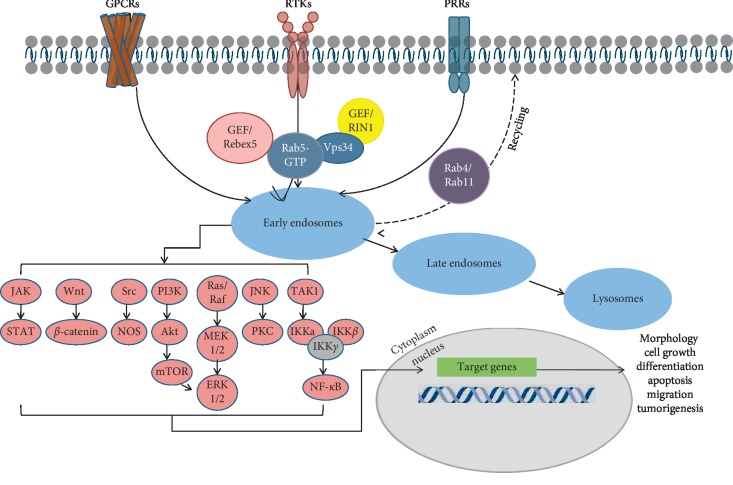
Rab5 affects the internalization and intracellular transport of receptors, such as receptor tyrosine kinases (RTKs), G protein-coupled receptors (GPCRs), and antigen recognition receptors by recruiting Rab5 effectors. The signal transduction of receptors occurs in early endosomes, further affecting gene transcription and ultimately affecting cell morphology, growth, differentiation, apoptosis, and disease development as shown in Figure 2.
Figure 2.
Roles of Rab5 in the internalization of receptors and signaling pathways. The receptors are sorted through the early endosome. The GTP-Rab5 recruits GEF Rabex5, which stabilizes Rab5, and Vps34 regenerates phosphatidylinositol 3-phosphate. Most of the receptor enter into the late endosomes and then degrade after reaching the lysosomes, and some recycle to the cell membrane via Rab4 and Rab11. Rab5 involves numerous signaling pathways, which may influence the cell progress and disease development (see the text for further details).
4.1. Rab5 and RTKs
RTKs are a large superfamily of receptors that can bind with ligands and phosphorylate tyrosine residues of the target proteins through tyrosine kinase domains. They have similar structures, including the extracellular glycosylated peptides, which are responsible for binding to ligands, hydrophobic transmembrane domain, and the intracellular region with tyrosine kinase activity [44, 45]. RTKs have important physiological functions, regulating cell proliferation, cell differentiation, tumorigenesis, and tumor development [46, 47]. These receptors are categorized into several families according to the similarity of their peptide sequences and other structural characteristics, mainly including the epidermal growth factor (EGF) receptor family, platelet-derived growth factor (PDGF) receptor family, nerve growth factor receptor family, fibroblast growth factor receptor family, vascular endothelial growth factor (VEGF) receptor family, and hepatocyte growth factor receptor (c-MET) family.
Endocytosis of RTKs includes internalization, transport, sorting, and degradation [48, 49], which stimulates downstream signals and regulates cellular processes, such as cell proliferation, migration, and morphological changes. The internalization of RTKs mainly depends on clathrin. After stimulation by the ligands, cell surface invaginates and the adapter molecules recruit RTKs to clathrin-coated pits [50], which then enter into the cell. With catalyzation by dynamin [51], RTKs are transported into the cytoplasm to form clathrin vesicles and fuse with early endosomes (mainly Rab5/EEA1-positive early endosomes). Then, RTKs are transported to the late endosomes together with the early endosomes, promoting the formation of the multivesicular body. Subsequently, the multivesicular body enters into the late endosomes and finally degraded after reaching the lysosomes via the endosomal sorting complex required for transport to terminate the RTK signal [52]. RTKs, sorted through the early endosome, can be also recycled to the cell membrane via Rab4- and Rab11-positive endosomes [53].
The EGF receptor is the most widely studied molecules among RTKs. The extracellular region of the EGF receptor consists of 622 amino acid residues, which bind multiple kinds of ligands including EGF and TGFα [54]. In addition to the important function of Rab5 in the clathrin vesicle formation, Rab5 promotes the formation of the early endosomes by regulating vesicle fusion [55]. Knockdown of Rab5 inhibits EGF receptor internalization and trafficking, resulting in decreased EGF receptor degradation and sustained signaling transduction. In addition, Rab5-Q79L or the EGF receptor kinase inhibitor, AG1478, may inhibit the formation of Rab5-positive early endosomes, reduce the colocalization of the EGF receptor and Rab5, and further suppress endosome fusion. Rab5 GEF Rin1 restores the inhibitory effect of the AG1478 or Rab5-Q79L mutant on endosome fusion to a certain extent [56].
GEFs or interacting proteins of Rab5, such as phospholipase D (PLD), hypoxia inducible factor (HIF), neuropilin-2 (NRP2)/WDFY1 axis, and leucine-rich repeat kinase 2 (LRRK2), may also regulate the internalization, transport, and downstream signaling pathways of the EGF receptor [57]. In addition, our previous study found that CMTM3, a tumor suppressor gene, decreased EGF receptor expression and EGF-mediated tumorigenicity by promoting Rab5 activity in gastric cancer [58].
