Abstract
The cyclic side chain of the amino acid proline confers unique conformational restraints on its backbone and side chain dihedral angles. This affects two equilibria—one at the backbone (cis/trans) and the other at the side chain (endo/exo). Substitutions on the proline ring impose additional steric and stereoelectronic effects that can further modulate both these equilibria, which in turn can also affect the backbone dihedral angle (ϕ, ψ) preferences. In this review, we have explored the conformational landscape of several termini capped mono-(2-, 3-, 4-, and 5-) substituted proline derivatives in the Cambridge Structural Database, correlating observed conformations with the nature of substituents and deciphering the underlying interactions for the observed structural biases. The impact of incorporating these derivatives within model peptides and proteins are also discussed for selected cases. Several of these substituents have been used to introduce bioorthogonal functionality and modulate structure-specific ligand recognition or used as spectroscopic probes. The incorporation of these diversely applicable functional groups, coupled with their ability to define an amino acid conformation via stereoelectronic effects, have a broad appeal among chemical biologists, molecular biophysicists, and medicinal chemists.
Electronic supplementary material
The online version of this article (10.1007/s12551-020-00621-8) contains supplementary material, which is available to authorized users.
Keywords: Proline, Substituted proline, Conformational restriction, Collagen, Exo-endo, Cis-trans
Introduction
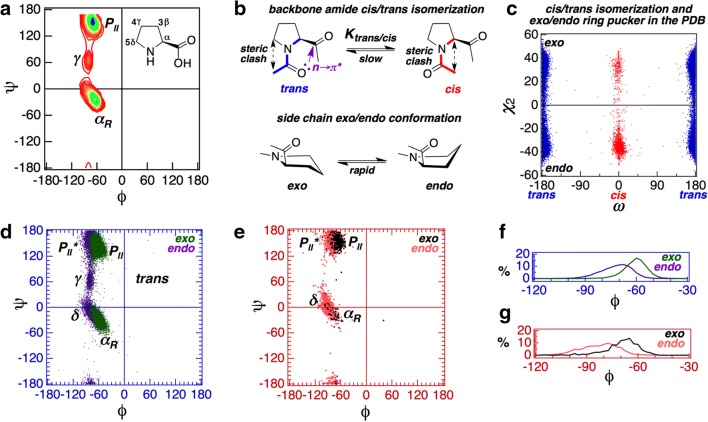
The local backbone conformation of a polypeptide chain is determined by three dihedral angles (ϕ, ψ, and ω). While ω is almost always restricted to ~ 180° (trans conformation), the energetically allowed ϕ-ψ conformational space is given by the Ramachandran map (Sasisekharan and Ramachandran 1957; Ramachandran et al. 1963; Ramachandran and Sasisekharan 1968). The imino acid proline (Pro) contains a cyclic side chain fused with its backbone (Fig. 1). This imposes a severe constraint on the dihedral angle ϕ (~ − 65°) yielding a Pro-specific Ramachandran map with two major conformational basins—a right-handed helix (which includes both helix and turn) and a left-handed polyproline II helix (Fig. 2a). The ϕ-ψ map corresponding to residues preceding Pro also deviates significantly (MacArthur and Thornton 1991; Ho and Brasseur 2005) from the canonical Ramachandran map. The lack of an amide hydrogen atom precludes Pro from participating in backbone hydrogen bond which is why Pro rarely appears in the middle of an α-helix or in non-edge region of a β-sheet (Chou and Fasman 1974). The most favorable conformation of Pro is in tight turns (Venkatachalam 1968; Lewis et al. 1973; Richardson 1981; Wilmot and Thornton 1988). The cyclic side chain is responsible for two types of isomerization equilibria in Pro (Fig. 2b): (i) imide cis/trans isomerism that is associated with the main chain dihedral angle ω and (ii) exo/endo ring puckering that is associated with the side chain dihedral angles χ1 and χ2.
Fig. 1.
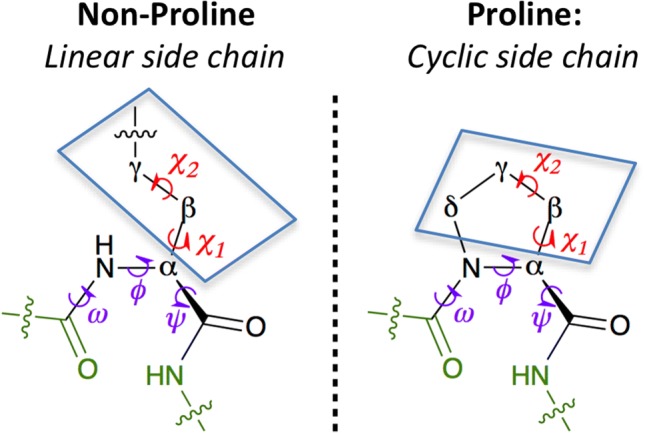
A schematic depicting a generic non-proline α-amino acid (with linear side chain) and proline (with cyclic side chain). The amino acids are shown as part of a polypeptide chain where the main chain dihedral angles (ω, ϕ, and ψ) and the side chain dihedral angles (χ1, χ2, etc.) are shown in purple and red, respectively. Atoms of the preceding and following residues are shown in green, while the side chain atoms are boxed
Fig. 2.
a Observed distribution of ϕ-ψ dihedral angles for Pro in PDB. Atom numbering of Pro side chain is shown in the inset. b Two distinct isomerization equilibria in Pro: cis/trans and exo/endo. Both steric and stereoelectronic effects operative in the cis and the trans states are shown. c Observed distribution of ω-χ2 angles for Pro in PDB. The angle χ2 reflects endo (χ2 < 0)/exo (χ2 > 0) ring puckering, while the angle ω reflects cis (ω ~ 0)/trans (ω ~ ± 180) imide conformation. d Observed distributions of ϕ-ψ dihedral angles for trans Pro (exo and endo) in PDB. e Observed distributions of ϕ-ψ dihedral angles for cis Pro (exo and endo) in PDB. f Distribution of ϕ dihedral angles for trans-exo and trans-endo conformers (from panel d). g Distribution of ϕ dihedral angles for cis-exo and cis-endo conformers (from panel e)
For the imide isomerization, both steric and stereo-electronic factors determine the relative stability of the cis and the trans conformers (Ramachandran and Mitra 1976; Newberry and Raines 2017). A non-Pro amino acid prefers the trans conformation since the corresponding cis conformation is associated with steric repulsion between its Cα atom and the Cα atom of the amino acid preceding it. For Pro, both cis and the trans conformations are associated with steric repulsions (Cα-Cδ in trans and Cα-Cα in cis) (Ramachandran and Mitra 1976). Therefore, for Xaa-Pro units, the cis conformation becomes experimentally detectable, although the trans conformation is still preferred due to the delocalization of the non-bonding electrons from the carbonyl oxygen (n) of the residue preceding Pro to the anti-bonding π* orbital of Pro carbonyl carbon (the n → π* interaction; see Fig. 2b) (Bartlett et al. 2010; Zondlo 2010; Newberry and Raines 2017). The enhanced cis-content of Xaa-Pro can be further influenced by the presence of neighboring aromatic amino acids (Grathwohl and Wüthrich 1976; Hetzel and Wüthrich 1979; Yao et al. 1994; Wu and Raleigh 1998; Pal and Chakrabarti 1999; Meng et al. 2006; Thomas et al. 2006; Zondlo 2013). For example, in a series of designed peptides containing -Xaa-Pro-, the cis-content was found to be 37% when Xaa was Trp compared with 7% when Xaa was Ala (Wu and Raleigh 1998; Reimer et al. 1998). Similarly, analysis of Xaa-Pro in folded proteins showed a significantly higher cis-content of Xaa-Pro when Xaa was aromatic (12.4% versus 5% for Xaa = Trp and Ala, respectively). Non-contiguous C–H/ π interactions from aromatic groups can also significantly alter the cis-content of Xaa-Pro (Kemmink and Creighton 1993, 1995; Nardi et al. 2000; Dasgupta et al. 2007; Ganguly et al. 2012, 2013).
Substitutions on the Pro ring can affect both the cis/trans and the endo/exo equilibria. A classic example is the effect of 4R-hydroxyproline (Hyp) (Fischer 1902), a naturally occurring imino acid (Plimmer 1912) derived from the post-translational modification (PTM) of Pro by the enzyme prolyl 4-hydroxylase (Gorres and Raines 2010). Hyp is mostly found in collagen, the most abundant protein in humans. Collagen contains three polypeptide chains consisting of (Xaa-Yaa-Gly)n repeats (n ~ 300) (Fietzek and Kühn 1975) that winds around each other to form a right-handed triple helix providing significant tensile strength (Sasisekharan and Ramachandran 1957). While the Xaa is mostly Pro, either a Pro or a Hyp often occupies the position Yaa. While hydroxylation of Pro is known to increase the stability of collagen (Berg and Prockop 1973), the frequency of Hyp (at Yaa position) directly contributes to thermal stability of collagen. Originally it was hypothesized that water molecule–mediated hydrogen bonding between the hydroxyl group of Hyp and the carbonyl oxygen from the adjacent chains contributed to the enhanced stability of the collagen triple helix structure (Hospital et al. 1979). However, using 4R-fluroproline (Flp), a synthetic Pro analog, Raines and coworkers disproved this hypothesis by generating a hyperstable collagen mimic with Pro-Flp-Gly repeats (Holmgren et al. 1999). The hyperstability was observed despite fluorine being a weaker hydrogen bond acceptor than the hydroxyl oxygen in Hyp. Raines et. al. and other groups firmly established that a stereoelectronic effect, via the hydroxyl group in Hyp (reviewed in detail later), pre-organizes the Yaa position to an exo ring pucker and trans amide conformation thereby imparting stabilization (Holmgren et al. 1999; Bretscher et al. 2001; Jenkins and Raines 2002; DeRider et al. 2002; Hodges and Raines 2003; Shoulders et al. 2006, 2009; Shoulders and Raines 2009a, b). This method of investigation, i.e., to use substituted proline residues, was later extended to other systems as well.
Because of the ease of synthesis from commercially cheap starting materials (mainly Hyp), the use of 4-substituted Pro analogs in the field of chemical and structural biology has increased significantly in the past few decades. Such modifications are achievable by making Fmoc/Boc protected 4-substituted Pro analogs using Hyp in solution phase and incorporating them into peptides via standard solution-phase or solid-phase peptide synthesis protocols or even more enticingly, via the alluring “proline editing” (Thomas et al. 2005; Pandey et al. 2013) method on a pre-synthesized sequence on resin. Coupled with the increasing interest in 19F NMR (Dorai 2015; Dahanayake et al. 2018) and a promising future of 19F MRI (Dahanayake et al. 2018; Rose-Sperling et al. 2019) probes, 4-fluoroprolines, or fluorine-containing proline analogs in general, have justifiably dominated research in chemical biology. Recently, the impact of conformationally defined 4-fluoroprolines and other fluorine containing proline analogs on peptide structure and their application as 19F NMR probes were comprehensively reviewed (Verhoork et al. 2018). Other 4-substituted proline analogs (mainly fluoro- and methylprolines) that are related to collagen stabilization/destabilization were also reviewed (Shoulders and Raines 2009b; Kubyshkin 2019). A suite of modifications and substitutions on prolines other than fluoroproline or merely 4-subtituted prolines are available. Each of these substitutions is unique in their ability to control the conformational landscape (ω, ϕ, ψ, χ1, and χ2) that is accessible to the proline scaffold. This review gives an overview of the conformational preference for the substituted prolines in the minimal amino acid context (singly or doubly capped Pro) as well as within a short peptide or protein context, where available.
