Abstract
Cannabis use disorder (CUD) is prevalent and demand for treatment is increasing, yet few individuals engage in formal treatment and the efficacy of established interventions for CUD is modest. Existing clinical trials evaluating psychosocial and pharmacological treatments for CUD have incorporated a wide variety of measures for assessing cannabis use outcomes, including abstinence, self-reported frequency and quantity used, withdrawal, use/dependence severity, and other psychosocial outcomes. The heterogeneity of measures and outcomes has limited quantitative analyses of the comparative effectiveness of existing interventions. The purpose of this systematic review is to: 1) identify and characterize approaches for measuring cannabis use in existing CUD intervention trials, including abstinence, frequency and quantity of use, and 2) summarize measures used to assess treatment efficacy in other outcome domains (e.g., cannabis use severity, psychosocial functioning, cannabis withdrawal), and provide a platform for future research to evaluate which outcome measures are most likely to reflect treatment efficacy and clinically significant improvement in other outcome domains.
Keywords: Cannabis use disorder, Dependence, Abuse, Treatment, Randomized controlled trial, Outcomes
1. Introduction
Cannabis is the most widely-used internationally regulated drug worldwide (Degenhardt and Hall, 2012; United Nations Office on Drugs and Crime (UNODC, 2017). In the United States (US) in 2016, approximately 8.9% of individuals aged 12 or older reported past month use of cannabis (SAMHSA, 2017a, b). A growing body of evidence has documented a range of adverse consequences of acute and chronic cannabis use, including emergence of cannabis use disorder (CUD; Broyd et al., 2016; Crane et al., 2013; Hall, 2015; Macleod et al., 2004; Volkow et al., 2016). The public health burden of CUD is substantial; an estimated 1.5% of US citizens 12 or older (four million individuals) have a current (past year) CUD, and cannabis was the primary substance reported in 14% of substance use disorder (SUD) treatment admissions in 2015 (third behind opioids and alcohol; SAMHSA, 2017a, b). Evidence-based psychosocial treatments have been developed for CUD, but most individuals that formally enter treatment relapse within a year (Budney et al., 2007; Gates et al., 2016). To date, no pharmacotherapies have been approved for CUD (Brezing and Levin, 2018; Marshall et al., 2014; Sherman and McRae-Clark, 2016). Taken together, these findings underscore the clear need for a continued focus on improving treatments for CUD, which includes determining the criteria of success, optimal study methods, and outcome measures used to evaluate efficacy in clinical trials.
Consideration of clinically meaningful outcome measures is an integral step in the development of psychosocial and pharmacological treatments for CUD. Substantial progress has been made in establishing consensus for clinical endpoints in other substances of abuse, namely tobacco and alcohol. Efficacy endpoints for tobacco trials are based on the extent to which an intervention results in prolonged abstinence from cigarette smoking, since risks are largely related to the long-term adverse health effects of smoking and no known level of smoking is considered safe (Hughes et al., 2003). Abstinence is also considered a primary clinical endpoint for alcohol. Unlike tobacco, however, heavy alcohol use is associated with a myriad of adverse short-term consequences, including negative health-related effects, legal problems (e.g., driving while intoxicated), and deterioration in psychosocial functioning. Conversely, moderate levels of alcohol consumption are not strongly associated with adverse short-term consequences and may even confer minor beneficial health-related effects. As such, the outcome of reduced alcohol use, percentage of subjects with no heavy drinking days (PSNHDD), defined as days in which at least four or five drinks per day are consumed by women and men, respectively, was endorsed by the U.S. Food and Drug Administration (FDA) as a meaningful clinical endpoint in medications development trials for alcohol use disorder (Sobell et al., 2003). This outcome was subsequently validated in studies demonstrating that elimination of heavy drinking days resulted in beneficial effects on short- and long-term psychosocial functioning (e.g., Falk et al., 2010; Kline-Simon et al., 2013, 2017).
Consensus agreement on substance use outcomes has not been established for substances other than tobacco and alcohol. Multiple expert panels have convened in recent years with the aim of determining clinically meaningful outcomes in clinical trials for SUDs (Donovan et al., 2012; Kiluk et al., 2016; Tiffany et al., 2012). Across panels, discussions highlighted numerous challenges encountered when attempting to define and measure an indicator of clinically significant change in substance use both during and following treatment. A key challenge to establishing efficacious SUD interventions is the heterogeneity with which substance use outcomes are measured and reported, and inclusion (or lack thereof) of other relevant outcome domains (e.g., self-efficacy, craving, psychosocial functioning, quality of life, social network and social supports; Donovan et al., 2012; Tiffany et al., 2012). Trials evaluating interventions for SUDs have incorporated a wide variety of outcomes to measure change in substance use, including use of multiple dichotomous and continuous measures of abstinence, and reduction in frequency and quantity of use. Both self-report assessments and biological matrices have been utilized to evaluate change in use, either as primary indicators or to confirm self-reported use. Many of these challenges in measurement of clinically relevant outcomes are relevant to CUD trials. For example, measuring self-reported cannabis use presents several challenges, particularly with obtaining accurate measurements of use across wide variations in potency and route of administration (Cuttler and Spradlin, 2017; Gray et al., 2009). In addition, the long half-life of cannabis limits the sensitivity of biochemical matrices to detect short durations of abstinence, and reduction in use.
Obtaining accurate measures of cannabis use frequency and quantity is important since both are independently associated with cannabis-related problems (Chen et al., 1997; Coffey et al., 2002; Norberg et al., 2012; c.f. Buu et al., 2017) and may differentially inform treatment efficacy (Brezing et al., 2018). While abstinence is the most common primary outcome for SUD clinical trials, reduction in use may be a meaningful outcome if the reduction results in a clinically significant improvement in functioning. Over the past decade, declining perceptions of risks and harms of cannabis use (Compton et al., 2016; Pacek et al., 2015), and increasing acceptance of legalized cannabis for medical and recreational use in the U.S. and Canada has increased efforts to determine guidelines for “low risk” use to guide prevention and treatment efforts (Fischer et al., 2017), though evidence to inform a specific level of low risk use has yet to be determined.
Consideration of other outcome domains that might be sensitive to an overall reduction in negative physical or psychosocial symptoms of cannabis use is an integral step in evaluating optimal outcomes of abstinence and reduction in use. Though outcome domains have not been standardized in CUD treatment research, one previous study identified several domains integral to determining clinically significant improvement, including severity of cannabis use, and psychosocial functioning (Peters et al., 2011). In addition, relief of cannabis withdrawal-related symptoms has been a primary focus of multiple trials evaluating pharmacotherapies for CUD and might be an important indicator for development of efficacious medications and psychoeducation on withdrawal and craving for CUD interventions (Brezing and Levin, 2018).
The purpose of this systematic review is to provide a comprehensive overview of measures and outcomes for cannabis use and other domains reported in existing psychosocial and pharmacological treatments for CUD. This review will primarily focus on characterizing the frequency and methodology with which self-report assessments and biological matrices are used to assess cannabis abstinence and reduction in frequency and quantity of use both within and following treatment completion. Key endpoints for other clinically relevant domains (e.g., cannabis withdrawal and craving, severity of use and dependence, psychosocial functioning) will also be identified and summarized. The overall goal of this review is to provide an overview of the current state of cannabis use outcome measurements used in CUD treatment trials, discuss the strengths and weaknesses of each approach, and provide a platform for subsequent discussions directed at establishing a research agenda to standardize outcomes across CUD intervention trials.
2. Method
2.1. Identification of trials
The following electronic databases were searched in September 2017 for published randomized controlled trials for CUD, inclusive of all date ranges up to the date of search:
PubMed, Embase, Cochrane Central, Cochrane Reviews, Cochrane Other Reviews, CINAHL, and PsycINFO. A search strategy was first developed in PubMed by a medical librarian and subsequently adapted for the other databases (see Supplementary Table 1 for the PubMed search strategy). The search strategy included keywords and MeSH terms to specify the population (e.g., cannabis, marijuana smoking, marijuana abuse), interventions (e.g., psychotherapy, psychosocial, pharmacotherapy, substance withdrawal syndrome) and trial design characteristics (e.g., randomized controlled trial, controlled clinical trial, placebo, groups) relevant to the current review. Reference lists for eligible trials were also reviewed in addition to recently published systematic reviews evaluating psychosocial and pharmacological interventions for CUD (i.e., Brezing and Levin, 2018; Cooper et al., 2015; Gates et al., 2016; Marshall et al., 2014; Sherman and McRae-Clark, 2016).
2.2. Inclusion/exclusion criteria
The PICOS (Population, Intervention, Comparison, Outcome, Setting) process was utilized to identify specific inclusion/exclusion criteria with the overall aim of maximizing inclusion of all relevant clinical trials. To be included, study samples in each identified trial were required to comprise current (i.e., past 30-day) adolescent or adult cannabis users who (a) were seeking treatment for cannabis use or reported a desire to reduce or quit use, and/or (b) met DSM-IV, 5, or ICD-10 diagnostic criteria for cannabis abuse, dependence, or CUD. These criteria were selected to include trials in clinical populations with substantial cannabis-related problems who would be most likely to benefit from receiving treatment to reduce or quit use, while excluding trials evaluating brief prevention/early interventions and laboratory studies in non-treatment seeking opportunistic samples.
2.2.1. Intervention
All psychosocial and pharmacological interventions for CUD were considered for inclusion. Examples include: cognitive behavioral therapy, motivational interviewing/motivational enhancement therapy, drug counseling and/or education, contingency management, mindfulness-based meditation, relapse prevention, technology/telephone-assisted interventions, and pharmacological treatments targeting reduction/cessation of cannabis use and/or reducing symptoms of cannabis withdrawal.
2.2.2. Comparison
Trials that included any comparison condition(s) were considered for inclusion (e.g., no/minimal treatment, delayed treatment, placebo control, any alternative psychosocial or pharmacological intervention).
2.2.3. Outcomes
Outcome measures of cannabis use were the primary focus of the review, and trials were required to have at least one primary or secondary outcome assessment of cannabis abstinence or reduction to be included. Acceptable cannabis use outcomes included biologically-verified cannabis abstinence (e.g., negative urinalyses) and/or self-report measures of cannabis abstinence, frequency or quantity. Other outcome domains, including assessments of cannabis use severity, cannabis withdrawal, psychosocial functioning, self-efficacy, and use of other substances (self-report and observer-rated) were extracted for synthesis and compilation; however, no inclusion/exclusion criteria were specified for these outcomes.
2.2.4. Other inclusion/exclusion criteria
All trials were required to be published in English in a peer-reviewed journal. Articles published as editorials, letters, case studies/series, commentaries, or conference abstracts/proceedings were excluded. Trials were also excluded if: (a) they examined cannabis/cannabinoids as therapeutic agents for any physical, mental, or substance use disorder other than cannabis, or (b) evaluated an intervention designed to treat a mental and/or substance use disorder with no specific intervention tailored for cannabis use.
2.3. Data extraction
Titles and abstracts of citations identified by the search strategy were screened and critically appraised by two independent assessors (DL and NS). Full texts for potentially eligible trials were independently assessed by both reviewers. Each reviewer completed data extraction for eligible trials included in the review, and a second reviewer checked all data extracted for accuracy. Points of disagreement were discussed until consensus was reached at each step of the identification/data extraction process. Data extracted for each trial included trial design and sample characteristics (i.e., intervention type and length, sample size and targeted age, number and duration of follow-up assessments, and use of cannabis and other substance use at baseline). For each outcome, data extracted by reviewers included details on declaration as a primary/secondary outcome, outcome domain, type and details of outcome measure (including unit of measurement and method of aggregation), time-point for assessing the reported outcome (i.e., within/post-treatment), and methods of analysis. For biological outcomes, each measure was reviewed and classified as either a primary/secondary outcome, or confirmation of self-reported use. Additional information was extracted to provide data on the level of concordance between self-reported outcomes and biological confirmation outcomes. All selection of trials and data extraction occurred in Covidence Systematic Review Software and Microsoft Excel.
2.4. Data synthesis
The primary purpose of the review was to compile and provide a narrative synthesis of a comprehensive list of instruments and outcome measures used to assess cannabis use and other outcome domains in psychosocial and pharmacological interventions for CUD, including self-report and biological matrices. Measures of cannabis use were classified by domain (i.e., abstinence or reduction in frequency/quantity) and variable type (e.g., dichotomous or continuous), then compiled based upon classification as a primary or secondary outcome and intervention type (psychosocial and pharmacological) to provide an assessment of the frequency of each outcome measure. Outcome measures in other domains were compiled in a similar manner to provide an assessment of the prevalence of trials that included measures in each domain. Each section also included a summary of instruments, and where possible, comparisons were made between psychosocial and pharmacological interventions for common outcomes to highlight differences between each trial type.
