Abstract
It is now appreciated that in addition to the immunoglobulin (Ig)M and D isotypes fish also make the mucosal IgT. In this study we sequenced the full length of Ig τ as well as μ in the commercially important Thunnus orientalis (Pacific bluefin tuna), the first molecular analysis of these two Ig isotypes in a member of the order Perciformes. Tuna IgM and IgT are each composed of four constant (CH) domains. We cloned and sequenced 48 different variable (VH) domain gene rearrangements of tuna immunoglobulins and grouped the VH gene sequences to four VH gene segment families based on 70% nucleotide identity. Three VH gene families were used by both IgM and IgT but one group was only found to be used by IgM. Most interestingly, both μ and τ clones appear to use the same diversity (DH) segment, unlike what has been described in other species, although they have dedicated IgT and IgM joining (JH) gene segments. We complemented this repertoire study with phylogenetic and tissue expression analysis. In addition to supporting the development of humoral vaccines in this important aquaculture species, these data suggest that the DH-JH recombination rather than the VH-DH recombination may be instructive for IgT versus IgM/D bearing lymphocyte lineages in some fish.
Keywords: immunoglobulin, IgZ/T, isotype exclusion, evolution, tuna
1. Introduction
The immunoglobulin (Ig) superfamily-based adaptive immune system evolved in cartilaginous fish (sharks and skates) and is maintained in all jawed vertebrates (Flajnik and Rumfelt, 2000). One of the major characteristics of this adaptive immune system is the production of a repertoire of antibodies through somatic V(D)J recombination of the loci that encode them. While mammals possess five functionally distinct Ig isotypes (IgM, IgD, IgG, IgA and IgE), teleost fish have only three: IgM, IgD and IgT (Danilova et al., 2005; Fillatreau et al., 2013; Hansen et al., 2005; Wilson et al., 1997).).
IgT was concomitantly discovered in trout (Oncorhynchus mykiss) and zebrafish (Danio rerio, where it was given the appellative IgZ) and IgT or forms of Ig with IgT domains have since been described in fugu (Fugu rubripes) (Savan et al., 2005b), carp (Cyprinus carpio) (Savan et al., 2005a), and stickleback (Gasterosteus aculeatus) (Gambon-Deza et al., 2010). IgT perhaps exists in most teleost groups, although it has yet to be found in catfish (Bengten et al., 2006; Salinas et al., 2011) and medaka (Magadan-Mompo et al., 2011). So far IgT is an isotype restricted to bony fish, and sequence characteristics (Hansen et al., 2005), gut localization and functional work (Zhang et al., 2010) have suggested that it is a dedicated mucosal isotype (Zhang et al., 2011), functionally analogous but not orthologous with IgX/A of tetrapods (Mashoof et al., 2013). IgT was found to be expressed in gill of Chinese perch (Siniperca chuatsi) (Tian et al., 2009), IgT positive cells were identified in the epithelium of trout gill lamellae (Olsen et al., 2011), and clonal IgT responses were induced to trout viral pathogens (Castro et al., 2013), all further supporting the idea of this isotype filling a mucosal role in teleost humoral adaptive immunity. The IgT encoding DH-JH-CH elements are located 5’ of the μ and δ DH-JH-CH regions in the fish genomes in which it has been studied, with most or all VH genes 5’ to the τ block (Danilova et al., 2005; Gambon-Deza et al., 2010; Savan et al., 2005b). Although class switch recombination has been described in shark (Zhu et al., 2012) and fish activation-induced cytidine deaminase (AID) is competent to induce somatic hypermutation and class switch in mammalian cells (Wakae et al., 2006), it does not appear that teleosts employ this for Ig heavy (H) chain isotype switching, instead they use deletional VH(DH)JH rearrangement to remove τ in IgM and IgD expressing cells and differential RNA splicing to control expression of IgM and IgD (Hikima et al., 2011), the τ/μ rearrangement appearing to have influence on lineage commitment similarly to the mechanism operating at the T cell receptor αδ locus.
We recently turned our attentions to the expressed IgH transcripts of the Pacific bluefin tuna (Thunnus orientalis). Thunnus species are the most valuable global aquaculture product (Ottolenghi, 2008), yet infections from several groups of parasites plague high intensity tuna mariculture ranches (Fromentin and Powers, 2005), impeding the industry from optimal relief of fishing pressures upon wild adult stocks. In addition to their economic importance, the extreme physiological specializations of these migratory apex predators made their Ig of interest to us. Tuna are among the fastest fish and have countercurrent heat exchangers that minimize convective heat loss to maintain a form of endothermy distinct from that of birds and mammals (Block et al., 2001; Jusup et al., 2011). Specifically, we were curious whether tuna Ig harbored any special adaptations evident in their primary amino acid sequence to this rare form of fish endothermy.
Here we report the first full-length μ and τ sequences from tuna. We have analyzed representative clones of the expressed variable domain repertoire of these isotypes, performed phylogenetic analysis of the IgH genes of this modern teleost, and analyzed their relative expression in tuna primary and secondary lymphoid tissues, including the mucosal gill. Our results demonstrate that these fish employ the same Ig VH gene families as other teleosts, can use the same VH genes in both IgM and IgT heavy chains, make diverse IgH complementarity determining region (CDR)3 regions, and surprisingly employ the same DH segment in both τ and μ rearrangements in what appears to be a previously undescribed mechanism of B cell isotype determination.
2. Methods
2.1. Animals and collection of tissues
Sample tissues of spleen, gill and kidney from ranched T. orientalis were collected during the regular slaughter process from two different commercial tuna facilities located off the coast of Ensenada, Baja California, Mexico. At the time of harvest, fish weight and fork length were 16.2 ± 6.5 kg and 96.3 ± 14.3 cm, respectively. Samples were placed in RNAlater (Qiagen, Valencia CA), frozen in liquid nitrogen, shipped to Texas A&M on dry ice and stored at −80°C until further use.
2.2. Total RNA isolation and cDNA synthesis
Total RNA was purified from spleen, gill and head kidney (pronephros, or anterior kidney) (35 mg from each tissue) using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instruction. The quantity and quality of the RNA samples were assessed by NanoDrop 2000c spectrophotometer (Thermo Scientific, Wilmington, DE) and Agilent 2100 Bioanalyzer (Agilent, Santa Clara CA) respectively. Message representation of RNA was assessed by PCR of common (β-actin) and less common transcripts (TNF-α, IL1-β), using previously published primer sets (Mladineo and Block, 2009). The GeneRacer kit (Life Technologies, Grand Island NY) with GeneRacer oligo dT and gene specific primers was used to produce 5’ rapid amplification of cDNA ends (RACE) PCR products. Pools of 3’ RACE products were synthesized by Superscript III First-Strand Synthesis SuperMix kit (Life Technologies) using the oligo dT primer.
2.3. IgH RACE PCR, cloning, and sequencing
5’ and 3’ RACE products were amplified by standard PCR using various combinations of 5’ GeneRacer (as forward primer in 5’RACE), Oligo dT (as reverse primer in 3’RACE), and specifically designed primers for the conserved regions encoding the C domains of T. orientalis IgM and IgT (as forward or reverse for 3’ RACE or 5’ RACE, respectively). Primers are listed in Supplemental Table 1. The PCR conditions were as follows: one cycle of 95°C for 2 minutes, 35 cycles of 95°C for 30 seconds, 50–53°C for 30 seconds, 72°C for 2 minutes, followed by one cycle of 72°C for 7 minutes. The amplicons were purified from a 0.8% agarose gel after electrophoresis in tris/acetic acid/EDTA (TAE) buffer, cloned into pCR II vector with the TOPO TA cloning kit (Life Technologies), and transformed into chemically competent TOP10 Escherichia coli cells (Invitrogen). Colonies were picked based on blue/white screening produced by X-Gal (Sigma-Aldrich, Saint Louis MO). The plasmid DNA was purified using Zyppy Plasmid Miniprep kit (Zymo Research Corporation, Irvine CA) and was digested with EcoRI (Promega, Madison, WI) to identify clones with inserts. Products for sequencing were amplified using either M13 forward or reverse primers, purified using ABI BigDye X terminator purification kit (Life Technologies), and sequenced by the DNA Technologies Core lab of the Department of Veterinary Pathobiology at Texas A&M University.