Conformational preference of unsubstituted proline
Before discussing the conformational landscape of substituted prolines, here, we briefly summarize the key conformational features associated with unsubstituted proline. As mentioned in the previous section, the origin of specific conformational features of Pro lies in its cyclic side chain. The cyclic side chain not only restricts the back bone angle ϕ to approximately − 65° but also, uniquely, makes two side chain conformation states available to Pro: endo and exo. In addition, the cyclic side chain also allows Pro to access the cis amide (imide in case of Pro) isomer in addition to the canonical trans isomer. The occurrence of the endo and the exo side chain conformations is correlated with the backbone cis and trans conformations. This is evident from Fig. 2c which shows that the endo conformation strongly favors the cis isomer but the exo conformation has no such preference. Similarly, Fig. 2d, where the ϕ-ψ distribution of trans Pro (in proteins) are shown, shows that the endo and the exo conformations prefer slightly different ϕ-ψ distributions. The γ-basin, occupied by a small fraction of endo-trans Pro is empty for the corresponding exo-trans Pro. Similarly, as shown in Fig. 2e, the endo and the exo conformations prefer slightly different ϕ-ψ distribution for cis Pro (in proteins) as well. Specifically, compared with the exo conformations, the endo conformations in both trans (Fig. 2f) and cis (Fig. 2g) Pro prefer a broader distribution of ϕ with the mean shifted towards lower values (trans-endo − 70.75° ± 9.21°; trans-exo − 59.43° ± 7.09°; cis-endo − 81.62° ± 11.37°; cis-exo − 67.43° ± 11.31°). In summary, the side chain ring puckering of Pro can affect its backbone conformational preference. Therefore, any perturbation on the ring, like substitution of ring hydrogen atoms, can affect the conformational landscape of Pro. In the following sections, we have surveyed the conformational landscape of α(2)-, β-(3), γ(4)-, and δ(5)-substituted prolines using structures from the Cambridge Structural Database (CSD).
α-substituted proline
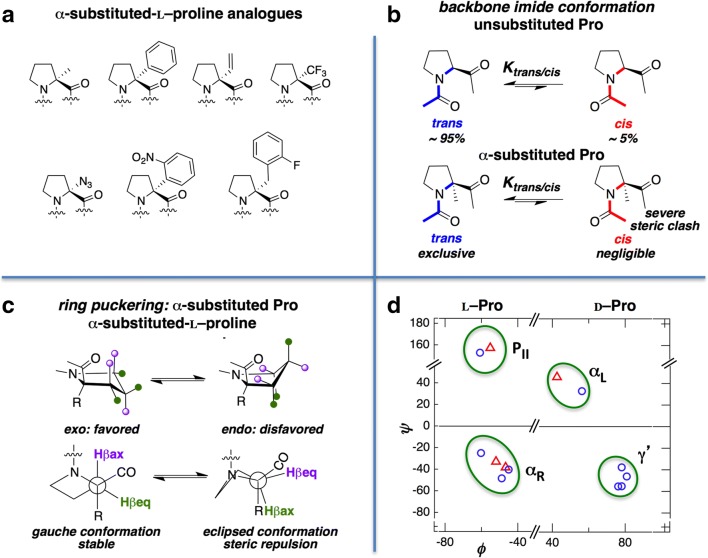
A variety of α-substituted Pro (Fig. 3a) have been investigated by several research groups, mostly using X-ray crystallography. Here we survey representative Pro α-substitutions as summarized in Table S1. Substitutions at the Cα position favors the trans over the cis imide conformation due to steric clash between the α-substituent and the side chain of the preceding residue (Torbeev et al. 2012) (see Fig. 3b). Solution 1H NMR data on N-acetylated αMep and α-trifluoromethylproline expectedly show near negligible cis amide population in D2O (Kubyshkin et al. 2018). In addition, α-substitution can also influence Pro ring puckering. The exo ring pucker is favored for L-Pro while the endo ring pucker is favored for D-Pro. This is because the alternate puckering states (exo for D-Pro and endo for L-Pro) are in all-eclipsed conformations and therefore sterically disfavored (Fig. 3c). Substitutions at the Cα position are also expected to impose restrictions on the accessible ϕ-ψ phase space in a manner analogous to that of α-amino-iso-butyric acid (Aib). Crystallographic (Flippen-Anderson et al. 1983; Torbeev et al. 2012; Mykhailiuk et al. 2017) and spectroscopic data, coupled with theoretical calculations, indeed show that the back bone preferences of α-methylproline (αMep) are restricted to the helical region of the Ramachandran plot (De Poli et al. 2009).
Fig. 3.
a Representative α-substituted L-Pro analogs discussed in this article (see Table S1 for structural details). b A schematic showing the effect (steric clash in the cis conformation) of α-substitution on cis/trans equilibrium. c A schematic showing the effect (steric clash in the endo puckered state) of α-substitution on endo/exo equilibrium. d Ramachandran plot for α-substituted L-Pro and D-Pro analogs (circle trans; triangle cis)
The geometric restriction of the αMep was exploited to build several structural scaffolds in conjunction with other amino acids. Toniolo and co-workers have designed several scaffolds with β-II, β-II’, β-III, and β-III’ conformation using a combination of αMep, Aib, and Ala in homochiral and heterochiral peptides (Moretto et al. 2008). In a suite of systematic studies, they showed that although αMep largely prefers the compact 310- and α-helical conformations (Toniolo 1989; Crisma and Toniolo 2015; Drouillat et al. 2018), it also has the tendency to explore the semi-extended PII conformation (Kang and Park 2014). Interestingly, compared with unsubstituted proline, αMep showed a remarkable tendency to promote a β-turn conformation when present at the i + 1 position (De Poli et al. 2009).
Similar to αMep, α-vinylproline exhibited the αR conformation (Reuter et al. 2015). However, incorporation of a phenyl group at the Cα position results in conformations associated with slightly lower ϕ and higher ψ values (Fig. 3d). Except for Boc-α-phenylproline, which also crystallized in a cis conformer (Tamazyan et al. 2004), α-phenyl-D-proline (Tamazyan et al. 2002, 2008) and α-(4-chlorophenyl)-D-proline (Polindara-García and Miranda 2012) appear in the γ’-basin (Fig. 2d) with an endo ring pucker. The nitrophenyl substitution on L-proline however, exemplified as α-(4-nitrophenyl) proline, expectedly showed αR conformation with a trans amide bond (Foschi et al. 2010). Finally, two more independent studies on azido- and benzylic- substituted Pro, α-azido-L-proline and α-(2-fluorobenzyl)-L-proline, yielded PII conformation (Lynch et al. 1995; Rajalakshmi et al. 2013). It is to be noted that for α-substituted proline, only αMep has been studied systematically. The conformational data for the other proline analogs were generated from non-systematic independent studies, often with results from synthesis that generated racemic mixtures of these “monopeptides.” In many cases, the absolute stereochemistries from the resulting “monopeptides” were not determined via X-ray crystallography.
Among the α-substituted proline analogs, αMep is the most popular choice for biochemical and structural biology applications. This is expected as among all the α-substituted prolines, αMep has the least effect on the hydrophobicity of the unsubstituted proline, promotes trans amide conformation, and offers restricted ϕ-ψ space. Similar to the polymeric chain of proline, poly-αMep chains strongly favor the left-handed polyproline conformation (PII) in solution (Kang and Park 2014). αMep has been incorporated into several polypeptide chains and proteins of biological significance including the hormone bradykinin (positions 3 and 7) (Welsh et al. 1992), the Asn-Pro-Asn-Ala (NPNA) repeat motif of CS Surface protein in P. falciparium (Nanzer et al. 1997), mimotope of apolipoprotein H (Sem et al. 1998), and the antimicrobial peptide buforin 2 (BF2) (Kobayashi et al. 2004). These substitutions provided valuable mechanistic insights about the structure-function relationships. In bradykinin, substitution of either Pro3 or Pro7 with αMep induces disorder-to-order transition nucleating a type II β-turn (Juvvadi et al. 1992; Welsh et al. 1992; Pierson et al. 2013). Similar results were obtained for Pro to αMep substitution in the NPNA repeat motifs (Bisang et al. 1995). The Pro to αMep substitution for the mimotope of apolipoprotein H resulted in the antigenic peptide to adopt a distorted type I β-turn and improved its binding to anti-cardiolipin antibodies. The same substitution obliterated the cis-trans isomerism in the antimicrobial peptide BF2 but had no effect on its translocation via lipid membranes implying that the cis-trans isomerization is not mechanistically involved in BF2’s translocation (Kobayashi et al. 2004). Substitution of Pro32 by αMep in β2-microglobulin, a 99-residue human protein implicated in dialysis-related amyloidsis, increases the tendency of the protein to oligomerize (Azinas et al. 2011). Using the Pro32αMep mutation, it was shown that Pro32 is constrained to the trans amide conformation initiating a misfolding that is directly responsible for the amyloid nucleation, providing a window of opportunity to study and target dialysis-related amyloidosis in the early stages (Torbeev et al. 2015).
4-substituted prolines
Consequences of substituting the hydrogen atoms at γ-carbon
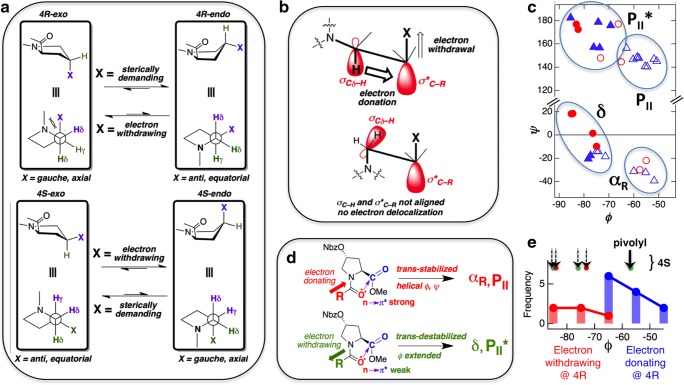
Substitution at the γ-carbon (position 4) of Pro yields two diastereoisomers, 4S and 4R. As shown in Fig. 4a, each of these can be present either in the endo or the exo ring puckered state. The relative stabilities of the endo/exo conformations depend on the nature of the substitution (X) and the particular diastereoisomeric state. When X is bulky, sterics become the key determinant and 4R-endo is favored over 4R-exo while 4S-exo is favored over 4S-endo. The origin of this can be understood from the orientation of X in the pyrrolidine ring. The relative orientation of the imide nitrogen atom and X is gauche in 4R-exo and anti in 4R-endo. Since gauche is more sterically crowded than anti, steric factors would favor the 4R-endo (anti) conformation. The steric bias against 4R-exo is further augmented by the fact that X is axial in 4R-exo but equatorial in 4R-endo. Similarly, 4S-exo is sterically favored over 4S-endo due to the fact that the relative orientation of the imide nitrogen atom and X is anti (and X is equatorial) in 4S-endo. The situation is reversed if the substituent X is electron-withdrawing in nature.
Fig. 4.
a Schematic showing the effects of a sterically demanding or an electron-withdrawing substituent at position 4 of Pro. b A schematic depicting the gauche effect—a stereoelectronic effect that stabilizes the gauche conformer when an electron-withdrawing group is present at position 4 of Pro. c Ramachandran plot for γ-substituted L-Pro analogs (triangle trans; circle cis; filled symbols represent endo while open symbols represent exo). More details about the structures used are given in Table S2. d A schematic depicting the effect of electron-withdrawing versus electron-donating substitutions at the N-cap position of 4-nitrobenzoatehydroxyproline on backbone dihedral angles, mediated by n → π* interactions. The nitrobenzoate group (NBz) is an electron-withdrawing substituent and hence it favors the exo ring pucker when substituted at the 4R position and endo ring pucker when substituted at the 4S position. e Distribution of dihedral angle ϕ in 4R-substituted proline, separated in two groups: one with electron-donating (blue) and the other with electron-withdrawing (red) N-cap. The corresponding 4S variants are also shown (arrows on the top) where all except the pivolyl N-cap show extended ϕ
When X is electron-withdrawing, the C–X and the C–N bonds will show a preference for the gauche conformation. The origin of this “gauche effect” (O’Hagan et al. 2000; Mooney et al. 2002) is hyperconjugative interaction. As shown in Fig. 4b, the electron-withdrawing group X will render the antibonding orbital associated with the C–X bond (σ*C–X) highly electron deficient. The gauche conformation will be favored over the anti conformation because only in the gauche conformation can the electron deficient σ*C–X orbital participate in hyperconjugative interaction with bonding orbitals associated with the Cδ–H or the Cβ–H bonds (σCδ–H or σCβ–H) (Fig. 4b). In summary, this leads to a strong preference for the exo pucker of the pyrrolidine ring with an electron-withdrawing group as a 4R substituent or an endo pucker of the pyrrolidine ring with an electron-withdrawing group as a 4S substituent. It should be noted that the exo ring pucker is associated with compact secondary structures, e.g., αR, PPII while the endo favors extended conformation (Dunbrack and Karplus 1994). The endo ring pucker is also strongly associated with the cis imide conformation. Pyrroline ring pucker correlates with the Pro main chain conformation, and therefore, the chemical nature and the stereotopic position of the 4-substituent can be directly exploited in molecular designs.
