Abstract
Lower socioeconomic status (SES) is consistently related to higher obesity risk, especially in women living in developed countries such as the United States and Western Europe. Prevailing theories to describe this relationship have focused primarily on proximate level factors such as the generally poorer food environment (e.g. relative lack of healthy food options and higher concentrations of fast food restaurants) found in lower vs. higher SES neighborhoods and the higher financial costs associated with purchasing healthy, nutrient-dense foods compared to unhealthy, energy-dense foods. These factors are hypothesized to preclude the purchase of these foods by lower SES individuals. Unfortunately, public health interventions aimed at improving the food environment of lower SES communities and to provide financial resources for purchasing healthy foods have had limited success in reducing overall energy intake and body weight. Some evidence suggests these interventions may even exacerbate obesity. More recent hypotheses have shifted the focus to ultimate (or adaptive) factors that view increased energy intake and accrual of body fat among individuals of lower social status as adaptive strategies to protect against potential prolonged food scarcity. The purpose of this review is integrate past research at the proximate and ultimate levels with a consideration of how social status and SES during development (in utero through adolescence) may moderate the relationships between social status, eating behavior, and obesity. Utilizing an evolutionary framework that incorporates life history theory can lead to more integrative and thorough interpretations of past research and allow researchers to better elucidate the complex set of environmental, physiological, psychological, and behavioral factors that influence obesity risk among individuals of lower social status.
Keywords: evolution, social status, eating, obesity, life history theory, socioeconomic status
1 Introduction
Obesity is a complex condition that is responsible for substantial public health (Malnick, 2006) and financial costs (Finkelstein, Trogdon, Cohen, & Dietz, 2009). Despite billions of dollars in spending on research and commercial and clinical weight loss programs, the obesity epidemic continues unabated (MacLean et al., 2015), and behavioral treatment options offer only slim prospects for long-term clinically-significant weight loss (Barte et al., 2010; Franz et al., 2007; Lowe, Kral, & Miller-Kovach, 2008; MacLean et al., 2015). The current food and physical activity environment is commonly referred to as being “obesogenic” and is purported to be a critical factor in the etiology and continuation of high rates of obesity in the United States and other wealthy countries (Hall, 2018; Swinburn et al., 2011). Support for the importance of environmental factors driving obesity risk comes from multiple lines of evidence including observed increased per capita food availability (Hall, Guo, Dore, & Chow, 2009; Vandevijvere, Chow, Hall, Umali, & Swinburn, 2015), consumption of larger portion sizes (Duffey & Popkin, 2011), and the ready availability and consumption of highly-palatable and energy dense foods (Swinburn et al., 2011). One of the most consistent findings among observational studies of obesity risk is that people of color and lower socioeconomic status (SES) are disproportionately affected by obesity across multiple populations and especially in women (Darmon & Drewnowski, 2015; Djalalinia, Peykari, Qorbani, Larijani, & Farzadfar, 2015; Swinburn et al., 2011; Wang & Beydoun, 2007). For example, non-Hispanic black women are at ~2 times greater odds (OR [95% CI]: 2.10 [1.77, 2.50]) for obesity compared to non-Hispanic white women, and having greater than a high school education was associated with reduced risk of obesity among women (OR [95% CI]: 0.68 [0.54, 0.87]) (Flegal, Kruszon-Moran, Carroll, Fryar, & Ogden, 2016).
Numerous hypotheses have been put forth to describe the mechanism by which living at a lower SES increases the risk for obesity, but none seem to sufficiently describe the phenomenon. We propose that an evolutionarily-informed approach can lead to a more complete understanding of the relationships between social status, eating behavior, and obesity by providing a framework to organize existing perspectives and research to guide the development of novel hypotheses. In the following sections, we present this framework, and briefly summarize some of the prevailing hypotheses and empirical research regarding the relationship between social and economic status and obesity risk in the context of this framework. In the final section, we detail how life history theory integrates developmental and current social status to describe how social status influences physiological and psychological systems in predictable, historically adaptive ways that promote overeating and increase obesity risk among lower social status populations. This perspective delineates why social status and SES during developmental periods (in utero through adolescence) may moderate the relationship between current social status and obesity risk. We propose that the relative lack of accounting for developmental social status and SES in past research may help explain some of the inconsistencies in the literature as regards the effectiveness of public health interventions to positively impact eating behavior and reduce obesity risk among people of lower SES.
2 Evolutionary Perspective
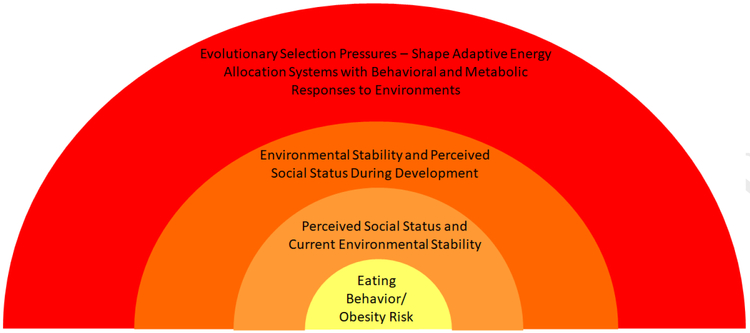
Evolutionary theory provides a framework that can help integrate the complex set of factors that are known to affect behaviors and physiology in ways that increase obesity risk, and lead to novel hypotheses that may not be considered in the absence of evolutionary theory. In 1963, Nikolaas Tinburgen, an evolutionary biologist, outlined an organizational framework for utilizing evolutionary theory to better understand traits and behavior (1963). This framework involves understanding four levels of analysis (proximate, ultimate, phylogenic, and developmental) that together lead to a more complete understanding of a trait or behavior. The proximate level examines observable, measureable factors that are closely or immediately linked to the behavior or outcome of interest. In the current case, social status has been treated as a proximate factor to predict obesity as well as the physiological, psychological, or behavioral mechanisms that influence eating behavior and obesity risk. Most researchers are familiar with the proximate level, because it is where science primarily operates. Evolutionarily-informed research aims to unpack these proximate predictors further to understand if, why, and how the relationship between the proximate factor(s) and the behavior currently serve, or historically served, a functional or adaptive purpose. This ultimate level of analysis is much more difficult to delineate, but, when properly utilized, can generate novel hypotheses, and lead to more thorough interpretation, integration, and application of empirical findings. The phylogenetic or comparative level of analysis can help researchers understand whether and/or how long a trait, behavior, or the relationship between proximate predictors and the trait or behavior have been preserved over evolutionary time by examining if it they exist in related species. Animal researchers are familiar with this level of analysis, though the evolutionary implications of this approach are not often made explicit. An evolutionarily-informed phylogenetic perspective also seeks to predict and understand how relationships between predictors and behaviors in animal models would or would not exist in humans. These predictions are based on known differences (e.g., in genetics, physiology, lifespan, or social structure) between humans and non-human animals. Finally, the developmental level of analysis examines the ways developmental environments (including in utero) interact with genetic predispositions to shape a range of possible phenotypes that maximize the likelihood of reproductive success and survival in environments similar to developmental ones. Ideally, more research within and across each of these levels leads to a more complete understanding of a trait or behavior. Hypotheses can be derived from work on one level regarding what can be expected at another, or can be aimed at integrating across levels more directly. This organizational framework can be particularly useful for understanding obesity and eating behavior because both are complex and influenced by a broad array of factors at each level. In the following section we describe each level in greater detail and summarize the relevant research regarding social status, eating behavior, and obesity in the context of this framework (Figure 1).
Figure 1:
Conceptual model depicting how an evolutionary framework widens then lens for seeing a more complete picture of the factors that influence eating behavior and obesity risk. As depicted in the model, eating behavior and obesity risk are embedded within and influenced by perceived social status and current environmental stability. These perceptions have been shaped and influenced by cues of how harsh and unpredictable energetic resources were in developmental environments (particularly in utero and early childhood). Natural selection has shaped the information that is relevant to communicate about developmental environments (e.g., energetic resource availability/predictability), the cues that communicate them (e.g., through the umbilical cord, breastmilk, and food) and the appropriate behavioral and metabolic ‘strategy’ to adopt given those cues (e.g., in environments where food availability fluctuates, one should eat as much as possible when available, ignore satiety signals, and store fat to compensate for fluctuations) because those strategies predictably led to greater survival and/or reproductive success in such environments over long periods of evolutionary history.
3 Proximate Level of Analysis
Traditional approaches and hypotheses examining immediate factors and mechanisms between social status, SES and BMI exemplify a proximate approach. Two common hypotheses and research taking a proximate approach are summarized briefly below. The relationship between educational attainment and obesity is also typically investigated using a proximate level of analysis. While lower educational attainment has been consistently associated with higher BMI and low diet quality in high income countries (Cohen, Rai, Rehkopf, & Abrams, 2013; Yu, 2012), we believe a more integrated approach – specifically using life history theory – may allow for a more complete understanding of the observed relationship between education and obesity and is discussed in Life History Theory – Integrating Developmental, Proximate and Ultimate Levels of Analysis.
3.1 The Availability Hypothesis
The simplest explanation for the relationship between lower social status and SES with higher obesity risk is related to the availability of healthy food and fast-food/”less healthy” food outlets in lower SES neighborhoods. The overall premise of this hypothesis is simply that greater exposure to energy dense/nutrient poor foods results in greater consumption of these foods and higher risk of obesity. A major limitation of the Availability Hypothesis is that it does not explain how and/or why individuals living in poor food environments choose to consume less healthy and/or energy dense foods. As noted by Dhurandhar (2016), the presence of high calorie, palatable foods may be an important permissive factor in the development of obesity, but it does not mechanistically explain overeating and weight gain in the population.
