Abstract
The sulfur biochemistry of the thiol group endows cysteines with a number of highly specialized and unique features that enable them to serve a variety of different functions in the cell. Typically highly conserved in proteins, cysteines are predominantly found in functionally or structurally crucial regions, where they act as stabilizing, catalytic, metal-binding and/or redox-regulatory entities. As highly abundant low molecular weight thiols, cysteine thiols and their oxidized disulfide counterparts are carefully balanced to maintain redox homeostasis in various cellular compartments, protect organisms from oxidative and xenobiotic stressors and partake actively in redox-regulatory and signaling processes. In this review, we will discuss the role of protein thiols as scavengers of hydrogen peroxide in antioxidant enzymes, use thiol peroxidases to exemplify how protein thiols contribute to redox signaling, provide an overview over the diverse set of low molecular weight thiol-based redox systems found in biology, and illustrate how thiol-based redox systems have evolved not only to protect against but to take full advantage of a world full of molecular oxygen.
1. Introduction
The transition towards an oxygenated atmosphere was likely the greatest challenge that organisms experienced during evolution. A particular threat of aerobic life is the incomplete reduction of molecular oxygen, which generates reactive oxygen species (ROS), including superoxide anions () and hydrogen peroxide (H2O2) [1,2]. Electrons can escape from the flavin groups or iron-sulfur centers of complex I, II, and III of the electron transport chain and monovalently reduce O2 to [3]. Enzymes, such as flavoprotein oxidases and monooxygenases [4] as well as lipoxygenases and cyclooxygenases involved in prostaglandin, prostacyclin, and leukotriene synthesis [5], generate , H2O2 or hydroperoxides. Moreover, NADPH oxidase family members produce and in some cases H2O2, not only in activated macrophages and neutrophils but also in non-phagocytic cell types [6]. Once formed, is usually rapidly converted to H2O2 by superoxide dismutase. However, in the presence of nitric oxide, which is produced by activated nitric oxide synthases, can form peroxynitrite, a highly reactive nitrogen species (RNS) [7]. H2O2 is able to readily react with free iron or copper (i.e., Fenton reaction) to form hydroxyl radicals, which are highly reactive and short-lived species [8,9].
The constant generation of cellular ROS and RNS, which can cause irreversible damage to proteins, lipids and DNA [9,10], demands efficient antioxidant systems to ensure organismal survival [8,11,12]. However, by the same mechanism by which uncontrolled widespread production of reactive oxygen and nitrogen species can lead large-scale oxidative damage, its controlled and more localized production offers unique regulatory opportunities in the cell [13–17]. It is now well established that spatiotemporally produced H2O2 serves as second messenger that oxidizes proteins with redox-sensitive thiols to induce distinct cellular signaling pathways [18,19]. In this review, we will provide insights into how cells utilize the versatility of the thiol group to protect themselves against and, at the same time, make use of cellular oxidants.
2. Cysteine thiols in proteins
Cysteines are unique among naturally occurring amino acids because of their thiol-containing side chain, which can undergo a variety of different nucleophilic reactions. The sulfhydryl group makes cysteines a popular choice for active sites in enzymes, attachment sites for prenylation and palmitoylation tags, as well as high-affinity binding sites of metals, such zinc or iron [20]. Likely the most defining feature of cysteine thiols, however, is their ability to undergo reversible and irreversible oxidation reactions. Deprotonation of the sulfhydryl group to the corresponding thiolate anion increases its reactivity in nucleophilic reactions. Although the general ability of a molecule in a given ionization state to act as a nucleophile drops with decreasing pKa [20,21], the reactivity of the thiol group depends at least in part on its pKa value [20]. In free cysteine, the pKa value of the thiol group is 8.45, which predicts that only a small proportion of cysteines are deprotonated under physiological pH conditions (Table 1) [22]. However, in proteins, reactive cysteines often exhibit significantly lower pKa values due to vicinal polar or positively charged amino acids stabilizing the thiolate anion [23,24]. Moreover, the localization of cysteines within secondary structure elements of proteins affects their reactivity. The dipole character of alpha-helices, for instance, lowers the pKa value and renders cysteine residues located towards the N-terminal end in general more reactive than cysteines positioned towards the C-terminal end [25]. The structural context of the thiol matters as well as since it defines the accessibility of a cysteine residue within the three-dimensional protein structure. In cellular systems, however, kinetic parameters determine the ability of a protein thiol to react with other molecules, and allow even thermodynamically unfavorable reactions to occur [26,27]. Hence, the prediction of reactive thiols in the proteome is still challenging and quantitative redox proteomic techniques are indispensable for identifying oxidation-sensitive cysteine thiols in living organisms [28–30].
Table 1.
Thiol-dependent redox systems.
| Thiol-specific reductase (NADPH-dependent) | Reduction potential in mVa | Thiol pKa | Thiol-specific oxidoreductase | Organism | |
|---|---|---|---|---|---|
| Cys | ND | −223 [35] | 8.5 [22] | ND | Giardia duodenalis, Entamoeba histolytica and others [36] |
| γ-Glu-Cys | Bis-γ-glutamyl-cystine reductase [37] | −234 [38] | 9.9 [39] | ND | Lactic acid bacteria [36], Halobacterium spp [40]. |
| GSH | Glutathione reductase (GR) [41] | −240 [42] | 8.9 [42] | Glutaredoxin (Grx) [43] | Eukaryotes, some bacteria (mainly Gram-negative) |
| T(SH)2 | Trypanothione reductase (TR) [44] | −242 [44] | 7.4 [45] | Trypanoredoxin (Tpx) [46] | Trypanosomatids, Euglena gracilis, Entamoeba histolytica, Naegleria fowleri [45] |
| MSH | Mycothiol disulfide reductase (Mtr) [47] | −230 [48] | 8.8 [48] | Mycoredoxin (Mrxl) [49] | Actinomycetes [47] |
| BSH | Disulfide reductase (YpdA) [50] | −221 [51] | 8.0 [51] | Bacilliredoxin (Brx) A and B [52] | Firmicutes [53], Deinococus-Thermus [54], Chlorobiaceaeb and Bacterioideteb [54] |
| CoA | CoA-disulfide reductase (CoA-DR) [55] | −234 [35] | 9.8 [56] | ND | Staphylococcus aureus, Bacillus anthracis, B. megaterium, Borrelia burgdorferi and others [57,58] |
| ESH | ND | − 60 [59] | 10.8 [60] | ND | Actinomycetes, cyanobacteria, methylbacteria, fungi [61] |
| OSH | ND | −90 [62] | 1.4 [63] | ND | Marine invertebrates; some Algae, bacteria, and trypanosomatids [63] |
ND = not determined.
For calculating thiol reduction potentials, E0’ (GSSG/GSH) = − 240 mV is commonly used as reference.
These organisms synthesize N-methyl-BSH.
Cysteines are among the four most frequently conserved amino acids (together with glycine, proline, and tryptophan) in proteins [31]. Genome-wide sequence comparisons revealed that when cysteines are conserved, the degree of conversation is often 90% or higher, while poorly conserved cysteines are typically less than 10% conserved. Such a high degree of conservation has not been exceeded by any of the other 19 amino acids, and likely emphasizes the strong selective pressure for keeping cysteine residues in functionally important locations while removing them from others [31]. Although more than 90% of eukaryotic proteins contain one or more cysteine residues, their occurrence is significantly below the theoretical value determined for amino acids encoded by two codons (i.e., 3.3%) [32]. Moreover, and in contrast to all other amino acids, the number of cysteines in proteins increases with the complexity of organisms, starting with only 0.4% in the archaeal and reaching 2.3% in the mammalian proteome. The lowest cysteine content has been observed in the halophilic archaea Haloarcula marismortui, in which fewer than 50% proteins contain a cysteine residue [32]. The overall phenomenon might be at least partially explained by the fact that the amount of non-cytosolic proteins harboring structural disulfide bonds goes up with increasing organismal complexity. However, given the important redox-regulatory role of cysteines, it is also tempting to speculate that increasing the complexity of organisms raises the need for additional ways to post-translationally regulate protein activity.
Cysteine distribution studies also revealed that in all organisms except plants, cysteines are preferably arranged in a CXXC motif, a signature motif typically associated with metal-binding and/or redox reactions [33,34]. In fact, every fifth human protein contains at least one CXXC motif [32]. Interestingly, the number of cysteines arranged in this pattern increases with decreasing numbers of total cysteines in the proteome. In S. cerevisiae, which has one of the lowest protein cysteine contents among eukaryotes, more than 5% of its total cysteines are arranged in CXXC motifs, and more than 21% of cysteines are arranged in this pattern in archaea [32]. These findings likely reflect the important and evolutionary conserved role of vicinal cysteines.
3. Reversible and irreversible thiol oxidation processes
Cells spend considerable energetic efforts to prevent non-specific thiol oxidation reactions while allowing the controlled formation of protein disulfide bonds or other oxidative thiol modifications that play physiologically relevant roles. The formation of structural disulfide bonds in proteins, for instance, is a tightly organized process catalyzed by specialized enzymes like oxidoreductases and thiol oxidases [64] and occurs mostly in dedicated cellular compartments, such as the periplasmic space, the endoplasmic reticulum (ER) and the mitochondrial intermembrane space (IMS) [64–66]. The cytosol, nucleus and mitochondrial matrix, on the other hand, maintain an overall reducing environment that ensures that protein thiols are kept reduced. However, presence of reactive oxygen, nitrogen or chlorine species (R–O/N/C–S) can either directly or indirectly cause a variety of different thiol modifications [67–69], which affect enzymatic activities, protein-protein interactions, susceptibility towards other post-translational modifications, protein turnover and/or sub-cellular localizations [16,70,71]. While protein thiol oxidations have, for many years, been considered to be undesirable side reactions of oxidative stress, an increasing number of redox-regulated proteins have been identified that use reversible thiol modifications of structurally or functionally important cysteine thiols to adjust their activity to the prevailing redox conditions of the environment [72–74]. Identifying and (potentially) predicting the functional consequences of thiol modifications in proteins, however, remains a major challenge in the field, and still requires careful case-by-case biochemical studies.
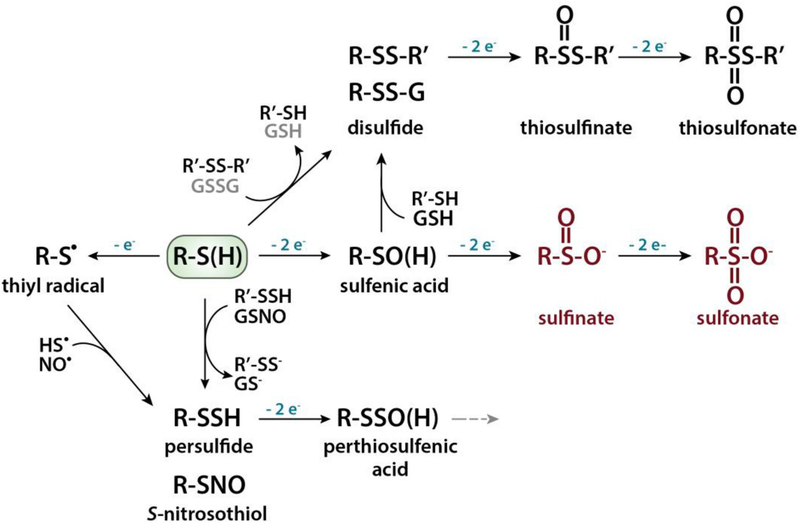
The two-electron oxidation of a thiol(ate) residue by reactive oxygen or nitrogen species, such as H2O2 or peroxynitrite, yields a cysteine-sulfenic acid (SOH) intermediate that is highly reactive and metastable [15,75]. Nevertheless, selective dimedone-based probes have been developed to detect these intermediates both in vitro and in cells [76]. Sulfenic acids readily react with near-by thiols to form intra- or intermolecular protein disulfide bonds (S–S) or mixed disulfides with LMW thiols, such as glutathione (S-SG) (Fig. 1) [20,70,77]. These oxidative thiol modifications are fully reversible, and their reduction is mediated by thiol-based redox systems, most prominently the glutathione (GSH)/glutathione reductase (GR) and thioredoxin (Trx)/thioredoxin reductase (TrxR) system. Both of these systems use NADPH as final electron donor [78]. Further oxidation of the sulfenic acid to a sulfinic or sulfonic acid species is generally considered an irreversible overoxidation and thought to lead to premature degradation (Fig. 1). One known exemption is the sulfinic acid formation at the catalytic cysteine of peroxiredoxin (Prx), which is reduced by the ATP-dependent Prx-specific repair enzyme sulfiredoxin [15,75].
Fig. 1.
Oxidative thiol modifications. Deprotonation of a protein thiol (RSH) leads to the formation of a highly reactive thiolate anion (RS−), which can undergo a variety of different oxidative modifications. Two-electron oxidation results in a sulfenic acid intermediate, which can react with another protein thiol to form an intra- or intermolecular disulfide bond or with a LMW thiol, such as glutathione (GSH) to form a mixed disulfide (i.e., S-glutathionylation). Further oxidation of the sulfenic acid first to sulfinic and then to sulfonic acid is mostly irreversible (shown in red). One known exemption is the sulfinic acid formed at the catalytic cysteine of peroxiredoxin, which can be reduced by sulfiredoxin. One-electron oxidation of the thiolate anion results in the formation of a thiyl radical, which can form a persulfide (i.e., S-sulfhydration) or an S-nitrosothiol. Oxidation of a persulfide will generate perthiosulfenic acid intermediate, which can get further oxidized (grey dashed arrow). Thiolate anions can also directly react with protein disulfides or oxidized LMW thiols such as GSSG through thiol-disulfide exchange reactions. The scheme represents a subset of physiological relevant modifications.
The one-electron oxidation of a thiol(ate) group generates a thiyl radical, which gives rise to a diverse range of oxidation products, including S-nitrosothiols (SNO) and persulfides (S-SH) [20] (Fig. 1). Protein persulfides can also form when endogenous hydrogen sulfide (H2S) reacts with oxidized thiol species such as sulfenic acid intermediates, S-nitrosothiols, or disulfides [16,70,71]. Moreover, hydrogen polysulfides (H2Sn) readily sulfurate cysteine thiols, and several oxidative modifications originally suggested to be induced by H2S have now been demonstrated to involve H2Sn [79]. Inorganic polysulfides and protein persulfides appear to be reduced by the Trx/TrxR1 and GSH/GR systems as well [80]. Recently, it has also been shown that protein persulfides can be further oxidized to perthiosulfenic acid (S–SOH) (Fig. 1). It is of note that perthiosulfenic acid species will also react with dimedone-based reagents. However, in contrast to sulfenic acid intermediates, the reaction products between perthiosulfenic acid and dimedone-based reagents can be cleaved by DTT [81]. The recent discovery that a significant amount of dimedone-bound proteins in cells is indeed susceptible to cleavage by DTT suggests that the cellular formation of perthiosulfenic acid intermediates is more pronounced than originally thought [81].