2.4. Sequence analysis of μ and τ gene rearrangements in Pacific bluefin tuna
BLASTX (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and visual inspection were used to identify the Ig isotype as well as VH, JH and CH sequences of tuna amplicons based upon homology to those from representative fish and other vertebrates. The amino acid sequences were blasted to discriminate the VH segments and CH domains. SignalP 4.1 was used to determine the leader peptides (Emanuelsson et al., 2007). Three prediction methods concurred upon the cleavage site of the representative μVH (after the 18th residue) and were in less agreement for τVH (after the 20th residue) as shown in Supplemental Figures 1 and 2. Sequences were translated with Expasy translate tool (http://web.expasy.org/translate/), and the Clustal W program in Bioedit was employed to align amino acid sequences (http://www.mbio.ncsu.edu/bioedit/bioedit.html) for figures. Sequences were managed and assembled in Bioedit and have been deposited in Genbank under accession numbers KF713322-KF713372. CDR3 length was calculated using the “CDR3 length = exclusive number of amino acids from C (of VH segment Y×C) to F (of JH segment FG×G)” IMGT formula (Lefranc et al., 2003).
2.5. Phylogenetic studies
Amino acid alignments were made with ClustalW employing gap opening penalties of 10 and gap extension penalties of 0.1 for pairwise alignments, then 0.2 for multiple alignments using a Dayhoff matrix based method (Schwarz and Dayhoff, 1979). Phylogenetic trees were constructed using MEGA 5 software (Tamura et al., 2011). Neighbor joining trees using the substitution method of Jones, Taylor and Thornton (Jones et al., 1992) and pairwise deletion of empty positions were constructed from alignments of VH and CH domain sequences.. Trees were bootstrapped 1000 times (Koichiro Tamura, 2011) and were viewed and adjusted using the Treeview Software (Page, 2002).
2.6. Real time quantitative PCR
Oligo-dT transcribed cDNA samples from spleen, gill and anterior kidney were assayed for levels of μ and τ message using β-actin as a constitutively expressed control. Real-time PCR reactions were performed using 25 and 50ng of cDNA with SYBR Advantage qPCR Premix (Clontech, Mountain View, C A) per the manufacturer’s instructions. Primers were designed to span across introns. Using a Roche LightCycler 480 a three-step thermal cycling program was followed: 1 cycle at 95°C for 5 minutes, then 45 cycles of 95°C for 10 seconds, then 60°C for 5 seconds, then 72°C for 5 seconds. The Roche LightCycler software was utilized for raw data acquisition and calculation of Ct (threshold cycle) values. Changes in gene expression were estimated using the 2−ΔΔCt method (Livak and Schmittgen, 2001), with β-actin utilized as the stable reference gene for all experimental situations. The fold changes in gene expression were calculated with respect to the expression level of the genes in the anterior kidney (the primary B lymphopoietic tissue of bony fish).
3. Results
3.1. Characterization of μ cDNA of T. orientalis
The initial full length tuna μ was cloned and sequenced using a cDNA RACE library that was obtained from RNA pooled from several tuna anterior kidney, spleen and gill samples. The secretory tuna μ sequence shown in Supplemental Figure 1 is an 1827 bp open reading frame which encodes a 609 amino acid protein containing a leader peptide of 18 residues, one Ig VH and four CH domains. The primary amino acid sequence showed two cysteine residues (and intervening tryptophan) conserved for intra-domain disulfide bond formation present in each of the Ig domains with the cysteines being spaced by approximately 70 residues in the VH domain and 60 in the CH domains. The amino-terminal cysteine in the CH1 domain forms an interdomain disulfide bond between the IgH chain to the IgL chain. The potential N-linked glycosylation site near the carboxyl terminus of the IgM chain w as found at this position of the tuna IgM (Danilova N, 2005).
3.2. Characterization of tuna IgT
While sequencing 3’ RACE PCR products employing VH primers designed from μ clones we found other clones with Ig CH region amino acid sequences distinct from IgM, although they often shared a VH domain highly homologous with μ clones. The CH1 domain of these clones shares 56% amino acid identity with the CH1 of Siniperca chuatsi. More primers allowed the complete cloning of the IgT encoding cDNAs, with CH3 proving to be even more definitively of the isotype (60% identical amino acids to S. chuatsi) (Supplemental Figure 2).
The secretory tuna τ cDNA is composed of 1614 base pairs translating to 539 amino acids forming a leader peptide, VH domain and four CH domains. As in tuna IgM, two conserved cysteine residues and one tryptophan were identified in the VH and each CH domain which are important for folding of the β-sandwich Ig domains. There is also one conserved cysteine in the CH1 domain which forms a disulfide covalent linkage between the IgH chain to the IgL chain. The secretory tail of tuna IgT is composed of 12 amino acids.
