Abstract
The Klotho (KL) gene was first identified as a potent aging suppressor. The KL family currently comprises of three proteins: α-Klotho (KLA), β-Klotho (KLB), and γ-Klotho (KLG). Many studies have shown that KLA and KLB participate in tumor progression or suppression, depending on the type of cancer; however, the relationship between KLG and prostate cancer has not yet been studied. Some studies have claimed that KL is correlated to sensitivity to chemotherapy. Here, we investigated the oncogenic potential of KLG in castration-resistant prostate cancer (CRPC). Immunohistochemical analysis using prostate biopsy specimens revealed that patients with high KLG expression in primary prostate cancer tissue had a significantly poor prognosis for overall survival. In addition, the prostate-specific antigen response rate after docetaxel (DTX) therapy in patients with high KLG expression was lower than that in patients with low KLG expression. To evaluate the potential of KLG as a therapeutic target in human prostate cancer, we generated a xenograft model of human CRPC cell line (PC-3) in male athymic mice. The animals were randomly divided into four groups as follows: i) control group (vehicle only); ii) DTX group (intraperitoneal administration); iii) small interfering RNA targeting KLG (KLG siRNA) group (intratumoral administration); and iv) a combination group (DTX plus KLG siRNA). After 3 weeks of treatment, the tumor weight and tumor Ki-67 labeling index were significantly lower in the KLG siRNA group and the combination group than in the control group. Sensitivity to DTX was increased upon treatment with KLG siRNA. These findings suggest that KLG expression in primary prostate cancer lesions is associated with resistance to DTX in CRPC and has potential as a diagnostic and therapeutic target for patients with CRPC.
Keywords: KLG, CRPC, DTX, OS, xenograft model, PC-3
Introduction
Prostate cancer is the second most commonly diagnosed malignant tumor in men and a major cause of mortality. It is estimated that there will be almost 1.3 million new cases of prostate cancer and 359,000 prostate cancer-associated deaths worldwide in 2018 (1). Once a patient reaches a state of castration-resistant disease, no curative treatment option is available. Castration-resistant prostate cancer (CRPC) has a poor prognosis despite recent therapeutic advances, including docetaxel (DTX), cabazitaxel, sipuleucel-T, abiraterone acetate, enzalutamide, and radium-223 therapies (2). Presently, DTX is established as an effective treatment and is widely used for patients with CRPC (3,4). According to the National Comprehensive Cancer Network guidelines, DTX is still recommended as a first-line chemotherapeutic agent (5). However, not all patients with CRPC receive clinical benefit from DTX treatment. Therefore, to choose an optimal treatment and to prolong patient survival, the identification of predictive markers for DTX treatment in patients with CRPC is urgently required.
The Klotho (KL) gene was originally identified as an anti-aging gene in 1997 (6). KL is a transmembrane protein belonging to the glycosyl hydrolase 1 family. The KL family comprises of three proteins, α-Klotho (KLA), β-Klotho (KLB), and γ-Klotho (KLG). KLs act as cofactors of fibroblast growth factors (FGFs)19, 21 and 23 (7–9). The FGF pathway performs a vital role in various stages of cancer development, including mitogenesis, cell differentiation, and angiogenesis (10–12).
Recently, several studies have reported the association between KLs and cancer development. Many researchers have treated KLA as a new biomarker for cancer (13). Most reports have revealed that KLA serves as a tumor suppressor, via the promotion of apoptosis and inhibition of transforming factor-β1 signaling involved in epithelial-mesenchymal transition (EMT) (14–18). Contrarily, the role of KLB in cancer development is still controversial (19–21). We have previously reported the association between KLA/KLB and bladder cancer and showed that KLB contributes to cancer development (22). However, the relationship between KLG and cancer progression remains unclear. Currently, there are very few reports on the association between KLG and cancer (9,23,24). One study reported that KLA enhances the activity of chemotherapeutic drugs on pancreatic cancer cells (25). It has been suggested that KLA may be correlated with the sensitivity to chemotherapy. KLG acts mainly with FGF19 and FGF receptor 4 (FGFR4) (26). FGF19/FGFR4 signaling promotes cancer progression together with the expression of KLA and/or KLB as cofactors in prostate cancer (20,27). In this study, we aimed to investigate the effect of KLG on tumor growth in prostate cancer, and thereby to determine its importance in therapeutic strategies.
Materials and methods
Patients
A total of 65 patients with CRPC who received DTX chemotherapy from December 2004 to April 2015 at our institution were eligible for inclusion in our study. We excluded patients with hormone-sensitive prostate cancer, those diagnosed with nonadenocarcinoma histology such as small-cell carcinoma, and those with no prostate biopsy specimen available for immunohistochemistry (IHC) staining. Overall, 36 patients were included in this study (Table I). This study was retrospective and the Ethics Committee of Nara Medical University approved the study protocol (reference ID: 1256). Written informed consent was obtained from all participants.
Table I.
Clinicopathological background of 36 patients with castration-resistant prostate cancer who were administered DTX at our hospital and comparison of the variables with low and high KLG expression.