Quantitative mass spectrometry-based redox proteomic techniques have contributed to the identification of a large number of pro- and eukaryotic proteins that harbor redox-sensitive cysteine residues, which form sulfenic acid intermediates, disulfide bonds, or become S-glutathionylated, S-nitrosylated or persulfidated in response to endogenous and exogenous stimuli [29,82–86]. At this point, we are still far away from fully understanding what makes particular cysteine residues in proteins highly oxidation sensitive and others apparently redox inert. However, the increasing number of characterized redox-sensitive and redox-regulated proteins will hopefully provide us with the necessary information to generate algorithms and prediction programs in the near future. We would like to refer the readers to several recent reviews, which have expertly summarized quantitative proteomic approaches and discussed the functional implications of oxidizing redox-sensitive target proteins in the cell [70,71,87–90].
4. Thiol-based antioxidant enzymes
To prevent the uncontrolled oxidative modification of proteins, lipids and DNA and protect cells against irreversible and toxic damage, cells evolved a number of highly efficient and specific antioxidant enzymes. Superoxide dismutase catalyzes the conversion of into H2O2 and O2. H2O2 molecules are further eliminated by catalase, which catalyzes decomposition of H2O2 to H2O and O2 or by thiol peroxidases, such as glutathione peroxidases and Prxs [91,92], which catalyze the reduction of H2O2 and/or organic hydroperoxides to water and the corresponding alcohols, respectively [93]. In contrast to catalases and superoxide dismutases, peroxidases use a thiol-based reaction mechanism to detoxify hydroperoxides and their reduced state needs to be restored by the GSH/GR or the Trx/TrxR system under the consumption of NADPH (or in some special case NADH) [93].
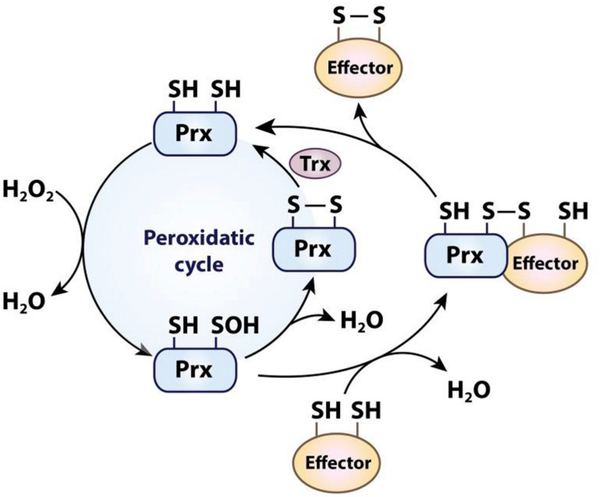
Prxs have a very high affinity for H2O2, which is based on their strictly conserved active site. The Thr/Cys/Arg triad facilitates the reduction of H2O2 by stabilizing the transition state and polarizing the O–O peroxyl bond, which promotes the transfer of electrons from the catalytic cysteine [94–96]. This mechanism results in a reaction rate of 105-108 M−1 s−1 with H2O2 [97], which is several orders of magnitude higher than the typical reaction rate of protein thiols with H2O2 [75,98]. The sulfenic acid intermediate formed at the catalytic cysteine readily reacts in 2-Cys Prxs with the resolving cysteine to release water under the formation of a disulfide bond (Fig. 2). The cycle is completed when the free thiol state is restored by the interaction with Trx-like proteins or other reductants like GSH. In the case of 1-Cys Prx, the resolving cysteine is absent and the sulfenic acid formed upon reaction of the catalytic cysteine with H2O2 is reduced by GSH in conjunction with glutathione S-transferase [99].
Fig. 2.
The dual function of peroxiredoxin as peroxidase and H2O2-signal transducer. The reaction of the catalytic cysteine in peroxiredoxins (Prx) with H2O2 leads to the formation of a sulfenic acid intermediate. In the peroxidatic cycle (left), the sulfenic acid forms a disulfide with the resolving cysteine which is reduced by oxidoreductases like thioredoxin (Trx) or by GSH. In the Prx-redox relay (right), the sulfenic acid intermediate forms an intermolecular disulfide with the thiol group of an effector protein. Reduced Prx is released upon the formation of an intramolecular disulfide in the effector protein, leading to the activation or inactivation of the effector protein. It should be noted that typical 2-Cys Prxs function as obligate homodimers, which is not shown for simplification.
5. Peroxiredoxin-based redox relay
High concentrations of reactive oxygen and nitrogen species are considered to be hazardous. Nevertheless, some oxidants including nitric oxide, peroxynitrite, and H2O2 have been shown to act as second messengers, affecting a number of different signaling pathways through the oxidative modification of redox-regulated key proteins [100,101]. However, the fast reaction rates of Prxs with H2O2 together with their high intracellular abundance put redox-regulated proteins at a clear disadvantage with respect to direct reactions with H2O2 [102,103]. To overcome this kinetic challenge and promote H2O2-mediated thiol oxidation of effector molecules, organisms have evolved several different mechanisms [19]. The mechanism that appears to be responsible for the majority of thiol oxidation events in mammalian cells involves the Prx-based redox relay [104]. In this relay, the catalytic cysteine of Prx first reacts with H2O2 as part of its regular catalytic cycle. However, instead of reacting with a second cysteine in Prx (i.e., the resolving cysteine), the sulfenic acid intermediate then condenses with a cysteine residue of a target protein forming an intermolecular disulfide (Fig. 2). This disulfide bond is then attacked by a second cysteine of the target protein, resulting in an intramolecular disulfide bond in the target protein and the release of reduced Prx (Fig. 2) [19,102,105–107]. Although these findings do not exclude Prx-independent H2O2 signaling mechanisms, they clearly show that Prxs act as sensitive and common transducers of H2O2-derived oxidizing equivalents in redox signaling [18,19].
One of the best-known examples of a peroxidase-based redox relay is the yeast glutathione peroxidase-like enzyme Orp1 (Gpx3), which transfers oxidative equivalents to the yeast oxidative stress transcription factor Yap1 via the formation of an intermolecular disulfide bond [108–110]. The Orp1-Yap1 disulfide is subsequently resolved by a second cysteine in Yap1. The formation of an intramolecular disulfide triggers the translocation of Yap1 into the nucleus, where it induces expression of antioxidant enzymes until reducing conditions are restored [108]. A similar peroxidase-mediated redox relay has been described for the mammalian Prx1, which accelerates oxidation of the apoptosis signaling kinase 1 (ASK1) via transient formation of a Prx1-ASK1 disulfide [111]. In addition, Prx2 was found to oxidize the transcription factor STAT3, which, in turn, converts into oxidized dimers and tetramers with attenuated activity [112].
Considering the peroxidatic cycle of Prx, in which the sulfenic acid formed at the catalytic cysteine reacts with the resolving second cysteine (Fig. 2), the question arises as to how the kinetically unfavorable formation of an intermolecular disulfide with an effector protein can compete with the intramolecular disulfide bond formation of Prx (Fig. 2). Recent studies suggested that the Orp1-mediated oxidation of Yap1 in yeast requires a third interaction partner, Ybp1, which acts as scaffolding protein [110]. Ybp1 apparently brings Orp1 and Yap1 together in a ternary complex, which allows the sulfenylated cysteine of Orp1 to react with one of the six cysteines of Yap1 while inhibiting the formation of the intramolecular disulfide bond [110]. These studies suggest that Prx-catalyzed thiol oxidation provides H2O2-dependent signaling with spatiotemporal precision and target specificity. Given the fact, that Prxs are virtually found in every subcellular compartment, the individual role and regulation of Prx-mediated H2O2-signaling as well as the identification of effector molecules at the specific locations will be an exciting objective for future studies.
6. Low-molecular-weight thiols
Organisms produce millimolar concentrations of distinct low-molecular-weight (LMW) thiols that serve as cofactor for thiol-dependent enzymes [92], form covalent linkages with protein thiols to protect against overoxidation [77,113], and reduce existing oxidative modifications in proteins [78,114]. Given the redox reactivity of cysteine thiols, one might have anticipated that free cysteine prevailed as major LMW thiol maintaining the cellular redox homeostasis. However, the cellular concentration of free cysteine is generally kept very low (100–200 μM) [35]. Cysteines rapidly undergo auto-oxidation and form disulfide bonds with other cysteines(i.e., cystine), a process that is further accelerated by transition metal ions like copper and iron [115,116]. Cystine is largely insoluble, which makes its effective reduction an essential requirement in vivo. So far, however, only very few enzymes have been found to show cystine reductase activity in vitro, and their physiological roles remain to be determined [117].
Instead, the tripeptide γ-glutamylcysteinyl glycine (glutathione, GSH) appears to maintain the cellular redox homeostasis in most eukaryotes, Gram-negative bacteria, as well as some Gram-positive bacteria [27]. Other cysteine-containing LMW thiols that have evolved include the sugar-based cysteinyl derivates mycothiol and bacillithiol in Gram-positive bacteria [54,118] and trypanothione [T(SH)2] in Euglenozoa [119] (see section below). In almost all organisms, NADPH-dependent reductases catalyze the re-reduction of the LMW thiols [120]. The key component in all of these redox systems is the thiol group, which is essential for conducting the sulfur biochemistry.
7. Glutathione, the prevalent cellular low-molecular-weight thiol
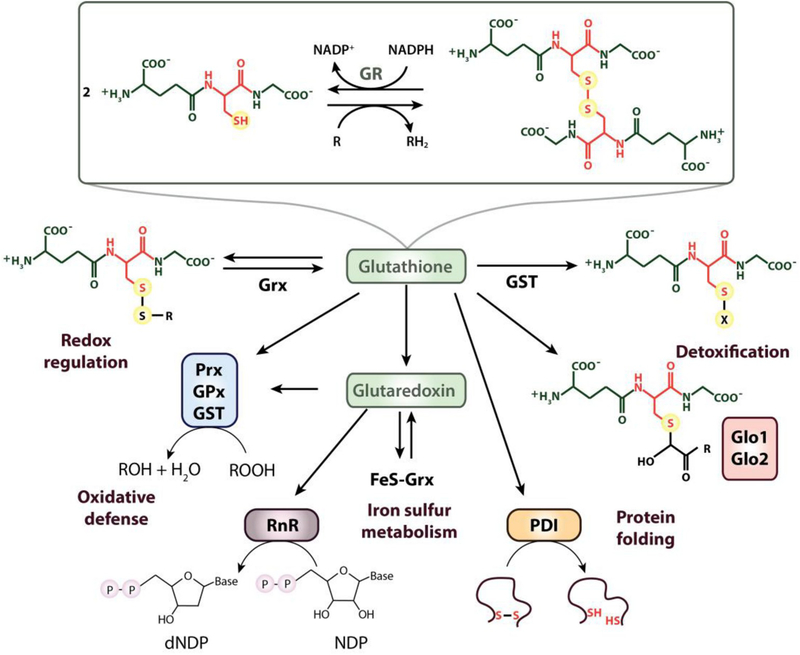
Based on its ability to reduce sulfur to hydrogen sulfide, Joseph de Rey-Pailhade originally named glutathione “philothion” from the Greek words for love and sulfur [121]. In 1921, Frederick G. Hopkins revealed its physiological significance and narrowed down the chemical composition to glutamate and cysteine, resulting in the name “glutathione” [122]. A few years later, the complete structure of the tripeptide was solved, revealing the uncommon γ-peptide linkage connecting the carboxyl group of the glutamate side chain with the amine group of the cysteine (Fig. 3) [123]. While the cysteine residue confers GSH with its chemical reactivity, the γ-glutamyl peptide bond and the negative net charge make GSH a specific substrate for glutathione-dependent enzymes. One of these enzymes is the FAD-dependent glutathione reductase (GR), which transfers electrons from NADPH to glutathione disulfide (GSSG), thereby regenerating two molecules of GSH (Fig. 3).
Fig. 3.
Overview over the biological functions of glutathione. GSH is ubiquitously distributed throughout all kingdoms and represents the most widely used LMW thiol. The NADPH-dependent flavoenzyme glutathione reductase (GR) regenerates GSH from its disulfide-bonded form (GSSG). GSH forms mixed disulfide bonds with proteins to regulate their activity and/or protect against irreversible overoxidation. Deglutathionylation of GSH-mixed disulfides is catalyzed by glutaredoxin (Grx). GSH regenerates reduced Grx, enabling it to support iron-sulfur cluster biogenesis and maintain enzymes such as ribonucleotide reductase (RnR) in their reduced state. Glutathione peroxidases (GPx) and peroxiredoxins (Prx), catalyzing the reduction of hydroperoxides, obtain their reducing equivalents either from GSH or Trx (not shown here). Glutathione transferase (GST) uses GSH as a cofactor in the detoxification of drugs and xenobiotics. Glyoxalase (Glo) 1 and 2 catalyze the isomerization and hydrolysis of glutathione thioesters, respectively, to detoxify 2-oxoaldehydes. GSH delivers electrons for protein disulfide isomerase (PDI)-catalyzed reduction and isomerization of disulfides.
Some lactic acid bacteria [36] and several halophilic archaea [40] have been found to omit the second ATP-dependent step of the GSH biosynthesis, and accumulate millimolar concentrations of gamma-glutamyl cysteine (γ-Glu-Cys) instead (Table 1). Although the free cysteinyl carboxylate is highly sensitive towards metal-catalyzed autooxidation, high intracellular salt concentration in these organisms appears to mitigate this reaction [37]. To maintain an overall reducing cytosol, H. halobium expresses the NADPH-dependent bis-γ-glutamyl cystine reductase (Table 1), a protein that is highly similar to GR but reduces disulfide bonded γ-Glu-Cys instead of GSSG [37].
7.1. The redox properties of glutathione
The reduction potential, or less formally called redox potential, as calculated by the Nernst equation, describes the driving force behind reduction reactions of redox pairs such as the reduced and oxidized form of glutathione (i.e., GSH/GSSG) [42,124]. Generally, a redox pair with a low reduction potential acts as the electron donor in reactions with redox pairs of higher redox potential. However, biological systems are not at equilibrium and kinetic parameters will ultimately determine which reactions occur in vivo, even though they are thermodynamically unfavorable [26,27].
Measurements of GSH and GSSG concentrations in whole-cell extracts revealed a GSH:GSSG ratio of ≥100:1 in unstressed cells [42]. Assuming a total cytosolic glutathione concentration of 10 mM, this molar ratio would correspond to a redox potential of − 240 mV. However, glutathione measurements based on whole-cell extracts reflect the total cellular glutathione pools, and do not provide information about compartment-specific GSH:GSSG ratios. With the development of genetically encoded fluorescent redox probes, we have now obtained detailed insights into compartment-specific GSH:GSSG ratios in intact cells [125–127]. These measurements revealed that in yeast cells, the cytosolic GSH:GSSG ratio is at least 1000 times higher and the glutathione redox potential significantly more reducing (~−320 mV) than previously assumed [126]. The mechanism that yeast cells employ to keep cytosolic GSSG levels low involves a combination of effective GSSG reduction and the active sequestration of GSSG into the yeast vacuole, which explains the apparent discrepancy between whole-cell and cytosolic-specific measurements [126]. Another mechanism that contributes to maintaining a stable GSH:GSSG ratio in the cytosol involves the selective degradation of glutathione [128].
