Chen et al. review the effects of type-I IFNs and the potential of anti–type-I IFN therapies in atherosclerosis.
Abstract
The contribution of dyslipidemia and inflammation in atherosclerosis is well established. Along with effective lipid-lowering treatments, the recent success of clinical trials with anti-inflammatory therapies and the accelerated atherosclerosis in many autoimmune diseases suggest that targeting inflammation may open new avenues for the prevention and the treatment for cardiovascular diseases (CVDs). In the past decades, studies have widened the role of type-I interferons (IFNs) in disease, from antivirus defense to autoimmune responses and immuno-metabolic syndromes. While elevated type-I IFN level in serum is associated with CVD incidence in patients with interferonopathies, experimental data have attested that type-I IFNs affect plaque-residing macrophages, potentiate foam cell and extracellular trap formation, induce endothelial dysfunction, alter the phenotypes of dendritic cells and T and B lymphocytes, and lead to exacerbated atherosclerosis outcomes. In this review, we discuss the production and the effects of type-I IFNs in different atherosclerosis-associated cell types from molecular biology studies, animal models, and clinical observations, and the potential of new therapies against type-I IFN signaling for atherosclerosis.
Introduction
Cardiovascular diseases (CVDs) are the leading cause of death worldwide (Hess et al., 2017; Lozano et al., 2012; Wang et al., 2016). Atherosclerosis, the main underlying causal factor of CVDs, is a chronic inflammatory disease driven by lipid accumulation in the arterial intima where modified low-density lipoprotein (mLDL) deposition provokes the recruitment of blood-derived immune cells and triggers inflammatory cascades (Rafieian-Kopaei et al., 2014; Tabas et al., 2007). Pro-atherogenic lipids are taken up mainly by smooth muscle cells (SMCs) and monocytes/macrophages, which subsequently secrete pro-inflammatory cytokines and chemokines (den Brok et al., 2018; Owsiany et al., 2019). In lipid-laden macrophage-derived foam cells, endoplasmic reticulum stress-associated apoptosis can be induced by high cholesterol, mLDL-triggered pattern recognition receptor (PRR) signaling, and increased inflammatory cytokines in the plaques (Seimon and Tabas, 2009). As such, atherosclerotic lesion development is characterized by the recruitment of monocytes and macrophages, accumulating pro-apoptotic foam cells and SMCs, infiltrating leukocytes, plaque-stabilizing collagen deposition, and phagocytes responding to engulfed cellular debris (Hansson, 2005; Moore and Tabas, 2011; Williams et al., 2019). As the activation hierarchy progresses to a chronic process, the spatiotemporal homeostasis between inflammation and disease-suppressing resolution pathways is disrupted. Unresolved inflammation, together with subsequently impaired efferocytosis, leads to cell necrosis, microvessel formation, fibrous cap thinning, and destabilization of the advanced atherosclerotic plaques (Kojima et al., 2017; Rafieian-Kopaei et al., 2014). Various cell types with high heterogeneity are involved in this pathogenic process. Notably important are differentially activated monocytes and macrophages, dendritic cells, neutrophils, T and B lymphocytes, endothelial cells (ECs), and SMCs (Döring et al., 2017; Ketelhuth and Hansson, 2016; Stöger et al., 2012).
The unstable advanced plaques are prone to rupture, increasing the risk of thrombosis and consequent ischemic heart diseases and stroke. Traditional pharmacological strategies for atherosclerosis prevention and treatment focus mainly on reducing plasma low-density lipoprotein (LDL) levels. Given the success of cholesterol-lowering therapy, mainly by statins for secondary prevention of CVDs, new therapeutic approaches usually develop on top of the widespread statin treatment. However, emerging evidence from both clinical and experimental studies reinforced the beneficial effect of dampening inflammation in atherothrombosis where the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study, applying anti–IL-1β antibody therapy, exhibited a significantly reduction of the risk of recurrent cardiovascular events, independent of lipid-levels (Bäck and Hansson, 2015; Hansson, 2005; Libby et al., 2009; Ridker et al., 2017, 2018; Zhang and Reilly, 2018).
IFNs are a group of cytokines named by their characteristics of viral interference (Isaacs et al., 1957; Pestka, 2007). IFNs can be classified into three families (type-I, -II, and -III) according to the protein structure and the receptors they signal through. Type-I IFNs are important immune modulator altering both innate and adaptive immunity (González-Navajas et al., 2012; Kopitar-Jerala, 2017; Trinchieri, 2010). Accumulating evidence from both human and murine studies supports their role in atherogenesis and linked clinical manifestations. Experimental data show that systemic or intraplaque type-I IFNs deteriorate atherogenesis by activating endothelium and immune cells, promoting foam cell formation, altering progenitor cell function, and enhancing pro-inflammatory leukocyte recruitment to arteries. Further, individuals suffering from autoimmune diseases with elevated type-I IFN signatures, such as systemic lupus erythematosus (SLE), are predisposed to accelerated atherosclerosis leading to increased risk of CVDs and cardiovascular (CV)-associated mortality. The syndromic concurrences of interferonopathy and cardiovascular manifestations may result from shared pathogenic processes (Ganguly, 2018).
In this review, we focus on the role of type-I IFNs in atherogenesis and discuss the potential opportunities to dampen inflammation for prevention and therapeutic intervention of atherosclerosis. In particular, we specify the effect of type-I IFNs on various atherogenic cell types (Table 1, provided at the end of the review), highlight their involvement in accelerated atherosclerosis in patients with IFN-associated autoimmune diseases, and discuss how anti–type-I IFN treatments could serve as multi-target therapy in disease.
Table 1. Summary of the production and the effect of type-I IFNs in atherosclerosis-associated cells.
| Cell/tissue | Type-I IFN production/induction | Responses to type-I IFNs | Human sample | Murine model | Reference |
|---|---|---|---|---|---|
| Aortic tissue | Increased SiglecH and Ifna expression in the aortic tissue and elevated IFN-α in the serum in mice with HFD | Apoe−/− (in vivo) aorta, HFD vs. ND for 12 wk | Döring et al., 2012 | ||
| B cell | Increased type-I IFN expression/secretion in B cell stimulated by Mtb (in vivo and in vitro) | Reduced type-I IFN production in Ifnar1−/− B cells | Ifnar1−/−, Sting−/−, WT pulmonary tissue, pleural fluid, and spleen | Bénard et al., 2018 | |
| B cell | Ifnar1−/− mice showed reduced auto-Ab production | Ifnar1−/−, WT BM chimera | Domeier et al., 2018 | ||
| B cell | Increased anti-dsDNA Ab serum levels in symptomatic vs. asymptomatic carotid artery stenosis patients | Carotid artery stenosis patients | Döring et al., 2012 | ||
| B cell | Elevated anti-dsDNA Ab in IFN-α/CpG-treated/HFD-fed mice and dampened by pDC depletion by PDCA1 Ab in Apoe−/− mice or in Cramp−/− mice (Ldlr−/−) | Apoe−/− (in vivo), HFD vs. ND, Ldlr−/− Cramp+/+ vs. Cramp−/−, ± IFN-α/CpG treatment | Döring et al., 2012 | ||
| B cell | Reduced anti-dsDNA Ab in tofacitinib treated mice | MRL/lpr mice ± tofacitinib | Furumoto et al., 2017 | ||
| B cell | Ifnb1−/− B cells showed suboptimal responses toward TLR7 stimulation | Reduced cytokine production in Ifnar1−/− B cell upon TLR stimulation, exogenous type-I IFNs strengthen the responses | Ifnar1−/−, Ifnb1−/−, WT spleen | Green et al., 2009 | |
| B cell | Transitional B cell subsets express type-I IFNs | Endogenous IFN-β promotes survival and development of transitional (autoreactive) B cells | Ifnb1−/−, Rag1−/−, WT | Hamilton et al., 2017 | |
| B cell | Autoantibodies against neutrophil antimicrobial peptides is associated with seurm IFN-α in SLE patients | SLE patients | Lande et al., 2011 | ||
| B cell | Reduced Ab production as a response to poly(I:C) in Ifnar1−/− B cells | Ifnar1−/−, WT | Swanson et al., 2010 | ||
| B cell | B cells from SLE patients produce IFN-α | SLE PBMC | Ward et al., 2016 | ||
| EC | SLE serum induced more ISGs compared with HC, IFN-α suppressed NO production and increases CCL2 and VCAM-1 expression and neutrophil migration | HUVEC, SLE patients vs. HC sera | Buie et al., 2017 | ||
| EC | Interrupted CD31 staining (EC damage) in vasculature of mice expressing IFN-α | In vivo Ifna5 expressing model (plasmid transduced, 3 wk) vs. WT | Diao et al., 2016 | ||
| EC | IFN-β1a induces membrane-bound ICAM protein expression | HUVEC | Giorelli et al., 2002 | ||
| EC | IFN-β but not IFN-α inhibits HUVEC proliferation and survival, both type-I IFNs reduce HUVEC NO production | HUVEC | Jia et al., 2018 | ||
| EC | ICAM-1, VCAM-1 adnd osinophil adhesion was significantly augmented by IFN-β in the presence of TNF-α but not in its absence | HUVEC | Kobayashi et al., 2008 | ||
| EC | Type-I IFNs induce CCL5, CX3CL1 production via JAK signaling | HUVEC, HPAEC, HAEC, HLMVEC | Nakano et al., 2012 | ||
| EC | IFNα2b does not affect HAEC proliferation and NO production | HAEC | Reynolds et al., 2014 | ||
| EC | Type-I IFNs inhibit the growth factor deprivation- or oxidative cytotoxicity-induced cell death | HAEC | Sano et al., 2012 | ||
| EC | Type-I IFNs alone did not affect the expression of E-selectin, P-selectin, VCAM-1, and ICAM-1 | HUVEC | Shen et al., 1997 | ||
| EC | TNFR1, TNFR2 signaling induce IRF1 expression and IFN-β production in MHEC | IFN-β increased VCAM-1, CXCR3 chemokines (Cxcl9, Cxcl10) expression in MHEC supporting monocyte recruitment | Ifnar1−/−, WT MHEC with/without IFN-β treatment | Venkatesh et al., 2013 | |
| EC/EPC | SLE serum/IFN-α prevents monolayer formation and maturation from EPC and induces apoptosis, SLE EPC restores a normal phenotype with IFNA(R) blockade | SLE patient serum, EPC | Denny et al., 2007 | ||
| EC/EPC | Improved endothelium-dependent vasorelaxation, EPC differentiation in tofacitinib treated mice | MRL/lpr mice ± tofacitinib, aorta | Furumoto et al., 2017 | ||
| EC/EPC | IFN-α suppresses EPC differentiation | Murine bone marrow and spleen EPC | Thacker et al., 2010 | ||
| EC/EPC | Loss of type-I IFN signaling improves EPC number and EC function in lupus-prone mice while additional IFN-α worsens EC function and EPC differentiation | IFNαβR−/− or IFNαβR+/+ and lupus-prone vs. normal mice, ± Ifna-expressing virus, Apoe−/− IFNαβR−/− mice WD for 10 wk | Thacker et al., 2012 | ||
| Eosinophil | oxLDL up-regulates IFN-α and IFN-β (CD36 dependent), reduce IL-4/IL13 expression | BM-derived eosinophils (in vitro), ± anti-CD36 Mab, ± Cd36 siRNA | Qin et al., 2017 | ||
| EPC | Increased IFN signature of PBMC and reduced differentiation capacity of EPC in APS patients or EPC treated with APS sera, which could be rescued by anti-IFNAR Ab | APS/SLE patients vs. HC PBMC | Grenn et al., 2017 | ||
| mDC | Pro-IL-1β synthesis and IL-1β maturation are unaffected by type-I IFNs | WT BMDC ± type-I IFNs | Guarda et al., 2011 | ||
| mDC | IFN-α increases TNF expression upon LPS stimulation | MoDC (in vitro) | Niessner et al., 2007 | ||
| mDC | Combining IFN-α with LPS amplifies TNF expression while IFN-α alone does not affect TNF expression (JAK/STAT, NF-κB dependent) but increases TLR4 expression | MoDC (in vitro) | Niessner et al., 2007 | ||
| Monocyte | Tofacitinib and JAK1 inhibitor increase IL6 and reduce CXCL10, TNF production in monocyte stimulated with LPS+IFNγ | HC monocyte ± tofacitinib/JAK1 inhibitor/JAK3 inhibitor | De Vries et al., 2019 | ||
| Monocyte | Reduced recruitment to peritoneal cavity in WT mice upon poly(I:C) followed by TLR4 intraperitoneal injection, but the reduction is reduced in Ifnar1−/− mice | Ifnar1−/−, WT mice ± poly(I:C) followed by alum intraperitoneal injection | Guarda et al., 2011 | ||
| Monocyte | In vitro IFN-β priming or IFN-β treatment in MS patients suppresses IL-1β production in monocyte upon LPS/Alum stimulation | treated MS vs. HC monocyte ± IFN-β, LPS, Alum | Guarda et al., 2011 | ||
| Monocyte | Increased oxLDL uptake in SLE patient monocyte | SLE patients vs. HC | Li et al., 2011a | ||
| Monocyte | IFN-α increases TNF expression upon LPS stimulation | THP1 (in vitro) | Niessner et al., 2007 | ||
| Monocyte | Increased lipid content and LDL uptake via upregulation of SR-A in HIV patients or HC with IFN-α treatment (correlates with MX1, CXCL10 expression) | HIV patients vs. HC, ± IFN-α | Pulliam et al., 2014 | ||
| Monocyte | Ifna1high Ly-6C− monocyte subsets identified | Apoe−/− (in vivo) plaque | Fig. 3 in Winkels et al., 2018 | ||
| Monocyte/Mφ | Increased CCL5-dependent leukocyte arrest in the carotid arteries upon IFN-β treatment | Apoe−/− (in vivo) plaque, ± IFN-β 1 d, ± Met-Rantes, HFD 3 wk | Goossens et al., 2010 | ||
| Mφ | IFN-α treatment altered gene expression enriched in metabolism pathways, such as lipid metabolism | MDM (in vitro) ± IFN-α | BMM (in vitro) | Ahmed et al., 2018 | |
| Mφ | Mtb-treated B cell–conditioned media induce expression of Cox2, Nos2, PDL-1 in WT BMMs which is abrogated in Ifnar−/− BMMs | Ifnar1−/−, WT BMM | Bénard et al., 2018 | ||
| Mφ | Increased foam cell formation via upregulation of SR-A with IFN-β treatment | BMM (in vitro), PM (Ldlr−/−, HFD 10 wk, in vivo) | Boshuizen et al., 2016 | ||
| Mφ | TNF is restricted by IFN-γ priming but potentiated by IFN-β priming, the effect of timing is gene- and stimulus-specific | Cheng et al., 2019 | |||
| Mφ | Tofacitinib and JAK1 inhibitor reduce IL6, CXCL10, TNF production and pro-inflammatory gene expression in BMM stimulated with LPS+IFNγ | WT BMM ± tofacitinib/JAK1 inhibitor/JAK3 inhibitor | De Vries et al., 2019 | ||
| Mφ | Increased IFNAR1/STAT1-dependent CCR2, CCR5, CCL5 expression, EC adhesion upon IFN-α/β treatment | BMM (in vitro, WT, Ifnar1−/−, Stat1−/−) | Goossens et al., 2010 | ||
| Mφ | Increased CCR5, CCL5 expression/secretion upon IFN-α/β treatment | MDM (in vitro) | Goossens et al., 2010 | ||
| Mφ | IFN-β suppresses pro-IL-1β synthesis and IL-1β maturation via IL10 and STAT3 signaling, and suppresses NLRP3 inflammasome activation via STAT1 | Ifnar1−/−, Stat3−/−, Stat1−/−, Il10−/−, Asc−/−, Nlrp3−/− WT BMM, ± type-I IFNs | Guarda et al., 2011 | ||