Nevertheless, a recent longitudinal study found that the momentary food environment (assessed using ecological momentary assessment) predicted eating behavior in adults with overweight/obesity. There was an increased likelihood of eating high-energy snacks when participants were near fast food restaurants (OR = 1.89, 95% CI = 1.22, 2.93) whereas the likelihood of consuming low-energy snacks was higher when a supermarket was nearby (OR = 2.93, 95% CI = 1.38, 3.82) (Elliston, Ferguson, Schüz, & Schüz, 2017). Compared to higher SES neighborhoods, those of lower SES are characterized by relative lack of access to retail food outlets with healthy and fresh foods (e.g. fresh fruits and vegetables), a greater reliance on small food outlets or convenience stores for grocery shopping among community members, and higher concentrations of fast food restaurants compared to higher SES neighborhoods (Laxy, Malecki, Givens, Walsh, & Nieto, 2015). The generally poor food environment of lower SES neighborhood would therefore be expected to promote consumption of higher-energy foods, overeating, and increase the risk for weight gain and obesity. However, data linking the poorer food environments in low SES neighborhoods to eating behaviors and obesity risk among low SES individuals are inconclusive (Flint, Cummins, & Matthews, 2013; Hawkesworth et al., 2017; Laxy, Malecki, Givens, Walsh, & Nieto, 2015; Zagorsky & Smith, 2017).
The Survey of the Health of Wisconsin (SHOW) study (Laxy et al., 2015) calculated a food environment index consisting of a ratio of the distance from participants’ homes to the 3 closest supermarkets relative to the distance to the 3 closest convenience stores or fast food restaurants (higher number indicates less favorable food environment). Results from the SHOW study demonstrated that low SES neighborhoods had a less favorable food environment (index = 2.53) compared to higher SES neighborhoods (index = 1.77, p < 0.01), but the food environment index was not consistently linked to obesity risk. Regardless of SES, frequent consumption of fast foods (≥2 times/week) was only weakly associated with increased odds of being obese (OR = 1.35, p = 0.06), and the relationship between neighborhood access to fast food and its consumption was weak and only borderline-significant. Another observational study of over 8000 participants found that higher net wealth (total assets minus total debt) was associated with slightly less overall fast food consumption, but that higher income (rather than “wealth”) was associated with a greater number of fast food meals in the past 7 days (Zagorsky & Smith, 2017). However, the authors noted that the associations were weak and unlikely to have a large public health impact. As an example, a $1 million increase in net wealth was associated with consuming only 0.7 less fast food meals per week.
If eating behavior and obesity risk are strongly affected by the mere availability of healthy/unhealthy foods, then interventions to increase access to healthy food options (or decrease the availability of unhealthy foods) in poor food environments should improve eating behaviors and reduce obesity. A new neighborhood supermarket was constructed in a low SES neighborhood with a poor food environment in Philadelphia as part of the Pennsylvania Fresh Food Financing Initiative. The objective of this natural experiment was to investigate whether increasing the availability of healthy foods increased perceptions of healthy food access, increased fruit and vegetable intake, and decreased BMI (Cummins, Flint, & Matthews, 2014). Results of this quasi-experimental study demonstrated a moderate increase in perceptions of food access but fruit and vegetable intake and BMI were not affected. Point-of-sale interventions at existing retail food outlets (e.g. product placement, promotions, financial incentives, and awareness campaigns) are another avenue for potentially increasing consumption of healthy foods and reducing obesity, but results of these interventions have been mixed and generally suffer from a lack of rigorous scientific methodology to provide clear evidence of causality. While a variety of methods and outcome measures have been utilized in point-of-purchase interventions, the most effective interventions for increasing the purchase of healthy foods appear to be those that provide a financial incentive (e.g. decreased price of “healthy” foods) (Adam & Jensen, 2016; An, 2013; Karen Glanz, Bader, & Iyer, 2012; Liberato, Bailie, & Brimblecombe, 2014), which suggests that it may not be availability per se that influences eating behavior but rather the cost (real or perceived) of healthy food items (e.g. whole grains, fruits, vegetables, lean meats) compared to unhealthy foods (e.g. refined grains, processed foods with added sugar and fat). The proposed influence of food prices on eating behavior is discussed in the following section.
3.2 The Economic Hypothesis
Price could act as a barrier to purchasing healthy foods among low SES populations even if these foods are “available” in retail food outlets in close proximity to their homes. Taste is commonly cited as the principal driver of food choices, but price is the second strongest predictor of food choices and is an especially important factor among individuals of lower SES (K Glanz, Basil, Maibach, Goldberg, & Snyder, 1998). Furthermore, nutritional concerns (including the importance of weight control) were generally less important for determining food choices among Americans except among the most health conscious cluster of individuals (Glanz et al., 1998). Multiple analyses have demonstrated that healthy foods and dietary patterns that include items such as fruits, vegetables, and whole grains are more expensive than less healthy options such as refined grain products and processed foods with added sugars and fats (Darmon & Drewnowski, 2015; Drewnowski, Darmon, & Briend, 2004; Lopez et al., 2009; McAllister, Baghurst, & Record, 1994; Monsivais, Aggarwal, & Drewnowski, 2011). A 2015 systematic review by Darmon and Drewnowski (2015) based on 151 observational studies of the relationships between food prices, dietary quality, and SES concluded that diet costs are inversely associated with the energy density of the diet (i.e. higher-energy, nutrient-poor diets costs less than lower-energy, nutrient-dense diets) and that higher-energy diets are preferentially selected by individuals of lower SES. It is also apparent that the food budgets of many people of lower SES are insufficient to purchase a healthy diet that meets established dietary recommendations (Darmon & Drewnowski, 2015; Lopez et al., 2009; Monsivais, Aggarwal, & Drewnowski, 2011).
Further, the price of many staple food items (e.g. dairy, fruit, grains, and some protein foods) are more expensive at small food stores and convenience stores compared to supermarkets (Caspi et al., 2017; Krukowski, West, Harvey-Berino, & Elaine Prewitt, 2010; Leone et al., 2011), which may disproportionately affect lower SES populations where convenience stores and “corner markets” are more concentrated (Cannuscio et al., 2013; Cannuscio, Hillier, Karpyn, & Glanz, 2014; D. M. Gibson, 2011). Indeed, higher neighborhood median household income was positively associated with the overall availability of healthy foods (r = 0.27, p < 0.05), and healthy foods were less expensive at larger retail food outlets (r = 0.40, p < 0.01) (Krukowski, West, Harvey-Berino, & Elaine Prewitt, 2010). The availabilities of low-fat milk and lean beef were examined in greater detail. Both racial composition and median household income were associated with the availability of these products. When racial composition and income were both included in the regression model, neighborhood racial composition explained 49% of variance in low-fat milk availability and neighborhood household income explained 29% of the variance in lean beef availability (Krukowski et al., 2010). Thus, there is a strongly held belief among the scientific and lay communities that the increased cost of healthy, nutrient dense foods and dietary patterns drive people of lower SES to purchase and consume less healthy foods with lower nutrient densities and higher energy densities, which promotes overeating and weight gain.
Results from qualitative and self-report research indicate that price is a substantial barrier to purchasing and consuming healthy diets among people of lower SES (Dachner, Ricciuto, Kirkpatrick, & Tarasuk, 2010; Hampson, Martin, Jorgensen, & Barker, 2009; Trumbo, Schlicker, Yates, & Poos, 2002; Waterlander, de Mul, Schuit, Seidell, & Steenhuis, 2010; Wiig & Smith, 2009). Education and awareness of healthy diets does not appear to be the major issue in this population because fresh produce, fish, and dairy products are generally recognized as “healthy,” and respondents commonly report a desire to purchase more “healthy foods” given the financial means to do so (Inglis, Ball, & Crawford, 2009; Wiig Dammann & Smith, 2009). These findings seem to suggest that providing individuals of lower SES with the financial resources to purchase and consume a healthier diet should improve overall diet quality, reduce the energy density of the diet, reduce overall energy intake, and positively influence body weight. A major assumption of this deductive reasoning is that potential energy from “healthy foods” would displace energy from “unhealthy foods” rather than simply adding additional energy to the diet. Without sufficient replacement, overall diet quality may rise but energy intake may also increase and lead to further weight gain rather than weight stability or loss. In fact, significant weight gain was observed when either money or a food basket were provided to women of lower SES in rural Mexico, and women that were already obese gained more weight than women with a BMI between 25 and 30 kg/m2 (Leroy, Gadsden, González de Cossío, & Gertler, 2013). While not conclusive, these findings suggest that providing additional financial resources or the direct provision of healthy foods to lower SES populations may not have the intended effect of replacing energy dense foods with healthier, lower energy foods. Rather, it appears that providing these extra resources has the potential to exacerbate obesity in low SES populations because the energy from purchased/provided foods may be additive vs. substitutive in the diet.
The majority of past research exploring the relationships among SES/social status, eating behavior, and obesity have utilized a proximate level of analysis with a principal focus on current or experimentally manipulated SES/social status (experimental studies are discussed in the Adaptive Strategy Hypotheses section). These studies have yielded important findings regarding how neighborhood-level food availability and the price of healthy foods potentially influence eating behaviors among lower SES/social status individuals. However, public health interventions to improve availability or provide financial resources for healthy foods have yielded inconsistent results (Cummins, Flint, & Matthews, 2014; Jones & Frongillo, 2006; Leroy, Gadsden, González de Cossío, & Gertler, 2013). More recent hypotheses, detailed in the next section, have therefore shifted the focus to a potential adaptive response whereby overeating and accrual of body fat under may act as a buffer against future actual or perceived threats to food security/availability (Dhurandhar, 2016; Kaiser, Smith, & Allison, 2012; Nettle, Andrews, & Bateson, 2017; Smith, Stoddard, & Barnes, 2007). We believe these “Adaptive Hypotheses” represent a major leap forward for shifting the perspective for why social status influences eating behavior and obesity risk to a more ultimate level of analysis.