By targeting the genetically encoded fluorescent GSH/GSSG probes into specific subcellular compartments, insights into the glutathione pools of specific subcellular compartments have been obtained as well [129–131]. For instance, live-cell GSH/GSSG measurements in the periplasmic space of Gram-negative bacteria and the endoplasmic reticulum revealed redox potentials of −165 mV and − 208 mV, respectively, providing the quantitative support for previous conclusions that these compartments are much more oxidizing than the cytosol [42,132–134]. With the constant development of even more sophisticated and sensitive probes, it will be only a matter of time before we will have a complete picture about the cellular and subcellular glutathione pools, and be able to monitor the flux of GSH and/or GSSG between those pools under non-stress and stress conditions.
7.2. Cellular functions of glutathione
Although GSH is best known for its role as physiological antioxidant, the rate with which it reacts with peroxide under physiological conditions is 3–5 orders of magnitude slower compared to the reaction rates of dedicated peroxidases with H2O2 [26,27]. Moreover, due to its thiol pKa value of 8.9 (Table 1), which is substantially above the physiological pH of the cytosol, only 2.9% of GSH are present as reactive thiolate anion at pH 7.4 [135]. Indeed, GSH works primarily by providing the reducing equivalents for antioxidant enzymes, including glutathione peroxidases [136] and ascorbate peroxidases [137] (Fig. 3). In addition, GSH delivers the electrons for phospholipid hydroperoxidase GPx4, an enzyme that protects mammalian cells from lipid peroxide formation and iron-induced cell death (i.e., ferroptosis) [138]. GSH has also been shown to protect cells against potentially harmful xenobiotics by interacting with electrophilic substrates, a reaction usually catalyzed by glutathione-S-transferases (Fig. 3) [27]. GSH-dependent glyoxalase 1 and 2 mediate the detoxification of 2-oxoaldehydes, such as methylglyoxal, which is an electrophilic by-product of glycolysis that reacts spontaneously with proteins, lipids, and nucleic acids, causing irreversible damage [139]. Moreover, GSH forms complexes with reactive metal ions (e.g. Hg2+, Ag+, Cd2+, Pb2+), thereby reducing metal toxicity (Fig. 3) [9].
Under oxidizing conditions, GSH readily forms mixed disulfides with protein cysteines, an oxidative protein modification commonly known as protein S-glutathionylation (Fig. 3). Whereas in non-stressed cells, less than 0.1% of total protein cysteines are S-glutathionylated, the number increases to up to 15% under distinct oxidative stress conditions [69]. It is now well accepted that the reversible formation of GSH-mixed disulfides serves to protect protein thiols against irreversible overoxidation, and plays an essential role in regulating redox-dependent cellular functions by translating oxidative or nitrosative stimuli into cellular responses [77,140,141]. In addition, S-glutathionylation might also contribute to maintaining the cellular GSH:GSSG homeostasis under oxidative stress conditions [69,142].
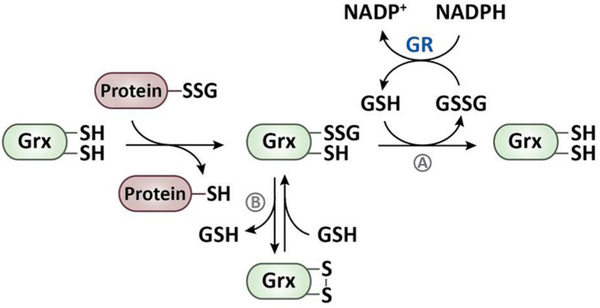
The removal of GSH from a protein-mixed disulfide occurs either directly via a thiol-disulfide exchange reaction with another GSH molecule [42] or is catalyzed by glutaredoxin (Grx) via a monothiol or a dithiol mechanism [143–145] (Fig. 4). Grx-mediated protein deglutathionylation results in the transient formation of a Grx-GSH-mixed disulfide, which, in the monothiol mechanism, is resolved by a second GSH molecule to restore the reduced form of Grx [146]. GSSG, generated in this reaction, is efficiently reduced by GR [43]. In the dithiol mechanism, the transiently formed Grx-GSH-mixed disulfide is attacked by the second active-site cysteine of Grx leading to the formation of an intramolecular disulfide bond (Fig. 4) [147,148]. Disulfide-bonded Grx is then reduced by two molecules of GSH in two consecutive reactions (Fig. 4) [143]. Although the dithiol mechanism appears to be non-productive, it presumably plays an important cellular role [145,149]. Considering that S-glutathionylation serves to protect proteins from irreversible overoxidation, Grx-catalyzed deglutathionylation would be counterproductive under stress conditions. Disulfide bond formation in Grx might therefore be used as mechanism to transiently inactivate Grx as GSH levels drop during oxidative stress. Once the cellular GSH:GSSG ratio has been restored, the intramolecular disulfide in Grx is reduced and deglutathionylation can resume [147,149].
Fig. 4.
Glutaredoxin-catalyzed reduction of S-glutathionylated proteins. The reduction of S-glutathionylated proteins is catalyzed by glutaredoxin (Grx), which, in the first step, forms a mixed disulfide with GSH, thereby releasing of the protein in its reduced state. (A) In the monothiol mechanism, the Grx-GSH-mixed disulfide reacts with free GSH to regenerate the dithiol form of Grx. (B) In the dithiol mechanism, Grx forms an intramolecular disulfide bond and releases GSH. The oxidized Grx is then reduced by GSH under the formation of a Grx-GSH-mixed disulfide, which is then reduced by a second GSH molecule. GSSG, which is formed in both of the mechanisms, is reduced by the NADPH-dependent glutathione reductase (GR).
Genetic approaches in various model organisms revealed that the Trx/TrxR system can functionally substitute for the lack of the GSH/GR system and vice versa [78,150]. For instance, studies in S. cerevisiae demonstrated that mutants lacking either both cytoplasmic Trx1 and Trx2 [151] or both dithiol Grxs [152] are viable, whereas the combined deletion of GR, Trx1, and Trx2 [153] or GR and TrxR [154] are lethal. Remarkably, however, deletion of γ-glutamyl cysteine synthase (GSH1), which catalyzes the first, rate-limiting step in GSH biosynthesis, cannot be complemented by the Trx system, and gsh1 deletion mutants require exogenous GSH supplementation for growth [155,156]. The finding that strict anaerobic growth conditions failed to rescue the growth defect ruled out that the antioxidant function of GSH is crucial for cell survival [157]. Instead, follow-up studies in yeast suggested that the essential role of GSH is associated with the maturation of extra-mitochondrial iron-sulfur cluster proteins [158].
7.3. Roles of glutathione in oxidative protein folding
Oxidative protein folding is defined as the formation of one or more disulfide bonds in proteins during the folding process [159]. Since not all disulfide bonds that are initially formed during de novo folding connect the native cysteine pair, oxidative protein folding also involves the reduction of non-native disulfide bonds and re-formation of the correct ones (i.e., disulfide isomerization). The enzymes involved in these processes are dedicated oxidases (e.g., DsbAB system in bacteria, Ero1 and Erv1 in eukaryotic cells) and isomerases (e.g., DsbC/D system in bacteria, PDI in eukaryotic cells) [133]. Oxidative protein folding typically occurs in dedicated subcellular compartments, particularly the periplasmic space of Gram-negative bacteria and the endoplasmic reticulum (ER) in eukaryotic cells. These compartments, which have GSH:GSSG ratios that are much lower than in the cytosol, provide environments conducive for oxidative protein folding but yet contain sufficient GSH to assist in isomerization reactions [160]. Since GSH biosynthesis takes place exclusively in the cytosol and in chloroplasts, and GR activity is absent in these subcellular compartments, the GSH redox potential is most likely controlled by the import and export of GSH and GSSG [160–162].
To transport GSH into the periplasmic space, Gram-negative bacteria use the ATP-binding cassette-type transporter CydCD [163]. Depletion of cellular GSH or inactivation of CydCD turns the periplasm into an even more oxidizing compartment with a redox potential of −125 mV [132,133]. CydCD deletion strains of E. coli reveal a host of phenotypes, reminiscent of mutants that are either defective in components of the oxidizing (DsbA, DsbB) or reducing (DsbC, DsbD) pathway of disulfide bond formation [163]. These results suggest that deletion of CydCD causes an overall disturbance of the periplasmic redox homeostasis accompanied by oxidative protein folding defects. Although the exact contribution of GSH to oxidative protein folding in the bacterial periplasm remains still elusive, the finding that the addition of GSH to the medium mitigated the protein folding defects of a cydCD deletion strain supports the essential role that GSH plays in the periplasm [160].
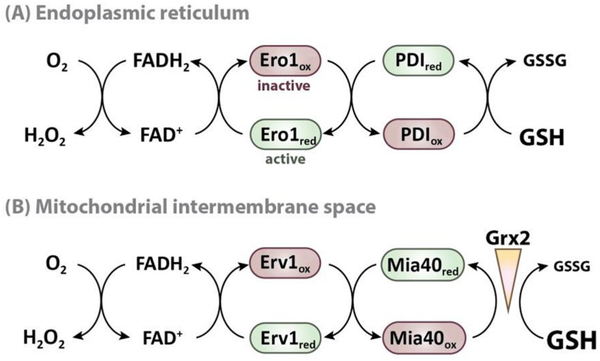
In eukaryotic cells, disulfide bond formation is catalyzed by protein disulfide isomerase (PDI), which, in the ER, receives its oxidizing equivalents from Ero1 [164,165]. Ero1 is an FAD-dependent thiol oxidase, which transfers its electrons onto molecular oxygen, thereby generating considerable amounts of H2O2 (Fig. 5A) [166]. In the reduced form, PDI acts as disulfide bond isomerase, resolving non-native disulfide bonds and reintroducing correct ones. GSH in the ER appears to primarily function as a reductant of PDI (Fig. 5A), although more recent studies have now suggested the involvement of the cytosolic Trx pathway in the reduction of PDI as well [167]. In addition, GSH seems to be involved in the redox regulation of Ero1 [168,169]. When the ER becomes too oxidizing, Ero1 has been shown to form regulatory disulfide bonds between non-catalytic cysteines, which lock the thiol oxidase in an inactive conformation and prevent further production of H2O2. Although GSH does not directly reduce Ero1, it provides the electrons to recycle oxidized PDI, which, in turn, reduces Ero1 [160,169] (Fig. 5A). These findings suggest that GSH paradoxically promotes ER oxidation by providing the reducing equivalents required for Ero1 reduction and activation. It is of note that GSH-mediated activation of Ero1 also provides a feedback mechanism to regulate GSH import. Recently, Ponsero et al. showed that GSH enters the ER by facilitated diffusion through the Sec61 protein-conducting channel. As GSH activates Ero1, the production of H2O2 causes the oxidation of Bip (Kar2), which, in turn, inhibits further GSH import [162]. While this negative regulatory feedback loop appears to control the levels of GSH, it is still unclear how the GSSG concentration is regulated in order to maintain a stable redox potential.
Fig. 5.
Model for the role of glutathione in oxidative protein folding. The pathways for oxidative protein folding in the ER and mitochondrial IMS are conceptually highly similar. The FAD-dependent oxidases Ero1 and Erv1 oxidize the oxidoreductases PDI and Mia40, respectively, which introduce disulfides into target proteins during protein folding. GSH directly affects oxidative protein folding by reducing the oxidoreductases or their protein substrates. Therefore, the reducing power of the GSH pool and the activity of the oxidizing machinery need to be carefully balanced to maintain the redox poise. (A) In the ER, the GSH:GSSG ratio is tightly regulated via the selective import of GSH. The activity of Ero1 is controlled by the formation of inhibitory disulfide bonds, which are reduced by GSH via PDI. Hence, GSH actually promotes ER oxidation by activating Ero1 and Ero1-mediated H2O2 production. (B) In contrast to the ER, the GSH pool of the mitochondrial IMS is as reducing as in the cytosol, since the tripeptide can pass the porins of the outer mitochondrial membrane. To prevent the rapid reduction of Mia40 and its substrates, limited glutaredoxin (Grx) activity is required to maintain the balance between Mia40 and the GSH pool.
Like the ER, mitochondria lack enzymes to synthesize GSH and the negatively charged tri-peptide must be imported into both the IMS and matrix [129]. Whereas porins allow molecules smaller than ~5kDa to diffuse through the outer mitochondrial membrane, the inner mitochondrial membrane is impermeable for glutathione. Dedicated transporters have been suggested to mediate the transport of GSH and/ or GSSG (for recent review, please see Calabrese et al. [129]). However, since glutathione can freely pass the outer mitochondrial membrane, the IMS has the same reducing redox potential as the cytosol [42,170]. Yet, it has been shown that the import and folding of many cysteine-containing IMS proteins rely on the introduction of intramolecular disulfide bonds in the IMS, a process that is facilitated by the IMS-resident oxidoreductase Mia40 [66,171,172]. Mia40 itself is kept oxidized by the FAD-dependent sulfhydryl oxidase Erv1 [66], which transfers electrons onto molecular oxygen either directly or via cytochrome c [173,174] (Fig. 5B). Somewhat surprisingly, oxidation of Mia40 substrates was found to be accelerated in vitro by physiological concentrations of GSH, suggesting that GSH might be required to reduce long-lived reaction intermediates, which otherwise impair Mia40 activity [175]. Another regulator of Mia40 in S. cerevisiae is cytosolic Grx2, which is imported into the IMS, where it reacts specifically with GSH. It was found that both Grx2 overexpression and deletion shifted the equilibrium of Mia40 towards its reduced state, and decreased the rate of oxidative protein import and folding [176]. One possible explanation for this finding is that limited Grx activity is needed to maintain the correct balance between Mia40 and the GSH pool, and that Grx2 – speculatively by catalyzing GSH-dependent disulfide bond isomerization or reduction of disulfides – provides a kinetic barrier that prevents GSH-mediated reduction of Mia40 substrates (Fig. 5B) [176]. The exact mechanism how Grx/GSH affects the protein folding machinery in the IMS still needs to be unraveled. In either case, this example illustrates how, by balancing sub-cellular thiol pools, efficient oxidative protein folding can occur even within a reducing redox environment.
8. Trypanothione – A dithiolic derivative of glutathione
The thiol redox metabolism of trypanosomatids, including human pathogenic Trypanosoma and Leishmania species, is distinctly different from the redox systems of the host, and reflects the evolutionary adaption to a parasitic lifestyle [45,119]. The parasites lack genes encoding GR and TrxR, and have significant lower amounts of cellular GSH compared to other eukaryotes. To maintain their thiol redox homeostasis, they contain millimolar concentrations of the dithiolic polyamine-conjugate N1, N8-bis(glutathionyl)spermidine, known as trypanothione [T(SH)2] (Fig. 6) [177]. Trypanothione is kept reduced by the NADPH-dependent flavoprotein trypanothione reductase, which is essential for parasite survival and different enough from the host GR to serve as a major drug target [178,179].
