Abstract
Objective
Endoplasmic reticulum (ER) stress is involved in the pathogenesis of various ophthalmic diseases, and ER stress-mediated degradation systems play an important role in maintaining ER homeostasis during ER stress. The purpose of this review is to explore the potential relationship between them and to find their equilibrium sites.
Design
This review illustrates the important role of reasonable regulation of the protein degradation system in ER stress-mediated ophthalmic diseases. There were 128 articles chosen for review in this study, and the keywords used for article research are ER stress, autophagy, UPS, ophthalmic disease, and ocular.
Data sources
The data are from Web of Science, PubMed, with no language restrictions from inception until 2019 Jul.
Results
The ubiquitin proteasome system (UPS) and autophagy are important degradation systems in ER stress. They can restore ER homeostasis, but if ER stress cannot be relieved in time, cell death may occur. However, they are not independent of each other, and the relationship between them is complementary. Therefore, we propose that ER stability can be achieved by adjusting the balance between them.
Conclusion
The degradation system of ER stress, UPS and autophagy are interrelated. Because an imbalance between the UPS and autophagy can cause cell death, regulating that balance may suppress ER stress and protect cells against pathological stress damage.
Keywords: ER stress, Autophagy, UPS, Ophthalmic disease, Ocular
Introduction
The endoplasmic reticulum (ER) is a highly dynamic and important organelle of eukaryotic cells that has many functions, such as mediating free calcium storage, regulating lipid/sterol synthesis, and participating in the synthesis, processing, and transportation of proteins (Fregno & Molinari, 2018). When the internal environment of the ER is destroyed, the accumulation of improperly folded proteins therein eventually leads to ER stress (Li et al., 2017a). In order to inhibit ER stress and coordinate the recovery of ER function, cells have integrated signaling systems, including unfolded protein response (UPR) and the ER-associated degradation (ERAD) pathway (Fujita et al., 2007).
During ER stress, the UPR is activated and performs physiological functions that include enhancement of protein folding ability, stasis of translation of most proteins, and acceleration of protein degradation (Kroeger et al., 2019; Labbadia & Morimoto, 2015). Moreover, ERAD pathways composed by ubiquitin (Ub)—proteasome-dependent and autophagy—lysosome-dependent ERAD are also activated to participate in the removal of improperly folded proteins in order to restore the function of the ER (Schroder & Kaufman, 2005a).
Although ER stress, autophagy, and the ubiquitin proteasome system (UPS) have been fully studied in ophthalmic diseases, the relationship among them requires further examination. The purpose of this article is to explore the relationship between them in order to find new opportunities for future research directions and treatment of diseases. We suggest that diseases caused by ER stress can be blocked by regulating the balance between autophagy and the UPS, and especially by removing the pathogenic factor that results in ER stress that cannot be effectively removed.
Survey methodology
This review focuses on the relationship between ER stress, autophagy, and the UPS and their interactions in ocular diseases. Academic articles were searched in journal databases such as Web of Science, PubMed, and ER stress, autophagy, UPS, ophthalmic disease, and ocular were the search terms used for article research. The inclusion criteria for the selected articles required that articles be related to ophthalmic diseases and focused on the relationship between ER stress, autophagy, or the UPS.
ER stress and the UPR
The ER is an important site for protein synthesis, and therefore, if the function of the ER is disrupted by various pathological factors, the excessive accumulation of unfolded or misfolded proteins in the ER may eventually lead to ER stress. Many pathological conditions can lead to ER stress, such as hypoxia, oxidative stress, aging, or metabolic disorders (Lenox et al., 2015; Rozpedek et al., 2016; Rutkowski & Hegde, 2010; Zhu et al., 2018). When ER stress happens, the UPR is activated as a protective mechanism to restore the balance of the ER environment. The UPR involves 3 ER transmembrane proteins: activated transcription factor 6 (ATF6), ER to nuclear signaling 1 (ERN1; also known as inositol-requiring enzyme 1 [IRE1]), and eukaryotic translation initiation factor 2–kinase 3 (EIF2AK3; also known as protein kinase R–like endoplasmic-reticulum kinase [PERK]) (Ron & Walter, 2007). Under normal physiological conditions, these proteins bind to the 78 kDa glucose regulatory protein (Grp78; also known as binding immunoglobulin protein [BiP]) in the ER with lumen. During ER stress, BiP is separated from the sensor so that the UPR signals become activated. IRE1 then becomes phosphorylated, which activates endoribonuclease activity, splices the 26-nucleotide (nt) sequence from X-box binding protein (XBP1) messenger ribonucleic acid (mRNA) and produces functional XBP1(S), which is transferred to the nucleus and activates transcription of the genes encoding the ER chaperone and ERAD (Wakabayashi & Yoshida, 2013). PERK is phosphorylated and activated, which in turn phosphorylates eukaryotic translation initiation factor 2 (eIF2), thereby inhibiting protein translation and reducing protein synthesis (Dan et al., 2017). ATF6 is transported to the Golgi in the form of vesicles and is then cleaved by protease (site 1 and site 2 protease [S1P and S2P]) to produce a transcriptionally active polypeptide (Chen, Shen & Prywes, 2002). Activated ATF6 translocates to the nucleus and activates the gene transcription of proteins such as ER chaperones that increase ER protein folding (Yamamoto et al., 2007).
Interestingly, recent studies have shown that the three signaling pathways of the ER transmembrane proteins may interfere with each other. For example, the IRE1α and PERK pathways are not mutually independent, because knocking out IRE1α can alter the PERK pathway and also lead to decreased eIF2α expression (Storniolo et al., 2018). ATF6 is associated with IRE1, and ATF6 knockdown can result in unchecked IRE1 reporter activity that increases the splicing of XBP1 (Franziska et al., 2018).
ER stress and the UPS
The UPS is an important protein degradation pathway in eukaryotic cells. Protease and ubiquitin (Ub) in collaboration are responsible for non-lysosomal protein hydrolysis, which may remove abnormal proteins and prevent the accumulation of nonfunctional and harmful proteins in cells, therefore maintaining cellular homeostasis (Angele et al., 1999; Coux, Tanaka & Goldberg, 1996). The UPS may participate in a variety of biological processes, such as cell cycle, transcription, signaling, trafficking, and protein quality control (Jiang, Zhao & Qiu, 2018; Rousseau & Bertolotti, 2018). Ub, the smallest protein found in all eukaryotic cells (8 kDa), covalently conjugates many proteins to label them for downstream effector recognition (Cohen-Kaplan, Ciechanover & Livneh, 2017). Ub plays an important role in cells, including DNA repair, kinase activation, secretion, and protein transport in endocytic pathways (Kostova, Tsai & Weissman, 2007).
The conjugation of Ub protein with a substrate is a multi-step reaction that requires the participation of many enzymes and consumes energy (Glickman & Ciechanover, 2002; Pickart & Eddins, 2004). Targeted proteins undergo Ub–proteasome degradation by the 26S proteasome, which is highly conserved as a 2.5-MDa complex responsible for selective ATP-dependent degradation of ubiquitinated proteins in eukaryotic cells (Zwickl, Voges & Baumeister, 1999). It is composed of 2 large subcomplexes consisting of a 28-subunit 20S protease and a 19-subunit PA700 compound (also called a 19S complex or an S-regulating particle) (Liu & Jacobson, 2013). The 20S proteasome is responsible for substrate degradation, and its 19S subunit assists in degradation primarily by recognizing the substrate (Glickman, 2000). The 19S complex plays an important role in processing ubiquitinated substrates because it binds, ubiquitinates, and unfolds ubiquitinated protein, which is then transferred to the proteolysis chamber of the 20S proteasome for degradation (Zwickl, Voges & Baumeister, 1999).
