Abstract
Recent studies have shown that colorectal serrated lesions, which include sessile serrated adenomas (SSAs) and traditional serrated adenomas (TSAs), are precursors of colorectal cancer. However, the molecular mechanisms underlying the carcinogenesis, particularly in TSAs, remain largely uncharacterized. To clarify their molecular and clinicopathological characteristics, we performed mutation and methylation analyses of cancer-associated genes in 78 serrated lesions, including TSAs, SSAs and microvesicular hyperplastic polyps. Target exon sequence analysis was performed with 39 genes, including genes known to be frequently mutated in colorectal cancers and/or serrated lesions. We also used bisulfite pyrosequencing to assess the methylation status of various cancer-associated genes and marker genes of the CpG island methylator phenotype (CIMP). The prevalence of mutations in genes associated with Wnt signaling was significantly higher in TSAs than SSAs (65% vs. 28%, p < 0.01). Among those, RNF43 mutations were observed in 38% of TSAs and 17% of SSAs. In immunohistochemical studies of 39 serrated lesions, the prevalence of abnormal nuclear β-catenin accumulation was significantly higher in TSAs (57%) than SSAs (8%) (P = 0.01). SMOC1 methylation was detected in 54% of TSAs but in no SSAs (p < 0.01). Additionally, SMOC1 methylation was more prevalent among TSAs with KRAS mutation (82%) than with BRAF mutation (38%, p = 0.03). Lesions with CIMP-high or RNF43 mutations were detected only in TSAs with BRAF mutation, suggesting two distinct carcinogenic pathways in TSAs. Mutations in genes associated with Wnt signaling play a greater role in the carcinogenesis of TSAs than SSAs.
Introduction
Colorectal cancer (CRC) is a major cause of cancer-related death worldwide. Recent molecular pathological studies, including The Cancer Genome Atlas project, have shown that CRCs are heterogeneous diseases that arise via different molecular pathways [1,2]. Most (80–85%) sporadic CRCs are classified as non-hypermutated or microsatellite stable (MSS) tumors and develop through accumulation of multiple genetic and epigenetic alterations [1,2], including mutation of oncogenes and tumor suppressor genes, as well as chromosomal instability [3,4]. The remaining 15–20% of sporadic CRCs are classified as hypermutated tumors and mainly exhibit microsatellite instability (MSI) and concurrent hypermethylation in multiple loci [2], which is referred to as the CpG island methylator phenotype (CIMP) and is closely associated with BRAF mutation [5–7].
Recent studies of the molecular and clinicopathological characteristics of colorectal premalignant lesions have provided insight into the pathogenesis of CRCs as well as clues to prevention and treatment [8–10]. Since establishing the pathological classification of serrated colorectal lesions as hyperplastic polyps, traditional serrated adenomas (TSAs) or sessile serrated adenomas (SSAs) [11–13], a number of studies have demonstrated that SSAs are associated with BRAF mutation and CIMP, and that they are precursors of MSI-positive CRCs, which are frequently located in the proximal colon [6,9,10,14–17]. However, less is known about the biological and clinical characteristics of TSAs, although they are also considered to be premalignant lesions and reportedly exhibit BRAF or KRAS mutations and aberrant DNA methylation [16–21]. In addition, several recent studies have reported PTPRK-RSPO3 fusion and somatic mutations of RNF43 in TSAs [22–24]. Because only a small number of analyses have investigated gene mutations in serrated lesions [23,25,26], the molecular mechanisms underlying carcinogenesis, especially in TSAs, are still not well characterized. Although it has been suggested based on immunohistochemical studies that dysregulation of the Wnt signaling pathway contributes to carcinogenesis in serrated lesions [20,21,23,27–29], mutation of individual gene within this pathway have not been investigated.
To clarify the molecular and clinicopathological characteristics of colorectal serrated lesions, we assessed the mutation of genes associated with Wnt signaling as well as other genes reportedly mutated in serrated lesions [26] and advanced CRCs [2]. We also investigated mutations in genes associated with oncogene-induced senescence, which have been reported as germline mutations in patients with multiple SSAs [30], and we performed immunohistochemical studies of β-catenin expression to assess activation of the Wnt signaling pathway. Finally, we investigated DNA methylation of cancer-associated genes including SMOC1 as well as CIMP marker genes in TSAs because it was reported that SMOC1 is specifically methylated in TSAs [31].
Materials and methods
Patients and tissue samples
Specimens of colorectal serrated lesions (n = 78) were obtained from 78 Japanese patients who underwent endoscopic mucosal resection at Nagoya City University Hospital, Fukui Prefectural Hospital, or Komatsu Municipal Hospital. This study was approved by the Institutional Review Board at each hospital as well as Kanazawa University and Sapporo Medical University.
Endoscopic analysis
High-resolution magnifying endoscopes (CF260AZI; Olympus, Tokyo, Japan) were used for all colonoscopic examinations. The morphology of colorectal lesions was determined according to the Paris classification [32]. All lesions detected during colonoscopy were observed at high magnification using indigo carmine dye, after which samples were collected through endoscopic mucosal resection for histological analysis. Tumor locations were defined as proximal colon (cecum, ascending colon, transverse colon) or distal colon (descending colon, sigmoid colon, and rectum).
Histological analysis
Histological diagnosis of tumors was done at each facility, after which the histological findings for all specimens were reviewed by a board certified pathologist (Sugai T) who was blinded to the clinical and molecular information. Serrated lesions, including microvesicular hyperplastic polyps (MVHPs), SSAs and TSAs, were classified according to WHO classification criteria [33]. Mixed serrated lesion composed of TSA and tubulovillous adenoma was classified as TSA in our analyses. The clinicopathological features of the lesions are summarized in Table 1.
Table 1. Clinicopathological features of the serrated lesions in this study.
| Patients (n = 78) | |
| Age (y, mean ± SD) | 65.3 ± 10.9 |
| Sex, n (%) | |
| Male | 51 (65) |
| Female | 27 (35) |
| Lesions (n = 78) | |
| Location, n (%) | |
| Proximal | 42 (54) |
| Distal | 36 (46) |
| Bowel subsites, n (%) | |
| Cecum | 13 (17) |
| Ascending colon | 22 (28) |
| Transverse colon | 7 (9) |
| Descending colon | 5 (6) |
| Sigmoid colon | 20 (26) |
| Rectum | 11 (14) |
| Morphology, n (%) | |
| 0-Ip | 15 (19) |
| 0-Is | 47 (60) |
| 0-IIa | 16 (21) |
| Histology, n (%) | |
| MVHP | 23 (30) |
| TSA | 36 (46) |
| TSA + TVA | 1 (1) |
| SSA | 18 (23) |
MVHP, microvesicular hyperplastic polyp; TSA, traditional serrated adenoma; TVA, tubulovillous adenoma; SSA, sessile serrated adenoma.