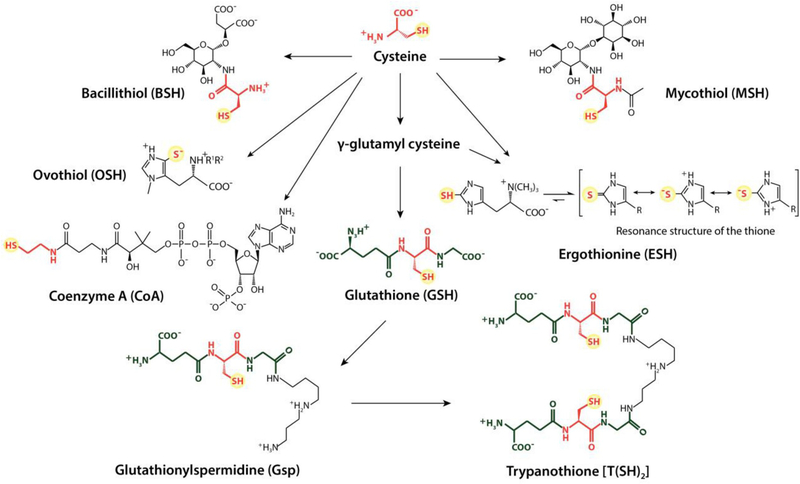
Fig. 6.
Commonly found low-molecular-weight thiols. All LMW thiol species that are known to contribute to the maintenance of the thiol redox homeostasis have the cysteine-derived thiol group in common. The tripeptide glutathione (GSH) acts as a precursor molecule for the biosynthesis of glutathionylspermidine (Gsp). The polyamine derivatives are generated by amide bond formation between the carboxyl group of the glycine residue of GSH and the free amine group of spermidine. Gsp can react with a second GSH molecule resulting in the dithiol trypanothione (T(SH)2). For the biosynthesis of mycothiol (MSH), the cysteine is attached to D-myoinosityl 2-acetamido-2-deoxy-α-D-glucopyranoside followed by the acetylation of the amine group. In the case of bacillithiol (BSH), the cysteine is modified by L-cysteinyl-D-glucosamine linked to L-malic acid (R1 = R2 = H). Recently, N-methylated BSH has been discovered (R1 = H, R2 = CH3). During the biosynthesis of Coenzyme A (CoA), the cysteine becomes decarboxylated after its incorporation. Both ergothionine (ESH) and ovothiol (OSH) are histidine-based LMW thiols. OSH can exist as any one of three derivatives, which differ by the number of methyl groups at the a-amino group: (A) R1 = R2 = H; (B) R1 = CH3, R2 = H; (C) R1 = R2 = CH3.
T(SH)2 is synthesized from two GSH molecules linked by spermidine in two ATP-dependent consecutive steps [180]. Monoglutamylspermidine (Gsp) is formed as an intermediate of T(SH)2 biosynthesis (Fig. 6). Despite their similar redox potentials (Table 1), T(SH)2 is a much more potent reducing agent than GSH or Gsp. Oxidation of T(SH)2 results in the formation of an intramolecular disulfide (TS2), which is kinetically favored compared to the intermolecular disulfide formed in GSSG [44]. The thiol pKa of T(SH)2 is 7.4, and hence significantly lower than that of GSH (Table 1) [45]. This promotes thioldisulfide exchange reactions at physiological pH conditions [181]. T(SH)2 serves as the electron donor for the Trx-like oxidoreductase tryparedoxin, which has a pKa of 7.2. Since the thiol redox potentials of tryparedoxin (− 249 mV) and T(SH)2 (− 242 mV) are nearly identical, the concentration of both reaction partners ultimately determines the electron flow [182,183].
The T(SH)2/tryparedoxin redox couple serves as the electron donor for several essential enzymes, including ribonucleotide reductase, methionine sulfoxide reductase, and various tryparedoxin peroxidases [45]. Therefore, it is not surprising that depletion of either T(SH)2 [182] or tryparedoxin [183] causes severe growth defects and increases the parasite’s sensitivity towards oxidative agents.
Although T(SH)2 is the predominant LMW thiol in trypanosomatids, African trypanosomes also contain up to 100 μM GSH, which becomes protein-bound under stress conditions [142]. The deglutathionylation of these proteins is catalyzed by the non-essential oxidoreductase Grx [142,184,185]. However, the observation that protein dethiolation occurs even in the absence of Grx1 strongly suggests that GSH-mixed disulfides can be reduced by T(SH)2 as well [142]. Although T(SH)2 was originally thought to not form stable protein-mixed disulfides due to its dithiol character, a recent study showed that all three LMW thiols, GSH, Gsp, and T(SH)2, can form protein-mixed disulfides in African trypanosomes upon exogenous and endogenous stress conditions [142]. The in vitro trypanothionylation of a parasite peroxidase further demonstrated that even a dithiol can become stably attached to protein thiols if the structure of the protein prevents the immediate attack of the second thiol in TSH2 [142].
9. Alternative thiol-based redox systems
Not all organisms synthesize GSH, but rely instead on other LMW thiols as co-factors to maintain their cellular redox homeostasis. Here, we will provide an overview over well-known alternative LMW thiol systems (for summary see Table 1) and refer the reader to recent comprehensive review articles highlighting their roles beyond their functions as antioxidants.
9.1. Mycothiol
Actinomycetes, such as Mycobacteria, Corynebacteria, and Streptomyces, synthesize the cysteinyl pseudo-disaccharide mycothiol (MSH) as major LMW thiol [47]. Oxidation of MSH results in the formation of mycothiol disulfide (MSSM), which is efficiently reduced by the NADPH-dependent flavoenzyme mycothiol disulfide reductase (Mtr) to maintain a high intracellular MSH:MSSM ratio [47,186]. The reactive group in MSH is N-acetylcysteine, which is linked to the sugar moiety 1D-myo-Inosityl 2-acetamido-2-deoxy-a-D-glucopyranoside (GlcN-Ins) [135,187] (Fig. 6). The N-acetyl and GlcN-Ins moieties shield the amino and carboxyl groups, respectively, making MSH highly resistant towards metal-catalyzed oxidation [67]. Some marine Actinomycetes produce an N-acyl homologue of MSH, in which the acetyl group is replaced by a propionyl group [188].
The redox potential of the MSH/MSSM couple (− 230 mV) is 10 mV higher than the redox potential reported for the GSH/GSSG couple (Table 1). The thiol pKa of MSH was recently determined to be 8.76 ± 0.02 [48] and differs only marginally from that of GSH (8.9, Table 1). Although its pKa value suggests that MSH is mainly present in its less reactive thiol form at physiological pH, the millimolar cellular concentration of MSH makes the MSH thiolate the predominant LMW thiolate in mycobacteria [135,189].
MSH biosynthesis is not essential in Mycobacterium smegmatis, which compensates for the loss of MSH by an increased production of ergothioneine (ESH, see below) and expression of the thiol-dependent organic hydroperoxide resistance (Ohr) protein [190,191]. In contrast, M. tuberculosis strains lacking Ohr homologs require MSH biosynthesis for growth unless catalase is added to the medium [135]. Lowering the cellular concentration of MSH causes a higher susceptibility of cells towards various stresses [192], consistent with its cellular role in detoxifying ROS, heavy metals, and antibiotics (for recent review, please see Ref. [135]).
Exposure of MSH-containing bacteria to hypochlorite stress results in mixed disulfide bond formation between MSH and protein thiols, a process termed as S-mycothiolation [193,194]. Many proteins that were found to be S-mycothiolated in Actinomycetes like Corynebacterium glutamicum, C. diphtheria and M. smegmatis are involved in metabolic pathways such as glycolysis, gluconeogenesis, glycogen and maltodextrin degradation as well as fatty acid, amino acid and nucleotide biosynthesis [189,193–195]. Protein S-mycothiolation is reversible and dethiolation is catalyzed by mycoredoxin-1 (Mrx1), a Grx analog that functions exclusively in MSH-dependent electron transfer reactions [196]. Since mutating the second cysteine of the active site CGYC motif does not affect its dethiolation activity, Mrx1 likely uses a monothiol mechanism for the reduction of S-mycothiolated proteins [49,197]. In some instances, such as the mycothiol peroxidase (Mpx), dethiolation is also catalyzed by Trx via a dithiol mechanism [198]. Although the reaction rate is slightly slower, Trx-mediated dethiolation might function as an alternative reduction mechanism in case the MSH-specific Mrx-1 pathway is impaired.
In addition to its role as redox moiety, MSH has been shown to function as a sulfur donor in the biosynthesis of lincomycin A, a sulfur-containing lincosamide antibiotic that has been widely used to treat Gram-positive bacterial infections [199].
9.2. Bacillithiol
Compared to some of the other LMW thiols discussed here, the discovery of bacillithiol (BSH) in Gram-positive bacteria such as Bacillus spp., Staphylococcus spp., and Streptococcus agalactiae was a relatively recent event [57,58]. During studies on coenzyme A-related thiols in low-GC Gram-positive bacteria, a unique thiol compound was identified and later characterized as a glycoside of l-cysteine-glucosamine and l-malate, termed bacillithiol [58] (Fig. 6). More recently, a N-methylated derivative of BSH (N-Me-BSH) has been identified in Chlorobaculum tepidum. The N-methylation of the cysteine amino group is catalyzed by N-methyl-bacillithiol synthase [54]. A comparative genomic analysis searching for orthologues of genes for known LMW thiol biosynthetic pathways suggests that BSH and its derivative N-Me-BSH might be “the most broadly distributed LMW in biology” [54]. Analysis of the thiol content of Thermus thermophiles (Deinococcus-Thermus) and Polaribacter sp. (Bacterioidetes) confirmed the predicted presence of BSH and N-Me-BSH, respectively (Table 1) [54] but a system-wide validation for this conclusion is still missing.
Like other classical LMW thiols, BSH is readily oxidized to bacillithiol disulfide (BSSB). Although the NADPH-dependent disulfide reductase YpdA has been proposed to catalyze the regeneration of BSH, experimental evidence still needs to be provided [50]. The cellular BSH concentration in B. subtilis is about 5 mM and the content of BSSB is kept very low under non-stress conditions, resulting in a standard thiol-redox potential of − 221 mV (Table 1) [51]. The thiol group of BSH has a pKa of 8.0 and is thus more acidic than the thiol group of GSH (Table 1). Accordingly, more BSH is present in its reactive thiolate state at physiological pH. Methylation of the BSH cysteinyl amine presumably stabilizes the thiolate anion which may further lower the thiol pKa [54]. However, the precise biophysical properties of N-Me-BSH still need to be investigated.
In addition to its function in maintaining the cellular redox homeostasis in Gram-positive bacteria, BSH appears to exert a number of other important roles [53,200,201]. For instance, BSH has been shown to serve as an essential cofactor of glyoxalases and thiol S-transferases (e.g., FosB) [141,202]. These enzymes catalyze the conjugation of BSH to toxic electrophiles and antibiotics, such as methylglyoxal and fosfomycin for their effective detoxification. BSH also plays a role in Fe–S cluster biogenesis and functions in copper and zinc storage [203,204]. Moreover, BSH is essential for defending pathogens, such as Staphylococcus aureus against macrophage-induced oxidative burst, and BSH depletion from clinical MRSA strains has been shown to strongly impair their survival in human whole-blood survival assays [205]. These results suggest that the BSH-specific machinery might be promising alternative drug target to treat persistent MRSA infections [201].
Quantitative thiol redox proteomics in hypochlorous acid-treated B. subtilis or S. aureus revealed the accumulation of S-bacillithiolated proteins [206,207]. As highlighted by Imber et al. in a recent review, many of the identified S-bacillithiolated proteins are involved in cellular metabolism, protein translation, redox regulation and antioxidant defense mechanisms [189].
The reduction of S-bacillithiolated proteins is catalyzed by bacilliredoxins (BrxA and BrxB), which belong to the DUF1094 family of small, Trx-like proteins. Instead of the classical CXXC-motif present in Trx family members, DUF1094 proteins contain an invariant CGC active site, and likely use a monothiol mechanism to reduce S-bacillithiolated cysteines [52,207]. Whereas deletion of the brxB gene causes a slight increase in the B. subtilis sensitivity towards cumene hydroperoxide, no difference in growth or levels of bacillithiolated test proteins was observed in brxA and/or brxB deletion strains in response to hypochlorous acid treatment [52]. These results suggest that other oxidoreductases, such as Trx may contribute to the reversion of S-bacillithiolation in vivo.
9.3. Coenzyme A
Coenzyme A (CoA), a thiol-containing central metabolite and essential cofactor in all living organisms, has been shown to take over the role of reducing agents in bacteria that lack GSH or MSH (Fig. 6) [208,209]. The NAD(P)H-dependent CoA disulfide reductase (CoADR), which is an oxidoreductase with a single redox-active cysteine, reduces CoA-disulfides via the formation of a protein-CoA-mixed disulfide [55]. Deletion of CoADR in B. burgdorferi results in increased sensitivity to tertbutyl hydroperoxide treatment but not to H2O2 stress, suggesting that other antioxidant systems protect the bacteria against peroxide [210].
Although the redox potential of CoA is similar to that of GSH, the pKa of 9.8 indicates that CoA is primarily present in its unreactive thiol form at physiological pH (Table 1) [56]. Recently, CoA-thiolation (i.e., CoAlation) of proteins has been shown to occur as a reversible post-translational modification induced by oxidizing agents and metabolic stress conditions in S. aureus as well as in mammalian cells and tissues [211,212]. In vitro studies showed that CoAlation of the active site cysteine in S. aureus glyceraldehyde-3-phosphate dehydrogenase (GAPDH) inhibits the enzyme activity while protecting the cysteine against irreversible H2O2-mediated overoxidation [211]. This redox-regulated mechanism is very similar to the mechanism previously reported for GSH and other LMW thiols [195,213,214]. The finding that certain bacteria like S. aureus contain both, the CoA/CoADR and the BSH redox systems, suggests that the two systems are interchangeable and might reflect the demand for backup-systems necessary to survive the oxidative challenges of the innate immune response.
9.4. Ergothioneine
Millimolar concentrations of Ergothioneine (ESH), a 2-mercaptohistidine (Fig. 6), are found in certain bacteria, fungi, plants, animals, and humans [61]. While most organisms seem to obtain ESH from external sources, Actinomycetes are known to possess enzymes for ESH biosynthesis. Synthesis of ESH starts with the tri-methylation of the amino group in histidine, forming hercynine. Cysteine or γ-Glu-Cys provide the sulfur, which is transferred to the imidazole side chain of hercynine [61].