As an important branch of the ERAD pathway, most of the unfolded and misfolded proteins in the ER are degraded by the UPS pathway (Nakatsukasa & Brodsky, 2008; Zattas & Hochstrasser, 2015). The UPS plays an important role in maintaining ER homeostasis, and impaired Ub–proteasome function can lead to ER stress (Shruthi et al., 2016). Because the UPS can remove proteins from the ER, it is vital to be able to correctly identify improperly folded proteins in the ER. There are at least two monitoring mechanisms for ER protein folding, consisting of one for the luminal domain (soluble or membrane proteins), and the other for the cytoplasmic domain (membrane proteins) (Vashist & Ng, 2004). The UPS removes proteins in the ER through four tightly coupled steps: (1) substrate selection, (2) retro translocation to the cytosol, (3) C-conjugated covalent polyubiquitination, and (4) proteasome degradation (Olzmann, Kopito & Christianson, 2013).
Substrate degradation begins with molecular chaperones, which identify proteins to be degraded by detecting abnormal disulfide bonds or hydrophobic fragments exposed by unassembled protein complexes (Vembar & Brodsky, 2008). In addition, another marker used to identify misfolded proteins is the presence of high mannose (Man5–8GlcNAc2) glycan (Mallinger et al., 2012). The substrates are targeted to the retrotranslocation machinery and then translocated to the cytoplasm through the retrotranslocation channel (Vembar & Brodsky, 2008).
In addition, ubiquitination is a complex process that requires the participation of three enzymes: ubiquitin activating enzyme E1 (ubiquitin activating enzyme), ubiquitin binding enzyme E2 (ubiquitin-conjugating enzyme, E2), and E3 (ubiquitin ligase) (Pickart, 2001). At first, E1 forms a high-energy thioester bond with ubiquitin in an ATP-dependent manner, activating the ubiquitin molecule. Then, the activated ubiquitin molecule is transported to E2, acquires the function of recognizing the target protein, and finally binds the target protein under the catalysis of E3 (Glickman & Ciechanover, 2002). After repeated enzymatic reactions, the polyubiquitin chain binds to the target protein, which is recognized by the 26S proteasome, and then degraded (Hershko & Ciechanover, 1998; Voges, Zwickl & Baumeister, 1999).
There are many types of E2 enzymes involved in ubiquitination, but not all E2 types are involved in ERAD. The three types involved in ERAD are ubiquitin-conjugating enzymes J1, J2, and G2 (UBE2J1, UBE2J2, and UBE2G2, respectively) (Christianson & Ye, 2014). Similarly, not all E3 enzymes are involved in ERAD. In yeast, Doa10p and Hrd1p are E3 ligases, and they have participated in the degradation of all substrates that have been studied (Cui et al., 2012). However, E3 ligases found in mammals, such as Hrd1/synoviolin, gp78, TEB4/MARCHVI, RNF5, HRD1, RNF-12, and RNF185, are involved in protein degradation in the ERAD pathway (Darom, Bening-Abu-Shach & Broday, 2010; El Khouri et al., 2013; You et al., 2016). It was reported that the UPS is regulated by the UPR pathway; for example, the PERK signal can increase the expression of RNF-121 to further enhance the UPS during ER stress (Darom, Bening-Abu-Shach & Broday, 2010). In addition, XBP1, the downstream factor of IRE1, is required for Nrf2 expression which is the central regulator of cell-protective genes ubiquitous expressed in cells, while Nrf2 interacts with the Cullin3-based E3 ubiquitin ligase adaptor to promote the proteasome (Chen et al., 2018; Ding et al., 2017; Tonelli, Chio & Tuveson, 2018). This indicates that the Nrf2 factor may be a link by which the IRE1 pathway regulates the UPS. Moreover, rapamycin is the core of proteasome assembly regulation, while ATF6 is essential for mediating ER stress to activate the mammalian target of the rapamycin (mTOR) pathway (Dylan & Jin, 2018). Therefore, mTOR may be the intermediate factor for ATF6 to activate UPS.
ER stress and autophagy
Autophagy is another metabolic pathway that regulates the degradation of long-lived proteins, organelles, and other cellular contents (Liu et al., 2015). Autophagy can be divided into macrophagy, microautophagy, and molecular chaperone-mediated autophagy (CMA) (Bejarano & Cuervo, 2010; Mijaljica, Prescott & Devenish, 2011; Parzych & Klionsky, 2014). Macroautophagy is divided into non-selective and selective autophagy. Non-selective autophagy is usually induced by nutrient deprivation and often involve the mTORC1 and protein kinase AMP–activated catalytic subunit alpha (PRKAA)/adenosine monophosphate–activated protein kinase (AMPK) pathways (Ganley et al., 2009). Selective autophagy targets specific substrates, including protein aggregates and damaged organelles such as mitochondria and peroxisomes (Lamark & Johansen, 2012). Macroautophagy is a continuous process involving the formation of autophagosomes, the fusion of autophagosomes with lysosomes, and the dynamic process of lysosomal degradation (Baehrecke, 2005). It is an important metabolic pathway in eukaryotic cells that is often used to resist stress and maintain intracellular homeostasis (Boya et al., 2016; Lin & Kuang, 2014; Shi et al., 2013). In the process of microautophagy, the lysosomal membrane acts as a concave protuberance or membrane, allowing a small portion of the cytoplasmic volume to enter the lysosomal cavity, which degrades the substrate (Li, Li & Bao, 2012). Molecular CMA does not require vacuolar formation and is tightly regulated by chaperone heat shock cognate 71 kDa protein (Hsc70) and its receptor, and it is associated with the PERK pathway in ER stress (Li et al., 2017b).
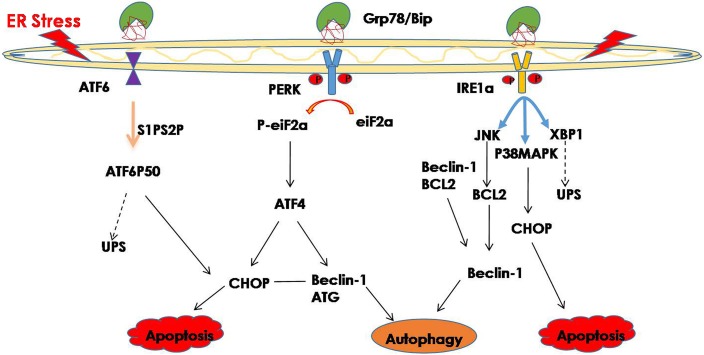
Hence, improperly folded proteins are not only degraded through the UPS pathway, but autophagy is also involved in protein degradation, and an increasing number of studies have shown that ER stress may trigger autophagy (Bachar-Wikstrom et al., 2013; Chandrika et al., 2015). Misfolded proteins and protein aggregates are cleared under stress by autophagy, especially when the other cellular repair and cellular clearance processes, namely molecular CMA and the UPS, fail (Libby & Gould, 2010; Pandey et al., 2007). In general, ER stress induces autophagy through the IRE1 and PERK signaling pathway (Ogata et al., 2007; Wafa et al., 2013). The downstream factor JNK is activated through the IER1 signaling pathway and further promotes autophagy during ER stress (Corazzari et al., 2017). Phosphorylated IRE1 also activates the MAPK8/JNK1/MAPK9/MAPK10 pathway, thereby upregulating autophagy (Yan et al., 2018). In addition, spliced XBP1 is reported to be involved in the activation of autophagy by upregulating the transcription of BECN1 (Christen & Fent, 2012). The PERK signaling pathway is activated during ER stress, and its downstream factor eIF2a phosphorylates, while phosphorylated eIF2a activates deoxyribonucleic acid (DNA) damage—inducible transcript 3 (DDIT3)/ATF4, thereby promoting tribbles pseudokinase 3 (TRIB3) to induce autophagy by inhibiting Akt1/mTORC1 (Salazar et al., 2009; Tang et al., 2015). Additionally, ATF4, which is the downstream factor of PERK, may function as a transcription factor regulating the expression of various autophagy-related genes (Wafa et al., 2013) (Fig. 1). Studies have shown that ER stress-related autophagy is mainly mediated by the IRE1a and PERK pathways, while the ATF6 signaling pathway can indirectly regulate autophagy by upregulating the expression of XBP1 and CHOP.
Figure 1. ER stress and its degradation pathways.
When ER stress occurs, in order to restore the function of the ER, the UPR is activated, and the UPS and autophagy are activated to suppress ER stress. However, if they still cannot restore the function of the ER, cell death may result.
