Buffone and Weaver discuss how the structure of the backbones and glycans of the tumor glycocalyx governs cell–matrix interactions and directs cancer progression.
Abstract
Mechanical interactions between tumors and the extracellular matrix (ECM) of the surrounding tissues have profound effects on a wide variety of cellular functions. An underappreciated mediator of tumor–ECM interactions is the glycocalyx, the sugar-decorated proteins and lipids that act as a buffer between the tumor and the ECM, which in turn mediates all cell-tissue mechanics. Importantly, tumors have an increase in the density of the glycocalyx, which in turn increases the tension of the cell membrane, alters tissue mechanics, and drives a more cancerous phenotype. In this review, we describe the basic components of the glycocalyx and the glycan moieties implicated in cancer. Next, we examine the important role the glycocalyx plays in driving tension-mediated cancer cell signaling through a self-enforcing feedback loop that expands the glycocalyx and furthers cancer progression. Finally, we discuss current tools used to edit the composition of the glycocalyx and the future challenges in leveraging these tools into a novel tractable approach to treat cancer.
Introduction
The mechanical interactions between a cell and the ECM tissue that encompasses it control nearly all aspects of cellular fate (Daley et al., 2008). The glycocalyx, the thick mixture of protein, lipids, and their post-translational sugar structures, surrounds all living cells and acts as a buffer between the cell and the ECM, especially in terms of mechanics (Butler and Bhatnagar, 2019). In cancer, the size of the tumor cell glycocalyx as a whole is significantly increased (Pavelka and Roth, 2010), and this in turn alters all aspects of tumor progression including transmembrane receptor function, cellular tension, integrin-mediated signaling, cell–cell and cell–ECM interactions, and immune recognition (Uchimido et al., 2019). On the other hand, the composition of the glycan structures decorating the protein and lipid backbones during cancer is context dependent, as the glycan trees are either elongated or truncated based on the specific cancer (Munkley and Elliott, 2016). Regardless, the composition of these sugar structures in the glycocalyx plays an important role in regulating both the overall phenotype and mechanics of the tumor (Martinez-Seara Monne et al., 2013). This review will discuss both the protein and lipid backbones that comprise the glycocalyx and also the critical glycan structures attached to these backbones, which are altered during cancer progression. Furthermore, we will detail how mechanics modulates the structure and function of the cancer glycocalyx and how this drives a “feedback loop” which drives malignancy. Finally, we will discuss current strategies to “prune” the glycocalyx in a specific manner to modulate cancer progression.
Key protein and lipid backbones of the glycocalyx in vivo
The composition and structure of the glycocalyx, a heterogeneous mixture of proteins and lipids that extend away from the cell membrane to which they are anchored, affect nearly all interactions between the cell and the extracellular environment. The height of the glycocalyx varies widely between cells and tissues but in general ranges from tens of nanometers to several micrometers thick (Möckl et al., 2019). The proteins and lipids of the glycocalyx have bulky post-translational sugar structures decorating their surface that extend the height and bulkiness of the glycocalyx and give it a strong negative charge (Reitsma et al., 2007). Cell surface chemokine receptors and integrins that are encompassed by the glycocalyx are much shorter (∼10 nm; Ye et al., 2010) and must navigate this negative charge and the repulsion between the ECM and glycocalyx, in order for cellular adhesion, migration, signaling, and most any cell-surface interactions to occur (Hammer and Tirrell, 1996).
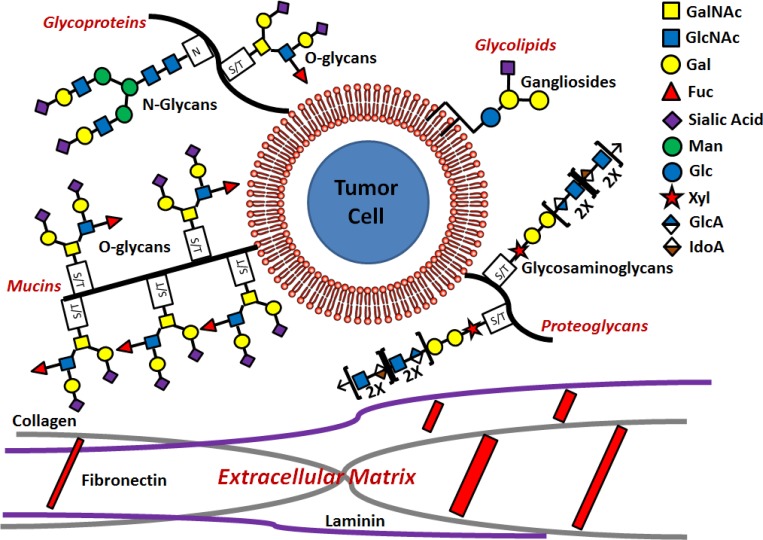
The protein and lipid backbones of the cellular glycocalyx comprise four main classes with unique glycosylation patterns (Fig. 1): mucins, which are glycoproteins with bulky O-linked glycan attachments that influence integrin function and cell signaling; trafficking glycoproteins, which primarily regulate cell adhesion through N- and O-linked structures; glycolipids, which consist of ganglioside attachments to ceramides; and proteoglycans, which are characterized by glycosaminoglycans (GAG) attachments. Each of these classes is discussed separately below.
Figure 1.
Structure of the tumor cell glycocalyx. The glycocalyx is the first line of contact between the tumor cell and the components of the ECM such as fibronectin, collagens, and laminin. The cancer cell glycocalyx consists of four main glycan branches on four distinct types of protein or lipid backbone: O-glycans attached to glycoproteins and mucins at serine/threonine sites, N-glycans attached to glycoproteins at asparagine sites, gangliosides attached to ceramide glycolipids, and GAGs characterized by the Xyl-Glc-Glc motif attached to a protein at a serine/threonine site on proteoglycans.
Mucins
The mucins are critical glycoprotein components of the glycocalyx that form a gel-like mucus on the surface of cells that modulates a variety of cellular interactions including integrin clustering, tension sensing, and signaling (Kufe, 2009). Mucins consist of rather lightly glycosylated N- and C-terminal domains flanking a central region containing a massive amount of O-glycosylation, which increases the overall molecular weight of the glycoprotein and makes mucins uniquely resistant to degradation (Bansil et al., 1995). The sheer amount of glycosylation on mucins makes them especially susceptible to changes during cancer progression as there are countless sites for aberrant glycans. In particular, the mucins MUC1 and MUC16 have been implicated in driving cancer progression. MUC1 is a key oncomarker and is critical for maintaining the cancer stem cell population (Nath and Mukherjee, 2014). During breast and other cancers, MUC1 becomes even more heavily glycosylated than in normal tissues, with many more O-glycan sites occupied, and adds to the overall bulk of the glycocalyx (Müller et al., 1997; Taylor-Papadimitriou et al., 1999). Furthermore, MUC16 is critical for shielding cancer cells from natural killer (NK) cells during innate immune surveillance (Gubbels et al., 2010). Recent work has described how synthetic glycopolymers that truncate either MUC1 or MUC16 serve to de-bulk the glycocalyx and slow cancer progression in response to mechanical stimuli (Paszek et al., 2014; Woods et al., 2017).
PSGL-1, CD43, and CD44, the glycoprotein regulators of cell adhesion
The glycoproteins P-selectin glycoprotein ligand-1 (PSGL-1), CD43 (leukosialin), and CD44 are components of the glycocalyx that act as regulators of cell–cell, cell–endothelial, and cell–ECM interactions in cancer (Spertini et al., 2019). PSGL-1 is a cell surface glycoprotein found on all leukocytes and some cancers that is a critical regulator of selectin binding to the endothelial surface (McEver and Cummings, 1997). It binds to E-, P-, and L-selectin at varying affinities and can carry both sialyl Lewis x (sLex) and sialyl Lewis a (sLea) glycans to confer this binding (Moore, 1998). PSGL-1 has been shown to have profound effects on immune recognition and cancer progression as its deletion has been shown to up-regulate macrophage cytokine production and colorectal cancer formation (Li et al., 2017), prevent T cell exhaustion and improve outcomes in melanoma (Tinoco et al., 2016), and attenuate trafficking to the lung and platelet adhesion in epithelial cancers (Kim et al., 1998).
CD43 or leukosialin is a glycoprotein constituent of the glycocalyx that acts as a selectin-dependent trafficking receptor (Matsumoto et al., 2007) and is expressed on all hematopoietic subsets except some B cell populations (Carlsson and Fukuda, 1986; Fukuda and Carlsson, 1986). Aberrant glycosylation of CD43, detected by the biomarker UN1, is a hallmark of many lymphoid cancers (Tuccillo et al., 2014) and also solid tumors in the breast, colon, gastrointestinal tract, and lung (Tassone et al., 2002). Furthermore, overexpression of CD43 has been implicated in an increase in the p53 tumor suppressor and enhanced p53-mediated cell death of colon cancer (Kadaja et al., 2004).
Finally, CD44 is a glycoprotein expressed on pancreatic and breast tumors (Li et al., 2014) that binds its principal ligand, hyaluronan (hyaluronic acid), but also collagen and fibronectin in the ECM (Chen et al., 2018). CD44-HA binding of tumors leads to an up-regulation in cell proliferation and cell motility (Ponta et al., 2003) in the tumor through activation of the MAPK and phosphoinositide 3-kinase pathways (Lv et al., 2016). Changes in the association of CD44 with the actin cytoskeleton through the Ezrin-Radixin-Moesin protein Ezrin have been implicated in both breast (Donatello et al., 2012) and pancreatic (Meng et al., 2010) cancer progression through both loss of cell–cell contacts and altered growth factor signaling (Clucas and Valderrama, 2014). Furthermore, CD44 has up to 10 splice variants termed CD44v1–10 that are alternatively expressed in other cancers, such as head and neck (Reategui et al., 2006), prostate (Ni et al., 2014), colorectal (Todaro et al., 2014), bladder (Kobayashi et al., 2016), and gastric (Lau et al., 2014), and are correlated with increased cancer progression and poorer patient outcomes (Mulder et al., 1994). Finally, CD44 is able to switch between the CD44s and CD44v forms (Brown et al., 2011), and epithelial to mesenchymal transition (EMT) in pancreatic and breast tumors requires a switch from CD44v to CD44s (Zhao et al., 2016).
Glycolipids
In addition to the glycan structures attached to proteins that we have discussed, glycans attached to lipids play a critical role in cancer progression and are susceptible to manipulation during cancer progression (Daniotti et al., 2013). There are two main classes of glycolipids: glyceroglycolipids, which contain a glycerol and a fatty acid as the lipid, and glycosphingolipids (GSLs), which carry a sphingosine as the lipid (Lopez and Schnaar, 2006). Since glyceroglycolipids are only found in plants, we will focus on GSLs, which are divided into several subclasses, including cerebrosides, which are singly glycosylated GSLs (Glc-Cer or Gal-Cer), globosides, which are poly-glycosylated GSLs (Lac-Cer), and gangliosides, which contain at least one sialic acid attached to the Lac-Cer structure (Schnaar, 2019; Schnaar and Kinoshita, 2015). GSLs play a critical role in tumor progression in the brain, an organ with complex and abundant gangliosides (Hirabayashi, 2012), but also in the bone, skin, and lung (Daniotti et al., 2016). GSLs also play roles in escaping tumor immunosurveilance by repressing monocyte cytokine release (Heitger and Ladisch, 1996), preventing release of IgG and IgM from B cells (Kimata and Yoshida, 1994), and limiting CD8+ T cell production (McKallip et al., 1999). Furthermore, GSLs increase tumor cell trafficking as they carry sLex structures, which confer E-selectin binding, the master regulator of leukocyte and tumor cell trafficking, through the endothelium (Mondal et al., 2016; Nimrichter et al., 2008).
Proteoglycans
Proteoglycans are the last major component of the glycocalyx and are characterized by a protein backbone with abundant O-linked GAG attachments (Nikitovic et al., 2018). The most abundant GAG attachments include heparin sulfate, chondroitin sulfate, or keratin sulfate and are characterized by the galactose-galactose-xylose motif (Gal-Gal-Xyl; Pomin and Mulloy, 2018). Several tumor-associated proteoglycans have been implicated in cancer signaling and progression, including the Syndecan (Cheng et al., 2016a) and Glypican (Li et al., 2018) families, and their roles are discussed in great detail in the cited reviews.