Crystal structures of terminally blocked 4-substituted proline
A number of crystal structures have been solved for 4-substituted Pro (see Table S2 for details). The ϕ-ψ angle distribution of these structures is shown in Fig. 4c. In general, they are distributed into four clusters (αR, δ, PII, and PII*) in the Ramachandran map without much difference in trends between trans and cis conformations. However, endo ring puckered structures (filled symbols in Fig. 4c) show a clear tendency to appear at lower ϕ and higher ψ values. The most common 4-substituted Pro is the naturally occurring Hyp. With the electron-withdrawing hydroxyl group occupying the 4R position, Hyp prefers the exo ring pucker and adopts either the αR (Clegg et al. 2003; Lübben et al. 2014) or the PII conformation (Panasik et al. 1994), exhibiting the trans imide bond. In contrast, 4S-hydroxyproline (hyp), which does not occur in nature, prefers the endo ring pucker with a cis amide bond and appears mostly in the δ-basin (Shoulders et al. 2010). Consistent with hydroxyproline, other electron-withdrawing groups at the 4R position (e.g., methoxy-, aryloxy-, fluro-, nitrobenzoate, and azido-) exhibit an exo ring pucker and are associated with either the αR or the PII conformation (Panasik et al. 1994; Sonntag et al. 2006; Kotch et al. 2008; Ghosh et al. 2014; Pandey et al. 2014; Forbes et al. 2016). When present at the 4S position, the electron-withdrawing groups favors the endo ring pucker and the δ-/PII* basin in the Ramachandran plot (Webb and Eigenbrot 1991; Anderson et al. 1996; Shoulders et al. 2010; Siebler et al. 2015; Szcześniak et al. 2015; Forbes et al. 2016).
The non-electron-withdrawing methyl, vinyl, or phenyl groups, when present as a 4R substituent, exhibit the endo ring pucker appearing in the PII*/δ region of the Ramachandran plot, while the same groups when present as 4S substituents exhibit the exo ring pucker and αR/PII conformation (Flippen-Anderson et al. 1983; Tamaki et al. 2001; Valle and Goodman 2002; Jones et al. 2011; Hack et al. 2013). Interestingly, despite chlorine being more electronegative than carbon, and therefore qualifying as an electron-withdrawing group, chloroproline behaves differently than fluoroprolines. The 4R-chloroproline (Clp) exhibits an endo ring pucker in the δ-basin, as opposed to Flp (or Hyp) which appears in the αR/PII-basin with exo ring pucker. The 4S-chloroproline (clp) exhibits the exo ring pucker in PII-basin, similar to 4S-alkylprolines and in sharp contrast to flp or hyp. This is possibly due to the large size of the chlorine atom and the steric necessity in this case outcompete the electronic effects that would have resulted from its electronegativity alone (Shoulders et al. 2008; Park et al. 2012). Interestingly, for the 4R-azidoproline (Azp) crystal, both cis and trans isomers were observed in the same asymmetric unit (Sonntag et al. 2006). The cis and the trans isomers are associated with the endo and the exo ring pucker, respectively, as expected. The cis-endo crystal exhibits the PII* conformation, while the trans-exo crystal exhibits the PII conformation.
Tuning structure-function of peptides and proteins using 4-substituted proline
Selective tuning of the main chain conformation via stereospecific substitution at the γ-carbon of the proline residue has been justifiably exploited to alter/modify protein conformation and function. With Hyp being a cheap commercial source, chemists have conveniently designed several synthetic routes to make enantiopure 4-substituted proline derivatives in high yields using classical solution-phase synthesis and “editing” proline residues on the solid-phase within peptides (Thomas et al. 2005; Pandey et al. 2013). The free 4-substituted amino acids were genetically incorporated within proteins either via the use of appropriate auxotrophs or via unnatural amino acid incorporation approaches based on clever use of the amber stop codon/tRNA suppressors (Kim et al. 2004; Chalker and Davis 2010; Wals and Ovaa 2014; Kato 2015; Smolskaya and Andreev 2019). The incorporation of these 4-substituted proline residues provides valuable insights into protein structure and function, thereby subsequently leading to the design of important compounds for therapeutics including conformationally biased biological probes, inhibitors, and peptidomimetic drugs (Tressler and Zondlo 2014). A few selected examples of the use of 4-substituted prolines are discussed below.
In the individual strand of the collagen repeats [Xaa-Yaa-Gly]n, modified proline residues at either the Xaa or the Yaa position can significantly affect the stability of the triple helix assembly. The 4-substituted prolines with preference for the exo ring pucker, e.g., Hyp, Flp, Mop (4R-methoxyproline), and mep (4S-methylproline), increase the stability of the model collagen triple helix. Incorporation of 4-substituted proline residues with a preference for endo ring pucker leads to destabilization of the collagen assembly (Holmgren et al. 1999; Hodges and Raines 2003; Kotch et al. 2008). Other than providing mechanistic insights into the stability of the native collagen triple helix, 4-substituted prolines can be used in design and development of non-native collagen with tunable mechanical properties (Kubyshkin 2019). A detailed discussion of the mechanism collagen stability explored via 4-substituted proline has been reviewed elsewhere (Jenkins and Raines 2002; Shoulders and Raines 2009b).
Rational design of structures via the incorporation of 4-substituted proline residues in place of proline has also been investigated in proteins and model peptides other than collagen. The consensus pentaresidue repeat sequence in elastin, VPGVG, undergoes a reversible temperature-dependent assembly that is driven by its intrinsic hydrophobicity (Urry et al. 1997). During this assembly process, the proline-glycine motif within these pentaresidue repeats forms type II β-turn structures and is critical to the phase separation event (Urry et al. 1985). Three repeats containing the cyclic version of the VPGVG sequence show that the proline residue exhibits an exo ring pucker, and the main chain dihedral angles (ϕ,ψ = −53°, + 140°) are compatible with the (i + 1)th position of a β-turn (Cook et al. 1980). Replacement of the proline residues with Flp, that favor the exo ring pucker, selected the type II β-turn conformation and induced elastin transition at a lower temperature (favorable mechanism). In contrast, flp induced multiple conformational states, disfavored the type II β-turn conformation, and increased the transition temperature for the elastin assembly (unfavorable mechanism) (Kim et al. 2005). In the Trp-cage miniprotein, the incorporation of the strongly exo ring pucker promoting 4R-(4-nitrobenzoate)-hydroxyproline and 4R-(4-trifluromethylbenzoate)-hydroxyproline at the Pro12 position resulted in a dramatic increase of the stability of the protein, where both Flp and Hyp only modestly increased the protein stability (Naduthambi and Zondlo 2006). This finding advocates for the use of 4-substituted derivatives other than the commonly used fluoroprolines or hydroxyprolines to modulate protein structure and function.
The global replacement of 32 proline residues with Flp in KlenTaq DNA polymerase did not result in any loss of function for the protein despite causing a slight loss in the thermostablity of the protein (Holzberger and Marx 2010; Holzberger et al. 2012). Similarly, replacement of 10 proline residues with flp in EGFP resulted in faster refolding of the protein due to better accommodation of the endo ring puckered substituted proline in the engineered variant (Steiner et al. 2008; Moroder and Budisa 2010). Interestingly, attempts to express the KlenTaq DNA polymerase with flp and EGFP with Flp were unsuccessful probably because the misfolding of these protein variants rendered them insoluble (Holzberger and Marx 2010). All three proline residues in ubiquitin—Pro19, Pro37, and Pro38—were replaced with Flp leading to a greater stability of the engineered protein (Crespo and Rubini 2011). In the WW domain of Pin1, Pro37 exhibits an endo ring pucker (Mortenson et al. 2018). The Pro37 is crucial to the structural stability in the hydrophobic core of the Pin1 WW domain; a P37A mutation resulted in an unstable protein (Jäger et al. 2009). The mutation of Pro37 with a 4-substituted proline residue provided valuable insights into the role of designing the preference of the proline ring pucker and exploiting the effects of hydrophobicity on the stability of the WW domain and subsequently its ligand binding ability. While the exo pucker promoting mutations, P37Flp, P37Hyp and P37Mop, were destabilizing, the endo pucker promoting P37flp mutation had a stabilizing effect on the structure of the Pin1 WW domain. Interestingly, although the endo pucker-favoring P37hyp and P37mop variants exhibited greater stability for the WW domain than their corresponding exo ring-promoting P37Hyp and P37Mop mutations, the P37hyp and P37mop mutants were less stable than the wild type Pin1 WW domain due to solvation and steric restraints. Nonetheless, the overall stability of the Pin1 WW domain positively correlated with its ability to bind the specific phospho-peptide (Huang and Horng 2015), advocating the role of 4-substituted proline derivatives to provide important molecular insights into the structure-activity relationship of protein-protein interactions (Tang et al. 2014). However, it should be pointed out that the thermodynamic consequences of using a 4-substituted proline residue with a preorganized ring puckered state may become complex if other non-covalent interactions including solvation play a dominant role. (Kantharaju et al. 2009; Rubini et al. 2013).
The residue Pro62 present in the villin headpiece protein exhibits an exo ring pucker, and its Hγ atom is involved in a C–H…π interaction with the Trp64 (Bhattacharyya and Chakrabarti 2003). Surprisingly, all exo pucker promoting 4-substituted proline derivatives (Hyp, Flp, and Mop) destabilized the villin headpiece protein and so did all endo pucker promoting substituents (hyp and mop) (Zheng et al. 2010). Substitution of the Hγ at Pro62 possibly weakens the proline-aromatic C–H…π interaction, which is crucial in maintaining the local structure. Only flp (which promotes the endo ring pucker) at position 62 marginally increased the stability of the villin headpiece protein, which is attributed to its hydrophobic effect (Hsu et al. 2015). The HPK1-derived proline rich sequence (PPPLPPKPKF) that recognizes and binds to the SH3 domain of the murine cortactin (SH3m–cort) via formation of the left-handed polyproline structure (Feng et al. 1994; Rubini et al. 2010) did not show a gain in function when exo ring pucker promoting, and PII stabilizing 4-substituted prolines were incorporated in place of the proline residues (Borgogno and Ruzza 2013).
4-substituted proline in polyproline helices
Proline peptides can exist in two conformations: (i) the left-handed polyproline helix (PPII) with all trans peptide bonds and (ii) the right-handed polyproline helix (PPI) with all cis peptide bonds (Cowan et al. 1955; Kurtz et al. 1956; Traub and Shmueli 1963). While the PPII conformation is common in protein structures and is stabilized in aqueous environment, PPI helix is rarely encountered in proteins and can form only in organic solvents. The 4R-substituted prolines with electron-withdrawing substituents promote the exo ring pucker and increase the population of n → π* interaction-driven trans peptide bonds. The exo ring pucker is also associated with a more compact ϕ distribution for the proline residue (~ − 60° to − 70°) and is therefore expected to contribute favorably to the stability of a PPII helix. In contrast, the PPI helix should be preferred by cis peptide bond promoting endo ring pucker-favored electron-withdrawing substituents at the 4S position of the proline. Expectedly, Flp, Hyp, Mop, and 4R-azidoproline (Azp) were found to stabilize the PPII conformation compared with unsubstituted proline in homo-polymeric peptides (Horng and Raines 2006). Interestingly, (Flp)10 and (Hyp)10 peptides are resistant to form PPI structures even after long incubation in organic solvents while (Pro)10 showed significant PPI conformation under identical conditions (Chiang et al. 2009). The peptides with 4S-substituents (e.g., (flp)10, (hyp)10, (azp)9) showed more stable PPI structure in organic solvents and less stable PPII structure in aqueous solution compared with their all proline analog (Chiang et al. 2009).