UPR and ophthalmic diseases
The UPR is known to be an adaptive cellular response to ER dysfunction that suppresses ER stress and promotes cell survival. Many studies have found that proper UPR response plays an important role in maintaining the normal physiological function of cells (Sano & Reed, 2013; Schroder & Kaufman, 2005b). Insufficient activation of the UPR response to ER stress is the pathogenic factor of age-related retinal neurodegeneration. Thus, the lack of X-box binding protein 1 (XBP1), which is an important component of the UPR, may accelerate age-related retinal neurodegenerative diseases (Mclaughlin et al., 2018). It was also reported that in retinal pigmented epithelium (RPE)-specific XBP1 KO mice showed a 33% reduction in retinal cone cells and reduced the thickness of the outer nuclear layer (ONL), suggesting that XBP1 plays an important role in maintaining ER homeostasis and normal RPE cell function (Zhong et al., 2012). Moreover, it was shown that PERK activation is a protective response that increases the survival of photoreceptors in a P23H-1 transgenic rat model, indicating that UPR plays an important role as the first line of defense against protein-toxic cellular stress (Athanasiou et al., 2017). In addition, the ATF6 pathway is crucial to human color vision, and ATF6 mutation causes autosomal-recessive color blindness (Ansar et al., 2015).
UPR plays an important role in maintaining the normal physiological functions of cells under ER stress; however, if UPR still cannot suppress ER stress, it may result in cell death. Thus, if ER stress cannot be effectively controlled, phosphorylated IRE1α forms a complex with TRAF2 and ASK1 and activates the downstream factor JNK, which may activate caspase-12 to promote apoptosis (Ron & Hubbard, 2008). PERK activation further increases the expression of ATF4 and CHOP, promotes transcription of genes involved in oxidative stress and apoptosis, and further leads to cell death (Lu, 2004; Rzymski et al., 2009). The activated ATF6 translocates into the nucleus, where it binds the ER stress response elements to activate target genes, including XBP-1 and CHOP, which directly or indirectly result in cell death (Adachi et al., 2008; Guo et al., 2014; Hirsch et al., 2014).
An increasing number of studies have shown that ER stress is a factor in the pathology of many ophthalmic diseases, such as chronic glaucoma, glucocorticosteroid-induced glaucoma, cataract, DR, optic-nerve (ON) degeneration, and AMD (Doh et al., 2010; Elmasry et al., 2018; Ojino et al., 2015; Palsamy & Shinohara, 2017; Salminen et al., 2010; Zhou, Bennett & Shiels, 2016; Zode et al., 2014). For this reason, suppressing ER stress and quickly restoring the ER homeostasis play vital roles in the treatment of those diseases. For example, a recent study indicated that ER stress is detrimental for retinas in the early stages of DR, and suppressing ER stress may protect retinas against visual deficits caused by hyperglycemia (Raji et al., 2018). Moreover, in a mice model of TON induced by optic nerve crush (ONC), ER stress results in RGC death, however suppressing ER stress through GRP78 overexpression is an effective way to protect RGC from death (Ha et al., 2018). This suggests that reducing the pathological factors that result in ER stress and maintaining cell homeostasis are key factors in sustaining cell physiological functions. Therefore, UPS and autophagy, as important mechanisms of ERAD, play vital roles in restoring ER homeostasis during ER stress.
UPS and ophthalmic diseases
The UPS is essential for eye health and is involved in organ development and maintenance of lens function (Liu, 2015; Wride, 2011). In addition, it was reported that the proteasome can accelerate protein renewal and efficiently accelerate the degradation of rhodopsin T17M mutant (Jiang, Xiong & Xia, 2014). Inadequate UPS function is involved in the development of ophthalmic diseases. In glaucoma, decreased ubiquitination in the optic nerve may increase the level of proapoptotic proteins that are normally degraded by proteasomes, leading to axonal degeneration after increased intraocular pressure (IOP) (Dibas et al., 2008). However, overactivation of the UPS also impairs eye health. For example, tumor necrosis factor (TNF) destroys the interstitial connections in human corneal fibroblasts in a manner dependent on the UPS degradation of connexin 43 (Cx43) (Kimura & Nishida, 2010). A study reported that UPS participated in the degradation of rhodopsin and impairs visual function during retinal inflammation (Ozawa et al., 2008). Therefore, it was reported that UPS is involved in LPS-induced rat endotoxic uveitis (EIU); intravitreal resolvin D1 (RvD1) can inhibit uveitis by reducing the local level of Ub–proteasome (Rossi et al., 2015).
Given that the UPS is an important part of the ERAD pathway, and that it is involved in removing intracellular proteins, the UPS plays an important role in the inhibition of ER stress-mediated ophthalmic diseases. The importance of the UPS has been demonstrated in a large number of diseases associated with protein misfolding (Guerriero & Brodsky, 2012). It was shown that ER stress is the pathogenic factor involved in granular corneal dystrophy type 2 (GCD2), while the UPS activated by melatonin can accelerate the degradation of TGF- β-inducible protein (TGFBI) to suppress ER stress, and thus prevent the death of GCD2 cells (Choi et al., 2017). In addition, a growing number of studies have shown that if the UPS cannot restore the homeostasis of ER during ER stress, many ophthalmic diseases may occur. For example, in pseudo-exfoliation (PEX) disease, ER stress is overactivated, and the UPS is unable to remove harmful substances, resulting in secondary glaucoma (Hayat et al., 2019). Analysis of retinal proteins in patients with high IOP showed the existence of ER stress, and if the UPS cannot inhibit overactivated ER stress, this could eventually lead to retinal damage (Yang et al., 2015).
Autophagy and ophthalmic diseases
Under normal physiological conditions, autophagy, which plays an important role in maintaining normal cell function, is maintained at a relatively low level. Neuronal cells, for example, control cytoskeleton and organelle turnover through the autophagy process, allowing neurons to survive and regenerate after distal axon dissection or nerve suture (David, Crish & Inman, 2015; Tatiana et al., 2018). Some studies have shown that autophagy deficiency is a pathological factor leading to many ophthalmic diseases such as corneal opacification, elevated IOP, retinal dystrophy, mucopolysaccharide storage disease type VI, and AMD (Claudepierre et al., 2010; Golestaneh et al., 2017; Karnati et al., 2016; Lőrincz et al., 2016). Moreover, it was reported that exposure of ex-vivo mice retinal explants to high glucose resulted in the death of retinal neuronal cells, while treatment the explants with octreotide may protect neuronal cells against high glucose damage by enhancing autophagy (Amato et al., 2018). Although proper autophagy is beneficial to cell survival under stress, overactivated autophagy may lead to cell death, which is called autophagic cell death (ACD) (Liu & Levine, 2015; Vegliante & Ciriolo, 2018). A study demonstrated that the over-activated autophagy lead to the death of photoreceptors and inhibition of autophagy with 3MA may protect photoreceptors against photodamage (Zhang et al., 2014). Thus, as a double-edged sword, autophagy may either promote cell survival or lead to cell death, depending on the duration and intensity of pathology.
In general, autophagy, as another component mechanism of the ERAD pathway, is a survival mechanism to protect cells against stress, and a large number of studies have shown that autophagy can suppress ER stress and attenuate the pathological damage caused by stress. In glaucoma, enhanced ER stress-mediated autophagy may accelerate myosin clearance in trabecular meshwork cells, thus protecting them against damage. Sulforaphane (SFN) reduces the incidence of posterior cataracts by increasing ER stress-mediated autophagy (Liu et al., 2017). It was also reported that neurons in the lesioned cortex undergo apoptosis after traumatic brain injury, however, treatment with sevoflurane may enhance ER stress-mediated autophagy and inhibit neuronal apoptosis (He et al., 2018). However, ER stress-mediated autophagy also acts as a double-edged sword. For example, It has been shown that in diabetic retinopathy ER stress-mediated autophagy caused by a low concentration of oxidized glycosylated low-density lipoprotein (HOG-LDL) may attenuate the loss of peripheral blood cells, while prolonged ER stress-mediated autophagy caused by a higher concentration of HOG-LDL may promote the death of peripheral blood cells (Fu et al., 2016). Hence, excessive ER stress-induced autophagy may also lead to cell death. It was shown that the protective effect of mini- αA on NaIO3-induced retinal degeneration was achieved by inhibiting ER stress and autophagy (Zhang et al., 2015a). A recent study showed that in a mouse model of retinal degeneration induced by a P23H rhodopsin gene mutation, the accumulation of misfolded proteins in retinal photoreceptor cells activated ER stress and excessive autophagy, while inhibition of autophagy via deleting the autophagy-activating gene Atg5 decreased photoreceptor death and improved retinal function (Yao et al., 2018). Therefore, whether ER stress-induced autophagy is protective or damaging depends on disease conditions.
