Abstract
The aim of this article is to explore neurosurgeons' knowledge and understanding of the physiology of zinc and provide current information about the role zinc plays in post-neurological wound healing. We review several medical journals and bring together the most updated information related to lesion-healing after surgery.
Keywords: zinc deficiency, delayed wound healing, zinc excess, trace mineral, mineral, micro-nutrient
Introduction and background
Monitoring and treating wounds in the post-neurosurgical procedure is crucial due to the high cost of treatment to the healthcare system in the US. Post-neurological wound-healing process could be complicated and complex due to the prevalence of deficiencies in anti-oxidants, trace minerals, vitamins, and micro-elements in patients [1].
Post-neurosurgical wound-healing involves a multilayered procedure administered by chronological steps plus inflammation, proliferation, and remodeling phases [2]. During the post-neurosurgical procedure, there is an exposure of the skull, dura mater, pia, arachnoid villi, sub-endothelium, and collagen. Collagen and material factors do trigger aggregation of the platelet, which results in chemokines release and endothelial developmental features to form fibrin lumping [3]. Neutrophil initially appears at the site of injury. It gets marginalized from the center of blood flow to the periphery. Neutrophil cleans debris and bacteria and provides good homeostasis for wound healing. Macrophage phagocytoses the bacteria and damaged tissue [4]. The inflammation phase does not usually exceed 96 hours.
The accretion of cells and tissues is considered to be the common factor that characterizes the proliferative stage. The post-neurosurgical wound at this phase includes keratinocytes, endothelial cells, and fibroblasts. A granulation tissue is formed, which is composed of extracellular matrix (ECM) and which replaces the fibrin clot. The ECM is formed from collagen, elastin, and proteoglycans [5]. There are many developmental factors and cytokines that participate in this phase, converting development factor-beta, vascular endothelial factor developmental, and interleukins (IL), which facilitates the angiogenesis process. This phase continues until the sixth week [6].
The makeover phase is the last stage in post-neurosurgical wound healing. It requires a good balance between the creation of new cells and apoptosis. Immature type III collagen, gradual degradation of ECM, and developed type I collagen are dangerous in this stage, which lasts for a considerably longer time [6,7]. Any deficiency in anti-oxidants, trace minerals, vitamins, and microelements impairs the lesion-healing process at this stage. While there have been a few comprehensive studies on monitoring and treatment of post-neurosurgical wound healing, this study aims to focus on the process of post-neurosurgical wound healing, monitoring, and how deficiencies in vitamins, antioxidants, trace minerals, and micronutrients play a vital role in compounding the problem. A huge part of this article will be dedicated to the role vitamins, anti-oxidants, trace nutrients, and micronutrients play in post-neurosurgical wound healing.
Review
Our research methods involved reviewing and analyzing information available in medical journals about wound healing. The information gathered from healthcare journals was sorted out to develop a proper understanding of wound healing, including pathophysiology, biochemistry, and scientific analysis of wound healing. We hope to fill some of the gaps that exist in our understanding of wound healing and identify and address more gaps.
Our review and analysis of the journals have led us to conclude that deficiency of zinc is a prominent factor in delayed wound healing after neurological surgery [8]. We have sought to emphasize that many post-surgical wounds are not healing promptly due to zinc deficiency. Neurosurgeons around the world do not fully understand the wound-healing process and it has been neglected for far too long. Several published studies have clearly illustrated that zinc is a vital micronutrient for post-neurosurgical wound healing.
The pathophysiology of zinc in wound healing
The essential micronutrient zinc plays a major role in wound healing. The human body contains less than 50 mg/kg of zinc. It is a key factor related to immune function, central nervous system, wound healing, and bone metabolism [8]. Zinc accounts for over 10% of DNA programmed by the human genome (~3,000 DNA/enzyme). Zinc-dependent DNA aids in gene transcription regulation, DNA repair, cell death, physiological processes, extracellular regulation, and antioxidant defense [9-13].