DNA preparation
DNA was isolated from formalin-fixed, paraffin-embedded (FFPE) tissue sections using a QIAamp DNA FFPE Tissue kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. A TaqMan RNase P Detection Reagents kit (Thermo Fisher Scientific, Waltham, MA) was used to quantify the purified DNA.
Semiconductor-based next-generation sequencing
A customized panel, encompassing all the exons of 39 cancer-related genes, including genes frequently reported to be involved in advanced CRCs and serrated lesions [2,25,26], was created using the Ion Torrent System with an Ion AmpliSeq Designer (Thermo Fisher Scientific) (S1 Fig). Genes within oncogene-induced senescence pathways detected in patients with multiple serrated polyps were also investigated [30]. The assay design consisted of 1,455 amplicons ranging from 125 to 175 bp in length, covering 94% of the 112.8 kb target sequence.
Library preparation and sequencing with the Ion Torrent sequencer were performed as previously described [34–36]. The templates were sequenced after emulsion PCR was performed with 24 samples per Ion PI chip using the Ion PI HI-Q Chef kit (Thermo Fisher Scientific).
Identification of somatic mutations and copy number variations (CNVs)
Human genome build 19 (hg19) was used as a reference. Signal processing, mapping to the hg19 reference, and quality control were performed using Torrent Suite version 5.0 (Thermo Fisher Scientific). Somatic mutations (point mutations, insertions, and deletions) were detected using Ion Reporter Software 5.0 (Thermo Fisher Scientific). Because matched normal controls were not available, the control sequence data provided by Thermo Fisher Scientific were used as a control. Pathogenic status of the variant was stated if it was a missense variant with < 0.1% global minor allele frequency in dbSNP or the 1000 Genomes Project database and/or the variant was registered as pathogenic in ClinVar or COSMIC databases. Variants with allele frequencies between 0.4 and 0.6 or > 0.9 were considered germline variants unless listed as a pathogenic variant. Additionally, if the same variants were detected in multiple samples, these variants were considered germline variants unless occurring at a known hotspot variant in databases. Integrative Genomics Viewer (IGV) software (http://software.broadinstitute.org/software/igv/) was used to filter out possible strand-specific errors, such as a mutation that was detected in the forward or reverse DNA strand but not in both strands. CNV detection was also performed with the Ion Reporter Software using an algorithm based on the Hidden Markov Model. Recurrent genomic regions with CNVs were identified using copy numbers greater than 3 and less than 1 for gains and losses, respectively.
DNA methylation analysis
DNA methylation was analyzed using bisulfite pyrosequencing as described previously [37,38]. Briefly, genomic DNA (1 μg) was modified with sodium bisulfite using an EpiTect Bisulfite kit (Qiagen). Pyrosequencing was then carried out using a PSQ 96MA system (Qiagen) with a Pyro Gold Reagent kit (Qiagen), and the results were analyzed using Pyro Q-CpG software (Qiagen). A cutoff value of 15% was used to define genes as methylation-positive. Using five classic CIMP markers (MINT1, MINT2, MINT12, MINT31 and MLH1) and CDKN2A (p16), tumors were defined as CIMP-positive (three or more loci showed methylation) or CIMP-high (CIMP-H, four or more loci showed methylation). Methylation of SMOC1, GALNT14, SFRP1, SFRP2, IGFBP7, SOX5 and long interspersed nucleotide element 1 (LINE-1) was also analyzed using bisulfite pyrosequencing. The primer sequences used were as previously reported [9,31,39].
Immunohistochemistry
Immunohistochemical studies of β-catenin expression were performed as previously described with 39 serrated lesions, including 14 TSAs, 13 SSAs and 12 MVHPs [40]. A mouse anti-β-catenin monoclonal antibody (1:1000 dilution, Clone 14; BD Biosciences, San Jose, CA) was used. β-catenin expression was semi-quantitatively evaluated in tumor cells with β-catenin-positive nuclei, and positive nuclear accumulation was defined as staining of more than 10% of tumor cell nuclei throughout the lesions, as reported previously [40]. All slides were evaluated by two independent pathologists (YK and TM) who were blinded to the clinical and molecular data.
Statistical analysis
Continuous data were analyzed using t-tests (for two groups) or ANOVA with a post hoc Tukey’s HSD test (for more than two groups). Fisher’s exact test and logistic regression were used to assess the association between categorical variables. Values of P < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS 20 (IBM Corporation, Somers, NY) and GraphPad Prism 6 (GraphPad Software, La Jolla, CA).
Results
Clinicopathological characteristics of serrated lesions
The clinicopathological and molecular characteristics of the colorectal serrated lesions analyzed in this study are summarized in Tables 1 and 2. The majority of MVHPs (17/23, 74%) and SSAs (15/18, 83%) were located in the proximal colon, from the cecum to the transverse colon, while TSAs were more prevalent in the distal colon (27/37, 73%), especially the sigmoid colon and rectum (14 cases and 11 cases, respectively). On endoscopic observation, more than one-third (14/37, 38%) of TSAs were protruding, pedunculated (0-Ip) lesions and differed significantly from SSAs, most of which were protruding, sessile (0-Is) lesions (16/18, 88%) (Table 2).
Table 2. Clinicopathological and molecular characteristics of the respective serrated lesion.
