ABSTRACT
Chlamydiae are common, important pathogens for humans and animals alike. Despite recent advancement in genetics, scientists are still searching for efficient tools to knock out or knock down the expression of chromosomal genes. We attempted to adopt a dCas9-based CRISPR interference (CRISPRi) technology to conditionally knock down gene expression in Chlamydia trachomatis using an anhydrotetracycline (ATC)-inducible expression system. Surprisingly, expression of the commonly used Streptococcus pyogenes dCas9 in C. trachomatis causes strong inhibition in the absence of any guide RNA (gRNA). Staphylococcus aureus dCas9 also shows strong toxicity in the presence of only an empty gRNA scaffold. Toxicity of the S. pyogenes dCas9 is readily observed with as little as 0.2 nM ATC. Growth inhibition by S. aureus dCas9 is evident starting at 1.0 nM ATC. In contrast, C. trachomatis growth was not affected by methionine-tRNA ligase overexpression induced with 10 nM ATC. We conclude that S. pyogenes and S. aureus dCas9 proteins in their current forms have limited utility for chlamydial research and suggest strategies to overcome this problem.
Keywords: Chlamydia, CRISPR, CRISPRi, dCas9, conditional gene knock-down, essential gene knock-down, toxicity
Expression of two deactivated Cas proteins (dCas) commonly used for CRISPRi gene-silencing both cause unintended toxicity in the sexually transmitted human pathogen Chlamydia trachomatis.
INTRODUCTION
Chlamydiae are broadly distributed pathogenic bacteria. For example, Chlamydia trachomatis is one of the most prevalent sexually transmitted pathogens worldwide (WHO 2012). In the United States, cases of sexually transmitted C. trachomatis infection consistently account for 60% of the total infected cases reported to the Centers for Diseases and Prevention (CDC 2018). Common complications from C. trachomatis infection include infertility, abortion, ectopic pregnancy and pelvic inflammatory syndrome (Zhong et al.2017). Unfortunately, an effective vaccine has yet to be developed despite numerous efforts by the scientific community over the past 60 years (de la Maza, Zhong and Brunham 2017; Zhong et al.2017). There is a clear need to better understand the Chlamydia biology to develop effective preventives.
Chlamydiae are obligate intracellular bacteria with a unique developmental cycle that is characterized by two contrasting cellular forms (Abdelrahman and Belland 2005). The small, electron-dense and non-dividing elementary body (EB) attaches to the host cell and enters a host membrane-derived vacuole through endocytosis (Hybiske and Stephens 2007). Within the vacuole, termed chlamydial inclusion, the EB transitions to the non-infectious reticulate body (RB), which is larger and less electron dense than the EB. RBs undergo several rounds of replication before asynchronously transitioning back into EBs, which exit the host cell (Abdelrahman and Belland 2005; Lee et al.2018).
Progression of the chlamydial developmental cycle is dictated by transcription (Belland et al.2003; Nicholson et al.2003; Humphrys et al.2013). Studies using animal models indicate that expression of specific genes is required for chlamydiae to colonize different tissues and/or organs, even though these genes may not affect chlamydial growth in cell culture (Yang et al.2017; Koprivsek et al.2019). However, the exact roles that many genes play in these processes have yet to be defined.
The C. trachomatis genome carries fewer than 1000 genes, of which nearly a third encode hypothetical proteins. Many of these encoding genes are unique to chlamydiae and require robust genetic tools to study their functions (Stephens et al.1998). Since the first report of reproducible transformation of C. trachomatis with an engineered plasmid (Wang et al.2011), methodologies for conditional gene expression and inactivation of non-essential genes have been developed (Agaisse and Derre 2013; Wickstrum et al.2013; Bauler and Hackstadt 2014; Key and Fisher 2017; Mueller, Wolf and Fields 2017). More recently, a strategy to conditionally knock down chlamydial genes using CRISPR interference (CRISPRi) was reported. The strategy relies on a catalytically-deactivated Cas9 (dCas9) from Staphylococcus aureus to block the transcription of a C. trachomatis gene that encodes a inclusion protein termed IncA (Ouellette 2018). If proven reliable, this technology would provide great power for studying essential genes, even though IncA is non-essential. However, in our attempt to adopt this technology, we found that conditional expression of the staphylococcal dCas9 (Sa-dCas9) strongly inhibits chlamydial growth in the absence of any specific guide RNA (gRNA). We also detected serious, nonspecific toxicity of a Streptococcus pyogenes dCas9 (Sp-dCas9) in C. trachomatis. These findings suggest new strategies are needed to conditionally knock down essential genes in this important pathogen.