4 Ultimate Level of Analysis
Evolutionary approaches are based on the premise that proximate factors are often directly or indirectly related to an ultimate adaptive function. Adaptive traits, behaviors and mechanisms persist because they improved the likelihood of reproductive success or survival in predictable circumstances across evolutionary history. Ultimate explanations consider how behaviors and their underlying mechanisms were shaped by natural selection to derive hypotheses. Natural selection operates on differential reproductive success, and those who reproduce more have greater reproductive fitness because their genes are passed on at a higher rate than those who reproduce less. The forces that determine the traits and behaviors that improve Darwinian fitness are called selection pressures. Selection continues to act on traits, behaviors, and their underlying psychological and physiological mechanisms as long as they exhibit heritable variation that influences reproductive success. This is not to say that all existing proximate factors are adaptive, they can also be benign factors that are linked to a trait or behavior that is adaptive. They can also be traits that do not affect reproductive fitness or survival, and thus have not been selected out, but have also not been specifically selected for. It is important to note that adaptive traits, behaviors and their underlying mechanisms need not improve reproductive success universally to have been selected for through natural selection. In fact, though it seems counterintuitive, natural selection can lead to characteristics, traits, and behaviors that are detrimental to health and survival when environments change rapidly. Historically adaptive traits, characteristics, and behaviors are then said to be “mismatched” to current environments where the adaptive advantage is not relevant, or even harmful in the changed environment. Mismatches are particularly relevant for deriving hypotheses using ultimate explanations in research on eating behavior and obesity risk because our environments have changed vastly and rapidly over the last 200 years in terms of food availability, storage and production, and the (lack of) physical activity necessary to acquire food.
In general, humans (and other non-human primates) are expected to have evolved behavioral and physiological mechanisms that favor fat storage in order to fuel our large, metabolically expensive brains, even during times of food scarcity (Al-Shawaf, 2016; Shively, Register, & Clarkson, 2009; Wells, 2006; Wells, 2010). Hedonic and homeostatic mechanisms that regulate intake and energy balance, hunger, satiation, overeating, frequent eating, and eating in the absence of need are all potential mechanisms that have evolved in humans to favor fat accumulation and storage because they would be adaptive in environments that lacked the food abundance that characterize modern environments (Ahlstrom, Dinh, Haselton, & Tomiyama, 2017; Al-Shawaf, 2016). In modern environments, those mechanisms instead increase the risk of obesity and chronic disease.
Humans and other primates are predicted to be particularly sensitive to cues of resource scarcity and environmental unpredictability and shift metabolic and behavioral strategies that favor fat accumulation, storage, and defense when environments require it. Moreover, humans are unique, even among primates, in their sociality and social structure. Humans are an extraordinarily social species with an unusual propensity to cooperate, and even share food among extended kin and non-kin networks (Kaplan, Hill, Lancaster, & Hurtado, 2000). Humans are therefore predicted to be particularly sensitive to cues of social status, social stress and being socially ostracized, and respond to threats in these domains with changes in eating behaviors and energy metabolism. The loss of this social safety net could have devastating energetic consequences, and the fear of losing this social safety net has been posited as a driving force behind increases intake and fat accumulation among populations that experience social discrimination (Neuberg & Kenrick, 2018). Pepper and Nettle (2014) further argue that social status is linked to perceived mortality risk, which is shown to influence the likelihood of investing in preventive health behaviors because the costs to perform them do not outweigh long-term benefits when the future is less certain. Some of the leading hypotheses that incorporate an adaptive or ultimate level of analysis are summarized in the following section.
4.1 Adaptive Strategy Hypotheses
More recent hypotheses have begun to shift the focus to “adaptive strategies” to explain the observed relationship between lower SES and obesity (see “The Insurance Hypothesis” (Nettle, Andrews, & Bateson, 2017), “Economic Insecurity Hypothesis” (Smith et al., 2007), “Resource Scarcity Hypothesis” (Dhurandhar, 2016), and a review by Kaiser, Smith, and Allison (2012)). The overarching premise of the Adaptive Strategy Hypotheses are that physiological and behavioral mechanisms promote overeating and accrual of body fat under conditions of actual and/or perceived food uncertainty in order to buffer against the potential of prolonged food scarcity. These hypotheses are based in evolutionary theory and – in our opinion – represent a major improvement over past hypotheses to describe the mechanisms relating lower SES or social status to obesity risk.
Evidence in support of the Adaptive Strategy Hypotheses for mechanistically describing the observed relationships among lower SES/social status, eating behavior, and obesity risk comes from naturalistic and highly controlled animal studies as well as quasi-experimental and randomized clinical trials in humans. As will be described in detail in Phylogeny: Animal models examining social status, eating behavior and obesity, studies in animals models including birds (Gosler, 1996), rodents (Bartolomucci et al., 2009; Foster, Solomon, Huhman, & Bartness, 2006; Li, Cope, Johnson, Smith Jr, & Nagy, 2010; Melhorn et al., 2010; Moles et al., 2006; Tamashiro et al., 2007), and non-human primates (Arce, Michopoulos, Shepard, Ha, & Wilson, 2010; Michopoulos, Diaz, & Wilson, 2016; Michopoulos & Wilson, 2011; Moore et al., 2013; Wilson et al., 2008) consistently demonstrate that lower social status animals or animals exposed to food uncertainty are more likely to consume greater amounts of food energy from high or low energy diets (Wilson et al., 2008) and become obese compared to their higher status counterparts.
The Moving to Opportunity (MTO) project is an intriguing example of a quasi-experimental trial that demonstrates the potential importance of social status (as compared to strictly SES) on obesity risk. From 1994 – 1998, the Department of Housing and Urban Development randomly assigned 4498 women with children living in public housing in urban census tracts with ≥40% of residents below the federal poverty threshold to receive either 1) housing vouchers for moving to a low-poverty census tract (≤10% poverty), 2) unrestricted housing vouchers, or 3) no voucher (control) (Ludwig et al., 2011). The design of the MTO project is especially important for determining the relative importance of SES/financial status vs. social status on obesity risk because both treatment groups received a one-time financial resource to relocate, but only the group that was required to move to a low-poverty census tract experienced a change in their surrounding economic and social environments. Results of the follow-up analysis demonstrated that only the group receiving the voucher for exclusive use in low-poverty areas reduced the prevalence of BMI ≥35 kg/m2 (4.61% reduction, 95% CI: −8.54, −0.69) and hemoglobin A1c (4.31% reduction, 95% CI: −7.82, −0.80) (Ludwig et al., 2011). These results indicate that moving from a high-poverty to a low-poverty neighborhood – which would be expected to improve subjective relative social status – is sufficient to reduce rates of obesity independently of changes in overall SES, education, and income.
A limited number of randomized clinical trials have attempted to test how manipulations in subjective social status influence short-term eating behaviors and/or food preference in humans (Bratanova, Loughnan, Klein, Claassen, & Wood, 2016; Cardel et al., 2016; Cheon & Hong, 2017; Pavela, Lewis, Dawson, Cardel, & Allison, 2017; Sim, Lim, Forde, & Cheon, 2018; Swaffield & Roberts, 2015). Randomized clinical trials provide the benefit of providing strong causal evidence for the relationship between social status and eating behavior, but are limited in some important ways. For example, most randomized clinical trials will be limited to an investigation of short-term eating behavior, which necessitates extrapolating findings from acute behaviors to long-term obesity risk. Secondly, it is unlikely that experimental attempts to manipulate perceptions of social status among participants are able to recapitulate the experiences of individuals actually living (or having lived in the past) under conditions of lower SES/social status.
With these limitations in mind, the majority of human clinical trials reviewed (except Pavela et al., 2017) found that experimentally manipulating perceived social status (Bratanova et al., 2016; Cardel et al., 2016; Cheon & Hong, 2017; Sim et al., 2018) or exposing participants to cues of environmental harshness (Swaffield & Roberts, 2015) increased total energy intake and/or enhanced preferences for high-calorie foods. Importantly, eating behaviors were altered by manipulating only the perception of SES or social status, or environmental harshness without actual changes in access to financial resources (Bratanova et al., 2016; Cheon & Hong, 2017). Brotanova et al. (2016) demonstrated that pairing participants and fostering the belief they were poorer or wealthier than their partner caused feelings of social anxiety, which led to increased food intake especially in those who reported a strong need to belong. Social anxiety and stress could provide a causal mechanism relating perceived social status and eating behavior. Cortisol is an established biomarker of stress and has been implicated in the regulation of eating behavior in women (Epel, Lapidus, McEwen, & Brownell, 2001). Low social status has been associated with alterations in basal and stress-induced cortisol levels compared to higher social status individuals (Dhurandhar, 2016). These preliminary findings from human clinical studies provide a strong scientific premise for future studies to further investigate the influence of perceived social status on eating behavior and the potential mechanistic role of basal and/or stress-induced cortisol in mediating this relationship.
These Adaptive Strategy Hypotheses are useful for improving our understanding of the relationships between SES/social status and obesity risk. However, we believe the approaches to date have been limited by their principal focus on a proximate analysis of SES and social status, and do not fully account for the potential impact of SES/social status during development (in utero through adolescence) on later life obesity risk. This developmental impact will be detailed in the Developmental Level of Analysis section.
5 Phylogenetic Level of Analysis
A phylogenetic or comparative level of analysis includes research utilizing animal models. Traditionally, animal models are used to test mechanisms and experimental paradigms that are not ethical, possible, or much more difficult to do in humans. This implicitly relies on the mechanisms in animals being similar to humans because we share an evolutionary history. An evolutionary interpretation of this research may go a step further to more explicitly estimate the evolutionary trajectory of a trait or behavior, based on how far back it is preserved in the phylogenetic tree (e.g.., is it found among primates, mammals, vertebrates, etc.). Known differences between species (e.g., in brain size or sociality) can also be leveraged to make predictions about when a trait is expected to be seen or not in a given species. In the following section, we present an overview of the animal model research on the effect of social status and eating behavior/obesity risk that has been conducted thus far.
5.1 Animal models examining social status, eating behavior and obesity
Low ranking individuals in social species with naturally formed hierarchies face higher mortality risks because they have less access to food and non-food resources, are vulnerable to aggression from higher ranking members, and are less likely to be protected from aggression by outgroup members. Low social status is associated with higher markers of chronic stress (higher cortisol and inflammation). It is intuitive to hypothesize that these differences between lower and higher ranking members of social species would have adaptive shifts in behavior to increase intake and metabolism that lead to fat accumulation in lower ranking members to allow them to buffer against times of resource scarcity. Where low social status influences access to food, resource scarcity is much more dangerous for those with lower social status. Evidence from birds and primates clearly support this hypothesis (Gosler, 1996; Moore et al., 2013; Wilson et al., 2008), but findings among rodent models present a more complicated picture (e.g., Melhorn et al., 2010; Moles et al., 2006; Tamashiro et al., 2007).
