Abstract
Na,K-ATPase is a membrane protein which plays a vital role. It pumps Na+ and K+ ions across the cellular membranes using energy from ATP hydrolysis, and is responsible for maintaining the osmotic equilibrium and generating the membrane potential. Moreover, Na,K-ATPase has also been involved in cell signaling, interacting with partner proteins. Cardiotonic steroids bind specifically to Na,K-ATPase triggering a number of signaling pathways. Because of its importance, many efforts have been employed to study the structure and function of this protein. Difficulties associated with its removal from natural membranes and the concomitant search for appropriate replacement conditions to keep the protein in solution have presented a challenge that had to be overcome prior to carrying out biophysical and biochemical studies in vitro. In this review, we summarized all of the methods and techniques applied by our group in order to obtain information about Na,K-ATPase in respect to solubilization, reconstitution into mimetic system, influence of lipid composition, stability, oligomerization, and aggregation.
Keywords: Na,K-ATPase; Membrane protein; Proteoliposome; Solubilization; Oligomerization
Structure and physiological importance
The discovery of the Na,K-ATPase (NKA) by Skou in 1957 (Skou 1957), for which he was awarded the 1997 Nobel Prize in Chemistry, was an important step in improving our understanding of the cell as basic unit for animal life (Skou 1957; Skou 1998). NKA is a membrane transport protein which belongs to the P-type family of active cation transport proteins and it can be found in the plasma membrane of practically all animal cells. NKA is responsible for the translocation of three Na+ ions out of the cell, whereas it pumps two K+ ions into the cell, against their concentration gradients, with this ion transport coupled with ATP (adenosine triphosphate) hydrolysis (Skou and Esmann 1992). The electrochemical gradient generated by NKA drives many other transport processes like co-transporters (sodium glucose), exchangers (Na+/Ca2+), amino acids, and vitamin transport into the cells (Lingrel 2010). The electrochemical gradient is also important for other physiological processes such as electrical excitability, nerve transmission, and muscle contraction (Bagrov et al. 2009; Reinhard et al. 2013).
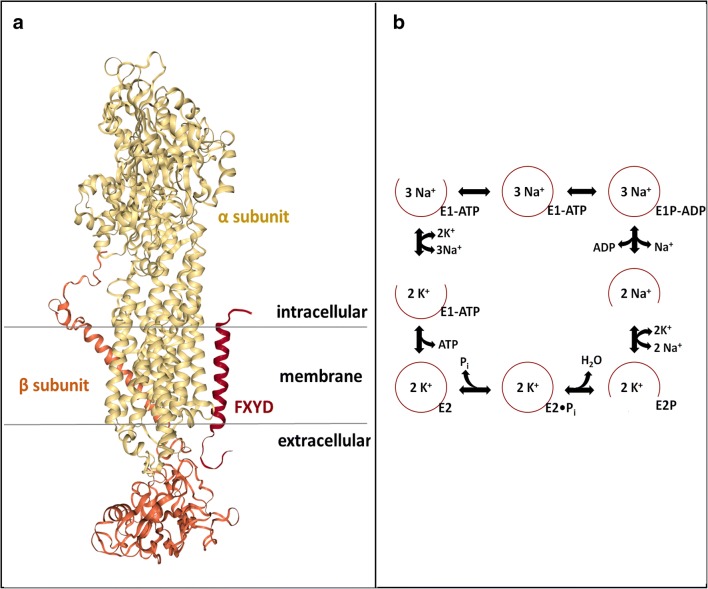
There are two main polypeptide chains (see Fig. 1a): the α (~ 110 kDa) and β subunit (~ 35–50 kDa). The α subunit consists of three domains (actuator, nucleotide binding, and phosphorylation site) and undergoes conformational changes while facilitating ion transportation through the membrane (Kaplan 2002; Skou and Esmann 1992). The presence of the β subunit is needed for translation, stability, and correct insertion of protein into the membrane (Barwe et al. 2007; Kaplan 2002; Skou and Esmann 1992). Also, the β subunit seems to have inherent functions such as cell adhesion and motility (Geering 2008). A third subunit is also reported, which is a small (~ 7–12 kDa) and hydrophobic protein (FXYD2 or γ subunit in kidney). It modulates the enzymatic activity by changing the Na+, K+, and ATP apparent affinity (Beguin et al. 1997; Geering 2008).
Fig. 1.
a Structural architecture of Na,K-ATPase [ProteinDataBank (PDB) ID: 3WGU] (Kanai et al., 2013). The three subunits are represented in different colors: yellow, α subunit; orange, β subunit; red, FXYD. b The Post-Albers catalytic cycle of Na,K-ATPase with the two main states: E1 and E2 (Albers, 1967; Post et al., 1969)
In general, there are two main conformational states (see Fig. 1b): the sodium-bound E1 state and the potassium-bound E2 state, which have been well elucidated by the corresponding crystallographic structures (Kanai et al. 2013; Morth et al. 2007).
It should be highlighted that there exist other cellular functions related to NKA beyond its role in ion pumping. For instance, there is much evidence that NKA plays an important role as a signal transducer, controlling a number of vital cell functions (Aperia et al. 2016). Some studies have suggested that NKA can interact with other proteins, and ouabain-dependent signaling can be mediated within caveolae by those protein complexes (Yosef et al. 2016). Another work compared NKA from caveolae and non-caveolae pools and concluded that even though the caveolar located enzyme plays a signaling role, it still retains its enzymatic activity alongside its ion pumping function (Liu et al. 2011).
Cardiotonic steroids (CS) (or cardiac glycosides), such as ouabain are well-characterized drugs previously used in the treatment of heart failure (Whayne 2018). They are specific NKA ligands, which bind to the extracellular domain of all NKA α subunit isoforms inhibiting its activity (Aperia et al. 2016). The inhibition of NKA increases the intracellular sodium concentration, activating the Na+/Ca2+- exchanger, which thus causes higher intracellular calcium concentration and, consequently, enhances the cardiac muscle contractility (Goncalves-de-Albuquerque et al. 2017).
In addition, recognition of CS as an antitumor molecule turned NKA into a promising drug target in the field of oncology (Alevizopoulos et al. 2014; Durlacher et al. 2015; Khajah et al. 2018). Several cell signaling pathways are activated upon the binding of CS to NKA, resulting in proliferation, differentiation, and promotion of autophagy or apoptosis. These effects are cell type and CS concentration dependent. Abnormal expression of NKA has been observed in cancer cells with higher activity exhibited during the transformation of malignant cells (Goncalves-de-Albuquerque et al. 2017). However, recent work has shown conflicting results indicating that cancer mortality was not significantly different for users of cardiac antiarrhythmia drugs (Kaapu et al. 2018). CS have also been described as being anti-inflammatory agents through studies in different animal models of acute and chronic inflammation (Fürst et al. 2017) and they have been recently reported as anti-viral agents (Amarelle and Lecuona 2018).
Furthermore, the pathogenesis of various diseases may be related to changes in NKA expression and/or malfunction. In fact, changes in NKA expression and activity were observed in patients with neurological diseases, diabetes, and hypertension (Chen et al. 2006; Clausen et al. 2017; Isaksen and Lykke-Hartmann 2016). Besides, there is evidence showing the involvement of NKA in cardiovascular and renal system function, sperm capacitation, rheumatoid arthritis, sepsis, pulmonary edema, and preeclampsia (Suhail 2010).
For instance, it is known that the kidneys of mammals are responsible for the homeostasis of extracellular compartments and the NKA is highly abundant in the renal epithelial cells, more specifically on the basolateral membrane where it plays an important role in salt regulation. There is a quantitative correlation between NKA levels and sodium reabsorption capacity, which is the reason that kidneys are abundant in this protein (therefore constituting a rich protein source for purification). NKA is involved in hormonal control of sodium transport in the kidney being an important molecular target of hormonal regulation (Féraille and Doucet 2001).
Considering NKA is one of the most abundant proteins in the brain, consuming 40–50% of energy in the ATP form, it is reasonable to associate its malfunction to a series of cellular abnormalities (Ferreira et al. 2011). In particular, the reduction of NKA activity causes a deficiency in cellular energy, which is commonly observed in neurodegenerative diseases (Kinoshita et al. 2016), such as Alzheimer’s disease (AD) (Zhang et al. 2013). It has been described that beta-amyloid (Aβ) peptide, which is found in the early stages of AD, interacts directly with NKA and inhibits its activity (Petrushanko et al. 2016). A suggestion raised by the authors in regard to the mechanism is that the exposition of NKA to β-amyloid and oxidative stress alter its function by redox modifications (Lakunina et al. 2017). It is believed that the interaction of NKA with β-amyloid oligomers (AβO) is the initial step that occurs at the cell surface, bringing about the pathological consequences (DiChiara et al. 2017; Ohnishi et al. 2015). The discovery about the role of oxidative stress in the impairment of NKA function and its relation with degenerative diseases may shed light in the development of proper treatments for these diseases (Omotayo et al. 2015).
NKA activity is known to be dependent on the redox state of the cells. Several studies have shown that the protein is redox-sensitive and may respond to changes in the level of intracellular glutathione (Lakunina et al. 2017; Mitkevich et al. 2016). Although the oxidative damage of NKA has been reported in the literature, the mechanism of redox-induced regulation is not fully understood (Petrushanko et al. 2006). Studies showed that the α subunit of NKA has a basal glutathionation, which is not abrogated by reducing agents. However, the function of this basal glutathionation is unknown, and the investigation is hampered by the absence of crystallographic structures in which the glutathione reactive amino acids were identified (Mitkevich et al. 2016).
NKA also has a role in adapting organisms to hypoxia or anoxia, although it does not have a specific site for oxygen (O2). The sensitivity of NKA to O2 occurs through different modifications such as S-glutathionation, S-nitrosylation, and redox-sensitive phosphorylation (Bogdanova et al. 2016). Four cysteine residues are found in the α subunit of NKA and these amino acids can be subjected to regulatory glutathionation induced by the increase of oxidized glutathione. This leads to a reversible inactivation of the enzyme, preventing the depletion of ATP in cells under oxidative stress (Mitkevich et al. 2016).
The third subunit of NKA belongs to the family of seven FXYD proteins. They are not essential for NKA function, but exhibit a regulatory role (Geering 2005). There are two conserved cysteines in the C-terminus of FXYD proteins and they have also been related to NKA regulatory glutathionylation (Bibert et al. 2011). Bibert et al. (2011) demonstrated that the cysteine residue on FXYD is susceptible to glutathionylation and the NKA inhibition caused by glutathionylation on β-subunit can be reversed by the presence of FXYD protein.