Apart from affecting the stability of the PPII and PPI helices, incorporation of 4-substituted proline residues in polyproline peptides modulate the transitional barrier of the PPI/PPII interconversion. While incorporation of a single Flp residue registers an increase in the transition barrier of PPII → PPI conversion (compared with Hyp, Mop, and Pro), incorporation of a flp residue registers the largest decrease in the PPI → PPII transition energy (compared with hyp, mop, and Pro) (Chiang et al. 2009). Therefore, both the chemical nature and the stereochemistry of the substituent modulate the thermodynamic and kinetic properties of the polyproline oligomers. Encouragingly, homo and hetero functionalization of the 4-azidoproline via Huisgen’s 1,3-dipolar cycloaddition (popularly referred as “click chemistry”) in polyproline peptides showed that the PPII helical fold remains intact following the reaction. This has the potential for developing proline-based structural scaffolds with their use in bioorthogonal applications and medicinal chemistry (Kümin et al. 2007; Siebler et al. 2015). Potential applications for utilization of 4-substitued prolines with defined impact of peptide backbone conformation have been demonstrated. Cell-penetrating peptides with 4-substituted prolines were successfully designed where the structure of the peptide was stabilized in the PPII conformation (Fillon et al. 2005). With the incorporation of both the 4R and 4S perfluoro-tert-butyl ethers of hydroxyproline in peptides, the potential for developing conformationally restrained and highly sensitive 19F NMR (9 equivalent 19F nuclei) probes is getting realized (Tressler and Zondlo 2014; Verhoork et al. 2018). Replacement of the central proline residue with 4S-azidoproline in the peptide catalyst DPro-Pro-Glu, which catalyzes C–C bond formation, resulted in an increase of the enantiopurity of the product (Schnitzer and Wennemers 2018).
Tuning n → π* interaction with 4-substituted proline
The main chain conformation of a proline residue can be further controlled via differential N-capping groups that can directly modulate the n → π··interaction (Fig. 4d). This was achieved via the utilization of “monopeptides”—4-substituted nitrobenzoate esters of 4-hydroxyproline. The 4R-(4-nitrobenzoate)-hydroxyproline (Hnb) is known to prefer the exo ring pucker, while the 4S-(4-nitrobenzoate)-hydroxyproline (hnb) prefers the endo ring pucker, consistent with the gauche effect (Pandey et al. 2014). Electron-donating groups at the N-terminal of the exo pucker-promoting Hnb, e.g., pivaloyl, iso-butyryl, propionyl, and acetyl, strengthened the n → π* interaction (Fig. 4d), thereby leading to a compact helical backbone (PPII or αR) (Fig. 4e) and even stabilized α-helical conformations without any hydrogen bond (Wenzell et al. 2019). In contrast, electron-withdrawing groups at the N-terminal of Hnb (e.g., fluoroacetyl, trifluoroacetyl) weakened n → π* interactions, thereby promoting extended conformations (Fig. 4e).
In contrast to Hnb, hnb is associated with the endo ring pucker and promotes cis imide conformation, the latter incompatible with n → π* interaction. Therefore, in general, hnb promotes extended conformations (δ, PII*), independent of the identity of its N-cap. The only exception to this is the pivaloyl N-cap group, which, due to its unusually strong electron-donating property, shows evidence of an n → π* interaction and occupies the PII conformational basin (Costantini et al. 2019) (Fig. 4e).
3-substituted prolines
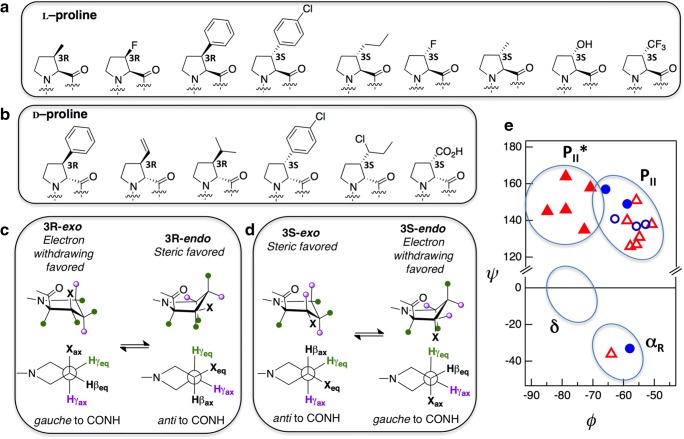
As shown in Fig. 5 a and b, a number of 3-substituted L-Pro (as well as D-Pro) analogs have been studied, both at 3R and 3S positions (see Table S3 for details). Substitutions at the 3R and 3S positions have effects on the exo/endo puckering equilibrium analogous to that of 4-substituted prolines. Thus, an electron-withdrawing substituent at 3R favors the exo ring pucker while the same group at 3S favors the endo ring pucker, consistent with the “gauche” effect (Fig. 4b). Similarly, a bulky alkyl or aryl substituent at 3R favors the endo ring pucker while the same group at 3S favors the exo ring pucker, due to differential steric repulsion effects associated with the axial/equatorial positions (Fig. 5c–d).
Fig. 5.
a Representative 3-substituted L-Pro analogs discussed in this article (see Table S3 for structural details). The substituents are labeled according to the stereochemistry of the prochiral hydrogen atom. b Representative 3-substituted D-Pro analogs discussed in this article (see Table S3 for structural details). c Schematic showing the differential effect of 3R substitution (bulky steric group versus electron-withdrawing group). d Schematic showing the differential effect of 3S substitution (bulky steric group versus electron-withdrawing group). e Ramachandran plot for 3-substituted L-Pro analogs (triangle trans; circle cis; open symbols exo; filled symbols endo)
In examining the effect of the mono-substitution at the 3-position of Pro, care should be taken with the nomenclature. Several substituents alter the stereochemical nomenclature, both at positions 3 and 2, depending on the priority of the substituent group according to the Cahn-Ingold-Prelog nomenclature for the determination of the absolute stereochemistry. Thus, the replacement of the pro-3R hydrogen atom with a methyl group results in a (2S,3R)-methylproline, while a phenyl group substitution at the same position would result in a (2S,3S)-phenylproline. Substitution of any of the two β-hydrogen atoms by a fluorine atom results in a change of nomenclature to 2R for L-proline, instead of 2S.
The endo ring pucker is exhibited by (2S,3R)-methylproline and (2S,3S)-phenylproline (in both cases, the substituents occupy the pro-3R position) (Flippen-Anderson et al. 1983; Flamant-Robin et al. 2002), while (2S,3S)-methylproline, (2S,3S)-(n-propyl)-proline, (2S,3R)-phenylproline, and (2S,3R)-(4-chlorophenyl)-proline exhibit the exo ring pucker (all substituents occupy the pro-3S position) (Flippen-Anderson et al. 1983; Zhong and Carlson 2006; Tran et al. 2008; Reuter et al. 2011; Huy et al. 2011; Fatás et al. 2011). This is consistent with the favorable placement of the sterically demanding group in the pseudo-equatorial position; in contrast, the electron-withdrawing substituents (–OH, and –F) occupy the pseudo-axial position (Fig. 4 c and d). This is also consistent with the “gauche effect,” via which the pro-3S substitutions on β-carbon ((2R,3S)-fluoroproline and (2S,3S)-hydroxyproline) exhibit the endo ring pucker (Jenkins et al. 2003; Kim et al. 2006; Davies et al. 2018) and the pro-3R substitutions on β-carbon ((2R,3R)-fluoroproline) exhibits the exo ring pucker (Kim et al. 2006). Such 3-substituted fluoroproline derivatives were used as molecular probes to study prolyl cis-trans isomerization via 19F NMR (Kim et al. 2006; Thomas et al. 2009). Since fluorine and hydrogen atoms are nearly isosteric and the trifluoromethyl group is only modestly electron-withdrawing (comparable to either hydroxyl and fluorine), (2S,3S)-trifluoromethylproline exhibits multiple conformations that access both the exo and the endo ring pucker conformations, with multiple backbone conformations within the same crystal structure (Tolmachova et al. 2018).
The ϕ-ψ distribution for 3-substituted L-Pro analogs is shown in Fig. 5e. Compared with the ϕ-ψ distribution for 4-substituted L-Pro analogs (Fig. 4c), there are two main differences. The δ-basin is empty for 3-substituted Pro (this could be due to less number of total 3-substituted structures). The other difference is that for 3-substituted Pro, all cis-endo conformations show compact ϕ (> − 70°), while all trans-endo conformations show extended ϕ (< − 70°). Among the 3-substituted alkyl and aryl prolines, β-phenylprolines were used as β-turn scaffolds (Fatás et al. 2011). With a strong electron–donating and n → π* interaction-promoting pivaloyl group at the N-terminus of the proline analog, the β-phenylprolines were locked in a PII conformation that is compatible with i + 1 position of a type II turn. Coupled with a DPhe residue at its C-terminal, β-phenylprolines, via aromatic-aromatic interactions, stabilize and promote turn conformations with trans amide bonds. In a peptide template, MT-II (Ac-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-NH2]) replacement of the His residue with β-phenylproline analogs, where the phenyl ring is substituted by H, -Cl, -Br, -CF3, and -OMe, showed a suite of conformations. The conformations with a compact ϕ (> − 70°) had a slightly lower IC50 values for melanocortin receptors, while the extended ϕ (< − 70°) had much lower IC50. All peptides with β-pheylproline analogs had a much lower IC50 value than with the peptide containing unsubstituted proline residue, advocating the role of 3-substituted proline residues and their conformational importance in medicinal chemistry (Cai et al. 2004).
3-hydroxyproline and collagen stability
Although rare, trans-3-hydroxyproline (2S,3S)-hydroxyproline (3-Hyp), like Hyp, is also found in nature, produced via proline PTM. The formation of 3-Hyp is not due to the promiscuity of prolyl-4-hydroxylase, as was initially hypothesized. Rather, proline PTM by prolyl 3-hydroxylase (P3H) specific to Pro-4Hyp-Gly sequences incorporates the 3-Hyp residues (Hudson and Eyre 2013). Interestingly, proline residues that are substrates of P3H are strongly conserved (Hudson et al. 2014).
Only one to two residues of 3-Hyp are found in type I and type II collagen, while this number marginally increases to 3–6 residues in types V and XI collagen. Type IV collagens have the highest 3-Hyp content (10% of all the hydroxyproline variants) (Weis et al. 2010; Eyre et al. 2011; Hudson et al. 2012). Surprisingly, the presence of 3-Hyp destabilizes the collagen triple helix assembly. Although the triple helices from (3Hyp-4Hyp-Gly)n sequences are more stable than the unmodified (Pro-Pro-Gly)n sequences, the triple helix assembly is slightly unstable compared with (Pro-4Hyp-Gly)n sequences. The extent of destabilization of the triple helix assembly is much less if 3-Hyp is in position Xaa of the (Xaa-Yaa-Gly)n sequence rather than when at position Yaa. The position Yaa strongly prefers an exo ring pucker, and because of the “gauche effect,” the endo ring pucker is preferred for 3-Hyp. This is the reason behind the observed decrease in the stability of (Xaa-3Hyp-Gly)n collagen peptides (Jenkins et al. 2003).
5-substituted proline
Substitution of Pro at the 5 (or δ) position is unique since this is the only ring position where substitutions are devoid of any amide “gauche effect,” as applicable for 3- or 4-substituted Pro. In other words, for 5-substituted Pro, none of the electron donating C–H bonds are aligned with the σ* of the Cδ–X bond. Therefore, only steric repulsions guide the pyrrolidine ring pucker (Fig. 6a). In unsubstituted Pro, the pro-5R hydrogen atom is pseudo-equatorial in the endo ring pucker conformation and pseudo-axial in the exo ring pucker conformation. Therefore, substituents at pro-5R position favor the endo ring pucker in the pyrrolidine ring. Similarly, pro-5S substituents prefer the exo ring pucker. The crystal structures of the “monopeptides” that were explored (Table S4) from the Cambridge Structural Database are consistent with this hypothesis (Masumi et al. 1982; Flippen-Anderson et al. 1983; Lynch et al. 1995; Gilchrist et al. 1997; Arndt et al. 1997; Soave et al. 1997; Mulzer et al. 2000; Hussaini and Moloney 2003; Matsumura et al. 2006; Moloney et al. 2006; Onomura et al. 2008; Reuter et al. 2011; Rodríguez et al. 2015; Beatty et al. 2016; Salih et al. 2016; Kubyshkin and Budisa 2017).