Perhaps the clearest evidence supporting this hypothesis was demonstrated among great tits (Parus major) in study examining social status and fat accumulation in the wild comparing seasons with high and low resource availability (Gosler, 1996). Fat accumulation was demonstrated to be strategic, occurring only when it was colder, during seasons of resource scarcity but not abundance, to a greater degree in less dominant birds, and it predicted survival among less dominant birds in the season with scarce resource availability. Existing research among primates tends to mirror the human RCTs summarized previously. In socially housed macaques, subordinate females show signs of chronic stress, eat more than dominants, and eat during the day and night, while dominants restrict intake to during the day (Wilson et al., 2008). In another study among macaques (Moore et al., 2013), subordinate females who had prior experience with a highly palatable diet consumed more calories from snacks than those who did not have prior exposure to a highly palatable diet.
In contrast to humans, who tend to eat more nutrient-dense foods when stressed (Torres & Nowson, 2007), rodents who are experimentally stressed in the lab tend to reduce food intake (Tamashiro, Hegeman, & Sakai, 2006). This is true for several types of stressors, including social stress in the visual burrow system (VBS), where both dominant and subordinate male rats consistently decrease intake and lose weight during and immediately following the establishment of a dominance hierarchy (Foster et al., 2006; Tamashiro et al., 2006). One study has shown that the rats regain weight during recovery from the social stress paradigm, but that subordinate rats regain relatively more fat mass, which would predispose them to obesity (Tamashiro et al., 2007). However, subordinate, but not dominant rats also lose lean mass (Tamashiro et al., 2006). Dulloo et al. (1996) demonstrated that losing lean mass during restriction leads to fat overshooting during recovery because feedback signals from losses in fat (i.e., adipostats) and lean mass (i.e., proteinstats) modulate energy intake and adaptive thermogenesis to regain weight until lean mass losses are recovered. Fat mass is recovered at a faster rate, and is therefore higher than baseline levels following recovery. Thus, it is the loss of lean mass observed in subordinate rats during energy restriction that leads to greater accumulation in fat mass during recovery. When Syrian hamsters, a naturally aggressive, non-social species, are group housed in the laboratory, they respond with increases in food intake, body weight and fat mass overall (Bartness, 1996; Borer, Pryor, Conn, Bonna, & Kielb, 1988; Foster et al., 2006; Gattermann, Fritzsche, Weinandy, & Neumann, 2002). These responses may be moderated by social status, because males subjected to resident-intruder interactions and lose multiple interactions showed greater increases in energy intake, body weight, and adipose tissue relative to winners of those interactions (Foster et al., 2006). Importantly, to our knowledge, there are no studies on the effect of social status on intake or obesity risk among female rats, but female Syrian hamsters that are socially housed eat more and gain weight over time (Meisel, Hays, Del Paine, & Luttrell, 1990). In mice, social stress leads to increases in body weight in subordinates, but this effect was not driven by increases in energy intake among subordinantes, but rather a lower level of physical activity (Moles et al., 2006).
In order to draw meaningful conclusions about the effect of social status on eating behavior and obesity risk in humans from animal models, differences in physiology and social structure between species, as well as how ecologically valid the experimental paradigm is for establishing a hierarchy be taken into account. For example, primates (and humans in particular) have abnormally large brains relative to body size, which requires metabolic and behavioral adaptations to protect against starvation and defend fat stores to fuel large and energetically costly brains (Shively et al., 2009; Shively et al., 2009; Wells, 2006). This helps to interpret the differences between humans and rodents (whose brain to body size ratios are consistent with mammalian norms) in the acute stress and social response. In rats, both dominant and to a greater degree subordinate rats consistently decrease intake and lose weight in response being experimentally forced to establish a social hierarchy (Tamashiro et al., 2006). In contrast, humans and non-human primates with relatively low social status are observed to acutely increase intake (Arce et al., 2010; Bratanova et al., 2016; Cardel et al., 2016; Cheon & Hong, 2017; Michopoulos et al., 2016; Michopoulos & Wilson, 2011; Moore et al., 2013; Pavela et al., 2017; Sim et al., 2018; Swaffield & Roberts, 2015; Wilson et al., 2008), particularly when environmental harshness or instability are experimentally manipulated (S. E. Hill, Prokosch, DelPriore, Griskevicius, & Kramer, 2016; Laran & Salerno, 2013; Maner, Dittmann, Meltzer, & McNulty, 2017). Given how uniquely social and cooperative humans are in food production and sharing compared to rodents, it is not surprising that humans respond to cues of low social status with increasing intake or storing more fat to buffer against lower access to resources in response. Furthermore, when comparing humans to Syrian hamsters, it is important to keep in mind that the latter are not naturally social species, and therefore would not be expected to respond the way social species (e.g., humans, macaques, and birds) respond to social stress/status. This may partially account for why findings are equivocal with regards to the relationship between social stress and eating behavior among Syrian hamsters (Bartness, 1996; Borer et al., 1988; Foster et al., 2006; Gattermann et al., 2002). Recall that adaptive behavioral responses (increasing intake in response to low social status) can only evolve in reliable social environments where time and again, reacting in the adaptive way (by increasing intake because being low social status resulted in lower access to resources) increased the likelihood of reproductive success and/or survival. Although rodent models have many benefits, they also have limitations for understanding the effect of social status on eating behaviors and obesity risk. Differences between species must be considered to utilize evidence from animal models in order to guide more relevant and directed hypotheses and experimental designs in humans.
6 Developmental Level of Analysis
The developmental level of analysis includes the developmental trajectory of a trait or behavior throughout the life course, as well as developmental plasticity. Developmental plasticity describes how an organism’s genotype can give rise to a range of phenotypes depending on the environmental conditions experienced throughout development (West-Eberhard, 2003). Some environmental factors that are predicted to influence reaction norms in eating behavior and obesity are resource availability, the energy required to extract resources, exposure to pathogens and infections, and social and cultural factors. A range of phenotypes can be expressed from a given genotype that do not require changes in gene frequency in order to evolve. These phenotypes are not necessarily heritable, but rather reflect the level of adaptability to varying environmental conditions.
For example, humans are particularly adaptive to variable environments. The human lineage began in Africa but humans have spread to every major ecosystem possible through physiological, morphological, behavioral, and cultural adaptations. Humans have even successfully adapted to living at high altitudes with hypoxic stress, or a reduction in oxygen availability (Antón, Leonard, & Robertson, 2002; Leonard, 2015). Populations living at high altitudes around the world exhibit relatively larger lung volumes in adulthood, which is achieved through relatively slower growth rates overall, but faster growth of the organs associated with oxygen transportation (heart and lungs) during childhood and adolescence (Leonard 2015). Physiological and psychological factors that influence eating behavior and obesity are affected by environments and experiences faced during development (including in utero), as well as those that shaped these mechanisms ancestrally, and even those that shaped our lineage before diverging from a common ancestor with chimpanzees, bonobos, and other primates. An evolutionary perspective that incorporates each of these categories of analysis allows for a more complete understanding of diet and physical activity and their underlying mechanisms that is not possible from other perspectives.
6.1 Developmental Origins of Adult Disease
Several lines of research have demonstrated that energetic stress and poor nutrition during prenatal and childhood development, which is much more likely among those of low SES, has a profound impact on risk of obesity and related chronic diseases later in life. Epidemiological evidence has consistently shown that early life under-nutrition, indexed by low birth weight (alone and when paired with postnatal “catch-up fat”), is related to high blood pressure, obesity, type 2 diabetes, cardiovascular disease, and stroke in adulthood (Barker, 1989, 1994, 2004; Bleker et al., 2004; Curhan et al., 1996; Dulloo, Jacquet, Seydoux, & Montani, 2006; Ravelli, Stein, & Susser, 1976). After observing these patterns, researchers termed this phenomenon the fetal or developmental origins of adult disease (reviewed in Barker, 2004). It remains unclear whether this phenomenon is truly an adaptive response to energetic stress during development, as in the Predictive Adaptive Response and the Thrifty Phenotype and Thrifty ‘catch-up ’ phenotype (Dulloo et al., 2006), or whether a thrifty predisposition instead reflects limitations of developing under poorer conditions (Bogin, Varela Silva, & Rios, 2007), or a combination of both (Wells, 2010).
6.2 Predictive Adaptive Response and a Thrifty Phenotype
Hales & Barker (1992) revised Neel’s thrifty gene hypothesis (1962) to include the importance of the interaction of genes with developmental environments to shape phenotypes. They hypothesized that poor fetal and post-natal nutrition can lead to a “thrifty phenotype” that results in impaired development of the pancreas and endocrine disruptions that lead to increased risk of ype 2 diabetes. Evolutionary researchers have proposed that when faced with energetic stress prenatally and in infancy, developmentally plastic energy allocation systems make predictive adaptive responses (PARs) and shape more thrifty energy allocation systems later in life (Bleker et al., 2004; Dulloo et al., 2006; Ellison, 1990; Ellison & Jasienska, 2007; Gluckman, Hanson, & Spencer, 2005; Hales & Barker, 1992; Kuzawa, 2005; Kuzawa, Gluckman, Hanson, & Beedle, 2007; Lipson, 2001; Ravelli et al., 1976). It is hypothesized that maternal cues of constrained resource availability were likely to signify future environments with unstable resource availability, and therefore it was adaptive to shift reaction norms to develop a suite of metabolic characteristics that help buffer against hard times with: increases in fat accumulation, lower muscle mass, insulin and leptin resistance, lower vascular density, compact body shape, and earlier reproductive age. Proponents of the PAR hypothesis contend that over the course of human evolution, later environments predictably did resemble prenatal environments, and selection would favor energy saving mechanisms. Ancestrally, errors were unlikely to produce deleterious health outcomes because all environments required high levels of obligatory physical activity and had limited food availability even in the best of times (Gluckman et al., 2005; Kuzawa, 2007).