Critical glycan moieties aberrantly expressed in cancer
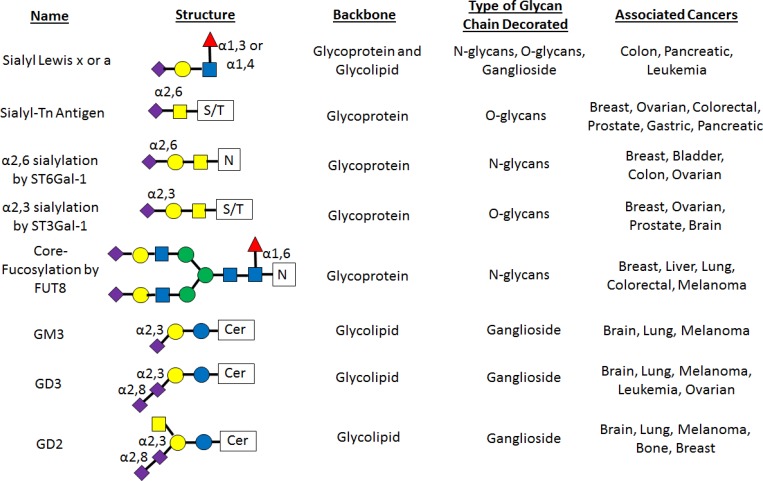
In terms of the glycocalyx content, it is not only the protein and lipid backbones that are important but also the post-translational sugar structures attached to the backbones (Pinho and Reis, 2015). After the proteins and lipids are synthesized ER, they move to the Golgi, where they are post-translationally modified by the combination of activated sugar donors and enzymes called glycosyltransferases before being transported to the cell surface (Stanley, 2011). This leads to the diverse set of glycan structures which, when presented on the surface of the protein and lipid backbones, form the typical “brush”-like structure of the glycocalyx (Kabedev and Lobaskin, 2018). There are several main glycosylation types on the backbones of the glycocalyx. Glycoprotein glycosylation comprises two main types: the branched N-glycan chains attached to asparagine and the step-wise constructed O-glycan chains attached to either serine or threonine. Finally, glycolipids have glycans attached to a lipid and are initiated by a glucosamine attached to the ceramide backbone (Glc-cer; Varki, 2017). The composition of these glycan structures is not only critical in regulating tumor cell function as a whole but also modified in tumors as compared with normal cells. To this end, specific aberrant glycan structures have been implicated as playing a regulatory role in cancer progression (Fig. 2), and they are outlined in the next section.
Figure 2.
Principal glycan structures associated with cancer. A listing of the most common glycosylation structures seen during cancer including sLex and sLea, sialyl-Tn antigen, α2,6 sialylation by ST6Gal1, α2,3 sialylation by ST3Gal1, core-fucosylation by FUT8, and the GM3, GD3, and GD2 structures. The name, structure, which glycocalyx backbone it decorates, class of glycan, and which cancers it is associated with are listed for each glycan.
Terminal sialylation with ST3Gal-1, ST6Gal-1, and ST6GalNAcs
Sialylation is a critical modification to glycan chains as the addition of a sialic acid both terminates further glycosylation on the chain and is the only sugar that carries a (negative) charge (Wopereis et al., 2006). Altered sialylation is a hallmark of cancer progression, and which class of sialyltransferase adds the sialic acid plays a key role to this progression. To this end, the sialyltransferases ST3Gal-1, ST6Gal-1, and the ST6GalNAcs all add sialic acid to specific glycan structures and have been implicated in cancer. ST3Gal-1 is a sialyltransferase that catalyzes the terminal addition of a sialic acid in a 2,3 linkage to the galactose and terminates the O-glycan. High levels of these short core 1 O-glycan structures have been implicated in a variety of cancers including breast (Yeo et al., 2019), ovarian (Wu et al., 2018), prostate (Tzeng et al., 2018), and brain (Chong et al., 2015). ST3Gal 1 is thought to be up-regulated during tumorigenesis (Picco et al., 2010), and the truncated glycan created interacts with galectin-4, which promotes metastatic signaling (Tzeng et al., 2018).
ST6Gal-1, which adds a sialic acid in a 2,6 linkage on N-linked glycans, causes drastically different functions in cancer, especially in terms of resistance to treatment and survival (Garnham et al., 2019). Sialylation by ST6Gal-1 is thought to maintain a more stem-like state in cancer cells (Schultz et al., 2016) and has been implicated in bladder (Antony et al., 2014), colon (Zhang et al., 2017), breast (Lu et al., 2014), and ovarian cancers (Christie et al., 2008). Multiple works have demonstrated how ST6Gal-1 both confers resistance to chemotherapy (Britain et al., 2018; Schultz et al., 2013) and protects cancer cells from apoptosis by sialylation of Fas (Swindall and Bellis, 2011) and tumor necrosis factor receptor (Holdbrooks et al., 2018). Interestingly, ST6Gal-1 is also the only glycosyltransferase to date in which its soluble form can “extrinsically” glycosylate outside the cell (Manhardt et al., 2017). This mechanism describes how donor sugar from platelets (Lee-Sundlov et al., 2016; Lee et al., 2014) and ST6Gal-1 from B cells (Irons and Lau, 2018; Irons et al., 2019) can sialylate progenitors in the bone marrow (Nasirikenari et al., 2014) and prevent granulopoiesis (Dougher et al., 2017). In fact, this “extrinsic” sialylation mechanism has recently been implicated in colon cancer progression, as ST6Gal-1 loaded into exomeres can both sialylate β1 integrins and promote cancer organoid growth (Zhang et al., 2019).
Finally the ST6GalNAc family of sialyltransferases catalyzes the addition of a 2,6 sialic acid to the initial GalNAc instead of the galactose-like ST3Gal-1 and ST6Gal-1 and creates the sialyl-Tn antigen that is expressed in a wide variety of cancers (Marcos et al., 2004). ST6GalNAcs are thought to be regulators of malignancy as they form the binding epitopes for galectin-3 and other galectins as well (Dimitroff, 2015, 2019) and compete away possible core 2 O-glycan extensions such as sLex (Lo et al., 2013b). In sum, the interplay between these three structures in preemptively terminating O- and N-glycosylation affects almost all aspects of cancer progression.
Sialyl Lewis antigens
The sialyl Lewis antigens, especially sLex and sLea, form the binding epitopes to the endothelial selectins on immune cells (Buffone et al., 2013; Mondal et al., 2015) and circulating tumor cells (Burdick et al., 2012; Li and King, 2012). These tetrasaccharide structures, consisting of sialic acid bound to a lactosamine (Gal-GlcNAc) in a 2,3 linkage and a fucose bound in either a 1,3 or 1,4 linkage (Trinchera et al., 2017), are highly implicated in the metastasis of tumors to secondary sites through the bloodstream (Blanas et al., 2018; Mondal et al., 2018). They are strongly up-regulated in a variety of tumors, and sLea (CA19.9) and sLex (NCC-ST-439) are prognostic markers for both colon and pancreatic cancer (Shiozaki et al., 2011). Furthermore, enforced fucosylation of hematopoietic stem and progenitor cells (or other immune cells; Buffone et al., 2017; Videira et al., 2018) generates sLeX structures on CD44 to create the hematopoietic cell E- and L-selectin ligand epitope, which is the principal regulator of trafficking to the bone marrow compartment (Dimitroff et al., 2001a,b; Sackstein, 2012; Zhao et al., 2016).
Core fucosylation of N-glycans
Another critical regulator of cancer invasiveness and progression is the core fucosylation of N-glycans. The addition of a 1,6 linked fucose to the chain initiating GlcNAc on complex N-glycans is catalyzed by the fucosyltransferase, FUT8 (Yang et al., 2017). FUT8-mediated core-fucoylation has been implicated as a driver of cancer invasiveness in breast cancer by fucosylation of TGF-β (Tu et al., 2017), in colorectal cancer through a p53-mediated mechanism (Noda et al., 2018), in melanoma by fucosylation of L1CAM (Agrawal et al., 2017), through a β-catenin– and LEF-1–dependent mechanism in nonsmall cell lung cancer (Chen et al., 2013), and through miRNA regulation of fucosylation in hepatocellular carcinoma (Cheng et al., 2016b).
GM3, GD2, and GD3 gangliosides
Mammalian GSL glycosylation is characterized by the initial addition of a glucosamine (Glc-Cer) to the ceramide followed by a galactose to form Lac-Cer (Merrill, 2011). It is from this basic glycan structure that all of the gangliosides, or sialic acid–containing GSLs, are formed (Maccioni, 2007). The shortest of the cancer associated glycolipids is GM3, which is has one sialic acid attached to the Lac-Cer structure (Birklé et al., 2003), and is highly up-regulated in brain, lung, and skin cancers (Zheng et al., 2019; Gu et al., 2008). GD2 and GD3 are longer glycans that are characterized by two sialic acids apiece and differ by an extra GalNAc structure on GD2 (Liu et al., 2018a). GD3 is a structure that is seen in low levels in healthy adults, and increases in GD3 correlate with a treatment resistant brain cancer stem cell population (Yeh et al., 2016). Elevated levels are seen in melanomas, acute lymphoblastic leukemias, and lung cancers, where it regulates tumor growth and proliferation (Furukawa et al., 2012; Merritt et al., 1994). Furthermore, the GD3 structure has been shown to bind and suppress both T helper and NKT cell function in brain and ovarian cancers (Mycko et al., 2014; Webb et al., 2012). GD2 is also a well-known ganglioside that is up-regulated in in melanoma, lung cancers, sarcomas, neuroblastomas, and triple-negative breast cancers (Dobrenkov et al., 2016; Orsi et al., 2017; Terzic et al., 2018; Yanagisawa et al., 2011). GD2 associates with the VLA-2 integrin and up-regulates the binding of neuroblastomas to ECM proteins such as collagens. In fact, anti-GD2 therapies (dinutuximab) are currently approved for use in the clinic to treat neuroblastomas and osteosarcomas (Greenwood and Foster, 2017; Roth et al., 2014).
Glycocalyx function in EMT, mechanics, and anti-tumor immunity
The glycocalyx plays a critical role in mediating the interactions between the tumor and its extracellular environment and has profound effects on EMT transition, mechanosensing, signaling, metastasis, and immune evasion, all of which will be discussed in the following sections.
Composition changes of the glycocalyx during EMT in cancer
The transition of cancer cells from an epithelial phenotype to a more mobile mesenchymal phenotype, the EMT, has in many instances been correlated with more invasive (Moustakas and de Herreros, 2017) and treatment-resistant cancers (Brabletz et al., 2018), and overall poorer patient outcomes (George et al., 2017). This a source of open debate, though, as not all cancers display EMT as a prerequisite to cancer metastasis and the link to human cancers in vivo is complex (Ledford, 2011). Even so, a major change associated with the EMT is the change to a more bulky and taller phenotype in the structure of the tumor glycocalyx (Lange-Consiglio et al., 2014; Mitchell and King, 2014; Paszek et al., 2014; Zeng et al., 2016).
For complex and hybrid N-glycans, a major change during the EMT is the loss of the bisecting GlcNAc structure and the accompanying up-regulation of β1,6 branches (Xu et al., 2017). The glycosyltransferase MGAT5 regulates the β1,6 branching, and studies in MGAT5-deficient breast tumors showed that ablation of MGAT5 and loss of β1,6 branching correlated with pronounced reductions in tumor growth and activation due to increased immune response from CD4+ T cells and macrophages (Li et al., 2008). Furthermore, EMT in liver carcinoma (hepatocellular carcinoma) correlated with up-regulated core-fucose and β1,6 branching on N-glycans and sLex and T-antigens (Gal-GalNAc) on O-glycans (Li et al., 2013), all of which are markers of cancer as discussed in the previous section. MUC1 O-glycosylation is also specifically up-regulated during EMT in a variety of cancers (Freire-de-Lima, 2014). Deletion of the MUC1 cytoplasmic tail leads to a subsequent decrease in EMT and metastasis in pancreatic cancer through loss of its association with β-catenin, which is required for nuclear localization and transcription (Roy et al., 2011). Finally, glycolipid composition is also modified during EMT as sialylated gangliosides such as GM2, GD2, and GD3 are present at high levels in brain cancers while absent in normal tissues (Hakomori, 1996). More detailed information can be found in the comprehensive review of the changes seen in the glycocalyx during cancer EMT (Li et al., 2016).