Fig. 6.
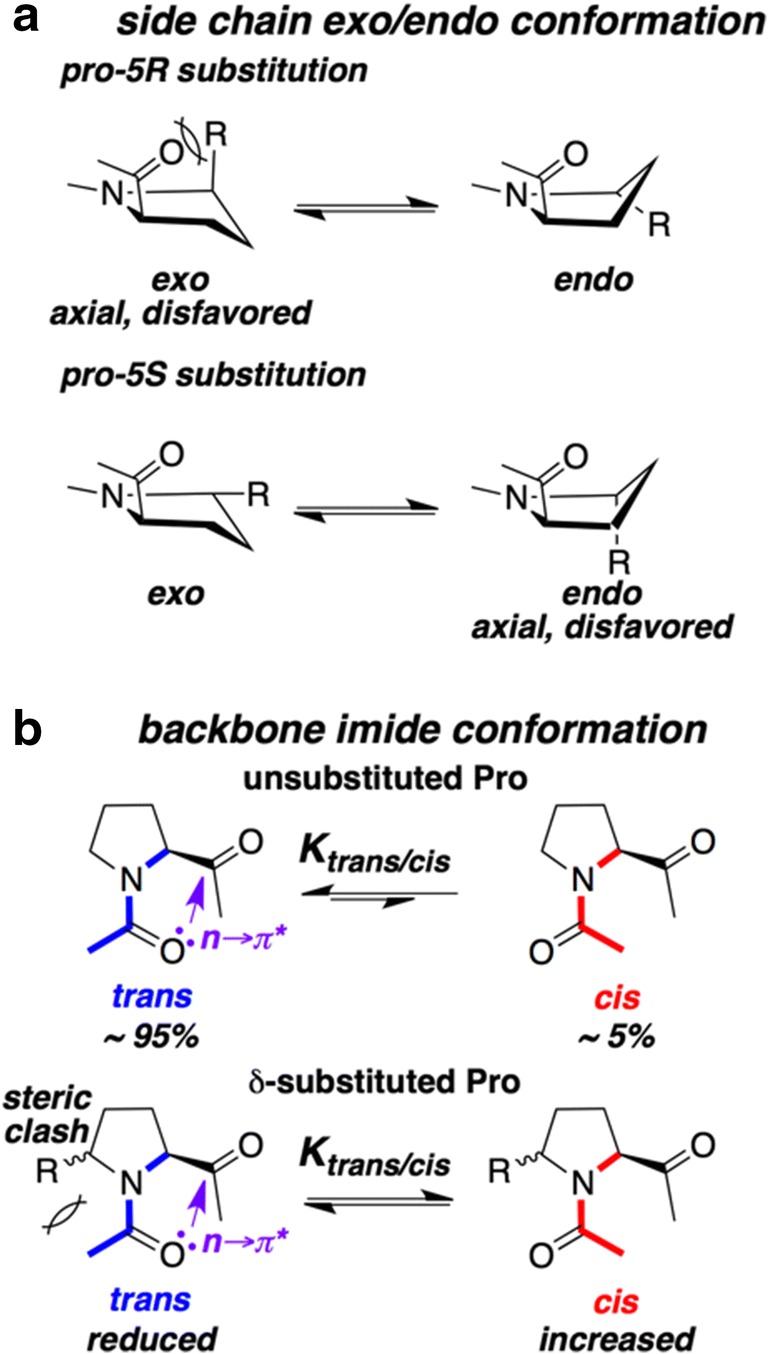
a A schematic showing how steric clash in 5-substituted Pro increases the endo-content for 5R substitution while the same steric effect increases the exo-content for 5S substitution. b A schematic showing how steric clash in 5-substituted Pro increases the cis-content
In addition, substitutions at the Cδ position also enhance the population of the cis imide conformation (Fig. 6b). This is due to the increased steric repulsion for the trans isomer, making it more disfavored compared with the unsubstituted proline. The Ktrans/cis value for Xaa-Pro unit in model peptides is around 19 (95% of the prolyl amides in trans conformation). Incorporation of methyl, trifluoromethyl, or tert-butyl groups at position 5 results in a monotonic decrease of Ktrans/cis (4.6, 2.8, and 0.82, respectively) with the degree of substitution (Lummis et al. 2005; Kubyshkin et al. 2018). In other words, tert-butyl substitution at the Cδ-position produces almost equal populations of cis and trans isomers. The trans → cis isomerization of the conserved hinge proline residue in the M2-M3 loops of the cation-selective nicotinic aetylcholine and 5-HT3 receptors opens the pore of the neurotransmitter ion-gated channel. The use of 5-substituted proline residues, which increases the population of the cis imide bond, provides valuable mechanistic insights for these receptors (Lummis et al. 2005).
Summary and future outlook
The backbone ϕ angle of Pro is severely constrained due to the cyclic nature of its side chain. In addition, only Pro, among all amino acids, can access the cis backbone isomer. The unique conformational restrictions of Pro can be modulated further by suitable substitutions at various ring positions. Two such variants, 4-Hyp and 3-Hyp, are found to occur naturally in the protein collagen where they alter collagen stabilities. The mechanism behind this is a special stereoelectronic effect. In addition to the natural variants, a large number of Pro substitutions (at all the four positions: α-, 3-, 4-, and 5-) have been studied over the past two decades. For α- and 5-substitutions, steric factors play the dominant role in determining the conformational fate of the molecule. On the other hand, for 3- and 4-substituted Pro, the final conformational bias is determined by an interplay of steric interactions and the stereoelectronic gauche effect. Depending on the nature of substitution and the resulting dominant isomer (cis/trans and exo/endo), 3-,4- and 5-substituted Pro can assume either a compact (appearing in the αR- or the PII-basin) or a more extended (appearing in the δ- or the PII*-basin) conformation. In general, the endo pucker favors an extended conformation while the exo pucker favors a more compact conformation. α-substituted Pro, on the other hand, is always restricted to the compact conformation (appearing in the αR- or the PII-basin). In all cases, the trans-exo conformation is associated with a stronger n → π* interaction compared with the trans-endo conformation. Substituted Pro has a wide range of applications. Substituted Pro has been used in studies where a specific conformation (of a naturally occurring Pro) was designed to be stabilized (or destabilized). They have been used as 19F NMR probes, and seleno-proline derivatives hold promise as a 77Se NMR probe. They can be useful in medicinal chemistry due to the stereochemical control they offer in controlling distance between attached functional groups. The stereochemical control added with capacity to work as a sensitive 19F NMR probe makes them attractive to probe intrinsically disordered proteins. Although we have discussed only singly substituted prolines, multi-substituted proline offers added steric and stereoelectronic controls.
Electronic supplementary material
(PDF 169 kb)
Authors’ contribution
HKG performed the literature search. HKG and GB analyzed the data and wrote the review.
Funding information
This work was supported by an intramural grant to GB from Bose Institute.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Himal Kanti Ganguly, Email: h.k.ganguly@gmail.com.
Gautam Basu, Email: gautamda@gmail.com.
References
- Anderson NG, Lust DA, Colapret KA, et al. Sulfonation with inversion by Mitsunobu reaction: an improvement on the original conditions. J Org Chem. 1996;61:7955–7958. doi: 10.1021/jo9609539. [DOI] [PubMed] [Google Scholar]
- Arndt H-D, Polborn K, Koert U. Stereoselective synthesis of a terpyrrolidine unit, a potential building block for anion recognition. Tetrahedron Lett. 1997;38:3879–3882. [Google Scholar]
- Azinas S, Colombo M, Barbiroli A, et al. D-strand perturbation and amyloid propensity in beta-2 microglobulin. FEBS J. 2011;278:2349–2358. doi: 10.1111/j.1742-4658.2011.08157.x. [DOI] [PubMed] [Google Scholar]
- Bartlett GJ, Choudhary A, Raines RT, Woolfson DN. n→π* interactions in proteins. Nat Chem Biol. 2010;6:615–620. doi: 10.1038/nchembio.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty JW, Douglas JJ, Miller R, et al. Photochemical perfluoroalkylation with pyridine N-oxides: mechanistic insights and performance on a kilogram scale. Chem. 2016;1:456–472. doi: 10.1016/j.chempr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RA, Prockop DJ. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun. 1973;52:115–120. doi: 10.1016/0006-291x(73)90961-3. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya R, Chakrabarti P. Stereospecific interactions of proline residues in protein structures and complexes. J Mol Biol. 2003;331:925–940. doi: 10.1016/s0022-2836(03)00759-9. [DOI] [PubMed] [Google Scholar]
- Bisang C, Weber C, Inglis J, et al. Stabilization of type-I β-turn conformations in peptides containing the NPNA-repeat motif of the plasmodium falciparum circumsporozoite protein by substituting proline for (S)-α-methylproline. J Am Chem Soc. 1995;117:7904–7915. [Google Scholar]
- Borgogno A, Ruzza P. The impact of either 4-R-hydroxyproline or 4-R-fluoroproline on the conformation and SH3m-cort binding of HPK1 proline-rich peptide. Amino Acids. 2013;44:607–614. doi: 10.1007/s00726-012-1383-y. [DOI] [PubMed] [Google Scholar]
- Bretscher LE, Jenkins CL, Taylor KM, et al. Conformational stability of collagen relies on a stereoelectronic effect. J Am Chem Soc. 2001;123:777–778. doi: 10.1021/ja005542v. [DOI] [PubMed] [Google Scholar]
- Cai M, Cai C, Mayorov AV, et al. Biological and conformational study of β-substituted prolines in MT-II template: steric effects leading to human MC5 receptor selectivity. J Pept Res. 2004;63:116–131. doi: 10.1111/j.1399-3011.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- Chalker JM, Davis BG. Chemical mutagenesis: selective post-expression interconversion of protein amino acid residues. Curr Opin Chem Biol. 2010;14:781–789. doi: 10.1016/j.cbpa.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Chiang Y-C, Lin Y-J, Horng J-C. Stereoelectronic effects on the transition barrier of polyproline conformational interconversion. Protein Sci. 2009;18:1967–1977. doi: 10.1002/pro.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou PY, Fasman GD. Prediction of protein conformation. Biochemistry. 1974;13:222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Clegg W, Deboves HJC, Elsegood MRJ. 1-tert-butyl 2-methyl 4-(R)-hydroxypyrrolidine-1,2-(2S)-dicarboxylate. Acta Cryst E. 2003;59:o1987–o1989. [Google Scholar]
- Cook WJ, Einspahr H, Trapane TL, et al. Crystal structure and conformation of the cyclic trimer of a repeat pentapeptide of elastin, cyclo-(L-valyl-L-prolylglycyl-L-valylglycyl)3. J Am Chem Soc. 1980;102:5502–5505. [Google Scholar]
- Costantini NV, Ganguly HK, Martin MI, et al. The distinct conformational landscapes of 4S-substituted prolines that promote an endo ring pucker. Chem Eur J. 2019;25:11356–11364. doi: 10.1002/chem.201902382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan PM, Mcgavin S, North AC. The polypeptide chain configuration of collagen. Nature. 1955;176:1062–1064. doi: 10.1038/1761062a0. [DOI] [PubMed] [Google Scholar]
- Crespo MD, Rubini M. Rational design of protein stability: effect of (2S,4R)-4-fluoroproline on the stability and folding pathway of ubiquitin. PLoS One. 2011;6:e19425. doi: 10.1371/journal.pone.0019425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisma M, Toniolo C. Helical screw-sense preferences of peptides based on chiral, Cα-tetrasubstituted α-amino acids. Pept Sci. 2015;104:46–64. doi: 10.1002/bip.22581. [DOI] [PubMed] [Google Scholar]
- Dahanayake JN, Kasireddy C, Karnes JP, et al (2018) Chapter five-progress in our understanding of 19F chemical shifts. In: Webb GA (ed) Annual Reports on NMR Spectroscopy. Academic Press, pp 281–365
- Dasgupta B, Chakrabarti P, Basu G. Enhanced stability of cis Pro-Pro peptide bond in Pro-Pro-Phe sequence motif. FEBS Lett. 2007;581:4529–4532. doi: 10.1016/j.febslet.2007.08.039. [DOI] [PubMed] [Google Scholar]
- Davies SG, Fletcher AM, Linsdall SM, et al. Asymmetric syntheses of (2R,3S)-3-hydroxyproline and (2S,3S)-3-hydroxyproline. Org Lett. 2018;20:4135–4139. doi: 10.1021/acs.orglett.8b01736. [DOI] [PubMed] [Google Scholar]
- De Poli M, Moretto A, Crisma M, et al. Is the backbone conformation of Cα-methyl proline restricted to a single region? Chem Eur J. 2009;15:8015–8025. doi: 10.1002/chem.200900688. [DOI] [PubMed] [Google Scholar]
- DeRider ML, Wilkens SJ, Waddell MJ, et al. Collagen stability: insights from NMR spectroscopic and hybrid density functional computational investigations of the effect of electronegative substituents on prolyl ring conformations. J Am Chem Soc. 2002;124:2497–2505. doi: 10.1021/ja0166904. [DOI] [PubMed] [Google Scholar]
- Dorai K (2015) Chapter 4 - investigations of biomolecular conformation and dynamics using 19F NMR. In: ur-Rahman A, Choudhary MI (eds) Applications of NMR Spectroscopy: Volume 3. Bentham Science Publishers, pp 116–149
- Drouillat B, Peggion C, Biondi B, et al. A novel peptide conformation: the γ-bend ribbon. Org Biomol Chem. 2018;16:7947–7958. doi: 10.1039/c8ob02279h. [DOI] [PubMed] [Google Scholar]
