Abstract
The scavenger receptor stabilin-1 has been reported to be expressed by tumor-associated macrophages (TAMs) and to facilitate tumor growth and metastasis in mouse models of breast carcinoma and melanoma. However, to the best of our knowledge, its expression and association with prognosis in human gastric cancer has not been evaluated. The present study investigated the expression of stabilin-1 and its association with clinicopathological parameters in patients with gastric cancer. The expression of stabilin-1 was evaluated by immunohistochemical staining of gastric cancer tissue samples of 371 Chinese patients with primary gastric adenocarcinoma. Confocal laser scanning microscopy was used to determine the cellular source of stabilin-1 in the gastric cancer tissues using anti-CD68, anti-CD163, anti-stabilin-1 and anti-secreted protein acidic and rich in cysteine antibodies. A higher number of stabilin-1-positive cells were observed in the cancer tissues of primary gastric adenocarcinoma compared with adjacent non-cancerous tissues of primary gastric adenocarcinoma (P<0.001); the majority of stabilin-1-positve cells were CD68+/CD163+ macrophages. Poorly-differentiated gastric cancer tissue had fewer stabilin-1-positive cells compared with medium- and well-differentiated gastric cancer (P=0.030). A higher number of stabilin-1-positive cells were found in the early Tumor-Node-Metastasis (TNM) stage (TNM I stage) of primary gastric adenocarcinoma (P=0.038) compared with TNM stage IV. For patients with TNM stage I disease, a higher number of stabilin-1-positive TAMs was associated with shorter cumulative survival (P<0.05). Overall, stabilin-1 was found to be expressed by CD68+ TAMs in human gastric cancer. The high expression of stabilin-1 in TNM stage I disease was associated with poor patient survival, indicating the clinical significance of stabilin-1 in gastric cancer.
Keywords: tumor-associated macrophages, gastric cancer, stabilin-1, secreted protein acidic and rich in cysteine
Introduction
Gastric cancer is one of the leading causes of cancer-associated mortality worldwide, accounting for 8.2% of cancer-associated mortality in 2018 (1). Therefore, novel diagnostic, as well as prognostic, biomarkers for this disease are urgently required.
During tumor progression, the interplay between tumor cells and both the cellular and acellular stromal components is required for the regulation of tumor growth, invasion and metastasis (2). Among the cellular components of the tumor microenvironment (TME), the composition and phenotype of infiltrating immune cells has been shown to have prognostic value in several types of cancer, including gastric cancer (3,4). Tumor-associated macrophages (TAMs) are one of the most abundant immune cell types in the TME of solid tumors, such as breast, prostate, lung and gastric tumors (5–7). Of note, the association between TAM density and disease outcome has been widely reported (8,9); TAMs have been routinely detected by immunohistochemistry using the pan-macrophage marker CD68. The elevated density of macrophages in the tumor mass is typically associated with negative prognosis in breast cancer, non-small cell lung cancer, thyroid cancer, esophageal cancer and other cancer types (10–13). Not only the overall density of CD68+ TAMs, but also the expression levels of several TAM-associated receptors have been reported to influence cancer prognosis. For example, an increase in the expression of endocytic and scavenger receptors (SRs), including CD206, CD163 and CD204, predicts a negative outcome in ovarian cancer, lung cancer and hepatocellular carcinoma (14–16). In gastric cancer, a high infiltration of CD163+ TAMs in the stromal compartment is associated with poor overall survival (17), whereas a high density of CD204+ TAMs is associated with adverse clinicopathological parameters and poor cancer-specific survival (18).
Previously, the expression of the type 1 transmembrane receptor stabilin-1, a member of SR superfamily, has been found in TAMs in several types of murine and human cancer (19–22). In mouse models of B16 melanoma and breast cancer, the expression of stabilin-1 in TAMs facilitates tumor growth and metastasis, although the tumor-promoting mechanism of stabilin-1 expression has not been completely clarified (19,21). One of these studies indicated that the tumor-promoting effect of stabilin-1 is associated with increased endocytic clearance of a soluble component of extracellular matrix (secreted protein acidic and rich in cysteine; SPARC), which is known to inhibit breast cancer growth (19). In humans, the expression of stabilin-1 has been reported in breast cancer, melanoma and glioblastoma (19,20). Specifically, stabilin-1 is co-expressed by a fraction of CD68+ TAMs in breast cancer, and its expression is more pronounced in the early tumor stages of breast cancer and glioblastoma (19,20). However, to the best of our knowledge, the expression of stabilin-1 and its localization in specific TAM subsets in gastric cancer tissues has not yet been analyzed. The data from mouse tumor models suggests that the expression of stabilin-1 in the tumor mass may have prognostic significance for disease progression and patient survival, due to the involvement of stabilin-1 in the regulation of tumor growth and metastatic spread (21). Indeed, a high number of peritumoral stabilin-1-positive macrophages in human colorectal cancer has been reported to be correlated with improved disease-specific survival (DSS), whereas a higher number of stabilin-1-positive TAMs in stage IV of the disease predicted shorter DSS (23). To the best of our knowledge, there are currently no reports regarding the role of stabilin-1 as a prognostic marker in human gastric cancer.
In the present study, the expression of stabilin-1 in human gastric cancer and its co-expression with other macrophage markers, including CD68 and CD163, in the TME were assessed. Furthermore, the association of stabilin-1 expression with clinicopathological characteristics and cumulative survival were examined in a cohort of patients with primary gastric cancer.