Mechanosensing through the glycocalyx and its impact on integrins
The glycocalyx is the principal structure in contact with the ECM (Lahir, 2016); therefore, it plays a critical role in regulating both the adhesions of integrins to the ECM and the mechanics of the tumor cell in response to stiffness (Sun et al., 2016). Recent works were the first to demonstrate that the glycocalyx is intrinsically coupled to integrin clustering and mechanosensing with the ECM (Paszek et al., 2009, 2014). Initially, a computational model made two main predictions: first, that cell-bound integrins cluster and bind to the ECM more avidly with increasing stiffness, and, second, that the size and bulk of the glycocalyx limits the ability of integrins to cluster and bind due to the intrinsic repulsion between the glycocalyx and the ECM. According to this model, the composition and size of the cellular glycocalyx modulate the degree of mechanosensing that cell-bound integrins undergo when in contact with the ECM (Paszek et al., 2009). A second study experimentally examined this computational model, demonstrating that in cancer, the glycocalyx and especially bulky cancer-related glycoproteins such as MUC1, drive integrin clustering and mechanosensing. The overall mechanism involves bulky glycoproteins acting as physical barriers that funnel integrins to cluster in the adhesive zones in contact with the ECM. Finally, the bulky glycoproteins are able to reinforce or enhance mechanosensing, and this in turn promotes cancer cell growth and survival (Paszek et al., 2014).
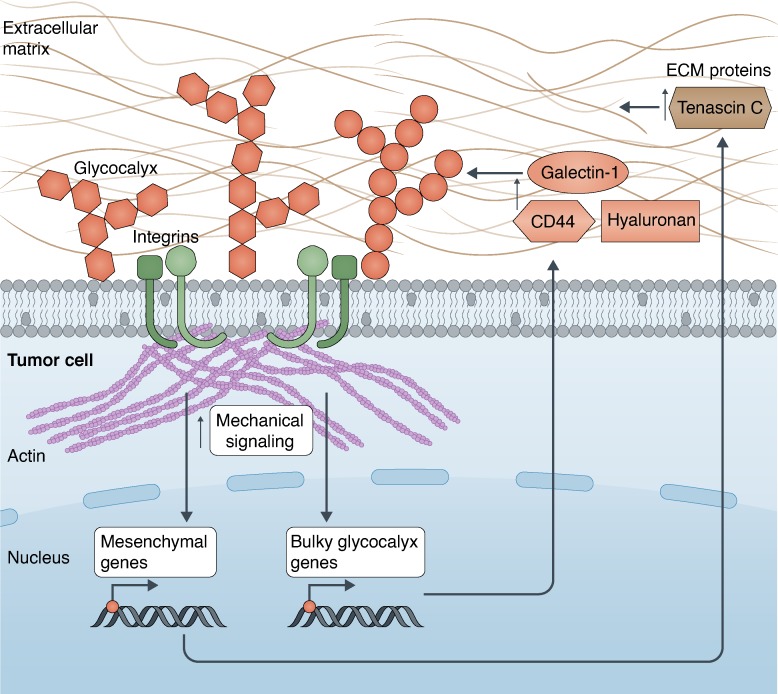
The role of the glycocalyx in cancer progression was expanded by recent studies demonstrating that a bulky glycocalyx drives the metastatic potential by increasing cell cycle progression through the phosphoinositide 3-kinase–AKT axis and mechanosensing through integrin-FAK interplay (Woods et al., 2017). Furthermore, increasing the size of the glycocalyx with the MUC1 ecto-domain was sufficient to drive metastatic potential in an in vivo model of breast cancer (Woods et al., 2017). A subsequent study found that in glioblastoma multiforme, a mesenchymal phenotype is linked with greater aggression, and this is regulated by increased mechanosensing through stiffer ECM substrates (Barnes et al., 2018). Overall, a bulkier glycocalyx is linked with a more mesenchymal and aggressive cancer phenotype, and mechanosensing up-regulates both mesenchymal and bulky glycocalyx-related genes, most principally galectin-1, to drive aggression (Barnes et al., 2018). This work was the first to describe a tension-mediated glycocalyx–integrin feedback loop wherein mechanical signaling through the FAK pathway up-regulates both mesenchymal and bulky glycocalyx genes, which in turn increases the glycocalyx size and further enhances integrin mechanosignaling (Fig. 3). Knockdown of either the glycocalyx component galectin-1 or FAK was able to decrease both glycocalyx bulkiness and the glioblastoma multiforme aggressiveness (Barnes et al., 2018).
Figure 3.
Tension mediates a glycocalyx-integrin feedback loop in cancer. The glycocalyx drives enhanced mechanical signaling between the integrin–actin axis on the tumor cell and the ECM. This mechanical signaling promotes the up-regulation of genes in the nucleus driving both a more mesenchymal phenotype (production of ECM proteins such as Tenascin C) and a bulkier glycocalyx (such as CD44, hyaluronic acid, and galectin-1) in the tumor. Together, these drive glioma aggressiveness in a tension-dependent feedback loop, which is self-enforcing. Illustration by Neil Smith (http://www.neilsmithillustration.co.uk).
The role of the glycocalyx in promoting migration, invasion, and metastasis
A larger and bulkier glycocalyx has been implicated in both increased migration and metastasis during cancer (Lahir, 2016). As discussed, a taller, bulkier glycocalyx is associated with both increased migration (Barnes et al., 2018) and metastatic potential of cancers (Woods et al., 2017). Furthermore, the cancer cell glycocalyx itself acts as a mechanosensor that responds to the interstitial flow coming from the comprised tumor vasculature, which can increase the migration and invasion of the tumor (Tarbell and Pahakis, 2006; Tarbell and Shi, 2013; Zeng and Tarbell, 2014). The glycocalyx responds to the mechanical force generated by the shear flow by secreting matrix metalloproteinases into the ECM to degrade it. This promotes tumor migration, as the degraded ECM is easier to move through, and eventual movement of the tumor out of the tissue and into the vasculature to colonize secondary sites (Qazi et al., 2011, 2013, 2016). The ability of the glycocalyx to act as a mechanosensor to degrade the ECM holds therapeutic potential as manipulating the glycocalyx structure could influence the mechanical response to fluid stress (Tarbell and Cancel, 2016). Furthermore, it could impact whether tumor cells can migrate against the direction of shear flow to reach the vasculature, a phenomenon so far only seen in various immune cell subsets that allows for faster and more effective trans-endothelial migration (Anderson et al., 2019; Buffone et al., 2018, 2019; Dominguez et al., 2015; Tedford et al., 2017; Valignat et al., 2013).
Sialic acid shielding evades anti-tumor immunity
The final major way in which the cancer cell glycocalyx is known to affect tumor progression is by shielding the tumor from immune surveillance and the subsequent immune response (Kim et al., 2007; Xiao et al., 2016). The tumor glycocalyx accomplishes this by tricking the immune system into thinking the tumor is part of its normal, healthy tissue via an increase in terminal sialic acids (Büll et al., 2014; Varki and Gagneux, 2012). High sialic acid content of the cancer glycocalyx has been implicated in both decreased anti-tumor activity and poorer survival outcomes (Brossart et al., 2001). Perhaps most critically, the increased sialic acid content in tumor cells increases the amount of ligands for self-inhibitory siglecs on the immune cell surface (Crocker et al., 2007). Increased sialylated glycans “trick” the responding NK cell into thinking the tumor is healthy tissue by activating both Siglec-7 and -9 (Avril et al., 2004; Hudak et al., 2014; Jandus et al., 2014). Blocking the function of Siglec-7 or -9 with antibodies or removing the terminal sialic acids from the tumor has been shown to restore NK-mediated tumor killing (Jandus et al., 2014; Nicoll et al., 2003).
To conclude, the specific content and structure of the cancer cell glycocalyx has a critical role in mediating all aspects of tumor fate, from mechanosensing, signaling, migration, and metastasis to immune evasion. To this end, in order to realize the full therapeutic potential of manipulating the cancer cell glycocalyx, precise editing of the glycocalyx at the monosaccharide level is needed. In the final section, we will discuss the currently used methods for editing the glycocalyx, and potential strategies to prune the specific glycans of the glycocalyx as if it were a tree.
Tools for specifically pruning the glycocalyx
As discussed, the structure, content, and bulkiness of the glycocalyx have profound effects on the mechanics surrounding tumor growth and signaling, along with immune evasion and EMT. What is lacking are precise tools to specifically manipulate the structure of the glycocalyx. A variety of approaches have been attempted to manipulate the glycans; however, they still lack the precision needed to make specific alterations.
Small molecule inhibitors and mimetics
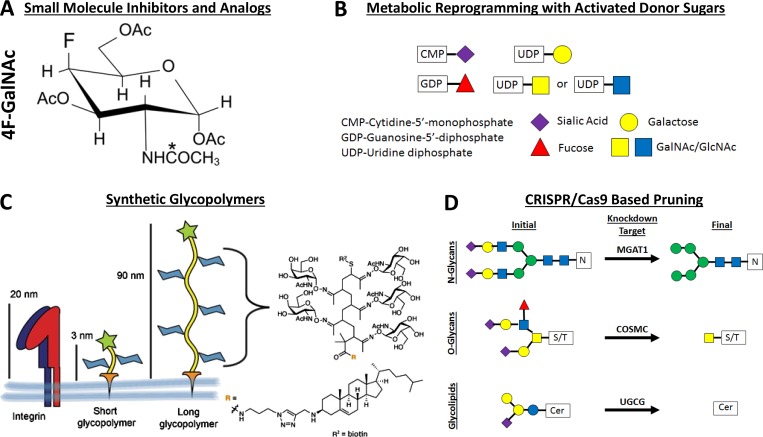
The basic premise of small molecule inhibitors is to design them in such a way that they either incorporate into the native glycan during synthesis to stop further elongation of the glycan or act as a natural competition for the glycan recognition site to stop the binding and signaling processes associated with the glycan (Jacob, 1995; Esko and Schnaar, 2017). In terms of the incorporation of a nonnative sugar, these act mainly as metabolic decoys that hijack native glycosyltransferase activity and incorporate themselves into the native glycan trees (Fig. 4 A). To this effect, many different synthetic sugars have been generated to reprogram N- and O-glycan biosynthesis, including analogues to sialic acids (Macauley et al., 2014; van den Bijgaart et al., 2019), fucoses (Okeley et al., 2013), GalNAcs (Marathe et al., 2010), GlcNAcs (Barthel et al., 2011; Gainers et al., 2007), and xylose (Garud et al., 2008). Furthermore, inhibitors have been developed to modify other major glycosylation pathways including O-GlcNAc pathways in mucin biosynthesis (Liu et al., 2018b) and glycolipids (Nimrichter et al., 2008). The drawback to these inhibitors is that most have minimal incorporation into cells at relatively high millimolar concentrations, which limits their therapeutic potential in the clinic (Kudelka et al., 2016). Some newly described glycosylation mimetics such as thioglycosides (Wang et al., 2018) may be able to overcome the hurdle of how to get high incorporation at lower concentrations.
Figure 4.
Methods for editing the cancer glycocalyx. (A–C) Representation of the various methods used to edit the cancer glycocalyx including small molecule analogues such as 4F-GalNAc, which incorporate into and truncate glycan chains (A); addition of the activated sugar donors to cells to metabolically reprogram glycan synthesis by manipulating the amount of substrate for glycosyltransferases (B), and synthetic glycopolymers that attach to the cell membrane and can change the height of the glycocalyx (C), which affects integrin binding and clustering. The asterisk denotes the acetylation site. (D) Genetic knockdown of MGAT1, COSMC, or UGCG can precisely truncate N-glycan, O-glycan, and ganglioside biosynthesis. Panel C was adapted from Woods et al. (2017).
On the other hand, several glycan mimetics that compete away binding activity of the native glycan receptors have found great promise as therapeutics and are either on their way to or in the clinic. Mimetics against the sLex tetrasaccharide along with other ligands have been used in clinical trials for pan selectin inhibition (Chang et al., 2010; Morikis et al., 2017) to treat sickle cell crises, E-selectin inhibition to treat acute myeloid leukemia in bone marrow (Winkler et al., 2012), and combined E-selectin and CXCR4 inhibition (Price et al., 2016) to treat dormant breast cancer. Furthermore, glycan-based therapeutics to cleave sialic acid ligands on cancer cells to prevent siglec-based shielding from immunosurveilance are also entering clinical trials (Haas et al., 2019; Stanczak et al., 2018). While small molecule inhibitors and mimetics have therapeutic applications, they still represent a brute force approach to manipulating glycosylation and lack the precision needed to specifically edit the glycocalyx.