The impairment in NKA activity has been associated with the presence of certain diseases and also observed as a result of aging. In muscle cells, reduction in NKA enzymatic activity alters the membrane potential and consequently affects the muscle contraction strength, impairing physical activity in elderly people, who lose quality of life (Wyckelsma and McKenna 2016). A study comparing erythrocyte membranes from young and old rats detected a lower NKA activity coupled with increased lipid peroxidation in older rats (Rebrova et al. 2016). This may indicate that these processes (reduction of NKA activity and lipid peroxidation) are dependent on aging. However, it is not known whether they occur independently or whether the functional change of NKA is a consequence of membrane oxidation. Even though the structure of the protein may be a target of oxidative modifications, it is reasonable to assume that, as it is a membrane protein, alterations of membrane properties due to lipid oxidation (Itri et al. 2014) may also affect NKA activity. Studies have reported that lipid peroxidation together with reduced NKA activity is involved in the pathophysiology of bipolar disorder (Banerjee et al. 2012; Lichtstein et al. 2018a; Lichtstein et al. 2018b) and schizophrenia (Roy et al. 2016). The same was observed for coronary artery disease when the level of lipid peroxidation and NKA activity was compared in erythrocytes of sick patients and healthy individuals (Namazi et al. 2015).
Bearing in mind all these important aspects about NKA, the study of the isolated protein is an essential approach to achieve information about its structure and function. Because NKA is a membrane protein, its isolation and maintenance in solution is not a trivial task. Therefore, many efforts have been employed by our and other groups to obtain NKA in experimental conditions which allow studies on the enzymatic kinetics, protein–lipid interaction, structural stability, oligomerization, and aggregation as will be reported in the following sections.
Solubilization and purification
Proteins associated to membranes can be divided into three main groups: amphitropic, peripheral, and integral. Amphitropic proteins are weakly and reversibly linked to membrane through a conformational change that exposes a binding site guided by either phosphorylation or a ligand binding. Therefore, these proteins can be found released in the cytosol or attached to the membrane. Peripheral proteins are associated to membrane through electrostatic forces and hydrogen bonding to hydrophilic moieties of integral proteins and to membrane lipids. The consequence of these interactions is that they are susceptible to ionic strength and pH variations, which makes mild treatments with buffers and salts easy options to purify and isolate them. Integral membrane proteins are intrinsically associated to membrane. They are amphiphilic and large biomolecules either crossing both layers or buried in one of the leaflets of the lipid bilayer. This characteristic arises from the side groups of the amino acids that compose the polar and nonpolar domains of the protein, and that is why it has always been challenging to work with them. Integral membrane proteins experience two distinct environments defined by the membrane: the aqueous media (extracellular media/cytosol) and the hydrophobic core of the bilayer where acyl chains of the membrane lipids are oriented. In general, the study of integral membrane proteins involves three main steps: (Adamian et al., 2011) mechanical disruption of the membrane; (Albers, 1967) protein solubilization and purification with an appropriate detergent; (Akera et al., 1976) subsequent reconstitution into liposomes.
The complex structure of membrane proteins discussed above is indispensable for the comprehension of the necessity of solubilization. Detergents are amphiphilic molecules that in solution are in equilibrium between molecules solvated by water and exposed to the air/water interface where the hydrophobic chains partially face outside the liquid. As the concentration of detergent rises, the critical micellar concentration (CMC) can be achieved. At this point, the detergent monomers in solution are rearranged into micelles to mitigate the hydrophobic effect from solvating their hydrocarbon chains and expose only the hydrophilic group of the detergent. According to their class and type, the different detergent CMCs can be strongly influenced by physical parameters such as temperature, ionic strength, and pH (Helenius and Simons 1975; Santos and Ciancaglini 2000; Tanford and Reynolds 1976).
The use of the detergents to solubilize membrane fragments and separate proteins from their native lipid membrane has been extensively investigated from the early 1970s (Jorgensen and Skou 1971; Pitts et al. 1973; Jorgensen, 1974a, b; Helenius and Simons 1975; Tanford and Reynolds 1976). The first trials used ionic surfactants, such as SDS (sodium dodecyl sulfate), DOC (deoxycholate), and CA (cholic acid), and successfully reported the extraction of membrane proteins, since they are capable of opening lipid vesicles and solubilizing the hydrophobic domains of the membrane proteins (Santos and Ciancaglini 2000). However, they can also strongly influence protein–protein interactions and can act like denaturants when interacting with polar moieties and water soluble proteins. Nowadays, they are considered a risky choice for purification and, more importantly, for solubilization and maintenance of protein in solution. To overcome the denaturation process from ionic detergents, a more suitable approach involves the use of nonionic detergents like Lubrol, Polyoxyethylene, Triton, Tween, or zwitterionic such as CHAPS/CHAPSO. Such detergents have been used alone or in combination with a previous treatment involving ionic detergents (Brotherus et al. 1983; Cornelius 1991; Helenius and Simons 1975; Koepsell 1986; Santos and Ciancaglini 2000; Silvius 1992; Tanford and Reynolds 1976).
The process of solubilization of membrane proteins from natural tissues involves an initial mechanical disruption followed by centrifugation steps. This procedure selects membrane fractions that rearrange into microsomes, preserving the native lipids and the target protein along with other contaminants. Thereafter, the solubilization steps begin and microsomes are disrupted by adding appropriated detergents to break the native lipid–lipid and lipid–protein interactions of the vesicles and isolate micelles with proteins embedded (Cornelius 2001).
NKA is a good example of a membrane protein that displays a high level of solubilization complexity. The protein structure is extremely sensitive to several denaturing elements, from common ones such as temperature, ionic strength, pH, presence of native lipids, cholesterol levels, and the detergent concentration. Several authors have tried different methodologies and many have had some degree of success. Some researchers believe that pump activity and membrane organization are best studied in membrane-bound preparations due to preservation of native lipids and less perturbation of protein structure. In this way, the solubilization strategy is designed for the maintenance of NKA structure. In contrast, other researchers suggest that fully solubilized enzymes retain sufficient characteristics of the native enzyme while also allowing assays to be performed with high purity and without inappropriate interference from unknown biological factors. Both camps have sound scientific reasons for their viewpoints. In the end, experiments performed with these two types of enzyme preparations often produce results that are complementary and which can be used together as a type of internal experimental control. However, the best procedure to work with solubilized NKA still remains a matter for debate, since for each approach there is an enormous variety of methods, each one with positive and negative points that extend well beyond diversity of starting material tissues with singular specific activity (Jorgensen 1974a; Jorgensen 1974b; Jørgensen 1988).
In order to purify a high concentration of NKA pump, starting tissues must be selected based on their likely involvement in the active transport of Na+ across the membrane. As such, mammalian kidneys, brain tissue, heart, the electric organ of eels, and shark rectal glands are commonly the main source of the pump preparation (Jorgensen 1974a). Depending on the original tissue, successful preparations have yielded NKA over 90% purity after only one solubilization step, separating the solubilized enzyme from aggregates and insoluble byproducts. Although the extracts are pure, they can commonly be contaminated with another ATPase, the Mg+-ATPase, which requires activity tests using ouabain binding assays to properly differentiate the enzymes and the purity level of samples (summarized in Table 1).
Table 1.
NKA preparation methods in solubilized and membrane-bound purification
| Authors | Detergent treatment | Solubilization | Chromatography | Source | Specific activity (μmol mg−1 min−1) |
|---|---|---|---|---|---|
| (Jorgensen and Skou 1971) | Deoxycholate | No | No | Rabbit kidney | 4.5 |
| (Pitts et al. 1973) | Deoxycholate | No | No | Dog heart | 3.3 |
| (Jorgensen 1974a) | Deoxycholate or SDS | No | No | Mammalian kidney | 25–36 |
| (Jorgensen 1974b) | SDS | No | No | Mammalian kidney | 32–37 |
| (Akera et al. 1976) | Deoxycholate | No | No | Rat brain/Dog heart | 350 |
| (Hayashi et al. 1977) | Deoxycholate | SDS | No | Canine kidney | 26.7–30 |
| (Akera et al. 1978) | Deoxycholate | No | No | Dog heart | 2.5 |
| (Skou and Esmann 1979) | Deoxycholate | No | no | Shark rectal glands | 40–43.3 |
| (Esmann et al. 1979) | C12E8 | C12E8 | Gel | Shark rectal glands | 38.3 |
| (Brotherus et al. 1983) | SDS | C12E8 | Gel | Pig kidney | 35–48 |
| (Ottolenghi et al. 1986) | SDS | C12E8 | Cellulose | Pig kidney | 32 |
| (Esmann 1988) | Deoxycholate | C12E8 | Gel | Shark rectal glands | 8.3 |
| (Jørgensen 1988) | SDS | C12E8 | no | Mammalian kidney | 35–48 |
| (Morohashi et al. 1988) | Lubrol and SDS | Chaps | Chaps | Shrimp | 8.3 |
| (Peterson and Hokin 1988) | Lubrol and SDS | No | No | Shrimp | 10 |
| (Smith 1988) | Lubrol and SDS | No | No | Duck salt gland | 26.7–28.3 |
| (Hayashi et al. 1989) | SDS | C12E8 | Gel | Canine kidney | 3.3 |
| (Mimura et al. 1993) | SDS | C12E8 | Gel | Canine kidney | 38–48 |
| (Kessi et al. 1994) | SDS | – | No | Mammalian kidney | – |
| (Venter et al. 1997) | Deoxycholate | Lubrol | Gel | Sheep heart | 90 |
| (Mohraz 1999) | SDS | No | Wheat germ | Lamb/dog kidney | 25–30 |
| (Santos et al. 2002) | C12E8 | C12E8 | Gel | Rabbit kidney | 0.6–0.7 |
| (Laughery et al. 2004) | Immunoprecip. | DDM | No | Recombinant | 0.01–0.12 |
| (Cohen et al. 2005) | DDM | DDM | Affinity and gel | Recombinant | 0.025–0.040 |
| Ghosh et al. (Ghosh et al. 2009) |
DHPC or C12E8 or TX-100 |
DHPC/C12E8/ TX-100 |
Wheat germ, gel and immun. | Bovine pulm. artery | 12/8/6 |
| (Habeck et al. 2009) | DDM | C12E8 | Affinity | Recombinant alpha1/alpha2 | 14.1/8.5 |
Shark rectal glands are a great source of NKA protein and yield high concentrations of protein when using Hokin’s protocol (Hokin et al. 1973), although with less specific activity mostly related to precipitation with (NH4)2SO4 during the last step of purification. So, to overcome the loss of activity, Skou and Esmann (1979) used a methodology first developed for mammalian kidney outer medulla by Jorgensen and Skou (1971) and further adapted this protocol in 1974 (Jorgensen 1974b).