Materials and methods
Patient characteristics and tissue samples
A total of 371 Chinese patients with primary gastric adenocarcinoma, including 278 men and 93 women (median age, 60 years; age range 26–83 years), were included in the present study. All patients underwent gastrectomy at the Department of Gastrointestinal Surgery in The First Affiliated Hospital of Anhui Medical University of China between June 2009 and September 2012, and did not receive any preoperative chemotherapy or radiotherapy. Patients who died of surgical complications were excluded from the study. Tumor tissue samples were obtained from all patients enrolled in the study. Non-cancerous gastric tissues were derived from 40 out of the 371 samples with primary gastric adenocarcinoma (4 cm off tumor margin). The demographic characteristics of the 40-selected control samples and 331 non-selected samples are provided in the Table SI. The basic clinical and pathological features of the patients, such as age, sex, tumor location, tumor size and distant metastasis were retrospectively collected. The depth of invasion, nodal metastasis, cancer embolus, differentiation degree, pathohistological type and TNM stage of the tumors were evaluated by two independent pathologists, according to the American Joint Committee on Cancer (7th edition) (24). The survival follow-up was successfully completed for 169 of 371 patients until November 2015, by completing a questionnaire via two nurses over the telephone. The remaining 202 patients were lost to contact due to an invalid telephone number or as a result of disconnecting the follow-up phone calls over three times. The comparison of the demographic and clinical information between the 169 follow-up patients and 202 lost-to-follow-up patients is presented in Table SII. This study was approved by the Ethics Committee of the Anhui Medical University (approval no. 20080253). Informed consent forms explaining the usage of tissue samples were collected from all the patients.
Immunohistochemical staining and data evaluation
Gastric cancer and non-cancerous tissues were fixed in 10% formalin at room temperature for at least 24 h before being embedded in paraffin. Immunohistochemical staining of CD68 and stabilin-1 was performed at room temperature on formalin-fixed, paraffin-embedded tissue sections (4-µm thick) of gastric cancer and non-cancerous tissues. The tissue sections were incubated with 3% peroxide blocking solution for 10 min at room temperature before adding primary antibody. The mouse anti-human CD68 monoclonal antibody (1:50; cat. no. MSK055 KP-1; Zytomed Systems GmbH) or rabbit anti-human stabilin-1 polyclonal antibody (2 µg/ml; 1:800; clone number #27606), which was kindly provided by Professor Julia Kzhyshkowska (Institute of Transfusion Medicine and Immunology, Medical Faculty Mannheim, University of Heidelberg, Germany), were added to each section at room temperature for 1.5 h. The sections were washed three times with PBS, and were incubated with HRP-conjugated goat anti-mouse IgG antibody (1:400; cat. no. 115-035-062; Dianova GmbH) or HRP-conjugated donkey anti-rabbit IgG antibody (1:200; cat. no. sc-2020; Santa Cruz Biotechnology, Inc.) diluted in 0.01M PBS (pH 7.4; Gibco; Thermo Fisher Scientific, Inc.)/1% BSA buffer at room temperature for 45 min. The CD68+ and stabilin-1-positive cells were evaluated using a light microscope (magnification, ×400) by two independent pathologists, who were blinded to each other's findings. The mean number of CD68+ and stabilin-1+ cells at five random views was calculated for each section.
Immunofluorescence analysis
Immunofluorescence staining was performed on formalin-fixed, paraffin-embedded tissue sections (4-µm thick) of 5 patients with primary gastric cancer with representative pathohistological types and various status of local nodal metastasis (from N0 to N3), including 1 case of well-differentiated papillary adenocarcinoma, 3 cases of moderately-differentiated tubular adenocarcinoma and 1 case of poorly-differentiated adenocarcinoma. The tissue sections were incubated with 3% BSA (Sigma-Aldrich; Merck KGaA) for 45 min at room temperature before adding primary antibody. The following primary antibodies were used: Mouse anti-human CD68 monoclonal antibody (1:50; cat. no. MSK055 KP-1; Zytomed Systems GmbH), rabbit anti-human stabilin-1 polyclonal antibody (2 µg/ml; 1:800; clone number #27606), kindly provided by Professor Julia Kzhyshkowska (Institute of Transfusion Medicine and Immunology, Medical Faculty Mannheim, University of Heidelberg, Germany), goat anti-human CD163 antibody (1:200; cat. no. 1607; R&D Systems Europe, Ltd.) and goat anti-human SPARC polyclonal antibody (1:60; cat. no. AF941; R&D Systems, Inc.). The primary antibodies were diluted in 0.01 M PBS (pH 7.4)/1% BSA buffer. Sections were incubated with primary antibody at room temperature for 1.5 h. The following secondary antibodies were used (all purchased from Dianova GmbH): Alexa Fluor 488-conjugated donkey anti-rabbit IgG (1:400; cat. no. 711-545-152), Alexa Fluor 488-conjugated donkey anti-goat IgG (1:400; cat. no. 705-545-003), Alexa Fluor 647-conjugated donkey anti-mouse IgG (1:400; cat. no. 711-545-152), Cy3-conjugated donkey anti-mouse IgG (1:400; cat. no. 715-165-151;), Cy3-conjugated donkey anti-goat IgG (1:400; cat. no. 705-165-003), Cy3-conjugated donkey anti-rabbit IgG (1:400; cat. no. 711-167-003). The nuclei were visualized using DRAQ5 (1:1,000; cat. no. 4084; Cell Signaling Technology, Inc.). The secondary antibodies were diluted in 0.01 M PBS (pH 7.4, Gibco; Thermo Fisher Scientific, Inc.)/1% BSA buffer. Sections were incubated with mixed secondary antibodies with at room temperature for 45 min in the dark. The co-localization of CD68, CD163 and stabilin-1 was analyzed by confocal laser scanning microscopy using the Leica SP8 microscope. The number of CD68+, CD163+ and stabilin-1-positive cells was counted in ten random views at ×63 magnification.
Statistical analysis
The data were analyzed using GraphPad Prism 7.0 software (GraphPad Soft Inc.). Non-parametric Mann-Whitney U test and Dunn's multiple comparisons test following Kruskal-Wallis test were used to compare the number of CD68+ and stabilin-1-positive cells in gastric cancer and non-cancerous tissue. A χ2 test was used to analyze the significance between follow-up and lost-to-follow-up patients. The median number of CD68+ and stabilin-1+ cells was used as the cutoff value for determining high and low expression of CD68 and stabilin-1. Kaplan-Meier analysis was used to evaluate the cumulative survival. A log-rank test was used to compare the prognostic significance of the individual variables on survival. All statistical tests were two-sided, and P<0.05 was considered to indicate a statistically significant difference.