3. Results
3.1. Search results
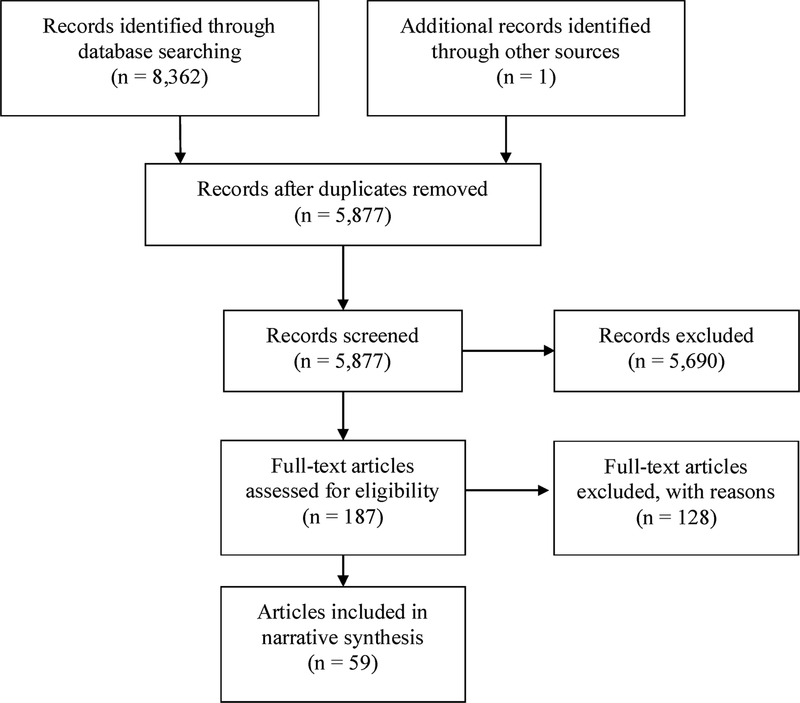
Fig. 1 displays the results of the search strategy used to identify relevant articles as recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The search strategy yielded 5877 unique records from which 59 reports, relating to 58 different randomized controlled trials, were included in the final narrative synthesis. Out of 187 full-text studies assessed for eligibility, 128 were excluded from the final review. The primary reasons for exclusion included: wrong population (i.e., not treatment seeking, no CUD; n = 68), wrong study type (i.e., pilot, single arm trial; n = 39), and wrong intervention (i.e., not focused on cannabis; n = 16).
Fig. 1.
PRISMA Flow Diagram
3.2. Description of trials
Characteristics of included trials are presented in Table 1. The 58 trials included in the review comprise 36 psychosocial interventions (PSY) and 22 pharmacological interventions (PHA). Participants were adults in 72% of PSY trials (n = 26), and 86% of PHA trials (n = 19). Participants were adolescents in 25% of PSY trials (n = 9), and both adults and adolescents were included in four trials (1 PSY, 3 PHA).
Table 1.
Characteristics of Included Trials.
| Trial and Intervention | N | Age | Cannabis use Inclusion Criteria | Follow-Up | Outcome |
|---|---|---|---|---|---|
| Adult Trials (PSY) | |||||
|
Barrowclough et al., 2014 (1) Brief or (2) Long MI-CBT |
110 | Adults | DSM-IV abuse or dependence; used cannabis at least 1 day per week in at least half the weeks in prior 3 months | 3 FU: 4.5, 9, 18 months post-randomization |
Cannabis Use Abstinence (TLFB) Quantity (TLFB) Confirmation of SR Use (Hair) Other BAI, CDS, GAF, PANSS, RTC, TLFB (other substances) |
|
Bonsack et al., 2011 (1) Motivational Intervention + TAU (2) TAU |
62 | Adults | Smoked at least 3 joints of cannabis/week in month preceding inclusion | 3 FU: 3, 6, 12 months post-randomization |
Cannabis Use Abstinence (TLFB) Frequency (TLFB, CASUAS) Quantity (TLFB) Other CASUAS (index of severity), PANSS, RTC, SOFAS |
|
Budney et al., 2000 (1) Motivational Enhancement (ME) (2) ME + behavioral coping skills (MBT) (3) MBT + voucher-based incentives (MBTV) |
60 | Adults | DSM-III-R dependence; used cannabis in past 30 days | ETX at 14 wks post-baseline |
Cannabis Use Abstinence (UA, TLFB + UA) Frequency (TLFB) Confirmation of SR Use (UA) Other ASI, BDI, BSI, MPS, SCQ, URICA |
|
Budney et al., 2006 (1) CBT + vouchers (2) CBT alone (3) Vouchers alone |
90 | Adults | DSM-IV dependence; used cannabis in past 30 days | 4 FU: 3, 6, 9, 12 months post-treatment |
Cannabis Use Abstinence (UA) Frequency (TLFB) Other ASI, BDI, BSI, MPS, Remission |
|
Budney et al., 2011 (1) Computer-delivered MET/CBT/CM (2) Therapist delivered MET/CBT/CM |
38 | Adults | DSM-IV abuse or dependence; report cannabis use on at least 50 of past 90 days | ETX at 12 wks post-baseline |
Cannabis Use Abstinence (UA) Frequency (TLFB) Other CSS, MPS, Self-Efficacy |
|
Budney et al., 2015 (1) Computer-delivered MET/CBT/CM (2) Therapist delivered MET/CBT/CM (3) MET only |
75 | Adults | DSM-IV abuse or dependence; report cannabis use on at least 50 of past 90 days | 2 FU: 3 and 9 months post-treatment |
Cannabis Use Abstinence (UA) Frequency (TLFB) Other CSS, MPS, Self-Efficacy |
|
Carroll et al., 2006 (1) MET/CBT + CM (2) MET/CBT (3) Drug Counseling + CM (4) Drug Counseling |
132 | Adults | DSM-IV dependence; cannabis-positive urine specimen at baseline | 2 FU: 3 and 12 months post-treatment |
Cannabis Use Abstinence (UA) Frequency (TLFB) Confirmation of SR Use (UA) Other ASI, Retention |
|
Carroll et al., 2012 (1) CM for abstinence (2) CBT + CM for abstinence (3) CBT (4) CBT + CM for CBT session attendance/homework completion |
127 | Adults | DSM-IV dependence | 4 FU: 3, 6, 9, 12 months post-treatment |
Cannabis Use Abstinence (UA + TLFB) Frequency (TLFB) Confirmation of SR Use (UA) Other N/A |
|
Copeland et al., 2001 (1) 6-session CBT (6CBT) (2) 1-session CBT (1CBT) |
229 | Adults | Expressed a desire to cease cannabis use | 1 FU: 24 weeks post-treatment |
Cannabis Use Abstinence (TLFB) Quantity (OTI): Confirmation of SR Use (UA) Other CPQ, SCL-90-R, SDS |
|
Copeland et al., 2017 (1) Brief or (2) extended feedback versions of “Grassessment” (a brief online intervention for cannabis users) |
287 | Adults | At least one symptom of DSM-IV cannabis abuse or dependence | 1 FU: 24 weeks posttreatment |
Cannabis Use Frequency (TLFB) Quantity (TLFB) Other SDS |
|
de Dios et al., 2012 (1) Motivational interviewing (MI) + Mindfulness meditation (MM) (2) Assessments only |
34 | Adults (all female) | Smoked cannabis at least three times in the past month; endorsed a desire to quit or reduce cannabis use; used cannabis to relax, relieve anxiety, or calm down | 3 FU: 1, 2, 3 months post-treatment |
Cannabis Use Abstinence (TLFB + UA) Frequency (TLFB) Confirmation of SR Use (UA) Other N/A |
|
Gates et al., 2012 (1) MI + CBT via phone (2) Delayed treatment control (DTC) |
160 | Adults | Use of cannabis in past month; not receiving other treatment for cannabis use | 2 FU; 4 and 12 weeks post-treatment |
Cannabis Use Abstinence (TLFB) Frequency (TLFB) Quantity (TLFB) Clinically significant improvement in cannabis use Other CPQ, TLFB (other drug use), SDS |
|
Hjorthoj et al., 2013 (1) CapOpus (MI + CBT) + TAU (2) TAU |
103 | Adults | CUD; cannabis was “primary substance of abuse” | 1 FU: 4 months post-treatment |
Cannabis Use Frequency (TLFB) Quantity (TLFB) Other Cognitive Battery, CSQ, EQ-5D, GAF, MANSA, PANSS, WHODAS |
|
Hoch et al., 2014 (1) CANDIS (MET + CBT + PPS) (2) DTC |
279 | Adults | Use of any cannabis product at least twice a week in past month; motivation to quit or reduce cannabis consumption | 2 FU; 3 and 6 months post-treatment |
Cannabis Use Abstinence (TLFB) Quantity (TLFB): Confirmation of SR Use (UA) Other CIDI, CPQ, CUPIT, SDS, TLFB, Retention |
|
Jungerman et al., 2007 (1) 4 weekly motivational interviewing and relapse prevention sessions over 1 month (1MIRP) (2) Same as above but over 3 months (3MIRP) |
160 | Adults | Smoked cannabis at least 40 times in 90 days prior to interview | 1 FU: 4 months post-randomization |
Cannabis Use Abstinence (TLFB) Frequency (TLFB) Quantity(TLFB) Confirmation of SR Use (UA) Other ASI, DSM Checklist, MPS, TLFB (other drug use) |
|
Kadden et al., 2007 (1) MET/CBT + CM (2) MET/CBT (3) CM |
240 | Adults | DSM-IV dependence | 4 FU; 3, 6, 9, 12 months post-treatment |
Cannabis Use Abstinence (TLFB) Quantity (TLFB) Other: Time to relapse Confirmation of SR Use (UA) Other ASI, MPS |
|
Litt et al., 2013 (1) MET/CBT + CM for treatment homework (2) MET/CBT + CM for abstinence |
215 | Adults | DSM-IV dependence or abuse | 4 FU; 3, 6, 9, 12 months post-treatment |
Cannabis Use Abstinence (TLFB/Form-90) Confirmation of SR Use (UA) Other CSS, MPS, Marijuana Self-Efficacy Questionnaire, RCTQ, Retention |
|
Madigan et al., 2013 (1) Group Psychological Intervention (GPI; MI + CBT) (2) TAU |
88 | Adults | Cannabis dependence | 2 FU; 3, 12 months post-treatment |
Cannabis Use Frequency (ASI) Other Birchwood Insight Scale, CDSS, DAI-30, DUP, GAF, SAPS/SANS, WHOQOL-BREF |
|
MTRPG, 2004 (1) MET (2) Multicomponent Treatment (CBT + Case Management + MET) |
450 | Adults | DSM-IV dependence; used cannabis on at least 40 of the past 90 days. | 3 FU: 4, 9, and 15 months post-randomization |
Cannabis Use Abstinence (TLFB) Frequency (TLFB) Quantity (TLFB): Confirmation of SR Use (UA + collateral reports) Other ASI, BDI, MPS, SCID, STAI, TLFB (alcohol use) |
|
Roffman et al., 1988 (1) Relapse Prevention (RP) (2) Social Support (SSP) |
110 | Adults | Used cannabis at least 50 times in past 90 days | 5 FU: 1, 3, 6, 9, 12 months post-treatment |
Cannabis Use Frequency (Calendar) Other |
|
Rooke et al., 2013 (1) Web-based intervention (6-modules) (2) Control condition |
225 | Adults | Used cannabis at least once in past month and desired to stop or reduce use | 2 FU: 6 weeks and 3 months post-baseline |
Cannabis Use Abstinence (TLFB) Frequency (TLFB) Quantity (TLFB) Other GAIN-I, SDS |
|
Sinha et al., 2003 (1) MET (2) MET + CM |
65 | Adults | All met criteria for CUD (25% abuse, 75% dependence) and provided THC-positive urine at baseline | 1 FU: 1 month post-treatment |
Cannabis Use Frequency (Calendar) Confirmation of SR Use (UA) Other ASI, SOCRATES, Treatment Engagement |
|
Stephens et al., 1994 (1) Relapse Prevention (RP) (2) Social Support (SSP) |
212 | Adults | Use of cannabis at least 50/past 90 days | 5 FU: 1, 3, 6, 9, 12 months post-treatment |
Cannabis Use Abstinence (Calendar) Frequency (Calendar) Confirmation of SR Use (UA + collateral reports) Other Calendar (other substance use), DAST |
|
Stephens et al., 2000 (1) Relapse Prevention Support Group (RPSG) (2) Individualized assessment and Intervention (IAI) |
291 | Adults | Use of cannabis at least 50 times/past 90 days | 5 FU: 1, 4, 7, 13, 16 months post-treatment |
Cannabis Use Abstinence (Calendar) Frequency (Calendar) Verification of SR Use (Collateral Reports) Other Calendar (other substance use), Drug-Related Problems, MDS |
|
Tossmann et al., 2011 Web-based intervention (counseling program, QTS) |
1292 | Adults | Looking to quit or reduce cannabis use within the next weeks | 1 FU: 3 months post-randomization |
Cannabis Use Frequency (SR) Quantity (SR) Other DTCQ, GDS, STAI |
|
Walker et al., 2015 9MET/CBT + Maintenance Check-Ups (MCU) |
74 | Adults | Cannabis dependence; use of cannabis on 50 or more/past 90 days | 2 FU: 3 and 9 months post-treatment |
Cannabis Use Frequency (TLFB) Confirmation of SR Use (UA) Other MPS, SCID-I, Self-Efficacy for Avoiding Cannabis, SWLS |
| Adolescent Trials (PSY) | |||||
|
Dennis et al., 2004 Trial 1: MET/CBT 5 sessions vs. MET/CBT 12 sessions vs. Family Support Network Trial 2: MET/CBT 5 sessions vs. ACRA vs. MDFT |
600 | Adolescents | Self-report one or more DSM-IV criteria for abuse or dependence | 4 FU; 3, 6, 9, 12 months post-treatment |
Cannabis Use Abstinence (GAIN) Confirmation of SR Use (UA + Collateral Reports) Other % of adolescents in recovery |
|
Hendriks et al., 2011 (1) MDFT (2) CBT (TAU) |
109 | Adolescents | DSM-IV CUD, use for at least 26 of past 90 days | 4 FU; 3, 6, 9, 12 months post-baseline |
Cannabis Use Frequency (TLFB) Quantity (TLFB) Confirmation of SR Use (UA) Other Treatment Responders (% of participants), In Recovery (% of participants), Self-Report Delinquency Scale, Treatment Retention |
|
Hoch et al., 2012 CANDIS (MET + CBT + PPS) |
122 | Adolescents & Adults (Age 16+) | Use of any cannabis product at least twice a week in past month; DSM-IV criteria for lifetime abuse or dependence | 2 FU; 3, 6 months post-treatment |
Cannabis Use Abstinence (TLFB) Frequency (TLFB) Confirmation of SR Use (UA) Other ASI, BSI, CIDI-I |
|
Kaminer et al., 2014 Voucher-based reinforcement therapy (VBRT) for abstinence + CBT |
59 | Adolescents | DSM-IV abuse or dependence, positive drug test for THC | 1 FU: 3 months post-treatment |
Cannabis Use Abstinence (TLFB) Confirmation of SR Use (UA) Other CRI-Y, SCQ-39 |
|
Kaminer et al., 2017 Phase 1: MET/CBT-7 Phase 2: (1) Individualized enhanced CBT (2) ACRA |
161 | Adolescents | DSM-IV CUD | 5 FU: over 1 year post-phase 2 |
Cannabis Use Abstinence (TLFB) Confirmation of SR Use (UA) Other Attendance at Week 17 (%) |
|
Killeen et al., 2012 Abstinence-based CM + TAU |
31 | Adolescents | Primary CUD, past 45 day cannabis use | 1 FU: 3 months |
Cannabis Use Abstinence (UA) Other BIS, MCQ, Retention |
|
Lascaux et al., 2016 (1) TAU (formalized) (2) TAU |
73 | Adolescents | DSM-IV CUD | 4 FU; 3, 6, 9, 12 months post-randomization |
Cannabis Use Frequency (TLFB) Other ADI-Light, CBCL + YSR, FES, SS |
|
Rigter et al., 2013 MDFT |