MATERIALS AND METHODS
Vectors
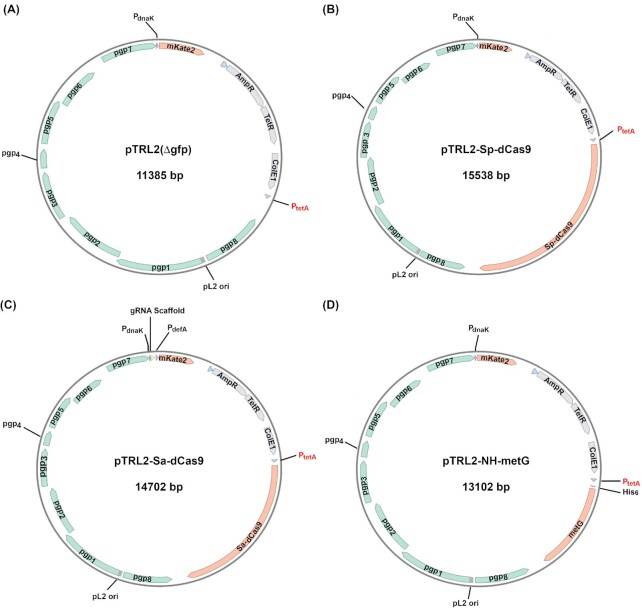
DNA primers for vector construction and/or sequencing (Table S1, Supporting Information) were custom-synthesized at Millipore Sigma. Q5 high fidelity DNA polymerase (used for amplifying DNA fragments; catalog # E5520S), Q5 site-directed mutagenesis kit (New England Biolabs catalog E0554) and NEBuilder HiFi DNA Assembly Cloning Kit (used for vector construction; catalog # M0491) were purchased from New England Biolabs. Plasmid pTRL2(Δgfp) (Fig. 1A) was derived by deleting the GFP gene from the shuttle vector pASK-GFP-L2-mkate2 (Wickstrum et al.2013). Primers Del-gfp-pASK-GFP-F and Del-gfp-pASK-GFP-R (Table S1, Supporting Information) were used to amplify the remaining portion of pASK-GFP-L2-mkate2. The amplified fragment was circled using the Q5 site-directed mutagenesis kit.
Figure 1.
Structures of pTRL2(Δgfp) (A), pTRL2-Sp-dCas9 (B), pTRL2-Sp-dCas9 (C) and pTRL2-metG (D). Abbreviations: Sp, S. pyogenesis; Sa, S. aureus; dCas9, deactivated Cas9; MetG, methionine-tRNA ligase; Pgp, chlamydial plasmid-encoded protein; PdnaK, dnaK promoter; mKate, a monomeric basic far-red fluorescence protein; gRNA, guide RNA; PdefA, defA promoter; AmpR, penicillin/ampicillin-resistance gene; tetR, tet repressor; ColE1, E. coli replication origin E1; PtetA, tetracycline/anhydrotetracycline (ATC)-activated promoter. pL2 ori, CtL2 plasmid replication origin.
Plasmid pTRL2-Sp-dCas9 (Fig. 1B) was constructed by assembling two DNA fragments using the NEBuilder HiFi DNA Assembly Cloning Kit. Fragment 1, essentially a linearized shuttle vector pTRL2(Δgfp), was amplified using pASK-GFP-L2-mkate2 as the template, primer TRL2-Frag-2-F and primer TRL2-Frag-1-R (Table S1, Supporting Information). Fragment 2, which contained the open reading frame of Sp-dCas9, was amplified using pTDH3-dCas9 as the template and primers PTRL2-dCas9-F and PTRL2-dCas9-R (Table S1, Supporting Information). pTDH3-dCas9 was a gift from Stanley Qi & Jonathan Weissman (Addgene plasmid # 46920; http://n2t.net/addgene:46920; RRID:Addgene_46920).
Plasmid pTRL2-Sa-dCas9 (Fig. 1C) was constructed by assembling four DNA fragments using the NEBuilder HiFi DNA Assembly Cloning Kit. Fragment 1 contained the pASK-GFP-L2-mkate2-originated mKate gene, ampicillin-resistance gene, tet-regulated transcription repressor (tetR), E. coli replication origin ColE1 and the tet-inducible promoter (PtetA). The fragment was amplified using pASK-GFP-L2-mkate2 as the template, and primers PTRL2-Frag1-F and PTRL2-Sa-dCas9-Frag1-R (Table S1, Supporting Information). Fragment 2, which contained all components of the CtL2 plasmid and a mutated dnaK promoter (Wickstrum et al.2013), was amplified using pTRL2 as the template, and primers PTRL2-Sa-dCas9-Frag2-F and PTRL2-Sa-dCas9-Frag2-R (Table S1, Supporting Information). Fragment 3, which contained a gRNA scaffold sequence followed by the transcription termination signal of ctl0608 and the defA (ctl0607) promoter (PdefA), was amplified using pUC57-Kan-GrgA gRNA-scaffold-TTS-PdefA as the template, and primers PTRL2-gRNA-C-F and PTRL2-gRNA-R (Table S1, Supporting Information). Fragment 4, which contained the Sa-dCas9 open reading frame, was amplified using pX603-AAV-CMV::NLS-dSaCas9 (D10A, N580A)-NLS-3xHA-bGHpA as the template, and primers Sa-dCas9-pTRL2-F and Sa-dCas9-pTRL2-R (Table S1, Supporting Information). pX603-AAV-CMV::NLS-dSaCas9 (D10A, N580A)-NLS-3xHA-bGHpA was a gift from Feng Zhang (Addgene plasmid # 61594; http://n2t.net/addgene:61594; RRID:Addgene_61594).