PLD can directly affect the upstream molecules of the EGF receptor and interact with GAP proteins, during which, Rab5 regulates EGF receptor endocytosis, clathrin vesicles formation, and finally affects EGF receptor function [31]. By downregulating the expression of Rab5 effector rabaptin-5, HIF inhibits EGF receptor degradation, resulting in prolonging EGF receptor signaling and promoting cell proliferation and survival. Pleckstrin homology (PH) domain of PLD1 may be associated with HIF and restore the decreased rabaptin-5 expression and the inhibited EGF receptor degradation [31, 59]. WDFY1, a downstream molecule of NRP2 colocalizes with EEA1 and promotes the maturation of endosomes, which affect the transport and degradation of the EGF receptor. NRP2 deletion results in a large accumulation of EEA1/Rab5 in early endosomes, downregulating late endosomes marker Rab7, delaying the maturation process of early endosomes to late endosomes, and finally inhibiting the formation of lysosomes. Moreover, NRP2/WDFY1 axis plays an important role in cancer cell endocytosis. In cancer cells, the expression of NRP2 is negatively correlated with WDFY1. NRP2 deletion leads to abnormal activation of Erk signaling pathway and causes cell death [60]. LRRK2 interacts with Rab5 to coregulate vesicle formation, during which, LRRK2 phosphorylates Thr6 of Rab5 enhances Rab5 activity and promotes EGF receptor degradation [61].
C-MET is the receptor for HGF, which is involved in cell proliferation, differentiation, and signal transduction and regulation of cytoskeleton rearrangement. C-MET is closely associated with tumorigenesis and development of various cancers. Rab5 is also involved in the transport and signal transduction of c-MET. PTP1B interacts with c-MET, EGF, and PDGF receptors, affecting their internalization. Deletion of PTP1B promotes the phosphorylation of NSF and reduces the formation of phosphatidylinositol 3-phosphate-positive early endosomes and the activation of Rab5, resulting in inhibition of c-MET and EGF receptor transport and degradation [62]. Knockdown of NSF influences signal transduction and recirculation of c-MET, EGF receptor, integrin, and IGF-1 receptor, leading to restraining of the receptors in vesicles instead of entering the nucleus and an ultimately sustained activation of c-MET/MEK1/2 and EGF receptor/MEK1/2 signaling pathways [63, 64].
In addition, Rab5 plays a role in PDGF receptor internalization and trafficking [65]. P85, a subunit of phosphatidylinositol 3-phosphate with GAP activity [22], regulates the endosome transport, recirculation, and downstream signal activation of receptors and maintains the balance of the receptors [66]. The p85 mutant p85-R274 reverses p85 activity, inducing the accumulation of Rab5 in the cytoplasm and promoting the internalization of the PDGF receptor in a Rab5-dependent manner. Stable overexpression of p85-R274 in NIH3T3 cells reduces Rab5 activity, inhibits the degradation of the PDGF receptor, and activates downstream PI3K/Akt signaling pathway, resulting in changing in the cell morphology, promoting cell proliferation, and increase in the risk of cancer [23, 67]. However, overexpression of Rab5-S34N mutant can reverse these effect [23]. The classic Rho GTPases family member RhoD is located in early endosomes and recycling endosomes and is an interaction protein of rabankyrin-5 (a Rab5 effector) [36]. RhoD is involved in the transport of the endosome and affects PDGF receptor internalization and its downstream PLC and Akt signaling pathways [65].
Rab5 affects the internalization, trafficking, and signal transduction of the VEGF receptor and colony-stimulating factor 1 receptor. Overexpression of Rab5-Q79L in endothelial cells increases the size of early endosomes and induces the colocalization of EEA1 and VEGF receptors in endosomes, while knockdown of Rab5 enhances the activation of VEGF receptor (Y1175)/MAPK p42/44 signaling pathway [68]. Colony-stimulating factor 1 receptor colocalizes with Rab5 in macrophages. Knockdown of Rab5 inactivates p110δ (a catalytic subunit of Class I PI3K) and inhibits colony-stimulating factor 1 receptor downstream Akt signaling pathway, ultimately affecting the function of macrophages [69].
4.2. Rab5 and GPCRs
GPCR family, the largest and the most important membrane receptor superfamily in human, has more than 2000 members and is involved in virtually all life activities. The structure of GPCRs includes extracellular N-terminal domain, seven transmembrane helices (TM1-TM7) [70], intracellular C-terminal domain, three extracellular loops (ECL1-ECL3), and three or four intracellular loops (ICL1-ICL4). The amino acids of the transmembrane helical region of GPCRs are relatively conservative, while the amino acids of C-terminal, N-terminal, and loop regions are various. The abnormal expression of GPCRs may cause many diseases, such as Alzheimer's disease, Parkinson's disease, dwarfism, and color blindness, and it may affect tumorigenesis and tumor development [71].
Upon ligands stimulation, GPCRs are phosphorylated rapidly by GPCR kinases, and they bind to adapter protein β-arrestins to (1) inhibit the interaction of GPC receptors with G proteins, resulting in signal termination and (2) promoting endocytosis of GPC receptors, most of which are mediated by clathrin and catalyzed by dynamin [72, 73]. The endocytosis, trafficking, and functions of GPCRs are regulated by Rab GTPases. β-Arrestin induces the trafficking of GPCRs to the coated pits via β2-adaptin and clathrin [74]. After internalization, GPCRs are dephosphorylated in endosomes and then recycled to the cell membrane or stay in the early endosomes, followed by transporting into late endosomes and lysosomes for degradation [75, 76].