| Mφ | oxLDL loading suppresses Ifnb1 expression | PM | Jongstra-Bilen et al., 2017 | ||
| Mφ | Macrophage cluster with upregulated ISGs is identified | Ldlr−/− (in vivo) plaque | Kim et al., 2018 | ||
| Mφ | Increased oxLDL uptake, foam cell formation via upregulation of SR-A with IFN-α treatment (could be blocked by anti-IFN-α Ab, B18R or anti-SRA Ab) | THP1-derived macrophage, MDM, ± IFN-α, ± B18R | Li et al., 2011a | ||
| Mφ | IFN signaturehigh macrophage subset enriched in progressing plaque | Ldlr−/− (in vivo) plaque | Lin et al., 2019 | ||
| Mφ | IFN-α abrogates TNF-mediated tolerance, increases Ifnb1 expression. Similar ATAC-seq profile resembling IFN-α in vitro could be found in SLE monocytes | MDM (in vitro), SLE monocytes | Park et al., 2017 | ||
| Mφ | Tofacitinib restore IFN-γ-inhibited ABCA1 protein expression and IFN-γ–increased lipid accumulation | THP-1 ± tofacitinib ± IFN-γ ± HFD rabbit serum or oxLDL | Pérez-Baos et al., 2017 | ||
| Mφ | oxLDL down-regulates IFN-α and IFN-β | PM (in vitro) | Qin et al., 2017 | ||
| Mφ | IFN stimulated gene Ch25h−/− macrophages produce more IL-1β | Ch25h−/−, WT BMM | Reboldi et al., 2014 | ||
| Mφ | HFD suppresses Irf1, Ifnb1 in Ldlr−/− PM | PM (Ldlr−/−, HFD vs. NHD 12 wk) | Table S1 B in Spann et al., 2012 | ||
| Mφ | Tofacitinib treatment supresses pro-inflammatory gene expression and increases ABCA1 and anti-inflammatory gene expression reducing foam cell formation | WT ± tofacitinib, PM, + oxLDL | Wang et al., 2017 | ||
| Mφ | Tofacitinib treatment reduces pro-inflammatory and increase anti-inflammatory PM cell number, gene expression (in vivo) | Apoe−/− mice ± tofacitinib, atherogenic diet, PM | Wang et al., 2017 | ||
| Neutrophil | Increased type-I IFN production in LDGs | SLE patients | Denny et al., 2010 | ||
| Neutrophil | Decreased NET formation in tofacitinib treated bone marrow–derived neutrophils | MRL/lpr mice ± tofacitinib | Furumoto et al., 2017 | ||
| Neutrophil | Increased NET formation in SLE neutrophils could promote type-I IFN induction from pDCs | Increased IFN signaling pathway in neutrophil from SLE patients or treated with SLE serum | SLE patients vs. HC | Garcia-Romo et al., 2011 | |
| Neutrophil | IFN-α treatment/SLE serum induce TLR7 expression | HC ± IFN-α | Garcia-Romo et al., 2011 | ||
| Neutrophil | Reduced recruitment to peritoneal cavity in WT mice upon poly(I:C) followed by TLR4 intraperitoneal injection, but the reduction is ablogated in Ifnar1−/− mice | Ifnar1−/−, WT mice ± poly(I:C) followed by alum intraperitoneal injection | Guarda et al., 2011 | ||
| Neutrophil | Increased NET formation in SLE neutrophils/SLE serum, immune complexes, or monomeric Ig could promote type-I IFN production from pDCs | SLE patients vs. HC | Lande et al., 2011 | ||
| Neutrophil | Increased NET formation, mtROS in LDGs could promote type-I IFN induction in vivo | SLE/CGD patients | WT, Tmem173−/−, Myd88−/− (in vivo induction of type-I IFNs) | Lood et al., 2016 | |
| Neutrophil | Increased NETosis in SLE neutrophils which could promote IFN-α induction from pDCs, and induce apoptosis in ECs partially via NET | SLE patients vs. HC neutrophil/LDG ± Mnase, Gen2.2, HUVEC | Villanueva et al., 2011 | ||
| PBMC | upregulated SRA expression in their PBMC (positively correlates with ISGs: MX1, OAS1) | SLE patients vs. HC | Li et al., 2011a | ||
| PBMC/monocyte | NET-derived 8-OHdG+ DNA is a potent inducer of IFNB1 in PBMC and THP-1 | PBMC, THP1 | Lood et al., 2016 | ||
| pDC | Exacerbated atherosclerosis with unaltered IFN-α serum levels in pDC-depleted mice (by 120G8 mAb administration) | Ldlr−/− (in vivo) plaque, ± 120G8, HFD + carotid artery bilateral placement of semiconstrictive collars | Daissormont et al., 2011 | ||
| pDC | Increased pDC mRNA sigatures/LL37 and BDCA2 staining in the advanced plaques | Early vs. advanced carotid artery specimens | Döring et al., 2012 | ||
| pDC | Decreased plaque sizes, anti-dsDNA Ab titers, and IFN-α serum levels in pDC-depleted mice (by anti-PDCA1 Ab injection) | Apoe−/− (in vivo) plaque, ± anti-PDCA1, HFD | Döring et al., 2012 | ||
| pDC | Cramp/DNA complexes and high-anti-dsDNA Ab-titer serum induce pDC-dependent IFN-α production | Apoe−/− (in vivo) Cramp/DNA complexes injection three times/wk for 4 wk, ± anti-PDCA1 | Döring et al., 2012 | ||
| pDC | Increased IFN-α production upon treatment with serum containing high anti-dsDNA Ab titers | isolated pDC (in vitro) | Döring et al., 2012 | ||
| pDC | Anti-dsDNA IgE trigger pDC IFN-α production | HC PBMC, SLE sera | Henault et al., 2016 | ||
| pDC | Decreased plaque sizes (reduced macrophage area, increased collagen) in pDC-depleted mice, but serum and plaque IFN-α was undetectable | Apoe−/− (in vivo) plaque, ± anti-PDCA1, HFD | Macritchie et al., 2012 | ||
| pDC | Expressing IFN-α in the plaque | Plaque (IHC staining) | Niessner et al., 2006 | ||
| pDC | pDC from hydroxychloroguine-treated SLE patients showed decreased IFN-α production upon TLR7/9 stimulation | SLE vs. HC pDC ± TLR7/9 ligands | Sacre et al., 2012 | ||
| pDC | Upon TLR9 in vivo/in vitro challenge, isolated, in vivo expended aortic pDC secret IFN-α, native aortic pDC expressed PDC-TREM and Ifnb1 | WT aorta, Ldlr−/−, Humanized mice (in vivo) plaque, WD for 10 wk | Yun et al., 2016 | ||
| Plaque | Upregulated IFN signaling pathways in ruptured plaques | Ruptured vs. stable carotid endarterectomy specimens | Goossens et al., 2010 | ||
| Plaque | Increased plaque size in IFN-α treated mice | Ldlr−/−, HFD ± IFN-α treatment for 5 wk | Levy et al., 2003 | ||
| Plaque | Increased IFNA expression is associated with instability without treatment, TLR9 ligands trigger IFN-α production in the plaque | Plaque | Niessner et al., 2006 | ||
| Plaque | TLR9 ligands trigger IFN-α secretion | IFN-α increases LPS-triggered TNF secretion | Plaque | Niessner et al., 2007 | |
| Plaque | CpG treatment increases IFN-α+ cells and secreted IFN-α | Combining IFN-α with LPS amplifies TNF, IL12, IL23, MMP9 expression while IFN-α alone does not affect the expression | Plaque (IHC staining) | Niessner et al., 2007 | |
| Plaque | No changes in plaque sizes, neutrophil, T cell counts, collagen, necrosis | Ldlr−/− or Apoe−/− ± anti-IFNAR1 Ab for 4 wk, HFD for 10 wk | Teunissen et al., 2015 | ||
| Plaque (DCs) | pDC and mDC are present in the shoulder region of human atherosclerotic plaques | Human plaque | Niessner et al., 2007 | ||
| Plaque (Mφ) | Increased macrophage area in plaque with IFN-α injection | Apoe−/− (in vivo) plaque, ± IFN-α two times per wk for 4 wk, HFD | Döring et al., 2012 | ||
| Plaque (Mφ) | Increased macrophage area in plaque with IFN-β injection | Ldlr−/− (in vivo) plaque, ± IFN-β for 3 wk, HFD for 6 wk | Goossens et al., 2010 | ||
| Plaque (Mφ) | Reduced macrophage area in plaque from Ifnar−/− BMT | Myeloid Ifnar−/− vs. WT BMT to Ldlr−/−, HFD 11 wk | Goossens et al., 2010 | ||
| Plaque (Mφ) | Increased macrophage area, decreased apoptosis, no differences in pro-/anti-inflammatory macrophage gene expression | Ldlr−/− ± anti-IFNAR1 Ab for 4 wk, HFD for 10 wk | Teunissen et al., 2015 | ||
| Plaque (Mφ) | Tofacitinib treatment reduces plaque macrophage and lipid area | Apoe−/− mice ± tofacitinib, atherogenic diet | Wang et al., 2017 | ||
| Plaque (necrosis) | Reduced necrotic area in plaque from Ifnar−/− BMT, no differences in IFN-β treated mice, compared with WT untreated controls | Ldlr−/− (in vivo) plaque, ± IFN-β for 3 wk, myeloid Ifnar−/− vs. WT BMT to Ldlr−/− | Goossens et al., 2010 | ||
| Plaque (neutrophil) | NET detected in the vicinity of EGFP+ neutrophils in the plaque in the monocyte depleted mice as early as 2 wk after HFD | Clodronate-containing liposome injection-induced monocyte-depleted LysmEGFP/EGFPApoe−/−, HFD | Döring et al., 2012 | ||
| Plaque (neutrophil) | Increased Cramp mRNA and CRAMP protein in the vicinity of the segment-nucleated neutrophils in the plaques | Apoe−/− (in vivo) aorta, HFD vs. ND for 12 wk | Döring et al., 2012 | ||
| Plaque (neutrophil) | Reduced neutrophil area in plaque from Ifnar−/− BMT, but no changes in IFN-β treated mice | Myeloid Ifnar−/− vs. WT BMT to Ldlr−/−, ± IFN-β, HFD for 11 wk | Goossens et al., 2010 | ||
| Plaque (neutrophil) | Increased Ifna expression, NET formation in arteries from HFD old mice, which could be inhibited by Cl−amidine | Atheroprotective Cl−amidine treatment is NET-IFNAR dependent | Apoe−/− (in vivo) plaque, ± Cl−amidine for 11 wk | Knight et al., 2014 | |
| Plaque (pDC) | Unchanged Ifna expression after pDC-selective deprivation | Ldlr−/− (in vivo) plaque, WD for 7 wk | Yun et al., 2016 | ||
| Platelet | Increased protein expression of CD58, CD69, IFITM1 and PRKRA, increased activation markers (Annexin V binding and platelet–monocyte complexes) | SLE patients (platelet) | Lood et al., 2010 | ||
| Platelet | Reduced time of cloting, increase secreted P-selectin in mice | IFNαβR−/− or IFNαβR+/+ and lupus-prone vs. normal mice, ± Ifna-expressing virus, Apoe−/− IFNαβR−/− mice WD for 10 wk | Thacker et al., 2012 | ||
| SMC/SMPC | IFN-α affects maturation of SMPC (in vivo and in vitro), increases pre-atherosclerotic-like lesions but no significant changes on medial SMC density or thickness (in vivo) | In vivo Ifna5 expressing model (plasmid transduced, 3 wk) vs. WT, IFN-I (in vitro), WT vs. Ifnar−/−, PBMC | Diao et al., 2016 | ||
| T cell | Loss of IFNAR signaling promotes T reg function and proliferation | T reg–specific (Foxp3) IFNAR deficient | Gangaplara et al., 2018 | ||
| T cell | IFN-α suppresses T reg activation, SLE-plasma exert comparable results which could be rescued by IFN-α/β receptor blocking Ab | PBMC, SLE plasma | Golding et al., 2010 | ||
| T cell | IFN-α and IFN-β, but not IFN-γ induce TRAIL expression on CD4+/CD8+ T cells, improving cytotoxicity against tumor cell lines | HC peripheral blood T | Kayagaki et al., 1999 | ||
| T cell | Reduced number of Ifnar−/− T subsets in mixed BM chimeras models, T regs lack of IFNAR show an impairment of immunomodulating function and survival | T reg–specific (Foxp3)/full IFNAR deficient | Metidji et al., 2015 | ||
| T cell | TRAIL colocalizes with IFN-α in the plaque, IFN-α–primed plaque-isolated/blood-derived T cells enhances SMC apoptosis | Plaque (IHC staining, T cell isolation), PBMC | Niessner et al., 2006 | ||
| T cell | Type-I IFNs suppress T reg activation and proliferation and promote other effector T cells' function | Trex1−/−, Trex1−/−Rag2−/−, Trex1−/−Ifnar1−/− (in vivo, colitis) | Srivastava et al., 2014a | ||
| T cell | IFN-β-IFNAR signaling inhibits T reg proliferation | Ifnar1−/− vs. WT | Srivastava et al., 2014b |
Ab, antibody; BMDC, bone marrow–derived dendritic cell; BMM, bone marrow–derived macrophage; BMT, bone marrow transplantation; CGD, chronic granulomatous disease; DC, dendritic cell; HFD, high-fat diet; HLMVEC, human lung microvascular EC; HPAEC, human pulmonary artery EC; Mφ, macrophage; MoDC, monocyte-derived dendritic cell; Mtb, Mycobacterium tuberculosis; mtROS, mitochondrial ROS; ND, normal diet; PM, peritoneal macrophage; SMPC, smooth muscle progenitor cell; WD, Western diet.
IFN families and type-I IFNs
IFNs have been known for their antiviral activity for decades. Studies have broadened the range of their biological roles in metabolic rewiring, viral and nonviral infections, cancer, and autoimmunity (Ganguly, 2018; Ivashkiv, 2018; Trinchieri, 2010). Each of the three IFN family members signals through distinct receptors to exert their function. The type-I IFN receptor (IFNAR) is a heterodimer of IFNAR1 and IFNAR2 (Mogensen et al., 1999; Fig. 1); the type-II IFN receptor (IFNGR) consists of subunits IFNGR1 and IFNGR2 (Walter et al., 1995); and the type-III IFN receptor (IFNLR) is formed by IFNLR1 and IL-10 receptor β. The last is also a subunit of IL-10 receptor (Kotenko et al., 2003; Sheppard et al., 2003). The type-II IFN family has one single member, IFN-γ, with no structural similarity to type-I and -III IFNs (Epstein, 1982). IFN-γ is mainly produced by innate lymphoid cells, T cells, and natural killer cells coordinating cell-mediated and innate immune responses against viruses, bacteria, and tumors (Boehm et al., 1997; Ivashkiv, 2018; Schroder et al., 2004). In contrast, type-I and -III IFNs include various subtypes with some protein sequence homology (∼15–19% amino acid identity), and part of the antiviral activities of these two families can be compensated by either one (Crotta et al., 2013; Sheppard et al., 2003). While the three IFN families share some similar properties and signaling cascades (Raza et al., 2014; Waddell et al., 2010), how different cell types regulate the expression and decipher distinct IFN signals is not completely clear and has been shown to be pathogen dependent (Cheng et al., 2019; Lazear et al., 2019; Mesev et al., 2019; Singhania et al., 2019). In this review, we will focus on type-I IFNs. An overview of the three types of IFNs has been extensively reviewed elsewhere (Borden et al., 2007; González-Navajas et al., 2012; Kim et al., 2016; Lazear et al., 2019; Mesev et al., 2019; Wack et al., 2015).
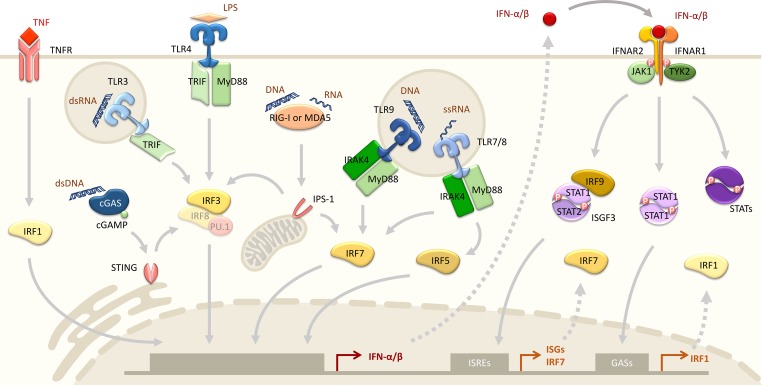
Figure 1.
Simplified schematic of type-I IFN induction and major IFNAR signaling pathways. Type-I IFNs are induced by nucleic acid or LPS activation of a variety of PRRs, including cytosolic nucleic acid sensors and TLRs. Activation of PRRs results in nuclear translocation of IRFs, which bind to the promoter region of type-I IFNs. IRF3-mediated IFN expression could be induced by STING (via cGAS), RIG-1, MDA5, TLR3, and TLR4 (through TRIF) while ligand engagement of TLR7/8 and TLR9 activate IRF7 and/or IRF5 via MyD88. Cytokines such as TNF can also induce type-I IFN expression through the TNFR-IRF1 signaling pathway. Secreted type-I IFNs bind autocinely/paracrinely to the IFNAR complex composed of IFNAR1 and IFNAR2, which consequently triggers cross-phosphorylation of TYK2 and JAK1 and activates different STAT homo/heterodimers controlling distinct transcription programs. ISGF3 consists of STAT1, STAT2, and IRF9 and binds to IFN-stimulated response element (ISRE) sequences upstream of ISGs and IRF7, while STAT1 homodimer induces IRF1 and pro-inflammatory gene expression via GASs binding. dsRNA, double-stranded RNA; ssRNA, single-stranded RNA.