Initially in the 1960s, the pump was considered purified as membranous preparations, where crude tissue fractions were poorly solubilized with Lubrol, Deoxycholate, or SDS in concentrations above the CMC and separated by differential, isopynical or rate-zonal centrifugation, glycerol precipitation, or NaI treatment. Some of these procedures were briefly summarized by Jorgensen (1974a) who also described three methods of NKA isolation based on similar steps for mammalian kidney preparations. The first of these three methods involved cutting the red dark outer medulla from kidneys and preparing a homogenate which is centrifuged at 6000g for 15 min twice. Then, the supernatant is further centrifuged at 48,000g for 30 min and the pellet is selected and resuspended in sucrose buffer. The result is a microsomal-enriched NKA fraction. This fraction is then incubated with SDS or deoxycholate—a crucial step to break lipid–lipid and lipid–protein interactions thereby solubilizing the protein from its native environment in the membrane. The protein was considered to be solubilized when it remained in the supernatant after 1 h of centrifugation at 100,000–280,000g. Considering that the ionic detergents have strong denaturing effects on proteins, ATP was successfully used to protect membrane-bound NKA with purity estimated of 58% (Jorgensen 1974a). In 1988, Jørgensen (1988) extended this procedure rendering membrane-bound pump 95–100% pure when using centrifugation with a metrizamide gradient at 5900g for 2 h followed by incubation with SDS-ATP and a final centrifugation in a sucrose gradient. An angular rotor centrifugation assay after SDS-ATP treatment and without the metrizamide and sucrose gradient was also prepared, but this only yielded 40–60% purity. The procedures described by Jorgensen were widely adapted by several authors when isolating NKA from mammalian kidneys. In order to achieve the proper detergent molar ratio and prevent protein denaturation during isolation or solubilization, the strategy used by all researchers is to prepare ATPase activity vs detergent concentration assays as well as to check protein concentration in the supernatant along with protein activity following a 100,000g centrifugation step.
Previously mammalian heart tissues were often prepared using an adaptation of Pitts’ method which was based on homogenization with deoxycholate followed by two centrifugation steps where the pellets were resuspended in the presence of deoxycholate presence and further treated with NaI. The NaI-treated enzyme was then solubilized with deoxycholate once again, then centrifuged and the supernatant treated with glycerol 20%, which was then again centrifuged and the pellet homogenized in a non-detergent buffer (Akera et al. 1976; Pitts et al. 1973).
In 1994, DHPC (diheptanoylphosphatidylcholine) was introduced as a mild detergent capable of solubilizing plasma and organelle membrane constituents. It is a short chain phosphatidylcholine which has the dual properties of spreading among the lipids and breaking the membrane into micelles while also preserving the native phospholipids surrounding the proteins. Due to this dual action, DHPC showed a powerful ability to solubilize a greater amount of membrane-bound NKA than was previously prepared by using the Jorgensen method (Jørgensen 1988; Kessi et al. 1994). Later on, Ghosh and collaborator (Ghosh et al. 2009) isolated NKA from caveolae vesicles of bovine pulmonary artery smooth muscle plasma membrane and tested its solubilization against Triton X-100 1:1, C12E8 (octaethylene glycol monododecyl ether)1:1 and DHPC 1.5:1 ratio (w/w detergent:protein). Protein was then purified using sequential 30% and 50% ammonium sulfate precipitation steps. Wheat germ affinity chromatography and gel filtration with 0.005 mg/mL detergent was performed prior to a final immunoaffinity chromatography step using an anti α2 antibody in the presence of 0.05 mg/mL detergent. This concluded a very long systematic procedure in which DHPC was shown to be a superior choice for this tissue, targeting the specific α2β1 subunit of 155 kDa, which is wholly consistent with the expected association product between the alpha (110 kDa) and beta (45 kDa) subunits.
Since 2002, our group (Santos et al. 2002) has been using the homogenized red dark outer medulla from rabbit kidney to obtain membrane fractions with NKA as in (Jørgensen 1988), but with some modifications and without the addition of SDS. Solubilization was carried out exclusively using the nonionic detergent C12E8 with a maximum in both the recovery of protein and its specific activity obtained when using a protein/detergent mass ratio of 1:1. These remarkably simple conditions produced overall better performance—only a single detergent addition was required before the 100,000g centrifugation with this step directly followed by a single chromatographic step involving a Sepharose gel column. This optimized approach allows for a considerable saving of time in the production of highly purified solubilized protein. The specificity and purity of the sample was confirmed through use of the specific inhibitor ouabain with complete inhibition at 5 mM (99.1%). However, different from other preparations discussed, the kinetic results for NKA solubilized revealed two classes of ATP hydrolyzing sites. One of them in the micromolar range (high-affinity site) and another one in the millimolar range (low-affinity site). High-affinity sites correspond to approximately 15% of total activity, and low-affinity sites account for 85% of total activity. Such results suggest that the protein oligomerizes in the solubilized final sample. More details on this oligomerization will be provided in subsequent sections.
Reconstitution into lipid bilayer
It is known that membrane proteins are directly affected by lipid bilayer properties (Habeck et al. 2017). For the investigation of structure, stability, interaction, and kinetic properties of NKA, it is crucial to have it in a purified form, preferably in a lipid microenvironment, so as to achieve the nearest physiological medium as possible. Also, when a purified protein is incorporated into lipid vesicles, interference from other membrane constituents can be avoided. Another advantage is the possibility to control the lipid composition to analyze the role of different lipids in protein structure and function (Ciancaglini et al. 2012; Cornelius et al. 2015).
Obtainment of bilayer lipid vesicles with the integral protein incorporated (proteoliposome) is achieved by the co-solubilization method, which consists of first solubilizing the lipid/protein in detergent, followed by detergent removal (usually by using a hydrophobic resin) resulting in the spontaneous formation of proteoliposome (Ciancaglini et al. 2012).
Phospholipid chain length, degree of saturation, and presence of cholesterol are all crucial parameters of the lipid bilayer which must be explored to ensure NKA integrity and reconstitution (de Lima Santos et al. 2005). The lipid composition and the ratio among protein, detergent, and lipid (P:D:L) must be also explored in order to check the enzyme incorporation and activity recovery. The ratio (P:D:L) of 10:1:12.5 (w/w/w), corresponding to a molar ratio of 1:7730:4500 was found to be the best result (de Lima Santos et al. 2005). Another important condition was the incubation time of enzyme in the detergent-phospholipid solution. It must be lower than 10 min to minimize enzyme inactivation because of detergent excess (de Lima Santos et al. 2005).
Length variation in the fatty acid chain of PC (phosphatidylcholine) lipids played an effect on the differential incorporation of protein into the lipid phase and its resultant measured ATPase activity. Fatty acyl chains of 12 and 14 carbons (DLPC - 1,2-dilauroyl-sn-glycero-3-phosphocholine and DMPC - 1,2-dimyristoyl-sn-glycero-3-phosphocholine) achieved the lowest reconstitution degree, with a high percentage of open vesicles and protein aggregates. Incorporation of PC increased when the fatty acyl chain was increased to 16 carbons (DPPC - 1,2-dipalmitoyl-sn-glycero-3-phosphocholine), leading to the recovery of about 75% of ATPase activity. However, an opposite result was obtained upon further increase in the length of the phospholipid to 18 carbons (DSPC - 1,2-distearoyl-sn-glycero-3-phosphocholine) (de Lima Santos et al. 2005) confirming that the hydrophobic thickness is a crucial parameter for the optimal activity of the transporter protein. Dependence of NKA recovery and activity on the fatty acyl chain length of lipids was also reported by other groups. For instance, Cornelius (2001) reported that for saturated PC, the optimal recovery of protein took place with 16 carbons in the acyl chain, while for mono-unsaturated chains, the optimal length was 20 carbons. The inclusion of cholesterol along with mono-unsaturated PC improved the reconstitution for all lengths, mainly for the smaller acyl chains. The optimal activity was also compared when the number of carbons in the acyl chain ranged from 14 to 21 in the presence or absence of cholesterol. Inclusion of cholesterol changed the optimal recorded NKA ATPase activity from a 22 to 18 carbon chain length, with a general increase in activity when compared with cholesterol-free systems (Cornelius 2001). Besides PC lipids, a combination of one, two, or three lipids was tested, including DPPS (1,2-dipalmitoyl-sn-glycero-3-phospho-L-serine), DPPE (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine), DLOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine), and cholesterol. The presence of DPPS impaired the enzyme incorporation, while systems with phosphatidylcholine (PC) and phosphatidylethanolamine (PE) resulted in enhanced percentage of reconstitution, depending on the L:P ratio. If the ratio between DPPC and DLOPE increased from 1:1 (w/w) to 1:3 and 1:6 (w/w), there was a reduction in NKA incorporation. The system DPPC:DPPE achieved the highest enzymatic activity among the combinations tested and the inclusion of cholesterol favored the recovery of activity (de Lima Santos et al. 2005). The presence of cholesterol (10 to 40% mol) was also related to changes in thermostability as reported below and the activity was maximum at 20% mol of cholesterol (Yoneda et al. 2014). In fact, cholesterol seems to have a relevant role since it was demonstrated that its depletion inhibited NKA activity in a near-native membrane environment (Garcia et al. 2019).
Another important fact which should be highlighted is that the function of membrane proteins like NKA can be dependent on both general and specific lipid–protein interactions (Cornelius et al. 2015; Haviv et al. 2013). For instance, in the crystallographic structure of NKA obtained from shark, one cholesterol molecule appeared specifically bound to the protein structure, localized between α and β subunits (Shinoda et al. 2009). The presence of cholesterol in the NKA structure may play an important role in defining the interaction between its subunits (Adamian et al. 2011). Furthermore, phosphatidylserine (PS) has been described as a modulator of NKA activity via specific binding to NKA, although its site of action was not identified in any crystallographic structure as for cholesterol (Cornelius et al. 2015; Habeck et al. 2017; Haviv et al. 2013). Therefore, it is worth noting that not only bulk lipids but also specific lipids are essential for maintaining the function of membrane proteins (Adamian et al. 2011; Cornelius et al. 2015; Habeck et al. 2017).
Kinetic studies were performed using the DPPC:DPPE-NKA system, for which ATP hydrolysis site is located at the external side of the lipid bilayer vesicle (inside-out orientation). A potassium ion concentration of 150 mM was placed in the aqueous inner compartment. Two classes of ATP hydrolyzing sites at pH 7.5 were detected: a high-affinity site (K0.5 = 6.0 μM) and low-affinity site (K0.5 = 0.4 mM) which accounted for 54% and 46% of total activity, respectively (K0.5 = apparent dissociation constant) (Santos et al. 2006). The liposome/proteoliposome size was also evaluated by DLS (dynamic light scattering). There was a twofold increase in liposome diameter when the fatty acyl chain of PC varied from 12 to 18 carbons. For all samples, the corresponding proteoliposomes were larger (1.5–3-fold) compared with the lipid vesicles (liposomes) (de Lima Santos et al. 2005).
Further to these findings, the integrity of vesicles could be analyzed by fluorescent release assay, using calcein, which is a self-quenched fluorescent molecule. When calcein is entrapped inside the liposome or proteoliposome, there is no fluorescent signal. If there is the rupture of membrane, the calcein can be released and it will be diluted in the outer solution, which enhances its fluorescence signal. This effect was observed for DPPC:DPPE proteoliposomes with calcein entrapped, suggesting that the vesicles were closed before (no fluorescence) but then they were disrupted with the addition of detergent or alamethicin, thereby increasing the fluorescent signal (de Lima Santos et al. 2005).