- Dunbrack RL, Karplus M. Conformational analysis of the backbone-dependent rotamer preferences of protein sidechains. Nat Struct Mol Biol. 1994;1:334–340. doi: 10.1038/nsb0594-334. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Weis M, Hudson DM, et al. A novel 3-Hydroxyproline (3Hyp)-rich motif marks the triple-helical C terminus of tendon type I collagen. J Biol Chem. 2011;286:7732–7736. doi: 10.1074/jbc.C110.195768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatás P, Jiménez AI, Calaza MI, Cativiela C. β-Phenylproline: the high β-turn forming propensity of proline combined with an aromatic side chain. Org Biomol Chem. 2011;10:640–651. doi: 10.1039/c1ob06561k. [DOI] [PubMed] [Google Scholar]
- Feng S, Chen JK, Yu H, et al. Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science. 1994;266:1241–1247. doi: 10.1126/science.7526465. [DOI] [PubMed] [Google Scholar]
- Fietzek PP, Kühn K. Information contained in the amino acid sequence of the alpha1(I)-chain of collagen and its consequences upon the formation of the triple helix, of fibrils and crosslinks. Mol Cell Biochem. 1975;8:141–157. doi: 10.1007/BF01792765. [DOI] [PubMed] [Google Scholar]
- Fillon YA, Anderson JP, Chmielewski J. Cell penetrating agents based on a polyproline helix scaffold. J Am Chem Soc. 2005;127:11798–11803. doi: 10.1021/ja052377g. [DOI] [PubMed] [Google Scholar]
- Fischer E. Über eine neue Aminosäure aus Leim. Chem Ber. 1902;35:2660–2665. [Google Scholar]
- Flamant-Robin C, Wang Q, Chiaroni A, Sasaki NA. An efficient method for the stereoselective synthesis of cis-3-substituted prolines: conformationally constrained α-amino acids. Tetrahedron. 2002;58:10475–10484. [Google Scholar]
- Flippen-Anderson JL, Gilardi R, Karle IL, et al. Crystal structures, molecular conformations, infrared spectra, and carbon-13 NMR spectra of methylproline peptides in the solid state. J Am Chem Soc. 1983;105:6609–6614. [Google Scholar]
- Forbes CR, Pandey AK, Ganguly HK, et al. 4R- and 4S-iodophenyl hydroxyproline, 4R-pentynoyl hydroxyproline, and S-propargyl-4-thiolphenylalanine: conformationally biased and tunable amino acids for bioorthogonal reactions. Org Biomol Chem. 2016;14:2327–2346. doi: 10.1039/c5ob02473k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foschi F, Landini D, Lupi V, et al. Enantioselective rearrangement of proline sulfonamides: an easy entry to enantiomerically pure α-aryl quaternary prolines. Chem Eur J. 2010;16:10667–10670. doi: 10.1002/chem.201000989. [DOI] [PubMed] [Google Scholar]
- Ganguly HK, Kaur H, Basu G. Local control of cis-peptidyl–prolyl bonds mediated by CH···π interactions: the Xaa-pro-Tyr motif. Biochemistry. 2013;52:6348–6357. doi: 10.1021/bi4007918. [DOI] [PubMed] [Google Scholar]
- Ganguly HK, Majumder B, Chattopadhyay S, et al. Direct evidence for CH···π interaction mediated stabilization of pro- cis pro bond in peptides with pro-pro-aromatic motifs. J Am Chem Soc. 2012;134:4661–4669. doi: 10.1021/ja209334v. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Mallick A, Díaz DD. Crystal structure of (2S, 4R)-2-benzyl 1-tert-butyl 4-(tosyloxy)pyrrolidine- 1,2-dicarboxylate, C24H29NO7S. Zeitschrift für Kristallographie - New Crystal Structures. 2014;227:361–362. [Google Scholar]
- Gilchrist TL, Lemos A, Ottaway CJ. Azabicyclo[3.2.0]heptan-7-ones (carbapenams) from pyrrole. J Chem Soc Perkin Trans. 1997;1:3005–3012. [Google Scholar]
- Gorres KL, Raines RT. Prolyl 4-hydroxylase. Crit Rev Biochem Mol Biol. 2010;45:106–124. doi: 10.3109/10409231003627991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grathwohl C, Wüthrich K. The X-Pro peptide bond as an nmr probe for conformational studies of flexible linear peptides. Biopolymers. 1976;15:2025–2041. doi: 10.1002/bip.1976.360151012. [DOI] [PubMed] [Google Scholar]
- Hack V, Reuter C, Opitz R, et al. Efficient α-helix induction in a linear peptide chain by N-capping with a bridged-tricyclic diproline analogue. Angew Chem Int Ed. 2013;52:9539–9543. doi: 10.1002/anie.201302014. [DOI] [PubMed] [Google Scholar]
- Hetzel R, Wüthrich K. Conformational energy studies of linear dipeptides H-X-L-Pro-OH. Biopolymers. 1979;18:2589–2606. [Google Scholar]
- Ho BK, Brasseur R. The Ramachandran plots of glycine and pre-proline. BMC Struct Biol. 2005;5:14. doi: 10.1186/1472-6807-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JA, Raines RT. Stereoelectronic effects on collagen stability: the dichotomy of 4-fluoroproline diastereomers. J Am Chem Soc. 2003;125:9262–9263. doi: 10.1021/ja035881z. [DOI] [PubMed] [Google Scholar]
- Holmgren SK, Bretscher LE, Taylor KM, Raines RT. A hyperstable collagen mimic. Chem Biol. 1999;6:63–70. doi: 10.1016/S1074-5521(99)80003-9. [DOI] [PubMed] [Google Scholar]
- Holzberger B, Marx A. Replacing 32 Proline residues by a noncanonical amino acid results in a highly active DNA polymerase. J Am Chem Soc. 2010;132:15708–15713. doi: 10.1021/ja106525y. [DOI] [PubMed] [Google Scholar]
- Holzberger B, Obeid S, Welte W, et al. Structural insights into the potential of 4-fluoroproline to modulate biophysical properties of proteins. Chem Sci. 2012;3:2924–2931. [Google Scholar]
- Horng J-C, Raines RT. Stereoelectronic effects on polyproline conformation. Protein Sci. 2006;15:74–83. doi: 10.1110/ps.051779806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hospital M, Courseille C, Leroy F, Roques BP. The role of water in the crystal structure of N-acetyl-L-4-hydroxyproline. Biopolymers. 1979;18:1141–1148. [Google Scholar]
- Hsu W-L, Shih T-C, Horng J-C. Folding stability modulation of the villin headpiece helical subdomain by 4-fluorophenylalanine and 4-methylphenylalanine. Biopolymers. 2015;103:627–637. doi: 10.1002/bip.22689. [DOI] [PubMed] [Google Scholar]
- Huang K-Y, Horng J-C. Modulating the affinities of phosphopeptides for the human Pin1 WW domain using 4-substituted proline derivatives. Biochemistry. 2015;54:6186–6194. doi: 10.1021/acs.biochem.5b00880. [DOI] [PubMed] [Google Scholar]
- Hudson DM, Eyre DR. Collagen prolyl 3-hydroxylation: a major role for a minor post-translational modification? Connect Tissue Res. 2013;54:245–251. doi: 10.3109/03008207.2013.800867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson DM, Kim LS, Weis M, et al. Peptidyl 3-hydroxyproline binding properties of type I collagen suggest a function in fibril supramolecular assembly. Biochemistry. 2012;51:2417–2424. doi: 10.1021/bi2019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson DM, Werther R, Weis M, et al. Evolutionary origins of C-terminal (GPP) n 3-hydroxyproline formation in vertebrate tendon collagen. PLoS One. 2014;9:e93467. doi: 10.1371/journal.pone.0093467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussaini SR, Moloney MG. 2,5-disubstituted pyrrolidines: versatile regioselective and diastereoselective synthesis by enamine reduction and subsequent alkylation. Org Biomol Chem. 2003;1:1838–1841. doi: 10.1039/b303789d. [DOI] [PubMed] [Google Scholar]
- Huy P, Neudörfl J-M, Schmalz H-G. A practical synthesis of trans-3-substituted proline derivatives through 1,4-addition. Org Lett. 2011;13:216–219. doi: 10.1021/ol102613z. [DOI] [PubMed] [Google Scholar]
- Jäger M, Dendle M, Kelly JW. Sequence determinants of thermodynamic stability in a WW domain—an all-β-sheet protein. Protein Sci. 2009;18:1806–1813. doi: 10.1002/pro.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins CL, Bretscher LE, Guzei IA, Raines RT. Effect of 3-hydroxyproline residues on collagen stability. J Am Chem Soc. 2003;125:6422–6427. doi: 10.1021/ja034015j. [DOI] [PubMed] [Google Scholar]
- Jenkins CL, Raines RT. Insights on the conformational stability of collagen. Nat Prod Rep. 2002;19:49–59. doi: 10.1039/a903001h. [DOI] [PubMed] [Google Scholar]
- Jones RCF, Howard KJ, Snaith JS, et al. An enantioselective route to pyrrolidines: removal of the chiral template from homochiral pyrroloimidazoles. Tetrahedron. 2011;67:8925–8936. [Google Scholar]
- Juvvadi P, Dooley DJ, Humblet CC, et al. Bradykinin and angiotensin II analogs containing a conformationally constrained proline analog. Int J Pept Protein Res. 1992;40:163–170. doi: 10.1111/j.1399-3011.1992.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Kantharaju RS, Raghavender US, et al. Conformations of heterochiral and homochiral proline-pseudoproline segments in peptides: context dependent cis–trans peptide bond isomerization. Pept Sci. 2009;92:405–416. doi: 10.1002/bip.21207. [DOI] [PubMed] [Google Scholar]
- Kato Y. An engineered bacterium auxotrophic for an unnatural amino acid: a novel biological containment system. PeerJ. 2015;3:e1247. doi: 10.7717/peerj.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YK, Park HS. Conformational preferences of the 2-methylproline residue and its role in stabilizing β-turn and polyproline II structures of peptides. New J Chem. 2014;38:2831–2840. [Google Scholar]
- Kemmink J, Creighton TE. Local conformations of peptides representing the entire sequence of bovine pancreatic trypsin inhibitor and their roles in folding. J Mol Biol. 1993;234:861–878. doi: 10.1006/jmbi.1993.1631. [DOI] [PubMed] [Google Scholar]
- Kemmink J, Creighton TE. The physical properties of local interactions of tyrosine residues in peptides and unfolded proteins. J Mol Biol. 1995;245:251–260. doi: 10.1006/jmbi.1994.0021. [DOI] [PubMed] [Google Scholar]
- Kim W, George A, Evans M, Conticello VP. Cotranslational incorporation of a structurally diverse series of proline analogues in an Escherichia coli expression system. ChemBioChem. 2004;5:928–936. doi: 10.1002/cbic.200400052. [DOI] [PubMed] [Google Scholar]
- Kim W, Hardcastle KI, Conticello VP. Fluoroproline Flip-flop: regiochemical reversal of a stereoelectronic effect on peptide and protein structures. Angew Chem Int Ed. 2006;45:8141–8145. doi: 10.1002/anie.200603227. [DOI] [PubMed] [Google Scholar]
- Kim W, McMillan RA, Snyder JP, Conticello VP. A stereoelectronic effect on turn formation due to proline substitution in elastin-mimetic polypeptides. J Am Chem Soc. 2005;127:18121–18132. doi: 10.1021/ja054105j. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Chikushi A, Tougu S, et al. Membrane translocation mechanism of the antimicrobial peptide Buforin 2. Biochemistry. 2004;43:15610–15616. doi: 10.1021/bi048206q. [DOI] [PubMed] [Google Scholar]