450 | Adolescents | DSM-IV CUD | 4 FU: 3, 6, 9, 12 months post-randomization |
Cannabis Use Frequency (TLFB) Other ADI-Light, Therapy Completion, Treatment Completion |
|
Stanger et al., 2009 (1) MET/CBT + CM for abstinence or abstinence-based parent training |
69 | Adolescents | Reported use of cannabis/past 30 days or THC-positive urine test | 3 FU: 3, 6, 9 months post-treatment |
Cannabis Use Abstinence (TLFB/Parent Report) Confirmation of SR Use (UA) Other APQ, CBCL + YSR, VSDI |
|
Stanger et al., 2015 (1) MET/CBT + CM (2) MET/CBT + CM + Parent Training |
153 | Adolescents | DSM-IV CUD | 4 FU: 3, 6, 9, 12 months post-treatment |
Cannabis Use Abstinence (UA) Frequency (TLFB) Other APQ, CBCL |
| Adult Trials (PHA) | |||||
|
Allsop et al., 2014 Nabiximols (maximum daily dose: 86.4mg THC, 80 mg CBD) |
51 | Adults | Met DSM-IV criteria for dependence; experienced withdrawal during previous quit attempts; desired to reduce or quit cannabis use | 9-day inpatient period and 1 FU 28 days after discharge |
Cannabis Use Abstinence (TLFB) Quantity (TLFB) Confirmation of SR Use (UA) Other CPQ, CWS, SDS, Adverse Events, Retention |
|
Brunette et al., 2011 Clozapine (400 mg/day) |
31 | Adults | Diagnosis of current CUD; cannabis use on at least 5 days over 3 wks prior to screening | none |
Cannabis Use Quantity (TLFB) Confirmation of SR Use (UA) Other BPRS, CGI, SANS, TLFB (other substance use) |
|
Carpenter et al., 2009 (1) Bupropion (150 mg bid) (2) Nefazodone (300 mg bid) |
106 | Adults | Met DSM-IV criteria for current dependence; smoked > 5 joints per week | none |
Cannabis Use Abstinence (SR + UA) Frequency (SR): # of days used Quantity (SR) Other Adherence, CGI, HAM-A, Side Effects, SMHSQ, Snaith |
|
Gray et al., 2017 N-Acetylcysteine (1200 mg bid) |
302 | Adults | Met DSM-IV criteria for dependence; positive UCT at screen | 1 FU: 4 wks post-treatment |
Cannabis Use Abstinence (UA) Frequency (TLFB) Other Safety/Tolerability |
|
Hill et al., 2017 Nabilone (0.5 mg/day for first 7 days; increased to 1 mg/day for 7 days; increased to 2mg/day for 4 weeks; reverse titration for final 3 weeks) |
18 | Adults | Met DSM-IV criteria for dependence | FU at weeks 11–14 after completion of 10 wk treatment phase |
Cannabis Use Abstinence (UA) Frequency (TLFB/Daily Diaries) Quantity (TLFB/Daily Diaries) Change in urine cannabinoid levels (UA) Other BAI, MCQ, QIDS, Adverse Events |
|
Johnston et al., 2014 Lithium Carbonate (500 mg bid) |
38 | Adults | Met DSM-IV criteria for dependence; self-reported withdrawal symptoms as a barrier to achieving abstinence from cannabis in previous quit attempts | 3 FU: 14, 30, and 90 days post-discharge |
Cannabis Use Abstinence (SR) Frequency (SR) Quantity (SR) Confirmation of SR Use (UA) Cannabinoid Levels: Plasma cannabinoid levels measured at baseline and days 2, 4, and 7 Other CPQ, CWS, DASS-21, SDS, SF-12, WHOQOL-BREF |
|
Levin et al., 2004 Divalproex sodium (250 mg bid for 8 days; raised to 500 mg bid for 6 days; raised to single daily dose of 1500 mg for 4 wks); max dose 2000 mg for pts with blood levels below 50 ng/ml |
25 | Adults | Met DSM-IV criteria for dependence; use of at least 5 joints per week in the month prior to enrollment | 6 wk cross-over phase to assess effectiveness (following 6 wks of medical/ placebo intervention) |
Cannabis Use Abstinence (SR + UA) Frequency (SR) Quantity (SR) Confirmation of SR Use (UA) Other: Weekly clinician-rated global impression assessment for marijuana use Other ASI, HSC, Snaith, VAS |
|
Levin et al., 2011 Dronabinol (10 mg/day, increased to 20 mg bid; gradual dose tapering at wks 9–10, placebo at wks 11–12) |
156 | Adults | Met DSM-IV criteria for dependence; report using cannabis at least 5 days/week during past 28 days; THC-positive urine screen at study entry | None |
Cannabis Use Abstinence (SR) Frequency (SR) Quantity (SR) Confirmation of SR Use (UA) Other MWC, Medication Adherence, SAFTEE, Retention |
|
Levin et al., 2013 Venlafaxine-XR (titrated to target dose of 225 mg daily; max dose of 375 mg daily if well tolerated) |
103 | Adults | Met DSM-IV criteria for dependence; report that cannabis was primary drug of abuse | None |
Cannabis Use Abstinence (UA + TLFB) Change in urine cannabinoid levels (UA) Confirmation of SR Use (UA) Other HAMD, SAFTEE, Treatment Compliance |
|
Levin et al., 2016 Lofexidine-dronabinol combination (Lofex was titrated in 0.2 mg increments, Dron was given in 10 mg increments until reaching max tolerated dose): Dron, 20 mg 3 times/day, Lofex, 0.6 mg 3 times/day |
122 | Adults | Met DSM-IV criteria for dependence; cannabis use at least 5 days/wk; THC-positive urine at study entry | None |
Cannabis Use Abstinence (TLFB) Frequency (TLFB) Other MWC, Time-to-Dropout |
|
Mason et al., 2012 Gabapentin (1200 mg/day; titrated by increasing by 300 mg/day until max dose reached; dosing schedule: 300 mg morning and midday, 600 mg evening) |
50 | Adults | Met DSM-IV criteria for dependence; seeking research-based outpatient treatment for cannabis dependence involving daily medication; smoked cannabis at least once in week prior to randomization | 1 FU: 1 wk after treatment completion |
Cannabis Use Quantity (TLFB/Smoking Diaries): Other: Change in urine cannabinoid levels (UA) Frequency (TLFB/Smoking Diaries): Other: Point prevalence of new cannabis use (UA) Other BDI-II, MPS, MWC, PSQI, SAFTEE-GI |
|
McRae-Clark et al., 2009 Buspirone (60 mg/day; achieved by gradually increasing 5 mg bid starting dose by 5–10 mg every 3–4 days) |
93 | Adults | Met DSM-IV criteria for dependence | None |
Cannabis Use Abstinence (UA) Frequency (TLFB) Quantity (TLFB) Other HAM-A, MCQ, MWC |
|
McRae-Clark et al., 2010 Atomoxetine (starting dosage of 25 mg daily, increasing to 40 mg in wk 2, 80 mg in wk 3 as tolerated, up to 100 mg) |
78 | Adults | Met DSM-IV criteria for dependence | None |
Cannabis Use Abstinence (UA) Frequency (TLFB) Frequency/Quantity (TLFB) Other CAARS-Self, CGI, WRAADS |
|
McRae-Clark et al., 2015 Buspirone (initiated at 5 mg bid, increased by 5–10 mg every 3–4 days as tolerated; max dose 60 mg daily) |
175 | Adults | Met DSM-IV criteria for dependence | None |
Cannabis Use Abstinence (UA) Other 5-HT1A Receptor Genotype, HAM-A, MCQ |
|
McRae-Clark et al., 2016 Vilazodone (initiated at 10 mg/day for 7 days, 20 mg/day for 7 days, 40 mg/day as tolerated) |
76 | Adults | Met DSM-IV criteria for dependence | None |
Cannabis Use Abstinence (UA) Frequency (TLFB) Quantity (TLFB) Other MCQ |
|
Penetar et al., 2012 Bupropion (150 mg/day on days 1–3, 150 mg bid on days 4–21) |
22 | Adults | Minimum of 3 years of heavy use (smoke 5/7 days per wk/ greater than 25 days per month); experienced 2 or more negative symptoms in previous quit attempts | None |
Cannabis Use Other: Point prevalence of new cannabis use (UA) Other BAI, BDI, Cognitive Performance Battery, MWC, Sleep Diary/Actigraphy, URICA |
|
Sherman et al., 2017 MET + Oxytocin (40 IUs administered intranasally 30 min prior to first 2 of 3 MET sessions) |
16 | Adults | Cannabis dependence | None |
Cannabis Use Frequency (TLFB) Quantity (TLFB) Other: Urine and saliva samples used to confirm recent abstinence (data not reported in manuscript) Other N/A |
|
Trigo et al., 2018 Nabiximols (as-needed, up to 113.4 mg THC/105 mg CBD) |
40 | Adults | Met DSM-IV criteria for dependence; report cannabis as primary drug of abuse; report cannabis use at least 5 days/week for at least 1 month; cannabis positive urine screen; smoked less than or equal to the equivalent of 4 joints/day (or 4 g/day) | None |
Cannabis Use Abstinence (TLFB/Smoking Diary) Frequency (TLFB/Smoking Diary) Quantity (TLFB/Smoking Diary) Other: Results of TLFB and smoking diaries compared for consistency Cannabinoid Concentrations (Blood Plasma and UA). Other MCQ, Medication Use, MWC, SMHSQ, Tolerability, Vitals |
|
Weinstein et al., 2014 Escitalopram (10 mg/day) |
52 | Adults | Met DSM-IV criteria for dependence | 1 FU: 6-month post-treatment |
Cannabis Use Abstinence (UA) Other ASI, BDI, CIWA (Modified), STAI |
| Adolescent Trials (PHA) | |||||
|
Cornelius et al., 2010 & 2012 Fluoxetine (20 mg/day) |
70 | Adolescents & Adults (14–25) | Met DSM-IV criteria for dependence or abuse; current cannabis use (i.e. past 30 days) | None |
Cannabis Use Frequency (TLFB) Other BDI, SCID (Cannabis Abuse/Dependence Symptoms, Alcohol, MDD), HAM-D-27, Side Effects, TLFB (alcohol use) |
|
Gray et al., 2012 N-acetylcysteine (1200 mg bid) |
116 | Adolescents & Adults (15–21) | Used cannabis regularly; met criteria for cannabis dependence expressed interest in CUD TX | 1 FU: 4 wks post-treatment |
Cannabis Use Abstinence (UA) Frequency (TLFB) Other Safety/Tolerability |
|
Miranda et al., 2017 Topiramate (titrated over 4 wks, stabilized at 200 mg/day for 2 wks) |
66 | Adolescents & Adults (15–24) | Heavy cannabis use; some clinically significant problems associated with cannabis use | None |
Cannabis Use Abstinence (UA) Frequency (TLFB) Quantity (TLFB) Other BDI, Neurocognitve Test Battery, SAFTEE |
Notes: Outcomes in bold text were identified as primary outcomes. Abbreviations: ADI-Light, Adolescent Diagnostic Interview-Light; APQ, Alabama Parenting Questionnaire; ASI, Addiction Severity Index; BAI, Beck Anxiety Inventory; BDI/BDI-II, Beck Depression Inventory; BSI, Brief Symptom Inventory; BIS, Barratt Impulsivity Scale; BPRS, Brief Psychiatric Rating Scale; CAARS-Self, Conners Adult ADHD Rating Scale-Self; CBCL, Child Behavior Checklist; CDSS, Calgary Depression Scale for Schizophrenia; CGI, Clinical Global Impression; CIDI, Munich Composite International Diagnostic Interview; CIWA, Clinical Institute Withdrawal Assessment Scale; CPQ, Cannabis Problems Questionnaire; CRI-Y, Coping Response Inventory-Youth; CSS, Coping Strategies Scale; CSQ, Client Satisfaction Questionnaire; CUPIT, Cannabis Use Problems Identification Test; CWS, Cannabis Withdrawal Scale; DAI-30, Drug Attitude Inventory-30; DASS-21, Depression, Anxiety, and Stress Scale; DAST, Drug Abuse Screening Test; DTCQ-8, Drug-Taking Confidence Questionnaire; DUP, Duration of Untreated Psychosis; ETX, End-of-Treatment; EQ-5D, EuroQol’s Quality of Life Interview; FES, Family Environment Scale; GAF, Global Assessment of Functioning; GAIN-I, Global Appraisal of Individual Needs-Initial; GDS, General Depression Scale; HAM-A, Hamilton Anxiety Scale; HAM-D, Hamilton Rating Scale for Depression; HSC, Hopkins Symptom Checklist; MANSA, Manchester Short Assessment of Quality of Life; MCQ, Marijuana Craving Questionnaire; MDS, Marijuana Dependence Scale; MPS, Marijuana Problem Scale; MWC, Marijuana Withdrawal Checklist; PANSS, Positive and Negative Syndrome Scale; PSQI, Pittsburgh Sleep Quality Index; QIDS, Quick Inventory for Depressive Symptoms; RTC, Readiness to Change Questionnaire; SAFTEE, Modified Systematic Assessment for Treatment and Emergent Events; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; SCID, Structured Clinical Interview for DSM-IV; SCL-90, The Symptom Checklist-90; SCQ, Situational Confidence Questionnaire; SDS, Severity of Dependence Scale; SF-12, Short Form-12; SMHSQ, St. Mary’s Hospital Sleep Questionnaire; Snaith, Snaith Irritability Scale; SOCRATES, Stages of Change Readiness and Treatment Eagerness Scale; SOFAS, Social and Occupational Functioning Scale; SS, Satisfaction Scale; STAI, State-Trait Anxiety Inventory; SWLS, Satisfaction with Life Scale; TLFB, Timeline Followback; TX, Treatment; UA, Urinalysis; URICA, University of Rhode Island Change Assessment; VAS, Visual Analog Scale; VSDI, Vermont Structured Diagnostic Interview; WHODAS, World Health Organization Disability Assessment Schedule; WHOQOL-BREF-World Health Organization Quality of Life Assessment; WRAADS, Wender-Reimherr Adult Attention Deficit Disorder Scale; YSR, Youth Self-Report.