Plasmid pTRL2-NH-metG (Fig. 1D) was constructed by assembling two DNA fragments using the NEBuilder HiFi DNA Assembly Cloning Kit. Fragment 1, which contained all CtL2 plasmid encoding genes, mKate gene, ampicillin-resistance, tetR, ColE1, PtetA and a polyhistidine tag, was amplified using pTRL2-NH-GrgA (unpublished work) as the template, primers metG-NH-pTRL-F and metG-NH-pTRL-R (Table S1, Supporting Information). Fragment 2, which contained the open reading frame of metG gene, was amplified using CtL2 genomic DNA as the template and primers NH-pTRL-metG-F and NH-pTRL-metG-R (Table S1, Supporting Information).
All four plasmids constructed for this study were sequenced almost entirely using services provided by MacrogenUSA or Genscript. The only portions that were not sequenced were ampR and ColE 1. Sequences of the constructed plasmids were aligned against source sequences. None of the final plasmid used for experiments contained any nucleotide changes.
Chlamydiae
Chlamydia trachomatis L2 (CtL2) strain 434/BU was purchased from American Type Culture Collection (cat. # VR-902B) and maintained with L929 host cells (Bao et al.2014). CtL2 transformation and selection of transformants were carried out as previously described (Xu et al.2013).
Chlamydia trachomatis growth determination
L929 cells grown on 6-well plates at about 90% confluency were inoculated with CtL2 transformants and cultured at 37°C with or without ATC (Millipore Sigma, catalog # 37919). Bright field and mKate images were acquired on an Olympus IX51 fluorescence microscope using constant exposure time for each channel 30 hours post-inoculation. Image overlay was performed using the PictureFrame software. To quantify progeny EB formation, cultures were terminated 30 hours post-inoculation. Cells were disrupted by sonication to release chlamydiae. 1:10-serially diluted cell lysates were inoculated onto L929 cells grown on 96-well plates. Recoverable inclusion-forming units were scored 30 hours later (Gong et al.2014).
Immunostaining
L929 cells grown on 24-well plates were infected with pTRL2-Sa-dCas9 transformants and cultured at 37°C for 17 hours. Sa-dCas9 expression was induced for 4 hours following addition of ATC to the media. Cells were fixed sequentially with paraformaldehyde and cold methanol as previously described. The double-fixation protocol preserves mKate signal (Zhang et al.2017). The fixed cells were sequentially reacted with a mouse monoclonal anti-Sa-Cas9 primary antibody (Boster Biological Technology, catalog # M30929–3) and an FITC-conjugated anti-mouse IgG secondary antibody (Millipore Sigma, catalog # F9137). Evan Blue and Hochst dye 33342 were used to counter stain non-nuclear proteins and DNA, respectively, before microscopy. Images were acquired using constant exposure time for each channel.