Rab5 is involved in the internalization and trafficking of GPCRs by regulating vesicle fusion and receptor sorting in early endosomes [77]. The transport of NK1R is regulated by Rab5, which promotes the accumulation of NK1R in the perinuclear early endosomes. Next, NK1R enters into the late endosomes and lysosomes. However, Rab5-S34N induces the retention of NK1R in early endosomes on the membrane [78]. Blocking NK1R suppresses the phosphorylation of p70S6K and 4E-BP1/2, resulting in inhibition of classical Wnt signaling pathway, which ultimately inhibits cell proliferation [79]. These findings illustrate that Rab5 not only plays a key role in the regulation of NK1R transport but also affects the related signaling pathways to make a contribution to tumorigenesis and tumor development.
Lysophosphatidic acid (LPA) is involved in metabolism, signal transduction, regulation of organ function, and is associated with inflammation [80, 81] and cancer [82, 83]. Rab5-S34N inhibits the internalization of the LPA receptor and the activation of serum response factor that is dependent on LPA [84], which further suppresses downstream signaling pathways and inhibits tumor cell motility and migration [85]. CB2 is phosphorylated via ligand stimulation [86], which promotes cell proliferation [87, 88]. Overexpression of Rab5-S34N inhibits the internalization of CB2, but has no obvious effect on CB2 recycling [89]. Leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5) is involved in Wnt signaling pathway and plays an important role in a variety of tissue stem cells. After internalization, LGR5 migrates from clathrin-coated pits, enters rapidly into EEA1/Rab5-positive early endosomes, and colocalizes with Rab5. After binding with R-spondins, LGR activates Wnt/β-catenin signaling pathway and affects disease development [90, 91].
In addition, Rab5 colocalizes with the oxytocin receptor [92], CXCR2 [93], and other various GPCRs. The study of its mechanism will help us understand the occurrence of disease and provide new ideas for disease treatment.
4.3. Rab5 and Antigen Recognition Receptors
In addition to the aforementioned receptors, Rab5 is involved in the transport and signal transduction of antigen recognition receptors, such as pattern-recognition receptors (PRRs) in innate immune cells, T-cell receptor (TCR), and B-cell receptor (BCR) in adaptive immune cells.
PRRs can recognize pathogen-associated molecular patterns, which can activate a series of signaling pathways and trigger innate immune responses. PRRs include toll-like receptors (TLRs), C-type lectin receptors, NOD-like receptors, RIG-I-like receptor, and DNA-sensing molecules in the cytoplasm [94].
By binding with TLR4, lipopolysaccharide activates inflammatory-related cells and leads to inflammation [95]. The colocalization of TLR4 and Rab5 can be observed in bone marrow-derived macrophages and hematopoietic stem cells, and progenitor cells upon lipopolysaccharide stimulation [96]. Rab5 affects both TLR4 downstream NF-κB signaling and the downstream of target genes, such as Hif-1 and CCL2, ultimately promoting the amplification of bone marrow-derived multifunctional hematopoietic stem cells [97].
Mannose and scavenger receptors are macrophage surface receptors, which participate in pathogen recognition, antigens presentation, and maintain homeostasis [98, 99]. Both mannose and scavenger receptors colocalize with Rab5 [100–102]. IL4/PGE2 stimulation significantly upregulates the expression of the mannose receptor, Rab5, and Rab5 GEF Rin1 in mouse bone marrow-derived macrophages, eventually promoting phagocytosis of mouse bone marrow-derived macrophages [103].
In addition, Rab5 is involved in the transport and signal transduction of TCR and BCR. TCR forms complex with Rab5 in early endosomes and accumulates in Rab5-positive early endosomes [104]. The reduced activity of Rab5 inhibits TCR degradation and enhances TCR signaling pathways. In mouse Th2 cells, knockdown of Rab5 selectively affects TCR downstream signaling and promotes the production of the corresponding cytokines [105]. It was reported that the number of CD4+CD8+ thymocytes is obviously reduced in T-cell-specific Rab5-N133I transgenic mice, suggesting that Rab5 plays a key role in TCR transport and signal transduction [106].
The internalization of BCR and BCR-mediated signal transduction establish a series of checkpoints in B cells to ensure B-cell maturation, BCR receptor formation, and humoral immune response generation [107]. Upon antigen stimulation, BCR transmits signals to extend cell morphology, and the clathrin-coated pits are generated in BCR-antigen clusters [108]. Rab5 promotes the formation of early endosomes in the internalized vesicle fusion and triggers Erk, p38, JNK, and Akt signal to affect further the life processes of cells.
Overall, there are two methods we suggest to treat diseases according to the current Rab5-related studies. First, a direct interaction with Rab5, such as Rab5-targeted therapies, that can transform the activation of Rab5 and influence receptor internalization and trafficking, leading to physiological changes of patients. Second, an indirect way to regulate Rab5 by influencing Rab5 effectors, GEFs, or GAPs is another potential strategy via influencing the aberrant expression, internalization, trafficking, and degradation of receptors. At present, the clinical cancer treatment, for instance, is difficult to achieve satisfactory prognosis of cancer patients because of the high recurrence and metastasis tendency after surgery, and the resistance to radiotherapy and chemotherapy. Thus, regulation of the Rab5 strategy may relieve the cancer patient's distress and provide us a novel idea for cancer therapy.
5. Conclusions
Rab5 is a key factor in regulating early endocytosis. Rab5, recruits its effectors to early endosomes, is involved in the transport of endosomes, and affects membrane receptor internalization, trafficking, and related signaling pathways, which contribute to gene transcription and the biological processes of cells. The mutation of Rab5 can cause abnormal cell morphology and function, suggesting that the structure of Rab5 is closely related to its function and occurrence and development of diseases. However, the mechanisms of Rab5 in diseases are not fully understood and need further investigation.