3.3. Primary outcome reporting
Primary outcome measure(s) for cannabis use were explicitly declared in 67% of trials (n = 39; 25 PSY, 14 PHA). In trials with no clearly stated declaration of primary cannabis use outcomes, primary outcomes were inferred from the aims/hypotheses or statistical analysis/results sections when possible (n = 16; 10 PSY, 6 PHA). Primary outcomes were not identifiable in three trials, each of which had broad exploratory aims (Levin et al., 2004; Penetar et al., 2012; Roffman et al., 1988). Of the 39 trials that declared primary outcome measures, a single primary outcome was declared in 20 trials (9 PSY, 11 PHA), two primary outcomes were declared in 13 trials (10 PSY, 3 PHA), with the remaining six trials (all PSY interventions) declaring three or more primary outcomes. For this review, all primary outcomes were listed and described in Tables and text as declared or inferred from each trial.
3.4. Cannabis use measures
3.4.1. Self-report instruments
Overall, 91% of trials (53 total) included one or more self-report measures as primary or secondary outcome measures to assess cannabis use within or post-treatment. Of the 53 trials, 43 (81%) used a Timeline Follow-back calendar (TLFB; Sobell and Sobell, 1992). Four trials reported use of a retrospective use diary or calendar (Roffman et al., 1989; Sinha et al., 2003; Stephens et al., 1994, 2000), and four trials reported use of a self-report instrument but did not provide additional details (Carpenter et al., 2009; Johnston et al., 2014; Levin et al., 2004; Tossmann et al., 2011). Two trials (Dennis et al., 2004; Madigan et al., 2013) used single-item measures from other scales, the Global Appraisal of Individual Needs (GAIN; Dennis et al., 2004), and the cannabis abuse subscale of the Addiction Severity Index (ASI; McLellan et al., 1992).
3.4.2. Toxicology
Objective testing for THC or its metabolites in urine, blood, saliva, or hair was conducted in 79% of trials (46 total; 26 PSY, 20 PHA). The outcome of toxicological analysis was a standalone primary or secondary outcome measure in 22 trials (9 PSY, 13 PHA), was used only to confirm self-reported abstinence in 19 trials (14 PSY, 5 PHA), and in five trials toxicological testing was used as both a standalone outcome and to confirm self-reported cannabis use measures (3, PSY, 2 PHA). Testing urine for THC−COOH, a metabolite of THC, was the most predominant method used for toxicological testing (45 trials). Federal drug testing guidelines currently recommend “screening” with rapid immunoassay tests at a cut-off of 50 ng/ml THC−COOH, followed by confirmation of screening “positive” samples via gas chromatography mass spectrometry (GC/MS) or liquid chromatography coupled with tandem mass spectrometry (LC/MS/MS) at a cut-off of 15 ng/mL. Among published CUD treatment trials, urine testing was limited to qualitative “screening” tests (use of “rapid” onsite immunoassay tests to determine whether a sample was “positive” or “negative”) or semi-quantitative immunoassay tests in 23 trials. Quantitative testing using GC/MS or LC/MS/MS was used in 11 trials; to confirm “positive” rapid immunoassay screening tests in six trials, or as the only source of toxicology testing in five trials. Details for urine toxicology test methods were not specified in 11 trials. Among the trials that used immunoassay screening tests, there was inconsistency in the cut-off level used to determine a positive versus negative result. The federally recommended cutoff of 50 ng/ml THC−COOH was used in 18 of the completed trials. Three trials used a higher cutoff (100 ng/mL; Budney et al., 2000; Carpenter et al., 2009; Levin et al., 2013), one trial used a lower cutoff (20 ng/ml; Penetar et al., 2012), and one trial evaluated multiple cutoffs (20, 50, 100 ng/mL; Levin et al., 2004). Cutoffs were not specified in five trials (Babor et al., 2004; Levin et al., 2011; Hoch et al., 2014, 2012; Stephens et al., 1994). Only five trials used biological matrices other than urinalysis; three pharmacological trials (two inpatient) used blood plasma to quantify THC and cannabinoid metabolite concentrations (Allsop et al., 2014; Johnston et al., 2014; Trigo et al., 2018), and two used hair (Barrowclough et al., 2014) or saliva (Sherman et al., 2017) to confirm self-reported cannabis use during and after treatment.
3.4.3. Use of toxicology to confirm self-reported abstinence
Concordance between self-reported use and toxicology was reported in 18 out of 24 trials that used toxicological testing to confirm self-reported abstinence. Concordance rates were high overall, consistent with findings from a previous systematic review that evaluated the validity of TLFB for cannabis and other substances (Hjorthoj et al., 2012). Percent agreement between self-report and urine tests (reported in 10 trials) ranged between 80 and 100% (MTPRG, 2004; Budney et al., 2000; Carroll et al., 2006, 2012; de Dios et al., 2012 Johnston et al., 2014; Jungerman et al., 2007; Sinha et al., 2003; Stephens et al., 1994; Walker et al., 2015). Kappa values (reported in five trials) generally ranged between 0.6 to 0.9 (Barrowclough et al., 2014; Copeland et al., 2001; Dennis et al., 2004; Hoch et al., 2012), except for one trial that reported a lower kappa of 0.26 at post-treatment, which increased to 0.8 at a later follow-up (Kadden et al., 2007). Carpenter et al. (2009) reported good concordance with high sensitivity (0.73) and specificity (0.86) between urine specimens and self-reported use. Two trials did not report quantitative statistics, but reported a strong association between self-report and urine toxicology tests (Hendriks et al., 2011; Levin et al., 2011).
3.5. Cannabis use outcome domains
Table 2 presents cannabis use outcome domains separated by pre-specified domain (i.e., abstinence, reduction in frequency, quantity) for psychosocial and pharmacological intervention trials. All trials included at least one cannabis use outcome measure.
Table 2.
Cannabis Use Outcome Measures.
| # | Domain/Measure | Timeframe | Type | Trials Reporting Outcome (# Primary) | Citations | ||
|---|---|---|---|---|---|---|---|
| Abstinence | PSY | PHA | PSY | PHA | |||
| 1 | Proportion of participants with specific durations of continuous abstinence | During TX: cumulative at each week; > 2, 3, 4, 6, 8, 10 consecutive weeks; first/final 2 weeks; final week; continuous during TX. Post-TX: past month, continuous between ETX and/or follow-ups | D | 12 (8) | 7 (5) | Budney et al., 2000, 2006, 2011; Copeland et al., 2001; Jungerman et al., 2007; Litt et al., 2013; MTPRG, 2004; Rooke et al., 2013; Stanger et al., 2009, 2015; Stephens et al., 1994, 2000 | Carpenter et al., 2009; Johnston et al., 2014; Levin et al., 2004, 2011; 2013; 2016; Weinstein et al., 2014 |
| 2 | Point prevalence abstinence | During TX: final week Post-TX: ETX, follow-ups |
D | 10 (5) | 7 (2) | Budney et al., 2000, 2006, 2015; de Dios et al., 2012; Hoch et al., 2012, 2014; Kaminer et al., 2017; Stanger et al., 2009, 2015; Stephens et al., 1994 | Carpenter et al., 2009; Gray et al., 2012, 2017; Johnston et al., 2014; McRae-Clark et al., 2015; Trigo et al., 2018; Weinstein et al., 2014 |
| 3 | Longest duration of continuous abstinence | During TX: max consecutive negative urine specimens, SR days, or weeks | C | 10 (9) | 1 (0) | Budney et al., 2000, 2006, 2011, 2015; Carroll et al., 2006, 2012; Kadden et al., 2007; Kileen et al., 2012; Stanger et al., 2009, 2015 | Levin et al., 2011 |
| 4 | Proportion of cannabis negative or positive urine specimens | During TX: each week, total across TX Post-TX: final TX session to follow-up |
C | 5 (4) | 7 (6) | Budney et al., 2006; Carroll et al., 2006, 2012; Kaminer et al., 2014; Kileen et al., 2012 | Gray et al., 2012, 2017; McRae-Clark et al., 2009, 2010, 2015, 2016; Miranda et al., 2017 |
| 5 | Proportion of days abstinent | Post-TX: past month, 90 days, final TX session to follow-up | C | 5 (4) | 0 | Bonsack et al., 2011; Copeland et al., 2001; Gates et al., 2012; Kadden et al., 2007; Litt et al., 2013 | |
| 6 | Number of negative urine specimens | During TX: mean total across TX | C | 1 (1) | 0 | Carroll et al., 2006 | |
| 7 | Number of days or weeks of abstinence | During TX: mean total Post-TX: mean days (past 30, by quarter) |
C | 2 (2) | 1 (0) | Barrowclough et al., 2014; Dennis et al., 2004 | Levin et al., 2004 |
| 8 | Time to first negative UA | During TX | C | 0 | 2 (0) | Gray et al., 2012; McRae-Clark et al., 2009 | |
| 9 | Time to relapse | Post-TX | C | 1 (0) | 1 (0) | Kadden et al., 2007 | Allsop et al., 2014 |
| Total: | 46 (33) | 26 (13) | 27 Trials | 16 Trials | |||
| Frequency | |||||||
| 1 | Number of days/weeks used | During TX: mean total weeks, mean number of days each week, final TX week, During/Post-TX: past 7, 30, 90 days | C | 18 (14) | 8 (2) | Budney et al., 2000, 2006; Carroll et al., 2006, 2012; Copeland et al., 2017; de Dios et al., 2012; Gates et al., 2012; Hendriks et al., 2011; Hjorthoj et al., 2013; Lascaux et al., 2016; Madigan et al., 2013; Rigter et al., 2013; Roffman et al., 1988; Rooke et al., 2013; Sinha et al., 2003; Stephens et al., 1994, 2000; Tossman et al., 2011 | Carpenter et al., 2009; Cornelius et al., 2010; Hill et al., 2017; Johnston et al., 2014; Levin et al., 2004, 2011; Mason et al., 2012; McRae-Clark et al., 2010 |
| 2 | Proportion of days used | During/Post-TX: past 28, 30, 90 days, weekly during TX, Final TX week, ETX to follow-up | C | 8 (5) | 7 (1) | Budney et al., 2011, 2015; Carroll et al., 2006, 2012; Jungerman et al., 2007; MTPRG 2004; Stanger et al., 2015; Walker et al., 2015 | Gray et al., 2012, 2017; Levin et al., 2016; McRae-Clark et al., 2009, 2010; Miranda et al., 2017; Trigo et al., 2018 |
| 3 | Number of periods or times per use day | During/Post-TX: past 7, 30, 90 days, last 30 days TX, weekly during TX | C | 4 (1) | 3 (1) | Budney et al., 2006; Jungerman et al., 2007; MTPRG 2004; Stephens et al., 2000 | Hill et al., 2017; McRae-Clark et al., 2016; Sherman et al., 2017 |
| 4 | Frequency of cannabis use (Categorical: daily, weekly, monthly) | Post-TX: prior to each follow-up | CAT | 2 (0) | 0 | Bonsack et al., 2011; Roffman et al., 1988 | |
| Total: | 32 (20) | 18 (4) | 25 Trials | 16 Trials | |||
| Quantity | |||||||
| 1 | Grams | During/Post-TX: total (past 30 days, mean use per day/week (past 28, 30 days) | C | 3 (1) | 8 (3) | Barrowclough et al., 2014 (total + mean weight per day); Tossman et al., 2011 | Allsop et al., 2014; Carpenter et al., 2009; Johnston et al., 2014; Mason et al., 2012; McRae-Clark et al., 2016; Miranda et al., 2017; Sherman et al., 2017; Trigo et al., 2018 |
| 2 | Joints/cones | During/Post-TX: total (past week, month), mean use per day/week (past 7, 28, 30, 90 days) | C | 6 (4) | 2 (1) | Bonsack et al., 2011; Gates et al., 2012; Hendriks et al., 2011; Jungerman et al., 2007; Kadden et al., 2007; MTPRG, 2004 | Brunette et al., 2011; Levin et al., 2004 |