| MVHP | SSA | TSA | p value (SSA vs TSA) | |
|---|---|---|---|---|
| No. of cases | 23 | 18 | 37 | |
| Sex (male/female) | 16/7 | 9/9 | 26/11 | 0.24 |
| Age (y, mean ± SD) | 66 ± 9.1 | 63.3 ± 10.6 | 65.8 ± 11.8 | 0.44 |
| Tumor location, n (bowel subsite) | ||||
| Proximal (C/A/T) | 17 (6/10/1) | 15 (6/5/4) | 10 (1/7/2) | <0.01 |
| Distal (D/S/R) | 6 (1/5/0) | 3 (2/1/0) | 27 (2/14/11) | |
| Tumor size (mm, mean ± SD) | 11.3 ± 7.0 | 11.2 ± 4.7 | 12.2 ± 5.4 | 0.5 |
| Morphology, n (%) | ||||
| 0-Ip | 0 (0) | 1 (6) | 14 (38) | 0.01 |
| 0-Is | 13 (57) | 16 (88) | 18 (49) | |
| 0-IIa | 10 (43) | 1 (6) | 5 (13) | |
| Gene mutation/epigenetic alteration, n (%) | ||||
| BRAF V600E mutation | 14 (61) | 14 (78) | 24 (65) | 0.37 |
| KRAS mutation | 4 (17) | 1 (6) | 11 (30) | 0.08 |
| RNF43 mutation | 3 (13) | 3 (17) | 14 (38) | 0.13 |
| APC mutation | 1 (4) | 0 (0) | 7 (19) | 0.08 |
| WNT signaling associated genes | 9 (39) | 5 (28) | 24 (65) | <0.01 |
| CIMP | 9 (39) | 8 (44) | 16 (43) | 1 |
| CIMP-high | 5 (22) | 4 (22) | 10 (27) | 1 |
| SMOC1 methylation | 0 (0) | 0 (0) | 20 (54) | <0.01 |
MVHP, microvesicular hyperplastic polyp; SSA, sessile serrated adenoma; TSA, traditional serrated adenoma; C, cecum; A, ascending colon; T, transverse colon; D, descending colon; S, sigmoid colon; R, rectum; CIMP, CpG island methylator phenotype
Targeted amplicon sequencing of colorectal serrated lesions
We performed semiconductor sequencing of all exons in 39 cancer-related genes that were previously detected in 78 colorectal serrated lesions and advanced CRCs. The sequencing overview, including reads, coverage, and uniformity of the read coverage distribution, is shown in S1 Table. Each FFPE sample underwent an average of 2.7 million sequencing reads after quality filtering. A mean coverage depth of 1722.5 reads (100.2–5126.0) per base was observed.
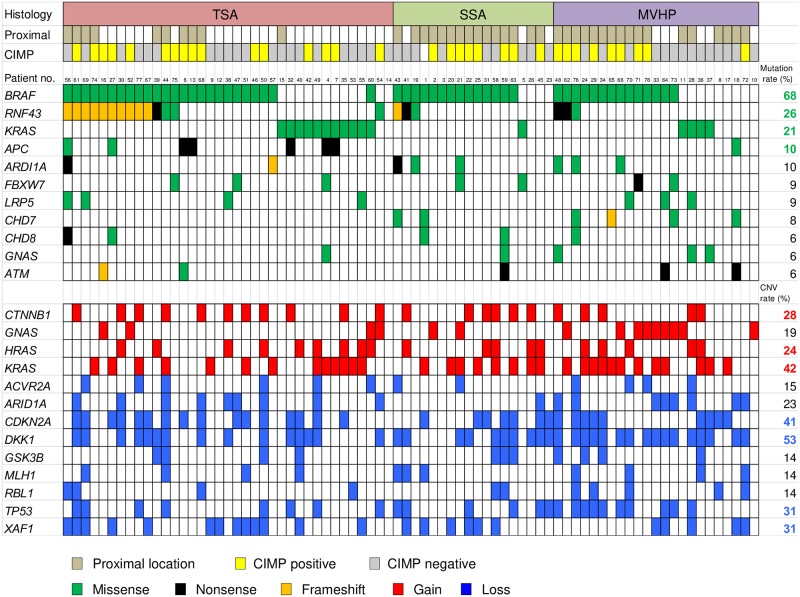
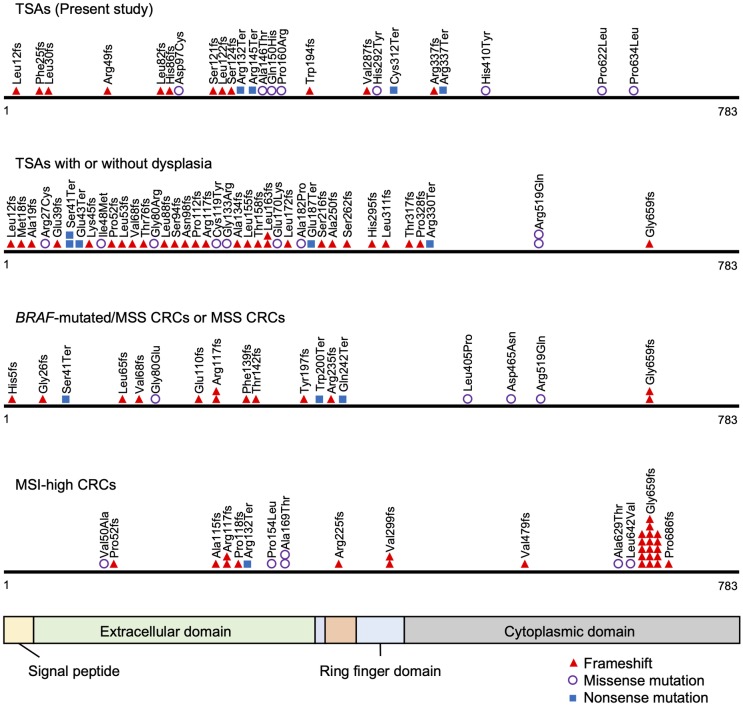
All single nucleotide variants (SNVs) and insertions and deletions (InDels) detected through bioinformatics analysis underwent visual inspection using the IGV for confirmation. We identified a mean of 2.4 somatic nonsynonymous mutations (range 0–13) per sample (S2 Table). The 11 most commonly mutated genes in serrated lesions are depicted in Fig 1. BRAF was the most frequently mutated gene in serrated lesions (68%, 53 of 78 cases), followed by RNF43 (26%), KRAS (21%), and APC (10%). BRAF V600E mutations were detected in 52 samples, and an N581S mutation was detected in 1 sample accompanied by a KRAS Q61H mutation (Patient 60 in Fig 1). The BRAF V600E mutation and KRAS mutation appeared to be mutually exclusive, as they were never detected in the same sample (Fig 1). RNF43 mutations were detected in 38% of TSAs and 17% of SSAs. Nonsense or frameshift mutations in RNF43 were seen in 30% of TSAs and 11% of SSAs. Most of the mutations were located upstream of the ring finger domain of RNF43 (Fig 2). We also found APC mutations in 7 of 37 TSAs (19%), but none in SSAs. Protein-truncating (nonsense and frameshift) mutations of APC were detected in 14% of TSAs (Fig 1). Overall, the frequency of mutations in Wnt pathway components was significantly higher in TSAs than in SSAs (65% vs. 28%, P < 0.01) (Fig 3, Table 2). In addition, a GNAS R201H mutation was found in 1 TSA, while 1 SSA and 3 MVHPs harbored a GNAS V334G mutation, though the biological significance of the latter is unknown (Fig 3A, S2 Table).