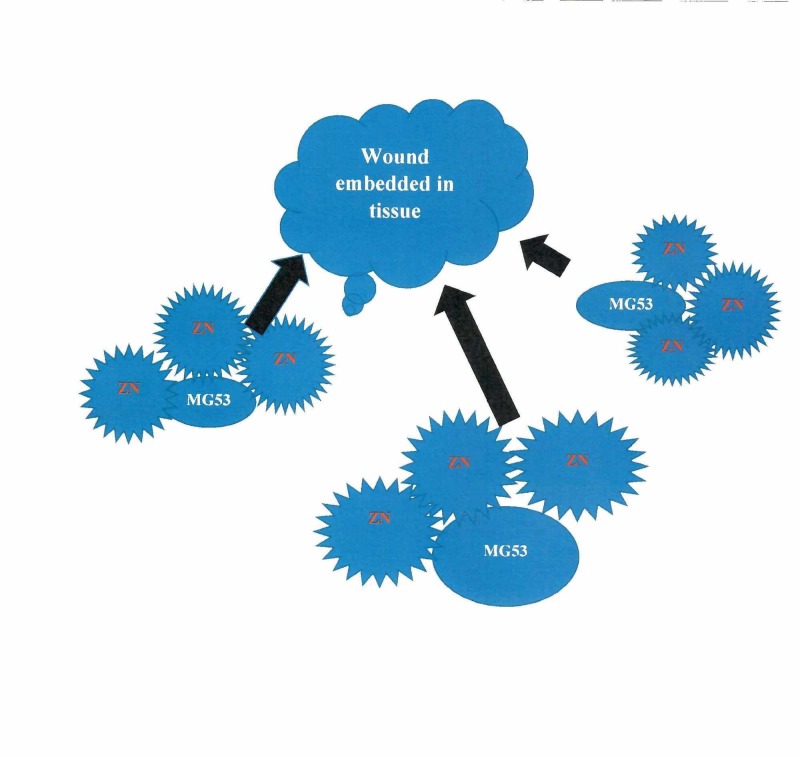
Zinc, a trace mineral, is found to be in low concentration in tissues and across cell membranes in post-neurosurgical wound-healing patients [8]. As such, zinc is firmly regulated through gene transcription rule, ion carriers, cellular homeostasis, and extracellular supplies [14]. During physiological processes, there is a small quantity of zinc in extracellular vesicles. The zinc transporter protein (ZIP) usually takes up zinc in intracellular vesicles [15,16]. Free zinc ions are found in the cytosol and have been identified as secondary messengers that are capable of targeting proteins to regulate numerous chemical and physiological pathways. Therefore, the availability of zinc and its regulation are essential components of cellular physiology [17,18]. The mechanism of action of zinc in wound healing is illustrated below (Figure 1).
Figure 1. The mechanism of zinc in wound healing.
Zn: zinc; MG53: Mitsugumin 53
Zinc deficiency is common in daily food intake. Dietary zinc is kept in human allium by a carrier-mediated process. The majority of zink found within the human body is in skeletal muscle (62%), followed by bone (29%), skin and liver (5%), and various other organs (2-3%) [19]. Due to the multilayered nature of zinc in our diets, the effects are extensive and involve several organ arrangements and matters. Globally, research dating back to 1970 has shown that zinc is a “common plight” to tissues [20]. Malnutrition is a known cause of zinc deficiency, and this has led to dietary problems that can manifest clinically as gastrointestinal (GI) malabsorption syndrome, liver and renal diseases, aging, immune dysfunction, mental and growth retardation, hypogonadism, and impaired healing of wounds [21-28]. Zinc shortage has been blamed for delays in wound healing [29,30]. Zinc deficiency plays a role in inflammation, mainly by elevating inflammatory response as well as causing damage to host tissue [31]. Zinc supplements have been given to post-neurosurgical and severely ill patients, patients with severe burn injury, hypodermic sore, insignificant surgery, and pressure ulcer [32-38]. Table 1 lays out statistical data, which have been discussed in other research studies, about the role zinc plays in wound healing.