The important role of balance between autophagy and UPS during ER stress
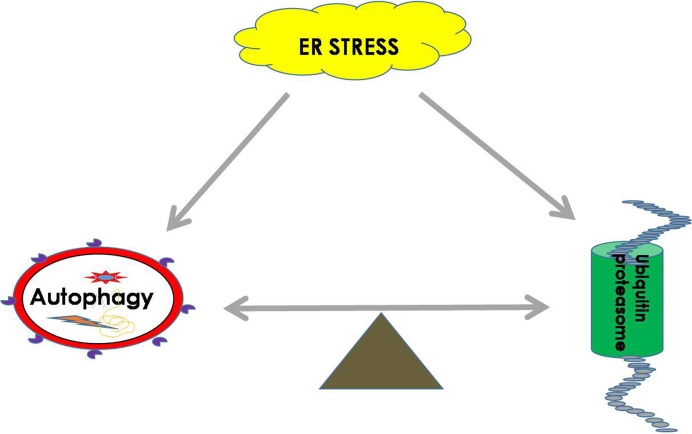
Both the UPS and autophagy play important roles in maintaining the balance of cellular proteins, and each has its own advantages. The UPS is responsible for the degradation of both short-lived proteins and misfolded proteins, while autophagy can degrade misfolded proteins and damaged organelles (Li et al., 2016). It was found that there is a certain relationship between the UPS and autophagy. It is known that sequestosome 1 (SQSTM1) is a multitasking bridging protein that regulates multiple signaling pathways, and the UPS and autophagy are correlated with each other through P62 protein (Jorge & Diaz-Meco, 2009; Milan et al., 2015). In addition to p62, other adaptors, such as neighbors of type 1 breast cancer (NBR1), can also recognize ubiquitinated substrates and localize them to autophagosomes (Cohen-Kaplan et al., 2016). In general, Ub ligase E3 is mainly degraded and regulated by proteasomes or by the recycling of its own ubiquitination. However, a recent study demonstrated that etoposide-induced protein 2.4 homolog (EI24) recognizes the RING domain existing in most E3 ligases and degrades them via the autophagic pathway (De Bie & Ciechanover, 2011; Nam et al., 2017). In addition, autophagic inhibition impairs the UPS function and leads to ER stress (Zhang et al., 2015b). It was shown that the functions of autophagy and the UPS are complementary in some conditions, and passive regulation of the functions between them is necessary to maintain cell protein homeostasis (Jung et al., 2019) (Fig. 2).
Figure 2. ER homeostasis can be achieved by balancing the UPS and autophagy pathways during ER stress.
During ER stress, the UPS and autophagy will be activated to remove harmful substrates such as misfolded proteins or protein aggregates to maintain the normal function of the ER. The balance between the UPS and autophagy is extremely important for restoring cell homeostasis.
A growing number of studies have shown that the UPS and autophagy may restore cellular homeostasis through mutual regulation. For instance, low levels of proteasome inhibitors in the treatment of oxidative stress injury of RPE cells can inhibit the PI3K/AKT/mTOR pathway and activate autophagy, thus protecting RPE cells against oxidative damage (Tang et al., 2014). In addition, it was reported that inhibition of autophagy, especially in the case of adequate nutrition, can enhance the activity of proteasomes, which are activated as a compensatory form of protein degradation (Wang et al., 2013). Moreover, Zacks et al. reported that in a mouse model of retinal degeneration caused by a gene mutation in P23H rhodopsin, ER stress-related autophagy led to photoreceptor death, while the treatment of P23H mice with selective phosphodiesterase-4 inhibitor (rolipram) to increase proteasome activity could effectively inhibit ER stress-related autophagy and reduce the rate of retinal degeneration (Qiu et al., 2019). Therefore, the balance between UPS and autophagy is very important, and these two systems have irreplaceable effects on cellular health.
Conclusion
Autophagy and the UPS are normal phenomena that manage the health of the living eukaryotic cell. However, deficiency and excessive activation of both autophagy and proteasomes are not conducive to cellular health. Many diseases are related to ER stress, and the UPS and autophagy play an important role in suppressing ER stress and maintaining ER homeostasis. We should take this information into consideration while also removing pathogenic factors, especially pathogenic factors such as genetic diseases that cannot be removed by current medical treatments. Then, we may be able to inhibit diseases by simultaneously regulating autophagy and the UPS in order to achieve intracellular homeostasis.
Acknowledgments
We thank LetPub for its linguistic assistance during the preparation of this manuscript.
Funding Statement
The study was funded by the National Natural Science Foundation of China (No. 81570864) and the Natural Science Foundation of Jilin Province (No. 20160101004JC; No. 20160414045GH; No. 2016J041). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Jing-Yao Song analyzed the data, prepared figures, authored drafts of the paper, approved the final draft.
Xue-Guang Wang and Lin Che collected the data, drafted the paper, approved the final draft.
Zi-Yuan Zhang and Bin Fan collected the data, prepared figures, approved the final draft.
Guang-Yu Li analyzed the data, reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
This is a literature review article and did not generate raw data.
References
- Adachi et al. (2008).Adachi Y, Yamamoto K, Okada T, Yoshida H, Mori K. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Structure & Function. 2008;33:75–89. doi: 10.1247/csf.07044. [DOI] [PubMed] [Google Scholar]
- Amato et al. (2018).Amato R, Catalani E, Dal Monte M, Cammalleri M, Di Renzo I, Perrotta C, Cervia D, Casini G. Autophagy-mediated neuroprotection induced by octreotide in an ex vivo model of early diabetic retinopathy. Pharmacological Research. 2018;128:167–178. doi: 10.1016/j.phrs.2017.09.022. [DOI] [PubMed] [Google Scholar]
- Angele et al. (1999).Angele MK, Smail N, Ayala A, Cioffi WG, Bland KI, Chaudry IH. L-arginine: a unique amino acid for restoring the depressed macrophage functions after trauma-hemorrhage. Journal of Trauma and Injury. 1999;46:34–41. doi: 10.1097/00005373-199901000-00006. [DOI] [PubMed] [Google Scholar]
- Ansar et al. (2015).Ansar M, Santos-Cortez RL, Saqib MA, Zulfiqar F, Lee K, Ashraf NM, Ullah E, Wang X, Sajid S, Khan FS, Amin-ud Din M, Smith JD, Shendure J, Bamshad MJ, Nickerson DA, Hameed A, Riazuddin S, Ahmed ZM, Ahmad W, Leal SM. Mutation of ATF6 causes autosomal recessive achromatopsia. Human Genetics. 2015;134:941–950. doi: 10.1007/s00439-015-1571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiou et al. (2017).Athanasiou D, Aguila M, Bellingham J, Kanuga N, Adamson P, Cheetham ME. The role of the ER stress-response protein PERK in rhodopsin retinitis pigmentosa. Human Molecular Genetics. 2017;26:4896–4905. doi: 10.1093/hmg/ddx370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachar-Wikstrom et al. (2013).Bachar-Wikstrom E, Wikstrom JD, Kaiser N, Cerasi E, Leibowitz G. Improvement of ER stress-induced diabetes by stimulating autophagy. Autophagy. 2013;9:626–628. doi: 10.4161/auto.23642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehrecke (2005).Baehrecke EH. Autophagy: dual roles in life and death? Nature Reviews Molecular Cell Biology. 2005;6:505–510. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- Bejarano & Cuervo (2010).Bejarano E, Cuervo AM. Chaperone-mediated autophagy. Proceedings of the American Thoracic Society. 2010;7:29–39. doi: 10.1513/pats.200909-102JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya et al. (2016).Boya P, Esteban-Martinez L, Serrano-Puebla A, Gomez-Sintes R, Villarejo-Zori B. Autophagy in the eye: development, degeneration, and aging. Progress in Retinal and Eye Research. 2016;55:206–245. doi: 10.1016/j.preteyeres.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Chandrika et al. (2015).Chandrika BB, Yang C, Ou Y, Feng X, Muhoza D, Holmes AF, Theus S, Deshmukh S, Haun RS, Kaushal GP. Endoplasmic reticulum stress-induced autophagy provides cytoprotection from chemical hypoxia and oxidant injury and ameliorates renal ischemia-reperfusion injury. PLOS ONE. 2015;10:e0140025. doi: 10.1371/journal.pone.0140025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2018).Chen C, Zhong Y, Wang JJ, Yu Q, Plafker K, Plafker S, Zhang SX. Regulation of Nrf2 by X box-binding protein 1 in retinal pigment epithelium. Frontiers in Genetics. 2018;9 doi: 10.3389/fgene.2018.00658. Article 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Shen & Prywes (2002).Chen X, Shen J, Prywes R. The luminal domain of atf6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. Journal of Biological Chemistry. 2002;277:13045–13052. doi: 10.1074/jbc.M110636200. [DOI] [PubMed] [Google Scholar]