RESULTS
Toxicity of Sp-dCas9 in C. trachomatis
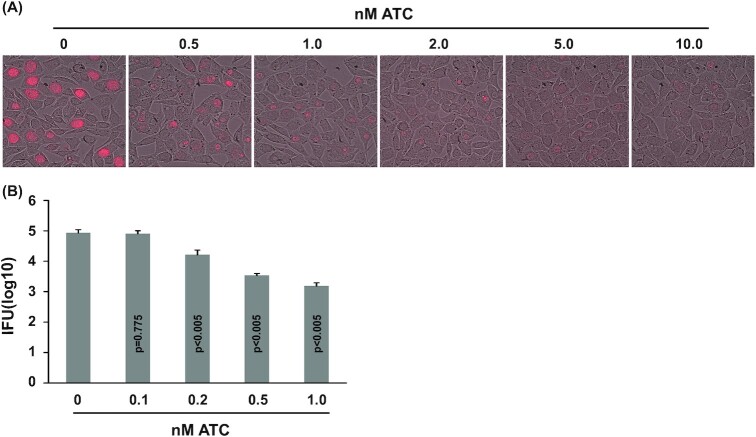
We initially chose Sp-dCas9 for CRISPRi in C. trachomatis because (i) it is the most extensively used dCas in the literature (Jiang and Doudna 2017) and (ii) it recognizes a short protospacer adjacent motif (PAM) sequence of only three nucleotides (NGG where N is any nucleotide). This notably short PAM sequence consequently allows for numerous targeting sites. We constructed a C. trachomatis expression vector for Sp-dCas9 termed pTRL2-Sp-dCas9 (Fig. 1A), in which the Sp-dCas9 sequence is placed downstream of an ATC-inducible promoter (Wickstrum et al.2013). This vector also carries an mKate gene, which encodes a far-red fluorescence protein under the control of a mutated dnaK promoter (PdnaK) (Wickstrum et al.2013). After a single-time transformation with pTRL2-Sp-dCas9, mKate-expressing inclusions were observed in 2 wells of a 6-well plate of L929 cells infected with transformed EBs at passage two of selection with penicillin. Both populations demonstrated severely reduced mKate signals in their inclusions 30 hours after inducing Sp-dCas9 expression with 10 nM ATC. One population was further analyzed using various concentrations of ATC. Reduced mKate inclusion signals were readily observed with 0.5 nM ATC, with progressively decreased signal intensity observed at higher concentrations (Fig. 2A). An 80% inhibition of progeny EB production was observed with even 0.2 nM ATC (Fig. 2B). The inhibition increased to 96 and 98%, with 0.5 nM and 1.0 nM ATC, respectively (Fig. 2B).
Figure 2.
Chlamydia trachomatis L2 (CtL2) growth inhibition by ATC-induced Sp-dCas9 expression. (A) Microscopic images showing reduced mKate expression in inclusions formed by pTRL2-Sp-dCas9 transformants of CtL2 cultured with indicated ATC concentrations. (B) ATC concentration-dependent inhibition of EB production by the transformants. Data are averages ± standard deviations of triplicate experiments. P values are of two-tailed t tests. (A, B) ATC was added at the time of inoculation. Microscopy and determination of recoverable inclusion-forming units were performed at 30 hours post-inoculation.
Toxicity of Sa-dCas9 in C. trachomatis
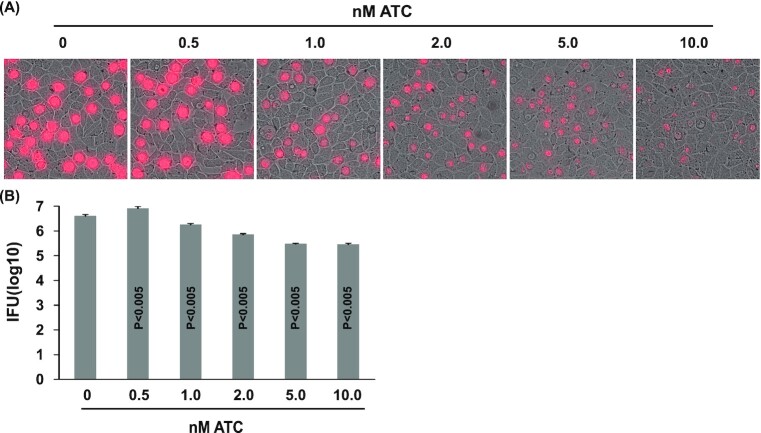
In light of a recent publication showing Sa-dCas9-mediated IncA expression inhibition (Ouellette 2018), we decided to adopt the same system by constructing the pTRL2-Sa-dCas9 vector with the addition of an mKate gene. In pTRL2-Sa-dCas9, PdnaK controls expression of a gRNA scaffold carrying no specific targeting sequence; a peptide deformylase gene promoter (PdefA) drives mKate expression (Fig. 2B). After a single-time transformation with pTRL2-Sp-dCas9, mKate-expressing inclusions emerged in 5 wells of a 6-well plate of L929 cells infected with transformed EBs at passage two of selection with penicillin. Disappointingly, the transformants also showed growth inhibition following Sa-dCas9 induction as evidenced by a dose-dependent decrease in both mKate expression (Fig. 3A) and progeny EB production (Fig. 3B). Although 0.5 nM ATC did not exhibit an inhibitory effect, 1, 2, 5 and 10 nM ATC inhibited EB production by 55, 82, 93 and 93%, respectively.
Figure 3.
CtL2 growth inhibition by ATC-induced Sa-dCas9 expression. (A) Microscopic images showing reduced mKate expression in inclusions formed by pTRL2-Sa-dCas9 transformants cultured with indicated ATC concentrations. (B) ATC concentration-dependent inhibition of EB production by the transformants. Data are averages ± standard deviations of triplicate experiments. P values are of two-tailed t tests. (A, B) ATC was added at the time of inoculation. Microscopy and determination of recoverable inclusion-forming units were performed at 32 hours post-inoculation.