In humans, type-I IFNs consist of 13 IFN-α isoforms (14 in mice), IFN-β, IFN-ε, IFN-κ, IFN-δ, and IFN-ω, which all signal through IFNAR (Kopitar-Jerala, 2017; Ng et al., 2016). As IFN-α and IFN-β are the most characterized and the most predominantly expressed type-I IFNs, we mainly discuss these two subtypes in this review. Most nucleated cells in the body are able to produce type-I IFNs in response to viral pathogens and the bacterial cell wall component LPS. This process is driven by activation of PRRs that induce cGAMP synthase (cGAS), stimulator of IFN genes (STING), retinoic acid–inducible gene I (RIG-I), MDA5, and IPS-1, which subsequently provoke nuclear translocation of IFN regulatory factor 3 (IRF3) and IRF7 (Fig. 1; Honda et al., 2006; Kawai and Akira, 2010; Kawai et al., 2005; Li and Chen, 2018; Zhao et al., 2015). Innate immune cells, including monocytes and plasmacytoid dendritic cells (pDCs), can secrete type-I IFNs by triggering TLR3, TLR4, and TLR7–9, which activate TRIF, MyD88, IRF1, 3, 5, 7, and 9, and myeloid-specific IRF8 (Blasius and Beutler, 2010; Borden, 2019; Li et al., 2011b; Muskardin and Niewold, 2018). In addition, TNF induces IFN-β expression by activating TNF receptors (TNFRs) and IRF1 in macrophages and ECs (Yarilina et al., 2008). Notably, pDCs are considered to be the predominant producers of IFN-α, while other cell types, including lymphocytes, dendritic cells, macrophages, ECs, and fibroblasts, mainly produce IFN-β (Gilliet et al., 2008; Izaguirre et al., 2003; Kerkmann et al., 2003; Takauji et al., 2002).
Upon type-I IFN stimulation, IFNAR1 and IFNAR2 employ tyrosine kinase 2 (TYK2) and JAK1, respectively, which results in the dimerization and activation of STAT; Hervas-Stubbs et al., 2011; Fig. 1). The most important STAT is the STAT1–STAT2 heterodimer, which assembles with IRF9 to form the ISGF3 complex (Stark and Darnell, 2012) provoking the transcription of IFN-stimulated genes (ISGs; Budhwani et al., 2018). In addition to STAT1 and STAT2, type-I IFNs also activate STAT3–6 that can form different homo- or heterodimers with distinct and sometimes even opposing biological functions. The detailed roles and regulation of different IFN signaling pathways are reviewed elsewhere (Ivashkiv and Donlin, 2014; Mesev et al., 2019; Schreiber, 2017). The varied and pleiotropic IFNAR signaling pathways suggest that the effects of type-I IFNs are cell type– and stimulus-specific, highlighting their complex crosstalk in coordinating the immune system.
Type-I IFN–associated cells in atherogenesis
Monocytes and macrophages
Plaque-residing monocytes and macrophages are highly heterogeneous populations with different functions during atherosclerosis development. Accumulated lipids in the arterial intima are mainly taken up by these cells. Type-I IFN treatment facilitates lipid uptake in both human and mouse monocytes/macrophages through up-regulation of scavenger receptors class A, leading to increased foam cell formation (Table 1, Fig. 2, and Fig. 3; Ahmed et al., 2018; Boshuizen et al., 2016; Li et al., 2011a; Pulliam et al., 2014). Elevated scavenger receptors class A expression can also be found in human peripheral blood mononuclear cells (PBMCs) from subjects with elevated IFN signatures, such as SLE patients and HIV-infected individuals (Table 1 and Fig. 3; Li et al., 2011a; Pulliam et al., 2014). While SLE and HIV infection are associated with accelerated atherogenesis (Kearns et al., 2017; McMahon and Skaggs, 2014), these findings hint on a causal link between type-I IFN–accelerated foam cell formation and the increased CVD risk. Consistently, mice receiving IFN-β treatment showed increased atherosclerotic macrophage area and larger plaque size (Table 1; Goossens et al., 2010).
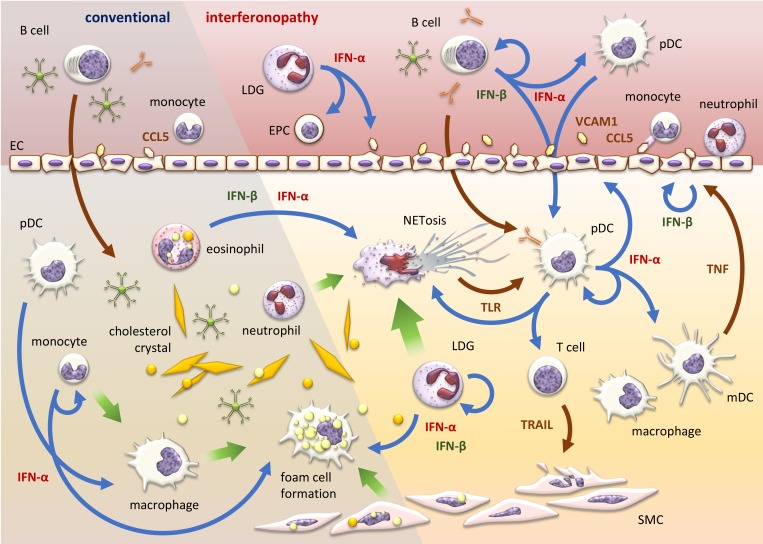
Figure 2.
Type-I IFNs affect atherosclerosis. Type-I IFNs can be produced by monocytes, macrophages, pDCs, eosinophils, and B cells and LDGs in autoimmune patients. Locally and systemically elevated type-I IFNs result in increased foam cell formation, EC dysfunction, suppressed EPC maturation, enhanced NETosis, increased monocyte and neutrophil recruitment, and elevated immune cell activation. IFN-stimulated T cells exert stronger cytotoxic function to SMC via TRAIL. Myeloid APCs including macrophages and mDCs secrete higher levels of TNF, leading to IFN-β expression in ECs. Type-I IFN priming of IFN-producing cells such as pDCs, monocytes, B cells, ECs, and LDGs results in an autocrine feedback loop that exacerbates the pro-inflammatory microenvironment.
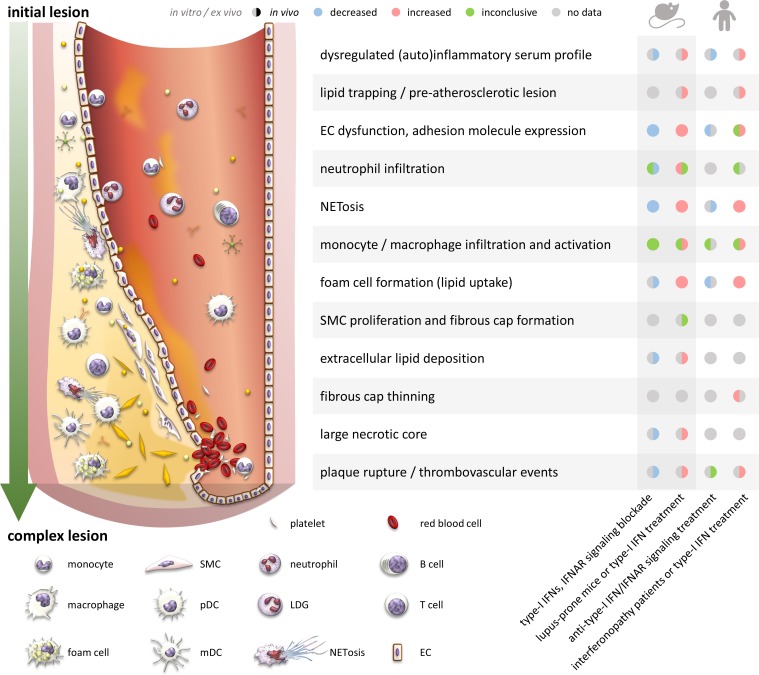
Figure 3.
The effect of type-I IFNs during atherosclerosis development in different models. Atherosclerosis is driven by predisposing risk factors such as dysregulated lipids, pro-inflammatory stimuli, and cytokines. The development of the lesion is characterized by lipid trapping, leukocyte infiltration and activation, foam cell formation, fibrous cap, and extracellular lipid core formation. In unstable, complex plaques, fibrous cap thinning and necrosis take place, which lead to plaque rupture. The dots, from left to right, represent the effect on the corresponding event due to “type-I IFNs, IFNAR signaling blockade in mice,” “lupus-prone mice or type-I IFN treatment in mice,” “anti-type-I IFN/IFNAR treatment in human,” and “interferonopathy patients or type-I IFN treatment in human,” respectively. For each dot, the left hemisphere of the dot indicates in vitro or ex vivo data, while the right means in vivo studies.
Another role of monocytes and macrophages in atherogenesis is their pro- and anti-inflammatory function. Single-cell transcriptomic studies deconvoluted the complex spectrum of blood-derived monocytes and macrophages in the murine plaque where distinct subpopulations were distinguished. A Ly-6C− monocyte subset expressing high Ifna1 and Il12b and low anti-inflammatory Il10 mRNA was identified by single-cell RNA sequencing (scRNA-seq) of murine atherosclerotic plaques (Table 1 and Fig. 3; Winkels et al., 2018). Whereas some monocyte and macrophage subsets might serve as a source of type-I IFNs in the murine plaques, cytokine production and IFN-responsive genes are actually suppressed in mLDL-treated or cholesterol-laden peritoneal macrophages (Table 1; Jongstra-Bilen et al., 2017; Qin et al., 2017; Spann et al., 2012). Consistently, lipid-probe based analysis suggests that intimal nonfoamy macrophages, rather than foamy cells, exhibit more pro-inflammatory characters in the murine atherosclerotic lesions (Table 1; Kim et al., 2018).
In addition to clusters of cells exhibiting pro-inflammatory NF-κB signaling signatures (M1-like) or expressing genes associated with type 2 cytokine activation (M2-like), macrophages showing up-regulated ISGs were also identified in mouse plaques, suggesting a type-I IFN responsive subset (Table 1; Kim et al., 2018; Lin et al., 2019). This IFN-associated subpopulation was enriched in progressing lesions compared with the regressing ones (Lin et al., 2019), implying the involvement of local type-I IFN signaling in the complex cytokine crosstalk during atherosclerosis development. Of note, type-I IFN–stimulated macrophages can exert both pro- and anti-inflammatory phenotypes (Cheng et al., 2019). Type-I IFNs have been shown to suppress both mouse and human macrophages’ inflammatory cytokine production and inflammasome activity through anti-inflammatory cytokine induction, such as IL-10, epigenetic regulation affecting chromatin accessibility, and metabolic rewiring, for instance, oxysterol 25-hydroxycholesterol production (Table 1 and Fig. 3; Ahmed et al., 2018; Cheng et al., 2019; Guarda et al., 2011; Reboldi et al., 2014). By contrast, upon type-I IFN stimulation, human peripheral monocyte-derived macrophages (MDMs) restore their inflammatory function silenced by TNF-induced LPS tolerance through reprogramming the accessibility of NF-κB–associated chromatin regions (Table 1; Park et al., 2017). These in vitro data corroborate the observation that isolated myeloid dendritic cells (mDCs) and macrophages from IFN-α–treated human plaque tissue ex vivo show markedly enhanced LPS-triggered TNF secretion as well as other pro-atherogenic cytokines such as IL-12, IL-23, and MMP-9 production (Table 1, Fig. 2, and Fig. 3; Niessner et al., 2007).
Neutrophils
Neutrophils are infiltrating leukocytes present in both mouse and human plaques (van Dijk et al., 2016; van Leeuwen et al., 2008; Zernecke et al., 2008). During human atherosclerosis development, their number significantly increases in the vulnerable lesions (van Dijk et al., 2016). Particularly, neutrophil distribution positively correlates with lipid core size, macrophage numbers, and micro-vessels and negatively with collagen content and SMC numbers in human plaques (Ionita et al., 2010; Maracle et al., 2018). In murine atherosclerosis models, intraplaque neutrophils distribute mainly in the shoulder regions (Rotzius et al., 2010) and accumulate in the early high-fat diet period; then the number gradually reduces over time (Drechsler et al., 2010; Rotzius et al., 2010; van Leeuwen et al., 2008). In advanced murine atherosclerotic lesions, neutrophils correlate with necrosis, plaque size, and vulnerability, and inversely correlate with SMCs and fibrous cap thickness, while no correlation was found to the collagen content, macrophage, and EC activation, which is in contrast to human data (Maracle et al., 2018; Silvestre-Roig et al., 2019).
Neutrophil recruitment to the arterial wall has been shown to be driven by CCL5 in mice (Drechsler et al., 2010), which is induced in type-I IFN–stimulated macrophages and ECs (Table 1, Fig. 2, and Fig. 3; Goossens et al., 2010; Nakano et al., 2012). While the function of neutrophils in atherosclerosis is not completely clear (Drechsler et al., 2010), over recent years, emerging evidence has revealed the contributory role of neutrophils in atherogenesis and plaque instability (Teixeira and Tam, 2018). As a phagocytic granulocyte, neutrophils are capable of discharging granule proteins, proteases, and reactive oxygen species (ROS) and producing neutrophil extracellular traps (NETs; Döring et al., 2017). NETs consist of web-like chromatin with cytosolic and granule proteins that can trap pathogenic microbes and have been shown to be involved in many (chronic) inflammatory diseases (Papayannopoulos, 2018), including rheumatoid arthritis (RA), vasculitis, and SLE.
The presence of NET structures has been shown in atherosclerotic plaques and arterial thrombi in humans and mice, especially in cholesterol-rich or erosion-prone areas (Fig. 2; Franck et al., 2018; Quillard et al., 2015; Warnatsch et al., 2015). Consistently, cholesterol crystals can induce NET formation (NETosis) in vitro (Warnatsch et al., 2015). Studies proposing the mechanistic relation of NETs and atherogenesis show the ability to induce endothelial cytotoxicity, cause high-density lipoprotein (HDL) oxidative modifications, promote macrophage accumulation and cytokine release, activate T helper (Th) 17 cells, and trigger type-I IFN production from pDCs (Table 1, Fig. 2, and Fig. 3; Döring et al., 2012; Knight et al., 2014; Smith et al., 2014; Villanueva et al., 2011; Warnatsch et al., 2015). Of note, several studies suggest an imbalance between NETosis and NET degradation in autoimmune diseases, which may in part account for the increased CVD risk in these patients (Carmona-Rivera et al., 2015; Kessenbrock et al., 2009; Khandpur et al., 2013). While a recent study shows inhibiting NETosis via Pad4 deficiency in Ldlr−/− mice does not impede atherogenesis but prevents EC injury and thrombus formation (Franck et al., 2018), applying myeloid-specific Pad4-deficient model on Apoe−/− mice exerts reduced atherosclerosis burden (Liu et al., 2018). Similarly, inhibiting NETosis by pan-protein-arginine deiminase (PAD)–inhibitor chloramidine treatment before starting the high-fat diet feeding decreased atherosclerosis and mitigated Ifna expression in Apoe−/− mice, suggesting an initiating role of NETs in atherogenesis. Furthermore, the atheroprotective effects of chloramidine could not be reproduced in Apoe−/− Ifnar1−/− mice, indicating a causal role of type-I IFN signaling in NET-driven atherogenesis (Knight et al., 2014).
Low-density granulocytes (LDGs), an immature, pro-inflammatory subtype of neutrophils identified in SLE individuals, display increased expression of IFNA and are associated with IFN signature enrichment in these patients (Denny et al., 2010; Kegerreis et al., 2019). Further, LDGs are capable of inducing an IFN signature in ECs, thus disrupting their differentiation from the progenitors (Table 1 and Fig. 2; Denny et al., 2010). In addition to affecting endothelial maturation directly, LDGs are also capable of inducing mitochondrial ROS and NETs upon stimulation (Table 1; Kaplan, 2011; Lood et al., 2016; Villanueva et al., 2011), leading to increased plaque vulnerability. Several studies have shown that LDG NETs are the main inducer of type-I IFN secretion in pDCs via TLR9 signaling in SLE patients (Table 1; Garcia-Romo et al., 2011; Lande et al., 2011; Villanueva et al., 2011). Moreover, after adjustment for traditional risk factors, noncalcified plaque burden in SLE patients is directly associated with LDG levels (Carlucci et al., 2018). In addition to this particular neutrophil subset LDG, atherosclerotic mouse models applying myeloid-specific deletion of Ifnar1, which mainly affects neutrophils and monocytes/macrophages, revealed smaller lesions, reduced neutrophil and macrophage recruitment, and less necrosis (Fig. 3; Goossens et al., 2010). Of note, while the plasma CCL5 was increased in mice treated with IFN-β, these animals did not show a difference in neutrophil area in the plaque when compared with the untreated controls (Table 1 and Fig. 3; Goossens et al., 2010). These data reinforce the concept of positive feedback between NET formation and type-I IFNs in neutrophils and other cell types during atherosclerosis progression.
Eosinophils
Though the direct role of eosinophils in atherogenesis remains unclear, clinical studies reveal that absolute eosinophil count, intraplaque eosinophil markers, and eosinophil-specific chemokines are associated with increased risk of CVDs (Haley et al., 2000; Kitano et al., 2017; Lee et al., 2001; Niccoli et al., 2010; Tanaka et al., 2012). While EG2+ eosinophils are considered to be absent during the atherosclerosis development according to immunohistochemistry (IHC) staining of human plaques (van Dijk et al., 2016), the occurrence of eosinophil extracellular trap was identified in human thrombosed atherosclerotic specimen by double staining of eosinophil major basic protein and citrullinated histone H3 (Pertiwi et al., 2019), which might trigger IFN-α production in pDCs. Circulating eosinophils can affect atherogenesis through their cytokine production. In vitro data revealed that oxidized LDL (oxLDL) triggers the expression of type-I IFNs in cultured mouse eosinophils via CD36 and suppresses Il4 expression, and subsequently polarizes macrophages to a pro-inflammatory phenotype (Table 1 and Fig. 2; Qin et al., 2017).
pDCs and mDCs
pDCs are known to have the ability to rapidly produce massive amounts of type-I IFNs and are present in both murine and human atherosclerotic lesions as well as intact aorta (Chistiakov et al., 2014; Cole et al., 2018; Döring et al., 2012; Yun et al., 2016). Single-cell data revealed an increased amount of pDCs in aortas from Apoe−/− mice fed a high-fat diet compared with mice fed normal chow (Cole et al., 2018). In human plaques, CD123+ pDCs localize closely to T cells and mDCs in the unstable shoulder region (Chistiakov et al., 2014; Niessner et al., 2006, 2007) and accumulate during atherogenesis (Fig. 2; Li et al., 2017).