Metabolic reprogramming of sugar donors
Another method of editing the content and structures of the glycocalyx includes manipulating the metabolic pathways involved in the synthesis of the activated sugar donors needed for glycoyltransferases to catalyze their addition to growing glycan chains (Fig. 4 B). This includes the activated sugars CMP-Neu5Ac, GDP-Fuc, and UDP-GlcNAc. Loss of the CMP–sialic acid transporter (Martinez-Duncker et al., 2005) and the GDP-fucose transporter, called leukocyte adhesion deficiency II (Lübke et al., 2001), are both congenital disorders of glycosylation seen in humans, and are characterized by defects in the SLC35A1 and SLC35C1 genes, respectively. Researchers have used knowledge from these disorders to disruptively manipulate the sialylation and fucosylation patterns of cancer cells. Manipulation of the amount of GlcNAc has been done both by feeding it into the diet of mice or adding it exogenously to PymT tumor cells to up-regulate UDP-GlcNAc and rescue the branching of their N-glycans in the absence of MGAT5. This in turn worsens their cancer prognosis as the more complex N-glycans correlate with more growth factor signaling and galectin-3 binding (Mendelsohn et al., 2007; Ryczko et al., 2016). Another major way that the metabolic pathways involved in glycosylation have been modified is the use of tunicamycin to block proper N-glycosylation (Merlie et al., 1982). Tunicamycin inhibits GlcNAc phosphotransferase, which transfers GlcNAc-1-phosphoate to dolichol phosphate, during the first steps of N-glycan synthesis (Wyszynski et al., 2012). Thus, tunicamycin has been used as a chemical tool in research as a way to study the effect of disrupted N-glycosylation. In sum, while metabolic regulation of the donor sugars is a viable mechanism to modify the glycocalyx, it lacks the level of specificity needed to manipulate the cancer glycocalyx at the single-sugar level.
Modifying the protein backbone with recombinant and synthetic glycoproteins
Another viable approach to modifying the structure of the glycocalyx has involved modifying the protein and lipid backbones that carry the glycans themselves (Fig. 4 C). For example, modification of both the height and bulk of the glycocalyx through the use of mucin mutants of varying lengths has been accomplished to tune the response to stiffness in cancer (Barnes et al., 2018; Kramer et al., 2015; Paszek et al., 2014; Woods et al., 2017). Modified glycoproteins that tune glycosylation have also been used as a way to enforce selectin-binding activity in mesenchymal stem cells (Abdi et al., 2015; Lo et al., 2013a, 2016), to present antigens to activate dendritic cells (García-Vallejo et al., 2013), and to increase NK-mediated killing of cancer cells by competing away siglec binding (Hudak et al., 2014). Again, while these methods are elegant in their control of the protein backbone, they are only able to control the glycosylation by loss or gain of potential glycosylation sites. This leaves manipulating glycan structures at the single sugar level out of the scope of these tools.
CRISPR-based pruning of specific glycosyltransferases
While the other approaches discussed here rely on bulk disruption of the glycan structures by incorporation of a modified sugar, removing or increasing the activated donor sugar, or actually manipulating the protein or lipid backbone, disruption of the glycosyltransferase enzyme associated with the addition of a specific monosaccharide represents an elegant method for the specific truncation of glycan structures (Steentoft et al., 2014). This approach is two-pronged as it can be used to either remove the enzymatic function of a specific glycan (Buffone et al., 2013; Mondal et al., 2015) or target the chain-initiating enzyme of the entire glycan tree (Mondal et al., 2016; Vester-Christensen et al., 2013). In fact, specific removal of N-glycosylation has been used as a clinical tool for anti–PD-L1 antibodies to more robustly recognize PD-L1 and enhance treatment of breast cancer (Lee et al., 2019). Two recent toolkits have been developed in order to specifically manipulate the composition and structure of the glycocalyx. The first is a comprehensive toolkit for manipulating the characteristics of the O-glycome on cancer cells at both the glycan and protein backbone levels (Shurer et al., 2018). This toolkit has allowed them to elegantly quantify the biophysics of the glycocalyx and manipulate its shape and function (Shurer et al., 2019). The second toolkit allows for the elegant quantification of the relative function of specific glycan classes (Stolfa et al., 2016). The knockdown of MGAT1, COSMC, and UGCG to specifically truncate complex and hybrid N-linked glycoproteins, O-linked glycoproteins, and glycolipids can precisely quantity the relative function of each type of glycan (Fig. 4 D). Although this was applied in the context of leukocyte recruitment to the sites of inflammation (Stolfa et al., 2016), this toolkit holds great potential in a wide variety of settings, most notably in determining the critical glycans regulating the size and bulk of the glycocalyx, and in turn the mechanics, in cancer.
Conclusions and future outlook
To summarize, the tumor cell glycocalyx is critical in regulating tumor cell–ECM mechanosensing as it acts as a buffer for interactions between the cell surface receptors and surrounding tissues. The glycocalyx composition affects all aspects of tumor cell progression including cellular tension, integrin signaling, migration, metastasis, and immune recognition and also drives a feedback loop to increase the height and bulk of the glycocalyx and sustain the pro-cancer phenotype. While de-bulking the glycocalyx through the use of synthetic mucins can disrupt this cancer feedback loop by attenuating mechanical signaling, better tools are needed to prune the glycocalyx in a more precise manner. Being able to quantify the relative contribution of the N-linked, O-linked, and glycolipid components of the glycocalyx represents a significant step forward in attributing a specific glycan moiety to regulation of the tension-feedback loop. With this said, in order to truly unlock the potential of controlling the glycocalyx-integrin mechanosensing feedback loop to halt cancer progression, tools must developed to prune the glycans at a single-sugar level.
Acknowledgments
The authors thank Connor Stashko, Gretchen Ford, and Dhruv Thakar (University of California, San Francisco, San Francisco, CA) for their feedback on this review.
This work was supported by the National Cancer Institute (2R01CA227942-18A1) and the National Institute of Neurological Disorders and Stroke (1R01NS109911-01 to V.M. Weaver). This work was funded by the National Institute of General Medical Sciences (1R21GM133060-01 to A. Buffone Jr.).
The authors declare no competing financial interests.
Author contributions: A. Buffone Jr. and V.M. Weaver both conceived and wrote the review.
References
- Abdi R., Moore R., Sakai S., Donnelly C.B., Mounayar M., and Sackstein R.. 2015. HCELL Expression on Murine MSC Licenses Pancreatotropism and Confers Durable Reversal of Autoimmune Diabetes in NOD Mice. Stem Cells. 33:1523–1531. 10.1002/stem.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal P., Fontanals-Cirera B., Sokolova E., Jacob S., Vaiana C.A., Argibay D., Davalos V., McDermott M., Nayak S., Darvishian F., et al. 2017. A Systems Biology Approach Identifies FUT8 as a Driver of Melanoma Metastasis. Cancer Cell. 31:804–819.e7. 10.1016/j.ccell.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N.R., Buffone A. Jr., and Hammer D.A.. 2019. T lymphocytes migrate upstream after completing the leukocyte adhesion cascade. Cell Adhes. Migr. 13:163–168. 10.1080/19336918.2019.1587269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony P., Rose M., Heidenreich A., Knüchel R., Gaisa N.T., and Dahl E.. 2014. Epigenetic inactivation of ST6GAL1 in human bladder cancer. BMC Cancer. 14:901 10.1186/1471-2407-14-901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avril T., Floyd H., Lopez F., Vivier E., and Crocker P.R.. 2004. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J. Immunol. 173:6841–6849. 10.4049/jimmunol.173.11.6841 [DOI] [PubMed] [Google Scholar]
- Bansil R., Stanley E., and LaMont J.T.. 1995. Mucin biophysics. Annu. Rev. Physiol. 57:635–657. 10.1146/annurev.ph.57.030195.003223 [DOI] [PubMed] [Google Scholar]
- Barnes J.M., Kaushik S., Bainer R.O., Sa J.K., Woods E.C., Kai F., Przybyla L., Lee M., Lee H.W., Tung J.C., et al. 2018. A tension-mediated glycocalyx-integrin feedback loop promotes mesenchymal-like glioblastoma. Nat. Cell Biol. 20:1203–1214. 10.1038/s41556-018-0183-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel S.R., Antonopoulos A., Cedeno-Laurent F., Schaffer L., Hernandez G., Patil S.A., North S.J., Dell A., Matta K.L., Neelamegham S., et al. 2011. Peracetylated 4-fluoro-glucosamine reduces the content and repertoire of N- and O-glycans without direct incorporation. J. Biol. Chem. 286:21717–21731. 10.1074/jbc.M110.194597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birklé S., Zeng G., Gao L., Yu R.K., and Aubry J.. 2003. Role of tumor-associated gangliosides in cancer progression. Biochimie. 85:455–463. 10.1016/S0300-9084(03)00006-3 [DOI] [PubMed] [Google Scholar]
- Blanas A., Sahasrabudhe N.M., Rodríguez E., van Kooyk Y., and van Vliet S.J.. 2018. Fucosylated Antigens in Cancer: An Alliance toward Tumor Progression, Metastasis, and Resistance to Chemotherapy. Front. Oncol. 8:39 10.3389/fonc.2018.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T., Kalluri R., Nieto M.A., and Weinberg R.A.. 2018. EMT in cancer. Nat. Rev. Cancer. 18:128–134. 10.1038/nrc.2017.118 [DOI] [PubMed] [Google Scholar]
- Britain C.M., Holdbrooks A.T., Anderson J.C., Willey C.D., and Bellis S.L.. 2018. Sialylation of EGFR by the ST6Gal-I sialyltransferase promotes EGFR activation and resistance to gefitinib-mediated cell death. J. Ovarian Res. 11:12 10.1186/s13048-018-0385-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossart P., Schneider A., Dill P., Schammann T., Grünebach F., Wirths S., Kanz L., Bühring H.-J., and Brugger W.. 2001. The epithelial tumor antigen MUC1 is expressed in hematological malignancies and is recognized by MUC1-specific cytotoxic T-lymphocytes. Cancer Res. 61:6846–6850. [PubMed] [Google Scholar]
- Brown R.L., Reinke L.M., Damerow M.S., Perez D., Chodosh L.A., Yang J., and Cheng C.. 2011. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J. Clin. Invest. 121:1064–1074. 10.1172/JCI44540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone A. Jr., Mondal N., Gupta R., McHugh K.P., Lau J.T.Y., and Neelamegham S.. 2013. Silencing α1,3-fucosyltransferases in human leukocytes reveals a role for FUT9 enzyme during E-selectin-mediated cell adhesion. J. Biol. Chem. 288:1620–1633. 10.1074/jbc.M112.400929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone A. Jr., Nasirikenari M., Manhardt C.T., Lugade A., Bogner P.N., Sackstein R., Thanavala Y., Neelamegham S., and Lau J.T.Y.. 2017. Leukocyte-borne α(1,3)-fucose is a negative regulator of β2-integrin-dependent recruitment in lung inflammation. J. Leukoc. Biol. 101:459–470. 10.1189/jlb.3A0516-215RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone A., Anderson N.R., and Hammer D.A.. 2018. Migration against the direction of flow is LFA-1-dependent in human hematopoietic stem and progenitor cells. J. Cell Sci. 131:jcs205575 10.1242/jcs.205575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone A. Jr., Anderson N.R., and Hammer D.A.. 2019. Human neutrophils will crawl upstream on ICAM-1 if Mac-1 is blocked. Biophys. J. 117:1393–1404. 10.1016/j.bpj.2019.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büll C., Stoel M.A., den Brok M.H., and Adema G.J.. 2014. Sialic acids sweeten a tumor’s life. Cancer Res. 74:3199–3204. 10.1158/0008-5472.CAN-14-0728 [DOI] [PubMed] [Google Scholar]