Another issue related to membrane protein incorporation into the bilayer involves the orientation acquired by the protein. Vesicles with inhomogeneous orientation of protein can be formed depending on the method used. The orientation of NKA can be studied by taking advantage of the fact that ouabain and vanadate have specific binding sites located on opposite regions of the enzyme structure. The major advantage of this is that no radioactive material is needed. The inhibition of DPPC:DPPE proteoliposome by ouabain was dependent on the detergent concentration, while by vanadate, it was not. For the purified and solubilized enzyme, where both sites were exposed, there was no difference. These results are consistent with the enzyme being preferentially oriented inside-out (opposite to natural orientation on cells) the DPPC:DPPE proteoliposome (de Lima Santos et al. 2005). The dependence of NKA orientation on different lipid composition was also verified by Hickey and Buhr (2011) when they incorporated NKA into liposome made by lipids extracted from bull sperm membranes. Cornelius (2001) also described an orientation dependence on the length of acyl chains in the PL. In the presence or absence of cholesterol, inside-out orientation was maximal at an acyl chain of 16 carbons (Cornelius 2001).
Atomic force microscopy (AFM) has been recently used to confirm the orientation detected by enzymatic activity assays described above (Sebinelli et al., 2019). In these studies DPPC and DPPC:DPPE proteoliposomes were prepared and their surfaces were analyzed by using the AFM. Interestingly, images showed some protrusions that could be related to the presence of NKA microdomains. Depending on the lipid composition (DPPC or DPPC:DPPE), the dimensions of protuberances varied in diameter and height. For DPPC-proteoliposomes, the average diameter was 74 nm and the average height was 2.1 nm, while for DPPC:DPPE proteoliposomes, the average diameter was 38 nm and height was 0.7 nm (Sebinelli et al. 2019). Because of the difference in size of extra or intracellular domains, it was possible to estimate the orientation of protein inside the lipid membranes. Intracellular and extracellular parts have approximately 4 nm and 8 nm, respectively, so if the enzyme is inside-out oriented, the height expected for the protrusion on the surface is smaller than when protein has a right side-out orientation. DPPC-proteoliposome resulted in primarily right side-out orientation, while for DPPC:DPPE-system, inside-out orientation was predominant. These measurements of the extramembranous domains of protein performed by using AFM corroborated the enzymatic activity assay findings with respect to protein orientation dependent on lipid composition (de Lima Santos et al. 2005; Sebinelli et al. 2019).
A different approach for probing NKA position and orientation within a complex multi-domain lipid environment was developed by Bhatia and coworkers (Bhatia et al. 2016a, b). Their technique involved the incorporation of protein into GUVs (giant unilamellar vesicles). For instance, binary and ternary lipid systems capable of forming single- and two-phase systems (liquid-ordered and liquid-disordered) within the membrane were used and the authors suggested the preferential NKA localization in the interface between two phases, stabilizing the domain. On the other hand, a homogeneous NKA distribution in the lipid bilayer was observed in the single phase system (Bhatia et al. 2016a. b).
Planar lipid systems, such as nanodiscs, have recently been described as another tool for analyzing membrane protein in a different fashion to the use of closed vesicles (Denisov and Sligar 2016; Lee et al. 2016). This kind of system holds interest since both extra and intracellular regions of protein are exposed, eliminating any problems associated with different orientation of protein inside the vesicles and also display a more homogeneous array in terms of NKA orientation and size (Denisov and Sligar 2016; Lee et al. 2016). Another advantage of the nanodisc planar system is the possibility to maintain the endogenous lipids around the protein so as to infer the natural membrane constitution surrounding the protein. Nanodiscs are also an attractive platform for obtaining high-resolution structures of membrane proteins, being recently applied as object of study in cryo-electron microscopy (Sun and Gennis 2019).
Microenvironment and protein stability: microcalorimetry studies
Calorimetry is a biophysical tool that is widely used in the study of proteins and lipid membranes and which is capable of providing thermodynamic parameters characterizing the thermal denaturation or phase transition induced by temperature. We have used DSC (differential scanning calorimetry) for direct measurement of NKA thermal stability, exploring how different factors such as detergent concentration, presence of ions (Na+ and K+), substrate (ATP), lipids, and the γ subunit can influence the whole protein stability (Yoneda et al. 2013).
Membrane-bound NKA (meaning the crude extract obtained from the membrane fractions) obtained from the outer medulla of rabbit kidneys was analyzed. Deconvolution of its endotherm showed a five-step thermal denaturation, with transition temperatures (Tt) of 47.9, 52.9, 57.7, 62.9, and 69.0 °C. When the solubilization was carried out with the detergent C12E8, the profile from temperature scanning indicated a curve which could be decomposed in three separate transition temperatures: 55.0, 62.7, and 69.5 °C. The integral under the DSC curve gives the total enthalpy variation (∆H), which was larger for the membrane-bound than for the solubilized protein, suggesting a higher thermal stability for the first sample (Yoneda et al. 2013). Two other groups adopted a similar approach for studying the thermal denaturation of NKA extracted from different sources. Grinberg et al. (2001) found a three-step denaturation process with respective transition temperatures of 47.5, 54.3, and 58.4 °C for membrane-bound NKA extracted from pig kidney. Fodor et al. (2008) reported respective transition temperatures of 33.2, 41.5, 45.1, and 50.2 °C for membranous NKA from the shark and 49.0, 52.7, 54.6, 62.7, and 70.1 °C for membranous NKA from pig kidney. The shifts in the number and value of the transition temperatures observed between the pig kidney and shark rectal derived samples are likely due to the different sources of proteins and experimental conditions. During the catalytic cycle, NKA undergoes conformational changes between two main conformations: E1 and E2. It is worth noting that the former sample had K+ ions, which induce the E2 conformation. After removal of K+ from sample, the unfolding temperatures were decreased by up to 5 °C, evidencing the protector effect of K+ ions. Inclusion of Na+ ions in the sample induces E1 conformation, and a concomitant modification of the thermal denaturation curve was observed. By comparison of the total change in enthalpy (∆H) for both conformations, the E1-promoted sample (Na+ stabilized) can be shown to be less stable than the E2-promoted sample (K+ stabilized) (Yoneda et al. 2013).
The influence of detergent/protein (D/P) molar ratio on protein thermostability was also ascertained using DSC (Yoneda et al. 2013). The results showed the increase in D/P ratio resulted in a change in thermostability concomitant with the reduction of enzymatic activity. In conclusion, the D/P ratio has an important role to maintain the protein in a functional state following solubilization (Yoneda et al. 2013).
As described in the introduction, there is a third subunit which is not necessary for NKA pump function but which acts in a regulatory role. This third subunit is named gamma (γ) subunit in kidney-derived preparations. Because the γ subunit is small and hydrophobic, it is often lost during the solubilization/purification process along with much of the native lipid population. In order to investigate the effect of the gamma subunit (γ) on NKA thermal stability, it must be separately extracted from the membrane fractions and incubated with a sample of solubilized NKA (αβ) to obtain (αβγ) samples. Interestingly, its addition enhanced the enzymatic activity by approximately 15% and changed the thermal stability profile, especially in the beginning of thermal denaturation process, leading to the conclusion that the first step in the denaturation of NKA probably occurs in a region which interacts with the γ subunit (Yoneda et al. 2013).
In the NKA catalytic cycle, K+ ions release is accompanied by a detachment of cytoplasmic domains in the form E2(K+)2 in the presence of the substrate ATP, consequently leading to a decrease in cooperativity among the protein domains. This lower cooperativity was detected using DSC by observing peaks with higher resolution in the endotherm, demonstrating that the closed and open states are different enough to modify the thermal unfolding profile of NKA (Yoneda et al. 2013).
The effect of lipids was also evaluated by comparison of DSC traces recorded between the detergent-solubilized and lipid-incorporated NKA protein. DPPC/DPPE proteoliposome was shown to be more thermally resistant than solubilized NKA, as expected, since it is well known that membrane proteins interact directly with lipid surrounding in membrane, which is necessary for keeping the functional topology of this protein class. The important protein–lipid interaction is probably governed by a specific combination of lipids. This was shown by more detailed experiments involving DSC measurement of samples with different lipids bound to the protein and also by experiments that systematically changed lipid membrane properties (such as fluidity) (Cornelius et al. 2015; Habeck et al. 2017; Haviv et al. 2013; Yoneda et al. 2013).
All of these noted differences in NKA thermal stability are probably a consequence of the change in microenvironment around the NKA structure. This change in microenvironment may take the form of substitution of the endogenous lipid membrane by detergent molecules or artificial membrane, loss of the γ subunit during solubilization, and the different conformations induced by K+, Na+, or ATP (Fig. 2) (Yoneda et al. 2013).
Fig. 2.
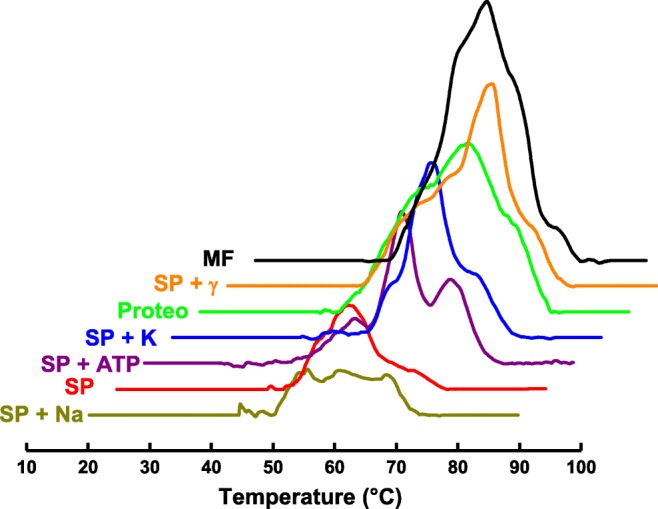
Thermal denaturation of NKA at different conditions. MF, membrane fraction; SP+γ, solubilized protein plus gamma subunit; Proteo, proteoliposome (DPPC:DPPE:NKA), SP+K, solubilized protein with 15 mM of potassium ions; SP+ATP, solubilized protein plus 3 mM of ATP; SP, solubilized protein in the absence of sodium and potassium ions; SP+Na, solubilized protein plus 15 mM of sodium ions
A variety of lipids in the plasma membrane as well as the lipid domain formation play important roles in the regulation of NKA (Cornelius 2008). The effect of cholesterol has been studied, since it is an abundant lipid in cellular membrane and has important roles on membrane properties as fluidity, permeability, and hydrophobicity (Ermilova and Lyubartsev 2018; Subczynski et al. 2017). Cholesterol acts to limit lipid motion by increasing the packing and thickness of the membrane (Ohvo-Rekila et al. 2002) and it is associated with lipid raft formation (Lingwood and Simons 2010). Cholesterol was included from 10 to 40 mol% in proteoliposome preparations in order to determine its influence on the thermal stability and function of NKA as measured by DSC. The variation resulted in a change of enzymatic activity, reaching a maximum at 20 mol% of cholesterol. The structural thermostability was shown to be highly dependent on the amount of cholesterol present. In absence of cholesterol, there were three peaks in the thermogram as seen for solubilized protein. When cholesterol was added into the system, there was a displacement of some temperature transitions, probably due to a conformational change producing different thermostability. This alteration corroborated the observed variation in ATPase activity depending on the cholesterol content. This work confirmed that changes in membrane lipid directly affect the protein conformation and consequently its thermal stability and function (Yoneda et al. 2014).