Results
Density of CD68+ and stabilin-1-positive cells is increased in gastric cancer tissue
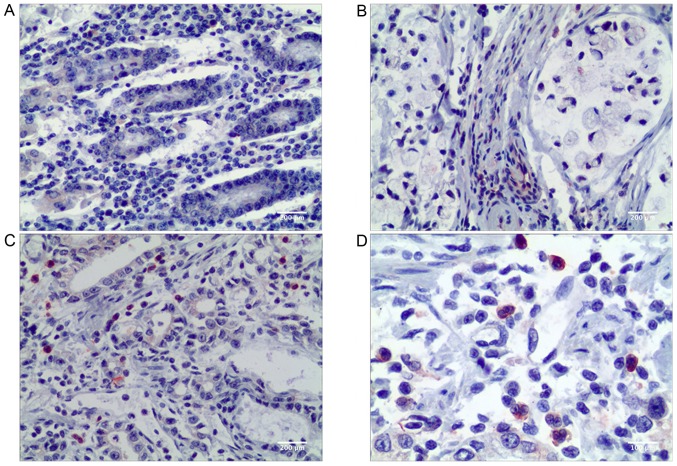
In the present study, CD68 was used as a pan-macrophage marker to visualize TAMs in the microenvironment of gastric cancer. CD68+ TAMs were found both in gastric non-cancerous tissue and in primary gastric cancer tissue. A higher number of TAMs was observed in the stromal areas of gastric cancer compared with the gastric non-cancerous tissue (P<0.001; Table I). Visually, more stabilin-1-positive cells were present in gastric cancer tissue as indicated in Fig. 1, and the difference in the number of stabilin-1-positive cells between cancer tissue and remote non-cancerous tissue of primary gastric carcinoma was statistically significant (P=0.018; Table I).
Table I.
Comparison of number of CD68+ and stabilin-1-positive cells in cancer tissue and remote non-cancerous tissue of primary gastric cancer.
| Tissue type | Cases (n) | CD68+ cells, median (interquartile range) | P-value | Stabilin-1-positive cells, median (interquartile range) | P-value |
|---|---|---|---|---|---|
| Non-cancerous tissue | 40 | 6.2 (4.4–9.2) | <0.001 | 5.5 (3.6–8.1) | 0.018 |
| Cancer tissue | 371 | 16.2 (10.4–25.6) | 8.0 (3.7–13.0) |
Mann-Whitney U test was used to compare the differences.
Figure 1.
Expression of stabilin-1 in human gastric non-cancerous and adenocarcinoma tissues demonstrated by immunohistochemical staining. (A) Stabilin-1 positive cells in normal gastric cancer tissue. Scale bar, 200 µm. (B) Stabilin-1 positive cells in poorly differentiated adenocarcinoma. Scale bar, 200 µm. (C) Stabilin-1 positive cells in medium-well differentiated adenocarcinoma. Scale bar, 200 µm. (D) Stabilin-1 positive cells in medium-well differentiated adenocarcinoma. Scale bar, 100 µm.
Stabilin-1 is expressed in CD68+ TAMs in gastric cancer tissues
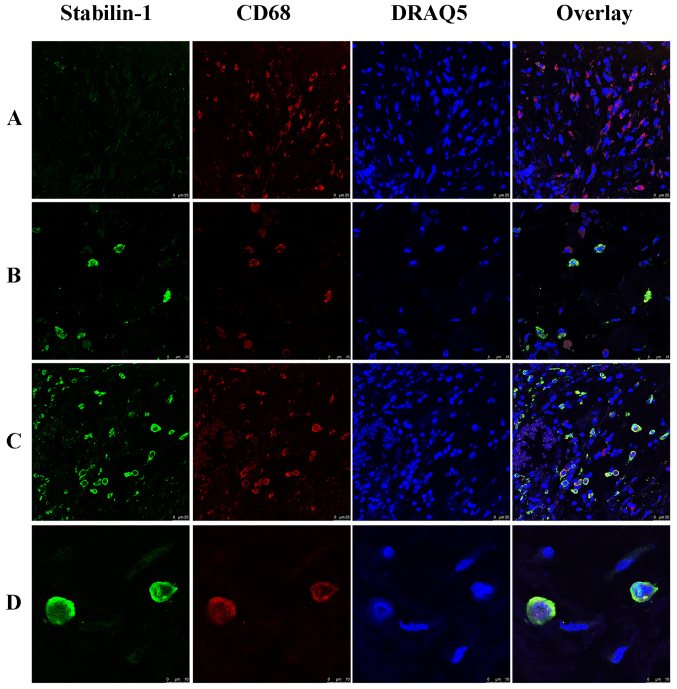
In order to analyze whether stabilin-1 was expressed on TAMs, double immunofluorescence staining for CD68 and stabilin-1 was performed. CD68+ cells were more abundant in the tumor mass compared with stabilin-1-positive cells (Fig. 2A), and almost all stabilin-1-positive cells were CD68+ (Fig. 2B). However, few stabilin-1-positive cells that only weakly expressed CD68 (Fig. 2C) were also observed, which accounted for 6% of total stabilin-1-positive cells in the tumor tissue. In terms of phenotype, ~31% of macrophages in the tumor mass were CD68+/stabilin-1-positive (Fig. 2C), whereas 67% of macrophages were CD68+/stabilin-1-negative, indicating that stabilin-1 was not a general marker of TAM in gastric cancer tissue. Typical CD68+/stabilin-1-positive cells are shown in Fig. 2D
Figure 2.
Expression of stabilin-1 by CD68+ macrophages in human gastric adenocarcinoma. Stabilin-1 was detected by rabbit anti-human stabilin-1 polyclonal antibody and Alexa Fluor 488-conjugated donkey anti-rabbit IgG (indicated in green). CD68 was detected by mouse anti-human CD68 monoclonal antibody and Cy3-conjugated donkey anti-mouse IgG (indicated in red). Nuclei were stained with DRAQ5 (indicated in blue). (A) Stabilin-1-negative/CD68+ cells in poorly gastric adenocarcinoma. Scale bar, 25 µm. (B) Stabilin-1-negative/CD68+ and stabilin-1-positive/CD68+ cells in moderately differentiated gastric adenocarcinoma. Scale bar, 25 µm. (C) Stabilin-1-positive/CD68+ cells in well differentiated gastric adenocarcinoma. Scale bar, 25 µm. (D) Typical stabilin-1-positive/CD68+ cell in moderately differentiated adenocarcinoma. Scale bar, 10 µm.