Fig 1. Summary of somatic mutations and CNVs across 78 serrated lesions.
Profiles of gene mutations and CNVs within individual samples are grouped with respect to histological types. In the upper panel, the top row indicates the histology, the second row indicates the tumor location, and the third row indicates the CIMP status. Columns correspond to the individual cases. In the middle panels, frequently mutated genes, colored to indicate the type of mutation, and their mutational frequency are shown. In the lower panels, CNVs frequently detected in colorectal serrated lesions are shown.
Fig 2. Distributions of RNF43 mutations in detected in TSAs in the present study.
Previously reported TSA mutations with or without dysplasia as well as MSS and MSI-high CRCs are shown for reference [23,24,41].
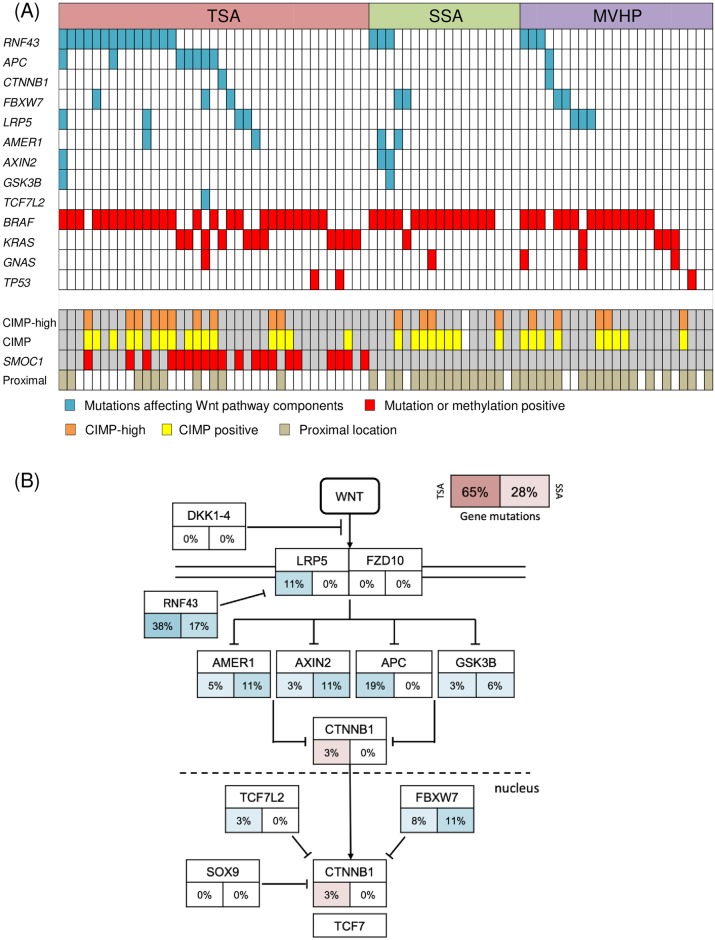
Fig 3. Gene mutations leading to dysregulation of Wnt signaling pathway in serrated lesions.
(A) Representative mutation and methylation profile across 78 serrated lesions. Blue coloration indicates gene mutations affecting Wnt pathway components, while red coloration indicates positivity for gene mutations or methylation. (B) Frequencies of gene mutations leading to dysregulation of Wnt signaling in TSAs and SSAs. Alteration frequencies are expressed as percentages of all cases. Frequencies of gene mutations in TSAs are shown on the left, while those in SSAs are shown on the right. Red denotes activated genes and blue denotes inactivated genes.
Target amplicon sequencing detects CNVs
We also detected CNVs in segments of the genome that could be duplicated or deleted from the sequencing data (Fig 1). In all samples, the genes most frequently affected by copy number gains were KRAS (42%), CTNNB1 (28%), and HRAS (24%), while the genes most frequently affected by copy number losses were DKK1 (53%), CDKN2A (41%), TP53 (31%), and XAF1 (31%). In addition, copy number loss at the APC gene locus was seen in one TSA (S2 Fig). In total, at least one CNV (loss or gain) affecting a Wnt pathway component was found in 49% of TSAs and 67% of SSAs. When considered together with the gene mutations, at least one genomic abnormality affecting genes associated with Wnt signaling was seen in 84% of TSAs and 78% of SSAs. Among these, the most frequently affected genes associated with Wnt signaling were CTNNB1 (gain at 3p22.1), which encodes β-catenin, and DKK1 (loss at 10q21.1), which encodes Dkk-1, a negative regulator of Wnt signaling (S2 Fig).
Methylation analysis of CIMP markers, cancer-associated genes, and LINE-1
We next assessed methylation of CIMP markers and genes known to be frequently methylated in CRCs (S3 Table) [9]. When comparing TSA and SSA, the prevalence of CIMP statuses (CIMP-positive or CIMP-H) did not significantly differ (Table 2, Fig 3A). Notably, however, SMOC1 methylation was detected in 20 of 37 TSAs (54%) but in none of the 18 SSAs tested (P < 0.01) (Table 2, Fig 3A). By contrast, the frequencies of lesions showing methylation of other cancer-associated genes did not significantly differ between TSAs and SSAs. There were no lesions in which MLH1 methylation was positive in the present study.
We found that the levels of SMOC1, SFRP1 and SFRP2 methylation were significantly higher in TSAs than in SSAs or MVHPs (S3 Fig). In addition, the level of SOX5 methylation was significantly higher in TSAs than in MVHPs. Levels of LINE-1 methylation, which was measured to evaluate global DNA hypomethylation in the lesions, did not correlate with the histological types of serrated lesions (S3 Fig).
Clinicopathological and molecular characteristics of TSAs with KRAS or BRAF mutation
We also compared clinicopathological and molecular characteristics of TSAs with KRAS or BRAF (V600E) mutation. It appears that TSAs with BRAF mutation were more likely to be located in the proximal colon than those with KRAS mutation, though the difference was not statistically significant (P = 0.12) (Table 3). Regarding gene mutations, RNF43 mutation was frequently detected in TSAs with BRAF mutation, but not in TSAs with KRAS mutation (P < 0.01). Lesions with CIMP-H were also found only in TSAs with BRAF mutations (38% vs. 0%, P = 0.03). Although lesions positive for SMOC1 methylation were seen in both groups, SMOC1 methylation was significantly more prevalent among TSAs with KRAS mutations than those with BRAF mutations (82% vs. 38%, P = 0.03) (Table 3). In addition, levels of SFRP1 methylation were significantly higher in TSAs with KRAS mutation than in those with BRAF mutation. On the other hand, levels of IGFBP7 methylation was significantly higher in TSAs with BRAF mutation than those with KRAS mutation (S4 Fig).