- Kotch FW, Guzei IA, Raines RT. Stabilization of the collagen triple helix by O-methylation of hydroxyproline residues. J Am Chem Soc. 2008;130:2952–2953. doi: 10.1021/ja800225k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubyshkin V. Stabilization of the triple helix in collagen mimicking peptides. Org Biomol Chem. 2019;17:8031–8047. doi: 10.1039/c9ob01646e. [DOI] [PubMed] [Google Scholar]
- Kubyshkin V, Budisa N. Amide rotation trajectories probed by symmetry. Org Biomol Chem. 2017;15:6764–6772. doi: 10.1039/c7ob01421j. [DOI] [PubMed] [Google Scholar]
- Kubyshkin V, Pridma S, Budisa N. Comparative effects of trifluoromethyl- and methyl-group substitutions in proline. New J Chem. 2018;42:13461–13470. [Google Scholar]
- Kümin M, Sonntag L-S, Wennemers H. Azidoproline containing helices: stabilization of the polyproline II structure by a functionalizable group. J Am Chem Soc. 2007;129:466–467. doi: 10.1021/ja067148o. [DOI] [PubMed] [Google Scholar]
- Kurtz J, Berger A, Katchalski E. Mutarotation of poly-L-proline. Nature. 1956;178:1066–1067. [Google Scholar]
- Lewis PN, Momany FA, Scheraga HA. Chain reversals in proteins. Biochimica et Biophysica Acta (BBA) - Protein Structure. 1973;303:211–229. doi: 10.1016/0005-2795(73)90350-4. [DOI] [PubMed] [Google Scholar]
- Lübben J, Volkmann C, Grabowsky S, et al. On the temperature dependence of H-Uiso in the riding hydrogen model. Acta Cryst A. 2014;70:309–316. doi: 10.1107/S2053273314010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lummis SCR, Beene DL, Lee LW, et al. Cis–trans isomerization at a proline opens the pore of a neurotransmitter-gated ion channel. Nature. 2005;438:248–252. doi: 10.1038/nature04130. [DOI] [PubMed] [Google Scholar]
- Lynch VM, Hulme C, Magnus P, Davis BE. Novel 2- and 5-Azido-N-(diphenylcarbamoyl) proline methyl esters. Examples of a novel proline oxidation. Acta Cryst C. 1995;51:2598–2601. doi: 10.1107/s0108270195010237. [DOI] [PubMed] [Google Scholar]
- MacArthur MW, Thornton JM. Influence of proline residues on protein conformation. J Mol Biol. 1991;218:397–412. doi: 10.1016/0022-2836(91)90721-h. [DOI] [PubMed] [Google Scholar]
- Masumi F, Takeuchi H, Kondo S, et al. Synthesis of L-tryptophan from L-glutamic acid. Chemical & Pharmaceutical Bulletin. 1982;30:3831–3833. [Google Scholar]
- Matsumura Y, Ogura K, Kouchi Y, et al. New efficient organic activators for highly enantioselective reduction of aromatic ketones by trichlorosilane. Org Lett. 2006;8:3789–3792. doi: 10.1021/ol0613822. [DOI] [PubMed] [Google Scholar]
- Meng HY, Thomas KM, Lee AE, Zondlo NJ. Effects of i and i+3 residue identity on Cis–Trans isomerism of the aromatici+1–prolyli+2 amide bond: implications for type VI β-turn formation. Pept Sci. 2006;84:192–204. doi: 10.1002/bip.20382. [DOI] [PubMed] [Google Scholar]
- Moloney MG, Panchal T, Pike R. trans-2,5-disubstituted pyrrolidines: rapid stereocontrolled access from sulfones. Org Biomol Chem. 2006;4:3894–3897. doi: 10.1039/b611583g. [DOI] [PubMed] [Google Scholar]
- Mooney SD, Kollman PA, Klein TE. Conformational preferences of substituted prolines in the collagen triple helix. Biopolymers. 2002;64:63–71. doi: 10.1002/bip.10123. [DOI] [PubMed] [Google Scholar]
- Moretto A, Terrenzani F, Crisma M, et al. Cα-methyl proline: a unique example of split personality. Biopolymers. 2008;89:465–470. doi: 10.1002/bip.20839. [DOI] [PubMed] [Google Scholar]
- Moroder L, Budisa N. Synthetic biology of protein folding. ChemPhysChem. 2010;11:1181–1187. doi: 10.1002/cphc.201000035. [DOI] [PubMed] [Google Scholar]
- Mortenson DE, Kreitler DF, Thomas NC, et al. Evaluation of β-amino acid replacements in protein loops: effects on conformational stability and structure. ChemBioChem. 2018;19:604–612. doi: 10.1002/cbic.201700580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulzer J, Schülzchen F, Bats J-W. Rigid dipeptide mimetics. Stereocontrolled synthesis of all eight stereoisomers of 2-Oxo-3-(N-Cbz-amino)-1-azabicyclo[4.3.0]nonane-9-carboxylic acid ester. Tetrahedron. 2000;56:4289–4298. [Google Scholar]
- Mykhailiuk PK, Kubyshkin V, Bach T, Budisa N. Peptidyl-prolyl model study: how does the electronic effect influence the amide bond conformation? J Org Chem. 2017;82:8831–8841. doi: 10.1021/acs.joc.7b00803. [DOI] [PubMed] [Google Scholar]
- Naduthambi D, Zondlo NJ. Stereoelectronic tuning of the structure and stability of the Trp cage miniprotein. J Am Chem Soc. 2006;128:12430–12431. doi: 10.1021/ja0648458. [DOI] [PubMed] [Google Scholar]
- Nanzer Alain P, Torda Andrew E, Bisang Christian, Weber Christoph, Robinson John A, van Gunsteren Wilfred F. Dynamical studies of peptide motifs in the Plasmodium falciparum circumsporozoite surface protein by restrained and unrestrained MD simulations. Journal of Molecular Biology. 1997;267(4):1012–1025. doi: 10.1006/jmbi.1997.0911. [DOI] [PubMed] [Google Scholar]
- Nardi F, Kemmink J, Sattler M, Wade RC. The cisproline(i - 1)-aromatic(i) interaction: folding of the Ala-cisPro-Tyr peptide characterized by NMR and theoretical approaches. J Biomol NMR. 2000;17:63–77. doi: 10.1023/a:1008380807603. [DOI] [PubMed] [Google Scholar]
- Newberry RW, Raines RT. The n→π* interaction. Acc Chem Res. 2017;50:1838–1846. doi: 10.1021/acs.accounts.7b00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hagan D, Bilton C, Howard JAK, et al. The preferred conformation of N-β-fluoroethylamides. Observation of the fluorine amide gauche effect. J Chem Soc Perkin Trans. 2000;2:605–607. [Google Scholar]
- Onomura O, Kirira PG, Tanaka T, et al. Diastereoselective arylation of l-proline derivatives at the 5-position. Tetrahedron. 2008;64:7498–7503. [Google Scholar]
- Pal D, Chakrabarti P. Cis peptide bonds in proteins: residues involved, their conformations, interactions and locations. J Mol Biol. 1999;294:271–288. doi: 10.1006/jmbi.1999.3217. [DOI] [PubMed] [Google Scholar]
- Panasik N, Eberhardt ES, Edison AS, et al. Inductive effects on the structure of proline residues. Int J Pept Protein Res. 1994;44:262–269. doi: 10.1111/j.1399-3011.1994.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Pandey AK, Naduthambi D, Thomas KM, Zondlo NJ. Proline editing: a general and practical approach to the synthesis of functionally and structurally diverse peptides. Analysis of steric versus stereoelectronic effects of 4-substituted prolines on conformation within peptides. J Am Chem Soc. 2013;135:4333–4363. doi: 10.1021/ja3109664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Yap GPA, Zondlo NJ. (2S,4R)-4-hydroxyproline(4-nitrobenzoate): strong induction of stereoelectronic effects via a readily synthesized Proline derivative. Crystallographic observation of a correlation between torsion angle and bond length in a hyperconjugative interaction. J Org Chem. 2014;79:4174–4179. doi: 10.1021/jo500367d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Byun BJ, Motooka D, et al. Conformational preferences of 4-chloroproline residues. Biopolymers. 2012;97:629–641. doi: 10.1002/bip.22054. [DOI] [PubMed] [Google Scholar]
- Pierson NA, Chen L, Russell DH, Clemmer DE. Cis–trans isomerizations of proline residues are key to bradykinin conformations. J Am Chem Soc. 2013;135:3186–3192. doi: 10.1021/ja3114505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plimmer RHA. The chemical constitution of the proteins: analysis. Green & Company: Longmans; 1912. [Google Scholar]
- Polindara-García LA, Miranda LD. Two-step synthesis of 2,3-dihydropyrroles via a formal 5-endo cycloisomerization of Ugi 4-CR/propargyl adducts. Org Lett. 2012;14:5408–5411. doi: 10.1021/ol3024727. [DOI] [PubMed] [Google Scholar]
- Rajalakshmi P, Srinivasan N, Krishnakumar RV, et al. N-tert-butoxycarbonyl-α-(2-fluorobenzyl)-l-proline. Acta Cryst E. 2013;69:o1297–o1297. doi: 10.1107/S1600536813019788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran GN, Mitra AK. An explanation for the rare occurrence of cis peptide units in proteins and polypeptides. J Mol Biol. 1976;107:85–92. doi: 10.1016/s0022-2836(76)80019-8. [DOI] [PubMed] [Google Scholar]
- Ramachandran GN, Ramakrishnan C, Sasisekharan V. Stereochemistry of polypeptide chain configurations. J Mol Biol. 1963;7:95–99. doi: 10.1016/s0022-2836(63)80023-6. [DOI] [PubMed] [Google Scholar]
- Ramachandran GN, Sasisekharan V (1968) Conformation of polypeptides and proteins* In: Anfinsen CB, Anson ML, Edsall JT, Richards FM (eds) Advances in Protein Chemistry. Academic Press, pp 283–437 [DOI] [PubMed]
- Reimer Ulf, Scherer Gerd, Drewello Mario, Kruber Susanne, Schutkowski Mike, Fischer Gunter. Side-chain effects on peptidyl-prolyl cis/trans isomerisation. Journal of Molecular Biology. 1998;279(2):449–460. doi: 10.1006/jmbi.1998.1770. [DOI] [PubMed] [Google Scholar]
- Reuter C, Huy P, Neudörfl J-M, et al. Exercises in pyrrolidine chemistry: gram scale synthesis of a pro–pro dipeptide mimetic with a polyproline type II helix conformation. Chem Eur J. 2011;17:12037–12044. doi: 10.1002/chem.201101704. [DOI] [PubMed] [Google Scholar]
- Reuter C, Opitz R, Soicke A, et al. Design and stereoselective synthesis of ProM-2: a spirocyclic diproline mimetic with polyproline type II (PPII) helix conformation. Chem Eur J. 2015;21:8464–8470. doi: 10.1002/chem.201406493. [DOI] [PubMed] [Google Scholar]
- Richardson JS. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Rodríguez I, Calaza MI, Jiménez AI, Cativiela C. Synthesis of enantiomerically pure δ-benzylproline derivatives. New J Chem. 2015;39:3310–3318. [Google Scholar]
- Rose-Sperling D, Tran MA, Lauth LM, et al. 19F NMR as a versatile tool to study membrane protein structure and dynamics. Biol Chem. 2019;400:1277–1288. doi: 10.1515/hsz-2018-0473. [DOI] [PubMed] [Google Scholar]
- Rubini C, Ruzza P, Spaller MR, et al. Recognition of lysine-rich peptide ligands by murine cortactin SH3 domain: CD, ITC, and NMR studies. Pept Sci. 2010;94:298–306. doi: 10.1002/bip.21350. [DOI] [PubMed] [Google Scholar]
- Rubini M, Schärer MA, Capitani G, Glockshuber R. (4R)- and (4S)-fluoroproline in the conserved cis-prolyl peptide bond of the thioredoxin fold: tertiary structure context dictates ring puckering. ChemBioChem. 2013;14:1053–1057. doi: 10.1002/cbic.201300178. [DOI] [PubMed] [Google Scholar]