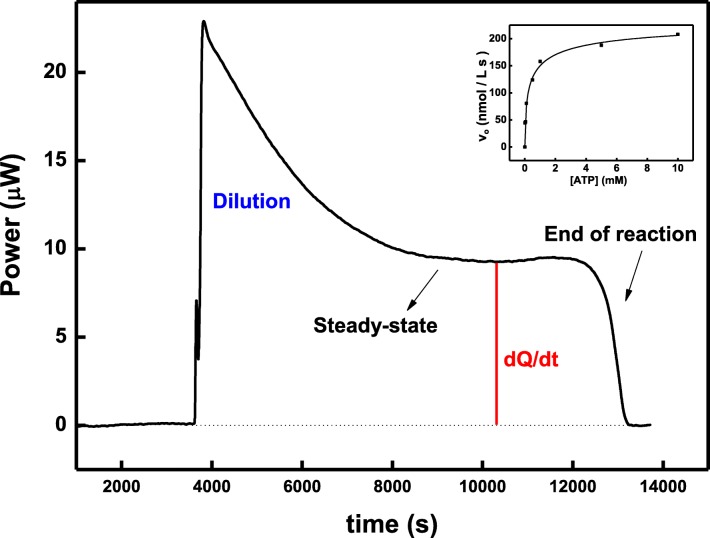
Another microcalorimetry technique, ITC (isothermal titration calorimetry) has also been used to measure enzymatic activity of NKA (Yoneda, J.S; unpublished data). The steady-state enzyme activity of the NKA can be evaluated by calorimetry since ATP hydrolysis is coupled with heat generation. In general, calorimetry (both ITC and DSC) have a number of advantages compared with normally used techniques to study kinetics of an enzyme. Other procedures such as discontinuous colorimetric enzyme assays, which means the reaction is stopped after a determined time followed by colorimetric methods used to determine the rate of inorganic phosphate released (Heinonen and Lahti 1981), have been widely used. These discontinuous approaches can be made continuous by using other enzymes to couple ATP hydrolysis to NADH oxidation producing an absorbance change detected spectrophotometrically (Schwartz et al. 1971). Also, radioactive-labeled substrate is commonly used for these purposes, however, with the disadvantage of contamination (Noske et al. 2010). As calorimetry does not require any of these additional complications, it is an appropriate technique for kinetic studies of enzymes. For ITC-based measurements, a single injection method was carried out at different ATP concentrations and the kinetic parameters were determined (Yoneda, JS; unpublished data). The enzyme solution was placed in the syringe and titrated into the reactional medium contained in the cell. It was possible to observe an initial peak, which is related to dilution. The apparent enthalpy variation for the reaction, ∆Happ, is given by the area under the isotherm curve. The dilution contribution was taken into account for the calculation of ∆Happ. After the peak of dilution, it is possible to observe a constant signal associated to the steady-state activity, which vanished after a certain time indicating the end of the reaction (Fig. 3).
Fig. 3.
Calorimetric signal related to steady-state activity of Na,K-ATPase after injection of 30 μL of protein solution (8375 nM) into the cell containing 914-μL reactional medium (HEPES 50 mM, pH 7.4, with ATP 1 mM, KCl 10 mM, MgCl2 5 mM e NaCl 50 mM). Inset: rate (mol/L s) vs subtract concentration (ATP)
For the ATP concentration equal to 1 mM (n = 0.914 μmol) the total heat was 110,094 μJ. The value of 42,039 μJ due to dilution was discounted. Therefore, the apparent enthalpy variation (∆Happ) was 74.4 kJ mol−1. The power (dQ/dt) was obtained as indicated in the Fig. 3 for each initial ATP concentration (only one curve is shown for clarification). The amount of heat associated with converting n moles of substrate to product is given by:
where V is the volume of solution in the reaction cell, [P] is the concentration of product generated, and ΔH is the molar enthalpy variation experimentally determined.
Therefore,
Rearranging,
Repeating the same procedure, varying the initial ATP concentration inside the cell, it was possible to obtain the rate of reaction depending on substrate concentration (Fig. 3, inset).
The kinetic parameters were then determined: the Michaelis constant at a submillimolar range as expected, Km = 0.41 mM, and the maximum velocity of reaction Vmax = 243 nmol/L s (Yoneda, JS; unpublished data).
Stoichiometry and subunits aggregation of Na,K-ATPase
Different stoichiometries are observed between α and β subunits of NKA depending on the method employed to solubilize and purify the enzyme (Hayashi et al. 1983; Kaya et al. 2003; Kobayashi et al. 2007; Mimura et al. 1993; Yoneda et al. 2016). It is well established that protomer (αβ) is enough to transport ions through cell membranes (Hayashi et al. 1989; Takeda and Kawamura 2001). Thus, there is no structural reason for the protein to oligomerize into dimers or higher oligomers for ion transportation. Nevertheless, several authors have suggested the involvement of diprotomer, tetraprotomer, or higher oligomers as constituting the nature of the NKA (Kobayashi et al. 2007; Laughery et al. 2004; Mimura et al. 2008; Taniguchi et al. 2001). Our group has also put effort into investigating this problem. However, the true nature of the oligomeric organization in membranes remains an open question. From the experimental point of view, many factors can contribute to aggregation of a membrane protein when it is submitted to solubilization/purification procedures. The parameters used in the process such as buffer composition, concentrations of enzyme, and detergent probably affect directly the association between polypeptide chains leading to either enhancement or repression of the formation of oligomers and/or aggregates in solution.
In order to investigate such an issue, a number of key parameters were controlled. For NKA solubilized with the detergent C12E8, the ratio between detergent and protein was varied followed by detection of possible structural changes by means of various biophysical techniques. CD (circular dichroism), for instance, did not show significant differences in spectra of NKA when its concentration varied from 0.1 to 0.6 mg mL−1 maintaining constant the C12E8 concentration equal to 0.005 mg mL−1. However, the CD profile and enzymatic activity were sensitive to surfactant concentration confirming that it is necessary to control this parameter to maintain the protein in a functional form (Rigos et al. 2008).
Measurements of surface tension and dilatational surface elasticity were also performed to gain information about the effect of detergent on protein and vice versa. The presence of enzyme (0.05, 0.2, or 0.5 mg mL−1) decreases the surface tension of C12E8 in the phospholipid bilayer. For low protein concentrations (0.05 and 0.2 mg mL−1), the surface tension in the phospholipid bilayer was virtually unchanged over the range of detergent concentration studied. On the other hand, at high enzyme concentration (0.5 mg mL−1), the surface tension significantly increased for low C12E8 concentration, before reaching an asymptotic limit at high C12E8 amounts. All these changes were hypothesized to be related to different oligomer species depending on the protein concentration (Rigos et al. 2008).
SAXS (small angle X-ray scattering) measurements were also performed to measure the size of the NKA aggregates as a function of surfactant amount. At low C12E8 concentration (0.005 mg mL−1) in 2 mg mL−1 enzyme solution, data analysis of SAXS profile suggested that NKA is associated as oligomers larger than (αβ)2, while at very high C12E8 concentration (2.7 mg mL−1), the enzyme dissociates with a subsequent self-assembling of α subunit into α4 aggregates (Souza Barbosa et al. 2010).
NKA aggregation was measured in protein reconstituted in liposomes by means of FTIR (Fourier transformed infrared) and fluorescence spectroscopy with or without proteolytic digestion of protein. The infrared spectrum of digested protein (which remained bound to the lipids) displayed a remarkably different profile as compared with the whole protein spectrum. However, by increasing the temperature to 60 °C, unfolding and aggregation were observed but not for the trypsinized sample, leading to the conclusion that the cytoplasmic part of α-subunit is the responsible for the NKA association (Rigos et al. 2010).
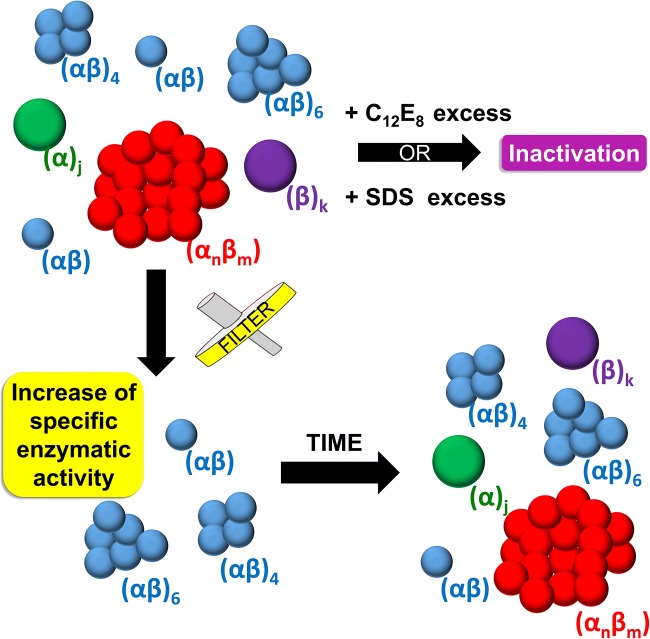
Measurements of DLS and AUC (analytical ultracentrifugation) confirmed that NKA, when solubilized and purified, presents as a heterogeneous particle size distribution in solution with C12E8(Yoneda et al. 2016). After the chromatography purification, seven different population sizes were identified by AUC, with monomers and tetramers corresponding to 55% of the total protein mass in solution. If the protein concentration was further increased, this percentage is decreased to 40%, indicating that other higher-order aggregates were formed. A simple filtration, using 220 nm pore filter of sample, was able to remove the higher-order oligomer/aggregate species with concomitant increase of the specific enzymatic activity, indicating the large species were inactive. However, once removed, the aggregation process started again after a 20-h time period. This finding revealed that C12E8-solubilized NKA is in a dynamic equilibrium of monomers, tetramers, and high-order oligomers/subunit aggregates. It was also shown that high amount of detergent leads to the dissociation of NKA into smaller aggregates with impaired enzymatic activity. In conclusion, increasing detergent concentration is not a good strategy to separate the large oligomer species (Fig. 4) (Yoneda et al. 2016).
Fig. 4.
Illustration scheme of the oligomerization and aggregation that possibly occur after the solubilization and purification of NKA with C12E8 detected by AUC, DLS, SAXS, and enzymatic activity assays
Conclusions
The study of isolated membrane proteins is necessary to determine particular information while avoiding interference of other components. To make these experiments feasible, necessary procedures are required to effect solubilization and purification. Biophysical tools employed to study NKA in vitro can provide relevant information dependent upon the success of its extraction from a natural source and subsequent purification. Here, we have reviewed results which describe how the experimental conditions and/or the environment around NKA can influence its measured properties. Controlling quality of sample is essential to avoid data misinterpretation, especially because the protein can associate in a variety of oligomer species when it is removed from the natural membrane. Our sincere hope is that this Review will help to promote best practice in the experimental preparation and experimental utilization of NKA in future biophysical investigations.
Acknowledgments
The authors thank Gustavo Scanavachi for the contributions with figure preparation and discussions.
