Abstract
Phoneix dactylifera L. commonly called date palm is a highly valuable horticultural cash crop for arid and semi-arid regions. The availability of offshoots and their survival during plantation are major concern. Being dioecious tree, seed propagation in date palm do not produce true-to-type offspring and tissue culture propagation is the only viable option to supply quality-planting propagules. Hereby, we report callus culture and plantlet regeneration in female date palm using in vitro-derived adventitious shoot bud tissues as explants. Explants (89.33 ± 2.67%) produced callus culture on 0.8% agar-gelled Murashige and Skoog’s basal medium containing 100.0 mg l−1 each polyvinylpyrrolidone, ascorbic acid and glutamine, 50.0 mg l−1 each citric acid, adenine sulphate and l-arginine as additives, 0.1% activated charcoal (AC), 100 mg l−1 2,4-dichlorophenoxyacetic acid (2,4-D) and 3.0 mg l−1 2-isopentenyladenine (2-iP). Callus culture were amplified on medium containing 3.0 mg l−1 2-iP along with 50 mg l−1 2,4-D for 2 passages and 10 mg l−1 2,4-D for 2 passages. Cultures grew moderately, organized and subsequently regenerated into shoot bud like structures during gradual transfer from medium containing higher concentration of 2,4-D to lower concentration. Plantlets were developed by sub-culturing of differentiated buds on (1) hormone free medium supplied with 10.0% sucrose and (2) medium containing 100.0 mg l−1 each ascorbic acid and glutamine, 50.0 mg l−1 each citric acid, adenine sulphate and l-arginine as additives, 1.0 mg l−1 each 6-benzylaminopurine, kinetin, 2-iP and α-naphthaleneacetic acid. Plantlets were developed on medium containing 0.1% AC, 1.0 mg l−1 each indole-3-acetic acid and indole-3-butyric acid. Rooted plantlets were soil-transplanted and acclimatized through gradual exposure from in vitro to in vivo conditions. Simple adoption, higher culture regeneration and simultaneous production of rooted plantlets in a cyclic manner render the protocol useful for mass scale propagation of elite genotype of female date palm.
Keywords: Dioecious, Female plant, Genetic improvement, Indirect regeneration, Organogenesis and tissue culture
Introduction
Phoenix dactylifera L. commonly called date palm is a member of Arecaceae family and considered as a key horticultural cash crop for arid and semi-arid regions. It is one of the oldest cultivated fruit trees and is being cultivated as a cash crop since pre-historic days. It is a multi-valued crop for arid/semi-arid regions of the world. Worldwide, more than 3000 cultivars of date palm are distributed wherever hot arid climate prevails. However, approximately 70% of total date production takes places in Middle East countries. Globally, date production in 2015–16 was approximately more than 8.5 million tonnes (FAO 2015–16). Dates are highly nutritious and eaten at Khalala, Rutab and Tamar stage. At Khalala stage, fruits gain maximum weight, turn gradually sweeter due to sucrose accumulation and exhibit shiny colour and hard texture. Rutab fruits lose water and become soft, sweeter and darker in colour. Rutab fruits contain more amount of reducing sugar as compared to Khalala. Fruits at tamar stage gains maximum total solids, sweetness and become wrinkled in shape. Tamar stage are rich in reducing sugar and contain least or no sucrose. Date syrup is also available in market and used as a source of sugar. In addition to fruits, date palm derived seeds, trunk, leaves and value added products (tree sap/syrup, ice creams, baby foods, jams, soft drinks and alcoholic beverages) generate additional income to the date palm farmers. Date palm is habituated to harsh environmental conditions of arid desert and creates a suitable microclimate to grow other agricultural crops as intercrops. Further RNA-Seq transcriptome analysis in date palm revealed multi-dimensional responses of date palm to salinity stress (Radwan et al. 2015), which renders it as a valuable genomic resource for identifying abiotic stress tolerance genes in tree system. Considering economic gain from date palm and comparative suitability under arid and semi-arid regions, this horticultural crop has potential of income generation as well as foreign exchange from unfertile tracts. It’s climate resilience, limited inputs for crop production, long-term productivity and multiple benefits make it as a key stone species for arid and semi-arid climate.
Phoneix dactylifera and P. sylvestris are two important species of palm in India. With higher economic potential, date palm possess a huge potential for development of rural areas and improvement of livelihoods of resource-poor farmers in arid and semi-arid areas. Date palm can be propagated sexually through seeds and vegetatively through offshoots. Date palm is a dioecious crop and seed raised progeny produce 50% male and 50% female (Tissert 1981). There are no means to identify female plants till flowering stage, thus plantation of seed raised date palm seedlings can cause considerable loss to farmers. Further due to heterozygous nature, seed progeny exhibit considerable variations and therefore desired characteristics of the mother palm may be lost, if propagated through seeds. Offshoot production is the most common method to multiply elite female genotype of date palm. Offshoots are produced in limited numbers during juvenile phase of tree (Sharma et al. 1986) and some of the cultivars do not produce offshoots. The availability of offshoots and their survival during plantation are major concern. Long-lived nature of date palm and lack of genetic description of germplasm (Chaluvadi et al. 2014) slowed the progress of breeding, genetics, crop improvement and expansion of commercial plantings. Clonal propagules are not available in sufficient quantity for commercial planting and large-scale productive plantation need high quality female planting propagules. As a non-GM biotechnology, in vitro cultures and plantlet regeneration systems are alternative production systems for mass-scale propagation of desired genotypes of plant (Rathore et al. 2011a) and supply of quality clonal propagules for commercial planting (Rathore et al. 2011b). Maximum utilization of in vitro culture techniques is rapid and mass-scale multiplication of elite genotype of plants, where natural regeneration does not meet the required demand (Rathore et al. 2011a, 2015a, b). In date palm, in vitro cultures are the best suited option to make available the planting propagules of elite female plants which have been proven to be suitable for horticulture in arid and semi-arid areas.