- Choi et al. (2017).Choi SI, Lee E, Akuzum B, Jeong JB, Maeng YS, Kim TI, Kim EK. Melatonin reduces endoplasmic reticulum stress and corneal dystrophy-associated TGFBIp through activation of endoplasmic reticulum-associated protein degradation. Journal of Pineal Research. 2017;63:e12426. doi: 10.1111/jpi.12426. [DOI] [PubMed] [Google Scholar]
- Christen & Fent (2012).Christen V, Fent K. Silica nanoparticles and silver-doped silica nanoparticles induce endoplasmatic reticulum stress response and alter cytochrome P4501A activity. Chemosphere. 2012;87:423–434. doi: 10.1016/j.chemosphere.2011.12.046. [DOI] [PubMed] [Google Scholar]
- Christianson & Ye (2014).Christianson JC, Ye Y. Cleaning up in the endoplasmic reticulum: ubiquitin in charge. Nature Structural & Molecular Biology. 2014;21:325–335. doi: 10.1038/nsmb.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudepierre et al. (2010).Claudepierre T, Paques M, Simonutti M, Buard I, Sahel J, Maue RA, Picaud S, Pfrieger FW. Lack of Niemann-Pick type C1 induces age-related degeneration in the mouse retina. Molecular and Cellular Neuroscience. 2010;43:164–176. doi: 10.1016/j.mcn.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Cohen-Kaplan, Ciechanover & Livneh (2017).Cohen-Kaplan V, Ciechanover A, Livneh I. Stress-induced polyubiquitination of proteasomal ubiquitin receptors targets the proteolytic complex for autophagic degradation. Autophagy. 2017;13:759–760. doi: 10.1080/15548627.2016.1278327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kaplan et al. (2016).Cohen-Kaplan V, Livneh I, Avni N, Cohen-Rosenzweig C, Ciechanover A. The ubiquitin-proteasome system and autophagy: coordinated and independent activities. International Journal of Biochemistry & Cell Biology. 2016;79:403–418. doi: 10.1016/j.biocel.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Corazzari et al. (2017).Corazzari M, Gagliardi M, Fimia GM, Piacentini M. Endoplasmic reticulum stress, unfolded protein response, and cancer cell fate. Frontiers in Oncology. 2017;7:78. doi: 10.3389/fonc.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coux, Tanaka & Goldberg (1996).Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annual Review of Biochemistry. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- Cui et al. (2012).Cui F, Liu L, Zhao Q, Zhang Z, Li Q, Lin B, Wu Y, Tang S, Xie Q. Arabidopsis ubiquitin conjugase UBC32 is an ERAD component that functions in brassinosteroid-mediated salt stress tolerance. The Plant Cell. 2012;24:233–244. doi: 10.1105/tpc.111.093062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan et al. (2017).Dan L, Laura K, Ove E, Sulev KK. Recent insights into the role of unfolded protein response in er stress in health and disease. Frontiers in Cell & Developmental Biology. 2017;5 doi: 10.3389/fcell.2017.00048. Article UNSP 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darom, Bening-Abu-Shach & Broday (2010).Darom A, Bening-Abu-Shach U, Broday L. RNF-121 is an endoplasmic reticulum-membrane E3 ubiquitin ligase involved in the regulation of -integrin. Molecular Biology of the Cell. 2010;21:1788–1798. doi: 10.1091/mbc.e09-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David, Crish & Inman (2015).David K, Crish SD, Inman DM. Decreased energy capacity and increased autophagic activity in optic nerve axons with defective anterograde transport. Investigative Ophthalmology & Visual Science. 2015;56:8215–8227. doi: 10.1167/iovs.15-17885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bie & Ciechanover (2011).De Bie P, Ciechanover A. Ubiquitination of E3 ligases: self-regulation of the ubiquitin system via proteolytic and non-proteolytic mechanisms. Cell Death & Differentiation. 2011;18:1393–1402. doi: 10.1038/cdd.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibas et al. (2008).Dibas A, Yang MH, He S, Bobich J, Yorio T. Changes in ocular aquaporin-4 (AQP4) expression following retinal injury. Molecular Vision. 2008;14:1770–1783. [PMC free article] [PubMed] [Google Scholar]
- Ding et al. (2017).Ding H, Wang X, Wang H, Zhu L, Wang Q, Jia Y, Wei W, Zhou C, Wu H, Ding K. Nrf2-ARE signaling provides neuroprotection in traumatic brain injury via modulation of the ubiquitin proteasome system. Neurochemistry International. 2017;111:32–44. doi: 10.1016/j.neuint.2017.04.016. [DOI] [PubMed] [Google Scholar]
- Doh et al. (2010).Doh SH, Kim JH, Lee KM, Park HY, Park CK. Retinal ganglion cell death induced by endoplasmic reticulum stress in a chronic glaucoma model. Brain Research. 2010;1308:158–166. doi: 10.1016/j.brainres.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Dylan & Jin (2018).Dylan A, Jin S. ER stress activates the TOR pathway through Atf6. Journal of Molecular Signaling. 2018;13:1. doi: 10.5334/1750-2187-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khouri et al. (2013).El Khouri E, Le Pavec G, Toledano MB, Delaunay-Moisan A. RNF185 is a novel E3 ligase of endoplasmic reticulum-associated degradation (ERAD) that targets cystic fibrosis transmembrane conductance regulator (CFTR) Journal of Biological Chemistry. 2013;288:31177–31191. doi: 10.1074/jbc.M113.470500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmasry et al. (2018).Elmasry K, Ibrahim AS, Saleh H, Elsherbiny N, Elshafey S, Hussein KA, Al-Shabrawey M. Role of endoplasmic reticulum stress in 12/15-lipoxygenase-induced retinal microvascular dysfunction in a mouse model of diabetic retinopathy. Diabetologia. 2018;61:1220–1232. doi: 10.1007/s00125-018-4560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franziska et al. (2018).Franziska W, Aisling O, Caoimhín C, Heiko D, Jochen P. ER stress signalling has an activating transcription factor 6α (ATF6)-dependent ’off-switch’. The Journal of Biological Chemistry. 2018;293(47):18270–18284. doi: 10.1074/jbc.RA118.002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregno & Molinari (2018).Fregno I, Molinari M. Endoplasmic reticulum turnover: ER-phagy and other flavors in selective and non-selective ER clearance. F1000 Research. 2018;7:454–462. doi: 10.12688/f1000research.13968.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu et al. (2016).Fu D, Yu JY, Yang S, Wu M, Hammad SM, Connell AR, Du M, Chen J, Lyons TJ. Survival or death: a dual role for autophagy in stress-induced pericyte loss in diabetic retinopathy. Diabetologia. 2016;59:2251–2261. doi: 10.1007/s00125-016-4058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita et al. (2007).Fujita E, Kouroku Y, Isoai A, Kumagai H, Misutani A, Matsuda C, Hayashi YK, Momoi T. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II) Human Molecular Genetics. 2007;16:618–629. doi: 10.1093/hmg/ddm002. [DOI] [PubMed] [Google Scholar]
- Ganley et al. (2009).Ganley IG, Du HL, Wang J, Ding X, Chen S, Jiang X. ULK1⋅ATG13⋅FIP200 complex mediates mtor signaling and is essential for autophagy. Journal of Biological Chemistry. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman (2000).Glickman MH. Getting in and out of the proteasome. Seminars in Cell and Developmental Biology. 2000;11:149–158. doi: 10.1006/scdb.2000.0161. [DOI] [PubMed] [Google Scholar]