Funding information
This work is financially supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Grants 2016/21236-6), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 304021/2017-2). JSY received CNPq fellowship grant 142248/2010-0 and currently receives FAPESP fellowship grant 2018/07194-9, HGS received CAPES PhD grant (88882328490/2019-01) and CAPES PhD sandwich grant (88887.368020/2019-00), RI and PC are recipients of CNPq research fellowships.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Juliana Sakamoto Yoneda and Heitor Gobbi Sebinelli contributed equally to this work.
References
- Adamian L, Naveed H, Liang J. Lipid-binding surfaces of membrane proteins: evidence from evolutionary and structural analysis. Biochim Biophys Acta. 2011;1808:1092–1102. doi: 10.1016/j.bbamem.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers RW. Biochemical aspects of active transport. Ann Biochem. 1967;36:727–756. doi: 10.1146/annurev.bi.36.070167.003455. [DOI] [PubMed] [Google Scholar]
- Akera T, Ku D, Tobin T, Brody TM. The complexes of ouabain with sodium- and potassium-activated adenosine triphosphatase formed with various ligands: relationship to the complex formed in the beating heart. Mol Pharmacol. 1976;12:101–114. [PubMed] [Google Scholar]
- Akera T, Temma K, Wiest SA, Brody TM. Reduction of the equilibrium binding of cardiac glycosides and related compounds to Na+,K+-ATPase as a possible mechanism for the potassium-induced reversal of their toxicity. Naunyn Schmiedeberg's Arch Pharmacol. 1978;304:157–165. doi: 10.1007/bf00495552. [DOI] [PubMed] [Google Scholar]
- Alevizopoulos K, Calogeropoulou T, Lang F, Stournaras C. Na+/K+ ATPase inhibitors in cancer. Curr Drug Targets. 2014;15:988–1000. doi: 10.2174/1389450115666140908125025. [DOI] [PubMed] [Google Scholar]
- Amarelle L, Lecuona E. The antiviral effects of Na,K-ATPase inhibition: a minireview. Int J Mol Sci. 2018;19:2154. doi: 10.3390/ijms19082154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aperia A, Akkuratov EE, Fontana JM, Brismar H. Na+-K+-ATPase, a new class of plasma membrane receptors. Am J Phys Cell Phys. 2016;310:C491–C495. doi: 10.1152/ajpcell.00359.2015. [DOI] [PubMed] [Google Scholar]
- Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61:9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee U, Dasgupta A, Rout JK, Singh OP. Effects of lithium therapy on Na+-K+-ATPase activity and lipid peroxidation in bipolar disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;37:56–61. doi: 10.1016/j.pnpbp.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Barwe SP, Kim S, Rajasekaran SA, Bowie JU, Rajasekaran AK. Janus model of the Na,K-ATPase beta-subunit transmembrane domain: distinct faces mediate alpha/beta assembly and beta-beta homo-oligomerization. J Mol Biol. 2007;365:706–714. doi: 10.1016/j.jmb.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguin P, Wang X, Firsov D, Puoti A, Claeys D, Horisberger JD, Geering K. The gamma subunit is a specific component of the Na,K-ATPase and modulates its transport function. EMBO J. 1997;16:4250–4260. doi: 10.1093/emboj/16.14.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia T, Cornelius F, Brewer J, Bagatolli L, Simonsen A, Ipsen J, Mouritsen O. Spatial distribution and activity of Na+/K+-ATPase in lipid bilayer membranes with phase boundaries. Biochim Biophys Acta. 2016;1858:1390–1399. doi: 10.1016/j.bbamem.2016.03.015. [DOI] [PubMed] [Google Scholar]
- Bhatia T, Cornelius F, Ipsen JH. Exploring the raft-hypothesis by probing planar bilayer patches of free-standing giant vesicles at nanoscale resolution, with and without Na,K-ATPase. Biochim Biophys Acta. 2016;1858:3041–3049. doi: 10.1016/j.bbamem.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Bibert S, Liu CC, Figtree GA, Garcia A, Hamilton EJ, Marassi FM, Sweadner KJ, Cornelius F, Geering K, Rasmussen HH. FXYD proteins reverse inhibition of the Na+-K+ pump mediated by glutathionylation of its beta1 subunit. J Biol Chem. 2011;286:18562–18572. doi: 10.1074/jbc.M110.184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova A, Petrushanko IY, Hernansanz-Agustín P, Martínez-Ruiz A. “Oxygen sensing” by Na,K-ATPase: these miraculous thiols. Front Physiol. 2016;7:314. doi: 10.3389/fphys.2016.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherus JR, Jacobsen L, Jorgensen PL. Soluble and enzymatically stable (Na+ + K+)-ATPase from mammalian kidney consisting predominantly of protomer alpha-beta-units: preparation, assay and reconstitution of active Na+,K+ transport. Biochim Biophys Acta. 1983;731:290–303. doi: 10.1016/0005-2736(83)90021-4. [DOI] [PubMed] [Google Scholar]
- Chen JQ, et al. Sodium/potassium ATPase (Na+, K+-ATPase) and ouabain/related cardiac glycosides: a new paradigm for development of anti-breast cancer drugs? Breast Cancer Res Treat. 2006;96:1–15. doi: 10.1007/s10549-005-9053-3. [DOI] [PubMed] [Google Scholar]
- Ciancaglini P, et al. Proteoliposomes in nanobiotechnology. Biophys Rev. 2012;4:67–81. doi: 10.1007/s12551-011-0065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen MV, Hilbers F, Poulsen H. The structure and function of the Na,K-ATPase isoforms in health and disease. Front Physiol. 2017;8:371. doi: 10.3389/fphys.2017.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Goldshleger R, Shainskaya A, Tal DM, Ebel C, le Maire M, Karlish SJ. Purification of Na+,K+-ATPase expressed in Pichia pastoris reveals an essential role of phospholipid-protein interactions. J Biol Chem. 2005;280:16610–16618. doi: 10.1074/jbc.M414290200. [DOI] [PubMed] [Google Scholar]
- Cornelius F. Functional reconstitution of the sodium pump kinetics of exchange reactions performed by reconstituted Na/K-ATPase. Biochim Biophys Acta. 1991;1071:19–66. doi: 10.1016/0304-4157(91)90011-k. [DOI] [PubMed] [Google Scholar]
- Cornelius F. Modulation of Na,K-ATPase and Na-ATPase activity by phospholipids and cholesterol. I. Steady-state kinetics. Biochem. 2001;40:8842–8851. doi: 10.1021/bi010541g. [DOI] [PubMed] [Google Scholar]
- Cornelius F. Cholesterol-dependent interaction of polyunsaturated phospholipids with Na,K-ATPase. Biochem. 2008;47:1652–1658. doi: 10.1021/bi702128x. [DOI] [PubMed] [Google Scholar]
- Cornelius F, Habeck M, Kanai R, Toyoshima C, Karlish SJ. General and specific lipid-protein interactions in Na,K-ATPase. Biochim Biophys Acta. 2015;1848:1729–1743. doi: 10.1016/j.bbamem.2015.03.012. [DOI] [PubMed] [Google Scholar]
- de Lima Santos H, Lopes ML, Maggio B, Ciancaglini P. Na,K-ATPase reconstituted in liposomes: effects of lipid composition on hydrolytic activity and enzyme orientation. Colloids Surf B: Biointerfaces. 2005;41:239–248. doi: 10.1016/j.colsurfb.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Denisov I, Sligar S. Nanodiscs for structural and functional studies of membrane proteins. Nat Struct Mol Biol. 2016;23:481–486. doi: 10.1038/nsmb.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiChiara T, et al. Alzheimer’s toxic amyloid beta oligomers: unwelcome visitors to the Na/K ATPase alpha3 docking station. Yale J biol med. 2017;90:45–61. [PMC free article] [PubMed] [Google Scholar]
- Durlacher CT, Chow K, Chen XW, He ZX, Zhang X, Yang T, Zhou SF. Targeting Na+/K+-translocating adenosine triphosphatase in cancer treatment. Clin Exp Pharmacol Physiol. 2015;42:427–443. doi: 10.1111/1440-1681.12385. [DOI] [PubMed] [Google Scholar]
- Ermilova I, Lyubartsev AP. Cholesterol in phospholipid bilayers: positions and orientations inside membranes with different unsaturation degrees. Soft Matter. 2018;15:78–93. doi: 10.1039/c8sm01937a. [DOI] [PubMed] [Google Scholar]
- Esmann M. Solubilization of Na+,K+-ATPase. Methods Enzymol. 1988;156:72–79. doi: 10.1016/0076-6879(88)56010-x. [DOI] [PubMed] [Google Scholar]
- Esmann M, Skou JC, Christiansen C. Solubilization and molecular weight determination of the (Na+ + K+)-ATPase from rectal glands of Squalus acanthias. Biochim Biophys Acta. 1979;567:410–420. doi: 10.1016/0005-2744(79)90127-x. [DOI] [PubMed] [Google Scholar]
- Féraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev. 2001;81:345–418. doi: 10.1152/physrev.2001.81.1.345. [DOI] [PubMed] [Google Scholar]
- Ferreira AG, et al. Role of antioxidants on Na(+),K (+)-ATPase activity and gene expression in cerebral cortex of hyperprolinemic rats. Metab Brain Dis. 2011;26:141–147. doi: 10.1007/s11011-011-9243-0. [DOI] [PubMed] [Google Scholar]
- Fodor E, Fedosova N, Ferencz C, Marsh D, Pali T, Esmann M. Stabilization of Na,K-ATPase by ionic interactions. Biochimica Et Biophysica Acta-Biomembranes. 2008;1778:835–843. doi: 10.1016/j.bbamem.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Fürst R, Zündorf I, Dingermann T. New knowledge about old drugs: the anti-inflammatory properties of cardiac glycosides. Planta Med. 2017;83:977–984. doi: 10.1055/s-0043-105390. [DOI] [PubMed] [Google Scholar]
- Garcia A, et al. Cholesterol depletion inhibits Na+,K+-ATPase activity in a near-native membrane environment. J Biol Chem. 2019;294:5956–5969. doi: 10.1074/jbc.RA118.006223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering K. Function of FXYD proteins regulators of Na,K-ATPase. J Bioenerg Biomembr. 2005;37:387–392. doi: 10.1007/s10863-005-9476-x. [DOI] [PubMed] [Google Scholar]
- Geering K. Functional roles of Na,K-ATPase subunits. Curr Opin Nephrol Hypertens. 2008;17:526–532. doi: 10.1097/MNH.0b013e3283036cbf. [DOI] [PubMed] [Google Scholar]
- Ghosh B, Chakraborti T, Kar P, Dey K, Chakraborti S. Solubilization, purification, and reconstitution of alpha 2 beta 1 isozyme of Na+/K+ -ATPase from caveolae of pulmonary smooth muscle plasma membrane: comparative studies with DHPC, C12E8, and Triton X-100. Mol Cell Biochem. 2009;323:169–184. doi: 10.1007/s11010-008-9977-0. [DOI] [PubMed] [Google Scholar]