| KLG expression | ||||
|---|---|---|---|---|
| Variables | Number of patients, n=36 | Low, n=13 | High, n=23 | P-value |
| Age, years | 68.5 (62.8, 73.3) | 65 (60, 73) | 71 (65.5, 73.5) | 0.24 |
| PSA at diagnosis, ng/ml | 102 (30.1, 398.3) | 33.9 (19.3, 170) | 251 (50.7, 714.5) | 0.016b |
| Total Gleason Score | 0.19 | |||
| 6 | 1 | 1 | 0 | |
| 7 | 3 | 2 | 1 | |
| 8- | 32 | 10 | 22 | |
| T stage | 0.44 | |||
| T2 | 6 | 3 | 3 | |
| T3-4 | 30 | 10 | 20 | |
| N stage | 0.029a | |||
| N0 | 19 | 10 | 9 | |
| N1-2 | 17 | 3 | 14 | |
| M stage | 0.17 | |||
| M0 | 14 | 7 | 7 | |
| M1 | 22 | 6 | 16 | |
| Primary therapy | ||||
| RP | 6 | 4 | 2 | 0.031a |
| RT | 4 | 3 | 1 | |
| ADT | 26 | 6 | 20 | |
| Survival after diagnosis, months | 54.5 (39.8, 88) | 80 (51, 100) | 50 (33, 68) | 0.048b |
| Period from ADT to DTX, months | 27 (16.8, 44.3) | 37 (22, 50) | 25 (11, 37.5) | 0.16 |
| Change rate of PSA, % | −83.7 (−97.8, −35.3) | −94.4 (−99.3, −65.8) | −57.9 (−95.9, −12.7) | 0.045b |
| DTX cycle, n | 8 (4, 11) | 10 (7, 12) | 6 (3.5, 10) | 0.051 |
Patients with low KLG expression had significantly lower initial PSA values (P=0.016), fewer lymph node metastases (P=0.029), higher rate of radical prostatectomy as primary therapy, longer OS (P=0.048), and better PSA response rate after DTX (P=0.045) than patients with high KLG expression. Data are presented as the median (interquartile range).
χ2 test or Fisher's exact test
Mann-Whitney U test. KLG, γ-Klotho; PSA, prostate specific antigen; RP, radical prostatectomy; RT, radiation therapy; ADT, androgen-deprivation therapy; DTX, docetaxel.
IHC analysis of prostate biopsy specimens
IHC was carried out to evaluate the expression levels of KLA, KLB, and KLG. Tissue sections were obtained from paraffin-embedded tissue blocks, which were sliced and placed on Super Frost Plus microscope slides (Thermo Fisher Scientific, Yokohama, Japan). The tissue sections were deparaffinized and antigen retrieval was carried out in citric acid buffer (pH 6.0) in an autoclave. We performed IHC staining using a Histofine ABC kit (Nichirei). The slides were treated with 1% hydrogen peroxide in methanol to block endogenous peroxidase activity. The slides were then incubated overnight at 4°C with anti-KLA antibody (sc-22220; rabbit polyclonal, dilution 1:500), anti-KLB antibody (sc-74343, rabbit polyclonal, dilution 1:200), and anti-KLG antibody (sc-137559; goat polyclonal, dilution 1:500). All these antibodies were purchased from Santa Cruz Biotechnology, Inc.. The slides were counterstained with hematoxylin, dehydrated, and mounted on a cover slide. We evaluated each slide using IHC scores (IHC score=intensity score + population score; intensity: None=0, low=1, intermediate=2, and high=3; population: None=0; 0–25%=1; 25–50%=2; 50–75%=3; and 75–100%=4). KLA/KLB/KLG expression was classified as low or high based on the IHC score (low=IHC score≤4; high=IHC score 5 or 6) (22).
Cell culture
Three human prostate cancer cell lines, PC-3, DU145, and LNCaP, were purchased from the American Type Culture Collection. Cells were maintained in RPMI-1640 medium (Nacalai Tesque, Kyoto, Japan), supplemented with 10% fetal bovine serum (JRH) and 1% penicillin-streptomycin (Thermo Scientific, Yokohama, Japan) in a standard humidified incubator at 37°C in an atmosphere of 5% CO2.
Reverse transcription-quantitative PCR (RT-qPCR)
RT-qPCR was performed to measure the expression level of KLG mRNA in cell lines. Cells were seeded in 6-well plates at a density of 1×105 cells/well in growth medium and incubated for 24 h. Total RNA was extracted using an RNeasy Mini kit (Qiagen), according to the manufacturer's instructions. Conversion to cDNA was performed using a High Capacity cDNA Reverse Transcription kit (Invitrogen; Thermo Fisher Scientific, Inc.). When we converted to cDNA in RT-PCR, concentration of RNA was 1,000 ng. Quantitative RT-PCR was performed using the cDNA, 0.2 µM of each primer, and 10 µl of AmpliTaq Gold® PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) under the following conditions: Denaturation at 95°C for 10 min; 40 cycles of denaturation at 95°C for 15 s; annealing and a final extension at 60°C for 1 min. PCR products were then electrophoresed in a 1.5% agarose gel and visualized using a transilluminator. Subsequent to verifying the mRNA expression of KLG, semi-quantitative RT-PCR for this gene was performed in cell lines. The gene-specific primer used in this study was Hs01385107_m1 for human KLG. Primer sequence of KLG was not available. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a control.
Western blot analysis of human prostate cell lines
Total cellular protein lysate was prepared as described previously (28). Protein was extracted from PC-3 and DU145 cells and concentration of proteins was 10 µg. In brief, tumor samples were minced and incubated in lysis buffer (250 mmol/l Tris-HCl (pH 6.8), 2% Sodium Dodecyl Sulphate (SDS), and 10% glycerol) and protein inhibitor cocktail (Sigma-Aldrich, St. Louis, US) for protein extraction. The membranes were incubated for 1 h with primary anti-KLG antibody (Santa Cruz Biotechnology, Inc.; sc-137559, goat polyclonal, dilution 1:200) or anti-GAPDH mouse monoclonal antibody (dilution 1:10,000) as an internal loading control, followed by 1 h with horseradish peroxidase-conjugated anti-rabbit IgG (1:5,000) or anti-mouse IgG antibody (1:20,000).
Animals
Animal care was conducted in compliance with the recommendations of The Guide for Care and Use of Laboratory Animals (National Research Council). This study was approved by the animal facility committee at Nara Medical University (protocol ID: 11896). Male athymic BALB/c nu/nu mice, 6 to 8 weeks old, were purchased from Oriental Bio Service. All mice were maintained under pathogen-free conditions and provided with sterile food and water. We monitored the dietary intake and body weight of mice every day and stop experiment if we observe marked loss of appetite and weight loss. During the experiment, if weight loss of 20% or more occurs within 2 to 3 days or weight loss of 25% or more occurs within 7 days, euthanasia will be performed.