The role of pDCs in atherosclerosis is still a subject of discussion. Both whole human plaque tissue and isolated pDCs respond to TLR9 agonists with increased IFN-α transcription and secretion, amplifying pro-inflammatory responses by mDCs and cytolytic T cells (Table 1 and Fig. 2; Niessner et al., 2006, 2007; Yun et al., 2016). Also, sorted mouse pDCs treated with oxLDL show an enhanced capacity to phagocytose and to stimulate antigen-specific T cell responses without altering IFN-α/β secretion (Döring et al., 2012). However, pDC-selective deletion in different atherosclerosis murine models showed conflicting results. Some studies found that antibody-mediated cell ablation or inducible pDC depletion in Ldlr−/− mouse deteriorated atherogenesis, suggesting an atheroprotective character of pDCs. This observation was specifically linked to the pDCs’ ability of tuning T cell activation and proliferation in murine models (Table 1; Daissormont et al., 2011; Yun et al., 2016). On the contrary, studies applying similar techniques showed a more favorable phenotype in pDC-depleted Apoe−/− mice with reduced lesion size, more stable plaques, and a reduction in IFN-α serum levels, supporting the pro-atherogenic role for pDCs in murine atherosclerosis (Table 1; Döring et al., 2012; Macritchie et al., 2012).
Of note, without CpG stimulation, IFN-α protein levels in the intact aorta or atherosclerotic plaques in mice are low or undetectable (Macritchie et al., 2012; Yun et al., 2016). This implies that without interferogenic stimuli, the effect of IFN-α–mediated atherogenesis might be minor or localized to restricted regions in these murine model settings. On the other side, NETs have been demonstrated to be present in human plaques and may serve as stimuli triggering pDC type-I IFN production via TLR signaling, which subsequently primes neutrophils for NET formation (Fig. 2). This positive feedback between NETs and type-I IFNs produced by pDCs might be the causal factor exacerbating atherosclerosis in autoimmune diseases with elevated IFN signatures. Moreover, mice stimulated with type A CpGs showed an advanced atherosclerotic plaque phenotype in a pDC-dependent manner (Döring et al., 2012), implying a central role of pDCs in autoimmune-associated atherogenesis. Taken together, the responsiveness of pDCs to TLR7/TLR9 activation and type-I IFN induction corroborates the link between accelerated atherosclerosis and chronic inflammation, and thus serves as additional pathogenic mechanism for increased cardiovascular events in interferonopathies.
ECs
EC dysfunction is an important player in the initiation of atherosclerosis, where the permeation and accumulation of lipoprotein particles take place at the arterial lesion-prone areas (Davignon and Ganz, 2004; Gimbrone and García-Cardeña, 2016). During plaque progression, ECs activated by oxLDL produce chemokines that attract circulating monocytes into the arterial intima. Inflammatory factors expressed by recruited immune cells further increase plaque instability by modulating EC phenotype and extracellular matrix (Gimbrone and García-Cardeña, 2016).
Type-I IFN–treated HUVECs produce more adhesion molecules and chemokines, such as VCAM1 and MCP1 (Fig. 2), possibly through the suppression of NOS3 (encoding, endothelial nitric oxide synthase) expression and insulin-mediated NO production (Table 1, Fig. 2, and Fig. 3; Buie et al., 2017; Jia et al., 2018). Though the effect on ICAM1 is not clear, studies showed that more human neutrophils attach to the type-I IFN–treated HUVECs (Table 1 and Fig. 2; Buie et al., 2017; Giorelli et al., 2002; Jia et al., 2018; Kobayashi et al., 2008; Shen et al., 1997). However, some studies revealed conflicting results. In vitro IFNα2b exposure of human aortic ECs (HAEC) was shown not to alter the proliferation, tubule formation, or NO production while IFN-signature genes were induced (Table 1; Reynolds et al., 2014). Also, both IFN-α and IFN-β, but not IFN-γ, show a protective effect on growth factor deprivation-induced apoptosis of HAECs in vitro (Table 1; Sano et al., 2012).
In murine atherosclerosis models, IFN-β enhances leukocyte arrest via EC-macrophage CCL5-CCR5 interaction, thus promoting plaque destabilization (Fig. 2; Goossens et al., 2010). The reciprocal interaction between type-I IFNs and other inflammatory mediators exacerbates this process. TNF triggers IFN-β production via TNFR2-IRF1–dependent signaling in ECs (Fig. 1). The secreted IFN-β serves as an autocrine signal triggering HUVECs to generate chemotactic factors, such as VCAM1, that promote monocyte recruitment (Table 1, Fig. 1, and Fig. 2; Venkatesh et al., 2013). IFN-α exposure was shown to disrupt the endothelium, and impair endothelial vasorelaxation and endothelial progenitor cell (EPC) function, resulting in accelerated thrombosis and platelet activation in lupus-prone and non–lupus-prone mice (Table 1 and Fig. 3; Diao et al., 2016; Thacker et al., 2012). In line with this, lupus-prone mice showed impaired function and increased type-I IFN signatures in the EPC compartments already without additional IFN-α application (Thacker et al., 2010, 2012). Corroborating the in vitro and animal data, type-I IFN signatures or high serum IFN-α are associated with impaired EPC and EC function, reduced flow-mediated dilation, and elevated endothelial activation markers, such as sVCAM1 and endothelial microparticles, in individuals with autoimmune diseases including SLE, antiphospholipid syndrome (APS), and RA (Table 1, Fig. 2, and Fig. 3; Denny et al., 2007; Somers et al., 2012; Grenn et al., 2017; Lee et al., 2007; Rodríguez-Carrio et al., 2014; Tydén et al., 2017). Many of the characteristics of EPC dysfunction in these patients could be overcome by neutralization of type-I IFNs or their receptor ex vivo (Table 1 and Fig. 3; Denny et al., 2007; Grenn et al., 2017). Comparable results were found in animal models. Ifnar1 deficiency in lupus-prone mice exhibit an improved EPC function and endothelial vasorelaxation whereas Apoe−/− Ifnar1−/− mice fed with high-fat diet showed a more preferable atherosclerosis phenotype (Table 1 and Fig. 3; Thacker et al., 2012).
T lymphocytes
T lymphocytes are adaptive immune cells present in both human and murine atherosclerotic plaques (Witztum and Lichtman, 2014). During plaque development, accumulated innate immune cells activate their pro-inflammatory response, exerting deleterious effects. The majority of plaque-residing T cells are CD4+, whereas the remaining are mainly CD8+ (Jonasson et al., 1986). Among the CD4+ T cell subsets, the role of Th17 and Th2 cells in atherosclerosis are still debated, while Th1 and regulatory T (T reg) cells are considered to be pro- and anti-atherogenic, respectively. Details of different subsets and functions are reviewed elsewhere (Ketelhuth and Hansson, 2016; Lahoute et al., 2011). Of note, T cell subset differentiation is not irreversible, as some T cells can acquire different properties under certain (chronic) inflammatory conditions (Geginat et al., 2014; Sakaguchi et al., 2013).
Many animal studies have verified the T reg to Th1 cell plasticity driven by atherosclerotic and hypercholesterolemic environments, in which T reg cells lost the immunosuppressive characteristics and acquired a pro-inflammatory phenotype (Tabas and Lichtman, 2017). Interestingly, the instability of T reg cells can also be found in many autoimmune diseases, implying a possible shared pathophysiology driving both atherosclerosis and autoimmunity (Dominguez-Villar and Hafler, 2018). Plasma from SLE patients with type-I IFN activity impaired T reg cell activation and function in vitro (Golding et al., 2010). Moreover, limited T reg cell generation and increased IFN-γ+ T cell proliferation were observed in activated SLE PBMCs (Table 1; Golding et al., 2010). Consistent with this observation, in a murine colitis model, Trex1 deficiency induced type-I IFN expression and attenuated T reg cell activation and proliferation while promoting Foxp3low/neg T cell expansion and pro-inflammatory function (Table 1; Srivastava et al., 2014a). IFN-α/β directly inhibits both human and murine T reg cell proliferation in vitro, while the murine Ifnar1-deficient counterpart is unaffected. This type-I IFN signaling-mediated inhibition of T reg cells can be observed in murine lymphocytic choriomeningitis virus infection and tumor models (Gangaplara et al., 2018; Srivastava et al., 2014b). However, studies also showed IFNAR signaling to be important for T reg cells exerting their immunomodulation function as IFNAR deficient T reg cells failed to dampen the effector T cell activation in a mouse model of inflammation (Metidji et al., 2015). These studies suggest that type-I IFNs can alter the pro- versus anti-inflammatory balance of T cell subsets.
Besides the effect of phenotype switching on T reg cells, IFN-α may directly modulate human CD4+ T cells’ cytotoxic function by increasing the expression of TNF-related apoptosis-inducing ligand (TRAIL) on the T cell surface (Table 1 and Fig. 2; Kayagaki et al., 1999; Niessner et al., 2006). Further, experiments applying human plaque-derived or peripheral blood-isolated CD4+ T cells to coronary SMC monolayers showed TRAIL-dependent apoptosis of SMCs, which could be amplified by IFN-α pretreatment of CD4+ T cells (Fig. 2 and Fig. 3). Of note, IFN-α alone did not induce SMC apoptosis in this study (Niessner et al., 2006).
As mentioned in a previous section, IFN-α–producing pDCs colocalize with T cells in human atherosclerotic plaques (Fig. 2; Niessner et al., 2006). The pro-atherogenic effect of type-I IFNs might be through paracrine signaling and exacerbate in autoimmune patients with elevated systemic IFN levels.
B lymphocytes
B cell subsets affect the atherosclerotic process through both cellular and humoral immunity functions. While whole blood gene expression profile implies an association between B cells and coronary heart diseases (Huan et al., 2013), the functional characteristics of B cell subtypes, the antibody targets, and different immunoglobulin classes in atherosclerosis are still elusive (Tsiantoulas et al., 2014). Two major subfamilies of B cells, B1 and B2 cells, process distinct machineries in plaque formation (Domeier et al., 2018; Tsiantoulas et al., 2015). In general, B1 cells are considered to be atheroprotective, as they can secrete natural IgM against oxidation-specific epitopes, thus limiting EC dysfunction and immune cell activation (Fig. 2; Tsiantoulas et al., 2014). B2 subsets, the majority of B cells in the body, show pro-atherogenic properties in some animal models, though the underlying mechanism remains under debate (Srikakulapu and McNamara, 2017; Tsiantoulas et al., 2015).
Atherosclerotic plaques contain apoptotic and necrotic cell debris, endogenous nucleic acids, and oxidized lipid species, which all can serve as a source of modified self-antigen and TLR ligands (Miller et al., 2011) triggering autoantibody production and adaptive immune responses. Murine atherosclerosis studies have also revealed an increase of anti–double-stranded DNA (dsDNA) antibody titers in high-fat diet–fed Apoe−/− mice (Table 1 and Fig. 2; Döring et al., 2012), again suggesting a shared pathophysiological continuum between autoimmunity and atherosclerosis. Detailed information on the role of B cells and immunoglobulins in atherogenesis is reviewed elsewhere (Sage et al., 2019; Tsiantoulas et al., 2015).
Besides the production of various antibodies, stimulated and early transitional stage B cells can secret type-I IFNs, which are important for B cell development and which might contribute to the pathogenesis of some autoimmune diseases (Table 1; Bénard et al., 2018; Green et al., 2009; Hamilton et al., 2017; Ward et al., 2016). This type-I IFN–IFNAR autocrine loop promotes autoreactive B cell development in the germinal center and strengthens follicular B cells’ ability to produce antibodies in a T cell–independent manner (Table 1; Domeier et al., 2018; Swanson et al., 2010). On the other hand, self-reactive anti-dsDNA IgE is associated with SLE disease activity and potentiates IFN-α production in human pDCs in vitro through promoting phagocytosis of DNA and TLR9 signaling (Table 1 and Fig. 2; Henault et al., 2016). As both IgE and pDCs are present in human atherosclerotic plaques (Wang et al., 2011), the aforementioned mechanisms might exacerbate atherogenesis and plaque instability, especially in individuals with systemically elevated type-I IFN levels or signaling signatures. Since B cells are capable of producing and responding to type-I IFNs, the complex nature of B cells in atherosclerosis and how type-I IFNs shape this process appear to be valuable subjects for further investigation.
Clinical implications and cardiovascular manifestations
While type-I IFN administration in murine models exacerbates atherosclerosis, the effects of type-I IFNs in humans, both direct and indirect to the plaque-residing cells, could result in clinical manifestation, especially in individuals with elevated IFN signatures. Therapeutic IFN treatment has been applied in cancer and chronic viral infection patients. In addition to the antivirus and antitumor effects, several clinical studies observed treatment-associated hypocholesterolemia, together with a decreased level of HDL, in these patients (Robertson and Ghazal, 2016). Further, the use of pegylated IFN and ribavirin as an anti–hepatitis C virus therapy is associated with risk factors of atherosclerosis such as insulin resistance (Brandman et al., 2012).
Compared with atheroma-free carotid wall samples, human atherosclerotic plaques show higher IFNA expression, and the level is even higher in inflamed and thrombosed lesions (Table 1 and Fig. 3; Niessner et al., 2006). Similarly, elevated IFN levels and/or its signatures are also observed in some autoimmune diseases, such as RA and SLE. The association between antiviral IFN-α therapy and the presence of autoantibodies indicated the casual role of type-I IFNs in systemic autoimmune pathogenesis (Fabris et al., 1992; Gota and Calabrese, 2003; Mavrogiannis et al., 2001; Mayet et al., 1989). Many clinical and epidemiological studies showed the concordance of accelerated atherosclerosis or increased risk of cardiovascular events in these interferonopathy-associated autoimmune diseases (Parker and Bruce, 2013; Pereira et al., 2009). A significant increase in aortic wall inflammation with mild to moderate disease activity is observed in SLE patients (Carlucci et al., 2018). In addition, type-I IFN–regulated proteins, such as IFITM1 and RPKRA, are increased in platelets isolated from SLE patients, and are associated with previous CV events including myocardial infarction and venous and arterial thrombosis (Table 1; Lood et al., 2010). Similarly, increased platelet activation was also observed in lupus-prone or normal mice receiving Ifna-expressing virus and reduced in Ifnar−/− mice (Table 1 and Fig. 3; Thacker et al., 2012). Gene expression differential analysis of PBMC or blood leukocytes from SLE patients also revealed a strong enrichment of IFN signaling signatures (Baechler et al., 2003; Bennett et al., 2003; Carlucci et al., 2018).
Of note, type-I IFNs play distinct roles in different autoimmune diseases. The response to type-I IFNs and the signature gene expression are usually lower in mononuclear cells from untreated multiple sclerosis (MS) patients compared with heathy controls (Feng et al., 2002). Opposite SLE and RA, many symptoms of MS benefit from recombinant IFN-β as a disease-modifying treatment (Reder and Feng, 2013). MS patients receiving IFN-β usually show a decrease in total cholesterol and an increase in triglyceride plasma level (Fig. 3; Morra et al., 2004; Robertson and Ghazal, 2016; Sena et al., 2000; Uher et al., 2017), similar to the effect of type-I IFN therapies in individuals with cancers or viral infections. In line with this, IFN-β treatment is associated with increased CV risk factors including lower HDL level and higher diastolic blood pressure (Sternberg et al., 2014). However, the increased prevalence of cardiovascular events such as myocardial infarction in MS patients cannot be fully explained by traditional risk factors, and its correlation with disease-modifying treatments was not clear (Marrie et al., 2019). Notably, applying type-I IFNs in murine atherosclerosis models showed increased or unchanged plasma cholesterol or triglyceride levels (Goossens et al., 2010; Levy et al., 2003).
Similarly, traditional risk factors for cardiovascular events including age, gender, smoking, hypertension, and dyslipidemia have been correlated with but cannot fully explain the exacerbated atherogenesis in SLE and RA patients (Bruce et al., 2003; Castañeda et al., 2015; Esdaile et al., 2001). While the serum type-I IFN activity is independently associated with biomarkers of atherosclerosis development in lupus patients (Somers et al., 2012), some studies reported that preventive therapies for traditional risk factors, such as hypercholesterolemia and hypertension, failed to significantly reduce the incidence of CVDs in lupus patients (Petri et al., 2011; Schanberg et al., 2012; Tselios et al., 2016; Wigren et al., 2015). Notably, increased subclinical atherosclerosis prevalence is observed in individuals with primary (i.e., antineutrophil cytoplasmic antibody–associated vasculitis) or secondary (i.e., associated with SLE or RA) vasculitis (Argyropoulou et al., 2018; Chironi et al., 2007; Guillevin and Dörner, 2007). Whether vasculitis and atherosclerosis share common pathogenic mechanisms and whether IFNs play a causal role in this disease continuum warrant further investigation.