Tissue culture of date palm have been attempted and reviewed by several workers from all over the world (Gantait et al. 2018). Numerous reviews and research papers appeared during last 25 years on tissue culture of date palm. A few researchers did extensive studies on tissue culture of date palm (Krikorian 1994; Tissert 1984; Al-Khayri and Al-Bahrany 2004a, b). Fki et al. (2003) optimized protocols for plant regeneration through establishment of embryogenic suspension culture. Sudhersan and El-Nil (2004) demonstrated production of axillary shoots in micropropagated date palms of different cultivars. Zouine and El Hadrami (2004) studied effects of sucrose contents on proteins, sugars, phenolics and peroxidases activity in cell suspension culture. Zouine et al. (2005) optimized protocol for proliferation of embryogenic suspension culture and germination of somatic embryos. A few workers attempted in vitro propagation of date palm in India (Sharma et al. 1986; Bhansali et al. 1988; Dass et al. 1989; Singh and Shekhawat 2008). Beside numerous reports on date palm tissue culture, scaling up of tissue culture protocol is hindered due to various reasons. Kumar et al. (2010a, b) established genetic stability in somatic embryo produced date palm plantlets using RAPD, ISSR and microsatellite markers. Date palm tissue culture is highly genotype specific and it is necessary to develop alternative method of tissue culture for propagating the high quality planting material for elite genotypes. In vitro organogenesis is influenced by both extrinsic and intrinsic factors and has potentials to be employed in genetic transformation, phyto-pharming, germplasm restoration program and mass-scale propagation of planting propagules. An efficient and reproducible regeneration protocol also fulfills requirements to induce somaclonal variations and any genetic transformation studies. The aim of present work was to standardize protocol for callus culture and plantlet regeneration in elite female genotype of date palm.
Materials and methods
Plant material and surface sterilization
Young (2–5 years old) offshoots of female date palm were collected from date palm growing field, Kutch, Gujarat (India). Offshoots were detached from mother plant and stored under greenhouse conditions till explant harvest. Offshoots were processed by removing old and mature leaves acropetally using saw and knife until the apical shoot buds covered with 2–3 layers of leaves appeared. During processing, care was taken to avoid any damage to apical shoot bud. Harvested apical shoot buds were used as explants. Explant harvesting and surface disinfection was performed following Singh and Shekhawat (2008). Harvested explants were immediately immersed in chilled and sterile solution of anti-oxidants (150.0 mg l−1 each of citric acid and l-ascorbic acid, 100.0 mg l−1 Polyvinylpyrrolidone; PVP). Explants were pre-treated with 0.2% each of bavistin (a systemic fungicide) and streptomycin (an antibiotic) solution for 40 minutes (min) and washed 3–5 times with autoclaved water. Pre-treated explants surface sterilized with 0.4% mercuric chloride (HgCl2) solution for 40 min. Surface disinfected explants were extensively washed with sterile water and finally dipped in chilled sterile anti-oxidant solution for 10 min. Prior to final processing, explants were immersed in 70% ethanol for 40–60 s and subsequently air dried under laminar air flow bench. Finally outer 3 whorls of leaves were removed with the help of sterile scalpel leaving apical shoot bud comprised of 3–4 primordial leaves.
Bud culture establishment
Intact apical shoot buds were cultured on 0.8% agar-gelled Murashige and Skoog’s (MS) basal medium (Murashige and Skoog 1962) supplied with additives (100.0 mg l−1 each of ascorbic acid and PVP, 50.0 mg l−1 of citric acid, 25.0 mg l−1 each of adenine sulphate, arginine), 1.0% activated charcoal (AC) and 4.0% sucrose (Singh and Shekhawat 2008). Medium was supplied with 10 mg l−1 6-benzylaminopurine (BAP), 3.0 mg l−1 2-isopentyladenine (2-iP), 1.0 mg l−1 α-naphthaleneacetic acid (NAA) and 1.0 mg l−1 indole-3-actic acid (IAA) for activation of meristem. Before meristem activation, the cultures were incubated in a culture room at 26 ± 1 °C and 60–70% RH and after activation, these were shifted under 12 hd−1 photoperiod of 30–35 µ mol m−2 s−1 spectral flux photon in a culture room. After 6–8 months, the cultured shoot buds grew and showed enlargement (Fig. 1a) and after 10–12 months these produced multiple adventitious shoot buds (Fig. 1b). Subsequently, the shoot bud culture were amplified and regularly maintained on MS medium supplied with additives (100.0 mg l−1 each of ascorbic acid, 50.0 mg l−1 each citric acid and adenine sulphate, 25.0 mg l−1 l-arginine, 100 mg l−1 glutamine), 4.0% sucrose and 1.0% each glucose and maltose (Singh and Shekhawat 2008). Culture amplification medium was supplied with 1.0 mg l−1 each BAP, 2-iP, NAA and Kinetin (Kin).