A metabolic phenotype that buffers against hard times would only lead to harmful health outcomes when there is a mismatch between energetic stress during fetal and infant development, paired with later life environments with abundant resources and much lower levels of obligatory physical activity. This is particularly relevant for populations undergoing transition from a more traditional lifestyle to one that is integrated with a market economy. This integration is typically accompanied by increased access to high-calorie, fatty, processed foods including refined carbohydrates that were not previously available. Some researchers have argued that rather than being an adaptive response, correlations between later life health and early-life nutritional stressors merely reflect individuals ‘making the best of a bad start’ (e.g., Bogin et al., 2007), or may be adaptive, but also limited by metabolic capacity, which is lower among populations that have been nutritionally stressed, and this increases the disease risk associated with modern lifestyles (Wells, 2010). It remains an open question whether metabolic changes reflect an adaptive shift to a more conservative energy allocation strategy, or are due more to disruptions during development that lead to poorer health outcomes. Regardless, a considerable body of epidemiological and experimental evidence supports the relationship between resource scarcity and energetic stress during development (e.g., birthweight, family SES, or perceptions of energy stress during development) are clearly and consistently related to and changes in developmental trajectory and metabolism (for a review see Gluckman et al., 2005), and are important to include in a more complete understanding of the influence of social status on eating behavior and obesity risk. Thrifty physiologic changes that predispose individuals to greater obesity and disease risk could be the result of developmental limitations, rather than adaptive shifts, but behavioral adaptations (e.g., increased intake during times of social stress or being socially ostracized) are more likely to reflect adaptive shifts in behavior.
We propose that taking a more holistic approach that integrates evidence across proximate, ultimate, phylogenetic, and developmental levels provides a useful theoretical framework for testing evolutionarily-informed hypotheses and will ultimately lead to a more complete understanding of the impact of social status on eating behavior and obesity risk. For example, a recent, phylogenetic meta-analysis integrated across these levels by testing the impact of prenatal stress (developmental) on offspring glucocorticoid levels (proximate/mechanistic stress response) across 14 vertebrate species (phylogenetic) (Thayer, Wilson, Kim, & Jaeggi, 2018). An overall positive relationship was found, suggesting a greater sensitivity to stress in response to prenatal stress is evolutionarily ancient, and will be consistent across a wide range of vertebrates. Another integrative approach utilizes Life History Theory, and is detailed in the following section.
7 Life History Theory – Integrating Developmental, Proximate and Ultimate Levels of Analysis
Another perspective that sheds light on the developmental origins of adult disease is life history theory (LHT). LHT is a theoretical framework from evolutionary biology that aims to explain differences in age-schedules of growth, reproduction, and mortality (Charnov, 1993; Stearns, 1992). LHT proposes that humans, like other species, face trade-offs in how to optimally allocate the limited resources of time and energy among growth, reproduction, and maintenance (e.g., immune function, survival, cellular repair). There are inherent trade-offs between how to allocate energy between these demands across the lifespan. The optimal allocations between these demands are expected to depend on individual and ecological parameters that influence resource production and availability (including consistency or predictability), as well as mortality and other factors (K. Hill & Kaplan, 1999). LHT posits that there have been long-term evolutionary pressures to balance energetic effort and channel resources and behaviors in ways that help to maximize the predicted reproductive success across the lifespan.
The way that energy is allocated between these competing demands forms coordinated patterns or life history ‘strategies’ that vary on a continuum from ‘slow’ to ‘fast’ (Ellis, Figueredo, Brumbach, & Schlomer, 2009). These strategies are the result of interactions between genes and environmental factors, including the harshness and unpredictability of the environment. This helps determine the optimal rates of growth and reproduction, and timing of reproductive maturation and senescence. Slow strategies are characterized by later age of reproductive maturation and first reproduction (including sexual debut, fewer sexual partners). This strategy is advantageous in predictable, stable environments with plentiful access to resources and fewer extrinsic mortality risks. In harsher, more unpredictable environments, where the future is less certain, a ‘fast’ strategy is more advantageous because it would be relatively more risky to delay reproduction, or behave in ways that generally value future gains over more immediate ones. Developmental environments that are characterized by all that comes with having low social and economic status (i.e., relatively more harsh and unpredictable) leads to a more fast life history trajectory, physiology, and behaviors that are more likely improve the chances of surviving and reproducing under such conditions. A life history perspective is therefore particularly important for understanding energy balance, weight management, and obesity because it helps to integrate the ultimate (improving the likelihood of reproductive fitness), developmental, and proximate (physiological, psychological and behavioral) factors that influence energy balance and obesity.
The physiological mechanisms that regulate fuel utilization, energy storage, and homeostasis at a more immediate level are intricately related to the endocrine mechanisms that coordinate the allocation of energy between the competing demands relevant for life history at the level of the lifespan. Those with a more ‘fast’ life history strategy are more likely to behave in ways that would have been beneficial for reproductive success in ancestral environments where food was seasonal and difficult to come by. But a ‘fast’ strategy can be detrimental for weight status and metabolic health in current, obesogenic environments where access to nutrient-dense resources are plentiful, and removed from physical effort that historically would have been necessary to obtain them. Hypotheses about the effect of life history on weight management and obesity are largely are limited to observational studies, as life histories cannot be experimentally assigned. To our knowledge, very few observational studies explicitly examine life history parameters and increased risk of overweight/obesity, but the evidence from the developmental origins of adult disease fit within this framework, and one study explicitly used a self-report questionnaire of life history trajectory to predict obesity risk and dysregulated eating (Maner et al., 2017). Other longitudinal studies that have measures of several life history parameters (e.g., birth weight, perceptions of the harshness and unpredictability of developmental/current environments, age of pubertal maturation) can test whether those with a more ‘fast’ strategy have an increased risk of overweight/obesity in adulthood. Some experimental studies have shown that life history strategy moderates the effect of experimental manipulations designed to evoke stressful, unpredictability of environments on inhibition, a cognitive ability needed to override hedonic food choices, and eating behavior directly.
A small number of empirical studies have utilized LHT as a guiding theoretical framework for the design of hypotheses and formation of hypotheses regarding social status, eating behavior, and obesity risk (Hill et al., 2016; Laran & Salerno, 2013; Maner et al., 2017). Laran & Salerno (Laran & Salerno, 2013) demonstrated that experimentally manipulated perceptions of environmental harshness led to increases in intake of calorie-dense foods, and a preference for calorie-dense foods. In a similar experimental paradigm, Hill et al., (2016) found that an experimental paradigm that influenced perceptions of environmental harshness influenced eating in the absence of need, but this effect was moderated by perceptions of resource scarcity in childhood. Those who reported having lower socioeconomic status during childhood, were more likely to eat in the absence of hunger when experimentally stressed compared to those with perceptions of relatively higher SES in childhood. In a longitudinal study, Maner et al (2017) demonstrated that lower SES during childhood was related to higher adult BMI. In a follow-up study, they tested a mediational model that demonstrated that childhood SES was related to perceptions of resource scarcity in childhood, which were in turn predictive of measure of life history strategy on a slow-fast continuum. Life history strategy related to BMI, but only through a measure of dysregulated eating. Another potential mediator between life history strategy and obesity is a predisposition to have cognition that is more present over future orientation. Processes such as inhibition, that is required to inhibit prepotent response in favor of goal oriented behavior (e.g., avoiding palatable foods to lose weight) have been demonstrated to be lower among individuals who perceived they had lower SES during childhood (Mittal, Griskevicius, Simpson, Sung, & Young, 2015). Together, the results of a limited number of studies support the theoretically based notion that developing in stressful/unpredictable environments leads to a ‘fast’ life history strategy, which alters cognition and behavior in ways that favor a more present over future orientation, that are advantageous in stressful, harsh and unpredictable environments (Frankenhuis, Panchanathan, & Nettle, 2016; Mittal et al., 2015), but increase the risk for obesity in modern, resource rich environments.
Thus far, research has shown that perceptions of the harshness and unpredictability of developmental environments are more predictive than current perceptions in shaping eating behavior and obesity risk generally (Maner et al., 2017) and were found to moderate the effect of perceptions of current environments on eating behavior (S. E. Hill et al., 2016). Perceptions of developmental environments warrant consideration in RCTs and observational research designed to measure the impact of SES/social status on eating behavior and obesity risk. Perceptions of low SES/social status currently may influence the eating behavior of those who also experienced low SES/social status during development to a greater degree than those who experienced stable, predictable environments during development.
The observed relationship between educational attainment and obesity risk represents an intriguing opportunity for integrating evidence with a life history framework. Past research has been conducted largely at the proximate level and often attributes the increased risk of obesity and poorer diet quality of less educated persons to a lack of knowledge of how diet influences health and/or which foods are “healthy” vs. “unhealthy” (Yu, 2012). As previously mentioned, people of lower social status, which tracks closely with educational attainment, are able to identify healthy foods and demonstrate a desire to purchase these foods given the financial resources to do so (Inglis et al., 2009; Wiig Dammann & Smith, 2009). Considering the massive public health campaigns promoting consumption of fruits and vegetables, low and non-fat dairy, and whole grains, it is not surprising that knowledge of the components of a healthy diet are widely-known even among people with the lowest levels of formal education. Thus, past research at the proximate level does not sufficiently explain the causal mechanisms linking education and obesity risk. Considering this relationship within the context of LHT offers potential explanations to describe the reasons why less educated people are predisposed to obesity and consume lower quality diets. Individuals who were raised and/or are currently living in a stable and predictable environment are able to place a greater emphasis on higher levels of education because it is less risky to wait for the delayed benefits, such as higher lifetime earnings, reflecting a ‘slow’ strategy. Taking this ‘slow’ strategy necessitates a relative degree of confidence that one will live a long and healthy enough life to take full advantage of the benefits of higher education. On the other hand, an individual reared and/or currently living in an unstable and unpredictable environment is predicted to use a ‘fast’ strategy that de-emphasizes education and its far off benefits. Rather, s/he might assign a higher value to more immediate rewards and opportunities (e.g., dropping out of high school for a low-paying job) due to the relative uncertainty of living long enough to benefit from more education, and the relatively greater need to begin making money immediately to fulfill basic needs. Within this context, higher levels of educational attainment could serve as a marker of one’s life history strategy more broadly rather than being directly and causally related to obesity risk.