Xenograft model and intratumoral treatment
After allowing mice to acclimate to the animal facility for 1 week, PC-3 (5×105/tumor) cells were inoculated subcutaneously in 50 µl RPMI-1640 medium, together with 50 µl of Matrigel (Corning Incorporated). When the tumors reached 5 mm in diameter, we randomly allocated the mice into four groups (n=4 mice per group): Control (vehicle only), DTX (intraperitoneal injection, 5 mg/kg), KLG siRNA [intratumoral injection, 10 µg of siRNA with 1.2 µl of in vivo-jetPEI (Polyplus-transfection Inc.) at an N/P ratio of 6, according to the manufacturer's protocol], and a combination group (DTX plus KLG siRNA). DTX was administered twice-a-week for 3 weeks and KLG siRNA was administered once-a-week for 3 weeks. In vivo-jet PEI was used in conjunction with siRNA to ensure optimal delivery in the xenograft tumor tissue (29). We underwent subcutaneous administration of siRNA from various direction around the tumor, taking care of not to sting the tumor. The mice of the control group were received intraperitoneal and intratumoral injection of PBS. We used isoflurane (induction 4%, maintenance 2%) as inhalation anesthesia during inoculation of cancer cells subcutaneously and administration of therapeutic agents. Tumor diameters were measured once-a-week with electronic calipers. Tumor volume was calculated using the following formula: {(width)2 × length}/2 (mm3). Five weeks after inoculation, all mice were euthanized by exsanguination under anesthesia with isoflurane (4%); tumors were harvested for IHC analysis. After harvest, we performed cervical dislocation as the additional euthanasia procedure to verify the death of the mice.
IHC analysis of xenograft tumors
Tumors were examined using IHC staining analysis in the same manner as described above. Anti-KLG antibody and anti-Ki-67 antibody (clone MIB-1, ready to use; Dako) were used for IHC analysis. Ki-67 was scored as the percentage of nuclei-stained cells among all cancer cells, regardless of the intensity (30).
Statistical analysis
All statistical analyses were performed using PASW Statistics 17.0 (SPSS, Inc.) and Prism software 5.00 (GraphPad Software, Inc.). Figures were constructed using GraphPad Prism 5.0. Comparisons between time points for individual data were performed using a Student's t-test or Mann-Whitney U test and comparisons between treatment groups were performed using a Kruskal-Wallis test followed by a Steel-Dwass test. P<0.05 was considered to indicate a statistically significant difference. The Cox proportional-hazards model was used for univariate and multivariate analyses, to identify prognostic factors of overall survival (OS) in patients with CRPC. For multivariate analysis, those variables with P<0.05 in the univariate analysis were selected. We determined cutoff values for all variables from ROC curves. The OS and disease-specific survival (DSS) curves were obtained using the Kaplan-Meier method and compared using the log-rank test for each prognostic variable.
Results
Association between expression levels of KLs and survival of patients with CRPC
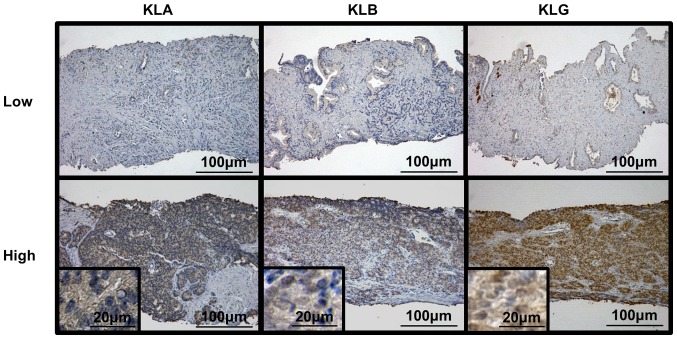
We performed IHC analysis for KLs using human prostate biopsy samples and explored the relationship between the expression levels of KLs and clinicopathological variables. Fig. 1 shows representative images of low and high expression levels of each KL. We calculated the IHC score for each slide and divided these into two groups, according to the scores. Of the 36 patients, 17 (47.2%) had low KLA expression and 19 (52.8%) had high KLA expression; 15 (41.7%) patients had low KLB expression and 21 (58.3%) had high KLB expression. Regarding expression of KLG, 13 (36.1%) patients showed low expression and 23 (63.9%) had high KLG expression. Table I shows the clinicopathological background of the 36 patients with CRPC who were administered DTX at our hospital and comparison of the variables according to low and high KLG expression.
Figure 1.
Typical images of low and high expression of Klotho genes. KLA, α-Klotho; KLB, β-Klotho; KLG, γ-Klotho.
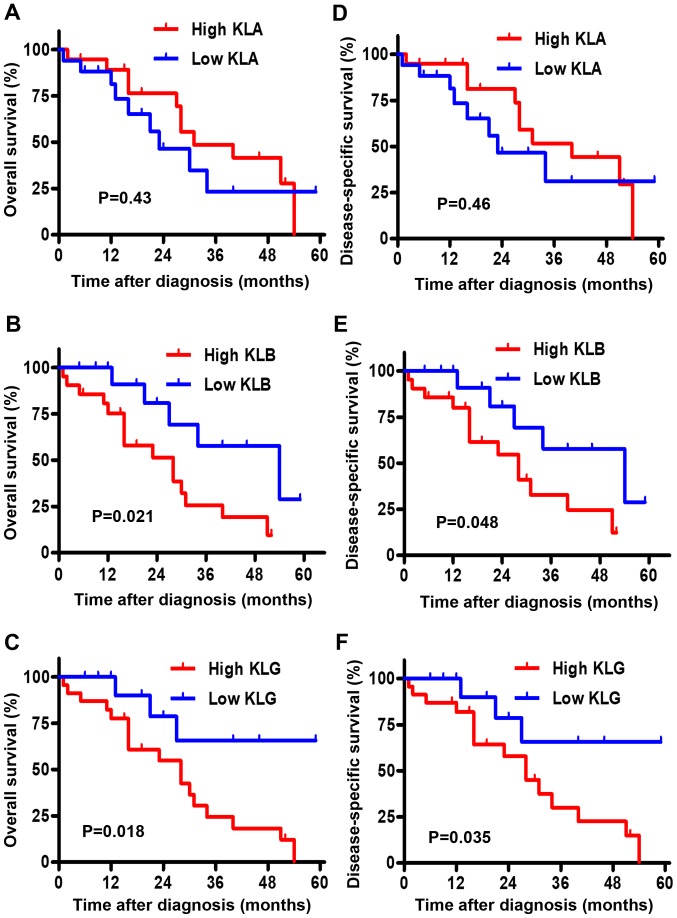
The Kaplan-Meier curves for the association between OS and the expression level of each KL are depicted in Fig. 2A-C. Although there was no significant difference in OS between the low and high groups of KLA expression (P=0.43), high expression levels of KLB and KLG were associated with shorter OS than low expression levels (P=0.021 and P=0.018, respectively). The Kaplan-Meier curves for the association between DSS and the expression level of each KL are shown in Fig. 2D-F. Similarly, there was no significant difference in DSS between the low and high groups of KLA expression (P=0.46); high expression levels of KLB and KLG were associated with shorter DSS than low expression levels (P=0.048 and P=0.035, respectively).