Fig. 1.
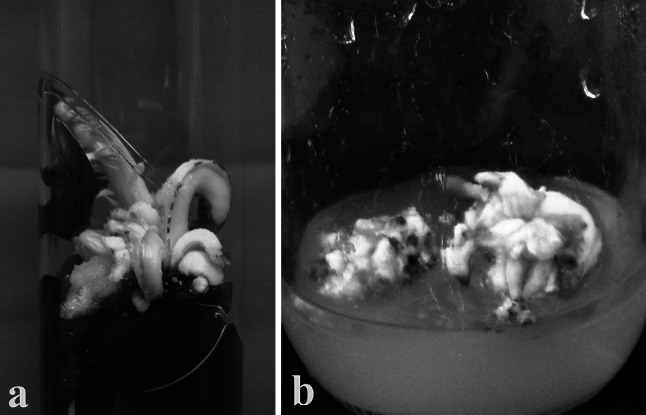
Culture of apical shoot bud on medium for induction of adventitious shoot bud cultures (a) and proliferating adventitious shoot bud culture (b)
Callus culture establishment and amplification
In vitro–derived adventitious buds were cut into pieces (ranging from 0.5–0.75 cm length) using sterile scalpel and cultured on 0.8% agar gelled MS medium augmented with 0.3% AC, 30 gl−1 sucrose, 10–100 mg l−1 2,4-dichlorphenoxyacetic acid (2,4-D) and 0.0–3.0 mg l−1 2-iP. Induced callus were sub-cultured on MS medium augmented with 0.1% AC, 40–50 g l−1 sucrose, 10–50 mg l−1 2,4-D and 0.0–3.0 mg l−1 2-iP. Cultures were incubated in dark at 26 ± 1 °C and 60% RH in a culture room and transferred on to fresh medium after regular interval of every 4 weeks.
Plantlet regeneration
Callus culture showing globular structures were sub-cultured on MS medium containing 0.1% AC, 40 g l−1 sucrose, 0.0–50 mg l−1 2,4-D and 0.0–3.0 mg l−1 2-iP. Cultures were incubated under 12 h d−1 photoperiod of 30–35 µ mol m−2 s−1 spectral flux photon, 60–70% RH and 26 ± 1 °C in a culture room. Cultures were sub-cultured on to fresh medium at a regular interval of every 4 weeks. Rooted shoot buds were cultured on MS medium supplied with additives (50.0–100.0 mg l−1 ascorbic acid, 25.0–50.0 mg l−1 each citric acid and adenine sulphate, 25.0 mg l−1 l-arginine, 50 mg l−1 glutamine), 3.0% sucrose and different concentrations (0.0–2.0 mg l−1 each BAP, 2-iP, Kin, IAA and IBA) of hormones.
Plantlet acclimatization
Rooted seedlings were removed from culture bottles and washed with autoclaved water to clean the adhered agar-medium to prevent microbial contamination. Plantlets were soil transplanted in a mixture of garden soil and sand (1:1 v/v) in plastic cups. Soil transplanted sapling were covered with transparent polythene bags to maintain high RH. Plantlets were incubated under culture room conditions and nutrified with half strength of MS macro-salt solution. After certain level of acclimatization of plantlets on soil, polythene bags were made porous for gradual exposure of plantlets to culture room conditions. After 16–20 weeks of acclimatization under culture room conditions, plantlets were shifted under greenhouse conditions. After 10–12 weeks of acclimatization in greenhouse, plantlets were transplanted into larger nursery poly-bags or earthen pots containing garden soil and farmyard fertilizer in 1:1 (v/v) ratio; and transferred under nursery conditions.
Experimental design and data analysis
Experiments were set up in a Randomized Block Design (RBD). All experiments were repeated thrice with minimum 15 replicates for each treatment for callus induction and plantlet regeneration. SPSS ver 7.5 (SPSS Inc., Chicago, USA) was used to analyze data statically and data were presented as mean ± SE.