Table 1. Studies that discuss zinc's role in wound healing.
| Number of studies | Author name | Year of publication | Country of origin of study | Findings |
| 1 | Cereda E, et al. [39] | 2009 | Italy | There was a significant reduction in the size of ulcers after 84 days of supplementation of zinc, arginine, anti-oxidants, and high protein formula ( 8-20mg zinc daily). |
| 6 | Wilkinson EA, et al. [9] | 2012 | UK | Zinc oxide paste-medicated dressing with a concentration between 6-15% for chronic venous leg ulcer improved wound healing. |
| 1 | Sakae K, et al. [40] | 2013 | Japan | A study of 42 patients with ulcers treated with zinc-containing polaprezinc versus oral L-carnosine (at 34 mg per day) showed no difference in healing. |
| 1 | Attia EA, et al. [10] | 2014 | Egypt | Ninety non-diabetics patients with uncomplicated wounds treated with 0.2 mg/100 mL per 10cm2 of zinc chloride solution reported significant improvement in wound healing. |
The biochemistry of zinc in wound healing
Tripartite motif family (TRIM) proteins and an N-terminal ring zinc finger domain play important biochemical roles in regulating biochemical processes associated with wound healing and normal physiological processes. TRIM protein, Mitsugumin 53, and TRIM72 are implicated in tissue repair after injury. Vascular endothelial and transforming growth factors facilitate wound healing, and these growth factors require zinc for normal physiological functions [41,42]. The significant development of homeostasis is rapidly achieved when micronutrients are in the right proportions in the serum.
Economic implications
When wounds do not heal promptly, there is an increase in hospital visits; and it increases the burden on our healthcare insurance industry. Many patients become devastated and it affects their quality of life and economic prospects. Increased awareness about zinc supplements' ability to help heal wounds faster will expedite the treatment process and help reduce the occurrence of hospital visits after neurosurgical procedures.
Clinical implications
There is substantial evidence that without zinc a post-neurosurgical wound will take more time to heal. This touches on the value we place on ensuring patients a decent quality of life after surgery. Non-usage of zinc increases patient visits to the clinic and may lead to financial and mental distress. It will be beneficial for neurosurgeons to check the levels of antioxidants and zinc before surgery to help curb hospital re-admissions.
Scientific analysis
Of the many studies read, reviewed, and analyzed, the article by Pei-Hui Lin et al was a prominent source of reliable information because it presented detailed facts and scientific evidence to substantiate that zinc is critical to wound healing [8]. Neurosurgeons should be encouraged to use topical zinc ointment more often to help wounds heal faster.
Unanswered questions
Despite overwhelming evidence that delays in wound healing can be prominently caused by trace elements like zinc, other anti-oxidants like Vitamin A, C, and E have been implicated as well [43-46]. Many issues still remain unaddressed pertaining to the subject under review, such as the prevalence of inordinate delay in wound healing and the absence of reliable data on the economic burden it places on society in general and insurance industry in particular. There is insufficient scientific and statistical data involving the general population and sample size with regard to the role zinc plays in wound healing.