Stabilin-1 is predominantly expressed by CD68+/CD163+ TAMs in human gastric cancer
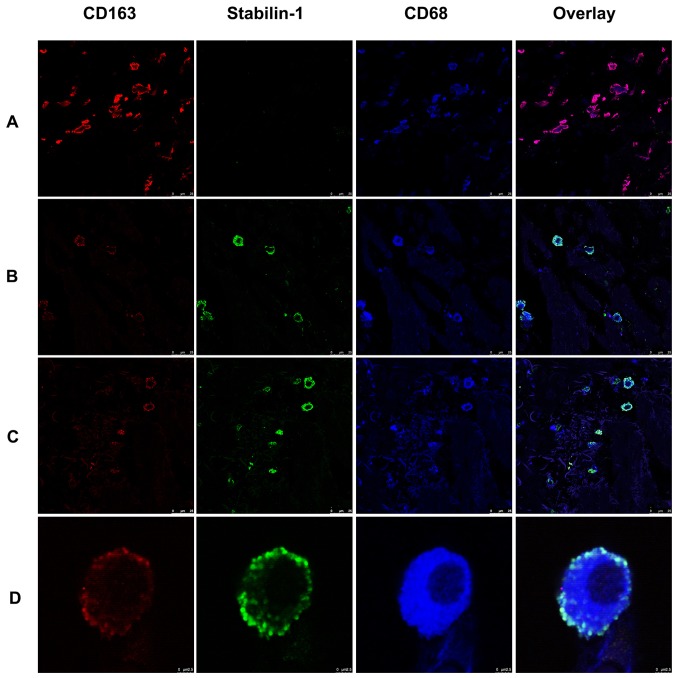
Since stabilin-1 has previously been proposed as a marker of alternatively activated macrophages (21,23,25), the expression of CD163 was assessed in stabilin-1-positive cells, as a typical marker of alternatively activated macrophages (26,27). Triple immunofluorescence staining for stabilin-1, CD163 and CD68 on five sections of gastric cancer tissues was performed to characterize the predominant phenotype of TAMs. As shown in Fig. 3A, some of the CD163+ cells found in the tumor mass were stabilin-1-negative. Stabilin-1-positive cells did not lack in the expression of CD163, although some of the cells that strongly expressed stabilin-1 were only weakly positive for CD163 expression (Fig. 3B). Stabilin-1 expression was mainly observed in CD68+/CD163+ cells, although CD68+/CD163+/stabilin-1-positive cells accounted only for ~7% of the total TAM population (Fig. 3C). Typical CD68+/CD163+/stabilin-1-positive macrophages are shown in Fig. 3D.
Figure 3.
Expression of stabilin-1 by CD68+/CD163+ macrophages in human gastric adenocarcinoma demonstrated by immunofluorescence staining. Stabilin-1 was detected by rabbit anti-human stabilin-1 polyclonal antibody and Alexa Fluor 488-conjugated donkey anti-rabbit IgG (indicated in green). CD163 was detected by goat anti-human CD163 antibody Cy3-conjugated donkey anti-goat IgG (indicated in red). CD68 was detected by mouse anti-human CD68 monoclonal antibody and Alexa Fluor 647-conjugated donkey anti-mouse IgG (indicated in blue). (A) CD68+/CD163+/stabilin-1- negative macrophages in gastric adenocarcinoma. Scale bar, 25 µm. (B) CD68+/CD163low/stabilin-1-positive macrophages in gastric adenocarcinoma. Scale bar, 25 µm. (C) CD68+/CD163low/stabilin-1-positive and CD68+/CD163+/stabilin-1-positive cells in gastric adenocarcinoma. Scale bar, 25 µm. (D) Single CD68+/CD163+/stabilin-1-positive cell in gastric adenocarcinoma. Scale bar, 2.5 µm.
A fraction of stabilin-1-positive TAMs in gastric cancer express SPARC
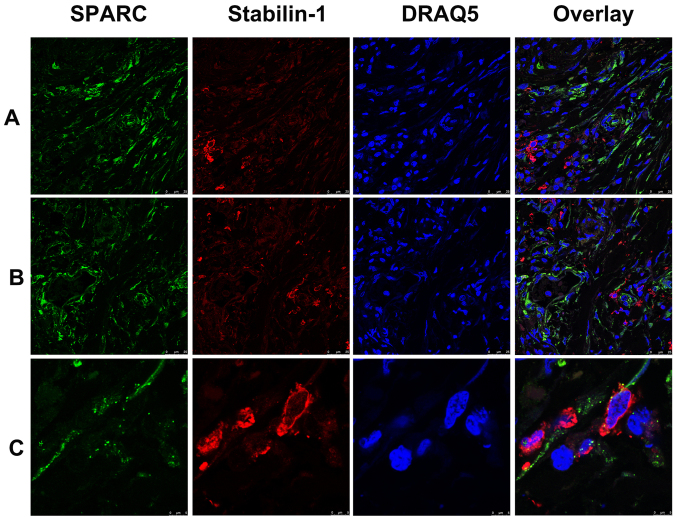
SPARC was previously identified as an endocytic ligand of stabilin-1 receptor and its clearance from extracellular space is associated with increased growth of mammary adenocarcinoma in a mouse model (19,28). Since both SPARC and stabilin-1 proteins are involved in the regulation of tumor growth, their expression was assessed in gastric cancer using double immunofluorescence staining. Abundant SPARC expression was detected in the tumor tissue of both poorly- and well-differentiated gastric adenocarcinoma (Fig. 4A and B). Although in most cases SPARC protein was not expressed by stabilin-1-positive cells, SPARC was detectable in a subset of stabilin-1-positive TAMs (Fig. 4C).
Figure 4.