Results and discussion
Date palm tissue culture is an important process and both extrinsic and intrinsic factors influence the in vitro organogenesis. In vitro organogenesis could be employed for genetic transformation, phyto-pharming, germplasm restoration program, and mass-scale multiplication of economically important and elite genotypes. In vitro callus culture and plantlet regeneration have been attempted in elite genotype of female date palm, which could be further scaled up to supply large-scale quality planting propagules for this commercially important crop. Explants were disinfected and adventitious shoot bud culture were established following Singh and Shekhawat (2008). The pre-established adventitious shoot bud culture (Fig. 1a, b) served as source of explants. In present case, in vitro–derived adventitious shoot buds (ranging 1.0–1.5 cm. in length) were used as explant for callus culture establishment. Compared with different parts of an offshoot (leaf, rachis, leaf base, roots, axillary shoot buds etc.), the in vitro–derived explants responded best and produced the sterile cultures. Pre-conditioning of explant tissues, developmental stages, endogenous level of plant hormones and their metabolism, secondary metabolism and better response to exogenous and endogenous signals might be reasons for better response of in vitro-derived explants as compared to in vivo-derived tissues (Rathore et al. 2016). Explants responded on medium containing 2,4-D and 42.67 ± 1.33–89.33 ± 2.67% explants produced callus on medium containing additives (100.0 mg l−1 each polyvinylpyrrolidone, ascorbic acid and glutamine, 50.0 mg l−1 each citric acid, adenine sulphate and l-arginine), 10–100 mg l−1 2,4-D along with 1.0–3.0 mg l−1 2-iP (Table 1). On medium containing additives, 100 mg l−1 2,4-D and 3.0 mg l−1 2-iP, 89.33 ± 2.67% explants produced callus culture (Fig. 2a) after 8–12 weeks. Delayed response was observed on medium containing lower concentration of 2,4-D. The 2,4-D is known to be a robust performing plant growth regulator as compared to other plant hormones for induction of callus culture (Rathore et al. 2011a). Induction of callus culture requires the presence of auxin in the medium and it is a pre-requisite for in vitro plantlet regeneration and somatic embryogenesis (Gueye et al. 2009a, b). Present results confirmed the requirement of higher concentration of 2,4-D in cultre medium for date palm callogenesis (Kumar et al. 2010a, b). Use of anti-oxidants in medium prevented leaching of phenolic substances, browning of medium and tissues in the culture system. Induced callus culture differed in texture and morphology therefore, selection of regenerative callus was observed as a critical factor. At callus induction stage, sometime callus appeared mucilaginous and resembled microbial contaminations, thus the selection of regenerative culture was considered as critical factor during culture induction. Anti-oxidant and AC in medium has been reported to reduce the lethal effect of phenolic compounds, browning of tissue and culture medium (Rathore et al. 2011a). AC and diffused light helped in early meristem activation and culture proliferation. Regular sub-culturing of cultures on fresh medium was performed after an interval of every 4 weeks.
Table 1.
Effect of different concentrations of 2,4-D and 2-iP on callus induction from in vitro—derived explants on 0.8% agar-gelled MS medium
| Hormones (mgl−1) | Percent callusing ± SE | |
|---|---|---|
| 2,4-D | 2-iP | |
| 0 | 0 | 0.00 ± 0.00f |
| 10 | – | 42.67 ± 1.33e |
| 50 | – | 66.67 ± 1.33d |
| 100 | – | 82.67 ± 1.33ab |
| 50 | 1.0 | 70.67 ± 5.33 cd |
| 50 | 1.5 | 72.00 ± 4.00 cd |
| 50 | 3.0 | 77.33 ± 3.53bc |
| 100 | 1.0 | 77.33 ± 2.67bc |
| 100 | 1.5 | 86.67 ± 1.33a |
| 100 | 3.0 | 89.33 ± 2.67a |
Mean ± SE followed by the same letter in same column are not significantly different by the Duncan’s multiple range test at 0.05% probability level
Fig. 2.
Induction of callus cultures (a), amplification of callus culture (b), callus culture differentiation (c), callus-differentiated buds under growing phase (d), embryo-like culture buds (e), clumps of unrooted adventitious buds (f) and rooted adventitious buds (g)