Figure 2.
Association between KL expression and OS/DSS. (A-C) Association between OS of 36 patients with CRPC and KL expression. (A) There was no significant association between KLA and OS. (B and C) OS was significantly lower in patients with high KLB and high KLG expression than that in patients with low KLB and low KLG expression, respectively. (D-F) Association between DSS of 36 patients with CRPC and KL expression. (D) There was no significant association between KLA and DSS. (E and F) DSS was significantly lower in patients with high KLB and high KLG expression than that in patients with low KLB and low KLG expression, respectively. KL, Klotho; OS, overall survival; DSS, disease-specific survival; CRPC, castration-resistant prostate cancer; KLA, α-Klotho; KLB, β-Klotho; KLG, γ-Klotho.
Expression level of KLG is a prognostic factor in patients with CRPC
Table II shows the univariate and multivariate analyses of prognostic factors for OS after the initiation of DTX in patients with CRPC. High expressions of KLB and KLG were prognostic factors for OS in the univariate analysis (P=0.032 and P=0.029, respectively). The multivariate analysis revealed that only high expression of KLG was an independent prognostic factor (P=0.012).
Table II.
Univariate and multivariate analysis of prognostic factors of OS in patients with castration-resistant prostate cancer who were administered DTX.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age, years | ||||||
| <70 | 1 | 0.33 | ||||
| ≥70 | 1.6 | 0.6–3.8 | ||||
| PSA at diagnosis, ng/ml | ||||||
| <70 | 1 | 0.49 | ||||
| ≥70 | 0.7 | 0.3–1.8 | ||||
| Total Gleason Score | ||||||
| Up to 4+4 | 1 | 0.075 | ||||
| 4+5, 5+4, 5+5 | 2.7 | 0.9–8.2 | ||||
| Clinical T stage | ||||||
| <3 | 1 | 0.91 | ||||
| ≥3 | 1.1 | 0.3–3.8 | ||||
| Clinical N stage | ||||||
| <1 | 1 | 0.14 | ||||
| ≥1 | 1.9 | 0.8–4.9 | ||||
| Clinical M stage | ||||||
| 0 | 1 | 0.83 | ||||
| 1 | 0.9 | 0.4–2.3 | ||||
| RP as Primary therapy | ||||||
| No | 1 | 0.4 | ||||
| Yes | 0.59 | 0.2–2.1 | ||||
| Period from ADT to DTX, months | ||||||
| <25 | 1 | 0.17 | ||||
| ≥25 | 0.5 | 0.2–1.3 | ||||
| PSA nadir of ADT, ng/ml | ||||||
| <2 | 1 | 0.69 | ||||
| ≥2 | 1.2 | 0.5–3.0 | ||||
| Time from PSA nadir of ADT to DTX, months | ||||||
| <20 | 1 | 0.68 | ||||
| ≥20 | 0.83 | 0.3–2.1 | ||||
| PSA at starting of DTX | ||||||
| <50 | 1 | 0.77 | ||||
| ≥50 | 0.9 | 0.4–2.1 | ||||
| KLA | ||||||
| Low | 1 | 0.43 | ||||
| High | 0.7 | 0.3–1.7 | ||||
| KLB | ||||||
| Low | 1 | 0.032 | 1 | 0.27 | ||
| High | 3.3 | 1.1–10.1 | 2 | 0.6–7.3 | ||
| KLG | ||||||
| Low | 1 | 0.029 | 1 | 0.012 | ||
| High | 3.9 | 1.1–13.5 | 3.9 | 1.1–13.4 | ||
KLB and KLG were prognostic factors of OS in univariate analysis. In multivariate analysis, KLG was an independent prognostic factor. OS, overall survival; PSA, prostate specific antigen; RP, radical prostatectomy; ADT, androgen-deprivation therapy; DTX, docetaxel; KLA, α-Klotho; KLB, β-Klotho; KLG, γ-Klotho; HR, hazard ratio; CI, confidence interval.
Association of KLG expression with clinicopathological variables and response change rate of prostate-specific antigen (PSA) after DTX therapy in CRPC
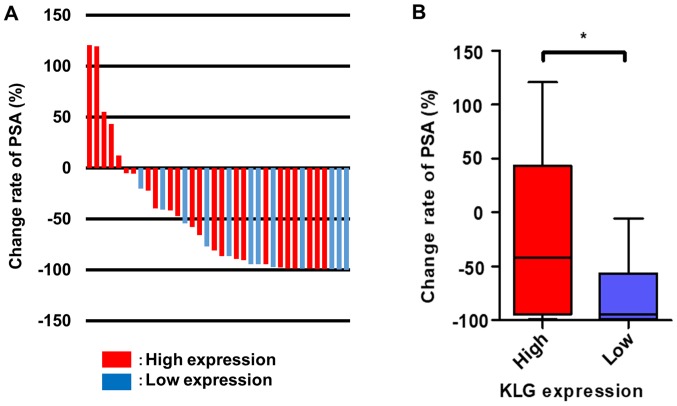
Based on the result of prognostic factors, we focused on the association between the expression level of KLG and CRPC. In comparison with patients who had high KLG expression, those with low KLG expression had significantly lower initial PSA at diagnosis (P=0.016), negative lymph node metastasis (P=0.029), higher proportion of radical prostatectomy as primary therapy (P=0.031), longer OS (P=0.048), and better PSA response rate after the initiation of DTX (P=0.045). In addition, patients in the high expression group received ADT as primary therapy rather than those in the low expression group (P=0.031) (Table I). Fig. 3A represents a waterfall plot of the response change rate of PSA. Patients with high KLG expression had a poorer PSA response rate after the initiation of DTX therapy than patients with low expression (P=0.043; Fig. 3B).