8 Future Directions
Obesity is complex and multifactorial disease that will likely require similarly complex and multifactorial interventions to treat effectively. We believe that a principal focus on single-level interventions (e.g. diet macronutrient content, aerobic exercise, changes to the built environment) is an important factor leading to the lack of long-term success in past obesity treatment interventions. Consideration of the ultimate/adaptive (e.g. responses to perceived or actual resource scarcity) and developmental (e.g. early childhood SES/social status) levels in addition to the more traditionally studied proximate factors related to obesity are expected to lead to more complete understanding of the etiology and treatment of obesity. Using this integrated approach is well-suited for the development and testing of multi-level and adaptive obesity treatment interventions. In particular, the use of innovative experimental designs such as Sequential Multiple Assignment Randomized Trials (SMART) and Multiphase Optimization Strategies (MOST) (Almirall, Nahum-Shani, Sherwood, & Murphy, 2014; Collins, Murphy, & Strecher, 2007; Kidwell & Hyde, 2016) could lead to powerful, pragmatic, and translational interventions to more effectively treat obesity.
Importantly, SMART and MOST trials can be designed to optimize intervention strategies based on known baseline factors hypothesized to influence intervention effectiveness. One potential example would be to design an intervention that is tailored to participants’ predicted or measured life history parameters (via markers of birth weight, age of reproductive maturation, along with delay discounting, etc). Individuals reared in a harsh and unpredictable environments would be expected to have a ‘fast’ life history strategy and thus place greater value on immediate vs. long-term rewards. On the other hand, growing up in a stable and predictable environment fosters a ‘slow’ life history strategy that places greater value on long-term benefits and rewards. One could design an adaptive intervention that tailored treatment strategies, rewards, and motivational tools to the individuals’ personal life history strategies to test whether these ultimate and developmental factors impact the success of behavioral weight management programs. The addition of surveys and questionnaires that address developmental SES and social status to a traditional randomized clinical trial could be easily implemented to determine if these factors influence the intervention response. Such an approach may be a simpler approach compared to SMART and MOST designs, and is consistent with current initiatives such as the National Institutes of Health-supported ADOPT (Accumulating Data to Optimally Predict Obesity Treatment) working group (MacLean et al., 2018).
9 Conclusion
In conclusion, an evolutionary and life history perspective can lead to more integrative and thorough interpretations of existing empirical studies that integrate across the four levels of analysis outlined by Tinbergen. We believe utilizing this approach will allow researchers to better elucidate the complex set of environmental, physiological, psychological and behavioral factors that influence obesity and lead to novel hypotheses that can be tested in future experimental or observational research examining the relationship between social status, eating behavior, and obesity.
Acknowledgments
This work was supported by the National Heart Lung and Blood Institute [T32 HL HL116276] and the University of Colorado Nutrition Obesity Research Center [P30 DK048520]
Abbreviations
- SES
Socioeconomic status
- LHT
Life History Theory
- BMI
Body Mass Index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam A, & Jensen JD (2016). What is the effectiveness of obesity related interventions at retail grocery stores and supermarkets? —a systematic review. BMC Public Health, 16(1), 1247 10.1186/s12889-016-3985-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlstrom B, Dinh T, Haselton MG, & Tomiyama AJ (2017). Understanding eating interventions through an evolutionary lens. Health Psychology Review, 11(1), 72–88. 10.1080/17437199.2016.1260489 [DOI] [PubMed] [Google Scholar]
- Al-Shawaf L (2016). The evolutionary psychology of hunger. Appetite, 105, 591–595. 10.1016/j.appet.2016.06.021 [DOI] [PubMed] [Google Scholar]
- Almirall D, Nahum-Shani I, Sherwood NE, & Murphy SA (2014). Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Translational Behavioral Medicine, 4(3), 260–274. 10.1007/s13142-014-0265-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An R (2013). Effectiveness of subsidies in promoting healthy food purchases and consumption: a review of field experiments. Public Health Nutrition, 16(07), 1215–1228. 10.1017/S1368980012004715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antón SC, Leonard WR, & Robertson ML (2002). An ecomorphological model of the initial hominid dispersal from Africa. Journal of Human Evolution, 43(6), 773–785. 10.1053/jhev.2002.0602 [DOI] [PubMed] [Google Scholar]
- Arce M, Michopoulos V, Shepard KN, Ha Q-C, & Wilson ME (2010). Diet choice, cortisol reactivity, and emotional feeding in socially housed rhesus monkeys. Physiology & Behavior, 101(4), 446–455. 10.1016/j.physbeh.2010.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP (1989). Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. British Medicine Journal, 298, 564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP (1994). Mothers, babies, and disease in later life. London: BMJ. [Google Scholar]
- Barker DJP (2004). The Developmental Origins of Adult Disease. Journal of the American College of Nutrition, 23(sup6), 588S–595S. 10.1080/07315724.2004.10719428 [DOI] [PubMed] [Google Scholar]
- Barte JCM, Ter Bogt NCW, Bogers RP, Teixeira PJ, Blissmer B, Mori TA, & Bemelmans WJE (2010). Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obesity Reviews, 11(12), 899–906. 10.1111/j.1467-789X.2010.00740.x [DOI] [PubMed] [Google Scholar]
- Bartness TJ (1996). Photoperiod, sex, gonadal steroids, and housing density affect body fat in hamsters. Physiology & Behavior, 60(2), 517–529. 10.1016/S0031-9384(96)80027-8 [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, Berra D, … Palanza P (2009). Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLoS ONE, 4(1), e4331 10.1371/journal.pone.0004331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleker OP, Roseboom TJ, Ravelli ACJ, van Montfrans GA Osmond C, & Barker DJP (2004). Cardiovascular disease in survivors of the Dutch famine. Nestle Nutrition Workshop Series, Pediatric Program, Vol. 55 10.1159/000082602 [DOI] [PubMed] [Google Scholar]
- Bogin B, Varela Silva MI, & Rios L (2007). Life history trade-offs in human growth: adaptation or pathology?. American Journal of Human Biology, 19, 631–642. [DOI] [PubMed] [Google Scholar]
- Borer KT, Pryor A, Conn CA, Bonna R, & Kielb M (1988). Group housing accelerates growth and induces obesity in adult hamsters. The American Journal of Physiology, 255(1 Pt 2), R128–33. [DOI] [PubMed] [Google Scholar]
- Bratanova B, Loughnan S, Klein O, Claassen A, & Wood R (2016). Poverty, inequality, and increased consumption of high calorie food: Experimental evidence for a causal link. Appetite, 100, 162–171. 10.1016/j.appet.2016.01.028 [DOI] [PubMed] [Google Scholar]
- Cannuscio CC, Hillier A, Karpyn A, & Glanz K (2014). The social dynamics of healthy food shopping and store choice in an urban environment. Social Science & Medicine, 122, 13–20. 10.1016/j.socscimed.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Cannuscio CC, Tappe K, Hillier A, Buttenheim A, Karpyn A, & Glanz K (2013). Urban food environments and residents’ shopping behaviors. American Journal of Preventive Medicine, 45(5), 606–614. 10.1016/j.amepre.2013.06.021 [DOI] [PubMed] [Google Scholar]
- Cardel MI, Johnson SL, Beck J, Dhurandhar E, Keita AD, Tomczik AC, … Allison DB (2016). The effects of experimentally manipulated social status on acute eating behavior: A randomized, crossover pilot study. Physiology & Behavior, 162, 93–101. 10.1016/j.physbeh.2016.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi C, Pelletier J, Harnack L, Erickson D, Lenk K, & Laska M (2017). Pricing of staple foods at supermarkets versus small food stores. International Journal of Environmental Research and Public Health, 14(12), 915 10.3390/ijerph14080915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov E (1993). Life history invariants: some explorations of symmetry in evolutionary ecology. Oxford: Oxford University Press. [Google Scholar]
- Cheon BK, & Hong Y-Y (2017). Mere experience of low subjective socioeconomic status stimulates appetite and food intake. Proceedings of the National Academy of Sciences, 114(1), 72–77. 10.1073/pnas.1607330114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AK, Rai M, Rehkopf DH, & Abrams B (2013). Educational attainment and obesity: a systematic review. Obesity Reviews : An Official Journal of the International Association for the Study of Obesity, 14(12), 989–1005. 10.1111/obr.12062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Murphy SA, & Strecher V (2007). The Multiphase Optimization Strategy (MOST) and the Sequential Multiple Assignment Randomized Trial (SMART). American Journal of Preventive Medicine, 32(5), S112–S118. 10.1016/j.amepre.2007.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins S, Flint E, & Matthews SA (2014). New neighborhood grocery store increased awareness of food access but did not alter dietary habits or obesity. Health Affairs, 33(2), 283–291. 10.1377/hlthaff.2013.0512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curhan GC, Chertow GM, Willett WC, Spiegelman D, Colditz G. a, Manson JE, … Stampfer MJ (1996). Birth weight and adult hypertension and obesity in women. Circulation, 94, 1310–1315. 10.1161/01.CIR.94.6.1310 [DOI] [PubMed] [Google Scholar]
- Dachner N, Ricciuto L, Kirkpatrick SI, & Tarasuk V (2010). Food purchasing and food insecurity: Among low-income families in Toronto. Canadian Journal of Dietetic Practice and Research, 71(3), e50–e56. 10.3148/71.3.2010.127 [DOI] [PubMed] [Google Scholar]