In contrast to other LMW thiols, ESH exists as a tautomer between its thiol and thione form (Fig. 6). In the thione state, mesomeric release of the lone pair of electrons on the nitrogen atom of the amino group generates a thiolate anion explaining the weak thiol-like character of ESH with a thiol pKa of 10.8 (Table 1 [60]). The thione form is predominant at physiological pH, and prevents ESH from undergoing autooxidation [215]. The high redox potential of − 60 mV (Table 1) further contributes to ESH’s resistance towards autooxidation. In fact, whereas other LMW thiols in aerobic organisms are susceptible to metal-catalyzed autooxidation, the sulfur atom of ESH coordinates metal ions such as Cu2+ and Fe2+, thereby forming a non-redox active complex composed of two molecules of ergothioneine and one metal ion [216,217].
ESH has been shown to serve as an efficient antioxidant, which quenches singlet oxygen and scavenges hydroxyl radicals, hypochloric acid and peroxynitrite at biological significant rates [218,219]. Although disulfide bonded ESSE can be reduced by GSH via thiol-disulfide exchange [220], it most commonly decomposes to ESH and a highly reactive sulfenic acid intermediate (ESOH) [221]. Two ESOH molecules combine to form one molecule of ESH and a sulfinic acid intermediate (ESO2H), which is subsequently converted to hercynine and H2SO4. Thus, the regeneration of ESH from ESSE can occur partially without any reducing agent [221].
Biosynthesis of ESH has been shown to protect both pathogenic and nonpathogenic microbes against diverse environmental stresses, including oxidative stress, heavy metals, as well as nutritional limitations [218,219]. However, ESH-producing microbes often also synthesize other LMW thiols, such as GSH and MSH that assist with the maintenance of the cellular redox homeostasis [222]. For instance, Mycobacterium tuberculosis contains MSH, ESH and γ-Glu-Cys, which appear to be all able to compensate for each other, and ensure survival and fitness under oxidative stress conditions [223].
9.5. Ovothiol (OSH)
Ovothiols are a group of structurally related 1-methyl-4-thiol-histidines, found in a variety of marine invertebrates, halotolerant algae as well as in some trypanosomatids [63]. Ovothiol A, the first identified member of this group, was originally isolated from sea urchin eggs. Produced in high amounts, it protects the eggs against H2O2 generated during the formation of the fertilization envelope [224]. The ovothiol derivatives B and C contain one and two additional methyl groups at the amine, respectively (Fig. 6). Due to the very low pKa of the imidazole-linked thiol (pKa = 1.4), OSH is exclusively present as a thiolate anion under physiological conditions, which makes OSH an excellent scavenger of peroxides [225]. The redox potential of OSH strongly depends on the protonation state of the imidazole ring and is at physiological pH considerably more positive (E0’ = −67 mV) than the redox potential of GSH (Table 1). Thus, GSH maintains ovothiol almost completely in its reduced form by thiol-disulfide exchange reaction [62]. Accordingly, most ovothiol-producing organisms contain at least one alternative thiol-redox system, such as the GSH/GR or T(SH)2/TR system providing the electrons for the regeneration of OSH.
10. Conclusion and outlook
Thiol-based redox systems arose early in evolution among all phylogenetic branches, a finding that was long proposed to reflect the necessity of organisms to defend themselves against the constant threat of molecular oxygen-mediated ROS production and damage. However, the fact that LMW thiols and domains of some glutathione-dependent enzymes such as Grx and glutathione-S-transferase have also been found in anaerobic bacteria and archaea, suggests functions that go beyond their ability to counteract oxidative damage [27]. Indeed, glutathione has been shown to be essential for the maturation of Fe–S clusters in yeast, whereas its function in maintaining the cytosolic redox balance serves only as a backup for the Trx system [157]. With the repertoire of available genetic tools, it will be exciting to learn about the many other roles that these systems might play in organisms.
It is now well-established that thiol-based post-translational protein modifications are crucial to not only protect cysteines against irreversible overoxidation, but also to regulate protein activity in response to oxidative stimuli [16,17,70,103]. A main future goal will therefore be to unravel the mechanisms and kinetics that mediate the controlled and targeted oxidation of thiols within the highly reducing environment of the cytosol as well as the compartment-specific function(s) of thiol-based redox systems. We already know that the subcellular GSH:GSSG ratios are carefully balanced in the cell but the mechanisms by which this is achieved are still largely unknown. Genetically encoded redox probes that can be targeted to defined subcellular compartments and even specific locations within one compartment will help to map redox-active species in the context of intact cells [131,226]. These tools together with genetic interventions will hopefully help to uncover the regulatory network underlying cellular redox changes.
Acknowledgements
This work was supported by grants from the National Institutes of Health (GM122506) and the Priority Program SPP 1710 of the Deutsche Forschungsgemeinschaft (Schw823/3-2).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.freeradbiomed.2019.05.035.
References
- [1].Sies H, Role of metabolic H2O2 generation: redox signaling and oxidative stress, J. Biol. Chem 289 (13) (2014) 8735–8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Imlay JA, Pathways of oxidative damage, Annu. Rev. Microbiol 57 (2003) 395–418. [DOI] [PubMed] [Google Scholar]
- [3].Shadel GS, Horvath TL, Mitochondrial ROS signaling in organismal homeostasis, Cell 163 (3) (2015) 560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kelley EE, Khoo NK, Hundley NJ, Malik UZ, Freeman BA, Tarpey MM, Hydrogen peroxide is the major oxidant product of xanthine oxidase, Free Radic. Biol. Med 48 (4) (2010) 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cordray P, Doyle K, Edes K, Moos PJ, Fitzpatrick FA, Oxidation of 2-Cys-peroxiredoxins by arachidonic acid peroxide metabolites of lipoxygenases and cyclooxygenase-2, J. Biol. Chem 282 (45) (2007) 32623–32629. [DOI] [PubMed] [Google Scholar]
- [6].Lambeth JD, Neish AS, Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited, Ann. Rev. Pathol 9 (2014) 119–145. [DOI] [PubMed] [Google Scholar]
- [7].Bartesaghi S, Radi R, Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration, Redox Biol. 14 (2018) 618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chance B, Sies H, Boveris A, Hydroperoxide metabolism in mammalian organs, Physiol. Rev 59 (3) (1979) 527–605. [DOI] [PubMed] [Google Scholar]
- [9].Imlay JA, Chin SM, Linn S, Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro, Science 240 (4852) (1988) 640–642. [DOI] [PubMed] [Google Scholar]
- [10].Ray PD, Huang BW, Tsuji Y, Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling, Cell. Signal 24 (5) (2012) 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Winterbourn CC, Reconciling the chemistry and biology of reactive oxygen species, Nat. Chem. Biol 4 (5) (2008) 278–286. [DOI] [PubMed] [Google Scholar]
- [12].Imlay JA, Cellular defenses against superoxide and hydrogen peroxide, Annu. Rev. Biochem 77 (2008) 755–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].D’Autreaux B, Toledano MB, ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis, Nat. Rev. Mol. Cell Biol 8 (10) (2007) 813–824. [DOI] [PubMed] [Google Scholar]
- [14].Bae YS, Oh H, Rhee SG, Yoo YD, Regulation of reactive oxygen species generation in cell signaling, Mol. Cells 32 (6) (2011) 491–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Poole LB, Karplus PA, Claiborne A, Protein sulfenic acids in redox signaling, Annu. Rev. Pharmacol. Toxicol 44 (2004) 325–347. [DOI] [PubMed] [Google Scholar]
- [16].Forman HJ, Ursini F, Maiorino M, An overview of mechanisms of redox signaling, J. Mol. Cell. Cardiol 73 (2014) 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bolduc JA, Collins JA, Loeser RF, Reactive Oxygen Species, Aging and Articular Cartilage Homeostasis, Free radical biology & medicine, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stocker S, Van Laer K, Mijuskovic A, Dick TP, The conundrum of hydrogen peroxide signaling and the emerging role of peroxiredoxins as redox relay hubs, Antioxidants Redox Signal. 28 (7) (2018) 558–573. [DOI] [PubMed] [Google Scholar]
- [19].Rhee SG, Woo HA, Kang D, The role of peroxiredoxins in the transduction of H2O2 signals, Antioxidants Redox Signal. 28 (7) (2018) 537–557. [DOI] [PubMed] [Google Scholar]
- [20].Poole LB, The basics of thiols and cysteines in redox biology and chemistry, Free Radic. Biol. Med 80 (2015) 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sardi F, Manta B, Portillo-Ledesma S, Knoops B, Comini MA, Ferrer-Sueta G, Determination of acidity and nucleophilicity in thiols by reaction with mono-bromobimane and fluorescence detection, Anal. Biochem 435 (1) (2013) 74–82. [DOI] [PubMed] [Google Scholar]
- [22].Clement GE, Hartz TP, Determination of the microscopic ionization constants of cysteine, J. Chem. Educ 48 (6) (1971) 395–397. [DOI] [PubMed] [Google Scholar]
- [23].Ferrer-Sueta G, Manta B, Botti H, Radi R, Trujillo M, Denicola A, Factors affecting protein thiol reactivity and specificity in peroxide reduction, Chem. Res. Toxicol 24 (4) (2011) 434–450. [DOI] [PubMed] [Google Scholar]
- [24].Salsbury FR Jr., S.T. Knutson, L.B. Poole, J.S. Fetrow, Functional site profiling and electrostatic analysis of cysteines modifiable to cysteine sulfenic acid, Protein Sci. : Pub. Protein Soc 17 (2) (2008) 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kortemme T, Creighton TE, Ionisation of cysteine residues at the termini of model alpha-helical peptides. Relevance to unusual thiol pKa values in proteins of the thioredoxin family, J. Mol. Biol 253 (5) (1995) 799–812. [DOI] [PubMed] [Google Scholar]
- [26].Flohe L, The fairytale of the GSSG/GSH redox potential, Biochim. Biophys. Acta 1830 (5) (2013) 3139–3142. [DOI] [PubMed] [Google Scholar]
- [27].Deponte M, The incomplete glutathione puzzle: just guessing at numbers and figures? Antioxidants Redox Signal. 27 (15) (2017) 1130–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brandes N, Reichmann D, Tienson H, Leichert LI, Jakob U, Using quantitative redox proteomics to dissect the yeast redoxome, J. Biol. Chem 286 (48) (2011) 41893–41903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Leichert LI, Gehrke F, Gudiseva HV, Blackwell T, Ilbert M, Walker AK, Strahler JR, Andrews PC, Jakob U, Quantifying changes in the thiol redox proteome upon oxidative stress in vivo, Proc. Natl. Acad. Sci. U.S.A 105 (24) (2008) 8197–8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Baez NO, Reisz JA, Furdui CM, Mass spectrometry in studies of protein thiol chemistry and signaling: opportunities and caveats, Free Radic. Biol. Med 80 (2015) 191–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Marino SM, Gladyshev VN, Cysteine function governs its conservation and degeneration and restricts its utilization on protein surfaces, J. Mol. Biol 404 (5) (2010) 902–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Miseta A, Csutora P, Relationship between the occurrence of cysteine in proteins and the complexity of organisms, Mol. Biol. Evol 17 (8) (2000) 1232–1239. [DOI] [PubMed] [Google Scholar]
- [33].Fomenko DE, Gladyshev VN, Genomics perspective on disulfide bond formation, Antioxidants Redox Signal. 5 (4) (2003) 397–402. [DOI] [PubMed] [Google Scholar]
- [34].Hall DO, Cammack R, Rao KK, Role for ferredoxins in the origin of life and biological evolution, Nature 233 (5315) (1971) 136–138. [DOI] [PubMed] [Google Scholar]
- [35].Keire DA, Strauss E, Guo W, Noszal B, Rabenstein DL, Kinetics and equilibria of thiol/disulfide interchange reactions of selected biological thiols and related molecules with oxidized glutathione, J. Org. Chem 57 (1992) 123–127. [Google Scholar]
- [36].Kim EK, Cha CJ, Cho YJ, Cho YB, Roe JH, Synthesis of gamma-gluta-mylcysteine as a major low-molecular-weight thiol in lactic acid bacteria Leuconostoc spp, Biochem. Biophys. Res. Commun 369 (4) (2008) 1047–1051. [DOI] [PubMed] [Google Scholar]
- [37].Sundquist AR, Fahey RC, The function of gamma-glutamylcysteine and bis-gamma-glutamylcystine reductase in Halobacterium halobium, J. Biol. Chem 264 (2) (1989) 719–725. [PubMed] [Google Scholar]
- [38].Birtic S, Colville L, Pritchard HW, Pearce SR, Kranner I, Mathematically combined half-cell reduction potentials of low-molecular-weight thiols as markers of seed ageing, Free Radic. Res 45 (9) (2011) 1093–1102. [DOI] [PubMed] [Google Scholar]
- [39].Benesch RE, Benesch RB, The acid strength of the -SH group in cysteine and related compounds, J. Am. Chem. Soc 77 (22) (1955) 5877–5881. [Google Scholar]
- [40].Newton GL, Javor B, gamma-Glutamylcysteine and thiosulfate are the major low-molecular-weight thiols in halobacteria, J. Bacteriol 161 (1) (1985) 438–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schulz GE, Schirmer RH, Sachsenheimer W, Pai EF, The structure of the flavoenzyme glutathione reductase, Nature 273 (5658) (1978) 120–124. [DOI] [PubMed] [Google Scholar]
- [42].Schafer FQ, Buettner GR, Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple, Free Radic. Biol. Med 30 (11) (2001) 1191–1212. [DOI] [PubMed] [Google Scholar]
- [43].Gravina SA, Mieyal JJ, Thioltransferase is a specific glutathionyl mixed disulfide oxidoreductase, Biochemistry 32 (13) (1993) 3368–3376. [DOI] [PubMed] [Google Scholar]