Longer maintenance (more than 16–20 weeks, four–five passages) of callus culture on induction medium lead to hyper-hydration of cultures and callus become glossy, watery and non-regenerative. Hyper-hydration resulted due to higher concentration of hormones and water potential in the culture medium (Rathore et al. 2011a; Correll et al. 2000). Hyper-hydration is considered as a physiological disorder of in vitro cultures and adversely affects the growth and regeneration capabilities of cultures leading to production of difficult to handle cultures. Therefore, after tissue rejuvenation and establishment of regenerative culture, it is suggested to optimize media formulations, hormonal combinations and media supplements to maintain the regenerative potential of cultures. In present case, 2,4-D concentration was lowered down in medium to maintain the regeneration potential of cultures and amplify the callus culture. Calli were sub-cultured on to medium supplemented with 50 mg l−1 2,4-D for 2–3 passages and subsequently on to medium with 10 mg l−1 2,4-D for 2–3 passages along 3.0 mg l−1 2-iP and additives. Lower concentrations of 2,4-D have been reported to support good growth and maintain regenerative potential of cultures after initiation of cultures on medium containing higher amount of 2,4-D (Fei et al. 2002; Rathore et al. 2011a, 2014). During the transfer, callus amplified and exhibited moderate growth rate and texture of regenerative culture (Fig. 2b). Callus grew, transformed in globular structures and subsequently regenerated into elongated regenerative shoot bud like structures during gradual transfer from higher concentration of 2,4-D to lower concentrations. Hormonal concentration in culture medium strongly influence culture development, regeneration frequency and plantlets recovered following their sub-culture onto hormone free medium (Rathore et al. 2014). As callus showed regeneration, the juvenile and elongated shoot buds were transferred on fresh hormone-free medium containing 10.0% sucrose. Sucrose was reported as essential for embryo development having a strong effect on embryogenesis (Veramendi and Navarro 1996). Higher concentration of sucrose in culture medium helped maturation of cultures. Differentiating shoot buds like cultures from hormone free medium were transferred on to medium containing 5.0% carbohydrate, 0.1% AC, additives (100.0 mg l−1 each ascorbic acid and glutamine, 50.0 mg l−1 each citric acid, adenine sulphate and l-arginine), 1.0 mg l−1 each BAP, Kin, 2-iP and NAA. On this medium, the regenerated culture buds organized, grew as cream coloured shoot buds and showed growth (Fig. 2c). BAP, Kin and 2-iP were found to have synergic effect on adventitious shoot bud proliferation, as omissions of any one of these cytokinins resulted in slow growth of cultures. Further, these buds were cultured on fresh medium devoid of AC. On this medium, shoot buds exhibited growth and showed amplification/multiplication (Fig. 2d). Regular sub-culturing was done on fresh medium after an interval of every 4–6 weeks. On this medium, culture produced embryo like culture buds, shoot bud clumps and rooted shootlets (Fig. 2e–g). Culture buds multiplied and shootlets elongated on this medium (Fig. 3a). A well-maintained culture exhibited four to fivefold rate of shoot bud multiplication in cultures (Figs. 2d, 3a). Culture buds, shoot bud clumps and rooted shootlets were produced on same medium. It must be noted that when rooting in shoot buds were achieved along with culture amplification, the protocol saved time to induce rooting separately. Thus it saves time, resources and labor for plantlet production. Regeneration of higher number of rooted shoot buds is important for a commercially viable protocol and in present case, non-synchronized development of in vitro tissues was observed as a major limitation. Further improvement in regeneration of rooted shootlets would have a great impact on commercial applicability of any protocol (Rathore et al. 2015a, b). Irregular and asynchronous development of different stages of shoot bud tissues in cultures indicated the complexity of developmental process during culture regeneration. The rooted shootlets were sub-cultured and grown for 2 passages on MS medium supplied with 0.1% AC, 0.5–1.0 mg l−1 each IAA and IBA to elongate shoot buds and strengthen the roots of shootlets (Fig. 3b).