Although the role for type-I IFN in the pathogenesis and instability of atherosclerosis is supported by studies in both human and mouse models (Table 1; Goossens et al., 2010; Levy et al., 2003; Niessner et al., 2006), there is no statistically significant association between the risk of coronary artery events and IFN-α production–correlated single nucleotide polymorphisms (Nelson et al., 2015). Alternatively, mutations in some components of the IFN regulation and signaling, such as IRF8 and JAK2, are associated with the increased risk of cardiovascular events in different populations (Jaiswal et al., 2017; Leonard et al., 2013).
Anti-IFN/IFNAR signaling therapies
Immunomodulating therapies that dampen IFN signatures have been applied in many autoimmune diseases. Whereas the relation between immunosuppressive steroids and atherosclerosis remains controversial (Wu et al., 2016), hydroxychloroquine (HCQ), an alkalinizing lysosomatropic drug for SLE treatment, has been attested to be atheroprotective and is effective in dampening disease activity and mortality, reducing required steroids dosages, and preventing organ damage accrual and thrombovascular events (Fig. 3; Fasano et al., 2017; Ponticelli and Moroni, 2017; Yang et al., 2019). Mechanistically, the atheroprotective effects of HCQ may be due to interfering with IFN-α and TNF production by TLR7/TLR9-activated pDCs in SLE individuals (Table 1 and Fig. 3; Sacre et al., 2012). In addition to immune cell modulation and the prevention of EC dysfunction, HCQ offers beneficial effects on other traditional CV risk factors, such as dyslipidemia and diabetes (Floris et al., 2018), which are also shown to be associated with systemic type-I IFNs. Similarly, chloroquine, a closely related anti-malarial compound that may also be used in SLE treatment, is shown to be able to inhibit NETosis and the subsequent HDL oxidation in human neutrophils and LDGs from patients in vitro (Smith et al., 2014).
Inhibitors directly targeting downstream pathways of IFN signaling, such as JAK–STAT pathways and IRFs, have been proposed as a potential treatment strategy in CVD (Schwartz et al., 2017; Szelag et al., 2016). RA patients receiving tofacitinib, one of the first-generation JAK inhibitors, showed an increase in plasma total cholesterol, LDL, and HDL without changes in atherogenic index (Kang et al., 2018). In agreement with this observation, in vitro experiments and in vivo animal models revealed that tofacitinib up-regulated ABCA1 expression, promoted anti-inflammatory macrophage polarization, improved EC function, and attenuated atherosclerosis (Table 1 and Fig. 3; De Vries et al., 2019; Furumoto et al., 2017; Pérez-Baos et al., 2017; Wang et al., 2017). However, recently the Oral Surveillance study revealed a statistically significant and clinically relevant increase in pulmonary embolism and an increased mortality in patients older than 50 yr with an increased CV risk when treated with a tofacitinib dose of 10 mg twice daily (Fig. 3; FDA, 2019; Pfizer, 2019). In this respect, more selective JAK1 inhibitors may demonstrate a more favorable safety profile. Another anti-IFN approach is to inhibit type-I IFNs and IFNAR signaling directly. Blocking IFNAR1 in mice has been shown to be cardioprotective in MI (King et al., 2017) and could stimulate mouse arteriogenesis without affecting atherosclerosis burden after 4-wk treatment (Table 1 and Fig. 3; Teunissen et al., 2015). The potential beneficial effects of inhibiting type-I IFNs and their signaling have been applied to clinic trials. Anifrolumab, a fully human IgG1κ monoclonal antibody blocking IFNAR1 signaling, decreased disease activity, reduced NETosis, dampened chemokine serum level elevation, and improved cholesterol efflux capacity in SLE patients (Fig. 3; Casey et al., 2018; Furie et al., 2017; White et al., 2018). Further, sifalimumab, an anti–IFN-α monoclonal antibody, also yielded promising results in SLE patients (Khamashta et al., 2016), though the effect on atherosclerosis and cardiovascular outcome has not been investigated yet.
Given the positive outcomes of these anti-IFN and/or immunomodulating medicines dampening IFN signaling, it is of great interest to further investigate their effects on atherogenesis in autoimmune diseases, from premature, subclinical atherosclerosis to severe CV comorbidities.
Concluding remarks
Dyslipidemia and inflammation are central in the development of atherosclerosis. In addition to the great breakthrough of lipid-lowering therapy, there has been progress in understanding the role of inflammation that leads to plaque development and clinical complications. The causal role of chronic inflammation accelerating atherosclerosis in several systemic autoimmune diseases is well established. Studies applying elegant single-cell profiling approaches and functional studies have advanced our understanding on how type-I IFNs and the IFNAR signaling are regulated in different cell types involved in the pathogenesis, and helped to guide therapy development. As a pleiotropic cytokine modulating pro- and anti-inflammatory phenotypes in various cell types, the putative role of type-I IFNs in atherogenesis and autoimmunity has been verified, though still much remains to be investigated about the complex crosstalk of IFN signaling in the immune system and metabolism (Fig. 3). Future studies should continue to investigate the delicate homeostasis between IFNs’ antitumor and anti-pathogen effects and the pro-inflammatory and systemic lipid-altering effects.
We envision that type-I IFNs and their signaling pathways will be shared therapeutic targets in both atherosclerosis and rheumatic diseases. However, because type-I IFNs mediate important immune responses against infections and cancers, there is reason for caution for applying long-term anti-IFN therapy for CVD prevention. Advanced understanding of the genetic and epigenetic regulation and the biological underpinnings of IFN signaling in cardiovascular and autoimmune diseases might enable us to intervene in these diseases via fine-tuned modulation of type-I IFN signaling using targeted pharmacological technologies, which might serve as a promising and safe treatment strategy for atherosclerosis in patients with autoimmune diseases, and might be generalizable to the nonautoimmune population as well.
Acknowledgments
This work was supported by the Netherlands Heart Foundation (CVON 2011/B019, CVON 2017-20), Spark-Holding BV (2015B002), the European Union (Innovative Training Networks grant EPIMAC SEP-210163258), Leducq Foundation (Transatlantic Network Grant, 16CVD01), REPROGRAM (EU Horizon 2020, 667837), and the Amsterdam University Medical Centers fellowship.
The authors declare no competing financial interests.
Author contributions: H.J. Chen and M.P.J. de Winther decided on the topics included in the manuscript. H.J. Chen conceptualized and wrote the manuscript and prepared the figures and the table. S.W. Tas and M.P.J. de Winther conceptualized the article and edited the manuscript. All authors contributed to manuscript editing, and read and approved the final version.
References
- Ahmed D., Jaworski A., Roy D., Willmore W., Golshani A., and Cassol E.. 2018. Transcriptional Profiling Suggests Extensive Metabolic Rewiring of Human and Mouse Macrophages during Early Interferon Alpha Responses. Mediators Inflamm. 2018:5906819 10.1155/2018/5906819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyropoulou O.D., Protogerou A.D., and Sfikakis P.P.. 2018. Accelerated atheromatosis and arteriosclerosis in primary systemic vasculitides: current evidence and future perspectives. Curr. Opin. Rheumatol. 30:36–43. 10.1097/BOR.0000000000000453 [DOI] [PubMed] [Google Scholar]
- Bäck M., and Hansson G.K.. 2015. Anti-inflammatory therapies for atherosclerosis. Nat. Rev. Cardiol. 12:199–211. 10.1038/nrcardio.2015.5 [DOI] [PubMed] [Google Scholar]
- Baechler E.C., Batliwalla F.M., Karypis G., Gaffney P.M., Ortmann W.A., Espe K.J., Shark K.B., Grande W.J., Hughes K.M., Kapur V., et al. 2003. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA. 100:2610–2615. 10.1073/pnas.0337679100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénard A., Sakwa I., Schierloh P., Colom A., Mercier I., Tailleux L., Jouneau L., Boudinot P., Al-Saati T., Lang R., et al. 2018. B Cells Producing Type I IFN Modulate Macrophage Polarization in Tuberculosis. Am. J. Respir. Crit. Care Med. 197:801–813. 10.1164/rccm.201707-1475OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett L., Palucka A.K., Arce E., Cantrell V., Borvak J., Banchereau J., and Pascual V.. 2003. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197:711–723. 10.1084/jem.20021553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius A.L., and Beutler B.. 2010. Intracellular toll-like receptors. Immunity. 32:305–315. 10.1016/j.immuni.2010.03.012 [DOI] [PubMed] [Google Scholar]
- Boehm U., Klamp T., Groot M., and Howard J.C.. 1997. Cellular responses to interferon-γ. Annu. Rev. Immunol. 15:749–795. 10.1146/annurev.immunol.15.1.749 [DOI] [PubMed] [Google Scholar]
- Borden E.C. 2019. Interferons α and β in cancer: therapeutic opportunities from new insights. Nat. Rev. Drug Discov. 18:219–234. 10.1038/s41573-018-0011-2 [DOI] [PubMed] [Google Scholar]
- Borden E.C., Sen G.C., Uze G., Silverman R.H., Ransohoff R.M., Foster G.R., and Stark G.R.. 2007. Interferons at age 50: past, current and future impact on biomedicine. Nat. Rev. Drug Discov. 6:975–990. 10.1038/nrd2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshuizen M.C., Hoeksema M.A., Neele A.E., van der Velden S., Hamers A.A., Van den Bossche J., Lutgens E., and de Winther M.P.. 2016. Interferon-β promotes macrophage foam cell formation by altering both cholesterol influx and efflux mechanisms. Cytokine. 77:220–226. 10.1016/j.cyto.2015.09.016 [DOI] [PubMed] [Google Scholar]
- Brandman D., Bacchetti P., Ayala C.E., Maher J.J., and Khalili M.. 2012. Impact of insulin resistance on HCV treatment response and impact of HCV treatment on insulin sensitivity using direct measurements of insulin action. Diabetes Care. 35:1090–1094. 10.2337/dc11-1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce I.N., Urowitz M.B., Gladman D.D., Ibañez D., and Steiner G.. 2003. Risk factors for coronary heart disease in women with systemic lupus erythematosus: the Toronto Risk Factor Study. Arthritis Rheum. 48:3159–3167. 10.1002/art.11296 [DOI] [PubMed] [Google Scholar]
- Budhwani M., Mazzieri R., and Dolcetti R.. 2018. Plasticity of Type I Interferon-Mediated Responses in Cancer Therapy: From Anti-tumor Immunity to Resistance. Front. Oncol. 8:322 10.3389/fonc.2018.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buie J.J., Renaud L.L., Muise-Helmericks R., and Oates J.C.. 2017. IFN-α Negatively Regulates the Expression of Endothelial Nitric Oxide Synthase and Nitric Oxide Production: Implications for Systemic Lupus Erythematosus. J. Immunol. 199:1979–1988. 10.4049/jimmunol.1600108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlucci P.M., Purmalek M.M., Dey A.K., Temesgen-Oyelakin Y., Sakhardande S., Joshi A.A., Lerman J.B., Fike A., Davis M., Chung J.H., et al. 2018. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight. 3:e99276 10.1172/jci.insight.99276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Rivera C., Zhao W., Yalavarthi S., and Kaplan M.J.. 2015. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann. Rheum. Dis. 74:1417–1424. 10.1136/annrheumdis-2013-204837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey K.A., Guo X., Smith M.A., Wang S., Sinibaldi D., Sanjuan M.A., Wang L., Illei G.G., and White W.I.. 2018. Type I interferon receptor blockade with anifrolumab corrects innate and adaptive immune perturbations of SLE. Lupus Sci. Med. 5:e000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda S., Martín-Martínez M.A., González-Juanatey C., Llorca J., García-Yébenes M.J., Pérez-Vicente S., Sánchez-Costa J.T., Díaz-Gonzalez F., and González-Gay M.A.. CARMA Project Collaborative Group . 2015. Cardiovascular morbidity and associated risk factors in Spanish patients with chronic inflammatory rheumatic diseases attending rheumatology clinics: Baseline data of the CARMA Project. Semin. Arthritis Rheum. 44:618–626. 10.1016/j.semarthrit.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Cheng Q., Behzadi F., Sen S., Ohta S., Spreafico R., Teles R., Modlin R.L., and Hoffmann A.. 2019. Sequential conditioning-stimulation reveals distinct gene- and stimulus-specific effects of Type I and II IFN on human macrophage functions. Sci. Rep. 9:5288 10.1038/s41598-019-40503-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chironi G., Pagnoux C., Simon A., Pasquinelli-Balice M., Del-Pino M., Gariepy J., and Guillevin L.. 2007. Increased prevalence of subclinical atherosclerosis in patients with small-vessel vasculitis. Heart. 93:96–99. 10.1136/hrt.2006.088443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov D.A., Orekhov A.N., Sobenin I.A., and Bobryshev Y.V.. 2014. Plasmacytoid dendritic cells: development, functions, and role in atherosclerotic inflammation. Front. Physiol. 5:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J.E., Park I., Ahern D.J., Kassiteridi C., Danso Abeam D., Goddard M.E., Green P., Maffia P., and Monaco C.. 2018. Immune cell census in murine atherosclerosis: cytometry by time of flight illuminates vascular myeloid cell diversity. Cardiovasc. Res. 114:1360–1371. 10.1093/cvr/cvy109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotta S., Davidson S., Mahlakoiv T., Desmet C.J., Buckwalter M.R., Albert M.L., Staeheli P., and Wack A.. 2013. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. PLoS Pathog. 9:e1003773 10.1371/journal.ppat.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daissormont I.T., Christ A., Temmerman L., Sampedro Millares S., Seijkens T., Manca M., Rousch M., Poggi M., Boon L., van der Loos C., et al. 2011. Plasmacytoid dendritic cells protect against atherosclerosis by tuning T-cell proliferation and activity. Circ. Res. 109:1387–1395. 10.1161/CIRCRESAHA.111.256529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davignon J., and Ganz P.. 2004. Role of endothelial dysfunction in atherosclerosis. Circulation. 109(23, Suppl 1):III27–III32. [DOI] [PubMed] [Google Scholar]
- De Vries L.C.S., Duarte J.M., De Krijger M., Welting O., Van Hamersveld P.H.P., Van Leeuwen-Hilbers F.W.M., Moerland P.D., Jongejan A., D’Haens G.R., De Jonge W.J., and Wildenberg M.E.. 2019. A JAK1 Selective Kinase Inhibitor and Tofacitinib Affect Macrophage Activation and Function. Inflamm. Bowel Dis. 25:647–660. 10.1093/ibd/izy364 [DOI] [PubMed] [Google Scholar]
- den Brok M.H., Raaijmakers T.K., Collado-Camps E., and Adema G.J.. 2018. Lipid Droplets as Immune Modulators in Myeloid Cells. Trends Immunol. 39:380–392. 10.1016/j.it.2018.01.012 [DOI] [PubMed] [Google Scholar]
- Denny M.F., Thacker S., Mehta H., Somers E.C., Dodick T., Barrat F.J., McCune W.J., and Kaplan M.J.. 2007. Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood. 110:2907–2915. 10.1182/blood-2007-05-089086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny M.F., Yalavarthi S., Zhao W., Thacker S.G., Anderson M., Sandy A.R., McCune W.J., and Kaplan M.J.. 2010. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 184:3284–3297. 10.4049/jimmunol.0902199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao Y., Mohandas R., Lee P., Liu Z., Sautina L., Mu W., Li S., Wen X., Croker B., and Segal M.S.. 2016. Effects of Long-Term Type I Interferon on the Arterial Wall and Smooth Muscle Progenitor Cells Differentiation. Arterioscler. Thromb. Vasc. Biol. 36:266–273. 10.1161/ATVBAHA.115.306767 [DOI] [PubMed] [Google Scholar]
- Domeier P.P., Chodisetti S.B., Schell S.L., Kawasawa Y.I., Fasnacht M.J., Soni C., and Rahman Z.S.M.. 2018. B-Cell-Intrinsic Type 1 Interferon Signaling Is Crucial for Loss of Tolerance and the Development of Autoreactive B Cells. Cell Reports. 24:406–418. 10.1016/j.celrep.2018.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Villar M., and Hafler D.A.. 2018. Regulatory T cells in autoimmune disease. Nat. Immunol. 19:665–673. 10.1038/s41590-018-0120-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring Y., Manthey H.D., Drechsler M., Lievens D., Megens R.T., Soehnlein O., Busch M., Manca M., Koenen R.R., Pelisek J., et al. 2012. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation. 125:1673–1683. 10.1161/CIRCULATIONAHA.111.046755 [DOI] [PubMed] [Google Scholar]
- Döring Y., Soehnlein O., and Weber C.. 2017. Neutrophil Extracellular Traps in Atherosclerosis and Atherothrombosis. Circ. Res. 120:736–743. 10.1161/CIRCRESAHA.116.309692 [DOI] [PubMed] [Google Scholar]
- Drechsler M., Megens R.T., van Zandvoort M., Weber C., and Soehnlein O.. 2010. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 122:1837–1845. 10.1161/CIRCULATIONAHA.110.961714 [DOI] [PubMed] [Google Scholar]
- Epstein L.B. 1982. Interferon-gamma: success, structure and speculation. Nature. 295:453–454. 10.1038/295453a0 [DOI] [PubMed] [Google Scholar]
- Esdaile J.M., Abrahamowicz M., Grodzicky T., Li Y., Panaritis C., du Berger R., Côte R., Grover S.A., Fortin P.R., Clarke A.E., and Senécal J.-L.. 2001. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 44:2331–2337. [DOI] [PubMed] [Google Scholar]
- Fabris P., Betterle C., Floreani A., Greggio N.A., de Lazzari F., Naccarato R., and Chiaramonte M.. 1992. Development of type 1 diabetes mellitus during interferon alfa therapy for chronic HCV hepatitis. Lancet. 340:548 10.1016/0140-6736(92)91744-S [DOI] [PubMed] [Google Scholar]
- Fasano S., Pierro L., Pantano I., Iudici M., and Valentini G.. 2017. Longterm Hydroxychloroquine Therapy and Low-dose Aspirin May Have an Additive Effectiveness in the Primary Prevention of Cardiovascular Events in Patients with Systemic Lupus Erythematosus. J. Rheumatol. 44:1032–1038. 10.3899/jrheum.161351 [DOI] [PubMed] [Google Scholar]
- FDA 2019. FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR). Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and (accessed October 3, 2019).