| 3 | Standard cannabis units (defined as 3 cones OR a joint containing 0.5 grams) | Post-TX: total (past month), use per day | C | 3 (2) | 0 | Copeland et al., 2017; Hjorthoj et al., 2013; Rooke et al., 2013 | |
| 4 | Waterpipes | Post-TX: mean use per day (past month) | C | 1 (1) | 0 | Copeland et al., 2001 | |
| 5 | Total cannabis units consumed (by ROA) | Post-TX: past 7 days | C | 2 (1) | 0 | Hoch et al., 2012, 2014 | |
| 6 | Inhalations per day | During TX: past 7 days | C | 0 | 1 (0) | Hill et al., 2017 | |
| 7 | “Amount” per use day | During TX: past 7 days | C | 0 | 1 (0) | McRae-Clark et al., 2009 | |
| 8 | Total money spent on cannabis | During TX: per day, per week | C | 0 | 2 (0) | Carpenter et al., 2009; Levin et al., 2011 | |
| Total: | 15 (9) | 14 (4) | 14 Trials | 13 Trials | |||
| Other | |||||||
| 1 | Change in quantitative cannabinoid levels | During TX: change from baseline to ETX, mean weekly levels during TX | C | 0 | 8 (1) | Hill et al., 2017; Johnston et al., 2014; Levin et al., 2004, 2013; Mason et al., 2012; McRae-Clark et al., 2015, 2016; Trigo et al., 2018 | |
| 2 | New use (operationalized at > 50% increase in THC-COOH from prior level) | During TX: each week | D | 0 | 2 (0) | Mason et al., 2012; Penetar et al., 2012 | |
| Total: | 0 (0) | 10 (1) | 9 Trials | ||||
Note: Citations in bold text reported measure as primary outcome. Abbreviations: C, continuous measure, CAT, categorical measure, D, dichotomous measure, ETX, end of treatment, ROA, route of administration; TX, treatment.
3.5.1. Abstinence
Cannabis abstinence was reported as an outcome measure in 74% of trials (n = 43; 27 PSY, 16 PHA), and was identified as a primary outcome in 32 trials (20 PSY, 12 PHA). However, there was variability in how abstinence was measured and reported. Abstinence was assessed using both dichotomous and continuous outcome measures, which were collected using biological (i.e., urinalysis), self-report (i.e., TLFB), or a combination of self-reports confirmed by a biological assay. Dichotomous outcomes included: (a) proportion of participants with specific durations of continuous abstinence (reported in 19 trials −13 as a primary outcome), and (b) point-prevalence abstinence at end of treatment (ETX) and post-treatment follow-up visits (reported in 17 trials – seven as a primary outcome). Continuous outcomes included: (a) longest duration of continuous abstinence (reported in 11 trials – nine as a primary outcome), (b) proportion of cannabis negative/positive urine specimens (reported in 12 trials −10 as a primary outcome), (c) proportion of days abstinent (reported in five trials – four as a primary outcome), (d) mean number of negative urine specimens (reported as a primary outcome in one PSY trial), (e) mean number of days or weeks of abstinence (reported in three trials – two as a primary outcome), (f) time to first negative urinalysis (UA; reported as secondary outcome in 2 PHA trials, and (g) time to relapse (reported as secondary outcome in two trials).
3.5.2. Frequency
Reduction in frequency of cannabis use was included as an outcome measure in 71% of trials (n = 41; 25 PSY and 16 PHA), and was identified as a primary outcome in 21 trials (17 PSY and 4 PHA). Frequency was assessed using continuous and categorical outcome measures collected via self-report assessments. The most commonly used frequency outcome measure was number of days/weeks that the participant used cannabis; 26 trials assessed this outcome (16 as a primary outcome) either during treatment or at post-treatment follow-ups. However, while this measure was commonly reported across trials, it is important to note that the reference point for assessing the measure varied widely between trials (e.g., mean total weeks, mean number of days within each treatment week, total days during final week of treatment, past 7, 30, 90 days post-treatment), which limited ability to compare frequency outcomes across trials. Other frequency outcomes included mean proportion of days used (reported in 15 trials – six as a primary outcome), number of periods or times per use day (reported in seven trials – two as primary outcome), and frequency of cannabis use using a categorical variable to assess whether cannabis use occurred daily, weekly, or monthly on average (reported as a secondary outcome in two trials).
3.5.3. Quantity
Reduction in quantity of cannabis use was included as an outcome measure in 47% of trials (n = 27; 14 PSY and 13 PHA), and was identified as a primary outcome in 13 trials (9 PSY and 4 PHA). Reduction in quantity was assessed using eight different units of measurement. The two most prevalent units of measurement were grams (11 trials – 3 PSY, eight PHA) and joints or cones (eight trials – six PSY, two PHA). Other units of measurement included standard cannabis units (defined as three “cones” or a joint containing 0.5 g of cannabis; three trials – all PSY), waterpipes (one trial – PSY), total cannabis consumed by route of administration (e.g., joints, bongs, pipes; two PSY trials), inhalations per day (one PHA trial), “amount” per use day (no unit of measurement specified; one PHA trial), and total money spent on cannabis (two trials – both PHA).
Changes in quantitative cannabinoid levels were assessed in eight trials (all PHA), while two PHA trials included an assessment of new use (defined as any urine specimen with > 50% increase in THC−COOH levels since the previous specimen collection).
3.6. Measures for other outcome domains
3.6.1. Cannabis withdrawal and craving
Cessation of cannabis following a prolonged period of heavy use is linked to the onset of a clinically significant withdrawal syndrome that is associated with a range of negative consequences, including impairment in functioning and increased risk for relapse (Allsop et al., 2012; Budney et al., 2004; Haney, 2005). Cannabis withdrawal is formally recognized in the most recent Diagnostic and Statistical Manual of Mental Disorders (DSM-5; APA, 2013). Diagnostic symptoms of cannabis withdrawal include irritability, anxiety, sleep disturbance, decreased appetite/weight loss, restlessness, depressed mood, and other physical symptoms that elicit significant discomfort (i.e., abdominal pain, shakiness/tremors, sweating, fever, chills, headache). Recognition of the clinical significance of the cannabis withdrawal syndrome has increased efforts to identify pharmacotherapies that specifically target suppression of withdrawal-related symptoms as a primary outcome (Brezing and Levin, 2018).
Cannabis withdrawal was assessed as a primary or secondary outcome in nine trials included in the current review, all of which were pharmacological interventions (Table 3). Measures of withdrawal were identified as the primary outcome in two inpatient trials (Allsop et al., 2014; Johnston et al., 2014) and was the primary focus of the outpatient trial by Penetar et al. (2012). The Marijuana Withdrawal Checklist (MWC; Budney et al., 1999) was the measure used most frequently to assess withdrawal (included in six trials). Two trials used the Cannabis Withdrawal Scale (CWS; Allsop et al., 2011), both of which identified the CWS as the primary outcome measure (Allsop et al., 2014; Johnston et al., 2014). The Clinical Institute Withdrawal Assessment Scale (originally created for alcohol and adapted for cannabis; Sullivan et al., 1989) was used in one trial. Individual symptoms of withdrawal (e.g., sleep disturbance and irritability) were assessed in seven PHA trials. Withdrawal-related sleep disturbance was assessed in four trials (Carpenter et al., 2009; Mason et al., 2012; Penetar et al., 2012; Trigo et al., 2018) using the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989), St. Mary’s Hospital Sleep Questionnaire (SMHSQ; Ellis et al., 1981), and a combination of sleep diaries and actigraphy (Penetar et al., 2012). Withdrawal-related irritability was assessed in two trials (Carpenter et al., 2009; Levin et al., 2004) using the Snaith Irritability Scale (Snaith et al., 1978) and the irritability items from the Hopkins Symptom Checklist (Derogatis et al., 1974).
Table 3.
Cannabis Withdrawal and Craving Outcome Measures.
| # | Domain/Instrument | # Trials (# primary) | Citations | ||
|---|---|---|---|---|---|
| PSY | PHA | PSY | PHA | ||
| 1 | Marijuana Withdrawal Checklist | 0 | 6 (0) | N/A | Levin et al., 2011, 2016; Mason et al., 2012; McRae-Clark et al., 2009; Penetar et al., 2012; Trigo et al., 2018 |
| 2 | Cannabis Withdrawal Scale | 0 | 2 (2) | Allsop et al., 2014; Johnston et al., 2014 | |
| 3 | Clinical Institute Withdrawal Assessment Scale (adapted for cannabis) | 0 | 1 (0) | Weinstein et al., 2014 | |
| 4 | Marijuana Craving Questionnaire | 1 (0) | 5 (0) | Kileen et al., 2012 | Hill et al., 2017; McRae-Clark et al., 2009, 2015, 2016; Trigo et al., 2018 |
| 5 | Visual Analog Scale (Intensity and Desire for Cannabis) | 0 | 1 (0) | Levin et al., 2004 | |
| Totals: | 1 (0) | 15 (2) | |||
Note: Citations in bold text reported measure as primary outcome.
Craving was identified as an outcome domain of interest in eight trials (one PSY, seven PHA). The Marijuana Craving Questionnaire (Heishman et al., 2009) was the measure most frequently used to assess craving (included in six trials, one PSY, five PHA); other measures of craving included a visual analog scale that assessed intensity and desire to use cannabis (included in one PHA trial; Levin et al., 2004), and a single-item measure of craving included in the MWC (included in one PHA trial; Penetar et al., 2012).
3.6.2. Other relevant outcome domains
Measures used to assess clinically significant improvement in other outcome domains as a function of cannabis abstinence or reduction in use were highly heterogeneous depending on the population (i.e., co-occurring mental disorders, adolescent versus adult population), and aims of each trial (see Table 1 for full list of outcome measures used in each trial). The domains with the highest prevalence of reported outcome measures included the following: (1) Presence or Severity of Dependence, which was assessed in 21 trials (14 PSY, seven PHA) using 11 different instruments, (2) Mood (i.e., depression and anxiety), which was assessed in 20 trials (five PSY, 15 PHA) using eight different instruments, (3) Psychosocial Functioning, which included assessments of global functioning (assessed in 17 trials – 14 PSY, three PHA), and population-specific measures in youth (four trials, all PSY), and psychopathology (five trials, all PSY) using 17 different instruments, (4) Cannabis-related Problems, which were assessed in 15 trials (12 PSY, three PHA) using three different instruments, (5) Readiness to Change/Self-Efficacy, which was assessed in 11 trials (10 PSY, one PHA) using seven different instruments, (6) Alcohol and Other Drug Use, which was assessed in eight trials (six PSY, two PHA) using two different instruments, and (7) Quality of Life, which was assessed in four trials (three PSY, one PHA) using five different instruments.