- Darmon N, & Drewnowski A (2015). Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutrition Reviews, 73(10), 643–660. 10.1093/nutrit/nuv027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhurandhar EJ (2016). The food-insecurity obesity paradox: A resource scarcity hypothesis. Physiology & Behavior, 162, 88–92. 10.1016/j.physbeh.2016.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djalalinia S, Peykari N, Qorbani M, Larijani B, & Farzadfar F (2015). Inequality of obesity and socioeconomic factors in Iran: a systematic review and meta-analyses. Medical Journal of the Islamic Republic of Iran, 29, 241. [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A, Darmon N, & Briend A (2004). Replacing fats and sweets with vegetables and fruits—A question of cost. American Journal of Public Health, 94(9), 1555–1559. 10.2105/AJPH.94.9.1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffey KJ, & Popkin BM (2011). Energy density, portion size, and eating occasions: Contributions to increased energy intake in the United States, 1977–2006. PLoS Medicine, 8(6). 10.1371/journal.pmed.1001050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulloo AG, Jacquet J, & Girardier L (1996). Autoregulation of body composition during weight recovery in human: the Minnesota Experiment revisited. International Journal of Obesity, 20, 393–405. 10.1017/CBO9781107415324.004 [DOI] [PubMed] [Google Scholar]
- Dulloo AG, Jacquet J, Seydoux J, & Montani J-P (2006). The thrifty ‘catch-up fat’ phenotype: its impact on insulin sensitivity during growth trajectories to obesity and metabolic syndrome. International Journal of Obesity, 30(S4), S23–S35. 10.1038/sj.ijo.0803516 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Figueredo AJ, Brumbach BH, & Schlomer GL (2009). Fundamental dimensions of environmental risk: The impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Human Nature, 20(2), 204–268. 10.1007/s12110-009-9063-7 [DOI] [PubMed] [Google Scholar]
- Ellison PT (1990). Human ovarian function and reproductive ecology. American Anthropologist, 92, 933–952. [Google Scholar]
- Ellison PT, & Jasienska G (2007). Constraint, pathology, and adaptation: How can we tell them apart? American Journal of Human Biology, 19, 622–630. [DOI] [PubMed] [Google Scholar]
- Elliston KG, Ferguson SG, Schüz N, & Schuz B (2017). Situational cues and momentary food environment predict everyday eating behavior in adults with overweight and obesity. Health Psychology, 36(4), 337–345. 10.1037/hea0000439 [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, & Brownell K (2001). Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology, 26(1), 37–49. 10.1016/S0306-4530(00)00035-4 [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Trogdon JG, Cohen JW, & Dietz W (2009). Annual medical spending attributable to obesity: Payer-and service-specific estimates. Health Affairs, 28(5). 10.1377/hlthaff.28.5.w822 [DOI] [PubMed] [Google Scholar]
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, & Ogden CL (2016). Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA, 315(21), 2284 10.1001/jama.2016.6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint E, Cummins S, & Matthews S (2013). Do perceptions of the neighbourhood food environment predict fruit and vegetable intake in low-income neighbourhoods? Health & Place, 24, 11–15. 10.1016/j.healthplace.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster MT, Solomon MB, Huhman KL, & Bartness TJ (2006). Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 290(5), R1284–R1293. 10.1152/ajpregu.00437.2005 [DOI] [PubMed] [Google Scholar]
- Frankenhuis WE, Panchanathan K, & Nettle D (2016). Cognition in harsh and unpredictable environments. Current Opinion in Psychology, 7, 76–80. 10.1016/j.copsyc.2015.08.011 [DOI] [Google Scholar]
- Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, … Pronk NP (2007). Weight-Loss Outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. Journal of the American Dietetic Association, 107(10), 1755–1767. 10.1016/j.jada.2007.07.017 [DOI] [PubMed] [Google Scholar]
- Gattermann R, Fritzsche P, Weinandy R, & Neumann K (2002). Comparative studies of body mass, body measurements and organ weights of wild-derived and laboratory golden hamsters (Mesocricetus auratus). Laboratory Animals, 36(4), 445–454. 10.1258/002367702320389125 [DOI] [PubMed] [Google Scholar]
- Gibson DM (2011). The neighborhood food environment and adult weight status: Estimates from longitudinal data. American Journal of Public Health, 101(1), 71–78. 10.2105/AJPH.2009.187567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanz K, Bader MDM, & Iyer S (2012). Retail grocery store marketing strategies and obesity. American Journal of Preventive Medicine, 42(5), 503–512. 10.1016/j.amepre.2012.01.013 [DOI] [PubMed] [Google Scholar]
- Glanz K, Basil M, Maibach E, Goldberg J, & Snyder D (1998). Why americans eat what they do. Journal of the American Dietetic Association, 98(10), 1118–1126. 10.1016/S0002-8223(98)00260-0 [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, & Spencer HG (2005). Predictive adaptive responses and human evolution. TRENDS in Ecology and Evolution, 20, 527–533. [DOI] [PubMed] [Google Scholar]
- Gosler AG (1996). Environmental and social determinants of winter fat storage in the great tit Parus major. The Journal of Animal Ecology, 65(1), 1 10.2307/5695 [DOI] [Google Scholar]
- Hales CN, & Barker DJP (1992). Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. International Journal of Epidemiology, 35, 595–601. 10.1093/ije/dyt133 [DOI] [PubMed] [Google Scholar]
- Hall KD (2018). Did the Food Environment Cause the Obesity Epidemic? Obesity, 26(1), 11–13. 10.1002/oby.22073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KD, Guo J, Dore M, & Chow CC (2009). The progressive increase of food waste in America and its environmental impact. PLoS ONE, 4(11). 10.1371/journal.pone.0007940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson SE, Martin J, Jorgensen J, & Barker M (2009). A social marketing approach to improving the nutrition of low-income women and children: an initial focus group study. Public Health Nutrition, 12(09), 1563 10.1017/S1368980009004868 [DOI] [PubMed] [Google Scholar]
- Hawkesworth S, Silverwood RJ, Armstrong B, Pliakas T, Nanchahal K, Sartini C, … Lock K (2017). Investigating the importance of the local food environment for fruit and vegetable intake in older men and women in 20 UK towns: a cross-sectional analysis of two national cohorts using novel methods. International Journal of Behavioral Nutrition and Physical Activity, 14(1), 128 10.1186/s12966-017-0581-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, & Kaplan H (1999). Life history traits in humans: Theory and empirical studies. Annual Review of Anthropology, 28, 397–430. 10.1146/annurev.anthro.28.1.397 [DOI] [PubMed] [Google Scholar]
- Hill SE, Prokosch ML, DelPriore DJ, Griskevicius V, & Kramer A (2016). Low Childhood Socioeconomic Status Promotes Eating in the Absence of Energy Need. Psychological Science, 27(3), 354–364. 10.1017/CBO9781107415324.004 [DOI] [PubMed] [Google Scholar]
- Inglis V, Ball K, & Crawford D (2009). Does modifying the household food budget predict changes in the healthfulness of purchasing choices among low- and high-income women? Appetite, 52(2), 273–279. 10.1016/j.appet.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Jones SJ, & Frongillo EA (2006). The modifying effects of food stamp program participation on the relation between food insecurity and weight change in women. The Journal of Nutrition, 136(4), 1091–1094. 10.1093/jn/136.4.1091 [DOI] [PubMed] [Google Scholar]
- Kaiser KA, Smith DL, & Allison DB (2012). Conjectures on some curious connections among social status, calorie restriction, hunger, fatness, and longevity. Annals of the New York Academy of Sciences, 1264(1), 1–12. 10.1111/j.1749-6632.2012.06672.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan HS, Hill KR, Lancaster JB, & Hurtado AM (2000). A Theory of Human Life History Evolution: Diet, Intelligence, and Longevity. Evolutionary Anthropology, 9(156–185). [Google Scholar]
- Kidwell KM, & Hyde LW (2016). Adaptive Interventions and SMART Designs: Application to Child Behavior Research in a Community Setting. American Journal of Evaluation, 37(3), 344–363. 10.1177/1098214015617013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukowski RA, West DS, Harvey-Berino J, & Elaine Prewitt T (2010). Neighborhood impact on healthy food availability and pricing in food stores. Journal of Community Health, 35(3), 315–320. 10.1007/s10900-010-9224-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW (2005). Fetal origins of developmetnal plasticity: are fetal cues reliable predictors of future nutritional environments? American Journal of Human Biology, 17, 5–21. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW (2007). Developmental origins of life history: Growth, productivity, and reproduction. American Journal of Human Biology, 19, 654–661. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Gluckman PD, Hanson MA, & Beedle AS (2007). Evolution, developmental plasticity, and metabolic disease In Stearns SC, Koella JC (Ed.), Evolution in health and disease (2nd ed.). Oxford: Oxford University Press. [Google Scholar]
- Laran J, & Salerno A (2013). Life-History Strategy, Food Choice, and Caloric Consumption. Psychological Science, 24(2), 167–173. 10.1177/0956797612450033 [DOI] [PubMed] [Google Scholar]
- Laxy M, Malecki KC, Givens ML, Walsh MC, & Nieto FJ (2015). The association between neighborhood economic hardship, the retail food environment, fast food intake, and obesity: findings from the Survey of the Health of Wisconsin. BMC Public Health, 15(1), 237 10.1186/s12889-015-1576-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard WR (2015). Physiological adaptations to environmental stressors In Muehlenbein MP (Ed.), Basics in Human Evolution (pp. 251–272). Academic Press. [Google Scholar]
- Leone AF, Rigby S, Betterly C, Park S, Kurtz H, Johnson MA, & Lee JS (2011). Store Type and Demographic Influence on the Availability and Price of Healthful Foods, Leon County, Florida, 2008. Preventing Chronic Disease, 8(6), A140–. https://doi.org/A140 [pii] [PMC free article] [PubMed] [Google Scholar]
- Leroy JL, Gadsden P, González de Cossío T, & Gertler P (2013). Cash and in-kind transfers lead to excess weight gain in a population of women with a high prevalence of overweight in rural mexico. The Journal of Nutrition, 143(3), 378–383. 10.3945/jn.112.167627 [DOI] [PubMed] [Google Scholar]
- Li X, Cope MB, Johnson MS, Smith DL Jr, & Nagy TR (2010). Mild calorie restriction induces fat accumulation in female C57BL/6J mice. Obesity, 78(3), 456–462. 10.1038/oby.2009.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberato SC, Bailie R, & Brimblecombe J (2014). Nutrition interventions at point-of-sale to encourage healthier food purchasing: a systematic review. BMC Public Health, 14(1), 919 10.1186/1471-2458-14-919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson SF (2001). Metabolism, maturation, and ovarian function In Ellison PT (Ed.), Reproductive ecology and human evolution. New york: Aldine de gruyter. [Google Scholar]