- [44].Fairlamb AH, Cerami A, Metabolism and functions of trypanothione in the Kinetoplastida, Annu. Rev. Microbiol 46 (1992) 695–729. [DOI] [PubMed] [Google Scholar]
- [45].Krauth-Siegel RL, Comini MA, Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism, Biochim. Biophys. Acta 1780 (11) (2008) 1236–1248. [DOI] [PubMed] [Google Scholar]
- [46].Reckenfelderbaumer N, Krauth-Siegel RL, Catalytic properties, thiol pK value, and redox potential of Trypanosoma brucei tryparedoxin, J. Biol. Chem 277 (20) (2002) 17548–17555. [DOI] [PubMed] [Google Scholar]
- [47].Newton GL, Arnold K, Price MS, Sherrill C, Delcardayre SB, Aharonowitz Y, Cohen G, Davies J, Fahey RC, Davis C, Distribution of thiols in microorganisms: mycothiol is a major thiol in most actinomycetes, J. Bacteriol 178 (7) (1996) 1990–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sharma SV, Van Laer K, Messens J, Hamilton CJ, Thiol redox and pKa properties of mycothiol, the predominant Low-Molecular-Weight thiol cofactor in the actinomycetes, Chembiochem : Eur. J. Chem. Biol 17 (18) (2016) 1689–1692. [DOI] [PubMed] [Google Scholar]
- [49].Van Laer K, Buts L, Foloppe N, Vertommen D, Van Belle K, Wahni K, Roos G, Nilsson L, Mateos LM, Rawat M, van Nuland NA, Messens J, Mycoredoxin-1 is one of the missing links in the oxidative stress defence mechanism of Mycobacteria, Mol. Microbiol 86 (4) (2012) 787–804. [DOI] [PubMed] [Google Scholar]
- [50].Tung QN, Linzner N, Loi VV, Antelmann H, Application of genetically encoded redox biosensors to measure dynamic changes in the glutathione, bacillithiol and mycothiol redox potentials in pathogenic bacteria, Free Radic. Biol. Med 128 (2018) 84–96. [DOI] [PubMed] [Google Scholar]
- [51].Sharma SV, Arbach M, Roberts AA, Macdonald CJ, Groom M, Hamilton CJ, Biophysical features of bacillithiol, the glutathione surrogate of Bacillus subtilis and other firmicutes, Chembiochem : Eur. J. Chem. Biol 14 (16) (2013) 2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gaballa A, Chi BK, Roberts AA, Becher D, Hamilton CJ, Antelmann H, Helmann JD, Redox regulation in Bacillus subtilis: the bacilliredoxins BrxA(YphP) and BrxB(YqiW) function in de-bacillithiolation of S-bacillithiolated OhrR and MetE, Antioxidants Redox Signal. 21 (3) (2014) 357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chandrangsu P, Loi VV, Antelmann H, Helmann JD, The role of bacillithiol in Gram-positive firmicutes, Antioxidants Redox Signal. 28 (6) (2018) 445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hiras J, Sharma SV, Raman V, Tinson RAJ, Arbach M, Rodrigues DF, Norambuena J, Hamilton CJ, Hanson TE, Physiological studies of Chlorobiaceae suggest that bacillithiol derivatives are the most widespread thiols in bacteria, mBio 9 (6) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Luba J, Charrier V, Claiborne A, Coenzyme A-disulfide reductase from Staphylococcus aureus: evidence for asymmetric behavior on interaction with pyridine nucleotides, Biochemistry 38 (9) (1999) 2725–2737. [DOI] [PubMed] [Google Scholar]
- [56].Keire DA, Robert JM, Rabenstein DL, Microscopic protonation equilibria and solution conformations of coenzyme A and coenzyme A disulfides, J. Org. Chem 57 (16) (1992) 4427–4431. [Google Scholar]
- [57].Gaballa A, Newton GL, Antelmann H, Parsonage D, Upton H, Rawat M, Claiborne A, Fahey RC, Helmann JD, Biosynthesis and functions of bacillithiol, a major low-molecular-weight thiol in Bacilli, Proc. Natl. Acad. Sci. U.S.A 107 (14) (2010) 6482–6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Newton GL, Rawat M, La Clair JJ, Jothivasan VK, Budiarto T, Hamilton CJ, Claiborne A, Helmann JD, Fahey RC, Bacillithiol is an antioxidant thiol produced in Bacilli, Nat. Chem. Biol 5 (9) (2009) 625–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cheah IK, Halliwell B, Ergothioneine; antioxidant potential, physiological function and role in disease, Biochim. Biophys. Acta 1822 (5) (2012) 784–793. [DOI] [PubMed] [Google Scholar]
- [60].Stanovnik B, Tisler M, Dissociation constants and structure of ergothioneine, Anal. Biochem 9 (1964) 68–74. [DOI] [PubMed] [Google Scholar]
- [61].Cumming BM, Chinta KC, Reddy VP, Steyn AJC, Role of ergothioneine in microbial physiology and pathogenesis, Antioxidants Redox Signal. 28 (6) (2018) 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Weaver KH, Rabenstein DL, Thiol/disulfide exchange reactions of ovothiol A with glutathione, J. Org. Chem 60 (6) (1995) 1904–1907. [Google Scholar]
- [63].Castellano I, Seebeck FP, On Ovothiol Biosynthesis and Biological Roles: from Life in the Ocean to Therapeutic Potential, Natural Product Reports, (2018). [DOI] [PubMed] [Google Scholar]
- [64].Herrmann JM, Dick TP, Redox biology on the rise, Biol. Chem 393 (9) (2012) 999–1004. [DOI] [PubMed] [Google Scholar]
- [65].Mamathambika BS, Bardwell JC, Disulfide-linked protein folding pathways, Annu. Rev. Cell Dev. Biol 24 (2008) 211–235. [DOI] [PubMed] [Google Scholar]
- [66].Riemer J, Bulleid N, Herrmann JM, Disulfide formation in the ER and mitochondria: two solutions to a common process, Science 324 (5932) (2009) 1284–1287. [DOI] [PubMed] [Google Scholar]
- [67].Reddie KG, Carroll KS, Expanding the functional diversity of proteins through cysteine oxidation, Curr. Opin. Chem. Biol 12 (6) (2008) 746–754. [DOI] [PubMed] [Google Scholar]
- [68].Brandes N, Schmitt S, Jakob U, Thiol-based redox switches in eukaryotic proteins, Antioxidants Redox Signal. 11 (5) (2009) 997–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hansen RE, Roth D, Winther JR, Quantifying the global cellular thiol-disulfide status, Proc. Natl. Acad. Sci. U.S.A 106 (2) (2009) 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Klomsiri C, Karplus PA, Poole LB, Cysteine-based redox switches in enzymes, Antioxidants Redox Signal. 14 (6) (2011) 1065–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Paulsen CE, Carroll KS, Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery, Chem. Rev 113 (7) (2013) 4633–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Groitl B, Jakob U, Thiol-based redox switches, Biochim. Biophys. Acta 1844 (8) (2014) 1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Leichert LI, Dick TP, Incidence and physiological relevance of protein thiol switches, Biol. Chem 396 (5) (2015) 389–399. [DOI] [PubMed] [Google Scholar]
- [74].Herrmann JM, Becker K, Dick TP, Highlight: dynamics of thiol-based redox switches, Biol. Chem 396 (5) (2015) 385–387. [DOI] [PubMed] [Google Scholar]
- [75].Winterbourn CC, Hampton MB, Thiol chemistry and specificity in redox signaling, Free Radic. Biol. Med 45 (5) (2008) 549–561. [DOI] [PubMed] [Google Scholar]
- [76].Nelson KJ, Klomsiri C, Codreanu SG, Soito L, Liebler DC, Rogers LC, Daniel LW, Poole LB, Use of dimedone-based chemical probes for sulfenic acid detection methods to visualize and identify labeled proteins, Methods Enzymol. 473 (2010) 95–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mieyal JJ, Chock PB, Posttranslational modification of cysteine in redox signaling and oxidative stress: focus on S-glutathionylation, Antioxidants Redox Signal. 16 (6) (2012) 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Holmgren A, Johansson C, Berndt C, Lonn ME, Hudemann C, Lillig CH, Thiol redox control via thioredoxin and glutaredoxin systems, Biochem. Soc. Trans 33 (Pt 6) (2005) 1375–1377. [DOI] [PubMed] [Google Scholar]
- [79].Kimura H, Hydrogen sulfide and polysulfide signaling, Antioxidants Redox Signal. 27 (10) (2017) 619–621. [DOI] [PubMed] [Google Scholar]
- [80].Doka E, Pader I, Biro A, Johansson K, Cheng Q, Ballago K, Prigge JR, Pastor-Flores D, Dick TP, Schmidt EE, Arner ES, Nagy P, A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems, Sci. Adv 2 (1) (2016) e1500968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Heppner DE, Hristova M, Ida T, Mijuskovic A, Dustin CM, Bogdandi V, Fukuto JM, Dick TP, Nagy P, Li J, Akaike T, van der Vliet A, Cysteine perthiosulfenic acid (Cys-SSOH): a novel intermediate in thiol-based redox signaling? Redox Biol. 14 (2018) 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].McDonagh B, Ogueta S, Lasarte G, Padilla CA, Barcena JA, Shotgun redox proteomics identifies specifically modified cysteines in key metabolic enzymes under oxidative stress in Saccharomyces cerevisiae, J. Proteomics 72 (4) (2009) 677–689. [DOI] [PubMed] [Google Scholar]
- [83].Lind C, Gerdes R, Hamnell Y, Schuppe-Koistinen I, von Lowenhielm HB, Holmgren A, Cotgreave IA, Identification of S-glutathionylated cellular proteins during oxidative stress and constitutive metabolism by affinity purification and proteomic analysis, Arch. Biochem. Biophys 406 (2) (2002) 229–240. [DOI] [PubMed] [Google Scholar]
- [84].Su D, Gaffrey MJ, Guo J, Hatchell KE, Chu RK, Clauss TR, Aldrich JT, Wu S, Purvine S, Camp DG, Smith RD, Thrall BD, Qian WJ, Proteomic identification and quantification of S-glutathionylation in mouse macrophages using resin-assisted enrichment and isobaric labeling, Free Radic. Biol. Med 67 (2014) 460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Le Moan N, Clement G, Le Maout S, Tacnet F, Toledano MB, The Saccharomyces cerevisiae proteome of oxidized protein thiols: contrasted functions for the thioredoxin and glutathione pathways, J. Biol. Chem 281 (15) (2006) 10420–10430. [DOI] [PubMed] [Google Scholar]
- [86].Go YM, Roede JR, Walker DI, Duong DM, Seyfried NT, Orr M, Liang Y, Pennell KD, Jones DP, Selective targeting of the cysteine proteome by thioredoxin and glutathione redox systems, Mol. Cell. Proteom. : MCP 12 (11) (2013) 3285–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Duan J, Gaffrey MJ, Qian WJ, Quantitative proteomic characterization of redox-dependent post-translational modifications on protein cysteines, Mol. Biosyst 13 (5) (2017) 816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lennicke C, Rahn J, Heimer N, Lichtenfels R, Wessjohann LA, Seliger B, Redox proteomics: methods for the identification and enrichment of redox-modified proteins and their applications, Proteomics 16 (2) (2016) 197–213. [DOI] [PubMed] [Google Scholar]
- [89].McDonagh B, Detection of ROS induced proteomic signatures by mass spectrometry, Front. Physiol 8 (2017) 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Furdui CM, Poole LB, Chemical approaches to detect and analyze protein sulfenic acids, Mass Spectrom. Rev 33 (2) (2014) 126–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Flohe L, Toppo S, Cozza G, Ursini F, A comparison of thiol peroxidase mechanisms, Antioxidants Redox Signal. 15 (3) (2011) 763–780. [DOI] [PubMed] [Google Scholar]
- [92].Brigelius-Flohe R, Maiorino M, Glutathione peroxidases, Biochim. Biophys. Acta 1830 (5) (2013) 3289–3303. [DOI] [PubMed] [Google Scholar]
- [93].Ursini F, Maiorino M, Brigelius-Flohe R, Aumann KD, Roveri A, Schomburg D, Flohe L, Diversity of glutathione peroxidases, Methods Enzymol. 252 (1995) 38–53. [DOI] [PubMed] [Google Scholar]
- [94].Nakamura T, Kado Y, Yamaguchi T, Matsumura H, Ishikawa K, Inoue T, Crystal structure of peroxiredoxin from Aeropyrum pernix K1 complexed with its substrate, hydrogen peroxide, J. Biochem 147 (1) (2010) 109–115. [DOI] [PubMed] [Google Scholar]
- [95].Hall A, Parsonage D, Poole LB, Karplus PA, Structural evidence that peroxiredoxin catalytic power is based on transition-state stabilization, J. Mol. Biol 402 (1) (2010) 194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Rhee SG, Overview on peroxiredoxin, Mol. Cells 39 (1) (2016) 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Winterbourn CC, Peskin AV, Kinetic approaches to measuring peroxiredoxin reactivity, Mol. Cells 39 (1) (2016) 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Marinho HS, Real C, Cyrne L, Soares H, Antunes F, Hydrogen peroxide sensing, signaling and regulation of transcription factors, Redox Biol. 2 (2014) 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Manevich Y, Feinstein SI, Fisher AB, Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST, Proc. Natl. Acad. Sci. U.S.A 101 (11) (2004) 3780–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Holmstrom KM, Finkel T, Cellular mechanisms and physiological consequences of redox-dependent signalling, Nat. Rev. Mol. Cell Biol 15 (6) (2014) 411–421. [DOI] [PubMed] [Google Scholar]
- [101].Sies H, Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress, Redox Biol. 11 (2017) 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Perkins A, Nelson KJ, Parsonage D, Poole LB, Karplus PA, Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling, Trends Biochem. Sci 40 (8) (2015) 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Winterbourn CC, Hampton MB, Redox biology: signaling via a peroxiredoxin sensor, Nat. Chem. Biol 11 (1) (2015) 5–6. [DOI] [PubMed] [Google Scholar]
- [104].Stocker S, Maurer M, Ruppert T, Dick TP, A role for 2-Cys peroxiredoxins in facilitating cytosolic protein thiol oxidation, Nat. Chem. Biol 14 (2) (2018) 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Netto LE, Antunes F, The roles of peroxiredoxin and thioredoxin in hydrogen peroxide sensing and in signal transduction, Mol. Cells 39 (1) (2016) 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Randall LM, Ferrer-Sueta G, Denicola A, Peroxiredoxins as preferential targets in H2O2-induced signaling, Methods Enzymol. 527 (2013) 41–63. [DOI] [PubMed] [Google Scholar]
- [107].Flohe L, The impact of thiol peroxidases on redox regulation, Free Radic. Res 50 (2) (2016) 126–142. [DOI] [PubMed] [Google Scholar]
- [108].Delaunay A, Pflieger D, Barrault MB, Vinh J, Toledano MB, A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation, Cell 111 (4) (2002) 471–481. [DOI] [PubMed] [Google Scholar]