Instruments used to assess other relevant outcome domains included a heterogeneous mix of self-report questionnaires and clinician-administered interviews (59 unique instruments in total). No self-report instrument emerged as highly prevalent across trials (see Table 1 for details on instruments in all trials). Examples of self-report instruments included the Severity of Dependence Scale (SDS; Gossop et al., 1995), Beck Depression Inventory (BDI; Beck et al., 1988), Marijuana Problem Scale (MPS; Stephens et al., 2000), Readiness to Change Questionnaire (RTCQ; Rollnick et al., 1992), Marijuana Self-Efficacy Scale (Litt et al., 2005), and World Health Organization Quality of Life Assessment (WHOQOL-BREF; Skevington et al., 2004). The most common clinician-administered interviews were the Addiction Severity Index (ASI; McLellan et al., 1992), which was included in 12 trials (nine PSY, three PHA) to assess psychosocial functioning, and the TLFB, which was included in eight trials (six PSY, two PHA) to assess alcohol and other drug use.
3.7. Significant outcome measures
There was substantial heterogeneity of treatment type, the timeframe with which an end point provided assessment (e.g., cannabis use in the last 7, 30, or 90 days), and the outcome measures reported across trials. Moreover, in many studies, a primary outcome measure was not explicitly designated. As a result, a comparative analysis of outcome measures with respect to sensitivity of detecting treatment effects was not possible. In an attempt to provide some guidance for selecting outcomes in future studies, an overview of statistically significant outcome measures from both psychosocial and pharmacological trials is provided below.
Twenty-four of 35 psychosocial trials reported statistically-significant outcomes on at least one measure. Significant differences were reported on measures of cannabis abstinence in 12 trials, six of which reported significant effects on more than one cannabis abstinence outcome measure. The cannabis abstinence outcome measures that were sensitive to treatment effects were: longest duration of continuous abstinence (six trials), proportion of participants with specific durations of continuous abstinence (five trials), point-prevalence abstinence (four trials), proportion of days abstinent (three trials), and number of abstinent days/negative urine specimens (two trials). Twelve trials reported significant differences on measures of cannabis use frequency, two of which reported two or more frequency measures. Specific cannabis frequency outcome measures sensitive to treatment effects were: number of days/weeks in which cannabis was used (nine trials), proportion of days cannabis was used (four trials), and number of periods or times used per day (one trial). Eight trials reported significant differences on measures of cannabis quantity used, with joints/cones (three trials) and standard cannabis units (three trials) the most frequently reported (grams and waterpipes were measured in two trials). Measures in other outcome domains (i.e., severity of cannabis dependence, cannabis-related problems, improvement in functioning, treatment engagement) were reported as sensitive to treatment effects in six trials.
Five of the 20 pharmacological trials reported one or more statistically-significant outcome measures. In two trials, significant differences were reported for the proportion of participants that achieved > 1 cannabis-negative urine specimen (measure of cannabis abstinence). In two trials, self-reported number of times cannabis was used per day (measure of cannabis frequency) was significantly reduced. Two trials reported a significant reduction in the concentration of THC-COOH (a metabolite of THC) in urine as well as reduced self-reported grams of cannabis used per day or week. In addition, one trial reported a significant reduction in cannabis withdrawal as assessed by the Marijuana Withdrawal Checklist in the experimental drug condition versus placebo.
4. Discussion
4.1. Summary
This review provides a comprehensive summary of the outcome measures used in clinical trials evaluating psychosocial and pharmacological interventions for CUD, and upon review of the findings, several conclusions and discussion points emerge. First, techniques used to measure cannabis consumption were fairly consistent across trials; most included self-report assessments, primarily the TLFB, and urine toxicology testing as a standalone outcome or to validate self-report data. Second, cannabis use outcome measures derived from self-report and toxicology tests were highly heterogeneous between trials. Third, and converging with the alcohol treatment literature, reductions in cannabis use were commonly reported outcomes. However, current assessments of cannabis use lack a standardized approach for measuring quantity of cannabis use and utilize a wide range of temporal reference points (e.g., day, week, month). Frequently, a primary outcome measure was not explicitly defined, particularly in trials testing psychosocial treatments. Finally, assessment of outcome domains beyond that of cannabis use vary widely with minimal standardization; trials employed a range of self-report and clinician-administered instruments with varying frequency to assess other outcome domains.
4.2. Consideration of self-report versus toxicology testing to measure cannabis abstinence and reduction
Self-report and biological verification of abstinence both present unique strengths and limitations for measuring cannabis use. Strengths of self-report measures of cannabis use include: (a) efficiency and ease of data acquisition; that is, information can be collected in-person or over phone/online with minimal burden to participants, (b) sensitivity to subtle changes in patterns of consumption, especially when use patterns are collected with high temporal resolution, such as daily use (i.e., reduction in days of use, or occasions per day), (c) ability to measure consumption across multiple routes of administration, and (d) changes in patterns of cannabis use can be collected retrospectively over extended time intervals. Limitations of self-report measures of cannabis use include: (a) validity – retrospective recall of past cannabis use may yield reduced precision due to recall bias, (b) deliberate inaccuracies resulting from demand characteristics or socially-desirable response patterns, especially when contingencies are provided dependent upon abstinence, and (c) lack of an established standardized unit for cannabis administration, which decreases the ability to compare self-reported use across trials.
Across trials, self-reports of cannabis abstinence and reductions in use were primarily assessed via the TLFB. The TLFB is a popular method used by researchers and clinicians to obtain frequency and quantity estimates of alcohol, tobacco, and other drug use. The TLFB is traditionally administered in person by an interviewer, but concurrent validity has been established for administration on the Internet, by telephone, or unassisted completion using a computer (Pedersen et al., 2012; Sobell et al., 1996). The psychometric properties for collecting self-reported cannabis use via the TLFB have demonstrated high test-retest reliability across intervals ranging from 30 to 360 days (ICCs between 0.78 to 0.96; Robinson et al., 2014), and appropriate validity, interpreted via high concordance between TLFB data and urine cannabinoid toxicology tests (lowest and highest agreement rates ranged between 87.3% and 90.9%; Hjorthoj et al., 2012).
Urine toxicology provides an objective verification of abstinence with a high level of accuracy that is widely accepted in treatment trials and allows for cross-comparisons to be made between trials. Urine specimens can be assessed using qualitative, semi-quantitative, and/or quantitative analyses. Strengths of qualitative urinalyses include: (a) generally low cost (relative to quantitative testing and testing in other biological matrices), (b) results obtained quickly (usually within a few minutes depending on test type), and (c) no specialized equipment or shipments needed to a specialized laboratory for analysis. However, the lengthy delay (often several weeks) between initiation of abstinence and a “negative” urine toxicology test is a substantial disadvantage of this approach, as is the invasiveness of collecting observed urine specimens from study participants. Quantitative urinalysis, though costlier and more prone to a longer delay between specimen collection and analysis, has the potential to confirm self-reported abstinence prior to a “negative” sample with the use of algorithms designed to differentiate new use from residual cannabinoids (i.e., Schwilke et al., 2011). The limitation of this approach is that existing algorithms cannot fully account for the considerable variability across individuals in the rate of cannabinoid elimination, and thus are prone to false positive and false negative determinations. The best solution may be to utilize a combination of self-reported cannabis use, along with both qualitative and quantitative urine toxicology testing until a more sensitive approach is developed. Accurate and immediate detection of recent cannabis use is especially relevant to interventions that use incentives to reinforce abstinence contingent upon biological verification of cannabis abstinence (i.e., contingency management), or programs with other consequences (i.e., legal implications) for positive urine screens.
4.3. Heterogeneity in cannabis use outcome measures
Results from the narrative synthesis of cannabis use outcome measures provided in the current review highlighted the heterogeneity in type and unit of measurement across cannabis use outcome domains. Abstinence was reported as an outcome in 67% of trials, while 76% included a self-report outcome measuring reduction in frequency, and 45% reported an outcome measuring reduction in quantity of use. Outcome measures for abstinence included nine distinct categories assessed both during and post-treatment, including dichotomous (i.e., proportion of participants achieving specific durations of abstinence, point prevalence abstinence) and continuous measurements (i.e., longest duration of continuous abstinence). Reduction in frequency of use was measured primarily using proportion or mean number of days or weeks of cannabis use or abstinence, whereas a greater number of approaches were used to assess reduction in quantity (eight total, depending on unit of measurement). In addition to the variety in type of outcome measurements, it is important to note that the timeframe for assessing change in use varied widely across trials. For example, data for frequency and quantity of use were aggregated across multiple timeframes (e.g., past 7, 30, 90 days), with similar heterogeneity in timeframes for measuring specific durations of cannabis abstinence, (e.g., > 2, 3, 4, 6, 8, 10 weeks of consecutive abstinence, continuous abstinence throughout treatment or between follow-ups). Taken together, over 60 unique cannabis use outcome measures were reported across trials when accounting for outcome domain, measurement type, and timeframe for aggregating data. Limiting heterogeneity by standardizing outcomes reported across trials has important implications for current and future development of CUD interventions, including increasing the sensitivity of meta-analyses evaluating the comparative efficacy of interventions.
4.4. Challenges in assessing reduction in cannabis use
While abstinence is the most common primary outcome for CUD intervention trials, reduction in use is frequently reported as a primary or secondary outcome in CUD interventions, and reduction attempts are more common among cannabis users attempting to change cannabis use patterns (Hughes et al., 2016). The majority of trials included in the current review reported an outcome measuring reduction in frequency and quantity of use, and nearly 50% of trials included reduction as a primary outcome. Outcomes measuring use frequency primarily assessed reduction in proportion or mean number of days or weeks of use. However, outcomes assessing reduction in quantity varied widely depending on unit of measurement. Quantity of use was most frequently assessed in grams or joints/cones per use day; however, other units were also used (i.e., standard cannabis units, waterpipes, inhalations, and total money spent per day). Determining an optimal measure of quantity presents several unique challenges for cannabis, unlike tobacco and alcohol where units of measurement are more clearly defined (e.g., cigarettes per day, number of standard drinks per day). Accounting for potency, route of administration, and other potential factors (e.g., number of individuals administering cannabis from a shared joint/blunt/pipe etc., inhalation intensity) is necessary to obtain an accurate measurement of cannabis quantity, which can be prohibitive for clinical research using self-report measures such as the TLFB to assess cannabis use each day during and post-treatment. One potential approach to supplement collection of daily use via the TLFB might be to assess quantity via a detailed screening measure, such as the recently developed and psychometrically sound Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (DFAQ-CU; Cuttler and Spradlin, 2017) which accounts for multiple aspects of use and can be extrapolated to calculate an average frequency. However, this approach might not be sensitive to subtle changes in quantity across a short period of time. Other daily self-report modalities, such as Ecological Momentary Assessment, and/or Interactive Voice Response might also be considered as approaches to maximize accuracy of data collection while balancing participant burden.
4.5. Considerations for standardizing outcome measures in other domains
As indicated in the synthesis of extant trials, the secondary outcome domains of interest in individual trials seem to be driven by unique features of a given population under study (e.g., externalizing behavior in an adolescent population) and by additional objectives of the clinical trial. With that said, there appear to be common domains of interest across trials (e.g., severity of use and dependence, mood, psychosocial functioning), but no clear consensus on the optimal instrument for assessing outcomes in each domain. One approach to reconcile this issue would be to convene an expert workgroup of researchers to decide which ancillary domains are of broad interest for assessment in CUD treatment trials, and to select “core” assessments to be used across trials for each domain. Emphasis should be placed on use of validated “gold standard” assessments for each domain, but also to select assessments that are freely available, are relevant across cultures and have been translated into multiple languages, and that are short, to minimize participant burden. Standard measures in online collaborative databases such as the PhenX Toolkit (https://www.phenxtoolkit.org/) could be emphasized, where relevant, to increase access to measures and further enhance standardization efforts. Depending on unique features of a given population (e.g., adults with schizophrenia and CUD), additional measures specific to the population could be added to supplement the “gold standard” assessments. This would streamline the decision-making process during protocol preparation and would greatly enhance the ability to conduct meta-analyses across trials.
4.6. Limitations and final conclusions
There are several potential limitations of this review that warrant discussion. First, the overall aim of this review was to maximize inclusion of all relevant clinical trials for CUD, but this may have resulted in inclusion of trials with potential methodological weaknesses. Second, we excluded randomized controlled trials of brief interventions in nondependent, and/or non-treatment seeking cannabis users. While brief intervention trials are integral to provide psychoeducation and/or motivational enhancement in cannabis users that are ambivalent/not interested in reducing or quitting cannabis use, population characteristics and outcomes related to goals of treatment may differ substantially for these trials (e.g., reduction might be a more acceptable and/or preferred outcome, whereas other outcome domains would not be relevant to assess improvement in functioning). Finally, this review did not include detailed quantitative comparisons of outcomes between interventions. We attempted to do so, but after considerable effort we concluded that the wide variability in methods of included trials rendered a meta-analysis impossible. Although significant outcome measures were most frequently reported on measures of cannabis abstinence (14 trials) and reduction in frequency (14 trials), outcome measures are dependent on multiple factors such as trial design and data analysis methodology. As such, statistically significant results do not necessarily equate with sensitivity to clinically relevant changes. Evaluating the comparative effectiveness of CUD interventions is important, but was beyond the scope of the paper. Rather, this review was focused primarily on providing a summary of outcome measures to inform standardization of outcomes, which is necessary for improving the power of future quantitative comparisons.