- Burdick M.M., Henson K.A., Delgadillo L.F., Choi Y.E., Goetz D.J., Tees D.F.J., and Benencia F.. 2012. Expression of E-selectin ligands on circulating tumor cells: cross-regulation with cancer stem cell regulatory pathways? Front. Oncol. 2:103 10.3389/fonc.2012.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P.J., and Bhatnagar A.. 2019. Mechanobiology of the abluminal glycocalyx. Biorheology. 56:101–112. 10.3233/BIR-190212 [DOI] [PubMed] [Google Scholar]
- Carlsson S.R., and Fukuda M.. 1986. Isolation and characterization of leukosialin, a major sialoglycoprotein on human leukocytes. J. Biol. Chem. 261:12779–12786. [PubMed] [Google Scholar]
- Chang J., Patton J.T., Sarkar A., Ernst B., Magnani J.L., and Frenette P.S.. 2010. GMI-1070, a novel pan-selectin antagonist, reverses acute vascular occlusions in sickle cell mice. Blood. 116:1779–1786. 10.1182/blood-2009-12-260513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-Y., Jan Y.-H., Juan Y.-H., Yang C.-J., Huang M.-S., Yu C.-J., Yang P.-C., Hsiao M., Hsu T.-L., and Wong C.-H.. 2013. Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer. Proc. Natl. Acad. Sci. USA. 110:630–635. 10.1073/pnas.1220425110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zhao S., Karnad A., and Freeman J.W.. 2018. The biology and role of CD44 in cancer progression: therapeutic implications. J. Hematol. Oncol. 11:64 10.1186/s13045-018-0605-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B., Montmasson M., Terradot L., and Rousselle P.. 2016a Syndecans as Cell Surface Receptors in Cancer Biology. A Focus on their Interaction with PDZ Domain Proteins. Front. Pharmacol. 7:10 10.3389/fphar.2016.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Gao S., Song X., Dong W., Zhou H., Zhao L., and Jia L.. 2016b Comprehensive N-glycan profiles of hepatocellular carcinoma reveal association of fucosylation with tumor progression and regulation of FUT8 by microRNAs. Oncotarget. 7:61199–61214. 10.18632/oncotarget.11284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong Y.K., Sandanaraj E., Koh L.W.H., Thangaveloo M., Tan M.S.Y., Koh G.R.H., Toh T.B., Lim G.G.Y., Holbrook J.D., Kon O.L., et al. 2015. ST3GAL1-Associated Transcriptomic Program in Glioblastoma Tumor Growth, Invasion, and Prognosis. J. Natl. Cancer Inst. 108:djv326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie D.R., Shaikh F.M., Lucas J.A. IV, Lucas J.A. III, and Bellis S.L.. 2008. ST6Gal-I expression in ovarian cancer cells promotes an invasive phenotype by altering integrin glycosylation and function. J. Ovarian Res. 1:3 10.1186/1757-2215-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clucas J., and Valderrama F.. 2014. ERM proteins in cancer progression. J. Cell Sci. 127:267–275. 10.1242/jcs.133108 [DOI] [PubMed] [Google Scholar]
- Crocker P.R., Paulson J.C., and Varki A.. 2007. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7:255–266. 10.1038/nri2056 [DOI] [PubMed] [Google Scholar]
- Daley W.P., Peters S.B., and Larsen M.. 2008. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 121:255–264. 10.1242/jcs.006064 [DOI] [PubMed] [Google Scholar]
- Daniotti J.L., Vilcaes A.A., Torres Demichelis V., Ruggiero F.M., and Rodriguez-Walker M.. 2013. Glycosylation of glycolipids in cancer: basis for development of novel therapeutic approaches. Front. Oncol. 3:306 10.3389/fonc.2013.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniotti J.L., Lardone R.D., and Vilcaes A.A.. 2016. Dysregulated Expression of Glycolipids in Tumor Cells: From Negative Modulator of Anti-tumor Immunity to Promising Targets for Developing Therapeutic Agents. Front. Oncol. 5:300 10.3389/fonc.2015.00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitroff C.J. 2015. Galectin-Binding O-Glycosylations as Regulators of Malignancy. Cancer Res. 75:3195–3202. 10.1158/0008-5472.CAN-15-0834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitroff C.J. 2019. I-branched carbohydrates as emerging effectors of malignant progression. Proc. Natl. Acad. Sci. USA. 116:13729–13737. 10.1073/pnas.1900268116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitroff C.J., Lee J.Y., Rafii S., Fuhlbrigge R.C., and Sackstein R.. 2001a CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J. Cell Biol. 153:1277–1286. 10.1083/jcb.153.6.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitroff C.J., Lee J.Y., Schor K.S., Sandmaier B.M., and Sackstein R.. 2001b differential L-selectin binding activities of human hematopoietic cell L-selectin ligands, HCELL and PSGL-1. J. Biol. Chem. 276:47623–47631. 10.1074/jbc.M105997200 [DOI] [PubMed] [Google Scholar]
- Dobrenkov K., Ostrovnaya I., Gu J., Cheung I.Y., and Cheung N.-K.V.. 2016. Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatr. Blood Cancer. 63:1780–1785. 10.1002/pbc.26097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez G.A., Anderson N.R., and Hammer D.A.. 2015. The direction of migration of T-lymphocytes under flow depends upon which adhesion receptors are engaged. Integr. Biol. 7:345–355. 10.1039/C4IB00201F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donatello S., Babina I.S., Hazelwood L.D., Hill A.D.K., Nabi I.R., and Hopkins A.M.. 2012. Lipid raft association restricts CD44-ezrin interaction and promotion of breast cancer cell migration. Am. J. Pathol. 181:2172–2187. 10.1016/j.ajpath.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougher C.W.L., Buffone A. Jr., Nemeth M.J., Nasirikenari M., Irons E.E., Bogner P.N., and Lau J.T.Y.. 2017. The blood-borne sialyltransferase ST6Gal-1 is a negative systemic regulator of granulopoiesis. J. Leukoc. Biol. 102:507–516. 10.1189/jlb.3A1216-538RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko J.D.B.C., and Schnaar R.L.. 2017. Chemical Tools for Inhibiting Glycosylation. In Essentials of Glycobiology. Cold Spring Harbor Laboratory, Press, Cold Spring Harbor, NY. [Google Scholar]
- Freire-de-Lima L. 2014. Sweet and sour: the impact of differential glycosylation in cancer cells undergoing epithelial-mesenchymal transition. Front. Oncol. 4:59 10.3389/fonc.2014.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., and Carlsson S.R.. 1986. Leukosialin, a major sialoglycoprotein on human leukocytes as differentiation antigens. Med. Biol. 64:335–343. [PubMed] [Google Scholar]
- Furukawa K., Hamamura K., Ohkawa Y., Ohmi Y., and Furukawa K.. 2012. Disialyl gangliosides enhance tumor phenotypes with differential modalities. Glycoconj. J. 29:579–584. 10.1007/s10719-012-9423-0 [DOI] [PubMed] [Google Scholar]
- Gainers M.E., Descheny L., Barthel S.R., Liu L., Wurbel M.-A., and Dimitroff C.J.. 2007. Skin-homing receptors on effector leukocytes are differentially sensitive to glyco-metabolic antagonism in allergic contact dermatitis. J. Immunol. 179:8509–8518. 10.4049/jimmunol.179.12.8509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Vallejo J.J., Ambrosini M., Overbeek A., van Riel W.E., Bloem K., Unger W.W.J., Chiodo F., Bolscher J.G., Nazmi K., Kalay H., and van Kooyk Y.. 2013. Multivalent glycopeptide dendrimers for the targeted delivery of antigens to dendritic cells. Mol. Immunol. 53:387–397. 10.1016/j.molimm.2012.09.012 [DOI] [PubMed] [Google Scholar]
- Garnham R., Scott E., Livermore K.E., and Munkley J.. 2019. ST6GAL1: A key player in cancer. Oncol. Lett. 18:983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garud D.R., Tran V.M., Victor X.V., Koketsu M., and Kuberan B.. 2008. Inhibition of heparan sulfate and chondroitin sulfate proteoglycan biosynthesis. J. Biol. Chem. 283:28881–28887. 10.1074/jbc.M805939200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J.T., Jolly M.K., Xu S., Somarelli J.A., and Levine H.. 2017. Survival Outcomes in Cancer Patients Predicted by a Partial EMT Gene Expression Scoring Metric. Cancer Res. 77:6415–6428. 10.1158/0008-5472.CAN-16-3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood K., and Foster J.H.. 2017. Dinutuximab for the treatment of pediatric patients with neuroblastoma. Drugs Today (Barc). 53:469–476. 10.1358/dot.2017.53.9.2693023 [DOI] [PubMed] [Google Scholar]
- Gu Y., Zhang J., Mi W., Yang J., Han F., Lu X., and Yu W.. 2008. Silencing of GM3 synthase suppresses lung metastasis of murine breast cancer cells. Breast Cancer Res. 10:R1 10.1186/bcr1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels J.A.A., Felder M., Horibata S., Belisle J.A., Kapur A., Holden H., Petrie S., Migneault M., Rancourt C., Connor J.P., and Patankar M.S.. 2010. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol. Cancer. 9:11 10.1186/1476-4598-9-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas Q., Boligan K.F., Jandus C., Schneider C., Simillion C., Stanczak M.A., Haubitz M., Jafari S.M.S., Zippelius A., Baerlocher G.M., et al. 2019. Siglec-9 Regulates an Effector Memory CD8+ T-cell Subset That Congregates in the Melanoma Tumor Microenvironment. Cancer Immunol. Res. 7:707–718. 10.1158/2326-6066.CIR-18-0505 [DOI] [PubMed] [Google Scholar]
- Hakomori S. 1996. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 56:5309–5318. [PubMed] [Google Scholar]
- Hammer D.A., and Tirrell M.. 1996. Biological Adhesion at Interfaces. Annu. Rev. Mater. Sci. 26:651–691. 10.1146/annurev.ms.26.080196.003251 [DOI] [Google Scholar]
- Heitger A., and Ladisch S.. 1996. Gangliosides block antigen presentation by human monocytes. Biochim. Biophys. Acta. 1303:161–168. 10.1016/0005-2760(96)00091-4 [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y. 2012. A world of sphingolipids and glycolipids in the brain-novel functions of simple lipids modified with glucose. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 88:129–143. 10.2183/pjab.88.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdbrooks A.T., Britain C.M., and Bellis S.L.. 2018. ST6Gal-I sialyltransferase promotes tumor necrosis factor (TNF)-mediated cancer cell survival via sialylation of the TNF receptor 1 (TNFR1) death receptor. J. Biol. Chem. 293:1610–1622. 10.1074/jbc.M117.801480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak J.E., Canham S.M., and Bertozzi C.R.. 2014. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 10:69–75. 10.1038/nchembio.1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons E.E., and Lau J.T.Y.. 2018. Systemic ST6Gal-1 Is a Pro-survival Factor for Murine Transitional B Cells. Front. Immunol. 9:2150 10.3389/fimmu.2018.02150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons E.E., Lee-Sundlov M.M., Zhu Y., Neelamegham S., Hoffmeister K.M., and Lau J.T.Y.. 2019. B cells suppress medullary granulopoiesis by an extracellular glycosylation-dependent mechanism. eLife. 8:e47328 10.7554/eLife.47328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob G.S. 1995. Glycosylation inhibitors in biology and medicine. Curr. Opin. Struct. Biol. 5:605–611. 10.1016/0959-440X(95)80051-4 [DOI] [PubMed] [Google Scholar]
- Jandus C., Boligan K.F., Chijioke O., Liu H., Dahlhaus M., Démoulins T., Schneider C., Wehrli M., Hunger R.E., Baerlocher G.M., et al. 2014. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J. Clin. Invest. 124:1810–1820. 10.1172/JCI65899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabedev A., and Lobaskin V.. 2018. Structure and elasticity of bush and brush-like models of the endothelial glycocalyx. Sci. Rep. 8:240 10.1038/s41598-017-18577-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadaja L., Laos S., and Maimets T.. 2004. Overexpression of leukocyte marker CD43 causes activation of the tumor suppressor proteins p53 and ARF. Oncogene. 23:2523–2530. 10.1038/sj.onc.1207359 [DOI] [PubMed] [Google Scholar]