- Glickman & Ciechanover (2002).Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiological Reviews. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Golestaneh et al. (2017).Golestaneh N, Chu Y, Xiao YY, Stoleru GL, Theos AC. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death & Disease. 2017;8:e2537. doi: 10.1038/cddis.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero & Brodsky (2012).Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiological Reviews. 2012;92:537–576. doi: 10.1152/physrev.00027.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo et al. (2014).Guo FJ, Xiong Z, Lu X, Ye M, Han X, Jiang R. ATF6 upregulates XBP1S and inhibits ER stress-mediated apoptosis in osteoarthritis cartilage. Cellular Signalling. 2014;26:332–342. doi: 10.1016/j.cellsig.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Ha et al. (2018).Ha Y, Liu W, Liu H, Zhu S, Xia F, Gerson JE, Azhar NA, Tilton RG, Motamedi M, Kayed R, Zhang W. AAV2-mediated GRP78 transfer alleviates retinal neuronal injury by downregulating ER stress and tau oligomer formation. Investigative Ophthalmology & Visual Science. 2018;59:4670–4682. doi: 10.1167/iovs.18-24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat et al. (2019).Hayat B, Padhy B, Mohanty PP, Alone DP. Altered unfolded protein response and proteasome impairment in pseudoexfoliation pathogenesis. Experimental Eye Research. 2019;181:197–207. doi: 10.1016/j.exer.2019.02.004. [DOI] [PubMed] [Google Scholar]
- He et al. (2018).He H, Weifeng L, Yingying Z, Yibin L, Peiqing W, Yasong L, Huangde F. Sevoflurane post-conditioning attenuates traumatic brain injury-induced neuronal apoptosis by promoting autophagy via the PI3K/AKT signaling pathway. Drug Design Development & Therapy Volume. 2018;12:629–638. doi: 10.2147/DDDT.S158313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko & Ciechanover (1998).Hershko A, Ciechanover A. The ubiquitin system. Annual Review of Biochemistry. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hirsch et al. (2014).Hirsch I, Weiwad M, Prell E, Ferrari DM. ERp29 deficiency affects sensitivity to apoptosis via impairment of the ATF6-CHOP pathway of stress response. Apoptosis. 2014;19:801–815. doi: 10.1007/s10495-013-0961-0. [DOI] [PubMed] [Google Scholar]
- Jiang, Xiong & Xia (2014).Jiang H, Xiong S, Xia X. Retinitis pigmentosa-associated rhodopsin mutant T17M induces endoplasmic reticulum (ER) stress and sensitizes cells to ER stress-induced cell death. Molecular Medicine Reports. 2014;9:1737–1742. doi: 10.3892/mmr.2014.1987. [DOI] [PubMed] [Google Scholar]
- Jiang, Zhao & Qiu (2018).Jiang TX, Zhao M, Qiu XB. Substrate receptors of proteasomes. Biological reviews of the Cambridge Philosophical Society. 2018;93:1765–1777. doi: 10.1111/brv.12419. [DOI] [PubMed] [Google Scholar]
- Jorge & Diaz-Meco (2009).Jorge M, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137(6):1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung et al. (2019).Jung HL, Seoyoung P, Eunkyoung K, Min J. Negative-feedback coordination between proteasomal activity and autophagic flux. Autophagy. 2019;15(4):726–728. doi: 10.1080/15548627.2019.1569917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnati et al. (2016).Karnati R, Talla V, Peterson K, Laurie GW. Lacritin and other autophagy associated proteins in ocular surface health. Experimental Eye Research. 2016;144:4–13. doi: 10.1016/j.exer.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura & Nishida (2010).Kimura K, Nishida T. Role of the ubiquitin-proteasome pathway in downregulation of the gap-junction protein Connexin43 by TNF-{alpha} in human corneal fibroblasts. Investigative Ophthalmology & Visual Science. 2010;51:1943–1947. doi: 10.1167/iovs.09-3573. [DOI] [PubMed] [Google Scholar]
- Kostova, Tsai & Weissman (2007).Kostova Z, Tsai YC, Weissman AM. Ubiquitin ligases, critical mediators of endoplasmic reticulum-associated degradation. Seminars in Cell and Developmental Biology. 2007;18:770–779. doi: 10.1016/j.semcdb.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger et al. (2019).Kroeger H, Chiang WC, Felden J, Nguyen A, Lin JH. ER stress and unfolded protein response in ocular health and disease. The FEBS Journal. 2019;286:399–412. doi: 10.1111/febs.14522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbadia & Morimoto (2015).Labbadia J, Morimoto RI. Repression of the heat shock response is a programmed event at the onset of reproduction. Molecular Cell. 2015;59(4):639–650. doi: 10.1016/j.molcel.2015.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamark & Johansen (2012).Lamark T, Johansen T. Aggrephagy: selective disposal of protein aggregates by macroautophagy. International Journal of Cell Biology. 2012;2012 doi: 10.1155/2012/736905. Article 736905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenox et al. (2015).Lenox AR, Bhootada Y, Gorbatyuk O, Fullard R, Gorbatyuk M. Unfolded protein response is activated in aged retinas. Neuroscience Letters. 2015;609:30–35. doi: 10.1016/j.neulet.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2017a).Li F, Yang Y, Yang L, Wang K, Zhang X, Zong Y, Ding Y, Wang C, Zhang L, Ji G. Resveratrol alleviates FFA and CCl4 induced apoptosis in HepG2 cells via restoring endoplasmic reticulum stress. Oncotarget. 2017a;8:43799–43809. doi: 10.18632/oncotarget.16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Li & Bao (2012).Li WW, Li J, Bao JK. Microautophagy: lesser-known self-eating. Cellular and Molecular Life Sciences. 2012;69:1125–1136. doi: 10.1007/s00018-011-0865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2017b).Li W, Zhu J, Dou J, She H, Tao K, Xu H, Yang Q, Mao Z. Phosphorylation of LAMP2A by p38 MAPK couples ER stress to chaperone-mediated autophagy. Nature Communications. 2017b;8 doi: 10.1038/s41467-017-01609-x. Article 1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2016).Li X, Zhu F, Jiang J, Sun C, Zhong Q, Shen M, Wang X, Tian R, Shi C, Xu M, Peng F, Guo X, Hu J, Ye D, Wang M, Qin R. Simultaneous inhibition of the ubiquitin-proteasome system and autophagy enhances apoptosis induced by ER stress aggravators in human pancreatic cancer cells. Autophagy. 2016;12:1521–1537. doi: 10.1080/15548627.2016.1191722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby & Gould (2010).Libby RT, Gould DB. Endoplasmic reticulum stress as a primary pathogenic mechanism leading to age-related macular degeneration. Advances in Experimental Medicine & Biology. 2010;664:403–409. doi: 10.1007/978-1-4419-1399-9_46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin & Kuang (2014).Lin WJ, Kuang HY. Oxidative stress induces autophagy in response to multiple noxious stimuli in retinal ganglion cells. Autophagy. 2014;10:1692–1701. doi: 10.4161/auto.36076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2015).Liu C, DeRoo EP, Stecyk C, Wolsey M, Szuchnicki M, Hagos EG. Impaired autophagy in mouse embryonic fibroblasts null for Kruppel-like factor 4 promotes DNA damage and increases apoptosis upon serum starvation. Molecular Cancer. 2015;14 doi: 10.1186/s12943-015-0373-6. Article 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu & Jacobson (2013).Liu CW, Jacobson AD. Functions of the 19S complex in proteasomal degradation. Trends in Biochemical Sciences. 2013;38:103–110. doi: 10.1016/j.tibs.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2017).Liu H, Smith AJ, Ball SS, Bao Y, Bowater RP, Wang N, Michael Wormstone I. Sulforaphane promotes ER stress, autophagy, and cell death: implications for cataract surgery. Journal of Molecular Medicine. 2017;95:553–564. doi: 10.1007/s00109-016-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu (2015).Liu K. Altered ubiquitin causes perturbed calcium homeostasis, hyperactivation of calpain, dysregulated differentiation, and cataract. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:1071–1076. doi: 10.1073/pnas.1404059112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu & Levine (2015).Liu Y, Levine B. Autosis and autophagic cell death: the dark side of autophagy. Cell Death & Differentiation. 2015;22:367–376. doi: 10.1038/cdd.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lőrincz et al. (2016).Lőrincz P, Takáts S, Kárpáti M, Juhász G. IFly: the eye of the fruit fly as a model to study autophagy and related trafficking pathways. Experimental Eye Research. 2016;144:90–98. doi: 10.1016/j.exer.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Lu (2004).Lu DP. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. Journal of Cell Biology. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallinger et al. (2012).Mallinger A, Wen HM, Dankle GM, Glenn KA. Using a ubiquitin ligase as an unfolded protein sensor. Biochemical and Biophysical Research Communications. 2012;418:44–48. doi: 10.1016/j.bbrc.2011.12.109. [DOI] [PubMed] [Google Scholar]