- Feng X., Petraglia A.L., Chen M., Byskosh P.V., Boos M.D., and Reder A.T.. 2002. Low expression of interferon-stimulated genes in active multiple sclerosis is linked to subnormal phosphorylation of STAT1. J. Neuroimmunol. 129:205–215. 10.1016/S0165-5728(02)00182-0 [DOI] [PubMed] [Google Scholar]
- Floris A., Piga M., Mangoni A.A., Bortoluzzi A., Erre G.L., and Cauli A.. 2018. Protective Effects of Hydroxychloroquine against Accelerated Atherosclerosis in Systemic Lupus Erythematosus. Mediators Inflamm. 2018:3424136 10.1155/2018/3424136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck G., Mawson T.L., Folco E.J., Molinaro R., Ruvkun V., Engelbertsen D., Liu X., Tesmenitsky Y., Shvartz E., Sukhova G.K., et al. 2018. Roles of PAD4 and NETosis in Experimental Atherosclerosis and Arterial Injury: Implications for Superficial Erosion. Circ. Res. 123:33–42. 10.1161/CIRCRESAHA.117.312494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie R., Khamashta M., Merrill J.T., Werth V.P., Kalunian K., Brohawn P., Illei G.G., Drappa J., Wang L., and Yoo S.. CD1013 Study Investigators . 2017. Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 69:376–386. 10.1002/art.39962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumoto Y., Smith C.K., Blanco L., Zhao W., Brooks S.R., Thacker S.G., Abdalrahman Z., Sciumè G., Tsai W.L., Trier A.M., et al. 2017. Tofacitinib Ameliorates Murine Lupus and Its Associated Vascular Dysfunction. Arthritis Rheumatol. 69:148–160. 10.1002/art.39818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangaplara A., Martens C., Dahlstrom E., Metidji A., Gokhale A.S., Glass D.D., Lopez-Ocasio M., Baur R., Kanakabandi K., Porcella S.F., and Shevach E.M.. 2018. Type I interferon signaling attenuates regulatory T cell function in viral infection and in the tumor microenvironment. PLoS Pathog. 14:e1006985 10.1371/journal.ppat.1006985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly D. 2018. Do Type I Interferons Link Systemic Autoimmunities and Metabolic Syndrome in a Pathogenetic Continuum? Trends Immunol. 39:28–43. 10.1016/j.it.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Garcia-Romo G.S., Caielli S., Vega B., Connolly J., Allantaz F., Xu Z., Punaro M., Baisch J., Guiducci C., Coffman R.L., et al. 2011. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3:73ra20 10.1126/scitranslmed.3001201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geginat J., Paroni M., Maglie S., Alfen J.S., Kastirr I., Gruarin P., De Simone M., Pagani M., and Abrignani S.. 2014. Plasticity of human CD4 T cell subsets. Front. Immunol. 5:630 10.3389/fimmu.2014.00630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliet M., Cao W., and Liu Y.J.. 2008. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 8:594–606. 10.1038/nri2358 [DOI] [PubMed] [Google Scholar]
- Gimbrone M.A. Jr., and García-Cardeña G.. 2016. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 118:620–636. 10.1161/CIRCRESAHA.115.306301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorelli M., De Blasi A., Defazio G., Avolio C., Iacovelli L., Livrea P., and Trojano M.. 2002. Differential regulation of membrane bound and soluble ICAM 1 in human endothelium and blood mononuclear cells: effects of interferon beta-1a. Cell Commun. Adhes. 9:259–272. 10.1080/15419060216305 [DOI] [PubMed] [Google Scholar]
- Golding A., Rosen A., Petri M., Akhter E., and Andrade F.. 2010. Interferon-alpha regulates the dynamic balance between human activated regulatory and effector T cells: implications for antiviral and autoimmune responses. Immunology. 131:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Navajas J.M., Lee J., David M., and Raz E.. 2012. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 12:125–135. 10.1038/nri3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens P., Gijbels M.J., Zernecke A., Eijgelaar W., Vergouwe M.N., van der Made I., Vanderlocht J., Beckers L., Buurman W.A., Daemen M.J., et al. 2010. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab. 12:142–153. 10.1016/j.cmet.2010.06.008 [DOI] [PubMed] [Google Scholar]
- Gota C., and Calabrese L.. 2003. Induction of clinical autoimmune disease by therapeutic interferon-α. Autoimmunity. 36:511–518. 10.1080/08916930310001605873 [DOI] [PubMed] [Google Scholar]
- Green N.M., Laws A., Kiefer K., Busconi L., Kim Y.-M., Brinkmann M.M., Trail E.H., Yasuda K., Christensen S.R., Shlomchik M.J., et al. 2009. Murine B cell response to TLR7 ligands depends on an IFN-beta feedback loop. J. Immunol. 183:1569–1576. 10.4049/jimmunol.0803899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenn R.C., Yalavarthi S., Gandhi A.A., Kazzaz N.M., Núñez-Álvarez C., Hernández-Ramírez D., Cabral A.R., McCune W.J., Bockenstedt P.L., and Knight J.S.. 2017. Endothelial progenitor dysfunction associates with a type I interferon signature in primary antiphospholipid syndrome. Ann. Rheum. Dis. 76:450–457. 10.1136/annrheumdis-2016-209442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarda G., Braun M., Staehli F., Tardivel A., Mattmann C., Förster I., Farlik M., Decker T., Du Pasquier R.A., Romero P., and Tschopp J.. 2011. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 34:213–223. 10.1016/j.immuni.2011.02.006 [DOI] [PubMed] [Google Scholar]
- Guillevin L., and Dörner T.. 2007. Vasculitis: mechanisms involved and clinical manifestations. Arthritis Res. Ther. 9(Suppl 2):S9 10.1186/ar2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley K.J., Lilly C.M., Yang J.-H., Feng Y., Kennedy S.P., Turi T.G., Thompson J.F., Sukhova G.H., Libby P., and Lee R.T.. 2000. Overexpression of eotaxin and the CCR3 receptor in human atherosclerosis: using genomic technology to identify a potential novel pathway of vascular inflammation. Circulation. 102:2185–2189. 10.1161/01.CIR.102.18.2185 [DOI] [PubMed] [Google Scholar]
- Hamilton J.A., Wu Q., Yang P., Luo B., Liu S., Hong H., Li J., Walter M.R., Fish E.N., Hsu H.-C., et al. 2017. Cutting Edge: Endogenous IFN-β Regulates Survival and Development of Transitional B Cells. J. Immunol. 199:2618–2623. 10.4049/jimmunol.1700888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G.K. 2005. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352:1685–1695. 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- Henault J., Riggs J.M., Karnell J.L., Liarski V.M., Li J., Shirinian L., Xu L., Casey K.A., Smith M.A., Khatry D.B., et al. 2016. Self-reactive IgE exacerbates interferon responses associated with autoimmunity. Nat. Immunol. 17:196–203. 10.1038/ni.3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervas-Stubbs S., Perez-Gracia J.L., Rouzaut A., Sanmamed M.F., Le Bon A., and Melero I.. 2011. Direct effects of type I interferons on cells of the immune system. Clin. Cancer Res. 17:2619–2627. 10.1158/1078-0432.CCR-10-1114 [DOI] [PubMed] [Google Scholar]
- Hess P.L., Al-Khalidi H.R., Friedman D.J., Mulder H., Kucharska-Newton A., Rosamond W.R., Lopes R.D., Gersh B.J., Mark D.B., Curtis L.H., et al. 2017. The Metabolic Syndrome and Risk of Sudden Cardiac Death: The Atherosclerosis Risk in Communities Study. J. Am. Heart Assoc. 6:e006103 10.1161/JAHA.117.006103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Takaoka A., and Taniguchi T.. 2006. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 25:349–360. 10.1016/j.immuni.2006.08.009 [DOI] [PubMed] [Google Scholar]
- Huan T., Zhang B., Wang Z., Joehanes R., Zhu J., Johnson A.D., Ying S., Munson P.J., Raghavachari N., Wang R., et al. Coronary ARteryDIsease Genome wide Replication and Meta-analysis (CARDIoGRAM) Consortium, International Consortium for Blood Pressure GWAS (ICBP) . 2013. A systems biology framework identifies molecular underpinnings of coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 33:1427–1434. 10.1161/ATVBAHA.112.300112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionita M.G., van den Borne P., Catanzariti L.M., Moll F.L., de Vries J.P., Pasterkamp G., Vink A., and de Kleijn D.P.. 2010. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler. Thromb. Vasc. Biol. 30:1842–1848. 10.1161/ATVBAHA.110.209296 [DOI] [PubMed] [Google Scholar]
- Isaacs A., Lindenmann J., and Andrewes Christopher H.. 1957. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 147:258–267. 10.1098/rspb.1957.0048 [DOI] [PubMed] [Google Scholar]
- Ivashkiv L.B. 2018. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 18:545–558. 10.1038/s41577-018-0029-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkiv L.B., and Donlin L.T.. 2014. Regulation of type I interferon responses. Nat. Rev. Immunol. 14:36–49. 10.1038/nri3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguirre A., Barnes B.J., Amrute S., Yeow W.-S., Megjugorac N., Dai J., Feng D., Chung E., Pitha P.M., and Fitzgerald-Bocarsly P.. 2003. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 74:1125–1138. 10.1189/jlb.0603255 [DOI] [PubMed] [Google Scholar]
- Jaiswal S., Natarajan P., Silver A.J., Gibson C.J., Bick A.G., Shvartz E., McConkey M., Gupta N., Gabriel S., Ardissino D., et al. 2017. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 377:111–121. 10.1056/NEJMoa1701719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H., Thelwell C., Dilger P., Bird C., Daniels S., and Wadhwa M.. 2018. Endothelial cell functions impaired by interferon in vitro: Insights into the molecular mechanism of thrombotic microangiopathy associated with interferon therapy. Thromb. Res. 163:105–116. 10.1016/j.thromres.2018.01.039 [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., and Hansson G.K.. 1986. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 6:131–138. 10.1161/01.ATV.6.2.131 [DOI] [PubMed] [Google Scholar]
- Jongstra-Bilen J., Zhang C.X., Wisnicki T., Li M.K., White-Alfred S., Ilaalagan R., Ferri D.M., Deonarain A., Wan M.H., Hyduk S.J., et al. 2017. Oxidized Low-Density Lipoprotein Loading of Macrophages Downregulates TLR-Induced Proinflammatory Responses in a Gene-Specific and Temporal Manner through Transcriptional Control. J. Immunol. 199:2149–2157. 10.4049/jimmunol.1601363 [DOI] [PubMed] [Google Scholar]
- Kang E.H., Liao K.P., and Kim S.C.. 2018. Cardiovascular Safety of Biologics and JAK Inhibitors in Patients with Rheumatoid Arthritis. Curr. Rheumatol. Rep. 20:42 10.1007/s11926-018-0752-2 [DOI] [PubMed] [Google Scholar]
- Kaplan M.J. 2011. Neutrophils in the pathogenesis and manifestations of SLE. Nat. Rev. Rheumatol. 7:691–699. 10.1038/nrrheum.2011.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., and Akira S.. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11:373–384. 10.1038/ni.1863 [DOI] [PubMed] [Google Scholar]
- Kawai T., Takahashi K., Sato S., Coban C., Kumar H., Kato H., Ishii K.J., Takeuchi O., and Akira S.. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6:981–988. 10.1038/ni1243 [DOI] [PubMed] [Google Scholar]
- Kayagaki N., Yamaguchi N., Nakayama M., Eto H., Okumura K., and Yagita H.. 1999. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: A novel mechanism for the antitumor effects of type I IFNs. J. Exp. Med. 189:1451–1460. 10.1084/jem.189.9.1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns A., Gordon J., Burdo T.H., and Qin X.. 2017. HIV-1-Associated Atherosclerosis: Unraveling the Missing Link. J. Am. Coll. Cardiol. 69:3084–3098. 10.1016/j.jacc.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegerreis B.J., Catalina M.D., Geraci N.S., Bachali P., Lipsky P.E., and Grammer A.C.. 2019. Genomic Identification of Low-Density Granulocytes and Analysis of Their Role in the Pathogenesis of Systemic Lupus Erythematosus. J. Immunol. 202:3309–3317. 10.4049/jimmunol.1801512 [DOI] [PubMed] [Google Scholar]
- Kerkmann M., Rothenfusser S., Hornung V., Towarowski A., Wagner M., Sarris A., Giese T., Endres S., and Hartmann G.. 2003. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J. Immunol. 170:4465–4474. 10.4049/jimmunol.170.9.4465 [DOI] [PubMed] [Google Scholar]
- Kessenbrock K., Krumbholz M., Schönermarck U., Back W., Gross W.L., Werb Z., Gröne H.-J., Brinkmann V., and Jenne D.E.. 2009. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 15:623–625. 10.1038/nm.1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelhuth D.F., and Hansson G.K.. 2016. Adaptive Response of T and B Cells in Atherosclerosis. Circ. Res. 118:668–678. 10.1161/CIRCRESAHA.115.306427 [DOI] [PubMed] [Google Scholar]
- Khamashta M., Merrill J.T., Werth V.P., Furie R., Kalunian K., Illei G.G., Drappa J., Wang L., and Greth W.. CD1067 study investigators . 2016. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 75:1909–1916. 10.1136/annrheumdis-2015-208562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandpur R., Carmona-Rivera C., Vivekanandan-Giri A., Gizinski A., Yalavarthi S., Knight J.S., Friday S., Li S., Patel R.M., Subramanian V., et al. 2013. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 5:178ra40 10.1126/scitranslmed.3005580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.-H., Chee J.D., Bradfield C.J., Park E.-S., Kumar P., and MacMicking J.D.. 2016. Interferon-induced guanylate-binding proteins in inflammasome activation and host defense. Nat. Immunol. 17:481–489. 10.1038/ni.3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Shim D., Lee J.S., Zaitsev K., Williams J.W., Kim K.W., Jang M.Y., Seok Jang H., Yun T.J., Lee S.H., et al. 2018. Transcriptome Analysis Reveals Nonfoamy Rather Than Foamy Plaque Macrophages Are Proinflammatory in Atherosclerotic Murine Models. Circ. Res. 123:1127–1142. 10.1161/CIRCRESAHA.118.312804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King K.R., Aguirre A.D., Ye Y.-X., Sun Y., Roh J.D., Ng R.P. Jr., Kohler R.H., Arlauckas S.P., Iwamoto Y., Savol A., et al. 2017. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat. Med. 23:1481–1487. 10.1038/nm.4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano T., Nezu T., Shiromoto T., Kubo S., Uemura J., Wada Y., and Yagita Y.. 2017. Association Between Absolute Eosinophil Count and Complex Aortic Arch Plaque in Patients With Acute Ischemic Stroke. Stroke. 48:1074–1076. 10.1161/STROKEAHA.116.016436 [DOI] [PubMed] [Google Scholar]
- Knight J.S., Luo W., O’Dell A.A., Yalavarthi S., Zhao W., Subramanian V., Guo C., Grenn R.C., Thompson P.R., Eitzman D.T., and Kaplan M.J.. 2014. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ. Res. 114:947–956. 10.1161/CIRCRESAHA.114.303312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Takaku Y., Yokote A., Miyazawa H., Soma T., Hagiwara K., Kanazawa M., and Nagata M.. 2008. Interferon-β augments eosinophil adhesion-inducing activity of endothelial cells. Eur. Respir. J. 32:1540–1547. 10.1183/09031936.00059507 [DOI] [PubMed] [Google Scholar]
- Kojima Y., Weissman I.L., and Leeper N.J.. 2017. The Role of Efferocytosis in Atherosclerosis. Circulation. 135:476–489. 10.1161/CIRCULATIONAHA.116.025684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopitar-Jerala N. 2017. The Role of Interferons in Inflammation and Inflammasome Activation. Front. Immunol. 8:873 10.3389/fimmu.2017.00873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotenko S.V., Gallagher G., Baurin V.V., Lewis-Antes A., Shen M., Shah N.K., Langer J.A., Sheikh F., Dickensheets H., and Donnelly R.P.. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4:69–77. 10.1038/ni875 [DOI] [PubMed] [Google Scholar]
- Lahoute C., Herbin O., Mallat Z., and Tedgui A.. 2011. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat. Rev. Cardiol. 8:348–358. 10.1038/nrcardio.2011.62 [DOI] [PubMed] [Google Scholar]
- Lande R., Ganguly D., Facchinetti V., Frasca L., Conrad C., Gregorio J., Meller S., Chamilos G., Sebasigari R., Riccieri V., et al. 2011. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3:73ra19 10.1126/scitranslmed.3001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear H.M., Schoggins J.W., and Diamond M.S.. 2019. Shared and Distinct Functions of Type I and Type III Interferons. Immunity. 50:907–923. 10.1016/j.immuni.2019.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.D., Folsom A.R., Nieto F.J., Chambless L.E., Shahar E., and Wolfe D.A.. 2001. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: atherosclerosis risk in communities study. Am. J. Epidemiol. 154:758–764. 10.1093/aje/154.8.758 [DOI] [PubMed] [Google Scholar]