Fig. 3.
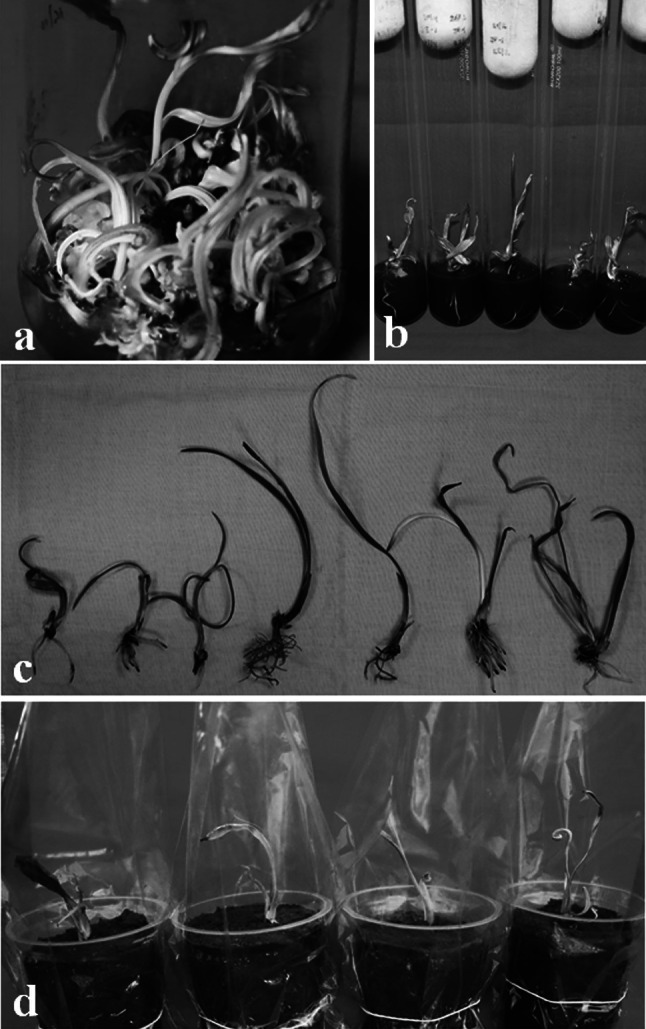
Callus-differentiated adventitious buds under elongation phase (a), rooted adventitious buds (b), rooted adventitious buds ready for soil transplantation (c) and soil transplanted tissue culture plants of female date palm
Elongated shootlets with sturdy root system on MS medium containing 0.5 mg l−1 each IAA and IBA were removed and washed thoroughly with autoclaved water to remove adhered medium to prevent microbial growth (Fig. 3c). Auxins, in present case IBA and IAA promoted induction of lateral roots and primordial growth (Fukaki and Tasaka 2009; Patel et al. 2014). Acclimatization of in vitro propagated plantlets is crucial step of regeneration protocol to ensure proper hardening before transplanting them to in situ conditions. Rooted plantlets were transplanted in plastic cups containing sterile soil and nutrified with half-strength of MS macro-salts. Use of AC during root development and half-strength of MS macro-salts during acclimatization has been suggested advantageous. Plastic cups containing plantlets were covered with inverted polythene bags and incubated in culture room. After 12–16 weeks of growth under culture room, polythene bags were punched gradually to allow gas exchange and subsequent acclimatization (Fig. 3d). After certain level (24–28 weeks) of adaptation under culture room conditions, the tissue culture raised plantlets were transferred under greenhouse conditions. Plantlets were nutrified twice in a week with half strength of MS macro-salts solution. After 4–8 weeks of growth the inverted polythene bags were removed completely and plantlets were allowed to grow for further 6–8 months. These were transferred in larger polythene bags and after 2 weeks of growth these soil transplanted plantlets were transferred to nursery. These plantlets were allowed to grow under nursery conditions to achieve suitable growth (10–12 leaf stage) so that they may be transplanted in field. Higher frequency of culture regeneration along production of rooted shootlets in cyclic manner renders the present protocol as an effective mass-scale propagation system for elite genotype of female date palm (Fig. 4).
Fig. 4.
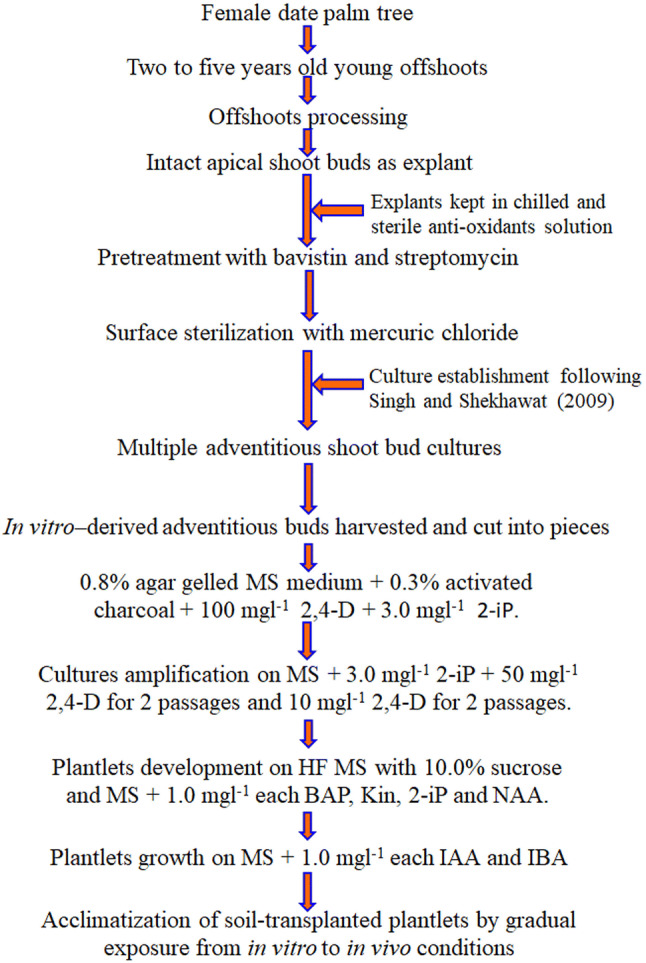
A step-wise diagrammatic representation of regeneration protocol for elite genotype of female date palm
Conclusion
Present study report a protocol for callus culture and in vitro plantlet regeneration in elite genotype of female date palm. Explants (segments of in vitro-derived adventitious shoot buds) produced callus, cultured on MS medium with 2,4-D, 2-iP and other supplements. Callus culture induced on MS medium with higher concentration of 2,4-D were transferred step by step on medium with lower level of 2,4-D along with 2-iP and other supplements. During transfers on medium with higher to lower concentration of 2,4-D cultures organized and differentiated into shoot buds, which were further developed on medium containing BAP, Kin, 2-iP and NAA. Elongated and rooted shoot buds were transplanted on to sterile soil and acclimatized by slow changes in their growth conditions from high humidity and lower temperature to low humidity and higher temperature. Due to dioecious nature of date palm and its huge economic potential in arid and semi-arid regions, tissue culture protocol for micropropagation of elite genotype of female plants offer huge opportunity to generate quality planting propagules at mass-scale and revenue generation for resource-poor farmers of arid and semi-arid regions. The simple adoption, higher culture regeneration and simultaneous production of rooted plantlets in a cyclic manner make the protocol superior to other reported protocols.