Table 3. Clinicopathological and molecular characteristics of the KRAS- and BRAF- mutant TSAs.
| BRAF mutant TSA | KRAS mutant TSA | p value | |
|---|---|---|---|
| No. of cases | 24 | 11 | |
| Sex (male/female) | 16/8 | 9/2 | 0.45 |
| Age (y, mean ± SD) | 65.9 ± 8.7 | 63.1 ± 15.9 | 0.61 |
| Tumor location, n (bowel subsite) | |||
| Proximal (C/A/T) | 9 (1/7/1) | 1 (0/0/1) | 0.12 |
| Distal (D/S/R) | 15 (2/9/4) | 10 (0/4/6) | |
| Tumor size (mm, mean ± SD) | 11.9 ± 4.5 | 12.1 ± 5.9 | 0.92 |
| Morphology, n (%) | |||
| 0-Ip | 10 (42) | 3 (27) | 0.26 |
| 0-Is | 12 (50) | 5 (46) | |
| 0-IIa | 2 (8) | 3 (27) | |
| Gene mutation/epigenetic alteration, n (%) | |||
| RNF43 mutation | 13 (54) | 0 (0) | < 0.01 |
| APC mutation | 4 (17) | 3 (27) | 0.65 |
| WNT signaling associated genes | 17 (71) | 6 (55) | 0.35 |
| CIMP | 12 (50) | 3 (27) | 0.28 |
| CIMP-high | 9 (38) | 0 (0) | 0.03 |
| SMOC1 methylation | 9 (38) | 9 (82) | 0.03 |
TSA, traditional serrated adenoma; C, cecum; A, ascending colon; T, transverse colon; D, descending colon; S, sigmoid colon; R, rectum; CIMP, CpG island methylator phenotype
Immunohistochemistry
Of 39 serrated lesions analyzed, 12 (31%) showed positive nuclear accumulation, while 27 (69%) showed membranous expression of β-catenin. The abnormal nuclear accumulation was observed in 8/14 TSAs (57%), 1/13 (8%) of SSAs, and 3/12 (25%) MVHPs (S4 Table, S5 Fig). When compared with SSAs, the nuclear accumulation of β-catenin was more prevalent in TSAs (57% vs. 8%, P = 0.01). Across all lesions, the prevalence of nuclear β-catenin accumulation was significantly higher in lesions with RNF43 mutations than in those without mutations (83% vs. 21%, P < 0.01). When we focused only on TSAs, the prevalence of nuclear β-catenin accumulation was significantly higher in TSAs with a BRAF V600E mutation than those with KRAS mutation (7/8 vs. 1/5, P = 0.01).
Discussion
In the present study, we used targeted next-generation sequencing to assess the mutation of genes associated with Wnt signaling. We found that these mutations occurred with higher frequency in TSAs than SSAs, which is suggestive of the importance of Wnt signaling in the pathogenesis of TSAs. Sekine et al. reported genetic alterations that included PTPRK-RSPO3 fusions among Wnt pathway components in 71% of TSAs [23]. If PTPRK-RSPO3 fusions had also been investigated in the present study, the frequency of genetic alterations would have been even higher. RNF43 encodes E3 ubiquitin ligase, which negatively regulates Wnt signaling. We detected RNF43 mutations in 38% of TSAs and 17% of SSAs, which is consistent with earlier reports [23,24,26]. Most mutations, especially protein-truncating mutations, were situated upstream of the ring finger domain without clustering, as was shown in previous studies of TSAs with or without dysplasia [23,24]. Two major frameshift mutations (Arg117fs and Gly659fs) have been reported in CRCs with MSI [41], while CRCs with BRAF mutation/MSS had mutation profiles similar to TSAs [24,41]. This finding may indicate that TSAs belong to a carcinogenic pathway that is distinct from the SSA pathway (serrated-neoplasia pathway), making them possible precursors of BRAF mutated/MSS CRCs. We also sequenced the entire region of the APC gene and found protein-truncating mutations in 14% of TSAs, which is consistent with a report from Sekine et al. (13%) [23], though they only investigated frequently mutated regions of the gene. Protein truncating mutations of RNF43 and APC were found to be mutually exclusive, suggesting the importance of both genes to Wnt signaling during carcinogenesis in TSAs. By contrast, the other genes encoding Wnt pathway components, including CTNNB1, FBXW7, LRP5, AMER1, and AXIN2, harbored mutations in only small fractions of tumors.
Previous studies have shown that there is wide variation in the rate of β-catenin positivity in both TSAs [21,23,27–29] and SSAs [29]. We showed in the present study that nuclear β-catenin accumulation is significantly more prevalent in TSAs than SSAs. This is likely associated with a high prevalence of mutations in the Wnt signaling pathway, especially in RNF43. These results may thus support the greater significance of RNF43 mutation and Wnt signaling pathway activation in the carcinogenesis of TSAs than SSAs. Alternatively, it is possible that RNF43 mutation represents subgroups in which Wnt signaling is activated by other genetic or epigenetic mechanisms.
Although an earlier study using a comparative genomic hybridization (CGH) microarray reported that CNVs were found only infrequently in colorectal precursor lesions including serrated lesions [9], CNVs in colorectal serrated lesions have not been thoroughly investigated. The present study demonstrated the frequent occurrence of CNVs at the CTNNB1 and DKK1 loci among Wnt signaling pathway associated genes. In addition, copy number losses at 17p (the TP53 locus) are reportedly associated with progression of tumors from conventional-type adenoma to carcinoma and are frequently found in advanced CRCs [2,3]. This result may indicate the importance of TP53 to carcinogenesis of serrated lesions. Because recent studies suggest that somatic CNVs at oncogenic loci are not always associated with gene expression [2,42,43], validation of the effect of CNVs through comparison with expression data is needed.