Conclusions
Delayed wound healing after surgery has been the frontline worry for neurosurgeons in recent times. Whiles other anti-oxidants and trace elements like zinc are heavily implicated, zinc supplementation has proven to be an overwhelming success in managing this condition. Further studies are needed under rigorous conditions to substantiate the role zinc plays in wound healing after neurosurgical procedures.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Biology and biomarkers for wound healing. Lindley LE, Stojadinovic O, Pastar I, Tomic-Canic M. Plast Reconstr Surg. 2016;138:18–28. doi: 10.1097/PRS.0000000000002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Mol Med. 2011;17:113–125. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Berman B, Maderal A, Raphael B. Dermatol Surg. 2017;43:0–18. doi: 10.1097/DSS.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 4.Nonsteroidal anti-inflammatory drugs for wounds: pain relief or excessive scar formation? Su WH, Cheng MH, Lee WL, Tsou TS, Chang WH, Chen CS, Wang PH. Media Inflamm. 2010;2010:413238. doi: 10.1155/2010/413238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regeneration of fat cells from myofibroblasts during wound healing. Plikus MV, Guerrero-Juarez CF, Ito M, et al. Science. 2017;355:748–752. doi: 10.1126/science.aai8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesenchymal stem cell in wound healing and regeneration. Tsai HW, Wang PH, Tsui KH. J Chin Med Assoc. 2018;81:223–224. doi: 10.1016/j.jcma.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Zinc and its importance for human health: an integrative review. Roohani N, Hurrell R, Kelishadi R, Schulin R. https://www.ncbi.nlm.nih.gov/pubmed/23914218. J Res Med Sci. 2013;18:144–157. [PMC free article] [PubMed] [Google Scholar]
- 8.Zinc in wound healing modulation. [Jan;2020 ];Lin PH, Sermersheim M, Li H, Lee PHU, Steinberg SM, Ma J. https://www.ncbi.nlm.nih.gov/pubmed/29295546. Nutrients. 2017 10:0. doi: 10.3390/nu10010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oral zinc for arterial and venous leg ulcers. [Jan;2020 ];Wilkinson EA. https://www.ncbi.nlm.nih.gov/pubmed/22895918. Cochrane Database Syst Rev. 2012 8:0. doi: 10.1002/14651858.CD001273.pub2. [DOI] [PubMed] [Google Scholar]
- 10.A pilot trial using topical regular crystalline insulin vs. aqueous zinc solution for uncomplicated cutaneous wound healing; impact on quality of life. [Dec;2019 ];Attia EA, Belal DM, El Samahy MH, El Hamamsy MH. Wound Repair Regen. 2014 22:52–57. doi: 10.1111/wrr.12122. [DOI] [PubMed] [Google Scholar]
- 11.Disease-specific, versus, standard, nutritional support for the treatment of pressure ulcer in institutionalized older adults: a randomized controlled trial. [Dec;2019 ];Cereda E, Gini A, Pedrolli C, Vanotti A. J Am Geriatr Soc. 2009 57:1395–1402. doi: 10.1111/j.1532-5415.2009.02351.x. [DOI] [PubMed] [Google Scholar]
- 12.Structure of human MDM2 complexed with RPL11 reveals the molecular basis of p53 activation. Zheng J, Lang Y, Zhang Q, et al. Genes Dev. 2015;29:1524–1534. doi: 10.1101/gad.261792.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ZNF224, Krüppel like zinc finger protein, induces cell growth and apoptosis-resistance by down-regulation of p21 and p53 via miR-663a. [Jan;2020 ];Cho JG, Park S, Lim CH, et al. https://www.ncbi.nlm.nih.gov/pubmed/27105517. Oncotarget. 2016 7:31177–31190. doi: 10.18632/oncotarget.8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Synthesis and structure of [Zn(OMe)(L)] x Zn(OH)(L)] x 2(BPh4), L = cis,cis-1,3,5-tris[(E,E)-3-(2-furyl)acrylideneamino]cyclohexane: structural models of carbonic anhydrase and liver alcohol dehydrogenase. Cronin L, Walton PH. Chem Commun (Camb) 2003;13:1572–1573. doi: 10.1039/b302895j. [DOI] [PubMed] [Google Scholar]