- Kim Y.J., Borsig L., Varki N.M., and Varki A.. 1998. P-selectin deficiency attenuates tumor growth and metastasis. Proc. Natl. Acad. Sci. USA. 95:9325–9330. 10.1073/pnas.95.16.9325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R., Emi M., and Tanabe K.. 2007. Cancer immunoediting from immune surveillance to immune escape. Immunology. 121:1–14. 10.1111/j.1365-2567.2007.02587.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata H., and Yoshida A.. 1994. Differential effects of gangliosides on Ig production and proliferation by human B cells. Blood. 84:1193–1200. 10.1182/blood.V84.4.1193.1193 [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Matsumoto H., Matsuyama H., Fujii N., Inoue R., Yamamoto Y., and Nagao K.. 2016. Clinical significance of CD44 variant 9 expression as a prognostic indicator in bladder cancer. Oncol. Rep. 36:2852–2860. 10.3892/or.2016.5061 [DOI] [PubMed] [Google Scholar]
- Kramer J.R., Onoa B., Bustamante C., and Bertozzi C.R.. 2015. Chemically tunable mucin chimeras assembled on living cells. Proc. Natl. Acad. Sci. USA. 112:12574–12579. 10.1073/pnas.1516127112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudelka M.R., Antonopoulos A., Wang Y., Duong D.M., Song X., Seyfried N.T., Dell A., Haslam S.M., Cummings R.D., and Ju T.. 2016. Cellular O-Glycome Reporter/Amplification to explore O-glycans of living cells. Nat. Methods. 13:81–86. 10.1038/nmeth.3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufe D.W. 2009. Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer. 9:874–885. 10.1038/nrc2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahir Y. 2016. Understanding the basic role of glycocalyx during cancer. J. Radiat. Cancer Res. 7:79–84. 10.4103/0973-0168.197974 [DOI] [Google Scholar]
- Lange-Consiglio A., Accogli G., Cremonesi F., and Desantis S.. 2014. Cell Surface Glycan Changes in the Spontaneous Epithelial-Mesenchymal Transition of Equine Amniotic Multipotent Progenitor Cells. Cells Tissues Organs (Print). 200:212–226. 10.1159/000433420 [DOI] [PubMed] [Google Scholar]
- Lau W.M., Teng E., Chong H.S., Lopez K.A.P., Tay A.Y.L., Salto-Tellez M., Shabbir A., So J.B.Y., and Chan S.L.. 2014. CD44v8-10 is a cancer-specific marker for gastric cancer stem cells. Cancer Res. 74:2630–2641. 10.1158/0008-5472.CAN-13-2309 [DOI] [PubMed] [Google Scholar]
- Ledford H. 2011. Cancer theory faces doubts. Nature. 472:273 10.1038/472273a [DOI] [PubMed] [Google Scholar]
- Lee M.M., Nasirikenari M., Manhardt C.T., Ashline D.J., Hanneman A.J., Reinhold V.N., and Lau J.T.Y.. 2014. Platelets support extracellular sialylation by supplying the sugar donor substrate. J. Biol. Chem. 289:8742–8748. 10.1074/jbc.C113.546713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-H., Wang Y.-N., Xia W., Chen C.-H., Rau K.-M., Ye L., Wei Y., Chou C.-K., Wang S.-C., Yan M., et al. 2019. Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell. 36:168–178.e4. 10.1016/j.ccell.2019.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Sundlov M.M., Ashline D.J., Hanneman A.J., Grozovsky R., Reinhold V.N., Hoffmeister K.M., and Lau J.T.Y.. 2016. Circulating blood and platelets supply glycosyltransferases that enable extrinsic extracellular glycosylation. Glycobiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., and King M.R.. 2012. Adhesion receptors as therapeutic targets for circulating tumor cells. Front. Oncol. 2:79 10.3389/fonc.2012.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Li Y., Wu X., Li Q., Yu J., Gen J., and Zhang X.-L.. 2008. Knockdown of Mgat5 inhibits breast cancer cell growth with activation of CD4+ T cells and macrophages. J. Immunol. 180:3158–3165. 10.4049/jimmunol.180.5.3158 [DOI] [PubMed] [Google Scholar]
- Li S., Mo C., Peng Q., Kang X., Sun C., Jiang K., Huang L., Lu Y., Sui J., Qin X., and Liu Y.. 2013. Cell surface glycan alterations in epithelial mesenchymal transition process of Huh7 hepatocellular carcinoma cell. PLoS One. 8:e71273 10.1371/journal.pone.0071273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Hao X., Qin J., Tang W., He F., Smith A., Zhang M., Simeone D.M., Qiao X.T., Chen Z.-N., et al. 2014. Antibody against CD44s inhibits pancreatic tumor initiation and postradiation recurrence in mice. Gastroenterology. 146:1108–1118.e12. 10.1053/j.gastro.2013.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang X., Tan Z., Chen S., and Guan F.. 2016. Role of Glycans in Cancer Cells Undergoing Epithelial-Mesenchymal Transition. Front. Oncol. 6:33 10.3389/fonc.2016.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhou Z., Zhang X., Zheng L., He D., Ye Y., Zhang Q.-Q., Qi C.-L., He X.-D., Yu C., et al. 2017. Inflammatory Molecule, PSGL-1, Deficiency Activates Macrophages to Promote Colorectal Cancer Growth through NFκB Signaling. Mol. Cancer Res. 15:467–477. 10.1158/1541-7786.MCR-16-0309 [DOI] [PubMed] [Google Scholar]
- Li N., Gao W., Zhang Y.-F., and Ho M.. 2018. Glypicans as Cancer Therapeutic Targets. Trends Cancer. 4:741–754. 10.1016/j.trecan.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zheng X., Pang X., Li L., Wang J., Yang C., and Du G.. 2018a Ganglioside GD3 synthase (GD3S), a novel cancer drug target. Acta Pharm. Sin. B. 8:713–720. 10.1016/j.apsb.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.-W., Zandberg W.F., Gloster T.M., Deng L., Murray K.D., Shan X., and Vocadlo D.J.. 2018b Metabolic Inhibitors of O-GlcNAc Transferase That Act In Vivo Implicate Decreased O-GlcNAc Levels in Leptin-Mediated Nutrient Sensing. Angew. Chem. Int. Ed. Engl. 57:7644–7648. 10.1002/anie.201803254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C.Y., Antonopoulos A., Dell A., Haslam S.M., Lee T., and Neelamegham S.. 2013a The use of surface immobilization of P-selectin glycoprotein ligand-1 on mesenchymal stem cells to facilitate selectin mediated cell tethering and rolling. Biomaterials. 34:8213–8222. 10.1016/j.biomaterials.2013.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C.Y., Antonopoulos A., Gupta R., Qu J., Dell A., Haslam S.M., and Neelamegham S.. 2013b Competition between core-2 GlcNAc-transferase and ST6GalNAc-transferase regulates the synthesis of the leukocyte selectin ligand on human P-selectin glycoprotein ligand-1. J. Biol. Chem. 288:13974–13987. 10.1074/jbc.M113.463653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C.Y., Weil B.R., Palka B.A., Momeni A., Canty J.M. Jr., and Neelamegham S.. 2016. Cell surface glycoengineering improves selectin-mediated adhesion of mesenchymal stem cells (MSCs) and cardiosphere-derived cells (CDCs): Pilot validation in porcine ischemia-reperfusion model. Biomaterials. 74:19–30. 10.1016/j.biomaterials.2015.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez P.H.H., and Schnaar R.L.. 2006. Determination of glycolipid-protein interaction specificity. Methods Enzymol. 417:205–220. 10.1016/S0076-6879(06)17015-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Isaji T., Im S., Fukuda T., Hashii N., Takakura D., Kawasaki N., and Gu J.. 2014. β-galactoside α2,6 sialyltranferase 1 promotes transforming growth factor-β-mediated epithelial-mesenchymal transition. J. Biol. Chem. 289:34627–34641. 10.1074/jbc.M114.593392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübke T., Marquardt T., Etzioni A., Hartmann E., von Figura K., and Körner C.. 2001. Complementation cloning identifies CDG-IIc, a new type of congenital disorders of glycosylation, as a GDP-fucose transporter deficiency. Nat. Genet. 28:73–76. 10.1038/ng0501-73 [DOI] [PubMed] [Google Scholar]
- Lv L., Liu H.G., Dong S.Y., Yang F., Wang Q.X., Guo G.L., Pan Y.F., and Zhang X.H.. 2016. Upregulation of CD44v6 contributes to acquired chemoresistance via the modulation of autophagy in colon cancer SW480 cells. Tumour Biol. 37:8811–8824. 10.1007/s13277-015-4755-6 [DOI] [PubMed] [Google Scholar]
- Macauley M.S., Arlian B.M., Rillahan C.D., Pang P.-C., Bortell N., Marcondes M.C.G., Haslam S.M., Dell A., and Paulson J.C.. 2014. Systemic blockade of sialylation in mice with a global inhibitor of sialyltransferases. J. Biol. Chem. 289:35149–35158. 10.1074/jbc.M114.606517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni H.J.F. 2007. Glycosylation of glycolipids in the Golgi complex. J. Neurochem. 103(s1, suppl 1):81–90. 10.1111/j.1471-4159.2007.04717.x [DOI] [PubMed] [Google Scholar]
- Manhardt C.T., Punch P.R., Dougher C.W.L., and Lau J.T.Y.. 2017. Extrinsic sialylation is dynamically regulated by systemic triggers in vivo. J. Biol. Chem. 292:13514–13520. 10.1074/jbc.C117.795138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marathe D.D., Buffone A. Jr., Chandrasekaran E.V., Xue J., Locke R.D., Nasirikenari M., Lau J.T.Y., Matta K.L., and Neelamegham S.. 2010. Fluorinated per-acetylated GalNAc metabolically alters glycan structures on leukocyte PSGL-1 and reduces cell binding to selectins. Blood. 115:1303–1312. 10.1182/blood-2009-07-231480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos N.T., Pinho S., Grandela C., Cruz A., Samyn-Petit B., Harduin-Lepers A., Almeida R., Silva F., Morais V., Costa J., et al. 2004. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 64:7050–7057. 10.1158/0008-5472.CAN-04-1921 [DOI] [PubMed] [Google Scholar]
- Martinez-Duncker I., Dupré T., Piller V., Piller F., Candelier J.J., Trichet C., Tchernia G., Oriol R., and Mollicone R.. 2005. Genetic complementation reveals a novel human congenital disorder of glycosylation of type II, due to inactivation of the Golgi CMP-sialic acid transporter. Blood. 105:2671–2676. 10.1182/blood-2004-09-3509 [DOI] [PubMed] [Google Scholar]
- Martinez-Seara Monne H., Danne R., Róg T., Ilpo V., and Gurtovenko A.. 2013. Structure of Glycocalyx. Biophys. J. 104:251a 10.1016/j.bpj.2012.11.1412 [DOI] [Google Scholar]
- Matsumoto M., Shigeta A., Furukawa Y., Tanaka T., Miyasaka M., and Hirata T.. 2007. CD43 collaborates with P-selectin glycoprotein ligand-1 to mediate E-selectin-dependent T cell migration into inflamed skin. J. Immunol. 178:2499–2506. 10.4049/jimmunol.178.4.2499 [DOI] [PubMed] [Google Scholar]
- McEver R.P., and Cummings R.D.. 1997. Perspectives series: cell adhesion in vascular biology. Role of PSGL-1 binding to selectins in leukocyte recruitment. J. Clin. Invest. 100:485–491. 10.1172/JCI119556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKallip R., Li R., and Ladisch S.. 1999. Tumor gangliosides inhibit the tumor-specific immune response. J. Immunol. 163:3718–3726. [PubMed] [Google Scholar]
- Mendelsohn R., Cheung P., Berger L., Partridge E., Lau K., Datti A., Pawling J., and Dennis J.W.. 2007. Complex N-glycan and metabolic control in tumor cells. Cancer Res. 67:9771–9780. 10.1158/0008-5472.CAN-06-4580 [DOI] [PubMed] [Google Scholar]
- Meng Y., Lu Z., Yu S., Zhang Q., Ma Y., and Chen J.. 2010. Ezrin promotes invasion and metastasis of pancreatic cancer cells. J. Transl. Med. 8:61 10.1186/1479-5876-8-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J.P., Sebbane R., Tzartos S., and Lindstrom J.. 1982. Inhibition of glycosylation with tunicamycin blocks assembly of newly synthesized acetylcholine receptor subunits in muscle cells. J. Biol. Chem. 257:2694–2701. [PubMed] [Google Scholar]
- Merrill A.H., Jr 2011. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 111:6387–6422. 10.1021/cr2002917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt W.D., Der-Minassian V., and Reaman G.H.. 1994. Increased GD3 ganglioside in plasma of children with T-cell acute lymphoblastic leukemia. Leukemia. 8:816–822. [PubMed] [Google Scholar]