Compared to pTRL2-Sp-dCas9 transformants (Fig. 2), pTRL2-Sa-dCas9 transformants were inhibited by ATC somewhat less efficiently (Fig. 3). We hoped that there would be a Sa-dCas9 expression range induced by ATC concentrations that allowed for CRISPRi without nonspecific toxicity. To assess this possibility, we performed immunofluorescence assays using a monoclonal anti-Sa-dCas9 antibody to analyze Sa-dCas9 expression following culture with different concentrations of ATC for 4 hours. pTRL2-Sa-dCas9 transformants induced with 0.5 and 1.0 nM ATC had indistinguishable staining profiles as the non-induced control. Specific dCas9 signals became definitively evident only with ≥2 nM ATC (Fig. 4), even though toxicity was readily detectable in cultures treated with 1 nM ATC (Fig. 3). Together, these findings do not support the existence of a practical ATC concentration range within which efficient gene transcription interference can be induced without nonspecific toxicity.
Figure 4.
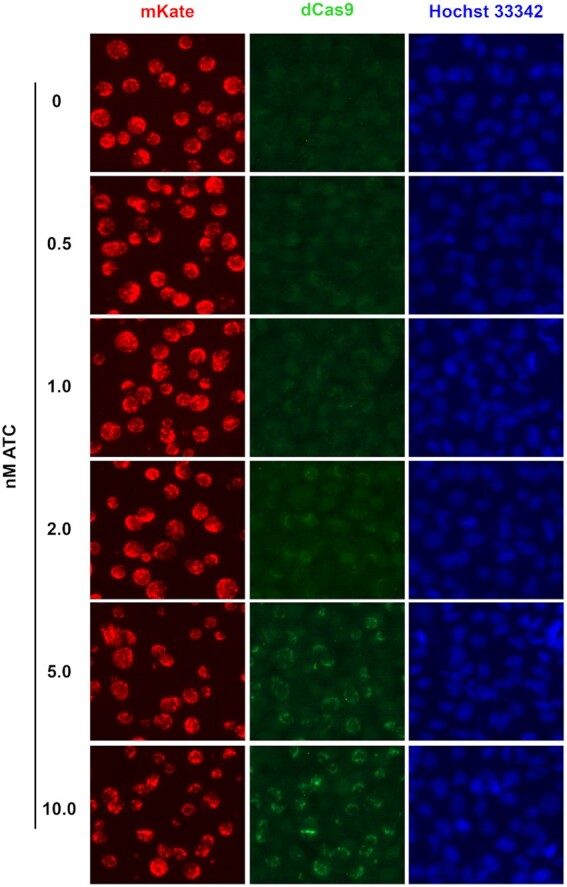
ATC concentration-dependent induction of Sa-dCas9 expression in pTRL2-Sa-dCas9 transformants. ATC was added at 17 hours posti-noculation. Cultures were fixed at 21 hours for Sa-dCas9 expression analysis using immunofluorescence.
Lack of toxicity by ATC
ATC is derived from tetracycline, a potent and known antichlamydial. To eliminate the possibility that residual antibacterial ATC activity was responsible for the observed toxicities following dCas9-overexpression, we determined the effects of ATC on the growth of CtL2 transformed with control plasmid pTRL2(Δgfp) (Fig. 1A). When compared to a non-treated control, mock-induction with 10 nM ATC did not demonstrate any inhibition of inclusion size or the mKate intensity (Fig. 5A). Furthermore, subsequent EB formation was also un-impeded (Fig. 5B). These data are consistent with findings by Wickstrum et al., that show a lack of inhibition by 10 nM ATC in wild-type CtL2 (Wickstrum et al.2013).
Figure 5.