Figure 3.
Association between KLG expression and change rate of PSA. Change rate of PSA in patients with castration-resistant prostate cancer after DTX. (A) Waterfall plot of change rate of PSA. (B) Patients with high KLG expression had a poorer PSA response rate after DTX treatment than patients with low expression. Mann-Whitney U test was conducted. *P<0.05. KLG, γ-Klotho; PSA, prostate-specific antigen; DTX, docetaxel.
PC-3 cells expressing endogenous KLG and KLG siRNA could knock out endogenous KLG in vitro
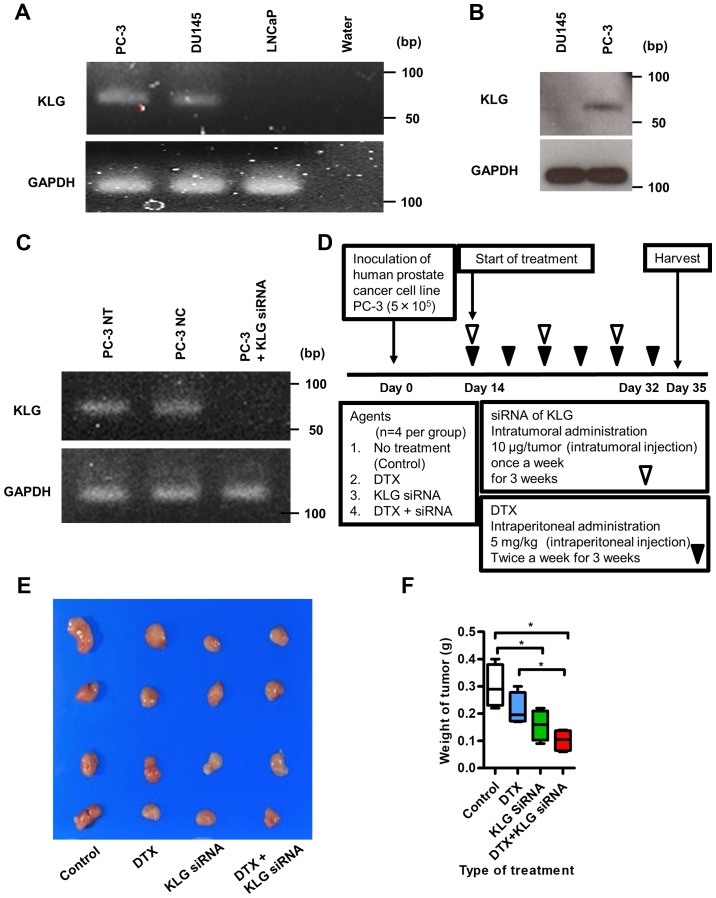
To check the expression levels of KLG in human PC-3, DU145, and LNCaP cell lines, we performed RT-PCR analysis. PC-3 and DU145 human CRPC cells expressed endogenous KLG mRNA; LNCaP cells did not express endogenous KLG mRNA (Fig. 4A). We performed western blot analysis to examine the expression level of KLG in PC-3 and DU145 cell lines; KLG protein was expressed in PC-3 cells (Fig. 4B). RT-PCR analysis revealed that expression of KLG in PC-3 cells in vitro was knocked down in the presence of KLG siRNA (Fig. 4C).
Figure 4.
Reverse transcription PCR and western blot analysis of KLG in PC-3 cells, and xenograft model. (A) Reverse transcription-PCR analysis revealed that PC-3 and DU145 cells expressed KLG RNA. (B) Western blot analysis showed that KLG protein was expressed in PC-3 cells. (C) Expression of KLG was knocked downed with KLG siRNA as shown in the reverse transcription-PCR analysis. (D) Schematic diagram illustrating the study workflow. Mice were injected with PC-3 cells (5×105/tumor) together with Matrigel. After 2 weeks of inoculation, mice were randomly divided into four groups [n=4 per group; control (no treatment), DTX, KLG siRNA and DTX + KLG siRNA]. Mice were treated for 3 weeks. After 5 weeks of inoculation, mice were euthanized and xenografts were harvested. (E) Subcutaneous tumors were removed from the mouse xenograft model. (F) Tumor weight was significantly lower in the KLG siRNA and KLG siRNA + DTX treated groups than in the control and DTX only groups. Kruskal-Wallis test was conducted. *P<0.05. KLG, γ-Klotho; siRNA, small interfering RNA; DTX, docetaxel; siRNA, small interfering RNA.
KLG siRNA treatment inhibits tumor growth in vivo
We inoculated PC-3 cells subcutaneously into the flanks of male athymic BALB/c nu/nu mice as shown in Fig. 4D. Five weeks after inoculation, all subcutaneous tumors were resected (Fig. 4E). The median body weights of the mice at the beginning of this study in the control group, the DTX treated group, the KLG siRNA treated group and the DTX + KLG siRNA treated group were 18.5 (18–19) g, 20.5 (18–22) g, 19 (18–21) g and 20 (18–21) g, respectively. And the median body weights of the mice at the endpoint of this study were 17 (16–17) g, 19 (18–20) g, 18.5 (17–20) g and 18 (17–20) g, respectively. Significant body weight loss was not observed in any of the treated groups (data not shown).