Taken together, this review provided an in-depth assessment of the vast heterogeneity in outcome measures used to assess change in cannabis use and other outcome domains in CUD interventions. There is a clear need for a research agenda to focus on providing systematic data in order to standardize outcome measures for cannabis use and other domains. One key recommendation to assist in this need is for future studies to clearly identify a single primary outcome measure as well as explicitly differentiating a-priori secondary outcome measures versus exploratory outcomes. Another recommendation is the conduct of secondary analyses of data across existing CUD trials to determine cannabis use outcome measures that are most closely associated with clinically significant improvement in functioning. Such analyses have recently been conducted in stimulant trials, which has improved evidence of cocaine use outcome measures most associated with sustained improvement in functioning (i.e., Carroll et al., 2014; Kiluk et al., 2014, 2017). Similar trials and analyses across CUD interventions are needed to determine if reduction in use could be a reasonable alternative to complete abstinence, especially if individuals with CUD are refractory to complete abstinence. Future research efforts will need to provide a systematic assessment across frequency and quantity of use outcomes to determine a specific rate of reduction that is associated with clinically significant improvement in functioning that can be sustained over time. For abstinence, determining a specific duration within and/or post-treatment that best predicts prolonged abstinence and significant improvement in functioning is needed to further inform standardization of other outcome domains. Overall, given the substantial risks associated with CUD and continued demand for treatment, efforts aimed at improving standardization and rigor of CUD trials are warranted in order to streamline development of efficacious psychosocial interventions and identification of FDA-approved medications to treat CUD.
Supplementary Material
Acknowledgements
We thank Erin Martin and Sarah Moshman for their assistance in the preparation of tables and figures for the manuscript.
Role of the funding source
Financial support for this project was provided by the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) public-private partnership with the U.S. Food and Drug Administration (FDA), which has received research contracts, grants, or other revenue from the FDA, multiple pharmaceutical and device companies, philanthropy, and other sources.
Conflict of interest
Authors DCL, NJS, ENP, RD, and DT have no conflicts of interest to disclose. Author ECS has served as a consultant or served on advisory boards for Analgesic Solutions, Indivior, The Oak Group, Egalet Pharmaceuticals, Caron, Innocoll, Otsuka, and Pinney Associates, and has received research funding through his university from Alkermes. Author RV has been a paid consultant to Zynerba Pharmaceuticals, Insys Therapeutics, Inc, Battelle Memorial Institute, and Canopy Health Innovations Inc. in the past year.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.drugalcdep.2018.10.020.
References
- Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ, 2011. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 119, 123–129. [DOI] [PubMed] [Google Scholar]
- Allsop DJ, Copeland J, Norberg MM, Fu S, Molnar A, Lewis J, Budney AJ, 2012. Quantifying the clinical significance of cannabis withdrawal. PLoS One 7, e44864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, Rivas GR, Holland RM, Muhleisen P, Norberg MM, Booth J, McGregor IS, 2014. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry 71, 281–291. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (APA), 2013. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Publishing, Arlington, VA. [Google Scholar]
- Babor TF, Carroll K, Christiansen K, Donaldson J, Herrell J, Kadden R, Litt M, McRee B, Miller M, Roffman R, Solowji N, Steinberg K, Stephens R, Vendetti J, 2004. Brief treatments for cannabis dependence: findings from a randomized multisite trial. J. Consult. Clin. Psychol 72, 455–466. [DOI] [PubMed] [Google Scholar]
- Barrowclough C, Marshall M, Gregg L, Fitzsimmons M, Tomenson B, Warburton J, Lobban F, 2014. A phase-specific psychological therapy for people with problematic cannabis use following a first episode of psychosis: a randomized controlled trial. Psychol. Med 44, 2749–2761. [DOI] [PubMed] [Google Scholar]
- Bonsack C, Gibellini Manetti S, Favrod J, Montagrin Y, Besson J, Bovet P, Conus P, 2011. Motivational intervention to reduce cannabis use in young people with psychosis: a randomized controlled trial. Psychother. Psychosom 80, 287–297. [DOI] [PubMed] [Google Scholar]
- Brezing CA, Levin FR, 2018. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology 43, 173–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezing CA, Choi CJ, Pavlicova M, Brooks D, Mahony AL, Mariani JJ, Levin FR, 2018. Abstinence and reduced frequency of use are associated with improvements in quality of life among treatment-seekers with cannabis use disorder. Am. J. Addict 27, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, van Hell HH, Beale C, Yucel M, Solowij N, 2016. Acute and chronic effects of cannabinoids on human cognition: a systematic review. Biol. Psychiatry 79, 557–567. [DOI] [PubMed] [Google Scholar]
- Brunette MF, Dawson R, O’Keefe CD, Narasimhan M, Noordsy DL, Wojcik J, Green AI, 2011. A randomized trial of clozapine versus other antipsychotics for cannabis use disorder in patients with schizophrenia. J. Dual Diag 7, 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR, 1999. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction 94, 1311–1322. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL, 2000. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J. Consult. Clin. Psychol 68, 1051–1061. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R, 2004. Review of the validity and significance of cannabis withdrawal syndrome. Am. J. Psychiatry 161, 1967–1977. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST, 2006. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J. Consult. Clin. Psychol 74, 307–316. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Roffman R, Stephens RS, Walker D, 2007. Marijuana dependence and its treatment. Addict. Sci. Clin. Pract 4, 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Fearer S, Walker DD, Stanger C, Thostenson J, Grabinski M, Bickel WK, 2011. An initial trial of a computerized behavioral intervention for cannabis use disorder. Drug Alcohol Depend. 115, 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Stanger C, Tilford JM, Scherer EB, Brown PC, Li Z, Li Z, Walker DD, 2015. Computer-assisted behavioral therapy and contingency management for cannabis use disorder. Psychol. Addict. Behav 29, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buu A, Hu YH, Pampati S, Arterberry BJ, Lin HC, 2017. Predictive validity of cannabis consumption measures: results from a national longitudinal study. Addict. Behav 73, 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ, 1989. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, McDowell D, Brooks DJ, Cheng WY, Levin FR, 2009. A preliminary trial: double-blind comparison of nefazodone, bupropion-SR, and placebo in the treatment of cannabis dependence. Am. J. Addict 18, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, Ford HL, Vitolo SA, Doebrick CA, Rounsaville BJ, 2006. The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. J. Consult. Clin. Psychol 74, 955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Lapaglia DM, Peters EN, Easton CJ, Petry NM, 2012. Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: less can be more, more or less. Addiction 107, 1650–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kiluk BD, Nich C, DeVito EE, Decker S, LaPaglia D, Duffey D, Babuscio TA, Ball SA, 2014. Toward empirical identification of a clinically meaningful indicator of treatment outcome: features of candidate indicators and evaluation of sensitivity to treatment effects and relationship to one year follow up cocaine use outcomes. Drug Alcohol Depend. 137, 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Kandel DB, Davies M, 1997. Relationships between frequency and quantity of marijuana use and last year proxy dependence among adolescents and adults in the United States. Drug Alcohol Depend. 46 (1), 53–67. [DOI] [PubMed] [Google Scholar]
- Coffey C, Carlin JB, Degenhardt L, Lynskey M, Sanci L, Patton GC, 2002. Cannabis dependence in young adults: an Australian population study. Addiction 97 (2), 187–194. [DOI] [PubMed] [Google Scholar]
- Compton WM, Han B, Jones CM, Blanco C, Hughes A, 2016. Marijuana use and use disorders in adults in the USA, 2002–14: analysis of annual cross-sectional surveys. Lancet Psychiatry 3, 954–964. [DOI] [PubMed] [Google Scholar]
- Cooper K, Chatters R, Kaltenthaler E, Wong R, 2015. Psychological and psychosocial interventions for cannabis cessation in adults: a systematic review short report. Health Technol. Assess. (Rockv) 19, 1–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J, Swift W, Roffman R, Stephens R, 2001. A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. J. Subst. Abuse Treat 21, 55–64 discussion 65–66. [DOI] [PubMed] [Google Scholar]
- Copeland J, Rooke S, Rodriquez D, Norberg MM, Gibson L, 2017. Comparison of brief versus extended personalised feedback in an online intervention for cannabis users: short-term findings of a randomised trial. J. Subst. Abuse Treat 76, 43–48. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Bukstein OG, Douaihy AB, Clark DB, Chung TA, Daley DC, Wood DS, Brown SJ, 2010. Double-blind fluoxetine trial in comorbid MDD-CUD youth and young adults. Drug Alcohol Depend. 112, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R, 2013. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol. Rev 23, 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttler C, Spradlin A, 2017. Measuring cannabis consumption: psychometric properties of the daily sessions, frequency, age of onset, and quantity of Cannabis use inventory (DFAQ-CU). PLoS One 12, e0178194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Dios MA, Herman DS, Britton WB, Hagerty CE, Anderson BJ, Stein MD, 2012. Motivational and mindfulness intervention for young adult female marijuana users. J. Subst. Abuse Treat 42, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, 2012. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet 379, 55–70. [DOI] [PubMed] [Google Scholar]
- Dennis M, Godley SH, Diamond G, Tims FM, Babor T, Donaldson J, Liddle H, Titus JC, Kaminer Y, Webb C, Hamilton N, Funk R, 2004. The Cannabis youth treatment (CYT) study: main findings from two randomized trials. J. Subst. Abuse Treat 27, 197–213. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L, 1974. The hopkins symptom checklist (HSCL): a self-report symptom inventory. Behav. Sci 19, 1–15. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Bigelow GE, Brigham GS, Carroll KM, Cohen AJ, Gardin JG, Hamilton JA, Huestis MA, Hughes JR, Lindblad R, Marlatt GA, Preston KL, Selzer JA, Somoza EC, Wakim PG, Wells EA, 2012. Primary outcome indices in illicit drug dependence treatment research: systematic approach to selection and measurement of drug use end-points in clinical trials. Addiction 107, 694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BW, Johns MW, Lancaster R, Raptopoulos P, Angelopoulos N, Priest RG, 1981. The St. mary’s hospital sleep questionnaire: a study of reliability. Sleep 4, 93–97. [DOI] [PubMed] [Google Scholar]
- Falk D, Wang XQ, Liu L, Fertig J, Mattson M, Ryan M, Johnson B, Stout R, Litten RZ, 2010. Percentage of subjects with no heavy drinking days: evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol. Clin. Exp. Res 34, 2022–2034. [DOI] [PubMed] [Google Scholar]
- Fischer B, Russell C, Sabioni P, van den Brink W, Le Foll B, Hall W, Rehm J, Room R, 2017. Lower-risk cannabis use guidelines: a comprehensive update of evidence and recommendations. Am. J. Public Health 107, e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates PJ, Norberg MM, Copeland J, Digiusto E, 2012. Randomized controlled trial of a novel cannabis use intervention delivered by telephone. Addiction 107, 2149–2158. [DOI] [PubMed] [Google Scholar]
- Gates PJ, Sabioni P, Copeland J, Le Foll B, Gowing L, 2016. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst. Rev, Cd005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Watson NL, Christie DK, 2009. Challenges in quantifying marijuana use. Am. J. Addict 18, 178–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, McRae-Clark AL, Brady KT, 2012. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am. J. Psychiatry 169, 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Sonne SC, McClure EA, Ghitza UE, Matthews AG, McRae-Clark AL, Carroll KM, Potter JS, Wiest K, Mooney LJ, Hasson A, Walsh SL, Lofwall MR, Babalonis S, Lindblad RW, Sparenborg S, Wahle A, King JS, Baker NL, Tomko RL, Haynes LF, Vandrey RG, Levin FR, 2017. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend. 177, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, 2015. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction 110, 19–35. [DOI] [PubMed] [Google Scholar]
- Haney M, 2005. The marijuana withdrawal syndrome: diagnosis and treatment. Curr. Psychiatry Rep 7, 360–366. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA, 2009. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug Alcohol Depend. 102, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks V, van der Schee E, Blanken P, 2011. Treatment of adolescents with a cannabis use disorder: main findings of a randomized controlled trial comparing multidimensional family therapy and cognitive behavioral therapy in the Netherlands. Drug Alcohol Depend. 119, 64–71. [DOI] [PubMed] [Google Scholar]
- Hill KP, Palastro MD, Gruber SA, Fitzmaurice GM, Greenfield SF, Lukas SE, Weiss RD, 2017. Nabilone pharmacotherapy for cannabis dependence: a randomized, controlled pilot study. Am. J. Addict. 26, 795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorthoj CR, Hjorthoj AR, Nordentoft M, 2012. Validity of Timeline Follow-Back for self-reported use of cannabis and other illicit substances: systematic review and meta-analysis. Addict. Behav 37, 225–233. [DOI] [PubMed] [Google Scholar]
- Hjorthoj CR, Fohlmann A, Larsen AM, Gluud C, Arendt M, Nordentoft M, 2013. Specialized psychosocial treatment plus treatment as usual (TAU) versus TAU for patients with cannabis use disorder and psychosis: the CapOpus randomized trial. Psychol. Med 43, 1499–1510. [DOI] [PubMed] [Google Scholar]