- Goncalves-de-Albuquerque C, Silva A, da Silva C, Castro-Faria-Neto H, Burth P. Na/K pump and beyond: Na/K-ATPase as a modulator of apoptosis and autophagy. Molecules. 2017;22:578. doi: 10.3390/molecules22040578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg A, Gevondyan N, Grinberg N, Grinberg V. The thermal unfolding and domain structure of Na+/K+-exchanging ATPase. A scanning calorimetry study. Eur J Biochem. 2001;268:5027–5036. doi: 10.1046/j.0014-2956.2001.02436.x. [DOI] [PubMed] [Google Scholar]
- Habeck M, Cirri E, Katz A, Karlish SJ, Apell HJ. Investigation of electrogenic partial reactions in detergent-solubilized Na,K-ATPase. Biochemistry. 2009;48:9147–9155. doi: 10.1021/bi901148k. [DOI] [PubMed] [Google Scholar]
- Habeck M, Kapri-Pardes E, Sharon M, Karlish S. Proceedings of the National Academy of Sciences of the United States of America. 2017. Specific phospholipid binding to Na,K-ATPase at two distinct sites; pp. 2904–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haviv H, Habeck M, Kanai R, Toyoshima C, Karlish S. Neutral phospholipids stimulate Na,K-ATPase activity a specific lipid-protein interaction. J Biol Chem. 2013;288:10073–10081. doi: 10.1074/jbc.M112.446997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Kimimura M, Homareda H, Matsui H. Purification and characteristics of (Na+, K+)-ATPase from canine kidney by zonal centrifugation in sucrose density gradient. Biochim Biophys Acta. 1977;482:185–196. doi: 10.1016/0005-2744(77)90365-5. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Mimura K, Matsui H, Takagi T. Minimum enzyme unit for Na+/K+-ATPase is the alpha beta-protomer determination by low-angle laser light scattering photometry coupled with high-performance gel chromatography for substantially simultaneous measurement of ATPase activity and molecular weight. Biochim Biophys Acta. 1989;983:217–229. doi: 10.1016/0005-2736(89)90237-X. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Takagi T, Maezawa S, Matsui H. Molecular weights of alpha beta-protomeric and oligomeric units of soluble (Na+, K+)-ATPase determined by low-angle laser light scattering after high-performance gel chromatography. Biochim Biophys Acta. 1983;748:153–167. doi: 10.1016/0167-4838(83)90291-1. [DOI] [PubMed] [Google Scholar]
- Heinonen JK, Lahti RJ. A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal Biochem. 1981;113:313–317. doi: 10.1016/0003-2697(81)90082-8. [DOI] [PubMed] [Google Scholar]
- Helenius A, Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975;415:29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hickey KD, Buhr MM. Lipid bilayer composition affects transmembrane protein orientation and function. J Lipids. 2011;2011:208457. doi: 10.1155/2011/208457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin LE, Dahl JL, Deupree JD, Dioxon JF, Hackney JF, Perdue JF. Studies on the characterization of the sodium-potassium transport adenosine triphosphatase X. Purification of the enzyme from the rectal gland of Squalus acanthias. J Biol Chem. 1973;248:2593–2605. [PubMed] [Google Scholar]
- Isaksen TJ, Lykke-Hartmann K. Insights into the pathology of the α2-Na(+)/K(+)-ATPase in neurological disorders: lessons from animal models. Front Physiol. 2016;7:161. doi: 10.3389/fphys.2016.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri R, Junqueira HC, Mertins O, Baptista MS. Membrane changes under oxidative stress: the impact of oxidized lipids. Biophys Rev. 2014;6:47–61. doi: 10.1007/s12551-013-0128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen PL. Isolation of (Na+ plus K+)-ATPase. Methods Enzymol. 1974;32:277–290. doi: 10.1016/0076-6879(74)32029-0. [DOI] [PubMed] [Google Scholar]
- Jorgensen PL. Purification and characterization of (Na+ plus K+ )-ATPase. 3. Purification from the outer medulla of mammalian kidney after selective removal of membrane components by sodium dodecylsulphate. Biochim Biophys Acta. 1974;356:36–52. doi: 10.1016/0005-2736(74)90292-2. [DOI] [PubMed] [Google Scholar]
- Jørgensen PL. Purification of Na+,K+-ATPase: enzyme sources, preparative problems, and preparation from mammalian kidney. Methods Enzymol. 1988;156:29–43. doi: 10.1016/0076-6879(88)56005-6. [DOI] [PubMed] [Google Scholar]
- Jorgensen PL, Skou JC. Purification and characterization of (Na+ + K+)-ATPase. I. The influence of detergents on the activity of (Na+ + K+)-ATPase in preparations from the outer medulla of rabbit kidney. Biochim Biophys Acta. 1971;233:366–380. doi: 10.1016/0005-2736(71)90334-8. [DOI] [PubMed] [Google Scholar]
- Kaapu KJ, Rantaniemi L, Talala K, Taari K, Tammela TLJ, Auvinen A, Murtola TJ. Cancer mortality does not differ by antiarrhythmic drug use: a population-based cohort of Finnish men. Sci Rep. 2018;8:10308. doi: 10.1038/s41598-018-28541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Ogawa H, Vilsen B, Cornelius F, Toyoshima C. Crystal structure of a Na+-bound Na+,K+-ATPase preceding the E1P state. Nature. 2013;502:201–206. doi: 10.1038/nature12578. [DOI] [PubMed] [Google Scholar]
- Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- Kaya S, et al. Oligomeric structure of P-type ATPases observed by single molecule detection technique. Ann N Y Acad Sci. 2003;986:278–280. doi: 10.1111/j.1749-6632.2003.tb07185.x. [DOI] [PubMed] [Google Scholar]
- Kessi J, Poirée JC, Wehrli E, Bachofen R, Semenza G, Hauser H. Short-chain phosphatidylcholines as superior detergents in solubilizing membrane proteins and preserving biological activity. Biochemistry. 1994;33:10825–10836. doi: 10.1021/bi00201a033. [DOI] [PubMed] [Google Scholar]
- Khajah MA, Mathew PM, Luqmani YA. Na+/K+ ATPase activity promotes invasion of endocrine resistant breast cancer cells. PLoS One. 2018;13:e0193779. doi: 10.1371/journal.pone.0193779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita PF, Leite JA, Orellana AM, Vasconcelos AR, Quintas LE, Kawamoto EM, Scavone C. The influence of Na(+), K(+)-ATPase on glutamate signaling in neurodegenerative diseases and senescence. Front Physiol. 2016;7:195. doi: 10.3389/fphys.2016.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Tahara Y, Takenaka H, Mimura K, Hayashi Y. Na+- and K+-dependent oligomeric interconversion among alphabeta-protomers, diprotomers and higher oligomers in solubilized Na+/K+-ATPase. J Biochem. 2007;142:157–173. doi: 10.1093/jb/mvm150. [DOI] [PubMed] [Google Scholar]
- Koepsell H. Methodological aspects of purification and reconstitution of transport proteins from mammalian plasma membranes. Rev Physiol Biochem Pharmacol. 1986;104:65–137. doi: 10.1007/bfb0031013. [DOI] [PubMed] [Google Scholar]
- Lakunina VA, Petrushanko IY, Burnysheva KM, Mitkevich VA, Makarov AA. Alzheimer’s disease Aβ 42 peptide induces an increase in Na,K-ATPase glutathionylation. Dokl Biochem Biophys. 2017;473:114–117. doi: 10.1134/S1607672917020077. [DOI] [PubMed] [Google Scholar]
- Laughery M, Todd M, Kaplan JH. Oligomerization of the Na,K-ATPase in cell membranes. J Biol Chem. 2004;279:36339–36348. doi: 10.1074/jbc.M402778200. [DOI] [PubMed] [Google Scholar]
- Lee S, et al. A method for detergent-free isolation of membrane proteins in their local lipid environment. Nat Protoc. 2016;11:1149–1162. doi: 10.1038/nprot.2016.070. [DOI] [PubMed] [Google Scholar]
- Lichtstein D, Ilani A, Rosen H, Horesh N, Singh SV, Buzaglo N, Hodes A. Na+, K+-ATPase signaling and bipolar disorder. Int J Mol Sci. 2018;19:2314. doi: 10.3390/ijms19082314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtstein D, Ilani A, Rosen H, Horesh N, Singh SV, Buzaglo N, Hodes A. Na+, K+-ATPase signaling and bipolar disorder. Int J Mol Sci. 2018;19:2314. doi: 10.3390/ijms19082314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingrel JB. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu Rev Physiol. 2010;72:395–412. doi: 10.1146/annurev-physiol-021909-135725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- Liu L, Ivanov AV, Gable ME, Jolivel F, Morrill GA, Askari A. Comparative properties of caveolar and noncaveolar preparations of kidney Na+/K+-ATPase. Biochemistry. 2011;50:8664–8673. doi: 10.1021/bi2009008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura K, Matsui H, Takagi T, Hayashi Y. Change in oligomeric structure of solubilized Na+/K(+)-ATPase induced by octaethylene glycol dodecyl ether, phosphatidylserine and ATP. Biochim Biophys Acta. 1993;1145:63–74. doi: 10.1016/0005-2736(93)90382-A. [DOI] [PubMed] [Google Scholar]
- Mimura K, Tahara Y, Shinji N, Tokuda E, Takenaka H, Hayashi Y. Isolation of stable (alpha beta)(4)-tetraprotomer from Na+/K+-ATPase solubilized in the presence of short-chain fatty acids. Biochemistry. 2008;47:6039–6051. doi: 10.1021/Bi800445f. [DOI] [PubMed] [Google Scholar]
- Mitkevich VA, Petrushanko IY, Poluektov YM, Burnysheva KM, Lakunina VA, Anashkina AA, Makarov AA. Basal glutathionylation of Na,K-ATPase α-subunit depends on redox status of cells during the enzyme biosynthesis. Oxidative Med Cell Longev. 2016;2016:9092328. doi: 10.1155/2016/9092328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohraz M. Reconstitution of detergent-solubilized Na,K-ATPase and formation of two-dimensional crystals. J Struct Biol. 1999;125:76–85. doi: 10.1006/jsbi.1998.4067. [DOI] [PubMed] [Google Scholar]
- Morohashi M, Konishi K, Kawamura M. Effects of Na+ and K+ on Artemia salina (Na,K)-ATPase solubilized with a zwitterionic detergent, CHAPS. J Biochem. 1988;103:1073–1077. doi: 10.1093/oxfordjournals.jbchem.a122382. [DOI] [PubMed] [Google Scholar]
- Morth JP, et al. Crystal structure of the sodium–potassium pump. nature. 2007;450:1043–1049. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- Namazi G, Jamshidi Rad S, Attar AM, Sarrafzadegan N, Sadeghi M, Naderi G, Pourfarzam M. Increased membrane lipid peroxidation and decreased Na+/K+-ATPase activity in erythrocytes of patients with stable coronary artery disease. Coron Artery Dis. 2015;26:239–244. doi: 10.1097/MCA.0000000000000196. [DOI] [PubMed] [Google Scholar]