- 15.Three matrix metalloproteinases are required in vivo for macrophage migration during embryonic development. Tomlinson ML, Garcia-Morales C, Abu-Elmagd M, Wheeler GN. Mech Dev. 2008;125:1059–1070. doi: 10.1016/j.mod.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 16.The alteration in Cu/Zn superoxide dismutase and adhesion molecules concentrations in diabetic patients with chronic kidney disease: the effect of dialysis treatment. Pawlak K, Mysliwiec M, Pawlak D. Diabetes Res Clin Pract. 2012;98:264–270. doi: 10.1016/j.diabres.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Zinc’ing sensibly: controlling zinc homeostasis at the transcriptional level. Choi S, Bird AJ. Metallomics. 2014;6:1198–1215. doi: 10.1039/c4mt00064a. [DOI] [PubMed] [Google Scholar]
- 18.Cytosolic zinc buffering and muffling: their role in intracellular zinc homeostasis. Colvin RA, Holmes WR, Fontaine CP, Maret W. Metallomics. 2010;2:306–317. doi: 10.1039/b926662c. [DOI] [PubMed] [Google Scholar]
- 19.Zinc transporters and signaling in physiology and pathogenesis. Hojyo S, Fukada T. Arch Biochem Biophys. 2016;611:43–50. doi: 10.1016/j.abb.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 20.The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Kambe T, Tsuji T, Hashimoto A, Itsumura N. Physiol Rev. 2015;95:749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- 21.Free zinc ions outside a narrow concentration range are toxic to a variety of cells in vitro. Bozeman RA, Chimienti F, Giblin LJ, et al. Exp Biol Med (Maywood) 2010;235:741–750. doi: 10.1258/ebm.2010.009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Structural insights of ZIP4 extracellular domain critical for optimal zinc transport. [Jan;2020 ];Zhang T, Sui D, Hu J. https://www.ncbi.nlm.nih.gov/pubmed/27321477. Nat Commun. 2016 7:11979. doi: 10.1038/ncomms11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stable-isotope dilution measurement of zinc and lead in rat hippocampus and spinal cord. Frederickson CJ, Manton WI, Frederickson MH, Howell GA, Mallory MA. Brain Res. 1982;246:338–341. doi: 10.1016/0006-8993(82)91188-x. [DOI] [PubMed] [Google Scholar]
- 24.Zinc-containing neurons. Frederickson CJ, Moncrieff DW. Biol Signals. 1994;3:127–139. doi: 10.1159/000109536. [DOI] [PubMed] [Google Scholar]
- 25.Competition for zinc among serum albumin and amino acids. Giroux EL, Henkin RI. Biochim Biophys Acta. 1972;273:64–72. doi: 10.1016/0304-4165(72)90191-2. [DOI] [PubMed] [Google Scholar]
- 26.The effects of insulin on the electroretinogram of the bovine retina in vitro. Gosbell A, Favilla I, Jablonski P. Curr Eye Res. 1996;15:1132–1137. doi: 10.3109/02713689608995145. [DOI] [PubMed] [Google Scholar]
- 27.Zinc deficiency in man: its origins and effects. Hambidge KM. Philos Trans R Soc Lond B Biol Sci. 1981;294:129–144. doi: 10.1098/rstb.1981.0094. [DOI] [PubMed] [Google Scholar]
- 28.Zinc homeostasis and gut function in children with celiac disease. Tran CD, Katsikeros R, Manton N, Krebs NF, Hambidge KM, Butler RN, Davidson GP. Am. J. Clin. Nutr. 2011;94:1026–1032. doi: 10.3945/ajcn.111.018093. [DOI] [PubMed] [Google Scholar]
- 29.Low plasma zinc is associated with higher mitochondrial oxidative stress and faster liver fibrosis development in the Miami Adult Studies in HIV cohort. Martinez SS, Campa A, Li Y, Fleetwood C, Stewart T, Ramamoorthy V, Baum MK. J Nutr. 2017;147:556–562. doi: 10.3945/jn.116.243832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Effects of zinc supplementation on antioxidant enzyme activities in healthy old subjects. Mariani E, Mangialasche F, Feliziani FT, et al. Exp Gerontol. 2008;43:445–451. doi: 10.1016/j.exger.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Zinc in infection and inflammation. [Jan;2020 ];Gammon NZ, Rink L. https://www.ncbi.nlm.nih.gov/pubmed/28629136. Nutrients. 2017 9:0. doi: 10.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Severe infant rash resistant to therapy due to zinc deficiency. Mohammed J, Mehrotra S, Schulz H, Lim R. Pediatr Emerg Care. 2017;33:582–584. doi: 10.1097/PEC.0000000000001218. [DOI] [PubMed] [Google Scholar]