3.3. IgHμ and τ VH, DH and JH segments
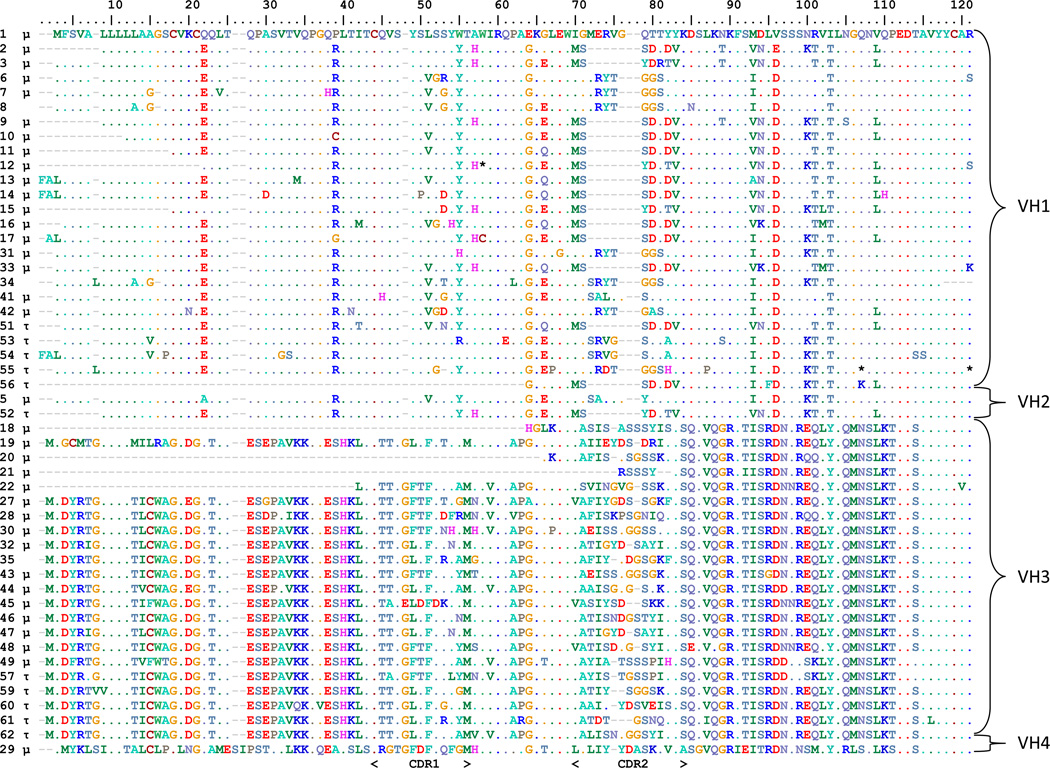
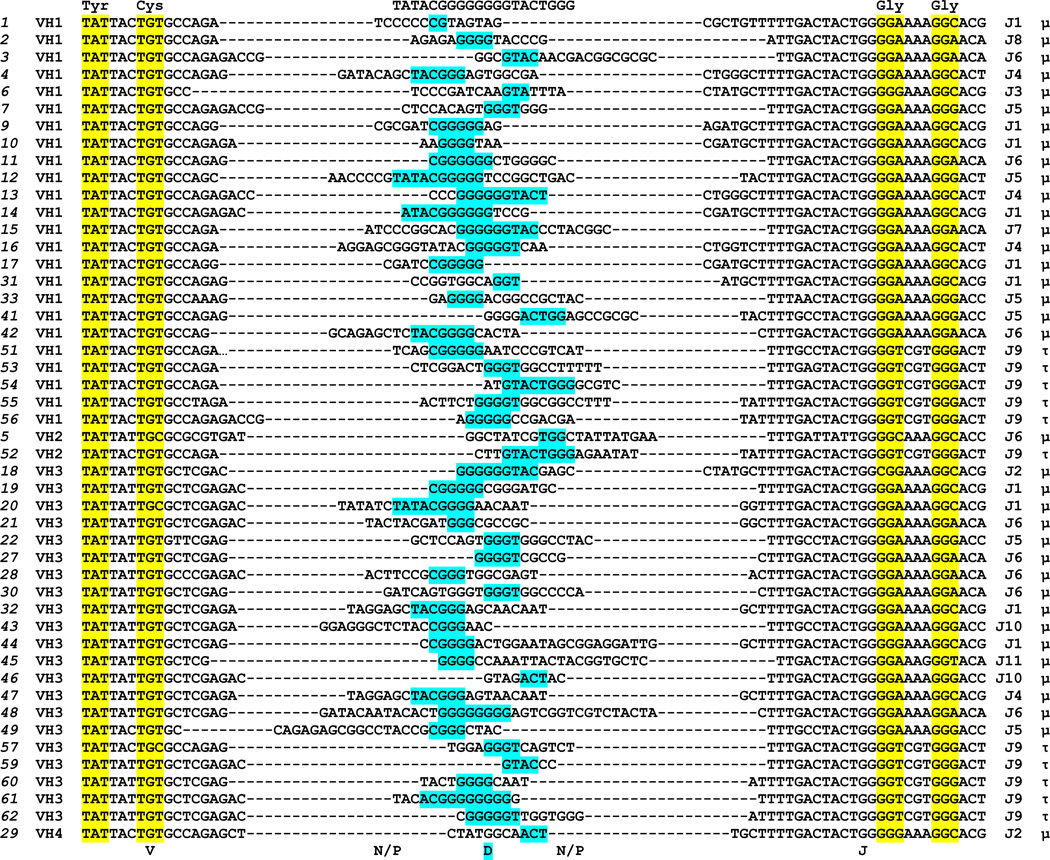
The same cDNA pools were used to examine tuna IgH μ and τ VH(DH)JH rearrangement diversity. In total 50 different sequences encoding VH domains (Figure 1) that possessed full or partial unique VH regions were cloned, 36 spliced to μ CH regions and 11 with τ (three contained complete VH regions but were incompletely rearranged or did not splice to a CH). Based on percent identity the VH segment sequences were divided into four separate families of IgH V genes. Members of each family were more than 70% identical in their nucleotide sequences (Brodeur and Riblet, 1984; Pascual and Capra, 1991) (Supplemental Figure 3). Analysis of the carboxy-terminal portion of the VH domains gave insight into the DH and JH gene segments used to rearrange mature VH exons. We predicted 11 different JH segments used in these clones and one DH segment (TATACGGGGGGGGTACTGGG) could be identified in the 48 unique CDR3 encoding rearrangements analyzed (Figure 2). The one DH segment apparently was employed by both isotopes, as various stretches of the sequence (including portions at each end) are found in both μ and τ clones. The predicted DH germline nucleotide contribution to the final expressed CDR3 encoding sequence ranges from 3 to 10 with a mean of 5.5 base pairs. All three reading frame of the D segment were used (Figure 3). The τ clones all used a dedicated JH segment (J9).
Figure 1. Four VH families used by tuna IgH.
Amino acid alignment of VH segment encoded sequences found within T. orientalis μ and τ cDNAs. Clone numbers are shown to the left and VH family designations are shown to the right. VH gene segment families were ascribed based upon 70% nucleotide identity in a pairwise matrix (Supplemental Figure 2). Gaps introduced into the alignments are indicated by dashes (“-“) and identity to the first sequence is indicated by a period (“.”) in the column. CDR1 and CDR2 are indicated below the alignment. If clone contained CH region encoding region, μ or τ is indicated at left of sequence after the clone name.
Figure 2. Tuna VH(DH)JH junctional diversity.
Nucleotide alignment arranged by VH family VH(DH)JH junctional region. Clone names and VH family are denoted on the left, JH gene and CH region is given to the right. Conserved tyrosine and cysteine codons of Y×C motif of VH segment as well as G×G glycines of JH gene are highlighted in yellow. Predicted DH segment is highlighted in blue.
Figure 3. Translated complementarity determining region (CDR) 3 repertoire sampling of tuna IgH.
Amino acid alignment arranged by JH gene of the VH(DH)JH junctional region. Clone names and VH family are denoted on the left, JH gene, reading frame of DH used and CH region is given to the right. Conserved tyrosine and cysteine of Y×C motif of VH segment as well as G×G glycines of JH gene are highlighted in yellow. Predicted DH segment is highlighted in green, blue or magenta depending on the use of reading frame one, two or three, respectively, in panel B. Amino acids were assigned to VH, DH or JH based on at least two bases of codon matching consensus, grey highlighting indicates a residue partially encoded by D consensus that does not encode consensus amino acid.
IgH CDR3 is the crucial loop in the paratope of most antibody antigen interactions. This sample of the tuna Ig heavy chains expressed at the mRNA level allowed an initial analysis of the length of IgH CDR3 of μ and τ. Table 1 shows that tuna μ clones display a broader range of CDR3 lengths (from 9 to 18aa) as well as an average of one amino acid longer CDR3 length than those found in tuna τ.
Table 1.
CDR3 Lengths in amino acids
| IgM | IgT | |
|---|---|---|
| Maximum | 18 | 13 |
| Minimum | 9 | 8 |
| Range | 9 | 5 |
| Median | 12 | 11 |
| Mean | 12.49 | 11.00 |
| Variance | 3.31 | 2.00 |
3.4. IgM and IgT CH regions of tuna
The tuna IgM CH region amino acid sequence showed the most identity to the mandarin fish (S. chuatsi, also known as the Chinese perch and also a member of the Order Perciformes) with 53.6% and then to the rainbow trout with 39.8% identity and presented the least with chicken (23.8% identity) amongst the sequences we included in our analysis (Figure 4). The tuna IgT CH region has the highest identity also to that of the mandarin fish with 52.5% and the least to grass carp with 20.4% (Figure 5). Unlike the cyprinid grass carp and zebrafish, the CH3 domain of tuna IgT conforms to the canonical immunoglobulin domain fold with cysteines and tryptophans in positions common for β-sandwich tertiary structure.
Figure 4. Amino acid sequence alignment of the heavy chain of IgM in different vertebrate species.