The prevalence of CIMP-positive or CIMP-H lesions did not significantly differ between SSAs and TSAs in this study. CIMP was detected in up to 79% of TSAs in previous studies [16,17]. One recent study reported that the prevalence of CIMP-H was significantly higher in SSAs than TSAs [44], which is inconsistent with our results. This difference likely reflects differences in the sample cohort, or may be due to a difference in the CIMP markers analyzed or the method used for methylation analysis (pyrosequencing vs. MethyLight). Interestingly, levels of DNA methylation in SFRP1 and SFRP2 were higher in TSAs than in SSAs or MVHPs. Epigenetic inactivation of SFRP family genes, including SFRP1 and SFRP2, occurs early during CRC progression and enables constitutive Wnt signaling in CRCs [45]. It was recently reported that levels of SFRP1 and SFRP2 transcription correlate inversely with the methylation levels in samples of gastric mucosa, with or without H. pylori infection, as well as background mucosa in gastric cancers [46]. It is possible that SFRP1 and SFRP2 methylation contributes more significantly to carcinogenesis in TSAs than SSAs, although these phenomena may be influenced by the sample cohort, the degree of contamination by non-neoplastic cells, and methodology. We also analyzed the methylation status of SMOC1 in a patient cohort different from the one examined in an earlier study [31]. We found that SMOC1 methylation was highly specific for TSAs, and the methylation level was significantly higher in TSAs than SSAs. Thus, SMOC1 methylation may be a potential marker to distinguish TSAs from other serrated polyps.
Recent reports suggest that TSAs belong to a heterogenous category and develop through at least two different neoplastic progression pathways. It is also suggested that lesions with BRAF mutation and those with KRAS mutation exhibit different clinicopathological and molecular characteristics [18–21]. In the present study, TSAs with BRAF mutation were preferentially located in the proximal colon, while those with KRAS mutation were preferentially located in the distal colon and rectum. In addition, TSAs with BRAF mutation were more likely to be CIMP-positive than those with KRAS mutation. CIMP-H positivity was only detected in TSAs with BRAF mutation, which is consistent with an earlier study [21]. Lesions with RNF43 mutation were also found only in TSAs with BRAF mutation, while the prevalence of SMOC1 methylation was significantly higher in TSAs with KRAS mutation than with BRAF mutation. The level of IGFBP7 methylation is significantly higher in TSAs with BRAF mutation than with KRAS mutation, though the frequency of methylation-positive IGFBP7 cases did not significantly differ between the two groups. IGFBP7 has been shown to play a central role in BRAF-induced senescence and to be a direct target of TP53 [47]. Although methylation of IGFBP7 has been investigated in specific histological types of serrated lesions [10,48], IGFBP7 methylation status has not been compared between these two TSA subtypes. In addition to these differences in genetic and epigenetic alterations, the difference in the prevalence of β-catenin expression further supports there being two different neoplastic pathways for TSAs. Determining whether TSAs with KRAS or BRAF mutation belong to different neoplastic pathways with different malignant potentials will require further clinicopathological and molecular analyses.
The present study has several limitations, including a relatively small sample size, lack of data on fusion genes, and a lack of normal background samples for mutational analysis to rule out single nucleotide polymorphisms. Nonetheless, we were able to make several important observations. First, the mutational status of genes involved in Wnt signaling differs among colorectal serrated polyps, depending on TSA histology, which likely results in the differences in nuclear β-catenin expression. Second, we confirmed that SMOC1 methylation is very specific to TSAs. Third, we detected significant differences in clinicopathological and molecular variables between TSAs with KRAS or BRAF mutation, which may indicate the presence of separate carcinogenic pathways among TSAs. By comparing gene expression data from CRCs and SSAs, an earlier study found that a particular subtype of CRCs with a poor-prognosis developed from serrated lesions [49]. Additionally, previous studies have also shown that there are similarities between the gene expression profiles of SSAs and those of MVHPs and MSI CRCs, which supports the concept of a serrated-neoplasia pathway [50,51]. Comprehensive gene expression studies together with analyses of the genetic and epigenetic alterations in TSAs and comparison of those data with other serrated polyps or CRCs could potentially establish the molecular carcinogenesis pathway in TSAs.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(A, B) SSA showing membranous localization of β-catenin. (C, D) TSA showing nuclear accumulation of β-catenin.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors thank Mutsumi Toyota, Akina Omori, Toshiki Kenuka, Aki Iwata and Shigenori Wakita for their excellent technical assistance; Takahito Katano, Mamoru Tanaka, Hirotaka Nishiwaki, Keiji Ozeki, Hironobu Tsukamoto, Tsutomu Mizoshita, Yoshinori Mori, Hiroyuki Aoyagi, Shigetsugu Tsuji, Masashi Yoshimitsu, Kazuhiro Miwa, Yoshinori Goto and Yutaka Matano for providing samples; and Dr. William F. Goldman for editing the manuscript.
Abbreviations
- A
ascending colon
- C
cecum
- CGH
comparative genomic hybridization
- CIMP
CpG island methylator phenotype
- CIMP-H
CpG island methylator phenotype-high
- CIN
chromosomal instability
- CNV
copy number variation
- CRC
colorectal cancer
- D
descending colon
- FFPE
formalin-fixed, paraffin-embedded
- IGV
Integrative Genomics Viewer
- InDel
insertion and deletion
- LINE
long interspersed nucleotide elements
- MSI
microsatellite instability
- MSS
microsatellite stable
- MVHP
microvesicular hyperplastic polyp
- R
rectum
- S
sigmoid colon
- SNV
single nucleotide variant
- SSA
sessile serrated adenoma
- T
transverse colon
- TSA
traditional serrated adenoma
Data Availability
Sequence data has been deposited at the Japanese Genotype-phenotype Archive (JGA, http://trace.ddbj.nig.ac.jp/jga), which is hosted by the DNA DataBank of Japan (DDBJ), under accession number JGAS00000000217.