Acknowledgements
CSIR-CSMCRI PRIS 164/2018. Authors thankfully acknowledge the SERB, Govt. of India for financial support (SERB/SB/SO/PS/02/2014; GAP-2013) under Extra Mural Research Funding Project. Authors acknowledge Mr. Mahesh Saini and Mr. Darshan Patel for their initial work in this project. SAS acknowledge University Grant Commission, Govt. of India financial support in the form of UGC-JRF and AcSIR, New Delhi for registration in Ph.D. program.
Author’s contribution
MSR: Conceptualization, experiment planning and fund raising; PP, SAS and MSR: execution of plan, culture maintenance, manuscript writing; MSR: finalization of manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/21/2022
A Correction to this paper has been published: 10.1007/s12298-022-01199-z
References
- Al-Khayri JM, Al-Bahrany AM. Genotype-dependent in vitro response of date palm (Phoenix dactylifera L.) cultivars to silver nitrate. Sci Hortic. 2004;99(2):153–162. doi: 10.1016/S0304-4238(03)00091-8. [DOI] [Google Scholar]
- Al-Khayri JM, Al-Bahrany AM. Growth, water content, and proline accumulation in drought-stressed callus of date palm. Biol Plant. 2004;48(1):105–108. doi: 10.1023/B:BIOP.0000024283.74919.4c. [DOI] [Google Scholar]
- Bhansali RR, Kaul RK, Dass HC. Mass cloning of date palm plantlet through repetitive somatic embryogenesis. J Plant Anat Morphol. 1988;5:73–79. [Google Scholar]
- Chaluvadi SR, Khanam S, Aly MA, Bennetzen JL. Genetic diversity and population structure of native and introduced date palm (Phoenix dactylifera) germplasm in the United Arab Emirates. Trop Plant Biol. 2014;7(1):30–41. doi: 10.1007/s12042-014-9135-7. [DOI] [Google Scholar]
- Correll MJ, Wu Y, Weathers PJ. Controlling hyperhydration of carnations (Dianthus caryophyllus L.) grown in a mist reactor. Biotechnol Bioeng. 2000;71(4):307–314. doi: 10.1002/1097-0290(2000)71:4<307::AID-BIT1019>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Dass HC, Kaul RK, Joshi SP, Bhansali RR. In vitro regeneration of date-palm plantlets. Curr Sci. 1989;58(1):22–24. [Google Scholar]
- FAO (2015–16). http://www.fao.org/faostat/en/#data/QC/visualize
- Fei SZ, Riordan T, Read P. Stepwise decrease of 2, 4-D and addition of BA in subculture medium stimulated shoot regeneration and somatic embryogenesis in buffalograss. Plant Cell Tissue Organ Cult. 2002;70(3):275–279. doi: 10.1023/A:1016513516130. [DOI] [Google Scholar]
- Fki L, Masmoudi R, Drira N, Rival A. An optimised protocol for plant regeneration from embryogenic suspension cultures of date palm, Phoenix dactylifera L., cv. Deglet Nour. Plant Cell Rep. 2003;21(6):517–524. doi: 10.1007/s00299-002-0558-5. [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tasaka M. Hormone interactions during lateral root formation. Plant Mol Biol. 2009;69:437–449. doi: 10.1007/s11103-008-9417-2. [DOI] [PubMed] [Google Scholar]
- Gantait S, El-Dawayati MM, Panigrahi J, Labrooy C, Verma SK. The retrospect and prospect of the applications of biotechnology in Phoenix dactylifera L. Appl Microbiol Biotechnol. 2018;102(19):8229–8259. doi: 10.1007/s00253-018-9232-x. [DOI] [PubMed] [Google Scholar]
- Gueye B, Morcillo F, Collin M, Gargani D, Overvoorde P, Aberlenc-Bertossi F, et al. Acquisition of callogenic capacity in date palm leaf tissues in response to 2,4-D treatment. Plant Cell Tissue Organ Cult. 2009;99(1):35–45. doi: 10.1007/s11240-009-9573-3. [DOI] [Google Scholar]
- Gueye B, Saïd-Ahmed H, Morcillo F, Borgel A, San D, Hilbert JL, et al. Callogenesis and rhizogenesis in date palm leaf segments: Are there similarities between the two auxin-induced pathways? Plant Cell Tissue Organ Cult. 2009;98(1):47–58. doi: 10.1007/s11240-009-9537-7. [DOI] [Google Scholar]
- Krikorian AD. In vitro culture of plantation crops. In: Vasil IK, Thorpe TA, editors. Plant cell and tissue culture. Dordrecht: Kluwer Academic Publishers; 1994. [Google Scholar]