Expression of stabilin-1 and SPARC in gastric cancer tissue demonstrated by immunofluorescence staining. SPARC was detected by goat anti-human SPARC polyclonal antibody and Alexa Fluor 488-conjugated donkey anti-goat IgG (indicated in green). Stabilin-1 was detected by rabbit anti-human stabilin-1 polyclonal antibody and Cy3-conjugated donkey anti-rabbit IgG indicated in red). Nuclei were stained with DRAQ5 (indicated in blue). The distribution of SPARC-positive and stabilin-1-positive cells in (A) poorly differentiated gastric adenocarcinoma and (B) well differentiated gastric adenocarcinoma. Scale bar, 25 µm. (C) SPARC expression in stabilin-1-positive cells in gastric carcinoma. Scale bar, 5 µm. SPARC, secreted protein acidic and rich in cysteine.
Association between stabilin-1 expression in gastric cancer and clinicopathological characteristics
The association between CD68+ cells and clinicopathological characteristics in gastric cancer is demonstrated in Table SII. No difference was found between the number of CD68+ cells and TNM stages, which was similar to previous studies (4,13). The association between stabilin-1 expression and the clinicopathological characteristics of patients with gastric cancer was subsequently investigated. Poorly-differentiated tumors were found to have fewer stabilin-1-positive cells compared with medium- and well-differentiated tumors (P=0.030; Table II). Furthermore, pathological type was found to influence the density of infiltrating stabilin-1-positive cells (P=0.031; Table II). Specifically, the highest number of stabilin-1-positive cells was observed in tubular papillary adenocarcinoma, whereas fewer stabilin-1-positive cells were found in poorly differentiated adenocarcinoma (Table II). The highest density of stabilin-1-positive cells was found in patients with T1 stage gastric adenocarcinoma, whereas fewer stabilin-1-positive cells were found in T2-4 stage tumors (Table II). The accumulation of stabilin-1 cells among different TNM stages demonstrated statistical significance (P=0.038; Table II). Specifically, more stabilin-1-positive cells were found in the early stage (TNM stage I) of gastric cancer tissues compared with that of late stage (TNM stage IV) gastric cancer tissues (P<0.05; Table II). Thus, in patients with gastric cancer stabilin-1 was preferentially expressed in the early tumor stages.
Table II.
Associations between clinicopathological features and the number of stabilin-1-positive cells in primary gastric adenocarcinoma.
| Clinicopathological feature | Cases (n) | Stabilin-1-positive cells, median (interquartile range) | P-value |
|---|---|---|---|
| Sex | 0.818 | ||
| Male | 278 | 8.1 (4.0–13.5) | |
| Female | 93 | 9.2 (2.7–14.4) | |
| Age | 0.846 | ||
| <60 years | 143 | 8.2 (4.0–13.0) | |
| ≥60 years | 228 | 8.4 (3.9–13.5) | |
| Diameter | 0.053 | ||
| <5 cm | 179 | 9.4 (4.2–14.8) | |
| ≥5 cm | 192 | 8.0 (3.7–12.2) | |
| Tumor location | 0.795 | ||
| Cardia | 199 | 8.6 (3.8–13.6) | |
| Corpora ventriculi | 85 | 8.0 (3.6–13.0) | |
| Sinuses ventriculi | 87 | 8.0 (4.7–14.9) | |
| Differentiation degree | 0.030 | ||
| Poor | 171 | 7.2 (3.0–12.6) | |
| Medium and well | 200 | 9.4 (4.5–14.4) | |
| Pathological type | 0.031a | ||
| Poorly differentiated adenocarcinoma | 103 | 7.0 (2.8–12.0) | |
| Tubular papillary adenocarcinoma | 188 | 9.5 (4.6–14.5) | |
| Signet ring cell and mucinous adenocarcinoma | 80 | 7.7 (3.7–12.7) | |
| Cancer embolus | 0.358 | ||
| Negative | 299 | 8.4 (4.0–14.0) | |
| Positive | 72 | 8.5 (3.85–11.9) | |
| Nodal metastasis | 0.893 | ||
| No | 141 | 8.0 (4.3–12.7) | |
| Yes | 230 | 8.5 (3.8–13.8) | |
| Serosa invasion | 0.080 | ||
| No | 74 | 10.6 (4.8–15.5) | |
| Yes | 297 | 8.0 (3.8–13.0) | |
| T stages | 0.099 | ||
| T1 | 31 | 12.0 (5.8–18.8) | |
| T2 | 43 | 8.4 (3.8–15.4) | |
| T3 | 174 | 9.0 (4.0–13.7) | |
| T4 | 123 | 7.4 (3.4–11.4) | |
| N stages | 0.962 | ||
| N0 | 141 | 8.0 (4.3–12.7) | |
| N1 | 86 | 8.0 (4.0–13.4) | |
| N2 | 73 | 8.8 (3.4–13.8) | |
| N3 | 71 | 8.8 (4.4–14.4) | |
| Distant metastasis | 0.135 | ||
| M0 | 319 | 8.6 (4.0–13.8) | |
| M1 | 52 | 6.9 (3.8–11.4) | |
| TNM stage | 0.038b | ||
| I | 59 | 11.6 (5.8–16.4) | |
| II | 116 | 8.4 (3.4–12.7) | |
| III | 144 | 8.4 (4.2–14.6) | |
| IV | 52 | 6.6 (3.7–11.4) | |
| Smoking | 0.564 | ||
| No | 186 | 7.8 (3.6–14.2) | |
| Yes | 166 | 9.0 (4.2–13.0) | |
| Alcohol consumption | 0.114 | ||
| No | 208 | 7.6 (3.4–13.2) | |
| Yes | 144 | 9.4 (4.8–13.3) | |
| Family history | 0.350 | ||
| No | 312 | 8.2 (3.8–13.2) | |
| Yes | 40 | 9.2 (4.5–13.4) |
TNM, Tumor-Node-Metastasis. Mann-Whitney U test or Dunn's multiple comparisons test following Kruskal-Wallis were used to evaluate the significance differences.
Comparison of stabilin-1-positive cells between poorly differentiated adenocarcinoma and tubular papillary adenocarcinoma.