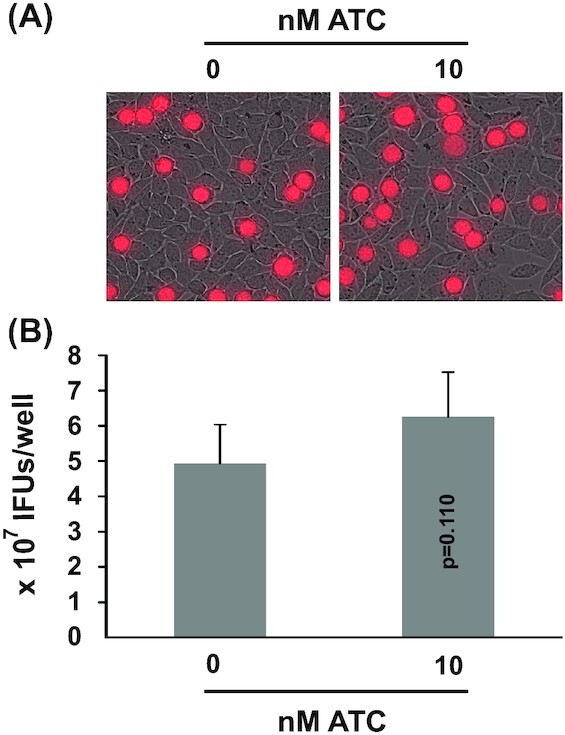
Growth of CtL2 transformed with control pTRL2(Δgfp) plasmid unaffected by ATC. (A) Microscopic images showing mKate-expressing inclusions formed in the presence of 0 or 10 nM ATC. (B) Recoverable IFUs formed in the presence of 0 or 10 nM ATC. Data are averages ± standard deviations of triplicate experiments. P values are of two-tailed t tests. (A, B) ATC was added at the time of inoculation. Microscopy and determination of recoverable inclusion-forming units were performed at 35 hours post-inoculation.
Lack of toxicity by methionine-tRNA ligase (MetG) overexpression
We considered the possibility that overexpression of any protein in CtL2 may induce toxicity, for which we constructed the plasmid pTRL2-NH-metG to overexpress N-terminally (His)6-tagged MetG (NH-MetG) under the control of PtetA (Fig. 1D). For transformants cultured in the absence of ATC, western blot using anti-(His)6 tag antibody detected only a low level of NH-MetG. However, when cultured in the presence of 10 nM ATC, a markedly increased level of NH-MetG was detected (Fig. 6A). Further analysis of inclusion size, mKate intensity (Fig. 6B) and EB production (Fig. 6C) corroborate the absence of growth inhibition following ATC-induced NH-MetG overexpression. Taken together, these data indicate Sp-dCas9 and Sa-dCas9 are themselves responsible for observed growth inhibition rather than the ATC used to induce protein expression.
Figure 6.
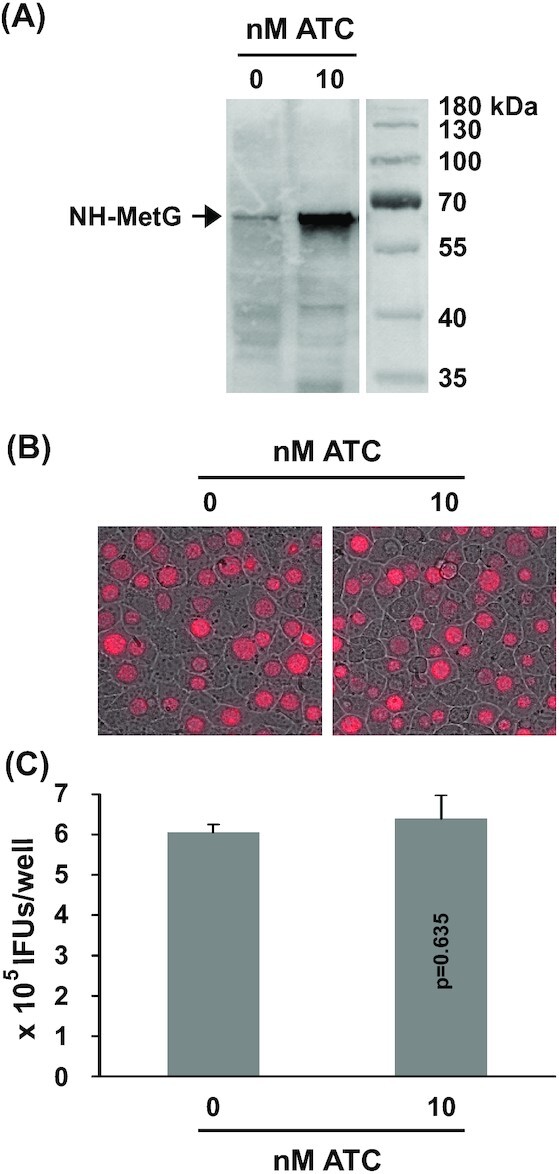
CtL2 growth unaffected by ATC-induced NH-MetG expression. (A) Western blot detecting the 65 kDa NH-MetG in pTRL2-NH-metG transformants of CtL2 cultured in the presence of 0 or 10 nM ATC. (B) mKate-expressing inclusions formed in the presence of 0 or 10 nM ATC. (C) Recoverable IFUs formed in the presence of 0 or 10 nM ATC. Data are averages ± standard deviations of triplicate experiments. P values are of two-tailed t tests. (A-C) ATC was added at the time of inoculation. Microscopy and determination of recoverable inclusion-forming units were performed at 30 hours post-inoculation.