- [109].Delaunay A, Isnard AD, Toledano MB, H2O2 sensing through oxidation of the Yap1 transcription factor, EMBO J. 19 (19) (2000) 5157–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Bersweiler A, D’Autreaux B, Mazon H, Kriznik A, Belli G, Delaunay-Moisan A, Toledano MB, Rahuel-Clermont S, A scaffold protein that chaperones a cysteinesulfenic acid in H2O2 signaling, Nat. Chem. Biol 13 (8) (2017) 909–915. [DOI] [PubMed] [Google Scholar]
- [111].Jarvis RM, Hughes SM, Ledgerwood EC, Peroxiredoxin 1 functions as a signal peroxidase to receive, transduce, and transmit peroxide signals in mammalian cells, Free Radic. Biol. Med 53 (7) (2012) 1522–1530. [DOI] [PubMed] [Google Scholar]
- [112].Sobotta MC, Liou W, Stocker S, Talwar D, Oehler M, Ruppert T, Scharf AN, Dick TP, Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling, Nat. Chem. Biol. 11 (1) (2015) 64–70. [DOI] [PubMed] [Google Scholar]
- [113].Dalle-Donne I, Rossi R, Colombo G, Giustarini D, Milzani A, Protein S-glutathionylation: a regulatory device from bacteria to humans, Trends Biochem. Sci 34 (2) (2009) 85–96. [DOI] [PubMed] [Google Scholar]
- [114].Allen EM, Mieyal JJ, Protein-thiol oxidation and cell death: regulatory role of glutaredoxins, Antioxidants Redox Signal. 17 (12) (2012) 1748–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Giles NM, Watts AB, Giles GI, Fry FH, Littlechild JA, Jacob C, Metal and redox modulation of cysteine protein function, Chem. Biol 10 (8) (2003) 677–693. [DOI] [PubMed] [Google Scholar]
- [116].Wang XF, Cynader MS, Pyruvate released by astrocytes protects neurons from copper-catalyzed cysteine neurotoxicity, J. Neurosci. : Off. J. Soc. Neurosci 21 (10) (2001) 3322–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Anderson CL, Iyer SS, Ziegler TR, Jones DP, Control of extracellular cysteine/cystine redox state by HT-29 cells is independent of cellular glutathione, Am. J. Physiol. Regul. Integr. Comp. Physiol 293 (3) (2007) R1069–R1075. [DOI] [PubMed] [Google Scholar]
- [118].Van Laer K, Hamilton CJ, Messens J, Low-molecular-weight thiols in thiol-disulfide exchange, Antioxidants Redox Signal. 18 (13) (2013) 1642–1653. [DOI] [PubMed] [Google Scholar]
- [119].Manta B, Bonilla M, Fiestas L, Sturlese M, Salinas G, Bellanda M, Comini MA, Polyamine-based thiols in trypanosomatids: evolution, protein structural adaptations, and biological functions, Antioxidants Redox Signal. 28 (6) (2018) 463–486. [DOI] [PubMed] [Google Scholar]
- [120].Stoll VS, Simpson SJ, Krauth-Siegel RL, Walsh CT, Pai EF, Glutathione reductase turned into trypanothione reductase: structural analysis of an engineered change in substrate specificity, Biochemistry 36 (21) (1997) 6437–6447. [DOI] [PubMed] [Google Scholar]
- [121].Meister A, On the discovery of glutathione, Trends Biochem. Sci 13 (5) (1988) 185–188. [DOI] [PubMed] [Google Scholar]
- [122].Hopkins FG, On an autoxidisable constituent of the cell, Biochem. J 15 (2) (1921) 286–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Nicolet BH, The structure of glutathione, Science 71 (1849) (1930) 589–590. [DOI] [PubMed] [Google Scholar]
- [124].Deponte M, Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes, Biochim. Biophys. Acta 1830 (5) (2013) 3217–3266. [DOI] [PubMed] [Google Scholar]
- [125].Meyer AJ, Dick TP, Fluorescent protein-based redox probes, Antioxidants Redox Signal. 13 (5) (2010) 621–650. [DOI] [PubMed] [Google Scholar]
- [126].Morgan B, Ezerina D, Amoako TN, Riemer J, Seedorf M, Dick TP, Multiple glutathione disulfide removal pathways mediate cytosolic redox homeostasis, Nat. Chem. Biol 9 (2) (2013) 119–125. [DOI] [PubMed] [Google Scholar]
- [127].Gutscher M, Pauleau AL, Marty L, Brach T, Wabnitz GH, Samstag Y, Meyer AJ, Dick TP, Real-time imaging of the intracellular glutathione redox potential, Nat. Methods 5 (6) (2008) 553–559. [DOI] [PubMed] [Google Scholar]
- [128].Bachhawat AK, Kaur A, Glutathione degradation, Antioxidants Redox Signal. 27 (15) (2017) 1200–1216. [DOI] [PubMed] [Google Scholar]
- [129].Calabrese G, Morgan B, Riemer J, Mitochondrial glutathione: regulation and functions, Antioxidants Redox Signal. 27 (15) (2017) 1162–1177. [DOI] [PubMed] [Google Scholar]
- [130].Ezerina D, Morgan B, Dick TP, Imaging dynamic redox processes with genetically encoded probes, J. Mol. Cell. Cardiol 73 (2014) 43–49. [DOI] [PubMed] [Google Scholar]
- [131].Schwarzlander M, Dick TP, Meyer AJ, Morgan B, Dissecting redox biology using fluorescent protein sensors, Antioxidants Redox Signal. 24 (13) (2016) 680–712. [DOI] [PubMed] [Google Scholar]
- [132].Goldman BS, Gabbert KK, Kranz RG, The temperature-sensitive growth and survival phenotypes of Escherichia coli cydDC and cydAB strains are due to deficiencies in cytochrome bd and are corrected by exogenous catalase and reducing agents, J. Bacteriol 178 (21) (1996) 6348–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Messens J, Collet JF, Van Belle K, Brosens E, Loris R, Wyns L, The oxidase DsbA folds a protein with a nonconsecutive disulfide, J. Biol. Chem 282 (43) (2007) 31302–31307. [DOI] [PubMed] [Google Scholar]
- [134].Birk J, Meyer M, Aller I, Hansen HG, Odermatt A, Dick TP, Meyer AJ, Appenzeller-Herzog C, Endoplasmic reticulum: reduced and oxidized glutathione revisited, J. Cell Sci 126 (Pt 7) (2013) 1604–1617. [DOI] [PubMed] [Google Scholar]
- [135].Reyes AM, Pedre B, De Armas MI, Tossounian MA, Radi R, Messens J, Trujillo M, Chemistry and redox biology of mycothiol, Antioxidants Redox Signal. 28 (6) (2018) 487–504. [DOI] [PubMed] [Google Scholar]
- [136].Toppo S, Flohe L, Ursini F, Vanin S, Maiorino M, Catalytic mechanisms and specificities of glutathione peroxidases: variations of a basic scheme, Biochim. Biophys. Acta 1790 (11) (2009) 1486–1500. [DOI] [PubMed] [Google Scholar]
- [137].Rouhier N, Lemaire SD, Jacquot JP, The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation, Annu. Rev. Plant Biol 59 (2008) 143–166. [DOI] [PubMed] [Google Scholar]
- [138].Maiorino M, Conrad M, Ursini F, GPx4, lipid peroxidation, and cell death: discoveries, rediscoveries, and open issues, Antioxidants Redox Signal. 29 (1) (2018) 61–74. [DOI] [PubMed] [Google Scholar]
- [139].Rabbani N, Thornalley PJ, The glyoxalase system-from microbial metabolism, through ageing to human disease and multidrug resistance, Semin. Cell Dev. Biol 22 (3) (2011) 261. [DOI] [PubMed] [Google Scholar]
- [140].Dalle-Donne I, Colombo G, Gagliano N, Colombo R, Giustarini D, Rossi R, Milzani A, S-glutathiolation in life and death decisions of the cell, Free Radic. Res 45 (1) (2011) 3–15. [DOI] [PubMed] [Google Scholar]
- [141].Loi VV, Rossius M, Antelmann H, Redox regulation by reversible protein S-thiolation in bacteria, Front. Microbiol 6 (2015) 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Ulrich K, Finkenzeller C, Merker S, Rojas F, Matthews K, Ruppert T, Krauth-Siegel RL, Stress-induced protein S-glutathionylation and S-trypanothionylation in African trypanosomes - a quantitative redox proteome and thiol Analysis, Antioxidants Redox Signal. 27 (9) (2017) 517–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Lillig CH, Berndt C, Glutaredoxins in thiol/disulfide exchange, Antioxidants Redox Signal. 18 (13) (2013) 1654–1665. [DOI] [PubMed] [Google Scholar]
- [144].Shelton MD, Mieyal JJ, Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases, Mol. Cells 25 (3) (2008) 332–346. [PMC free article] [PubMed] [Google Scholar]
- [145].Ukuwela AA, Bush AI, Wedd AG, Xiao Z, Glutaredoxins employ parallel monothiol-dithiol mechanisms to catalyze thiol-disulfide exchanges with protein disulfides, Chem. Sci 9 (5) (2018) 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Gallogly MM, Starke DW, Leonberg AK, Ospina SM, Mieyal JJ, Kinetic and mechanistic characterization and versatile catalytic properties of mammalian glutaredoxin 2: implications for intracellular roles, Biochemistry 47 (42) (2008) 11144–11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Peltoniemi MJ, Karala AR, Jurvansuu JK, Kinnula VL, Ruddock LW, Insights into deglutathionylation reactions. Different intermediates in the glutaredoxin and protein disulfide isomerase catalyzed reactions are defined by the gamma-linkage present in glutathione, J. Biol. Chem 281 (44) (2006) 33107–33114. [DOI] [PubMed] [Google Scholar]
- [148].Bushweller JH, Aslund F, Wuthrich K, Holmgren A, Structural and functional characterization of the mutant Escherichia coli glutaredoxin (C14——S) and its mixed disulfide with glutathione, Biochemistry 31 (38) (1992) 9288–9293. [DOI] [PubMed] [Google Scholar]
- [149].Mashamaite LN, Rohwer JM, Pillay CS, The glutaredoxin mono- and di-thiol mechanisms for deglutathionylation are functionally equivalent: implications for redox systems biology, Biosci. Rep 35 (1) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Lee S, Kim SM, Lee RT, Thioredoxin and thioredoxin target proteins: from molecular mechanisms to functional significance, Antioxidants Redox Signal. 18 (10) (2013) 1165–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Muller EG, Thioredoxin deficiency in yeast prolongs S phase and shortens the G1 interval of the cell cycle, J. Biol. Chem 266 (14) (1991) 9194–9202. [PubMed] [Google Scholar]
- [152].Luikenhuis S, Perrone G, Dawes IW, Grant CM, The yeast Saccharomyces cerevisiae contains two glutaredoxin genes that are required for protection against reactive oxygen species, Mol. Biol. Cell 9 (5) (1998) 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Muller EG, A glutathione reductase mutant of yeast accumulates high levels of oxidized glutathione and requires thioredoxin for growth, Mol. Biol. Cell 7 (11) (1996) 1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Trotter EW, Grant CM, Non-reciprocal regulation of the redox state of the glutathione-glutaredoxin and thioredoxin systems, EMBO Rep. 4 (2) (2003) 184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Wu AL, Moye-Rowley WS, GSH1, which encodes gamma-glutamylcysteine synthetase, is a targetgene foryAP-1 transcriptional regulation, Mol. Cell. Biol 14 (9) (1994) 5832–5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Grant CM, Dawes IW, Synthesis and role of glutathione in protection against oxidative stress in yeast, Redox Rep. : communications in free radical research 2 (4) (1996) 223–229. [DOI] [PubMed] [Google Scholar]
- [157].Kumar C, Igbaria A, D’Autreaux B, Planson AG, Junot C, Godat E, Bachhawat AK, Delaunay-Moisan A, Toledano MB, Glutathione revisited: a vital function in iron metabolism and ancillary role in thiol-redox control, EMBO J. 30 (10) (2011) 2044–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Sipos K, Lange H, Fekete Z, Ullmann P, Lill R, Kispal G, Maturation of cytosolic iron-sulfur proteins requires glutathione, J. Biol. Chem 277 (30) (2002) 26944–26949. [DOI] [PubMed] [Google Scholar]
- [159].Hudson DA, Gannon SA, Thorpe C, Oxidative protein folding: from thiol-disulfide exchange reactions to the redox poise of the endoplasmic reticulum, Free Radic. Biol. Med 80 (2015) 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Delaunay-Moisan A, Ponsero A, Toledano MB, Reexamining the function of glutathione in oxidative protein folding and secretion, Antioxidants Redox Signal. 27 (15) (2017) 1178–1199. [DOI] [PubMed] [Google Scholar]
- [161].Lu SC, Glutathione synthesis, Biochim. Biophys. Acta 1830 (5) (2013) 3143–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Ponsero AJ, Igbaria A, Darch MA, Miled S, Outten CE, Winther JR, Palais G, D’Autreaux B, Delaunay-Moisan A, Toledano MB, Endoplasmic reticulum transport of glutathione by Sec61 is regulated by Ero1 and Bip, Mol. Cell 67 (6) (2017) 962–973 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Pittman MS, Robinson HC, Poole RK, A bacterial glutathione transporter (Escherichia coli CydDC) exports reductant to the periplasm, J. Biol. Chem 280 (37) (2005) 32254–32261. [DOI] [PubMed] [Google Scholar]
- [164].Pollard MG, Travers KJ, Weissman JS, Ero1p: a novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum, Mol. Cell 1 (2) (1998) 171–182. [DOI] [PubMed] [Google Scholar]
- [165].Frand AR, Kaiser CA, Ero1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum, Mol. Cell 4 (4) (1999) 469–477. [DOI] [PubMed] [Google Scholar]
- [166].Tu BP, Weissman JS, The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum, Mol. Cell 10 (5) (2002) 983–994. [DOI] [PubMed] [Google Scholar]
- [167].Poet GJ, Oka OB, van Lith M, Cao Z, Robinson PJ, Pringle MA, Arner ES, Bulleid NJ, Cytosolic thioredoxin reductase 1 is required for correct disulfide formation in the ER, EMBO J. 36 (5) (2017) 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Sevier CS, Qu H, Heldman N, Gross E, Fass D, Kaiser CA, Modulation of cellular disulfide-bond formation and the ER redox environment by feedback regulation of Ero1, Cell 129 (2) (2007) 333–344. [DOI] [PubMed] [Google Scholar]
- [169].Zhang L, Niu Y, Zhu L, Fang J, Wang X, Wang L, Wang CC, Different interaction modes for protein-disulfide isomerase (PDI) as an efficient regulator and a specific substrate of endoplasmic reticulum oxidoreductin-1alpha (Ero1alpha), J. Biol. Chem 289 (45) (2014) 31188–31199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Kojer K, Bien M, Gangel H, Morgan B, Dick TP, Riemer J, Glutathione redox potential in the mitochondrial intermembrane space is linked to the cytosol and impacts the Mia40 redox state, EMBO J. 31 (14) (2012) 3169–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Deponte M, Hell K, Disulphide bond formation in the intermembrane space of mitochondria, J. Biochem 146 (5) (2009) 599–608. [DOI] [PubMed] [Google Scholar]