- Lee P.Y., Li Y., Richards H.B., Chan F.S., Zhuang H., Narain S., Butfiloski E.J., Sobel E.S., Reeves W.H., and Segal M.S.. 2007. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum. 56:3759–3769. 10.1002/art.23035 [DOI] [PubMed] [Google Scholar]
- Leonard D., Svenungsson E., Sandling J.K., Berggren O., Jönsen A., Bengtsson C., Wang C., Jensen-Urstad K., Granstam S.-O., Bengtsson A.A., et al. 2013. Coronary heart disease in systemic lupus erythematosus is associated with interferon regulatory factor-8 gene variants. Circ Cardiovasc Genet. 6:255–263. 10.1161/CIRCGENETICS.113.000044 [DOI] [PubMed] [Google Scholar]
- Levy Z., Rachmani R., Trestman S., Dvir A., Shaish A., Ravid M., and Harats D.. 2003. Low-dose interferon-α accelerates atherosclerosis in an LDL receptor-deficient mouse model. Eur. J. Intern. Med. 14:479–483. 10.1016/j.ejim.2003.08.010 [DOI] [PubMed] [Google Scholar]
- Li T., and Chen Z.J.. 2018. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 215:1287–1299. 10.1084/jem.20180139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Fu Q., Cui H., Qu B., Pan W., Shen N., and Bao C.. 2011a Interferon-α priming promotes lipid uptake and macrophage-derived foam cell formation: a novel link between interferon-α and atherosclerosis in lupus. Arthritis Rheum. 63:492–502. 10.1002/art.30165 [DOI] [PubMed] [Google Scholar]
- Li P., Wong J.J.-Y., Sum C., Sin W.-X., Ng K.-Q., Koh M.B.C., and Chin K.-C.. 2011b IRF8 and IRF3 cooperatively regulate rapid interferon-β induction in human blood monocytes. Blood. 117:2847–2854. 10.1182/blood-2010-07-294272 [DOI] [PubMed] [Google Scholar]
- Li S., Wu J., Zhu S., Liu Y.-J., and Chen J.. 2017. Disease-Associated Plasmacytoid Dendritic Cells. Front. Immunol. 8:1268 10.3389/fimmu.2017.01268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Ridker P.M., and Hansson G.K.. Leducq Transatlantic Network on Atherothrombosis . 2009. Inflammation in atherosclerosis: from pathophysiology to practice. J. Am. Coll. Cardiol. 54:2129–2138. 10.1016/j.jacc.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.-D., Nishi H., Poles J., Niu X., Mccauley C., Rahman K., Brown E.J., Yeung S.T., Vozhilla N., Weinstock A., et al. 2019. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 4:e124574 10.1172/jci.insight.124574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Carmona-Rivera C., Moore E., Seto N.L., Knight J.S., Pryor M., Yang Z.-H., Hemmers S., Remaley A.T., Mowen K.A., and Kaplan M.J.. 2018. Myeloid-Specific Deletion of Peptidylarginine Deiminase 4 Mitigates Atherosclerosis. Front. Immunol. 9:1680 10.3389/fimmu.2018.01680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lood C., Amisten S., Gullstrand B., Jönsen A., Allhorn M., Truedsson L., Sturfelt G., Erlinge D., and Bengtsson A.A.. 2010. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: up-regulation of the type I interferon system is strongly associated with vascular disease. Blood. 116:1951–1957. 10.1182/blood-2010-03-274605 [DOI] [PubMed] [Google Scholar]
- Lood C., Blanco L.P., Purmalek M.M., Carmona-Rivera C., De Ravin S.S., Smith C.K., Malech H.L., Ledbetter J.A., Elkon K.B., and Kaplan M.J.. 2016. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22:146–153. 10.1038/nm.4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 380:2095–2128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macritchie N., Grassia G., Sabir S.R., Maddaluno M., Welsh P., Sattar N., Ialenti A., Kurowska-Stolarska M., McInnes I.B., Brewer J.M., et al. 2012. Plasmacytoid dendritic cells play a key role in promoting atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 32:2569–2579. 10.1161/ATVBAHA.112.251314 [DOI] [PubMed] [Google Scholar]
- Maracle C.X., Agca R., Helder B., Meeuwsen J.A.L., Niessen H.W.M., Biessen E.A.L., de Winther M.P.J., de Jager S.C.A., Nurmohamed M.T., and Tas S.W.. 2018. Noncanonical NF-κB signaling in microvessels of atherosclerotic lesions is associated with inflammation, atheromatous plaque morphology and myocardial infarction. Atherosclerosis. 270:33–41. 10.1016/j.atherosclerosis.2018.01.032 [DOI] [PubMed] [Google Scholar]
- Marrie R.A., Garland A., Schaffer S.A., Fransoo R., Leung S., Yogendran M., Kingwell E., and Tremlett H.. 2019. Traditional risk factors may not explain increased incidence of myocardial infarction in MS. Neurology. 92:e1624–e1633. 10.1212/WNL.0000000000007251 [DOI] [PubMed] [Google Scholar]
- Mavrogiannis C., Papanikolaou I.S., Elefsiniotis I.S., Psilopoulos D.I., Karameris A., and Karvountzis G.. 2001. Ulcerative colitis associated with interferon treatment for chronic hepatitis C. J. Hepatol. 34:964–965. 10.1016/S0168-8278(01)00022-8 [DOI] [PubMed] [Google Scholar]
- Mayet W.-J., Hess G., Gerken G., Rossol S., Voth R., Manns M., and Meyer zum Büschenfelde K.H.. 1989. Treatment of chronic type B hepatitis with recombinant α-interferon induces autoantibodies not specific for autoimmune chronic hepatitis. Hepatology. 10:24–28. 10.1002/hep.1840100106 [DOI] [PubMed] [Google Scholar]
- McMahon M., and Skaggs B.. 2014. Pathogenesis and treatment of atherosclerosis in lupus. Rheum. Dis. Clin. North Am. 40:475–495: viii. 10.1016/j.rdc.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesev E.V., LeDesma R.A., and Ploss A.. 2019. Decoding type I and III interferon signalling during viral infection. Nat. Microbiol. 4:914–924. 10.1038/s41564-019-0421-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metidji A., Rieder S.A., Glass D.D., Cremer I., Punkosdy G.A., and Shevach E.M.. 2015. IFN-α/β receptor signaling promotes regulatory T cell development and function under stress conditions. J. Immunol. 194:4265–4276. 10.4049/jimmunol.1500036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Y.I., Choi S.H., Wiesner P., Fang L., Harkewicz R., Hartvigsen K., Boullier A., Gonen A., Diehl C.J., Que X., et al. 2011. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 108:235–248. 10.1161/CIRCRESAHA.110.223875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen K.E., Lewerenz M., Reboul J., Lutfalla G., and Uzé G.. 1999. The type I interferon receptor: structure, function, and evolution of a family business. J. Interferon Cytokine Res. 19:1069–1098. 10.1089/107999099313019 [DOI] [PubMed] [Google Scholar]
- Moore K.J., and Tabas I.. 2011. Macrophages in the pathogenesis of atherosclerosis. Cell. 145:341–355. 10.1016/j.cell.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra V.B., Coppola G., Orefice G., De Michele G., Vacca G., Filla A., and Bonavita V.. 2004. Interferon-β treatment decreases cholesterol plasma levels in multiple sclerosis patients. Neurology. 62:829–830. 10.1212/01.WNL.0000113750.11090.67 [DOI] [PubMed] [Google Scholar]
- Muskardin T.L.W., and Niewold T.B.. 2018. Type I interferon in rheumatic diseases. Nat. Rev. Rheumatol. 14:214–228. 10.1038/nrrheum.2018.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Fujii T., Hashimoto M., Yukawa N., Yoshifuji H., Ohmura K., Nakaizumi A., and Mimori T.. 2012. Type I interferon induces CX3CL1 (fractalkine) and CCL5 (RANTES) production in human pulmonary vascular endothelial cells. Clin. Exp. Immunol. 170:94–100. 10.1111/j.1365-2249.2012.04638.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.P., Schunkert H., Samani N.J., and Erridge C.. 2015. Genetic analysis of leukocyte type-I interferon production and risk of coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 35:1456–1462. 10.1161/ATVBAHA.114.304925 [DOI] [PubMed] [Google Scholar]
- Ng C.T., Mendoza J.L., Garcia K.C., and Oldstone M.B.A.. 2016. Alpha and Beta Type 1 Interferon Signaling: Passage for Diverse Biologic Outcomes. Cell. 164:349–352. 10.1016/j.cell.2015.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccoli G., Ferrante G., Cosentino N., Conte M., Belloni F., Marino M., Bacà M., Montone R.A., Sabato V., Schiavino D., et al. 2010. Eosinophil cationic protein: A new biomarker of coronary atherosclerosis. Atherosclerosis. 211:606–611. 10.1016/j.atherosclerosis.2010.02.038 [DOI] [PubMed] [Google Scholar]
- Niessner A., Sato K., Chaikof E.L., Colmegna I., Goronzy J.J., and Weyand C.M.. 2006. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-α. Circulation. 114:2482–2489. 10.1161/CIRCULATIONAHA.106.642801 [DOI] [PubMed] [Google Scholar]
- Niessner A., Shin M.S., Pryshchep O., Goronzy J.J., Chaikof E.L., and Weyand C.M.. 2007. Synergistic proinflammatory effects of the antiviral cytokine interferon-α and Toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation. 116:2043–2052. 10.1161/CIRCULATIONAHA.107.697789 [DOI] [PubMed] [Google Scholar]
- Owsiany K.M., Alencar G.F., and Owens G.K.. 2019. Revealing the Origins of Foam Cells in Atherosclerotic Lesions. Arterioscler. Thromb. Vasc. Biol. 39:836–838. 10.1161/ATVBAHA.119.312557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V. 2018. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 18:134–147. 10.1038/nri.2017.105 [DOI] [PubMed] [Google Scholar]
- Park S.H., Kang K., Giannopoulou E., Qiao Y., Kang K., Kim G., Park-Min K.-H., and Ivashkiv L.B.. 2017. Type I interferons and the cytokine TNF cooperatively reprogram the macrophage epigenome to promote inflammatory activation. Nat. Immunol. 18:1104–1116. 10.1038/ni.3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B., and Bruce I.. 2013. SLE and metabolic syndrome. Lupus. 22:1259–1266. 10.1177/0961203313502570 [DOI] [PubMed] [Google Scholar]
- Pereira R.M.R., de Carvalho J.F., and Bonfá E.. 2009. Metabolic syndrome in rheumatological diseases. Autoimmun. Rev. 8:415–419. 10.1016/j.autrev.2009.01.001 [DOI] [PubMed] [Google Scholar]
- Pérez-Baos S., Barrasa J.I., Gratal P., Larrañaga-Vera A., Prieto-Potin I., Herrero-Beaumont G., and Largo R.. 2017. Tofacitinib restores the inhibition of reverse cholesterol transport induced by inflammation: understanding the lipid paradox associated with rheumatoid arthritis. Br. J. Pharmacol. 174:3018–3031. 10.1111/bph.13932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertiwi K.R., de Boer O.J., Mackaaij C., Pabittei D.R., de Winter R.J., Li X., and van der Wal A.C.. 2019. Extracellular traps derived from macrophages, mast cells, eosinophils and neutrophils are generated in a time-dependent manner during atherothrombosis. J. Pathol. 247:505–512. 10.1002/path.5212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. 2007. The interferons: 50 years after their discovery, there is much more to learn. J. Biol. Chem. 282:20047–20051. 10.1074/jbc.R700004200 [DOI] [PubMed] [Google Scholar]
- Petri M.A., Kiani A.N., Post W., Christopher-Stine L., and Magder L.S.. 2011. Lupus Atherosclerosis Prevention Study (LAPS). Ann. Rheum. Dis. 70:760–765. 10.1136/ard.2010.136762 [DOI] [PubMed] [Google Scholar]
- Ponticelli C., and Moroni G.. 2017. Hydroxychloroquine in systemic lupus erythematosus (SLE). Expert Opin. Drug Saf. 16:411–419. 10.1080/14740338.2017.1269168 [DOI] [PubMed] [Google Scholar]
- Pulliam L., Calosing C., Sun B., Grunfeld C., and Rempel H.. 2014. Monocyte activation from interferon-α in HIV infection increases acetylated LDL uptake and ROS production. J. Interferon Cytokine Res. 34:822–828. 10.1089/jir.2013.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M., Wang L., Li F., Yang M., Song L., Tian F., Yukht A., Shah P.K., Rothenberg M.E., and Sharifi B.G.. 2017. Oxidized LDL activated eosinophil polarize macrophage phenotype from M2 to M1 through activation of CD36 scavenger receptor. Atherosclerosis. 263:82–91. 10.1016/j.atherosclerosis.2017.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillard T., Araújo H.A., Franck G., Shvartz E., Sukhova G., and Libby P.. 2015. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur. Heart J. 36:1394–1404. 10.1093/eurheartj/ehv044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafieian-Kopaei M., Setorki M., Doudi M., Baradaran A., and Nasri H.. 2014. Atherosclerosis: process, indicators, risk factors and new hopes. Int. J. Prev. Med. 5:927–946. [PMC free article] [PubMed] [Google Scholar]
- Raza S., Barnett M.W., Barnett-Itzhaki Z., Amit I., Hume D.A., and Freeman T.C.. 2014. Analysis of the transcriptional networks underpinning the activation of murine macrophages by inflammatory mediators. J. Leukoc. Biol. 96:167–183. 10.1189/jlb.6HI0313-169R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboldi A., Dang E.V., McDonald J.G., Liang G., Russell D.W., and Cyster J.G.. 2014. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science. 345:679–684. 10.1126/science.1254790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reder A.T., and Feng X.. 2013. Aberrant Type I Interferon Regulation in Autoimmunity: Opposite Directions in MS and SLE, Shaped by Evolution and Body Ecology. Front. Immunol. 4:281 10.3389/fimmu.2013.00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J.A., Ray D.W., Zeef L.A.H., O’Neill T., Bruce I.N., and Alexander M.Y.. 2014. The effect of type 1 IFN on human aortic endothelial cell function in vitro: relevance to systemic lupus erythematosus. J. Interferon Cytokine Res. 34:404–412. 10.1089/jir.2013.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S.D., et al. CANTOS Trial Group . 2017. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 377:1119–1131. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- Ridker P.M., MacFadyen J.G., Glynn R.J., Koenig W., Libby P., Everett B.M., Lefkowitz M., Thuren T., and Cornel J.H.. 2018. Inhibition of Interleukin-1β by Canakinumab and Cardiovascular Outcomes in Patients With Chronic Kidney Disease. J. Am. Coll. Cardiol. 71:2405–2414. 10.1016/j.jacc.2018.03.490 [DOI] [PubMed] [Google Scholar]
- Robertson K.A., and Ghazal P.. 2016. Interferon Control of the Sterol Metabolic Network: Bidirectional Molecular Circuitry-Mediating Host Protection. Front. Immunol. 7:634 10.3389/fimmu.2016.00634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Carrio J., de Paz B., López P., Prado C., Alperi-López M., Ballina-García F.J., and Suárez A.. 2014. IFNα serum levels are associated with endothelial progenitor cells imbalance and disease features in rheumatoid arthritis patients. PLoS One. 9:e86069 10.1371/journal.pone.0086069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotzius P., Thams S., Soehnlein O., Kenne E., Tseng C.-N., Björkström N.K., Malmberg K.-J., Lindbom L., and Eriksson E.E.. 2010. Distinct infiltration of neutrophils in lesion shoulders in ApoE-/- mice. Am. J. Pathol. 177:493–500. 10.2353/ajpath.2010.090480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacre K., Criswell L.A., and McCune J.M.. 2012. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res. Ther. 14:R155 10.1186/ar3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage A.P., Tsiantoulas D., Binder C.J., and Mallat Z.. 2019. The role of B cells in atherosclerosis. Nat. Rev. Cardiol. 16:180–196. 10.1038/s41569-018-0106-9 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Vignali D.A.A., Rudensky A.Y., Niec R.E., and Waldmann H.. 2013. The plasticity and stability of regulatory T cells. Nat. Rev. Immunol. 13:461–467. 10.1038/nri3464 [DOI] [PubMed] [Google Scholar]
- Sano E., Tashiro S., Tadakuma H., Takei T., Ueda T., and Tsumoto K.. 2012. Type 1 IFN inhibits the growth factor deprived apoptosis of cultured human aortic endothelial cells and protects the cells from chemically induced oxidative cytotoxicity. J. Cell. Biochem. 113:3823–3834. 10.1002/jcb.24259 [DOI] [PubMed] [Google Scholar]