In summary, the study of Rab5 will help us understand the regulation mechanisms of receptor internalization and trafficking and provide new ideas and targets for the treatment of related diseases.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81672133 and 81874010).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nature Reviews Molecular Cell Biology. 2009;10(8):513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 2.Stenmark H., Olkkonen V. M. The Rab GTPase family. Genome Biology. 2001;5:p. S3007. doi: 10.1186/gb-2001-2-5-reviews3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 4.Olchowik M., Miaczynska M. Effectors of GTPase Rab5 in endocytosis and signal transduction. Postepy Biochemii. 2009;2:171–180. [PubMed] [Google Scholar]
- 5.Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- 6.Gallwitz D., Donath C., Sander C. A yeast gene encoding a protein homologous to the human C-Has/Bas proto-oncogene product. Nature. 1983;306(5944):704–707. doi: 10.1038/306704a0. [DOI] [PubMed] [Google Scholar]
- 7.Touchot N., Chardin P., Tavitian A. Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: molecular cloning of YPT-related cDNAs from a rat brain library. Proceedings of the National Academy of Sciences. 1987;84(23):8210–8214. doi: 10.1073/pnas.84.23.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stroupe C., Brunger A. T. Crystal structures of a rab protein in its inactive and active conformations. Journal of Molecular Biology. 2000;304(4):585–598. doi: 10.1006/jmbi.2000.4236. [DOI] [PubMed] [Google Scholar]
- 9.Itzen A., Goody R. S. GTPases involved in vesicular trafficking: structures and mechanisms. Seminars in Cell & Developmental Biology. 2011;22(1):48–56. doi: 10.1016/j.semcdb.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer S. R. Rab GTPase regulation of membrane identity. Current Opinion in Cell Biology. 2013;25(4):414–419. doi: 10.1016/j.ceb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosshans B. L., Ortiz D., Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proceedings of the National Academy of Sciences. 2006;103(32):11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zahraoui A., Touchot N., Chardin P., Tavitian A. The human Rab genes encode a family of GTP-binding proteins related to yeast YPT1 and SEC4 products involved in secretion. Journal of Biological Chemistry. 1989;21:12394–12401. [PubMed] [Google Scholar]
- 13.Dumas J. J., Zhu Z., Connolly J. L., Lambright D. G. Structural basis of activation and GTP hydrolysis in rab proteins. Structure. 1999;7(4):413–s2. doi: 10.1016/s0969-2126(99)80054-9. [DOI] [PubMed] [Google Scholar]
- 14.Stenmark H., Parton R. G., Steele-Mortimer O., Lütcke A., Gruenberg J., Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. The EMBO Journal. 1994;13(6):1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenfeld J. L., Moore R. H., Zimmer K. P., et al. Lysosome proteins are redistributed during expression of a GTP-hydrolysis-defective Rab5a. Journal of Cell Science. 2001;24:4499–4508. doi: 10.1242/jcs.114.24.4499. [DOI] [PubMed] [Google Scholar]
- 16.Bos J. L., Rehmann H., Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129(5):865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Carney D. S., Davies B. A., Horazdovsky B. F. Vps9 domain-containing proteins: activators of Rab5 GTPases from yeast to neurons. Trends in Cell Biology. 2006;16(1):27–35. doi: 10.1016/j.tcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Dohlman H. G., Campbell S. L. Regulation of large and small G proteins by ubiquitination. Journal of Biological Chemistry. 2019;294(49):18613–18623. doi: 10.1074/jbc.rev119.011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato M., Sato K., Fonarev P., Huang C.-J., Liou W., Grant B. D. Caenorhabditis elegans RME-6 is a novel regulator of RAB-5 at the clathrin-coated pit. Nature Cell Biology. 2005;7(6):559–569. doi: 10.1038/ncb1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smythe E. Role of the Rab5 guanine nucleotide exchange factor, rme-6, in the regulation of clathrin-coated vesicle uncoating. Methods in Molecular Biology. 2015;1298:283–293. doi: 10.1007/978-1-4939-2569-8_24. [DOI] [PubMed] [Google Scholar]
- 21.Szíber Z., Liliom H., Morales C. O. O., et al. Ras and Rab interactor 1 controls neuronal plasticity by coordinating dendritic filopodial motility and AMPA receptor turnover. Molecular Biology of the Cell. 2017;28(2):285–295. doi: 10.1091/mbc.e16-07-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamberlain M. D., Berry T. R., Pastor M. C., Anderson D. H. The P85alpha subunit of phosphatidylinositol 3′-kinase binds to and stimulates the GTPase activity of Rab proteins. Journal of Biological Chemistry. 2004;279(47):48607–48614. doi: 10.1074/jbc.m409769200. [DOI] [PubMed] [Google Scholar]
- 23.Chamberlain M. D., Chan T., Oberg J. C., et al. Disrupted RabGAP function of the P85 subunit of phosphatidylinositol 3-kinase results in cell transformation. Journal of Biological Chemistry. 2008;283(23):15861–15868. doi: 10.1074/jbc.m800941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall J., Whitecross D. E., Mellor P., Anderson D. H. Impact of P85alpha alterations in cancer. Biomolecules. 2019;9(1):p. 29. doi: 10.3390/biom9010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas A. K., Fuchs E., Kopajtich R., Barr F. A. A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nature Cell Biology. 2005;7(9):887–893. doi: 10.1038/ncb1290. [DOI] [PubMed] [Google Scholar]