- Mitchell M.J., and King M.R.. 2014. Physical biology in cancer. 3. The role of cell glycocalyx in vascular transport of circulating tumor cells. Am. J. Physiol. Cell Physiol. 306:C89–C97. 10.1152/ajpcell.00285.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möckl L., Pedram K., Roy A.R., Krishnan V., Gustavsson A.-K., Dorigo O., Bertozzi C.R., and Moerner W.E.. 2019. Quantitative Super-Resolution Microscopy of the Mammalian Glycocalyx. Dev. Cell. 50:57–72.e6. 10.1016/j.devcel.2019.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal N., Buffone A. Jr., Stolfa G., Antonopoulos A., Lau J.T.Y., Haslam S.M., Dell A., and Neelamegham S.. 2015. ST3Gal-4 is the primary sialyltransferase regulating the synthesis of E-, P-, and L-selectin ligands on human myeloid leukocytes. Blood. 125:687–696. 10.1182/blood-2014-07-588590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal N., Stolfa G., Antonopoulos A., Zhu Y., Wang S.-S., Buffone A. Jr., Atilla-Gokcumen G.E., Haslam S.M., Dell A., and Neelamegham S.. 2016. Glycosphingolipids on Human Myeloid Cells Stabilize E-Selectin-Dependent Rolling in the Multistep Leukocyte Adhesion Cascade. Arterioscler. Thromb. Vasc. Biol. 36:718–727. 10.1161/ATVBAHA.115.306748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal N., Dykstra B., Lee J., Ashline D., Reinhold V.N., Rossi D.J., and Sackstein R.. 2018. Distinct human α(1,3)-fucosyltransferases drive Lewis-X/sialyl Lewis-X assembly in human cells. J. Biol. Chem. 293:7300–7314. 10.1074/jbc.RA117.000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.L. 1998. Structure and function of P-selectin glycoprotein ligand-1. Leuk. Lymphoma. 29:1–15. 10.3109/10428199809058377 [DOI] [PubMed] [Google Scholar]
- Morikis V.A., Chase S., Wun T., Chaikof E.L., Magnani J.L., and Simon S.I.. 2017. Selectin catch-bonds mechanotransduce integrin activation and neutrophil arrest on inflamed endothelium under shear flow. Blood. 130:2101–2110. 10.1182/blood-2017-05-783027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A., and de Herreros A.G.. 2017. Epithelial-mesenchymal transition in cancer. Mol. Oncol. 11:715–717. 10.1002/1878-0261.12094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J.W., Kruyt P.M., Sewnath M., Oosting J., Seldenrijk C.A., Weidema W.F., Offerhaus G.J., and Pals S.T.. 1994. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet. 344:1470–1472. 10.1016/S0140-6736(94)90290-9 [DOI] [PubMed] [Google Scholar]
- Müller S., Goletz S., Packer N., Gooley A., Lawson A.M., and Hanisch F.-G.. 1997. Localization of O-glycosylation sites on glycopeptide fragments from lactation-associated MUC1. All putative sites within the tandem repeat are glycosylation targets in vivo. J. Biol. Chem. 272:24780–24793. 10.1074/jbc.272.40.24780 [DOI] [PubMed] [Google Scholar]
- Munkley J., and Elliott D.J.. 2016. Hallmarks of glycosylation in cancer. Oncotarget. 7:35478–35489. 10.18632/oncotarget.8155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mycko M.P., Sliwinska B., Cichalewska M., Cwiklinska H., Raine C.S., and Selmaj K.W.. 2014. Brain glycolipids suppress T helper cells and inhibit autoimmune demyelination. J. Neurosci. 34:8646–8658. 10.1523/JNEUROSCI.0885-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasirikenari M., Veillon L., Collins C.C., Azadi P., and Lau J.T.Y.. 2014. Remodeling of marrow hematopoietic stem and progenitor cells by non-self ST6Gal-1 sialyltransferase. J. Biol. Chem. 289:7178–7189. 10.1074/jbc.M113.508457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath S., and Mukherjee P.. 2014. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 20:332–342. 10.1016/j.molmed.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J., Cozzi P.J., Hao J.L., Beretov J., Chang L., Duan W., Shigdar S., Delprado W.J., Graham P.H., Bucci J., et al. 2014. CD44 variant 6 is associated with prostate cancer metastasis and chemo-/radioresistance. Prostate. 74:602–617. 10.1002/pros.22775 [DOI] [PubMed] [Google Scholar]
- Nicoll G., Avril T., Lock K., Furukawa K., Bovin N., and Crocker P.R.. 2003. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via siglec-7-dependent and -independent mechanisms. Eur. J. Immunol. 33:1642–1648. 10.1002/eji.200323693 [DOI] [PubMed] [Google Scholar]
- Nikitovic D., Berdiaki A., Spyridaki I., Krasanakis T., Tsatsakis A., and Tzanakakis G.N.. 2018. Proteoglycans-Biomarkers and Targets in Cancer Therapy. Front. Endocrinol. (Lausanne). 9:69 10.3389/fendo.2018.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimrichter L., Burdick M.M., Aoki K., Laroy W., Fierro M.A., Hudson S.A., Von Seggern C.E., Cotter R.J., Bochner B.S., Tiemeyer M., et al. 2008. E-selectin receptors on human leukocytes. Blood. 112:3744–3752. 10.1182/blood-2008-04-149641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Okayama H., Kofunato Y., Chida S., Saito K., Tada T., Ashizawa M., Nakajima T., Aoto K., Kikuchi T., et al. 2018. Prognostic role of FUT8 expression in relation to p53 status in stage II and III colorectal cancer. PLoS One. 13:e0200315 10.1371/journal.pone.0200315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeley N.M., Alley S.C., Anderson M.E., Boursalian T.E., Burke P.J., Emmerton K.M., Jeffrey S.C., Klussman K., Law C.-L., Sussman D., et al. 2013. Development of orally active inhibitors of protein and cellular fucosylation. Proc. Natl. Acad. Sci. USA. 110:5404–5409. 10.1073/pnas.1222263110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi G., Barbolini M., Ficarra G., Tazzioli G., Manni P., Petrachi T., Mastrolia I., Orvieto E., Spano C., Prapa M., et al. 2017. GD2 expression in breast cancer. Oncotarget. 8:31592–31600. 10.18632/oncotarget.16363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek M.J., Boettiger D., Weaver V.M., and Hammer D.A.. 2009. Integrin clustering is driven by mechanical resistance from the glycocalyx and the substrate. PLOS Comput. Biol. 5:e1000604 10.1371/journal.pcbi.1000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek M.J., DuFort C.C., Rossier O., Bainer R., Mouw J.K., Godula K., Hudak J.E., Lakins J.N., Wijekoon A.C., Cassereau L., et al. 2014. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 511:319–325. 10.1038/nature13535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavelka M., and Roth J.. 2010. Glycocalyx Changes in Tumours. In Functional Ultrastructure: Atlas of Tissue Biology and Pathology. Pavelka M., and Roth J., editors. Springer Vienna, Vienna, Austria: 164–165. 10.1007/978-3-211-99390-3_86 [DOI] [Google Scholar]
- Picco G., Julien S., Brockhausen I., Beatson R., Antonopoulos A., Haslam S., Mandel U., Dell A., Pinder S., Taylor-Papadimitriou J., and Burchell J.. 2010. Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology. 20:1241–1250. 10.1093/glycob/cwq085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S.S., and Reis C.A.. 2015. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer. 15:540–555. 10.1038/nrc3982 [DOI] [PubMed] [Google Scholar]
- Pomin V.H., and Mulloy B.. 2018. Glycosaminoglycans and Proteoglycans. Pharmaceuticals (Basel). 11:27 10.3390/ph11010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponta H., Sherman L., and Herrlich P.A.. 2003. CD44: from adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 4:33–45. 10.1038/nrm1004 [DOI] [PubMed] [Google Scholar]
- Price T.T., Burness M.L., Sivan A., Warner M.J., Cheng R., Lee C.H., Olivere L., Comatas K., Magnani J., Kim Lyerly H., et al. 2016. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci. Transl. Med. 8:340ra73 10.1126/scitranslmed.aad4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi H., Shi Z.-D., and Tarbell J.M.. 2011. Fluid shear stress regulates the invasive potential of glioma cells via modulation of migratory activity and matrix metalloproteinase expression. PLoS One. 6:e20348 10.1371/journal.pone.0020348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi H., Palomino R., Shi Z.-D., Munn L.L., and Tarbell J.M.. 2013. Cancer cell glycocalyx mediates mechanotransduction and flow-regulated invasion. Integr. Biol. 5:1334–1343. 10.1039/c3ib40057c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi H., Shi Z.-D., Song J.W., Cancel L.M., Huang P., Zeng Y., Roberge S., Munn L.L., and Tarbell J.M.. 2016. Heparan sulfate proteoglycans mediate renal carcinoma metastasis. Int. J. Cancer. 139:2791–2801. 10.1002/ijc.30397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reategui E.P., de Mayolo A.A., Das P.M., Astor F.C., Singal R., Hamilton K.L., Goodwin W.J., Carraway K.L., and Franzmann E.J.. 2006. Characterization of CD44v3-containing isoforms in head and neck cancer. Cancer Biol. Ther. 5:1163–1168. 10.4161/cbt.5.9.3065 [DOI] [PubMed] [Google Scholar]
- Reitsma S., Slaaf D.W., Vink H., van Zandvoort M.A.M.J., and oude Egbrink M.G.. 2007. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 454:345–359. 10.1007/s00424-007-0212-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M., Linkowski M., Tarim J., Piperdi S., Sowers R., Geller D., Gill J., and Gorlick R.. 2014. Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer. 120:548–554. 10.1002/cncr.28461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy L.D., Sahraei M., Subramani D.B., Besmer D., Nath S., Tinder T.L., Bajaj E., Shanmugam K., Lee Y.Y., Hwang S.I.L., et al. 2011. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene. 30:1449–1459. 10.1038/onc.2010.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryczko M.C., Pawling J., Chen R., Abdel Rahman A.M., Yau K., Copeland J.K., Zhang C., Surendra A., Guttman D.S., Figeys D., and Dennis J.W.. 2016. Metabolic Reprogramming by Hexosamine Biosynthetic and Golgi N-Glycan Branching Pathways. Sci. Rep. 6:23043 10.1038/srep23043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackstein R. 2012. Glycoengineering of HCELL, the human bone marrow homing receptor: sweetly programming cell migration. Ann. Biomed. Eng. 40:766–776. 10.1007/s10439-011-0461-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaar R.L. 2019. The Biology of Gangliosides. Adv. Carbohydr. Chem. Biochem. 76:113–148. 10.1016/bs.accb.2018.09.002 [DOI] [PubMed] [Google Scholar]
- Schnaar R.L., and Kinoshita T.. 2015. Glycosphingolipids. In Essentials of Glycobiology. Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., Prestegard J.H., et al., editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: 125–135. [Google Scholar]
- Schultz M.J., Swindall A.F., Wright J.W., Sztul E.S., Landen C.N., and Bellis S.L.. 2013. ST6Gal-I sialyltransferase confers cisplatin resistance in ovarian tumor cells. J. Ovarian Res. 6:25 10.1186/1757-2215-6-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz M.J., Holdbrooks A.T., Chakraborty A., Grizzle W.E., Landen C.N., Buchsbaum D.J., Conner M.G., Arend R.C., Yoon K.J., Klug C.A., et al. 2016. The Tumor-Associated Glycosyltransferase ST6Gal-I Regulates Stem Cell Transcription Factors and Confers a Cancer Stem Cell Phenotype. Cancer Res. 76:3978–3988. 10.1158/0008-5472.CAN-15-2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki K., Yamaguchi K., Takahashi K., Moriya S., and Miyagi T.. 2011. Regulation of sialyl Lewis antigen expression in colon cancer cells by sialidase NEU4. J. Biol. Chem. 286:21052–21061. 10.1074/jbc.M111.231191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurer C.R., Colville M.J., Gupta V.K., Head S.E., Kai F., Lakins J.N., and Paszek M.J.. 2018. Genetically Encoded Toolbox for Glycocalyx Engineering: Tunable Control of Cell Adhesion, Survival, and Cancer Cell Behaviors. ACS Biomater. Sci. Eng. 4:388–399. 10.1021/acsbiomaterials.7b00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurer C.R., Kuo J.C.-H., Roberts L.M., Gandhi J.G., Colville M.J., Enoki T.A., Pan H., Su J., Noble J.M., Hollander M.J., et al. 2019. Physical Principles of Membrane Shape Regulation by the Glycocalyx. Cell. 177:1757–1770.e21. 10.1016/j.cell.2019.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spertini C., Baïsse B., Bellone M., Gikic M., Smirnova T., and Spertini O.. 2019. Acute Myeloid and Lymphoblastic Leukemia Cell Interactions with Endothelial Selectins: Critical Role of PSGL-1, CD44 and CD43. Cancers (Basel). 11:1253 10.3390/cancers11091253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczak M.A., Siddiqui S.S., Trefny M.P., Thommen D.S., Boligan K.F., von Gunten S., Tzankov A., Tietze L., Lardinois D., Heinzelmann-Schwarz V., et al. 2018. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. J. Clin. Invest. 128:4912–4923. 10.1172/JCI120612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. 2011. Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 3:a005199 10.1101/cshperspect.a005199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steentoft C., Bennett E.P., Schjoldager K.T.B.G., Vakhrushev S.Y., Wandall H.H., and Clausen H.. 2014. Precision genome editing: a small revolution for glycobiology. Glycobiology. 24:663–680. 10.1093/glycob/cwu046 [DOI] [PubMed] [Google Scholar]