The conserved (identical and similar) residues are highlighted in black. Arrows indicate CH1–CH4 and the secretory tail. An asterisk (*) is above the conserved cysteine that forms a disulfide bond with the light chain, a carrot (^) is above conserved cysteines that form intra-domain disulfide bonds. Gaps are indicated by dashes. Genbank accession numbers are: AAQ14846.1 Siniperca chuatsi (Chinese perch), A45804 Ictalurus punctatus (channel catfish), AF281480_1 Danio rerio (zebrafish), ADC45388.1 Latris lineata (striped trumpeter), AAW66973.1 Oncorhynchus mykiss (rainbow trout), AF226284_1 Paralichthys olivaceus (flounder), AAB24064.1 Salmo salar (salmon), AAU04507.1 Ginglymostoma cirratum (nurse shark), AAA49774.1 Xenopus laevis (African clawed frog), CAC43280.1 Anas platyrhyncho (duck), AAN60017.1 Bos taurus (cattle), and AAS01769.1 Homo sapiens (human).
Figure 5. Amino acid sequence alignment of the heavy chain of IgT CH domain.
The conserved (identical and similar) residues are marked in black. Arrows indicate CH1–CH4 and the secretory tail. An asterisk (*) is above the conserved cysteine that forms a disulfide bond with the light chain, a carrot (^) is above conserved cysteine of intra-domain bond. Gaps are indicated by dashes. Genbank accession numbers are: ACZ54909.1 Epinephelus coioides (grouper), ABF19723.1 Ctenopharyngodon idella (grass carp), AAY42141.1 S. chuatsi, ACX50291. S. salar, AAW66981.1 O. mykiss, and CAI20890.1 D. rerio.
3.5. Phylogenetic analysis
To assess the phylogenetic relationship of the tuna Ig VH gene segments with those of other teleosts we created dendrograms with their pairwise genetic distances (Figure 6). The four tuna IgH V gene families interleaved amongst those VH segment sequences used by the other fish, indicating that they are using members of the same ancient VH families that have been conserved by trans-species maintenance since at least the common ancestor of these divergent fish. However tuna families VH1 and VH2 appear to have arisen from a more recent duplication in the order Perciformes.
Figure 6. Tuna IgH V genes shared with other fish.
Phylogenetic analysis of representatives of the four tuna IgH V families (clone 3 for VH1, 5 for VH2, 57 for VH3 and 29 for VH4) with VH genes from two of the better studied teleost models, rainbow trout and zebrafish. Trout and zebrafish accession numbers are labeled at each branch terminus.
We also explored the relationship of these new tuna IgH C regions to those of other fish and other vertebrates (Figure 7). As expected, the tuna IgM grouped with that isotype from other fish, most closely the Perciforme trumpeter fish (Latris lineata). Within the IgM, teleosts group together as a sister group to all of the other vertebrates, including the shark which shares a more ancient common ancestor and would be expected to branch outside of teleosts and tetrapods. However this incongruence with the organisms’ natural history is not unusual for phylogenetic analyses of teleost antigen receptors, unless balancing numbers of operational taxonomic units fill the other vertebrate classes (Criscitiello and Flajnik, 2007). IgT of tuna clusters with that isotype from other representative fish.
Figure 7. Tuna IgM and IgT CH regions group with those isotypes of other teleosts.
Neighbor joining phylogeny using Dayhoff matrix and 1000 bootstrap replications. Alignment and accession numbers used in tree are shown in Figures 5 and 6.
3.6. IgH μ and τ relative tissue expression
Quantitative real-time PCR (Supplemental Figure 4) was used to assess the expression of these isotypes at the mRNA level in secondary lymphoid tissues relative to the anterior kidney (the chief primary lymphoid tissue of fish (Fillatreau et al., 2013; Lam et al., 2004; Trede et al., 2004)). Relative levels of μ were higher than τ in both spleen and gill, but μ did not predominate τ to as great an extent in gill as it did in spleen. The averaged ratio of HCμ to HCτ in tuna spleen was 7.35 compared to 2.89 in the gill.
4. Discussion
4.1. Repertoire
The 20–30% sequence disparity between some VH family members in tuna suggests either ample somatic hypermutation for affinity maturation of these fish antibodies or an older divergence date of VH family members than has been seen in some other teleosts such as stickleback (Gambon-Deza et al., 2010). Families VH1 and VH2 share between 52% and 64% nucleotide identity (Supplemental Figure 3) and appear recently diverged (Figure 6), perhaps within a Perciformes branch including tuna.
Despite the initial report that found shorter CDR3 in trout IgM than IgT (Hansen et al., 2005) we found a small skewing towards shorter IgT CDR3 (Table 1). We predict that this may be an effect of a different immunogenetic rearrangement mechanism involving a single shared DH gene segment that governs τ versus μ/δ in a clade including tuna and other fish (more below). IgH CDR3 often dominates antigen recognition properties of the six CDRs comprising the Fab paratope (Davis, 2004; Xu and Davis, 2000). The three reading frames usually supplied by DH gene segments therefore contribute significantly to the eventual translated repertoire of antigenic specificities. Additionally, extended length of IgH CDR3 has been crucial in many clinically important antibodies against viral scourges (Kwong and Wilson, 2009; McLellan et al., 2011), and the loop has evolved into an entirely new domain in some antibodies of cattle (Wang et al., 2013). Thus, restricting the entire repertoire to rearrangements based on a single DH would be expected to place constraints on antigen recognition.
As Perciformes, tuna belong to the largest order of vertebrates that accounts for approximately 40% of all bony fishes. As T. orientalis is the first Perciformes to have either their IgM and T repertoire or IgH locus analyzed immunogenetically, there may be a great many fish that employ this system for Ig isotype control and B lineage commitment. As successful as the Perciformes have been in radiating to occupy most fresh and saltwater niches on Earth, the potential restriction in CDR3 length variability must not have too great a toll on the fitness of these fish.
4.2. Genomic organization
The generalized translocon configuration of the teleost IgH locus with a set of VH genes and downstream μ and δ CH regions has been confirmed in many studies (Bengten et al., 2002; Jørgensen, 2000; Samuel Aparicio et al., 2002), but many deviations on the theme are present as catfish and medaka appear to lack τ and many fish have duplications of blocks of the locus (Fillatreau et al., 2013). Although reported in shark (Zhu et al., 2012), class switch recombination (CSR) has not been described in a teleost. However, one study showed that teleost AID could induce CSR in mouse (Vasco M. Barreto et al., 2005).