- 26.Xiao G.-H., Shoarinejad F., Jin F., Golemis E. A., Yeung R. S. The tuberous sclerosis 2 gene product, tuberin, functions as a Rab5 GTPase activating protein (GAP) in modulating endocytosis. Journal of Biological Chemistry. 1997;272(10):6097–6100. doi: 10.1074/jbc.272.10.6097. [DOI] [PubMed] [Google Scholar]
- 27.Law F., Rocheleau C. E. Vps34 and the armus/TBC-2 Rab GAPs: putting the brakes on the endosomal Rab5 and Rab7 GTPases. Cell Logist. 2017;7(4) doi: 10.1080/21592799.2017.1403530.e1403530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simonsen A., Lippe R., Christoforidis S., et al. EEA1 links PI (3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394(6692):494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 29.Mishra A., Eathiraj S., Corvera S., Lambright D. G. Structural basis for Rab GTPase recognition and endosome tethering by the C2H2 zinc finger of early endosomal autoantigen 1 (EEA1) Proceedings of the National Academy of Sciences. 2010;107(24):10866–10871. doi: 10.1073/pnas.1000843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stenmark H., Vitale G., Ullrich O., Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83(3):423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 31.Park M. H., Choi K.-Y., Min D. S. The pleckstrin homology domain of phospholipase D1 accelerates EGFR endocytosis by increasing the expression of the Rab5 effector, rabaptin-5. Experimental & Molecular Medicine. 2015;47(12):p. e200. doi: 10.1038/emm.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahajeng J., Caplan S., Naslavsky N. Common and distinct roles for the binding partners rabenosyn-5 and Vps45 in the regulation of endocytic trafficking in mammalian cells. Experimental Cell Research. 2010;316(5):859–874. doi: 10.1016/j.yexcr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miaczynska M., Christoforidis S., Giner A., et al. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116(3):445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 34.Kalaidzidis I., Miaczynska M., Brewińska-Olchowik M., et al. APPL endosomes are not obligatory endocytic intermediates but act as stable cargo-sorting compartments. The Journal of Cell Biology. 2015;211(1):123–144. doi: 10.1083/jcb.201311117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang C., Manes T. D., Liu L., et al. ZFYVE21 is a complement-induced Rab5 effector that activates non-canonical NF-kappaB via phosphoinosotide remodeling of endosomes. Nature Communications. 2019;10(1):p. 2247. doi: 10.1038/s41467-019-10041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fabrowski P., Necakov A. S., Mumbauer S., et al. Tubular endocytosis drives remodelling of the apical surface during epithelial morphogenesis in drosophila. Nature Communications. 2013;4(1):p. 2244. doi: 10.1038/ncomms3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu G., Zhai P., Liu J., Terzyan S., Li G., Zhang X. C. Structural basis of rab5-rabaptin5 interaction in endocytosis. Nature Structural & Molecular Biology. 2004;11(10):975–983. doi: 10.1038/nsmb832. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen E., Christoforidis S., Uttenweiler-Joseph S., et al. Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. The Journal of Cell Biology. 2000;151(3):601–612. doi: 10.1083/jcb.151.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawe D. C., Chawla A., Merithew E., et al. Sequential roles for phosphatidylinositol 3-phosphate and Rab5 in tethering and fusion of early endosomes via their interaction with EEA1. Journal of Biological Chemistry. 2002;277(10):8611–8617. doi: 10.1074/jbc.m109239200. [DOI] [PubMed] [Google Scholar]
- 40.Balderhaar H. J. K., Ungermann C. CORVET and HOPS tethering complexes—coordinators of endosome and lysosome fusion. Journal of Cell Science. 2013;126(6):1307–1316. doi: 10.1242/jcs.107805. [DOI] [PubMed] [Google Scholar]
- 41.Jovic M., Sharma M., Rahajeng J., Caplan S. The early endosome: a busy sorting station for proteins at the crossroads. Histology and Histopathology. 2010;25(1):99–112. doi: 10.14670/HH-25.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vitale G., Rybin V., Christoforidis S., et al. Distinct rab-binding domains mediate the interaction of rabaptin-5 with GTP-bound Rab4 and Rab5. The EMBO Journal. 1998;17(7):1941–1951. doi: 10.1093/emboj/17.7.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamber E. P., Siedenburg A.-C., Barr F. A. Rab regulation by GEFs and GAPs during membrane traffic. Current Opinion in Cell Biology. 2019;59:34–39. doi: 10.1016/j.ceb.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Hubbard S. R., Till J. H. Protein tyrosine kinase structure and function. Annual Review of Biochemistry. 2000;69(1):373–398. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- 45.Robinson D. R., Wu Y.-M., Lin S.-F. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19(49):5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- 46.Jin N., Bi A., Lan X., et al. Identification of metabolic vulnerabilities of receptor tyrosine kinases-driven cancer. Nature Communications. 2019;10(1):p. 2701. doi: 10.1038/s41467-019-10427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Won E., Basunia A., Chatila W. K., et al. Efficacy of combined VEGFR1-3, PDGFalpha/beta, and FGFR1-3 blockade using nintedanib for esophagogastric cancer. Clinical Cancer Research. 2019;25(13):3811–3817. doi: 10.1158/1078-0432.ccr-18-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mellman I. Membranes and sorting. Current Opinion in Cell Biology. 1996;8(4):497–498. doi: 10.1016/s0955-0674(96)80026-3. [DOI] [PubMed] [Google Scholar]