Comparison of stabilin-1-positive cells between TNM stage I and TNM stage IV.
The patients' characteristics such as sex (P=0.818), age (P=0.846), smoking (P=0.564), regular alcohol intake (P=0.114) and family history of gastric cancer (P=0.350), as well as tumor location (P=0.795), were not associated with the number of infiltrating stabilin-1-positive cells (Table II). Moreover, the density of stabilin-1-positive cells was similar in tumors with or without local nodal metastasis (P=0.893), cancer embolus (P=0.358), distant metastasis (P=0.135) and serosa invasion (P=0.080), as presented in Table II. Smaller tumors (<5 cm in size) had more stabilin-1-positove cells compared with tumors of larger size; however, this tendency did not reach statistical significance (P=0.053; Table II).
Association of CD68 and stabilin-1 expression with cumulative patient survival
A total of 169 patients with gastric cancer were enrolled between November 2009 and November 2015, in a follow-up survival study. There was no statistical significance between the demographical and clinical data, including age, sex, tumor size, tumor location, tumor differentiation, pathological type, TNM stage and lymph node metastasis between the follow-up and lost to follow-up groups (Table SIII). However, higher ratio of family history of gastric cancer was found in lost to follow-up group compared with follow-up group (P=0.008).
The Kaplan-Meier survival analysis was conducted for all follow-up patients, of which none died of surgical complications during follow-up. The log-rank test was used to compare the prognostic significance of individual variables on survival. The followed-up patients were divided into groups with high and low expression of stabillin-1, based on the median number of stabilin-1-positive cells in the tumor tissue. The sample that contained a lower number of stabilin-1-positive cells than the median value was assigned to the stabilin-1 low expression group, whereas the sample that contained a higher number of stabilin-1-positive cells than the median value was included in the stabilin-1 high expression group. High expression of stabilin-1 in patients with TNM stage I gastric cancer was associated with poor cumulative survival (P<0.05; Fig. 5A), however no significant differences were found for stages II–IV (Fig. S1). The number of CD68+ cells in patients with TNM stage I gastric cancer did not affect their survival (P=0.489; Fig. 5B).
Figure 5.
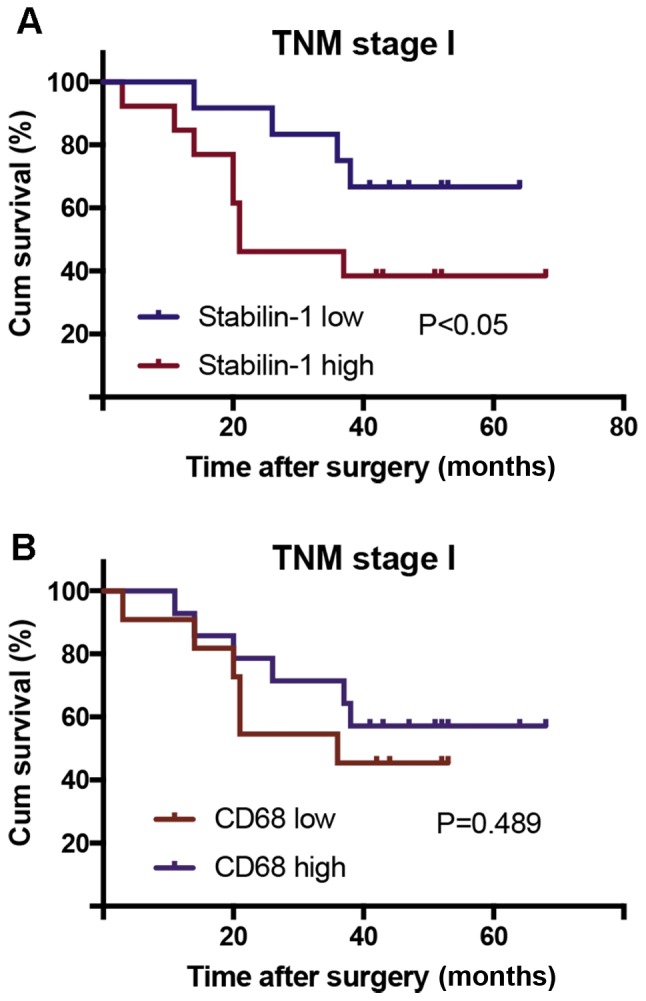
Association of stabilin-1 and CD68 expression in TNM stage I gastric cancer with cumulative patient survival. Kaplan-Meier analysis and a log rank test were used to compare patient survival with low and high numbers of intratumoral stabilin-1-positive and CD68+ cells. (A) The association of stabilin-1 expression with patient survival. (B) The association of CD68 expression with patient survival. The cutoff value for determining CD68 and stabilin-1 expression as low and high expression was 16.2 cells and 8.0 cells, respectively. TNM, Tumor-Node-Metastasis; Cum, cumulative.
Discussion
In the present study, the SR stabilin-1 was demonstrated to be primarily expressed by CD68+ TAMs in the TME of human gastric cancer tissues. Furthermore, the increased density of stabilin-1-positive cells at an early stage of gastric cancer (TNM stage I) was associated with poor cumulative patient survival. To the best of our knowledge, this is the first study to investigate the expression of stabilin-1 in gastric cancer and its association with clinicopathological parameters and survival. However, the follow-up study only included 25 patients with TNM stage I gastric carcinoma, and large sample studies are required to further confirm this conclusion. In previous studies, the intratumoral expression of stabilin-1 was studied in breast cancer, colorectal cancer, glioblastoma and melanoma (19–21,23,29). In human breast cancer, stabilin-1 is expressed by CD68+ TAMs, as well as CD68− cells and some CD31+ vessels (19). The expression of stabilin-1 in the intratumoral vasculature has also been observed in human melanoma and colorectal cancer (23,29). Phenotypically, stabilin-1-positive TAMs in mouse glioblastoma, human breast and colorectal cancer demonstrated co-expression of CD206 and CD163 markers (also known as markers of macrophage alternative activation) (19,20,23). In the present study, stabilin-1 was mainly expressed by CD68+ or CD68-low TAMs in gastric cancer tissues. In line with the previous findings in breast cancer (19), stabilin-1-positive TAMs in gastric cancer heterogeneously expressed CD163. For instance, CD163+/stabilin-1-positive and CD163−/stabilin-1-positive subpopulations of TAMs have been identified in human breast cancer (19), whereas CD163+/stabilin-1-positive and CD163-low/stabilin-1-positive TAMs were present in the TME of gastric cancer tissue in the present study. Thus, both studies indicated the presence of heterogeneous subsets of stabilin-1 expressing TAMs with indications of alternative activation in the TME of breast and gastric tumors.