Four weeks after inoculation, the KLG siRNA treated group and KLG siRNA + DTX treated group started to show significant antitumor effects compared with the control group (data not shown). The median final maximum tumor volumes at the end of this study in the control group, the DTX treated group, the KLG siRNA treated group and the DTX + KLG siRNA treated group were 277.8 (271.5–312.7) mm3, 234.9 (201.6–291.2) mm3, 207.3 (114.1–255.9) mm3 and 165.7 (140.9–210.5) mm3, respectively. And the median final weight of subcutaneous tumors were 0.29 (0.22–0.4) g, 0.2 (0.17–0.3) g, 0.16 (0.09–0.22) g and 0.11 (0.08–0.14) g, respectively. The final tumor weight was significantly lower in the KLG siRNA and KLG siRNA + DTX treated groups than in the control and DTX only groups at the end of the treatment (Fig. 4F).
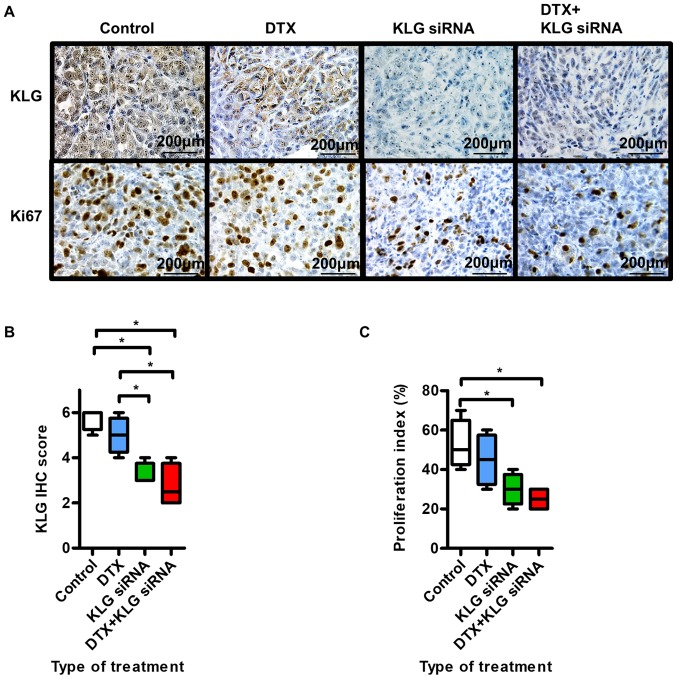
Fig. 5A shows representative images of IHC staining with anti-KLG and Ki-67 antibodies. In tumors treated with KLG siRNA and KLG siRNA + DTX, the expression levels of KLG and Ki-67 were significantly lower than those in the control group (Fig. 5B and C). Treatment with KLG siRNA led to decreased expression of KLG. These results suggested that tumor proliferation and sensitivity to DTX in PC-3 cells were decreased after treatment with KLG siRNA.
Figure 5.
Effect of KLG siRNA on subcutaneous tumor. (A) Immunohistochemistry of tumor with KLG and Ki-67 antibodies. (B) Treatment with KLG siRNA led to downregulation of KLG, as expected. (C) In the KLG siRNA and KLG siRNA + DTX treated groups, the proliferation index was lower than in the control and DTX only groups. Mann-Whitney U test was conducted. *P<0.05. KLG, γ-Klotho; siRNA, small interfering RNA; DTX, docetaxel.
Discussion
Our previous reports indicated that KLB and KLG act as tumor promoters and that KLA does not have an important role as a tumor suppressor or promoter in human bladder cancer (22,23). KLB and KLG work as co-receptors along with the classic FGF receptors (FGFRs) to activate FGF/FGFRs signaling. FGF/FGFRs signaling regulates important biological processes of tumor progression including tumor proliferation, angiogenesis, cell differentiation, and apoptosis (10–12). In prostate cancer, FGF19/FGFR4 signaling promotes cancer progression together with expression of KLA and/or KLB as cofactors (20,27). KLG acts mainly with FGF19 and FGFR4 (26); however, there have been no reports on the association of KLG with prostate cancer. Here, we have investigated a link between human prostate cancer and the KL gene family, including KLG.
Because KLG could be a prognostic factor of OS in patients with CRPC, we focused on KLG and found that KLG plays an important role in tumor progression in prostate cancer. Although the mechanism of tumor growth in relation to KLG is unclear, considering that KLG is a cofactor of FGF/FGFR signaling, tumor growth may be activated through FGF/FGFRs signaling. KLG siRNA had a cytoreductive effect on subcutaneous tumor in an in vivo xenograft model. In addition, KLG siRNA combined with DTX was more effective in the treatment of CRPC than DTX monotherapy. Therefore, we determined that sensitivity to DTX may be increased after treatment with KLG siRNA.
To our knowledge, there are only three reports on the association between KLG and cancer development (9,23,24). The present study is the first report of the relationship between KLG and prostate cancer. Our previous report describes the effects of KLG on tumor growth, including promotion of cell proliferation, inhibition of apoptosis, and enhancement of the EMT in human bladder cancer (23). Kim et al reported that KLG is an important factor in cell proliferation by activating ERK1/2 signaling pathway in colon cancer (9). Trošt et al reported that high expression level of KLG correlates with poor disease progression and that in vitro analysis indicated that KLG was a necessary factor for cell survival; depletion of KLG resulted in cell cycle arrest, apoptosis, and persistent activation of the ERK1/2 signaling pathway in patients with triple-negative breast cancer (24). Therefore, we speculate that KLG plays a role in the promotion of prostate cancer growth and that the balance of existing KLs affects activation of various pathways, including FGF/FGFR signaling.