- 49.Katzmann D. J., Odorizzi G., Emr S. D. Receptor downregulation and multivesicular-body sorting. Nature Reviews Molecular Cell Biology. 2002;3(12):893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 50.Traub L. M. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nature Reviews Molecular Cell Biology. 2009;10(9):583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 51.Mettlen M., Pucadyil T., Ramachandran R., Schmid S. L. Dissecting dynamin’s role in clathrin-mediated endocytosis. Biochemical Society Transactions. 2009;37(5):1022–1026. doi: 10.1042/bst0371022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raiborg C., Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458(7237):445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 53.Maxfield F. R., McGraw T. E. Endocytic recycling. Nature Reviews Molecular Cell Biology. 2004;5(2):121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 54.Morath I., Jung C., Lévêque R., et al. Differential recruitment of CD44 isoforms by ErbB ligands reveals an involvement of CD44 in breast cancer. Oncogene. 2018;37(11):1472–1484. doi: 10.1038/s41388-017-0030-1. [DOI] [PubMed] [Google Scholar]
- 55.Pagano A., Crottet P., Prescianotto-Baschong C., Spiess M. In vitro formation of recycling vesicles from endosomes requires adaptor protein-1/clathrin and is regulated by rab4 and the connector rabaptin-5. Molecular Biology of the Cell. 2004;15(11):4990–5000. doi: 10.1091/mbc.e04-04-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jozic I., Saliba S. C., Alejandro Barbieri M. Effect of EGF-receptor tyrosine kinase inhibitor on rab5 function during endocytosis. Archives of Biochemistry and Biophysics. 2012;525(1):16–24. doi: 10.1016/j.abb.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jékely G., Sung H.-H., Luque C. M., Rørth P. Regulators of endocytosis maintain localized receptor tyrosine kinase signaling in guided migration. Developmental Cell. 2005;9(2):197–207. doi: 10.1016/j.devcel.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Yuan W., Liu B., Wang X., et al. CMTM3 decreases EGFR expression and EGF-mediated tumorigenicity by promoting Rab5 activity in gastric cancer. Cancer Letters. 2017;386:77–86. doi: 10.1016/j.canlet.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y., Roche O., Yan M. S., et al. Regulation of endocytosis via the oxygen-sensing pathway. Nature Medicine. 2009;15(3):319–324. doi: 10.1038/nm.1922. [DOI] [PubMed] [Google Scholar]
- 60.Dutta S., Roy S., Polavaram N. S., et al. Neuropilin-2 regulates endosome maturation and EGFR trafficking to support cancer cell pathobiology. Cancer Research. 2016;76(2):418–428. doi: 10.1158/0008-5472.can-15-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heo H. Y., Kim K.-S., Seol W. Coordinate regulation of neurite outgrowth by LRRK2 and its interactor, Rab5. Experimental Neurobiology. 2010;19(2):97–105. doi: 10.5607/en.2010.19.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zarelli V. E. P., Ruete M. C., Roggero C. M., Mayorga L. S., Tomes C. N. PTP1B dephosphorylates N-ethylmaleimide-sensitive factor and elicits SNARE complex disassembly during human sperm exocytosis. Journal of Biological Chemistry. 2009;284(16):10491–10503. doi: 10.1074/jbc.m807614200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dagnell M., Cheng Q., Rizvi S. H. M., et al. Bicarbonate is essential for protein-tyrosine phosphatase 1B (PTP1B) oxidation and cellular signaling through EGF-triggered phosphorylation cascades. Journal of Biological Chemistry. 2019;294(33):12330–12338. doi: 10.1074/jbc.ra119.009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dietel E., Brobeil A., Gattenlohner S., Wimmer M. The importance of the right framework: mitogen-activated protein kinase pathway and the scaffolding protein PTPIP51. International Journal of Molecular Sciences. 2018;19(10):p. 3282. doi: 10.3390/ijms19103282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nehru V., Voytyuk O., Lennartsson J., Aspenström P. RhoD binds the Rab5 effector rabankyrin-5 and has a role in trafficking of the platelet-derived growth factor receptor. Traffic. 2013;14(12):1242–1254. doi: 10.1111/tra.12121. [DOI] [PubMed] [Google Scholar]
- 66.Chamberlain M. D., Oberg J. C., Furber L. A., et al. Deregulation of Rab5 and Rab4 proteins in p85R274A-expressing cells alters PDGFR trafficking. Cellular Signalling. 2010;22(10):1562–1575. doi: 10.1016/j.cellsig.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 67.Ignatiuk A., Quickfall J. P., Hawrysh A. D., Chamberlain M. D., Anderson D. H. The smaller isoforms of ankyrin 3 bind to the P85 subunit of phosphatidylinositol 3′-kinase and enhance platelet-derived growth factor receptor down-regulation. Journal of Biological Chemistry. 2006;281(9):5956–5964. doi: 10.1074/jbc.m510032200. [DOI] [PubMed] [Google Scholar]