Funding Statement
This study was supported in part by JSPS KAKENHI Grant Numbers JP16K09304 (TS), JP17K09374 (SI), and JP19K08367 (RO), Grant-in-Aid from the Japanese Foundation for Research and Promotion of Endoscopy (TS), Extramural Collaborative Research Grant of Cancer Research Institute, Kanazawa University (TS), and a Grant-in-Aid from the Takeda Science Foundation (TS). There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wang W, Kandimalla R, Huang H, Zhu L, Li Y, Gao F, et al. Molecular subtyping of colorectal cancer: Recent progress, new challenges and emerging opportunities. Semin Cancer Biol. 2019;55: 37–52. 10.1016/j.semcancer.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487: 330–337. 10.1038/nature11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61: 759–767. 10.1016/0092-8674(90)90186-i [DOI] [PubMed] [Google Scholar]
- 4.Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135: 1079–1099. 10.1053/j.gastro.2008.07.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;96: 8681–8686. 10.1073/pnas.96.15.8681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kambara T, Simms LA, Whitehall VL, Spring KJ, Wynter CV, Walsh MD, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53: 1137–1144. 10.1136/gut.2003.037671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38: 787–793. 10.1038/ng1834 [DOI] [PubMed] [Google Scholar]
- 8.Lochhead P, Chan AT, Giovannucci E, Fuchs CS, Wu K, Nishihara R, et al. Progress and opportunities in molecular pathological epidemiology of colorectal premalignant lesions. Am J Gastroenterol. 2014;109: 1205–1214. 10.1038/ajg.2014.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto E, Suzuki H, Yamano HO, Maruyama R, Nojima M, Kamimae S, et al. Molecular dissection of premalignant colorectal lesions reveals early onset of the CpG island methylator phenotype. Am J Pathol. 2012;181: 1847–1861. 10.1016/j.ajpath.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 10.Kimura T, Yamamoto E, Yamano HO, Suzuki H, Kamimae S, Nojima M, et al. A novel pit pattern identifies the precursor of colorectal cancer derived from sessile serrated adenoma. Am J Gastroenterol. 2012;107: 460–469. 10.1038/ajg.2011.457 [DOI] [PubMed] [Google Scholar]
- 11.Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990;14: 524–537. 10.1097/00000478-199006000-00003 [DOI] [PubMed] [Google Scholar]
- 12.Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27: 65–81. 10.1097/00000478-200301000-00008 [DOI] [PubMed] [Google Scholar]
- 13.Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP. Serrated polyps of the large intestine: a morphologic and molecular review of an evolving concept. Am J Clin Pathol. 2005;124: 380–391. 10.1309/V2EP-TPLJ-RB3F-GHJL [DOI] [PubMed] [Google Scholar]
- 14.Iino H, Jass JR, Simms LA, Young J, Leggett B, Ajioka Y, et al. DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol. 1999;52: 5–9. 10.1136/jcp.52.1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spring KJ, Zhao ZZ, Karamatic R, Walsh MD, Whitehall VL, Pike T, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131: 1400–1407. 10.1053/j.gastro.2006.08.038 [DOI] [PubMed] [Google Scholar]
- 16.Rosty C, Hewett DG, Brown IS, Leggett BA, Whitehall VL. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol. 2013;48: 287–302. 10.1007/s00535-012-0720-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien MJ, Yang S, Mack C, Xu H, Huang CS, Mulcahy E, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30: 1491–1501. 10.1097/01.pas.0000213313.36306.85 [DOI] [PubMed] [Google Scholar]
- 18.Kim KM, Lee EJ, Kim YH, Chang DK, Odze RD. KRAS mutations in traditional serrated adenomas from Korea herald an aggressive phenotype. Am J Surg Pathol. 2010;34: 667–675. 10.1097/PAS.0b013e3181d40cb2 [DOI] [PubMed] [Google Scholar]
- 19.Kim MJ, Lee EJ, Suh JP, Chun SM, Jang SJ, Kim DS, et al. Traditional serrated adenoma of the colorectum. Clinicopathologic implications and endoscopic findings of the precursor lesions. Am J Clin Pathol. 2013;140: 898–911. 10.1309/AJCPDJC9VC5KTYUS [DOI] [PubMed] [Google Scholar]
- 20.Tsai JH, Liau JY, Lin YL, Lin LI, Cheng YC, Cheng ML, et al. Traditional serrated adenoma has two pathways of neoplastic progression that are distinct from the sessile serrated pathway of colorectal carcinogenesis. Mod Pathol. 2014;27: 1375–1385. 10.1038/modpathol.2014.35 [DOI] [PubMed] [Google Scholar]
- 21.Bettington ML, Walker NI, Rosty C, Brown IS, Clouston AD, McKeone DM, et al. A clinicopathological and molecular analysis of 200 traditional serrated adenomas. Mod Pathol. 2015;28: 414–427. 10.1038/modpathol.2014.122 [DOI] [PubMed] [Google Scholar]
- 22.Yan HHN, Lai JCW, Ho SL, Leung WK, Law WL, Lee JFY, et al. RNF43 germline and somatic mutation in serrated neoplasia pathway and its association with BRAF mutation. Gut. 2017;66: 1645–1656. 10.1136/gutjnl-2016-311849 [DOI] [PubMed] [Google Scholar]
- 23.Sekine S, Yamashita S, Tanabe T, Hashimoto T, Yoshida H, Taniguchi H, et al. Frequent PTPRK-RSPO3 fusions and RNF43 mutations in colorectal traditional serrated adenoma. J Pathol. 2016;239: 133–138. 10.1002/path.4709 [DOI] [PubMed] [Google Scholar]
- 24.Tsai JH, Liau JY, Yuan CT, Lin YL, Tseng LH, Cheng ML, et al. RNF43 is an early and specific mutated gene in the serrated pathway, with increased frequency in traditional serrated adenoma and its associated malignancy. Am J Surg Pathol. 2016;40: 1352–1359. 10.1097/PAS.0000000000000664 [DOI] [PubMed] [Google Scholar]
- 25.Tahara T, Yamamoto E, Madireddi P, Suzuki H, Maruyama R, Chung W, et al. Colorectal carcinomas with CpG island methylator phenotype 1 frequently contain mutations in chromatin regulators. Gastroenterology. 2014;146: 530–538. 10.1053/j.gastro.2013.10.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakai E, Fukuyo M, Ohata K, Matsusaka K, Doi N, Mano Y, et al. Genetic and epigenetic aberrations occurring in colorectal tumors associated with serrated pathway. Int J Cancer. 2016;138: 1634–1644. 10.1002/ijc.29903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiao YF, Nakamura S, Sugai T, Yamada N, Habano W. Serrated adenoma of the colorectum undergoes a proliferation versus differentiation process: new conceptual interpretation of morphogenesis. Oncology. 2008;74: 127–134. 10.1159/000151359 [DOI] [PubMed] [Google Scholar]