- Lopez CN, Martinez-Gonzalez MA, Alonso A, Sanchez-Villegas A, de la Fuente C, & Bes-Rastrollo M (2009). Cost of compliance with daily recommended values of micronutrients among a cohort of Spanish university graduates: the SUN (Seguimiento Universidad de Navarra) Study. Public Health Nutrition, 12(11), 2092 10.1017/S1368980009005278 [DOI] [PubMed] [Google Scholar]
- Lowe MR, Kral TVE, & Miller-Kovach K (2008). Weight-loss maintenance 1, 2 and 5 years after successful completion of a weight-loss programme. British Journal of Nutrition, 99(4), 925–930. 10.1017/S0007114507862416 [DOI] [PubMed] [Google Scholar]
- Ludwig J, Sanbonmatsu L, Gennetian L, Adam E, Duncan GJ, Katz LF, … McDade TW (2011). Neighborhoods, obesity, and diabetes — A randomized social experiment. New England Journal of Medicine, 365(16), 1509–1519. 10.1056/NEJMsa1103216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PS, Rothman AJ, Nicastro HL, Czajkowski SM, Agurs-Collins T, Rice EL, … Loria CM (2018). The Accumulating Data to Optimally Predict Obesity Treatment (ADOPT) Core Measures Project: Rationale and Approach. Obesity (Silver Spring, Md.), 26 Suppl 2, S6–S15. 10.1002/oby.22154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnick SDH (2006). The medical complications of obesity. QJM, 99(9), 565–579. 10.1093/qjmed/hcl085 [DOI] [PubMed] [Google Scholar]
- Maner JK, Dittmann A, Meltzer AL, & McNulty JK (2017). Implications of life-history strategies for obesity. Proceedings of the National Academy of Sciences, 114(32), 8517–8522. 10.1073/pnas.1620482114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister M, Baghurst K, & Record S (1994). Financial costs of healthful eating: A comparison of three different approaches. Journal of Nutrition Education, 26(3), 131–139. 10.1016/S0022-3182(12)80387-6 [DOI] [Google Scholar]
- Meisel RL, Hays TC, Del Paine SN, & Luttrell VR (1990). Induction of obesity by group housing in female Syrian hamsters. Physiology & Behavior, 47(5), 815–817. 10.1016/0031-9384(90)90002-L [DOI] [PubMed] [Google Scholar]
- Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, & Sakai RR (2010). Meal patterns and hypothalamic NPY expression during chronic social stress and recovery. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 299(3), R813–R822. 10.1152/ajpregu.00820.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Diaz MP, & Wilson ME (2016). Social change and access to a palatable diet produces differences in reward neurochemistry and appetite in female monkeys. Physiology & Behavior, 162, 102–111. 10.1016/j.physbeh.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, & Wilson ME (2011). Body weight decreases induced by estradiol in female rhesus monkeys are dependent upon social status. Physiology & Behavior, 102(3–4), 382–388. 10.1016/j.physbeh.2010.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal C, Griskevicius V, Simpson JA, Sung S, & Young ES (2015). Cognitive adaptations to stressful environments: When childhood adversity enhances adult executive function. Journal of Personality and Social Psychology, 109(4), 604–621. 10.1037/pspi0000028 [DOI] [PubMed] [Google Scholar]
- Moles A, Bartolomucci A, Garbugino L, Conti R, Caprioli A, Coccurello R, … Damato F (2006). Psychosocial stress affects energy balance in mice: Modulation by social status. Psychoneuroendocrinology, 31(5), 623–633. 10.1016/j.psyneuen.2006.01.004 [DOI] [PubMed] [Google Scholar]
- Monsivais P, Aggarwal A, & Drewnowski A (2011). Following federal guidelines to increase nutrient consumption may lead to higher food costs for consumers. Health Affairs, 30(8), 1471–1477. 10.1377/hlthaff.2010.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CJ, Lowe J, Michopoulos V, Ulam P, Toufexis D, Wilson ME, & Johnson Z (2013). Small changes in meal patterns lead to significant changes in total caloric intake. Effects of diet and social status on food intake in female rhesus monkeys. Appetite, 62, 60–69. 10.1016/j.appet.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel JV (1962). Diabetes Mellitus: A "Thrifty" Genotype Rendered Detrimental by "Progress"? American Journal of Human Genetics, 14(4), 363–362. 10.1007/SpringerReference_98337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettle D, Andrews C, & Bateson M (2017). Food insecurity as a driver of obesity in humans: The insurance hypothesis. Behavioral and Brain Sciences, 40, e105 10.1017/S0140525X16000947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberg S, & Kenrick A (2018). Discriminating ecologies: A life history approach to stigma and health. Oxford University Press. [Google Scholar]
- Pavela G, Lewis DW, Dawson JA, Cardel M, & Allison DB (2017). Social status and energy intake: a randomized controlled experiment. Clinical Obesity, 7(5), 316–322. 10.1111/cob.12198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper GV, & Nettle D (2014). Socioeconomic disparities in health behaviour: An evolutionary perspective In Gibson MA & Lawson DW (Eds.), Applied evolutionary anthropology: Darwinian approaches to contemporary world issues (pp. 225–243). New York, NY: Springer. [Google Scholar]
- Ravelli GP, Stein ZA, & Susser MW (1976). Obesity in young men after famine exposure in utero and early infancy. New England Journal of Medicine, 295(7), 349–353. 10.1056/NEJM197608122950701 [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, & Clarkson TB (2009). Social stress, visceral obesity, and coronary artery atherosclerosis: product of a primate adaptation. American Journal of Primatology, 71(9), 742–751. 10.1002/ajp.20706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim AY, Lim EX, Forde CG, & Cheon BK (2018). Personal relative deprivation increases self-selected portion sizes and food intake. Appetite, 121, 268–274. 10.1016/j.appet.2017.11.100 [DOI] [PubMed] [Google Scholar]
- Smith TG, Stoddard C, & Barnes MG (2007). Why the poor get fat: Weight gain and economic insecurity. SSRN Electronic Journal. 10.2139/ssrn.979189 [DOI] [Google Scholar]
- Stearns SC (1992). The evolution of life histories. Oxford: Oxford University Press. [Google Scholar]
- Swaffield J, & Roberts SC (2015). Exposure to cues of harsh or safe environmental conditions alters food preferences. Evolutionary Psychological Science, 1(2), 69–76. 10.1007/s40806-014-0007-z [DOI] [Google Scholar]
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, & Gortmaker SL (2011). The global obesity pandemic: Shaped by global drivers and local environments. The Lancet, 378(9793), 804–814. 10.1016/S0140-6736(11)60813-1 [DOI] [PubMed] [Google Scholar]
- Tamashiro KLK, Hegeman MA, & Sakai RR (2006). Chronic social stress in a changing dietary environment. Physiology and Behavior, 89(4), 536–542. 10.1016/j.physbeh.2006.05.026 [DOI] [PubMed] [Google Scholar]
- Tamashiro KLK, Nguyen MMN, Ostrander MM, Gardner SR, Ma LY, Woods SC, & Sakai RR (2007). Social stress and recovery: implications for body weight and body composition. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 293(5), R1864–R1874. 10.1152/ajpregu.00371.2007 [DOI] [PubMed] [Google Scholar]
- Thayer ZM, Wilson MA, Kim AW, & Jaeggi AV (2018). Impact of prenatal stress on offspring glucocorticoid levels: A phylogenetic meta-analysis across 14 vertebrate species. Scientific Reports, 8(1), 4942 10.1038/s41598-018-23169-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinbergen N (1963). On aims and methods of ethology. Zeitschrift Für Tierpsychologie, 20, 410–433. [Google Scholar]
- Torres SJ, & Nowson CA (2007). Relationship between stress, eating behavior, and obesity. Nutrition. 10.1016/j.nut.2007.08.008 [DOI] [PubMed] [Google Scholar]
- Trumbo P, Schlicker S, Yates AA, & Poos M (2002). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Journal of the American Dietetic Association. 10.1016/S0002-8223(02)90346-9 [DOI] [PubMed] [Google Scholar]
- Vandevijvere S, Chow CC, Hall KD, Umali E, & Swinburn BA (2015). Increased food energy supply as a major driver of the obesity epidemic: a global analysis. Bulletin of the World Health Organization, 93(7), 446–456. 10.2471/BLT.14.150565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, & Beydoun MA (2007). The obesity epidemic in the United States gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiologic Reviews, 29(1), 6–28. 10.1093/epirev/mxm007 [DOI] [PubMed] [Google Scholar]
- Waterlander WE, de Mul A, Schuit AJ, Seidell JC, & Steenhuis IH (2010). Perceptions on the use of pricing strategies to stimulate healthy eating among residents of deprived neighbourhoods: a focus group study. International Journal of Behavioral Nutrition and Physical Activity, 7(1), 44 10.1186/1479-5868-7-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JC (2006). The evolution of human fatness and susceptibility to obesity: an ethological approach. Biol Rev Camb Philos Soc, 81(2), 183–205. 10.1017/S1464793105006974 [DOI] [PubMed] [Google Scholar]
- Wells JCK (2010). Maternal capital and the metabolic ghetto: An evolutionary perspective on the transgenerational basis of health inequalities. American Journal of Human Biology, 22(1), 1–17. 10.1002/ajhb.20994 [DOI] [PubMed] [Google Scholar]
- Wells JCK (2010). The evolutionary biology of human body fatness : thrift and control. Cambridge studies in biological and evolutionary anthropology (Vol. 58). [Google Scholar]
- West-Eberhard MJ (2003). Developmental plasticity and evolution. Nature. Oxford: Oxford University Press; 10.2002/ajpa.20219 [DOI] [Google Scholar]
- Wiig Dammann K, & Smith C (2009). Factors affecting low-income women’s food choices and the perceived impact of dietary intake and socioeconomic status on their health and weight. Journal of Nutrition Education and Behavior, 41(4), 242–253. 10.1016/j.jneb.2008.07.003 [DOI] [PubMed] [Google Scholar]
- Wiig K, & Smith C (2009). The art of grocery shopping on a food stamp budget: factors influencing the food choices of low-income women as they try to make ends meet. Public Health Nutrition, 12(10), 1726 10.1017/S1368980008004102 [DOI] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, & Bartness TJ (2008). Quantifying food intake in socially housed monkeys: Social status effects on caloric consumption. Physiology & Behavior, 94(4), 586–594. 10.1016/j.physbeh.2008.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y (2012). Educational differences in obesity in the United States: a closer look at the trends. Obesity (Silver Spring, Md.), 20(4), 904–908. 10.1038/oby.2011.307 [DOI] [PubMed] [Google Scholar]
- Zagorsky JL, & Smith PK (2017). The association between socioeconomic status and adult fast-food consumption in the U.S. Economics & Human Biology, 27, 12–25. 10.1016/j.ehb.2017.04.004 [DOI] [PubMed] [Google Scholar]