- Stolfa G., Mondal N., Zhu Y., Yu X., Buffone A. Jr., and Neelamegham S.. 2016. Using CRISPR-Cas9 to quantify the contributions of O-glycans, N-glycans and Glycosphingolipids to human leukocyte-endothelium adhesion. Sci. Rep. 6:30392 10.1038/srep30392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Guo S.S., and Fässler R.. 2016. Integrin-mediated mechanotransduction. J. Cell Biol. 215:445–456. 10.1083/jcb.201609037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindall A.F., and Bellis S.L.. 2011. Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J. Biol. Chem. 286:22982–22990. 10.1074/jbc.M110.211375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell J.M., and Cancel L.M.. 2016. The glycocalyx and its significance in human medicine. J. Intern. Med. 280:97–113. 10.1111/joim.12465 [DOI] [PubMed] [Google Scholar]
- Tarbell J.M., and Pahakis M.Y.. 2006. Mechanotransduction and the glycocalyx. J. Intern. Med. 259:339–350. 10.1111/j.1365-2796.2006.01620.x [DOI] [PubMed] [Google Scholar]
- Tarbell J.M., and Shi Z.-D.. 2013. Effect of the glycocalyx layer on transmission of interstitial flow shear stress to embedded cells. Biomech. Model. Mechanobiol. 12:111–121. 10.1007/s10237-012-0385-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone P., Bonelli P., Tuccillo F., Bond H.M., D’Armiento F.P., Galea E., Palmieri C., Tagliaferri P., Natali P.G., and Venuta S.. 2002. Differential expression of UN1, early thymocyte-associated sialoglycoprotein, in breast normal tissue, benign disease and carcinomas. Anticancer Res. 22:2333–2340. [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Burchell J., Miles D.W., and Dalziel M.. 1999. MUC1 and cancer. Biochim. Biophys. Acta. 1455:301–313. 10.1016/S0925-4439(99)00055-1 [DOI] [PubMed] [Google Scholar]
- Tedford K., Steiner M., Koshutin S., Richter K., Tech L., Eggers Y., Jansing I., Schilling K., Hauser A.E., Korthals M., and Fischer K.-D.. 2017. The opposing forces of shear flow and sphingosine-1-phosphate control marginal zone B cell shuttling. Nat. Commun. 8:2261 10.1038/s41467-017-02482-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzic T., Cordeau M., Herblot S., Teira P., Cournoyer S., Beaunoyer M., Peuchmaur M., Duval M., and Sartelet H.. 2018. Expression of Disialoganglioside (GD2) in Neuroblastic Tumors: A Prognostic Value for Patients Treated With Anti-GD2 Immunotherapy. Pediatr. Dev. Pathol. 21:355–362. 10.1177/1093526617723972 [DOI] [PubMed] [Google Scholar]
- Tinoco R., Carrette F., Barraza M.L., Otero D.C., Magaña J., Bosenberg M.W., Swain S.L., and Bradley L.M.. 2016. PSGL-1 Is an Immune Checkpoint Regulator that Promotes T Cell Exhaustion. Immunity. 44:1190–1203. 10.1016/j.immuni.2016.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro M., Gaggianesi M., Catalano V., Benfante A., Iovino F., Biffoni M., Apuzzo T., Sperduti I., Volpe S., Cocorullo G., et al. 2014. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 14:342–356. 10.1016/j.stem.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Trinchera M., Aronica A., and Dall’Olio F.. 2017. Selectin Ligands Sialyl-Lewis a and Sialyl-Lewis x in Gastrointestinal Cancers. Biology (Basel). 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C.-F., Wu M.-Y., Lin Y.-C., Kannagi R., and Yang R.-B.. 2017. FUT8 promotes breast cancer cell invasiveness by remodeling TGF-β receptor core fucosylation. Breast Cancer Res. 19:111 10.1186/s13058-017-0904-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuccillo F.M., Palmieri C., Fiume G., de Laurentiis A., Schiavone M., Falcone C., Iaccino E., Galandrini R., Capuano C., Santoni A., et al. 2014. Cancer-associated CD43 glycoforms as target of immunotherapy. Mol. Cancer Ther. 13:752–762. 10.1158/1535-7163.MCT-13-0651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng S.-F., Tsai C.-H., Chao T.-K., Chou Y.-C., Yang Y.-C., Tsai M.-H., Cha T.-L., and Hsiao P.-W.. 2018. O-Glycosylation-mediated signaling circuit drives metastatic castration-resistant prostate cancer. FASEB J. 32:fj201800687 10.1096/fj.201800687 [DOI] [PubMed] [Google Scholar]
- Uchimido R., Schmidt E.P., and Shapiro N.I.. 2019. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit. Care. 23:16 10.1186/s13054-018-2292-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valignat M.-P., Theodoly O., Gucciardi A., Hogg N., and Lellouch A.C.. 2013. T lymphocytes orient against the direction of fluid flow during LFA-1-mediated migration. Biophys. J. 104:322–331. 10.1016/j.bpj.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bijgaart R.J.E., Kroesen M., Wassink M., Brok I.C., Kers-Rebel E.D., Boon L., Heise T., van Scherpenzeel M., Lefeber D.J., Boltje T.J., et al. 2019. Combined sialic acid and histone deacetylase (HDAC) inhibitor treatment up-regulates the neuroblastoma antigen GD2. J. Biol. Chem. 294:4437–4449. 10.1074/jbc.RA118.002763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. 2017. Biological roles of glycans. Glycobiology. 27:3–49. 10.1093/glycob/cww086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A., and Gagneux P.. 2012. Multifarious roles of sialic acids in immunity. Ann. N. Y. Acad. Sci. 1253:16–36. 10.1111/j.1749-6632.2012.06517.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vester-Christensen M.B., Halim A., Joshi H.J., Steentoft C., Bennett E.P., Levery S.B., Vakhrushev S.Y., and Clausen H.. 2013. Mining the O-mannose glycoproteome reveals cadherins as major O-mannosylated glycoproteins. Proc. Natl. Acad. Sci. USA. 110:21018–21023. 10.1073/pnas.1313446110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira P.A., Silva M., Martin K.C., and Sackstein R.. 2018. Ligation of the CD44 Glycoform HCELL on Culture-Expanded Human Monocyte-Derived Dendritic Cells Programs Transendothelial Migration. J. Immunol. 201:1030–1043. 10.4049/jimmunol.1800188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.-S., Gao X., Solar V., Yu X., Antonopoulos A., Friedman A.E., Matich E.K., Atilla-Gokcumen G.E., Nasirikenari M., Lau J.T., et al. 2018. Thioglycosides Are Efficient Metabolic Decoys of Glycosylation that Reduce Selectin Dependent Leukocyte Adhesion. Cell Chem. Biol. 25:1519–1532.e5. 10.1016/j.chembiol.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb T.J., Li X., Giuntoli R.L. II, Lopez P.H.H., Heuser C., Schnaar R.L., Tsuji M., Kurts C., Oelke M., and Schneck J.P.. 2012. Molecular identification of GD3 as a suppressor of the innate immune response in ovarian cancer. Cancer Res. 72:3744–3752. 10.1158/0008-5472.CAN-11-2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I.G., Barbier V., Nowlan B., Jacobsen R.N., Forristal C.E., Patton J.T., Magnani J.L., and Lévesque J.-P.. 2012. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat. Med. 18:1651–1657. 10.1038/nm.2969 [DOI] [PubMed] [Google Scholar]
- Woods E.C., Kai F., Barnes J.M., Pedram K., Pickup M.W., Hollander M.J., Weaver V.M., and Bertozzi C.R.. 2017. A bulky glycocalyx fosters metastasis formation by promoting G1 cell cycle progression. eLife. 6:e25752 10.7554/eLife.25752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wopereis S., Lefeber D.J., Morava E., and Wevers R.A.. 2006. Mechanisms in protein O-glycan biosynthesis and clinical and molecular aspects of protein O-glycan biosynthesis defects: a review. Clin. Chem. 52:574–600. 10.1373/clinchem.2005.063040 [DOI] [PubMed] [Google Scholar]
- Wu X., Zhao J., Ruan Y., Sun L., Xu C., and Jiang H.. 2018. Sialyltransferase ST3GAL1 promotes cell migration, invasion, and TGF-β1-induced EMT and confers paclitaxel resistance in ovarian cancer. Cell Death Dis. 9:1102 10.1038/s41419-018-1101-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski F.J., Lee S.S., Yabe T., Wang H., Gomez-Escribano J.P., Bibb M.J., Lee S.J., Davies G.J., and Davis B.G.. 2012. Biosynthesis of the tunicamycin antibiotics proceeds via unique exo-glycal intermediates. Nat. Chem. 4:539–546. 10.1038/nchem.1351 [DOI] [PubMed] [Google Scholar]
- Xiao H., Woods E.C., Vukojicic P., and Bertozzi C.R.. 2016. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc. Natl. Acad. Sci. USA. 113:10304–10309. 10.1073/pnas.1608069113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Niu X., Wang W., Yang W., Du Y., Gu J., and Song L.. 2017. Specific N-glycan alterations are coupled in EMT induced by different density cultivation of MCF 10A epithelial cells. Glycoconj. J. 34:219–227. 10.1007/s10719-016-9754-3 [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Yoshimura S., and Yu R.K.. 2011. Expression of GD2 and GD3 gangliosides in human embryonic neural stem cells. ASN Neuro. 3:AN20110006 10.1042/AN20110006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Zhang R., Cai H., and Wang L.-X.. 2017. Revisiting the substrate specificity of mammalian α1,6-fucosyltransferase reveals that it catalyzes core fucosylation of N-glycans lacking α1,3-arm GlcNAc. J. Biol. Chem. 292:14796–14803. 10.1074/jbc.M117.804070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F., Hu G., Taylor D., Ratnikov B., Bobkov A.A., McLean M.A., Sligar S.G., Taylor K.A., and Ginsberg M.H.. 2010. Recreation of the terminal events in physiological integrin activation. J. Cell Biol. 188:157–173. 10.1083/jcb.200908045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh S.-C., Wang P.-Y., Lou Y.-W., Khoo K.-H., Hsiao M., Hsu T.-L., and Wong C.-H.. 2016. Glycolipid GD3 and GD3 synthase are key drivers for glioblastoma stem cells and tumorigenicity. Proc. Natl. Acad. Sci. USA. 113:5592–5597. 10.1073/pnas.1604721113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo H.L., Fan T.-C., Lin R.-J., Yu J.-C., Liao G.-S., Chen E.S.-W., Ho M.-Y., Lin W.-D., Chen K., Chen C.-H., et al. 2019. Sialylation of vasorin by ST3Gal1 facilitates TGF-β1-mediated tumor angiogenesis and progression. Int. J. Cancer. 144:1996–2007. 10.1002/ijc.31891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., and Tarbell J.M.. 2014. The adaptive remodeling of endothelial glycocalyx in response to fluid shear stress. PLoS One. 9:e86249 10.1371/journal.pone.0086249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y.E., Yao X.-H., Yan Z.-P., Liu J.-X., and Liu X.-H.. 2016. Potential signaling pathway involved in sphingosine-1-phosphate-induced epithelial-mesenchymal transition in cancer. Oncol. Lett. 12:379–382. 10.3892/ol.2016.4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Lu J., Xu Z., Zou X., Sun X., Xu Y., Shan A., Lu J., Yan X., Cui Y., et al. 2017. Differential expression of ST6GAL1 in the tumor progression of colorectal cancer. Biochem. Biophys. Res. Commun. 486:1090–1096. 10.1016/j.bbrc.2017.03.167 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Higginbotham J.N., Jeppesen D.K., Yang Y.-P., Li W., McKinley E.T., Graves-Deal R., Ping J., Britain C.M., Dorsett K.A., et al. 2019. Transfer of Functional Cargo in Exomeres. Cell Reports. 27:940–954.e6. 10.1016/j.celrep.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Chen C., Chang K., Karnad A., Jagirdar J., Kumar A.P., and Freeman J.W.. 2016. CD44 Expression Level and Isoform Contributes to Pancreatic Cancer Cell Plasticity, Invasiveness, and Response to Therapy. Clin. Cancer Res. 22:5592–5604. 10.1158/1078-0432.CCR-15-3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C., Terreni M., Sollogoub M., and Zhang Y.. 2019. Ganglioside GM3 and Its Role in Cancer. Curr. Med. Chem. 26:2933–2947. 10.2174/0929867325666180129100619 [DOI] [PubMed] [Google Scholar]