- Mclaughlin et al. (2018).Mclaughlin T, Falkowski M, Park JW, Keegan S, Zhang SX. Loss of XBP1 accelerates age-related decline in retinal function and neurodegeneration. Molecular Neurodegeneration. 2018;13 doi: 10.1186/s13024-018-0250-z. Article 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijaljica, Prescott & Devenish (2011).Mijaljica D, Prescott M, Devenish RJ. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy. 2011;7:673–682. doi: 10.4161/auto.7.7.14733. [DOI] [PubMed] [Google Scholar]
- Milan et al. (2015).Milan E, Perini T, Resnati M, Orfanelli U, Oliva L, Raimondi A, Cascio P, Bachi A, Marcatti M, Ciceri F, Cenci S. A plastic SQSTM1/p62-dependent autophagic reserve maintains proteostasis and determines proteasome inhibitor susceptibility in multiple myeloma cells. Autophagy. 2015;11:1161–1178. doi: 10.1080/15548627.2015.1052928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsukasa & Brodsky (2008).Nakatsukasa K, Brodsky JL. The recognition and retrotranslocation of misfolded proteins from the endoplasmic reticulum. Traffic. 2008;9:861–870. doi: 10.1111/j.1600-0854.2008.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam et al. (2017).Nam T, Han JH, Devkota S, Lee HW. Emerging paradigm of crosstalk between autophagy and the ubiquitin-proteasome system. Moleculer Cells. 2017;40:897–905. doi: 10.14348/molcells.2017.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata et al. (2007).Ogata M, Hino SI, Saito A, Morikawa K, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Molecular and Cellular Biology. 2007;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojino et al. (2015).Ojino K, Shimazawa M, Izawa H, Nakano Y, Tsuruma K, Hara H. Involvement of endoplasmic reticulum stress in optic nerve degeneration after chronic high intraocular pressure in DBA/2J mice. Journal of Neuroscience Research. 2015;93:1675–1683. doi: 10.1002/jnr.23630. [DOI] [PubMed] [Google Scholar]
- Olzmann, Kopito & Christianson (2013).Olzmann JA, Kopito RR, Christianson JC. The mammalian endoplasmic reticulum-associated degradation system. Cold Spring Harbor Perspectives in Biology. 2013;5:a013185–a013185. doi: 10.1101/cshperspect.a013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa et al. (2008).Ozawa Y, Nakao K, Kurihara T, Shimazaki T, Shimmura S, Ishida S, Yoshimura A, Tsubota K, Okano H. Roles of STAT3/SOCS3 pathway in regulating the visual function and ubiquitin-proteasome-dependent degradation of rhodopsin during retinal inflammation. Journal of Biological Chemistry. 2008;283:24561–24570. doi: 10.1074/jbc.M802238200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsamy & Shinohara (2017).Palsamy P, Shinohara T. Age-related cataracts: Role of unfolded protein response, Ca 2+ mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1 dependent cytoprotection. Progress in Retinal & Eye Research. 2017;60:1–19. doi: 10.1016/j.preteyeres.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey et al. (2007).Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA, Knight MA, Schuldiner O, Padmanabhan R, Hild M, Berry DL, Garza D, Hubbert CC, Yao TP, Baehrecke EH, Taylor JP. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- Parzych & Klionsky (2014).Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart (2001).Pickart CM. Mechanisms underlying ubiquitination. Annual Review of Biochemistry. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- Pickart & Eddins (2004).Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochimica et Biophysica Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Qiu et al. (2019).Qiu Y, Yao J, Jia L, Thompson DA, Zacks DN. Shifting the balance of autophagy and proteasome activation reduces proteotoxic cell death: a novel therapeutic approach for restoring photoreceptor homeostasis. Cell Death & Disease. 2019;10 doi: 10.1038/s41419-019-1780-1. Article 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji et al. (2018).Raji L, Nagy PG, Shanta A, Rao VR, Clauss MA, Kompella UB, Rajashekhar G. Critical role of endoplasmic reticulum stress in chronic endothelial activation-induced visual deficits in tie2-tumor necrosis factor mice. Journal of Cellular Biochemistry. 2018;119:8460–8471. doi: 10.1002/jcb.27072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron & Hubbard (2008).Ron D, Hubbard SR. How IRE1 reacts to ER stress. Cell. 2008;132:24–26. doi: 10.1016/j.cell.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Ron & Walter (2007).Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Rossi et al. (2015).Rossi S, Di Filippo C, Gesualdo C, Potenza N, Russo A, Trotta MC, Zippo MV, Maisto R, Ferraraccio F, Simonelli F, D’Amico M. Protection from endotoxic uveitis by intravitreal Resolvin D1: involvement of lymphocytes, miRNAs, ubiquitin-proteasome, and M1/M2 macrophages. Mediators of Inflammation. 2015;2015 doi: 10.1155/2015/149381. Article 149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau & Bertolotti (2018).Rousseau A, Bertolotti A. Regulation of proteasome assembly and activity in health and disease. Nature Reviews Molecular Cell Biology. 2018;19:697–712. doi: 10.1038/s41580-018-0040-z. [DOI] [PubMed] [Google Scholar]
- Rozpedek et al. (2016).Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl JA, Majsterek I. The role of the PERK/eIF2alpha/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Current Molecular Medicine. 2016;16:533–544. doi: 10.2174/1566524016666160523143937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski & Hegde (2010).Rutkowski DT, Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. Journal of Cell Biology. 2010;189:783–794. doi: 10.1083/jcb.201003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzymski et al. (2009).Rzymski T, Milani M, Singleton DC, Harris AL. Role of ATF4 in regulation of autophagy and resistance to drugs and hypoxia. Cell Cycle. 2009;8:3838–3847. doi: 10.4161/cc.8.23.10086. [DOI] [PubMed] [Google Scholar]
- Salazar et al. (2009).Salazar MCA, Salanueva IJ, Hernandez-Tiedra S, Lorente M, Egia A, Vazquez P, Blazquez C, Torres S, Garcia S, Nowak J, Fimia GM, Piacentini M, Cecconi F, Pandolfi PP, González-Feria L, Iovanna JL, Guzmán M, Boya P, Velasco G. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. Journal of Clinical Investigation. 2009;119:1359–1372. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen et al. (2010).Salminen A, Kauppinen A, Hyttinen JM, Toropainen E, Kaarniranta K. Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Molecular Medicine. 2010;16:535–542. doi: 10.2119/molmed.2010.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano & Reed (2013).Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochimica et Biophysica Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder & Kaufman (2005a).Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutation Research. 2005a;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Schroder & Kaufman (2005b).Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annual Review of Biochemistry. 2005b;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Shi et al. (2013).Shi X, Wu YC, Zhu XY, Sun XD. The latest advance of correlation between autophagy and optic neuritis. [Zhonghua yan ke za zhi] Chinese Journal of Ophthalmology. 2013;49:956–959. [PubMed] [Google Scholar]