- Salih N, Adams H, Jackson RFW. Synthesis of ω-Oxo amino acids and trans-5-substituted proline derivatives using cross-metathesis of unsaturated amino acids. J Org Chem. 2016;81:8386–8393. doi: 10.1021/acs.joc.6b01571. [DOI] [PubMed] [Google Scholar]
- Sasisekharan V, Ramachandran GN. Studies on collagen. Proc Indian Acad Sci. 1957;45:363–376. [Google Scholar]
- Schnitzer T, Wennemers H. Effect of γ-substituted proline derivatives on the performance of the peptidic catalyst H-dPro-pro-Glu-NH2. Synthesis. 2018;50:4377–4382. [Google Scholar]
- Sem DS, Baker BL, Victoria EJ, et al. Structural characterization and optimization of antibody-selected phage library mimotopes of an antigen associated with autoimmune recurrent thrombosis. Biochemistry. 1998;37:16069–16081. doi: 10.1021/bi9807207. [DOI] [PubMed] [Google Scholar]
- Shoulders MD, Guzei IA, Raines RT. 4-Chloroprolines: synthesis, conformational analysis, and effect on the collagen triple helix. Biopolymers. 2008;89:443–454. doi: 10.1002/bip.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulders MD, Hodges JA, Raines RT. Reciprocity of steric and stereoelectronic effects in the collagen triple helix. J Am Chem Soc. 2006;128:8112–8113. doi: 10.1021/ja061793d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulders MD, Kamer KJ, Raines RT. Origin of the stability conferred upon collagen by fluorination. Bioorg Med Chem Lett. 2009;19:3859–3862. doi: 10.1016/j.bmcl.2009.03.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulders MD, Kotch FW, Choudhary A, et al. The aberrance of the 4S diastereomer of 4-hydroxyproline. J Am Chem Soc. 2010;132:10857–10865. doi: 10.1021/ja103082y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulders MD, Raines RT. Modulating collagen triple-helix stability with 4-chloro, 4-fluoro, and 4-methylprolines. Adv Exp Med Biol. 2009;611:251. doi: 10.1007/978-0-387-73657-0_115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebler C, Trapp N, Wennemers H. Crystal structure of (4S)-aminoproline: conformational insight into a pH-responsive proline derivative. J Pept Sci. 2015;21:208–211. doi: 10.1002/psc.2734. [DOI] [PubMed] [Google Scholar]
- Smolskaya, Andreev Site-Specific Incorporation of Unnatural Amino Acids into Escherichia coli Recombinant Protein: Methodology Development and Recent Achievement. Biomolecules. 2019;9(7):255. doi: 10.3390/biom9070255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soave R, Roversi P, Destro R. Two 1,5-disubstituted proline esters. Acta Cryst C. 1997;53:933–936. [Google Scholar]
- Sonntag L-S, Schweizer S, Ochsenfeld C, Wennemers H. The “azido gauche effect”implications for the conformation of azidoprolines. J Am Chem Soc. 2006;128:14697–14703. doi: 10.1021/ja0654938. [DOI] [PubMed] [Google Scholar]
- Steiner T, Hess P, Bae JH, et al. Synthetic biology of proteins: tuning GFPs folding and stability with fluoroproline. PLoS One. 2008;3:e1680. doi: 10.1371/journal.pone.0001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szcześniak P, Maziarz E, Stecko S, Furman B. Synthesis of polyhydroxylated piperidine and pyrrolidine peptidomimetics via one-pot sequential lactam reduction/Joullié–Ugi reaction. J Org Chem. 2015;80:3621–3633. doi: 10.1021/acs.joc.5b00335. [DOI] [PubMed] [Google Scholar]
- Tamaki M, Han G, Hruby VJ. Synthesis of 4-cis-phenyl-l-proline via hydrogenolysis. J Org Chem. 2001;66:3593–3596. doi: 10.1021/jo005625u. [DOI] [PubMed] [Google Scholar]
- Tamazyan R, Ayvazyan A, Martirosyan A, et al. 1-(4-Bromobenzoyl)-2-phenylpyrrolidine-2-carboxamide. Acta Cryst E. 2008;64:o580–o580. doi: 10.1107/S1600536808003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamazyan R, Karapetyan H, Martirisyan A, et al. 1,2,5-substituted derivatives of 2-phenylpyrrolidine. Acta Cryst C. 2004;60:o390–o392. doi: 10.1107/S0108270104007930. [DOI] [PubMed] [Google Scholar]
- Tamazyan R, Karapetyan H, Martirosyan A, et al. 1-substituted derivatives of 2-phenylpyrrolidine-2-carboxamide. Acta Cryst C. 2002;58:o386–o388. doi: 10.1107/s0108270102008636. [DOI] [PubMed] [Google Scholar]
- Tang H-C, Lin Y-J, Horng J-C. Modulating the folding stability and ligand binding affinity of Pin1 WW domain by proline ring puckering. Proteins: Structure, Function, and Bioinformatics. 2014;82:67–76. doi: 10.1002/prot.24359. [DOI] [PubMed] [Google Scholar]
- Thomas CA, Talaty ER, Bann JG (2009) 3S-Fluoroproline as a probe to monitor proline isomerization during protein folding by 19F-NMR. Chem Commun:3366–3368 [DOI] [PMC free article] [PubMed]
- Thomas KM, Naduthambi D, Tririya G, Zondlo NJ. Proline editing: a divergent strategy for the synthesis of conformationally diverse peptides. Org Lett. 2005;7:2397–2400. doi: 10.1021/ol0506720. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Naduthambi D, Zondlo NJ. Electronic control of amide cis−trans isomerism via the aromatic−prolyl interaction. J Am Chem Soc. 2006;128:2216–2217. doi: 10.1021/ja057901y. [DOI] [PubMed] [Google Scholar]
- Tolmachova NA, Kondratov IS, Dolovanyuk VG, et al. Synthesis of new fluorinated proline analogues from polyfluoroalkyl β-ketoacetals and ethyl isocyanoacetate. Chem Commun. 2018;54:9683–9686. doi: 10.1039/c8cc05912h. [DOI] [PubMed] [Google Scholar]
- Toniolo C. Structure of conformationally constrained peptides: from model compounds to bioactive peptides. Biopolymers. 1989;28:247–257. doi: 10.1002/bip.360280125. [DOI] [PubMed] [Google Scholar]
- Torbeev V, Ebert M-O, Dolenc J, Hilvert D. Substitution of proline32 by α-methylproline preorganizes β2-microglobulin for oligomerization but not for aggregation into amyloids. J Am Chem Soc. 2015;137:2524–2535. doi: 10.1021/ja510109p. [DOI] [PubMed] [Google Scholar]
- Torbeev VY, Fumi E, Ebert M-O, et al. Cis-trans peptide-bond isomerization in α-methylproline derivatives. Helvetica Chimica Acta. 2012;95:2411–2420. [Google Scholar]
- Tran JA, Chen CW, Tucci FC, et al. Syntheses of tetrahydrothiophenes and tetrahydrofurans and studies of their derivatives as melanocortin-4 receptor ligands. Bioorg Med Chem Lett. 2008;18:1124–1130. doi: 10.1016/j.bmcl.2007.11.128. [DOI] [PubMed] [Google Scholar]
- Traub W, Shmueli U. Structure of poly- L -proline I. Nature. 1963;198:1165–1166. [Google Scholar]
- Tressler CM, Zondlo NJ. (2S,4R)- and (2S,4S)-perfluoro-tert-butyl 4-hydroxyproline: two conformationally distinct proline amino acids for sensitive application in 19F NMR. J Org Chem. 2014;79:5880–5886. doi: 10.1021/jo5008674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry DW, Luan C-H, Harris CM, Parker TM (1997) Protein-based materials with a profound range of properties and applications: the elastin ΔTt hydrophobic paradigm. Protein-Based Materials 133–178
- Urry DW, Shaw RG, Prasad KU. Polypentapeptide of elastin: temperature dependence of ellipticity and correlation with elastomeric force. Biochem Biophys Res Commun. 1985;130:50–57. doi: 10.1016/0006-291x(85)90380-8. [DOI] [PubMed] [Google Scholar]
- Valle JRD, Goodman M. Stereoselective synthesis of Boc-protected cis and trans-4-trifluoromethylprolines by asymmetric hydrogenation reactions. Angew Chem Int Ed. 2002;41:1600–1602. doi: 10.1002/1521-3773(20020503)41:9<1600::aid-anie1600>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Venkatachalam CM. Stereochemical criteria for polypeptides and proteins. V Conformation of a system of three linked peptide units Biopolymers. 1968;6:1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]
- Verhoork SJM, Killoran PM, Coxon CR. Fluorinated prolines as conformational tools and reporters for peptide and protein chemistry. Biochemistry. 2018;57:6132–6143. doi: 10.1021/acs.biochem.8b00787. [DOI] [PubMed] [Google Scholar]
- Wals K, Ovaa H (2014) Unnatural amino acid incorporation in E. coli: current and future applications in the design of therapeutic proteins. Front Chem 2:15 [DOI] [PMC free article] [PubMed]
- Webb TR, Eigenbrot C. Conformationally restricted arginine analogs. J Org Chem. 1991;56:3009–3016. [Google Scholar]
- Weis MA, Hudson DM, Kim L, et al. Location of 3-hydroxyproline residues in collagen types I, II, III, and V/XI implies a role in fibril supramolecular assembly. J Biol Chem. 2010;285:2580–2590. doi: 10.1074/jbc.M109.068726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JH, Zerbe O, von Philipsborn W, Robinson JA. β-Turns induced in bradykinin by (S)-α-methylproline. FEBS Lett. 1992;297:216–220. doi: 10.1016/0014-5793(92)80541-n. [DOI] [PubMed] [Google Scholar]
- Wenzell NA, Ganguly HK, Pandey AK, et al. Electronic and steric control of n→π* interactions: stabilization of the α-helix conformation without a hydrogen bond. ChemBioChem. 2019;20:963–967. doi: 10.1002/cbic.201800785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmot CM, Thornton JM. Analysis and prediction of the different types of β-turn in proteins. J Mol Biol. 1988;203:221–232. doi: 10.1016/0022-2836(88)90103-9. [DOI] [PubMed] [Google Scholar]
- Wu W-J, Raleigh DP. Local control of peptide conformation: stabilization of cis proline peptide bonds by aromatic proline interactions. Biopolymers. 1998;45:381–394. doi: 10.1002/(SICI)1097-0282(19980415)45:5<381::AID-BIP6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Yao J, Feher VA, Fabiola Espejo B, et al. Stabilization of a type VI turn in a family of linear peptides in water solution. J Mol Biol. 1994;243:736–753. doi: 10.1016/0022-2836(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Zheng T-Y, Lin Y-J, Horng J-C. Thermodynamic consequences of incorporating 4-substituted proline derivatives into a small helical protein. Biochemistry. 2010;49:4255–4263. doi: 10.1021/bi100323v. [DOI] [PubMed] [Google Scholar]
- Zhong H, Carlson HA. Conformational studies of polyprolines. J Chem Theory Comput. 2006;2:342–353. doi: 10.1021/ct050182t. [DOI] [PubMed] [Google Scholar]
- Zondlo NJ. Fold globally, bond locally. Nat Chem Biol. 2010;6:567–568. doi: 10.1038/nchembio.413. [DOI] [PubMed] [Google Scholar]
- Zondlo NJ. Aromatic–proline interactions: electronically tunable CH/π interactions. Acc Chem Res. 2013;46:1039–1049. doi: 10.1021/ar300087y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 169 kb)