- Kumar N, Modi AR, Singh AS, Gajera BB, Patel AR, Patel MP, Subhash N. Assessment of genetic fidelity of micropropagated date palm (Phoenix dactylifera L.) plants by RAPD and ISSR markers assay. Physiol Mol Biol Plants. 2010;16(2):207–213. doi: 10.1007/s12298-010-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Sing AS, Modi AR, Patel AR, Gajera BB, Subhash N. Genetic stability studies in micropropagated date palm (Phoenix dactylifera L.) plants using microsatellite marker. J For Environ Sci. 2010;26(1):31–36. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant. 1962;15:473–495. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Patel K, Phulwaria M, Rai MK, Gupta AK, Shekhawat S, Shekhawat NS. In vitro propagation and ex vitro rooting of Caralluma edulis (Edgew.) Benth. & Hook. f. An endemic and endangered edible plant species of the Thar Desert. Sci Hortic. 2014;165:175–180. doi: 10.1016/j.scienta.2013.10.039. [DOI] [Google Scholar]
- Radwan O, Arro J, Keller C, Korban SS. RNA-Seq transcriptome analysis in date palm suggests multi-dimensional responses to salinity stress. Trop Plant Biol. 2015;8(3–4):74–86. doi: 10.1007/s12042-015-9155-y. [DOI] [Google Scholar]
- Rathore MS, Chikara J, Mastan SG, Rahman H, Anand KGV, Shekhawat NS. Assessment of genetic stability and instability of tissue culture-propagated plantlets of Aloe vera L. by RAPD and ISSR markers. Appl Biochem Biotechnol. 2011;165(5–6):1356–1365. doi: 10.1007/s12010-011-9352-6. [DOI] [PubMed] [Google Scholar]
- Rathore MS, Chikara J, Shekhawat NS. Plantlet regeneration from callus cultures of selected genotype of Aloe vera L.—an ancient plant for modern herbal industries. Appl Biochem Biotechnol. 2011;163(7):860–868. doi: 10.1007/s12010-010-9090-1. [DOI] [PubMed] [Google Scholar]
- Rathore MS, Yadav P, Mastan SG, Prakash CR, Singh A, Agarwal PK. Evaluation of genetic homogeneity in tissue culture regenerates of Jatropha curcas L. using flow cytometer and DNA-based molecular markers. Appl Biochem Biotechnol. 2014;172(1):298–310. doi: 10.1007/s12010-013-0517-3. [DOI] [PubMed] [Google Scholar]
- Rathore MS, Paliwal N, Anand KV, Agarwal PK. Somatic embryogenesis and in vitro plantlet regeneration in Salicornia brachiata Roxb. Plant Cell Tissue Organ Cult. 2015;120(1):355–360. doi: 10.1007/s11240-014-0571-8. [DOI] [Google Scholar]
- Rathore MS, Yadav S, Yadav P, Kheni J, Jha B. Micropropagation of elite genotype of Jatropha curcas L. through enhanced axillary bud proliferation and ex vitro rooting. Biomass Bioenergy. 2015;83:501–510. doi: 10.1016/j.biombioe.2015.10.023. [DOI] [Google Scholar]
- Rathore MS, Mastan SG, Yadav P, Bhatt VD, Shekhawat NS, Chikara J. Shoot regeneration from leaf explants of Withania coagulans (Stocks) Dunal and genetic stability evaluation of regenerates with RAPD and ISSR markers. S Afr J Bot. 2016;102:12–17. doi: 10.1016/j.sajb.2015.08.003. [DOI] [Google Scholar]
- Sharma DR, Deepak S, Chaudhary JB. Regeneration of plantlets from somatic tissues of the date palm Phoenix dactylifera. Indian J Exp Biol. 1986;24:763–766. [Google Scholar]
- Singh M, Shekhawat NS. Tissue culture of date palm (Phoenix dactylifera)—a non-conventional approach. In: Shekhawat NS, Kumar A, editors. Plant tissue culture and molecular markers: their role in improving crop productivity. New Delhi: IK International Pvt Ltd.; 2008. [Google Scholar]
- Sudhersan C, El-Nil MA. Axillary shoot production in micropropagated date palm (Phoenix dactylifera) Curr Sci. 2004;86(6):771–773. [Google Scholar]
- Tissert B. Date palm tissue culture. Oakland: USDA-ARS Advances in Agricultural Technology; 1981. [Google Scholar]
- Tissert B. Propagation of date palm by shoot tip cultures. Hortic Sci. 1984;19:230–231. [Google Scholar]
- Veramendi J, Navarro L. Influence of physical conditions of nutrient medium and sucrose on somatic embryogenesis of date palm. Plant Cell Tissue Organ Cult. 1996;45(2):159–164. doi: 10.1007/BF00048760. [DOI] [Google Scholar]
- Zouine J, El Hadrami I. Somatic embryogenesis in Phoenix dactylifera L.: effect of exogenous supply of sucrose on proteins, sugars, phenolics and peroxidases activities during the embryogenic cell suspension culture. Biotechnology. 2004;3(2):114–118. doi: 10.3923/biotech.2004.114.118. [DOI] [Google Scholar]
- Zouine J, El Bellaj M, Meddich A, Verdeil JL, El Hadrami I. Proliferation and g ermination of somatic embryos from embryogenic suspension cultures in Phoenix dactylifera. Plant Cell Tissue Organ Cult. 2005;82(1):83–92. doi: 10.1007/s11240-004-6914-0. [DOI] [Google Scholar]