- Noske R, Cornelius F, Clarke RJ. Investigation of the enzymatic activity of the Na+,K+-ATPase via isothermal titration microcalorimetry. Biochim Biophys Acta. 2010;1797:1540–1545. doi: 10.1016/j.bbabio.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, et al. Na, K-ATPase α3 is a death target of Alzheimer patient amyloid-β assembly. Proc Natl Acad Sci U S A. 2015;112:E4465–E4474. doi: 10.1073/pnas.1421182112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohvo-Rekila H, Ramstedt B, Leppimaki P, Slotte J. Cholesterol interactions with phospholipids in membranes. Prog Lipid Res. 2002;41:66–97. doi: 10.1016/S0163-7827(01)00020-0. [DOI] [PubMed] [Google Scholar]
- Omotayo TI, Akinyemi GS, Omololu PA, Ajayi BO, Akindahunsi AA, Rocha JB, Kade IJ. Possible involvement of membrane lipids peroxidation and oxidation of catalytically essential thiols of the cerebral transmembrane sodium pump as component mechanisms of iron-mediated oxidative stress-linked dysfunction of the pump’s activity. Redox Biol. 2015;4:234–241. doi: 10.1016/j.redox.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi P, Nørby JG, Jensen J. Solubilization and further chromatographic purification of highly purified, membrane-bound Na,K-ATPase. Biochem Biophys Res Commun. 1986;135:1008–1014. doi: 10.1016/0006-291x(86)91028-4. [DOI] [PubMed] [Google Scholar]
- Peterson GL, Hokin LE. Preparation of Na+,K+-ATPase from brine shrimp. Methods Enzymol. 1988;156:48–65. doi: 10.1016/0076-6879(88)56008-1. [DOI] [PubMed] [Google Scholar]
- Petrushanko I, et al. Na-K-ATPase in rat cerebellar granule cells is redox sensitive. Am J Phys Regul Integr Comp Phys. 2006;290:R916–R925. doi: 10.1152/ajpregu.00038.2005. [DOI] [PubMed] [Google Scholar]
- Petrushanko IY, et al. Direct interaction of beta-amyloid with Na,K-ATPase as a putative regulator of the enzyme function. Sci Rep. 2016;6:27738. doi: 10.1038/srep27738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts BJ, Lane LK, Schwartz A. Nature of the transport ATPase-digitalis complex. VI. Purification of and ouabain binding to a highly active cardiac Na+, K+-ATPase. Biochem Biophys Res Commun. 1973;53:1060–1066. doi: 10.1016/0006-291x(73)90572-x. [DOI] [PubMed] [Google Scholar]
- Post RL, Kume S, Tobim T, Orcutt B, Sem AK. Flexibility of an active center in sodium-plus-potassium adenosine triphosphatase. J Gen Physiol. 1969;54:306–326. doi: 10.1085/jgp.54.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrova TY, Afanasiev SA, Popov SV. Age-dependent changes in Na(+),K(+)-ATPase activity and lipid peroxidation in membranes of erythrocytes during cardiosclerosis development in rats. Bull Exp Biol Med. 2016;161:235–236. doi: 10.1007/s10517-016-3384-4. [DOI] [PubMed] [Google Scholar]
- Reinhard L, Tidow H, Clausen MJ, Nissen P. Na(+),K (+)-ATPase as a docking station: protein-protein complexes of the Na(+),K (+)-ATPase. Cell Mol Life Sci. 2013;70:205–222. doi: 10.1007/s00018-012-1039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigos CF, Nobre TM, Zaniquelli ME, Ward RJ, Ciancaglini P. The association of Na,K-ATPase subunits studied by circular dichroism, surface tension and dilatational elasticity. J Colloid Interface Sci. 2008;325:478–484. doi: 10.1016/j.jcis.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Rigos CF, Santos HL, Yoneda JS, Montich G, Maggio B, Ciancaglini P. Cytoplasmatic domain of Na,K-ATPase alpha-subunit is responsible for the aggregation of the enzyme in proteoliposomes. Biophys Chem. 2010;146:36–41. doi: 10.1016/j.bpc.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Roy S, Dasgupta A, Banerjee U, Chowdhury P, Mukhopadhyay A, Saha G, Singh O. Role of membrane cholesterol and lipid peroxidation in regulating the Na+/K+-ATPase activity in schizophrenia. Indian J Psychiatry. 2016;58:317–325. doi: 10.4103/0019-5545.192023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos HL, Ciancaglini P. A practical approach to the choice of a suitable detergent and optimal conditions for solubilizing a membrane protein. Biochem Educ. 2000;28:178–182. doi: 10.1111/j.1539-3429.2000.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Santos HL, Lamas RP, Ciancaglini P. Solubilization of Na,K-ATPase from rabbit kidney outer medulla using only C12E8. Braz J Med Biol Res. 2002;35:277–288. doi: 10.1590/S0100-879X2002000300002. [DOI] [PubMed] [Google Scholar]
- Santos R, Rigos C, Clancaglini P. Kinetics behaviors of NaK-ATPase: comparison of solubilized and DPPC : DPPE-liposome reconstituted enzyme. Compar Biochem Physiol C-Toxicol Pharmacol. 2006;142:309–316. doi: 10.1016/j.cbpc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Schwartz A, Nagano K, Nakao M, Lindenmayer GE, Allen JC, Matsui H. The sodium- and potassium-activated adenotriphosphatase system meth. Pharmacol. 1971;1:361–388. [Google Scholar]
- Sebinelli HG, Borin IA, Ciancaglini P, Bolean M. Topographical and mechanical properties of liposome surfaces harboring Na,K-ATPase by means of atomic force microscopy. Soft Matter. 2019;15:2737–2745. doi: 10.1039/c9sm00040b. [DOI] [PubMed] [Google Scholar]
- Shinoda T, Ogawa H, Cornelius F, Toyoshima C. Crystal structure of the sodium-potassium pump at 2.4 A resolution. nature. 2009;459:446–450. doi: 10.1038/nature07939. [DOI] [PubMed] [Google Scholar]
- Silvius JR. Solubilization and functional reconstitution of biomembrane components. Annu Rev Biophys Biomol Struct. 1992;21:323–348. doi: 10.1146/annurev.bb.21.060192.001543. [DOI] [PubMed] [Google Scholar]
- Skou JC. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957;23:394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- Skou Jens C. The Identification of the Sodium-Potassium Pump (Nobel Lecture) Angewandte Chemie International Edition. 1998;37(17):2320–2328. doi: 10.1002/(SICI)1521-3773(19980918)37:17<2320::AID-ANIE2320>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Skou JC, Esmann M. Preparation of membrane-bound and of solubilized (Na+ + K+)-ATPase from rectal glands of Squalus acanthias. The effect of preparative procedures on purity, specific and molar activity. Biochim Biophys Acta. 1979;567:436–444. doi: 10.1016/0005-2744(79)90129-3. [DOI] [PubMed] [Google Scholar]
- Skou JC, Esmann M. The Na,K-ATPase. J Bioenerg Biomembr. 1992;24:249–261. doi: 10.1007/BF00768846. [DOI] [PubMed] [Google Scholar]
- Smith TW. Purification of Na+,K+-ATPase from the supraorbital salt gland of the duck. Methods Enzymol. 1988;156:46–48. doi: 10.1016/0076-6879(88)56007-x. [DOI] [PubMed] [Google Scholar]
- Souza Barbosa LR, Rigos CF, Yoneda JS, Itri R, Ciancaglini P. Unraveling the Na,K-ATPase alpha(4) subunit assembling induced by large amounts of C12E8 by means of small-angle X-ray scattering. J Phys Chem B. 2010;114:11371–11376. doi: 10.1021/jp1013829. [DOI] [PubMed] [Google Scholar]
- Subczynski WK, Pasenkiewicz-Gierula M, Widomska J, Mainali L, Raguz M. High cholesterol/low cholesterol: effects in biological membranes: a review. Cell Biochem Biophys. 2017;75:369–385. doi: 10.1007/s12013-017-0792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhail M. Na, K-ATPase: ubiquitous multifunctional transmembrane protein and its relevance to various pathophysiological conditions. J Clin Med Res. 2010;2:1–17. doi: 10.4021/jocmr2010.02.263w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Gennis RB. Single-particle cryo-EM studies of transmembrane proteins in SMA copolymer nanodiscs. Chem Phys Lipids. 2019;221:114–119. doi: 10.1016/j.chemphyslip.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Kawamura M. The functional unit of Na,K-ATPase is a monomeric alpha beta protomer. Biochem Biophys Res Commun. 2001;280:1364–1366. doi: 10.1006/bbrc.2001.4292. [DOI] [PubMed] [Google Scholar]
- Tanford C, Reynolds JA. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976;457:133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Kaya S, Abe K, Mardh S. The oligomeric nature of Na/K-transport ATPase. J Biochem. 2001;129:335–342. doi: 10.1093/oxfordjournals.jbchem.a002862. [DOI] [PubMed] [Google Scholar]
- Venter PA, Naudé RJ, Oelofsen W, Swan GE. Ovine cardiac Na,K-ATPase: isolation by means of selective solubilization in Lubrol and the effect of 1 alpha,2 alpha-epoxyscillirosidin on this enzyme. Int J Biochem Cell Biol. 1997;29:1103–1112. doi: 10.1016/s1357-2725(97)00046-0. [DOI] [PubMed] [Google Scholar]
- Whayne TF. Clinical use of digitalis: a state of the art review. Am J Cardiovasc Drugs. 2018;18:427–440. doi: 10.1007/s40256-018-0292-1. [DOI] [PubMed] [Google Scholar]
- Wyckelsma VL, McKenna MJ. Effects of age on Na(+),K(+)-ATPase expression in human and rodent skeletal muscle. Front Physiol. 2016;7:316. doi: 10.3389/fphys.2016.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda JS, Rigos CF, Aranda de Lourenco TF, Sebinelli HG, Ciancaglini P. Na,K-ATPase reconstituted in ternary liposome: the presence of cholesterol affects protein activity and thermal stability. Arch Biochem Biophys. 2014;564:136–141. doi: 10.1016/j.abb.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Yoneda JS, Rigos CF, Ciancaglini P. Addition of subunit gamma, K+ ions, and lipid restores the thermal stability of solubilized Na,K-ATPase. Arch Biochem Biophys. 2013;530:93–100. doi: 10.1016/j.abb.2012.12.022. [DOI] [PubMed] [Google Scholar]
- Yoneda JS, Scanavachi G, Sebinelli HG, Borges JC, Barbosa LRS, Ciancaglini P, Itri R. Multimeric species in equilibrium in detergent-solubilized Na,K-ATPase. Int J Biol Macromol. 2016;89:238–245. doi: 10.1016/j.ijbiomac.2016.04.058. [DOI] [PubMed] [Google Scholar]
- Yosef E, Katz A, Peleg Y, Mehlman T, Karlish S. Do Src kinase and caveolin interact directly with Na,K-ATPase? J Biol Chem. 2016;291:11736–11750. doi: 10.1074/jbc.M116.721084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LN, et al. Na+-K+-ATPase, a potent neuroprotective modulator against Alzheimer disease. Fundam Clin Pharmacol. 2013;27:96–103. doi: 10.1111/fcp.12000. [DOI] [PubMed] [Google Scholar]