In human lung cancer, increasing prevalence of cisplatin-resistant cancer has become a major problem. Wang et al demonstrated that KLA could improve the resistance of lung cancer cells to cisplatin (31). These authors suggested that KLA could inhibit the PI3K/Akt pathway and alleviate the resistance of lung cancer cells to cisplatin. In hepatocellular carcinoma (HCC), FGF19/FGFR4 signaling had an effect on HCC resistance to sorafenib therapy through the inhibition of reactive oxygen species generation and apoptosis (32). We suppose that KLs possibly have a potent effect in lowering resistance to anti-tumor drugs.
The prognostic factors of OS in patients with CRPC have been discussed. Some studies have claimed that various factors have been identified as independent prognostic factors of OS, such as age, neutrophil-to-lymphocyte ratio, serum PSA level at the start of chemotherapy, and anemia (33). In this study, KLG was found to be a prognostic factor for patients with CRPC in multivariate analysis. Thus, KLG may serve as a new prognostic factor in patients with CRPC. In addition, KLG was a predictor of resistance to DTX among patients with CRPC in this study. Overexpression of KLG in tissues from biopsy may be beneficial for patients if they are concurrently prescribed drugs that suppress KLG signaling or if they are not treated with DTX but with other agents, such as cabazitaxel, abiraterone or enzalutamide. KLG may be a new biomarker for diagnosis and/or a therapeutic target in patients with CRPC.
This study has some limitations. First, we only used an in vivo xenograft model in this study. As an ectopic model differs from the actual reaction in the human body, further investigation using an orthotopic model is necessary. Second, we have not been able to elucidate enough mechanism of signaling pathway and cell cycle. We did not evaluate the relationship between KLG and FGF/FGFR signaling in this study. Further experiments are needed to clarify which pathway in FGF/FGFR signaling is actually involved in the prostate tumorigenesis. And we should evaluate cell proliferation using not only Ki67 but also CD44, because CD44 are noted as a surface marker for cancer stem cells. We consider these experiments as our future studies. Third, our sample size was small with only 36 patients with CRPC. There were significant differences in PSA at diagnosis and proportion of patients with lymph node metastasis between low and high expression groups of KLG. This may be a bias of this study. It is necessary to conduct the analysis with a larger cohort of CRPC patients. Fourth, we only used tissues collected from biopsy at the time of diagnosis as human samples in this study. Tissues obtained from biopsy may be degenerated, in comparison with the actual nature of tissues in CRPC, owing to modification caused by treatment, such as hormone and radiation therapy. Further investigation using actual CRPC tissues, such as those from metastatic lesions, are needed.
In conclusion, our findings indicated that KLG has an important role in prostate tumorigenesis. The expression level of KLG was associated with cell proliferation of cancer cells in vivo by Ki-67 staining. In addition, KLG could be a predictor of resistance to DTX and a prognostic factor for mortality. KLG may become a new biomarker for diagnosis and/or a therapeutic target in patients with CRPC.
Acknowledgements
The authors would like to thank Mrs. Aya Asano (Department of Pathology, Nara Medical University) for providing substantial help with intratumoral treatment.
Glossary
Abbreviations
- KL
Klotho
- KLA
α-Klotho
- KLB
β-Klotho
- KLG
γ-Klotho
- CRPC
castration-resistant prostate cancer
- IHC
immunohistochemistry
- OS
overall survival
- DTX
docetaxel
- siRNA
small interfering RNA
- FGF
fibroblast growth factor
- EMT
epithelial-to-mesenchymal transition
- FGFR
fibroblast growth factor receptor
- DSS
disease-specific survival
- PSA
prostate-specific antigen
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
KO, MM, SH, YN and KF conceived the study. KO collected samples and drafted the manuscript. KO, KI, YM and SO conducted the laboratory work. KO, YT and NT performed the statistical analysis. All authors were involved in the preparation and revision of the manuscript, and approved the final manuscript.
Ethics approval and consent to participate
The Ethics Committee of Nara Medical University approved the patient study protocol (reference ID: 1256). Written informed consent was obtained from all participants. The animal study was approved by The Animal Facility Committee at Nara Medical University (protocol ID: 11896).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Lowrance WT, Roth BJ, Kirkby E, Murad MH, Cookson MS. Castration-resistant prostate cancer: AUA guideline amendment 2015. J Urol. 2016;195:1444–1452. doi: 10.1016/j.juro.2015.10.086. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.Carroll PR, Parsons JK, Andriole G, Bahnson RR, Castle EP, Catalona WJ, Dahl DM, Davis JW, Epstein JI, Etzioni RB, et al. NCCN guidelines insights: Prostate cancer early detection, version 2.2016. J Natl Compr Canc Netw. 2016;14:509–519. doi: 10.6004/jnccn.2016.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 7.Ito S, Fujimori T, Hayashizaki Y, Nabeshima Y. Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim Biophys Acta. 2002;1576:341–345. doi: 10.1016/S0167-4781(02)00281-6. [DOI] [PubMed] [Google Scholar]
- 8.Kurosu H, Kuro-O M. The Klotho gene family as a regulator of endocrine fibroblast growth factors. Mol Cell Endocrinol. 2009;299:72–78. doi: 10.1016/j.mce.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Eskiocak U, Stadler G, Lou Z, Kuro-o M, Shay JW, Wright WE. Short hairpin RNA screen indicates that Klotho beta/FGF19 protein overcomes stasis in human colonic epithelial cells. J Biol Chem. 2011;286:43294–43300. doi: 10.1074/jbc.M111.267641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiong KH, Mah LY, Leong CO. Functional roles of fibroblast growth factor receptors (FGFRs) signaling in human cancers. Apoptosis. 2013;18:1447–1468. doi: 10.1007/s10495-013-0886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlessinger J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science. 2004;306:1506–1507. doi: 10.1126/science.1105396. [DOI] [PubMed] [Google Scholar]