- [172].Koehler CM, Tienson HL, Redox regulation of protein folding in the mitochondrial intermembrane space, Biochim. Biophys. Acta 1793 (1) (2009) 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Herrmann JM, Kohl R, Catch me if you can! Oxidative protein trapping in the intermembrane space of mitochondria, J. Cell Biol 176 (5) (2007) 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Bihlmaier K, Mesecke N, Terziyska N, Bien M, Hell K, Herrmann JM, The disulfide relay system of mitochondria is connected to the respiratory chain, J. Cell Biol 179 (3) (2007) 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Bien M, Longen S, Wagener N, Chwalla I, Herrmann JM, Riemer J, Mitochondrial disulfide bond formation is driven by intersubunit electron transfer in Erv1 and proofread by glutathione, Mol. Cell 37 (4) (2010) 516–528. [DOI] [PubMed] [Google Scholar]
- [176].Kojer K, Peleh V, Calabrese G, Herrmann JM, Riemer J, Kinetic control by limiting glutaredoxin amounts enables thiol oxidation in the reducing mitochondrial intermembrane space, Mol. Biol. Cell 26 (2) (2015) 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Fairlamb AH, Blackburn P, Ulrich P, Chait BT, Cerami A, Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids, Science 227 (4693) (1985) 1485–1487. [DOI] [PubMed] [Google Scholar]
- [178].Leroux AE, Krauth-Siegel RL, Thiol redox biology of trypanosomatids and potential targets for chemotherapy, Mol. Biochem. Parasitol 206 (1–2) (2016) 67–74. [DOI] [PubMed] [Google Scholar]
- [179].Krieger S, Schwarz W, Ariyanayagam MR, Fairlamb AH, Krauth-Siegel RL, Clayton C, Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress, Mol. Microbiol 35 (3) (2000) 542–552. [DOI] [PubMed] [Google Scholar]
- [180].Comini M, Menge U, Flohe L, Biosynthesis of trypanothione in Trypanosoma brucei brucei, Biol. Chem 384 (4) (2003) 653–656. [DOI] [PubMed] [Google Scholar]
- [181].Gilbert HF, Molecular and cellular aspects of thiol-disulfide exchange, Adv. Enzymol. Relat. Area Mol. Biol 63 (1990) 69–172. [DOI] [PubMed] [Google Scholar]
- [182].Comini MA, Guerrero SA, Haile S, Menge U, Lunsdorf H, Flohe L, Validation of Trypanosoma brucei trypanothione synthetase as drug target, Free Radic. Biol. Med 36 (10) (2004) 1289–1302. [DOI] [PubMed] [Google Scholar]
- [183].Comini MA, Krauth-Siegel RL, Flohe L, Depletion of the thioredoxin homologue tryparedoxin impairs antioxidative defence in African trypanosomes, Biochem. J 402 (1) (2007) 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Musunda B, Benitez D, Dirdjaja N, Comini MA, Krauth-Siegel RL, Glutaredoxin-deficiency confers bloodstream Trypanosoma brucei with improved thermotolerance, Mol. Biochem. Parasitol 204 (2) (2015) 93–105. [DOI] [PubMed] [Google Scholar]
- [185].Ceylan S, Seidel V, Ziebart N, Berndt C, Dirdjaja N, Krauth-Siegel RL, The dithiol glutaredoxins of African trypanosomes have distinct roles and are closely linked to the unique trypanothione metabolism, J. Biol. Chem 285 (45) (2010) 35224–35237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Kumar A, Nartey W, Shin J, Manimekalai MSS, Gruber G, Structural and mechanistic insights into Mycothiol Disulphide Reductase and the Mycoredoxin-1-alkylhydroperoxide reductase E assembly of Mycobacterium tuberculosis, Biochimica et biophysica acta, General Sub. 1861 (9) (2017) 2354–2366. [DOI] [PubMed] [Google Scholar]
- [187].Newton GL, Buchmeier N, Fahey RC, Biosynthesis and functions of mycothiol, the unique protective thiol of Actinobacteria, Microbiol. Mol. Biol. Rev. : MMBR (Microbiol. Mol. Biol. Rev) 72 (3) (2008) 471–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Newton GL, Jensen PR, Macmillan JB, Fenical W, Fahey RC, An N-acyl homolog of mycothiol is produced in marine actinomycetes, Arch. Microbiol 190 (5) (2008) 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Imber M, Pietrzyk-Brzezinska AJ, Antelmann H, Redox regulation by reversible protein S-thiolation in Gram-positive bacteria, Redox Biol. 20 (2018) 130–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Alegria TG, Meireles DA, Cussiol JR, Hugo M, Trujillo M, de Oliveira MA, Miyamoto S, Queiroz RF, Valadares NF, Garratt RC, Radi R, Di Mascio P, Augusto O, Netto LE, Ohr plays a central role in bacterial responses against fatty acid hydroperoxides and peroxynitrite, Proc. Natl. Acad. Sci. U.S.A 114 (2) (2017) E132–E141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Ta P, Buchmeier N, Newton GL, Rawat M, Fahey RC, Organic hydroperoxide resistance protein and ergothioneine compensate for loss of mycothiol in Mycobacterium smegmatis mutants, J. Bacteriol 193 (8) (2011) 1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Buchmeier NA, Newton GL, Fahey RC, A mycothiol synthase mutant of Mycobacterium tuberculosis has an altered thiol-disulfide content and limited tolerance to stress, J. Bacteriol 188 (17) (2006) 6245–6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Chi BK, Busche T, Van Laer K, Basell K, Becher D, Clermont L, Seibold GM, Persicke M, Kalinowski J, Messens J, Antelmann H, Protein S-mycothiolation functions as redox-switch and thiol protection mechanism in Corynebacterium glutamicum under hypochlorite stress, Antioxidants Redox Signal. 20 (4) (2014) 589–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Hillion M, Bernhardt J, Busche T, Rossius M, Maass S, Becher D, Rawat M, Wirtz M, Hell R, Ruckert C, Kalinowski J, Antelmann H, Monitoring global protein thiol-oxidation and protein S-mycothiolation in Mycobacterium smegmatis under hypochlorite stress, Sci. Rep 7 (1) (2017) 1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Hillion M, Imber M, Pedre B, Bernhardt J, Saleh M, Loi VV, Maass S, Becher D, Astolfi Rosado L, Adrian L, Weise C, Hell R, Wirtz M, Messens J, Antelmann H, The glyceraldehyde-3-phosphate dehydrogenase GapDH of Corynebacterium diphtheria is redox-controlled by protein S-mycothiolation under oxidative stress, Sci. Rep 7 (1) (2017) 5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Ordonez E, Van Belle K, Roos G, De Galan S, Letek M, Gil JA, Wyns L, Mateos LM, Messens J, Arsenate reductase, mycothiol, and mycoredoxin concert thiol/disulfide exchange, J. Biol. Chem 284 (22) (2009) 15107–15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Hugo M, Van Laer K, Reyes AM, Vertommen D, Messens J, Radi R, Trujillo M, Mycothiol/mycoredoxin 1-dependent reduction of the peroxiredoxin AhpE from Mycobacterium tuberculosis, J. Biol. Chem 289 (8) (2014) 5228–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Si M, Xu Y, Wang T, Long M, Ding W, Chen C, Guan X, Liu Y, Wang Y, Shen X, Liu SJ, Functional characterization of a mycothiol peroxidase in Corynebacterium glutamicum that uses both mycoredoxin and thioredoxin reducing systems in the response to oxidative stress, Biochem. J 469 (1) (2015) 45–57. [DOI] [PubMed] [Google Scholar]
- [199].Zhao Q, Wang M, Xu D, Zhang Q, Liu W, Metabolic coupling of two small-molecule thiols programs the biosynthesis of lincomycin A, Nature 518 (7537) (2015) 115–119. [DOI] [PubMed] [Google Scholar]
- [200].Rosario-Cruz Z, Boyd JM, Physiological roles of bacillithiol in intracellular metal processing, Curr. Genet 62 (1) (2016) 59–65. [DOI] [PubMed] [Google Scholar]
- [201].Perera VR, Newton GL, Pogliano K, Bacillithiol: a key protective thiol in Staphylococcus aureus, Expert Rev. Anti-infect. Ther 13 (9) (2015) 1089–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Chandrangsu P, Dusi R, Hamilton CJ, Helmann JD, Methylglyoxal resistance in Bacillus subtÜis: contributions of bacillithiol-dependent and independent pathways, Mol. Microbiol 91 (4) (2014) 706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Kay KL, Hamilton CJ, Le Brun NE, Mass spectrometric studies of Cu(I)-binding to the N-terminal domains of B. subtilis CopA and influence of bacillithiol, J. Inorg. Biochem 190 (2019) 24–30. [DOI] [PubMed] [Google Scholar]
- [204].Ma Z, Chandrangsu P, Helmann TC, Romsang A, Gaballa A, Helmann JD, Bacillithiol is a major buffer of the labile zinc pool in Bacillus subtilis, Mol. Microbiol 94 (4) (2014) 756–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Posada AC, Kolar SL, Dusi RG, Francois P, Roberts AA, Hamilton CJ, Liu GY, Cheung A, Importance of bacillithiol in the oxidative stress response of Staphylococcus aureus, Infect. Immun 82 (1) (2014) 316–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Chi BK, Gronau K, Mader U, Hessling B, Becher D, Antelmann H, S-bacillithiolation protects against hypochlorite stress in Bacillus subtilis as revealed by transcriptomics and redox proteomics, Mol. Cell. Proteom. : MCP 10 (11) (2011) M111 009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Imber M, Loi VV, Reznikov S, Fritsch VN, Pietrzyk-Brzezinska AJ, Prehn J, Hamilton C, Wahl MC, Bronowska AK, Antelmann H, The aldehyde dehydrogenase AldA contributes to the hypochlorite defense and is redox-controlled by protein S-bacillithiolation in Staphylococcus aureus, Redox Biol. 15 (2018) 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Boylan JA, Hummel CS, Benoit S, Garcia-Lara J, Treglown-Downey J, Crane EJ 3rd, Gherardini FC, Borrelia burgdorferi bb0728 encodes a coenzyme A disulphide reductase whose function suggests a role in intracellular redox and the oxidative stress response, Mol. Microbiol 59 (2) (2006) 475–486. [DOI] [PubMed] [Google Scholar]
- [209].Harris DR, Ward DE, Feasel JM, Lancaster KM, Murphy RD, Mallet TC, Crane EJ 3rd, Discovery and characterization of a Coenzyme A disulfide reductase from Pyrococcus horikoshii. Implications for this disulfide metabolism of anaerobic hyperthermophiles, FEBS J. 272 (5) (2005) 1189–1200. [DOI] [PubMed] [Google Scholar]
- [210].Eggers CH, Caimano MJ, Malizia RA, Kariu T, Cusack B, Desrosiers DC, Hazlett KR, Claiborne A, Pal U, Radolf JD, The coenzyme A disulphide reductase of Borrelia burgdorferi is important for rapid growth throughout the enzootic cycle and essential for infection of the mammalian host, Mol. Microbiol 82 (3) (2011) 679–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Tsuchiya Y, Zhyvoloup A, Bakovic J, Thomas N, Yu BYK, Das S, Orengo C, Newell C, Ward J, Saladino G, Comitani F, Gervasio FL, Malanchuk OM, Khoruzhenko AI, Filonenko V, Peak-Chew SY, Skehel M, Gout I, Protein CoAlation and antioxidant function of coenzyme A in prokaryotic cells, Biochem. J 475 (11) (2018) 1909–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Tsuchiya Y, Peak-Chew SY, Newell C, Miller-Aidoo S, Mangal S, Zhyvoloup A, Bakovic J, Malanchuk O, Pereira GC, Kotiadis V, Szabadkai G, Duchen MR, Campbell M, Cuenca SR, Vidal-Puig A, James AM, Murphy MP, Filonenko V, Skehel M, Gout I, Protein CoAlation: a redox-regulated protein modification by coenzyme A in mammalian cells, Biochem. J 474 (14) (2017) 2489–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Grant CM, Quinn KA, Dawes IW, Differential protein S-thiolation of glycer-aldehyde-3-phosphate dehydrogenase isoenzymes influences sensitivity to oxidative stress, Mol. Cell. Biol 19 (4) (1999) 2650–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Imber M, Huyen NTT, Pietrzyk-Brzezinska AJ, Loi VV, Hillion M, Bernhardt J, Tharichen L, Kolsek K, Saleh M, Hamilton CJ, Adrian L, Grater F, Wahl MC, Antelmann H, Protein S-bacillithiolation functions in thiol protection and redox regulation of the glyceraldehyde-3-phosphate dehydrogenase Gap in Staphylococcus aureus under hypochlorite stress, Antioxidants Redox Signal. 28 (6) (2018) 410–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Kawano H, Otani M, Takeyama K, Kawai Y, Mayumi T, Hama T, Studies on ergothioneine. VI. Distribution and fluctuations of ergothioneine in rats, Chem. Pharm. Bull 30 (5) (1982) 1760–1765. [DOI] [PubMed] [Google Scholar]
- [216].Hanlon DP, Interaction of ergothioneine with metal ions and metalloenzymes, J. Med. Chem 14 (11) (1971) 1084–1087. [DOI] [PubMed] [Google Scholar]
- [217].Motohashi N, Mori I, Sugiura Y, Complexing of copper ion by ergothioneine, Chem. Pharm. Bull 24 (10) (1976) 2364–2368. [DOI] [PubMed] [Google Scholar]
- [218].Akanmu D, Cecchini R, Aruoma OI, Halliwell B, The antioxidant action of ergothioneine, Arch. Biochem. Biophys 288 (1) (1991) 10–16. [DOI] [PubMed] [Google Scholar]
- [219].Asmus KD, Bensasson RV, Bernier JL, Houssin R, Land EJ, One-electron oxidation of ergothioneine and analogues investigated by pulse radiolysis: redox reaction involving ergothioneine and vitamin C, Biochem. J 315 (Pt 2) (1996) 625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Arduini A, Eddy L, Hochstein P, The reduction of ferryl myoglobin by ergothioneine: a novel function for ergothioneine, Arch. Biochem. Biophys 281 (1) (1990) 41–43. [DOI] [PubMed] [Google Scholar]
- [221].Servillo L, Castaldo D, Casale R, D’Onofrio N, Giovane A, Cautela D, Balestrieri ML, An uncommon redox behavior sheds light on the cellular antioxidant properties of ergothioneine, Free Radic. Biol. Med 79 (2015) 228–236. [DOI] [PubMed] [Google Scholar]
- [222].Saini V, Cumming BM, Guidry L, Lamprecht DA, Adamson JH, Reddy VP, Chinta KC, Mazorodze JH, Glasgow JN, Richard-Greenblatt M, Gomez-Velasco A, Bach H, Av-Gay Y, Eoh H, Rhee K, Steyn AJC, Ergothioneine maintains redox and bioenergetic homeostasis essential for drug susceptibility and virulence of Mycobacterium tuberculosis, Cell Rep. 14 (3) (2016) 572–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Sao Emani C, Williams MJ, Van Helden PD, Taylor MJC, Carolis C, Wiid IJ, Baker B, Generation and characterization of thiol-deficient Mycobacterium tuberculosis mutants, Scientific data 5 (2018) 180184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Turner E, Hager LJ, Shapiro BM, Ovothiol replaces glutathione peroxidase as a hydrogen peroxide scavenger in sea urchin eggs, Science 242 (4880) (1988) 939–941. [DOI] [PubMed] [Google Scholar]
- [225].Holler TP, Hopkins PB, Ovothiols as biological antioxidants. The thiol groups of ovothiol and glutathione are chemically distinct, J. Am. Chem. Soc 110 (14) (1988) 4837–4838. [Google Scholar]
- [226].Morgan B, Sobotta MC, Dick TP, Measuring E(GSH) and H2O2 with roGFP2-based redox probes, Free Radic. Biol. Med 51 (11) (2011) 1943–1951. [DOI] [PubMed] [Google Scholar]