- 33.Behavioral impairments in animal models for zinc deficiency. [Jan;2020 ];Hagmeyer S, Haderspeck JC, Grabrucker AM. https://www.ncbi.nlm.nih.gov/pubmed/25610379. Front Behav Neurosci. 201 8:443. doi: 10.3389/fnbeh.2014.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Autosomal-recessive intellectual disability with cerebellar atrophy syndrome caused by mutation of the manganese and zinc transporter gene SLC39A8. Boycott KM, Beaulieu CL, Kernohan KD, et al. Am J Hum Genet. 2015;97:886–893. doi: 10.1016/j.ajhg.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Analysis of the relationship between the blood concentration of several metals, macro- and micronutrients and endocrine disorders associated with male aging. Rotter I, Kosik-Bogacka DI, Dolegowska B, Safranow K, Kuczyńska M, Laszczyńska M. Environ Geochem Health. 2016;38:749–761. doi: 10.1007/s10653-015-9758-0. [DOI] [PubMed] [Google Scholar]
- 36.Low serum zinc level as a predictive factor of delayed wound healing in total hip replacement. Zorrilla P, Gómez LA, Salido JA, Silva A, López-Alonso A. Wound Repair Regen. 2006;14:119–122. doi: 10.1111/j.1743-6109.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- 37.Zinc in wound healing: theoretical, experimental, and clinical aspects. Lansdown AB, Mirastschijski U, Stubbs N, Scanlon E, Agren MS. Wound Repair Regen. 2007;15:2–16. doi: 10.1111/j.1524-475X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 38.A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. Am J Hum Genet. 2002;71:66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Disease-specific, versus, standard, nutritional support for the treatment of pressure ulcer in institutionalized older adults: a randomized controlled trial. Cerada E, Gini A, Pedrolli C, Vanotti A. J Am Geriatr Soc. 2009;57:1395–1402. doi: 10.1111/j.1532-5415.2009.02351.x. [DOI] [PubMed] [Google Scholar]
- 40.Effects of L-carnosine and its zinc complex (Polaprezinc) on pressure ulcer healing. Sakae K, Agata T, Kamide R, Yanagisawa H. Nutr. Clin. Pract. 2013;28:609–616. doi: 10.1177/0884533613493333. [DOI] [PubMed] [Google Scholar]
- 41.Acrodermatitis enteropathica: case report and review of the literature. Perafán-Riveros C, França LF, Alves AC, Sanches JA Jr. Pediatr Dermatol. 2002;19:426–431. doi: 10.1046/j.1525-1470.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 42.Zinc and wound healing: a review of zinc physiology and clinical applications. Kogan S, Sood A, Garnick MS. https://europepmc.org/article/med/28448263. Wounds. 2017;29:102–106. [PubMed] [Google Scholar]
- 43.A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Besecker BY, Exline MC, Hollyfield J, Phillips G, Disilvestro RA, Wewers MD, Knoell DL. Am J Clin Nutr. 2011;93:1356–1364. doi: 10.3945/ajcn.110.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The role of antioxidant micronutrients in the rate of recovery of burn patients: a systematic review. Adjepong M, Agbenorku P, Brown P, Oduro I. Burns Trauma. 2016;4:18. doi: 10.1186/s41038-016-0044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heavy metal in the intensive care unit: a review of current literature on trace element supplementation in critically ill patients. Rech M, To L, Tobin A, Smoot T, Mlynarek M. Nutr Clin Pract. 2014;29:78–89. doi: 10.1177/0884533613515724. [DOI] [PubMed] [Google Scholar]
- 46.Trace element supplementation following severe burn injury: a systematic review and meta-analysis. Kurmis R, Greenwood J, Aromataris E. J Burn Care Res. 2016;37:143–159. doi: 10.1097/BCR.0000000000000259. [DOI] [PubMed] [Google Scholar]