Of note, the results of the present study identified an increased number of stabilin-1-positive TAMs at an early stage of gastric cancer (T1 and TNM stage I), which corroborated with previous studies in other cancer types, namely human breast cancer and mouse glioblastoma (19,20). In breast cancer, the highest intensity of stabilin-1 expression was observed in TNM stage I disease (19). In a mouse model of glioblastoma, stabilin-1 expression was pronounced at day 7 and decreased at day 14, following intracranial injection of GL26 glioma cells (20). These findings suggest that the TME at an early stage of gastric cancer, breast cancer and glioblastoma favors stabilin-1 expression in TAMs or facilitates the recruitment of monocytes that express stabilin-1. However, gradual molecular changes may decrease stabilin-1 expression or the recruitment of stabilin-1-positive monocytes at advanced cancer stages. The molecular factors responsible for the induction and suppression of stabilin-1 expression in the TME of gastric and other cancer types remain to be identified. In addition to the tumor stage, the density of stabilin-1-positive TAMs in the present study was significantly associated with pathological grade (P=0.031) and differentiation degree (P=0.030). However, the potential mechanism that promotes or inhibits the recruitment of stabilin-1-positive TAMs in gastric tumors, of different pathological types and differentiation degree, requires further characterization.
Although the expression of stabilin-1 has been identified in several types of human cancer, including breast cancer, melanoma and colorectal cancer, its association with patient survival was reported only in colorectal cancer (23). The density of intratumoral stabilin-1-positive TAMs is negatively associated with DSS in stage IV colorectal cancer (23). However, the association between the disease stage and the density of intratumoral stabilin-1-positive TAMs in colorectal cancer was not reported. In the present study, the highest density of stabilin-1-positive TAMs was observed in stage I gastric cancer and decreased in stages II–IV. Accordingly, stabilin-1 was found to be associated with reduced cumulative survival only in stage I gastric cancer, whereas no significant associations were found for stages II–IV. The results of the present study thus suggest that the high expression of stabilin-1 in TAMs is important for gastric cancer progression at an early stage, whereas the predominance of TAMs expressing another molecular phenotype may be crucial for regulating the progression of gastric cancer in the later stages of the disease. Notably, the total number of macrophages, as assessed by CD68 staining, was not associated with patient survival, which further highlighted the importance of specific macrophage subsets for gastric cancer progression.
In a previous study on breast cancer, it was shown that the effect of stabilin-1 on tumor progression could be mediated through the endocytic clearance of its extracellular ligands involved in the regulation of tumor growth, such as SPARC (19). As indicated in several previous studies, the expression of SPARC in gastric cancer is associated with a poor prognosis (30–32). However, one study reported lower microvascular density and decreased cancer cell proliferation in gastric tumors that have high expression of SPARC (33). In the present study, SPARC was found to be abundantly expressed in gastric cancer tissue and detected in some of the stabilin-1-positive TAMs. Thus, the involvement of SPARC clearance in the association between the density of stabilin-1-positive TAMs and gastric cancer prognosis cannot be excluded and deserves further investigation, due to the controversial role of SPARC in gastric cancer progression.
In summary, the present study demonstrated the association of stabilin-1 expression with clinicopathological parameters and cumulative patient survival in gastric cancer. This study offers new perspectives for further studies on stabilin-1 as a novel early prognostic marker of gastric cancer progression.
Supplementary Material
Acknowledgements
The authors would like to thank Professor Julia Kzhyshkowska (Institute of Transfusion Medicine and Immunology, Medical Faculty Mannheim, University of Heidelberg, Germany) for kindly providing the rabbit anti-human stabilin-1 polyclonal antibody.
Funding
The present study was supported by the Program of Culturing Academic Leader of the First Affiliated Hospital of Anhui Medical University (grant no. 1357).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
SPY designed the experiment, performed analysis of the data and drafted the final manuscript. YG and XSX performed immunohistochemistry. DDX performed immunofluorescence staining. VR was responsible for statistical analysis and language corrections. WDD designed all the experiments and edited the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Anhui Medical University (approval no. 20080253) and written inform was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jochems C, Schlom J. Tumor-infiltrating immune cells and prognosis: The potential link between conventional cancer therapy and immunity. Exp Biol Med (Maywood) 2011;236:567–579. doi: 10.1258/ebm.2011.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu K, Yang K, Wu B, Chen H, Chen X, Chen X, Jiang L, Ye F, He D, Lu Z. Tumor-infiltrating immune cells are associated with prognosis of gastric cancer. Medicine (Baltimore) 2015;94:e1631. doi: 10.1097/MD.0000000000001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noy R, Pollard JW. Tumor-associated macrophages: From mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016;2016:6058147. doi: 10.1155/2016/6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin S, Huang J, Li Z, Zhang J, Luo J, Lu C, Xu H, Xu H. The prognostic and clinicopathological significance of tumor-associated macrophages in patients with gastric cancer: A meta-analysis. PLoS One. 2017;12:e0170042. doi: 10.1371/journal.pone.0170042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: Functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35:585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei J, Xiao Z, Guo C, Pu Q, Ma L, Liu C, Lin F, Liao H, You Z, Liu L. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: A systemic review and meta-analysis. Oncotarget. 2016;7:34217–34228. doi: 10.18632/oncotarget.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. 2008;15:1069–1074. doi: 10.1677/ERC-08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugimura K, Miyata H, Tanaka K, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y. High infiltration of tumor-associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol. 2015;111:752–759. doi: 10.1002/jso.23881. [DOI] [PubMed] [Google Scholar]