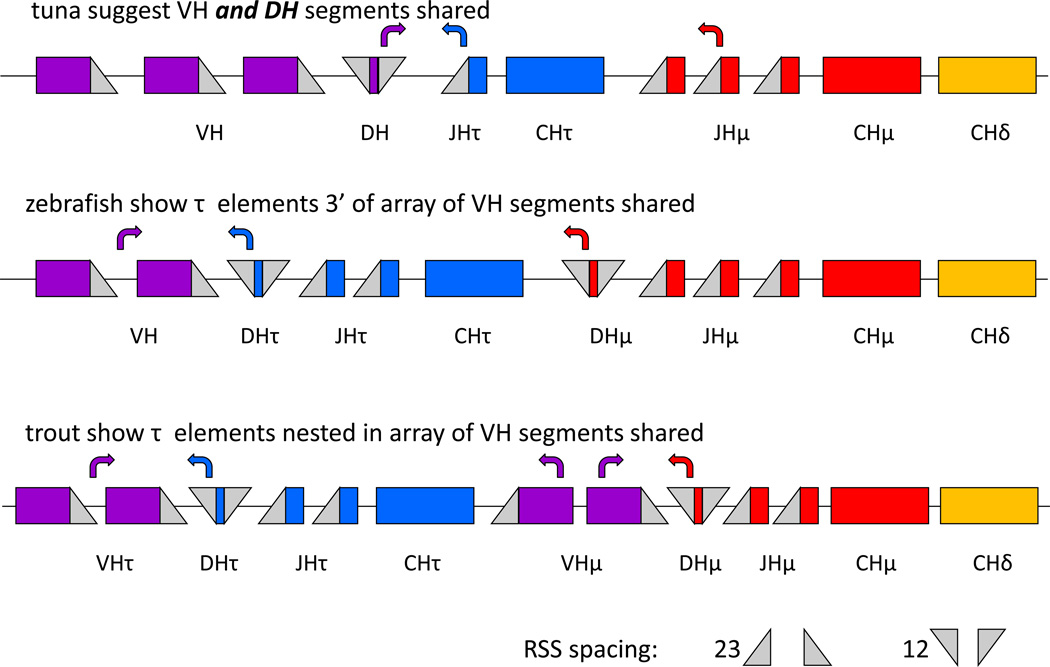
The IgH τ gene together with its dedicated DH and JH gene segments are located between the VH gene segment block and the (DH-JH-CH) μ cluster in zebrafish, fugu (Takifugu rubripes) and three-spined stickleback (Gasterosteus aculeatus) (Danilova et al., 2005; Danilova et al., 2011; Gambon-Deza et al., 2010; Hansen et al., 2005), or it is inserted within the VH gene segment array as in rainbow trout (John D. Hansen, 2005). Thus, in these fish the RAG mediated joining of a VH gene segment to either DH of τ or DH of μ/δ will determine whether the developing pro-B lymphocyte (using mammalian convention) becomes an IgT or IgM/D producer. Experiments in zebrafish (Schorpp et al., 2006) and trout (Zhang et al., 2010) have demonstrated heavy chain isotype exclusion at the cellular level in fish.
The repertoire data presented here suggest that something different may be occurring in tuna, however (Figure 8). Like in other fish, VH genes appear to be shared between both τ and μ/δ. Three of the four families we found expressed in these fish clearly were used in both μ and τ, although a fourth was only found with μ. This could easily be a case of low sampling depth as VH4 appeared as a singular use in the described clones. Since this is a more parsimonious explanation than a dedicated μ VH rearranging to a shared DH segment that rearranges to dedicated JH segments, Figure 8 depicts an array of VH gene segments that can be used in either primary transcript type.
Figure 8. Hypothetical organization of elements in the tuna IgH locus suggests a novel method of lineage determination at the fish IgH locus.
Simplified cartoon showing three paradigms in the locus organization and immunogenetic control of IgH τ vs μ/δ rearrangement.
However, unlike in other fish, both τ and μ rearrangements of tuna appear to employ the same DH gene segment. As all the tuna JH genes appear with only μ or τ (none seem to be shared), this points to an arrangement where a single shared DH can rearrange with JH segments upstream of either μ or τ to determine isotypic fate of the cell, and this DH’s rearrangement to several shared VH’s is not the event that stochastically determines isotype. So at least two possibilities of IgT vs. IgM/D lineage fate are now supported by data, one in which τ and μ/δ share VH genes from one block (as in zebrafish) or more than one array 5’ and 3’ to the τ elements (as in trout) but DH and JH are dedicated to isotype, and now the tuna paradigm where VH and DH are shared and JH is dedicated to isotype. In one instance (tuna) the DH-JH join would instruct lineage and in the other the VH-DH join would. Importantly, we note that genomic sequencing of the locus has not yet confirmed this organization in the tuna or the absence of additional DH that we did not sample. Interestingly, this hypothesized organization could also explain why in trout a significant difference was seen in CDR3 length and repertoire between τ and μ clones (each using dedicated DH and JH gene segments, (Castro et al., 2013)) while we do not see a great difference in tuna (sharing VH and DH and only having dedicated JH, Table 1 and Figure 3). Future work must determine if this is truly stochastic in lymphocyte development or if there are more complex control mechanisms instructing this important juncture determining the B cell’s fate.
4.3. CH regions
IgM is the most conserved isotype in jawed vertebrates and was thought to be omnipresent until the discovery of its absence in coelacanth (Amemiya et al., 2013). The tuna IgM CH region seems very consistent with its orthologs in other fish.
As also noted in other IgT sequences (Hansen et al., 2005), there are many prolines in the region of the tuna IgT CH1/CH2 juncture which may be indicative of hinge-like flexibility. Tuna IgT CH3 seems to conform to the classical Ig superfamily β-sandwich with canonical cysteines and tryptophan positions seen in the domain of the salmonids and grouper that are important in the folding of this domain (Figure 5) (Lesk and Chothia, 1982). The tryptophan to cysteine replacement seen in zebrafish and grass carp appears to be a cyprinid characteristic, and has been suggested by modeling to still allow an immunoglobulin superfamily domain fold (Danilova and Amemiya, 2009).
In this limited sampling, we found no evidence of the IgT hybrid molecule with two CH domains identified in the common carp Cyprinus carpio (Savan et al., 2005a), the IgM/D hybrids (with or without VH domains) found in catfish (Edholm et al., 2010), the IgMCH1/IgTCH4 variant IgT2 in carp (Ryo et al., 2010), nor the run-on transcription secreted IgD form of trout (Ramirez-Gomez et al., 2012).
In mammalian IgM a carboxyl terminal glycosylation site in the secretory tail is important in J chain polymerization (Hohman et al., 2003; Tacchi et al., 2013), but may have distinct physiology in teleost such as catfish and zebrafish that have it (Wiersma et al., 1997). This conserved N-linked glycosylation site is part of a larger sequence motif enabling polymerization of IgM and IgA of mammals but is not present in the secretory tail of tuna IgM or IgT, although there is a conserved cysteine in IgT shared with other teleosts. Trout IgT was found as a monomer in serum but a multimer in mucus (Zhang et al., 2010), however these IgT multimers did not appear to be covalently linked as they are known to be for trout IgM (Kaattari et al., 1998). More biochemical studies are necessary to resolve the stoichiometry and functional avidity of IgT.
4.4 Expression
Isotype expression studies in tuna echo what has been determined in other fish species: IgT and IgM both are present in primary and secondary lymphoid tissues, yet more IgM than IgT, however the gap closes at mucosal sites (Hansen et al., 2005; Ryo et al., 2010; Savan et al., 2005b; Xiao et al., 2010). IgT1 in adult zebrafish deviated from this pattern in being primarily in the head-kidney and thymus (Hu et al., 2010). The molecular data presented here could serve as a springboard for revisiting immunoglobulin studies in tuna at the protein level that were initiated in the southern bluefin (Thunnus maccoyii) (Watts et al., 2001). The work also opens gates to explorations of B lineage development and commitment, where molecular markers might could be adapted from fish species such as zebrafish (Zimmerman et al., 2011) and trout (Barr et al., 2011; Macmurray et al., 2013) where more work has been performed.
4.5. Conclusions
Endothermic birds and mammals employ immunoglobulin isotypes IgM, IgY, IgE and IgG in systemic immunity but have specialized IgA for mucosal immunity. Poikilothermic vertebrates lack IgA, although amphibians do have an orthologous mucosal isotype in their IgX. IgM had long been the primary functional immunoglobulin isotype recognized in teleost until the recent discovery of the mucosal specialization of IgT. Mucosal epithelia is the barrier breached or exploited by most internal pathogens of vertebrates, and also ectoparasites of fish (Xu et al., 2013). This penetration of mucosal defense is also true of many pathogens of concern in the tuna ranching industry, including sea lice (Hayward et al., 2009), betanodaviruses (Gomez et al., 2010) and gill platyhelminths (Colquitt et al., 2001). It is hoped that this basic molecular characterization of humoral immunity in these economically important endothermic fish will enable more studies of host-pathogen interactions and the feasibility of vaccine development for offshore ranches. Increasing the productivity of these operations by reducing infectious disease mortality will reduce pressures on wild tuna stocks and the fish species used to feed ranched tuna.