DISCUSSION
CRISPR-Cas9 technologies have armed scientists with unprecedented genome editing powers. As a result, genes can now be efficiently inactivated or modified in a wide range of organisms, whereas inducible dCas expression has been used to conditionally knock down essential genes (Jiang and Doudna 2017). Although there has been no report of successful Chlamydia genome editing with wild-type Cas9 (or any other Cas proteins), a recent study documented knock down of C. trachomatis IncA expression with Sa-dCas9 (Ouellette 2018). It showed decreased IncA expression in select cells following induction of the Sa-dCas9 with 10 nM ATC. However, the effect of Sa-dCas9 expression on chlamydial growth was not quantified (Ouellette 2018). Our data presented here clearly demonstrate that C. trachomatis is highly sensitive to both Sp-dCas9 and Sa-dCas9 in the absence of any gRNA. Accordingly, significant reduction in EB formation was detected with only 0.2 and 1.0 nM ATC to induce expression of Sp-dCas9 and Sa-dCas9, respectively.
Nonspecific dCas9 toxicities in Escherichia coli and other bacteria have also been reported, but only when very high concentrations of ATC or doxycycline were used to induce expression (Rock et al.2017; Cho et al.2018). For example, 1 µM ATC is needed to demonstrate toxicity of Sp-dCas9 in E. coli K-12 1655 (Cho et al.2018). Chlamydia trachomatis seems particularly susceptible to the toxicity of Sp-dCas9, where 0.2 nM ATC is sufficient for growth inhibition.
While the toxicity mechanism remains unclear, nonspecific binding of Sp-dCas9 at high concentrations to the PAM site in the absence of any gRNA is a potential mechanism (Jones et al.2017). The PAM sequences of Sp-dCas9 and Sa-dCas9 are NGG and NNGRRT, respectively. The C. trachomatis genome (or any other genome) thereby has a higher number of Sp-dCas9 PAM sites than Sa-dCas9 PAM sites. This may explain the need for lower ATC concentrations to induce the toxicity of Sp-dCas9. However, we think that the differential ATC susceptibility is more likely due to a lower expression efficiency of Sa-dCas9 than Sp-dCas9 although we have only performed Sa-dCas9 expression analysis. Even though expression of both dCas is driven by PtetA, the Sp-dCas9 gene may have a higher transcription efficiency if the Sa-dCas gene has a higher tendency for abortive initiation (Duchi et al.2016). The Sa-dCas9 mRNA may also have a lower translation efficiency if its codons are biased against by CtL2 (Heizer et al.2006).
In conclusion, nonspecific toxicities of Sp-dCas9 and Sa-dCas9 as documented in this report limit their utilities in Chlamydia biology. There are potential solutions to the problem. First, one might be able to select Chlamydia repressors that are no longer inhibited by either Sp-dCas9 or Sa-dCas9. Second, some of the strategies used to produce enzymatically active Sp-Cas9 variants with reduced toxicity could be used to generate dCas variants with less chlamydial toxicity (Bolukbasi et al.2015; Wright et al.2015). Finally, since a large number of Cas with dissimilar properties have been identified and characterized (Makarova, Wolf and Koonin 2018), the deactivated form of some of the alternative Cas may be tolerated by Chlamydia.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. P. Scott Hefty (University of Kansas) for supplying the pASK-GFP-L2-mkate2 plasmid (Wickstrum et al.2013) and our colleague Dr. Juan Carlos Collantes for discussing our findings.
FUNDING
This work was supported by the National Institute of Allergy and Infectious Diseases (Grant # AI122034 and AI140167) and New Jersey Health Foundation (Grant # PC 20–18). YH is a recipient of a China Scholarship Council scholarship. AMW was a Rutgers Aresty Scholar and a recipient of a Robert Wood Johnson Medical School Summer Research Fellowship funded by the New Jersey Health Foundation.
Conflict of interest None declared.