- Schanberg L.E., Sandborg C., Barnhart H.X., Ardoin S.P., Yow E., Evans G.W., Mieszkalski K.L., Ilowite N.T., Eberhard A., Imundo L.F., et al. Atherosclerosis Prevention in Pediatric Lupus Erythematosus Investigators . 2012. Use of atorvastatin in systemic lupus erythematosus in children and adolescents. Arthritis Rheum. 64:285–296. 10.1002/art.30645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber G. 2017. The molecular basis for differential type I interferon signaling. J. Biol. Chem. 292:7285–7294. 10.1074/jbc.R116.774562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K., Hertzog P.J., Ravasi T., and Hume D.A.. 2004. Interferon-γ: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75:163–189. 10.1189/jlb.0603252 [DOI] [PubMed] [Google Scholar]
- Schwartz D.M., Kanno Y., Villarino A., Ward M., Gadina M., and O’Shea J.J.. 2017. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 17:78 10.1038/nrd.2017.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seimon T., and Tabas I.. 2009. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J. Lipid Res. 50(Suppl):S382–S387. 10.1194/jlr.R800032-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena A., Pedrosa R., Ferret-Sena V., Almeida R., Andrade M.L., Morais M.G., and Couderc R.. 2000. Interferon beta1a therapy changes lipoprotein metabolism in patients with multiple sclerosis. Clin. Chem. Lab. Med. 38:209–213. 10.1515/CCLM.2000.030 [DOI] [PubMed] [Google Scholar]
- Shen J., T-To S.S., Schrieber L., and King N.J.. 1997. Early E-selectin, VCAM-1, ICAM-1, and late major histocompatibility complex antigen induction on human endothelial cells by flavivirus and comodulation of adhesion molecule expression by immune cytokines. J. Virol. 71:9323–9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard P., Kindsvogel W., Xu W., Henderson K., Schlutsmeyer S., Whitmore T.E., Kuestner R., Garrigues U., Birks C., Roraback J., et al. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4:63–68. 10.1038/ni873 [DOI] [PubMed] [Google Scholar]
- Silvestre-Roig C., Braster Q., Wichapong K., Lee E.Y., Teulon J.M., Berrebeh N., Winter J., Adrover J.M., Santos G.S., Froese A., et al. 2019. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature. 569:236–240. 10.1038/s41586-019-1167-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhania A., Graham C.M., Gabryšová L., Moreira-Teixeira L., Stavropoulos E., Pitt J.M., Chakravarty P., Warnatsch A., Branchett W.J., Conejero L., et al. 2019. Transcriptional profiling unveils type I and II interferon networks in blood and tissues across diseases. Nat. Commun. 10:2887 10.1038/s41467-019-10601-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.K., Vivekanandan-Giri A., Tang C., Knight J.S., Mathew A., Padilla R.L., Gillespie B.W., Carmona-Rivera C., Liu X., Subramanian V., et al. 2014. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 66:2532–2544. 10.1002/art.38703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers E.C., Zhao W., Lewis E.E., Wang L., Wing J.J., Sundaram B., Kazerooni E.A., McCune W.J., and Kaplan M.J.. 2012. Type I interferons are associated with subclinical markers of cardiovascular disease in a cohort of systemic lupus erythematosus patients. PLoS One. 7:e37000 10.1371/journal.pone.0037000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann N.J., Garmire L.X., McDonald J.G., Myers D.S., Milne S.B., Shibata N., Reichart D., Fox J.N., Shaked I., Heudobler D., et al. 2012. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 151:138–152. 10.1016/j.cell.2012.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikakulapu P., and McNamara C.A.. 2017. B cells and atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 312:H1060–H1067. 10.1152/ajpheart.00859.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Koch L.K., and Campbell D.J.. 2014a IFNαR signaling in effector but not regulatory T cells is required for immune dysregulation during type I IFN-dependent inflammatory disease. J. Immunol. 193:2733–2742. 10.4049/jimmunol.1401039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Koch M.A., Pepper M., and Campbell D.J.. 2014b Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection. J. Exp. Med. 211:961–974. 10.1084/jem.20131556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G.R., and Darnell J.E. Jr. 2012. The JAK-STAT pathway at twenty. Immunity. 36:503–514. 10.1016/j.immuni.2012.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg Z., Leung C., Sternberg D., Yu J., and Hojnacki D.. 2014. Disease modifying therapies modulate cardiovascular risk factors in patients with multiple sclerosis. Cardiovasc. Ther. 32:33–39. 10.1111/1755-5922.12049 [DOI] [PubMed] [Google Scholar]
- Stöger J.L., Gijbels M.J., van der Velden S., Manca M., van der Loos C.M., Biessen E.A., Daemen M.J., Lutgens E., and de Winther M.P.. 2012. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 225:461–468. 10.1016/j.atherosclerosis.2012.09.013 [DOI] [PubMed] [Google Scholar]
- Swanson C.L., Wilson T.J., Strauch P., Colonna M., Pelanda R., and Torres R.M.. 2010. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J. Exp. Med. 207:1485–1500. 10.1084/jem.20092695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szelag M., Piaszyk-Borychowska A., Plens-Galaska M., Wesoly J., and Bluyssen H.A.R.. 2016. Targeted inhibition of STATs and IRFs as a potential treatment strategy in cardiovascular disease. Oncotarget. 7:48788–48812. 10.18632/oncotarget.9195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., and Lichtman A.H.. 2017. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity. 47:621–634. 10.1016/j.immuni.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Williams K.J., and Borén J.. 2007. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 116:1832–1844. 10.1161/CIRCULATIONAHA.106.676890 [DOI] [PubMed] [Google Scholar]
- Takauji R., Iho S., Takatsuka H., Yamamoto S., Takahashi T., Kitagawa H., Iwasaki H., Iida R., Yokochi T., and Matsuki T.. 2002. CpG-DNA-induced IFN-α production involves p38 MAPK-dependent STAT1 phosphorylation in human plasmacytoid dendritic cell precursors. J. Leukoc. Biol. 72:1011–1019. [PubMed] [Google Scholar]
- Tanaka M., Fukui M., Tomiyasu K., Akabame S., Nakano K., Yamasaki M., Hasegawa G., Oda Y., and Nakamura N.. 2012. Eosinophil count is positively correlated with coronary artery calcification. Hypertens. Res. 35:325–328. 10.1038/hr.2011.191 [DOI] [PubMed] [Google Scholar]
- Teixeira V., and Tam L.-S.. 2018. Novel Insights in Systemic Lupus Erythematosus and Atherosclerosis. Front. Med. (Lausanne). 4:262 10.3389/fmed.2017.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen P.F., Boshuizen M.C., Hollander M.R., Biesbroek P.S., van der Hoeven N.W., Mol J.Q., Gijbels M.J., van der Velden S., van der Pouw Kraan T.C., Horrevoets A.J., et al. 2015. MAb therapy against the IFN-α/β receptor subunit 1 stimulates arteriogenesis in a murine hindlimb ischaemia model without enhancing atherosclerotic burden. Cardiovasc. Res. 107:255–266. 10.1093/cvr/cvv138 [DOI] [PubMed] [Google Scholar]
- Thacker S.G., Duquaine D., Park J., and Kaplan M.J.. 2010. Lupus-prone New Zealand Black/New Zealand White F1 mice display endothelial dysfunction and abnormal phenotype and function of endothelial progenitor cells. Lupus. 19:288–299. 10.1177/0961203309353773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker S.G., Zhao W., Smith C.K., Luo W., Wang H., Vivekanandan-Giri A., Rabquer B.J., Koch A.E., Pennathur S., Davidson A., et al. 2012. Type I interferons modulate vascular function, repair, thrombosis, and plaque progression in murine models of lupus and atherosclerosis. Arthritis Rheum. 64:2975–2985. 10.1002/art.34504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. 2010. Type I interferon: friend or foe? J. Exp. Med. 207:2053–2063. 10.1084/jem.20101664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tselios K., Gladman D.D., Su J., and Urowitz M.B.. 2016. Does Renin-Angiotensin System Blockade Protect Lupus Nephritis Patients From Atherosclerotic Cardiovascular Events? A Case-Control Study. Arthritis Care Res. (Hoboken). 68:1497–1504. 10.1002/acr.22857 [DOI] [PubMed] [Google Scholar]
- Tsiantoulas D., Diehl C.J., Witztum J.L., and Binder C.J.. 2014. B cells and humoral immunity in atherosclerosis. Circ. Res. 114:1743–1756. 10.1161/CIRCRESAHA.113.301145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiantoulas D., Sage A.P., Mallat Z., and Binder C.J.. 2015. Targeting B cells in atherosclerosis: closing the gap from bench to bedside. Arterioscler. Thromb. Vasc. Biol. 35:296–302. 10.1161/ATVBAHA.114.303569 [DOI] [PubMed] [Google Scholar]
- Tydén H., Lood C., Gullstrand B., Nielsen C.T., Heegaard N.H.H., Kahn R., Jönsen A., and Bengtsson A.A.. 2017. Endothelial dysfunction is associated with activation of the type I interferon system and platelets in patients with systemic lupus erythematosus. RMD Open. 3:e000508 10.1136/rmdopen-2017-000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher T., Fellows K., Horakova D., Zivadinov R., Vaneckova M., Sobisek L., Tyblova M., Seidl Z., Krasensky J., Bergsland N., et al. 2017. Serum lipid profile changes predict neurodegeneration in interferon-β1a-treated multiple sclerosis patients. J. Lipid Res. 58:403–411. 10.1194/jlr.M072751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk R.A., Rijs K., Wezel A., Hamming J.F., Kolodgie F.D., Virmani R., Schaapherder A.F., and Lindeman J.H.N.. 2016. Systematic Evaluation of the Cellular Innate Immune Response During the Process of Human Atherosclerosis. J. Am. Heart Assoc. 5:e002860 10.1161/JAHA.115.002860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen M., Gijbels M.J., Duijvestijn A., Smook M., van de Gaar M.J., Heeringa P., de Winther M.P., and Tervaert J.W.. 2008. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR-/- mice. Arterioscler. Thromb. Vasc. Biol. 28:84–89. 10.1161/ATVBAHA.107.154807 [DOI] [PubMed] [Google Scholar]
- Venkatesh D., Ernandez T., Rosetti F., Batal I., Cullere X., Luscinskas F.W., Zhang Y., Stavrakis G., García-Cardeña G., Horwitz B.H., and Mayadas T.N.. 2013. Endothelial TNF receptor 2 induces IRF1 transcription factor-dependent interferon-β autocrine signaling to promote monocyte recruitment. Immunity. 38:1025–1037. 10.1016/j.immuni.2013.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva E., Yalavarthi S., Berthier C.C., Hodgin J.B., Khandpur R., Lin A.M., Rubin C.J., Zhao W., Olsen S.H., Klinker M., et al. 2011. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187:538–552. 10.4049/jimmunol.1100450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wack A., Terczyńska-Dyla E., and Hartmann R.. 2015. Guarding the frontiers: the biology of type III interferons. Nat. Immunol. 16:802–809. 10.1038/ni.3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell S.J., Popper S.J., Rubins K.H., Griffiths M.J., Brown P.O., Levin M., and Relman D.A.. 2010. Dissecting interferon-induced transcriptional programs in human peripheral blood cells. PLoS One. 5:e9753 10.1371/journal.pone.0009753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M.R., Windsor W.T., Nagabhushan T.L., Lundell D.J., Lunn C.A., Zauodny P.J., and Narula S.K.. 1995. Crystal structure of a complex between interferon-γ and its soluble high-affinity receptor. Nature. 376:230–235. 10.1038/376230a0 [DOI] [PubMed] [Google Scholar]
- Wang J., Cheng X., Xiang M.-X., Alanne-Kinnunen M., Wang J.-A., Chen H., He A., Sun X., Lin Y., Tang T.-T., et al. 2011. IgE stimulates human and mouse arterial cell apoptosis and cytokine expression and promotes atherogenesis in Apoe-/- mice. J. Clin. Invest. 121:3564–3577. 10.1172/JCI46028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Naghavi M., Allen C., Barber R.M., Bhutta Z.A., Carter A., Casey D.C., Charlson F.J., Chen A.Z., Coates M.M., et al. GBD 2015 Mortality and Causes of Death Collaborators . 2016. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 388:1459–1544. 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wang S., Wang Z., Yun T., Wang C., and Wang H.. 2017. Tofacitinib ameliorates atherosclerosis and reduces foam cell formation in apoE deficient mice. Biochem. Biophys. Res. Commun. 490:194–201. 10.1016/j.bbrc.2017.06.020 [DOI] [PubMed] [Google Scholar]
- Ward J.M., Ratliff M.L., Dozmorov M.G., Wiley G., Guthridge J.M., Gaffney P.M., James J.A., and Webb C.F.. 2016. Human effector B lymphocytes express ARID3a and secrete interferon alpha. J. Autoimmun. 75:130–140. 10.1016/j.jaut.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnatsch A., Ioannou M., Wang Q., and Papayannopoulos V.. 2015. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 349:316–320. 10.1126/science.aaa8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White W.I., Seto N.L., Playford M.P., Casey K.A., Smith M.A., Carlucci P., Yu B., Wang L., Illei G., Mehta N., and Kaplan M.J.. 2018. OP0174 Alteration of mediators of vascular inflammation by anifrolumab in the phase iib muse study in sle. Ann. Rheum. Dis. 77:136. [Google Scholar]
- Wigren M., Nilsson J., and Kaplan M.J.. 2015. Pathogenic immunity in systemic lupus erythematosus and atherosclerosis: common mechanisms and possible targets for intervention. J. Intern. Med. 278:494–506. 10.1111/joim.12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.W., Huang L.H., and Randolph G.J.. 2019. Cytokine Circuits in Cardiovascular Disease. Immunity. 50:941–954. 10.1016/j.immuni.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkels H., Ehinger E., Vassallo M., Buscher K., Dinh H.Q., Kobiyama K., Hamers A.A.J., Cochain C., Vafadarnejad E., Saliba A.-E., et al. 2018. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ. Res. 122:1675–1688. 10.1161/CIRCRESAHA.117.312513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witztum J.L., and Lichtman A.H.. 2014. The influence of innate and adaptive immune responses on atherosclerosis. Annu. Rev. Pathol. 9:73–102. 10.1146/annurev-pathol-020712-163936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.-C., Liu H.-R., Leng R.-X., Li X.-P., Li X.-M., Pan H.-F., and Ye D.-Q.. 2016. Subclinical atherosclerosis in patients with systemic lupus erythematosus: A systemic review and meta-analysis. Autoimmun. Rev. 15:22–37. 10.1016/j.autrev.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Yang D.-H., Leong P.-Y., Sia S.-K., Wang Y.-H., and Wei J.C.-C.. 2019. Long-Term Hydroxychloroquine Therapy and Risk of Coronary Artery Disease in Patients with Systemic Lupus Erythematosus. J. Clin. Med. 8:796 10.3390/jcm8060796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarilina A., Park-Min K.-H., Antoniv T., Hu X., and Ivashkiv L.B.. 2008. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat. Immunol. 9:378–387. 10.1038/ni1576 [DOI] [PubMed] [Google Scholar]
- Yun T.J., Lee J.S., Machmach K., Shim D., Choi J., Wi Y.J., Jang H.S., Jung I.H., Kim K., Yoon W.K., et al. 2016. Indoleamine 2,3-Dioxygenase-Expressing Aortic Plasmacytoid Dendritic Cells Protect against Atherosclerosis by Induction of Regulatory T Cells. Cell Metab. 23:852–866. 10.1016/j.cmet.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Zernecke A., Bot I., Djalali-Talab Y., Shagdarsuren E., Bidzhekov K., Meiler S., Krohn R., Schober A., Sperandio M., Soehnlein O., et al. 2008. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 102:209–217. 10.1161/CIRCRESAHA.107.160697 [DOI] [PubMed] [Google Scholar]
- Zhang H., and Reilly M.P.. 2018. Who Done It? Macrophage Mayhem in Atherosclerosis. Circ. Res. 123:1106–1108. 10.1161/CIRCRESAHA.118.314006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G.-N., Jiang D.-S., and Li H.. 2015. Interferon regulatory factors: at the crossroads of immunity, metabolism, and disease. Biochim. Biophys. Acta. 1852:365–378. 10.1016/j.bbadis.2014.04.030 [DOI] [PubMed] [Google Scholar]
- Pfizer. 2019. Pfizer Announces Modification to Ongoing Tofacitinib FDA Post-Marketing Requirement Study in Patients with Rheumatoid Arthritis. Pfizer Press Release. Available at: https://investors.pfizer.com/investor-news/press-release-details/2019/Pfizer-Announces-Modification-to-Ongoing-Tofacitnib-FDA-Post-Marketing-Requirement-Study-in-Patients-with-Rheumatoid-Arthritis/default.aspx (accessed October 3, 2019).