Moreover, the apparent shift of isotype determination from VH-DH recombination to DH-JH recombination at the tuna IgH locus is interesting from a fundamental standpoint of lymphocyte antigen receptor immunogenetics, and begs many questions that must be verified and queried with new algorithms (Olivieri et al., 2013) at the levels of the tuna genome, the immunoglobulin proteins, tuna B cells, development in the pronephros, and the fish’s response to pathogen. If the single tuna DH gene is verified at the genome, it will be interesting to know whether this IgH locus orientation is found only within this clade of endothermic fish or a broader set of Perciformes. These studies should provide insight into the natural history and fundamental physiology of antibodies while providing much needed tools for managing the health of ranched, and thereby wild, tuna stocks.
Supplementary Material
Highlights.
-
-
Immunoglobulin (I)gH μ and Ig τ were analyzed in the Pacific bluefin tuna Thunnus orientalis
-
-
Junctional repertoire analysis shows distinct joining (JH) segments dedicated to IgT or IgM, but one shared diversity (DH) segment
-
-
IgT and IgM sharing the same DH segment suggests a deviation from the conserved IgH locus organization and isotype control mechanism of most teleost fish
-
-
Quantitative PCR shows a lower relative predominance of IgM in the gills to IgT
-
-
Ig μ and τ CDR3 lengths are similar in tuna, perhaps owing to the shared DH and distinct rearrangement mechanism compared to other fish
Acknowledgements
This work was supported by the NIH through grant AI56963 and the NSF through grant IOS1257829 to MFC. Funding support from the TAMU-CONACyT initiative to AB through grant 2010-006 is also gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sara Mashoof, Email: smashoof@cvm.tamu.edu.
Camilo Pohlenz, Email: cpohlenz@tamu.edu.
Patricia L. Chen, Email: pchen@cvm.tamu.edu.
Thaddeus C. Deiss, Email: tdeiss@cvm.tamu.edu.
Delbert Gatlin, III, Email: dgatlin@nature.tamu.edu.
Alejandro Buentello, Email: abuentello@schillgen.com.
Michael F. Criscitiello, Email: mcriscitielllo@cvm.tamu.edu.
References
- Amemiya CT, Alfoldi J, Lee AP, Fan S, Philippe H, Maccallum I, Braasch I, Manousaki T, Schneider I, Rohner N, Organ C, Chalopin D, Smith JJ, Robinson M, Dorrington RA, Gerdol M, Aken B, Biscotti MA, Barucca M, Baurain D, Berlin AM, Blatch GL, Buonocore F, Burmester T, Campbell MS, Canapa A, Cannon JP, Christoffels A, De Moro G, Edkins AL, Fan L, Fausto AM, Feiner N, Forconi M, Gamieldien J, Gnerre S, Gnirke A, Goldstone JV, Haerty W, Hahn ME, Hesse U, Hoffmann S, Johnson J, Karchner SI, Kuraku S, Lara M, Levin JZ, Litman GW, Mauceli E, Miyake T, Mueller MG, Nelson DR, Nitsche A, Olmo E, Ota T, Pallavicini A, Panji S, Picone B, Ponting CP, Prohaska SJ, Przybylski D, Saha NR, Ravi V, Ribeiro FJ, Sauka-Spengler T, Scapigliati G, Searle SM, Sharpe T, Simakov O, Stadler PF, Stegeman JJ, Sumiyama K, Tabbaa D, Tafer H, Turner-Maier J, van Heusden P, White S, Williams L, Yandell M, Brinkmann H, Volff JN, Tabin CJ, Shubin N, Schartl M, Jaffe DB, Postlethwait JH, Venkatesh B, Di Palma F, Lander ES, Meyer A, Lindblad-Toh K. The African coelacanth genome provides insights into tetrapod evolution. Nature. 2013;496:311–316. doi: 10.1038/nature12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr M, Mott K, Zwollo P. Defining terminally differentiating B cell populations in rainbow trout immune tissues using the transcription factor XbpI. Fish & shellfish immunology. 2011;31:727–735. doi: 10.1016/j.fsi.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengten E, Clem LW, Miller NW, Warr GW, Wilson M. Channel catfish immunoglobulins: repertoire and expression. Dev.Comp Immunol. 2006;30:77–92. doi: 10.1016/j.dci.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Bengten E, Quiniou SM, Stuge TB, Katagiri T, Miller NW, Clem LW, Warr GW, Wilson M. The IgH locus of the channel catfish, Ictalurus punctatus, contains multiple constant region gene sequences: different genes encode heavy chains of membrane and secreted IgD. J.Immunol. 2002;169:2488–2497. doi: 10.4049/jimmunol.169.5.2488. [DOI] [PubMed] [Google Scholar]
- Block BA, Dewar H, Blackwell SB, Williams TD, Prince ED, Farwell CJ, Boustany A, Teo SLH, Seitz A, Walli A, Fudge D. Migratory movements, depth preferences, and thermal biology of Atlantic bluefin tuna. Science. 2001;293:1310–1314. doi: 10.1126/science.1061197. [DOI] [PubMed] [Google Scholar]
- Brodeur PH, Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984;14:922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- Castro R, Jouneau L, Pham HP, Bouchez O, Giudicelli V, Lefranc MP, Quillet E, Benmansour A, Cazals F, Six A, Fillatreau S, Sunyer O, Boudinot P. Teleost fish mount complex clonal IgM and IgT responses in spleen upon systemic viral infection. PLoS pathogens. 2013;9:e1003098. doi: 10.1371/journal.ppat.1003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquitt SE, Munday BL, Daintith W. Pathological findings in southern bluefin tuna, Thunnus maccoyii (Castelnau), infected with Cardicola forsteri (Cribb, Daintith & Munday, 2000) (Digenea : Sanguinicolidae), a blood fluke. Journal of fish diseases. 2001;24:225–229. [Google Scholar]
- Criscitiello MF, Flajnik MF. Four primordial immunoglobulin light chain isotypes, including lambda and kappa, identified in the most primitive living jawed vertebrates. Eur.J.Immunol. 2007;37:2683–2694. doi: 10.1002/eji.200737263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilova N, Amemiya CT. Going adaptive: the saga of antibodies. Annals of the New York Academy of Sciences. 2009;1168:130–155. doi: 10.1111/j.1749-6632.2009.04881.x. [DOI] [PubMed] [Google Scholar]
- Danilova N, B J, Jekosch K, Steiner LA. The immunoglobulin heavy-chain locus in zebrafish: identification and expression of a previously unknown isotype, immunoglobulin Z. Nat Immunol. 2005;6:295–302. doi: 10.1038/ni1166. [DOI] [PubMed] [Google Scholar]
- Danilova N, Bussmann J, Jekosch K, Steiner LA. The immunoglobulin heavy-chain locus in zebrafish: identification and expression of a previously unknown isotype, immunoglobulin Z. Nat Immunol. 2005;6:295–302. doi: 10.1038/ni1166. [DOI] [PubMed] [Google Scholar]