- Hoch E, Noack R, Henker J, Pixa A, Hofler M, Behrendt S, Buhringer G, Wittchen HU, 2012. Efficacy of a targeted cognitive-behavioral treatment program for cannabis use disorders (CANDIS). Eur. Neuropsychopharmacol 22, 267–280. [DOI] [PubMed] [Google Scholar]
- Hoch E, Buhringer G, Pixa A, Dittmer K, Henker J, Seifert A, Wittchen HU, 2014. CANDIS treatment program for cannabis use disorders: findings from a randomized multi-site translational trial. Drug Alcohol Depend. 134, 185–193. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE, 2003. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob. Res 5, 13–25. [PubMed] [Google Scholar]
- Hughes JR, Naud S, Budney AJ, Fingar JR, Callas PW, 2016. Attempts to stop or reduce daily cannabis use: an intensive natural history study. Psychol. Addict. Behav 30, 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J, Lintzeris N, Allsop DJ, Suraev A, Booth J, Carson DS, Helliwell D, Winstock A, McGregor IS, 2014. Lithium carbonate in the management of cannabis withdrawal: a randomized placebo-controlled trial in an inpatient setting. Psychopharmacology 231, 4623–4636. [DOI] [PubMed] [Google Scholar]
- Jungerman FS, Andreoni S, Laranjeira R, 2007. Short term impact of same intensity but different duration interventions for cannabis users. Drug Alcohol Depend. 90, 120–127. [DOI] [PubMed] [Google Scholar]
- Kadden RM, Litt MD, Kabela-Cormier E, Petry NM, 2007. Abstinence rates following behavioral treatments for marijuana dependence. Addict. Behav 32, 1220–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminer Y, Burleson JA, Burke R, Litt MD, 2014. The efficacy of contingency management for adolescent cannabis use disorder: a controlled study. Subst. Abuse 35, 391–398. [DOI] [PubMed] [Google Scholar]
- Kaminer Y, Ohannessian CM, Burke RH, 2017. Adolescents with cannabis use disorders: adaptive treatment for poor responders. Addict. Behav 70, 102–106. [DOI] [PubMed] [Google Scholar]
- Killeen TK, McRae-Clark AL, Waldrop AE, Upadhyaya H, Brady KT, 2012. Contingency management in community programs treating adolescent substance abuse: a feasibility study. J. Child Adolesc. Psychiatr. Nurs 25, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk BD, Nich C, Witkiewitz K, Babuscio TA, Carroll KM, 2014. What happens in treatment doesn’t stay in treatment: cocaine abstinence during treatment is associated with fewer problems at follow-up. J. Consult. Clin. Psychol 82, 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk BD, Carroll KM, Duhig A, Falk DE, Kampman K, Lai S, Litten RZ, McCann DJ, Montoya ID, Preston KL, Skolnick P, Weisner C, Woody G, Chandler R, Detke MJ, Dunn K, Dworkin RH, Fertig J, Gewandter J, Moeller FG, Ramey T, Ryan M, Silverman K, Strain EC, 2016. Measures of outcome for stimulant trials: ACTTION recommendations and research agenda. Drug Alcohol Depend. 158, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk BD, Babuscio TA, Nich C, Carroll KM, 2017. Initial validation of a proxy indicator of functioning as a potential tool for establishing a clinically meaningful cocaine use outcome. Drug Alcohol Depend. 179, 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Simon AH, Falk DE, Litten RZ, Mertens JR, Fertig J, Ryan M, Weisner CM, 2013. Posttreatment low-risk drinking as a predictor of future drinking and problem outcomes among individuals with alcohol use disorders. Alcohol. Clin. Exp. Res 37 (Suppl. 1), E373–E380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Simon AH, Litten RZ, Weisner CM, Falk DE, 2017. Posttreatment low-risk drinking as a predictor of future drinking and problem outcomes among individuals with alcohol use disorders: a 9-year follow-up. Alcohol. Clin. Exp. Res 41, 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascaux M, Ionescu S, Phan O, 2016. Effectiveness of formalised therapy for adolescents with cannabis dependence: a randomised trial. Drugs 23, 404–409. [Google Scholar]
- Levin FR, McDowell D, Evans SM, Nunes E, Akerele E, Donovan S, Vosburg SK, 2004. Pharmacotherapy for marijuana dependence: a double-blind, placebo-controlled pilot study of divalproex sodium. Am. J. Addict 13, 21–32. [DOI] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV, 2011. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 116, 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Mariani J, Brooks DJ, Pavlicova M, Nunes EV, Agosti V, Bisaga A, Sullivan MA, Carpenter KM, 2013. A randomized double-blind, placebo-controlled trial of venlafaxine-extended release for co-occurring cannabis dependence and depressive disorders. Addiction 108, 1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Pavlicova M, Brooks D, Glass A, Mahony A, Nunes EV, Bisaga A, Dakwar E, Carpenter KM, Sullivan MA, Choi JC, 2016. Dronabinol and lofexidine for cannabis use disorder: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 159, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Stephens RS, 2005. Coping and self-efficacy in marijuana treatment: results from the Marijuana Treatment Project. J. Consult. Clin. Psychol 73 (6), 1015. [DOI] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Petry NM, 2013. Behavioral treatment for marijuana dependence: randomized trial of contingency management and self-efficacy enhancement. Addict. Behav 38, 1764–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod J, Oakes R, Copello A, Crome I, Egger M, Hickman M, Oppenkowski T, Stokes-Lampard H, Davey Smith G, 2004. Psychological and social sequelae of cannabis and other illicit drug use by young people: a systematic review of longitudinal, general population studies. Lancet 363, 1579–1588. [DOI] [PubMed] [Google Scholar]
- Madigan K, Brennan D, Lawlor E, Turner N, Kinsella A, O’Connor JJ, 2013. A multi-centre, randomised control trial of a group psychological intervention for psychosis with comorbid cannabis dependence over the early course of the illness. Schizophr. Res 143, 138–142. [DOI] [PubMed] [Google Scholar]
- Marijuana Treatment Project Research Group, 2004. Brief treatments for cannabis dependence: findings from a randomized multisite trial. J. Consult. Clin. Psychol 72, 455–466. [DOI] [PubMed] [Google Scholar]
- Marshall K, Gowing L, Ali R, Le Foll B, 2014. Pharmacotherapies for cannabis dependence. Cochrane Database Syst. Rev, Cd008940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, Buffkins K, Kyle M, Adusumalli M, Begovic A, Rao S, 2012. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology 37, 1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M, 1992. The fifth edition of the addiction severity index. J. Subst. Abuse Treat 9, 199–213. [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, Wahlquist AE, Simpson SA, Brady KT, 2009. A placebo-controlled trial of buspirone for the treatment of marijuana dependence. Drug Alcohol Depend. 105, 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, White KG, Brady KT, 2010. A placebo-controlled trial of atomoxetine in marijuana-dependent individuals with attention deficit hyperactivity disorder. Am. J. Addict 19, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Gray KM, Killeen TK, Wagner AM, Brady KT, DeVane CL, Norton J, 2015. Buspirone treatment of cannabis dependence: a randomized, placebo-controlled trial. Drug Alcohol Depend. 156, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Gray KM, Killeen T, Hartwell KJ, Simonian SJ, 2016. Vilazodone for cannabis dependence: a randomized, controlled pilot trial. Am. J. Addict 25, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R Jr, Treloar H, Blanchard A, Justus A, Monti PM, Chun T, Swift R, Tidey JW, Gwaltney CJ, 2017. Topiramate and motivational enhancement therapy for cannabis use among youth: a randomized placebo-controlled pilot study. Addict. Biol 22, 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg MM, Mackenzie J, Copeland J, 2012. Quantifying cannabis use with the Timeline Followback approach: a psychometric evaluation. Drug Alcohol Depend. 121 (3), 247–252. [DOI] [PubMed] [Google Scholar]
- Pacek LR, Mauro PM, Martins SS, 2015. Perceived risk of regular cannabis use in the United States from 2002 to 2012: differences by sex, age, and race/ethnicity. Drug Alcohol Depend. 149, 232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen ER, Grow J, Duncan S, Neighbors C, Larimer ME, 2012. Concurrent validity of an online version of the Timeline Followback assessment. Psychol. Addict. Behav 26, 672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penetar DM, Looby AR, Ryan ET, Maywalt MA, Lukas SE, 2012. Bupropion reduces some of the symptoms of marihuana withdrawal in chronic marihuana users: a pilot study. Subst. Abuse Res. Treat 6, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EN, Nich C, Carroll KM, 2011. Primary outcomes in two randomized controlled trials of treatments for cannabis use disorders. Drug Alcohol Depend. 118, 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigter H, Henderson CE, Pelc I, Tossmann P, Phan O, Hendriks V, Schaub M, Rowe CL, 2013. Multidimensional family therapy lowers the rate of cannabis dependence in adolescents: a randomised controlled trial in Western European outpatient settings. Drug Alcohol Depend. 130, 85–93. [DOI] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, Leo GI, 2014. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol. Addict. Behav 28, 154–162. [DOI] [PubMed] [Google Scholar]
- Roffman RA, Stephens RS, Simpson EE, Whitaker DL, 1988. Treatment of marijuana dependence: preliminary results. J. Psychoactive Drugs 20, 129–137. [DOI] [PubMed] [Google Scholar]
- Roffman RA, Stephens RS, Simpson EE, 1989. Relapse prevention with adult chronic marijuana smokers. J. Chem. Depend. Treat 2, 241–257. [Google Scholar]
- Rooke S, Copeland J, Norberg M, Hine D, McCambridge J, 2013. Effectiveness of a self-guided web-based cannabis treatment program: randomized controlled trial. J. Med. Internet Res 15, e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), 2017a. Key Substance Use and Mental Health Indicators in the United States: Results From the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17–5044, NSDUH Series H-52). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), Center for Behavioral Health Statistics and Quality, 2017b. Treatment Episode Data Set (TEDS): 2005–2015 National Admissions to Substance Abuse Treatment Services. BHSIS Series S-91, HHS Publication No. (SMA) 17–5037. Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Schwilke EW, Gullberg RG, Darwin WD, Chiang CN, Cadet JL, Gorelick DA, Pope HG, Huestis MA, 2011. Differentiating new cannabis use from residual urinary cannabinoid excretion in chronic, daily cannabis users. Addiction 106, 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BJ, McRae-Clark AL, 2016. Treatment of cannabis use disorder: current science and future outlook. Pharmacotherapy 36, 511–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BJ, Baker NL, McRae-Clark AL, 2017. Effect of oxytocin pretreatment on cannabis outcomes in a brief motivational intervention. Psychiatry Res. 249, 318–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Easton C, Renee-Aubin L, Carroll KM, 2003. Engaging young probation-referred marijuana-abusing individuals in treatment: a pilot trial. Am. J. Addict 12, 314–323. [PubMed] [Google Scholar]
- Snaith RP, Constantopoulos AA, Jardine MY, McGuffin P, 1978. A clinical scale for the self-assessment of irritability. Br. J. Psychiatry 132, 164–171. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline Follow-back: Measuring Alcohol Consumption. Springer, pp. 41–72. [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB, 1996. The reliability of the alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 42, 49–54. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Connors GJ, Agrawal S, 2003. Assessing drinking outcomes in alcohol treatment efficacy studies: selecting a yardstick of success. Alcohol. Clin. Exp. Res 27, 1661–1666. [DOI] [PubMed] [Google Scholar]
- Stanger C, Budney AJ, Kamon JL, Thostensen J, 2009. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug Alcohol Depend. 105, 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Scherer EA, Norton GE, Budney AJ, 2015. Clinic- and homebased contingency management plus parent training for adolescent cannabis use disorders. J. Am. Acad. Child Adolesc. Psychiatry 54, 445–453 e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE, 1994. Treating adult marijuana dependence: a test of the relapse prevention model. J. Consult. Clin. Psychol 62, 92–99. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L, 2000. Comparison of extended versus brief treatments for marijuana use. J. Consult. Clin. Psychol 68, 898–908. [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM, 1989. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for Alcohol Scale (CIWA-Ar). Br. J. Addict 84, 1353–1357. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Friedman L, Greenfield SF, Hasin DS, Jackson R, 2012. Beyond drug use: a systematic consideration of other outcomes in evaluations of treatments for substance use disorders. Addiction 107, 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tossmann HP, Jonas B, Tensil MD, Lang P, Struber E, 2011. A controlled trial of an internet-based intervention program for cannabis users. Cyberpsychol. Behav. Soc. Netw 14, 673–679. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Soliman A, Quilty LC, Fischer B, Rehm J, Selby P, Barnes AJ, Huestis MA, George TP, Streiner DL, Staios G, Le Foll B, 2018. Nabiximols combined with motivational enhancement/cognitive behavioral therapy for the treatment of cannabis dependence: a pilot randomized clinical trial. PLoS One 13, e0190768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC), 2017. World Drug Report 2017 . http://www.unodc.org/wdr2017/field/Booklet_3_Plantbased.pdf.
- Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, Bloomfield MA, Curran HV, Baler R, 2016. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry 73, 292–297. [DOI] [PubMed] [Google Scholar]
- Walker DD, Stephens RS, Towe S, Banes K, Roffman R, 2015. Maintenance checkups following treatment for cannabis dependence. J. Subst. Abuse Treat 56, 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein AM, Miller H, Bluvstein I, Rapoport E, Schreiber S, Bar-Hamburger R, Bloch M, 2014. Treatment of cannabis dependence using escitalopram in combination with cognitive-behavior therapy: a double-blind placebo-controlled study. Am. J. Drug Alcohol Abuse 40, 16–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.