- 14.Yuan X, Zhang J, Li D, Mao Y, Mo F, Du W, Ma X. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol Oncol. 2017;147:181–187. doi: 10.1016/j.ygyno.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Xu S. Tumor-associated CD204-positive macrophage is a prognostic marker in clinical stage I lung adenocarcinoma. Biomed Res Int. 2018;2018:8459193. doi: 10.1155/2018/8459193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren CX, Leng RX, Fan YG, Pan HF, Li BZ, Wu CH, Wu Q, Wang NN, Xiong QR, Geng XP, Ye DQ. Intratumoral and peritumoral expression of CD68 and CD206 in hepatocellular carcinoma and their prognostic value. Oncol Rep. 2017;38:886–898. doi: 10.3892/or.2017.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JY, Sung JY, Lee J, Park YK, Kim YW, Kim GY, Won KY, Lim SJ. Polarized CD163+ tumor-associated macrophages are associated with increased angiogenesis and CXCL12 expression in gastric cancer. Clin Res Hepatol Gastroenterol. 2016;40:357–365. doi: 10.1016/j.clinre.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Ichimura T, Abe H, Morikawa T, Yamashita H, Ishikawa S, Ushiku T, Seto Y, Fukayama M. Low density of CD204- positive M2-type tumor-associated macrophages in Epstein-Barr virus-associated gastric cancer: A clinicopathologic study with digital image analysis. Hum Pathol. 2016;56:74–80. doi: 10.1016/j.humpath.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Riabov V, Yin S, Song B, Avdic A, Schledzewski K, Ovsiy I, Gratchev A, Llopis Verdiell M, Sticht C, Schmuttermaier C, et al. Stabilin-1 is expressed in human breast cancer and supports tumor growth in mammary adenocarcinoma mouse model. Oncotarget. 2016;7:31097–31110. doi: 10.18632/oncotarget.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.David C, Nance JP, Hubbard J, Hsu M, Binder D, Wilson EH. Stabilin-1 expression in tumor associated macrophages. Brain Res. 2012;1481:71–78. doi: 10.1016/j.brainres.2012.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karikoski M, Marttila-Ichihara F, Elima K, Rantakari P, Hollmén M, Kelkka T, Gerke H, Huovinen V, Irjala H, Holmdahl R, et al. Clever-1/stabilin-1 controls cancer growth and metastasis. Clin Cancer Res. 2014;20:6452–6464. doi: 10.1158/1078-0432.CCR-14-1236. [DOI] [PubMed] [Google Scholar]
- 22.Schledzewski K, Falkowski M, Moldenhauer G, Metharom P, Kzhyshkowska J, Ganss R, Demory A, Falkowska-Hansen B, Kurzen H, Ugurel S, et al. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: Implications for the assessment of lymphangiogenesis. J Pathol. 2006;209:67–77. doi: 10.1002/path.1942. [DOI] [PubMed] [Google Scholar]
- 23.Algars A, Irjala H, Vaittinen S, Huhtinen H, Sundström J, Salmi M, Ristamäki R, Jalkanen S. Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer. 2012;311:864–873. doi: 10.1002/ijc.26457. [DOI] [PubMed] [Google Scholar]
- 24.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. Springer-Verlag; New York: 2009. AJCC Cancer Staging Manual, 7th edition; pp. 117–26. [Google Scholar]
- 25.Kzhyshkowska J. Multifunctional receptor stabilin-1 in homeostasis and disease. ScientificWorldJournal. 2010;10:2039–2053. doi: 10.1100/tsw.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harada K, Dong X, Estrella JS, Correa AM, Xu Y, Hofstetter WL, Sudo K, Onodera H, Suzuki K, Suzuki A, et al. Tumor-associated macrophage infiltration is highly associated with PD-L1 expression in gastric adenocarcinoma. Gastric Cancer. 2018;21:31–40. doi: 10.1007/s10120-017-0760-3. [DOI] [PubMed] [Google Scholar]
- 27.Pantano F, Berti P, Guida FM, Perrone G, Vincenzi B, Amato MM, Righi D, Dell'aquila E, Graziano F, Catalano V, et al. The role of macrophages polarization in predicting prognosis of radically resected gastric cancer patients. J Cell Mol Med. 2013;17:1415–1421. doi: 10.1111/jcmm.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kzhyshkowska J, Workman G, Cardó-Vila M, Arap W, Pasqualini R, Gratchev A, Krusell L, Goerdt S, Sage EH. Novel function of alternatively activated macrophages: Stabilin-1-mediated clearance of SPARC. J Immunol. 2006;176:5825–5832. doi: 10.4049/jimmunol.176.10.5825. [DOI] [PubMed] [Google Scholar]
- 29.Schönhaar K, Schledzewski K, Michel J, Dollt C, Gkaniatsou C, Géraud C, Kzhyshkowska J, Goerdt S, Schmieder A. Expression of stabilin-1 in M2 macrophages in human granulomatous disease and melanocytic lesions. Int J Clin Exp Pathol. 2014;7:1625–1634. [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao ZS, Wang YY, Chu YQ, Ye ZY, Tao HQ. SPARC is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2010;16:260–268. doi: 10.1158/1078-0432.CCR-09-1247. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Li AD, Xu L, Bai DW, Hou KZ, Zheng HC, Qu XJ, Liu YP. SPARC expression in gastric cancer predicts poor prognosis: Results from a clinical cohort, pooled analysis and GSEA assay. Oncotarget. 2016;7:70211–70222. doi: 10.18632/oncotarget.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franke K, Carl-McGrath S, Röhl FW, Lendeckel U, Ebert MP, Tänzer M, Pross M, Röcken C. Differential expression of SPARC in intestinal-type gastric cancer correlates with tumor progression and nodal spread. Transl Oncol. 2009;2:310–320. doi: 10.1593/tlo.09169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Yang M, Shan L, Qi L, Chai C, Zhou Q, Yao K, Wu H, Sun W. The role of SPARC protein expression in the progress of gastric cancer. Pathol Oncol Res. 2012;18:697–702. doi: 10.1007/s12253-012-9497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.