- Danilova N, Saunders HL, Ellestad KK, Magor BG. The zebrafish IgH locus contains multiple transcriptional regulatory regions. Dev Comp Immunol. 2011;35:352–359. doi: 10.1016/j.dci.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM. The evolutionary and structural 'logic' of antigen receptor diversity. Semin Immunol. 2004;16:239–243. doi: 10.1016/j.smim.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Edholm ES, Bengten E, Stafford JL, Sahoo M, Taylor EB, Miller NW, Wilson M. Identification of two IgD+ B cell populations in channel catfish, Ictalurus punctatus. J Immunol. 2010;185:4082–4094. doi: 10.4049/jimmunol.1000631. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Brunak S, von Heijne G, Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nature protocols. 2007;2:953–971. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Six A, Magadan S, Castro R, Sunyer JO, Boudinot P. The astonishing diversity of Ig classes and B cell repertoires in teleost fish. Frontiers in immunology. 2013;4:28. doi: 10.3389/fimmu.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik MF, Rumfelt LL. Early and natural antibodies in non-mammalian vertebrates. Curr.Top.Microbiol.Immunol. 2000;252:233–240. doi: 10.1007/978-3-642-57284-5_24. [DOI] [PubMed] [Google Scholar]
- Fromentin JM, Powers JE. Atlantic bluefin tuna: population dynamics, ecology, fisheries and management. Fish Fish. 2005;6:281–306. [Google Scholar]
- Gambon-Deza F, Sanchez-Espinel C, Magadan-Mompo S. Presence of an unique IgT on the IGH locus in three-spined stickleback fish (Gasterosteus aculeatus) and the very recent generation of a repertoire of VH genes. Developmental and Comparative Immunology. 2010;34:114–122. doi: 10.1016/j.dci.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Gomez DK, Mori K, Okinaka Y, Nakai T, Park SC. Trash fish can be a source of betanodaviruses for cultured marine fish. Aquaculture. 2010;302:158–163. [Google Scholar]
- Hansen JD, Landis ED, Phillips RB. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. Proc Natl Acad Sci U S A. 2005;102:6919–6924. doi: 10.1073/pnas.0500027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward CJ, Bott NJ, Nowak BF. Seasonal epizootics of sea lice, Caligus spp., on southern bluefin tuna, Thunnus maccoyii (Castelnau), in a long-term farming trial. Journal of fish diseases. 2009;32:101–106. doi: 10.1111/j.1365-2761.2008.01010.x. [DOI] [PubMed] [Google Scholar]
- Hikima J, Jung TS, Aoki T. Immunoglobulin genes and their transcriptional control in teleosts. Dev Comp Immunol. 2011;35:924–936. doi: 10.1016/j.dci.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Hohman VS, Stewart SE, Rumfelt LL, Greenberg AS, Avila DW, Flajnik MF, Steiner LA. J chain in the nurse shark: implications for function in a lower vertebrate. J Immunol. 2003;170:6016–6023. doi: 10.4049/jimmunol.170.12.6016. [DOI] [PubMed] [Google Scholar]
- Hu YL, Xiang LX, Shao JZ. Identification and characterization of a novel immunoglobulin Z isotype in zebrafish: implications for a distinct B cell receptor in lower vertebrates. Mol Immunol. 2010;47:738–746. doi: 10.1016/j.molimm.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Hansen John D, L ED, Phillips Ruth B. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. PNAS. 2005;102:6919–6924. doi: 10.1073/pnas.0500027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Computer applications in the biosciences : CABIOS. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- Jørgensen JSTØ. Immunoglobulin D (IgD) of Atlantic cod has a unique structure. Immunogenetics (2000) 2000;51:452–461. doi: 10.1007/s002510050644. 51, 452–461. [DOI] [PubMed] [Google Scholar]
- Jusup M, Klanjscek T, Matsuda H, Kooijman SA. A full lifecycle bioenergetic model for bluefin tuna. PLoS One. 2011;6:e21903. doi: 10.1371/journal.pone.0021903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaattari S, Evans D, Klemer J. Varied redox forms of teleost IgM: an alternative to isotypic diversity? Immunol Rev. 1998;166:133–142. doi: 10.1111/j.1600-065x.1998.tb01258.x. [DOI] [PubMed] [Google Scholar]
- Koichiro Tamura DP, Peterson Nicholas, Stecher Glen, Nei Masatoshi, Kumar Sudhir. MOLECULAR EVOLUTIONARY GENETICS ANALYSIS Authors. 2011 doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong PD, Wilson IA. HIV-1 and influenza antibodies: seeing antigens in new ways. Nat Immunol. 2009;10:573–578. doi: 10.1038/ni.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SH, Chua HL, Gong Z, Lam TJ, Sin YM. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev Comp Immunol. 2004;28:9–28. doi: 10.1016/s0145-305x(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Lefranc MP, Pommie C, Ruiz M, Giudicelli V, Foulquier E, Truong L, Thouvenin-Contet V, Lefranc G. IMGT unique numbering for immunoglobulin and T cell receptor variable domains and Ig superfamily V-like domains. Dev Comp Immunol. 2003;27:55–77. doi: 10.1016/s0145-305x(02)00039-3. [DOI] [PubMed] [Google Scholar]
- Lesk AM, Chothia C. Evolution of proteins formed by beta-sheets. II. The core of the immunoglobulin domains. J.Mol.Biol. 1982;160:325–342. doi: 10.1016/0022-2836(82)90179-6. [DOI] [PubMed] [Google Scholar]
- Macmurray E, Barr M, Bruce A, Epp L, Zwollo P. Alternative splicing of the trout Pax5 gene and identification of novel B cell populations using Pax5 signatures. Dev Comp Immunol. 2013;41:270–281. doi: 10.1016/j.dci.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magadan-Mompo S, Sanchez-Espinel C, Gambon-Deza F. Immunoglobulin heavy chains in medaka (Oryzias latipes) BMC evolutionary biology. 2011;11:165. doi: 10.1186/1471-2148-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashoof S, Goodroe A, Du CC, Eubanks JO, Jacobs N, Steiner JM, Tizard I, Suchodolski JS, Criscitiello MF. Ancient T-independence of mucosal IgX/A: gut microbiota unaffected by larval thymectomy in Xenopus laevis. Mucosal immunology. 2013;6:358–368. doi: 10.1038/mi.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O'Dell S, Patel N, Shahzad-ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang GY, Diwanji D, Georgiev I, Kwon YD, Lee D, Louder MK, Moquin S, Schmidt SD, Yang ZY, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang LX, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladineo I, Block BA. Expression of Hsp70, Na+/K+ ATP-ase, HIF-1 alpha, IL-1 beta and TNF-alpha in captive Pacific bluefin tuna (Thunnus orientalis) after chronic warm and cold exposure. J Exp Mar Biol Ecol. 2009;374:51–57. [Google Scholar]
- Olivieri D, Faro J, von Haeften B, Sanchez-Espinel C, Gambon-Deza F. An automated algorithm for extracting functional immunologic V-genes from genomes in jawed vertebrates. Immunogenetics. 2013;65:691–702. doi: 10.1007/s00251-013-0715-8. [DOI] [PubMed] [Google Scholar]
- Olsen MM, Kania PW, Heinecke RD, Skjoedt K, Rasmussen KJ, Buchmann K. Cellular and humoral factors involved in the response of rainbow trout gills to Ichthyophthirius multifiliis infections: molecular and immunohistochemical studies. Fish & shellfish immunology. 2011;30:859–869. doi: 10.1016/j.fsi.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Ottolenghi F. Capture-based aquaculture of blue-fin tuna. In: Lovatelli A, Holthus P, editors. Capture-based aquaculture. Rome: Food and Agriculture Organization of the United Nations; 2008. pp. 169–182. [Google Scholar]