- 68.Kofler N., Corti F., Rivera-Molina F., Deng Y., Toomre D., Simons M. The rab-effector protein RABEP2 regulates endosomal trafficking to mediate vascular endothelial growth factor receptor-2 (VEGFR2)-dependent signaling. Journal of Biological Chemistry. 2018;293(13):4805–4817. doi: 10.1074/jbc.m117.812172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lou J., Low-Nam S. T., Kerkvliet J. G., Hoppe A. D. Delivery of CSF-1R to the lumen of macropinosomes promotes its destruction in macrophages. Journal of Cell Science. 2014;127(24):5228–5239. doi: 10.1242/jcs.154393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Graaf C., Nijmeijer S., Wolf S., Ernst O. P. Handbook of Experimental Pharmacology. Berlin, Germany: Springer; 2016. 7TM domain structure of adhesion GPCRs; pp. 43–66. [DOI] [PubMed] [Google Scholar]
- 71.Ilter M., Mansoor S., Sensoy O. Utilization of biased G protein-coupled receptor signaling towards development of safer and personalized therapeutics. Molecules. 2019;24(11):p. 2052. doi: 10.3390/molecules24112052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takei K., McPherson P. S., Schmid S. L., Camilli P. D. Tubular membrane invaginations coated by dynamin rings are induced by GTP-gamma S in nerve terminals. Nature. 1995;374(6518):186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- 73.Nichols B. J., Lippincott-Schwartz J. Endocytosis without clathrin coats. Trends in Cell Biology. 2001;11(10):406–412. doi: 10.1016/s0962-8924(01)02107-9. [DOI] [PubMed] [Google Scholar]
- 74.Irannejad R., Tomshine J. C., Tomshine J. R., et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495(7442):534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pippig S., Andexinger S., Daniel K., et al. Overexpression of beta-arrestin and beta-adrenergic receptor kinase augment desensitization of beta 2-adrenergic receptors. The Journal of biological chemistry. 1993;268(5):3201–3208. [PubMed] [Google Scholar]
- 76.Ismail S., Gherardi M.-J., Froese A., et al. Internalized receptor for glucose-dependent insulinotropic peptide stimulates adenylyl cyclase on early endosomes. Biochemical Pharmacology. 2016;120:33–45. doi: 10.1016/j.bcp.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 77.Dores M. R., Trejo J. GPCR sorting at multivesicular endosomes. Sorting and Recycling Endosomes. 2015;130:319–332. doi: 10.1016/bs.mcb.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmidlin F., Déry O., DeFea K. O., et al. Dynamin and rab5a-dependent trafficking and signaling of the neurokinin 1 receptor. Journal of Biological Chemistry. 2001;276(27):25427–25437. doi: 10.1074/jbc.m101688200. [DOI] [PubMed] [Google Scholar]
- 79.Ilmer M., Garnier A., Vykoukal J., et al. Targeting the neurokinin-1 receptor compromises canonical Wnt signaling in hepatoblastoma. Molecular Cancer Therapeutics. 2015;14(12):2712–2721. doi: 10.1158/1535-7163.mct-15-0206. [DOI] [PubMed] [Google Scholar]
- 80.Dong Y.-L., Duan X.-Y., Liu Y.-J., et al. Autotaxin-lysophosphatidic acid axis blockade improves inflammation by regulating Th17 cell differentiation in DSS-induced chronic colitis mice. Inflammation. 2019;42(5):1530–1541. doi: 10.1007/s10753-019-01015-z. [DOI] [PubMed] [Google Scholar]
- 81.Velasco M., O’Sullivan C., Sheridan G. K. Lysophosphatidic acid receptors (LPARs): potential targets for the treatment of neuropathic pain. Neuropharmacology. 2017;113:608–617. doi: 10.1016/j.neuropharm.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 82.Xu M., Yin H., Cai Y., et al. Lysophosphatidic acid induces integrin beta6 expression in human oral squamous cell carcinomas cells via LPAR1 coupling to galphai and downstream SMAD3 and ETS-1 activation. Cellular Signalling. 2019;60:81–90. doi: 10.1016/j.cellsig.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 83.Im D.-S. Pharmacological tools for lysophospholipid GPCRs: development of agonists and antagonists for LPA and S1P receptors. Acta Pharmacologica Sinica. 2010;31(9):1213–1222. doi: 10.1038/aps.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murph M. M., Scaccia L. A., Volpicelli L. A., Radhakrishna H. Agonist-induced endocytosis of lysophosphatidic acid-coupled LPA1/EDG-2 receptors via a dynamin2- and rab5-dependent pathway. Journal of Cell Science. 2003;116(10):1969–1980. doi: 10.1242/jcs.00397. [DOI] [PubMed] [Google Scholar]
- 85.Iyoda T., Zhang F., Sun L., et al. Lysophosphatidic acid induces early growth response-1 (Egr-1) protein expression via protein kinase cdelta-regulated extracellular signal-regulated kinase (ERK) and c-jun N-terminal kinase (JNK) activation in vascular smooth muscle cells. Journal of Biological Chemistry. 2012;287(27):22635–22642. doi: 10.1074/jbc.m111.335695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bouaboula M., Dussossoy D., Casellas P. Regulation of peripheral cannabinoid receptor CB2 phosphorylation by the inverse agonist SR 144528. Implications for receptor biological responses. Journal of Biological Chemistry. 1999;274(29):20397–20405. doi: 10.1074/jbc.274.29.20397. [DOI] [PubMed] [Google Scholar]
- 87.Rao M., Chen D., Zhan P., Jiang J. MDA19, a novel CB2 agonist, inhibits hepatocellular carcinoma partly through inactivation of AKT signaling pathway. Biology Direct. 2019;14(1):p. 9. doi: 10.1186/s13062-019-0241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carrier E. J., Kearn C. S., Barkmeier A. J., et al. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Molecular Pharmacology. 2004;65(4):999–1007. doi: 10.1124/mol.65.4.999. [DOI] [PubMed] [Google Scholar]