- 28.Yachida S, Mudali S, Martin SA, Montgomery EA, Iacobuzio-Donahue CA. Beta-catenin nuclear labeling is a common feature of sessile serrated adenomas and correlates with early neoplastic progression after BRAF activation. Am J Surg Pathol. 2009;33: 1823–1832. 10.1097/PAS.0b013e3181b6da19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu X, Li L, Peng Y. Wnt signaling in the serrated neoplastic pathway of the colorectum: possible roles and epigenetic regulatory mechanisms. J Clin Pathol. 2012;65: 675–679. 10.1136/jclinpath-2011-200602 [DOI] [PubMed] [Google Scholar]
- 30.Gala MK, Mizukami Y, Le LP, Moriichi K, Austin T, Yamamoto M, et al. Germline mutations in oncogene-induced senescence pathways are associated with multiple sessile serrated adenomas. Gastroenterology. 2014;146: 520–529. 10.1053/j.gastro.2013.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aoki H, Yamamoto E, Takasawa A, Niinuma T, Yamano HO, Harada T, et al. Epigenetic silencing of SMOC1 in traditional serrated adenoma and colorectal cancer. Oncotarget. 2018;9: 4707–4721. 10.18632/oncotarget.23523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Endoscopic Classification Review Group. Update on the Paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37: 570–578. 10.1055/s-2005-861352 [DOI] [PubMed] [Google Scholar]
- 33.Snover DC, Ahnen DJ, Burt RW, Odze RD. Serrated polyps of the colon and rectum and serrated polyposis In: Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed Lyon: IARC; 2010: 160–165. [Google Scholar]
- 34.Nakagaki T, Tamura M, Kobashi K, Koyama R, Fukushima H, Ohashi T, et al. Profiling cancer-related gene mutations in oral squamous cell carcinoma from Japanese patients by targeted amplicon sequencing. Oncotarget. 2017;8: 59113–59122. 10.18632/oncotarget.19262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grasso C, Butler T, Rhodes K, Quist M, Neff TL, Moore S, et al. Assessing copy number alterations in targeted, amplicon-based next-generation sequencing data. J Mol Diagn. 2015;17: 53–63. 10.1016/j.jmoldx.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh RR, Patel KP, Routbort MJ, Reddy NG, Barkoh BA, Handal B, et al. Clinical validation of a next-generation sequencing screen for mutational hotpots in 46 cancer-related genes. J Mol Diagn. 2013;15: 607–622. 10.1016/j.jmoldx.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 37.Toyota M, Suzuki H, Sasaki Y, Maruyama R, Imai K, Shinomura Y, et al. Epigenetic silencing of microRNA-34b/c and B-cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer Res. 2008;68: 4123–4132. 10.1158/0008-5472.CAN-08-0325 [DOI] [PubMed] [Google Scholar]
- 38.Sawada T, Yamamoto E, Yamano HO, Nojima M, Harada T, Maruyama R, et al. Assessment of epigenetic alterations in early colorectal lesions containing BRAF mutations. Oncotarget. 2016;7: 35106–35118. 10.18632/oncotarget.9044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto E, Toyota M, Suzuki H, Kondo Y, Sanomura T, Murayama Y, et al. LINE-1 hypomethylation is associated with increased CpG island methylation in Helicobacter pylori-related enlarged-fold gastritis. Cancer Epidemiol Biomarkers Prev. 2008;17: 2555–2564. 10.1158/1055-9965.EPI-08-0112 [DOI] [PubMed] [Google Scholar]
- 40.Ougolkov AV, Yamashita K, Mai M, Minamoto T. Oncogenic β-catenin and MMP-7 (matrilysin) cosegregate in late-stage clinical colon cancer. Gastroenterology. 2002;122: 60–71. 10.1053/gast.2002.30306 [DOI] [PubMed] [Google Scholar]
- 41.Giannakis M, Hodis E, Jasmine Mu X, Yamauchi M, Rosenbluh J, Cibulskis K, et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat Genet. 2014;46: 1264–1266. 10.1038/ng.3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei R, Zhao M, Zheng CH, Zhao M, Xia J. Concordance between somatic copy number loss and down-regulated expression: A pan-cancer study of cancer predisposition genes. Sci Rep. 2016;6: 37358 10.1038/srep37358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roszik J, Wu CJ, Siroy AE, Lazar AJ, Davies MA, Woodman SE, et al. Somatic copy number alterations at oncogenic loci show diverse correlations with gene expression. Sci Rep. 2016;6: 19649 10.1038/srep19649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burnett-Hartman AN, Newcomb PA, Potter JD, Passarelli MN, Phipps AI, Wurscher MA, et al. Genomic aberrations occurring in subsets of serrated colorectal lesions but not conventional adenomas. Cancer Res. 2013;73: 2863–2872. 10.1158/0008-5472.CAN-12-3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36: 417–422. 10.1038/ng1330 [DOI] [PubMed] [Google Scholar]
- 46.Yang HJ, Kim SG, Lim JH, Choi JM, Kim WH, Jung HC. Helicobacter pylori-induced modulation of the promoter methylation of Wnt antagonist genes in gastric carcinogenesis. Gastric Cancer. 2018;21: 237–248. 10.1007/s10120-017-0741-6 [DOI] [PubMed] [Google Scholar]
- 47.Suzuki H, Igarashi S, Nojima M, Maruyama R, Yamamoto E, Kai M, et al. IGFBP7 is a p53-responsive gene specifically silenced in colorectal cancer with CpG island methylator phenotype. Carcinogenesis. 2010;31: 342–349. 10.1093/carcin/bgp179 [DOI] [PubMed] [Google Scholar]
- 48.Sambuudash O, Kim HM, Jo H, Kim HS, Lee KJ, Park HJ, et al. Molecular characteristics of colorectal serrated polyps and hyperplastic polyps: A STROBE compliant article. Medicine. 2016;95: e5592 10.1097/MD.0000000000005592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De F, Wang X, Jansen M, Fessler E, Trinh A, de Rooij LP, et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med. 2013;19: 614–618. 10.1038/nm.3174 [DOI] [PubMed] [Google Scholar]
- 50.Gonzalo DH, Lai KK, Shadrach B, Goldblum JR, Bennett AE, Downs-Kelly E, et al. Gene expression profiling of serrated polyps identifies annexin A10 as a marker of a sessile serrated adenoma/polyp. J Pathol. 2013;230: 420–429. 10.1002/path.4200 [DOI] [PubMed] [Google Scholar]
- 51.Kanth P, Bronner MP, Boucher KM, Burt RW, Neklason DW, Hagedorn CH, et al. Gene signature in sessile serrated polyps identifies colon cancer subtype. Cancer Prev Res. 2016;9: 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(A, B) SSA showing membranous localization of β-catenin. (C, D) TSA showing nuclear accumulation of β-catenin.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
Sequence data has been deposited at the Japanese Genotype-phenotype Archive (JGA, http://trace.ddbj.nig.ac.jp/jga), which is hosted by the DNA DataBank of Japan (DDBJ), under accession number JGAS00000000217.