- Page RD. Visualizing phylogenetic trees using TreeView. In: Baxevanis Andreas D, et al., editors. Current protocols in bioinformatics / editoral board. Unit 6 2. Chapter 6. 2002. [DOI] [PubMed] [Google Scholar]
- Pascual V, Capra JD. Human immunoglobulin heavy-chain variable region genes: organization, polymorphism, and expression. Adv Immunol. 1991;49:1–74. doi: 10.1016/s0065-2776(08)60774-9. [DOI] [PubMed] [Google Scholar]
- Ramirez-Gomez F, Greene W, Rego K, Hansen JD, Costa G, Kataria P, Bromage ES. Discovery and characterization of secretory IgD in rainbow trout: secretory IgD is produced through a novel splicing mechanism. J Immunol. 2012;188:1341–1349. doi: 10.4049/jimmunol.1101938. [DOI] [PubMed] [Google Scholar]
- Ryo S, Wijdeven RHM, Tyagi A, Hermsen T, Kono T, Karunasagar I, Rombout JHWM, Sakai M, Verburg-van Kemenade BML, Savan R. Common carp have two subclasses of bonyfish specific antibody IgZ showing differential expression in response to infection. Developmental and Comparative Immunology. 2010;34:1183–1190. doi: 10.1016/j.dci.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Salinas I, Zhang YA, Sunyer JO. Mucosal immunoglobulins and B cells of teleost fish. Dev Comp Immunol. 2011;35:1346–1365. doi: 10.1016/j.dci.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel Aparicio JC, Stupka Elia, Nik Putnam J-mC, Dehal Paramvir, Alan Christoffels SR, Hoon Shawn, Smit Arian, Sollewijn Maarten D, Gelpke Jared Roach TO, Ho Isaac Y, W M, Detter Chris, Verhoef Frans, Paul Predki AT, Lucas Susan, Richardson Paul, Smith, M.S.C Sarah F, Edwards Yvonne JK, Norman Doggett AZ, Tavtigian Sean V, Dmitry Pruss MB, Evans Cheryl, Baden Holly, Justin Powell GG, Rowen Lee, Leroy Hood YH, Tan GE, Hawkins Trevor, Byrappa Venkatesh DR, Brenner Sydney. Whole-Genome Shotgun Assembly and Analysis of the Genome of Fugu rubripes. Science. 2002;297:1301. doi: 10.1126/science.1072104. [DOI] [PubMed] [Google Scholar]
- Savan R, Aman A, Nakao M, Watanuki H, Sakai M. Discovery of a novel immunoglobulin heavy chain gene chimera from common carp (Cyprinus carpio L.) Immunogenetics. 2005a;57:458–463. doi: 10.1007/s00251-005-0015-z. [DOI] [PubMed] [Google Scholar]
- Savan R, Aman A, Sato K, Yamaguchi R, Sakai M. Discovery of a new class of immunoglobulin heavy chain from fugu. Eur J Immunol. 2005b;35:3320–3331. doi: 10.1002/eji.200535248. [DOI] [PubMed] [Google Scholar]
- Schorpp M, Bialecki M, Diekhoff D, Walderich B, Odenthal J, Maischein HM, Zapata AG, Boehm T. Conserved functions of Ikaros in vertebrate lymphocyte development: genetic evidence for distinct larval and adult phases of T cell development and two lineages of B cells in zebrafish. J Immunol. 2006;177:2463–2476. doi: 10.4049/jimmunol.177.4.2463. [DOI] [PubMed] [Google Scholar]
- Schwarz R, Dayhoff M. Matrices for detecting distant relationships. In: M D, editor. Atlas of protein sequences. National Biomedical Research Foundation; 1979. pp. 353–358. [Google Scholar]
- Tacchi L, Larragoite E, Salinas I. Discovery of J Chain in African Lungfish (Protopterus dolloi, Sarcopterygii) Using High Throughput Transcriptome Sequencing: Implications in Mucosal Immunity. PLoS One. 2013;8:e70650. doi: 10.1371/journal.pone.0070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JY, Sun BJ, Luo YP, Zhang YA, Nie P. Distribution of IgM, IgD and IgZ in mandarin fish, Siniperca chuatsi lymphoid tissues and their transcriptional changes after Flavobacterium columnare stimulation. Aquaculture. 2009;288:14–21. [Google Scholar]
- Trede NS, Langenau DM, Traver D, Look AT, Zon LI. The use of zebrafish to understand immunity. Immunity. 2004;20:367–379. doi: 10.1016/s1074-7613(04)00084-6. [DOI] [PubMed] [Google Scholar]
- Barreto Vasco M, Pan-Hammarstrom Qiang, Z Y, Hammarstrom Lennart, Misulovin Ziva, Nussenzweig aMC. AID from bony fish catalyzes class switch recombination. JEM. 2005;202:733–738. doi: 10.1084/jem.20051378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakae K, Magor BG, Saunders H, Nagaoka H, Kawamura A, Kinoshita K, Honjo T, Muramatsu M. Evolution of class switch recombination function in fish activation-induced cytidine deaminase, AID. Int Immunol. 2006;18:41–47. doi: 10.1093/intimm/dxh347. [DOI] [PubMed] [Google Scholar]
- Wang F, Ekiert DC, Ahmad I, Yu W, Zhang Y, Bazirgan O, Torkamani A, Raudsepp T, Mwangi W, Criscitiello MF, Wilson IA, Schultz PG, Smider VV. Reshaping antibody diversity. Cell. 2013;153:1379–1393. doi: 10.1016/j.cell.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts M, Munday BL, Burke CM. Isolation and partial characterisation of immunoglobulin from southern bluefin tuna Thunnus maccoyii Castelnau. Fish & shellfish immunology. 2001;11:491–503. doi: 10.1006/fsim.2000.0329. [DOI] [PubMed] [Google Scholar]
- Wiersma EJ, Chen F, Bazin R, Collins C, Painter RH, Lemieux R, Shulman MJ. Analysis of IgM structures involved in J chain incorporation. J Immunol. 1997;158:1719–1726. [PubMed] [Google Scholar]
- Wilson M, Bengten E, Miller NW, Clem LW, Du PL, Warr GW. A novel chimeric Ig heavy chain from a teleost fish shares similarities to IgD. Proc.Natl.Acad.Sci.U.S.A. 1997;94:4593–4597. doi: 10.1073/pnas.94.9.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao FS, Wang YP, Yan W, Chang MX, Yao WJ, Xu QQ, Wang XX, Gao Q, Nie P. Ig heavy chain genes and their locus in grass carp Ctenopharyngodon idella. Fish & shellfish immunology. 2010;29:594–599. doi: 10.1016/j.fsi.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Xu JL, Davis MM. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity. 2000;13:37–45. doi: 10.1016/s1074-7613(00)00006-6. [DOI] [PubMed] [Google Scholar]
- Xu Z, Parra D, Gomez D, Salinas I, Zhang YA, von Gersdorff Jorgensen L, Heinecke RD, Buchmann K, Lapatra S, Sunyer JO. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proc Natl Acad Sci U S A. 2013;110:13097–13102. doi: 10.1073/pnas.1304319110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YA, Salinas I, Li J, Parra D, Bjork S, Xu Z, LaPatra SE, Bartholomew J, Sunyer JO. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat Immunol. 2010;11:827–835. doi: 10.1038/ni.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YA, Salinas I, Oriol Sunyer J. Recent findings on the structure and function of teleost IgT. Fish & shellfish immunology. 2011;31:627–634. doi: 10.1016/j.fsi.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Lee V, Finn A, Senger K, Zarrin AA, Du Pasquier L, Hsu E. Origin of Immunoglobulin Isotype Switching. Current biology : CB. 2012 doi: 10.1016/j.cub.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AM, Moustafa FM, Romanowski KE, Steiner LA. Zebrafish immunoglobulin IgD: unusual exon usage and quantitative expression profiles with IgM and IgZ/T heavy chain isotypes. Mol Immunol. 2011;48:2220–2223. doi: 10.1016/j.molimm.2011.06.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.