- 89.Grimsey N. L., Goodfellow C. E., Dragunow M., Glass M. Cannabinoid receptor 2 undergoes rab5-mediated internalization and recycles via a rab11-dependent pathway. Biochimica et Biophysica Acta (BBA)—Molecular Cell Research. 2011;1813(8):1554–1560. doi: 10.1016/j.bbamcr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 90.de Lau W., Barker N., Low T. Y., et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476(7360):293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 91.Glinka A., Dolde C., Kirsch N., et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Reports. 2011;12(10):1055–1061. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Conti F., Sertic S., Reversi A., Chini B. Intracellular trafficking of the human oxytocin receptor: evidence of receptor recycling via a rab4/rab5 “short cycle”. American Journal of Physiology-Endocrinology and Metabolism. 2009;296(3):E532–E542. doi: 10.1152/ajpendo.90590.2008. [DOI] [PubMed] [Google Scholar]
- 93.Fan G.-H., Lapierre L. A., Goldenring J. R., Richmond A. Differential regulation of CXCR2 trafficking by Rab GTPases. Blood. 2003;101(6):2115–2124. doi: 10.1182/blood-2002-07-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tartey S., Takeuchi O. Pathogen recognition and toll-like receptor targeted therapeutics in innate immune cells. International Reviews of Immunology. 2017;36(2):57–73. doi: 10.1080/08830185.2016.1261318. [DOI] [PubMed] [Google Scholar]
- 95.Kagan J. C., Su T., Horng T., Chow A., Akira S., Medzhitov R. TRAM couples endocytosis of toll-like receptor 4 to the induction of interferon-beta. Nature Immunology. 2008;9(4):361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ghosh M., Subramani J., Rahman M. M., Shapiro L. H. CD13 restricts TLR4 endocytic signal transduction in inflammation. The Journal of Immunology. 2015;194(9):4466–4476. doi: 10.4049/jimmunol.1403133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goloviznina N. A., Verghese S. C., Yoon Y. M., Taratula O., Marks D. L., Kurre P. Mesenchymal stromal cell-derived extracellular vesicles promote myeloid-biased multipotent hematopoietic progenitor expansion via toll-like receptor engagement. Journal of Biological Chemistry. 2017;292(8):p. 3541. doi: 10.1074/jbc.a116.745653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stahl P., Schlesinger P. H., Rodman J. S., Doebber T. Recognition of lysosomal glycosidases in vivo inhibited by modified glycoproteins. Nature. 1976;264(5581):86–88. doi: 10.1038/264086a0. [DOI] [PubMed] [Google Scholar]
- 99.Lee S. J., Evers S., Roeder D., et al. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science. 2002;295(5561):1898–1901. doi: 10.1126/science.1069540. [DOI] [PubMed] [Google Scholar]
- 100.Vigerust D. J., Vick S., Shepherd V. L. Stable expression and characterization of an optimized mannose receptor. Journal of Clinical & Cellular Immunology. 2015;6(3) doi: 10.4172/2155-9899.1000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao X., Jiang C., Olufade R., Liu D., Emmett N. Kidney injury molecule-1 enhances endocytosis of albumin in renal proximal tubular cells. Journal of Cellular Physiology. 2016;231(4):896–907. doi: 10.1002/jcp.25181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mukherjee K., Parashuraman S., Krishnamurthy G., et al. Diverting intracellular trafficking of salmonella to the lysosome through activation of the late endocytic Rab7 by intracellular delivery of muramyl dipeptide. Journal of Cell Science. 2002;115(18):3693–3701. doi: 10.1242/jcs.00034. [DOI] [PubMed] [Google Scholar]
- 103.Wainszelbaum M. J., Proctor B. M., Pontow S. E., Stahl P. D., Barbieri M. A. IL4/PGE2 induction of an enlarged early endosomal compartment in mouse macrophages is rab5-dependent. Experimental Cell Research. 2006;312(12):2238–2251. doi: 10.1016/j.yexcr.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 104.Finetti F., Patrussi L., Masi G., et al. Specific recycling receptors are targeted to the immune synapse by the intraflagellar transport system. Journal of Cell Science. 2014;127(9):1924–1937. doi: 10.1242/jcs.139337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Che Y.-M., Zhang Y., Li M., Li X.-P., Zhang L.-L. In vitro and in vivo effect of PD-1/PD-L1 blockade on microglia/macrophage activation and T cell subset balance in cryptococcal meningitis. Journal of Cellular Biochemistry. 2018;119(4):3044–3057. doi: 10.1002/jcb.26432. [DOI] [PubMed] [Google Scholar]
- 106.Andre P., Boretto J., Hueber A. O., et al. A dominant-negative mutant of the Rab5 GTPase enhances T cell signaling by interfering with TCR down-modulation in transgenic mice. Journal of Immunology. 1997;159(11):5253–5263. [PubMed] [Google Scholar]
- 107.Buchner M., Swaminathan S., Chen Z., Müschen M. Mechanisms of pre-B-cell receptor checkpoint control and its oncogenic subversion in acute lymphoblastic leukemia. Immunological Reviews. 2015;263(1):192–209. doi: 10.1111/imr.12235. [DOI] [PubMed] [Google Scholar]
- 108.Hoogeboom R., Tolar P. Molecular mechanisms of B cell antigen gathering and endocytosis. Current Topics in Microbiology and Immunology. 2016;393(393):45–63. doi: 10.1007/82_2015_476. [DOI] [PubMed] [Google Scholar]