- Shruthi et al. (2016).Shruthi K, Reddy SS, Reddy PY, Shivalingam P, Harishankar N, Reddy GB. Amelioration of neuronal cell death in a spontaneous obese rat model by dietary restriction through modulation of ubiquitin proteasome system. Journal of Nutritional Biochemistry. 2016;33:73–81. doi: 10.1016/j.jnutbio.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Storniolo et al. (2018).Storniolo A, Alfano V, Carbotta S, Ferretti E, Di Renzo L. IRE1α deficiency promotes tumor cell death and eIF2α degradation through PERK dipendent autophagy. Cell Death Discovery. 2018;4 doi: 10.1038/s41420-017-0002-9. Article 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang et al. (2014).Tang B, Jingjing C, Lin S, Yiping L, Jia Q, Joy SB, Shengzhou W. Proteasome inhibitors activate autophagy involving inhibition of PI3K-Akt-mTOR pathway as an anti-oxidation defense in human RPE cells. PLOS ONE. 2014;9:e103364. doi: 10.1371/journal.pone.0103364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang et al. (2015).Tang B, Li Q, Zhao XH, Wang HG, Li N, Fang Y, Wang K, Jia YP, Zhu P, Gu J, Li JX, Jiao YJ, Tong WD, Wang M, Zou QM, Zhu FC, Mao XH. Shiga toxins induce autophagic cell death in intestinal epithelial cells via the endoplasmic reticulum stress pathway. Autophagy. 2015;11:344–354. doi: 10.1080/15548627.2015.1023682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatiana et al. (2018).Tatiana LR, David R-G, Sara M-M-A, Mireia H-G, Assumpció B, Joaquim F, Caty C. ATG5 overexpression is neuroprotective and attenuates cytoskeletal and vesicle-trafficking alterations in axotomized motoneurons. Cell Death & Disease. 2018;9 doi: 10.1038/s41419-018-0682-y. Article 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli, Chio & Tuveson (2018).Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashist & Ng (2004).Vashist S, Ng DT. Misfolded proteins are sorted by a sequential checkpoint mechanism of ER quality control. Journal of Cell Biology. 2004;165:41–52. doi: 10.1083/jcb.200309132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegliante & Ciriolo (2018).Vegliante R, Ciriolo MR. Autophagy and autophagic cell death: uncovering new mechanisms whereby dehydroepiandrosterone promotes beneficial effects on human health. Vitamins and Hormones. 2018;108:273–307. doi: 10.1016/bs.vh.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Vembar & Brodsky (2008).Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nature Reviews Molecular Cell Biology. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voges, Zwickl & Baumeister (1999).Voges D, Zwickl P, Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annual Review of Biochemistry. 1999;68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- Wafa et al. (2013).Wafa BC, Anne-Catherine M, Valérie C, Julien A, Céline J, Yuki M, Laurent P, Georges S, Pierre F, Alain B. The eIF2 α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Research. 2013;41(16):7683–7699. doi: 10.1093/nar/gkt563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi & Yoshida (2013).Wakabayashi S, Yoshida H. The essential biology of the endoplasmic reticulum stress response for structural and computational biologists. Computational and Structural Biotechnology Journal. 2013;6:e201303010. doi: 10.5936/csbj.201303010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2013).Wang XJ, Yu J, Wong SH, Cheng AS, Chan FK, Ng SS, Cho CH, Sung JJ, Wu WK. A novel crosstalk between two major protein degradation systems. Autophagy. 2013;9:1500–1508. doi: 10.4161/auto.25573. [DOI] [PubMed] [Google Scholar]
- Wride (2011).Wride AM. Lens fibre cell differentiation and organelle loss: many paths lead to clarity. Philosophical Transactions Biological Sciences. 2011;366:1219–1233. doi: 10.1098/rstb.2010.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto et al. (2007).Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6 α and XBP1. Developmental Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Yan et al. (2018).Yan B, Yunting P, Xiang Y, Gregory DB, Renu S, Howell SH, Bassham DC. IRE1B degrades RNAs encoding proteins that interfere with the induction of autophagy by ER stress in Arabidopsis thaliana. Autophagy. 2018;14(9):1562–1573. doi: 10.1080/15548627.2018.1462426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2015).Yang X, Hondur G, Li M, Cai J, Tezel G. Proteomics analysis of molecular risk factors in the ocular hypertensive human retina. Investigative Ophthalmology & Visual Science. 2015;56:5816–5830. doi: 10.1167/iovs.15-17294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao et al. (2018).Yao J, Qiu Y, Frontera E, Jia L, Khan NW, Klionsky DJ, Ferguson TA, Thompson DA, Zacks DN. Inhibiting autophagy reduces retinal degeneration caused by protein misfolding. Autophagy. 2018;14:1226–1238. doi: 10.1080/15548627.2018.1463121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You et al. (2016).You H, Shen Y, Yan C, Huang Y, Xu W. HRD1-mediated IGF-1R ubiquitination contributes to renal protection of resveratrol in db/db mice. 2016. [DOI] [PMC free article] [PubMed]
- Zattas & Hochstrasser (2015).Zattas D, Hochstrasser M. Ubiquitin-dependent protein degradation at the yeast endoplasmic reticulum and nuclear envelope. Critical Reviews in Biochemistry & Molecular Biology. 2015;50:1–17. doi: 10.3109/10409238.2014.959889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2015a).Zhang J, Zhao X, Cai Y, Li Y, Yu X, Lu L. Protection of retina by Mini-aA in NaIO3-induced retinal pigment epithelium degeneration mice. International Journal of Molecular Sciences. 2015a;16:1644–1656. doi: 10.3390/ijms16011644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2014).Zhang TZ, Fan B, Chen X, Wang W-J, Jiao Y-Y, Su G-F, Li G-Y. Suppressing autophagy protects photoreceptor cells from light-induced injury. Biochemical and Biophysical Research Communications. 2014;450:966–972. doi: 10.1016/j.bbrc.2014.06.082. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2015b).Zhang Y, Ye M, Chen LJ, Li M, Tang Z, Wang C. Role of the ubiquitin-proteasome system and autophagy in regulation of insulin sensitivity in serum-starved 3T3-L1 adipocytes. Endocrine Journal. 2015b;62:673–686. doi: 10.1507/endocrj.EJ15-0030. [DOI] [PubMed] [Google Scholar]
- Zhong et al. (2012).Zhong Y, Jingming L, Wang JJ, Chen C, Tran JT, Anisse S, Qiang Y, Yun-zheng L, Mandal MN, Anderson RE. X-box binding protein 1 is essential for the anti-oxidant defense and cell survival in the retinal pigment epithelium. PLOS ONE. 2012;7:e38616. doi: 10.1371/journal.pone.0038616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Bennett & Shiels (2016).Zhou Y, Bennett TM, Shiels A. Lens ER-stress response during cataract development in Mip-mutant mice. Biochimica et Biophysica Acta. 2016;1862:1433–1442. doi: 10.1016/j.bbadis.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (2018).Zhu X, Wang K, Zhou F, Zhu L. Paeoniflorin attenuates atRAL-induced oxidative stress, mitochondrial dysfunction and endoplasmic reticulum stress in retinal pigment epithelial cells via triggering Ca(2+)/CaMKII-dependent activation of AMPK. Archives of Pharmacal Research. 2018;41:1009–1018. doi: 10.1007/s12272-018-1059-6. [DOI] [PubMed] [Google Scholar]
- Zode et al. (2014).Zode GS, Sharma AB, Lin X, Searby CC, Bugge K, Kim GH, Clark AF, Sheffield VC. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. Journal of Clinical Investigation. 2014;124:1956–1965. doi: 10.1172/JCI69774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwickl, Voges & Baumeister (1999).Zwickl P, Voges D, Baumeister W. The proteasome: a macromolecular assembly designed for controlled proteolysis. Philosophical Transactions of the Royal Society B: Biological Sciences. 1999;354:1501–1511. doi: 10.1098/rstb.1999.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
This is a literature review article and did not generate raw data.