REFERENCES
- Abdelrahman YM, Belland RJ. The chlamydial developmental cycle. FEMS Microbiol Rev. 2005;29:949–59. [DOI] [PubMed] [Google Scholar]
- Agaisse H, Derre I. A C. trachomatis cloning vector and the generation of C. trachomatis strains expressing fluorescent proteins under the control of a C. trachomatis promoter. PLoS One. 2013;8:e57090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Gylfe A, Sturdevant GLet al.. Benzylidene acylhydrazides inhibit chlamydial growth in a type III secretion- and iron chelation-independent manner. J Bacteriol. 2014;196:2989–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauler LD, Hackstadt T. Expression and targeting of secreted proteins from Chlamydia trachomatis. J Bacteriol. 2014;196:1325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belland RJ, Zhong G, Crane DDet al.. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc Natl Acad Sci USA. 2003;100:8478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolukbasi MF, Gupta A, Oikemus Set al.. DNA-binding-domain fusions enhance the targeting range and precision of Cas9. Nat Methods. 2015;12:1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Sexually Transmitted Disease Surveillance 2016. Atlanta: U.S. Department of Health and Human Services, 2018. [Google Scholar]
- Cho S, Choe D, Lee Eet al.. High-level dCas9 expression induces abnormal cell morphology in Escherichia coli. ACS Synthetic Biology. 2018;7:1085–94. [DOI] [PubMed] [Google Scholar]
- de la Maza LM, Zhong G, Brunham RC. Update on Chlamydia trachomatis vaccinology. Clin Vaccine Immunol. 2017;24:e00543–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchi D, Bauer DLV, Fernandez Let al.. RNA polymerase pausing during initial transcription. Mol Cell. 2016;63:939–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Luna Y, Yu Pet al.. Lactobacilli inactivate Chlamydia trachomatis through lactic acid but not H2O2. PLoS One. 2014;9:e107758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizer EM Jr., Raiford DW, Raymer MLet al.. Amino acid cost and codon-usage biases in 6 prokaryotic genomes: a whole-genome analysis. Mol Biol Evol. 2006;23:1670–80. [DOI] [PubMed] [Google Scholar]
- Humphrys MS, Creasy T, Sun Yet al.. Simultaneous transcriptional profiling of bacteria and their host cells. PLoS One. 2013;8:e80597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hybiske K, Stephens RS. Mechanisms of Chlamydia trachomatis entry into nonphagocytic cells. Infect Immun. 2007;75:3925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Doudna JA. CRISPR–Cas9 structures and mechanisms. Annu Rev Biophys. 2017;46:505–29. [DOI] [PubMed] [Google Scholar]
- Jones DL, Leroy P, Unoson Cet al.. Kinetics of dCas9 target search in Escherichia coli. Science. 2017;357:1420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key CE, Fisher DJ. Use of group II Intron technology for targeted mutagenesis in Chlamydia trachomatis. Methods Mol Biol. 2017;1498:163–77. [DOI] [PubMed] [Google Scholar]
- Koprivsek J, Zhang T, Tian Qet al.. Distinct roles of chromosome- versus plasmid-encoded genital tract virulence factors in promoting Chlamydia muridarum colonization in the gastrointestinal tract. Infect Immun. 2019, DOI 10.1128/iai.00265-19: IAI.00265-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Enciso GA, Boassa Det al.. Replication-dependent size reduction precedes differentiation in Chlamydia trachomatis. Nat Commun. 2018;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova KS, Wolf YI, Koonin EV. Classification and nomenclature of CRISPR-Cas systems: where from here?. The CRISPR Journal. 2018;1:325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KE, Wolf K, Fields KA. Chlamydia trachomatis transformation and allelic exchange mutagenesis. Curr Protoc Microbiol. 2017;45:11A31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson TL, Olinger L, Chong Ket al.. Global stage-specific gene regulation during the developmental cycle of Chlamydia trachomatis. J Bacteriol. 2003;185:3179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette SP. Feasibility of a conditional knockout system for Chlamydia based on CRISPR interference. Front Cell Infect Mi. 2018;8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JM, Hopkins FF, Chavez Aet al.. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nature Microbiology. 2017;2:16274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RS, Kalman S, Lammel Cet al.. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kahane S, Cutcliffe LTet al.. Development of a transformation system for Chlamydia trachomatis: restoration of glycogen biosynthesis by acquisition of a plasmid shuttle vector. PLoS Pathog. 2011;7:e1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Global incidence and prevalence of selected curable sexually transmitted infections: 2008. Reprod Health Matters. 2012;20. [Google Scholar]
- Wickstrum J, Sammons LR, Restivo KNet al.. Conditional gene expression in Chlamydia trachomatis using the tet system. PLoS One. 2013;8:e76743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AV, Sternberg SH, Taylor DWet al.. Rational design of a split-Cas9 enzyme complex. Proc Natl Acad Sci. 2015;112:2984–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Battaglia L, Bao Xet al.. Chloramphenicol acetyltransferase as a selection marker for chlamydial transformation. BMC Res Notes. 2013;6:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Kari L, Sturdevant GLet al.. Chlamydia trachomatis ChxR is a transcriptional regulator of virulence factors that function in in vivo host-pathogen interactions. Pathog Dis. 2017;75:ftx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kunadia A, Lin Yet al.. Identification of a strong and specific antichlamydial N-acylhydrazone. PLoS One. 2017;12:e0185783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Brunham RC, de la Maza LMet al.. National Institute of Allergy and Infectious Diseases workshop report: “Chlamydia vaccines: the way forward”. Vaccine. 2017, 10.1016/j.vaccine.2017.10.075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.