- 12.Miyake M, Ishii M, Koyama N, Kawashima K, Kodama T, Anai S, Fujimoto K, Hirao Y, Sugano K. 1-tert-butyl-3-[6-(3,5-dimethoxy-phenyl)-2-(4-diethylamino-butylamino)-pyrido [2,3-d]pyrimidin-7-yl]-urea (PD173074), a selective tyrosine kinase inhibitor of fibroblast growth factor receptor-3 (FGFR3), inhibits cell proliferation of bladder cancer carrying the FGFR3 gene mutation along with up-regulation of p27/Kip1 and G1/G0 arrest. J Pharmacol Exp Ther. 2010;332:795–802. doi: 10.1124/jpet.109.162768. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X, Wang X. Klotho: A novel biomarker for cancer. J Cancer Res Clin Oncol. 2015;141:961–969. doi: 10.1007/s00432-014-1788-y. [DOI] [PubMed] [Google Scholar]
- 14.Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP, Rubinek T. Klotho: A tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27:7094–7105. doi: 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- 15.Chen B, Wang X, Zhao W, Wu J. Klotho inhibits growth and promotes apoptosis in human lung cancer cell line A549. J Exp Clin Cancer Res. 2010;29:99. doi: 10.1186/1756-9966-29-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–8665. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Y, Xu L, Zhang J, Xu W, Liu Y, Yin H, Lv T, An H, Liu L, He H, et al. Klotho suppresses tumor progression via inhibiting PI3K/Akt/GSK3β/Snail signaling in renal cell carcinoma. Cancer Sci. 2013;104:663–671. doi: 10.1111/cas.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie B, Zhou J, Yuan L, Ren F, Liu DC, Li Q, Shu G. Epigenetic silencing of Klotho expression correlates with poor prognosis of human hepatocellular carcinoma. Hum Pathol. 2013;44:795–801. doi: 10.1016/j.humpath.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Poh W, Wong W, Ong H, Aung MO, Lim SG, Chua BT, Ho HK. Klotho-beta overexpression as a novel target for suppressing proliferation and fibroblast growth factor receptor-4 signaling in hepatocellular carcinoma. Mol Cancer. 2012;11:14. doi: 10.1186/1476-4598-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng S, Dakhova O, Creighton CJ, Ittmann M. Endocrine fibroblast growth factor FGF19 promotes prostate cancer progression. Cancer Res. 2013;73:2551–2562. doi: 10.1158/0008-5472.CAN-12-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye X, Guo Y, Zhang Q, Chen W, Hua X, Liu W, Yang Y, Chen G. bKlotho suppresses tumor growth in hepatocellular carcinoma by regulating Akt/GSK-3β/cyclin D1 signaling pathway. PLoS One. 2013;8:e55615. doi: 10.1371/journal.pone.0055615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hori S, Miyake M, Onishi S, Tatsumi Y, Morizawa Y, Nakai Y, Anai S, Tanaka N, Fujimoto K. Clinical significance of α- and β-Klotho in urothelial carcinoma of the bladder. Oncol Rep. 2016;36:2117–2125. doi: 10.3892/or.2016.5053. [DOI] [PubMed] [Google Scholar]
- 23.Hori S, Miyake M, Tatsumi Y, Morizawa Y, Nakai Y, Onishi S, Onishi K, Iida K, Gotoh D, Tanaka N, Fujimoto K. Gamma-Klotho exhibits multiple roles in tumor growth of human bladder cancer. Oncotarget. 2018;9:19508–19524. doi: 10.18632/oncotarget.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trošt N, Peña-Llopis S, Koirala S, Stojan J, Potts PR, Fon Tacer K, Martinez ED. γKlotho is a novel marker and cell survival factor in a subset of triple negative breast cancers. Oncotarget. 2016;7:2611–2628. doi: 10.18632/oncotarget.6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abramovitz L, Rubinek T, Ligumsky H, Bose S, Barshack I, Avivi C, Kaufman B, Wolf I. KL1 internal repeat mediates klotho tumor suppressor activities and inhibits bFGF and IGF-I signaling in pancreatic cancer. Clin Cancer Res. 2011;17:4254–4266. doi: 10.1158/1078-0432.CCR-10-2749. [DOI] [PubMed] [Google Scholar]
- 26.Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ, Kliewer SA. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24:2050–2064. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagamatsu H, Teishima J, Goto K, Shikuma H, Kitano H, Shoji K, Inoue S, Matsubara A. FGF19 promotes progression of prostate cancer. Prostate. 2015;75:1092–1101. doi: 10.1002/pros.22994. [DOI] [PubMed] [Google Scholar]
- 28.Anai S, Goodison S, Shiverick K, Hirao Y, Brown BD, Rosser CJ. Knock-down of Bcl-2 by antisense oligodeoxynucleotides induces radiosensitization and inhibition of angiogenesis in human PC-3 prostate tumor xenografts. Mol Cancer Ther. 2007;6:101–111. doi: 10.1158/1535-7163.MCT-06-0367. [DOI] [PubMed] [Google Scholar]
- 29.Ito T, Shimada Y, Kan T, David S, Cheng Y, Mori Y, Agarwal R, Paun B, Jin Z, Olaru A, et al. Pituitary tumor-transforming 1 increases cell motility and promotes lymph node metastasis in esophageal squamous cell carcinoma. Cancer Res. 2008;68:3214–3224. doi: 10.1158/0008-5472.CAN-07-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li LT, Jiang G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer (Review) Mol Med Rep. 2015;11:1566–1572. doi: 10.3892/mmr.2014.2914. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Chen L, Huang G, He D, He J, Xu W, Zou C, Zong F, Li Y, Chen B, et al. Klotho sensitizes human lung cancer cell line to cisplatin via PI3k/Akt pathway. PLoS One. 2013;8:e57391. doi: 10.1371/journal.pone.0057391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao L, Wang X, Tang Y, Huang S, Hu CA, Teng Y. FGF19/FGFR4 signaling contributes to the resistance of hepatocellular carcinoma to sorafenib. J Exp Clin Cancer Res. 2017;36:8. doi: 10.1186/s13046-016-0478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao J, Zhu X, Zhao X, Li XF, Xu R. Neutrophil-to-lymphocyte ratio predicts PSA response and prognosis in prostate cancer: A systematic review and meta-analysis. PLoS One. 2016;11:e0158770. doi: 10.1371/journal.pone.0158770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.